Abstract
Adenosine is a key endogenous signaling molecule that regulates immune responses. A2B adenosine receptor (AR) is a relatively low-affinity receptor for adenosine, and the activation of A2BAR is believed to require pathological level of adenosine that is associated with ischemia, inflammation, trauma, or other types of stress. The role of A2BAR in the pathogenesis of multiple sclerosis (MS) is still unclear. In this study, we discovered that A2BAR was upregulated both in the peripheral blood leukocytes of MS patients and the peripheral lymphoid tissues of experimental autoimmune encephalomyelitis (EAE) mice. A2BAR-specific antagonists, CVT-6883 and MRS-1754, alleviated the clinical symptoms of EAE and protected the CNS from immune damage. A2BAR-knockout mice also developed less severe EAE. Further study indicated that blocking or deleting A2BAR inhibited Th17 cell differentiation by blocking IL-6 production from APCs such as dendritic cells. In dendritic cells, A2BAR was also upregulated during the development of EAE. CVT-6883 and genetic deletion of A2BAR significantly reduced adenosine-mediated IL-6 production. The phospholipase Cβ–protein kinase C and p38 MAPK pathways were found to be involved in the A2BAR-mediated IL-6 production. Our findings not only revealed the pathological role of A2BAR in EAE, but also suggested that this receptor might be a new therapeutic target for the development of anti-MS drugs.
Introduction
Multiple sclerosis (MS) is a T cell–mediated autoimmune disease that is characterized by immune-mediated demyelination and neurodegeneration of the CNS. Experimental autoimmune encephalomyelitis (EAE) is an animal model that shares many pathological and histological similarities with MS. Th1 cells had been considered the type of effector Th cells that mediate the pathogenesis of MS; subsequent studies have indicated that Th17 cells are the other major pathogenic T cells in EAE. T cell differentiation, migration and infiltration into CNS, and astrocytes and microglia activation are a few critical steps involved in EAE pathogenesis (1, 2). Elaborating factors mediating these processes might help the development of new therapeutic interventions for MS.
G protein–coupled receptors (GPCRs) mediate many important biological processes and are the most druggable targets on the market. Many GPCRs have been shown to mediate the pathogenesis of MS or EAE (3). Adenosine, an endogenous signaling molecule, accumulates in inflammation and ischemia (4) and elicits its actions via four GPCRs, denoted A1 adenosine receptor (AR), A2AAR, A2BAR, and A3AR; these receptors are expressed on many immune cells and modulate both innate and adaptive immune responses (5). A large number of studies support the notion that dysfunction of adenosinergic system is involved in the development of MS and EAE. A1AR-knockout (KO) mice developed severe EAE, and activation of A1AR with agonist adenosine amine congener attenuated demyelination in EAE (6). Chronic caffeine treatment has also been postulated to attenuate EAE via upregulation of A1AR (6, 7). A2AAR has also been recognized as a major mediator of anti-inflammatory responses. Activation of A2AAR has been reported to suppress key components of the inflammatory process (8). It seems adenosine might act mainly through A1AR and A2AAR to suppress inflammation. However, a recent study unexpectedly discovered that mice with a genetic deficiency in CD73, a nucleotidase critical for the generation of extracellular adenosine, are highly resistant to myelin oligodendrocyte glycoprotein (MOG)–induced brain and spinal cord injury (9), indicating a proinflammatory function of adenosine.
A2BAR is a relatively low-affinity receptor for adenosine. The activation of A2BAR requires high level of adenosine that is associated with pathological conditions. A2BAR plays proinflammatory roles in human asthma and chronic obstructive pulmonary disease (COPD) and murine colitis (10–12), but its functions in MS or EAE are still not clear. In this study, we found that A2BAR was upregulated in the PBLs of patients with relapsing remitting MS (RRMS) and the peripheral lymphoid tissues of EAE mice. Inhibition of A2BAR with two selective antagonists, CVT-6883 and MRS-1754, or genetic deletion of A2BAR attenuated the CNS infiltration of inflammatory cells and the clinical symptoms of EAE. We found adenosine might promote pathogenic Th17 differentiation by stimulating IL-6 production from dendritic cells (DCs). Blocking or deleting A2BAR largely eliminated adenosine-mediated IL-6 production. To our knowledge, our results demonstrated for the first time that A2BAR plays pathogenic roles in EAE, and blocking A2BAR might provide a new way to treat MS.
Materials and Methods
Study subjects
Subjects were patients from the outpatient clinic of Huashan Hospital (Shanghai, China) with clinically defined RRMS or age- and sex-matched healthy volunteers from personnel of Tongji University and Shanghai Institute of Biochemistry and Cell Biology. Informed consent was provided, and the sampling was in accordance with the guidelines of local institutional review boards.
Mice
C57BL/6 mice were purchased from Shanghai Laboratory Animal Center (Shanghai, China). A2BAR-KO mice on a C57BL/6 background were described in a previous report (13). All mice were maintained in pathogen-free condition with standard laboratory chow and water ad libitum. All experiments were approved and conducted in accordance with the guidelines of the Animal Care Committee of Tongji University.
EAE induction, drug treatment, and histopathological analysis
Female mice at 8–9 wk of age were immunized with 200 μg MOG35–55 in CFA containing 5 mg/ml heat-killed Mycobacterium tuberculosis H37RA. Pertussis toxin (200 ng/mouse) was injected i.p. on days 0 and 2. Mice were assessed daily for clinical signs by researchers blinded to experimental conditions and were assigned scores as follows: 0, no clinical signs; 1, paralyzed tail; 2, paresis; 3, paraplegia; 4, paraplegia with forelimb weakness or paralysis; and 5, moribund or death. CVT-6883 and MRS-1754 dissolved in saline were injected via i.p. once daily from day 3 or day 13 till the end of the study. Saline was given as vehicle control (100 μl/mouse). For histological staining, mice were anesthetized and perfused with PBS (pH 7.4), followed by 4% (w/v) paraformaldehyde. Spinal cord samples were then fixed in 4% (w/v) paraformaldehyde overnight. Paraffin-embedded sections were stained with H&E or luxol fast blue to analyze inflammation or demyelination.
Reverse transcription and real-time PCR
Total RNA was extracted from mouse tissues or human blood samples using TRIzol (Invitrogen). The RNA was subjected to reverse transcription with random hexamer primer and Moloney murine leukemia virus reverse transcriptase (Promega). Real-time PCR was conducted in the LightCycler quantitative PCR (qPCR) apparatus (Stratagene) using the SYBR Green JumpStart Taq ReadyMix kit (Sigma-Aldrich). Expression value was normalized to β-actin in the same sample and then normalized to the control. The sequences of the primer pairs are provided in Supplemental Table 1.
Isolation of CNS infiltrates and flow cytometry
Brain and spinal cord were homogenized in ice-cold tissue grinders and filtered through a 70-μm cell strainer, and the cells were collected by centrifugation at 500 × g for 10 min at 4°C. Cells were resuspended in 8 ml 37% isotonic Percoll and overlaid onto 4 ml 70% isotonic Percoll in 15-ml tubes. The gradient was centrifuged at 780 × g for 25 min at 25°C. Cells at the 37–70% Percoll interface were collected. Cells were incubated for 5 h at 37°C with PMA (50 ng/ml; Sigma-Aldrich), ionomycin (750 ng/ml; Sigma-Aldrich), and brefeldin A (10 μg/ml; Sigma-Aldrich). Surface markers were first stained with relevant Abs. Cells were then resuspended in fixation/permeabilization solution (Cytofix/Cytoperm kit; BD Pharmingen), and intracellular cytokines were stained with appropriate Abs. Guava easyCyte 8HT System and GuavaSoft software were used for the analysis.
ELISA of cytokines
Orbital blood was collected and incubated at 4°C for 30 min, and serum was collected after 10-min centrifugation at 4500 × g. Leukocytes isolated from the spleens of naive or EAE mice treated with vehicle or CVT-6883 were seeded onto 96-well plates at a density of 2 × 105/well/100 μl and restimulated with MOG35–55 (20 μg/ml) at 37°C for 48 h. Cytokines (IL-17a, IFN-γ, IL-4, TGF-β, and IL-6) in the culture supernatants after stimulation or the serum were quantified by ELISA, according to the manufacturer’s instructions.
CD4+ T cell separation and in vitro differentiation
Naive CD4+ T cells were prepared by magnetic cell separation (Invitrogen) from spleens of female C57BL/6 mice 8–9 wk of age. Cells were activated with anti-CD3 (2 μg/ml; 145-2C11) and anti-CD28 (2 μg/ml; 37.51) and were induced to differentiate into Th1 cells by supplementation with IL-12 (10 ng/ml) and anti–IL-4. For Th17 differentiation, cells received anti–IL-4 (10 μg/ml; 11B11) and anti–IFN-γ (10 μg/ml; XMG1.2) plus a Th17 mixture containing TGF-β1 (3 ng/ml), IL-6 (30 ng/ml), TNF-α (10 ng/ml), and IL-1β (10 ng/ml). Compounds were added with the cytokine mixture to assess their influence on T cell differentiation.
DC isolation, stimulation, and IL-6 measurement
Mouse CD11c+ DCs were isolated from the lymph nodes of C57BL/6 mice by MACS (Miltenyi Biotec). For human samples, PBMCs were first isolated from whole blood with density gradient centrifugation on Ficoll-Paque (Amersham), and DCs were then isolated with a human Blood DC Isolation Kit II (Miltenyi Biotec). Freshly isolated DCs (1 × 105) were incubated in 100 μl RPMI 1640 with 10% FBS and 10 μg/ml anti-CD40 in the presence of various compounds for 36 h at 37°C. CVT-6883 (30, 100, or 300 nM) or signaling inhibitors were added to the DCs 30 min before 5′-N-ethylcarboxamidoadenosine (NECA) to ensure the blocking effect. The supernatants were collected and subjected to ELISA for IL-6 measurement.
DC–T cell coculture
In DC–T cell coculture experiments, CD4+ T cells were mixed with CD11c+ DCs at a ratio of 3:1 for 72 h in the presence of anti-CD3 (2 μg/ml), anti-CD28 (2 μg/ml), and anti-CD40 (5 μg/ml). For Th17 differentiation, cells received anti–IL-4 (10 μg/ml), anti–IFN-γ (10 μg/ml), and TGF-β1 (3 ng/ml). The percentage of Th17 cells was analyzed by intracellular staining of IL-17a in the CD4+ gate.
Statistical analysis
Data are presented as means ± SEM. The statistical significance of the EAE clinical scores between treatments was analyzed with a two-way ANOVA test. Other analyses were assessed by Student t test. The p values <0.05 were considered statistically significant.
Results
A2BAR is upregulated in the peripheral lymphoid tissues during EAE pathogenesis
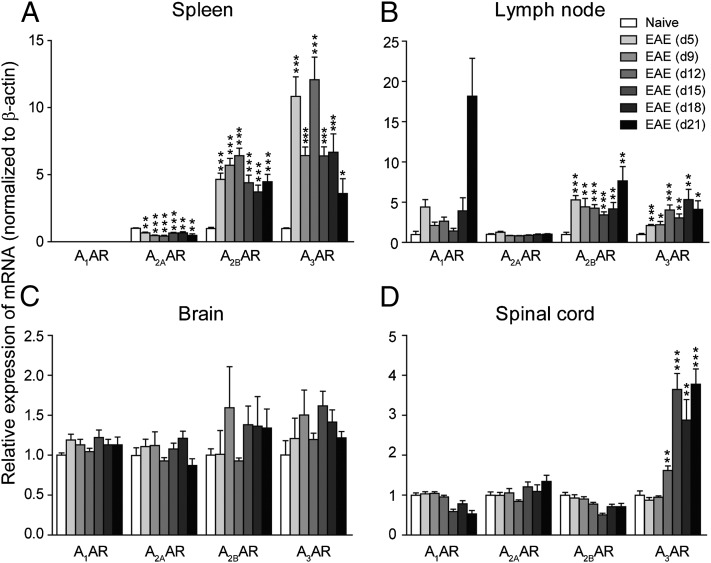
EAE was induced in C57BL/6 mice with MOG35–55, and the mRNA levels of four ARs were checked at days 5, 9, 12, 15, 18, and 21 postimmunization (PI). In spleen and lymph node (Fig. 1A, 1B), A2BAR and A3AR were significantly upregulated from day 5, the preclinical stage of the disease, and the upregulation was maintained for the duration of the study. A1AR was also upregulated in the lymph node, but due to the low expression level, it was not detected in the spleen. Interestingly, A2AAR was downregulated in the spleen of EAE mice. Mills et al. (14) reported that A2AAR−/− mice developed a more severe acute EAE phenotype. They discovered that A2AAR expression on nonimmune cells (most likely in the CNS) is required for efficient EAE development, whereas A2AAR expression on lymphocytes is essential for limiting the severity of the inflammatory response. So the downregulation of A2AAR in spleen may partially contribute to the onset of EAE. No significant change was found in the brain (Fig. 1C). However, in the spinal cord, A3AR was upregulated from day 12, the onset stage of EAE (Fig. 1D). Taken together, the upregulation of A2BAR and A3AR after MOG35–55 immunization indicates that these receptors may play a role in EAE pathogenesis.
FIGURE 1.
Expression change of ARs in EAE. Total RNA was isolated from spleen, lymph node, brain, and spinal cord of naive control mice and EAE animals on days 5, 9, 12, 15, 18, and 21 PI. qPCR was performed to analyze gene expression. Results were normalized to β-actin expression in the same sample and then normalized to the control. (A–D) Gene expression of A1, A2A, A2B, and A3 ARs in spleen (A), lymph node (B), brain (C), and spinal cord (D). Data are mean ± SEM (n = 6) and are representative of two independent experiments. *p < 0.05, **p < 0.01, and ***p < 0.001 versus naive.
Inhibition of A2BAR confers protection in EAE mice
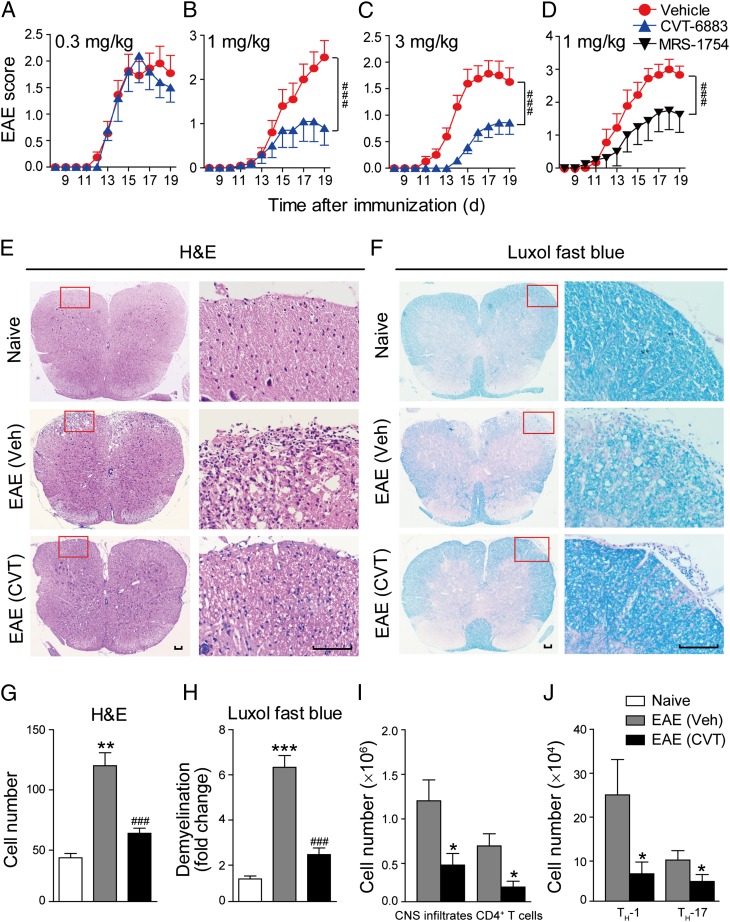
We then used CVT-6883 and MRS-1754, two selective A2BAR antagonists (15), to treat MOG-immunized EAE mice. The drugs were given once daily via i.p. injection from day 3 PI till the end of the experiment, and the control mice were injected with saline. When given at 1 or 3 mg/kg, CVT-6883 significantly reduced the peak severity and cumulative clinical score of EAE (Fig. 2A–C). MRS-1754 also significantly ameliorated the severity of EAE when given at 1 mg/kg (Fig. 2D). When given after the onset of EAE (day 13 PI), CVT-6883 still significantly reduced the clinical scores, although much less effectively compared with the prophylactic administration (Supplemental Fig. 1A). In contrast, A2BAR selective agonist BAY 60-6583 (16, 17) (Supplemental Fig. 1B) did not promote the development of EAE when given at 2 mg/kg (Supplemental Fig. 1C). However, at this dosage, BAY 60-6583 has been reported to elicit A2BAR-mediated biological responses (18, 19). The high level of endogenous adenosine in inflammatory conditions (20) might have masked the effect of the exogenous agonist. So knocking out the receptor (see below) or blocking the receptor with antagonists might be better ways to illustrate the function of A2BAR in EAE.
FIGURE 2.
A2BAR antagonists alleviate clinical symptoms of EAE. EAE mice were treated with CVT-6883 (0.3, 1, or 3 mg/kg/day) (A–C) or MRS-1754 (1 mg/kg/day) (D) once daily via i.p. injection from days 3 PI and were maintained on drug for the duration of the study. Control groups were given saline injection. Data are mean ± SEM (n = 10). ###p < 0.001 (two-way ANOVA test). (E) H&E staining and (F) Luxol fast blue staining of paraffin sections of spinal cords isolated from naive, vehicle, or CVT-6883 (3 mg/kg)–treated EAE mice on day 17. Scale bar, 100 μm. (G and H) Quantification of CNS infiltrates and the amount of demyelination presented in (E) and (F). Data are mean ± SEM. Three mice from each group were sacrificed, and 15 sections from each mouse were analyzed. **p < 0.01, ***p < 0.001 versus naive, ###p < 0.001 versus vehicle. (I and J) CNS infiltrates were isolated with 37–70% Percoll from EAE mice on day 17 PI, and the number of total infiltrates and CD4+ T (I), Th1, and Th17 cells (J) were analyzed by flow cytometry. Data are mean ± SEM (n = 6). *p < 0.05 versus vehicle.
Histological examination of the spinal cords was performed at day 17. Compared with vehicle control, CVT-6883 (3 mg/kg) caused a dramatic reduction of leukocyte infiltration in spinal cord (Fig. 2E, 2G). Luxol fast blue staining also revealed less extensive demyelination in CVT-6883–treated mice than in controls (Fig. 2F, 2H). Leukocytes infiltrated into the CNS were further quantified by flow cytometry at day 17 PI. Results again confirmed that both the total CNS infiltrates and the CD4+ T cells were decreased after CVT-6883 treatment (Fig. 2I). We also found that Th17 and Th1 cells, the main pathogenic CD4+ T effector cells in EAE, were also significantly decreased in the CNS of CVT-6883–treated mice (Fig. 2J). These data indicate that blocking A2BAR signaling with specific inhibitors significantly alleviates EAE severity accompanied by reduced CNS inflammation and demyelination.
Blocking A2BAR inhibits in vivo but not in vitro Th17 differentiation
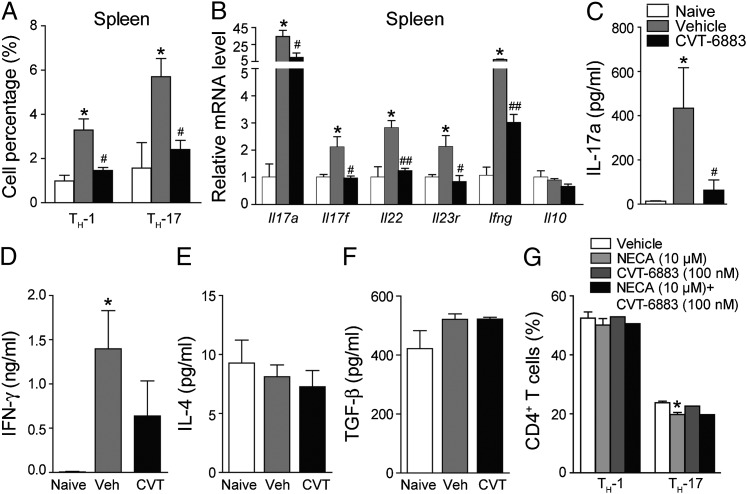
The upregulation of A2BAR occurred at the preclinical stage of EAE, so we speculated that A2BAR might play a role in T cell differentiation. In EAE mice, CVT-6883 did not significantly change the percentage of total leukocytes (CD45+), CD4+ T, CD8+ T, and B cells in both spleen and circulating blood (Supplemental Fig. 1D, 1E). However, the percentage of Th17 and Th1 cells in the CD4+ population was significantly lower in the spleen of CVT-6883–treated mice (Fig. 3A), which was close to the level of naive mice. Genes specific for the Th17 lineage, including Il17a, Il17f, Il22, and Il23r, which were upregulated in EAE, were reduced in CVT-6883–treated mice; and Ifn-γ, a Th1-related gene, was also reduced (Fig. 3B). Ag-specific Th17 response in CVT-6883–treated EAE mice was also significantly lowered, as shown by the measurement of IL-17a production from splenocytes after in vitro MOG restimulation (Fig. 3C). IFN-γ production was modestly reduced by CVT-6883 (Fig. 3D), but IL-4 and TGF-β were not altered (Fig. 3E, 3F).
FIGURE 3.
CVT-6883 inhibits in vivo, but not in vitro Th17 differentiation. Splenocytes were isolated from EAE mice treated with CVT-6883 (3 mg/kg) or vehicle on day 10 PI and analyzed with flow cytometry. (A) Th1 and Th17 cells were analyzed by intracellular staining of IFN-γ and IL-17a, respectively, in the CD4+ gate. Data are mean ± SEM (n = 10), *p < 0.05 versus naive, #p < 0.05 versus vehicle. (B) qPCR analysis of Th1- and Th17-related gene expression in spleen. Data are mean ± SEM (n = 6), *p < 0.05 versus naive, #p < 0.05, ##p < 0.01 versus vehicle. (C–F) Splenocytes from naive and EAE mice treated with CVT-6883 (3 mg/kg) were restimulated in vitro with MOG35–55 for 48 h, and IL-17a (C), IFN-γ (D), IL-4 (E), and TGF-β (F) in supernatants were detected with ELISA. Data are mean ± SEM (n = 10), *p < 0.05 versus naive, #p < 0.05 versus vehicle. (G) Naive CD4+ T cells were induced to differentiate into Th1 or Th17 cells in vitro, in the presence of NECA (10 μM), CVT-6883 (100 nM), or the combination of the two. Data are mean ± SEM (n = 3), *p < 0.05 versus vehicle.
ARs are expressed on the CD4+ T cells (21, 22). So we tested whether adenosine signaling directly affects T cell differentiation with the in vitro differentiation assay. NECA, a stable nonselective analog of adenosine, did not affect Th1 differentiation, but very slightly reduced Th17 differentiation. However, CVT-6883 did not affect Th1 and Th17 differentiation, either alone or in combination with NECA (Fig. 3G), indicating the small effect of NECA on Th17 differentiation is not mediated by A2BAR. Taken together, these data indicate that blocking A2BAR inhibits in vivo differentiation of Th17 cells, and to a lesser extent the differentiation of Th1 cells. And such effect is not likely mediated by the ARs on T cells.
Inhibition of A2BAR reduces IL-6 production from DCs
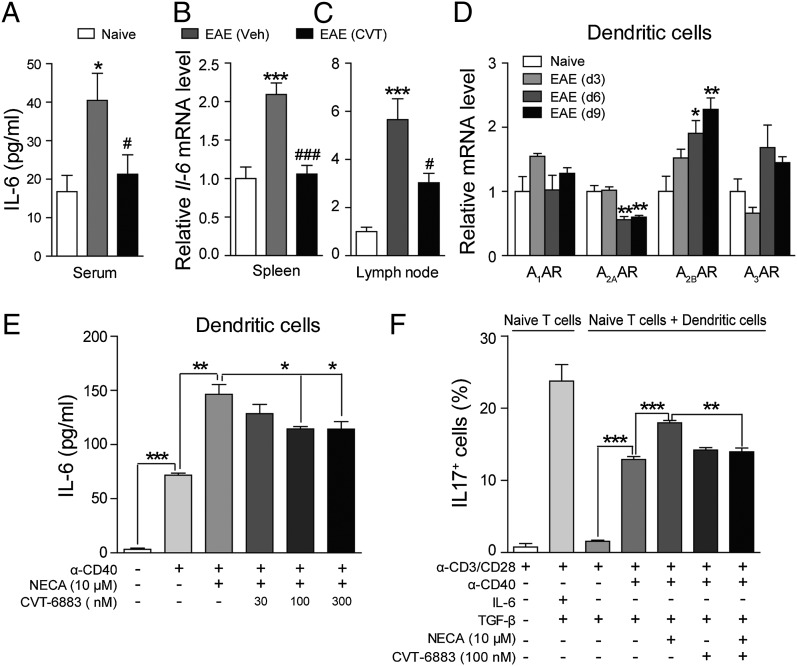
The development of Th cells is controlled largely by factors derived from APCs, such as DCs. Our data showed that CVT-6883 reduces Th17 differentiation in vivo, but not in vitro, indicating A2BAR might affect Th17 differentiation indirectly by modulating upstream cytokine production from APCs. IL-6 is a proinflammatory cytokine critical for Th17 development (23). The concentration of IL-6 was significantly higher in EAE mice (day 10 PI), but CVT-6883 reduced serum IL-6 level almost to the same as naive mice (Fig. 4A). The mRNA of IL-6 gene in both the spleen and lymph node was also significantly reduced by CVT-6883 (Fig. 4B, 4C). DCs are the main APCs in the periphery, which produce potent proinflammatory molecules, including IL-1, IL-6, IL-12, and TNF upon maturation (24). All four ARs were expressed in DCs, and A2BAR was significantly upregulated at day 6 and 9 PI, the preclinical stage of the disease (Fig. 4D). In contrast, A2AAR was significantly downregulated after the induction of EAE (Fig. 4D). It has been reported that A2AAR activation in mature DCs shifts their cytokine profile from a proinflammatory to an anti-inflammatory one, with reduced IL-12, IL-6, and IFN-α production and augmented IL-10 production (8, 25, 26), which suggests an immunosuppressive role of A2AAR. So A2AAR downregulation in DCs may partially contribute to EAE pathogenesis.
FIGURE 4.
CVT-6883 reduces IL-6 production both in vivo and in vitro. (A) Serum was collected from naive or EAE mice treated with CVT-6883 (3 mg/kg) on day 10 PI, and IL-6 level was measured. (B and C) qPCR analysis of IL-6 gene expression in leukocytes from spleen (B) and lymph node (C). Data are mean ± SEM (n = 6), *p < 0.05, ***p < 0.001 versus naive, #p < 0.05, ###p < 0.001 versus vehicle. (D) DCs were isolated from lymph node of EAE mice at day 3, 6, and 9 PI, and the expression change of ARs was monitored with qPCR. Data are mean ± SEM (n = 5), *p < 0.05, **p < 0.01 versus naive. (E) CD11c+ DCs were stimulated with anti-CD40 (10 μg/ml) for 36 h alone, or in combination with NECA (10 μM) in the presence of CVT-6883 (30, 100, or 300 nM) or not, and IL-6 production was measured. Data represent mean ± SEM (n = 6), *p < 0.05, **p < 0.01, ***p < 0.001. (F) Th17 differentiation (IL-17+) was monitored with FACS analysis in the in vitro DC–T cell coculture system in the presence of NECA (10 μM), CVT-6883 (100 nM), or the combination of the two. Data are mean ± SEM (n = 3). **p < 0.01, ***p < 0.001.
In the in vitro IL-6 production assay, DCs isolated from lymph nodes of naive mice were stimulated with anti-CD40 (10 μg/ml) alone, or in combination with NECA (10 μM) and CVT-6883 at various concentrations (30, 100, or 300 nM). NECA dramatically enhanced IL-6 production from DCs on top of the anti-CD40 Ab, and CVT-6883 at 100 and 300 nM significantly blocked the NECA-stimulated IL-6 production (Fig. 4E). In vitro Th17 differentiation can be achieved by stimulating naive T cells with anti-CD3/CD28 Abs in the presence of IL-6 and TGF-β (Fig. 4F, second bar). Then we tried to mimic in vivo T cell differentiation by coculturing DCs with naive T cells. Resting DCs did not induce Th17 differentiation even in the presence of TGF-β, but when stimulated with anti-CD40, the percentage of IL-17+ cells dramatically increased (Fig. 4F, third and fourth bars). NECA further enhanced Th17 differentiation in the coculture (Fig. 4F, fifth bar). CVT-6883 did not block anti-CD40–induced Th17 differentiation, but almost completely blocked the effect of NECA (Fig. 4F, sixth and seventh bars).
The Gq–phospholipase Cβ–protein kinase C and P38 MAPK pathways mediate A2BAR-stimulated IL-6 production in DCs
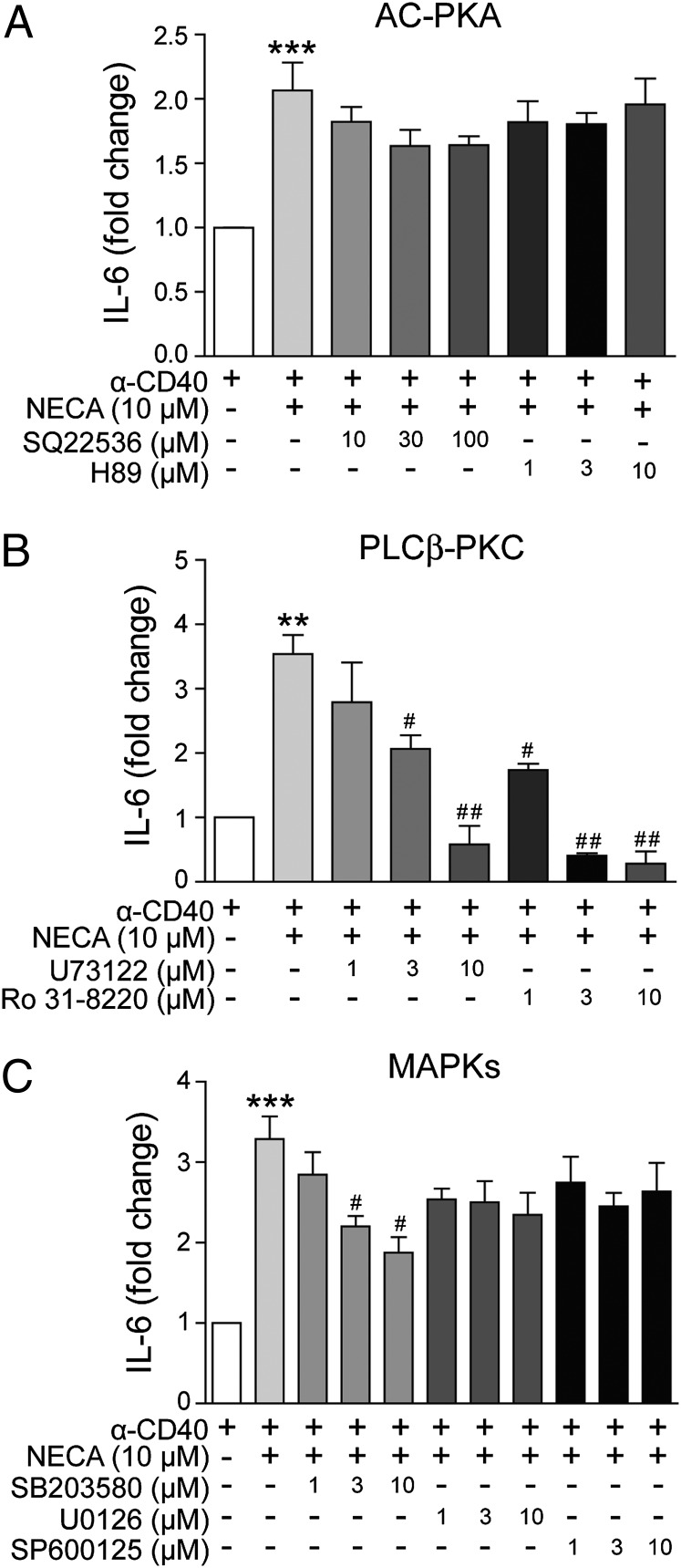
The A2BAR has been reported to couple to various signaling pathways, including the Gs–cAMP–kinase A (PKA), the Gq–phospholipase C (PLC)–protein kinase C (PKC), and the MAPK pathways (27). To investigate which of these pathways might be involved in NECA-induced IL-6 production in DCs, DCs were pretreated with a number of inhibitors before the stimulation with NECA and anti-CD40 Ab. Adenylate cyclase inhibitor SQ22536 and PKA inhibitors H-89 did not reduce the accumulation of IL-6 in the supernatant induced by NECA (Fig. 5A), indicating that the Gs pathway was not involved. In contrast, both the PLCβ inhibitor U73122 and the PKC inhibitor Ro 31-8220 dose dependently inhibited NECA-induced IL-6 production (Fig. 5B). Among the three MAPK inhibitors, only the p38 inhibitor SB203580 displayed significant and dose-dependent inhibition of the effect of NECA; the ERK inhibitor U0126 and JNK inhibitor SP600125 showed no effect at concentrations up to 10 μM (Fig. 5C).
FIGURE 5.
The pathways participate in A2BAR-mediated IL-6 production in DCs. CD11c+ DCs were isolated and stimulated with anti-CD40 (10 μg/ml) and NECA (10 μM) for 36 h in the presence of various pathway inhibitors, and IL-6 production was measured. (A–C) The effect of adenylate cyclase inhibitor SQ 22536 and PKA inhibitor H89 (A); PLCβ inhibitor U73122 and PKC inhibitor Ro 31-8220 (B); or three MAPK inhibitors, SB 203580, U0126, and SP 600125 (C) on NECA-induced IL-6 production from activated DCs. Data are mean ± SEM (n = 3). **p < 0.01, ***p < 0.001 versus anti-CD40 alone, #p < 0.05, ##p < 0.01 versus NECA treatment.
A2BAR-KO mice develop less severe EAE
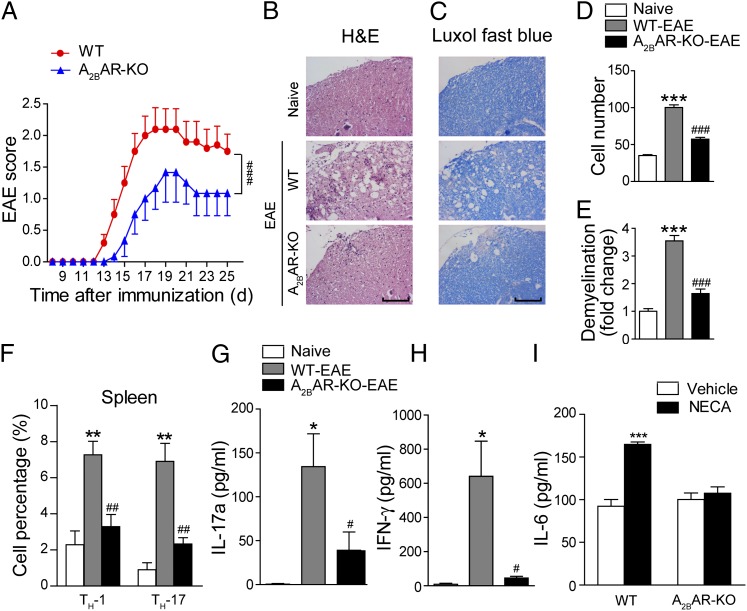
To avoid the possible off-target effects of CVT-6883, and to further investigate the importance of A2BAR signaling during EAE development, EAE was induced with MOG35–55 immunization in A2BAR-KO mice and their wild-type (WT) littermate controls. The A2BAR-KO mice exhibited significantly reduced peak severity and cumulative disease score of EAE (Fig. 6A). Histological examination of the spinal cords at day 17 PI revealed a dramatic reduction of leukocyte infiltration (Fig. 6B, 6D) and less extensive demyelination (Fig. 6C, 6E) in the A2BAR-KO mice. In vivo T cell differentiation was assessed at day 10 PI. The percentage of Th17 and Th1 cells in the CD4+ population was significantly lower in the spleen of A2BAR-KO mice (Fig. 6F). Ag-specific Th17 and Th1 responses in A2BAR-KO EAE mice were also significantly reduced, as shown by the measurement of IL-17a and IFN-γ production from the splenocytes after in vitro MOG restimulation (Fig. 6G, 6H).
FIGURE 6.
A2BAR-KO suppresses EAE pathogenesis. (A) Clinical scores of EAE induced in WT and A2BAR-KO mice. Data are mean ± SEM (n = 10). ###p < 0.001 (two-way ANOVA test). (B) H&E staining and (C) Luxol fast blue staining of the paraffin sections of the spinal cords isolated from naive and EAE-induced WT or A2BAR-KO mice on day 17 PI. Scale bar, 100 μm. (D and E) Quantification of CNS infiltrates and the amount of demyelination presented in (B) and (C). Data are mean ± SEM. Three mice from each group were sacrificed, and 15 sections from each mouse were analyzed. ***p < 0.001 versus naive, ###p < 0.001 versus WT-EAE. (F) Th1 and Th17 cells were analyzed by intracellular staining of IFN-γ and IL-17a, respectively, in the CD4+ gate. Data are mean ± SEM (n = 6), **p < 0.01 versus naive, ##p < 0.01 versus WT-EAE. (G and H) Splenocytes from naive and EAE-induced WT or A2BAR-KO mice were restimulated in vitro with MOG35–55 for 48 h. IL-17a (G) and IFN-γ (H) in the supernatants were detected with ELISA. Data are mean ± SEM (n = 6), *p < 0.05 versus naive, #p < 0.05 versus WT-EAE. (I) CD11c+ DCs isolated from WT or A2BAR-KO mice were stimulated with anti-CD40 (10 μg/ml) for 36 h alone or in combination with NECA (10 μM), and IL-6 production was measured. Data represent mean ± SEM (n = 3), ***p < 0.001.
As demonstrated earlier that blockade of A2BAR with CVT-6883 reduced NECA-stimulated IL-6 production from DCs, we tested whether A2BAR gene ablation affects IL-6 production from DCs. As shown in Fig. 6I, deletion of A2BAR almost completely abrogated NECA-induced IL-6 production from DCs, indicating that A2BAR is the main receptor on DCs that mediates adenosine-stimulated IL-6 production in vivo. The results generated from A2BAR-KO EAE mice were very similar to those from WT EAE mice treated with A2BAR antagonist CVT-6883, indicating that A2BAR plays an important role in controlling DC functions and subsequent T cell differentiation and EAE development.
A2BAR is upregulated in MS patients, and CVT-6883 blocks NECA-induced IL-6 production from human DCs
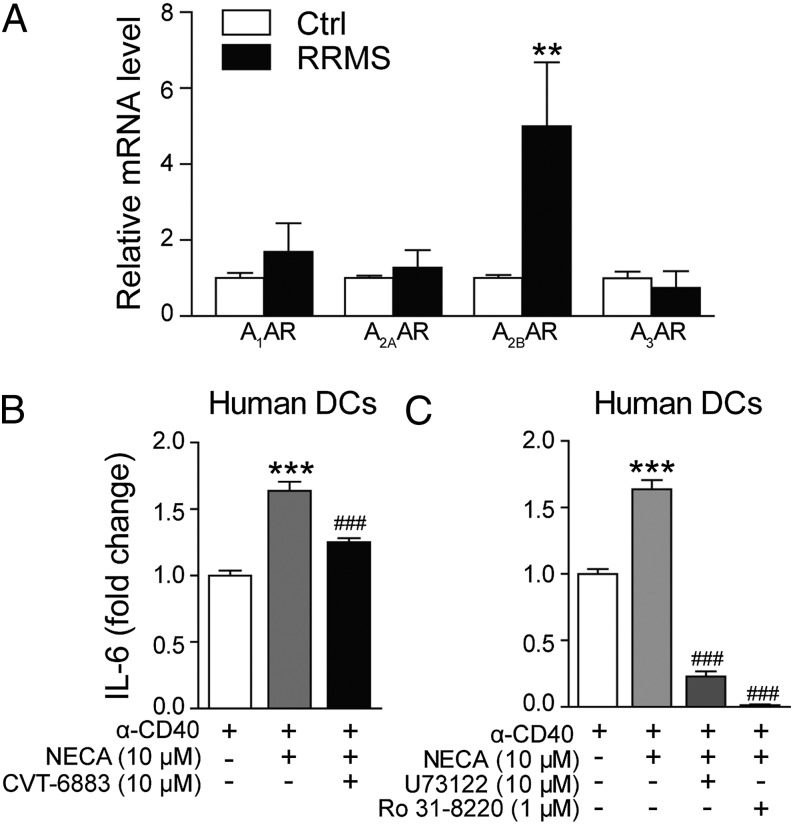
By using clinical samples (Table I), we found that A2BAR expression was significantly increased in the PBLs of patients with RRMS than in those of age-matched controls (Fig. 7A). In contrast, the expression of A1AR and A2AAR showed no substantial changes. Interestingly, A3AR, which was found to be upregulated in EAE mice, showed no significant change in RRMS patients (Fig. 7A), indicating a possible difference between the animal model and real disease. Next, we isolated DCs from PBMCs of healthy donors to see whether A2BAR signaling affects IL-6 production in human DCs. Similar to the results obtained with mouse DCs, we observed that NECA increased IL-6 production from human DCs, and this effect could be blocked by CVT-6883 (Fig. 7B). The production of IL-6 from human DCs also seems to be dependent on the PLCβ/PKC pathway, because PLCβ or PKC-specific inhibitors (U73122 or Ro 31–8220) completely abolished NECA-induced IL-6 release (Fig. 7C).
Table I. Characteristics of patients and controls.
| Control | RRMS | |
|---|---|---|
| Sample size | 20 | 10 |
| Age | 40 ± 11.8 | 39.6 ± 12.5 |
| Sex | ||
| Female | 10 (50%) | 6 (60%) |
| Male | 10 (50%) | 4 (40%) |
| EDSS score | — | 4.6 ± 2.4 |
| Drug treatment | No | No |
Relevant information about human subjects recruited for this study. Sample size is total number of subjects; age is presented in years ± SEM; sex is presented in total number (with percentage of group in parentheses); expanded disability status scale (EDSS) score is presented in mean ± SEM. –, Not applicable.
FIGURE 7.
Upregulation of A2BAR in MS patients and the effect of CVT-6883 on human DCs. (A) The expression of ARs in PBLs from normal controls (n = 20) and patients with RRMS (n = 10) was analyzed with qPCR. (B) Human DCs from healthy donors were stimulated with anti-CD4 (10 μg/ml) for 36 h alone, in combination with NECA (10 μM), or in combination with NECA and CVT-6883 (10 μM), and IL-6 production was measured. (C) Human DCs were incubated with PLCβ inhibitor U73122 (10 μM) or PKC inhibitor Ro 31-8220 (1 μM) for 30 min, then stimulated with anti-CD40 and NECA (10 μM) for 36 h, and IL-6 production was measured. Data are mean ± SEM (n = 3). **p < 0.01 versus naive control, ***p < 0.001 versus anti-CD40 alone, ###p < 0.001 versus NECA treatment.
Discussion
The understanding of the pathogenesis of MS is still very limited, and MS remains a devastating disorder. Drugs currently used in MS patients include those treating attacks, such as corticosteroids, and those modifying the disease course, such as IFN-β and glatiramer acetate (28). With the approval of fingolimod, a S1P1 receptor agonist, the function of GPCRs in MS and their potential role as therapeutic targets for MS are being reappreciated. In fact, many GPCRs are believed to participate in the pathogenesis of MS (3). In this study, we report that targeting A2BAR might provide a new way to treat MS.
Adenosine is an endogenous signaling molecule involved in ischemia, hypoxia, inflammation, and trauma (8). A1AR is believed to mediate the immunosuppressive roles of adenosine. A1AR-KO mice developed a severe form of EAE with extensive damage in CNS, and activation of A1AR with agonist adenosine amine congener attenuated demyelination in EAE mice (6). Recently, mice with a genetic deficiency of CD73, an enzyme critical for the generation of extracellular adenosine, were unexpectedly found to be highly resistant to EAE (9). The authors also found A2AAR antagonist SCH58261 protected mice from CNS injury in EAE. These results are quite controversial because A2AAR receptor is also recognized as a major mediator of anti-inflammatory responses (8, 29). And A2AAR agonists have already been clinically tested to treat inflammatory diseases such as COPD and diabetic foot ulcer (8). A recent paper indicated that although A2AAR expressed on the lymphocytes plays an anti-inflammatory role, the A2AAR in the CNS plays a proinflammatory role and is essential for EAE development (14), and this might explain the beneficial effect of A2AAR antagonist in EAE. The function of A3AR in MS or EAE is still not clear, although this receptor has been implicated to mediate the inhibition of TNF-α production by adenosine (30).
Activation of A2BAR generally requires adenosine levels exceed 10 μM, which is believed to occur in pathological conditions, including inflammation (20). Although its functions in MS or EAE are not clear, the proinflammatory role of A2BAR has been documented in asthma, COPD, and inflammatory bowel diseases (8, 11, 12). A2BAR selective antagonist CVT-6883 is under clinical investigation to treat COPD (8). Apart from ligand concentration, receptor density is also a key determinant in signaling. We found that A2BAR was significantly upregulated in the spleen and lymph node of EAE mice and the PBL samples from RRMS patients. These results suggested that A2BAR might mediate the peripheral immune cell development in EAE and MS. An interesting difference between A2BAR and A3AR was that A3AR was not upregulated in RRMS PBL samples like in EAE samples, suggesting a difference between human RRMS and MOG-EAE model, which has also been reported elsewhere (31). Focused on A2BAR, we found blocking the receptor with specific antagonists or knocking out the receptor effectively alleviated EAE severity.
Our in vivo and in vitro analysis of Th1 and Th17 percentage and cytokine production indicated that blocking or deleting A2BAR can block the development of Th17 cells in vivo, but such effect is not directly mediated by receptors on the T cells. So we hypothesized that A2BAR might be influencing the upstream factors that mediate Th17 differentiation. It is well established that TGF-β and IL-6 induce the differentiation of mouse naive T cells into Th17 by upregulating RORγt (32). Recent study revealed that IL-6 blockade inhibited the induction of Th17 and thus the development of EAE (33). Because we found no change of TGF-β production from MOG-specific cells after CVT-6883 treatment, we focused our effort on IL-6. Serum level of IL-6 was reduced by CVT-6883 treatment; so was the mRNA level in spleen and lymph node. In periphery, IL-6 is mainly produced by APCs such as macrophages and DCs. We evaluated the expression change of ARs on DCs after EAE induction, and A2BAR was significantly upregulated in DCs at the early phase of EAE pathogenesis (day 6), which is the time critical for T cell priming by APCs. In the in vitro DC culture, CVT-6883 or A2BAR-KO was found to reduce NECA-induced IL-6 production. DC–T cell coculture experiment also revealed that NECA enhances Th17 differentiation by enhancing IL-6 production from DCs, and CVT-6883 treatment could inhibit such effect by blocking A2BAR on DCs. By using various chemical inhibitors, we found that the Gq–PLC–PKC and the p38 MAPK pathways are critically involved in A2BAR-mediated IL-6 production from DCs in both mice and humans. Very recently, Wilson et al. (34) also reported that A2BAR mediated DC IL-6 production and promoted Th17 differentiation in vitro.
In summary, to our knowledge, our results demonstrated for the first time that A2BAR plays pathogenic roles in EAE and MS mainly by stimulating IL-6 production from DCs and enhancing Th17 differentiation. Genetic deletion of A2BAR or blocking A2BAR with specific inhibitors, such as CVT-6883, an anti-COPD drug currently under clinical evaluation, could block IL-6 production, Th17 differentiation, and thus the clinical symptoms of EAE. Because relocation of known drugs or compounds is now considered as a main source of new drug discovery, our results not only revealed part of the mechanisms underlying the onset of MS, but also provided new therapeutic targets for the clinical intervention.
Supplementary Material
Acknowledgments
We thank Drs. Ben Li and Li Chen from GinkgoPharma for providing BAY 60-6583.
This work was supported by National Natural Science Foundation of China Grants 31000399, 31171348, and 81202341; Ministry of Science and Technology of China Grants 2012CB910404 and 2009CB940900; Chinese Academy of Sciences Grant XDA01040301; and Shanghai Commission of Science and Technology Grant 12XD1402100.
The online version of this article contains supplemental material.
- AR
- adenosine receptor
- COPD
- chronic obstructive pulmonary disease
- DC
- dendritic cell
- EAE
- experimental autoimmune encephalomyelitis
- GPCR
- G protein–coupled receptor
- KO
- knockout
- MOG
- myelin oligodendrocyte glycoprotein
- MS
- multiple sclerosis
- NECA
- 5′-N-ethylcarboxamidoadenosine
- PI
- postimmunization
- PKA
- protein kinase A
- PKC
- protein kinase C
- PLC
- phospholipase C
- qPCR
- quantitative PCR
- RRMS
- relapsing remitting MS
- WT
- wild-type.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Sospedra M., Martin R. 2005. Immunology of multiple sclerosis. Annu. Rev. Immunol. 23: 683–747 [DOI] [PubMed] [Google Scholar]
- 2.Sun D., Wekerle H. 1986. Ia-restricted encephalitogenic T lymphocytes mediating EAE lyse autoantigen-presenting astrocytes. Nature 320: 70–72 [DOI] [PubMed] [Google Scholar]
- 3.Du C., Xie X. 2012. G protein-coupled receptors as therapeutic targets for multiple sclerosis. Cell Res. 22: 1108–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eltzschig H. K., Rivera-Nieves J., Colgan S. P. 2009. Targeting the A2B adenosine receptor during gastrointestinal ischemia and inflammation. Expert Opin. Ther. Targets 13: 1267–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haskó G., Cronstein B. N. 2004. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 25: 33–39 [DOI] [PubMed] [Google Scholar]
- 6.Tsutsui S., Schnermann J., Noorbakhsh F., Henry S., Yong V. W., Winston B. W., Warren K., Power C. 2004. A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J. Neurosci. 24: 1521–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen G. Q., Chen Y. Y., Wang X. S., Wu S. Z., Yang H. M., Xu H. Q., He J. C., Wang X. T., Chen J. F., Zheng R. Y. 2010. Chronic caffeine treatment attenuates experimental autoimmune encephalomyelitis induced by guinea pig spinal cord homogenates in Wistar rats. Brain Res. 1309: 116–125 [DOI] [PubMed] [Google Scholar]
- 8.Haskó G., Linden J., Cronstein B., Pacher P. 2008. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 7: 759–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mills J. H., Thompson L. F., Mueller C., Waickman A. T., Jalkanen S., Niemela J., Airas L., Bynoe M. S. 2008. CD73 is required for efficient entry of lymphocytes into the central nervous system during experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 105: 9325–9330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feoktistov I., Biaggioni I. 1997. Adenosine A2B receptors. Pharmacol. Rev. 49: 381–402 [PubMed] [Google Scholar]
- 11.Kolachala V. L., Vijay-Kumar M., Dalmasso G., Yang D., Linden J., Wang L., Gewirtz A., Ravid K., Merlin D., Sitaraman S. V. 2008. A2B adenosine receptor gene deletion attenuates murine colitis. Gastroenterology 135: 861–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolachala V., Asamoah V., Wang L., Obertone T. S., Ziegler T. R., Merlin D., Sitaraman S. V. 2005. TNF-alpha upregulates adenosine 2b (A2b) receptor expression and signaling in intestinal epithelial cells: a basis for A2bR overexpression in colitis. Cell. Mol. Life Sci. 62: 2647–2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Csóka B., Németh Z. H., Virág L., Gergely P., Leibovich S. J., Pacher P., Sun C. X., Blackburn M. R., Vizi E. S., Deitch E. A., Haskó G. 2007. A2A adenosine receptors and C/EBPbeta are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood 110: 2685–2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mills J. H., Kim D. G., Krenz A., Chen J. F., Bynoe M. S. 2012. A2A adenosine receptor signaling in lymphocytes and the central nervous system regulates inflammation during experimental autoimmune encephalomyelitis. J. Immunol. 188: 5713–5722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kalla, R. V., J. Zablocki, M. A. Tabrizi, and P. G. Baraldi. 2009. Recent developments in A2B adenosine receptor ligands. Handb. Exp. Pharmacol. 193: 99-122. [DOI] [PubMed]
- 16.Eckle T., Krahn T., Grenz A., Köhler D., Mittelbronn M., Ledent C., Jacobson M. A., Osswald H., Thompson L. F., Unertl K., Eltzschig H. K. 2007. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation 115: 1581–1590 [DOI] [PubMed] [Google Scholar]
- 17.Grenz A., Osswald H., Eckle T., Yang D., Zhang H., Tran Z. V., Klingel K., Ravid K., Eltzschig H. K. 2008. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 5: e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schingnitz U., Hartmann K., Macmanus C. F., Eckle T., Zug S., Colgan S. P., Eltzschig H. K. 2010. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J. Immunol. 184: 5271–5279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckle T., Grenz A., Laucher S., Eltzschig H. K. 2008. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J. Clin. Invest. 118: 3301–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredholm B. B. 2007. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 14: 1315–1323 [DOI] [PubMed] [Google Scholar]
- 21.Gessi S., Varani K., Merighi S., Cattabriga E., Avitabile A., Gavioli R., Fortini C., Leung E., MacLennan S., Borea P. A. 2004. Expression of A3 adenosine receptors in human lymphocytes: up-regulation in T cell activation. Mol. Pharmacol. 65: 711–719 [DOI] [PubMed] [Google Scholar]
- 22.Mirabet M., Herrera C., Cordero O. J., Mallol J., Lluis C., Franco R. 1999. Expression of A2B adenosine receptors in human lymphocytes: their role in T cell activation. J. Cell Sci. 112: 491–502 [DOI] [PubMed] [Google Scholar]
- 23.Zhou L., Ivanov I. I., Spolski R., Min R., Shenderov K., Egawa T., Levy D. E., Leonard W. J., Littman D. R. 2007. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8: 967–974 [DOI] [PubMed] [Google Scholar]
- 24.Comabella M., Montalban X., Münz C., Lünemann J. D. 2010. Targeting dendritic cells to treat multiple sclerosis. Nat. Rev. Neurol. 6: 499–507 [DOI] [PubMed] [Google Scholar]
- 25.Panther E., Idzko M., Herouy Y., Rheinen H., Gebicke-Haerter P. J., Mrowietz U., Dichmann S., Norgauer J. 2001. Expression and function of adenosine receptors in human dendritic cells. FASEB J. 15: 1963–1970 [DOI] [PubMed] [Google Scholar]
- 26.Panther E., Corinti S., Idzko M., Herouy Y., Napp M., la Sala A., Girolomoni G., Norgauer J. 2003. Adenosine affects expression of membrane molecules, cytokine and chemokine release, and the T-cell stimulatory capacity of human dendritic cells. Blood 101: 3985–3990 [DOI] [PubMed] [Google Scholar]
- 27.Schulte G., Fredholm B. B. 2003. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell. Signal. 15: 813–827 [DOI] [PubMed] [Google Scholar]
- 28.Polman C. H., Uitdehaag B. M. 2000. Drug treatment of multiple sclerosis. BMJ 321: 490–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milne G. R., Palmer T. M. 2011. Anti-inflammatory and immunosuppressive effects of the A2A adenosine receptor. ScientificWorldJournal 11: 320–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levy O., Coughlin M., Cronstein B. N., Roy R. M., Desai A., Wessels M. R. 2006. The adenosine system selectively inhibits TLR-mediated TNF-alpha production in the human newborn. J. Immunol. 177: 1956–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gold R., Linington C., Lassmann H. 2006. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain 129: 1953–1971 [DOI] [PubMed] [Google Scholar]
- 32.Korn T., Bettelli E., Oukka M., Kuchroo V. K. 2009. IL-17 and Th17 cells. Annu. Rev. Immunol. 27: 485–517 [DOI] [PubMed] [Google Scholar]
- 33.Serada, S. M. Fujimoto, M. Mihara, N. Koike, Y. Ohsugi, S. Nomura, H. Yoshida, T. Nishikawa, F. Terabe, T. Ohkawara, et al. 2008. IL-6 blockade inhibits the induction of myelin antigen-specific Th17 cells and Th1 cells in experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 105: 9041-9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson J. M., Kurtz C. C., Black S. G., Ross W. G., Alam M. S., Linden J., Ernst P. B. 2011. The A2B adenosine receptor promotes Th17 differentiation via stimulation of dendritic cell IL-6. J. Immunol. 186: 6746–6752 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.