Abstract
The filamentous fungus A. niger is a widely used strain in a broad range of industrial processes from food to pharmaceutical industry. One of the most intriguing and often uncontrollable characteristics of this filamentous organism is its complex morphology. It ranges from dense spherical pellets to viscous mycelia (Figure 1). Various process parameters and ingredients are known to influence fungal morphology 1. Since optimal productivity correlates strongly with a specific morphological form, the fungal morphology often represents the bottleneck of productivity in industrial production.
A straight forward and elegant approach to precisely control morphological shape is the addition of inorganic insoluble micro particles (like hydrous magnesium silicate, aluminum oxide or titanium silicate oxide) to the culture medium contributing to increased enzyme production 2-6. Since there is an obvious correlation between micro particle dependent morphology and enzyme production it is desirable to mathematically link productivity and morphological appearance. Therefore a quantitative precise and holistic morphological description is targeted.
Thus, we present a method to generate and characterize micro particle dependent morphological structures and to correlate fungal morphology with productivity (Figure 1) which possibly contributes to a better understanding of the morphogenesis of filamentous microorganisms.
The recombinant strain A. niger SKAn1015 is cultivated for 72 h in a 3 L stirred tank bioreactor. By addition of talc micro particles in concentrations of 1 g/L, 3 g/L and 10 g/L prior to inoculation a variety of morphological structures is reproducibly generated. Sterile samples are taken after 24, 48 and 72 hours for determination of growth progress and activity of the produced enzyme. The formed product is the high-value enzyme β-fructofuranosidase, an important biocatalyst for neo-sugar formation in food or pharmaceutical industry, which catalyzes among others the reaction of sucrose to glucose 7-9. Therefore, the quantification of glucose after adding sucrose implies the amount of produced β-fructofuranosidase. Glucose quantification is made by a GOD/POD-Assay 10, which is modified for high-throughput analysis in 96-well micro titer plates.
Fungal morphology after 72 hours is examined by microscope and characterized by digital image analysis. In doing so, particle shape factors for fungal macro morphology like Feret's diameter, projected area, perimeter, circularity, aspect ratio, roundness und solidity are calculated with the open source image processing program ImageJ. Relevant parameters are combined to a dimensionless Morphology number (Mn) 11, which enables a comprehensive characterization of fungal morphology. The close correlation of the Morphology number and productivity are highlighted by mathematical regression.
Keywords: Immunology, Issue 61, morphology engineering, Morphology number (Mn), filamentous fungi, fructofuranosidase, micro particles, image analysis
Protocol
1. Reactor Setup and Start of Cultivation
4 bioreactor cultivations in total are conducted.
Use a 3 L stirred tank bioreactor with a working volume of 2.2 L for the cultivation of A. niger SKAn1015.
Pour 72.6 g glucose monohydrate into the reactor and fill it up with 1.9 L deionized water.
Install the reactor equipment such as 3 baffles, a two six-bladed disk turbine impeller, a pH electrode, a gas inlet with air filter, a cooling finger, an exhaust air cooler with air filter, an immersion tube for sterile sampling with air filter and inlet hoses for medium, inoculum, acid and base.
Autoclave the reactor at 121 °C for 20 min.
Connect the reactor with the acid-base reservoir (2 M HCl, 2 M NaOH) and the corresponding pumps and set up a pH-value of 5.0 ± 0.05 with the pH control unit.
Link the reactor with the cooling-water system and put the heating jacket around the reactor vessel.
Install the temperature sensor with corresponding control unit and set up a temperature of 37 ± 0.1 °C at the temperature control unit.
Fit the agitation motor atop of the reactor and bring it into service with an agitation speed of 200 min-1.
Add the sterile minimal growth medium11 trough one of the sterile inlet hoses (250 mL). For medium preparation all components were autoclaved at 121 °C for 20 min and mixed together. For three cultivations the components include talc powder (3MgO•4SiO2•H2O) in the concentrations of 1 g/L (reactor 2), 3 g/L (reactor 3) and 10 g/L (reactor 4). Prior to use, re-suspend the micro particles in 50 mM sodium acetate buffer (pH 6.5) and add it to the sterile medium. In control cultures (without particles) replace the micro particle suspension by 50 mM sodium acetate buffer (pH 6.5).
Add the spore suspension (inoculum) as prepared in Wucherpfennig et. al (2011)11 trough one of the sterile inlet hoses (50 mL) so that the concentration of spores amounts to 1X106 mL-1. The inoculation marks the start of cultivation (h = 0 h).
Start the aeration with a rate of 1.0 L min-1.
2. Sterile Sampling after 24, 48 and 72 h of Cultivation
Take 50 mL of sterile culture broth into a flacon tube.
Use sample for determination of biomass dry weight, β-fructofuranosidase activity and microscopic analysis.
3. Determination of the Biomass Dry Weight after 24, 48 and 72 h of Cultivation
Biomass samples are to be taken at least in duplicate.
Weight a cellulose filter with a micro scales after drying in the desiccator and place the filter in a Büchner funnel with connected water-jet vacuum pump.
Filter a defined sample volume (e. g. 10 mL) and rinse the filter with 10 mL deionized water to remove medium compounds from the biomass.
Wrinkle the filter once in the middle, place it in a glass Petri dish and put it into a compartment dryer until weight constancy (at least 24 h).
Cool the filter in a desiccator and measure the weight.
Calculate the biomass dry weight as the difference between the weight of the filter with and without dried biomass divided by the used sample volume.
4. Determination of the Extracellular Enzymatic Activity of β-fructofuranosidase by GOD/POD-essay after 24, 48 and 72 h of Cultivation
Store the samples on ice constantly while working with them.
Filter 1.5 mL culture suspension trough a cellulose acetate filter by pressing the culture suspension with a syringe through the filter into a reaction tube. Store the reaction tube at -20 °C until usage.
For the reaction mixture use 20 μL sample and add 200 μL of 1.65 M sucrose dissolved in 0.05 M phosphate buffer (pH 5.4) to initiate the reaction from sucrose to glucose. Cary out the reaction at least in duplicate.
Incubate the reaction mixture at 40 °C for 20 min in a heating block. Stop the reaction by incubating at 95 °C for 10 min in a heating block. Cool the reaction mixture by storing it on ice and spin down the condensed water by centrifuge at 13.000 g for 10 min at 4 °C.
To account the pH and temperature dependent cleavage of sucrose to glucose, carry out a blank value by using 20 μL deionized water instead of 20 μL sample.
To account for residual glucose in the culture broth, carry out a negative control for each sample. To that end use 20 μL of the sample and inactivate β-fructofuranosidase by heating at 95 °C for 10 min prior to add the sucrose and incubate as described in step 4.3.
Dilute the samples such that the following measured adsorption is in the calibrated value range.
Carry out all enzymatic assays in triplicate. Apply 2 μL diluted sample in a micro titer plate well. Apply a standard range for calibration on the micro titer plate by each using 2 μL of ten glucose solutions with ten different know concentrations (from 1 mM to 15 mM) instead of 2 μL sample. For zero point calibration use 2 μL deionized water instead of 2 μL sample.
Add 200 μL of the reagent solution to each well by using a multi pipette.
Incubate the mixture for 10 min at room temperature.
Store the micro titer plate at 6 °C until measurement (a few hours at most).
Measure the absorption at 450 nm using a 96-well Sunrise micro plate reader and the Magellan data retrieval software. Set the mixing time up to 5 sec. and the rest period up to 1 sec.
Open the result chart with a spreadsheet and construct a calibration line by using the standard range: glucose concentration = a X absorption + b
Calculate the activity: activity = (absorption X dilution factor-blank value ) X a+b
Figure out the β-fructofuranosidase activity by calculating the difference between the activities of the sample and the appropriate negative control.
Calculate the specific productivity for following use by taking into account biomass dry weight and β-fructofuranosidase activity 11.
5. Microscopy and Automated Image Analysis after 72 h of Cultivation
Place around 3 mL culture suspension in a plastic Petri dish and dilute them with physiological sodium chloride solution until the morphological structures are separated.
Situate the Petri dish under a microscope, which features an integrated or connected camera. Acquire and save about 100 images (Figure 2) of morphological structures per sample. Pay attention that on each image at least one object is completely pictured.
Open all images of the same sample with the image processing program ImageJ12. Convert the images to black and white by using the process tool "Make Binary" (Figure 2). For applying the command to the whole series of images use a macro code as follows. run(,,Make Binary")
Open the processed binary images with ImageJ again. Calculate the shape factors Feret's diameter, projected area, perimeter, circularity, aspect ratio, roundness und solidity for every image with the analyze tool "Set Measurement". For applying the command to a series of images use a macro code as follows. run("8-bit"); run("Make Binary"); run("Set Scale...", "distance=X known=1000 pixel=1 unit=μm global"); run("Set Measurements...", "area perimeter shape feret's limit display redirect=None decimal=3"); run("Analyze Particles...", "size=10000-Infinity circularity=0.00-1.00 show=Outlines display"); Determine X as the number of pixels which correlates to 1000 μm by constructing a straight line across a scale bar. Correlate the number of pixels of the straight line with the length of the scale bar in μm.
Open the result chart containing shape factor values for each image with a spreadsheet. Calculate a Morphology number as follows for each image.
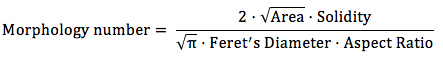
Calculate mean value and standard deviation for the Morphology number taking into account all images of one sample.
Use a graphing and data analysis program for graphical correlation of the Morphology number with the specific productivity and determine the mathematical relation by mathematical regression.
6. Representative Results
Through addition of talc micro particles A. niger SKAn 1015 morphology is changed from a true pellet morphology to a dispersed or even mycelial morphology. Whereas pellet morphology is exhibited at standard conditions a mycelial morphology is created by supplementation of medium with 10 g/L of talc micro particles (Figure 4.). Concurrently the activity of β-fructofuranosidase increases around 3 fold 3-5. A supplementation of 1 or 3 g/L of talc powder leads to a dispersed morphology, with a doubled fructofuranosidase activity (Figure 4.).
The micro particle dependent morphology can be comprehensively described by the Morphology number which can be calculated using parameters determined by automatic image analysis. Perfectly round and smooth pellets will in microscopic images appear as perfect circles. For such particles the Morphology number has a value of 1. The smallest fragment of mycelial morphology can be simplified as a one-dimensional line yielding a Morphology number of 0. All intermediate morphological forms like elongated irregular pellets or clumps will therefore have values between 0 and 1. Fairly large particles will result in a high, fungal particles with a large surface or elongated particles, in a rather low Morphology number 11.
At standard conditions the morphology in reactor 1 exhibits a Morphology number around 0.8. The morphology in reactor 4 with 10 g/L talc powder features a Mn around 0.1. The Morphology number for reactors 2 and 3, with talc powder concentrations of 1 and 3 g/L, lies between these extremes, demonstrating a dispersed morphology. Since micro particle dependent morphology is closely related with the β-fructofuranosidase productivity, a mathematical correlation of Morphology number and productivity similar to Figure 5 is obtained.
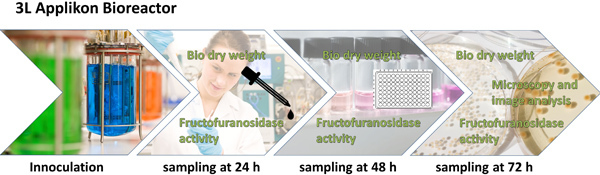 Figure 1. Overall scheme of the experimental design and the analytic procedure. A. niger is cultivated (with or without micro particles) in an 3 L stirred tank bioreactor for 72 h. After 24, 48 and 72 h a sample is taken for determination of biomass dry weight and β-fructofuranosidase activity, which again are used for calculation of the specific productivity. After 48 h the fungal morphology is examined by microscope and characterized by digital image analysis. Relevant parameters of image analysis are combined to a Morphology number, which is mathematical correlated with the specific productivity.
Figure 1. Overall scheme of the experimental design and the analytic procedure. A. niger is cultivated (with or without micro particles) in an 3 L stirred tank bioreactor for 72 h. After 24, 48 and 72 h a sample is taken for determination of biomass dry weight and β-fructofuranosidase activity, which again are used for calculation of the specific productivity. After 48 h the fungal morphology is examined by microscope and characterized by digital image analysis. Relevant parameters of image analysis are combined to a Morphology number, which is mathematical correlated with the specific productivity.
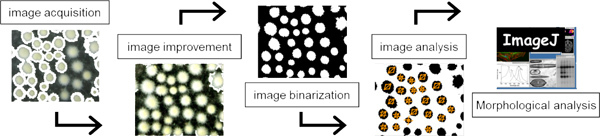 Figure 2. Steps of image processing for microscopic-generated images of morphological structures from A. niger. Step 1: image acquisition by microscope. Step 2: image improvement if necessary. Step 3: image binarization, black-and-white (binary) image generated in ImageJ. Step 4: the binary image is processed by and unwanted object are cleared. Step 5: morphological analysis is conducted with the "Analyze particles" function of the open source program ImageJ.
Figure 2. Steps of image processing for microscopic-generated images of morphological structures from A. niger. Step 1: image acquisition by microscope. Step 2: image improvement if necessary. Step 3: image binarization, black-and-white (binary) image generated in ImageJ. Step 4: the binary image is processed by and unwanted object are cleared. Step 5: morphological analysis is conducted with the "Analyze particles" function of the open source program ImageJ.
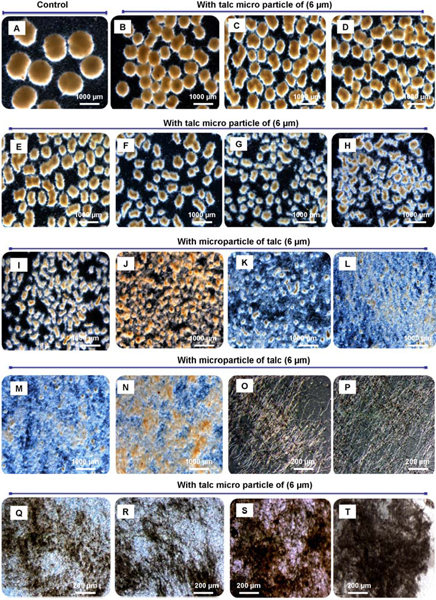 Figure 3. Different morphological forms of A. niger dependent on the concentration of added micro particles. With increasing concentration of micro particles added the pellet size can be precisely decreased down to small core-shell pellets, small flocks and even freely dispersed mycelium. Morphology engineering of Aspergillus niger SKAn1015 by micro particle supplementation in submerged culture. Without microparticles (A), 10 mg/L (B), 0.1 g/L (C), 0.2 g/L (D), 0.3 g/L (E), 0.6 g/L (F), 1.0 g/L (G), 1.5 g/L (H), 2.0 g/L (I), 2.5 g/L (J), 3.0 g/L (K), 3.5 g/L (L), 4.0 g/L (M), 4.5 g/L (N), 5.0 g/L (O), 10 g/L (P), 15 g/L (Q), 20 g/L (R), 30 g/L (S) and 40-50 g/L (T). Images were taken by light microscopy after 72 h of cultivation.
Figure 3. Different morphological forms of A. niger dependent on the concentration of added micro particles. With increasing concentration of micro particles added the pellet size can be precisely decreased down to small core-shell pellets, small flocks and even freely dispersed mycelium. Morphology engineering of Aspergillus niger SKAn1015 by micro particle supplementation in submerged culture. Without microparticles (A), 10 mg/L (B), 0.1 g/L (C), 0.2 g/L (D), 0.3 g/L (E), 0.6 g/L (F), 1.0 g/L (G), 1.5 g/L (H), 2.0 g/L (I), 2.5 g/L (J), 3.0 g/L (K), 3.5 g/L (L), 4.0 g/L (M), 4.5 g/L (N), 5.0 g/L (O), 10 g/L (P), 15 g/L (Q), 20 g/L (R), 30 g/L (S) and 40-50 g/L (T). Images were taken by light microscopy after 72 h of cultivation.
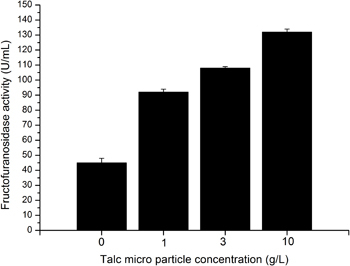 Figure 4. Fructofuranosidase activity in dependence of talc micro particle concentration 1 g/L (reactor 2), 3 g/L (reactor 3) and 10 g/L (reactor 4). Reactor 1 is not supplemented with micro particles; here the cultivation is conducted under standard conditions.
Figure 4. Fructofuranosidase activity in dependence of talc micro particle concentration 1 g/L (reactor 2), 3 g/L (reactor 3) and 10 g/L (reactor 4). Reactor 1 is not supplemented with micro particles; here the cultivation is conducted under standard conditions.
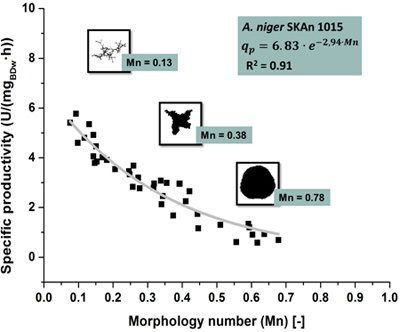 Figure 5. Representative good correlation (R2 = 0.91) of the Morphology number and the specific productivity. The Morphology number is plotted (abscissa) against the specific productivity (ordinate). Nonlinear regression yields the exponential correlation.
Figure 5. Representative good correlation (R2 = 0.91) of the Morphology number and the specific productivity. The Morphology number is plotted (abscissa) against the specific productivity (ordinate). Nonlinear regression yields the exponential correlation.
Discussion
The modification of fungal morphology has been of interest in biotechnology since many decades. Different studies have tried to vary selected process parameters such as pH value, power input, temperature, medium nutrients or inoculum concentration 1, but suffer from rather imprecise and incomplete control of morphology, high energy costs, inhibition effects or product instability, In contrast, the supplementation of micro particles allows a precise engineering of fungal morphology through fine-tuned variation of particle size and concentration. This opens new possibilities to use micro particles for optimization and for tailor-made design of high producing morphology in biotechnological production with A. niger and other filamentous microorganisms.
The digital image analysis is an easy reproducible method to characterize fungal macro morphology. However, the variety of parameters for size, shape and surface character of morphological structures described in literature makes quick assessment of fungal morphology complicated. The presented Morphology number as a combination of relevant parameters, avoids this deficiency and can be used not only for comprehensive characterization of morphological structures but also for direct mathematical correlation with productivity. This again renders an estimation of productivity by given morphology and therefore a customization of morphology for process needs possible.
Using the Morphology number, it is possible to distinguish between various pellet and clump morphologies 4,5. For further development of the Morphology number the consideration of the fractal dimension seems to be promising. A fractal dimension gives a measurement of the complexity and mass filling properties of an object 13 and is therefore predestinated for holistic characterization of mycelial morphology.
The creation of a high producing mycelial morphology, however, might lead to problems with process performance especially in large scale cultivation, because the mycelial growth form has been previously shown to exhibit much greater culture broth viscosities 2. This leads to problems with heat and mass transfer and formation of stagnant non mixed zones, which require a higher power input and make the cultivation more expensive to operate1. Therefore the relationship between fungal morphology and culture broth viscosity should be considered when changing the morphology and be incorporated in further models.
Disclosures
I have nothing to disclose.
Acknowledgments
The authors gratefully acknowledge financial support provided by the German Research Foundation (DFG) through the collaborative research center SFB 578 "From Gene to Product" at the Technische Universität Braunschweig, Germany.
References
- Wucherpfennig T, et al. Advances in Applied Microbiology. Vol. 72. Academic Press; 2010. pp. 89–136. [DOI] [PubMed] [Google Scholar]
- Driouch H, Hänsch R, Wucherpfennig T, Krull R, Wittmann C. Improved enzyme production by bio-pellets of Aspergillus niger: Targeted morphology engineering using titanate microparticles. Biotechnology and Bioengineering. 2012;109:462–471. doi: 10.1002/bit.23313. [DOI] [PubMed] [Google Scholar]
- Driouch H, Roth A, Dersch P, Wittmann C. Optimized bioprocess for production of fructofuranosidase by recombinant Aspergillus niger. Applied Microbiology and Biotechnology. 2010;87:2011–2024. doi: 10.1007/s00253-010-2661-9. [DOI] [PubMed] [Google Scholar]
- Driouch H, Roth A, Dersch P, Wittmann C. Filamentous fungi in good shape: Microparticles for tailor-made fungal morphology and enhanced enzyme production. Bioengineered Bugs. 2011;2:100–104. doi: 10.4161/bbug.2.2.13757. [DOI] [PubMed] [Google Scholar]
- Driouch H, Sommer B, Wittmann C. Morphology engineering of Aspergillus niger for improved enzyme production. Biotechnology and Bioengineering. 2010;105:1058–1068. doi: 10.1002/bit.22614. [DOI] [PubMed] [Google Scholar]
- Kaup B-A, Ehrich K, Pescheck M, Schrader J. Microparticle-enhanced cultivation of filamentous microorganisms: Increased chloroperoxidase formation by Caldariomyces fumago as an example. Biotechnology and Bioengineering. 2008;99:491–498. doi: 10.1002/bit.21713. [DOI] [PubMed] [Google Scholar]
- Hirayama M, Sumi N, Hidaka H. Purification and characterization of a fructooligosaccharide-producing beta-fructofuranosidase from Aspergillus niger ATCC 20611. Agricultural and Biological Chemistry. 2006;53:667–673. [Google Scholar]
- Rajoka MI, Yasmeen A. Improved productivity of beta-fructofuranosidase by a derepressed mutant of Aspergillus niger form conventional and non-conventional substrates. World Journal of Microbiology and Biotechnology. 2005;21:471–478. [Google Scholar]
- Zuccaro A, Götze S, Kneip S, Dersch P, Seibel J. Tailor-made fructooligosaccharides by a combination of substrate and genetic engineering. ChemBioChem. 2008;9:143–149. doi: 10.1002/cbic.200700486. [DOI] [PubMed] [Google Scholar]
- Huggett ASG, Nixon DA. Use of glucose oxidase, peroxidase, and o-dianisidin in determination of blood and urinary glucose. The Lancet. 1957. pp. 270–368. [DOI] [PubMed]
- Wucherpfenning T, Hestler T, Krull R. Morphology engineering - Osmolality and its effect on Aspergillus niger morphology and productivity. Microb. Cell Fact. 2011;10 doi: 10.1186/1475-2859-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband WS. ImageJ. Bethesda, Maryland, USA: National Institutes of Health; 1997. [Google Scholar]
- Papagianni M. Quantification of the fractal nature of mycelial aggregation in Aspergillus niger submerged cultures. Microbial Cell Factories. 2006;5:5. doi: 10.1186/1475-2859-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


