Abstract
Staphylococcus aureus is a commensal of the human skin or nares and a pathogen that frequently causes skin and soft tissue infections as well as bacteremia and sepsis. Recent efforts in understanding the molecular mechanisms of pathogenesis revealed key virulence strategies of S. aureus in host tissues: bacterial scavenging of iron, induction of coagulation pathways to promote staphylococcal agglutination in the vasculature, and suppression of innate and adaptive immune responses. Advances in all three areas have been explored for opportunities in vaccine design in an effort to identify the critical protective antigens of S. aureus. Human clinical trials with specific subunit vaccines have failed, yet provide important insights for the design of future trials that must address the current epidemic of S. aureus infections with drug-resistant isolates (MRSA, methicillin-resistant S. aureus).
Keywords: MRSA, Iron, Coagulation, Immunmodulation
Introduction
Staphylococcus aureus colonizes the skin and nares of 30–50% of the human population. Nasal colonization typically persists for long periods of time and represents a predisposition to future disease, typically skin and soft tissue infections (SSTIs) but also bacteremia and sepsis. A key feature of S. aureus SSTIs is recurrence, which occurs in 20–30 % of all cases even following antibiotic and/or surgical therapy [1]. Following the initial discovery and use of antibiotics, concern and frequency of S. aureus disease decreased significantly. However, the golden age of antibiotic therapy was followed by S. aureus acquisition of a wide variety of antibiotic resistance genes that provided escape from the most commonly used therapeutics [2]. Of particular concern is the emergence of methicillin-resistant S. aureus (MRSA), from community origins (community-acquired or CA-MRSA) and acquisition of additional antibiotic resistance including vancomycin (VRSA), often the antibiotic of last resort for infections with CA-MRSA [3–5]. S. aureus infections currently account for ~4% of all hospital admissions in the United States with the related mortality in the US exceeding that of any other infectious disease [6]. In addition, S. aureus infections are the leading cause of respiratory, skin and soft tissue, and bloodstream infections [6]. Considering that S. aureus has evolved drug-resistance against every antibiotic licensed for the therapy of staphylococcal infections [7], it seems highly unlikely that a miracle drug or silver bullet will be discovered addressing these issues.
Hygienic measures reduce the burden of staphylococcal infections. Although scientists have tried for decades to develop a vaccine that can protect against S. aureus infections, these efforts have not yet borne fruit and anti-staphylococcal vaccines are not available. An important obstacle in the development of vaccines is the clinical evidence for staphylococcal immune evasion. The very same individuals encounter recurrent infections with the same S. aureus strain, but are unable to mount protective immune responses [8]. The failure of a variety of subunit vaccines in late stage clinical trials highlights the formidable obstacles on the road towards a staphylococcal vaccine [7, 9–12]. Here we review recent work in three areas of S. aureus pathogenesis – iron scavenging, coagulation and immune evasion – and what this research has taught us about vaccine development.
I. Iron homeostasis
Iron in the host
Iron is an indispensable element for many organisms. In the human body iron is an essential component of hemoglobin, important for delivery and transport of oxygen through the blood to major organs and tissues. During cellular respiration, iron is important for energy generating redox reactions. The ability of iron to easily accept and donate electrons makes iron both essential and potentially toxic. Specifically, free, unregulated iron within the cell can catalyze the conversion of hydrogen peroxide into free radicals, having deleterious consequences. To prevent such harmful effects, the abundance and usage of iron in the body is tightly controlled, with free soluble iron concentrations kept at very low levels. As a result the majority of iron in the body is intracellular. 60–80% of the intracellular iron is located at the center of the porphyrin ring of heme [13, 14], a cofactor for hemoglobin in the blood or myoglobin in muscle tissue. Extracellular heme levels are controlled by the heme scavenging host protein hemopexin [15] while extracellular hemoglobin is bound by haptoglobin [16] and the complex removed by the reticuloendothelial system [17]. An additional 15–20% of iron is complexed with the storage molecule ferritin in non-erythrocyte cells [14]. The remaining extracellular iron is scavenged and tightly bound by transferrin in the plasma or lactoferrin in mucosal and similar secretions, aiding intercellular iron transport and preventing iron generated free radicals [18].
Iron homeostasis is regulated through the control of absorption and transport into cells. This occurs mainly through the effects of the small peptide hormone hepcidin which is made and released by the liver in response to iron levels in the body [19–22]. When iron levels are high, hepcidin levels increase and inhibit the uptake of transferrin iron from the plasma into iron storage cells (such as red blood cells) by binding to the Fe transporter ferriportin [23]. This results in the endocytosis and degradation of ferriportin and decreased iron absorption. Under low levels of iron, hepcidin levels are decreased and iron is more readily taken up by cells [23, 24]. Bacterial infection, one cause of inflammation in the host, also results in increased production of hepcidin [25, 26].
Host iron linked defense mechanisms
Much like in its human host, iron, required for cellular respiration, is also an essential element for S. aureus. As such, iron acquisition from the environment is vital to the survival of the pathogen. In addition to the usual tight control of free iron availability in the human body, the human host has a number of strategies, collectively referred to as the iron withholding defense system [27], to further limit iron availability to invading pathogens such as S. aureus. As mentioned, increased hepcidin levels during inflammation [28] function to suppress release of iron by macrophages and by duodenal enterocytes, concomitant with increased synthesis of macrophage ferritin [23, 29]. Additional iron withholding defenses are mediated by professional phagocytes called to the site of infection, including neutrophil release of apolactoferrin to bind iron [30], macrophage scavenging of hololactoferrin [31], release of bacterial siderophore binding lipocalins [32, 33], and release of nitric oxide which interferes with the pathogen’s iron metabolism [34]. Other described defenses include hepatic release of haptoglobin and hemopexin to bind and scavenge extracellular hemoglobin and heme [35] and B lymphocyte production of immunoglobulins to microbial cell surface receptors that mediate pathogen iron uptake [36]. These measures together limit free iron availability in human tissues to around 10−18 M or less [37], levels greatly below what is needed by the pathogen to continue to grow and cause disease.
Pathogen obstacles and strategies for obtaining and regulating iron
In order to successfully replicate and divide, bacterial pathogens have developed numerous mechanisms for iron scavenging, uptake, and transport of limited iron which circumvent the iron withholding defenses of the host. S. aureus is not an exception, and has developed a variety of mechanisms to acquire iron from its human host. These mechanisms include systems for binding transferrin, lactoferrin, and/or heme containing proteins by specific bacterial receptors and synthesis and secretion of siderophores, which in turn bind back to specific receptors on the bacterial surface. In addition to systems for acquiring iron, much like the host, bacterial pathogens must also tightly regulate the factors that influence their own intracellular iron levels. S. aureus uses a variety of regulatory systems to monitor iron levels and levels in the surrounding environment. This can occur directly via iron dependent regulators, which in the presence of iron bind target sequences located in the promoter region of bacterial iron acquisition proteins repressing their expression. S. aureus possesses homologues from two such negative repressor metalloregulatory protein families, Fur (ferric uptake regulator) and DtxR (diphtheria toxin repressor). Two Fur family homologues, Fur and PerR together with the DtxR homologue, MntR (also called SirR or FeoA) [38–41] co-ordinate S. aureus metal ion homeostasis and influence the expression of iron responsive proteins. Fur is directly responsive to iron as a Fe(II)-dependent regulator while PerR and DtxR regulation is indirect, mediated through their Mn-dependent roles. Through these three regulators, S. aureus has been shown to coordinate the intracellular availability of free iron with the level of antioxidant proteins and manganese present in the cell [39]. The global importance of iron metabolism and acquisition is demonstrated by reduced virulence of fur mutant staphylococci in both murine skin [42] and pneumonia infection models [43].
A second iron sensing system recently described for S. aureus is involved in directly sensing and regulating intracellular levels of heme. The two component signal transduction system, HssRS (heme sensor system), directly controls expression of the heme regulated ABC transporter HrtAB, which is thought to function as an efflux pump, removing excessive heme, or a toxic byproduct, from the bacterial cell [44, 45]. In the absence of the response regulator HssR or the ATPase component of the efflux pump HrtA, the staphylococci exhibit increased sensitivity to hemin toxicity. In vivo, intravenous infection of mice with either mutant strain exhibited liver specific increases in bacterial load and abscesses and an up-regulation of secreted virulence factors with important immunomodulatory functions [46]. The authors speculate that the hypervirulence in the liver is due to this change in expression, perhaps resulting from membrane damage following buildup of heme or a toxic byproduct in the absence of effective export [47].
Staphylococcus aureus iron acquisition systems
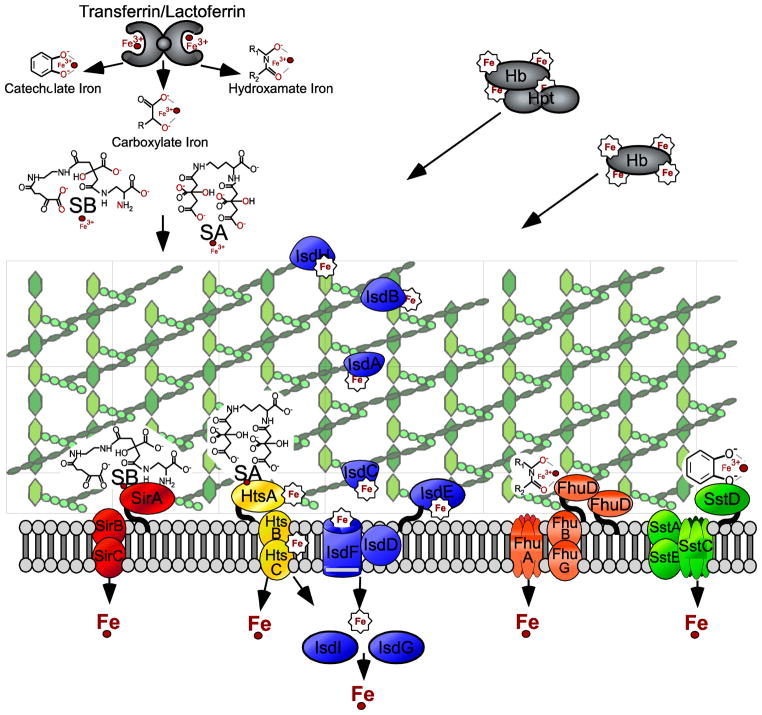
In addition to systems that sense and regulate bacterial iron levels, staphylococci have evolved diverse mechanisms to acquire iron and overcome the host defenses aimed at ing iron from the pathogen. These systems can be broadly divided into siderophore-mediated uptake mechanisms and heme transport mechanisms. Siderophores are secreted molecules of low molecular weight that function as high affinity iron chelators. Siderophores are synthesized by specialized enzymes and can involve non-ribosomal peptide synthetases (NRPS) or NRPS-independent siderophore (NIS) synthesis [48]. The latter synthesize siderophores via the condensation of dicarboxylic acids with diamines or amino alcohol substrates linked by amide or ester bonds [49]. The two S. aureus siderophores described to date employ the NIS system to synthesize staphyloferrin A (SA) and B (SB), which scavenge iron primarily from transferrin [50–52]. Following binding of the staphyloferrins to transferrin iron, specific receptors on the staphylococcal surface, HtsA (SA) and SirA (SB), bind the complex followed by permease import of the iron and/or siderophore-iron complex into the cell [53–55]. Mutation of htsB or htsC led to increased ratios of transferrin to heme-iron uptake, indicating that in addition to siderophore uptake, the Hts transporters can also function in heme-iron import. The same study also established a role for the Hts system in survival in host tissues, with mutations in htsB or htsC resulting in reduced staphylococcal load in organ tissues [56].
Two additional ABC transporters, Fhu and Sst, also contribute to staphylococcal iron acquisition and uptake. The FhuCBG ABC transporter and two lipoprotein receptors FhuD1 and D2 are important for import of Fe3+ hydroxamates [57, 58]. SstABCD has recently been shown to be required for growth promotion by catechol-type siderophores or chatecholamine liberated transferrin iron [59]. By possessing versatility in the class of siderophore transport systems available, S. aureus is better equipped to combat the limited iron availability within the host. The combined contribution of these seemingly redundant systems was experimentally confirmed as a triple mutant in sir, hts, and sst had significantly reduced bacterial cfu recovery from multiple organs in murine i.v. challenges compared to single or double mutants in these systems [59].
The S. aureus iron-regulated surface determinants (Isd) acquire iron from heme (Fe(III)-protoporphyrin IX). IsdB and IsdH are covalently attached to the cell wall peptidoglycan by the transpeptidase sortase A and displayed on the bacterial cell surface [60, 61]. IsdB and IsdH are receptors for host hemo-proteins, primarily hemoglobin or hemoglobin/haptoglobin complexes [62–64]. The sortase anchored proteins IsdA and IsdC facilitate the passage of heme from IsdB on the surface across the bacterial cell wall [60, 61, 65, 66]. Heme is then imported into the cell via the ABC transporters IsdDEF[67–69], where the iron is liberated by the mono-oxygenases IsdG and IsdI [70, 71]. Heme import across the plasma membrane may also be mediated by the ABC transporters Fhu or Hts [56].
Isd protein mediated protection and vaccine development
As discussed and detailed earlier (vide supra), a dichotomy exist for iron. While iron is required for respiratory processes, if uncontrolled cellular damage can result. This dual potential maintains that iron and the proteins involved in capturing, storing, and liberating it must be tightly regulated in most living organisms. In the case of bacterial pathogens, this ensures that specialized host-adapted iron acquisition systems like Isd are expressed only in iron-limiting environments as occurs during infection. Isd proteins are upregulated upon entry into the host, exposed to the immune system, and absent from non-invasive Coagulase-negative (CoNS) staphylococci. These proteins have been the focus of many S. aureus virulence and vaccine studies.
As the main heme transporter of S. aureus, the Isd pathway is one of the best characterized of the iron transport systems. Extensive biochemical and structural studies have been performed providing insight into the mechanism of hemoprotein binding, heme extraction and import into the cell, and extraction of iron from heme. In addition to the molecular and biochemical details, a vast amount of research has contributed to our understanding of the role of the Isd system in staphylococcal disease and use of the Isd surface components as protective antigens. Initial studies concerning the contribution of the Isd system to pathogenesis were directed at the role of the iron regulated transpeptidase sortase B and its single known substrate, the cell wall anchored surface protein IsdC. These studies established a role for srtB in later stages of infection, as a significant reduction in srtB mutant staphylococci recovered from infected tissues was only observed after 9 days [61]. Additional studies examining individual Isd surface receptor mutations, isdA, isdB, isdC, or isdH in murine renal abscess models of infection further corroborated a role for the Isd surface molecules in persistence. Mutants in isdA, isdB, and isdC, but not isdH, resulted in significant reductions in cfu recovered from renal tissue as well as reduction of abscesses present within renal organs [56, 72, 73]. These same mutants were also investigated for their role in a murine sepsis model. Though isd mutation did not alter the lethal outcome of S. aureus infection the mutants displayed delay in time to death [72]. IsdA has also been shown to contribute to colonization, with a mutation in isdA leading to the inability of the mutant staphylococci to colonize the nasopharynx of cotton rats [74].
Initial vaccination studies in murine models of disease together with the identification of IsdB reactive antibodies in infected patients further suggested that the Isd system components, in particular the surface receptors IsdB, IsdH, and IsdA, warranted further examination for the development of a staphylococcal vaccine. Following examination of immune sera from healthy colonized and non-colonized individuals against a large number of staphylococcal proteins, IsdA and IsdH were identified. Reactive antibodies to both proteins were significantly higher in non-colonized compared to colonized individuals. These proteins were then used for vaccination in a cotton rat nasal S. aureus colonization model and provided protection in this model [74].
Two studies independently confirmed the protective capacity of IsdB in active vaccination. The studies used different approaches to identify protective staphylococcal vaccine antigens. One group screened a library of recombinant surface displaced polypeptides using sera from patients suffering from acute staphylococcal infections [75]. IsdB immunized mice challenged with a lethal dose of S. aureus resulted in significant survival and produced high level titres both in mice and rhesus macaques [11]. In the second study, mice were vaccinated with 19 sortase anchored surface proteins followed by challenge with a renal abscess model of infection. IsdA (and IsdB to a lesser extent) provided the most significant protection in the renal abscess model. A combination vaccine was designed which included the four most promising antigens including IsdA and IsdB, and tested protection in a lethal challenge model. The combination vaccine provided significant protection against multiple MRSA strains [76]. Merck and Co. developed IsdB for active vaccination and the resulting vaccine, V710, showed promising results in early clinical trials [77]. Unfortunately, the most recent Phase II/III trials were ended prematurely after early data analysis failed to show a statistically significant clinical benefit. The failure of V710 could result from, among other possibilities, significant differences between hosts such as variations in development of humoral immunity and/or disease pathogenesis. In addition, a single-valency vaccine may not be sufficient to curtail severe invasive disease and instead a multi-target vaccine or therapy may be required.
In addition to establishment of IsdB as an effective antigen for active vaccination, the studies described above (vide supra) also led to development and investigation of antibodies against the Isd surface receptors. Antibodies against IsdB and IsdA have also provided insight into the mechanism of protection afforded by the Isd surface proteins and important conformational epitopes. In addition to V710, Merck also developed murine monoclonal antibodies (MAbs) and assessed them for binding diversity, efficacy in opsonophagocytic killing assays (OPK), and protection in three murine staphylococcal infection models. One promising antibody, 2H2 (both IgG1 and IgG2b isotypes), resulted in greater than fifty percent killing in the OPK assay and was protective in two lethal challenge models as well as in a sublethal indwelling catheter model [78]. A fully human monoclonal was later identified by screening a single chain variable fragment (scFv) antibody library. The human MAb recognized a conformational epitope, exhibited OPK activity similar to that of the mouse MAb 2H2, and displayed statistically significant protection in i.v. lethal challenge and central venous catheter (CVC) infection models in rats. In the CVC model, statistically significant protection was also achieved when administered therapeutically 1 hour post-challenge [79]. Studies with IsdA and IsdB rabbit polyclonal antibodies also demonstrated protection in murine renal abscess and lethal challenge models. Additionally, in vitro studies identified non-IsdB crosreacitve IsdA antibodies that interfered with IsdA heme-binding and non-IsdA crossreactive IsdB antibodies that significantly reduced hemoglobin binding to IsdB. Based on these in vitro assays, the authors speculate that protection mediated by IsdA and/or IsdB antibodies may in part be due to perturbation of Isd mediated heme acquisition by the pathogen [72]. Antibodies have also been used to develop clinical assays to assess protection in humans following vaccination via competitive ELISA, a strategy that could also be applied to other potential vaccine antigens [77, 80].
The importance of iron in the host in regards to Staphylococcus aureus infection is emphasized by iron use and storage genetic disorders which can serve to predispose patients to staphylococcal infections. These include hemochromatosis and thalassemia, both of which result in excessive absorption and storage of dietary iron [81]. In addition, host iron and iron binding proteins can also have secondary effects on inflammation and hemostatis. Hemoglobin liberated from red blood cells, lysed by bacterial hemolysins for example, can bind and inactivate nitric oxide (NO). This results in narrowed vessels and increased platelet aggregation, both of which contribute to thrombosis [82]. By reducing the available Fe2+ and limiting the generation of oxygen intermediates, the Isd proteins have also been linked to secondary effects resulting in resistance to phagocytic killing and H2O2 resistance within the phagosome [83, 84]. IsdB has also recently been shown to directly interact with the platelet receptor GPIIb/IIIa, slowing blood flow and contributing to platelet aggregation [85].
II. Blood Hemostasis
Host mechanisms for maintaining hemostasis
Most physiological processes of the human host, as already illustrated with iron homeostasis, are tightly regulated and interconnected. Blood hemostasis, the process of blood maintenance within vessels to ensure a fluid state in healthy endothelial tissue while mediating rapid initiation of the coagulation cascade and formation of a clot upon injury to the vasculature, most also be tightly controlled [82]. These events also influence one another. This is illustrated by extremely low levels of iron, as is the case with severe anemia, which affects the oxygenation state of the blood and can result in hypoxia in turn causeing an ischemic slowing of the blood flow [82]. On the reverse side, local iron excesses can result from blood hemorrhages and red blood cell lysis in damaged tissues (i.e. a bruise). Unrestricted and/or uncontrolled blood coagulation also trigger pathologies, for example thrombosis or embolism, while the inability to form an effective clot can lead to fatal blood loss, as is the case with hemophilia. Maintaining hemostasis involves three important components, the vascular wall (mainly the endothelium), platelets, and the coagulation cascade [82].
Upon injury and or inflammation of endothelia, inhibitory signals to prevent coagulation are removed and arteriolar vasoconstriction is signaled via reflex neurogenic mechanisms and release of endothelin [86]. Platelets are activated and recruited to the site of injury. Activation, involving secretory granule release, is primarily due to injury mediated exposure to ECM components like collagen and the glycoprotein vWF [82]. vWF mediates adhesion by bridging the platelets and the underlying endothelium through binding the platelet glycoprotein GpIb [87]. Additionally the platelet glycoproteins GpIIb and GpIIIa bind soluble fibrinogen [88, 89]. During these initial stages, a reversible hemostatic plug is formed at the site of trauma. Direct signals from the site of trauma trigger the coagulation cascase, series of serine zymogens that culminate in thrombin activation by the prothrombinase complex, which consists of factor Xa and factor Va [90]. In addition to injury, inflammatory cytokines and/or bacterial factors (such as endotoxin) can activate the coagulation cascade [91–93]. Initiation occurs via endothelial surface exposure of tissue factor (factor III/thromboplastin), the major activator of the extrinsic clotting cascade [94]. Polyanion contact dependent activation of the coagulation cascade intrinsic pathway can also occur, and this is mediated via the serum protease Factor XII [95].
Concomitant with platelet activation and recruitment, tissue factor and factor VIIa generate thrombin, which in turn cleaves soluble fibrinogen circulating in the blood to insoluble fibrin cables [95, 96]. Location of this reaction in the vicinity of the fibrinogen bound platelets stabilizes the clot and mediates platelet contraction, resulting in an irreversible mass or secondary hemostatic plug [82]. Speaking to its central role in blood clot formation, thrombin also serves a pro-inflammatory signaling role. Thrombin fibrinogen cleavage products result in chemotactic signaling of neutrophils to the site of infection while thrombin directly mediates adhesion of neutrophils and monocytes to the clot [97–99]. Lastly, a permanent plug to prevent further vascular leakage is formed following covalent crosslinks of the fibrin cables by Factor XIIIa [100]. The balance then swings back in the reverse direction to favor the anticoagulant mechanisms and assure that a clot only forms at the site of injury.
In order to maintain a hemostatic balance, Factor X conversion of prothrombin to thrombin and initiation of the coagulation cascade is normally inhibited within healthy vessels by endothelial cell secretion of heparan sulfate proteoglycans, thrombomodulin, and tissue factor pathway inhibitor [82]. Clot formation is further inhibited by nitric oxide and prostacyclin which prevent the aggregation of platelets [101]. In the absence of injury, endothelial cells may also cause the degradation of ADP within dense granules of platelets via adenosine diphosphatase secretion, further preventing platelet aggregation [102]. Under normal conditions, active fibrinolysis is also in effect and the protease tissue-type plasminogen activator (t-PA) activates plasmin to cleave fibrin clots [103]. In addition to preventing clot formation under steady state, many of these signaling cascades and mechanisms also function to terminate and resolve coagulation following formation of a stable clot.
Staphylococcus aureus, appropriating host hemostatic mechanisms
During S. aureus invasive disease, either at the site of primary infection or as the bacteria disseminate to other tissues and seed abscess formation, staphylococcal cells will come into contact with the host’s circulatory system [8]. S. aureus has been clinically distinguished from other staphylococcal species primarily based on bacterial molecular interactions with the host’s blood system. Of these clinical tests is the distinction between coagulase positive S. aureus from other CoNS. This clinical designation, referred to as the coagulase test, is historically based on S. aureus dependent coagulation of blood [104]. A related S. aureus dependent phenomenon, also exploited as a diagnostic tool, monitors the aggregation or agglutination of staphylococci exposed to calcium-chelated plasma on a glass slide [105, 106]. The slide agglutination test has further been reduced to the clumping or precipitation of bacteria in the presence of soluble fibrinogen [107]. These observations, mediated by molecular interactions between S. aureus and its host, have been studied for over 100 years. The major bacterial effectors of these observations have been known for some time, namely the staphylocoagulase Coa, and more recently identified, vWbp, which mediate host prothrombin and fibrinogen interactions [108, 109] and ClfA which mediates the clumping phenotype and binds to soluble fibrinogen [110, 111]. A handful of new studies have provided additional insight into these multi-component molecular interactions. Specifically, multi-gene mutation virulence studies and the identification of additional bacterial mediators have significantly furthered our understanding of S. aureus pathogenesis in blood hemostasis.
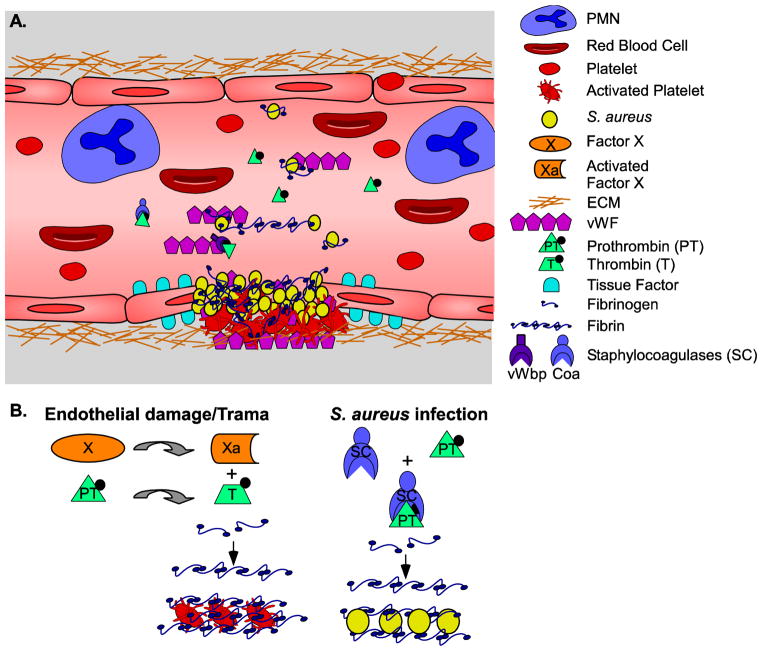
Early S. aureus studies on the bacterial dependent coagulation of blood/plasma were based primarily on the biochemical separation and isolation of this activity [112–114]. With the advancement of genetic and recombinant DNA technologies, the gene encoding staphylococcal coagulase (coa) was identified [115–118]. Later studies identified a second staphylococcal gene product that also possesses coagulase activity, von Willebrand factor binding protein (vWbp), first isolated based on its interactions with vWF [119, 120]. Both S. aureus coagulases bind to human prothrombin resulting in the zymogen dependent cleavage of the Aα and Bβ chains of fibrinogen to fibrin [108, 109]. It is this reaction that mediates the early observations of staphylococcal agglutination and coagulation.
A model delineating the mechanism for the coagulation and rapid conversion of fibrinogen to fibrin by the staphylococcal coagulases has been elegantly established following crystallization of the active staphylothrombin complex [108]. The N-terminal Ile1-Val2 of Coa insert into the Ile16 cleft of prothrombin while the remainder of the N-terminal D1D2 domain (present in both Coa and vWbp) binds and stabilizes the complex. Coagulase binding to prothrombin induces a conformational change resulting in a functionally active site [121]. Interestingly, while binding of the D2 region blocks the α– Thrombin fibrinogen recognition site, the Coa-Prothrombin complex is still able to bind fibrinogen with high affinity [108, 121]. In addition, coagulase induced formation of an active site in prothrombin in the absence of cleavage to thrombin and blockage of exosite1 also blocks the action of thrombin inhibitors such as heparins, vitamin K antagonists and the bivalent thrombin inhibitors such as hirudin [115]. In this same manner, the staphylococcal coagulases are also able to circumvent the regulatory processes of the coagulation cascade [122]. Univalent direct thrombin inhibitors (DTIs) such as argatroban, melagatran, and dabigatran are exceptions and are effective in inhibiting the Coa Prothrombin complex as they only target and bind the active site [123, 124]. Differences in zymogen activation [109, 125] and possessing very distinct C-termini, suggests that the role of the two coagulases is not simply redundant and that each provides a unique contribution to establishment of disease.
Contribution of staphylocoagulases to virulence and protection
The contribution of staphylococcal coagulation toward S. aureus pathogenesis had been a matter of debate. Conflicting reports on the importance of the staphylocoagulase in a variety of disease systems had left the role of coagulase during infection unresolved. However, clinical observations that infections caused by coagulase positive strains such as S. aureus are much more invasive and severe compared to CoNS such as S. epidermidis together with early experimental evidence that injection of CoNS with coagulase material enhances virulence support the inclusion of coagulase as a S. aureus virulence factor [113]. Initial studies of mutants isolated from chemical or transposon mutagenesis strategies found decreased virulence in experimental endocarditis, i.v. and i.p. LD50 infections, and murine mastitis [112, 126–129]. However the mode of mutagenesis and mutant selection may have also led to additional mutations, for example in important virulence regulatory genes. Generation of an isogenic coa mutant did not display any defect in skin abscess, endocarditis, or mastitis murine infection models [117, 130], while an independently generated isogenic coa mutant did exhibit a virulence defect in a hematogenous pulmonary infection model [131]. Based on the finding that staphylococcal vWbp also possesses coagulase activity, more recent work generated mutant strains in S. aureus Newman by allelic replacement of each coa and vWb genes or both in combination. The single mutants had significant defects in renal abscess formation, lethal bacteremia, as well as survival in blood. Importantly however, these phenotypes were greatly enhanced for the double mutant [132].
Studies aimed at further dissecting the formation of staphylococcal vegetations during acute endocarditis established that prothrombin bound to staphylocoagulase was found at the leading edge of the vegetations. Further, the survival of animals infected with the double coagulase mutant significantly improved and detection of staphylococci by positron emission tomography (PET) imaging revealed significantly reduced bacteria within vegetations [133]. The related biochemical reaction of agglutination employed citrate treated plasma and coagulase positive staphylococci, resulting in bacterial aggregation [105]. Distinct from the later reduction of this reaction, the fibrinogen clumping reaction, which employs only purified, soluble fibrinogen and bacteria, the agglutination reaction was proposed to require both fibrinogen and prothrombin [106, 107]. Mediating the fibrinogen clumping reaction, N2 and N3 of the A-domain of S. aureus ClfA, a staphylococcal sortase anchored surface protein, bind to soluble fibrinogen through the C-terminal ends of the γ chains [107, 111, 134] resulting in precipitation of staphylococci [107, 110, 135, 136] The N2 and N3 of a second staphylococcal clumping factor, ClfB which also binds to cytokeratin, bind to a short region of fibrinogen located within the C terminus of the α-chain [137]. The involvement of clumping factor as well as the coagulases in the agglutination reaction, more closely mimicking what may occur within the host, was recently confirmed. In addition, staphylococcal agglutination mediated by Coa, vWbp, and Clumping factor A (ClfA) in blood was shown to contribute to thromboembolic lesions in heart tissues and fatal outcome in a lethal i.v. infection model of bacteremia, as a triple coa, vWbp and clfA mutant resulted in complete survival [138].
Development of staphylocoagulase based therapies against S. aureus disease
The combined research investigating mutations in coa, vWbp, and clfA individually and in combination have contributed a great deal to furthering our understanding of the molecular events involved in staphylococcal disease and in the development of therapies aimed at prevention. As vaccines provide the greatest promise for treatment, investigation of antibodies raised against these factors has also provided significant insight into designing effective therapies. Specifically, active immunization and passive immunization with polyclonal antibodies raised against Coa and vWbp provide significant protection in S. aureus renal abscess and lethal models of infections, mimicking the virulence of the genetic deletions such that the combination of both antibodies provides increased protection compared to either alone [132]. In addition to providing significant protection in animal models, the antibodies were able to significantly extend the clotting time of human and mouse blood in vitro, again with the combination of both antibodies more significant than each alone. The antibodies were also able to block the association of the coagulases with fibrinogen (αvWbp) and prothrombin (αvWbp as well as αCoa) and inhibit the conversion of fibrinogen to fibrin [132].
Recent findings have shown that a new class of direct thrombin inhibitors including Pradaxa (dabigatran etexilate) are able to inhibit staphylocoagulase activity [124]. This finding together with the role of the coagulases and ClfA agglutination in murine lethal bacteremia were the basis of a successful combination therapy for treatment of murine acute S. aureus disease involving dabigatran and polyclonal ClfA antibodies [138]. Of interest, previous isolation of a monoclonal antibody to ClfA that interferes with fibrinogen γ–chain binding [139, 140] provided promising results in preclinical studies, however, no significant differences were detected between treatment and placebo groups in phase II end point analysis [141]. ClfA interactions with other host proteins, including complement regulatory factor I which mediates escape from phagocytic killing [142] or incomplete inhibition by the monoclonal if the gamma chain is not accessible following conversion to fibrin [143, 144], could help to explain the failure of Tefibazumab in phase II trials.
In addition to the specific contributions to survival and adaptation within the host blood system mediated by the staphylocoagulases and ClfA, S. aureus encodes an arsenal of additional gene products that aid in its ability to escape, grow, and survive the hemostatic pathway. These proteins, many of which also interact with fibrinogen, complement, and host mediators of inflammation, may also influence hemostatsis. A complete model for staphylococcal blood pathogenesis is thus not fully appreciated.
III. Immunmodulation
Upon breach of the epithelial surface barrier defenses, either as a result of trauma or other disease pathology, staphylococci gains access to deep tissues and/or the blood stream. There they encounter the host’s innate immune system, mainly composed of professional phagocytes (macrophages, dendritic cells, neutrophils and eosinophils) and antimicrobial proteins (cytokines, complement proteins and antimicrobial peptides). Immediate activation of complement proteins through recognition of foreign particles including pathogens is amplified by secretion of pro-inflammatory cytokines and recruitment of phagocytic cells. Neutrophils are the primary host defense against staphylococcal infection (reviewed in [145]). This is exemplified by the recurrence of S. aureus infection in patients suffering from agranulocytosis or chronic granulomatous disease (CGD), genetic disorders that result in deficiencies in NADPH activation and failed neutrophil respiratory bursts [146]. S. aureus possesses a variety of molecules and mechanisms to combat and diminish neutrophil defenses including secretion of proteins that inhibit the deposition and activation of complement, generation of molecules that prevent or deter neutrophil recruitment to the sites of infection, and molecules with lytic properties that target neutrophils and other phagocytic cells [145, 147–150].
Prevention of phagocytic killing and promotion of intracellular survival
Several bacterial factors also mediate interactions with phagocytes, aiding survival within the phagocyte. S. aureus is capable of secreting cytolytic toxins such as alpha-hemolysin, leukocidins and PVL, which as well as killing the phagocyte and liberating key nutrients such as heme bound iron for extracellular staphylococci, may provide intracellular staphylococci an escape from phagolysosomes. Recent identification and characterization of phenol soluble modulins (PSMs), which are short amphiphathic and alpha helical peptides generated by most S. aureus strains, implicated an additional mode of bacterial neutrophil killing. In addition to secreted factors to interfere with phagocytic cells, S. aureus also retains surface associated virulence factors that inhibit phagocytic uptake. Two sortase anchored proteins, ClfA and SpA, inhibit phagocytosis, partly due to their interactions with fibrinogen and the Fc portion of immunoglobulin, respectively.
A third recently characterized staphylococcal surface protein, AdsA, harbors two 5′-nucleotidase enzymatic domains that convert AMP to adenosine. Early experiments had provided that this protein was important in promoting staphylococcal survival in the phagolysosome of neutrophils. Following intravenous staphylococcal infection of mice, a significant Increase in extracellular adenosine was generated. Further confirming the role in generation of increased adenosine levels, a mutant strain expressing non-catalytic AdsA failed to produce a similar increase [151], While the exact details of this process are currently being examined, it is clear that the staphylococcal cell has adapted to hijack an immunosuppressive host signal for promotion of its own survival. Other staphylococcal strategies, including an additional potential role for adenosine, to prevent generation of a productive adaptive immune response will be discussed later in this review (vide infra). Staphylococci also elaborate a polysaccharide capsule and the polysaccharide intercellular adhesin (PIA, Poly-N-Acetyl-Glucosamine) both of which are thought to possess anti-opsonic properties [152, 153]. However, as mutant S. aureus incapable of expression of CPs and PIA did not exhibit decreased survival in naïve mouse blood, questions remain regarding the anti-opsonic role of these molecules (ref?).
S. aureus targets the intersection of the innate and adaptive immune response
Development of a pathogen specific adaptive immune response is a complex process whereby proper engagement of innate immune components and the proper cross-talk between T- and B-cells with phagocytic cells are essential [154–157]. While it is well established that the complement system is activated upon interaction with S. aureus in the blood stream, it was not fully appreciated until recently that the proper engagement and presentation of complement factors is critical to elicit a neutralizing immune response [158–161]. Not surprisingly, S. aureus produces an arsenal of proteins specifically aimed at targeting this important intersection between the innate and adaptive immune responses [162]. Three different pathways of complement activation (classical, alternative and lectin) converge at the formation of C3 convertases (C4bC2a for classical and lectin pathways and C3bBb for the alternative pathway). The C3 convertases enzymatically cleave C3 into its active products C3a and C3b. Release of the chemoattractant C3a reveals a reactive thioester in C3b that is necessary for covalent attachment and deposition onto the surface of foreign particles, including staphylococcal cells [163]. C3b deposition results in phagocytosis via complement receptor-containing cells. The secreted staphylococcal complement inhibitor (SCIN) protein interacts directly with both C3 convertases and stabilizes them, resulting in inhibition of further C3b deposition [148]. Staphylococci also interfere directly with the products of the C5 convertases. S. aureus superantigen-like protein 7 (SSL7) strongly inhibits C5a neutrophil recruitment through IgA dependent binding to C5, preventing its cleavage [164]. The staphylococcal secreted chemotaxis inhibitory protein (CHIPs) interacts directly with the C5a Receptor (C5aR) on neutrophils blocking C5a chemoattractant signaling [150].
S. aureus strains also express two immunoglobulin binding molecules, SpA and Sbi. These two proteins share significant amino acid homology in their Ig binding domains [165]. The interaction and sequestration of anti-staphylococcal immunoglobulins mediated by these proteins may severely limit host immunoglobulin mediated immune clearance of the pathogen. In contrast to SpA which is composed of four to five Ig binding domains (vide infra)[166], Sbi contains two Ig binding domains at the N-terminus followed by two domains that interact with complement protein C3 via C3d [165, 167]. C3d binds to antigens enhancing their uptake by dendritic cells and B cells[168]. The interaction with C3d is also mediated by two additional staphylococcal surface associated proteins, Efb and Ehp [169, 170]. Interestingly, mutational analysis revealed that the C3d residues involved in the interaction with complement receptor 2 (CR2) on B cells also participate in the interaction with the staphylococcal proteins [171]. Of note, CR2 can be found on the surface of follicular dendritic cells, B cells and T cells [168]. It is believed that synergistic B cell activation occurs through the simultaneous C3d-CR2 ligation and antigen specific immunoglobulin interactions [168].
Although complement activation is a necessary step to detect and eliminate invading pathogens, excessive complement activation can lead to increased, uncontrolled inflammation. In order to prevent uncontrolled activation, the host expresses complement factors H and I that downregulate complement fixation. Many pathogens have evolved to take advantage of these proteins by producing virulence factors that bind complement factors H and I. S. aureus is not an exception. The surface protein ClfA has been shown to capture and activate Factor I, enhancing the cleavage of nascent C3b into iC3b, ultimately reducing phagocytosis of bacteria by human neutrophils [147]. It was also suggested that Sbi binds Factor H and C3 simultaneously to form a tripartite complex, consuming a significant level of C3 in serum [167]. In order to promote its own survival, S. aureus has adapted to its human host in part by limiting complement mediated defenses which are essential for convergence of the innate and adaptive immune responses [162].
Preventing development of productive host adaptive immunity
As discussed earlier in this review (vide supra), AdsA supported delayed killing of S. aureus in blood neutrophils [151]. Though the exact mechanisms and strategies for survival of the staphylococci inside a professional phagocyte have not been determined, they would necessitate S. aureus circumventing and/or modulating a variety of bactericidal processes including reactive oxygen species (ROS) and hydrolytic enzymes. If one considers that S. aureus may possess mechanisms to evade the defense and killing strategies of professional phagocytes by altering phagocyte function, such strategies would also have downstream effects on the development of a proper adaptive immune response. In addition, as part of the anti-inflammatory signaling properties of adenosine, levels of MHC-II on macrophages and dendritic cells are reduced together with production of IL-12, a pivotal stimulus for Th1-type immune responses [172–174]. These signaling effects may also add to staphylococcal survival by interfering with the adaptive immune response, for example the generation of ineffective T-cell effector mechanisms.
As mentioned (vide supra), most human clinical isolates express two immunoglobulin binding proteins, protein A and Sbi. Protein A, which is one of the most abundant staphylococcal cell wall anchored proteins, harbors four to five immunoglobulin binding domains [166]. Individual Ig binding domains fold into triple helix bundles connected by short linkers [175]. The five successive Ig domains are followed by region X [176]. Protein A is well known for its binding to the Fcγ portion of most Ig subclasses [177]. Protein A specific interactions have more recently been expanded to include interactions with the F(ab)2 of VH3 type B cell receptors, von Willebrand factor, gC1qR/p33, and tumor necrosis factor receptor 1 (TNFR1) [178–181]. Protein A crosslinking to F(ab)2 B cell receptors results in B cell superantigen activity, apoptosis and deletion of VH3 type supra-clonal B cells. During staphylococcal infection, protein A is anchored to the peptidoglycan of staphylococci by the transpeptidase sortase [182]. Additional protein A is also released into the surrounding environment during peptidoglycan turnover resulting from normal cell growth and division. Both forms of protein A may interact with VH3+ BCR clones, which account for up to 50% of the total B cell population in humans [178, 183]. In mice, B-1 and marginal zone B cells, both innate like B cells which secrete natural antibodies, were the most susceptible B cells to soluble protein A treatment [184]. B-2 cells, follicular B cells which generate antigen specific antibodies, required more persistent superantigen activity [184]. Once depleted by protein A, the B cell subsets were not readily replenished, creating a deficit in the ability to elicit anti-staphylococcal immunity [185].
Staphylococcal immune evasion and vaccine design considerations
Despite being one of the most abundant staphylococcal surface proteins for potential interaction with the host immune surveillance system, wild-type Protein A makes an unattractive candidate for a S. aureus vaccine due to its toxicity and B cell superanitgen activity. Encouragingly however, Infection of mice with a Protein A knock out strain elicited protective immunity against secondary infection with wild-type S. aureus. This may be due to the resulting increased antibody responses [186, 187]. Subsequent generation of a non-toxigenic variant of Protein A with mutations in each one of five Ig binding domains (SpAKKAA) eliminated Fcg and F(ab)V(H)3 binding and prevented B cell apoptosis. Vaccination of mice with this antigen resulted in generation of high titre antibodies that promoted S. aureus clearance by opsonophagocytosis and protection of mice against the current US epidemic MRSA strain, LAC. Vaccination with the SpAKKAA also resulted in increased IgG titres to a variety of S. aureus antigens compared to adjuvant alone [186]. It is tempting to speculate that while also providing a new potential vaccine antigen, that SpAKKAA could also be thought of in an adjuvant capacity, administered in early childhood to help promote development of productive anti-staphylococcal immunity from natural infection.
Synthesis
S. aureus iron scavenging, coagulation and escape from innate and adaptive immune responses are key virulence strategies in the pathogenesis of skin and soft tissue infections, bacteremia or sepsis. Are these genetic determinants of staphylococcal disease also protective antigens that enable the development of efficacious vaccines? Research reviewed herein proposes several key virulence genes and presumed protective antigens but also describes the multi-factorial nature whereby staphylococci cause abscess lesions or sepsis. Thus, active or passive immunization strategies that focus on single antigens are more likely to fail than those that disrupt multiple pathogenetic processes. We therefore propose the development of combination vaccines that incorporate multiple protective determinants to raise immune responses that block staphylococcal iron uptake, interfere with coagulation and disrupt the immune evasion strategies of S. aureus.
Fig 1. Iron Acquisition and Transport in Staphylococcus aureus.
Five Iron import systems have been described to date in Staphylococcus aureus. The Isd system imports heme iron (Fe(III)-protoporphyrin IX). IsdB and IsdH are hemoprotein receptors, capturing hemoglobin (Hb) and hemoglobin-haptoglobin (Hpt) complexes, respectively. Following extraction from the hemoprotein, the heme moiety likely passes through the cell wall by increased binding interactions with IsdA, IsdC, and the lipoprotein IsdE, respectively. The heme is then imported into the cell by the IsdDEF and/or possibly the Hts or Fhu ABC transporters. The heme is then degraded and free iron liberated by the heme oxygenases IsdI and IsdG. Siderophore mediated transport systems include the Hts (multi-iron source import?), Sir, Sst, and Fhu (multi-iron source import?). These transporters acquire iron primarily from transferrin and/or lactoferrin and may demonstrate preferences for siderophore type (carboxylate, catecholate, or hydroxamate). HtsA and SirA are the receptors for the staphylococcal synthesized carboxylate-type siderophores Staphyloferrin A (SA) and Staphyloferrin B (SB), respectively. The Sst ABC transporter have been linked to catechol-type siderophore and chatecholamine iron sources and the Fhu transporter is important for hydroxamate-type iron sources. This diverse set of systems for acquiring iron from different sources in the host provides S. aureus with versatility and a better chance for survival in the iron limited environment within the host.
Fig 2. Staphylocoagulase mediated coagulation.
A. The S. aureus genome encodes for two gene products able to bind Prothrombin (PT) and mediate coagulation. In addition to PT, the staphylocoagulases (SC) Coa and the more recently discovered vWbp, mediate additional interactions to aid blood coagulation and clot formation. Normal clot formation is initiated by trama or damage to the endothelium, followed by release of tissue factor and exposure of ECM factors such as collagen and vWF. vWF bridges platelets and the underlying tissue, aiding in platelet activation, which bind soluble fibrinogen. S. aureus vWbp and Protein A both bind vWF and these interactions together with fibrinogen binding proteins such as ClfA, mediate accumulation of the products necessary to form fibrin cables and further reinforcement of the clot. The generation of thrombin serves additional pro-inflammatory signaling roles including neutrophil (PMNs) recruitment and covalent crosslinking of fibrin cables by Factor XIII helping to form a permanent clot and activation of fibrinolysis. These actions may be avoided or limited during staphylococcal coagulation. B. A mechanism and model for staphylococcal coagulase mediated conversion of fibrinogen to fibrin was established following crystallization of the active (Coa Prothrombin)2 complex. In this model, the N-terminal Ile1-Val2 of Coa (SC) inserts into the Ile16 cleft of prothrombin (PT). The D1D2 domain makes additional stabilization contacts with PT. A functional active site is formed following SC induced conformational changes and the complex binds fibrinogen with high affinity. By not requiring PT cleavage by Factor X (X-inactive, Xa-active) to thrombin (T) together with SC D2 blockage of the α-Thrombin recognition site for fibrinogen (●) on PT, the regulatory processes of the coagulation cascade are avoided
Acknowledgments
We kindly thank members of the Schneewind and Missiakas laboratories, in particular Molly McAdow, and Vilasack Thammavongsa for thoughtful comments and discussion of the manuscript and Yvonne Chan for artistic critique. Work described here was supported by grants from the National Institute of Allergy and Infectious Diseases (NIAID), Infectious Diseases Branch (AI52474 and AI92711 to O.S. and AI52767 to D.M.M.) and by Novartis Vaccines and Diagnostics (Siena, Italy). O.S. and D.M.M. acknowledge membership within and support from the Region V Great Lakes Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (NIH Award 1-U54-AI-057153).
Footnotes
Disclosure statement
Andrea DeDent, Hwan Keun Kim, Dominique Missiakas, and Olaf Schneewind are named inventors on a patent owned by the University of Chicago that is the subject of a license agreement with Novartis Vaccines and Diagnostics.
References
- 1.Peacock SJ, de Silva I, Lowy FD. What determines nasal carriage of Staphylococcus aureus? Trends Microbiol. 2001;9:605–10. doi: 10.1016/s0966-842x(01)02254-5. [DOI] [PubMed] [Google Scholar]
- 2.Neu HC. The crisis in antibiotic resistance. Science. 1992;257:1064–73. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- 3.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–41. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178–82. doi: 10.3201/eid0702.010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deleo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 375:1557–68. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klevens RM, Edwards JR, Gaynes RP. The impact of antimicrobial-resistant, health care-associated infections on mortality in the United States. Clin Infect Dis. 2008;47:927–30. doi: 10.1086/591698. [DOI] [PubMed] [Google Scholar]
- 7.Projan SJ, Nesin M, Dunman PM. Staphylococcal vaccines and immunotherapy: to dream the impossible dream? Curr Opin Pharmacol. 2006;6:473–9. doi: 10.1016/j.coph.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–32. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 9.Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, Yeoh H, Law D, Robbins JB, Schneerson R, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med. 2002;346:491–6. doi: 10.1056/NEJMoa011297. [DOI] [PubMed] [Google Scholar]
- 10.DeJonge M, Burchfield D, Bloom B, Duenas M, Walker W, Polak M, Jung E, Millard D, Schelonka R, Eyal F, et al. Clinical trial of safety and efficacy of INH-A21 for the prevention of nosocomial staphylococcal bloodstream infection in premature infants. J Pediatr. 2007;151:260–5. 265, e1. doi: 10.1016/j.jpeds.2007.04.060. [DOI] [PubMed] [Google Scholar]
- 11.Kuklin NA, Clark DJ, Secore S, Cook J, Cope LD, McNeely T, Noble L, Brown MJ, Zorman JK, Wang XM, et al. A novel Staphylococcus aureus vaccine: iron surface determinant B induces rapid antibody responses in rhesus macaques and specific increased survival in a murine S. aureus sepsis model. Infect Immun. 2006;74:2215–23. doi: 10.1128/IAI.74.4.2215-2223.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fattom AI, Horwith G, Fuller S, Propst M, Naso R. Development of StaphVAX, a polysaccharide conjugate vaccine against S. aureus infection: from the lab bench to phase III clinical trials. Vaccine. 2004;22:880–7. doi: 10.1016/j.vaccine.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 13.Finch CA. Body iron exchange in man. J Clin Invest. 1959;38:392–6. doi: 10.1172/JCI103813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huff RL, Hennessy TG, Austin RE, Garcia JF, Roberts BM, Lawrence JH. Plasma and red cell iron turnover in normal subjects and in patients having various hematopoietic disorders. J Clin Invest. 1950;29:1041–52. doi: 10.1172/JCI102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan WT, Liem HH, Sutor RP, Muller-Ebergard U. Transfer of heme from heme-albumin to hemopexin. Biochim Biophys Acta. 1976;444:435–45. doi: 10.1016/0304-4165(76)90387-1. [DOI] [PubMed] [Google Scholar]
- 16.Giblett ER. Recent advances in heptoglobin and transferrin genetics. Bibl Haematol. 1968;29:10–20. doi: 10.1159/000384590. [DOI] [PubMed] [Google Scholar]
- 17.Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 18.Uchida T, Akitsuki T, Kimura H, Tanaka T, Matsuda S, Kariyone S. Relationship among plasma iron, plasma iron turnover, and reticuloendothelial iron release. Blood. 1983;61:799–802. [PubMed] [Google Scholar]
- 19.Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loreal O. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276:7811–9. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 20.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276:7806–10. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 21.Krause A, Neitz S, Magert HJ, Schulz A, Forssmann WG, Schulz-Knappe P, Adermann K. LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett. 2000;480:147–50. doi: 10.1016/s0014-5793(00)01920-7. [DOI] [PubMed] [Google Scholar]
- 22.Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, Vaulont S. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci U S A. 2001;98:8780–5. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–3. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 24.Knutson MD, Oukka M, Koss LM, Aydemir F, Wessling-Resnick M. Iron release from macrophages after erythrophagocytosis is up-regulated by ferroportin 1 overexpression and down-regulated by hepcidin. Proc Natl Acad Sci U S A. 2005;102:1324–8. doi: 10.1073/pnas.0409409102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110:1037–44. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–3. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 27.Weinberg ED. Iron withholding: a defense against infection and neoplasia. Physiol Rev. 1984;64:65–102. doi: 10.1152/physrev.1984.64.1.65. [DOI] [PubMed] [Google Scholar]
- 28.Rivera S, Nemeth E, Gabayan V, Lopez MA, Farshidi D, Ganz T. Synthetic hepcidin causes rapid dose-dependent hypoferremia and is concentrated in ferroportin-containing organs. Blood. 2005;106:2196–9. doi: 10.1182/blood-2005-04-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pietrangelo A. Molecular insights into the pathogenesis of hereditary haemochromatosis. Gut. 2006;55:564–8. doi: 10.1136/gut.2005.078063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Snick JL, Masson PL, Heremans JF. The involvement of lactoferrin in the hyposideremia of acute inflammation. J Exp Med. 1974;140:1068–84. doi: 10.1084/jem.140.4.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Snick JL, Markowetz B, Masson PL. The ingestion and digestion of human lactoferrin by mouse peritoneal macrophages and the transfer of its iron into ferritin. J Exp Med. 1977;146:817–27. doi: 10.1084/jem.146.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fluckinger M, Haas H, Merschak P, Glasgow BJ, Redl B. Human tear lipocalin exhibits antimicrobial activity by scavenging microbial siderophores. Antimicrob Agents Chemother. 2004;48:3367–72. doi: 10.1128/AAC.48.9.3367-3372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–21. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 34.Ford FA, Mouratidou T, Wademan SE, Fraser RB. Effect of the introduction of ‘Healthy Start’ on dietary behaviour during and after pregnancy: early results from the ‘before and after’ Sheffield study. Br J Nutr. 2009;101:1828–36. doi: 10.1017/S0007114508135899. [DOI] [PubMed] [Google Scholar]
- 35.Eaton JW, Brandt P, Mahoney JR, Lee JT., Jr Haptoglobin: a natural bacteriostat. Science. 1982;215:691–3. doi: 10.1126/science.7036344. [DOI] [PubMed] [Google Scholar]
- 36.Moore DG, Earhart CF. Specific inhibition of Escherichia coli ferrienterochelin uptake by a normal human serum immunoglobulin. Infect Immun. 1981;31:631–5. doi: 10.1128/iai.31.2.631-635.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bullen JJ, Rogers HJ, Griffiths E. Role of iron in bacterial infection. Curr Top Microbiol Immunol. 1978;80:1–35. doi: 10.1007/978-3-642-66956-9_1. [DOI] [PubMed] [Google Scholar]
- 38.Horsburgh MJ, Clements MO, Crossley H, Ingham E, Foster SJ. PerR controls oxidative stress resistance and iron storage proteins and is required for virulence in Staphylococcus aureus. Infect Immun. 2001;69:3744–54. doi: 10.1128/IAI.69.6.3744-3754.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horsburgh MJ, Wharton SJ, Cox AG, Ingham E, Peacock S, Foster SJ. MntR modulates expression of the PerR regulon and superoxide resistance in Staphylococcus aureus through control of manganese uptake. Mol Microbiol. 2002;44:1269–86. doi: 10.1046/j.1365-2958.2002.02944.x. [DOI] [PubMed] [Google Scholar]
- 40.Hill PJ, Cockayne A, Landers P, Morrissey JA, Sims CM, Williams P. SirR, a novel iron-dependent repressor in Staphylococcus epidermidis. Infect Immun. 1998;66:4123–9. doi: 10.1128/iai.66.9.4123-4129.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiong A, Singh VK, Cabrera G, Jayaswal RK. Molecular characterization of the ferric-uptake regulator, fur, from Staphylococcus aureus. Microbiology. 2000;146(Pt 3):659–68. doi: 10.1099/00221287-146-3-659. [DOI] [PubMed] [Google Scholar]
- 42.Horsburgh MJ, Ingham E, Foster SJ. In Staphylococcus aureus, fur is an interactive regulator with PerR, contributes to virulence, and Is necessary for oxidative stress resistance through positive regulation of catalase and iron homeostasis. J Bacteriol. 2001;183:468–75. doi: 10.1128/JB.183.2.468-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torres VJ, Attia AS, Mason WJ, Hood MI, Corbin BD, Beasley FC, Anderson KL, Stauff DL, McDonald WH, Zimmerman LJ, et al. Staphylococcus aureus fur regulates the expression of virulence factors that contribute to the pathogenesis of pneumonia. Infect Immun. 78:1618–28. doi: 10.1128/IAI.01423-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stauff DL, Bagaley D, Torres VJ, Joyce R, Anderson KL, Kuechenmeister L, Dunman PM, Skaar EP. Staphylococcus aureus HrtA is an ATPase required for protection against heme toxicity and prevention of a transcriptional heme stress response. J Bacteriol. 2008;190:3588–96. doi: 10.1128/JB.01921-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stauff DL, Torres VJ, Skaar EP. Signaling and DNA-binding activities of the Staphylococcus aureus HssR-HssS two-component system required for heme sensing. J Biol Chem. 2007;282:26111–21. doi: 10.1074/jbc.M703797200. [DOI] [PubMed] [Google Scholar]
- 46.Torres VJ, Stauff DL, Pishchany G, Bezbradica JS, Gordy LE, Iturregui J, Anderson KL, Dunman PM, Joyce S, Skaar EP. A Staphylococcus aureus regulatory system that responds to host heme and modulates virulence. Cell Host Microbe. 2007;1:109–19. doi: 10.1016/j.chom.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Attia AS, Benson MA, Stauff DL, Torres VJ, Skaar EP. Membrane damage elicits an immunomodulatory program in Staphylococcus aureus. PLoS Pathog. 6:e1000802. doi: 10.1371/journal.ppat.1000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crosa JH, Walsh CT. Genetics and assembly line enzymology of siderophore biosynthesis in bacteria. Microbiol Mol Biol Rev. 2002;66:223–49. doi: 10.1128/MMBR.66.2.223-249.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Challis GL. A widely distributed bacterial pathway for siderophore biosynthesis independent of nonribosomal peptide synthetases. Chembiochem. 2005;6:601–11. doi: 10.1002/cbic.200400283. [DOI] [PubMed] [Google Scholar]
- 50.Cotton JL, Tao J, Balibar CJ. Identification and characterization of the Staphylococcus aureus gene cluster coding for staphyloferrin A. Biochemistry. 2009;48:1025–35. doi: 10.1021/bi801844c. [DOI] [PubMed] [Google Scholar]
- 51.Dale SE, Doherty-Kirby A, Lajoie G, Heinrichs DE. Role of siderophore biosynthesis in virulence of Staphylococcus aureus: identification and characterization of genes involved in production of a siderophore. Infect Immun. 2004;72:29–37. doi: 10.1128/IAI.72.1.29-37.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Modun B, Evans RW, Joannou CL, Williams P. Receptor-mediated recognition and uptake of iron from human transferrin by Staphylococcus aureus and Staphylococcus epidermidis. Infect Immun. 1998;66:3591–6. doi: 10.1128/iai.66.8.3591-3596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beasley FC, Vines ED, Grigg JC, Zheng Q, Liu S, Lajoie GA, Murphy ME, Heinrichs DE. Characterization of staphyloferrin A biosynthetic and transport mutants in Staphylococcus aureus. Mol Microbiol. 2009;72:947–63. doi: 10.1111/j.1365-2958.2009.06698.x. [DOI] [PubMed] [Google Scholar]
- 54.Dale SE, Sebulsky MT, Heinrichs DE. Involvement of SirABC in iron-siderophore import in Staphylococcus aureus. J Bacteriol. 2004;186:8356–62. doi: 10.1128/JB.186.24.8356-8362.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grigg JC, Cheung J, Heinrichs DE, Murphy ME. Specificity of Staphyloferrin B recognition by the SirA receptor from Staphylococcus aureus. J Biol Chem. 285:34579–88. doi: 10.1074/jbc.M110.172924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skaar EP, Humayun M, Bae T, DeBord KL, Schneewind O. Iron-source preference of Staphylococcus aureus infections. Science. 2004;305:1626–8. doi: 10.1126/science.1099930. [DOI] [PubMed] [Google Scholar]
- 57.Sebulsky MT, Hohnstein D, Hunter MD, Heinrichs DE. Identification and characterization of a membrane permease involved in iron-hydroxamate transport in Staphylococcus aureus. J Bacteriol. 2000;182:4394–400. doi: 10.1128/jb.182.16.4394-4400.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sebulsky MT, Heinrichs DE. Identification and characterization of fhuD1 and fhuD2, two genes involved in iron-hydroxamate uptake in Staphylococcus aureus. J Bacteriol. 2001;183:4994–5000. doi: 10.1128/JB.183.17.4994-5000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beasley FC, Marolda CL, Cheung J, Buac S, Heinrichs DE. Staphylococcus aureus transporters Hts, Sir, and Sst capture iron liberated from human transferrin by Staphyloferrin A, Staphyloferrin B, and catecholamine stress hormones, respectively, and contribute to virulence. Infect Immun. 79:2345–55. doi: 10.1128/IAI.00117-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mazmanian SK, Skaar EP, Gaspar AH, Humayun M, Gornicki P, Jelenska J, Joachmiak A, Missiakas DM, Schneewind O. Passage of heme-iron across the envelope of Staphylococcus aureus. Science. 2003;299:906–9. doi: 10.1126/science.1081147. [DOI] [PubMed] [Google Scholar]
- 61.Mazmanian SK, Ton-That H, Su K, Schneewind O. An iron-regulated sortase anchors a class of surface protein during Staphylococcus aureus pathogenesis. Proc Natl Acad Sci U S A. 2002;99:2293–8. doi: 10.1073/pnas.032523999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Torres VJ, Pishchany G, Humayun M, Schneewind O, Skaar EP. Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. J Bacteriol. 2006;188:8421–9. doi: 10.1128/JB.01335-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dryla A, Gelbmann D, von Gabain A, Nagy E. Identification of a novel iron regulated staphylococcal surface protein with haptoglobin-haemoglobin binding activity. Mol Microbiol. 2003;49:37–53. doi: 10.1046/j.1365-2958.2003.03542.x. [DOI] [PubMed] [Google Scholar]
- 64.Pishchany G, Dickey SE, Skaar EP. Subcellular localization of the Staphylococcus aureus heme iron transport components IsdA and IsdB. Infect Immun. 2009;77:2624–34. doi: 10.1128/IAI.01531-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muryoi N, Tiedemann MT, Pluym M, Cheung J, Heinrichs DE, Stillman MJ. Demonstration of the iron-regulated surface determinant (Isd) heme transfer pathway in Staphylococcus aureus. J Biol Chem. 2008;283:28125–36. doi: 10.1074/jbc.M802171200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu M, Tanaka WN, Zhu H, Xie G, Dooley DM, Lei B. Direct hemin transfer from IsdA to IsdC in the iron-regulated surface determinant (Isd) heme acquisition system of Staphylococcus aureus. J Biol Chem. 2008;283:6668–76. doi: 10.1074/jbc.M708372200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tiedemann MT, Muryoi N, Heinrichs DE, Stillman MJ. Iron acquisition by the haem-binding Isd proteins in Staphylococcus aureus: studies of the mechanism using magnetic circular dichroism. Biochem Soc Trans. 2008;36:1138–43. doi: 10.1042/BST0361138. [DOI] [PubMed] [Google Scholar]
- 68.Pluym M, Vermeiren CL, Mack J, Heinrichs DE, Stillman MJ. Heme binding properties of Staphylococcus aureus IsdE. Biochemistry. 2007;46:12777–87. doi: 10.1021/bi7009585. [DOI] [PubMed] [Google Scholar]
- 69.Mack J, Vermeiren C, Heinrichs DE, Stillman MJ. In vivo heme scavenging by Staphylococcus aureus IsdC and IsdE proteins. Biochem Biophys Res Commun. 2004;320:781–8. doi: 10.1016/j.bbrc.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 70.Wu R, Skaar EP, Zhang R, Joachimiak G, Gornicki P, Schneewind O, Joachimiak A. Staphylococcus aureus IsdG and IsdI, heme-degrading enzymes with structural similarity to monooxygenases. J Biol Chem. 2005;280:2840–6. doi: 10.1074/jbc.M409526200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skaar EP, Gaspar AH, Schneewind O. IsdG and IsdI, heme-degrading enzymes in the cytoplasm of Staphylococcus aureus. J Biol Chem. 2004;279:436–43. doi: 10.1074/jbc.M307952200. [DOI] [PubMed] [Google Scholar]
- 72.Kim HK, DeDent A, Cheng AG, McAdow M, Bagnoli F, Missiakas DM, Schneewind O. IsdA and IsdB antibodies protect mice against Staphylococcus aureus abscess formation and lethal challenge. Vaccine. 28:6382–92. doi: 10.1016/j.vaccine.2010.02.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng AG, Kim HK, Burts ML, Krausz T, Schneewind O, Missiakas DM. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009;23:3393–404. doi: 10.1096/fj.09-135467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Clarke SR, Brummell KJ, Horsburgh MJ, McDowell PW, Mohamad SA, Stapleton MR, Acevedo J, Read RC, Day NP, Peacock SJ, et al. Identification of in vivo-expressed antigens of Staphylococcus aureus and their use in vaccinations for protection against nasal carriage. J Infect Dis. 2006;193:1098–108. doi: 10.1086/501471. [DOI] [PubMed] [Google Scholar]
- 75.Etz H, Minh DB, Henics T, Dryla A, Winkler B, Triska C, Boyd AP, Sollner J, Schmidt W, von Ahsen U, et al. Identification of in vivo expressed vaccine candidate antigens from Staphylococcus aureus. Proc Natl Acad Sci U S A. 2002;99:6573–8. doi: 10.1073/pnas.092569199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stranger-Jones YK, Bae T, Schneewind O. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad Sci U S A. 2006;103:16942–7. doi: 10.1073/pnas.0606863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raedler MD, Heyne S, Wagner E, Shalkowski SK, Secore S, Anderson AS, Cook J, Cope L, McNeely T, Retzlaff M, et al. Serologic assay to quantify human immunoglobulin G antibodies to the Staphylococcus aureus iron surface determinant B antigen. Clin Vaccine Immunol. 2009;16:739–48. doi: 10.1128/CVI.00478-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown M, Kowalski R, Zorman J, Wang XM, Towne V, Zhao Q, Secore S, Finnefrock AC, Ebert T, Pancari G, et al. Selection and characterization of murine monoclonal antibodies to Staphylococcus aureus iron-regulated surface determinant B with functional activity in vitro and in vivo. Clin Vaccine Immunol. 2009;16:1095–104. doi: 10.1128/CVI.00085-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ebert T, Smith S, Pancari G, Clark D, Hampton R, Secore S, Towne V, Fan H, Wang XM, Wu X, et al. A fully human monoclonal antibody to Staphylococcus aureus iron regulated surface determinant B (IsdB) with functional activity in vitro and in vivo. Hum Antibodies. 19:113–28. doi: 10.3233/HAB-2010-0235. [DOI] [PubMed] [Google Scholar]
- 80.Smith JF, Kowalski R, Esser MT, Brown MJ, Bryan JT. Evolution of type-specific immunoassays to evaluate the functional immune response to Gardasil: a vaccine for human papillomavirus types 16, 18, 6 and 11. Hum Vaccin. 2008;4:134–42. doi: 10.4161/hv.4.2.5261. [DOI] [PubMed] [Google Scholar]
- 81.Khan FA, Fisher MA, Khakoo RA. Association of hemochromatosis with infectious diseases: expanding spectrum. Int J Infect Dis. 2007;11:482–7. doi: 10.1016/j.ijid.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 82.Kumar V, Abbas AK, Fausto N, Aster JC, editors. Robbins and Cotran Pathologic Basis of Disease. 8. Philadelphia: Saunders Elsevier; 2010. [Google Scholar]
- 83.Foster TJ. Colonization and infection of the human host by staphylococci: adhesion, survival and immune evasion. Vet Dermatol. 2009;20:456–70. doi: 10.1111/j.1365-3164.2009.00825.x. [DOI] [PubMed] [Google Scholar]
- 84.Palazzolo-Ballance AM, Reniere ML, Braughton KR, Sturdevant DE, Otto M, Kreiswirth BN, Skaar EP, DeLeo FR. Neutrophil microbicides induce a pathogen survival response in community-associated methicillin-resistant Staphylococcus aureus. J Immunol. 2008;180:500–9. doi: 10.4049/jimmunol.180.1.500. [DOI] [PubMed] [Google Scholar]
- 85.Miajlovic H, Zapotoczna M, Geoghegan JA, Kerrigan SW, Speziale P, Foster TJ. Direct interaction of iron-regulated surface determinant IsdB of Staphylococcus aureus with the GPIIb/IIIa receptor on platelets. Microbiology. 156:920–8. doi: 10.1099/mic.0.036673-0. [DOI] [PubMed] [Google Scholar]
- 86.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–5. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 87.Coller BS, Peerschke EI, Scudder LE, Sullivan CA. Studies with a murine monoclonal antibody that abolishes ristocetin-induced binding of von Willebrand factor to platelets: additional evidence in support of GPIb as a platelet receptor for von Willebrand factor. Blood. 1983;61:99–110. [PubMed] [Google Scholar]
- 88.Bennett JS, Shattil SJ, Power JW, Gartner TK. Interaction of fibrinogen with its platelet receptor. Differential effects of alpha and gamma chain fibrinogen peptides on the glycoprotein IIb-IIIa complex. J Biol Chem. 1988;263:12948–53. [PubMed] [Google Scholar]
- 89.Cook JJ, Trybulec M, Lasz EC, Khan S, Niewiarowski S. Binding of glycoprotein IIIa-derived peptide 217–231 to fibrinogen and von Willebrand factors and its inhibition by platelet glycoprotein IIb/IIIa complex. Biochim Biophys Acta. 1992;1119:312–21. doi: 10.1016/0167-4838(92)90219-4. [DOI] [PubMed] [Google Scholar]
- 90.Adams RL, Bird RJ. Review article: Coagulation cascade and therapeutics update: relevance to nephrology. Part 1: Overview of coagulation, thrombophilias and history of anticoagulants. Nephrology (Carlton) 2009;14:462–70. doi: 10.1111/j.1440-1797.2009.01128.x. [DOI] [PubMed] [Google Scholar]
- 91.Giesen PL, Rauch U, Bohrmann B, Kling D, Roque M, Fallon JT, Badimon JJ, Himber J, Riederer MA, Nemerson Y. Blood-borne tissue factor: another view of thrombosis. Proc Natl Acad Sci U S A. 1999;96:2311–5. doi: 10.1073/pnas.96.5.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Giesen PL, Nemerson Y. Tissue factor on the loose. Semin Thromb Hemost. 2000;26:379–84. doi: 10.1055/s-2000-8456. [DOI] [PubMed] [Google Scholar]
- 93.Lutz BR, Fulton GP, Akers RP. White thromboembolism in the hamster cheek pouch after trauma, infection and neoplasia. Circulation. 1951;3:339–51. doi: 10.1161/01.cir.3.3.339. [DOI] [PubMed] [Google Scholar]
- 94.Nemerson Y, Esnouf MP. Activation of a proteolytic system by a membrane lipoprotein: mechanism of action of tissue factor. Proc Natl Acad Sci U S A. 1973;70:310–4. doi: 10.1073/pnas.70.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Davie EW, Ratnoff OD. Waterfall Sequence for Intrinsic Blood Clotting. Science. 1964;145:1310–2. doi: 10.1126/science.145.3638.1310. [DOI] [PubMed] [Google Scholar]
- 96.Macfarlane RG. An Enzyme Cascade in the Blood Clotting Mechanism, and Its Function as a Biochemical Amplifier. Nature. 1964;202:498–9. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- 97.Senior RM, Skogen WF, Griffin GL, Wilner GD. Effects of fibrinogen derivatives upon the inflammatory response. Studies with human fibrinopeptide B. J Clin Invest. 1986;77:1014–9. doi: 10.1172/JCI112353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zimmerman GA, McIntyre TM, Prescott SM. Thrombin stimulates the adherence of neutrophils to human endothelial cells in vitro. J Clin Invest. 1985;76:2235–46. doi: 10.1172/JCI112232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cooper JA, Solano SJ, Bizios R, Kaplan JE, Malik AB. Pulmonary neutrophil kinetics after thrombin-induced intravascular coagulation. J Appl Physiol. 1984;57:826–32. doi: 10.1152/jappl.1984.57.3.826. [DOI] [PubMed] [Google Scholar]
- 100.Pisano JJ, Finlayson JS, Peyton MP. Cross-link in fibrin polymerized by factor 13: epsilon-(gamma-glutamyl)lysine. Science. 1968;160:892–3. doi: 10.1126/science.160.3830.892. [DOI] [PubMed] [Google Scholar]
- 101.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–49. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 102.Bakker WW, Willink EJ, Donga J, Hulstaert CE, Hardonk MJ. Antithrombotic activity of glomerular adenosine diphosphatase in the glomerular basement membrane of the rat kidney. J Lab Clin Med. 1987;109:171–7. [PubMed] [Google Scholar]
- 103.Nieuwenhuizen W, Vermond A, Voskuilen M, Traas DW, Verheijen JH. Identification of a site in fibrin(ogen) which is involved in the acceleration of plasminogen activation by tissue-type plasminogen activator. Biochim Biophys Acta. 1983;748:86–92. doi: 10.1016/0167-4838(83)90030-4. [DOI] [PubMed] [Google Scholar]
- 104.Loeb L. The Influence of certain Bacteria on the Coagulation of the Blood. J Med Res. 1903;10:407–19. [PMC free article] [PubMed] [Google Scholar]
- 105.Kolle WOR. Die Differenzierung der Staphylokokken mittelst der Agglutination. Z Hygiene. 1902;41:369–379. [Google Scholar]
- 106.Birch-Hirschfeld L. Über die Agglutination von Staphylokokken durch Bestandteile des Säugetierblutplasmas. Klinische Woschenschrift. 1934:13. [Google Scholar]
- 107.Hawiger J, Timmons S, Strong DD, Cottrell BA, Riley M, Doolittle RF. Identification of a region of human fibrinogen interacting with staphylococcal clumping factor. Biochemistry. 1982;21:1407–13. doi: 10.1021/bi00535a047. [DOI] [PubMed] [Google Scholar]
- 108.Friedrich R, Panizzi P, Fuentes-Prior P, Richter K, Verhamme I, Anderson PJ, Kawabata S, Huber R, Bode W, Bock PE. Staphylocoagulase is a prototype for the mechanism of cofactor-induced zymogen activation. Nature. 2003;425:535–9. doi: 10.1038/nature01962. [DOI] [PubMed] [Google Scholar]
- 109.Kroh HK, Panizzi P, Bock PE. Von Willebrand factor-binding protein is a hysteretic conformational activator of prothrombin. Proc Natl Acad Sci U S A. 2009;106:7786–91. doi: 10.1073/pnas.0811750106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McDevitt D, Francois P, Vaudaux P, Foster TJ. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol. 1994;11:237–48. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 111.Strong DD, Laudano AP, Hawiger J, Doolittle RF. Isolation, characterization, and synthesis of peptides from human fibrinogen that block the staphylococcal clumping reaction and construction of a synthetic clumping particle. Biochemistry. 1982;21:1414–20. doi: 10.1021/bi00535a048. [DOI] [PubMed] [Google Scholar]
- 112.Smith W, Hale JH, Smith MM. The role of coagulase in staphylococcal infections. Br J Exp Pathol. 1947;28:57–67. [PMC free article] [PubMed] [Google Scholar]
- 113.Ekstedt RD, Yotis WW. Studies on staphylococci. II. Effect of coagulase on the virulence of coagulase negative strains. J Bacteriol. 1960;80:496–500. doi: 10.1128/jb.80.4.496-500.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Duthie ES, Lorenz LL. Staphylococcal coagulase; mode of action and antigenicity. J Gen Microbiol. 1952;6:95–107. doi: 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]
- 115.Hemker HC, Bas BM, Muller AD. Activation of a pro-enzyme by a stoichiometric reaction with another protein. The reaction between prothrombin and staphylocoagulase. Biochim Biophys Acta. 1975;379:180–8. doi: 10.1016/0005-2795(75)90020-3. [DOI] [PubMed] [Google Scholar]
- 116.Kaida S, Miyata T, Yoshizawa Y, Kawabata S, Morita T, Igarashi H, Iwanaga S. Nucleotide sequence of the staphylocoagulase gene: its unique COOH-terminal 8 tandem repeats. J Biochem. 1987;102:1177–86. doi: 10.1093/oxfordjournals.jbchem.a122156. [DOI] [PubMed] [Google Scholar]
- 117.Phonimdaeng P, O’Reilly M, Nowlan P, Bramley AJ, Foster TJ. The coagulase of Staphylococcus aureus 8325–4. Sequence analysis and virulence of site-specific coagulase-deficient mutants. Mol Microbiol. 1990;4:393–404. doi: 10.1111/j.1365-2958.1990.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 118.Phonimdaeng P, O’Reilly M, O’Toole PW, Foster TJ. Molecular cloning and expression of the coagulase gene of Staphylococcus aureus 8325–4. J Gen Microbiol. 1988;134:75–83. doi: 10.1099/00221287-134-1-75. [DOI] [PubMed] [Google Scholar]
- 119.Bjerketorp J, Jacobsson K, Frykberg L. The von Willebrand factor-binding protein (vWbp) of Staphylococcus aureus is a coagulase. FEMS Microbiol Lett. 2004;234:309–14. doi: 10.1016/j.femsle.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 120.Bjerketorp J, Nilsson M, Ljungh A, Flock JI, Jacobsson K, Frykberg L. A novel von Willebrand factor binding protein expressed by Staphylococcus aureus. Microbiology. 2002;148:2037–44. doi: 10.1099/00221287-148-7-2037. [DOI] [PubMed] [Google Scholar]
- 121.Panizzi P, Friedrich R, Fuentes-Prior P, Richter K, Bock PE, Bode W. Fibrinogen substrate recognition by staphylocoagulase.(pro)thrombin complexes. J Biol Chem. 2006;281:1179–87. doi: 10.1074/jbc.M507956200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tager M, Drummond MC. Staphylocoagulase. Ann N Y Acad Sci. 1965;128:92–111. doi: 10.1111/j.1749-6632.1965.tb11632.x. [DOI] [PubMed] [Google Scholar]
- 123.Di Nisio M, Middeldorp S, Buller HR. Direct thrombin inhibitors. N Engl J Med. 2005;353:1028–40. doi: 10.1056/NEJMra044440. [DOI] [PubMed] [Google Scholar]
- 124.Vanassche T, Verhaegen J, Peetermans WE, Hoylaerts MF, Verhamme P. Dabigatran inhibits Staphylococcus aureus coagulase activity. J Clin Microbiol. 48:4248–50. doi: 10.1128/JCM.00896-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Panizzi P, Friedrich R, Fuentes-Prior P, Kroh HK, Briggs J, Tans G, Bode W, Bock PE. Novel fluorescent prothrombin analogs as probes of staphylocoagulase-prothrombin interactions. J Biol Chem. 2006;281:1169–78. doi: 10.1074/jbc.M507955200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Baddour LM, Tayidi MM, Walker E, McDevitt D, Foster TJ. Virulence of coagulase-deficient mutants of Staphylococcus aureus in experimental endocarditis. J Med Microbiol. 1994;41:259–63. doi: 10.1099/00222615-41-4-259. [DOI] [PubMed] [Google Scholar]
- 127.Hasegawa N, Kondo I, Hoshina S, Kurosaka K, Igarashi H. Effect of highly purified coagulase and culture filtrate on virulence and immunity of a coagulase-negative mutant of staphylococcus aureus BB. Infect Immun. 1983;39:1236–42. doi: 10.1128/iai.39.3.1236-1242.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jonsson P, Lindberg M, Haraldsson I, Wadstrom T. Virulence of Staphylococcus aureus in a mouse mastitis model: studies of alpha hemolysin, coagulase, and protein A as possible virulence determinants with protoplast fusion and gene cloning. Infect Immun. 1985;49:765–9. doi: 10.1128/iai.49.3.765-769.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Seki K, Ogasawara M, Sakurada J, Murai M, Masuda S. Altered virulence of a pleiotropic Staphylococcus aureus mutant with a low producibility of coagulase and other factors in mice. Microbiol Immunol. 1989;33:981–90. doi: 10.1111/j.1348-0421.1989.tb03156.x. [DOI] [PubMed] [Google Scholar]
- 130.Moreillon P, Entenza JM, Francioli P, McDevitt D, Foster TJ, Francois P, Vaudaux P. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect Immun. 1995;63:4738–43. doi: 10.1128/iai.63.12.4738-4743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sawai T, Tomono K, Yanagihara K, Yamamoto Y, Kaku M, Hirakata Y, Koga H, Tashiro T, Kohno S. Role of coagulase in a murine model of hematogenous pulmonary infection induced by intravenous injection of Staphylococcus aureus enmeshed in agar beads. Infect Immun. 1997;65:466–71. doi: 10.1128/iai.65.2.466-471.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cheng AG, McAdow M, Kim HK, Bae T, Missiakas DM, Schneewind O. Contribution of coagulases towards Staphylococcus aureus disease and protective immunity. PLoS Pathog. 6:e1001036. doi: 10.1371/journal.ppat.1001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Panizzi P, Nahrendorf M, Figueiredo JL, Panizzi J, Marinelli B, Iwamoto Y, Keliher E, Maddur AA, Waterman P, Kroh HK, et al. In vivo detection of Staphylococcus aureus endocarditis by targeting pathogen-specific prothrombin activation. Nat Med. 17:1142–6. doi: 10.1038/nm.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ganesh VK, Rivera JJ, Smeds E, Ko YP, Bowden MG, Wann ER, Gurusiddappa S, Fitzgerald JR, Hook M. A structural model of the Staphylococcus aureus ClfA-fibrinogen interaction opens new avenues for the design of anti-staphylococcal therapeutics. PLoS Pathog. 2008;4:e1000226. doi: 10.1371/journal.ppat.1000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McDevitt D, Francois P, Vaudaux P, Foster TJ. Identification of the ligand-binding domain of the surface-located fibrinogen receptor (clumping factor) of Staphylococcus aureus. Mol Microbiol. 1995;16:895–907. doi: 10.1111/j.1365-2958.1995.tb02316.x. [DOI] [PubMed] [Google Scholar]
- 136.McDevitt D, Nanavaty T, House-Pompeo K, Bell E, Turner N, McIntire L, Foster T, Hook M. Characterization of the interaction between the Staphylococcus aureus clumping factor (ClfA) and fibrinogen. Eur J Biochem. 1997;247:416–24. doi: 10.1111/j.1432-1033.1997.00416.x. [DOI] [PubMed] [Google Scholar]
- 137.Walsh EJ, Miajlovic H, Gorkun OV, Foster TJ. Identification of the Staphylococcus aureus MSCRAMM clumping factor B (ClfB) binding site in the alphaC-domain of human fibrinogen. Microbiology. 2008;154:550–8. doi: 10.1099/mic.0.2007/010868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.McAdow M, Kim HK, DeDent AC, Hendrickx APA, Missiakas DM, Schneewind O. Preventing Staphylococcus aureus sepsis through the inhibition of its agglutination in blood. PLoS Pathog. 2011 doi: 10.1371/journal.ppat.1002307. in Press, In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hall AE, Domanski PJ, Patel PR, Vernachio JH, Syribeys PJ, Gorovits EL, Johnson MA, Ross JM, Hutchins JT, Patti JM. Characterization of a protective monoclonal antibody recognizing Staphylococcus aureus MSCRAMM protein clumping factor A. Infect Immun. 2003;71:6864–70. doi: 10.1128/IAI.71.12.6864-6870.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vernachio J, Bayer AS, Le T, Chai YL, Prater B, Schneider A, Ames B, Syribeys P, Robbins J, Patti JM. Anti-clumping factor A immunoglobulin reduces the duration of methicillin-resistant Staphylococcus aureus bacteremia in an experimental model of infective endocarditis. Antimicrob Agents Chemother. 2003;47:3400–6. doi: 10.1128/AAC.47.11.3400-3406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Weems JJ, Jr, Steinberg JP, Filler S, Baddley JW, Corey GR, Sampathkumar P, Winston L, John JF, Kubin CJ, Talwani R, et al. Phase II, randomized, double-blind, multicenter study comparing the safety and pharmacokinetics of tefibazumab to placebo for treatment of Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2006;50:2751–5. doi: 10.1128/AAC.00096-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hair PS, Echague CG, Sholl AM, Watkins JA, Geoghegan JA, Foster TJ, Cunnion KM. Clumping factor A interaction with complement factor I increases C3b cleavage on the bacterial surface of Staphylococcus aureus and decreases complement-mediated phagocytosis. Infect Immun. 78:1717–27. doi: 10.1128/IAI.01065-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Donahue JP, Patel H, Anderson WF, Hawiger J. Three-dimensional structure of the platelet integrin recognition segment of the fibrinogen gamma chain obtained by carrier protein-driven crystallization. Proc Natl Acad Sci U S A. 1994;91:12178–82. doi: 10.1073/pnas.91.25.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ware S, Donahue JP, Hawiger J, Anderson WF. Structure of the fibrinogen gamma-chain integrin binding and factor XIIIa cross-linking sites obtained through carrier protein driven crystallization. Protein Sci. 1999;8:2663–71. doi: 10.1110/ps.8.12.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;3:948–58. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- 146.Gallin JI. Interferon-gamma in the management of chronic granulomatous disease. Rev Infect Dis. 1991;13:973–8. doi: 10.1093/clinids/13.5.973. [DOI] [PubMed] [Google Scholar]
- 147.Hair PS, Ward MD, Semmes OJ, Foster TJ, Cunnion KM. Staphylococcus aureus clumping factor A binds to complement regulator factor I and increases factor I cleavage of C3b. J Infect Dis. 2008;198:125–33. doi: 10.1086/588825. [DOI] [PubMed] [Google Scholar]
- 148.Rooijakkers SH, Wu J, Ruyken M, van Domselaar R, Planken KL, Tzekou A, Ricklin D, Lambris JD, Janssen BJ, van Strijp JA, et al. Structural and functional implications of the alternative complement pathway C3 convertase stabilized by a staphylococcal inhibitor. Nat Immunol. 2009;10:721–7. doi: 10.1038/ni.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chen H, Ricklin D, Hammel M, Garcia BL, McWhorter WJ, Sfyroera G, Wu YQ, Tzekou A, Li S, Geisbrecht BV, et al. Allosteric inhibition of complement function by a staphylococcal immune evasion protein. Proc Natl Acad Sci U S A. 107:17621–6. doi: 10.1073/pnas.1003750107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Postma B, Kleibeuker W, Poppelier MJ, Boonstra M, Van Kessel KP, Van Strijp JA, de Haas CJ. Residues 10–18 within the C5a receptor N terminus compose a binding domain for chemotaxis inhibitory protein of Staphylococcus aureus. J Biol Chem. 2005;280:2020–7. doi: 10.1074/jbc.M412230200. [DOI] [PubMed] [Google Scholar]
- 151.Thammavongsa V, Kern JW, Missiakas DM, Schneewind O. Staphylococcus aureus synthesizes adenosine to escape host immune responses. J Exp Med. 2009;206:2417–27. doi: 10.1084/jem.20090097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Thakker M, Park JS, Carey V, Lee JC. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect Immun. 1998;66:5183–9. doi: 10.1128/iai.66.11.5183-5189.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nilsson IM, Lee JC, Bremell T, Ryden C, Tarkowski A. The role of staphylococcal polysaccharide microcapsule expression in septicemia and septic arthritis. Infect Immun. 1997;65:4216–21. doi: 10.1128/iai.65.10.4216-4221.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Megiovanni AM, Sanchez F, Robledo-Sarmiento M, Morel C, Gluckman JC, Boudaly S. Polymorphonuclear neutrophils deliver activation signals and antigenic molecules to dendritic cells: a new link between leukocytes upstream of T lymphocytes. J Leukoc Biol. 2006;79:977–88. doi: 10.1189/jlb.0905526. [DOI] [PubMed] [Google Scholar]
- 155.van Gisbergen KP, Sanchez-Hernandez M, Geijtenbeek TB, van Kooyk Y. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J Exp Med. 2005;201:1281–92. doi: 10.1084/jem.20041276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Abi Abdallah DS, Egan CE, Butcher BA, Denkers EY. Mouse neutrophils are professional antigen-presenting cells programmed to instruct Th1 and Th17 T-cell differentiation. Int Immunol. 2011;23:317–26. doi: 10.1093/intimm/dxr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang CW, Strong BS, Miller MJ, Unanue ER. Neutrophils influence the level of antigen presentation during the immune response to protein antigens in adjuvants. J Immunol. 2010;185:2927–34. doi: 10.4049/jimmunol.1001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dempsey PW, Allison ME, Akkaraju S, Goodnow CC, Fearon DT. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–50. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- 159.Fischer MB, Goerg S, Shen L, Prodeus AP, Goodnow CC, Kelsoe G, Carroll MC. Dependence of germinal center B cells on expression of CD21/CD35 for survival. Science. 1998;280:582–5. doi: 10.1126/science.280.5363.582. [DOI] [PubMed] [Google Scholar]
- 160.Thornton BP, Vetvicka V, Ross GD. Function of C3 in a humoral response: iC3b/C3dg bound to an immune complex generated with natural antibody and a primary antigen promotes antigen uptake and the expression of co-stimulatory molecules by all B cells, but only stimulates immunoglobulin synthesis by antigen-specific B cells. Clin Exp Immunol. 1996;104:531–7. doi: 10.1046/j.1365-2249.1996.57761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kopf M, Abel B, Gallimore A, Carroll M, Bachmann MF. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat Med. 2002;8:373–8. doi: 10.1038/nm0402-373. [DOI] [PubMed] [Google Scholar]
- 162.Lambris JD, Ricklin D, Geisbrecht BV. Complement evasion by human pathogens. Nat Rev Microbiol. 2008;6:132–42. doi: 10.1038/nrmicro1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sahu A, Kozel TR, Pangburn MK. Specificity of the thioester-containing reactive site of human C3 and its significance to complement activation. Biochem J. 1994;302(Pt 2):429–36. doi: 10.1042/bj3020429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Langley R, Wines B, Willoughby N, Basu I, Proft T, Fraser JD. The staphylococcal superantigen-like protein 7 binds IgA and complement C5 and inhibits IgA-Fc alpha RI binding and serum killing of bacteria. J Immunol. 2005;174:2926–33. doi: 10.4049/jimmunol.174.5.2926. [DOI] [PubMed] [Google Scholar]
- 165.Atkins KL, Burman JD, Chamberlain ES, Cooper JE, Poutrel B, Bagby S, Jenkins AT, Feil EJ, van den Elsen JM. S. aureus IgG-binding proteins SpA and Sbi: host specificity and mechanisms of immune complex formation. Mol Immunol. 2008;45:1600–11. doi: 10.1016/j.molimm.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 166.Sjodahl J. Repetitive sequences in protein A from Staphylococcus aureus. Arrangement of five regions within the protein, four being highly homologous and Fc-binding. Eur J Biochem. 1977;73:343–51. doi: 10.1111/j.1432-1033.1977.tb11324.x. [DOI] [PubMed] [Google Scholar]
- 167.Haupt K, Reuter M, van den Elsen J, Burman J, Halbich S, Richter J, Skerka C, Zipfel PF. The Staphylococcus aureus protein Sbi acts as a complement inhibitor and forms a tripartite complex with host complement Factor H and C3b. PLoS Pathog. 2008;4:e1000250. doi: 10.1371/journal.ppat.1000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Toapanta FR, Ross TM. Complement-mediated activation of the adaptive immune responses: role of C3d in linking the innate and adaptive immunity. Immunol Res. 2006;36:197–210. doi: 10.1385/IR:36:1:197. [DOI] [PubMed] [Google Scholar]
- 169.Ricklin D, Tzekou A, Garcia BL, Hammel M, McWhorter WJ, Sfyroera G, Wu YQ, Holers VM, Herbert AP, Barlow PN, et al. A molecular insight into complement evasion by the staphylococcal complement inhibitor protein family. J Immunol. 2009;183:2565–74. doi: 10.4049/jimmunol.0901443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jongerius I, Kohl J, Pandey MK, Ruyken M, van Kessel KP, van Strijp JA, Rooijakkers SH. Staphylococcal complement evasion by various convertase-blocking molecules. J Exp Med. 2007;204:2461–71. doi: 10.1084/jem.20070818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Isenman DE, Leung E, Mackay JD, Bagby S, van den Elsen JM. Mutational analyses reveal that the staphylococcal immune evasion molecule Sbi and complement receptor 2 (CR2) share overlapping contact residues on C3d: implications for the controversy regarding the CR2/C3d cocrystal structure. J Immunol. 2010;184:1946–55. doi: 10.4049/jimmunol.0902919. [DOI] [PubMed] [Google Scholar]
- 172.Edwards CK, 3rd, Watts LM, Parmely MJ, Linnik MD, Long RE, Borcherding DR. Effect of the carbocyclic nucleoside analogue MDL 201,112 on inhibition of interferon-gamma-induced priming of Lewis (LEW/N) rat macrophages for enhanced respiratory burst and MHC class II Ia+ antigen expression. J Leukoc Biol. 1994;56:133–44. doi: 10.1002/jlb.56.2.133. [DOI] [PubMed] [Google Scholar]
- 173.Panther E, Idzko M, Herouy Y, Rheinen H, Gebicke-Haerter PJ, Mrowietz U, Dichmann S, Norgauer J. Expression and function of adenosine receptors in human dendritic cells. FASEB J. 2001;15:1963–70. doi: 10.1096/fj.01-0169com. [DOI] [PubMed] [Google Scholar]
- 174.Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marton A, Szabo C. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000;14:2065–74. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 175.Schneewind O, Fowler A, Faull KF. Structure of the cell wall anchor of surface proteins in Staphylococcus aureus. Science. 1995;268:103–6. doi: 10.1126/science.7701329. [DOI] [PubMed] [Google Scholar]
- 176.Guss B, Uhlen M, Nilsson B, Lindberg M, Sjoquist J, Sjodahl J. Region X, the cell-wall-attachment part of staphylococcal protein A. Eur J Biochem. 1984;138:413–20. doi: 10.1111/j.1432-1033.1984.tb07931.x. [DOI] [PubMed] [Google Scholar]
- 177.Lindmark R, Thoren-Tolling K, Sjoquist J. Binding of immunoglobulins to protein A and immunoglobulin levels in mammalian sera. J Immunol Methods. 1983;62:1–13. doi: 10.1016/0022-1759(83)90104-7. [DOI] [PubMed] [Google Scholar]
- 178.Sasso EH, Silverman GJ, Mannik M. Human IgM molecules that bind staphylococcal protein A contain VHIII H chains. J Immunol. 1989;142:2778–83. [PubMed] [Google Scholar]
- 179.Hartleib J, Kohler N, Dickinson RB, Chhatwal GS, Sixma JJ, Hartford OM, Foster TJ, Peters G, Kehrel BE, Herrmann M. Protein A is the von Willebrand factor binding protein on Staphylococcus aureus. Blood. 2000;96:2149–56. [PubMed] [Google Scholar]
- 180.Nguyen T, Ghebrehiwet B, Peerschke EI. Staphylococcus aureus protein A recognizes platelet gC1qR/p33: a novel mechanism for staphylococcal interactions with platelets. Infect Immun. 2000;68:2061–8. doi: 10.1128/iai.68.4.2061-2068.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gomez MI, Lee A, Reddy B, Muir A, Soong G, Pitt A, Cheung A, Prince A. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat Med. 2004;10:842–8. doi: 10.1038/nm1079. [DOI] [PubMed] [Google Scholar]
- 182.Mazmanian SK, Ton-That H, Schneewind O. Sortase-catalysed anchoring of surface proteins to the cell wall of Staphylococcus aureus. Mol Microbiol. 2001;40:1049–57. doi: 10.1046/j.1365-2958.2001.02411.x. [DOI] [PubMed] [Google Scholar]
- 183.Silverman GJ, Sasano M, Wormsley SB. Age-associated changes in binding of human B lymphocytes to a VH3-restricted unconventional bacterial antigen. J Immunol. 1993;151:5840–55. [PubMed] [Google Scholar]
- 184.Goodyear CS, Silverman GJ. Staphylococcal toxin induced preferential and prolonged in vivo deletion of innate-like B lymphocytes. Proc Natl Acad Sci U S A. 2004;101:11392–7. doi: 10.1073/pnas.0404382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Silverman GJ, Cary SP, Dwyer DC, Luo L, Wagenknecht R, Curtiss VE. A B cell superantigen-induced persistent “Hole” in the B-1 repertoire. J Exp Med. 2000;192:87–98. doi: 10.1084/jem.192.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kim HK, Cheng AG, Kim HY, Missiakas DM, Schneewind O. Nontoxigenic protein A vaccine for methicillin-resistant Staphylococcus aureus infections in mice. J Exp Med. 207:1863–70. doi: 10.1084/jem.20092514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kim HK, Kim HY, Schneewind O, Missiakas D. Identifying protective antigens of Staphylococcus aureus, a pathogen that suppresses host immune responses. FASEB J. 25:3605–12. doi: 10.1096/fj.11-187963. [DOI] [PMC free article] [PubMed] [Google Scholar]