Abstract
Purpose
Preoperative chemoradiotherapy (CRT) is part of the standard treatment of locally advanced rectal cancers. Tumour regression at the time of operation is desirable, but not much is known about the relationship between radiation dose and tumour regression. In the present study we estimated radiation dose-response curves for various grades of tumour regression after preoperative CRT.
Methods and Materials
A total of 222 patients, treated with consistent chemotherapy and radiotherapy techniques, were considered for the analysis. Radiotherapy consisted of a combination of external beam radiotherapy and brachytherapy. Response at the time of operation was evaluated from the histopathological specimen and graded on a five point scale (TRG1-5). The probability of achieving complete, major and partial response was analyzed using ordinal logistic regression, and the effect of including clinical parameters in the model was examined. The radiation dose response relationship for a specific grade of histopathological tumour regression was parameterized in terms of the dose required for 50% response, D50,i, and the normalized dose-response gradient, γ50,i.
Results
A highly significant dose–response relationship was found (p=0.002). For complete response (TRG1) the dose-response parameters were D50,TRG1=92.0 Gy (95% CI: 79.3 to 144.9 Gy), γ50,TRG1=0.982 (0.533 to 1.429), and for major response (TRG1-2) D50,TRG1&2=72.1 Gy (65.3 to 94.0 Gy), γ50,TRG1&2=0.770 (0.338 to 1.201). Tumour size and N-category both had a significant effect on the dose-response relationships.
Conclusions
This study has demonstrated a significant dose-response relationship for tumour regression after preoperative CRT for locally advanced rectal cancer for tumour dose levels in the range of 50.4 to 70 Gy, which is higher than the dose-range usually considered.
Keywords: Rectal cancer, chemoradiation, dose-response, tumour regression, dose escalation
Introduction
Preoperative chemoradiotherapy (CRT) is an integral part of the treatment of advanced rectal cancer [1]. Long course radiotherapy (RT) improves local control, and often causes downstaging of the tumour [2], but it has so far not been shown to affect long term survival [2,3]. Patients with a pathological complete response (pCR) at the time of operation have better disease-free survival [4]. It may, however, be due to responsive tumours having an inherently more favourable prognosis, and the value of pCR as surrogate endpoint has therefore not been conclusively demonstrated. Nevertheless, a subgroup of patients have so favourable response to CRT that they may be candidates for a watch-and-wait approach, omitting surgery. This is of particular interest to patients with a tumour location that does not allow for sphincter-preserving surgery [5].
Despite this, the effect of varying the radiation dose of the CRT regimen on tumour regression has so far not been quantified. A higher tumour dose (45 Gy or more) has been identified as an independent factor affecting the level of pCR [6], and phase II studies have indicated the presence of a dose-response relationship [7–9]. A new report [10] from our own institution confirmed these findings, but also showed that response level depends strongly on tumour size. Thus, current knowledge is based on a limited number of studies, in which variation in tumour stage, chemotherapy and RT technique may confound the analysis and makes extraction of an accurate dose-response relationship difficult.
In this study, we estimate a dose-response relationship for varying grades of tumour regression after preoperative CRT for locally advanced rectal cancer by analysing data on 222 patients treated with consistent RT techniques and chemotherapy regimens, well-defined pre-treatment tumour characteristics, and response evaluation by uniform pathological procedures.
Methods and materials
Patients participating in two previously published clinical trials [10,11] were eligible for inclusion: I) A phase II study of the effect of high-dose preoperative CRT for locally advanced T3 rectal cancers [11], in which RT consisted of external radiation (60 Gy/30 fractions to the tumour, 48.6 Gy/27 fractions to the lymph nodes in the posterior part of the pelvis), supplemented by a brachytherapy boost of 5 Gy in a single fraction. II) A phase III study evaluating the effect of adding a brachytherapy boost to the CRT regimen for T3 and T4 tumours [10], in which RT consisted of external radiation of 50.4 Gy/28 fractions to tumour and lymph nodes, and where patients were randomized to +/− a brachytherapy tumour boost of 10 Gy in 2 fractions.
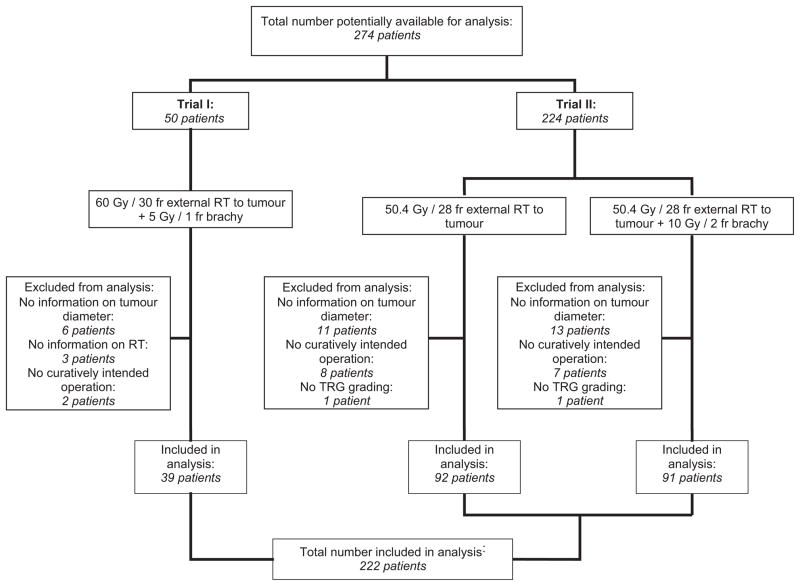
Only patients where full information on tumour size, delivered RT and tumour regression at the time of operation was available were included in the analysis. Figure 1 presents a flowchart of the patient selection.
Figure 1.
Flow-diagram of inclusion of patients in the analysis. For details on trial I and II, respectively, see [11] and [10]. RT: Radiotherapy. TRG: Tumour regression grade.
All tumours were histopathologically verified as adenocarcinomas, were less than 10 cm from the anal verge and had a circumferential margin on MRI of less than 5 mm. They were identified as T3-4, N0-2, M0 tumours based on MRI of the pelvis, rectal ultrasound, chest and abdominal CT scans or x-rays, and rectoscopy. Tumour size (maximum length and diameter) was estimated from MRI scans.
CT-based conformal treatment plans using 6 and/or 18 MV photon beams were used for external RT. Full tumour dose was given to a clinical target volume (CTV) consisting of tumour and rectum on the same level, plus a 1 cm margin to create the planning target volume (PTV). PTV coverage was optimized towards 95–107% of the prescribed dose. A secondary nodal volume was defined and treated according to the protocols, see [10,11] for details. The bladder, small intestine and femoral heads were defined as normal tissue at risk and spared to the highest extent possible while maintaining full CTV coverage. High dose-rate brachytherapy was delivered using a rigid, single channel endorectal applicator described in detail elsewhere [12] and a single 192Ir source. Applicator diameter (1.0 or 2.0 cm) was chosen on a patient-to-patient basis. Dose was prescribed 1.0 cm from the applicator surface, corresponding to 1.5 or 2.0 cm from the applicator central axes, and was planned so as to provide a uniform dose distribution along the tumour central axis. Orthogonal x-rays were used for verification of applicator position. Brachytherapy was scheduled for week 2 or 3 (study I) or weeks 4 and 6 (study II) during the treatment course. No external radiation was given on brachytherapy treatment days. Patients who could not comply with brachytherapy were prescribed an external boost of 6 or 12 Gy, delivered with 2 Gy per fraction, according to the protocols.
Chemotherapy consisted of daily peroral Tegafur-Uracil (UFT) 300 mg/m2 (split into three doses daily) and peroral L-leucovorin 7.5 mg × 3 during the whole treatment course. Both were given on treatment days (external RT and brachytherapy) only. Chemotherapy was discontinued in case of grade III toxicity.
Tumour regression at the time of operation was estimated during routine histopathological evaluation of the surgical specimens. Response was classified according to Mandard’s tumour regression grade (TRG) [13,14]: TRG1 corresponds to no residual tumour (complete response), TRG2 indicates only microscopic residual tumour (i.e. major response), TRG3 is a moderate response, TRG4 a minor response and TRG5 no response.
Estimation of tumour radiation dose
The total tumour dose was calculated on an individual patient basis, as a sum of external RT and brachytherapy. The total dose was based on information on the actual treatment delivered, and, in a separate analysis, according to intention to treat (ITT). If an external boost had been delivered to patients not complying with brachytherapy, external beam RT boost dose was used in the ITT analysis, as this procedure was according to protocol. For the external RT, a homogeneous tumour dose was assumed. All doses were converted to equivalent doses as given in 2 Gy-fractions (EQD2) using the linear quadratic model [15]
D is the total dose, d the dose per fraction and a generic α/β ratio of 10 Gy was assumed for the tumour. For the brachytherapy treatment, the radial dose fall-off was assumed to depend inversely on the distance, r. Hence the tumour dose for a single fraction at a distance r from the centre of the applicator was estimated as
where Dpres is the prescribed dose at distance Rpres from the applicator surface, and Rapp is the applicator diameter. We corrected the local dose to EQD2 and integrated over the entire tumour volume to estimate the average EQD2 from each brachytherapy fraction. Assuming that the tumour radius, R, is constant over the length of the tumour and that the tumour radius can be estimated from the pre-treatment MRI, we find
Here, is the estimated average radius of tumour and applicator (in cm) after the applicator has been positioned. We assumed that the rectal lumen was collapsed prior to the positioning of the applicator, and that the tumour, after positioning, stretched such as to preserve the total cross sectional area at each point along the applicator.
Statistical model
Tumour response was fitted to an ordinal logistic model using average EQD2 tumour dose as covariate. Lowest observed response grade was regarded as baseline, all other levels as responders. The model had the form
where TRPTRG≤i is the probability of response of at least grade TRGi, b0,i a parameter dependent on response grade and b1 a parameter describing the common dose-response. This provided a sigmoid response curve for each response grade, with one added extra variable per grade included in the analysis. The individual dose-response curves could be characterized by D50,i = −b0,i/b1, defined as the dose resulting in 50% response, and γ50,i = −0.25b0,i, defined as the normalized dose-response gradient at D50,i [16].
Additional clinical variables, such as tumour stage and size, were added to the model via the modification
where Yj could be either a binary or a continuous variable and was assumed to influence the probability of the various response grades in the same way.
All models were fitted in MATLAB® (2010b, The MathWorks Inc, Natick, MA) using maximum likelihood fitting, and confidence intervals (CIs) were estimated using a bootstrap procedure drawing 10,000 samples with replacement from the original dataset. The improvement in model fit from adding clinical parameters was evaluated using the likelihood ratio test. Two-tailed p-values of <0.05 were considered statistically significant.
Results
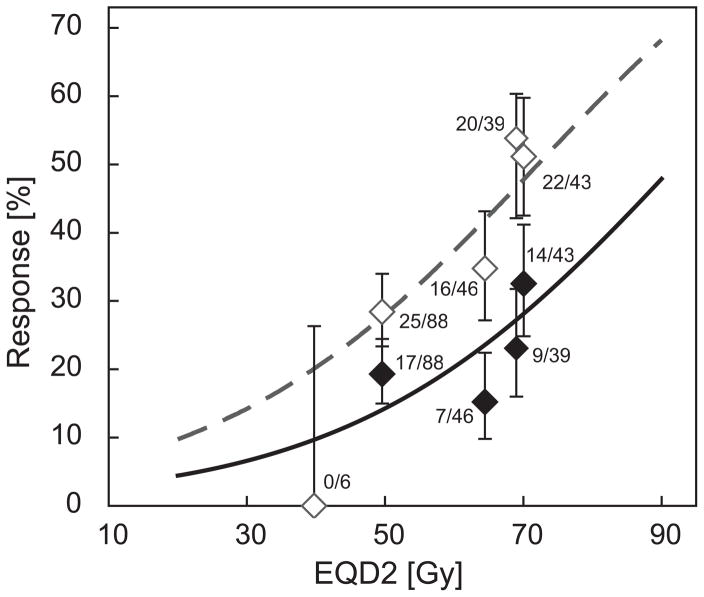
A total of 222 patients were available for analysis, consisting of 39 patients from trial I and 183 patients from trial II. See table 1 for a summary of patient characteristics and figure 1 for a flow diagram of patient inclusion. No patients presented a TRG5 response. Consequently, TRG4 was used as baseline, and positive response was regarded as either complete (TRG1), major (TRG1-2) or some (TRG1-3) response. The resulting model parameters can be found in table 2. Figure 2 shows the corresponding dose response curves for complete and major response, with clinical data added to illustrate the quality of the fit. The curves are characterized by D50,TRG1=92.0 Gy (95% CI: 79.3 to 144.9 Gy), γ50,TRG1=0.982 (0.533 to 1.429) for complete response and D50,TRG1&2=72.1 Gy (65.3 to 94.0 Gy), γ50,TRG1&2=0.770 (0.338 to 1.201) for major response.
Table 1.
Patient characteristics.
| Study I | Study II | |
|---|---|---|
| Age [years] | 63 (48–76) | 63 (35–78) |
|
| ||
| Gender | ||
| - Men/women | 31/8 | 113/70 |
|
| ||
| Tumour stage: | ||
| - T3/T4 | 39/0 | 149/34 |
| - N0/N1/N2 | 14/21/4 | 22/80/81 |
|
| ||
| Tumour size | ||
| - Diameter [cm] | 4.0 (2.3–5.0) | 3.5 (1.5–6.6) |
| - Length [cm] | 4.8 (2.5–8.0) | 4.5 (1.8–10) |
|
| ||
| Tumour regression after CRT | ||
| - TRG1/TRG2/TRG3/TRG4 | 9/12/16/2 | 38/25/106/14 |
All continuous values are medians, numbers in brackets indicate range. CRT: Chemoradiotherapy. TRG: Tumour regression grade.
Table 2.
Results for model fits
| Model with dose only | ||||
|---|---|---|---|---|
|
| ||||
| Actual dose delivered
|
Intention To Treat
|
|||
| b0,TRG1: | −3.928 (−5.787;−2.181) | b0,TRG1: | −3.511 (−5.417;−1.621) | |
| b0,TRG2: | −3.079 (−4.865;−1.392) | b0,TRG2: | −2.671 (−4.501;−0.814) | |
| b0,TRG3: | 0.085 (−1.591;1.693) | b0,TRG3: | 0.451 (−1.282;2.153) | |
| b1: | 0.0427 (0.0153;0.0712) | b1: | 0.0358 (0.0060;0.0648) | |
| Model with dose, tumour size and N-category
| ||||
|---|---|---|---|---|
|
| ||||
|
| ||||
| Actual dose delivered
|
Intention To Treat
|
|||
| b0,TRG1: | −2.634 (−4.836;0.600) | b0,TRG1: | −2.106 (−4.400;0.060) | |
| b0,TRG2: | −1.762 (−3.903;0.246) | b0,TRG2: | −1.240 (−3.514;0.856) | |
| b0,TRG3: | 1.463 (−0.641;3.404) | b0,TRG3: | 1.949 (−0.281;3.979) | |
| b1: | 0.0349 (0.0060;0.0641) | b1: | 0.0268 (−0.0039;0.0580) | |
| btumour-size: | −0.0058 (−0.0132;0.0008) | btumour-size: | −0.0061 (−0.0134;0.0008) | |
| bN-category: | −0.6252 (−1.3399;0.1449) | bN-category: | −0.6580 (−1.3920;0.1106) | |
TRPTRG≤i is the probability of tumour regression of at least grade TRGi. EQD2 is the equivalent average dose to the tumour in 2 Gy fractions. Yvol is the pre-treatment tumour volume, as estimated from the tumour length and diameter on the pre-treatment MRI scan and assuming cylindrical symmetry. YN-category is 0 for clinical N0 patients and 1 for N1-2 patients. The b1 coefficient has the unit of [Gy−1], the btumour-size coefficient the unit of [cm−3]. All other coefficients are dimensionless.
Figure 2.
Dose-response relationships for complete response (TRG1, solid line, filled squares) and major response (TRG1-2, dashed line, outlined squares) after pre-operative chemoradiotherapy (CRT) for rectal cancer. The model includes the full information on tumour regression grade (TRG1-4); however, for clarity only TRG1 and TRG2 responses are shown. Patient response data has been sorted into the following bins: 1) Patients not completing the full CRT course; 2) patients in trial II without brachytherapy; 3) patients in trial II with brachytherapy, below median tumour dose; 4) patients in trial II with brachytherapy, above median tumour dose; 5) patients in trial I. Error bars indicate 68% confidence intervals. EQD2: equivalent average dose to the tumour in 2 Gy fractions.
The results were tested for their sensitivity to the value of the α/β ratio. For α/β in the range of 5 to 15 Gy, D50,TRG1 varied between 93.3 and 89.1 Gy, D50,TRG1&2 between 75.5 and 70.7 Gy.
Clinical variables were included one by one and tested for significance in the model; all results are shown in table e1 (available online only). Tumour pre-treatment size (p=0.040, continuous variable) and N-category (p=0.039, binary variable) both significantly improved the fit when included individually. Tumour size was estimated from the diameter and length on the pre-treatment MRI scan, assuming cylindrical geometry; an increase in tumour volume of 50 cm3 corresponded to an odds ratio of 0.71 for positive response. N1-2 tumours had an odds ratio of 0.48 compared to N0 tumours. Adding the two clinical parameters (size and N-category) to the model simultaneously resulted in both parameters becoming not statistically significant (p ≈ 0.07 for both). However, since the model was still significantly better than a simple model with dose only, both clinical variables were maintained in the final model, see table 2. Neither T-category (p=0.161, binary variable), age (p=0.964, continuous variable) nor gender (p=0.823, binary variable) were significant. All analyses were repeated in the ITT analysis, with the optimal model fits reported in table 2. This did not result in any major changes to the above results, although the dose response became slightly shallower and D50 increased, as expected. The response curves for “intention to treat” doses were characterized by D50,TRG1=98.1 Gy (95% CI: 80.5 to 210.7 Gy), γ50,TRG1=0.878 (0.403 to 1.350) for complete response and D50,TRG1&2=74.6 Gy (65.9 to 120.3 Gy), γ50,TRG1&2=0.668 (0.211 to 1.128) for major response.
Discussion
This study has demonstrated a clear done-response relationship for tumour regression after preoperative CRT for rectal cancer. Previous studies have indicated the existence of such a relationship, but to our knowledge this is the first study providing a mathematical parameterization of the dose dependence in combination with clinical predictors.
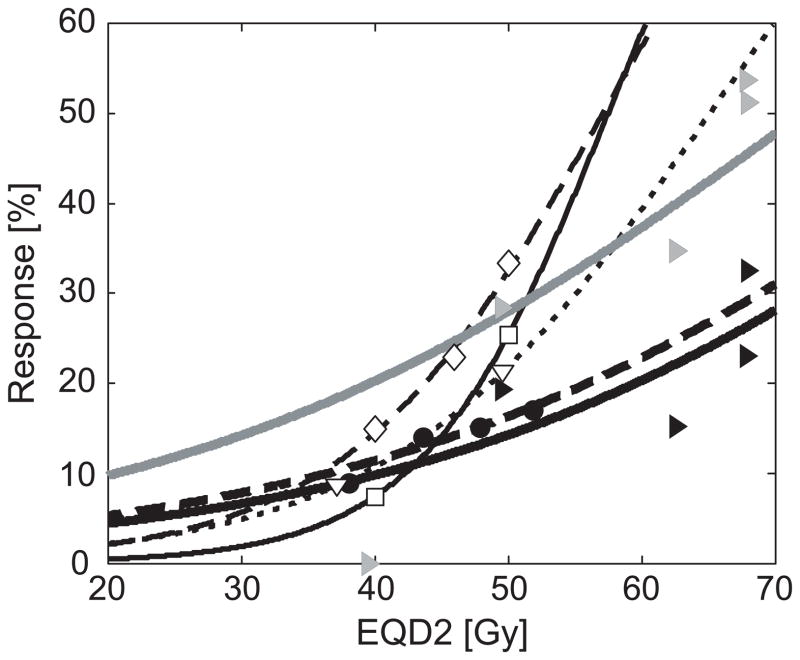
Chan et al [7] and Valentini et al [8] both demonstrated the effects of radiation dose escalation when combined with an intensification of the chemotherapy regimen, while Wiltshire et al [9] compared three radiation dose levels in three consecutive phase II trials with consistent chemotherapy for all patients. They all found an increased rate of pCR at higher dose levels, but neither of the studies investigated radiation doses above 60 Gy. The current study has confirmed the findings of these reports, using consistent chemotherapy regimens and modern 3D conformal RT techniques, with a range of tumour radiation doses (from 50.4 Gy to >70 Gy). Dose-response relationships for both complete response (D50,TRG1 = 92.0 Gy, γ50,TRG1 =0.98) and major response (D50,TRG1&2 = 72.1 Gy,γ50,TRG1&2=0.77) were established from individual patient doses and response grades. Figure 3 shows these response curves in the context of the results of the previously published studies [7–9]. Furthermore, the response-levels found in multivariate analysis in the review by Sanghera et al [6] are shown. The steep response curves from [7] and [8] can be explained by the additional chemotherapy in the high-dose groups. In the Wiltshire et al study there may have been an association between RT techniques and prescription dose, as higher dose regimens were given in the most recently treated patient groups.
Figure 3.
Comparison of reported dose-response relationships between EQD2 (equivalent dose in 2 Gy fractions) and complete response. All dose levels recalculated as EQD2, and logistic response-curves fitted for each study. Bold, dashed line; filled circles: Meta-analysis by Sanghera et al [6]. Thin, solid line; open squares: Chan et al [7]. Thin, dotted line; open triangles: Valentini et al [8]. Thin, dashed line; open diamonds: Wiltshire et al [9]. Bold, solid lines; filled triangles: Our study, with response curves for complete response (black line/triangles) and major response (grey line/triangles).
A number of trials have tested the addition of a second drug to the chemotherapy regimen (see e.g. [17] or [18]). However, while the response rates are encouraging, relatively high rates of acute toxicity have been reported. In order to match the complete tumour response of 17% reported in the STAR-01 trial, arm B [17], we estimate the need for a tumour dose of ~55 Gy for UFT based chemotherapy. Similarly, to match the 39% major response in the ACCORD trial, Capox 50 arm [18], a dose of ~62 Gy is needed. Given that the acute toxicity with radiotherapy dose escalation seems acceptable [11], this may be a viable alternative.
Most studies of preoperative RT for rectal cancer measure tumour response by the rate of pCR. Dichotomizing the response is associated with a loss of information and thereby potentially with a loss of statistical power. Moreover, tumour size may confound the evaluation of the endpoint, especially for advanced tumours where size alone might prevent observing a complete regression after CRT in many cases. Furthermore, the estimation of pCR from the pathological specimens might be influenced by the evaluation method used [19]. The variance in the response levels reported (see figure 3) could thus be a result of variations in pathological evaluation, rather than a true difference in response. The Mandard five-point grading system used in this study appears reproducible [20] and while maintaining the full grading information, it also facilitates subsequent pooling of response grades. This notion is supported by the fact that the response curves for complete and major response span the majority of the reported range of response. The ordinal logistic model employed here is a widely used, purely empirical model. It utilizes the assumption that the effect of radiation is similar for all tumours and can be parameterized by a logistic curve, but attempts no explanation of this. By using the fraction-size corrected dose as covariate, however, the model becomes sensitive to the choice of α/β ratio. We found, though, that our results were relatively insensitive to a change in α/β value – e.g. a choice of α/β=5 Gy resulted in D50,TRG1&2=75.5 Gy. Our analysis did not take dose recovery due to protraction of treatment time into account. If a value of 0.54 Gy recovered per day [21] is used to adjust for treatment time deviating from 39 days overall treatment time (corresponding to 50.4 Gy/28 fractions), a slightly lower D50,TRG1&2 of 70.2 Gy and slightly steeper γ50,TRG1&2 of 0.801 is derived.
That inclusion of tumour size in the model significantly improves the fit to data is unsurprising; but the underlying reason for this could be any combination of factors, such as larger tumours having a higher content of clonogenic cells, an increased radioresistance (e.g. because of hypoxia), generally more aggressive behaviour, etc. The model validity is independent of the true mechanism explaining the influence of tumour size or nodal status.
This study is a single institution study, thus maximizing the homogeneity in chemotherapy, RT techniques, pathology, and response evaluation. However, this could also affect the generalizability of the results. As an example, the margins used for generation of treatment planning volumes may not be consistent with emerging evidence from repeated imaging studies [22]. Similarly, only a relatively small number of patients were included, and the model constructed should be tested in a larger population, ideally containing patients from several institutions and treated with a variety of doses, applied both as external RT and brachytherapy. Estimation of tumour dose from a combined modality external and brachytherapy treatment can be difficult, not at least due to the potential breakdown of the linear quadratic model for high dose per fraction. In this study, a residual uncertainty in the dose estimate from the brachytherapy remains. Tumour diameter at the time of treatment was not available due to the lack of imaged based brachytherapy planning. Therefore a simplified assumption of constant maximum distance from the applicator to the outer edge of the tumour was employed. For future studies, a more accurate brachy therapy dose planning ought to be introduced, especially if CRT is to be applied as a curative modality without surgery for locally advanced rectal cancer, where the enhanced therapeutic ratio offered by brachytherapy may be a way to achieve sufficiently high doses.
Conclusion
This study has demonstrated the existence of a clear dose-response relationship for tumour regression after preoperative CRT for locally advanced rectal cancer, with response curves constructed both for complete and major response. Furthermore, it has confirmed the importance of tumour size in the prediction of treatment response grade.
Supplementary Material
Summary.
This study compared radiation dose and tumour regression at the time of operation in 222 rectal cancer patients. A significant correlation between dose and tumour regression was found when considering dose-levels in the range of 50.4 to 70 Gy, and dose-response relationships for different levels of tumour regression were established. Tumour size and N-category both had an influence on the dose-response. This may prove of interest for dose-escalation studies and watch-and-wait protocols.
Acknowledgments
ALA and IRV are supported by CIRRO - The Lundbeck Foundation Center for Interventional Research in Radiation Oncology and The Danish Council for Strategic Research. ALA acknowledges support from the Region of Southern Denmark. IRV is supported by the Global Excellence in Health program of the Capital Region of Denmark. The authors acknowledge and appreciate the assistance of Bjarke Mortensen in collecting radiation treatment details for the trial patients.
Footnotes
Meeting presentation
The results will be presented at the ESTRO 31 conference, on the 12th of May 2012 in Barcelona, Spain.
Conflicts of Interest Notification
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haustermans K, Debucquoy A, Lambrecht M. The ESTRO Breur Lecture 2010: Toward a tailored patient approach in rectal cancer. Radiother Oncol. 2011;100:15–21. doi: 10.1016/j.radonc.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 2.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 3.Gérard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620–4625. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 4.Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–844. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 5.O’Neill BD, Brown G, Heald RJ, et al. Non-operative treatment after neoadjuvant chemoradiotherapy for rectal cancer. Lancet Oncol. 2007;8:625–633. doi: 10.1016/S1470-2045(07)70202-4. [DOI] [PubMed] [Google Scholar]
- 6.Sanghera P, Wong DW, McConkey CC, et al. Chemoradiotherapy for rectal cancer: an updated analysis of factors affecting pathological response. Clin Oncol (R Coll Radiol) 2008;20:176–183. doi: 10.1016/j.clon.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Chan AK, Wong AO, Langevin J, et al. Preoperative chemotherapy and pelvic radiation for tethered or fixed rectal cancer: a phase II dose escalation study. Int J Radiat Oncol Biol Phys. 2000;48:843–856. doi: 10.1016/s0360-3016(00)00692-1. [DOI] [PubMed] [Google Scholar]
- 8.Valentini V, Coco C, Cellini N, et al. Ten years of preoperative chemoradiation for extraperitoneal T3 rectal cancer: acute toxicity, tumor response, and sphincter preservation in three consecutive studies. Int J Radiat Oncol Biol Phys. 2001;51:371–383. doi: 10.1016/s0360-3016(01)01618-2. [DOI] [PubMed] [Google Scholar]
- 9.Wiltshire KL, Ward IG, Swallow C, et al. Preoperative radiation with concurrent chemotherapy for resectable rectal cancer: effect of dose escalation on pathologic complete response, local recurrence-free survival, disease-free survival, and overall survival. Int J Radiat Oncol Biol Phys. 2006;64:709–716. doi: 10.1016/j.ijrobp.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Jakobsen A, Ploen J, Vuong T, et al. The dose-effect relationship in chemoradiation of locally advanced rectal cancer. A randomized trial comparing two radiation doses. Int J Radiat Oncol Biol Phys. 2012 doi: 10.1016/j.ijrobp.2012.02.006. in press. [DOI] [PubMed] [Google Scholar]
- 11.Jakobsen A, Mortensen JP, Bisgaard C, et al. Preoperative chemoradiation of locally advanced T3 rectal cancer combined with an endorectal boost. Int J Radiat Oncol Biol Phys. 2006;64:461–465. doi: 10.1016/j.ijrobp.2005.07.969. [DOI] [PubMed] [Google Scholar]
- 12.Hansen JW, Jakobsen A. The importance of applicator design for intraluminal brachytherapy of rectal cancer. Med Phys. 2006;33:3220–3224. doi: 10.1118/1.2207143. [DOI] [PubMed] [Google Scholar]
- 13.Mandard AM, Dalibard F, Mandard JC, et al. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680–2686. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 14.Bouzourene H, Bosman FT, Seelentag W, et al. Importance of tumor regression assessment in predicting the outcome in patients with locally advanced rectal carcinoma who are treated with preoperative radiotherapy. Cancer. 2002;94:1121–1130. [PubMed] [Google Scholar]
- 15.Joiner MC, Bentzen SM. Fractionation: the linear-quadratic approach. In: Joiner MC, van der Kogel A, editors. Basic clinical radiobiology. 4. London, UK: Hodder Arnold; 2009. pp. 102–119. [Google Scholar]
- 16.Bentzen SM, Tucker SL. Quantifying the position and steepness of radiation dose-response curves. Int J Radiat Biol. 1997;71:531–542. doi: 10.1080/095530097143860. [DOI] [PubMed] [Google Scholar]
- 17.Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773–80. doi: 10.1200/JCO.2010.34.4911. [DOI] [PubMed] [Google Scholar]
- 18.Gérard JP, Azria D, Gourgou-Bourgade S, et al. Comparison of two neoadjuvant chemoradiotherapy regimens for locally advanced rectal cancer: results of the phase III trial ACCORD 12/0405-Prodige 2. J Clin Oncol. 2010;28:1638–44. doi: 10.1200/JCO.2009.25.8376. [DOI] [PubMed] [Google Scholar]
- 19.Lindebjerg J, Ploen J, Rafaelsen S, et al. Step-sectioning of paraffin block from “completely regressed” rectal carcinoma after preoperative chemoradiation [Abstract] Ann Oncol. 2009;20(Suppl 7):vii59. [Google Scholar]
- 20.Lindebjerg J, Hansborg N, Ploen J, et al. Factors influencing reproducibility of tumour regression grading after high-dose chemoradiation of locally advanced rectal cancer. Histopathology. 2011;59:18–21. doi: 10.1111/j.1365-2559.2011.03888.x. [DOI] [PubMed] [Google Scholar]
- 21.Suwinski R, Taylor JM, Withers HR. Rapid growth of microscopic rectal cancer as a determinant of response to preoperative radiation therapy. Int J Radiat Oncol Biol Phys. 1998;42:943–51. doi: 10.1016/s0360-3016(98)00343-5. [DOI] [PubMed] [Google Scholar]
- 22.Nijkamp J, Swellengrebel M, Hollmann B, et al. Repeat CT assessed CTV variation and PTV margins for short- and long-course pre-operative RT of rectal cancer. Radiother Oncol. 2012;102:399–405. doi: 10.1016/j.radonc.2011.11.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.