Abstract
Down Syndrome (DS) is the most prevalent form of mental retardation caused by genetic abnormalities in humans. This has been successfully modeled in mice to generate the Ts65Dn mouse, a genetic model of DS. This transgenic mouse model shares a number of physical and functional abnormalities with people with DS, including changes in the structure and function of neuronal circuits. Significant abnormalities in noradrenergic (NE-ergic) afferents from the locus coeruleus to the hippocampus, as well as deficits in NE-ergic neurotransmission are detected in these animals.
In the current study we characterized in detail the behavioral phenotype of Ts65Dn mice, in addition to using pharmacological tools for identification of target receptors mediating the learning and memory deficits observed in this model of DS. We undertook a comprehensive approach to mouse phenotyping using a battery of standard and novel tests encompassing: i) locomotion (Activity Chamber, PhenoTyper, and CatWalk), ii) learning and memory (spontaneous alternation, delayed matching-to-place water maze, fear conditioning, and Intellicage), and iii) social behavior.
Ts65Dn mice showed increased locomotor activity in novel and home cage environments. There were significant and reproducible deficits in learning and memory tests including spontaneous alternation, delayed matching-to-place water maze, Intellicage place avoidance and contextual fear conditioning. Although Ts65Dn mice showed no deficit in sociability in the 3-chamber test, a marked impairment in social memory was detected. Xamoterol, a β1-adrenergic receptor (β1-ADR) agonist, effectively restored the memory deficit in contextual fear conditioning, spontaneous alternation and novel object recognition. These behavioral improvements were reversed by betaxolol, a selective β1-ADR antagonist.
In conclusion, our results demonstrate that this mouse model of Down Syndrome display cognitive deficits which is mediated by imbalance in noradrenergic system. In this experimental model of Down Syndrome a selective activation of β1-ADR does restore some of these behavioral deficits. Further mechanistic studies will be needed to investigate the failure of noradrenergic system and the role of β1-ADR in cognitive deficit and pathogenesis of DS in people. Restoring NE neurotransmission or a selective activation of β1-ADR need to be further investigated for development of any potential therapeutic strategies for symptomatic relieve of memory deficit in DS. Furthermore, due to the significant involvement of noradrenergic system in the cardiovascular function further safety and translational studies will be needed to ensure the safety and efficacy of this approach.
Keywords: Down Syndrome, behavior, Ts65Dn mouse, memory, social interaction, xamoterol, betaxolol, noradrenergic system, neurodegenerative disorder
Introduction
Down Syndrome (DS), a trisomy of chromosome 21 (HSA21), is the most prevalent form of mental retardation caused by genetic abnormalities in humans (Epstein et al., 1990). Extra copies of all or part of HSA21 affects a number of organs and in particular the central nervous system. In addition to intellectual dysfunction, people with DS may also suffer from congenital cardiac disease, immune and endocrine problems, genitourinary defects, gastrointestinal abnormalities, and orofacial malformations (Cleves et al., 2007; Greenwood and Nadas, 1976; Korenberg et al., 1994). An important characteristic of DS is the development of the neuropathological markers of Alzheimer’s disease (AD) by age 40, accompanied by the cognitive decline in later life (Burger and Vogel, 1973; Casanova et al., 1985; Mufson et al., 2002).
In spite of significant recent progress in understanding the neurobiology of DS (Belichenko et al., 2009a; Belichenko et al., 2004; Dierssen et al., 1997; Dierssen et al., 1996; Granholm et al., 2000; Holtzman et al., 1996; Lumbreras et al., 2006; Salehi et al., 2006; Salehi et al., 2007; Salehi et al., 2009) there is as yet little insight into the genes and mechanisms responsible for developmental and age-related changes in cognition and behavior. Genetic models are beneficial tools in helping to answer many of these questions because of the wealth of available mouse genetics data and the availability of tools to modify gene dosages.
Ts65Dn mice are the most commonly used mouse model of DS. This mouse is generated by Robertsonian segmental translocation of mouse chromosome 16 (MMU16) to the MMU17 centromere (Davisson et al., 1990). This chromosomal segment contains an extra copy of more than 100 gene homologues to HSA21 (Baxter et al., 2000; Kahlem et al., 2004; Reeves et al., 1995). Ts65Dn mice have shorter life expectancies and show morphological, neurological, and structural abnormalities that parallel those found in people with DS (Belichenko et al., 2004; Dierssen et al., 1997; Dierssen et al., 1996; Granholm et al., 2000; Holtzman et al., 1996; Lumbreras et al., 2006; Salehi et al., 2006; Salehi et al., 2007). They show changes in the structure and function of neuronal circuits, including deficits in hippocampal synaptic plasticity. These changes have been demonstrated in both cellular signaling and electrophysiological studies (Dierssen et al., 1997; Dierssen et al., 1996; Kleschevnikov et al., 2004; Siarey et al., 1999; Siarey et al., 2006; Siarey et al., 1997; Siarey et al., 2005). Ts65Dn mice also share behavioral abnormalities similar to those seen in DS. Along with increased locomotor activity (Coussons-Read and Crnic, 1996; Davisson et al., 1993; Escorihuela et al., 1995; Reeves et al., 1995; Stewart et al., 2007), the trisomic mice show increased repetitive and stereotypical movement in the home cage. Ts65Dn mice display impaired learning and memory, especially in hippocampus-dependent tasks such as the water maze spatial learning task (Demas et al., 1996; Escorihuela et al., 1995; Holtzman et al., 1996; Reeves et al., 1995), dry and water radial arm maze tests (Bimonte-Nelson et al., 2003; Demas et al., 1996; 1998; Hunter et al., 2004), and spontaneous alternation (Belichenko et al., 2007; Chang and Gold, 2008). However, in some learning and memory tests, such as the passive avoidance test, no significant difference was reported (Coussons-Read and Crnic, 1996; Holtzman et al., 1996; Rueda et al., 2008). It has been demonstrated that Ts65Dn mice have difficulty in learning the context discrimination task (Hyde et al., 2001b), as well as retrieving fear-dependent memories (Costa et al., 2008). Despite normal learning during the acquisition phase in the fear conditioning (FC) test, the Ts65Dn mouse demonstrates a selective and significant deficit in the contextual, but not the tone-dependent, retrieval test (Salehi et al., 2009). It has been shown that noradrenergic (NE-ergic) afferents from the locus coeruleus (LC) to the hippocampus play a key role in contextual retrieval in this test (Murchison et al., 2004). Furthermore, we have shown that Ts65Dn mice display abnormalities in these afferents; restoration of NE-ergic neurotransmission using a norepinephrine prodrug, L-threo-3,4-dihydroxyphenylserine (L-DOPS) restores the hippocampal-dependent retrieval in contextual testing (Salehi et al., 2009). Human studies have also shown a significant cell loss in LC (German et al., 1992) which is accompanied with a lower level of NE in cortical and subcortical areas of the brain (Godridge et al., 1987). It has also been shown that the Ts65Dn mice display an impaired β-adrenergic receptor mediated synaptic transmission (Dierssen et al., 1997). Xamoterol is a selective β1-adrenergic receptor (β1-ADR) partial agonist which has been clinically developed for cardiovascular indications (Marlow, 1990). It has been demonstrated that the xamoterol display central nervous system efficacy and can reverse the deficit observed in memory retrieval in dopamine hydroxylase knockout mice (Murchison et al., 2004). Furthermore, these authors have shown that the CNS effect of xamoterol on memory retrieval can be reversed by betaxolol, a selective β1-ADR antagonist. Conversely atenolol, which does not pass the blood-brain barrier (BBB), failed to block the xamoterol effect (Murchison et al., 2004).
In this study we aimed to identify robust and reproducible behavioral paradigms for the phenotyping of Ts65Dn mice with regards to motor function, learning and memory, as well as social behavior. We utilized pharmacological tools to target β1-ADR for restoration of learning and memory in this model of DS. This complete behavioral phenotype could be used to define effective pharmacological approaches for devising treatment for behavioral and cognitive disorders in people with DS and other neurodegenerative disorders.
Materials and Methods
Subjects
Unless otherwise specified, male and female Ts65Dn mice (B6EiC3Sn-a/A-Ts (1716)65Dn) and their age-matched normosomic (2N) littermates, aged 9-12 months, were used in this study. Before experimenataion, the genotypes of all animals were determined by real-time quantitative PCR. Mice that tested homozygous for the retinal degeneration1 mutant gene (Rd1) were excluded from this study. For social interaction experiments, 4-6 months old male and ovariectomized female C57Bl/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME). All animals were housed in a 12 hour dark/light cycle in a temperature- and humidity-controlled environment with ad libitum access to water and food; all tests were conducted in the light cycle. The same group of animals was used for the exploratory activity, CatWalk, spontaneous alternation, and fear conditioning tests. Another group of animals were used for Home cage behavioral monitoring tests. Different groups of mice were used for social interaction tests and afterward they were tested in Delayed Matching to Place (DMP) water maze tests. One group of female mice was tested for Intellicage. Different groups of mice were used for pharmacological experiments. All experiments were in accordance with protocols approved by the Institutional Animal Care and Use Committee of Stanford University and were performed based on the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All sufficient actions were considered for reducing pain or discomfort of subjects during all experiments.
Behavioral Tests
Exploratory Activity in a Novel Environment
The Activity Chamber (Med Associates Inc., St. Albans, VT) was used for the evaluation of general activity, gross locomotor activity and exploratory behavior. Assessment took place in a square arena (43.2×43.2 cm) that comprised three planes of infrared detectors within a specially designed sound attenuating chamber (66×55.9×55.9 cm) under dim light. The animal was placed in the center of the testing arena and allowed to move freely for 10 minutes while being tracked by an automated tracking system. Distance moved, velocity, resting time, and vertical count (rearing) were recorded.
Home Cage Behavioral Monitoring
PhenoTyper® (Noldus Information Technology, Wageningen, the Netherlands), an automated infrared video-based observation system, was used for the measurement of behavior of mice in their home cage (see (de Visser et al., 2006)(. The home cage environment minimizes stress or discomfort; the subjects are given ad libitum access to all accessories in the PhenoTyper chamber. Up to 16 PhenoTypers (each containing one mouse) were connected to a computer running Ethovision XT (Noldus Information Technology, Wageningen, the Netherlands), which acquires data over extended periods of time. After 3 days of baseline activity recording, a running wheel was placed in each cage. Time spent in the shelter, food zone, water zone, and running wheel were measured, as well as distance moved, velocity of ambulatory movement; these were reported separately for dark and light cycles.
CatWalk
The CatWalk® apparatus (Noldus Information Technology, Wageningen, the Netherlands) consists of a glass floor illuminated with beams of fluorescent light. Assessment in a dark room allows the paws to reflect light as they come in contact with the glass floor. The bright pixel images were recorded by a camera located directly below the glass walkway and digitally converted. The paw pixels were identified and analyzed by a blind observer, thus generating gait-related measurements (Starkey et al., 2005). Using home cage motivation, the mice were trained to traverse the CatWalk apparatus one day prior to gait assessment. On testing day, subjects were given three consecutive runs and allowed to return to their home cage each time. Runs in which an animal took more than 8 seconds to cross the end zone, walked backwards, walked in the reverse direction, or reared were excluded; the animal was returned to the home cage before being allowed to run again. The average of three runs for each animal was reported. For this study, general gait parameters (regularity index, stride pattern, and running duration) as well as individual paw parameters (intensity, paw area, stand duration, and stride length) were analyzed.
Three-Chamber Sociability and Social Novelty Test
A 3-chamber test (Crawley, 2007; Moy et al., 2004; Moy et al., 2007) was utilized to assess whether mice show interest in spending time with stranger mice. The rectangular testing box consisted of clear plastic divided into three adjacent chambers (each 20 cm long, 40.5 cm wide, 22 cm high) and connected by open doorways (10.2 cm wide, 5.4 cm high). Testing consisted of three consecutive 10-minute sessions with no inter-trial interval. In the first “habituation” session (session A), subject mice were allowed to freely investigate the 3-chamber box. This was followed by a “sociability” session (session B) where an unfamiliar C57Bl/6J male mouse was placed under an inverted pencil cup (stainless steel, 11 cm high, 10 cm diameter solid bottom, with stainless steel bars spaced at 1 cm intervals) in one of the side chambers. An identical inverted empty pencil cup was placed in the center of the other side chamber. The location of the stranger mouse was alternated from left to right across subject testing. In the “social novelty” session (session C), a second unfamiliar C57Bl/6J male mouse was placed under the second pencil cup. A plastic cup containing a heavy lead weight was placed on top of each pencil cup to prevent inadvertent movement and to prevent the subject from climbing onto the top of the wire cage. Between subjects, the box and pencil cup were cleaned with paper towels and 10% ethanol. Trials were videotaped for subsequent rating. Measured parameters were: i) number of entrances into the chambers, ii) time spent in chambers and iii) time spent sniffing the pencil cups.
Social Memory Testing
Prior to testing, randomly selected individually housed ovariectomized C57Bl/6J female mice (OEFs) were put into the home cages of subject mice 4 hours per day for 5-7 days to reduce sexual interest. Subject mice were 9 or 18 months old in all the social tests. Trials of all tests were videotaped and subsequently analyzed for olfactory investigation. Investigation was defined as nose-to-body contact of the test animal versus the intruder. Anogenital investigation, perioral investigation, and body investigation were scored in these tests.
2-Trial Test
An unfamiliar OEF was placed into the home cage of male Ts65Dn subject mice for 5 minutes and then removed. After an inter-trial interval (ITI) of 30 minutes, the same OEF was placed back in the home cage together with a novel unfamiliar OEF for 5 minutes. 5-Trial Test: A single OEF (SAME) was introduced into the home cage of an unfamiliar test animal for four 1-minute exposures with an ITI of 10 minutes for a total of 4 times. In a fifth trial, 10 minutes later, instead of the familiar OEF, a novel, unfamiliar OEF (NOVEL) was put into the home cage of the test animal for one minute.
Olfactory Habituation Test
The test consisted of 2-minute presentations of 6 different cotton swabs soaked with 100 μL of liquid separated by 3-minute ITIs. The tip of the cotton swab was placed 1 cm above the bedding in the home cage to allow investigation without rearing. After three presentations of distilled water, the animals received three presentations of either pure urine from an unfamiliar singly housed OEF mouse or almond scent (1:100 in distilled water). Trials were videotaped for subsequent scoring. Direct physical contact between the nose and the cotton swab was scored; chewing the cotton swab was excluded.
Spontaneous Alternation
Spontaneous alternation was measured using the Y-maze and T-maze. The Y-shaped maze was constructed with three symmetrical white solid plastic arms at a 120-degree angle (40 cm length, 8 cm width, and 15 cm height). Each session began with placement of the mouse in the center of the maze. The mouse was allowed to freely explore the three arms for 8 minutes. Arm entry was defined as all four limbs within the arm. A triad was defined as a set of three arm entries, when each entry was to a different arm of the maze. The maze was cleaned with 10% ethanol between sessions to eliminate odor traces. The number of arm entries and the number of triads were recorded in order to calculate the alternation percentage (generated by dividing the number of triads by the number of possible alternations and then multiplying by 100).
The T-maze had three equal arms (30 cm length, 10 cm width, and 20 cm height); the start arm and two goal arms had guillotine gates as previously described by Belichenko et al. (2009) and Deacon and Rawlins (2006) (Belichenko et al., 2009a; Deacon and Rawlins, 2006). This test is based on the rodents’ preference to experience a new arm of the T-maze instead of a familiar one (Gerlai, 1998). In each trial the mouse was placed in the start arm. The gate was opened and the mouse was able to freely explore the arms. As soon as the subject entered one goal arm, the sliding gate of the other goal arm closed. The mouse eventually returned to the start arm and the next trial was started. In the next trial, the mouse may visit the previously chosen goal arm (no alternation) or choose to explore a new arm (alternation). This trial was repeated 11 times per day for 3 consecutive days, for a total of 33 trials. The maze was cleaned with 10% ethanol between trials to eliminate odor traces. Percent of alternation (number of turns in each goal arm) was determined for analysis.
Intellicage
Home cage-based learning behaviors of socially housed mice were tested using the Intellicage® apparatus (NewBehavior AG, Zurich, Switzerland). Intellicage is an automated home cage-based system for the evaluation of place and operant learning {see (Galsworthy et al., 2005; Knapska et al., 2006) for details}. Animals were randomly assigned to Intellicages with 6-10 mice in each cage. The subjects were socially housed in these groups prior to the experiment. Forty-eight hours before introduction into the Intellicage, each animal was anesthetized by inhalation of isoflurane and injected subcutaneously with an RFID transponder (Datamars SA, Bedano, Switzerland). After general habituation to the cage, animals were subjected to the nose poke adaptation in order to learn to access the water during two drinking sessions every 24 hours. Following these adaptation periods, the animals were subjected to three different tests: i) place learning, ii) place avoidance and iii) entry to the novel satellite box.
In the place learning test, for 4 consecutive days each mouse had access to water in only one corner of the cage and learned to associate access to water with this specific corner of the cage. In all trials, percent of correct visits during drinking sessions was reported. Following the place learning session, all animals were removed from the Intellicage. This session was followed by a 72-hour delay before all animals were returned to the Intellicage for the probe trial to evaluate the total number of visits to the correct corner.
In the place avoidance test, animals learned to avoid a corner where they were met with the aversive stimulus of an air puff. After a 4-day training session, mice were removed from the apparatus for 72 hours and then returned to the Intellicage for a probe trial. During the probe trial, the animals received no air puffs. The percentage of visits to the previously punished corner versus all corners was reported as the percent of incorrect visits (errors) for each day.
In the novelty exploration test a smaller satellite box was attached with the entrance blocked on the end closest to the Intellicage. This was set up prior to housing of the animla in the Intellicage. The mice had access to water in all corners. The tunnel plug was removed and the animals were allowed to freely explore the novel satellite box. The latency to the first entrance to the satellite box and visit frequency was reported.
Delayed Matching-To-Place Water Maze
The delayed matching-to-place (DMP) water maze task was used to assess learning and memory as originally designed by Steel and Morris (1999) for rats (Steele and Morris, 1999). Subjects were given a series of four trials approximately 8-10 minutes apart in a large water tank (178 cm in diameter) filled with opaque water at a temperature of 22.0 ± 1.5°C. A 15 cm circular platform was submerged 1 cm below the water surface and placed randomly in the pool with daily changes in the position. The release point in the pool was changed based on the experimental setup. Each animal was given a maximum of 90 seconds to find the submerged platform. If they were unable to find the platform in that time, the animal was physically guided to it. After remaining on the platform for 10 seconds, the animals were removed and placed in a dry cage. This process was repeated for 7 days. After training in the DMP task, subjects were given visible platform training to ensure they had no gross sensorimotor or visual deficits. During visible platform training, the platform was marked with a black and white ping-pong ball attached to a 10-cm wooden stick. The swim paths of the animals were recorded with the Ethovision 3.1 computer-interfaced camera tracking system (Noldus Information Technology, Wageningen, the Netherlands) and subsequently analyzed. Throughout these tests the water was frequently changed and the tank disinfected.
Fear Conditioning and Startle Response Tests
Contextual and cued fear conditioning was conducted for evaluation of fear-dependent learning and retrieval in the study. The test was performed using chambers from Coulbourn Instruments (Whitehall, PA). On the first day animals were placed in a chamber (Context A) for 3 minutes for baseline recording, followed by five tone-shock pairings. The shock (0.5 mA, 2 sec) was delivered following the tone (70 dB, 2 kHz, 20 sec) in each conditional/unconditional stimulus pairing. On the second day a novel chamber (Context B; new room, new olfactory environment, new texture of floor, blue plastic inserts for walls, extra source of blue light, and visual cues) was used for cued testing. Following a 3-minute pre-tone period, three tones without shocks were presented to animals during a 3-minute testing period.. On the last day of the experiment, the mice were placed in Context A for 5 minutes without any conditional or unconditional stimulus {modified from the method described by (Saxe et al., 2006)}. Freezing was defined as the complete lack of motion for a minimum of 0.75 seconds as measured by FreezeFrame software (Actimetrics, Evanston, IL). The percentage of freezing in each period was reported.
For the startle response control test, an acoustic startle reflex apparatus (Med Associates Inc., St. Albans, VT) was used. The subjects were acclimated to the animal holder in the startle box for a total of 15 minutes over 3 consecutive days prior to the experiment. The animals were exposed to 25 different trials with 10-20 second randomly variable ITIs. Five different intensities of startle pulses (0, 90, 100, 110, and 120 dB) were chosen arbitrarily and each animal was randomly exposed five times to each intensity of the startle pulse. The duration of each startle pulse was 40 ms and the peak amplitude of the startle response in each trial was recorded for analysis. The holding cage on the apparatus was cleaned with 10% ethanol between each animal.
Pharmacological Experiments
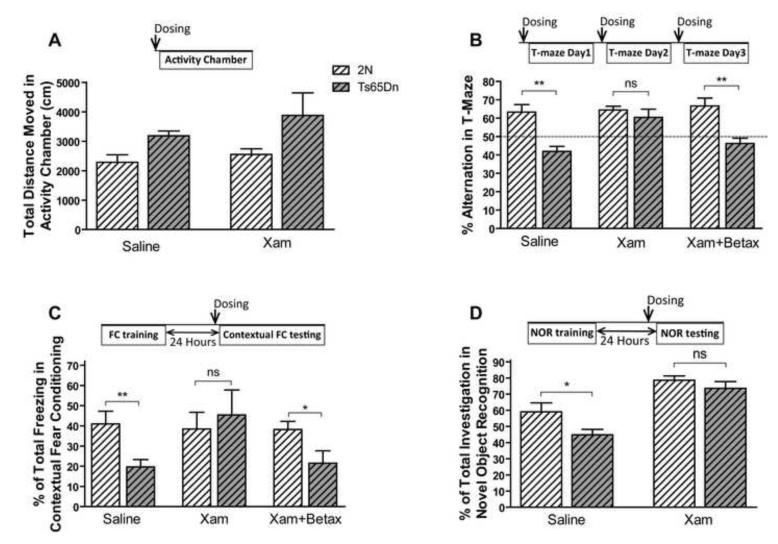
To test the role of β1-ADR in the cognitive deficits of Ts65Dn mice, xamoterol hemifumarate (Tocris Bioscience, Ellisville, MO), a selective β1 partial agonist, S-(−)-atenolol, and betaxolol HCl (Tocris Bioscience, Ellisville, MO), selective β1 antagonists, were injected subcutaneously. Xamoterol 3mg/kg and vehicle (saline) were injected one hour before the experiments. Betaxolol 1mg/kg and atenolol 3mg/kg were injected 30 minutes before the tests. Compounds were injected in a different room so as not to influence the behavioral outcome. Volume of the injection solution for all the injections was 10ml/kg. Pharmacological studies were performed using the activity chamber and three cognitive tests; the T-maze spontaneous alternation, contextual FC, and novel object recognition tests were performed. In T-maze spontaneous alternation and contextual FC tests, both Ts65Dn and 2N male mice were divided into three treatment groups - normal saline (Vehicle), xamoterol, and betaxolol plus xamoterol. In the novel object recognition test, both Ts65Dn and 2N male mice were divided into two treatment groups, normal saline and xamoterol. The T-maze was performed as previously described. Animals were injected once a day during the T-maze experiment. Since Ts65Dn mice showed a significant deficit in contextual FC, the tone cued testing paradigm was not performed in this part of the study. On the first day (training day), male Ts65Dn mice were placed in the FC chamber and after a 3-minute pre-shock period were exposed to five shocks (2s, 0.5 mA) with ITIs of 80 seconds and no tone. On the second day, the animals first received one dose of xamoterol, xamoterol plus betaxolol, or saline injection; they were placed one hour later in the same chamber for 5 minutes.
Similar to the Ts65Dn mice, another experiment was run on C57Bl/6J using different doses of xamoterol. In this dose-response experiment, animals received 1 mg/kg of betaxolol and xamoterol (0, 1, 3 or 10 mg/kg) one hour before contextual FC memory retrieval on the second day of the experiment. Additionally, a separate group of mice were treated with atenolol (3mg/kg) prior to the contextual testing. The novel object recognition task was conducted in an open field arena (40 cm × 40 cm). Mice were habituated in the testing room for one hour followed by 5 minutes of habituation inside the arena without any objects for 3 days. On the 4th day, animals were introduced to the arena three times with three identical objects for 10 minutes with 3-minute ITIs. On the 5th day (24 hours after training), one of the objects was replaced by a novel one, and the time spent sniffing each object was used for analysis. Finally, in order to identify if xamoterol can cross blood brain barrier (BBB), male C57Bl/6J mice received a single bolus injection of 3mg/kg of xamoterol subcutaneously and the plasma and brain samples were collected after 0.5, 1, and 2 hours. Xamoterol concentration in the brain and plasma were analyzed by LC-MS/MS. Analyses were performed on an Applied Biosystems API Sciex 3000 triple quadrupole mass spectrometer equipped with electrospray ion source and an Agilent 1200 HPLC system. The separation was performed on an Agilent Zorbax 300SB-C18 column (5 μm, 2.1 × 150 mm) using a gradient elution of mobile phases, 0.2% heptafluorobutyric acid in water and 0.2% heptafluorobutyric acid in methanol and used L-tyrosin-L-valine as an internal standard.
Statistical Analysis
All data were presented as Mean ±/+ SEM, and p<0.05 was considered statistically significant. Two-way ANOVA testing was used for the evaluation of the effect of genotype and sex in the Activity Chamber, CatWalk, T-maze, Y-maze, 3-chamber sociability and social novelty, and 2-trial social memory tests. Repeated measure two-way ANOVA was used for analyzing both the training period of place learning and place avoidance, and also the number of satellite box visits in the novelty exploration test in the Intellicage experiment. Repeated measure ANOVA was used for evaluation of the escape latency in DMP, fear conditioning, startle response, 5-trial social memory, and olfactory control tests. ANOVA was also used for analyzing the pharmacological experiment. The Bonferroni posthoc test was used when appropriate. For analyzing home cage monitoring data, three-way ANOVA testing was used for the main effects of genotype, sex, and light cycle. The Student’s t-test was used as a post test when appropriate. The Student’s t-test was also used for comparing Ts65Dn mice and 2N controls in probe trials and for the comparison of latency of the first visit to the satellite box in the Intellicage. Novelty exploration, time savings in DMP, and percent of freezing in fear conditioning were also examined using Student’s t-test. In the spontaneous alternation experiments, one sample t-test was used for comparison of the alternation to chance level (50%).
Results
Three groups of behavioral tests were conducted in this set of behavioral phenotyping assays. A condensed summary of behavioral outcomes in this study and structures involved in the behavioral tests have been shown in Table 1.
Table 1.
Results of activity, learning, and memory tests, and brain region involved in the task are presented (Anagnostaras et al., 1999; Deacon et al., 2002; Devenport et al., 1988; Galsworthy et al., 2005; Gerlai, 1998; Johnson et al., 1977; Kim and Fanselow, 1992; Lieben et al., 2006; Mumby et al., 2005; Steele and Morris, 1999; Takakusaki, 2008; Viggiano, 2008; Winters et al., 2004).
| Behavioral Parameter | Group | 2N Mean±SEM |
Ts65Dn Mean±SEM |
p Value * | Interpretation | Brain region involved ** With Reference(s) |
|
|---|---|---|---|---|---|---|---|
| Total Ambulatory Distance Moved in Activity Chamber (cm) |
Female | 2111±225 | 3862±594 | p<0.01 | Ts65Dn mice are hyperactive in a novel environment. |
Cerebellum, cerebral cortex, septum, striatum, thalamic reticular nucleus, spinal cord, hippocampus (Deacon et al., 2002; Viggiano, 2008) |
|
| Male | No Treatment | 2264±151 | 3638±388 | p<0.05 | |||
| Saline | 2287±253 | 3186±163 | ns for treatment | Xamoterol has no effect on the activity of the mice. |
|||
| Xam | 2554±192 | 3878±765 | |||||
| Total Vertical Count in Activity Chamber (n) |
Female | 68.9±13.8 | 145.2±27.8 | p<0.01 | Ts65Dn mice rear and jump more than 2N mice in a novel environment |
||
| Male | 75.0±6.1 | 147.7±7.3 | p<0.01 | ||||
| Baseline Distance Moved in Each Hour in Home Cage(cm) |
Female | Dark | 2031±508 | 4122±789 | p<0.05 | Ts65Dn mice are hyperactive in their home cage environment. |
Hippocampus, prefrontal cortex. striatum, cerebral cortex, cerebellum, brain stem, spinal cord (Takakusaki, 2008) |
| Light | 532±100 | 908±205 | ns | ||||
| Male | Dark | 1088±196 | 2358±350 | p<0.01 | |||
| Light | 366±27 | 511±96 | ns | ||||
| Distance Moved in the Running Wheel in Home Cage (cm) |
Female | Dark | 27663±8845 | 39204±7842 | ns | Ts65Dn mice are hyperactive in the running wheel in their home cage environment. |
|
| Light | 2298±1207 | 1812±971 | ns | ||||
| Male | Dark | 16711±6912 | 43645±8032 | p<0.05 | |||
| Light | 634±211 | 1762±750 | ns | ||||
| Baseline Velocity in the Home Cage (cm/s) |
Female | Dark | 7.32±0.71 | 8.65±0.89 | ns | Ts65Dn mice move faster in their home cage environment. |
|
| Light | 8.66±0.78 | 10.97±0.70 | p<0.05 | ||||
| Male | Dark | 6.57±0.56 | 7.23±0.66 | ns | |||
| Light | 7.87±0.37 | 11.07±1.02 | p<0.05 | ||||
| Velocity in Running Wheel in Home Cage (cm/s) |
Female | Dark | 23.16±5.75 | 28.02±4.23 | ns | Ts65Dn mice run faster in the running wheel in their home cage environment. |
|
| Light | 17.22±5.14 | 15.22±4.48 | ns | ||||
| Male | Dark | 10.90±3.12 | 30.33±4.36 | p<0.01 | |||
| Light | 4.15±0.55 | 11.80±2.48 | p<0.05 | ||||
| Time in Shelter in Each Hour During the Baseline Recording in Home Cage (s) |
Female | Dark | 2653±107 | 1630±136 | p<0.01 | Ts65Dn mice spend less time in the shelter during the dark cycle. |
|
| Light | 3238±42 | 3145±84 | ns | ||||
| Male | Dark | 2536±182 | 1591±298 | p<0.01 | |||
| Light | 3177±47 | 2752±331 | ns | ||||
| Time in Shelter in Each Hour During the Running Wheel in Home Cage (s) |
Female | Dark | 1492±256 | 665±252 | p<0.05 | Ts65Dn mice spend less time in the shelter after adding the running wheel. |
|
| Light | 3204±56 | 1568±579 | p<0.05 | ||||
| Male | Dark | 2025±156 | 1440±144 | p<0.05 | |||
| Light | 3189±63 | 3271±52 | ns | ||||
| % of Spontaneous Alternation in T-maze |
Female | 65.0±3.3 | 48.5±3.7 | p<0.01 | Ts65Dn mice have impaired spatial working memory. |
Hippocampus (Devenport et al., 1988; Gerlai, 1998; Johnson et al., 1977) |
|
| Male | No Treatment | 72.2±2.7 | 43.3±5.0 | p<0.001 | |||
| Saline | 63.3±4.0 | 41.9±2.7 | p<0.01 | Xamoterol can rescue the deficit in spatial working memory, and betaxolol prevents this effect. |
|||
| Xam | 64.4±2.1 | 60.4±4.5 | ns | ||||
| Xam + Betax | 66.7±4.2 | 46.3±2.8 | p<0.01 | ||||
| % of Correct Visit in Probe Trial after Place Learning in Intellicage |
Female | 60.3±2.7 | 66.2±4.0 | p=0.270 | Ts65Dn mice do not show a place learning deficit in a stress-free environment |
Hippocampus (Galsworthy et al., 2005) | |
| % of Visits to Previously Punished Corner in Intellicage |
Female | 5.3±1.9 | 13.4±3.4 | p<0.05 | Ts65Dn mice show spatial learning deficit in a stressful environment. |
Hippocampus, amygdale (Galsworthy et al., 2005) |
|
| Latency of First Satellite Box Entry in Intellicage (s) |
Female | 1172±175 | 587±75 | p<0.05 | Ts65Dn mice enter into the new compartment sooner. |
Hippocampus, amygdale (Galsworthy et al., 2005) |
|
| Latency of First Satellite Box Entry in Intellicage (s) |
Female | 1172±175 | 587±75 | p<0.05 | Ts65Dn mice enter into the new compartment sooner. |
Hippocampus (Galsworthy et al., 2005) | |
| Escape Latency in DMP (delayed matching-to-place ) Water Maze (s) |
Female | T3 | 50.97±6.80 | 76.42±3.16 | p<0.01 | Ts65Dn mice have a deficit in spatial working memory/episodic like-memory. |
Hippocampus (Steele and Morris, 1999) |
| Male | T2 | 55.98±5.18 | 77.29±4.18 | p<0.05 | |||
| T3 | 46.50±5.04 | 73.96±4.64 | p<0.001 | ||||
| T4 | 38.59±5.50 | 70.44±5.86 | p<0.001 | ||||
| % of Total Investigation in Novel Object Recognition |
Male | Saline | 59.0±5.5 | 44.9±3.3 | p<0.05 | Xamoterol can rescue the impairment of novel object recognition in Ts65Dn mice. |
Hippocampus, perirhinal cortex, raphe nuclei (Mumby et al.,2005; Winters et al., 2004; Lieben et al., 2006) |
| Xam | 78.6±2.6 | 73.6±4.2 | ns | ||||
| % Freezing in Tone-cued Fear Conditioning |
Female | 27.19±6.34 | 22.24±3.98 | ns | Ts65Dn do not have a deficit in tone-cued fear conditioning. |
Hippocampus, amygdale (Anagnostaras et al., 1999; Kim and Fanselow, 1992) |
|
| Male | 31.06±3.84 | 21.73±2.89 | ns | ||||
| % Freezing in Contextual Fear Conditioning |
Female | 16.37±5.30 | 4.65±1.66 | p<0.05 | Ts65Dn mice show a deficit in contextual fear conditioning. |
||
| Male | No Treatment | 19.33±4.16 | 7.93±2.87 | p<0.05 | |||
| Saline | 40.98±6.25 | 19.65±3.68 | p<0.01 | Xamoterol rescues the deficit in contextual fear conditioning, and betaxolol prevents this effect. |
|||
| Xam | 38.43±8.26 | 45.36±12.40 | ns | ||||
| Xam + Betax | 38.16±4.07 | 21.51±6.15 | p<0.05 | ||||
ns: nonsignificant, Xam: xamoterol, Betax: betaxolol
Brain region which is involved in each task is not limited to the reported one.
Exploratory Activity in a Novel Environment
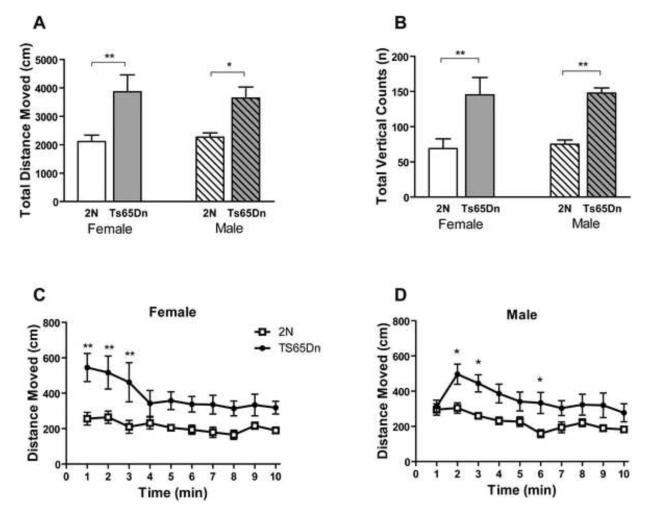
Exploratory behavior in a novel environment and general locomotor activity were assessed in automated Activity Chambers. Exploratory activity is defined as the total distance moved in the novel environment and the total number of vertical counts. As compared to 2N controls, both the male and female Ts65Dn mice showed increased distance traveled during a 10 minute testing period in the novel environment (Fig. 1A; effect of genotype, F1, 36=16.93, p=0.0002; effect of sex, F1, 36=0.009, p=0.925; effect of genotype in female, p<0.01 and in male, p<0.05). The distance moved by Ts65Dn mice was nearly twice that of 2N mice. In addition when minute to minute movement of animals in the novel box was studied, higher locomotor activity was observed in both male and females (Fig. 1C and D; effect of genotype for females, F1, 162=7.59, p=0.013; effect of genotype for males, F1, 162=7.93, p=0.011). Moreover, both male and female Ts65Dn exhibited significantly higher rearing in the novel environment compared to 2N mice (Fig.1B; effect of genotype, F1, 36=22.30, p<0.0001; effect of sex, F1, 36=0.531, p=0.47; effect of genotype both in female and male, p<0.01). The velocity of ambulatory movement in Ts65Dn mice was not significantly different from control littermates (Supplementary Fig. 1A). Consistent with this finding was that Ts65Dn mice had a significantly shorter resting time than control mice (Supplementary Fig. 1B).
Fig. 1. Exploratory activity in a novel environment in male and female Ts65Dn and 2N mice.
Ambulatory distance moved (A, C, and D) and total vertical count (B) recorded in the Activity Chamber. The results are presented as Mean ±/+ SEM. n=10 in all groups. Total ambulatory distance moved and total vertical count in Ts65Dn mice were both significantly higher than 2N control mice. Within groups comparison between Ts65Dn and 2N mice were analyzed and only significant differences are shown (*=p<0.05 and **=p<0.01).
Home Cage Monitoring
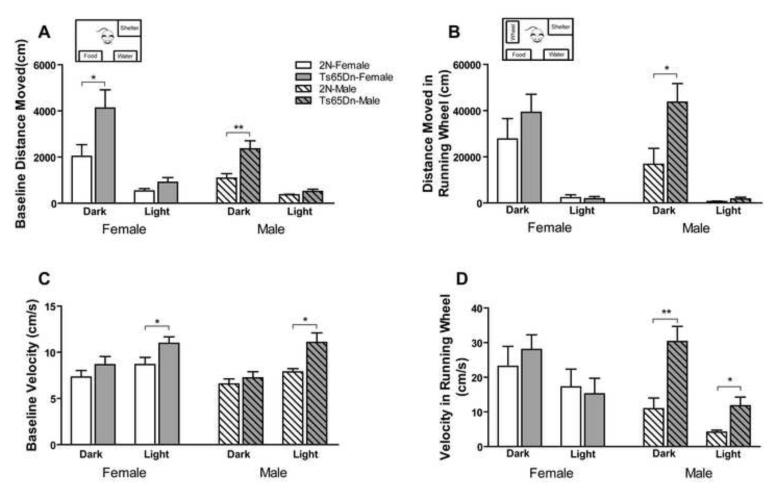
In order to further study locomotor activity of Ts65Dn mice in a stress-free environment, animals were monitored continuously in the home cage during both light and dark cycles using PhenoTyper. Recordings were conducted for 3 days at baseline and for 3 days following introduction of a running wheel. As in the novel environment, Ts65Dn mice were observed to move a greater distance than 2N controls during baseline monitoring. The distance moved during the dark cycle was significantly greater than that in the light cycle, and female subjects moved more than males (Fig. 2A; effect of genotype, F1, 64=13.66, p=0.001; effect of sex, F1, 64=9.70, p=0.003; effect of cycle, F1, 64=48.05, p<0.0001). The differences observed during the light cycle were not significant (p>0.05 for both males and females). Therefore, Ts65Dn mice are more active than 2N mice in the dark cycle but not in the light cycle. Introduction of the running wheel promoted a marked approximately 10-fold increase in locomotor activity in both Ts65Dn and 2N mice. As compared to 2N controls, the activity of Ts65Dn mice was significantly greater (Fig. 2B; effect of genotype, F1, 62=6.04, p=0.017; effect of cycle, F1, 62=57.76, p<0.0001; effect of sex, F1, 62=0.27, p=0.604). The velocity of movement during baseline recordings showed that Ts65Dn mice moved faster than 2N mice during the light cycle, and that the velocity during the light cycle was greater than the dark cycle (Fig. 2C; effect of genotype, F1, 64=13.05, p=0.001; effect of cycle, F1, 64=17.93, p<0.001; effect of sex, F1, 64=1.90, p=0.173). With the addition of the running wheel, the velocity of movement for male Ts65Dn mice was significantly higher than 2N mice during both the dark and light cycles (Fig. 2D; effect of genotype, F1, 60=6.74, p=0.012; effect of sex, F1, 60=5.26, p=0.026; effect of cycle, F1, 60=14.57, p<0.0001). Both 2N and Ts65Dn mice spent more time in the running wheel during the dark than the light cycle. However, only male Ts65Dn mice spent significantly more time in the running wheel during the dark cycle compared to 2N mice (Supplementary Fig. 2A). Ts65Dn mice spent less time in the shelter than 2N mice during the dark cycle and introduction of the running wheel to the cage resulted in reduced time spent in the shelter in Ts65Dn, as compared to 2N mice (Supplementary Fig. 2F and G).
Fig. 2. Automated home cage activity monitoring in male and female Ts65Dn and 2N mice.
Different activity parameters recorded during the dark and light cycles in home cage over 12 days, including baseline distance moved in each hour (A), distance moved in each hour after introducing running wheel (B), baseline velocity of movement (C), and velocity in running wheel (D), are presented. Both distance moved and velocity, during baseline and after introducing the running wheel, were higher in Ts65Dn mice than 2N mice (main effect of genotype). Mean + SEM is shown and n=8 for all groups. Within group comparison between each pair of genotypes was analyzed and only the significant differences are shown (*=p<0.05 and **=p<0.01).
CatWalk
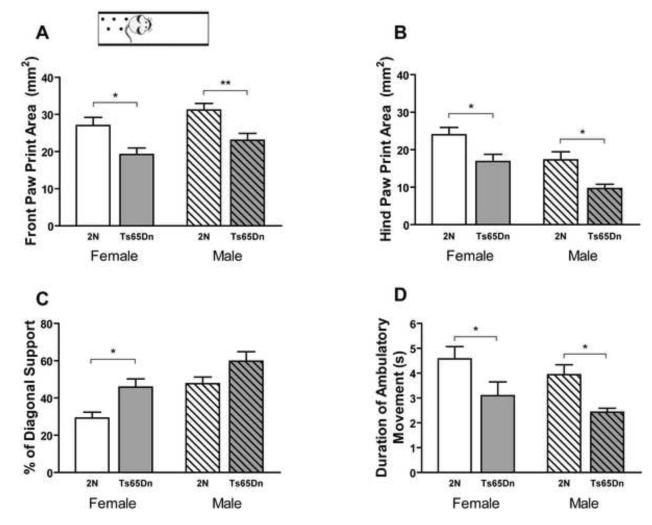
In order to examine subtle differences in locomotor activity, CatWalk, a quantitative gait analysis system for evaluating coordination and gait, was used. While the Activity Chamber and the PhenoTyper can assess gross locomotor deficit, the CatWalk system can show detailed impairment of each individual paw. Both female and male Ts65Dn mice had significantly smaller paw print areas compared to 2N mice, both in the front paw (Fig. 3A; effect of genotype, F1, 38=17.48, p=0.0002; effect of sex. F1, 38=4.417, p=0.042) and hind paw (Fig. 3B; effect of genotype, F1, 38=16.45, p=0.0002; effect of sex, F1, 38=14.49, p=0.0005). Statistical analysis showed that the intensity of both the front and hind paw print in Ts65Dn mice was lower than in 2N mice (Supplementary Fig. 3A and B). Since the body weight of animals can affect the paw print area, body weight of Ts65Dn and 2N mice were analyzed. The weight of male Ts65Dn mice was lower than 2N mice (38.23±1.37 and 46.52±2.09 grams respectively; p=0.004) but there was no difference between the body weight of the female Ts65Dn and 2N mice (34.55±2.43 and 34.2± 2.16 grams respectively, p=0.917).A significant difference was detected in the diagonal support of the Ts65Dn mice, which measures the percentage of time an animal’s right forepaw and left hind paw, or left forepaw and right hind paw, are simultaneously in contact with the floor during strides. Ts65Dn mice showed higher diagonal support than 2N mice and male mice showed a higher percentage of diagonal support compared to females (Fig. 3C; effect of genotype, F1, 38=11.72, p=0.0015; effect of sex, F1, 38=14.95, p=0.0004). Higher diagonal support indicates that Ts65Dn mice spend more time in ambulatory motion. The regularity index was another reported parameter. The regularity index expresses the number of normal step sequence patterns relative to the total number of paw placements. There was no significant difference in the regularity index between Ts65Dn and 2N mice or between male and female mice (Supplementary Fig. 3C). Rodents use six possible step sequence patterns during walking. Among these patterns, the Ab pattern is the most frequent. In this pattern the animals use in sequence: left front paw, right hind paw, right front paw, and left hind paw. To analyze the step pattern an inter-limb coordination parameter, the percent incidence of the Ab pattern between Ts65Dn and 2N mice during trials, was analyzed however no significant difference was observed (Supplementary Fig. 3D). There was no significant difference between Ts65Dn and 2N mice in the front and hind paw base of support, which is the distance between the center of the right and left paw (Supplementary Fig. 3E and F). Therefore, it was determined that these animals do not display a deficit in balance. There was no significant difference in stride length of the front paw or hind paw between male and female mice, and there was no effect of genotype on the stride length of the front paw, but stride length of the hind paw was longer in Ts65Dn than 2N mice (Supplementary Fig. 3G and H). Ts65Dn mice showed shorter stand duration in both front and hind paws (Supplementary Fig. 3I and J). Swing speed was not significantly different in Ts65Dn mice compared to 2N controls (Supplementary Fig. 3K and L). Total duration of ambulatory movement shows the time it takes for the mouse to move from the start to the finish zone. Total duration for the Ts65Dn was shorter compared to 2N mice (Fig. 3D; effect of genotype, F1, 38=12.21, p=0.001; effect of sex. F1, 38=2.29, p=0.138), indicating that Ts65Dn mice were walking across the walkway in a shorter time than the 2N mice.
Fig. 3. Automated gait analysis by CatWalk in male and female Ts65Dn and 2N mice.
Front paw print area (A), hind paw print area (B), percent of diagonal support (C), and total duration of ambulatory movement on walkway (D) are presented. Ts65Dn mice showed significantly smaller paw print area, a higher percentage of diagonal support, and shorter duration of ambulatory movement compared to 2N controls (main effect of genotype). Results are presented as Mean + SEM, n(male and female 2N)=10 and n(male and female Ts65Dn)=11. Within group comparison between Ts65Dn mice and 2N mice was analyzed and only the significant differences are shown (*=p<0.05 and **=p<0.01).
Social Interaction Tests
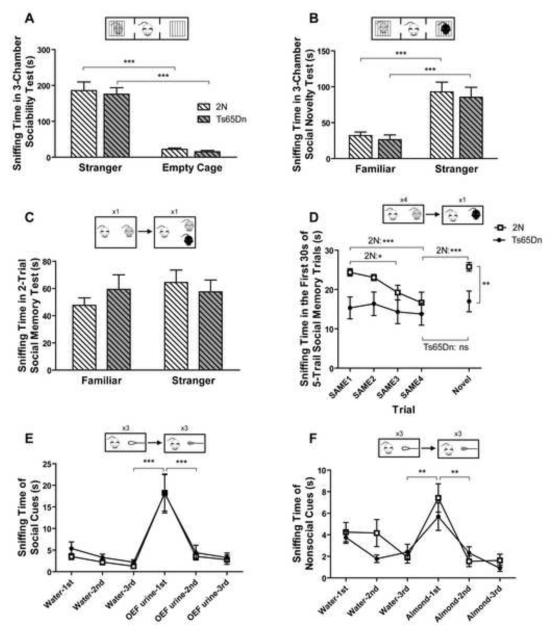
Social behavior was assessed using a 3-chamber test in which a subject mouse was first habituated to the test environment in session A (“habituation” session), tested for sociability in session B (“sociability” session) and finally tested for preference for social novelty in session C (“social novelty” session). Note that the three sessions are continuous with no ITI between sessions. During session A, Ts65Dn and 2N mice did not exhibit a side preference for the left or the right chamber (Supplementary Fig. 4A). During the subsequent session B, both Ts65Dn and 2N controls preferred sniffing at a cage containing a stranger mouse than sniffing at an empty cage as a nonsocial object (Fig. 4A; effect of genotype, F1, 18=0.34, p=0.567; effect of object, F1, 18= 97.71, p<0.0001). Both genotypes also spent significantly more time in the chamber with the stranger mouse versus the chamber with the empty cage (Supplementary Fig. 4B). In the first 5 minutes of session C, Ts65Dn and 2N mice spent significantly more time sniffing the novel stranger than the now-familiar mice (Fig. 4B; effect of genotype, F1, 18=0.26, p=0.616; effect of novelty of the social object, F1, 18= 59.17, p<0.0001). Both genotypes also preferred the chamber containing the stranger over the chamber containing the familiar mouse (Supplementary Fig. 4C).
Fig. 4. Sociability, social novelty, and social memory in 2N and Ts65Dn mice.
In the 3-chamber sociability test (A), mice spent more time investigating a caged intruder than an empty cage during session B. n=10 for both 2N and Ts65Dn mice. In the first 5 minute bin of the social novelty 3-chamber test during session C (B), animals spent more time sniffing at a cage with a novel intruder compared to sniffing a cage with a familiar intruder. n(2N)=9, n(Ts65Dn)=10. In the 2-trial social memory test (C), 30 minutes after the first interaction with an OEF, mice spent the same amount of time exploring both a familiar and a novel OEF intruder, n(2N)=9, n(Ts65Dn)=8. In the first 30 seconds of the 5-trial social memory test (D), Ts65Dn mice displayed no habituation, whereas 2N mice exhibited a significant habituation to the familiar OEF and a significant dishabituation to the novel OEF, n(2N) =9, n(Ts65Dn)=10. Olfactory habituation to social (E) and nonsocial cues (F) presented with cotton-tipped swabs was significant in both genotypes. In panels E and F, n(2N)=10 and n(Ts65Dn)=10. Results are presented as Mean ±/+ SEM (*=p<0.05, and **=p<0.01, ***=p<0.001, and ns=not significant).
To assess social memory, object mice were introduced into the home cages of Ts65Dn or 2N mice with ITIs between trials. In the 2-trial test, 30 minutes after a first 5-minute introduction of an OEF to the home cage of a subject mouse, the same OEF was reintroduced into the home cage together with a novel OEF for a second 5-minute encounter. Ts65Dn mice did not exhibit a preference for the novel versus the familiar intruder. Conversely 2N mice showed a trend for such a preference, although it was not statistically significant (Fig. 4C; effect of genotype, F1, 15= 0.053, p=0.821; effect of object mouse, F1, 15= 2.487, p=0.281). In the 5-trial social memory test, animals were subjected to four repeated pairings with the same OEF (SAME) followed by a pairing with a novel OEF (NOVEL). Analysis of the first 30-second bins revealed a significantly different result in 18 months old Ts65Dn and 2N mice (Fig. 4D; effect of genotype, F1, 68= 4.936, p=0.040; effect of trial, F4, 68=6.634, p<0.0001). 2N mice exhibited a significant habituation response to repeated exposures to the familiar OEF and a dishabituation response to a novel OEF (SAME 1 vs. SAME 3 p<0.05; SAME 1 vs. SAME 4 p<0.0001; SAME 4 vs. NOVEL p<0.0001). In contrast, Ts65Dn mice showed a persistent level of interest in both the familiar and novel stimulus OEF (p>0.05). Moreover, the Student’s t-test revealed a significantly different response of Ts65Dn and 2N mice to exposure to a NOVEL OEF (Ts65Dn NOVEL vs. 2N NOVEL p<0.01). Analysis of the final 30 seconds revealed a significant habituation response for both genotypes; significant dishabituation for 2N, and no significant dishabituation trend for Ts65Dn mice (Supplementary Fig. 4D). To determine whether the impairment observed was due to lack of sexual interest in Ts65Dn male mice, we repeated the 5-trial social memory experiment with male intruders and found a similar degree of social impairment in male-male interaction. This suggests that the deficit is not dependent on the sex of intruders (Supplementary Fig. 4 G). Furthermore, to test if the impairment is also present in younger mice, we tested a 9-month old cohort. We found a significant difference in the dishabituation response in both the first and second 30-second bins (Supplementary Fig. 4E and F, Ts65Dn NOVEL vs. 2N NOVEL p<0.05). Since recognition of individuality is thought to be mediated by olfactory cues we measured responses to both nonsocial and social olfactory cues. Ts65Dn mice were comparable to 2N mice in showing both a significant habituation response to social (Fig. 4E; effect of genotype, F1, 90=0.24, p=0.633) as well as to nonsocial olfactory cues (Fig. 4F; effect of genotype, F1, 90=0.97, p=0.337).
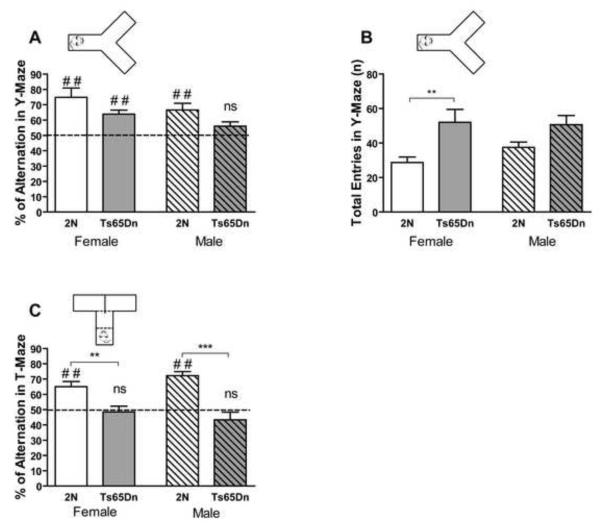
Spontaneous Alternation
The T-maze and Y-maze were used for assessment of spontaneous alternation for spatial working memory. Ts65Dn mice showed a significantly smaller rate of spontaneous alternation than 2N controls in the Y-maze; there was no significant effect of sex on alternation rate (Fig. 5A; effect of genotype, F1, 27=6.95, p=0.014; effect of sex, F1, 27=3.94, p=0.057). Comparing alternation rate with chance level (50%), a one sample t-test showed that female 2N and Ts65Dn mice and male 2N mice alternated significantly more than chance level (p<0.01). However, the alternation rate in male Ts65Dn mice was not significantly different from the chance level (p>0.05), indicating a deficit in spontaneous alternation. The number of entries to the arms of the Y-maze was significantly higher in Ts65Dn than 2N mice (Fig. 5B; effect of genotype, F1, 27=12.10, p=0.002; effect of sex, F1, 27=0.50, p=0.485). Increased number of entries to the arms of the maze signifies hyperactivity in the Ts65Dn mice.
Fig. 5. Y-maze and T-maze spontaneous alternation in male and female Ts65Dn and 2N mice.
Alternation rates in Y-maze (A) and T-maze (C) and total number of entries to arms of Y-maze (B) are presented. Results are presented as Mean + SEM. In the Y-maze study, n=7 for female Ts65Dn mice and n=8 for male Ts65Dn, female 2N, and female Ts65Dn mice. In the T-maze test, n=10 for female 2N mice, n=9 for female Ts65Dn mice, n=6 for male 2N mice, and n=7 for male Ts65Dn mice. Alternation rate in Y-maze and T-maze (A and C) was compared with the chance level (50%), and the significant and nonsignificant differences are shown (ns=not significant, # #=p<0.01 compared to chance level). Alternation rate in Ts65Dn mice was significantly lower than 2N mice in both Y-maze and T-maze. Within group comparison between each pair of genotypes was analyzed, and only the significant differences are shown (*=p<0.05, **=p<0.01, and ***=p<0.001).
Similar to the Y-maze, the alternation rate of Ts65Dn mice was significantly lower than 2N mice in the T-maze and there was no significant difference between female and male animals (Fig. 5C; effect of genotype, F1, 28=33.88, p<0.0001; effect of sex, F1, 28=0.07, p=0.796). Both female and male 2N control mice alternated significantly more than chance level (p=0.002, female, and p=0.0004, male, in one sample t-test compared to 50%). Spontaneous alternation in Ts65Dn mice was not statistically different from the chance level (p=0.702 for female and p=0.230 for male).
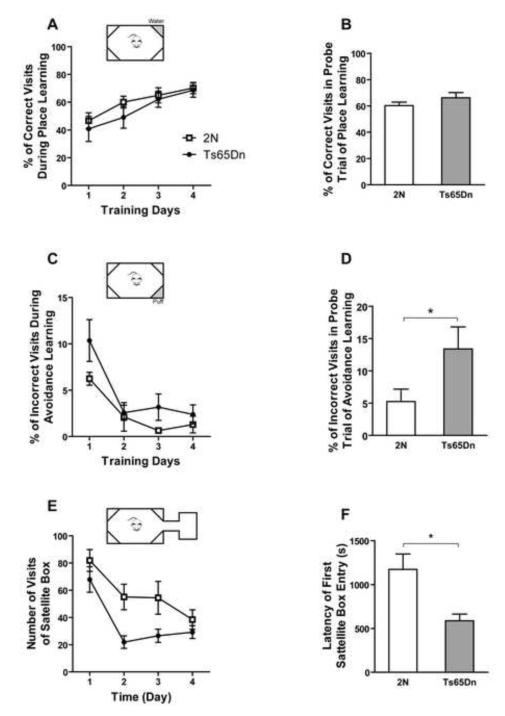
Intellicage
A novel automated system (Intellicage) was used to investigate place preference and place avoidance learning, as well as the exploratory behavior of female Ts65Dn mice in the home cage environment. Ts65Dn mice did not show a significant deficit in acquisition of place learning (Fig. 6A; effect of genotype, F1, 45=0.74, p=0.403; effect of training day, F3, 45=11.82, p=0.0005).They also showed no significant difference in the probe trial at 72 hours (Fig. 6B; p=0.21). In the avoidance learning test the Ts65Dn mice visited the punished corner as frequently as 2N mice during training days (Fig. 6C; effect of genotype, F1, 39=2.36, p=0.149; effect of training day, F3, 39=27.12, p<0.0001). However, Ts65Dn mice showed a significant deficit in the probe trial of the previously punished corner after 72 hours (Fig. 6D, p=0.042) in place avoidance learning.After opening the connection between the main compartment of the cage and the novel satellite box in the novelty exploration test, the Ts65Dn mice showed significantly fewer visits to the novel box as compared to 2N controls (Fig. 6E; effect of genotype, F1, 42=5.13, p=0.04 and for effect of time, F3, 42=8.22, p<0.0001). However, the latency for the first entry to satellite box was significantly shorter for the Ts65Dn mice than for 2N mice (Fig. 6F; p=0.027).
Fig. 6. Place learning, place avoidance, and novelty exploration tests in Intellicage.
In the place learning task, percent of correct visits (A) and probe trial after 72 hours (B) are presented. In both A and B, there was no significant effect of genotype. Genotype did not show any effect on percent of incorrect visits during avoidance learning (C), but after 72 hours retention, Ts65Dn mice had significantly more incorrect visits (D). After adding the satellite box to the Intellicage, the number of visits to the satellite box (E) and latency to the first visit of the box (F) are shown. Although there is a significant effect of genotype in E, the post hoc test did not show any significant difference between the genotype in each individual time point. In graphs B, D, and F, each pair of data was tested, and only the significant differences are shown (*=p<0.05). Mean ±/+ SEM is shown. In A and B, n(2N)=10 and n(Ts65Dn)=7. In C and D, n(2N)=9 and n(Ts65Dn)=6. In E and F, n(2N)=10 and n(Ts65Dn)=6. The experiment was done on female mice.
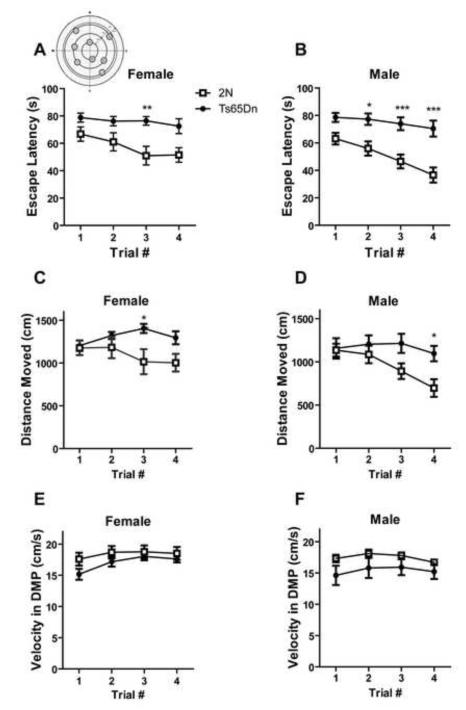
Delayed Matching-To-Place Water Maze
In the visible platform experiment both female and male Ts65Dn mice showed no significant difference in swimming velocity compared to 2N controls (Supplementary Fig. 5A and B). All female and male Ts65Dn and 2N mice found the visible platform, except for one female animal that was unable to find the visible platform during all four trials of the task; thisanimal was excluded from the study results.
The DMP water maze tests spatial working/episodic-like memory. Both female and male Ts65Dn mice showed significant differences in escape latency compared to controls, indicating a deficit in the spatial reference memory (Fig. 7A and B; effect of genotype, in female, F1, 42= 10.61, p=0.006 and in male, F1, 42=24.98, p=0.0002; effect of trial number, in female, F3, 42= 3.59, p= 0.021 and in male, F3, 42=7.82, p=0.0003). They also showed significant differences in distance moved to the hidden platform (Fig. 7C and D; effect of genotype, in female, F1, 42= 4.67, p=0.048 and in male, F1, 42=9.32, p=0.004). The velocity during the DMP water maze in Ts65Dn was not significantly different from 2N controls (Fig. 7E and F; effect of genotype, in female, F1, 42= 1.42, p=0.254 and in male, F1, 42=2.13, p=0.166).
Fig. 7. Delayed matching-to-place water maze task for testing spatial working memory/episodic-like memory.
Escape latency, distance moved, and velocity of swimming for female (A, C, and E) and male (B, D, and F) mice are presented. There was a significant effect of genotype in escape latency and distance moved to find the platform in DMP water maze test in both male and female mice. However, there was no effect of genotype in swimming velocity in female and male mice. The results of post hoc analysis for each time point is shown and only the significant differences are presented (*=p<0.05, **=p<0.01, and ***=p<0.001). In all groups, Mean ± SEM is shown and n=8.
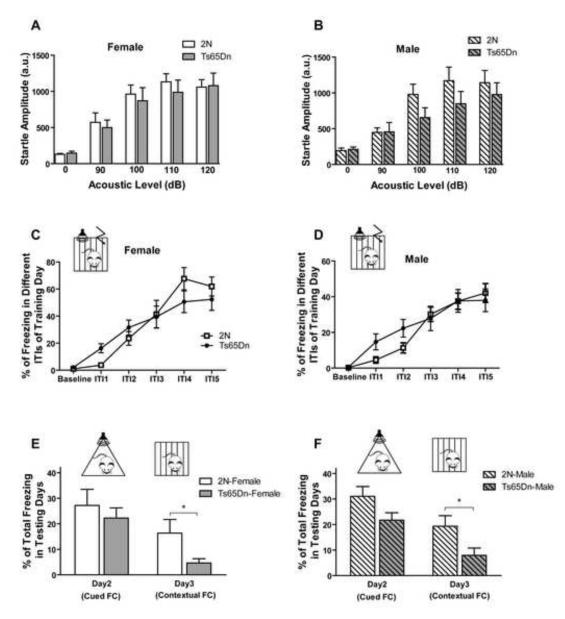
Fear Conditioning and Startle Response Tests
The startle response test was run to evaluate the ability of subjects to respond to tone cues in fear conditioning (FC). Neither female nor male Ts65Dn mice showed a significant deficit in the startle response test (Fig. 8A and B; effect of genotype in female, F1, 56=0.17, p=0.682; effect of genotype in male. F1, 56=1.13, p=0.306). Tone-cued and contextual FC was tested for evaluation of Pavlovian learning and memory. Figures 8C and D show the overall learning in the FC test during training day in different ITIs for both males and females. There was no significant difference in training day ITIs between Ts65Dn and 2N mice in both females (effect of genotype, F1, 90=0.03, p=0.861) and males (effect of genotype, F1, 180=0.29, p=0.59). In tone-cued FC in a novel context the Ts65Dn mice exhibited less freezing than 2N mice, however this difference was not statistically significant (Fig. 8E and F; p=0.517 for females and p=0.071 for males). In day three of the contextual retention test, both male and female Ts65Dn mice showed significantly less freezing (p=0.049 for females and p=0.038 for males) than their 2N littermates. These results indicate that Ts65Dn mice have a deficit in memory retrieval in contextual testing.
Fig. 8. Fear conditioning and startle response.
Freezing during the training day in different ITIs and in the baseline period for female (A) and male (B) Ts65Dn mice and 2N controls are presented. In both genders, there was no significant effect of genotype in acquisition of the task. Total freezing in testing days (C and D) indicates that there was no statistically significant difference between genotypes in tone-cued fear conditioning, but in both sexes, Ts65Dn mice showed deficit in contextual fear conditioning. In the fear conditioning test, n=10 for both 2N and Ts65Dn female mice and n=21 for male 2N and n=17 for male Ts65Dn mice (*=p<0.05). In the startle response test, no significant effect of genotype was seen in both female (E) and male (F) mice. In the startle response test, n=8 for all groups. Results are presented as Mean ±/+ SEM.
Pharmacological Experiments
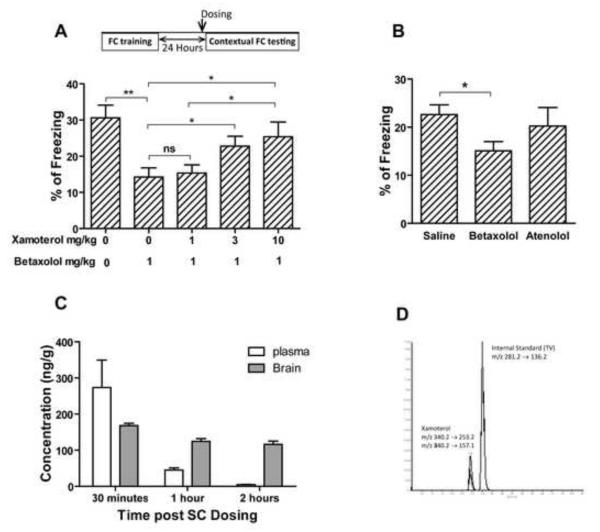
We have recently shown that restoration of norepinephrine levels using L-threo-3,4-dihydroxyphenylserine (L-DOPS) in Ts65Dn mice restored the hippocampus-mediated contextual deficit in FC and nesting behavior (Salehi et al., 2009). In order to explore the postsynaptic receptor mediating this effect, we used the selective β1-ADR agonist xamoterol and the β1 receptor antagonist betaxolol to study the role of β1-ADR in these behavioral effects. To control for the general effect of the compound dosed, the locomotor activity was monitored post dosing. Xamoterol did not show a significant effect on locomotor activity of male Ts65Dn (Fig. 9A; effect of treatment, F1, 30= 1.148, p=0.293; effect of genotype, F1, 30=6.168, p=0.019). However, xamoterol rescued the deficit observed in T-maze spontaneous alternation and its effect was blocked by the β1-ADR antagonist, betaxolol (Fig. 9.B; the effect of treatment, F2, 44=3.879, p=0.0281; the effect of genotype, F1, 44=27.38, p<0.0001). Xamoterol also improved the memory retrieval of Ts65Dn mice in contextual FC; the effect of xamoterol was completely blocked by betaxolol (Fig. 9C). The vehicle-treated Ts65Dn group showed a significant deficit in contextual FC (p<0.01) compared to the 2N control group, whilst no significant differences between the 2N control and xamoterol treated groups were observed (p=0.648). Simultaneous treatment with both betaxolol and xamoterol prevented the effect of xamoterol, in that there was a significant difference in percentage of freezing between 2N and Ts65Dn mice in this group (p<0.05). In novel object recognition tests, xamoterol corrected the novel object recognition deficit in Ts65Dn mice and had no effect on the performance of 2N mice (Fig. 9D, the effect of genotype, F1, 28=5.53, p=0.026; the effect of treatment, F1, 28=35.05, p<0.0001). In contrast, Xamoterol did not rescue the spatial working memory deficit in DMP water maze tests (Supplementary Fig. E and F). In order to further study the role of a β1-ADR and the selectivity and potency of the xamoterol, a dose response study was conducted in C57Bl/6J mice. In this experiment Betaxolol reduced the memory retrieval and xamoterol dose-dependently inversed the effect of betaxolol (Fig. 10A, the effect of dose, F4, 45=4.99, p=0.002). Atenolol, a β1-ADR antagonist which cannot cross the blood-brain barrier and has minimal CNS effect, had no significant effect on memory retrieval in contextual FC experiment in C57Bl/6J mice (p=0.552). However betaxolol, a β1-ADR antagonist which can cross the BBB significantly blocked the memory retrieval (Fig. 10B; p=0.024). In order to address the BBB permeability of xamoterol, the plasma and brain concentration of the xamoterol were measured using LC-Mass/Mass after a single SC bolus dose of 3mg/kg (Fig. 10C). Results obtained from this experiment demonstrate that xamoterol can cross the BBB and be detected in the brain after a single dose administration at concentrations (168 ng/g at 30 minutes), (125 ng/g at 60 minutes) and (116 ng/g at 120 minutes) with a brain/plasma (B/P) ratio of 0.61 at the peak plasma level. Our results demonstrate that while there is a rapid clearance of the drug from the plasma the brain concentration remains relatively high for at least two hours following a single dose. Fig. 10D displays the chromatogram of LC-Mass/Mass analysis for xamoterol and internal standard in brain sample.
Fig. 9. Xamoterol can rescue the learning and memory in Ts65Dn mice via interaction with β1 adrenergic receptors.
Analyzing the effect of xamoterol on total ambulatory distance moved in the Activity Chamber in male Ts65Dn mice and their control littermates (A) showed no significant effect of xamoterol in both genotypes. n(2N Saline)=9, n(Ts65Dn Saline)=7, n(2N Xamoterol)=9, and n(Ts65Dn xamoterol)=9. Xamoterol rescued the spontaneous alternation deficit in male Ts65Dn mice, and betaxolol prevents its effect (B). n(2N Saline)=10, n(Ts65Dn Saline)=7, n(2N xamoterol)=9, and n(the rest of experimental groups)=8. Xamoterol also rescued the memory retrieval deficit in contextual fear conditioning in male Ts65Dn, and betaxolol prevents its effect (C). n=9 for all 6 experimental groups. Treatment with xamoterol rescued the novel object recognition deficit in male Ts65Dn mice (D). n=8 for all 4 groups. In all experiments xamoterol 3mg/kg and betaxolol 1 mg/kg were injected subcutaneously. Results are shown as Mean + SEM. Within group comparison between Ts65Dn mice and 2N mice was analyzed, and the significant differences are shown (ns=not significant, *=p<0.05, and **=p<0.01).
Fig. 10. Xamoterol improves the memory retrieval dose-dependently by interaction with the adrenergic receptors in central nervous system.
Betaxolol impaired memory retrieval of contextual fear conditioning in C57Bl/6J mice and xamoterol reverse the impairment dose-dependently (A). Betaxolol which can cross blood-brain barrier impaired the memory retrieval in contextual fear conditioning but atenolol which cannot get access to brain, did not have such an effect (B). n=10 for all groups in the FC tests. In all experiments xamoterol 3mg/kg, atenolol 3 mg/kg, and betaxolol 1 mg/kg were injected subcutaneously. Analyzing of plasma and brain samples showed that xamoterol can get access to blood and brain after subcutaneous injection of 3mg/kg (C and D). n=3 for each experimental groups for the plasma and brain analyzing experiment. Results are shown as Mean + SEM (ns=not significant, *=p<0.05, and **=p<0.01).
Discussion
The Ts65Dn mouse model of DS with a segmental trisomy of chromosome 16 was introduced by Davisson et al. (Davisson et al., 1990; Davisson et al., 1993). For over two decades, this preclinical model has been used frequently in behavioral phenotyping and pharmacological screening studies. In this study we aimed to conduct a comprehensive phenotyping of these mice in order to identify robust and reproducible behavioral abnormalities in sensorimotor, exploratory, learning, memory, and social behavior. Our efforts identified the hippocampus-mediated learning and memory to be a severely affected phenotype in these animals. Since it has been shown that the norepinephrine (NE) projection to the hippocampus plays an important role in retrieval of contextual memory (Murchison et al., 2004), we used pharmacological tools to identify the target receptor mediating the action of NE in restoration of learning and memory in this model of DS. We explored the role of the β1-ADR as a therapeutic target for treatment of learning and memory deficits in this model of Down Syndrome.
Locomotion in Ts65Dn Mice
It has been reported that children between the ages of 6-11 years with DS are hyperactive compared to their typical peers (Pueschel et al., 1991). The Ts65Dn mice also displayed a higher overall locomotor activity, both in the home cage and a novel testing environment. It has been shown that prefrontal cortex and hippocampal lesions in rodents leads to hyperactivity (Deacon et al., 2002; Katsuta et al., 2003; Kolb, 1974; Takakusaki, 2008; Tani et al., 2001; Viggiano, 2008). Pathological and biochemical abnormalities observed in the hippocampus of Ts65Dn mice (Belichenko et al., 2004) or altered cholinergic neurotransmission (Granholm et al., 2000; Seo and Isacson, 2005) may be responsible for this observed hyperactivity in Ts65Dn mice. The hyperactivity could partially be due to the loss of working memory and therefore present as an inability to recall areas previously explored in the novel arena. It has been previously suggested that mice with hippocampus lesion have difficulties in formation of contextual map of the novel arena they are exposed to. The synaptic and neurotransmission abnormalities in the hippocampus and other region of the brain in the Ts65Dn mice may be responsible for the hyperactivity phenotype observed here. These results are in line with reports describing the hyperactivity of male and female Ts65Dn mice in open field and activity chamber tests (Coussons-Read and Crnic, 1996; Davisson et al., 1993; Reeves et al., 1995). Stewart et al. (2007) showed that Ts65Dn mice between the ages of 4-6 months were more active than their control littermates in the light cycle, while their activity in the dark cycle was not significantly different from 2N (Stewart et al., 2007). In contrast our results indicate a significantly higher home cage activity in trisomic mice compared to the 2N controls in the dark cycle, but not during the light cycle. These discrepancies could be due to differences in the age of animals tested or experimental conditions. In our study the activity of the animals was continuously monitored using a remotely controlled infrared video recording system, without any disturbance, as opposed to previous studies where snapshots of the animals’ behavior were taken in a novel context. In line with previous publications reporting the jumping and repetitive behavior of Ts65Dn animals (Reeves et al., 1995; Turner et al., 2001), we also found increased rearing and jumping behavior in both male and female Ts65Dn mice. Isolation of mice during testing in the Activity Chamber could be responsible for triggering this behavior and may indicate that isolation and single housing of Ts65Dn mice is an anxiety-inducing factor that leads to repetitive jumping behavior. When the running wheel was introduced to the home cage as a novel stimulus, male Ts65Dn mice showed longer wheel activity compared to the 2N mice. This increased activity on the running wheel agrees with the repetitive behavior observed in isolation as previously described (Stewart et al., 2007). Both Ts65Dn and 2N Female mice responded to the running wheel with no statistically significant difference between these two groups in contrary to the male 2N and Ts65Dn. This seems to be driven by higher interest of the female 2N mice in running wheel and could be due to hormonal differences in male and females 2N mice.
It has been shown that children with DS have gait abnormalities and impaired balance and postural control (Galli et al., 2008; Shumway-Cook and Woollacott, 1985). Observation of DS children’s walking patterns also revealed more flexion at the knees and hips accompanied by greater fluctuation of ankle movement (Parker et al., 1986). Correspondingly, it has been shown that Ts65Dn mice have a deficit in motor coordination in the rotarod test (Costa et al., 1999; Hyde et al., 2001a). In order to investigate this further in the Ts65Dn mice, we used the CatWalk automatic gait analysis system. Ts65Dn mice had a smaller paw print area and also applied lower pressure on their paws, which manifests as faster strides on the walkway; this can also be interpreted as toe walking. Hampton et al. (2004) also reported a faster walking speed, shorter stride length, and significant difference in gait dynamics in 10-12-week-old male Ts65Dn mice using a motorized treadmill (Hampton et al., 2004). In our study we found no significant difference in the step pattern or regularity index (which expresses the number of normal step sequences relative to the total number of paw placements) using this automated system. In summary, our results indicate that Ts65Dn mice show increased locomotor activity in the home cage as well the novel environment. The hyperactivity observed does not seem to be driven by the novelty of the testing environment. This may be a general nonspecific increase in activity that is also observed in the home cage and could be due to decreased inhibition.
Social Behavior in Ts65Dn Mice
DS in children is associated with abnormal social behavior (Coe et al., 1999). Moreover, approximately 10% of people suffering from DS also fulfill the criteria for autism spectrum disorder (Dykens, 2007). In order to further investigate this phenomenon in a mouse model of DS we tested sociability, preference for social novelty and social memory of Ts65Dn mice. We chose to test 9- and 18-month old mice because they could potentially exhibit greater social impairment than younger mice. In the 3-chamber test, we found no deficits in Ts65Dn mice both in session B (test for sociability), as well as in session C (test for preference for social novelty) (Crawley, 2007; Moy et al., 2004; Moy et al., 2007). However in the 5-trial social memory test during the first 30 seconds of the five trials the Ts65Dn mice exhibited the same level of interest in the familiar and novel intruder, whereas 2N mice showed a significant habituation response to the familiar OEF and a significant dishabituation response to the novel OEF. In contrast, in the final 30 seconds of the trials, both genotypes showed a significant habituation response. Habituation was also intact in both genotypes if mice were exposed to the urine of OEFs only. These findings suggest slower information processing of identity cues in Ts65Dn mice in the 5 trial social memory test, but intact memory if just urine from OEFs is presented in a non-social context. It appears that in Ts65Dn mice, identity recognition in a nonsocial context is less challenging compared to a social context. This is reminiscent of deficits shown in face recognition studies conducted in children with DS (Wishart and Pitcairn, 2000). It has been shown that DS children performed normally in the relatively simple task of matching photographs of simultaneously presented faces, but performed significantly worse in the more challenging task of matching faces to non-present people. Moreover, children with DS showed increased recognition latency when the photographs of the faces were rotated 90 or 180 degrees (Wishart and Pitcairn, 2000). In summary, our results demonstrate that both 9-month and 18-month old Ts65Dn mice display a robust social memory deficit with short intruder exposures and 10-minute ITIs, while displaying normal habituation and dishabituation to isolated social and nonsocial olfactory cues. The social memory impairment in 9-month old mice is milder than 18-month old ones. In addition, 18-month old Ts65Dn showed no deficit in the social novelty tests where the novel social contacts were presented for an extended time period with no delay between sessions. This further suggests that the deficit observed in social memory is selective and dependent on trial length, the time between trials, the context (social vs. nonsocial context), and the age of mice.
Learning and Memory in Ts65Dn Mice
Severe impairment and disruption in cognitive and verbal development is detected in people with DS (Abbeduto et al., 2007; Nadel, 2003; Pennington et al., 2003; Wishart et al., 1993). In addition, they specifically show deficits in spatial and hippocampus-dependent learning (Pennington et al., 2003). The Ts65Dn mouse model of DS displays similar behavioral deficits in spatial and hippocampus-dependent learning paradigms such as spontaneous alternation in T-maze, fear conditioning, Morris water maze, novel object recognition, and nesting behavior tests. In this study Ts65Dn mice were tested in both standard and novel learning and memory tests. The Intellicage (Galsworthy et al., 2005) was used to evaluate learning and memory in Ts65Dn mice under social housing in the home cage environment. Ts65Dn and 2N mice demonstrated identical place learning during acquisition and retrieval, which may indicate that these mice could have normal learning in a stress-free and simple environment. However, introduction of air puffs as an aversive stimulus in an avoidance protocol revealed a significant behavioral deficit in Ts65Dn mice. They showed no deficit in learning to avoid the punished corner, similar to 2N mice, but showed a significant deficit in memory retrieval after 72 hours. Our results suggest that the Ts65Dn mice do not exhibit learning and memory deficits in a stress-free home cage environment, as indicated by the place learning protocol used here. However, introduction of a stressful stimulus such as an air puff revealed the avoidance deficit in these mice. This may indicate that stress during standard behavior tests, such as water maze and FC tests, could be of great importance in identifying behavioral deficits in Ts65Dn mice and other transgenic mouse models of neurocognitive disorders. Interestingly after adding the satellite box, a completely novel environment to the Intellicage, Ts65Dn mice entered this new compartment sooner than 2N mice. These results highlight the lack of neophobia in these mice, which could potentially be used as a behavioral assay for anxiety. A significant deficit in spontaneous alternation in the T-maze has been reported in Ts65Dn mice (Belichenko et al., 2007; Fernandez et al., 2007). Spontaneous alternation is a highly hippocampus-dependent task (Devenport et al., 1988; Gerlai, 1998; Johnson et al., 1977), further highlighting the hippocampus-dependent deficit observed in this model of DS. This is in agreement with reported physiological abnormalities in the hippocampus of Ts65Dn mice, including reduced evoked LTP, which represents abnormal synaptic plasticity, reduced cAMP levels, and morphological changes in dendrites (Dierssen et al., 1996; Granholm et al., 2000; Kleschevnikov et al., 2004; Siarey et al., 1997). The novel object recognition assay is another hippocampus-dependent test for evaluation of recognition memory. In line with previous reports (Belichenko et al., 2009b; Fernandez et al., 2007) we did also show that the trisomic mice have severe deficits in recognition memory at 24 hours after training. It has been shown that Ts65Dn mice have difficulty learning the context discrimination task (Hyde et al., 2001b) as well as retrieval of fear-dependent memories (Costa et al., 2008). Despite normal learning during the acquisition phase in the FC test, the Ts65Dn mouse demonstrates a selective and significant deficit in contextual, but not tone-dependent, retrieval testing. Contextual memory in FC is believed to be a hippocampus-dependent task (Kim and Fanselow, 1992) whilst the response to the tone stimulus appears not to be mediated by the hippocampus (Anagnostaras et al., 1999). It has been shown that the LC and NE-energic system, via interaction with β1-ADR, plays an important role in hippocampus-mediated memory retrieval in FC (Murchison et al., 2004). The LC is the major source of norepinephrine in the brain and has neuronal projections to different areas of the brain including the hippocampus and frontal cortex (Loughlin et al., 1986). We have recently shown a significant degeneration of the LC in Ts65Dn mice and an increase in the β1-ADR immunoreactivity in the hippocampus (Salehi et al., 2009). In addition, we have shown that restoration of norepinephrine levels using L-DOPS in Ts65Dn mice restores this hippocampus-mediated contextual deficit in FC as well as nesting behavior (Salehi et al., 2009). In order to determine if these effects are selectively and exclusively mediated via β1-ADR, we used the selective β1-ADR partial agonist xamoterol and the β1-ADR antagonist betaxolol, in contextual FC, T-maze, novel object recognition, and locomotor activity in a novel environment. Our results show that xamoterol can completely rescue the impaired learning and memory of Ts65Dn mice in contextual FC, novel object recognition, and spontaneous alternation. These effects were effectively and completely reversed by using a β1-adrenergic antagonist, betaxolol. Interestingly, the β1-ADR agonist does not have any effect on the hyperactivity observed in our experimental animals. Moreover, Xamoterol did not have any significant effect on the behavioral deficit observed in the DMP task in the Ts65Dn mice. These results indicate that the β1-ADR does not play a role in the learning and memory process involved in the DMP or the hyperactivity observed in this model of DS. Betaxolol, a BBB permeable β1-ADR antagonist, could effectively block the memory retrieval in contextual FC; atenolol, a non BBB permeable peripheral β1-ADR antagonist, did not have such an effect. This result clearly demonstrates that the blockade of β1-ADR in CNS, but not in periphery, impairs the memory retrieval in the contextual FC. Further analysis of brain and plasma samples shows that xamoterol can cross the BBB and be found in detectable concentrations in the brain after a single dose administration. These results suggest that the β1-ADR may be involved in some of the hippocampus-related behavioral tasks conducted in this study for Ts65Dn mice.
In conclusion, we have shown that this mouse model of DS have robust behavioral deficit. Our results demonstrate that these cognitive deficits are mediated by imbalance in noradrenergic system. It is important to note that In addition to noreadrenergic system, deficits in other systems including cholinergic system can also contribute to the cognitive deficits in Ts65Dn mice. In this experimental model of Down Syndrome a selective activation of β1-ADR does restore these behavioral deficits. Further mechanistic studies will be needed to investigate the failure of noradrenergic system and the role β1-ADR in cognitive deficit and pathogenesis of DS in people. Restoring NE neurotransmission or a selective activation of β1-ADR need to be further investigated for development of any potential therapeutic strategies for symptomatic relieve of memory deficit in DS. Furthermore, due to significant involvement of noradrenergic system in the cardiovascular function further safety and translational studies will be needed to ensure the safety and efficacy of this approach.
Supplementary Material
Research Highlights.
Behavioral characterization of the Ts65Dn mice model of Down syndrome.
Ts65Dn mice have cognitive and sensorimotor abnormalities
β1-ADR is a key player in the learning and memory deficit inTs65Dn brain.
Xamoterol passes the BBB and can be found in detectable concentration in brain.
Xamoterol, a selective β1-ADR agonist, restores the cognitive function in Ts65Dn.
A selective antagonist of β1-ADR reversed this efficacy observed in Ts65Dn mice.
Acknowledgments
The authors wish to thank Jacqueline Pham, Josephine Valenzuela, and Janice Valletta for excellent technical assistance and critical contributions. Special thanks to Simret Beraki Tecle, for valuable scientific discussion. This work was supported by the Stanford School of Medicine, and Swiss National Science Foundation (PBBEA-121061 to P.L.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbeduto L, Warren SF, Conners FA. Language development in Down syndrome: from the prelinguistic period to the acquisition of literacy. Ment Retard Dev Disabil Res Rev. 2007;13:247–61. doi: 10.1002/mrdd.20158. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. J Neurosci. 1999;19:1106–14. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter LL, Moran TH, Richtsmeier JT, Troncoso J, Reeves RH. Discovery and genetic localization of Down syndrome cerebellar phenotypes using the Ts65Dn mouse. Hum Mol Genet. 2000;9:195–202. doi: 10.1093/hmg/9.2.195. [DOI] [PubMed] [Google Scholar]
- Belichenko NP, Belichenko PV, Kleschevnikov AM, Salehi A, Reeves RH, Mobley WC. The “Down syndrome critical region” is sufficient in the mouse model to confer behavioral, neurophysiological, and synaptic phenotypes characteristic of Down syndrome. J Neurosci. 2009a;29:5938–48. doi: 10.1523/JNEUROSCI.1547-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belichenko PV, Kleschevnikov AM, Masliah E, Wu C, Takimoto-Kimura R, Salehi A, Mobley WC. Excitatory-inhibitory relationship in the fascia dentata in the Ts65Dn mouse model of Down syndrome. J Comp Neurol. 2009b;512:453–66. doi: 10.1002/cne.21895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belichenko PV, Kleschevnikov AM, Salehi A, Epstein CJ, Mobley WC. Synaptic and cognitive abnormalities in mouse models of Down syndrome: exploring genotype-phenotype relationships. J Comp Neurol. 2007;504:329–45. doi: 10.1002/cne.21433. [DOI] [PubMed] [Google Scholar]
- Belichenko PV, Masliah E, Kleschevnikov AM, Villar AJ, Epstein CJ, Salehi A, Mobley WC. Synaptic structural abnormalities in the Ts65Dn mouse model of Down Syndrome. J Comp Neurol. 2004;480:281–98. doi: 10.1002/cne.20337. [DOI] [PubMed] [Google Scholar]
- Bimonte-Nelson HA, Hunter CL, Nelson ME, Granholm AC. Frontal cortex BDNF levels correlate with working memory in an animal model of Down syndrome. Behav Brain Res. 2003;139:47–57. doi: 10.1016/s0166-4328(02)00082-7. [DOI] [PubMed] [Google Scholar]
- Burger PC, Vogel FS. The development of the pathologic changes of Alzheimer’s disease and senile dementia in patients with Down’s syndrome. Am J Pathol. 1973;73:457–76. [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, Walker LC, Whitehouse PJ, Price DL. Abnormalities of the nucleus basalis in Down’s syndrome. Ann Neurol. 1985;18:310–3. doi: 10.1002/ana.410180306. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gold PE. Age-related changes in memory and in acetylcholine functions in the hippocampus in the Ts65Dn mouse, a model of Down syndrome. Neurobiol Learn Mem. 2008;89:167–77. doi: 10.1016/j.nlm.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleves MA, Hobbs CA, Cleves PA, Tilford JM, Bird TM, Robbins JM. Congenital defects among liveborn infants with Down syndrome. Birth Defects Res A Clin Mol Teratol. 2007;79:657–63. doi: 10.1002/bdra.20393. [DOI] [PubMed] [Google Scholar]
- Coe DA, Matson JL, Russell DW, Slifer KJ, Capone GT, Baglio C, Stallings S. Behavior problems of children with Down syndrome and life events. J Autism Dev Disord. 1999;29:149–56. doi: 10.1023/a:1023044711293. [DOI] [PubMed] [Google Scholar]
- Costa AC, Scott-McKean JJ, Stasko MR. Acute injections of the NMDA receptor antagonist memantine rescue performance deficits of the Ts65Dn mouse model of Down syndrome on a fear conditioning test. Neuropsychopharmacology. 2008;33:1624–32. doi: 10.1038/sj.npp.1301535. [DOI] [PubMed] [Google Scholar]
- Costa AC, Walsh K, Davisson MT. Motor dysfunction in a mouse model for Down syndrome. Physiol Behav. 1999;68:211–20. doi: 10.1016/s0031-9384(99)00178-x. [DOI] [PubMed] [Google Scholar]
- Coussons-Read ME, Crnic LS. Behavioral assessment of the Ts65Dn mouse, a model for Down syndrome: altered behavior in the elevated plus maze and open field. Behav Genet. 1996;26:7–13. doi: 10.1007/BF02361154. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Medicine. Testing hypotheses about autism. Science. 2007;318:56–7. doi: 10.1126/science.1149801. [DOI] [PubMed] [Google Scholar]
- Davisson MT, Schmidt C, Akeson EC. Segmental trisomy of murine chromosome 16: a new model system for studying Down syndrome. Prog Clin Biol Res. 1990;360:263–80. [PubMed] [Google Scholar]
- Davisson MT, Schmidt C, Reeves RH, Irving NG, Akeson EC, Harris BS, Bronson RT. Segmental trisomy as a mouse model for Down syndrome. Prog Clin Biol Res. 1993;384:117–33. [PubMed] [Google Scholar]
- de Visser L, van den Bos R, Kuurman WW, Kas MJ, Spruijt BM. Novel approach to the behavioural characterization of inbred mice: automated home cage observations. Genes Brain Behav. 2006;5:458–66. doi: 10.1111/j.1601-183X.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Bannerman DM, Kirby BP, Croucher A, Rawlins JN. Effects of cytotoxic hippocampal lesions in mice on a cognitive test battery. Behav Brain Res. 2002;133:57–68. doi: 10.1016/s0166-4328(01)00451-x. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Rawlins JN. T-maze alternation in the rodent. Nat Protoc. 2006;1:7–12. doi: 10.1038/nprot.2006.2. [DOI] [PubMed] [Google Scholar]
- Demas GE, Nelson RJ, Krueger BK, Yarowsky PJ. Spatial memory deficits in segmental trisomic Ts65Dn mice. Behav Brain Res. 1996;82:85–92. doi: 10.1016/s0166-4328(97)81111-4. [DOI] [PubMed] [Google Scholar]
- Demas GE, Nelson RJ, Krueger BK, Yarowsky PJ. Impaired spatial working and reference memory in segmental trisomy (Ts65Dn) mice. Behav Brain Res. 1998;90:199–201. doi: 10.1016/s0166-4328(97)00116-2. [DOI] [PubMed] [Google Scholar]
- Devenport LD, Hale RL, Stidham JA. Sampling behavior in the radial maze and operant chamber: role of the hippocampus and prefrontal area. Behav Neurosci. 1988;102:489–98. doi: 10.1037//0735-7044.102.4.489. [DOI] [PubMed] [Google Scholar]
- Dierssen M, Vallina IF, Baamonde C, Garcia-Calatayud S, Lumbreras MA, Florez J. Alterations of central noradrenergic transmission in Ts65Dn mouse, a model for Down syndrome. Brain Res. 1997;749:238–44. doi: 10.1016/s0006-8993(96)01173-0. [DOI] [PubMed] [Google Scholar]
- Dierssen M, Vallina IF, Baamonde C, Lumbreras MA, Martinez-Cue C, Calatayud SG, Florez J. Impaired cyclic AMP production in the hippocampus of a Down syndrome murine model. Brain Res Dev Brain Res. 1996;95:122–4. doi: 10.1016/0165-3806(96)00071-5. [DOI] [PubMed] [Google Scholar]
- Dykens EM. Psychiatric and behavioral disorders in persons with Down syndrome. Ment Retard Dev Disabil Res Rev. 2007;13:272–8. doi: 10.1002/mrdd.20159. [DOI] [PubMed] [Google Scholar]
- Epstein CJ, Berger CN, Carlson EJ, Chan PH, Huang TT. Models for Down syndrome: chromosome 21-specific genes in mice. Prog Clin Biol Res. 1990;360:215–32. [PubMed] [Google Scholar]
- Escorihuela RM, Fernandez-Teruel A, Vallina IF, Baamonde C, Lumbreras MA, Dierssen M, Tobena A, Florez J. A behavioral assessment of Ts65Dn mice: a putative Down syndrome model. Neurosci Lett. 1995;199:143–6. doi: 10.1016/0304-3940(95)12052-6. [DOI] [PubMed] [Google Scholar]
- Fernandez F, Morishita W, Zuniga E, Nguyen J, Blank M, Malenka RC, Garner CC. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat Neurosci. 2007;10:411–3. doi: 10.1038/nn1860. [DOI] [PubMed] [Google Scholar]
- Galli M, Rigoldi C, Brunner R, Virji-Babul N, Giorgio A. Joint stiffness and gait pattern evaluation in children with Down syndrome. Gait Posture. 2008;28:502–6. doi: 10.1016/j.gaitpost.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Galsworthy MJ, Amrein I, Kuptsov PA, Poletaeva II, Zinn P, Rau A, Vyssotski A, Lipp HP. A comparison of wild-caught wood mice and bank voles in the Intellicage: assessing exploration, daily activity patterns and place learning paradigms. Behav Brain Res. 2005;157:211–7. doi: 10.1016/j.bbr.2004.06.021. [DOI] [PubMed] [Google Scholar]
- Gerlai R. A new continuous alternation task in T-maze detects hippocampal dysfunction in mice. A strain comparison and lesion study. Behav Brain Res. 1998;95:91–101. doi: 10.1016/s0166-4328(97)00214-3. [DOI] [PubMed] [Google Scholar]
- German DC, Manaye KF, White CL, 3rd, Woodward DJ, McIntire DD, Smith WK, Kalaria RN, Mann DM. Disease-specific patterns of locus coeruleus cell loss. Ann Neurol. 1992;32:667–76. doi: 10.1002/ana.410320510. [DOI] [PubMed] [Google Scholar]
- Godridge H, Reynolds GP, Czudek C, Calcutt NA, Benton M. Alzheimer-like neurotransmitter deficits in adult Down’s syndrome brain tissue. J Neurol Neurosurg Psychiatry. 1987;50:775–8. doi: 10.1136/jnnp.50.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm AC, Sanders LA, Crnic LS. Loss of cholinergic phenotype in basal forebrain coincides with cognitive decline in a mouse model of Down’s syndrome. Exp Neurol. 2000;161:647–63. doi: 10.1006/exnr.1999.7289. [DOI] [PubMed] [Google Scholar]
- Greenwood RD, Nadas AS. The clinical course of cardiac disease in Down’s syndrome. Pediatrics. 1976;58:893–7. [PubMed] [Google Scholar]
- Hampton TG, Stasko MR, Kale A, Amende I, Costa AC. Gait dynamics in trisomic mice: quantitative neurological traits of Down syndrome. Physiol Behav. 2004;82:381–9. doi: 10.1016/j.physbeh.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Holtzman DM, Santucci D, Kilbridge J, Chua-Couzens J, Fontana DJ, Daniels SE, Johnson RM, Chen K, Sun Y, Carlson E, Alleva E, Epstein CJ, Mobley WC. Developmental abnormalities and age-related neurodegeneration in a mouse model of Down syndrome. Proc Natl Acad Sci U S A. 1996;93:13333–8. doi: 10.1073/pnas.93.23.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter CL, Bimonte-Nelson HA, Nelson M, Eckman CB, Granholm AC. Behavioral and neurobiological markers of Alzheimer’s disease in Ts65Dn mice: effects of estrogen. Neurobiol Aging. 2004;25:873–84. doi: 10.1016/j.neurobiolaging.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Hyde LA, Crnic LS, Pollock A, Bickford PC. Motor learning in Ts65Dn mice, a model for Down syndrome. Dev Psychobiol. 2001a;38:33–45. doi: 10.1002/1098-2302(2001)38:1<33::aid-dev3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Hyde LA, Frisone DF, Crnic LS. Ts65Dn mice, a model for Down syndrome, have deficits in context discrimination learning suggesting impaired hippocampal function. Behav Brain Res. 2001b;118:53–60. doi: 10.1016/s0166-4328(00)00313-2. [DOI] [PubMed] [Google Scholar]
- Johnson CT, Olton DS, Gage FH, 3rd, Jenko PG. Damage to hippocampus and hippocampal connections: effects on DRL and spontaneous alternation. J Comp Physiol Psychol. 1977;91:508–22. doi: 10.1037/h0077346. [DOI] [PubMed] [Google Scholar]
- Kahlem P, Sultan M, Herwig R, Steinfath M, Balzereit D, Eppens B, Saran NG, Pletcher MT, South ST, Stetten G, Lehrach H, Reeves RH, Yaspo ML. Transcript level alterations reflect gene dosage effects across multiple tissues in a mouse model of down syndrome. Genome Res. 2004;14:1258–67. doi: 10.1101/gr.1951304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuta K, Umemura K, Ueyama N, Matsuoka N. Pharmacological evidence for a correlation between hippocampal CA1 cell damage and hyperlocomotion following global cerebral ischemia in gerbils. Eur J Pharmacol. 2003;467:103–9. doi: 10.1016/s0014-2999(03)01573-5. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–7. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Kleschevnikov AM, Belichenko PV, Villar AJ, Epstein CJ, Malenka RC, Mobley WC. Hippocampal long-term potentiation suppressed by increased inhibition in the Ts65Dn mouse, a genetic model of Down syndrome. J Neurosci. 2004;24:8153–60. doi: 10.1523/JNEUROSCI.1766-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapska E, Walasek G, Nikolaev E, Neuhausser-Wespy F, Lipp HP, Kaczmarek L, Werka T. Differential involvement of the central amygdala in appetitive versus aversive learning. Learn Mem. 2006;13:192–200. doi: 10.1101/lm.54706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B. Dissociation of the effects of lesions of the orbital or medial aspect of the prefrontal cortex of the rat with respect to activity. Behav Biol. 1974;10:329–43. doi: 10.1016/s0091-6773(74)91924-5. [DOI] [PubMed] [Google Scholar]
- Korenberg JR, Chen XN, Schipper R, Sun Z, Gonsky R, Gerwehr S, Carpenter N, Daumer C, Dignan P, Disteche C, et al. Down syndrome phenotypes: the consequences of chromosomal imbalance. Proc Natl Acad Sci U S A. 1994;91:4997–5001. doi: 10.1073/pnas.91.11.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieben CK, Steinbusch HW, Blokland A. 5,7-DHT lesion of the dorsal raphe nuclei impairs object recognition but not affective behavior and corticosterone response to stressor in the rat. Behav Brain Res. 2006;168:197–207. doi: 10.1016/j.bbr.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Loughlin SE, Foote SL, Bloom FE. Efferent projections of nucleus locus coeruleus: topographic organization of cells of origin demonstrated by three-dimensional reconstruction. Neuroscience. 1986;18:291–306. doi: 10.1016/0306-4522(86)90155-7. [DOI] [PubMed] [Google Scholar]
- Lumbreras M, Baamonde C, Martinez-Cue C, Lubec G, Cairns N, Salles J, Dierssen M, Florez J. Brain G protein-dependent signaling pathways in Down syndrome and Alzheimer’s disease. Amino Acids. 2006;31:449–56. doi: 10.1007/s00726-005-0272-z. [DOI] [PubMed] [Google Scholar]
- Marlow HF. Review of clinical experience with xamoterol. Effects on exercise capacity and symptoms in heart failure. Circulation. 1990;81:III93–8. [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Counts SE, Ginsberg SD. Gene expression profiles of cholinergic nucleus basalis neurons in Alzheimer’s disease. Neurochem Res. 2002;27:1035–48. doi: 10.1023/a:1020952704398. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Tremblay A, Lecluse V, Lehmann H. Hippocampal damage and anterograde object-recognition in rats after long retention intervals. Hippocampus. 2005;15:1050–6. doi: 10.1002/hipo.20122. [DOI] [PubMed] [Google Scholar]
- Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA. A distinct role for norepinephrine in memory retrieval. Cell. 2004;117:131–43. doi: 10.1016/s0092-8674(04)00259-4. [DOI] [PubMed] [Google Scholar]
- Nadel L. Down’s syndrome: a genetic disorder in biobehavioral perspective. Genes Brain Behav. 2003;2:156–66. doi: 10.1034/j.1601-183x.2003.00026.x. [DOI] [PubMed] [Google Scholar]
- Parker AW, Bronks R, Snyder CW., Jr Walking patterns in Down’s syndrome. J Ment Defic Res. 1986;30(Pt 4):317–30. doi: 10.1111/j.1365-2788.1986.tb01327.x. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Moon J, Edgin J, Stedron J, Nadel L. The neuropsychology of Down syndrome: evidence for hippocampal dysfunction. Child Dev. 2003;74:75–93. doi: 10.1111/1467-8624.00522. [DOI] [PubMed] [Google Scholar]
- Pueschel SM, Bernier JC, Pezzullo JC. Behavioural observations in children with Down’s syndrome. J Ment Defic Res. 1991;35(Pt 6):502–11. doi: 10.1111/j.1365-2788.1991.tb00447.x. [DOI] [PubMed] [Google Scholar]
- Reeves RH, Irving NG, Moran TH, Wohn A, Kitt C, Sisodia SS, Schmidt C, Bronson RT, Davisson MT. A mouse model for Down syndrome exhibits learning and behaviour deficits. Nat Genet. 1995;11:177–84. doi: 10.1038/ng1095-177. [DOI] [PubMed] [Google Scholar]
- Rueda N, Florez J, Martinez-Cue C. Effects of chronic administration of SGS-111 during adulthood and during the pre- and post-natal periods on the cognitive deficits of Ts65Dn mice, a model of Down syndrome. Behav Brain Res. 2008;188:355–67. doi: 10.1016/j.bbr.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Salehi A, Delcroix JD, Belichenko PV, Zhan K, Wu C, Valletta JS, Takimoto-Kimura R, Kleschevnikov AM, Sambamurti K, Chung PP, Xia W, Villar A, Campbell WA, Kulnane LS, Nixon RA, Lamb BT, Epstein CJ, Stokin GB, Goldstein LS, Mobley WC. Increased App expression in a mouse model of Down’s syndrome disrupts NGF transport and causes cholinergic neuron degeneration. Neuron. 2006;51:29–42. doi: 10.1016/j.neuron.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Salehi A, Faizi M, Belichenko PV, Mobley WC. Using mouse models to explore genotype-phenotype relationship in Down syndrome. Ment Retard Dev Disabil Res Rev. 2007;13:207–14. doi: 10.1002/mrdd.20164. [DOI] [PubMed] [Google Scholar]
- Salehi A, Faizi M, Colas D, Valleta J, Laguna J, Takimoto-Kimura R, Kleschevnikov A, Wagner SL, Aisen P, Shamloo M, Mobley WC. Restoration of norepinephrine-modulated contextual memory in a mouse model of Down Syndrome. Science Translational Medicine. 2009;1:7ra17. doi: 10.1126/scitranslmed.3000258. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–6. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo H, Isacson O. Abnormal APP, cholinergic and cognitive function in Ts65Dn Down’s model mice. Exp Neurol. 2005;193:469–80. doi: 10.1016/j.expneurol.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Shumway-Cook A, Woollacott MH. Dynamics of postural control in the child with Down syndrome. Phys Ther. 1985;65:1315–22. doi: 10.1093/ptj/65.9.1315. [DOI] [PubMed] [Google Scholar]
- Siarey RJ, Carlson EJ, Epstein CJ, Balbo A, Rapoport SI, Galdzicki Z. Increased synaptic depression in the Ts65Dn mouse, a model for mental retardation in Down syndrome. Neuropharmacology. 1999;38:1917–20. doi: 10.1016/s0028-3908(99)00083-0. [DOI] [PubMed] [Google Scholar]
- Siarey RJ, Kline-Burgess A, Cho M, Balbo A, Best TK, Harashima C, Klann E, Galdzicki Z. Altered signaling pathways underlying abnormal hippocampal synaptic plasticity in the Ts65Dn mouse model of Down syndrome. J Neurochem. 2006;98:1266–77. doi: 10.1111/j.1471-4159.2006.03971.x. [DOI] [PubMed] [Google Scholar]
- Siarey RJ, Stoll J, Rapoport SI, Galdzicki Z. Altered long-term potentiation in the young and old Ts65Dn mouse, a model for Down Syndrome. Neuropharmacology. 1997;36:1549–54. doi: 10.1016/s0028-3908(97)00157-3. [DOI] [PubMed] [Google Scholar]
- Siarey RJ, Villar AJ, Epstein CJ, Galdzicki Z. Abnormal synaptic plasticity in the Ts1Cje segmental trisomy 16 mouse model of Down syndrome. Neuropharmacology. 2005;49:122–8. doi: 10.1016/j.neuropharm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Starkey ML, Barritt AW, Yip PK, Davies M, Hamers FP, McMahon SB, Bradbury EJ. Assessing behavioural function following a pyramidotomy lesion of the corticospinal tract in adult mice. Exp Neurol. 2005;195:524–39. doi: 10.1016/j.expneurol.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Steele RJ, Morris RG. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus. 1999;9:118–36. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Stewart LS, Persinger MA, Cortez MA, Snead OC., 3rd Chronobiometry of behavioral activity in the Ts65Dn model of Down syndrome. Behav Genet. 2007;37:388–98. doi: 10.1007/s10519-006-9119-y. [DOI] [PubMed] [Google Scholar]
- Takakusaki K. Forebrain control of locomotor behaviors. Brain Res Rev. 2008;57:192–8. doi: 10.1016/j.brainresrev.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Tani K, Iyo M, Matsumoto H, Kawai M, Suzuki K, Iwata Y, Won T, Tsukamoto T, Sekine Y, Sakanoue M, Hashimoto K, Ohashi Y, Takei N, Mori N. The effects of dentate granule cell destruction on behavioural activity and Fos protein expression induced by systemic methamphetamine in rats. Br J Pharmacol. 2001;134:1411–8. doi: 10.1038/sj.bjp.0704370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Presti MF, Newman HA, Bugenhagen P, Crnic L, Lewis MH. Spontaneous stereotypy in an animal model of Down syndrome: Ts65Dn mice. Behav Genet. 2001;31:393–400. doi: 10.1023/a:1012226603255. [DOI] [PubMed] [Google Scholar]
- Viggiano D. The hyperactive syndrome: metanalysis of genetic alterations, pharmacological treatments and brain lesions which increase locomotor activity. Behav Brain Res. 2008;194:1–14. doi: 10.1016/j.bbr.2008.06.033. [DOI] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. J Neurosci. 2004;24:5901–8. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart JG, MacLeod HA, Rowan C. Parents’ evaluations of pre-school services for children with Down syndrome in two Scottish regions. Child Care Health Dev. 1993;19:1–23. doi: 10.1111/j.1365-2214.1993.tb00709.x. [DOI] [PubMed] [Google Scholar]
- Wishart JG, Pitcairn TK. Recognition of identity and expression in faces by children with Down syndrome. Am J Ment Retard. 2000;105:466–79. doi: 10.1352/0895-8017(2000)105<0466:ROIAEI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.