Summary
Background
Astrocytic tumors are the primary brain tumors, which often progress to glioblastoma, a highly malignant neoplasm of the central nervous system. There is much new data regarding to the formation and progression of these tumors; however, glioblastoma remains one of the most fatal neoplasms in humans.
The aim of the study was to evaluate the role of c-erbB-2 protein expression in various groups of astrocytic tumors.
Material/Methods
65 cases of astrocytic tumors were divided into 3 groups: diffuse astrocytoma (group I; n=17 cases), anaplastic astrocytoma (group II; n=23 cases) and glioblastoma (group III; n=25 cases). C-erbB-2 protein expression was estimated semiquantitatively on immunohistochemically stained tissue sections using antibodies against c-erbB-2 protein. Statistical analysis was performed in all examined groups.
Results
The c-erbB-2 protein expression was observed in 15 out of 17 cases (88.3%) in group I, 22 out of 25 cases (88%) cases in group II, and in 19 out of 23 cases (82.6%) in group III. There were no statistically significant differences between the examined groups. The strongest c-erbB-2 immunoexpression was observed in low grade astrocytomas (diffuse astrocytomas G2); in the glioblastoma group the c-erbB-2 protein expression was weak and 17.4% of cases were negative.
Conclusions
C-erbB-2 protooncogene alteration is an early phenomenon in glial tumor development and progression.
Keywords: astrocytoma, glioblastoma, immunohistochemistry, c-erbB-2
Background
Astrocytic tumors are the most common primary glial tumors of the central nervous system, and are divided according to the degree of anaplasia and presence of necrosis into low grade astrocytomas (diffuse astrocytoma, pilocytic astrocytoma) or high grade astrocytomas (anaplastic astrocytoma, glioblastoma multiforme). Glioblastoma multiforme (GBM) represents 50% of all gliomas and 20% of all operated intracranial solid lesions. The median age for diagnosis for all primary brain tumors is 57 years, although GBMs are often diagnosed at slightly older ages. GBM is more common in men and Caucasians. Symptoms at presentation may be acute or subacute and include seizures, headaches, weakness or sensory disturbances, language dysfunction, changes in behavior or personality, or cognitive impairment. Magnetic resonance imaging is the preferred method for radiologic diagnosis, as these tumors are contrast enhancing, often with central necrosis and peritumoral edema. Imaging with positron emission tomography and magnetic resonance spectroscopy may allow for non-invasive grading of gliomas, but pathologic confirmation is needed in most cases [1–3].
The last decade has brought many advanced research discoveries such as the discovery of γ linolenic acid selective activity against tumor cells or the deciphering the role of cysteine protease inhibitors in brain tumor development [4,5]. Despite advances in therapeutic management of GBM, prognosis remains poor and the median overall survival rate is below 1 year. Even in the most favorable cases (young patients treated with radical surgery, radiotherapy and chemotherapy), death occurs in most cases within 2 years [1]. Several prognostic factors have been established: age, performance status, therapy (quality of surgery, radiotherapy, chemotherapy), and specific tumor characteristics such as location and nature (de novo or secondary to a low grade glioma; methylation of the MGMT gene promoter) [6–8]. At the time of tumor recurrence, treatment options are limited and survival is short. The infiltrative nature of GBMs may preclude repeat surgeries because of the risk of damage to adjacent areas [1,3,7,12].
HER-2/neu, also called c-erbB2 oncogene, is located on chromosome 17q21 and encodes for a 185 kD transmembrane glycoprotein with intracellular tyrosine kinase activity. The c-erbB-2 receptor belongs to the family of epidermal growth factor receptors that are crucial in the activation of subcellular signal transduction pathways controlling epithelial cell growth and differentiation [16]. Overexpression of c-erbB-2 protein is observed in 20% to 40% of breast cancers and other solid tumors and is generally associated with a poor prognosis. It has been reported to have a prognostic value in medulloblastoma and other neoplasms such as breast and lung cancers [9,10]. Recently, there have been some experimental approaches associated with anti c-erbB-2 receptor strategies as a gene therapeutic intervention in glioblastoma [9–12]. The c-erbB-2 protein was originally identified in the brain and is thought to play a critical role in neurogenesis. The overexpression of the c-erb protein appears to be correlated with aggressiveness of various tumors, including malignant gliomas [9,13,14].
The aim of the study was to evaluate c-erbB-2 protein expression in astrocytic tumors of various grades.
Material and Methods
We identified 65 patients with histopathological diagnosis of astrocytoma and glioblastoma. We retrospectively waived the 10% buffered formalin-fixed, paraffin-embedded sections. The patients were divided in 3 groups based on histopathologic diagnosis of their tumors: diffuse astrocytomas G2 (group I), anaplastic astrocytomas G3 (group II), glioblastomas G4 (group III) based on the criteria accepted by WHO (based on WHO Classification of tumors of the central nervous system by Louis DN et al Lyon, 2007).
Evaluation of c-erbB-2 protein expression was performed using immunohistochemical method. Following deparaffinization and rehydration, epitope retrieval was carried out in the EnVision Flex Target Retrieval Solution (DAKO) in high pH. Endogenous peroxidases were blocked by incubating the sections in methanol and 30% hydrogen peroxidase for 20 minutes. Next, slides were incubated with polyclonal antibody against c-erbB-2 protein (Novocastra NCL-c-erbB-2316) in 1:50 dilution overnight in 4°C. Visualization reagent (ABC Kit, Novostain Super) was applied for 30 minutes followed by DAB solution for 5 minutes. The slides were than counterstained with hematoxylin and examined under light microscope. Immunohistochemical evaluation of protein expression was performed by 2 independent pathologists. The intensity of immunostaining was evaluated in 10 random fields under 20× magnification. The results were expressed as the percentage of cells with a strong positive membrane staining as follows: ≤10% positive cells- negative (−), between 11–50% (+), and ≥51% positive cells (++) [3,9,10].
Appropriate positive and negative controls were performed. Negative controls were performed using a nonimmunized IgG replacing the primary antibody. Known c-erbB-2 protein expressing breast cancer and lymph node with metastatic disease were used as positive controls. The c-erbB-2 protein expression was evaluated and compared in all of the groups and the intensity of the staining was related to the nonneoplastic cerebral tissue containing neocortex and adjacent white matter (Figure 1).
Figure 1.
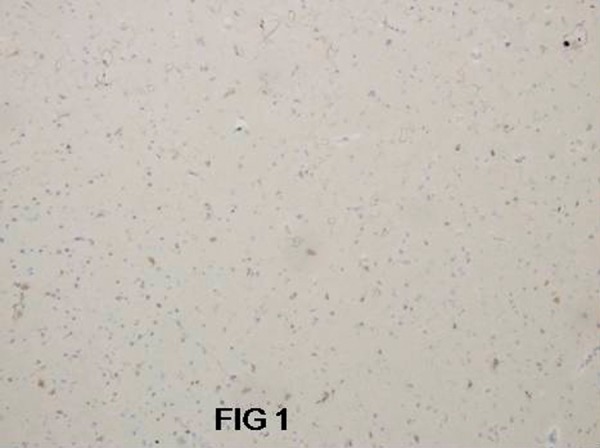
Nonneoplastic cerebral tissue with negative c-erbB-2 immunoexpression. Magn 200×.
Statistical analysis
Chi squared and Pearson’s correlation tests and Statistica 8.0 StatSoft software were used for statistical analysis. Values of p<0.05 were considered as statistically significant.
Results
Among 65 patients, 17 (26.1%) had diagnosis of diffuse astrocytoma (group I), 25 (38.5%) had anaplastic astrocytoma (group II) and 23 (35.4%) had a diagnosis of glioblastoma (group III). In the glioblastoma group, 19/23 patients had previous history of low grade astrocytic tumors. Demographic features and immunohistochemical findings of 65 patients are summarized in Table 1.
Table 1.
The c-erbB-2 protein expression in gliomas cases (p≤0.05).
| Feature | Astrocytoma G2 | Anaplastic astricytoma G3 | Glioblastoma G4 | P value | |||
|---|---|---|---|---|---|---|---|
| Age | 49.19 | 54.08 | 62.39 | P=0.0146 | |||
| Sex | Male 13 76.5% |
Female 4 23.5% |
Male 16 36.0% |
Female 9 64.0% |
Male 13 43.5% |
Female 10 56.5% |
P=0.426 |
| Negative for c-erbB-2 staining | 2/17 | 11.8% | 3/25 | 12% | 4/23 | 17.4% | P=0.829 |
| (+) positive for c-erbB-2 staining | 7/17 | 41.2% | 12/25 | 48% | 11/23 | 47.8% | P=0.892 |
| (++) for c-erbB-2 staining | 8/17 | 47.1% | 10/25 | 40% | 8/23 | 34.8% | P=0.736 |
In mean ages were 49.19 years in group I, 54.08 in group II, and 62.39 in group III. There was statistically significant correlation between age (p=0.0146) and sex (p=0.0426) in all 3 examined groups.
Diffuse astrocytomas (G2) occurred mainly in younger and in male patients, compared to glioblastoma which more commonly occurred in older patients.
In group I, c-erbB-2 protein expression was observed in 15/17 cases (88.3%); 7 (41.2%) were (+) positive, and 8 (47.1%) cases were (++) positive (Figure 2). In 8 cases the immunohistochemical reaction was very intense in 80–90% of the neoplastic cells.
Figure 2.
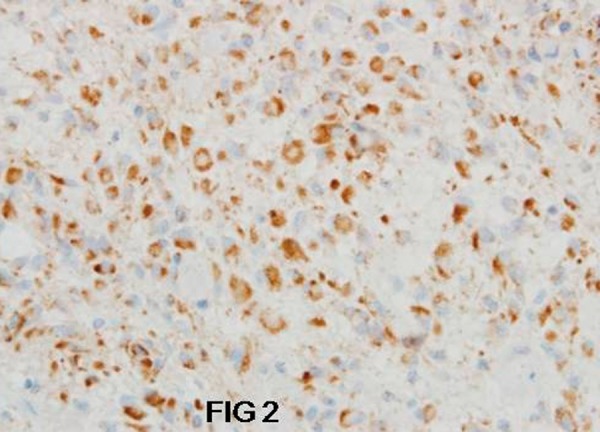
Strong positive c-erbB-2 immunoexpression in neoplastic cells of astrocytoma G2. Magn 400×.
In group II, c-erbB-2 protein expression was estimated in 22/25 cases (88%); 12 (48%) were (+) positive, and 10 (40%) were (++) positive (Figure 3) with strong membrane and cytoplasmatic granular reaction. The immunohistochemical staining was seen in 70–80% of the neoplastic cells.
Figure 3.
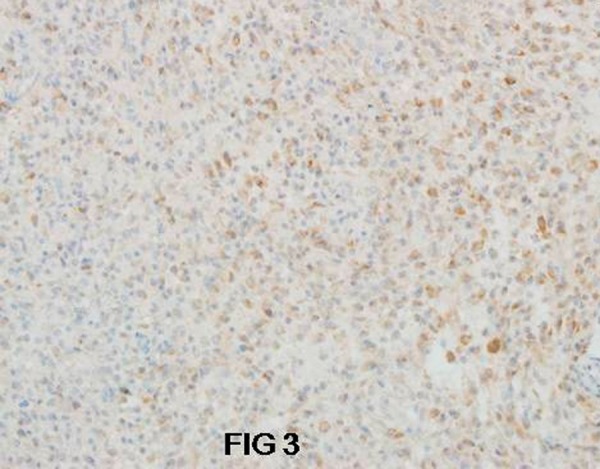
Diffused c-erbB-2 immunoexpression in anaplastic astrocytoma G3. Magn 200×.
In group III, c-erbB-2 protein expression was observed in 19/23 cases (82.6%); however, 11 (47.8%) were (+) positive for c-erbB-2 protein and only 8 (34.8%) tumors were (++) positive) (Figure 4). Even among this group only 50–60% of the neoplastic cells were positive for c-erbB-2 protein. It is worth to notice that in younger patients (23–48 years) with glioblastoma (12/23 cases) all cases were c-erbB-2 positive.
Figure 4.
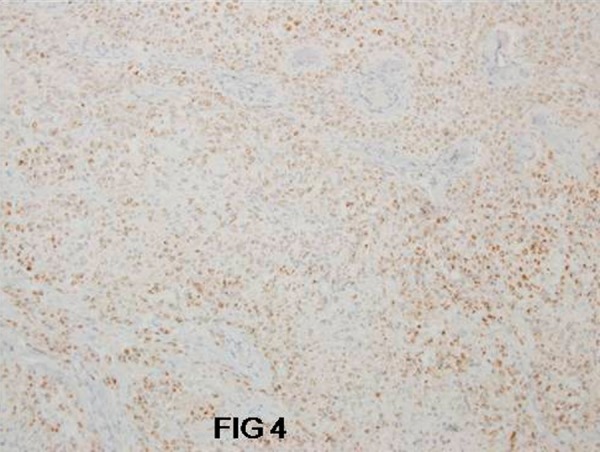
Diffused c-erbB-2 immunoexpression in glioblastoma G4. Magn 100×.
Comparing the percentage of all glial tumors cases negative for c-erbB-2 protein expression with the percentage of glial tumors with the strongest immunoreaction, it was discovered that the most evident positive staining was observed mainly in diffuse astrocytomas (47.1%), similar to anaplastic astrocytomas (40%), and less intensive in glioblastoma cases (34.8%). Only secondary glioblastomas (arising from low grade astrocytoma) were positive for c-erbB-2 protein, and all of the primary (arising “de novo”) glioblastoma were negative for c-erbB-2 protein.
Discussion
c-erbB-2 expression was the most significant in diffuse astrocytomas (G2) and anaplastic astrocytomas (G3), and less significant in glioblastoma (G4) cases.
In the glioblastoma group the intensity of the staining and the percentage of the positive cells were more suggestive in younger patients, although it was not statistically significant (p=0,829). In the glioblastoma group most cases showed weak positive immunostaining, estimated as (+) positive, and 17.4% of glioblastoma cases were negative for c-erbB-2 expression.
Astrocytic tumors are the most common primary central nervous system tumor classified according to tumor cell type and histological grade. For newly diagnosed patients with glioblastoma the standard treatment is maximal surgical resection followed by radiotherapy (RT). Chemotherapy is often used during or after RT. Recent data suggest that combining temozolomide with RT significantly improves median survival when compared with RT alone (from 12.1 to 14.6 months); however, survival still remains poor [1,8,15,16,18]. Recent studies suggest that aberrations of c-erbB-2 may be involved in astrocytic brain tumor development and progression, and its expression might be potentially significant owing to the role of recombinant monoclonal anti-HER-2/neu antibody trastuzumab (Herceptin) in the treatment of tumors [3,11,12,17,21].
There is a tremendous amount of recent literature on gene and microarrays on glial (including astrocytic) tumors, and many have presented EGFR, VEGF, and other markers as therapeutic targets; nevertheless, there are many inconsistencies, resulting in poor prognosis and failure of treatment in patients with primary brain tumors [6,7,15,19,20].
Similar to the results of the present study, Bian et al. [15] suggested that aberrations of c-erbB-2 might be merely an early event in the initiation and progression of astrocytomas, presenting, similarly to our results, the strongest c-erbB-2 expression in low grade astrocytomas. Happasalo et al. [16] observed negative immunoexpression for c-erbB2 protein in all 94 astrocytic neoplasms, similar to the results of Haynik et al. [10], who estimated all 49 glioblastoma tumors as negative for c-erbB-2 protein reaction, Torp et al. [14] who evaluated c-erbB-2 protein expression in 9 of 21 glioblastoma tumors (43%) and Duhem-Tonnelle et al. [17], who suggested that only erbB-3 may represent a new potential therapeutic target to gliomas.
Our results differ from those presented by Kristt et al. [13], who observed increased c-erbB-2 protein expression in glioblastomas, similarly to Liu et al. [2], who estimated c-erbB-2 expression in 76% of glioblastomas. The important role of c-erbB-2 expression in gliomas is also confirmed by Giles et al. [20] and Gao [21] et al., who observed that inhibition of c-erbB-2 greatly decreased glioma cell survival after irradiation, concluding that simultaneous inhibition of c-erbB-2 expression can promote potent antitumor activity and radiosensitizing activity in human glioma.
The results of our study show c-erbB-2 protein expression as an early phenomenon in astrocytic tumor development, as the strongest c-erbB-2 protein expression was observed in diffuse astrocytomas compared to the other examined glial tumors. Secondary glioblastomas, which in our study occurred mostly in younger patients, also showed very strong c-erbB-2 immunoreaction compared to primary glioblastomas. This might be also be evidence for a role of c-erbB-2 disorder in progression of astrocytic tumors. The c-erbB-2 protein expression differences between primary and secondary glioblastoma in our study might suggest that secondary glioblastoma development is associated with c-erbB-2 protein expression, but there are no clear data to confirm this suggestion.
Our research will be continued with a larger group of the patients and we will attempt to confirm the presented results using ISH methods.
Footnotes
Source of support: Departmental sources
References
- 1.Adamson C, Kanu OO, Mehta AI, et al. Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs. 2009;18:1061–83. doi: 10.1517/13543780903052764. [DOI] [PubMed] [Google Scholar]
- 2.Liu G, Ying H, Zeng G, et al. HER-2, gp100, and MAGE-1 are expressed in human glioblastoma and recognized by cytotoxic T cells cancer. Res. 2004;64:4980–86. doi: 10.1158/0008-5472.CAN-03-3504. [DOI] [PubMed] [Google Scholar]
- 3.Mineo JF, Bordron A, Baroncini M, et al. Low HER2-expressing glioblastomas are more often secondary to anaplastic transformation of low-grade glioma. J Neurooncol. 2007;85:281–87. doi: 10.1007/s11060-007-9424-1. [DOI] [PubMed] [Google Scholar]
- 4.Berdowska I, Siewiński A, Zarzycki-Reich A, et al. Activity of cysteine protease inhibitors in human brain tumors. Med Sci Monit. 2001;7(4):675–79. [PubMed] [Google Scholar]
- 5.Das UN. γ-linolenic acid therapy of human glioma-a review of in vitro, in vivo, and clinical studies. Med Sci Monit. 2007;13(7):RA119–31. [PubMed] [Google Scholar]
- 6.Tabatabai G, Stupp R, van den Bent MJ, et al. Molecular diagnostics of gliomas: the clinical perspective. Acta Neuropathol. 2010;120:585–92. doi: 10.1007/s00401-010-0750-6. [DOI] [PubMed] [Google Scholar]
- 7.Dunbar E, Yachnis AT. Glioma diagnosis: immunohistochemistry and beyond. Adv Anat Pathol. 2010;17:187–201. doi: 10.1097/PAP.0b013e3181d98cd9. [DOI] [PubMed] [Google Scholar]
- 8.Rivera AL, Pelloski CE, Gilbert MR, et al. MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro Oncol. 2010;12:116–21. doi: 10.1093/neuonc/nop020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lammering G, Hewit TH, Lin PS, et al. Anti-erB receptor strategy as a gene therapeutic intervention to improve radiotherapy in malignant humans tumor. Int J Radiat Biol. 2003;79:561–68. doi: 10.1080/0955300031000102632. [DOI] [PubMed] [Google Scholar]
- 10.Haynik DM, Roma AA, Prayson RA. HER-2/neu expression in glioblastoma multiforme. Appl Immunohistochem Mol Morphol. 2007;15:56–58. doi: 10.1097/01.pai.0000213133.09160.da. [DOI] [PubMed] [Google Scholar]
- 11.Potti A, Forseen SE, Koka VK, et al. Determination of HER-2/neu overexpression and clinical predictors of survival in a cohort of 347 patients with primary malignant brain tumors. Cancer Invest. 2004;22:537–44. doi: 10.1081/cnv-200026523. [DOI] [PubMed] [Google Scholar]
- 12.Raizer JJ. HER1/EGFR tyrosine kinase inhibitors for the treatment of glioblastoma multiforme. J Neuro-Oncology. 2005;74:77–86. doi: 10.1007/s11060-005-0603-7. [DOI] [PubMed] [Google Scholar]
- 13.Kristt DA, Yarden Y. Differences between phosphotyrosine accumulation and Neu/ErbB-2 receptor expression in astrocytic proliferative processes. Implications for glial oncogenesis. Cancer. 1996;78:1272–83. doi: 10.1002/(SICI)1097-0142(19960915)78:6<1272::AID-CNCR16>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 14.Torp SH, Gulati S, Johannessen E, Dalen A. Coexpression of c-erbB 1-4 receptor proteins in human glioblastomas. An immunohistochemical study. J Exp Clin Cancer Res. 2007;26:353–59. [PubMed] [Google Scholar]
- 15.Bian XW, Shi JQ, Liu FX. Pathologic significance of proliferative activity and oncoprotein expression in astrocytic tumors. Anal Quant Cytol Histol. 2000;22:429–37. [PubMed] [Google Scholar]
- 16.Haapasalo H, Hyytinen E, Sallinen P, et al. c-erbB-2 in astrocytomas: infrequent overexpression by immunohistochemistry and absence of gene amplification by fluorescence in situ hybridization. Br J Cancer. 1996;73:620–23. doi: 10.1038/bjc.1996.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duhem-Tonnelle V, Bieche I, Vacher S, et al. Differential distribution of erbB receptors in human glioblastoma multiforme: expression of erbB3 in CD133-positive putative cancer stem cells. J Neuropathol Exp Neurol. 2010;69:606–22. doi: 10.1097/NEN.0b013e3181e00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westphal M, Meima L, Szonyi E, et al. Heregulins and the ErbB-2/3/4 receptors in gliomas. J Neurooncol. 1997;35:335–46. doi: 10.1023/a:1005837122181. [DOI] [PubMed] [Google Scholar]
- 19.Andersson U, Schwartzbaum J, Wiklund, et al. A comprehensive study of the association between the EGFR and ERBB2 genes and glioma risk. Acta Oncol. 2010;49:767–75. doi: 10.3109/0284186X.2010.480980. [DOI] [PubMed] [Google Scholar]
- 20.Giles KM, Barker A, Zhang PM, et al. MicroRNA regulation of growth factor receptor signaling in human cancer cells. Methods Mol Biol. 2011;676:147–63. doi: 10.1007/978-1-60761-863-8_11. [DOI] [PubMed] [Google Scholar]
- 21.Gao L, Li F, Dong B, et al. Inhibition of STAT3 and ErbB2 suppresses tumor growth, enhances radiosensitivity, and induces mitochondria-dependent apoptosis in glioma cells. Int J Radiat Oncol Biol Phys. 2010;77:1223–31. doi: 10.1016/j.ijrobp.2009.12.036. [DOI] [PubMed] [Google Scholar]


