Abstract
Extracellular ATP is released from live cells in controlled conditions, as well as dying cells in inflammatory conditions, and, thereby, regulates T cell responses, including Th17 cell induction. The level of extracellular ATP is closely regulated by ATP hydrolyzing enzymes, such as ecto-nucleoside triphosphate diphosphohydrolases (ENTPDases). ENTPDase1/CD39, which is expressed in immune cells, was shown to regulate immune responses by downregulating the ATP level. In this study, we analyzed the immunomodulatory function of ENTPDase7, which is preferentially expressed in epithelial cells in the small intestine. The targeted deletion of Entpd7 encoding ENTPDase7 in mice resulted in increased ATP levels in the small intestinal lumen. The number of Th17 cells was selectively increased in the small intestinal lamina propria in Entpd7−/− mice. Th17 cells were decreased by oral administration of antibiotics or the ATP antagonist in Entpd7−/− mice, indicating that commensal microbiota-dependent ATP release mediates the enhanced Th17 cell development in the small intestinal lamina propria of Entpd7−/− mice. In accordance with the increased number of small intestinal Th17 cells, Entpd7−/− mice were resistant to oral infection with Citrobacter rodentium. Entpd7−/− mice suffered from severe experimental autoimmune encephalomyelitis, which was associated with increased numbers of CD4+ T cells producing both IL-17 and IFN-γ. Taken together, these findings demonstrate that ENTPDase7 controls the luminal ATP level and, thereby, regulates Th17 cell development in the small intestine.
Introduction
Extracellular ATP was shown to modulate cellular functions via purinergic receptors in the nervous, vascular, and immune system (1–3). In the immune system, the purinergic receptors, such as P2X7 and P2Y2, recognize ATP that is released from damaged and dying cells. P2X7-dependent sensing of ATP leads to activation of the NALP3 inflammasome that induces inflammation via production of IL-1β/IL-18 (4, 5). P2Y2 was shown to mediate recruitment of neutrophils and macrophages into inflammatory sites and clearance of apoptotic cells by phagocytes (6–8). Thus, the innate immune system recognizes extracellular ATP as danger signals to regulate inflammatory responses. In addition to ATP that is released from damaged cells, ATP is released from intact cells under normal conditions and modulates various immune-cellular functions, such as maturation of dendritic cells (DCs) and activation of B and T cells (3, 9). Recently, several reports indicated that ATP modulates mucosal immune responses by influencing the function of intestinal epithelial cells (ECs) and T cells (10–13). Extracellular ATP was also shown to directly modulate T cell responses through P2X receptors, leading to the induction of intestinal inflammation (14, 15).
Therefore, the level of extracellular ATP is closely regulated to prevent uncontrolled ATP-mediated cellular responses by surface-expressing enzymes that hydrolyze ATP, such as members of the ecto-nucleoside triphosphate diphosphohydrolase (ENTPDase) family, consisting of eight members (ENTPDase1–8) (16–18). Among them, ENTPDase1 (also known as CD39), which is highly expressed in immune cells, such as T cells, B cells, NK cells, DCs, and monocytes/macrophages (19, 20), was shown to possess anti-inflammatory activities through ATP hydrolysis. Indeed, severe inflammation was induced in mice lacking ENTPDase1/CD39 in several inflammatory models, including inflammatory bowel disease (21–24). Combinational activity of ENTPDases such as CD39 with CD73 ecto-5′-nucleotidase, which hydrolyzes AMP to adenosine, was also demonstrated in regulatory T cells and intestinal ECs (11, 20, 25). Thus, the immune-modulatory functions of ENTPDase1/CD39 have been well characterized. However, it remains unclear whether other ENTPDase family members are involved in the regulation of immune responses.
In this study, we analyzed the role of ENTPDase7, which was selectively expressed in ECs in the small intestine. Deletion of ENTPDase7 in mice resulted in increased ATP concentrations in the small intestinal lumen and increased numbers of IL-17–producing Th17 cells in the small intestinal lamina propria. Blockade of ATP action decreased the number of Th17 cells in the small intestine of ENTPDase7-deficient mice. In accordance with the increased Th17 cell number, ENTPDase7-deficient mice showed high resistance to the intestinal pathogen Citrobacter rodentium. These findings demonstrate that intestinal ECs participate in the regulation of Th17 cell responses by controlling intestinal ATP levels.
Materials and Methods
Real-time RT-PCR
RNA samples were prepared from various organs, epithelial layer, and lamina propria of C57BL/6J mice (CLEA Japan) using TRIzol reagent (Invitrogen), from single-cell suspensions using an RNeasy Mini Kit (QIAGEN), or from laser-microdissected tissue sections using an RNeasy Micro Kit (QIAGEN). Total RNA was reverse transcribed using Moloney murine leukemia virus reverse transcriptase (Promega) and random primers (Toyobo) after treatment with RQ1 DNase I (Promega). cDNA was analyzed by real-time RT-PCR using GoTaq qPCR Master Mix (Promega) in an ABI 7300 real-time PCR system (Applied Biosystems). Values were then normalized to the expression of Gapdh, and the fold difference in expression relative to that of Gapdh is shown. The following primer sets were used: Entpd1, 5′-TGGTGCAGCAGTTAGAGGAATG-3′ and 5′-CGCACCGATTTCATCTGTTTT-3′; Entpd7, 5′-CCCCTTTACATCCTCTGCAC-3′ and 5′-GTCAAACTCCAACGGCAAAT-3′; Muc2, 5′-ACATCACCTGTCCCGACTTC-3′ and 5′-GAGCAAGGGACTCTGGTCTG-3′; Krt7, 5′-ACGGCTGCTGAGAATGAGTT-3′ and 5′-CGTGAAGGGTCTTGAGGAAG-3′; and Gapdh, 5′-CCTCGTCCCGTAGACAAAATG-3′ and 5′-TCTCCACTTTGCCACTGCAA-3′.
Isolation of epithelium and lamina propria
Intestines were opened longitudinally, washed to remove fecal content, and incubated in PBS containing 30 mM EDTA for 5 min. Epithelial layer was peeled off from intestines and used as epithelium. For isolation of lamina propria, after removing the epithelial layer, fat tissue was also removed from intestines.
Laser microdissection
The frozen sections (10 μm) of the small intestine were fixed with acetic acid/ethyl alcohol (1:19) for 3 min, followed by H&E staining. Tissues containing >100 goblet cells, absorptive enterocytes, and lamina propria cells were collected by a laser microdissection device (DM6000B; Leica, Tokyo, Japan).
Generation of Entpd7-deficient mice
The targeting vector was constructed by replacement of a 1.0-kb fragment encoding the fourth and fifth exons of Entpd7 with a neomycin resistance gene cassette, and a gene encoding HSV thymidine kinase driven by a phosphoglycerate kinase promoter was inserted into the genomic fragment for negative selection. After the targeting vector was transfected into V6.5 embryonic stem cells, G418 and ganciclovir double-resistant colonies were selected and screened by PCR and Southern blot analysis. Homologous recombinants were microinjected into blastocysts of C57BL/6 female mice, and heterozygous F1 progeny mice were intercrossed to obtain Entpd7-deficient mice. Entpd7-deficient mice and their wild-type littermates from these intercrosses were confirmed by Southern blot analysis and Northern blot analysis and were used for experiments. Entpd7-deficient mice were backcrossed onto C57BL/6 mice for at least four generations, and Entpd7-deficient mice and their wild-type littermates from intercrosses of heterozygous mice were used for experiments. All animal experiments were conducted in accordance with the guidelines of the Animal Care and Use Committee of Osaka University.
Isolation of lymphocytes
To prepare single-cell suspensions from spleens, mesenteric lymph nodes (MLNs), and Peyer’s patches, the collected organs were ground between glass slides, and the cells were passed through 40-μm nylon meshes and suspended in PBS. Splenocytes were treated with RBC lysis buffer (0.15 M NH4Cl, 1 mM KHCO3, 0.1 mM EDTA) for 5 min before suspension. Naive CD4+ T cells were purified using a FACSAria system as CD4+CD25−CD44lowCD62Lhigh cells. For isolation of intraepithelial lymphocytes, intestines were opened longitudinally, washed to remove fecal content, and shaken in HBSS containing 5 mM EDTA for 20 min at 37°C. After filtration through nylon mesh, the EC fraction was washed with RPMI 1640 containing 4% FBS, resuspended in 5 ml 40% Percoll (GE Healthcare), and overlaid on 2.5 ml 80% Percoll in a 15-ml Falcon tube. Percoll-gradient separation was performed by centrifugation at 780 × g for 20 min at 25°C. The intraepithelial lymphocytes were collected at the interface of the Percoll gradient and washed with RPMI 1640 containing 10% FBS. For isolation of lamina propria lymphocytes, intestines were opened, washed to remove fecal content, shaken in HBSS containing 5 mM EDTA for 20 min at 37°C to remove ECs and fat tissue, cut into small pieces, and incubated with RPMI 1640 containing 4% FBS, 1 mg/ml collagenase D (Roche), 0.5 mg/ml dispase (Invitrogen), and 40 μg/ml DNase I (Roche) for 1 h at 37°C in a shaking water bath. The digested tissues were washed with HBSS containing 5 mM EDTA and subjected to Percoll density–gradient centrifugation as for isolation of intraepithelial lymphocytes. The lamina propria lymphocytes were collected at the interface of the Percoll gradient and washed with RPMI 1640 containing 10% FBS.
Intracellular cytokine staining
Intracellular expression of IL-17, IFN-γ, and IL-10 in CD4+ T cells was analyzed using a Cytofix/Cytoperm Kit Plus (with GolgiStop; BD Biosciences), according to the manufacturer’s instructions. In brief, lymphocytes obtained from the intestinal lamina propria, spleens, MLNs, or Peyer’s patches were incubated with 50 ng/ml PMA (Sigma), 5 μM calcium ionophore A23187 (Sigma), and GolgiStop at 37°C for 4 h. Surface staining was performed with anti–CD4-PerCP/Cy5.5 (BioLegend) for 20 min at 4°C, the cells were permeabilized with Cytofix/Cytoperm solution for 20 min at 4°C, and intracellular cytokine staining was performed with anti–IL-17A–Alexa Fluor 647 (BD Biosciences), anti–IL-10–PE (BD Biosciences), and anti–IFN-γ–FITC (BioLegend) for 20 min. For intracellular staining of Foxp3, cells were stained using the Foxp3 Staining Buffer set (eBiosciences).
Flow cytometry
The following Abs were used for flow cytometry: anti–CD4-PerCP/Cy5.5, anti–CD8α–Pacific Blue, anti–CD3-FITC, anti–TCRγδ-PE, anti–TCRβ-FITC, anti–CD8β–Alexa Fluor 647, and anti–CD4-PE/Cy7 (all from BioLegend); anti–B220-PE, anti–CD3-PE/Cy7, and anti–CD8α-PE (all from BD Biosciences); and anti–TCRγδ-FITC (eBioscience). Anti–Foxp3–Alexa Fluor 647 (eBioscience) was also used, according to the manufacturer’s instructions. Data were acquired using a FACSCanto II (BD Biosciences) and analyzed using FlowJo software (Tree Star).
Establishment of small intestinal EC lines
H-2Kb-tsA58–transgenic mice (26) were backcrossed to C57BL/6 mice for six generations. To establish the small intestinal EC lines from wild-type and Entpd7−/− mice, the mice were crossed with H-2Kb-tsA58–transgenic mice. Small intestinal ECs were isolated, as previously described (27), before incubation at 33°C. To confirm that they were intestinal ECs, a single-cell suspension was prepared and cytospun onto the glass slides. After fixation, the cells were incubated with polyclonal anti-cytokeratin Ab (1:500; Dako) and then treated with a ChemMate EnVision kit (Dako). DAB (Dako) was used as a chromogen. Images were taken using a BZ-9000 fluorescence microscope (Keyence).
Measurement of ATP
Feces from individual mice were collected, weighed, and gently suspended in PBS containing 0.01% NaN3. After centrifugation, the supernatants were collected, and the levels of ATP were determined with a luciferin-luciferase assay using the ATP assay kit (Toyo Ink), according to the manufacturer’s instructions. To analyze ATP levels in the small intestinal tissues, the small intestine was isolated and cut into quarters longitudinally. Each piece was weighed and lysed to measure ATP with a luciferin-luciferase assay. To analyze ATP levels in the EC lines, single-cell suspensions of the indicated cell lines were prepared. The cells were counted and lysed to measure ATP with a luciferin-luciferase assay. For determination of luminal ATP levels, the mice were fasted overnight and anesthetized by i.p. injection with 350 μl 0.5% pentobarbital sodium (Dainippon Sumitomo Pharma). The peritoneal cavity was opened, and the small intestine was ligated with nylon threads at 1.5 and 4.5 cm distal from the Treitz ligament (for the proximal region of the small intestine) or at 3 and 6 cm proximal from the ileum end (for the distal region of the small intestine) to make a closed intestinal loop. A total of 300 ml PBS or 1.5 mM ATP solution was applied luminally with a 29-G needle. The luminal fluid was recovered 15 min later using a 29-G needle and suspended in PBS. After centrifugation, the supernatants were collected, and the levels of ATP were determined with a luciferin-luciferase assay.
Measurement of NTP hydrolyzing activity
NTP (ATP, GTP, UTP, and CTP) hydrolyzing activity was measured in crude membranes from wild-type and Entpd7−/− small intestinal ECs, as previously described (28). Briefly, ECs were homogenized; after removing nuclei, the crude membrane fraction was separated from the cytosol by centrifugation at 100,000 × g for 30 min. To assay NTP hydrolyzing activity, the membrane fraction containing 10 μg total protein was suspended in reaction buffer (20 mM HEPES [pH 7.4], 120 mM NaCl, 5 mM KCl, 0.2 mM EDTA, 1 mM NaN3, and 0.5 mM Na3VO4, with or without 5 mM CaCl2). After incubation for 5 min at 37°C, 5 μl the reaction buffer containing 10 mM NTP was added and incubated for 30 min. NTP hydrolyzing activity was determined by measuring the inorganic phosphate, as described previously (28).
In vitro naive T cell differentiation
Naive T cells were grown for 4 d at 5 × 105 cells/ml with plate-bound anti-CD3 (2 mg/ml) in DMEM supplemented with 10% FBS, penicillin, and streptomycin under Th17-polarizing conditions (2 ng/ml TGF-β, 20 ng/ml IL-6, 5 μg/ml anti–IFN-γ, 5 μg/ml anti–IL-4) or Th0 conditions (5 μg/ml anti–IFN-γ, 5 μg/ml anti–IL-4). Then, cells were incubated with 50 ng/ml PMA (Sigma), 5 μM calcium ionophore A23187 (Sigma), and GolgiStop at 37°C for 4 h for flow cytometry analysis.
Treatment with antibiotics
Mice were given a combination of antibiotics containing 500 μg/ml vancomycin (Wako), 1 mg/ml metronidazole, 1 mg/ml ampicillin, and 1 mg/ml neomycin sulfate (all from Nacalai Tesque) in drinking water from birth for 8 wk prior to flow cytometric analysis of the small intestinal lamina propria CD4+ lymphocytes.
Isolation of bacterial DNA
The isolation of bacterial DNA was performed as previously described (29), with some modifications. Briefly, small intestines isolated from littermate mice at 10 wk of age were opened longitudinally, and intestinal contents were collected. Intestinal tissues were washed three times with PBS for 10 s to remove the mucus layer. To collect epithelium-associated bacteria, tissues were further treated by vigorous hand shaking three times for 20 s in PBS containing 0.5% Tween 20 (30). After centrifuging, pellets were suspended in 500 μl TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8]). Glass beads and extraction buffer containing TE-saturated phenol and NaDodSO4 solutions were added to the suspension. The mixture was shaken vigorously on a FastPrep FP100 A (BIO 101); this step was repeated after incubation for 10 min at 65°C. After centrifugation, bacterial DNA was precipitated with isopropanol, washed with 70% ethanol, and suspended in 50 μl TE buffer.
Quantitative real-time PCR amplification of 16S rRNA gene sequences
For quantitative analysis of specific bacterial groups in the luminal contents and epithelial layer of the small intestine, quantitative real-time PCR was performed using a LightCycler 480 II (Roche). Bacterial 16S rRNA genes extracted from luminal contents and epithelial surfaces were amplified by bacterial group–specific primers: all bacteria, 5′-ACTCCTACGGGAGGCAGCAGT-3′ and 5′-ATTACCGCGGCTGCTGGC-3′; Lactobacillaceae, 5′-AGCAGTAGGGAATCTTCCA-3′ and 5′-CACCGCTACACATGGAG-3′; segmented filamentous bacteria (SFB), 5′-GACGCTGAGGCATGAGAGCAT-3′ and 5′-GACGGCACGGATTGTTATTCA-3′; Bacteroides, 5′-GGTTCTGAGAGGAAGGTCCC-3′ and 5′-GCTGCCTCCCGTAGGAGT-3′; and Clostridiales, 5′-ACTCCTACGGGAGGCAGC-3′ and 5′-GCTTCTTAGTCAGGTACCGTCAT-3′ (31, 32). All reactions were performed in 20 μl using SYBR Green I Master Mix (Roche). Absolute numbers of bacterial 16S rRNA gene copies were determined from standard curves constructed by quantitative PCR of reference plasmids, including 16S rRNA genes isolated from Lactobacillus johnsonii, a type strain of Lactobacillus obtained from Japan Collection of Microorganisms (JCM No. 2012), murine intestinal Bacteroides, Clostridium, and SFB.
Treatment with oxidized ATP
Mice were given 100 μl 6 mM oxidized ATP (oATP; ATP periodate oxidized sodium salt; Sigma) i.v. daily for 2 wk prior to flow cytometric analysis of the small intestinal lamina propria CD4+ lymphocytes.
C. rodentium infection
C. rodentium (NBRC 105723T) was cultured in Luria–Bertani broth at 37°C for 16 h. Wild-type and Entpd7-deficient mice were infected orally with 2 × 109 C. rodentium in a total volume of 200 μl/mouse. Survival of infected mice was monitored. At 14 d after the infection, spleens were isolated, weighed, and homogenized. Serial dilutions of the homogenates with saline were spread onto MacConkey agar (Merck). After incubation at 37°C for 16 h, the colonies of the appropriate dilutions were counted, and the CFU of bacteria per gram of tissues was calculated. C. rodentium colonies were identified as pink colonies.
Experimental autoimmune encephalomyelitis induction in mice
For the induction of experimental autoimmune encephalomyelitis (EAE), mice were immunized s.c. with 100 μg myelin oligodendrocyte glycoprotein (MOG)35–55 (Biologica) in 100 μl CFA (Difco) divided among four sites, two on each hind flank. Then, the mice received 250 ng Bordetella pertussis toxin (List Biological Laboratories) i.p. on days 0 and 2. The CNS, especially the whole brain and spinal cord, was harvested 17 d after challenge, cut into pieces, and incubated in DMEM containing 2.5 mg/ml collagenase D (Roche) and 1 mg/ml DNase I (Roche) for 20 min at 37°C in a shaking water bath. The digested tissues were resuspended in 5 ml 37% Percoll (GE Healthcare) and then overlaid on 2.5 ml 70% Percoll in a 15-ml tube. Percoll-gradient separation was performed by centrifugation at 500 × g for 20 min at room temperature. Lymphocytes were collected at the Percoll gradient interface and washed with RPMI 1640 containing 10% FBS. Mice were assigned scores of 1 to 5 as follows: 0, no clinical signs of EAE; 1, paralyzed tail; 2, loss of coordinated movement; 3, both hind limbs paralyzed; 4, forelimbs paralyzed; and 5, moribund.
Statistical analysis
Differences between control and experimental groups were evaluated by the Student t test.
Results
Selective expression of Entpd7 in small intestinal epithelia
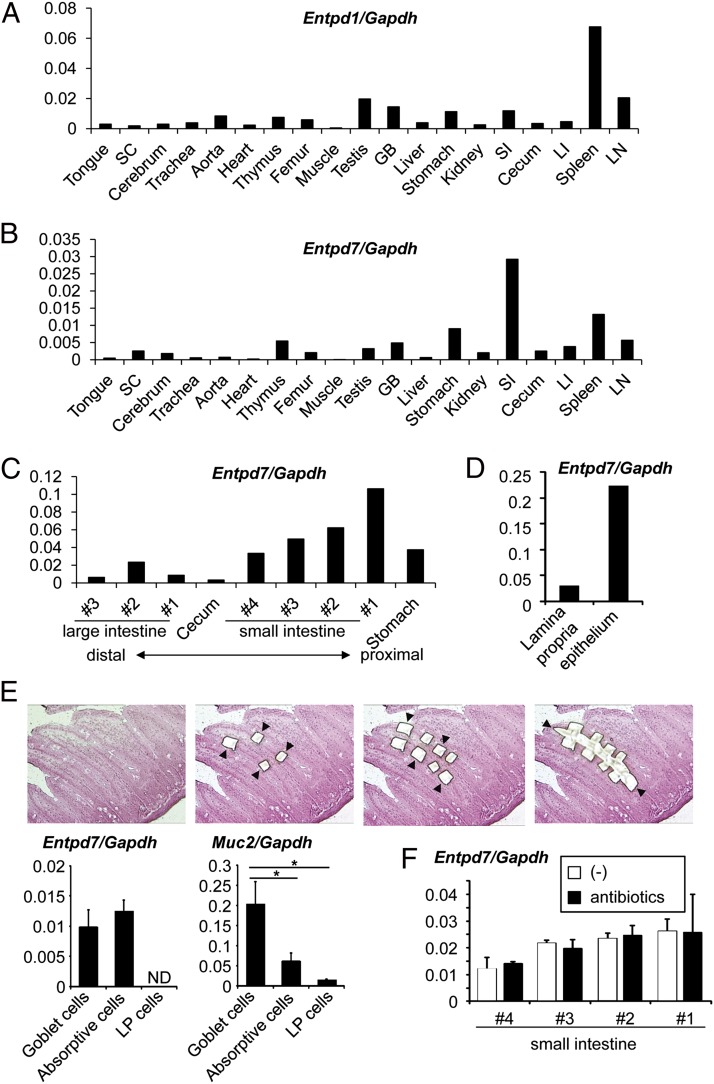
ENTPDase1/CD39 encoded by Entpd1 was shown to modulate inflammatory responses in addition to thrombopoiesis (24, 33, 34). Because the ENTPDase family consists of eight members, we analyzed tissue expression of Entpd gene family members. Entpd1 was preferentially expressed in lymphoid organs, such as the spleen and lymph nodes (Fig. 1A). Of the other Entpd genes, we focused on those that showed selective tissue-expression patterns. Entpd7 was highly expressed in the small intestine (Fig. 1B). The highest Entpd7 expression was observed in the proximal region of the small intestine, and its expression gradually decreased as the small intestines descended (Fig. 1C). We then analyzed expression of Entpd7 in the epithelial layers and lamina propria of the small intestine (Fig. 1D). Entpd7 was predominantly expressed in the ECs of the small intestine. We further analyzed which types of intestinal ECs (i.e., goblet cells or absorptive enterocytes) highly expressed Entpd7. Goblet cell–enriched, absorptive enterocyte-enriched, and lamina propria cell–enriched regions were isolated by laser microdissection, and expression of Entpd7 was analyzed (Fig. 1E). Entpd7 was highly expressed in absorptive enterocytes, as well as goblet cells characterized by high expression of Muc2. Thus, Entpd7 is highly expressed in all types of ECs of the small intestine. Expression of Entpd7 in the small intestine was not altered in mice treated with oral antibiotics, indicating that Enptd7 expression is not influenced by microbiota (Fig. 1F).
FIGURE 1.
High Entpd7 expression in epithelium of the small intestine. Real-time quantitative RT-PCR analysis of mRNA expression of Entpd1 (A) and Entpd7 (B) in various organs. RNA samples were prepared from various organs of C57BL/6J mice and analyzed by real-time RT-PCR. The values were normalized to that of Gapdh. (C) Real-time quantitative RT-PCR analysis of Entpd7 expression in the alimentary tract. The small intestine was cut transversely into four equal pieces, and the colon was cut into three equal pieces. The smaller number denotes the more proximal site of the intestine. Data are representative of three independent experiments. (D) Real-time quantitative RT-PCR analysis of Entpd7 expression in the epithelium and lamia propria of the small intestine. The values were normalized to that of Gapdh. Data are representative of three independent experiments. Real-time quantitative RT-PCR analysis of Entpd7 expression in goblet cells, absorptive ECs, and lamina propria (LP) cells (E) and the epithelium of the small intestine (F) in mice treated with oral antibiotics. Goblet cell–, absorptive cell–, and lamina propria cell–enriched regions were isolated by laser microdissection. Each region is indicated by arrowheads. H&E staining. Original magnification ×50. The expression of Muc2, encoding mucin-2, was also analyzed. The values were normalized to that of Gapdh. Data are representative of two independent experiments and represent mean + SD of three mice. *p < 0.05. GB, Gall bladder; LI, large intestine; LN, mesenteric lymph node; SC, spinal cord; SI, small intestine.
To assess the physiological function of ENTPDase7 encoded by Entpd7, we generated Entpd7−/− mice by gene targeting (Supplemental Fig. 1A, 1B). Entpd7−/− mice were born at the normal Mendelian ratios and grew healthily until 16 wk of age (Supplemental Fig. 1C). Normal lymphocyte development was observed in Entpd7−/− mice (Supplemental Fig. 1D). The composition of lymphocytes in the small and large intestine was not altered in Entpd7−/− mice (Supplemental Fig. 2).
Elevated ATP level in the small intestinal lumen of Entpd7−/− mice
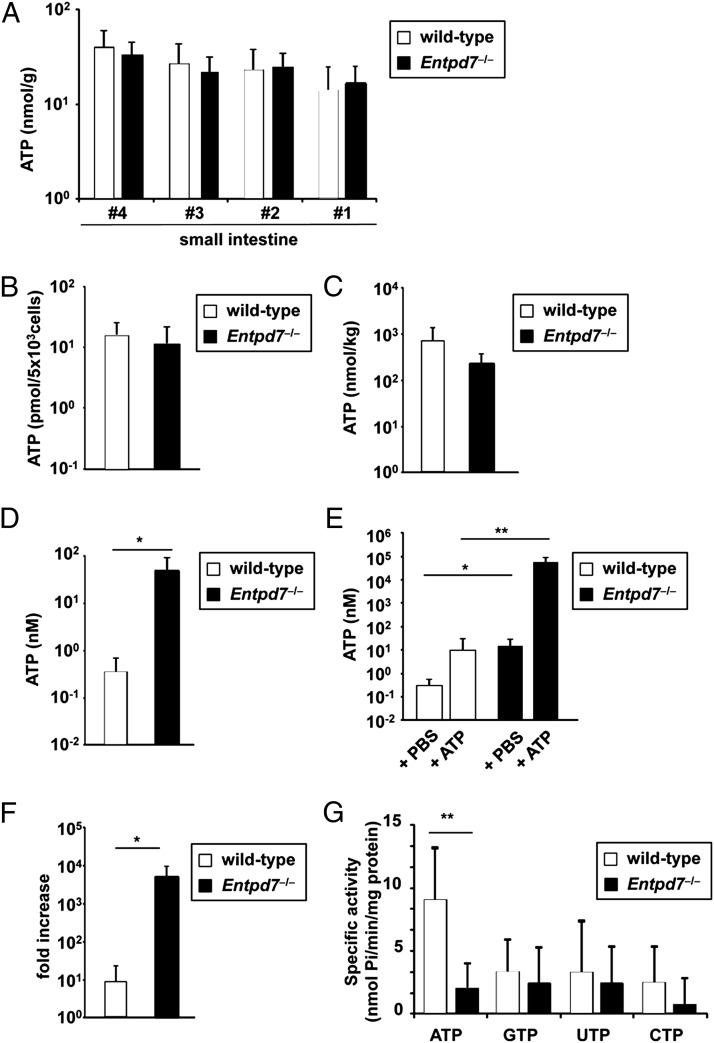
Because ENTPDase is an enzyme that hydrolyzes nucleoside triphosphates, and Entpd7 was selectively expressed in the small intestinal epithelia, we analyzed concentrations of ATP in the intestine. First, the small intestines were cut into four regions, and their lysates were analyzed for ATP concentration (Fig. 2A). The ATP level was not dramatically altered in any region of the small intestinal tissues between wild-type and Entpd7−/− mice. Because Entpd7 is highly expressed in ECs of the small intestine, we established intestinal EC lines from wild-type and Entpd7−/− mice using transgenic mice harboring a temperature-sensitive mutation of the SV40 large tumor Ag gene under the control of an IFN-γ–inducible H-2Kb promoter element to analyze ATP levels in the ECs (26, 35, 36). ECs from wild-type and Entpd7−/− mice expressed keratin proteins equally as well as Krt7 mRNA, indicating that these cells are ECs (Supplemental Fig. 3). Entpd7 was highly expressed in wild-type ECs but not in Entpd7−/− ECs (Supplemental Fig. 3). Intracellular ATP levels were not altered between wild-type and Entpd7−/− ECs (Fig. 2B). Fecal concentrations of ATP were not different in Entpd7−/− mice compared with wild-type mice (Fig. 2C). However, ATP levels in the luminal contents of the small intestine were substantially increased in Entpd7−/− mice (Fig. 2D). We then created a ligated intestinal loop model to analyze alterations in luminal ATP levels. The proximal regions of the small intestine were ligated to make a loop in wild-type and Entpd7−/− mice. Then, ATP or PBS was injected into the loop, and ATP levels were analyzed in the luminal contents 15 min later. ATP injection increased luminal ATP levels in wild-type and Entpd7−/− mice (Fig. 2E). There was a 10-fold increase observed in ATP-treated wild-type mice. In contrast, the ATP level was increased by >1000-fold in the luminal contents of Entpd7−/− mice (Fig. 2F). Thus, Entpd7−/− mice show an increased level of luminal ATP, possibly resulting from a serious defect in ATP-clearance activity in the small intestinal lumen. We then measured NTP (ATP, GTP, UTP, and CTP) hydrolyzing activity in membrane preparations of ECs (Fig. 2G). ATP was hydrolyzed most efficiently by the EC membrane fraction of wild-type mice. In addition, the ATP-hydrolyzing activity was severely impaired in the EC membrane fraction of Entpd7−/− mice. Thus, small intestinal ECs, which highly express ENTPDase7, have the ability to hydrolyze ATP. These findings indicate that ENTPDase7 is required for the maintenance of ATP levels in the small intestinal lumen.
FIGURE 2.
Increased luminal ATP levels in the small intestine of Entpd7−/− mice. (A) The small intestines of wild-type and Entpd7−/− mice were cut into quarters transversely. Each piece was weighed and lysed to measure ATP using a luciferin-luciferase assay. The smaller number denotes the more proximal site of the intestine. Data are representative of two independent experiments; means + SD. (B) Small intestinal ECs from wild-type and Entpd7−/− mice were lysed and analyzed for ATP levels, as described in (A). Data are representative of three independent experiments; means + SD. (C) Feces of wild-type and Entpd7−/− mice were dissolved in PBS, and ATP levels of the supernatants were measured as described in (A). Data are representative of three independent experiments and represent mean + SD of five mice. (D) Wild-type and Entpd7−/− mice were anesthetized, peritoneal cavities were opened, and the small intestine was ligated at 1.5 and 4.5 cm distal from the Treitz ligament to make a closed intestinal loop. PBS (300 μl) was injected into the lumen of the small intestinal loop, and the luminal fluid was recovered soon after the injection. ATP levels in the fluid were measured. Data are representative of three independent experiments and represent mean + SD of four mice. (E) ATP solution (1.5 mM) or PBS was injected into the intestinal loop, and the luminal fluid was collected 15 min later. ATP levels in the fluids were measured. (F) The fold increase in luminal ATP levels after ATP injection in the small intestine of wild-type and Entpd7−/− mice. Data are representative of three independent experiments and represent mean + SD of four mice. (G) NTP hydrolyzing activity in membrane preparations of intestinal EC lines. Activity for each of the four nucleoside triphosphates was assayed with crude membrane preparations from small intestinal ECs from wild-type and Entpd7−/− mice. Data are representative of three independent experiments; means + SD. *p < 0.05, **p < 0.01.
Increased number of Th17 cells in the small intestinal lamina propria in Entpd7−/− mice
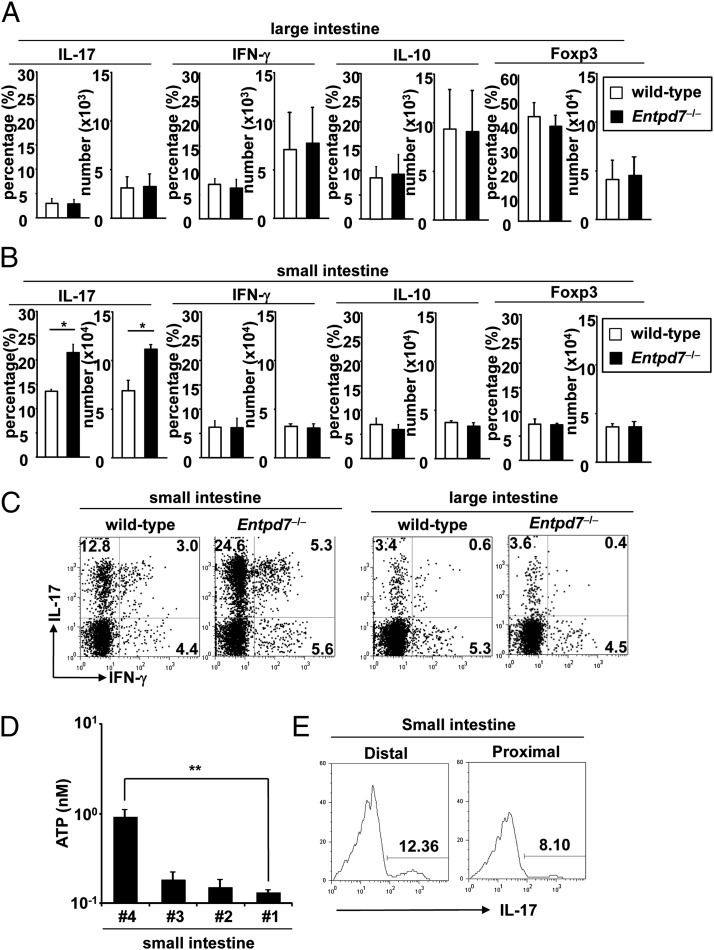
A previous study showed that luminal ATP in the intestine mediates Th17 cell development (10). In addition, extracellular ATP was shown to induce Th17 cell development via the inhibition of regulatory T cell functions (15). Therefore, we analyzed the number of CD4+ T cells expressing IL-17, IFN-γ, IL-10, and Foxp3 in the lamina propria of the small and large intestines. The numbers of IL-17–, IFN-γ–, IL-10–, and Foxp3-expressing CD4+ T cells were not altered in the large intestinal lamina propria of Entpd7−/− mice (Fig. 3A, 3C). In contrast, the number of IL-17–producing CD4+ T cells in the small intestinal lamina propria was markedly increased in Entpd7−/− mice compared with wild-type mice, although the numbers of IFN-γ+, IL-10+, and Foxp3+ T cells were not affected (Fig. 3B, 3C). The number of IL-17–producing CD4+ T cells was not increased in other lymphoid organs, such as the spleen, MLNs, and Peyer’s patches of Entpd7−/− mice (Supplemental Fig. 4). Thus, Entpd7−/− mice showed elevation of Th17 cells in the small intestinal lamina propria. Consistent with Entpd7-expression patterns in the small intestine, the level of luminal ATP was higher in the distal region than in the proximal region of the small intestine of wild-type mice (Fig. 3D); accordingly, the number of IL-17–producing CD4+ T cells was higher in the distal region (Fig. 3E).
FIGURE 3.
Enhanced Th17 cell development in the small intestine of Entpd7−/− mice. (A and B) The lamina propria lymphocytes were isolated from wild-type and Entpd7−/− mice, stimulated, permeabilized, stained for IL-17/IFN-γ/IL-10, and analyzed by flow cytometry. Percentages and total numbers of IL-17–, IFN-γ–, and IL-10–producing, as well as Foxp+ CD4+, cells in the large intestinal (A) and the small intestinal (B) lamina propria. Data are mean + SD of four mice. (C) Representative FACS dot plots gated on intestinal lamina propria CD4+ cells of wild-type and Entpd7−/− mice. (D) The level of luminal ATP in the small intestine. The smaller number denotes the more proximal site of the intestine. ATP levels in the luminal fluid in the indicated portions of the small intestine were measured. Data are representative of three independent experiments and represent mean + SD of four mice. (E) The numbers of IL-17–producing CD4+ cells in the small intestine. The small intestinal lamina propria lymphocytes were isolated from proximal and distal portions of small intestine of wild-type mice and analyzed for production of IL-17 from CD4+ T cells by flow cytometry. Representative FACS dot plots gated on small intestinal lamina propria CD4+ cells. *p < 0.05, **p < 0.01.
Commensal microbiota-dependent, ATP-dependent increase in Th17 cells in Entpd7−/− mice
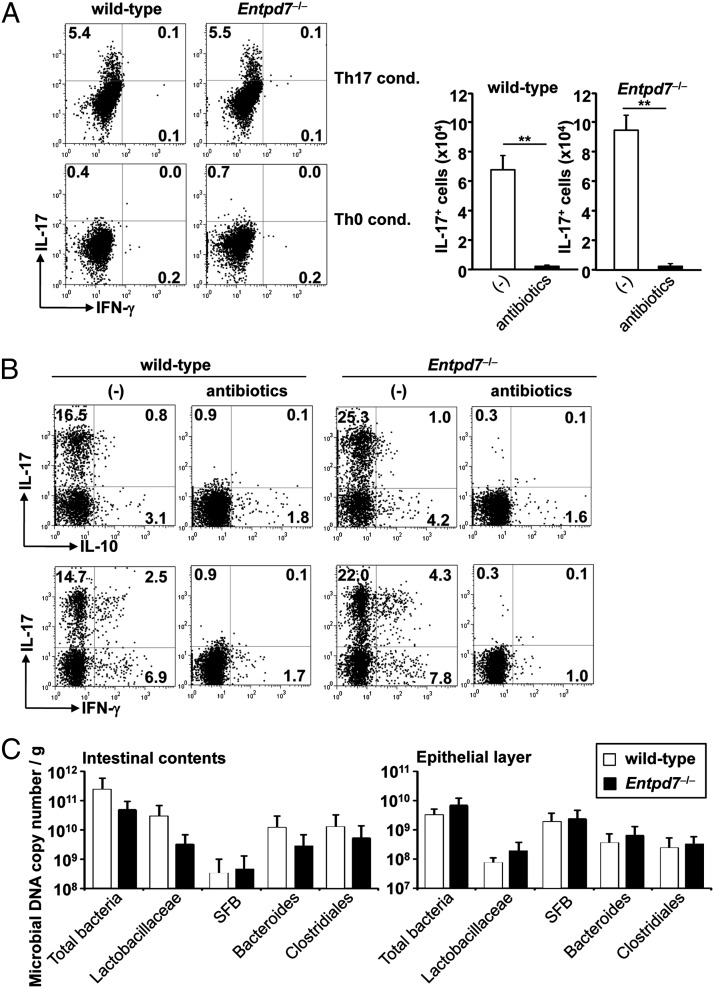
We analyzed whether increased Th17 cell development in the small intestine was intrinsic to the T cell itself or caused by extrinsic environmental factors. We first induced in vitro differentiation of splenic naive CD4+ T cells into Th17 cells. Naive CD4+ T cells were cultured in Th17 cell–skewing conditions and analyzed for IL-17 production (Fig. 4A). In vitro–differentiated CD4+ T cells from wild-type and Entpd7−/− mice produced almost equal amounts of IL-17, indicating that Entpd7−/− T cells were not intrinsically programmed to preferentially differentiate into Th17 cells. We then treated Entpd7−/− mice orally with combinations of four antibiotics (i.e., vancomycin, streptomycin, metronidazole, and ampicillin) from birth (Fig. 4B). In antibiotic-treated wild-type and Entpd7−/− mice, the number of IL-17–producing T cells, as well as IFN-γ– and IL-10–producing T cells, in the small intestinal lamina propria was dramatically reduced. These findings indicate that the augmentation of Th17 cells in Entpd7−/− mice was caused by altered environmental factors influenced by commensal microbiota. Recent data demonstrate that a specific microbiota, such as SFB, induces Th17 cell differentiation in the small intestine (37, 38). Therefore, we analyzed the number of intestinal bacteria in the luminal contents and epithelial layers of the small intestine of wild-type and Entpd7−/− mice (Fig. 4C). The number of intestinal bacteria was not altered in Entpd7−/− mice, indicating that Entpd7 deficiency did not cause alteration of microbiota.
FIGURE 4.
Decreased number of Th17 cells in antibiotic-treated Entpd7−/− mice. (A) Splenic naive CD4+ T lymphocytes were cultured for 4 d under Th17-polarizing conditions (TGF-β, IL-6, anti–IFN-γ, and anti–IL-4) or Th0 conditions (anti–IFN-γ and anti–IL-4). Then, lymphocytes were harvested, stimulated, permeabilized, stained for IL-17 and IFN-γ, and analyzed by flow cytometry. Data are representative of three independent experiments. (B) Wild-type (n = 4) and Entpd7−/− (n = 4) mice were administered vancomycin, metronidazole, ampicillin, and neomycin sulfate in drinking water from birth. The small intestinal lamina propria lymphocytes were isolated at 8 wk of age and analyzed for production of IL-17, IFN-γ, and IL-10 from CD4+ T cells by flow cytometry. Representative FACS dot plots and total numbers of cells gated on small intestinal lamina propria CD4+ cells are shown. (C) Intestinal bacteria in the luminal contents and epithelial layers of the small intestines of wild-type and Entpd7−/− mice. DNA isolated from the luminal contents and epithelial layers of the small intestines was analyzed by real-time quantitative PCR using primers for bacterial group–specific 16S rRNA genes. Data are representative of two independent experiments and are mean + SD of five mice.
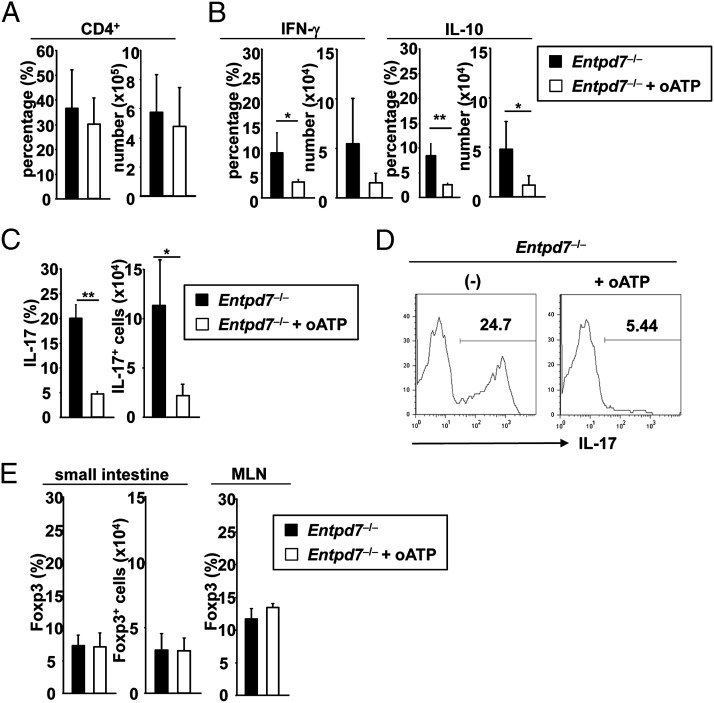
Because commensal microbiota were shown to influence luminal ATP level (10), we analyzed the effect of the blockade of ATP action. oATP, which antagonizes P2X receptors, was shown to be effective in modulating T cell responses in mice, especially Th17 cell responses (15). Therefore, Entpd7−/− mice were treated with oATP; however, the total number of CD4+ cells in the small intestinal lamina propria was not altered (Fig. 5A). In accordance with the previous finding that oATP inhibits T cell responses, such as cytokine production (14), the number of IFN-γ+ and IL-10+ CD4+ T cells was moderately reduced (Fig. 5B). Notably, the number of IL-17–producing CD4+ T cells was severely reduced in oATP-treated Entpd7−/− mice (Fig. 5C, 5D). In an intestinal inflammation model of immunocompromised Cd3e−/− mice transferred with conventional T cells, oATP treatment increased the number of Foxp3+ CD4+ T cells in MLNs of the diseased mice (14). However, Entpd7−/− mice treated with oATP did not show any increase in the number of Foxp3+ T cells in MLNs or the small intestinal lamina propria (Fig. 5E). Thus, the ATP antagonist severely decreased Th17 cells and moderately reduced IFN-γ– and IL-10–producing T cells. These findings indicate that the increased ATP level is responsible for enhanced Th17 cell development in the small intestine of Entpd7−/− mice.
FIGURE 5.
Decreased number of Th17 cells in oATP-treated Entpd7−/− mice. (A–C) Entpd7−/− mice were administered 100 μl of 6 mM oATP or PBS i.v. daily for 2 wk. The small intestinal lamina propria lymphocytes were then isolated and analyzed for production of IFN-γ, IL-10, and IL-17 from CD4+ T cells by flow cytometry. The percentages and total numbers of CD4+ T cells (A), IFN-γ and IL-10–producing CD4+ T cells (B), and IL-17–producing CD4+ T cells (C) in the small intestinal lamina propria of PBS- or oATP-treated Entpd7−/− mice. Data are representative of two independent experiments and are mean + SD of four mice. (D) Representative FACS graph showing IL-17 production gated on small intestinal lamina propria CD4+ T cells of the indicated mice. (E) The percentages and total numbers of Foxp3+ CD4+ T cells in the small intestinal lamina propria and MLNs of PBS- or oATP-treated Entpd7−/− mice. Data are representative of two independent experiments and are mean + SD of three mice. *p < 0.05, **p < 0.01.
Resistance to intestinal C. rodentium infection in Entpd7−/− mice
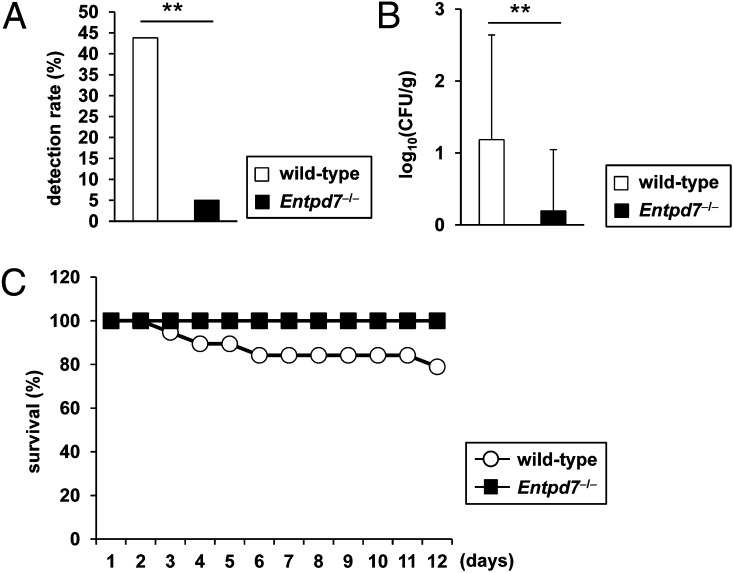
A previous study showed that development of Th17 cells in the small intestine provides the resistance to oral infection with C. rodentium (38). Therefore, we orally infected wild-type and Entpd7−/− mice with C. rodentium. The CFU titers of bacteria in the spleen were measured at day 14 after the infection (Fig. 6A, 6B). The number of spleens that was invaded with C. rodentium was dramatically decreased in Entpd7−/− mice. Accordingly, Entpd7−/− mice had decreased numbers of C. rodentium in the spleen compared with wild-type mice. In addition, although some wild-type mice died after the oral C. rodentium infection, none of the Entpd7−/− mice died (Fig. 6C). Thus, Entpd7−/− mice are resistant to the intestinal bacterium C. rodentium.
FIGURE 6.
Resistance to intestinal C. rodentium infection in Entpd7−/− mice. (A–C) Wild-type (n = 19) and Entpd7−/− (n = 19) mice were infected orally with C. rodentium. (A) Detection rate of C. rodentium in the spleen on day 14. The pooled data of two independent experiments are shown. (B) Log10 CFU of C. rodentium in spleens. (C) Survival rate of the mice at the indicated time points. The pooled data of two independent experiments are shown. **p < 0.01.
Deteriorated EAE in Entpd7−/− mice
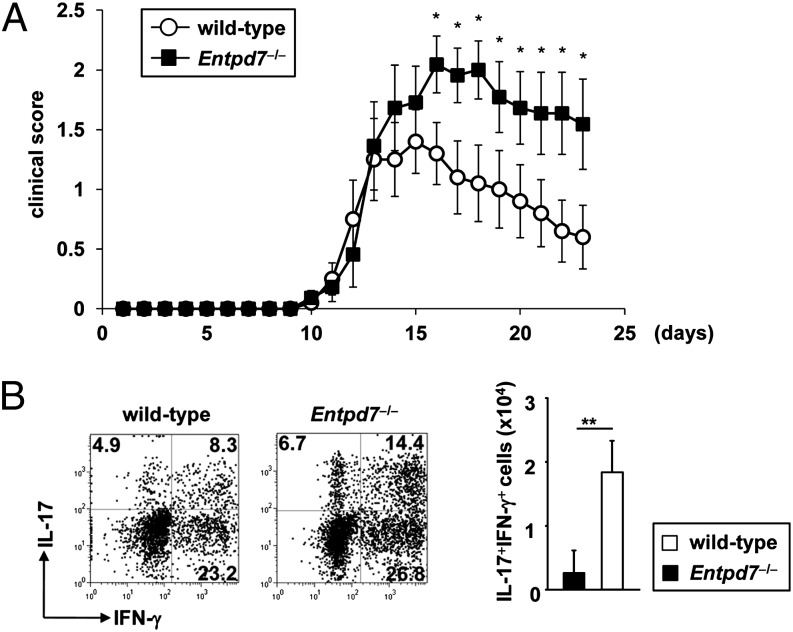
Enhanced Th17 responses are implicated in the development of several immune disorders, including EAE (39). Th17 cells, which develop in the small intestine, were also shown to induce inflammation in extraintestinal tissues, such as arthritis in the ankle joints (40). Furthermore, commensal microbiota were shown to be involved in the pathogenesis of EAE (41). Therefore, we used a MOG peptide–induced model of EAE in Entpd7−/− mice to determine the effect of ENTPDase7-mediated regulation of intestinal Th17 cells in inflammatory conditions in vivo. As shown in Fig. 7A, s.c. immunization of wild-type mice with the MOG peptide, together with pertussis toxin, induced encephalomyelitis associated with rapidly ascending paralysis appearing at approximately day 10–12. MOG peptide–immunized Entpd7−/− mice showed more severe clinical symptoms. We then analyzed cytokine production from CD4+ T cells infiltrated into the CNS of the diseased mice (Fig. 7B). In wild-type and Entpd7−/− mice, infiltration of IL-17– and IFN-γ–producing CD4+ T cells, as well as IL-17/IFN-γ double-producing cells, was observed. IL-17/IFN-γ double-producing CD4+ cells increased markedly in Entpd7−/− mice compared with diseased wild-type mice. Thus, in the absence of Entpd7, severe EAE developed that was accompanied by an increased infiltration of CD4+ T cells producing both IL-17 and IFN-γ.
FIGURE 7.
Severe EAE in Entpd7−/− mice. (A) Wild-type (n = 10) and Entpd7−/− (n = 11) mice were immunized with 100 μg MOG35–55 peptide in CFA; 100 ng of pertussis toxin was injected i.p. on days 0 and 2. The mean clinical score was calculated by averaging the scores of the mice in each group. Data are mean ± SEM at each time point. Experiments were performed twice with similar results. *p < 0.05. (B) Representative FACS dot plots gated on CD4+ cells of the CNS in the indicated mice at day 17 after EAE induction (left and middle panels). CNS lymphocytes were isolated from wild-type and Entpd7−/− mice 17 d after EAE induction and analyzed for the production of IFN-γ and IL-17 from CD4+ T cells by flow cytometry (right panel). Data are representative of five mice analyzed. **p < 0.05.
Discussion
In the current study, we analyzed the physiological function of ENTPDase7, which is preferentially expressed in ECs of the small intestine. ENTPDase7 deficiency in mice led to increased ATP levels in the small intestinal lumen, indicating that ENTPDase7 is responsible for the maintenance of luminal ATP levels. The number of IL-17–producing Th17 cells in the small intestinal lamina propria was increased in Entpd7−/− mice. The number of Th17 cells was decreased in Entpd7−/− mice in the absence of commensal microbiota or after ATP antagonist treatment. Entpd7−/− mice were resistant to infection with C. rodentium, against which Th17-related cytokines play a major role.
A previous report indicated that human ENTPDase7 is expressed in the membrane of intracellular compartments (28). The intracellular ATP level, which was analyzed using total-cell lysates, was not altered in Entpd7−/− ECs. However, given that the ATP concentration in the cytoplasm is >1 mM, whereas the ATP concentration in the extracellular compartment is usually <10 nM, ATP levels within the ENTPDase7-expressing cellular vesicles of ECs would be increased in the absence of ENTPDase7. Indeed, the membrane fraction of intestinal ECs had an enzymatic activity to hydrolyze ATP, and its activity was decreased in the absence of Entpd7. Because Entpd7 was highly expressed in goblet cells, as well as absorptive ECs, it is possible that Entpd7 is expressed in the membrane of mucin-containing vacuoles of goblet cells to control ATP levels in the vacuole. Given that goblet cells of the airway were shown to secrete ATP, as well as mucin (42), intestinal goblet cells might be a major source of luminal ATP, the level of which is closely regulated by ENTPDase7.
Human ENTPDase7 was shown to preferentially hydrolyze UTP, GTP, and CTP rather than ATP (28). However, the membrane fraction of mouse intestinal ECs effectively hydrolyzed ATP, and its activity was impaired by Entpd7 deficiency. Thus, mouse ENTPDase7, unlike human ENTPDase7, effectively hydrolyzes ATP. Indeed, apparent differences in amino acid sequences are observed in a domain between the second and third apyrase-conserved regions, supporting that mouse and human ENTPDase7 have different substrate affinities.
Luminal ATP is supposed to be derived from ECs, as discussed above. In addition, commensal microbiota are a source of luminal ATP (10). In this regard, commensal microbiota, especially SFB, mediate Th17 cell development in the small intestine, possibly through ATP-independent mechanisms (37, 43). Therefore, in the small intestine, luminal ATP may mediate Th17 cell development cooperatively with Th17-inducing commensal microbiota. There is controversy as to how luminal ATP is sensed and induces Th17 development. Intestinal CX3CR1+ DCs were shown to extrude their dendrites into the lumen to sample intestinal Ags (44, 45). These intestinal DCs might sense luminal ATP via purinergic receptors. Alternatively, as reported in several studies, ECs sense extracellular ATP (11, 25, 46). Therefore, intestinal ECs trigger inflammatory responses to activate T cell development via ATP sensing. Indeed, intestinal ECs were shown to control DC functions (47).
C. rodentium is an enteric bacterium that colonizes the intestine of mice postinfection. Clearance of C. rodentium is shown to be dependent on Th17-related cytokines, such as IL-17 and IL-22 (48, 49). Data showing that mice lacking IL-23, a critical cytokine for Th17 cell development, are highly susceptible to C. rodentium infection also indicate that Th17-related cytokines are critical for the resistance to intestinal C. rodentium infection (50). Consistent with these facts, Entpd7−/− mice showing an increased number of Th17 cells in the small intestine are highly resistant to intestinal infection with C. rodentium. IL-22 and IL-17, which induce production of antibacterial peptides (REGIIIγ and β-defensins) from intestinal ECs (48, 51), are produced from other cell populations, such as innate lymphoid cells and γδT cells (52, 53). Therefore, Th17 cells, together with an innate type of IL-17–producing cells, contribute to intestinal pathogens.
In an EAE model, CD4+ T cells producing both IL-17 and IFN-γ are observed in the CNS (54–56). It is still controversial whether these IL-17/IFN-γ double-producing T cells are Th1 or Th17 cells, but they do contribute to EAE pathogenesis (54). A study using Il23ra−/− mice, which showed a reduced number of Th17 cells, as well as IL-17/IFN-γ double-producing T cells and a normal number of Th1 cells, suggested that IL-17/IFN-γ double-producing T cells are derived from Th17 cells (57). Therefore, increased numbers of IL-17/IFN-γ double-producing T cells in the CNS of Entpd7−/− mice with EAE might be due to enhanced Th17 responses.
It remains unclear how Th17 cells residing in the small intestine mediate EAE. However, several lines of evidence indicate the relevance between gut immune cells and immune disorders in extraintestinal tissues: Th17 cells induced by SFB in the small intestinal lamina propria were shown to be responsible for the induction of autoimmune arthritis (40); alteration of the commensal flora composition influences the severity of EAE (41); and SFB-induced Th17 cells in the small intestine induce EAE (58). Thus, intestinal effector T cells are responsible for the induction of immune disorders in nongut tissues, including the CNS, and our present study demonstrates that enhanced intestinal Th17 responses can induce severe inflammatory conditions in these disease models.
In this study, we showed that an ENTPDase expressed by intestinal ECs regulates luminal ATP levels and, thereby, controls intestinal immune responses. Another ENTPDase, ENTPDase8, is selectively expressed by the ECs in the large intestine, as well as the small intestine (T. Kusu and K. Takeda, unpublished observations). Characterization of ENTPDase8 functions in terms of regulation of intestinal immune responses will be an interesting issue to be addressed in the future.
Supplementary Material
Acknowledgments
We thank C. Hidaka for secretarial assistance and Y. Magota for technical assistance.
This work was supported by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology; the Ministry of Health, Labour and Welfare; and the Osaka Foundation for the Promotion of Clinical Immunology.
The online version of this article contains supplemental material.
- DC
- dendritic cell
- EAE
- experimental autoimmune encephalomyelitis
- EC
- epithelial cell
- ENTPDase
- ecto-nucleoside triphosphate diphosphohydrolase
- MLN
- mesenteric lymph node
- MOG
- myelin oligodendrocyte glycoprotein
- oATP
- oxidized ATP
- SFB
- segmented filamentous bacteria.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Burnstock G. 2007. Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 87: 659–797 [DOI] [PubMed] [Google Scholar]
- 2.Vassort G. 2001. Adenosine 5′-triphosphate: a P2-purinergic agonist in the myocardium. Physiol. Rev. 81: 767–806 [DOI] [PubMed] [Google Scholar]
- 3.Junger W. G. 2011. Immune cell regulation by autocrine purinergic signalling. Nat. Rev. Immunol. 11: 201–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piccini A., Carta S., Tassi S., Lasiglié D., Fossati G., Rubartelli A. 2008. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc. Natl. Acad. Sci. USA 105: 8067–8072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu H. B., Finlay B. B. 2008. The caspase-1 inflammasome: a pilot of innate immune responses. Cell Host Microbe 4: 198–208 [DOI] [PubMed] [Google Scholar]
- 6.Chen Y., Corriden R., Inoue Y., Yip L., Hashiguchi N., Zinkernagel A., Nizet V., Insel P. A., Junger W. G. 2006. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314: 1792–1795 [DOI] [PubMed] [Google Scholar]
- 7.Elliott M. R., Chekeni F. B., Trampont P. C., Lazarowski E. R., Kadl A., Walk S. F., Park D., Woodson R. I., Ostankovich M., Sharma P., et al. 2009. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461: 282–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kronlage M., Song J., Sorokin L., Isfort K., Schwerdtle T., Leipziger J., Robaye B., Conley P. B., Kim H. C., Sargin S., et al. 2010. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci. Signal. 3: ra55. [DOI] [PubMed] [Google Scholar]
- 9.Trautmann A. 2009. Extracellular ATP in the immune system: more than just a “danger signal”. Sci. Signal. 2: pe6. [DOI] [PubMed] [Google Scholar]
- 10.Atarashi K., Nishimura J., Shima T., Umesaki Y., Yamamoto M., Onoue M., Yagita H., Ishii N., Evans R., Honda K., Takeda K. 2008. ATP drives lamina propria T(H)17 cell differentiation. Nature 455: 808–812 [DOI] [PubMed] [Google Scholar]
- 11.Weissmüller T., Campbell E. L., Rosenberger P., Scully M., Beck P. L., Furuta G. T., Colgan S. P. 2008. PMNs facilitate translocation of platelets across human and mouse epithelium and together alter fluid homeostasis via epithelial cell-expressed ecto-NTPDases. J. Clin. Invest. 118: 3682–3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivison S. M., Himmel M. E., Mayer M., Yao Y., Kifayet A., Levings M. K., Steiner T. S. 2011. The stress signal extracellular ATP modulates antiflagellin immune responses in intestinal epithelial cells. Inflamm. Bowel Dis. 17: 319–333 [DOI] [PubMed] [Google Scholar]
- 13.Heiss K., Jänner N., Mähnss B., Schumacher V., Koch-Nolte F., Haag F., Mittrücker H. W. 2008. High sensitivity of intestinal CD8+ T cells to nucleotides indicates P2X7 as a regulator for intestinal T cell responses. J. Immunol. 181: 3861–3869 [DOI] [PubMed] [Google Scholar]
- 14.Schenk U., Westendorf A. M., Radaelli E., Casati A., Ferro M., Fumagalli M., Verderio C., Buer J., Scanziani E., Grassi F. 2008. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci. Signal. 1: ra6. [DOI] [PubMed] [Google Scholar]
- 15.Schenk U., Frascoli M., Proietti M., Geffers R., Traggiai E., Buer J., Ricordi C., Westendorf A. M., Grassi F. 2011. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci. Signal. 4: ra12. [DOI] [PubMed] [Google Scholar]
- 16.Yegutkin G. G. 2008. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim. Biophys. Acta 1783: 673–694 [DOI] [PubMed] [Google Scholar]
- 17.Schetinger M. R., Morsch V. M., Bonan C. D., Wyse A. T. 2007. NTPDase and 5′-nucleotidase activities in physiological and disease conditions: new perspectives for human health. Biofactors 31: 77–98 [DOI] [PubMed] [Google Scholar]
- 18.Robson S. C., Sévigny J., Zimmermann H. 2006. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Purinergic Signal. 2: 409–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maliszewski C. R., Delespesse G. J., Schoenborn M. A., Armitage R. J., Fanslow W. C., Nakajima T., Baker E., Sutherland G. R., Poindexter K., Birks C., et al. 1994. The CD39 lymphoid cell activation antigen. Molecular cloning and structural characterization. J. Immunol. 153: 3574–3583 [PubMed] [Google Scholar]
- 20.Deaglio S., Dwyer K. M., Gao W., Friedman D., Usheva A., Erat A., Chen J. F., Enjyoji K., Linden J., Oukka M., et al. 2007. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204: 1257–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guckelberger O., Sun X. F., Sévigny J., Imai M., Kaczmarek E., Enjyoji K., Kruskal J. B., Robson S. C. 2004. Beneficial effects of CD39/ecto-nucleoside triphosphate diphosphohydrolase-1 in murine intestinal ischemia-reperfusion injury. Thromb. Haemost. 91: 576–586 [DOI] [PubMed] [Google Scholar]
- 22.Friedman D. J., Künzli B. M., A-Rahim Y. I., Sevigny J., Berberat P. O., Enjyoji K., Csizmadia E., Friess H., Robson S. C. 2009. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc. Natl. Acad. Sci. USA 106: 16788–16793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizumoto N., Kumamoto T., Robson S. C., Sévigny J., Matsue H., Enjyoji K., Takashima A. 2002. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat. Med. 8: 358–365 [DOI] [PubMed] [Google Scholar]
- 24.Enjyoji K., Kotani K., Thukral C., Blumel B., Sun X., Wu Y., Imai M., Friedman D., Csizmadia E., Bleibel W., et al. 2008. Deletion of cd39/entpd1 results in hepatic insulin resistance. Diabetes 57: 2311–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Synnestvedt K., Furuta G. T., Comerford K. M., Louis N., Karhausen J., Eltzschig H. K., Hansen K. R., Thompson L. F., Colgan S. P. 2002. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J. Clin. Invest. 110: 993–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jat P. S., Noble M. D., Ataliotis P., Tanaka Y., Yannoutsos N., Larsen L., Kioussis D. 1991. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc. Natl. Acad. Sci. USA 88: 5096–5100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans G. S., Flint N., Somers A. S., Eyden B., Potten C. S. 1992. The development of a method for the preparation of rat intestinal epithelial cell primary cultures. J. Cell Sci. 101: 219–231 [DOI] [PubMed] [Google Scholar]
- 28.Shi J. D., Kukar T., Wang C. Y., Li Q. Z., Cruz P. E., Davoodi-Semiromi A., Yang P., Gu Y., Lian W., Wu D. H., She J. X. 2001. Molecular cloning and characterization of a novel mammalian endo-apyrase (LALP1). J. Biol. Chem. 276: 17474–17478 [DOI] [PubMed] [Google Scholar]
- 29.Matsuki T., Watanabe K., Fujimoto J., Kado Y., Takada T., Matsumoto K., Tanaka R. 2004. Quantitative PCR with 16S rRNA-gene-targeted species-specific primers for analysis of human intestinal bifidobacteria. Appl. Environ. Microbiol. 70: 167–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong J., Forster R. J., Yu H., Chambers J. R., Sabour P. M., Wheatcroft R., Chen S. 2002. Diversity and phylogenetic analysis of bacteria in the mucosa of chicken ceca and comparison with bacteria in the cecal lumen. FEMS Microbiol. Lett. 208: 1–7 [DOI] [PubMed] [Google Scholar]
- 31.Bouskra D., Brézillon C., Bérard M., Werts C., Varona R., Boneca I. G., Eberl G. 2008. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 456: 507–510 [DOI] [PubMed] [Google Scholar]
- 32.Barman M., Unold D., Shifley K., Amir E., Hung K., Bos N., Salzman N. 2008. Enteric salmonellosis disrupts the microbial ecology of the murine gastrointestinal tract. Infect. Immun. 76: 907–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Enjyoji K., Sévigny J., Lin Y., Frenette P. S., Christie P. D., Esch J. S., II, Imai M., Edelberg J. M., Rayburn H., Lech M., et al. 1999. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat. Med. 5: 1010–1017 [DOI] [PubMed] [Google Scholar]
- 34.Robson S. C., Wu Y., Sun X., Knosalla C., Dwyer K., Enjyoji K. 2005. Ectonucleotidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin. Thromb. Hemost. 31: 217–233 [DOI] [PubMed] [Google Scholar]
- 35.Whitehead R. H., VanEeden P. E., Noble M. D., Ataliotis P., Jat P. S. 1993. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc. Natl. Acad. Sci. USA 90: 587–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saiga H., Nishimura J., Kuwata H., Okuyama M., Matsumoto S., Sato S., Matsumoto M., Akira S., Yoshikai Y., Honda K., et al. 2008. Lipocalin 2-dependent inhibition of mycobacterial growth in alveolar epithelium. J. Immunol. 181: 8521–8527 [DOI] [PubMed] [Google Scholar]
- 37.Gaboriau-Routhiau V., Rakotobe S., Lécuyer E., Mulder I., Lan A., Bridonneau C., Rochet V., Pisi A., De Paepe M., Brandi G., et al. 2009. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 31: 677–689 [DOI] [PubMed] [Google Scholar]
- 38.Ivanov I. I., Atarashi K., Manel N., Brodie E. L., Shima T., Karaoz U., Wei D., Goldfarb K. C., Santee C. A., Lynch S. V., et al. 2009. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139: 485–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El-behi M., Rostami A., Ciric B. 2010. Current views on the roles of Th1 and Th17 cells in experimental autoimmune encephalomyelitis. J. Neuroimmune Pharmacol. 5: 189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu H. J., Ivanov I. I., Darce J., Hattori K., Shima T., Umesaki Y., Littman D. R., Benoist C., Mathis D. 2010. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32: 815–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yokote H., Miyake S., Croxford J. L., Oki S., Mizusawa H., Yamamura T. 2008. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am. J. Pathol. 173: 1714–1723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okada S. F., Zhang L., Kreda S. M., Abdullah L. H., Davis C. W., Pickles R. J., Lazarowski E. R., Boucher R. C. 2011. Coupled nucleotide and mucin hypersecretion from goblet-cell metaplastic human airway epithelium. Am. J. Respir. Cell Mol. Biol. 45: 253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ivanov I. I., Littman D. R. 2010. Segmented filamentous bacteria take the stage. Mucosal Immunol. 3: 209–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rescigno M., Urbano M., Valzasina B., Francolini M., Rotta G., Bonasio R., Granucci F., Kraehenbuhl J. P., Ricciardi-Castagnoli P. 2001. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2: 361–367 [DOI] [PubMed] [Google Scholar]
- 45.Niess J. H., Brand S., Gu X., Landsman L., Jung S., McCormick B. A., Vyas J. M., Boes M., Ploegh H. L., Fox J. G., et al. 2005. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 307: 254–258 [DOI] [PubMed] [Google Scholar]
- 46.Schwiebert E. M., Zsembery A. 2003. Extracellular ATP as a signaling molecule for epithelial cells. Biochim. Biophys. Acta 1615: 7–32 [DOI] [PubMed] [Google Scholar]
- 47.Rescigno M., Lopatin U., Chieppa M. 2008. Interactions among dendritic cells, macrophages, and epithelial cells in the gut: implications for immune tolerance. Curr. Opin. Immunol. 20: 669–675 [DOI] [PubMed] [Google Scholar]
- 48.Ishigame H., Kakuta S., Nagai T., Kadoki M., Nambu A., Komiyama Y., Fujikado N., Tanahashi Y., Akitsu A., Kotaki H., et al. 2009. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30: 108–119 [DOI] [PubMed] [Google Scholar]
- 49.Zheng Y., Valdez P. A., Danilenko D. M., Hu Y., Sa S. M., Gong Q., Abbas A. R., Modrusan Z., Ghilardi N., de Sauvage F. J., Ouyang W. 2008. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat. Med. 14: 282–289 [DOI] [PubMed] [Google Scholar]
- 50.Mangan P. R., Harrington L. E., O’Quinn D. B., Helms W. S., Bullard D. C., Elson C. O., Hatton R. D., Wahl S. M., Schoeb T. R., Weaver C. T. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441: 231–234 [DOI] [PubMed] [Google Scholar]
- 51.Wolk K., Kunz S., Witte E., Friedrich M., Asadullah K., Sabat R. 2004. IL-22 increases the innate immunity of tissues. Immunity 21: 241–254 [DOI] [PubMed] [Google Scholar]
- 52.Sutton C. E., Lalor S. J., Sweeney C. M., Brereton C. F., Lavelle E. C., Mills K. H. 2009. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31: 331–341 [DOI] [PubMed] [Google Scholar]
- 53.Spits H., Di Santo J. P. 2011. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat. Immunol. 12: 21–27 [DOI] [PubMed] [Google Scholar]
- 54.Abromson-Leeman S., Bronson R. T., Dorf M. E. 2009. Encephalitogenic T cells that stably express both T-bet and ROR gamma t consistently produce IFNgamma but have a spectrum of IL-17 profiles. J. Neuroimmunol. 215: 10–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Korn T., Mitsdoerffer M., Croxford A. L., Awasthi A., Dardalhon V. A., Galileos G., Vollmar P., Stritesky G. L., Kaplan M. H., Waisman A., et al. 2008. IL-6 controls Th17 immunity in vivo by inhibiting the conversion of conventional T cells into Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. USA 105: 18460–18465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suryani S., Sutton I. 2007. An interferon-gamma-producing Th1 subset is the major source of IL-17 in experimental autoimmune encephalitis. J. Neuroimmunol. 183: 96–103 [DOI] [PubMed] [Google Scholar]
- 57.McGeachy M. J., Chen Y., Tato C. M., Laurence A., Joyce-Shaikh B., Blumenschein W. M., McClanahan T. K., O’Shea J. J., Cua D. J. 2009. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 10: 314–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee Y. K., Menezes J. S., Umesaki Y., Mazmanian S. K. 2011. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 108(Suppl. 1): 4615–4622 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.