Abstract
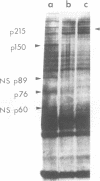
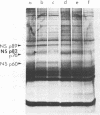
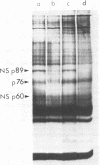
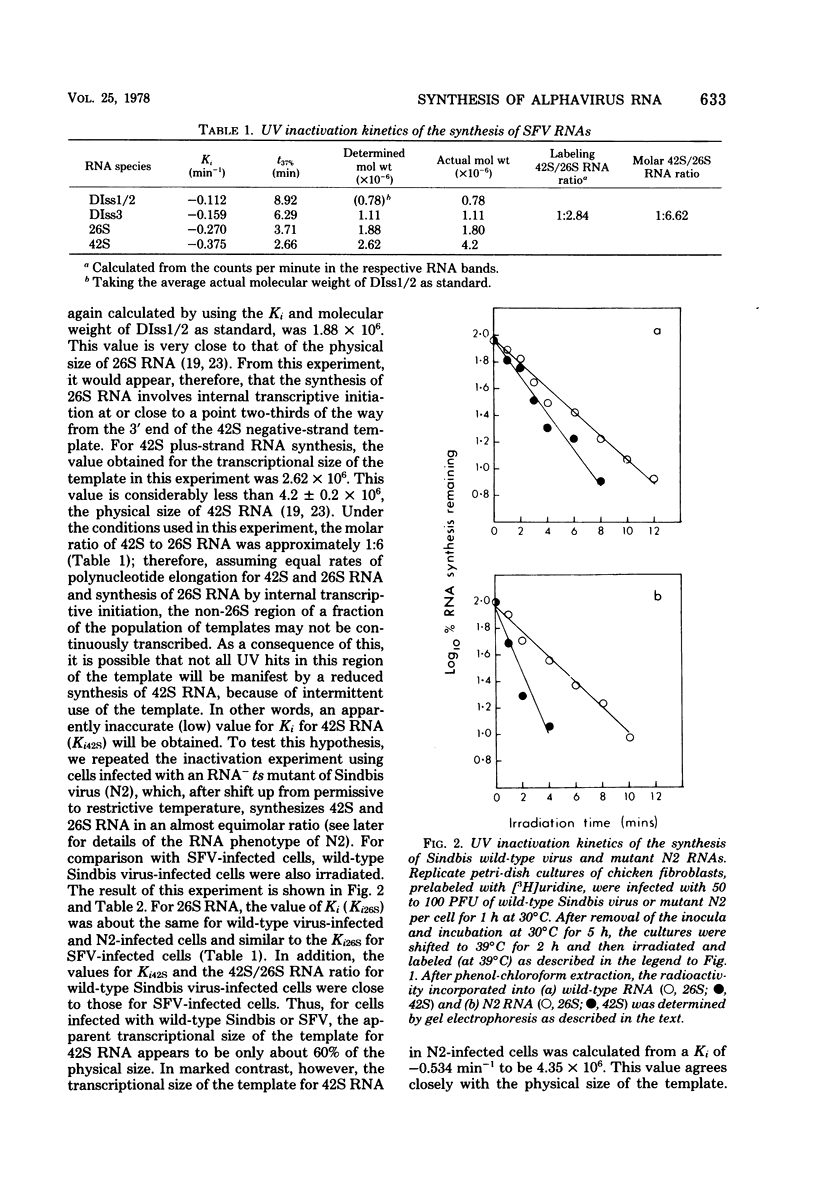
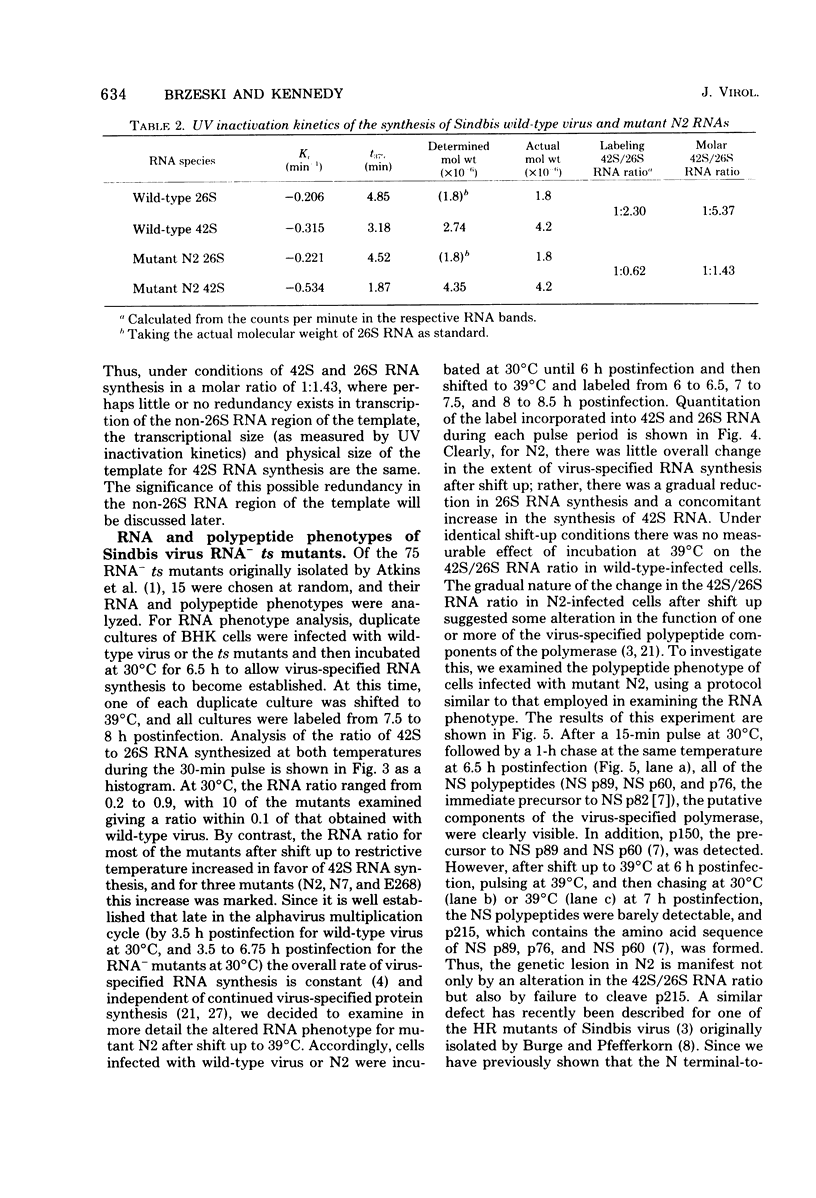
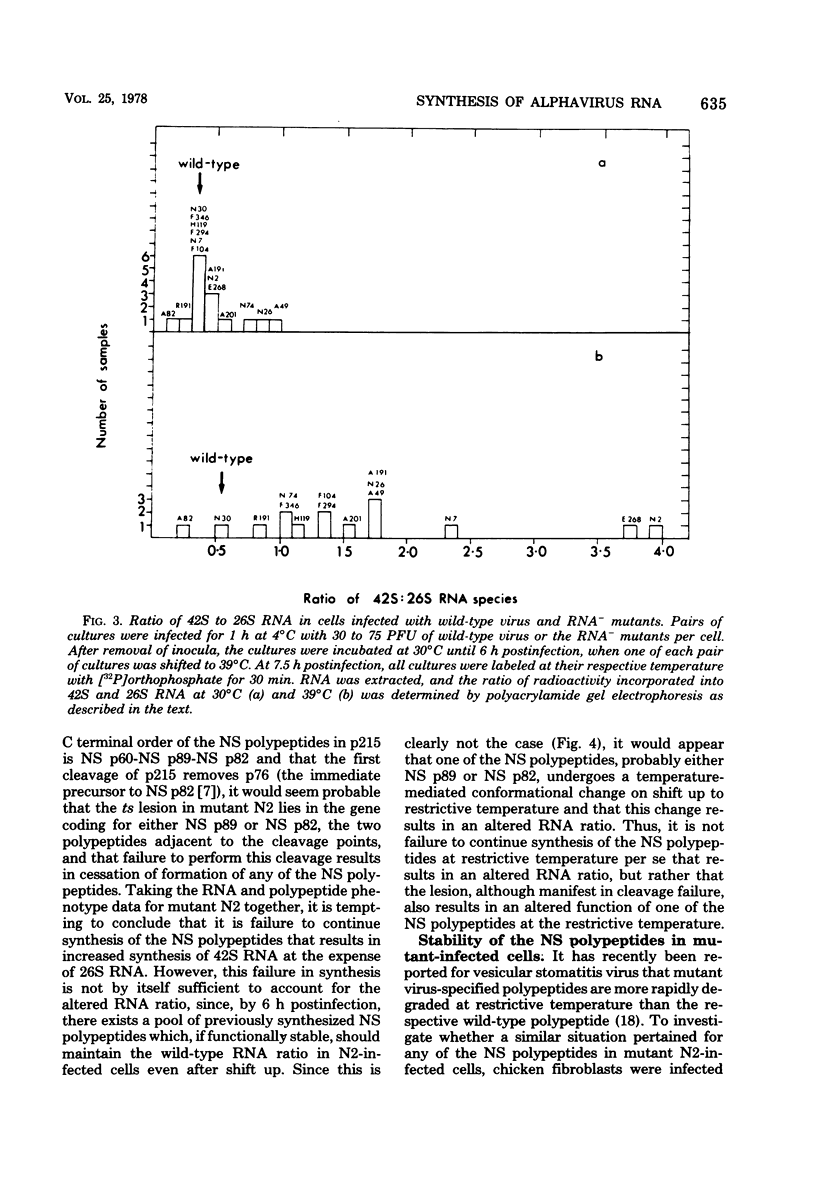
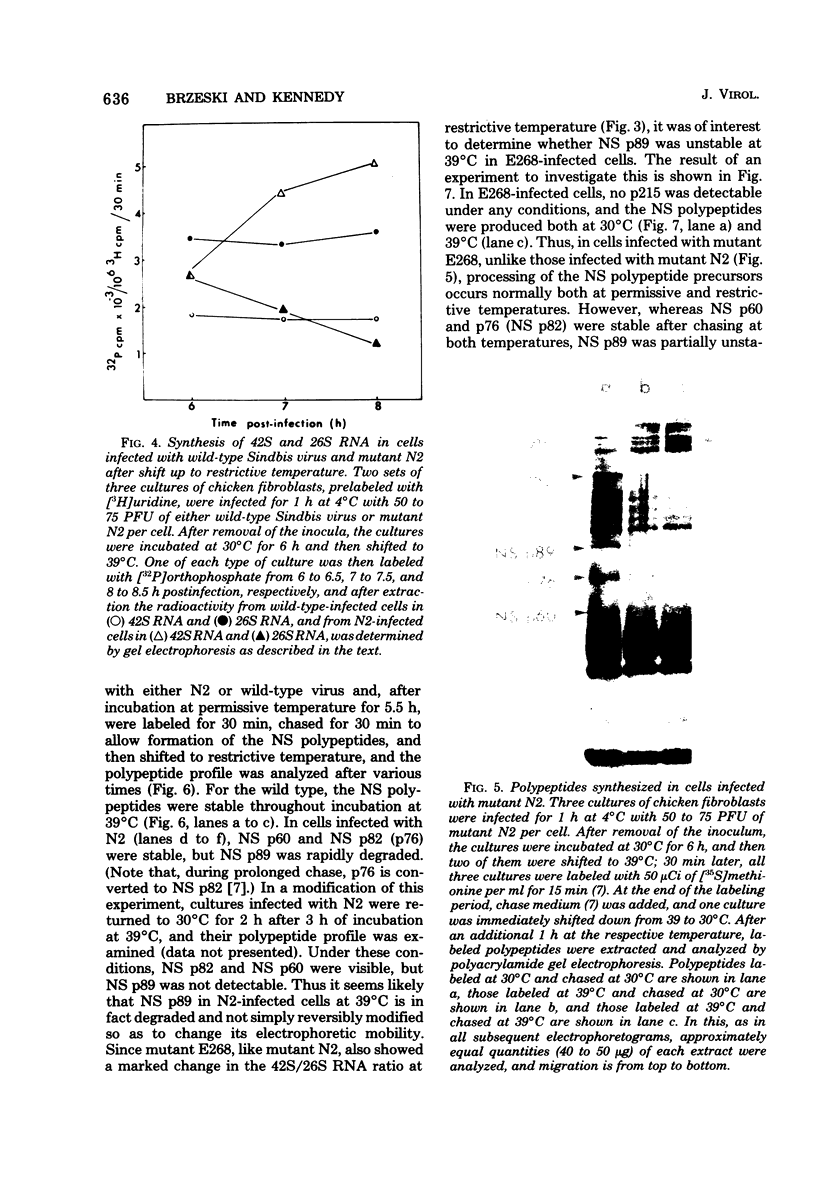
UV irradiation of chicken fibroblasts infected with Semliki Forest or Sindbis virus has been used to investigate the mechanism of synthesis of 42S and 26S RNA, the major plus-strand virus-specified RNAs formed during the multiplication of standard virus particles. From an analysis of the kinetics of UV inactivation of the synthesis of these two RNAs, we conclude (i) that 26S RNA is formed by internal transcriptive initiation from a point about two-thirds of the way from the 3′ end of the 42S negative-strand template; (ii) that there exists a population of plus-strand synthesizing complexes whose members are each capable of synthesizing both 42S and 26S RNA; and (iii) that, on a time-averaged basis, each complex in wild-type virus-infected cells contains one virus polymerase mediating 42S RNA synthesis and three mediating 26S RNA synthesis. The RNA phenotypes of 15 RNA−ts mutants of Sindbis virus have been examined after temperature shift to the restrictive temperature. Under these conditions, cells infected with three mutants, N2, N7, and E268, synthesized four to six times as much 42S RNA (relative to 26S RNA) as wild-type virus-infected cells. These studies were extended by examining, in detail, the RNA and polypeptide phenotypes of mutants N2 and E268. These experiments showed that, in N2- and E268-infected cells, one of the virus-specified nonstructural (NS) polypeptides (NS p89; H. Brzeski and S. I. T. Kennedy, J. Virol. 22:420-429, 1977) is thermolabile after shift up to restrictive temperature. This finding, together with the observation that, after shift, the 26S/42S RNA ratio in N2-infected cells changes markedly in favor of 42S RNA synthesis, leads us to conclude that, of the three NS polypeptides, NS p89 modulates 26S RNA synthesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins G. J., Samuels J., Kennedy S. I. Isolation and preliminary characterization of temperature-sensitive mutants of Sindbis virus strain AR339. J Gen Virol. 1974 Dec;25(3):371–380. doi: 10.1099/0022-1317-25-3-371. [DOI] [PubMed] [Google Scholar]
- Ball L. A. Transcriptional mapping of vesicular stomatitis virus in vivo. J Virol. 1977 Jan;21(1):411–414. doi: 10.1128/jvi.21.1.411-414.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracha M., Leone A., Schlesinger M. J. Formation of a Sindbis virus nonstructural protein and its relation of 42S mRNA function. J Virol. 1976 Dec;20(3):612–620. doi: 10.1128/jvi.20.3.612-620.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton C. J., Kennedy S. I. Defective-interfering particles of Semliki Forest Virus: structural differences between standard virus and defective-interfering particles. J Gen Virol. 1976 Jun;31(3):383–395. doi: 10.1099/0022-1317-31-3-383. [DOI] [PubMed] [Google Scholar]
- Bruton C. J., Kennedy S. I. Semliki Forest virus intracellular RNA: properties of the multi-stranded RNA species and kinetics of positive and negative strand synthesis. J Gen Virol. 1975 Jul;28(1):111–127. doi: 10.1099/0022-1317-28-1-111. [DOI] [PubMed] [Google Scholar]
- Bruton C. J., Porter A., Kennedy S. I. Defective-interfering particles of Semliki Forest virus: intracellular events during interference. J Gen Virol. 1976 Jun;31(3):397–416. doi: 10.1099/0022-1317-31-3-397. [DOI] [PubMed] [Google Scholar]
- Brzeski H., Kennedy S. I. Synthesis of Sindbis virus nonstructural polypeptides in chicken embryo fibroblasts. J Virol. 1977 May;22(2):420–429. doi: 10.1128/jvi.22.2.420-429.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge B. W., Pfefferkorn E. R. Isolation and characterization of conditional-lethal mutants of Sindbis virus. Virology. 1966 Oct;30(2):204–213. doi: 10.1016/0042-6822(66)90096-1. [DOI] [PubMed] [Google Scholar]
- Cancedda R., Swanson R., Schlesinger M. J. Effects of different RNAs and components of the cell-free system on in vitro synthesis of Sindbis viral proteins. J Virol. 1974 Sep;14(3):652–663. doi: 10.1128/jvi.14.3.652-663.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. C., Brzeski H., Kennedy S. I. RNA polymerase components in Semliki Forest virus-infected cells: synthesis from large precursors. J Gen Virol. 1976 Sep;32(3):413–430. doi: 10.1099/0022-1317-32-3-413. [DOI] [PubMed] [Google Scholar]
- Clegg J. C., Kennedy S. I. In vitro synthesis of structural proteins of Semliki Forest virus directed by isolated 26 S RNA from infected cells. FEBS Lett. 1974 Jun 15;42(3):327–330. doi: 10.1016/0014-5793(74)80757-x. [DOI] [PubMed] [Google Scholar]
- Clegg J. C., Kennedy S. I. Polyadenylic acid sequences in the virus RNA species of cells infected with Semliki Forest Virus. J Gen Virol. 1974 Mar;22(3):331–345. doi: 10.1099/0022-1317-22-3-331. [DOI] [PubMed] [Google Scholar]
- Clewley J. P., Kennedy S. I. Purification and polypeptide composition of Semliki Forest virus RNA polymerase. J Gen Virol. 1976 Sep;32(3):395–411. doi: 10.1099/0022-1317-32-3-395. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Levy H. B., Carter W. B. Replication of semliki forest virus: three forms of viral RNA produced during infection. Proc Natl Acad Sci U S A. 1966 Aug;56(2):440–446. doi: 10.1073/pnas.56.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett P. B., Sauerbier W. The transcriptional organization of the ribosomal RNA genes in mouse L cells. J Mol Biol. 1975 Jan 25;91(3):235–256. doi: 10.1016/0022-2836(75)90378-2. [DOI] [PubMed] [Google Scholar]
- Kennedy S. I. Sequence relationships between the genome and the intracellular RNA species of standard and defective-interfering Semliki Forest virus. J Mol Biol. 1976 Dec;108(2):491–511. doi: 10.1016/s0022-2836(76)80132-5. [DOI] [PubMed] [Google Scholar]
- Knipe D., Lodish H. F., Baltimore D. Analysis of the defects of temperature-sensitive mutants of vesicular stomatitis virus: intracellular degradation of specific viral proteins. J Virol. 1977 Mar;21(3):1140–1148. doi: 10.1128/jvi.21.3.1140-1148.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. A., Burke D. C. The replication of Semliki Forest virus. J Gen Virol. 1974 Jul;24(1):45–66. doi: 10.1099/0022-1317-24-1-45. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Plagemann P. G. Effect of ultraviolet light on mengovirus: formation of uracil dimers, instability and degradation of capsid, and covalent linkage of protein to viral RNA. J Virol. 1974 Mar;13(3):729–739. doi: 10.1128/jvi.13.3.729-739.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele C. M., Pfefferkorn E. R. Inhibition of interjacent ribonucleic acid (26S) synthesis in cells infected by Sindbis virus. J Virol. 1969 Aug;4(2):117–122. doi: 10.1128/jvi.4.2.117-122.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal S., Sreevalsan T. Sindbis virus replicative intermediates: purification and characterization. Virology. 1974 Jun;59(2):428–442. [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Replication of Sindbis virus. I. Relative size and genetic content of 26 s and 49 s RNA. J Mol Biol. 1972 Nov 28;71(3):599–613. [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Replication of Sindbis virus. II. Multiple forms of double-stranded RNA isolated from infected cells. J Mol Biol. 1972 Nov 28;71(3):615–631. doi: 10.1016/s0022-2836(72)80027-5. [DOI] [PubMed] [Google Scholar]
- Simmons D. T., Strauss J. H. Translation of Sindbis virus 26 S RNA and 49 S RNA in lysates of rabbit reticulocytes. J Mol Biol. 1974 Jun 25;86(2):397–409. doi: 10.1016/0022-2836(74)90027-8. [DOI] [PubMed] [Google Scholar]
- Wengler G., Beato M., Hackemack B. A. Translation of 26 S virus-specific RNA from Semliki Forest virus-infected cells in vitro. Virology. 1974 Sep;61(1):120–128. doi: 10.1016/0042-6822(74)90247-5. [DOI] [PubMed] [Google Scholar]
- Wengler G. Studies on the synthesis of viral RNA-polymerase-template complexes in BHK 21 cells infected with Semliki Forest virus. Virology. 1975 Jul;66(1):322–326. doi: 10.1016/0042-6822(75)90202-0. [DOI] [PubMed] [Google Scholar]
- Wengler G., Wengler G. Localization of the 26-S RNA sequence on the viral genome type 42-S RNA isolated from SFV-infected cells. Virology. 1976 Aug;73(1):190–199. doi: 10.1016/0042-6822(76)90073-8. [DOI] [PubMed] [Google Scholar]