Abstract
This review will summarize and interpret recent literature regarding the human CMV immune response, which is among the strongest measured and is the focus of attention for numerous research groups. CMV is a highly prevalent, globally occurring infection that rarely elicits disease in healthy immunocompetent hosts. The human immune system is unable to clear CMV infection and latency, but mounts a spirited immune-defense targeting multiple immune-evasion genes encoded by this dsDNA β-herpes virus. Additionally, the magnitude of cellular immune response devoted to CMV may cause premature immune senescence, and the high frequencies of cytolytic T cells may aggravate vascular pathologies. However, uncontrolled CMV viremia and life-threatening symptoms, which occur readily after immunosuppression and in the immature host, clearly indicate the essential role of immunity in maintaining asymptomatic co-existence with CMV. Approaches for harnessing the host immune response to CMV are needed to reduce the burden of CMV complications in immunocompromised individuals.
Keywords: adoptive transfer, atherosclerosis, CMV disease, CMV immunity, immune senescence, vaccines
Outlines of a paradigmatic interplay
Human CMV is an ancient β-herpes virus (type 5) that is ubiquitous in human populations, reaching a prevalence of 100% in Africa and Asia, and approximately 80% in Europe and the USA, depending on socioeconomic status [1]. Encoding approximately 165 genes, this dsDNA genome virus is the largest of the human herpes viruses. The millions of years (~108) of coevolution between CMV and the immune system of its host provides for a unique opportunity to study immune defense strategies and pathogen counterstrategies [2]. The most striking aspect of the immunobiology of CMV is the size of the cellular response elicited by the host [3]. However, in spite of the host attack, the virus efficiently adapts to the human immune system. As a result, CMV is never eliminated from the infected immunocompetent individual, in whom it causes a persistent asymptomatic infection. Multiple viral immune evasion genes have been identified, which profoundly interfere with both innate and adaptive compartments of the host immunity, preventing viral clearance [4]. Nonetheless, following subclinical primary CMV infection, the virus and the immune system are able to reach a homeostatic balance, and a lifelong latency is established primarily in cells of the myeloid lineage [5]. Reactivation events are likely, but their detection is relatively uncommon in immunocompetent individuals. Throughout the host life course, the enormous and continuous commitment of immune resources to controlling this one single virus exerts a heavy toll on the host system. In this context, a number of studies have indicated that CMV may contribute to the development of vascular diseases, including atherosclerosis, and have also outlined the deleterious effect of CMV on immune senescence and health outcomes in the elderly [6].
Significant suppression of host antiviral immunity can alter the life-or-death immune surveillance balance, allowing either CMV reactivation to become detectable or primary infection to cause clinical symptoms [7–11]. Uncontrolled viral replication and dissemination result in the development of life-threatening end-organ damage (CMV disease). Likewise, acquisition of CMV infection in utero or immediately after birth causes clinical complications that can be substantial and severe, as a consequence of the incapability of the immature host to adequately resolve the primary infection and convert the virus into latency.
Considering the profound effects of CMV infections on the health and quality of life of immunosuppressed individuals, the elderly and children with congenital infection, it is not surprising that the development of a vaccine against CMV or immunotherapeutic approaches to limit the incidence of CMV disease are considered a high priority [12–14]. Unfortunately, CMV replication is species-restricted and therefore no natural model exists for evaluating vaccine strategies directly applicable to human disease. Despite this difficulty, numerous clinical studies have been performed over the past three decades, although successful licensure of an effective vaccine formulation or immune strategy against CMV remains elusive [13,14].
To design effective therapeutics, a better understanding and characterization of the immunological mechanisms of primary and latent CMV infections, improved diagnostic and immune-monitoring tools, novel preventive and treatment options are of critical importance. The purpose of this review is to summarize and interpret the most relevant findings and recent literature regarding the complex interplay between the host immune system and CMV-mediated immune evasion, and the attempts to develop protective anti-CMV immune-strategies.
Virus entry & innate immunity
CMV displays a broad host cell range, being able to infect several cell types, such as endothelial cells, epithelial cells (including retinal cells), smooth muscle cells, fibroblasts, leukocytes and dendritic cells [15,16]. In healthy individuals, primary CMV infection initiates with replication in the mucosal epithelium; subsequently, the virus disseminates to monocytic cells of myeloid lineage including monocytes and CD34+ cells, where it establishes latent infection. Viral gene expression in these latently infected cells is restricted and markedly different from lytic infection. Genes that are abundantly expressed in lytic infection are expressed only transiently and weakly in in vitro experimental models of latency [17]. Thus, it has been presumed that cell immune surveillance in latently infected cells is limited. Nonetheless, a number of latency-associated transcripts, whose mechanistic role remains to be clarified, have now been described [18]. Interestingly, T-cell responses to latency-associated proteins have been shown, which indicate that latently infected cells could also potentially come under T-cell control [19].
The differentiation of virus-infected monocytes into macrophages can initiate productive infection. Infection of endothelial cells and hematopoietic cells facilitates systemic spread within the host, while infection of ubiquitous cell types, such as fibroblasts and smooth muscle cells, provides the platform for efficient proliferation of the virus [15,16]. As CMV enters cells to establish infection, the host recognizes the virions and activates several mechanisms and pathways of innate immune response. These include inflammatory cytokines, type I IFN and upregulation of costimulatory molecules that are crucial for slowing the pathogen and subsequently priming a high-quality adaptive immune response [20]. The innate immune responses are the first line of defense against CMV and allow the host to rapidly mount antiviral measures. Particularly during the perinatal period, innate immunity seems to be primarily responsible for host defenses against CMV infection, because of the immaturity of adaptive immunity [21].
Detection of pathogens by the innate immune system is carried out by a class of immune-sensor molecules termed pattern recognition receptors (PRRs). The Toll-like receptors are a class of PRRs that detect a broad range of pathogens, including bacteria and viruses [22]. Several studies from the Compton group have indicated that the very initial stages of CMV infection are subject to innate detection by Toll-like receptor 2. This ancient PRR has been shown to recognize CMV surface glycoproteins gB and gH, which leads to activation of an NF-κB-dependent signal transduction pathway [23]. The activation of the innate immune system also includes recruitment of professional APCs, phagocytes and NK cells [20]. Characterized by the lack of both CD3+ T- and CD19+ B-cell markers, in humans they usually express the surface markers CD16 (FcγRIII) and CD56. NK cells function as important sentinels of the immune system and are considered a bridge between the innate and adaptive immune systems because of their ability to provide rapid cytotoxic function similar to that of innate immunocytes (such as macrophages and granulocytes), and because this cytotoxic function (such as production of IFN-γ, granzymes and perforins) is closely aligned with that of T lymphocytes. It has been suggested that NK cells provide a cytokine milieu that supports and drives the subsequent maturation of adaptive immunity and in particular of T cells [24]. There is also increasingly compelling evidence that NK cells play a crucial role in host defense against viral infection [25,26]. While murine CMV infection provides direct evidence of the importance of NK cells in CMV clearance and protection [27], relatively little is known about the role of NK cells in the immune defense against human CMV. Complex arrangement of subsets expressing overlapping repertoires of invariant surface receptors, termed activating (aKIR) and inhibitory killer immunoglobulin-like receptor (iKIR) on NK cells are involved in the destruction of virally infected cells [28]. The role of aKIR genes seems associated with CMV reactivation, and in hematopoietic cell transplantation, certain haplotypes have recently been described to control CMV infection [29–31]. Additionally, patients with rare genetic defects involving overexpression of an iKIR can have serious recurrent episodes of CMV disease [32]. Finally, the extensive immune evasion mechanisms that CMV encodes to prevent NK cell activation indicate their importance in the innate response to CMV [33–35]. Recent studies in a rhesus macaque model of CMV infection have shown how the CMV-encoded viral IL-10 ortholog (vIL-10) attenuates innate immunity, establishing a long-term deficit of adaptive antiviral immunity [36]. In particular, vIL-10 alters the earliest host responses to primary CMV infection by reducing the size of the innate NK effector cell pool. This early viral strategy can modify the long-term adaptive immune responses to viral antigens, facilitating persistence in the immunocompetent host.
Control of CMV infection & adaptive immunity
Following establishment of primary CMV infection, virus particles or virus-associated dense bodies (defective enveloped particles that lack viral capsid and DNA [37]) are processed by professional APCs, which stimulate the antigen-specific immune response. The adaptive immune response to CMV is among the strongest ever documented in humans and fully engages both humoral and cellular immunity [38–40]. The development of adaptive immunity is needed to ultimately control primary CMV infection, after which CMV will enter a latency phase. The establishment of sustained and long-lasting adaptive immunity in healthy individuals is vital to maintain CMV latency and prevent productive (lytic) infection, which, in contrast, is common in immune-immature or immunocompromised individuals and patients on immunosuppressive medication, and often leads to uncontrolled replication, clinically serious CMV morbidity and mortality [7,10,11].
Humoral response
During CMV primary infection, antibodies specific for multiple CMV proteins are elicited in the host. They include structural tegument proteins (e.g., pp65 and pp150), envelope glycoproteins (predominantly gB and gH and gH/gL multimeric complexes), and nonstructural proteins such as the IE1 protein [15,41]. The role of antibodies for protection against and control of CMV has been debated. However, studies indicated that humoral immunity is crucial in restricting viral dissemination and probably contribute to minimizing the clinical manifestations of the disease [41].
Historically, the determination of the neutralizing activity of human sera from CMV-infected individuals has been carried out in in vitro assay systems using exclusively human fibroblasts, while the whole spectrum of antigens targeted by CMV-neutralizing antibodies remained poorly characterized [41]. More recently, this conventional approach has been challenged. Seminal studies have demonstrated that the entry pathway in fibroblasts, in which CMV replication differs from that in endothelial, epithelial and myeloid cells, is essential for the systemic spread of CMV [15,41]. In particular, the multimeric gH/gL/pUL complex has been shown to be required for infection of both endothelial and epithelial cells, while the glycoprotein complex gH/gL/gO is required for infection of human fibroblasts [42]. Importantly, it has been found that antibodies conformational to the gH/gL/pUL complex appear to display marked CMV-neutralizing and dissemination-inhibiting activity [39,41]. These results indicate the need for a revision of the fibroblast-based approach for CMV-neutralization studies to fully evaluate the epidemiological impact of neutralization antibodies against CMV in the human population.
Cellular response
The size of the CMV-specific T-cell response is the most striking aspect of the dynamic, life-long interaction between the host and CMV. The frequencies of CMV-specific T cells in healthy subjects are much higher than those observed for other human viruses (e.g., influenza, adenovirus, poxvirus, measles, mumps and other herpes viruses) and are similar to those of HIV-specific T cells in active HIV infection [3]. The properties of CMV that determine such large and long-lasting T-cell responses in the absence of detectable CMV viremia have not been elucidated. However the relatively prolonged, but reduced viral replication during primary infection may induce recruitment of CMV-specific T cells into the memory compartment. T cells are essential to restrain CMV viral replication and prevent disease, but do not eliminate the virus or preclude transmission. Broadly targeted CMV-specific T cells dominate the memory compartments of exposed subjects, comprising on average approximately 10% of both the CD4+ and CD8+ memory compartments in peripheral blood [3]. CMV-specific T-cell frequencies continue to inflate throughout life, and very large responses may be observed ex vivo against single epitopes contributing to a substantial fraction of memory T cells in healthy seropositive individuals (Figure 1) [3,6,43]. However, the extent to which high frequencies of CMV-specific T cells are required for viral control remains unclear. In fact, is not uncommon to find CMV-seropositive immunocompetent individuals who maintain lifetime protection from symptomatic CMV even with minimal CMV-specific T-cell responses [3,44].
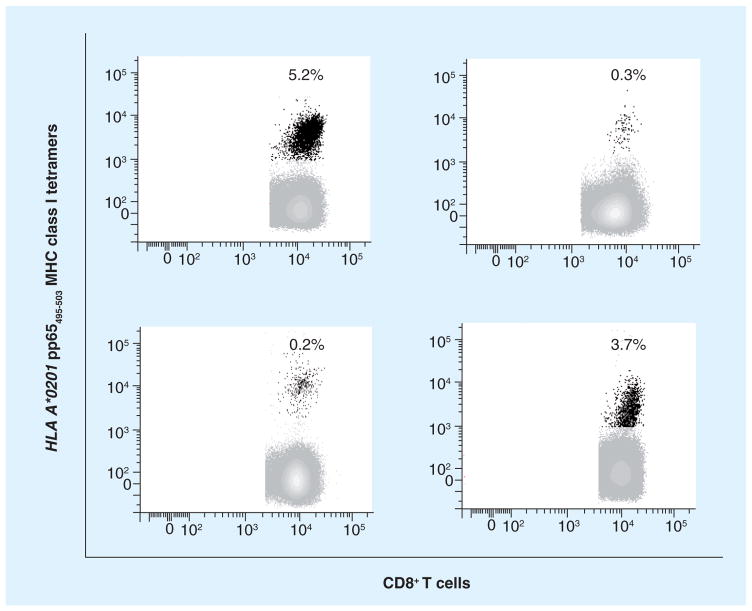
Figure 1. CMV-seropositive healthy adults.
Levels (expressed in percentages as shown inside each plot) of CD8+ T cells specific for MHC class I pp65495–503 tetramer (black dots) in four HLA A*0201 CMV-seropositive healthy adults (each plot represents a different subject). Peripheral blood mononuclear cells were stained with tetramers and analyzed by cytofluorometry as detailed [77]. Note the substantial portion of CD8+ T cells specific for a single HLA A*0201 CMV epitope from the pp65 protein among healthy people with no acute CMV infection [La Rosa et al., Unpublished Data].
The most comprehensive study in CMV-seropositive healthy individuals with various MHC class I types has identified CMV-specific responses from both CD4+ and CD8+ T cells to 213 predicted CMV-encoded open reading frames (ORFs) using 13,687 peptides. 151 ORFs elicited a CD4+ or CD8+ T-cell response in at least one donor. Cellular responses significantly differ among subjects, with some individuals recognizing only a single ORF and others as many as 39 [3]. Therefore few or multiple ORF-specific T-cell responses may not be representative of the overall individual’s CMV-specific T-cell response. Despite the large number of CMV-specific targets of the T-cell response, the vast majority of studies of the CMV-specific T-cell response in primary infection and long-term memory have measured immunity to pp65- and IE specific T cells, therefore limiting full understanding of the entire adaptive immune response to CMV and the correlates of protective immunity [3,45,46]. Though the in-depth ex vivo analyses by Sylwester et al. [3] indicated that UL83 (pp65) and UL123 (IE) were recognized by more than 50% of individuals, several other ORFs including UL48, UL55, UL122, UL48, UL32, UL123, UL99 and UL82 were also at the top of the recognition hierarchy for both CD4+ and CD8+ T-cell responses; however, their immune profiling remains to be fully characterized [47].
Several CMV-encoded gene products can cause disruption of the MHC class I and II antigen presentation pathway to avoid T-cell recognition (reviewed in [4]). Recently, a viral homolog of the immunomodulatory cytokine IL-10, expressed during latency (cmvLA IL-10), has been shown to reduce expression of MHC class I and II molecules and inhibit proliferation of peripheral blood mononuclear cells and the production of inflammatory cytokines [48].
The time course of the appearance of CMV-specific adaptive immune responses is difficult to follow in asymptomatic healthy subjects, since time of onset of primary infection typically goes unnoticed. Thus, the majority of the observations on CMV infection dynamics have been carried out in CMV-naive recipients of kidney transplant from CMV-seropositive donors (Figure 2). The patients chosen for these fundamental kinetic studies were able to control the initial viremia and CMV latency was induced [40]. Generally, 7 days after the peak of CMV replication, CD4+ T cells specific for the virus start to circulate, and synthesize Th1 cytokines (e.g., IFN-γ, TNF-α) [49,50]. Subsequently, CMV-specific CD8+ T cells appear in the peripheral blood. During primary CMV infection (Figure 2, left panels), a very high percentage of virus-specific T cells have phenotypes of recently activated naive T cells co-expressing CD45RA and CD45R0 surface cell markers, and the cell cycle-associated nuclear marker Ki67. In acquiring effector capacity they usually do not express the chemokine receptor CCR7, which is associated with homing to lymph nodes. They lose expression of the co-stimulatory molecule CD28 and have variable expression of CD27 [40]. The first CD8+ T cells that can be detected to have a CD45RA− CD28− CD27+ CCR7− cell surface phenotype express both perforin and granzyme B, and are capable of lysing CMV peptide-presenting target cells [44,51]. Following recovery from CMV infection, resting virus-specific CD8+ T cells express surface markers characteristic of memory T cells (CD45RA− CD45R0+). In the months after the primary response, CMV-specific CD8+ T cells gradually lost CD27 and re-acquire CD45RA expression, but still retain their HLA class I-restricted cytolytic potential (Figure 2).
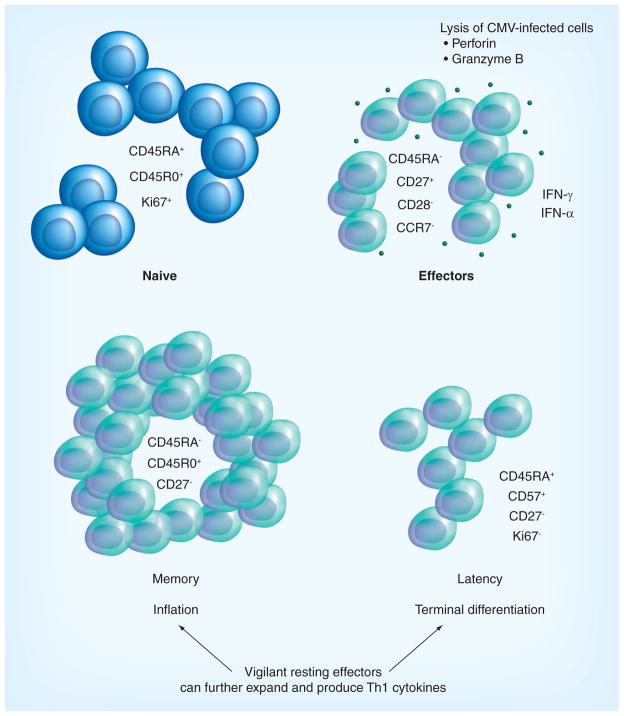
Figure 2. CMV-specific CD8+ T-cell phenotypes.
The peculiar differentiation stages of CMV-specific CD8+ T cells during primary infection, memory and latency [44]. Effector cells are markedly cytolytic and have a dominant Th1 cytokine production, which is the permanent CMV signature for both early- and late-stage CMV-specific T cells. In contrast to other viral infections, the memory pool does not contract after viral clearance, but keeps inflating and will eventually dominate the T-cell memory compartment. Although the phenotype of the CMV-specific CD8+ T cells during latency is typical of terminal differentiation, these cells can further expand and produce Th1 cytokines as if they were resting effectors [40]. This unique feature may be crucial in rapidly antagonizing virus replication upon reactivation of latent virus. On the downside, the high expression of IFN-γ may subtly increase the overall proinflammatory status, which may be causally related to pathogenic effects of CMV on the vasculature [55].
During CMV latency, CMV-specific cells can be characterized by the expression of CD57, and do not cycle as shown by the lack of Ki67 expression [52]. Though CD8+ CD45RA+ CD27− phenotype is typically associated with terminal differentiation of CD8+ T cells, the CMV ‘vigilant resting effector cells’ are not exhausted and can be induced to expand vigorously [44,53,54]. Importantly, the Th1 cytokine signature with predominant production of IFN-γ characterizes both early and late virus-specific T cells, which accumulate during latency. The constitutive and persistent upregulation of a number of IFN-γ genes is regulated by a combination of multiple transcription factors. These provide CD8+ effector-type T cells with the capacity to almost instantaneously secrete cytokines upon T-cell receptor ligation, a mechanism that may be essential to antagonize viral replication upon reactivation of latent virus (Figure 2) [44].
Atherosclerotic disease & immune senescence
The engagement of a substantial proportion of the immune system to CMV protects from CMV pathology, but may exert detrimental effects in immunocompetent individuals. The permanent high expression of IFN-γ and other Th1 cytokines by CMV-specific effector T cells induces a chronic proinflammatory state that has been hypothesized to be causally related to pathogenic effects on the vasculature [40]. Interestingly, a correlation was found between serum acute-phase response proteins, such as serum amyloid-A and CRP, and Th1 cytokines, such as IFN-γ, IL-18 and IFN-inducible protein-10 [38]. The high levels of CRP and IFN-γ typically measured in CMV-seropositive healthy individuals point to the involvement of both acute-phase response proteins and Th1 cytokines in generating athero-sclerotic disease, an age-related inflammatory state in which Th1 cytokines play an important role [55,56]. Increased serum IFN-γ levels promote macrophage and endothelial activation, resulting in increased expression of chemokines and adhesion molecules, finally leading to reduced plaque stability [57]. By the induction of a pool of highly differentiated cytotoxic cells capable of migrating to activated endothelium where they produce chemotactic cytokines, CMV could provide a positive feedback loop for the attraction and activation of immune effector cells to the vascular wall. A series of studies in octogenarian and nonagenarian individuals (reviewed in [6]) indicated that CMV-induced chronic systemic activation of the immune system may also contribute to the acceleration of immune senescence. Immune senescence is an age-associated impairment of immune functions, and is thought to significantly increase mortality and morbidity due to infections in people >65 years of age. CMV infection, and in particular the way the immune system deals with this persisting pathogen, may be a major driving force behind the phenomenon of immunosenescence. Typically after viral clearance, the pool of effector cells decreases, leaving a relatively small pool of long-lived memory cells. By contrast, CMV infection changes the composition of the T-cell pool by inducing a decrease of naive T cells and a permanent increase of highly differentiated T cells accumulates with increasing age. This shift is accompanied by a rapid and sustained decrease in the telomere length of the lymphocyte pool, which has been shown to be a risk factor for mortality in elderly people and for age-related diseases [58]. Finally, the inflation of a monoclonal/oligoclonal pool of terminally differentiated CMV-specific T cells, though fully functional and essential for CMV immunosurveillance, can come to dominate the entire T-cell repertoire in the elderly, and may constrict the ‘immunological space’, displacing or impairing T cells specific for other antigens [6,44,59]. The CMV-driven progressive loss of T-cell repertoire diversity is therefore considered to be one of the major contributors to the ‘immune risk phenotype’, which predicts 2-year mortality in very elderly individuals [60].
Immune immaturity & immune suppression
The effective homeostatic balance between CMV and the host prevents serious CMV complications in the healthy individual. However, in immunocompromised patients, and in the fetus and neonate, CMV infection can cause multiple types of clinical damage. Pre- and perinatal functional immaturity of the immune system, and impairment of T-cell immunity during post-transplant immune suppression profoundly affect control of CMV infection, leading to unchecked viral replication with consequent clinical disease and mortality.
Congenital CMV infection
As result of transplacental transmission, CMV constitutes the most commonly acquired congenital viral infection, causing major prenatal neurological damage (sensorineural hearing loss and other CNS abnormalities), which is particularly severe when primary maternal infection occurs during the first 16 weeks of pregnancy, when organs are developing and neuronal migration is occurring [11,21,61]. During gestation, anti-CMV antibodies in CMV-seropositive pregnant women play an important role in preventing congenital infection of the fetus [41]. Seronegative pregnant women carry an approximate 40% risk of CMV transmission to the fetus, though vertical transmission may also occur in seropositive pregnant women [11]. Clinical evidence has indicated that the neonatal cell-mediated immune response to primary infection is delayed when compared with that of adults with the same primary infection [41]. In in vitro studies, neonatal T cells have shown reduced cytolytic activity and capacity to respond to primary antigen, stimulatory monoclonal antibodies and toxic shock toxin, resulting in deficient IL-2 production [61]. Though independent primary humoral and cellular immunity were detected in CMV congenitally infected newborns, the mature CMV-specific CD8+ T-cell responses were characterized by lower levels of IFN-γ and higher IL-8 levels when compared with adults [11]. In summary, the reduced immune responsiveness, the characteristic neonatal bias towards a Th2 response (unable to fight intracellular pathogens), the lack of the typical CMV Th1 signature, and the sustained IL-8 levels (which can directly augment CMV replication) are among the factors that prevent the generation of a protective immune response, leading to uncontrolled viremia and severe clinical damage in congenitally infected newborns.
CMV infection in transplantation
CMV is a major opportunistic pathogen following allogeneic transplantation, reflecting the inability of the immune-depressed host to limit viral replication. CMV infection or reactivation is a frequent cause of morbidity and mortality in immunocompromised transplant recipients. Antirejection regimens administered to limit T-cell-mediated graft rejection induce significant suppression of host antiviral immunity, targeting both the CD8+ and CD4+ T-cell compartments that are critical for CMV immune containment. This profound deficit allows uncontrolled CMV replication, and may lead to dissemination and development of life-threatening end-organ damage (CMV disease, including pneumonitis, colitis and hepatitis). Antiviral chemotherapy limits CMV infection, though its use can have significant toxicity, and may expose transplant recipients to the risk of multiple infections [7,62]. Additionally, when antivirals are stopped or if immunodeficiency persists, patients may still develop late-onset CMV disease, often associated with antiviral resistance.
After allogeneic hematopoietic stem cell transplantation (HCT), a leading therapy for hematological malignancies, reconstitution of CMV-specific CD8+ T cells is essential to the control of CMV infection in CMV-positive recipients (R+) [63]. Donor CMV status seems to play a major role in the extent and quality of CMV-specific immunity, which has profound effects on patient outcomes [10,64]. Recent studies suggest that R+ of HCT from a CMV seropositive donor (D+) generate higher levels of multifunctional CMV-specific T-cells and require less antiviral therapy compared with D− R+ HCT recipients. These results highlight the benefit of immunocompetent D+ donors in whom a protective CMV response was developed in improving outcomes of R+ HCT recipients [65].
In solid organ transplantation (SOT), late CMV disease is most frequent and severe in R− recipients of a graft from a D+ donor, with about one-third of these D+ R− patients developing late CMV disease [7,66]. The development of a primary CMV response in symptomatic recipients has been studied, and various immune defects involved in CMV disease have been elucidated. In a cohort of D+ R− renal SOT recipients, specific CD8+ cytotoxic T-lymphocyte (CTL) and antibody responses developed regardless of clinical signs. Whereas in asymptomatic individuals the CMV-specific CD4+ T-cell response preceded CMV-specific CD8+ T-cell responses, the CMV-specific CD4+ T-cell response was delayed and only detectable after antiviral therapy in symptomatic individuals [67]. In more recent studies in D+ R− renal and liver transplant cohorts, dysregulated T-cell immune-phenotypes and cytokine responses were found to be associated with late CMV disease [68–72]. In a study from our group, we longitudinally monitored the negative immune-modulator PD-1 receptor on both CMV-specific CD4+ and CD8+ T cells in a liver transplant cohort. D+ R− patients who developed CMV disease expressed elevated levels of PD-1 on CMV-specific T cells (Figure 3) [68,70]. Upregulation of inhibitory molecules, such as PD-1 receptor, has been reported to impair T-cell immunity to persistent viruses [22,73–75]. Further studies will be required to assess whether PD-1 upregulation may affect other virus-specific T cells in individuals who progress to CMV disease. Additionally, in the plasma of liver and renal D+ R− patients diagnosed with CMV disease, increased levels of the immunosuppressive cytokine IL-10 were consistently detected (Figure 4) [68,71]. CMV-specific T cells were still functional when both PD-1 and IL-10 were upregulated; however, they showed a marked proliferation deficit, which may limit their ability to control viremia and clinically symptomatic CMV. Characterizing the post-transplant time course of immunosuppressive factors can lay the groundwork for a strategy to block negative immune modulators, restoring protective CMV immunity in immunocompromised SOT recipients [68,70].
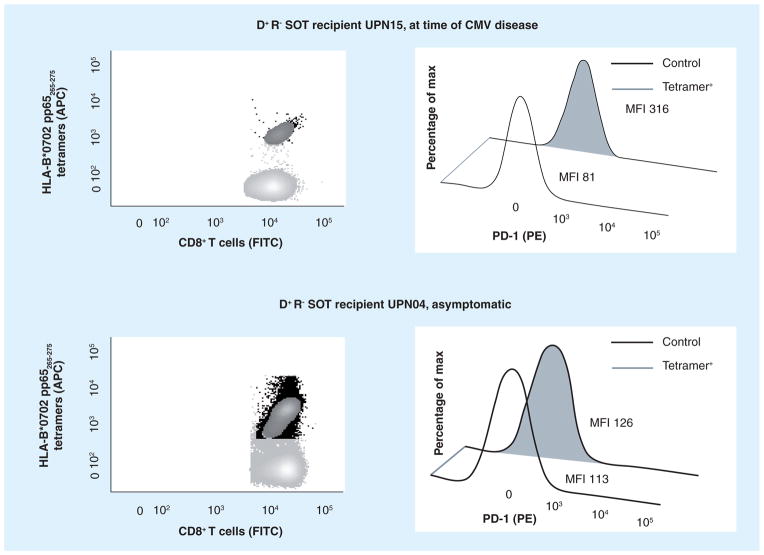
Figure 3. Primary CMV infection during immune suppression.
Data shown in FACS analysis panels are representative of HLA B*0702 R− receiving a SOT (orthotopic liver transplant) from a D+. These patients are prophylactically treated with antivirals, but are at high risk of developing life-threatening late CMV disease upon antiviral discontinuation. The upper panels report data from a CMV viremic D+ R− patient at time of CMV disease, and the lower panels are relative to a CMV viremic and asymptomatic D+ R− patient. A very high percentage of CD8+ T cells specific for MHC class I pp65265–275 tetramer (black dots, see Figure 1) was detected in both viremic recipients. However, in the case of the D+ R− patient with CMV disease, there are significantly higher levels of the negative immune-modulator PD-1 receptor on CMV-specific CD8+ T cells (gray filled curve, obtained as detailed in [70]). Importantly, PD-1 levels measured on CMV-specific T cells in healthy individuals were significantly lower than levels detected in the D+ R− cohort, exclusively when these patients were symptomatic for CMV disease [70]. Upregulation of PD-1 receptor impairs T-cell ability to contain viremia and can lead to CMV disease [68,70]. Blocking negative immune modulators may restore protective CMV immunity in SOT recipients [68,70] [La Rosa et al., Unpublished Data].
D+: CMV-seropositive donor; Max: Maximum; R−: CMV-naive recipient; SOT: Solid organ transplant.
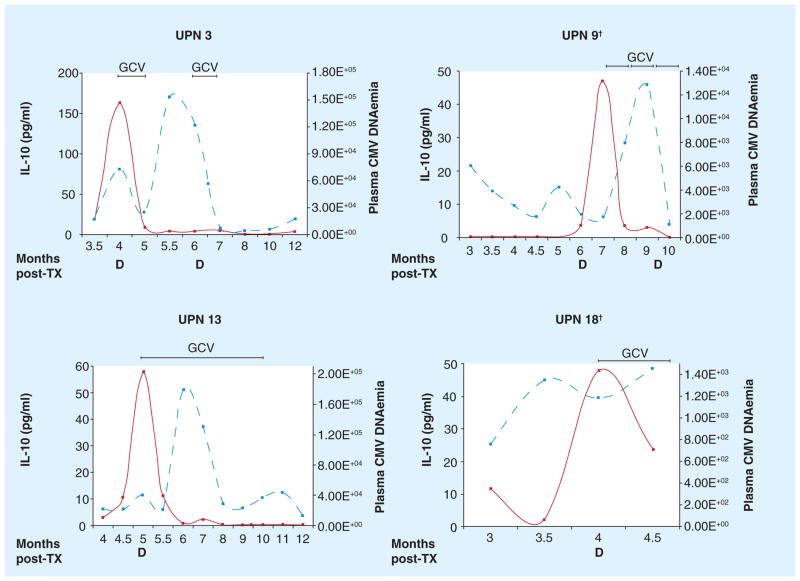
Figure 4. Immune dysregulation in solid organ transplant patients.
The plots represent data from the same cohort of patients described in Figure 3. All four patients shown progressed to CMV disease at the post-TX time point indicated on the x-axis. The left y-axes indicates the levels of IL-10 (pg/ml, solid line) measured in plasma, the right y-axes, the plasma DNAemia (copies/ml, dashed line) [68]. Very high levels of the immunosuppressive cytokine IL-10 were consistently detected in the plasma of liver recipients diagnosed with CMV disease [68,71]. IL-10 upregulation inhibits CMV-specific T-cell proliferation, which can lead to uncontrolled viremia and symptomatic complications [La Rosa et al., Unpublished Data].
†Patients died at 10 (UPN 9) and 4.5 (UPN 18) months post-TX.
D: CMV disease; GCV: Antiviral ganciclovir; TX: Transplant.
Harnessing the host immune response
The attractiveness of harnessing the extensive immune response to CMV in healthy CMV seropositive patients has been the focus of major investigations and clinical trials in the last 30 years. A number of approaches to stimulate the host immune response to CMV have been evaluated, though no vaccine/immunotherapeutic strategy to prevent or treat CMV infection or disease has yet been licensed [13,14]. The development of an anti-CMV vaccine remains a challenge, and the various immunogens formulated in the past three decades (reviewed in [13]) showed limited efficacy compared with the success in controlling CMV disease after HCT of the adoptive immunotherapy pilot trials (reviewed in [14]).
Vaccine
On the basis of the lifetime cost to the health care system and the impact of the virus on human suffering, the development of an effective prophylactic vaccine to prevent CMV symptomatic congenital disease and/or to prevent disease in immunocompromised individuals is a high priority and would be a highly cost-effective measure [13]. A successful vaccine strategy should ultimately aim to stimulate the innate and adaptive immune responses at the appropriate time. It is important to note that a vaccine approach to prevent congenital CMV disease and CMV-associated pathogenesis in transplant patients might be distinct, both humoral and cell-mediated immune responses might be necessary to prevent congenital disease, whereas cellular immune response alone might be sufficient to prevent virus-associated complications in transplant patients. In this perspective, as our knowledge of the immune response to CMV has progressed, various strategies have been developed, though a vaccine against CMV remains elusive. CMV vaccines has been obtained using attenuated or chimeric viruses, dense bodies, recombinant proteins, DNA, peptides and/or viral vectors (poxvirus/adenovirus) [13]. A number of subunit CMV vaccines tested in clinical trials targeted the abundant pp65 tegument protein, a major contributor to shaping the T-cell repertoire in CMV-exposed individuals [3]. CMV-infected cells express pp65 both early and late after infection, making it an appropriate vaccine target [76–78]. Importantly, it has been reported that T cells specific for CMV-pp65, a principal target for CTLs, can protect HCT recipients from CMV complications [3,63,76,78–81].
Our group has successfully completed a Phase Ib study in healthy volunteers [77], using two candidate CMV peptide vaccines composed of the HLA A*0201 pp65495–503 cytotoxic CD8+ T-cell epitope fused to two different universal T-helper epitopes (either the synthetic PADRE or a natural Tetanus sequence) [77,82]. HLA-restricted peptide epitopes are an untested option to develop a safe, noninfectious subunit CMV vaccine, avoiding CMV immune evasion mechanisms [3]. Results from this clinical study showed that the CMV peptide immunogens had acceptable safety profiles and elicited vaccine-driven expansion of pp65 T cells (Figure 5) [77]. We are currently planning a Phase II trial in HLA A*0201 CMV-positive allogeneic HCT recipients to evaluate the safety and efficacy of the peptide vaccine in protecting HCT recipients from CMV complications [63,80,83].
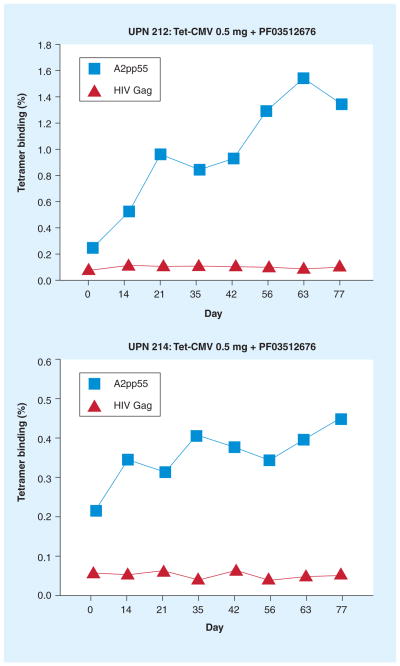
Figure 5. Immunogenicity of a CMV peptide vaccine.
The upper and lower plot show vaccine-induced expansion of pp65-specific T cells in two representative HLA A*0201 healthy adults (UPN 212 and 214) immunized with 0.5 mg Tet-CMV, a peptide vaccine developed by DJ Diamond, at the Division of Translational Vaccine Research (City of Hope, CA, USA). The CMV peptide vaccine consists of the HLA A*0201-specific CTL pp65495–503 epitope fused with the tetanus toxin P2 epitope (tt830–843), in the presence of PF03512676 adjuvant [71]. In this Phase Ib clinical trial, which was performed at City of Hope, healthy volunteers were vaccinated by subcutaneous injection, every 3 weeks for a total of four injections (days 0, 21, 42 and 63). Levels of CD8+ T cells in peripheral blood mononuclear cells of UPN 212 and 214 binding either to MHC class I pp65495–503 tetramer (in each plot, square symbol) or to a control HIVgag77–85 tetramer (in each plot, triangle symbol) are indicated on the y-axes (as percentages) [La Rosa et al., Unpublished Data].
Though pharmacologic agents, such as acyclovir, ganciclovir (GCV) or its oral form valganciclovir (VAL), and foscarnet, limit virus replication, CMV remains an important cause of mortality after HCT [84]. Furthermore, there are side effects of antiviral chemotherapy that affect its use in the HCT patient. For example, the use of GCV/VAL is associated with a higher proportion of recipients becoming neutropenic and increased numbers of concomitant fatal bacterial and fungal infections [62,84]. As GCV/VAL therapy has become ubiquitous in practice, delayed onset of CMV-pneumonia is more frequent, which suggests that GCV/VAL impairs immunologic reconstitution [10]. When antivirals are stopped or when virus resistance occurs, the same disease symptoms appear, only shifted to approximately 180 days post-HCT [63]. Thus, antivirals are at best a stop-gap measure, and their use does not address the major risks of late-onset CMV pneumonia, including early CMV reactivation and failure to reconstitute CMV-specific immunity [84]. In this context, our final goal is to develop a CMV vaccine that confers protective immunity early post-transplantation, until normal immunocompetence is re-established in the HCT recipient (6 months or earlier post-HCT), to reduce CMV morbidity and the use of antivirals [13].
Immunotherapy
A direct correlation between levels of CMV-specific CTL responses and improved control of CMV viremia has been documented in immuno-compromised HCT patients over the past several years [85,86]. Derived from these observations, the strategy of an adoptive transfer of CMV-specific T cells has been developed. Infusion of donor-derived CMV-specific CD8+ cytotoxic T-cell clones or cell lines can successfully transfer protective immunity [12,14,87]. Moreover, a number of investigations have indicated the importance of antiviral effector functions of T-helper cells in maintaining CTL responses after adoptive transfer, though few studies have actually tested this idea [12,88]. A broad variety of clinical protocols for CMV-specific immunotherapy have been published, which vary regarding the isolation procedure, composition of cellular product, number of transferred cells and treatment efficacy [14]. A number of relatively small Phase I/II studies have demonstrated the feasibility of transferring CMV-specific T cells [12,14,79,89]. Though presenting considerable technical challenges, they have all indicated that restoration of CMV-specific immunity can be accelerated without impacting graft-versus-host disease in those with CMV-seropositive donors. They also suggested that recovery of CMV-specific immunity correlates with clinical protection. These promising results confirm that cellular immunotherapy can accelerate long-term reconstitution of antiviral immunity in allogeneic transplant recipients, indicating that significant clinical benefits (such as reduction of secondary viral infection episodes) may be conferred, potentially reducing exposure to antiviral toxicities. In summary, cellular immunotherapy appears to be a fruitful area for investigation; however, randomized studies are strongly needed to establish the true efficacy of these promising strategies [14].
Future perspective
In the last decade, considerable progress has been made in our understanding of the immunobiology of CMV, and in the diagnosis and treatment of CMV disease, providing an excellent platform in the development of protective immune strategies. Nonetheless, CMV remains an unmet health problem of clinical relevance for a substantial portion of the human population. Future studies would need to focus on dissecting the humoral and cellular immune response to the whole viral antigenic repertoire, in order to finally identify CMV-protective antigens and to acquire in-depth understanding of the immune mechanisms leading to the control of this persistent virus. Additionally, investigations related to elucidating the immune correlate of protection are going to be essential to improve treatment and design novel immune therapeutics. In this perspective, further insight into the phenotypes, dynamics and mechanism(s) leading to the unique memory T-cell inflation, and into the role of the ‘vigilant resting effectors’ in long-term protection and age-related diseases would need to play a major role. The mechanism by which CMV may cause or exacerbate graft rejection in the transplant setting also needs to be investigated. Examination into immune evasion mechanisms needs to be augmented, since viral immune modulators (such as, vIL-10) could ultimately be exploited to combat CMV. Finally, enhanced understanding of the interplay between innate and adaptive immunity will be instrumental to elucidate the factors inducing CMV latency.
Executive summary.
Human CMV
Large, ubiquitous, double-stranded linear DNA, β-herpesvirus.
Elicits very strong immunity, which is effective in controlling viral replication.
Employs multiple immune-evasion strategies.
Homeostatic host–virus balance is reached in healthy individuals.
Lifelong latency is established.
Rarely symptomatic in healthy subjects.
Severe complications in immune-incompetent individuals.
First line of defense: innate immunity
Innate detection by Toll-like receptor 2.
Cytokines, type I IFN, upregulation of costimulatory molecules, NK cells.
Infection control: CMV-specific response
Neutralizing antibodies to multiple CMV proteins: inhibit viral dissemination.
Very large, broad and heterogeneous T-cell response: control of viremia.
Predominant production of Th1 type cytokines.
Induced latency.
Memory T cells continue to inflate, still cytolytic, though terminally differentiated.
CMV pathogenesis
May induce a chronic inflammation leading to atherosclerosis and age-related diseases.
In utero infection is usually severe, causing neurological damage.
High morbidity and mortality in immunosuppressed individuals.
Involved in graft rejection.
Immune therapeutic approaches
Vaccine: none licensed, various ongoing Phase I/II clinical trials.
Adoptive transfer of CMV-specific T cells: promising pilot studies, no randomized trials yet.
Future perspective
Protective antigens need to be identified and included in vaccine/immunotherapy strategies.
Immune correlates of protection are likely to be discovered.
More studies on immune evasion are needed to exploit viral immune modulators.
Acknowledgments
The administrative assistance of P Kwon and D Packer is gratefully acknowledged.
Footnotes
Financial & competing interests disclosure
DJ Diamond receives royalties from Beckman-Coulter and the Mayo Clinic. DJ Diamond and C La Rosa have been supported by grants from the National Cancer Institute (NCI; R01-CA77544 and P01-CA30206, Project III) and from the National Institute of Allergy and Infectious Diseases (R21 AI084019), respectively. The Division of Translational Vaccine Research at the City of Hope National Medical Center has been partly supported by The Edwin and Bea Wolfe Charitable Foundation. City of Hope Cancer Center is supported by P30 CA033572 from the NCI. MO1-RR0043-38 from the NCRR to COH General Clinical Research Center partially supported the CMV peptide clinical trial. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪ ▪ of considerable interest
- 1.Cannon MJ, Schmid DS, Hyde TB. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol. 2010;20(4):202–213. doi: 10.1002/rmv.655. [DOI] [PubMed] [Google Scholar]
- 2.Dolan A, Cunningham C, Hector RD, et al. Genetic content of wild-type human cytomegalovirus. J Gen Virol. 2004;85(Pt 5):1301–1312. doi: 10.1099/vir.0.79888-0. [DOI] [PubMed] [Google Scholar]
- 3▪▪.Sylwester AW, Mitchell BL, Edgar JB, et al. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202(5):673–685. doi: 10.1084/jem.20050882. The most comprehensive study in healthy individuals, which analyzed the broad and heterogeneous CMV-specific cellular immune response. These data provide the first insight into the rules governing immunodominance and cross-reactivity in complex human CMV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller-Kittrell M, Sparer TE. Feeling manipulated: cytomegalovirus immune manipulation. Virol J. 2009;6:4. doi: 10.1186/1743-422X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poole E, McGregor D, Sr, Colston J, Joseph RS, Sinclair J. Virally induced changes in cellular microRNAs maintain latency of human cytomegalovirus in CD34 progenitors. J Gen Virol. 2011;92(Pt 7):1539–1549. doi: 10.1099/vir.0.031377-0. [DOI] [PubMed] [Google Scholar]
- 6.Pawelec G, Akbar A, Beverley P, et al. Immunosenescence and cytomegalovirus: where do we stand after a decade? Immun Ageing. 2010;7:13. doi: 10.1186/1742-4933-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Limaye AP, Bakthavatsalam R, Kim HW, et al. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation. 2006;81(12):1645–1652. doi: 10.1097/01.tp.0000226071.12562.1a. [DOI] [PubMed] [Google Scholar]
- 8.Steininger C. Clinical relevance of cytomegalovirus infection in patients with disorders of the immune system. Clin Microbiol Infect. 2007;13(10):953–963. doi: 10.1111/j.1469-0691.2007.01781.x. [DOI] [PubMed] [Google Scholar]
- 9.Limaye AP, Kirby KA, Rubenfeld GD, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300(4):413–422. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffiths P, Whitley R, Snydman DR, Singh N, Boeckh M. Contemporary management of cytomegalovirus infection in transplant recipients: guidelines from an IHMF workshop, 2007. Herpes. 2008;15(1):4–12. [PubMed] [Google Scholar]
- 11.Revello MG, Zavattoni M, Furione M, Fabbri E, Gerna G. Preconceptional primary human cytomegalovirus infection and risk of congenital infection. J Infect Dis. 2006;193(6):783–787. doi: 10.1086/500509. [DOI] [PubMed] [Google Scholar]
- 12.Feuchtinger T, Opherk K, Bethge WA, et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood. 2010;116(20):4360–4367. doi: 10.1182/blood-2010-01-262089. [DOI] [PubMed] [Google Scholar]
- 13.Khanna R, Diamond DJ. Human cytomegalovirus vaccine: time to look for alternative options. Trends Mol Med. 2006;12(1):26–33. doi: 10.1016/j.molmed.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Peggs KS. Adoptive T-cell immunotherapy for cytomegalovirus. Expert Opin Biol Ther. 2009;9(6):725–736. doi: 10.1517/14712590902967588. [DOI] [PubMed] [Google Scholar]
- 15.Scrivano L, Sinzger C, Nitschko H, Koszinowski UH, Adler B. HCMV spread and cell tropism are determined by distinct virus populations. PLoS Pathog. 2011;7(1):E1001256. doi: 10.1371/journal.ppat.1001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinzger C, Digel M, Jahn G. Cytomegalovirus cell tropism. Curr Top Microbiol Immunol. 2008;325:63–83. doi: 10.1007/978-3-540-77349-8_4. [DOI] [PubMed] [Google Scholar]
- 17.Cheung AK, Abendroth A, Cunningham AL, Slobedman B. Viral gene expression during the establishment of human cytomegalovirus latent infection in myeloid progenitor cells. Blood. 2006;108(12):3691–3699. doi: 10.1182/blood-2005-12-026682. [DOI] [PubMed] [Google Scholar]
- 18.Goodrum F, Jordan CT, Terhune SS, High K, Shenk T. Differential outcomes of human cytomegalovirus infection in primitive hematopoietic cell subpopulations. Blood. 2004;104(3):687–695. doi: 10.1182/blood-2003-12-4344. [DOI] [PubMed] [Google Scholar]
- 19.Tey SK, Goodrum F, Khanna R. CD8+ T-cell recognition of human cytomegalovirus latency-associated determinant pUL138. J Gen Virol. 2010;91(Pt 8):2040–2048. doi: 10.1099/vir.0.020982-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isaacson MK, Juckem LK, Compton T. Virus entry and innate immune activation. Curr Top Microbiol Immunol. 2008;325:85–100. doi: 10.1007/978-3-540-77349-8_5. [DOI] [PubMed] [Google Scholar]
- 21.Gibson L, Dooley S, Trzmielina S, et al. Cytomegalovirus (CMV) IE1- and pp65-specific CD8+ T cell responses broaden over time after primary CMV infection in infants. J Infect Dis. 2007;195(12):1789–1798. doi: 10.1086/518042. [DOI] [PubMed] [Google Scholar]
- 22.Petrovas C, Casazza JP, Brenchley JM, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203(10):2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23▪.Boehme KW, Guerrero M, Compton T. Human cytomegalovirus envelope glycoproteins B and H are necessary for TLR2 activation in permissive cells. J Immunol. 2006;177(10):7094–7102. doi: 10.4049/jimmunol.177.10.7094. Study from the Compton group, who identified Toll-like receptor (TLR)2 as a host factor activating innate inflammatory cytokine secretion in response to CMV. Here, they define the molecular trigger for TLR2: gB and gH activate TLR2 and associate with TLR1 and TLR2. They also indicate that the functional immune sensor for CMV is a TLR2/TLR1 heterodimer. [DOI] [PubMed] [Google Scholar]
- 24.Moretta A, Marcenaro E, Parolini S, Ferlazzo G, Moretta L. NK cells at the interface between innate and adaptive immunity. Cell Death Differ. 2008;15(2):226–233. doi: 10.1038/sj.cdd.4402170. [DOI] [PubMed] [Google Scholar]
- 25.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320(26):1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 26.Vivier E, Raulet DH, Moretta A, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331(6013):44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crough T, Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev. 2009;22(1):76–98. doi: 10.1128/CMR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanier LL. Evolutionary struggles between NK cells and viruses. Nat Rev Immunol. 2008;8(4):259–268. doi: 10.1038/nri2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaia JA, Sun JY, Gallez-Hawkins GM, et al. The effect of single and combined activating killer immunoglobulin-like receptor genotypes on cytomegalovirus infection and immunity after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15(3):315–325. doi: 10.1016/j.bbmt.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cook M, Briggs D, Craddock C, et al. Donor KIR genotype has a major influence on the rate of cytomegalovirus reactivation following T-cell replete stem cell transplantation. Blood. 2006;107(3):1230–1232. doi: 10.1182/blood-2005-03-1039. [DOI] [PubMed] [Google Scholar]
- 31.Gallez-Hawkins GM, Franck AE, Li X, et al. Expression of activating KIR2DS2 and KIR2DS4 genes after hematopoietic cell transplantation: relevance to cytomegalovirus infection. Biol Blood Marrow Transplant. 2011;17(11):1662–1672. doi: 10.1016/j.bbmt.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gazit R, Garty BZ, Monselise Y, et al. Expression of KIR2DL1 on the entire NK cell population: a possible novel immunodeficiency syndrome. Blood. 2004;103(5):1965–1966. doi: 10.1182/blood-2003-11-3796. [DOI] [PubMed] [Google Scholar]
- 33.Wilkinson GW, Tomasec P, Stanton RJ, et al. Modulation of natural killer cells by human cytomegalovirus. J Clin Virol. 2008;41(3):206–212. doi: 10.1016/j.jcv.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson SE, Mason GM, Wills MR. Human cytomegalovirus immunity and immune evasion. Virus Res. 2011;157(2):151–160. doi: 10.1016/j.virusres.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 35.van Stijn A, Rowshani AT, Yong SL, et al. Human cytomegalovirus infection induces a rapid and sustained change in the expression of NK cell receptors on CD8+ T cells. J Immunol. 2008;180(7):4550–4560. doi: 10.4049/jimmunol.180.7.4550. [DOI] [PubMed] [Google Scholar]
- 36▪.Chang WL, Barry PA. Attenuation of innate immunity by cytomegalovirus IL-10 establishes a long-term deficit of adaptive antiviral immunity. Proc Natl Acad Sci USA. 2010;107(52):22647–22652. doi: 10.1073/pnas.1013794108. Focuses on viral IL-10 in rhesus macaque CMV infection, as a model for human CMV. The investigators provide evidence and mechanisms on how CMV utilizes viral IL-10 to subvert both innate and adaptive immune responses to viral challenge. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahlqvist J, Mocarski E. Cytomegalovirus UL103 controls virion and dense body egress. J Virol. 2011;85(10):5125–5135. doi: 10.1128/JVI.01682-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van de Berg PJ, Heutinck KM, Raabe R, et al. Human cytomegalovirus induces systemic immune activation characterized by a type 1 cytokine signature. J Infect Dis. 2010;202(5):690–699. doi: 10.1086/655472. [DOI] [PubMed] [Google Scholar]
- 39▪▪.Macagno A, Bernasconi NL, Vanzetta F, et al. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128–131A complex. J Virol. 2010;84(2):1005–1013. doi: 10.1128/JVI.01809-09. Results from this investigation have changed our knowledge of CMV humoral response. Previously unknown and unusually potent neutralizing antibodies against conformational epitopes of CMV are described. This study provides input for the design of a novel vaccine capable of inducing potent and broadly reactive neutralizing antibodies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40▪.van de Berg PJ, van Stijn A, ten Berge IJ, van Lier RA. A fingerprint left by cytomegalovirus infection in the human T cell compartment. J Clin Virol. 2008;41(3):213–217. doi: 10.1016/j.jcv.2007.10.016. Authors demonstrate that CMV infection induces a systemic inflammatory response with a clear type 1 cytokine signature during both primary infection and latency. This inflammatory state may be involved in the pathogenesis of chronic allograft rejection and may potentially contribute to the acceleration of chronic diseases. [DOI] [PubMed] [Google Scholar]
- 41.Gerna G, Sarasini A, Patrone M, et al. Human cytomegalovirus serum neutralizing antibodies block virus infection of endothelial/epithelial cells, but not fibroblasts, early during primary infection. J Gen Virol. 2008;89(Pt 4):853–865. doi: 10.1099/vir.0.83523-0. [DOI] [PubMed] [Google Scholar]
- 42.Wang D, Shenk T. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci USA. 2005;102(50):18153–18158. doi: 10.1073/pnas.0509201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Northfield J, Lucas M, Jones H, Young NT, Klenerman P. Does memory improve with age? CD85j (ILT-2/LIR-1) expression on CD8 T cells correlates with ‘memory inflation’ in human cytomegalovirus infection. Immunol Cell Biol. 2005;83(2):182–188. doi: 10.1111/j.1440-1711.2005.01321.x. [DOI] [PubMed] [Google Scholar]
- 44.Hertoghs KM, Moerland PD, van Stijn A, et al. Molecular profiling of cytomegalovirus-induced human CD8+ T cell differentiation. J Clin Invest. 2010;120(11):4077–4090. doi: 10.1172/JCI42758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lacey SF, Martinez J, Gallez-Hawkins G, et al. Simultaneous reconstitution of multiple cytomegalovirus-specific CD8+ cell populations with divergent functionality in hematopoietic stem-cell transplant recipients. J Infect Dis. 2005;191(6):977–984. doi: 10.1086/428136. [DOI] [PubMed] [Google Scholar]
- 46.Bunde T, Kirchner A, Hoffmeister B, et al. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J Exp Med. 2005;201(7):1031–1036. doi: 10.1084/jem.20042384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Z, Zhou W, Srivastava T, et al. A fusion protein of HCMV IE1 exon4 and IE2 exon5 stimulates potent cellular immunity in an MVA vaccine vector. Virology. 2008;377(2):379–390. doi: 10.1016/j.virol.2008.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jenkins C, Garcia W, Godwin MJ, et al. Immunomodulatory properties of a viral homolog of human interleukin-10 expressed by human cytomegalovirus during the latent phase of infection. J Virol. 2008;82(7):3736–3750. doi: 10.1128/JVI.02173-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49▪.van Leeuwen EM, Remmerswaal EB, Vossen MT, et al. Emergence of a CD4+ J Immunol. 2004;173(3):1834–1841. doi: 10.4049/jimmunol.173.3.1834. Elegantly describes how CMV infection induces acute and lasting changes in virus-reactive T cells. The authors conclude that persistent effector cell traits, together with the permanent changes in chemokine receptor usage of virus-specific, nonexhausted, long-lived CD8+ T cells, may be crucial in maintaining lifelong protection from CMV reactivation. [DOI] [PubMed] [Google Scholar]
- 50.van Leeuwen EM, ten Berge IJ, van Lier RA. Induction and maintenance of CD8+ T cells specific for persistent viruses. Adv Exp Med Biol. 2007;590:121–137. doi: 10.1007/978-0-387-34814-8_9. [DOI] [PubMed] [Google Scholar]
- 51.Gillespie GM, Wills MR, Appay V, et al. Functional heterogeneity and high frequencies of cytomegalovirus- specific CD8(+) T lymphocytes in healthy seropositive donors. J Virol. 2000;74(17):8140–8150. doi: 10.1128/jvi.74.17.8140-8150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Leeuwen EM, De Bree GJ, Remmerswaal EB, et al. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells. Blood. 2005;106(6):2091–2098. doi: 10.1182/blood-2005-02-0449. [DOI] [PubMed] [Google Scholar]
- 53.van Leeuwen EM, Remmerswaal EB, Heemskerk MH, ten Berge IJ, van Lier RA. Strong selection of virus-specific cytotoxic CD4+ T-cell clones during primary human cytomegalovirus infection. Blood. 2006;108(9):3121–3127. doi: 10.1182/blood-2006-03-006809. [DOI] [PubMed] [Google Scholar]
- 54.van Lier RA, ten Berge IJ, Gamadia LE. Human CD8(+) T-cell differentiation in response to viruses. Nat Rev Immunol. 2003;3(12):931–939. doi: 10.1038/nri1254. [DOI] [PubMed] [Google Scholar]
- 55.Hansson GK. Inflammatory mechanisms in atherosclerosis. J Thromb Haemost. 2009;7(Suppl 1):328–331. doi: 10.1111/j.1538-7836.2009.03416.x. [DOI] [PubMed] [Google Scholar]
- 56.Streblow DN, Dumortier J, Moses AV, Orloff SL, Nelson JA. Mechanisms of cytomegalovirus-accelerated vascular disease: induction of paracrine factors that promote angiogenesis and wound healing. Curr Top Microbiol Immunol. 2008;325:397–415. doi: 10.1007/978-3-540-77349-8_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6(7):508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 58.Brouilette SW, Moore JS, McMahon AD, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369(9556):107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 59.Pawelec G, Derhovanessian E. Role of CMV in immune senescence. Virus Res. 2011;157(2):175–179. doi: 10.1016/j.virusres.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 60.Blackman MA, Woodland DL. The narrowing of the CD8 T cell repertoire in old age. Curr Opin Immunol. 2011;23(4):537–542. doi: 10.1016/j.coi.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hassan J, Dooley S, Hall W. Immunological response to cytomegalovirus in congenitally infected neonates. Clin Exp Immunol. 2007;147(3):465–471. doi: 10.1111/j.1365-2249.2007.03302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hakki M, Riddell SR, Storek J, et al. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood. 2003;102(8):3060–3067. doi: 10.1182/blood-2002-11-3472. [DOI] [PubMed] [Google Scholar]
- 63▪▪.Gratama JW, Boeckh M, Nakamura R, et al. Immune monitoring with iTAg MHC Tetramers for prediction of recurrent or persistent cytomegalovirus infection or disease in allogeneic hematopoietic stem cell transplant recipients: a prospective multicenter study. Blood. 2010;116(10):1655–1662. doi: 10.1182/blood-2010-03-273508. Very important study in the context of CMV immune monitoring for allogeneic hematopoietic stem cell transplantation recipients. Data from this multicenter, prospective, longitudinal clinical trial indicate that CMV tetramer-based immune monitoring, in conjunction with virologic monitoring, is a crucial tool to assess risk of CMV-related complications and to guide preemptive therapeutic choices. [DOI] [PubMed] [Google Scholar]
- 64.Boeckh M, Nichols WG. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004;103(6):2003–2008. doi: 10.1182/blood-2003-10-3616. [DOI] [PubMed] [Google Scholar]
- 65.Zhou W, Longmate J, Lacey SF, et al. Impact of donor CMV status on viral infection and reconstitution of multifunction CMV-specific T cells in CMV-positive transplant recipients. Blood. 2009;113(25):6465–6476. doi: 10.1182/blood-2009-02-203307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Limaye AP, Bakthavatsalam R, Kim HW, et al. Late-onset cytomegalovirus disease in liver transplant recipients despite antiviral prophylaxis. Transplantation. 2004;78(9):1390–1396. doi: 10.1097/01.tp.0000145989.22373.03. [DOI] [PubMed] [Google Scholar]
- 67.Gamadia LE, Remmerswaal EB, Weel JF, Bemelman F, van Lier RA, ten Berge IJ. Primary immune responses to human CMV: a critical role for IFN-gamma -producing CD4+ T cells in protection against CMV disease. Blood. 2003;101(7):2686–2692. doi: 10.1182/blood-2002-08-2502. [DOI] [PubMed] [Google Scholar]
- 68.Krishnan A, Zhou W, Lacey SF, Limaye AP, Diamond DJ, La Rosa C. Programmed death-1 receptor and interleukin-10 in liver transplant recipients at high risk for late cytomegalovirus disease. Transpl Infect Dis. 2010;12(4):363–370. doi: 10.1111/j.1399-3062.2009.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sadeghi M, Daniel V, Naujokat C, et al. Dysregulated cytokine responses during cytomegalovirus infection in renal transplant recipients. Transplantation. 2008;86(2):275–285. doi: 10.1097/TP.0b013e31817b063d. [DOI] [PubMed] [Google Scholar]
- 70.La Rosa C, Krishnan A, Longmate J, et al. Programmed death-1 expression in liver transplant recipients as a prognostic indicator of cytomegalovirus disease. J Infect Dis. 2008;197(1):25–33. doi: 10.1086/523652. [DOI] [PubMed] [Google Scholar]
- 71.La Rosa C, Limaye AP, Krishnan A, Blumstein G, Longmate J, Diamond DJ. Primary response against cytomegalovirus during antiviral prophylaxis with valganciclovir, in solid organ transplant recipients. Transpl Int. 2011;24(9):920–931. doi: 10.1111/j.1432-2277.2011.01285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sester U, Presser D, Dirks J, Gartner BC, Kohler H, Sester M. PD-1 expression and IL-2 loss of cytomegalovirus- specific T cells correlates with viremia and reversible functional anergy. Am J Transplant. 2008;8(7):1486–1497. doi: 10.1111/j.1600-6143.2008.02279.x. [DOI] [PubMed] [Google Scholar]
- 73.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12(10):1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 74.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 75.Urbani S, Amadei B, Tola D, et al. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80(22):11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bernstein DI, Reap EA, Katen K, et al. Randomized, double-blind, Phase 1 trial of an alphavirus replicon vaccine for cytomegalovirus in CMV seronegative adult volunteers. Vaccine. 2009;28(2):484–493. doi: 10.1016/j.vaccine.2009.09.135. [DOI] [PubMed] [Google Scholar]
- 77.La Rosa C, Longmate J, Lacey S, et al. Clinical evaluation of safety and immunogenicity of PADRE-cytomegalovirus (CMV) and Tetanus-CMV fusion peptide vaccines with or without PF03512676 adjuvant. J Infect Dis. 2011 doi: 10.1093/infdis/jis107. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wloch MK, Smith LR, Boutsaboualoy S, et al. Safety and immunogenicity of a bivalent cytomegalovirus DNA vaccine in healthy adult subjects. J Infect Dis. 2008;197(12):1634–1642. doi: 10.1086/588385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Einsele H, Hebart H. CMV-specific immunotherapy. Hum Immunol. 2004;65(5):558–564. doi: 10.1016/j.humimm.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 80.Cobbold M, Khan N, Pourgheysari B, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005;202(3):379–386. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peggs KS, Mackinnon S. Immune reconstitution following haematopoietic stem cell transplantation. Br J Haematol. 2004;124(4):407–420. doi: 10.1046/j.1365-2141.2003.04767.x. [DOI] [PubMed] [Google Scholar]
- 82.La Rosa C, Wang Z, Brewer JC, et al. Preclinical development of an adjuvant-free peptide vaccine with activity against CMV pp65 in HLA transgenic mice. Blood. 2002;100(10):3681–3689. doi: 10.1182/blood-2002-03-0926. [DOI] [PubMed] [Google Scholar]
- 83.Rolland A. TransVax™, a therapeutic DNA vaccine for control of cytomegalovirus in hematopoietic cell transplant recipients Results of a Phase 2 Clinical Trial. Presented at: ASGCT 14th Annual Meeting.; Seattle, WA, USA. 18–21 May (2011). [Google Scholar]
- 84.Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Infect Dis Clin North Am. 2010;24(2):319–337. doi: 10.1016/j.idc.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 85.Reusser P, Riddell SR, Meyers JD, Greenberg PD. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78(5):1373–1380. [PubMed] [Google Scholar]
- 86.Li CR, Greenberg PD, Gilbert MJ, Goodrich JM, Riddell SR. Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood. 1994;83:1971–1979. [PubMed] [Google Scholar]
- 87.Riddell SR, Walter BA, Gilbert MJ, Greenberg PD. Selective reconstitution of CD8+ cytotoxic T lymphocyte responses in immunodeficient bone marrow transplant recipients by the adoptive transfer of T cell clones. Bone Marrow Transplant. 1994;14(Suppl 4):S78–S84. [PubMed] [Google Scholar]
- 88.Einsele H. Immunotherapy for CMV infection. Cytotherapy. 2002;4(5):435–436. doi: 10.1080/146532402320776080. [DOI] [PubMed] [Google Scholar]
- 89.Hill GR, Tey SK, Beagley L, et al. Successful immunotherapy of HCMV disease using virus-specific T cells expanded from an allogeneic stem cell transplant recipient. Am J Transplant. 2010;10(1):173–179. doi: 10.1111/j.1600-6143.2009.02872.x. [DOI] [PubMed] [Google Scholar]