Summary
Background
Monomers of methacrylates used in restorative dentistry have been recently reported to induce DNA double-strand breaks (DSBs) in human gingival fibroblasts (HGFs) in vitro. Because such monomers may penetrate the pulp and oral cavity due to the incompleteness of polymerization and polymer degradation, they may induce a similar effect in vivo. DSBs are the most serious type of DNA damage and if misrepaired or not repaired may lead to mutation, cancer transformation and cell death. Therefore, the protection against DSBs induced by methacrylate monomers released from dental restorations is imperative.
Material/Methods
We examined the protective action of chitosan oligosaccharide lactate (ChOL) against cytotoxic and genotoxic effects induced by monomers of the model adhesive consisting of 55% bisphenol A-diglycidyl dimethacrylate (Bis-GMA) and 45% 2-hydroxyethyl methacrylate (HEMA). We evaluated the extent of DSBs by the neutral comet assay and the phosphorylation of the H2AX histone test.
Results
ChOL increased the viability of HGFs exposed to Bis-GMA/HEMA as assessed by flow cytometry. ChOL decreased the extent of DSBs induced by Bis-GMA/HEMA as evaluated by neutral comet assay and phosphorylation of the H2AX histone. ChOL did not change mechanical properties of the model adhesive, as checked by the shear bond test. Scanning electron microscopy revealed a better sealing of the dentinal microtubules in the presence of ChOL, which may protect pulp cells against the harmful action of the monomers.
Conclusions
ChOL can be considered as an additive to methacrylate-based dental materials to prevent DSBs induction, but further studies are needed on its formulation with the methacrylates.
Keywords: dental restorative material, methacrylates, DNA damage, DNA double-strand breaks, gamma-H2AX histone
Background
Methacrylate-based materials are commonly used in restorative and aesthetic dentistry, as well as in orthodontics. They are an essential part of dental fillings, in which they occur as polymers, formed in situ from their monomers. The most common monomers used in dentistry are 2-hydroxyethyl methacrylate (HEMA), bisphenol A-diglycidyl dimethacrylate (Bis-GMA), urethane dimethacrylate (UDMA), and triethylene glycol dimethacrylate (TEGDMA). The process of polymerization is never complete, resulting in the release of free monomers into the oral cavity. The monomers can be also released from polymers as a consequence of chewing and the action of some enzymes present in the oral cavity. Moreover, the monomers may also migrate to the pulp through the microtubules present in the dentin, from where they can drift further with the bloodstream, reaching other tissues and organs. The presence of free methacrylate monomers provoked a question regarding the biocompatibility of methacrylate-based dental materials. Many clinical observations and laboratory research clearly indicate the potential health hazard associated with the use of such materials. Genotoxicity of methacrylate monomers is of a special concern because it may result in serious health complications, including cancer.
DNA damage is a hallmark of genotoxicity, and the ability of methacrylate monomers to induce DNA damage has been shown in several studies [1–4]. DNA double-strand breaks (DSBs) are the most serious type of DNA damage, which, if non-repaired or misrepaired, can lead to chromosomal breakage, cancer transformation and cell death. Urcan et al showed that Bis-GMA, HEMA, TEGDMA and UDMA induced DSBs in human gingival fibroblasts in vitro[5]. They showed this by using what is probably the most sensitive and reliable assay for DSBs – the H2AX histone phosphorylation test. These results are of great importance, because they suggest that a significant part of the adult human population may be at risk of serious health problem due to the ability of methacrylate monomers, constantly released from dental restoration, to induce DSBs. It is not easy to assess an actual risk, and further research should be performed. However, it should be taken into account that only 1 DSB, if not repaired, may lead to a chromosomal rearrangement, production of fusion genes and synthesis of a fusion protein, which can support cancer transformation [6]. Non-repaired DSBs may also result in the inactivation of tumor suppressor genes, activation of proto-oncogenes and changes in the functioning of mutator genes, which may promote cancer transformation [7].
The risk associated with the potential of methacrylates to induce DSBs should be taken into account in the technology of dental materials. First of all, substances displaying a protective effect against DNA-damaging agents can be considered as additives to dental fillings and adhesives, but at least 2 issues must be addressed with such an approach. First, such a protective substance should be released at a rate adjusted to the rate of release of the monomers, which is variable and difficult to predict. Second, such a substance should be compatible with the filling and adhesive, and it should not change their mechanical properties. Several substances can be considered as candidates to protect against the DSBs-inducing potential of methacrylate monomers. Chitosan is a polymer obtained by deacetylation of chitin, an abundant natural polysaccharide occurring mainly in the cell walls of fungi and yeasts and the exoskeletons of insects and arthropods [8]. Chitosan is a non-toxic, biocompatible and biodegradable substance and displays many useful physicochemical and biological properties. It has been applied in several branches of medicine, including dentistry. It possess amino groups, which capture hydrogen ions, resulting in its overall positive charge facilitating adhesion to negatively charged surfaces, including tooth enamel [9]. Chitosan decreased the viability of Streptococcus mutans isolated in dental plaque [10]. As it displays bactericidal and bacteriostatic properties, it was applied in the prevention of dental caries and treatment of periodontosis [11–13]. It has been suggested that chitosan may act as a barrier against acid penetration of the dental enamel, inhibiting its demineralization [14]. It is also considered as a bone substitute in tissue engineering, but the results obtained so far are ambiguous [15,16]. Chitosan was reported to improve properties of a tooth-paste [17]. We recently showed that chitosan derivatives decreased the DNA-damaging effect of HEMA and UDMA, displaying antioxidant properties [2,4]. Chitosan is also used in dental filling materials to seal the tooth cavity [18].
In the present work we investigated the protective potential of chitosan oligosaccharide lactate (ChOL) against DSBs induced by monomers of a model methacrylate adhesive, consisting of 55% Bis-GMA and 45% HEMA (Bis-GMA/HEMA) with 8% water content, as proposed by Kostoryz et al. (Figure 1) [19]. We evaluated the extent of DSBs by the neutral comet assay and the phosphorylation of the H2AX histone test. Additionally, we evaluated the impact of ChOL on the mechanical properties of the model adhesive and its interaction with dentin.
Figure 1.
The structure of 2-hydroxyethyl methacrylate (HEMA), bisphenol A-diglycidyl dimethacrylate (Bis-GMA) and chitosan oligosaccharide lactate (ChOL).
Material and Methods
Chemicals
HEMA, Bis-GMA, ChOL, Gradisol and RNase A, low melting point (LMP) and normal melting point (NMP) agarose, phosphate buffered saline (PBS), DAPI (4′,6-diamidino-2-phenylindole), dimethyl sulfoxide (DMSO), fetal bovine serum (FBS), MTT, lectin, penicillin, streptomycin, and Bradford reagent were from Sigma Chemicals (St. Louis, MO, USA). Quantum 333 medium, Dulbecco’s phosphate buffered saline (DPBS), trypsin and EDTA were from PAA Laboratories GmbH (Cölbe, Germany). Methanol-free formaldehyde solution was from Thermo Fisher Scientific (Worcester, MA, USA). Mouse monoclonal anti-γ-H2AX primary antibody, 1:100 dilution, anti-phospho-histone H2A.X (Ser139) clone JBW301, was obtained from Upstate (Charlottesville, VA, USA). Alexa Fluor 488 secondary antibody, 1:100 dilution, conjugated goat anti-mouse IgG was from Molecular Probes (Eugene, OR, USA). Self-cured acrylic resin Duracrol was from Sofa-Dental (Prague, Czech Republic). Cell viability kit was purchased from BD Biosciences (San Jose, CA, USA). All other chemicals were of the highest commercial grade available.
Cells and treatment
Human gingival fibroblasts (HGFs) cell line was purchased from Provitro (Berlin, Germany). The cells were grown in Quantum 333 medium containing L-glutamine and supplemented with 1% antibiotic-antimycotic solution (10,000 Units/ml penicillin, 10 mg/ml streptomycin sulphate, 25 μg/ml amphotericin B) in 75 cm2 cell culture flasks to approximately 75–80% confluence and maintained in an incubator with 5% CO2 atmosphere at 100% humidity at 37°C. After reaching confluence, the cells were washed with DPBS, detached from the flasks by a brief treatment with 0.05% trypsin – 0.02% EDTA.
The model adhesive consisted of HEMA and Bis-GMA at 45/55% w/w with 8% water based on the total final weight of the mixture [19]. To obtain a well-mixed resin we applied extensive shaking and sonication. The mixture was diluted with the cells medium to the concentrations desired in the experiments on DNA damage. HGFs were exposed to Bis-GMA/HEMA mixture at appropriate concentrations for 6 hours at 37°C. In the experiment with ChOL, the exposure to Bis-GMA/HEMA was preceded by 1 hr incubation with ChOL at 37°C. After the incubation, the suspension of the cells was centrifuged to remove free ChOL. Each DNA damage experiment included a positive control, which was hydrogen peroxide at 20 μM for 15 minutes on ice [20]. In the H2AX histone phosphorylation experiment the concentration of hydrogen peroxide was 1 mM.
Cell viability
HGFs were washed 3 times with PBS and then diluted in PBS to a concentration of 2.5×105 cells/ml. For preparation of dead cells (positive control), 1 sample was treated with 96% ethanol for 1 minute. All samples were centrifuged and cell pellets were suspended in 100 μl of 0.5 μM calcein-acetoxymethyl ester (cal AM)/10 μM propidium iodine (PI) in PBS. Cells were gently shaken and incubated with the dyes for 30 minutes at 37°C in a tissue culture incubator and then analyzed on a LSRII flow cytometer (Becton Dickinson, San Jose, USA) equipped with 488 nm laser excitation and BD FACS Diva software v 4.1.2. Each experiment was repeated in triplicate and 5×104 cells were analyzed.
DNA double-strand breaks assay
We used the neutral comet assay to screen for DSBs in HGFs [21]. A freshly prepared suspension of HGFs in 0.75% LMP agarose dissolved in DPBS was spread onto microscope slides precoated with 0.5% NMP agarose. The cells were then lysed for 1 hour at 4°C in a buffer consisting of 2.5 M NaCl, 100 mM EDTA, 1% Triton X-100, 10 mM Tris, pH 10. After lysis, the slides were placed in an electrophoresis unit and the DNA was allowed to unwind for 40 minutes in the electrophoretic solution consisting of 100 mM Tris and 300 mM sodium acetate at pH adjusted to 9.0 by glacial acetic acid. Electrophoresis was conducted at 4°C for 20 minutes at electric field strength of 0.73 V/cm (29 mA). The slides were then treated with 0.4 M Tris, pH 7.5, stained with 2 μg/ml DAPI and covered with cover slips. The slides were examined at 200× magnification in an Eclipse fluorescence microscope (Nikon, Tokyo, Japan) attached to a COHU 4910 video camera (Cohu, Inc., San Diego, CA) equipped with a UV filter block consisting of an excitation filter (359 nm) and barrier filter (461 nm) and connected to a personal computer-based image analysis system, Lucia-Comet v. 4.51 (Laboratory Imaging, Praha, Czech Republic). Fifty images were randomly selected from each sample and the comet tail DNA was measured. Two parallel tests with aliquots of the same sample of cells were performed for a total of 100 cells. Each experiment was repeated 3 times. The percentage of DNA in the tail (% tail DNA) was analyzed and it positively correlated with DNA double-strand breaks.
The ability of the methacrylate monomers of the Bis-GMA/HEMA model adhesive to induce DSBs was confirmed and further analyzed by the immunofluorescence assay for the phosphorylation of the H2AX histone [22]. HGFs were grown to approximately 75–80% confluence in 6-well plates. The medium was changed 24 hours before incubation with the mixture with Bis-GMA at 100 μM. After the incubation, the cells were trypsinized with 500 μl trypsin-EDTA, washed with 1 ml medium and collected in 1.5 ml tubes. For immunofluorescent staining, cells (1–2×106) were washed in DPBS by centrifugation (300×g for 5 minutes at room temperature), fixed by 1 ml ice-cold 1% methanol-free formaldehyde in DPBS and incubated on ice for 15 minutes. Cells were centrifuged (300×g, 5 minutes, room temperature) and permeabilized with 80% ethanol in distilled water and kept at −20°C for 2 hours until further staining. Cells were then washed 3 times with 1% BSA/0.2% Triton X-100/PBS (BTP) solution and stained with mouse monoclonal anti γ-H2AX primary antibody and incubated overnight at 4°C. Then, HGFs were washed 3 times with BTP solution and incubated with Alexa Fluor 488 secondary antibody for 1 hour at room temperature in the dark. After the incubation, cells were washed in BTP and counterstained with propidium iodide (PI, 5 μg/ml in DPBS in the presence of 100 μg/ml of RNase A) and incubated for 30 minutes at room temperature in the dark. Cells stained with Alexa Fluor 488 and PI were analyzed using an LSRII flow cytometer (Becton Dickinson Biologicals, San Jose, CA, USA) by measuring the intensity of green (530±20 nm) and red (>600 nm) fluorescence of the cells. DNA content (red fluorescence of DNA-bound PI) was plotted on the x-axis and the level of γ-H2AX immunofluorescence (green fluorescence – Alexa Fluor 488) was plotted on the y-axis. Logarithmic Alexa Fluor 488 fluorescence was plotted versus linear PI fluorescence using FlowJo analysis software (TreeStar, Ashland, OR, USA). Untreated controls were used to set the threshold gating to determine the percentage of γ-H2AX-positive cells. Intensity of cellular γ-H2AX immunofluorescence measured by flow cytometry is positively correlated with the level of DSBs and was used to quantify their extent [23,24].
Determination of shear bond strength
A total of 150 bovine incisors were extracted, washed with PBS and stored in 1% chloramine-B-hydrate. Each tooth was mounted in a 2-cm tube with the Duracrol resin (Spofa Dental, Prague, Czech Republic). The occlusal dentin surface was ground with a 600 grit SiC abrasive paper to produce a clinically relevant smear layer. A solution of 37% orthophosphoric acid as an etchant was applied to the dentin surface for 15 seconds, rinsed for 30 seconds and gently dried. Next the HEMA/BisGMA adhesive formulated with water and ChOL content was applied to the dentin surfaces, gently spread with air to remove its excess and light cured for 30 seconds under a halogen light curing unit (Elipar™ 2500, 3M ESPE, St. Paul, MN, USA) with an output intensity of 600 mW/cm2. The specimens were then stored for 24 hours in 0.9 NaCl solution and subjected to a universal Zwick testing machine (Zwick-Roell Z005, Zwick-Roell GmbH, Ulm, Germany) operating at a crosshead speed of 1.0 mm/minute to determine the strength of shear bond of the composite mediated by the bonding system. The Bis-GMA/HEMA model adhesive was modified with ChOL or was not modified. A knife-edge chisel was attached to the upper part of the machine and tests were performed at the velocity of 1 mm/minute and the strength of shear bond (MPa) was read directly from a computer with TestXpert Zwick software.
Scanning electron microscopy
After completion of the test for shear bond strength, the surface of the teeth was analyzed by scanning electron microscopy (SEM) to investigate the fracture pattern. Small fragments of fractured surfaces produced after shear testing were used for macro-analysis of the entire interface. After obtaining the macro SEM pictures, the occlusal dentin surfaces were gently ground with a 600 grit SiC in order to visualize orifices to dentin tubules. For better observation of the dentin coating materials that penetrated the dentinal tubules, the tooth surface was resolved by 6 M hydrochloric acid for 6 hours and rinsed with distilled water to remove the inorganic phase. Next, the organic phase was removed by treatment with 10% sodium hypochlorite for 5 minutes. These procedures allowed for a better observation of dentin coating materials that penetrated dentinal microchannels. The samples were dried in vacuum and gold sputtered before mounting on the stub of a Jeol JSM-35C scanning electron microscope (Jeol Ltd., Tokyo, Japan).
Data analysis
The data obtained from cell viability were expressed as mean ±S.D. The values in DNA DSBs study were expressed as mean ±S.E.M. from 3 experiments (ie, the data from 3 experiments were pooled and the statistical parameters were calculated, whereas there were 5 replications in the shear bond strength measurements). The Mann-Whitney test was used to determine differences between samples with distributions departing from normality. The differences between samples with normal distribution were evaluated by the Student’s t-test. Bond strengths means were analyzed by one-way ANOVA and Schefee’s post-hoc multiple comparison test. Data analysis was performed using SigmaStat software (v. 3.0.0, SPSS, Chicago, USA).
Results
Bis-GMA/HEMA reduces cell viability and chitosan oligosaccharide lactate decreases this effect
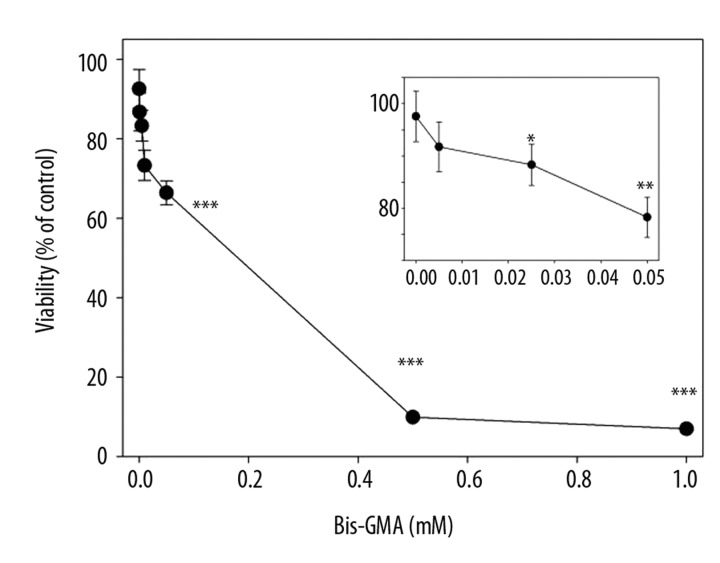
Bis-GMA/HEMA evoked a concentration-dependent decrease in the viability of HGFs with less than 10% of living cells at 0.5 and 1 mM (Figure 2). The EC50 value for this mixture was estimated to be 0.13 mM. In the next experiment, a 6-hour incubation with Bis-GMA/HEMA at 0.1 mM Bis-GMA was preceded by a 1-hour preincubation with ChOL at various concentrations. The results indicate a protective effect of ChOL against cytotoxic action of Bis-GMA/HEMA monomers (Figure 3). On the other hand, ChOL alone did not influence the viability of HGFs (Figure 3, inset).
Figure 2.
Viability of human gingival fibroblasts exposed for 6 h at 37°C to the mixture of methacrylate monomers containing 55% bisphenol A-diglycidyl dimethacrylate and 45% 2-hydroxyethyl methacrylate (w/w) (Bis-GMA/HEMA) in dependence on Bis-GMA concentration. The viability was measured by flow cytometry with thiazole orange and propidium iodide. Displayed is the mean of three experiments of 5×104 measurements each, error bars denote standard deviation. The inset presents the viability at low concentrations of Bis-GMA/HEMA. The radius of symbol is greater than the bars length at the highest concentrations of the mixture; *** p<0.001, ** p<0.01, * p<0.05 as compared with unexposed control.
Figure 3.
Viability of human gingival fibroblasts exposed for 6 h at 37°C to the mixture of methacrylate monomers containing 55% bisphenol A-diglycidyl dimethacrylate and 45% 2-hydroxyethyl methacrylate (w/w) (Bis-GMA/HEMA) at 0.1 mM Bis-GMA with or without a 1 h preincubation with chitosan oligosaccharide lactate (ChOL) at indicated concentrations. The viability was measured by flow cytometry with thiazole orange and propidium iodide. Displayed is the mean of three experiments of 5×104 measurements each, error bars denote standard deviation. The inset presents the viability of the cells exposed to ChOL singly. ** p<0.01, * p<0.05 as compared with control without preincubation with ChOL.
Bis-GMA/HEMA monomers induce DNA double-strand breaks and chitosan oligosaccharide lactate exerts a protective effect
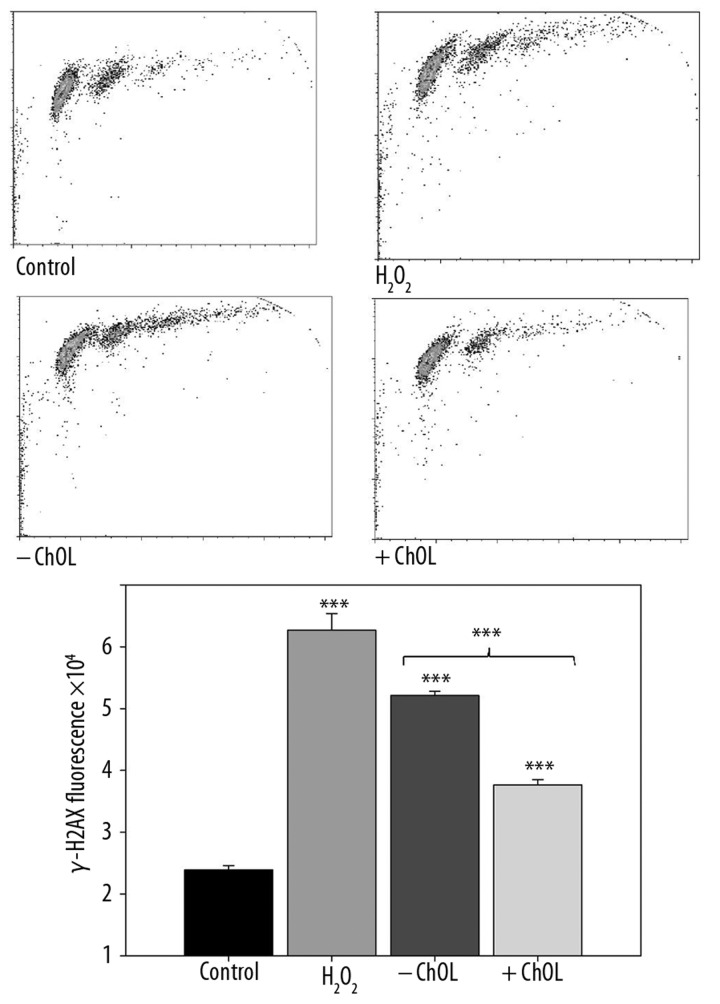
We observed a significant increase in the tail DNA in the comet of the neutral comet assay for all concentrations of Bis-GMA/HEMA monomers mixture (Figure 4). These results prompted us to employ the H2AX histone phosphorylation assay as a more reliable method to detect and quantify DSBs and assess the protective potential of ChOL against DSBs induced by the Bis-GMA/HEMA monomers. The results confirmed the ability of the methacrylates of the model adhesive at 0.2 mM Bis-GMA to induce DSBs and the protective action of 0.2% ChOL, which decreased DSBs-inducing effect of Bis-GMA/HEMA monomers (p<0.05) (Figure 5).
Figure 4.
DNA damage in human gingival fibroblasts exposed for 6 h at 37°C to the mixture of methacrylate monomers containing 55% bisphenol A-diglycidyl dimethacrylate and 45% 2-hydroxyethyl methacrylate (w/w) (Bis-GMA/HEMA) measured as percentage in the tail DNA in comets of neutral version of the comet assay in dependence on Bis-GMA concentration. The mean value for one hundred cells analyzed at each concentration in three independent experiments is displayed, error bars represent SEM, ** p<0.01, *** p<0.001 as compared with unexposed controls.
Figure 5.
DNA double strand breaks (DSBs) in human gingival fibroblasts exposed for 6 h at 37°C to the mixture of methacrylate monomers containing 55% bisphenol A-diglycidyl dimethacrylate and 45% 2-hydroxyethyl methacrylate (w/w) (Bis-GMA/HEMA) at 0.2 Bis-GMA with (+ ChOL) or without (– ChOL) 0.2% chitosan oligosaccharide lactate (ChOL) evaluated by the phosphorylation of the H2AX histone assay and compared with unexposed control (C, white bar). The intensity of fluorescence of the phosphorylated histone, γ-H2AX, is plotted and this quantity is positively correlated with the number of DSBs. Hydrogen peroxide at 1 mM was used as a positive control. The cells were incubated with appropriate antibodies, stained with Alexa Fluor and propidium iodine and analyzed by flow cytometry (upper diagrams). Error bars denote SEM, * p<0.05, ** p<0.01,*** p<0.001 as compared with unexposed control, an asterisk above the last pair of bars indicates the significance of the difference between effects induced by Bis-GMA/HEMA with and without ChOL.
Addition of chitosan oligosaccharide lactate does not change bond strength of the model adhesive
We observed a monotonic increase of shear bond strength with the increasing concentration of ChOL (Table 1). However, this increase was not significant (p>0.05). Therefore, ChOL did not improve mechanical properties of the model adhesive, but it did not worsen these properties.
Table 1.
Shear bond strength of the model dental adhesive consisting of 45% HEMA and 55% Bis-GMA modified or not with chitosan oligosaccharide lactate (ChOL) determined in the bovine teeth with the Zwick machine; presented is the mean ±SD for 30 specimen for each ChOL concentration.
| ChOL (%) | Shear bond strength (MPa, mean ±SD) |
|---|---|
| 0.0 | 14.00±3.18 |
| 0.1 | 14.27±2.74 |
| 0.2 | 14.96±2.20 |
| 0.3 | 15.53±2.34 |
| 0.5 | 15.59±2.06 |
Chitosan oligosaccharide lactate-modified model adhesive ensure better sealing of the dentin microtubules
Although ChOL did not change the shear bond strength of the model adhesive as evaluated by the Zwick machine, the ChOL-modified adhesive might differently interact with the dentin than the parental compound. Because dentin tubules are routes for the migration of released methacrylates, we were especially interested in the potential changes in their structure which might be induced by ChOL. SEM revealed that the ChOL-modified adhesive increased the sealing of the microtubules in a concentration-dependent manner (Figure 6). At the highest concentration of ChOL (0.5%) many tubules were completely sealed (6d).
Figure 6.

Debonded dentin surface of a model adhesive-treated specimen with or without modification with chitosan oligosaccharide lactate and after the shear bond strength test. The model adhesive was the mixture of methacrylate monomers containing 55% bisphenol A-diglycidyl dimethacrylate and 45% 2-hydroxyethyl methacrylate (w/w) (Bis-GMA/HEMA) with 8% of water. Presented is dentin surface non-exposed to any adhesive (A), exposed to the model adhesive only (B), exposed to the model adhesive modified with 0.2 (C) or 0.5% (D) chitosan oligosaccharide lactate.
Discussion
DNA double-strand breaks are the most serious type of DNA damage, which may result in chromosome aberrations, frequently producing a pathologic phenotype or cell death. Therefore, the potency of inducing DSBs by environmental factors or substances is of special importance, especially when this potency can be associated with chemicals which are of common, long-term use by a large portion of the population and are in close contact with humans. This feature has been attributed to methacrylate dental materials by Urcan et al. [5]. Due to the importance of the problem, there is an urgent need for research resulting in methods to reduce the chance of inducing DSBs by dental restorations.
In this study we showed that a chitosan derivative, ChOL, may reduce the extent of DSBs induced by mixture of HEMA and Bis-GMA, 2 methacrylates commonly used in restorative dental fillings. Chitosan oligosaccharides were reported to reduce lipolysaccharide-induced oxidative stress in mice [25]. This is in agreement with our results, as oxidative mechanisms underlie the effects of dental methacrylates [26]. Therefore, it seems that the use of chitosan in a suitable formulation may protect, at least in part, against DSBs induced by dental methacrylates.
We performed our research with relatively high concentrations of methacrylates. Although Schweikl et al suggested that local concentration in a human body of methacrylate monomers used in dentistry can be in the millimolar range [27], it is very difficult to estimate the actual local concentration anywhere in the body. In a recently published paper, Durner et al. questioned the possibility of high local concentrations of methacrylates originating from dental fillings [28]. According to their calculations, the realistic “worst case” concentration of Bis-GMA released from a commercial composite was 1.5 μM, a concentration 100-fold lower than the lowest observed concentration of Bis-GMA inducing DNA strand breaks in HGFs. However, their results were obtained after 24-hour incubation, whereas the real exposure may last dozen of years, increasing the probability of a DNA-damaging event. Genomic instability is associated with cancer development, but it can be induced with no evident dose response, similar to other non-targeted effects such as the bystander effects [29]. That is why we used higher concentrations of methacrylates than those which can be actually expected in the human body.
We performed an in vitro experiment, so it is important to assess the potential consequence of observed changes for the whole human body. However, this is a common problem in experimental toxicology. No clear evidence of the association of dental methacrylates with harmful health effects has been reported except for identifying them as occupational sensitizers with potential cross-reactivity in dental personnel and several other work-related harmful effects, including contact dermatitis [30,31]. The question about health consequences of in vitro genotoxic effects is a question of the carcinogenicity of these compounds and it cannot be answered at present. In an in vivo study, Di Pietro et al. [32] demonstrated the genotoxicity of dental restorative materials. It was suggested that the mechanisms of the observed effect were based on the production of reactive oxygen species able to damage DNA stimulated by those compounds, but these results cannot be directly transformed to clinically detected effects. No systematic research on cancer among dentists has been performed.
The results of our study indicate only a direction for further study and these results are not sufficient to draw a definite conclusion about the practical use of chitosan in clinical dentistry. First of all, we are not able to predict a protective effect of chitosan over a prolonged time. Methacrylate monomers can be released during the time their polymers are present in the tooth due to mechanical and enzymatic degradation. This process can last several dozen years and the local concentration of methacrylate monomers can be high enough to induce adverse biological effects. The highest local concentration of methacrylates is expected in the pulp and the oral cavity. The pulp cells can be affected by the monomers migrating through microtubules present in the dentine. This is possible due to the small size of the monomers. However, ChOL in the formulation we used in the present work is an oligomer and has an average of 5,000 Mn. The samples we used were a mixture of oligomers of various lengths and some parts of them might have a size exceeding the diameter of the dentin tubules. Therefore, a part of the ChOL molecules might have not been able to penetrate the pulp and protected pulp cells against DSBs induced by Bis-GMA/HEMA. Moreover, we do not know if ChOL is released from methacrylate-based chitosan-modified dental fillings. We expect that mechanical stress following from the chewing process and erosion may release both monomers and ChOL from such fillings. Methacrylate monomers contain ester groups, which may be targeted by esterases present in the saliva, resulting in the degradation of monomers and releasing the products of degradation into the oral cavity [19]. It seems that ChOL is resistant to esterases, which create an imbalance between the amount of released monomers and ChOL. Apart from enzymatic catalysis, degradation of dental composites may follow from a simple hydrolysis [33]. Therefore, each released molecule of methacrylate monomers or product of their degradation would be associated with the molecule(s) of a monomer of ChOL to protect against DSBs. This association would not necessarily be a binding between these 2 molecules resulting in their complex, but they should have a common target. The average diameter of the human dentinal tubules is 1–3 μm, allowing simultaneous passage of HEMA, Bis-GMA and ChOL [34]. However, such passage will be ensured if ChOL would contain only its monomers, so special formulation of ChOL should be prepared before using it as a protector in dental adhesives.
We observed a better sealing of dentin microtubules with the ChOL-modified adhesive than with the unmodified model adhesive (Figure 5). This may protect the pulp cells against methacrylate monomers and products of their degradation. It is also possible that ChOL may bind the monomers and other compounds, blocking or decreasing their transport into the pulp [35].
We do not know the actual mechanism underlying the protective action of ChOL against DSBs induced by HEMA or/and Bis-GMA. In particular, we do not know about the steric relationships occurring in such action. It may be carried out in a 1: 1 proportion, or 1 molecule of ChOL may protect against several molecules of HEMA or Bis-GMA. Sealing of dentinal microtubules with ChOL forms a barrier for methacrylates, preventing their pulp penetration and inducing any harmful effect. However, this process is probably caused by ChOL oligomers with a high number of monomers. If we assume that the protective effect of ChOL includes an equimolar interaction, then a monomer formulation of ChOL should be considered, but the problem of association of a ChOL molecule with Bis-GMA/HEMA remains. A physical joining of these molecules in a complex monomer for polymerization might be a solution, but the mechanism of such a complex is unknown. Making a nanoparticle containing methacrylate monomer could be considered, as nanoparticles are increasingly applied in various branches of medicine, including dentistry [36]. However, nanostructures are fundamentally different forms of matter than simple chemicals, and although chitosan may serve as a matrix for the assembly of nanoparticles, its action with methacrylates in the target sites may not be coordinated [37].
We prepared our model adhesive according to Kostoryz et al. [19], who formulated Bis-GMA/HEMA with various water contents. We formulated the adhesive with 8%, water content, although Kostoryz et al showed that the adhesive released smaller amount of HEMA with no water content compared to 8% and 15% water. However, water may be trapped within the methacrylate adhesive matrix during photopolymerization in the oral cavity or it can enter the matrix by diffusion into the loosely cross-linked or hydrophobic HEMA-rich domains [19]. The presence of water may facilitate the degradation of the adhesive and decrease its integrity and durability. However, water should not affect the protective effect of ChOL against DSBs induced by the components of the model adhesive.
Our study suggests the protective action of ChOL against DSBs, but the mechanism of this action is unknown. In general, we can consider 2 modes of ChOL activity leading to decreased ability of a chemical to induce DSBs. First, ChOL may interact with this chemical either directly, leading to its inactivation, or indirectly, with the involvement of cellular component(s). Second, ChOL may reduce the number of DSBs by stimulating or facilitating their repair. DSBs in human cells can be repaired mainly by non-homologous end joining (NHEJ) and homologous recombination (HRR). The third pathway, single-strand annealing (SSA), seems to play a minor role [38]. Briefly, NHEJ ligates together 2 DNA broken ends with little or no processing. Therefore, the structure of the ends, which are determined by its polarity (i.e., blunt, 5′- or 3′-single-strand overhang and the presence of various chemical residues) may be essential for entering the NHEJ pathway because some types of breaks cannot be joined in a simple ligation reaction. This structure is determined by the action of Bis-GMA/HEMA and its final form may be modulated by ChOL, which can also interact with main NHEJ proteins, Ku, DNA-PKCS, Artemis, Cernunnos, and XRCC4. The other main DSBs repair pathway, multi-protein HRR, uses an undamaged homologous DNA sequence as a template for accurate repair, which is initiated by a 5′ → 3′ degradation of 1 strand on both sides of the break. This reaction may be independent of the chemical nature of the end, so ChOL may not influence the efficacy of the repair by improving the substrate, but it may interact with its main proteins, RAD51/52, BRCA1/2, MDC1, as well as with the proteins of general reaction of the cell to DNA damage, ATM and ATR. All these speculations need to be experimentally verified in further research.
Conclusions
We confirmed the finding of Urcan et al. [5] that methacrylates used in dentistry are able to induce double-strand breaks in DNA, the most serious type of DNA damage, which, if not repaired or if misrepaired, may have a critical consequence for the cell and the whole organism. Chitosan oligosaccharide lactate may reduce the effect of the methacrylates without changing the mechanical properties of methacrylate-based composites. Further studies are needed to work out the formulation of a composite consisting of methacrylates and chitosan oligosaccharide lactate, ensuring their coordinated action.
Acknowledgements
We thank Anna Luczynska and Monika Kicinska for helping us preparing the manuscript and Dr. Poplawski for his help in the annotation of Figure 6.
Footnotes
Source of support: The study was supported by the grant N N403 188134 from the Polish Ministry of Science and Higher Education
References
- 1.Kleinsasser NH, Wallner BC, Harreus UA, et al. Genotoxicity and cytotoxicity of dental materials in human lymphocytes as assessed by the single cell microgel electrophoresis (comet) assay. J Dent. 2004;32:229–34. doi: 10.1016/j.jdent.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Pawlowska E, Poplawski T, Ksiazek D, et al. Genotoxicity and cytotoxicity of 2-hydroxyethyl methacrylate. Mutat Res. 2010;696:122–29. doi: 10.1016/j.mrgentox.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 3.Poplawski T, Pawlowska E, Wisniewska-Jarosinska M, et al. Cytytoxicity and genotoxicity of glycidyl methacrylate. Chem Biol Interact. 2009;180:69–78. doi: 10.1016/j.cbi.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Poplawski T, Loba K, Pawlowska E, et al. Genotoxicity of urethane dimethacrylate, a tooth restoration component. Toxicol in Vitro. 2010;24:854–63. doi: 10.1016/j.tiv.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Urcan E, Scherthan H, Styllou M, et al. Induction of DNA double-strand breaks in primary gingival fibroblasts by exposure to dental resin composites. Biomaterials. 2010;31:2010–14. doi: 10.1016/j.biomaterials.2009.11.065. [DOI] [PubMed] [Google Scholar]
- 6.Smith J, Tho LM, Xu N, et al. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res. 2010;108:73–112. doi: 10.1016/B978-0-12-380888-2.00003-0. [DOI] [PubMed] [Google Scholar]
- 7.Helleday T, Lo J, van Gent DC, et al. DNA double-strand break repair: from mechanistic understanding to cancer treatment. DNA Repair (Amst) 2007;6:923–35. doi: 10.1016/j.dnarep.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Aam BB, Heggset EB, Norberg AL, et al. Production of Chitooligosaccharides and Their Potential Applications in Medicine. Mar Drugs. 2010;8:1482–517. doi: 10.3390/md8051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pawlowska E. The assessment of influence of chitosan on the dental pulp in rats. In: Domard A, Roberts GAF, Varum KM, editors. Advances in chitin science. Lyon: Jacques Andre Publishers; 1997. pp. 705–10. [Google Scholar]
- 10.Pasquantonio G, Greco C, Prenna M, et al. Antibacterial activity and anti-biofilm effect of chitosan against strains of Streptococcus mutans isolated in dental plaque. Int J Immunopathol Pharmacol. 2008;21:993–97. doi: 10.1177/039463200802100424. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi Y, Ohara N, Ganno T, et al. Chewing chitosan-containing effectively inhibits the growth chariogenic bacteria. Arch Oral Biol. 2007;52:290–94. doi: 10.1016/j.archoralbio.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Fujiwara M, Hayashi Y, Ohara N. Inhibitory effect of watersoluble chitosan on growth of Streptococcus mutans. New Microbiol. 2004;27:83–86. [PubMed] [Google Scholar]
- 13.Akncbay H, Senel S, Ay ZY. Application of chitosan gel in the treatment of chronic periodontitis. J Biomed Mater Res B Appl Biomater. 2007;80:290–96. doi: 10.1002/jbm.b.30596. [DOI] [PubMed] [Google Scholar]
- 14.Arnaud TM, de Barros Neto B, Diniz FB. Chitosan effect on dental enamel de-remineralization: An in vitro evaluation. J Dent. 2010;38:848–52. doi: 10.1016/j.jdent.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Park JS, Choi SH, Moon IS, et al. Eight-week histological analysis on the effect of chitosan on surgically created one-wall intrabony defects in beagle dogs. J Clin Periodontol. 2003;30:443–53. doi: 10.1034/j.1600-051x.2003.10283.x. [DOI] [PubMed] [Google Scholar]
- 16.Spin-Neto R, Freitas RM, Pavone C, et al. Histological evaluation of chitosan-based biomaterials used for the correction of critical size defects in rat’s calvaria. J Biomed Mater Res. 2010;93A:107–14. doi: 10.1002/jbm.a.32491. [DOI] [PubMed] [Google Scholar]
- 17.Mohire NC, Yadav AV. Chitosan-based polyherbal toothpaste: as novel oral hygiene product. Indian J Dent Res. 2010;21:380–84. doi: 10.4103/0970-9290.70808. [DOI] [PubMed] [Google Scholar]
- 18.Lin JH, Lu PL, Lu CT, et al. Mechanical Property Evaluation of IRM®/Chitosan Fiber Composite Temporary Filling Material. Adv Mater Res. 2010;123:487–90. [Google Scholar]
- 19.Kostoryz EL, Dharmala K, Ye Q, et al. Enzymatic Biodegradation of HEMA/BisGMA Adhesives Formulated With Different Water Content. J Biomed Mater Res Part B: Appl Biomater. 2009;88B:394–401. doi: 10.1002/jbm.b.31095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tice RR, Agurell E, Anderson D, et al. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000;35:206–21. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 21.Singh NP, Stephens RE. Microgel electrophoresis: sensitivity, mechanisms and DNA electrostretching. Mutat Res. 1997;383:167–75. doi: 10.1016/s0921-8777(96)00056-0. [DOI] [PubMed] [Google Scholar]
- 22.Mah LJ, El-Osta A, Karagiannis TC. GammaH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia. 2010;24:679–86. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- 23.Rothkamm K, Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci USA. 2003;100:5057–62. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sedelnikova OA, Rogakou EP, Panyutin IG, et al. Quantitative detection of (125)IdU-induced DNA double-strand breaks with gamma-H2AX antibody. Radiat Res. 2002;158:486–92. doi: 10.1667/0033-7587(2002)158[0486:qdoiid]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 25.Qiao Y, Bai XF, Du YG. Chitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stress. Int Immunopharmacol. 2011;11:121–27. doi: 10.1016/j.intimp.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Chang MC, Chen LI, Chan CP, et al. The role of reactive oxygen species and hemeoxygenase-1 expression in the cytotoxicity, cell cycle alteration and apoptosis of dental pulp cells induced by BisGMA. Biomaterials. 2010;31:8164–71. doi: 10.1016/j.biomaterials.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 27.Schweikl H, Spagnuolo G, Schmalz G. Genetic and cellular toxicology of dental resin monomers. J Dent Res. 2006;85:870–77. doi: 10.1177/154405910608501001. [DOI] [PubMed] [Google Scholar]
- 28.Durner J, Debiak M, Burkle A, et al. Induction of DNA strand breaks by dental composite components compared to X-ray exposure in human gingival fibroblasts. Arch Toxicol. 2011;85:143–48. doi: 10.1007/s00204-010-0558-0. [DOI] [PubMed] [Google Scholar]
- 29.Streffer C. Strong association between cancer and genomic instability. Radiat Environ Biophys. 2010;49:125–31. doi: 10.1007/s00411-009-0258-4. [DOI] [PubMed] [Google Scholar]
- 30.Kanerva L. Cross-reactions of multifunctional methacrylates and acrylates. Acta Odontol Scand. 2001;59:320–29. doi: 10.1080/000163501750541200. [DOI] [PubMed] [Google Scholar]
- 31.Alanko K, Susitaival P, Jolanki R, et al. Occupational skin diseases among dental nurses. Contact Dermatitis. 2004;50:77–82. doi: 10.1111/j.0105-1873.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- 32.Di Pietro A, Visalli G, La Maestra S, et al. Biomonitoring of DNA damage in peripheral blood lymphocytes of subjects with dental restorative filling. Mut Res. 2008;650:115–22. doi: 10.1016/j.mrgentox.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 33.Santerre JP, Shajii L, Leung BW. Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Crit Rev Oral Biol Med. 2001;12:136–51. doi: 10.1177/10454411010120020401. [DOI] [PubMed] [Google Scholar]
- 34.Arends J, Stokroos I, Jongebloed WG, et al. The Diameter of Dentinal Tubules in Human Coronal Dentine after Demineralization and Air Drying. A Combined Light Microscopy and SEM Study. Caries Res. 1995;29:118–21. doi: 10.1159/000262052. [DOI] [PubMed] [Google Scholar]
- 35.Linden LA, Rabek J. Structures and mechanisms of formation of poly(acrylic acid)-iron(II and III)chloride gels in water and hydrogen peroxide. J Appl Polymer Sci. 1993;50:1331–41. [Google Scholar]
- 36.Chen MH. Update on dental nanocomposites. J Dent Res. 2010;89:549–60. doi: 10.1177/0022034510363765. [DOI] [PubMed] [Google Scholar]
- 37.Koev ST, Dykstra PH, Luo X, et al. Chitosan: an integrative biomaterial for lab-on-a-chip devices. Lab Chip. 2010;10:3026–42. doi: 10.1039/c0lc00047g. [DOI] [PubMed] [Google Scholar]
- 38.Hiom K. Coping with DNA double strand breaks. DNA Repair. 2010;9:1256–63. doi: 10.1016/j.dnarep.2010.09.018. [DOI] [PubMed] [Google Scholar]