Summary
Background
Nevirapine given once-daily for the first 6, 14, or 28 weeks of life to infants exposed to HIV-1via breastfeeding reduces transmission through this route compared with single-dose nevirapine at birth or neonatally. We aimed to assess incremental safety and efficacy of extension of such prophylaxis to 6 months.
Methods
In our phase 3, randomised, double-blind, placebo-controlled HPTN 046 trial, we assessed the incremental benefit of extension of once-daily infant nevirapine from age 6 weeks to 6 months. We enrolled breastfeeding infants born to mothers with HIV-1 in four African countries within 7 days of birth. Following receipt of nevirapine from birth to 6 weeks, infants without HIV infection were randomly allocated (by use of a computer-generated permuted block algorithm with random block sizes and stratified by site and maternal antiretroviral treatment status) to receive extended nevirapine prophylaxis or placebo until 6 months or until breastfeeding cessation, whichever came first. The primaryefficacy endpoint was HIV-1 infection in infants at 6 months and safety endpoints were adverse reactions in both groups. We used Kaplan-Meier analyses to compare differences in the primary outcome between groups. This study is registered with ClinicalTrials.gov, number NCT00074412.
Findings
Between June 19, 2008, and March 12, 2010, we randomly allocated 1527 infants (762 nevirapine and 765 placebo); five of whom had HIV-1 infection at randomisation and were excluded from the primary analyses. In Kaplan-Meier analysis, 1.1% (95% CI 0.3–1.8) of infants who received extended nevirapine developed HIV-1 between 6 weeks and 6 months compared with 2.4% (1.3–3.6) of controls (difference 1.3%, 95% CI 0–2.6), equating to a 54% reduction in transmission (p=0.049). However, mortality (1.2% for nevirapine vs 1.1% for placebo; p=0.81) and combined HIV infection and mortality rates (2.3% vs 3.2%; p=0.27) did not differ between groups at 6 months. 125 (16%) of 758 infants given extended nevirapine and 116 (15%) of 761 controls had serious adverse events, but frequency of adverse events, serious adverse events, and deaths did not differ significantly between treatment groups.
Interpretation
Nevirapine prophylaxis can safely be used to provide protection from mother-to-child transmission of HIV-1 via breastfeeding for infants up to 6 months of age.
Funding
US National Institutes of Health.
Introduction
Breastfeeding in sub-Saharan Africa provides vital nutrition for infants between birth and 24 months of age and provides protection against respiratory and diarrhoeal illnesses, leading to improved overall survival. However, breastfeeding up to 24 months of age in infants exposed to HIV can account for 30–40% of all mother-to-child transmission.1,2 Therefore, although prophylactic ante-partum and intrapartum antiretroviral regimens can substantially reduce in utero and intrapartum mother-to-child transmission, these substantial gains are lost in breastfeeding populations in whom infants have continued exposure to HIV-1 from breast milk.3,4
Extended regimens of 6, 14, and 28 weeks' nevirapine are effective in infants for reduction of postnatal HIV-1 transmission, as is 6 months of postnatal triple-drug prophylaxis for mothers.5–8 However, strategies for prevention of mother-to-child transmission (PMTCT) require extensive, coordinated services and postnatal preventionregimens can be costly. Moreover, in many settings infants exposed to HIV-1 are lost to follow-up after the first postpartum visit.9 Consequently, postnatal prophylactic antiretroviral regimens for PMTCT have been difficult to implement in many low-income settings, despite WHO guidelines recommending extended breastfeeding for infants exposed to HIV-1 until the age of 12 months together with antiretroviral prophylaxis for the mother or infant for the duration of breastfeeding.10,11
Implementation of the complete cascade of PMTCT services, including routine HIV screening of pregnant women, provision of antiretroviral interventions during pregnancy, labour, and delivery, and during breastfeeding, and linkage to long-term care and treatment services for HIV infected mothers in low-income settings is crucial to substantially reduce global perinatal HIV-1 infection.12 Implicit in such efforts is an assessment of the absolute effectiveness of every step of the PMTCT cascade, including antiretroviral inter ventions during breastfeeding. The duration of published infant prophylaxis regimens ranges from several weeks to several months, and the infrastructure investments needed for every regimen can vary widely, which might affect the feasibility of provision of these interventions. We aimed to assess the incremental efficacy and safety of extension of once-daily nevirapine until the age of 6 months in African infants exposed to HIV-1 during breastfeeding who had already received 6 weeks of once-daily nevirapine.
Methods
Study design and participants
HPTN 046 was a phase 3, randomised, double-blind, placebo-controlled trial that assessed the efficacy and safety of extension of once-daily nevirapine to 6 months of age or until cessation of breastfeeding (whichever came first) for prevention of transmission in HIV-1-exposed breastfeeding infants who had received nevirapine prophylaxis until the age of 6 weeks.
Women were recruited from antenatal clinics in Durban in South Africa, Dar es Salaam in Tanzania, Kampala in Uganda, and Chitungwiza in Zimbabwe, where HIV-1 testing, counselling, and the local standard of care antiretroviral regimen for PMTCT were provided. All women provided written informed consent. Eligible women and infants were enrolled within 7 days of delivery. Primary eligibility criteria included maternal age (≥18 years), infant HIV-1 DNA PCR negative from a specimen obtained within 7 days of birth, and birthweight of 2000 g or more. We excluded women and infants if either had a serious medical disorder that would interfere with study participation. Women receiving antiretroviral drugs for HIV-1 treatment or for PMTCT were eligible. Infant study visits were undertaken within 7 days postpartum, at 2, 5, 6, and 8 weeks, and at 3, 6, 9, 12, and 18 months. Infants who developed HIV-1 infections were taken off study drug and referred for additional care and treatment.
The study protocol was approved by at least one local ethics review committee affiliated with every study site, by committees affiliated with US collaborating institutions, and by other local regulatory bodies where applicable.
Randomisation and masking
All enrolled infants received once-daily open-label nevirapine (10 mg/mL oral suspension) for the first 6 weeks of life. After such treatment (at age 6–8 weeks), we randomly allocated eligible infants in a one-to-one ratio to receive extended nevirapine (treatment group) or placebo (control group) according to computer-generated permutated block algorithms by site with random block sizes. Because maternal receipt of anti retroviral drugs can affect postnatal transmission, infants were stratified by maternal antiretroviral treatment status at randomisation.8,13 Infants had to be HIV-1 DNA PCR negative on a specimen obtained within 21 days before randomisation and still breastfeeding, with the mother's intent to continue breastfeeding. An independent contractor in the USA provided identical, sealed, individual study drug kits, which were prepared and labelled centrally according to the random allocation assignment generated by the HPTN Statistical and Data Management Center (Seattle, WA, USA). These kits were sent to the study sites for sequential assignment to infants as they were randomised. Study staff and participants were masked to the study drug (nevirapine or placebo) assignment.
Procedures
Women gave study drug to their children with calibrated oral syringes. Randomly allocated infants started masked study drug and continued a once-daily dosing regimen until 6 months of age or until cessation of breastfeeding, whichever came first. The nevirapine dose was increased with age, ranging from 20 mg once-daily after 6–8 weeks of age to 28 mg once-daily after 5–6 months of age. Women were counselled to exclusively breastfeed for 6 months.
The primary efficacy endpoint was HIV-1 infection at age 6 months in infants who were uninfected at age 6 weeks in each study group. Primary safety endpoints were frequency and severity of adverse reactions in randomly allocated infants until age 6 months in each group for all infants who received at least one dose of study intervention. Secondary endpoints included HIV-1-free survival, relative rates of HIV-1 infection, and infant survival rates (mortality irrespective of HIV-1 infection) in the two study groups.
We tested infants for HIV-1 infection at 3, 6, 9, 12, and 18 months. Infant blood was stored at randomisation and tested retrospectively in those who had HIV-1-infection at the 3 month visit. Infants identified as HIV-1-infected at 6 weeks were excluded from analysis of primary and secondary endpoints. We defined HIV-1 infection as two separate peripheral blood specimens drawn on different days that were positive by HIV-1 DNA PCR. HIV-1 DNA was measured atsite laboratories with the Roche AMPLICOR HIV-1 DNA test, version 1.5 (Roche Molecular Systems, Branchburg, NJ, USA); all laboratories participated in the Virology Quality Assurance Program (Rush Medical College, Chicago, IL, USA) sponsored by the Division of AIDS (DAIDS) of the US National Institute of Allergy and Infectious Diseases (NIAID).
We graded clinical and laboratory adverse events according to the DAIDS table for grading of the severity of adult and paediatric adverse events (version 1.0; December, 2004, with clarification dated August, 2009). We used a separate grading scale for rash, malnutrition, and fever in infants. The three scales were adapted from different sources; the rash grading scale was adapted from standard NIAID/DAIDS rash grading; the malnutrition scale was adapted from the WHO definitions associated with malnutrition; and the fever scale was based on the standard NIAID/DAIDS grading, with adjustment for axillary measurement. We reported all adverse events (serious and non-serious) up to age 8 months (8 weeks after maximum study drug dosing); thereafter, only serious adverse events were reported. We assessed breastfeeding status at every infant visit and cessation was established by maternal report of last date of infant breast-milk exposure. All infants had reached age 6 months (for ascertainment of the primary study endpoints) by July, 2010, and 12 months of age by January, 2011. Our present analyses are for data obtained by the 12 month visit. Follow-up of infants is continuing to 18 months of age for further assessment of secondary endpoints.
Statistical analysis
On the basis of results from the Six Week Extended-Dose Nevirapine (SWEN) study,5 we assumed that the overall rate of HIV-1 infection in the placebo group would be about 2.6% at age 6 weeks and about 6.7% at age 6 months, giving a cumulative infection rate of 4.2% between these ages in the control group. We estimated that 1500 mother-infant pairs would provide 90% power to detect a reduction in HIV-1 infection from 4.2% to 1.4% at age 6 months with a Pearson χ2 test statistic and a one-sided false positive error rate of 0.025.
We summarised baseline characteristics with means and proportions. We calculated rates of cumulative infection, death, and HIV-1-free survival by randomisation group at 6, 9, and 12 months with the Kaplan-Meier method, and 95% CIs with Greenwood's formula. We compared cumulative rates with the Z statistic. Time of HIV-1 infection was defined as the midpoint between the last negative and the first positive HIV-1 test. For the cumulative-infection analysis, infants with no positive tests were censored at the time of last negative test. For HIV-1-freesurvival, the event time was the minimum of the infection time and the death time with censoring at the last negative test if no positive test or death was reported. We also created Kaplan-Meier curves of time to breastfeeding cessation, with comparisons between groups done with the log-rank test. Reported p values and CIs have not been adjusted for interim analyses or multiple comparisons. All statistical analyses were performed using SAS version 9.0 for UNIX.
This study is registered with ClinicalTrials.gov, number NCT00074412.
Role of the funding source
The sponsor (US National Institutes of Health) participated in the design and oversight of the study, interpretation of the results, and preparation of the manuscript. The sponsor also provided for an independent data and safety monitoring board to review the trial every 6 months. Members of the writing team had full access to the study data. The protocol chair, co-chairs, and sponsor representatives had final responsibility for the decision to submit for publication.
Results
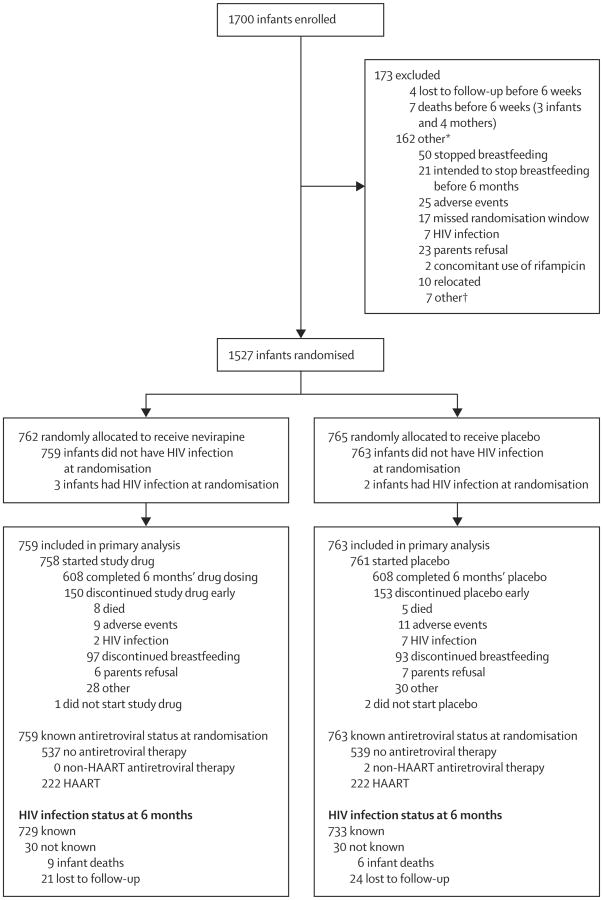
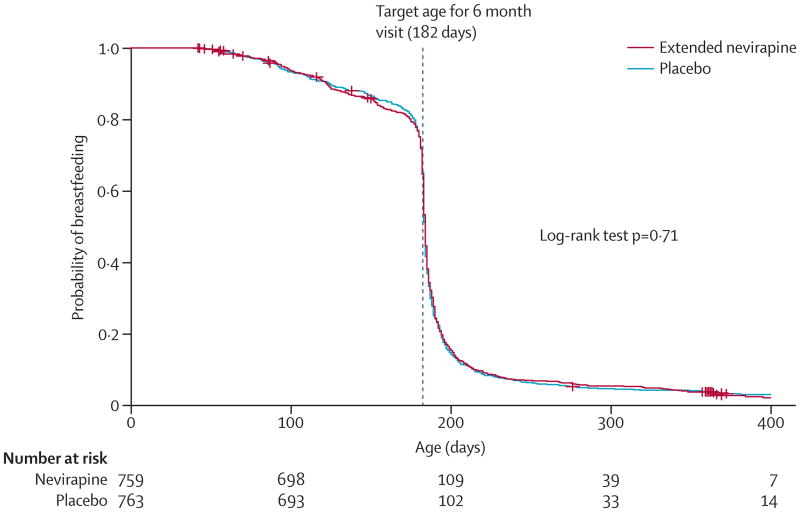
We randomly allocated 1527 infants aged 6 weeks, born to 1505 women with HIV-1 infection, between June 19, 2008, and March 12, 2010 (figure 1). Five infants had HIV-1 infection at the time of randomisation and were excluded from the primary analyses. Baseline characteristics of the infants and their mothers did not differ (table 1). At randomisation, 444 (30%) of 1505 mothers overall were on highly active antiretroviral therapy for their own health, increasing to 227 (31%) of 725 in the extended nevirapine group and 231 (32%) of 725 in the placebo group at 6 months. Median maternal CD4+ lymphocyte counts at randomisation at 6 weeks' postpartum were 528 cells per μL in the extended nevirapine group and 557 cells per μL in the placebo group. Breastfeeding status was assessed at each study visit; more than 95% of infants in both groups were no longer breastfed by the 9 month study visit. Time to breastmilk cessation differed by study site (log-rank p<0.0001; data not shown) but not by study group (p=0.71; figure 2).
Figure 1. Trial profile.
*Only main cause is listed. †Two first-born twins not eligible, one congenital heart defect, one mother in accident, two poor adherence to study procedures, and one mother could not dose competently.
Table 1. Baseline characteristics of mothers and infants.
| Nevirapine group | Placebo group | |
|---|---|---|
| Mothers | ||
|
| ||
| n | 752 | 753 |
| Median age (years) | 27 (23–30) | 27 (23–31) |
| Marital status | ||
| Never married or not living with partner | 235 (31%) | 226 (30%) |
| Married or living with partner | 495 (66%) | 504 (67%) |
| Separated or divorced | 9 (1%) | 11 (2%) |
| Widowed | 13 (2%) | 12 (2%) |
| On HAART at randomisation | 221 (29%) | 219 (29%) |
| Median CD4 cell count (cells per μl) | 528 (371–727) | 556.5 (400-755) |
| WHO classification at randomisation | ||
| Stage I | 616 (82 %) | 630 (84%) |
| Stage II | 105 (14%) | 96 (13%) |
| Stage III | 28 (4%) | 26 (4%) |
| Stage IV | 3 (<1%) | 1 (<1%) |
|
| ||
| Infants | ||
|
| ||
| n | 759 | 763 |
| Sex | ||
| Male | 363 (48%) | 398 (52%) |
| Female | 395 (52%) | 365 (48%) |
| Intersex | 1 (<1%) | 0 |
| Birthweight (g) | ||
| 2000–2499 | 50 (7%) | 52 (7%) |
| 2500–2999 | 214 (28%) | 211 (28%) |
| 3000–3499 | 329 (43%) | 336 (44%) |
| ≥3500 | 166 (22%) | 163 (21%) |
| Missing | 0 | 1 (<1%) |
| Median | 3100 (2800–3400) | 3100 (2800–3400) |
Data are n, median (IQR), or n (%). HAART=highly active antiretroviral therapy.
Figure 2. Kaplan-Meier analysis of breastfeeding duration, by study group.
Study drug adherence was measured as the proportion of infants who were reported to have received all scheduled doses since their last follow-up visit. Such adherence was 88–96% up to age 6 months and did not differ between sites or study groups. 1446 (97%) of 1496 infants expected to remain in the study did so at 6 months and 1180 (94%) of 1259 infants expected to remain in the study did so at 12 months; these proportions did not differ between study sites or groups.
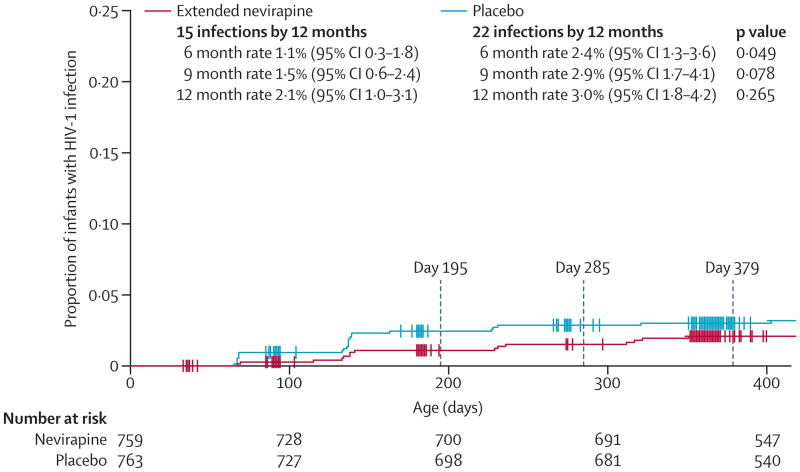
Table 2 shows rates of HIV-1 infection and death by study group. From Kaplan-Meier analysis, 1.1% (95% CI 0.3–1.8) of infants developed postnatal HIV-1 infection between ages 6 weeks and 6 months in the extended nevirapine group compared with 2.4% (1.3–3.6) of infants in the placebo group (difference 1.3%, 95% CI 0–2.6; p=0.049), equating to a 54% reduction in HIV-1 transmission (figure 3). Study interventions stopped at age 6 months. Differences between study groups were no longer significant at 9 months and 12 months of age.
Table 2. Cumulative incidence of HIV-1 infection or death in infants uninfected at age 6 weeks.
| Extended nevirapine (n=769) | Placebo group (n=753) | Relative-risk reduction | p value* | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Endpoints | Probability of endpoint† (95% CI) | Endpoints | Probability of endpoint† (95% CI) | |||
| All randomly allocated infants | ||||||
|
| ||||||
| HIV-1 infection | ||||||
| 6 months | 8/700 | 1.1% (0.3-1.8) | 18/699 | 2.4% (1.3-3.6) | 54% | 0.049 |
| 9 months | 11/692 | 1.5% (0.6-2.4) | 21/683 | 2.9% (1.7-4.1) | 48% | 0.078 |
| 12 months | 15/557 | 2.1% (1.0-3.1) | 22/545 | 3.0% (1.8-4.2) | 30% | 0.265 |
| Death | ||||||
| 6 months | 9/716 | 1.2% (0.4-2.0) | 8/725 | 1.1% (0.3-1.8) | 0% | 0.81 |
| 9 months | 16/703 | 2.2% (1.1-3.2) | 19/705 | 2.6% (1.4-37) | 15% | 0.61 |
| 12 months | 20/580 | 2.8% (1.6-4.0) | 26/572 | 3.6% (2.2-4.9) | 22% | 0.39 |
| HIV-1 infection or death | ||||||
| 6 months | 17/706 | 2.3% (1.2-3.4) | 24/707 | 3.2% (2.0-4.5) | 28% | 0.27 |
| 9 months | 26/693 | 3.6% (2.2-4.9) | 39/687 | 5.3% (37-6.9) | 32% | 0.10 |
| 12 months | 32/562 | 4.5% (3.0-6.0) | 46/547 | 6.3% (4.6-8.1) | 29% | 0.12 |
|
| ||||||
| Subgroup analyses | ||||||
|
| ||||||
| Infection from mothers on HAART at randomisation, independent of CD4 cell count | ||||||
| 6 months | 1/210 | 0.5% (0-1.4) | 0/203 | 0% | 0% | .. |
| 9 months | 1/169 | 0.5% (0-1.4) | 1/166 | 0.5% (0-1.4) | 0% | 0.976 |
| 12 months | 1/90 | 0.5% (0-1.4) | 1/81 | 0.5% (0-1.4) | 0% | 0.976 |
| Infection from mothers not on HAART at randomisation, independent of CD4 cell count | ||||||
| 6 months | 7/490 | 1.3% (0.4-2.3) | 18/492 | 34% (1.9-5.0) | 62% | 0.027 |
| 9 months | 10/483 | 2.0% (0.8-3.2) | 20/480 | 3.8% (2.2-5.5) | 47% | 0.071 |
| 12 months | 14/401 | 2.8% (1.3-4.2) | 21/398 | 4.0% (2.3-57) | 30% | 0.263 |
| Infection from mothers with high CD4 cell counts (≥350 cells per μL) and not on HAART at randomisation | ||||||
| 6 months | 3/418 | 0.7% (0-1.5) | 13/434 | 2.8% (1.3-44) | 75% | 0.014 |
| 9 months | 4/414 | 0.9% (0-1.8) | 15/422 | 3.3% (17-4.9) | 73% | 0.014 |
| 12 months | 7/340 | 1.7% (0.4-2.9) | 15/346 | 3.3% (1.7-4.9) | 48% | 0.116 |
| Infection from mothers with low CD4 cell counts (<350 cells per μL) and not on HAART at randomisation | ||||||
| 6 months | 4/71 | 4.8% (0.2-9.4) | 5/54 | 8.1% (1.3-14.8) | 41% | 0.438 |
| 9 months | 6/68 | 7.5% (1.7-13.3) | 5/54 | 8.1% (1.3-14.8) | 7% | 0.901 |
| 12 months | 7/59 | 8.9% (2.6-15.1) | 6/48 | 9.8% (2.3-17.3) | 9% | 0.850 |
Data are cumulative number of events/number at risk unless otherwise stated. HAART=highly active antiretroviral therapy.
Two-sided p value for Z statistic of the difference in probabilities.
Cumulative rates at 6, 9, and 12 months calculated with Kaplan-Meier methods.
Figure 3. Kaplan-Meier analysis of cumulative rates of HIV-1 infection, by study group.
When stratified by mothers who had highly active antiretroviral therapy at randomisation, infants born to these mothers had low rates of HIV-1 infection, which did not differ by study group at any age, whereas infants born to mothers who were not on such therapy had significantly lower rates of HIV-1 infection at 6 months with extended nevirapine (1.3%) compared with placebo (3.4%; p=0.027), but not at 9 or 12 months (table 2). Because WHO recommends treatment for women with low CD4 cell counts (ie, <350 cells per μL), we did a post-hoc analysis that assessed infection rates in infants born to mothers with high CD4 cell counts (≥350 cells per μL) who were not receiving highly active antiretroviral therapy at randomisation. In this subgroup, infants randomly allocated to the extended nevirapine group had a 6 month HIV-1 infection rate of 0.7%, which is much the same as that reported in infants of mothers on such therapy, compared with 2.8% for infants in the placebo group (p=0.014; difference 2.1%, 95% CI 0.4–3.9), for a 75% reduction in HIV-1 transmission. This difference continued to be significant at 9 months but not at 12 months. However, rates of HIV-1 infection did not differ in infants of women not receiving highly active antiretroviral therapy who had low CD4 cell counts. Overall survival and HIV-1-free survival did not differ in infants between study groups at 6, 9, or 12 months (table 2). However, most infant deaths occurred after age 6 months and cessation of breastfeeding.
Overall, 1259 (83%) of 1519 infants from both groups had adverse events (table 3). 125 (16%) of 758 infants given extended nevirapine and 116 (15%) of 761 controls had serious adverse events. Of 1519 infants who received at least one dose of nevirapine or placebo, the most common serious adverse events were gastroenteritis in 81 (5%) infants, pneumonia or bronchopneumonia in 73 (5%) infants, malaria in 54 (4%) infants, and kwashi or-kor in 12 (1%) infants, with no significant difference between study groups. In the 46 infant deaths reported by age 12 months (20 in the extended nevirapine group and 26 in the placebo group), the most frequent associated adverse events were gastroenteritis (11 infants) and pneumonia or bronchopneumonia (10 infants). Prevalence of laboratory abnormalities or rash did not differ between study groups (table 3).
Table 3. Adverse events, rash, and grade 3 or higher laboratory abnormalities in infants who received at least one dose of study drug.
| Overall (n=1519) | Extended nevirapine group (n=758) | Placebo group (n=761) | p value | |
|---|---|---|---|---|
| Adverse events | ||||
|
| ||||
| Infants with at least one adverse event | 1259 (83%) | 628 (83%) | 631 (83%) | 0.97 |
| Adverse event possibly related to study drug | 169 (11%) | 87 (12%) | 82 (11%) | 0.66 |
| Adverse event probably related to study drug | 16 (1%) | 10 (1%) | 6 (1%) | 0.31 |
| Grade 2B or worse rash | 1 (<1%) | 1 (<1%) | 0 | 0499 |
|
| ||||
| Abnormal laboratory values* | ||||
|
| ||||
| Infants with at least one laboratory assessment | 1514 (>99%) | 757 (>99%) | 757 (99%) | |
| Grade 3 or worse neutropenia | 294 (19%) | 161 (21%) | 133 (18%) | 0.07 |
| Grade 3 or higher ALT concentration | 6 (<1%) | 3 (<1%) | 3 (<1%) | 1.00 |
| Grade 3 or worse anaemia | 354 (23%) | 188 (25%) | 166 (22%) | 018 |
Data are n (%) unless otherwise stated. ALT=alanine aminotransferase.
Some laboratory values were also associated with an adverse event.
Discussion
After 6 weeks of treatment with once-daily nevirapine, continued use of nevirapine to age 6 months in uninfected infants of breastfeeding mothers with HIV-1 is safe, and results in a greater than 50% reduction in mother-to-child transmission from breastfeeding compared with placebo. No other study has directly assessed the incremental benefit of extension of nevirapine from age 6 weeks until 6 months to establish whether the extended period is more efficacious and whether there are increased safety issues associated with long-term treatment with nevirapine (panel). Our results, in addition to the published data for the efficacy of 6, 14, and 28 weeks of infant nevirapine, show the consistent benefit and safety of nevirapine for prevention of HIV-1 transmission via breastfeeding in the first 6 months of life.5–7 We also show that, once prophylaxis ends, trans mission risk returns at rates much the same as that reported without prophylaxis. These findings provide further evidence for the safety and efficacy of infant nevirapine prophylaxis for 6 months of exclusive breast feeding, as is recommended by the WHO. Furthermore, these results provide a proof of principle for the WHO recommendations to continue infant anti-retroviral prophylaxis throughout the period of breastfeeding, whether it lasts 6 months or longer.
The effect of the 6 month nevirapine regimen described in our study on reduction of HIV-1 breastfeeding transmission was especially striking in infants of women whose CD4+ cell counts were 350 cells per μL or more and who therefore would not qualify for highly active antiretroviral therapy for their own health according to present WHO criteria.11 In this population of mother-infant pairs, there was an overall four-fold decrease in HIV-1 breastfeeding transmission compared with transmission in infants who received placebo from age 6 weeks to 6 months (0.7% nevirapine vs 2.8% placebo). HIV-1 infection rates during extended nevirapine prophylaxis in infants born to women with high CD4 cell counts who were not receiving highly active antiretroviral therapy (0.7%) were much the same as infection rates reported in infants born to such women receiving this therapy (0.5%).
The importance of identification of and provision of therapy to women with low CD4 cell counts is shown by the high postnatal HIV-1 transmission rates reported in infants born to women not on highly active antiretroviral therapy with low CD4 cell counts (<350 cells per μL) irrespective of study group. In a study in Zambia,18 88% of perinatal and postnatal HIV-1 infections occurred in women who met the WHO criteria for receipt of treatment. Provision of highly active antiretroviral therapy seems to be very effective for reduction of postnatal infection in this population. Moreover, our study shows that extended nevirapine offers no additional benefit compared with 6 weeks of nevirapine in infants born to women receiving highly active antiretroviral therapy, supporting the present recommendation by the WHO for only 6 weeks of infant prophylaxis for infants of women on such treatement.
Previous studies suggested efficacy of other extended nevirapine regimens in infants for prevention of breastfeeding HIV-1 transmission. The SWEN study5 showed that once-daily infant nevirapine for 6 weeks resulted in a 53% reduction in postnatal HIV-1 infection at age 6 weeks compared with single-dose nevirapine (HIV-1 infection incidence of 2.5% vs 5.3%, respectively). The PEPI-Malawi study6,17 showed that once-daily infant nevirapine for 14 weeks resulted in a 69% reduction in the risk of postnatal HIV-1 infection at age 14 weeks compared with single-dose nevirapine and 1 week of infant zidovudine (2.6% vs 8.5%, respectively); however, follow-up to 24 months suggested continued transmission after cessation of nevirapine prophylaxis. The Breastfeeding and Nutrition study,7 which was undertaken in Malawi in infants born to mothers with CD4 cell counts of more than 250 cells per μL, showed that postnatal HIV-1 infection in infants uninfected at age 2 weeks was significantly reduced with 6 months of either maternal triple antiretroviral prophylaxis or extended infant nevirapine compared with single-dose nevirapine (2.9% for triple prophylaxis, 1.7% for extended nevirapine, and 5.7% for single-dose nevirapine); however, there was not a significant difference between the extended maternal and infant regimens. Efficacy of the infant nevirapine regimen in the Breastfeeding and Nutrition study7 was 70%, which is much the same as that reported with extended nevirapine in infants born to mothers with high CD4 cell counts (≥350 cells per μL) not receiving highly active antiretroviral therapy in our study (6-month HIV-1 infection rate of 1.1% with extended nevirapine vs 2.4% with placebo in infants receiving nevirapine up to 6 weeks of age). Therefore, the superiority of 6 months versus 6 weeks of nevirapine showed in HPTN 046 is consistent with the relative reductions in transmission reported in each of these studies.
Infant mortality did not differ significantly between study groups in our study. However, most infant deaths occurred after age 6 months and nearly all infants stopped breastfeeding by 6–9 months of age. Various studies have shown increased rates of infant mortality with early breastfeeding cessation before the age of 12 months, especially in infants born to mothers with low severity HIV-1 disease.19–21
Our analyses did not adjust for multiple statistical tests, thus increasing the risk of false-positive findings. However, because of the higher than expected rate of maternal highly active antiretroviral therapy use, we reported fewer infant infections than expected, which reduced our power to detect differences in HIV transmission risks between groups.
The major obstacles to PMTCT are the following factors: identification of pregnant women with HIV-1 infection;22 identification of and provision of highly active antiretroviral therapy to those women who need treatment for their own health (ie, those who have highest HIV-1 transmission risk); and provision of antiretroviral prophylaxis for those women who do not yet qualify for treatment based on WHO guidelines or for their infants. An analysis23 of the use of maternal triple antiretroviral prophylaxis compared with infant nevirapine prophylaxis for PMTCT in women who do not need therapy for their own health suggested that both interventions reduced the risk of transmission but that the cost of maternal prophylaxis was substantially greater than was the cost of infant nevirapine. Our study suggests the safety and incremental efficacy of extension of nevirapine to 6 months for prevention of postnatal HIV-1 infection in infants of women who do not need therapy and also shows the effectiveness of highly active antiretroviral therapy in reduction of mother-to-child trasmission in infants born to women with low CD4+ cell counts needing therapy. Global elimination of paediatric HIV-1 will require a multifaceted approach including primary prevention in women of childbearing age; prioritisation of efforts to identify and treat those pregnant women with HIV-1 who need therapy for their own health (which will benefit both the woman and prevent transmission to her infant); and provision of an effective prophylaxis regimen such as extended nevirapine to infants of women who do not need therapy to permit safe, long-term breastfeeding.
Panel: Research in Context
Systematic review
We searched PubMed for randomised trials published between September, 2001, and September, 2011, without language restriction for papers with the search terms “prevention of breastmilk HIV transmission” AND “antiretroviral” and “clinical trials to prevent breastmilk HIV transmission”. We identified three randomised trials that assessed antiretroviral prophylaxis in infants for 6, 14, or 28 weeks,5–7 and three trials that assessed antiretroviral prophylaxis in mothers for 24-28 weeks.7,8,13
Interpretation
Antiretroviral prophylaxis in infants and mothers strikingly reduces postnatal HIV transmission. Only one trial7 included infant and maternal antiretroviral prophylaxis groups; in this trial no significant difference in postnatal 28 week transmission rates was noted dependent on who received the treatment (1.7% for infant receipt vs 2.9% for maternal receipt). Although transmission is continuous during breastfeeding, the highest-risk period is probably the first 6-8 weeks of life as suggested by two randomised trials published in 2000-03.14,16The incremental efficacy of extension of prophylaxis beyond this high-risk period has not been assessed previously; the 14 week6,17 and 28 week7 infant prophylaxis trials were not designed to address whether longer prophylaxis was better in terms of efficacy, safety, feasibility, and cost, than was a 6 week regimen. HPTN 046 is the first randomised trial to show that extension of prophylaxis to 6 months provides significant benefit compared with shorter courses. WHO currently recommends life-long highly active antiretroviral therapy for women with low CD4 cell counts (<350 cells per μL) or WHO stage 3-4disease, and either maternal or infant antiretroviral therapy during breastfeeding for 12 months for mothers who do not need treatment for their own health.11 However, WHO acknowledged the low quality of evidence for this recommendation to extend prophylaxis to 12 months.10,11 HPTN 046 provides proof-of-principle data to support the WHO recommendation. Furthermore, infant antiretroviral prophylaxis is only recommended for infants of women with high CD4 cell counts (≥350 cells per μL) who are not currently eligible for highly active antiretroviral therapy. HPTN 046 also showed that infant nevirapine prophylaxis is very effective in this group, with postnatal transmission much the same as that in infants of mothers on highly active antiretroviral therapy.
Acknowledgments
The HIV Prevention Trials Network (HPTN) 046 study was funded by the US National Institutes of Health (NIH), initially through the HPTN and later through the International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) group. The HPTN (U01AI46749) has been funded by the National Institute of Allergy and Infectious Diseases (NIAID), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), National Institute of Drug Abuse (NIDA), and National Institute of Mental Health (NIMH). The IMPAACT Group (U01AI068632) has been funded by NIAID, NICHD, and NIMH. The study products were provided for free by Boehringer-Ingelheim. We thank the mothers and their children who participated in the study; the HPTN 046 study coordinators, counsellors, clinicians, pharmacists, data quality and laboratory staff, and those responsible for recruitment and retention for their dedication and hard work on site; Thomas R Fleming (University of Washington/Fred Hutchinson Cancer Research Center) for his contributions to study design and his strong support throughout study conduct; Scharla Estep (NIAID protocol pharmacist) for her help on pharmaceutical matters; and Avinash Shetty (Wake Forest University Health Sciences) for his contribution to study development and safety data review.
Footnotes
Contributors: HC (protocol chair) provided scientific leadership on study design, implementation, data interpretation, and manuscript preparation. ERB (lead statistician) oversaw the study analyses and contributed to the manuscript. YM, DM, and MGF (protocol co-chairs) contributed to the scientific leadership, study implementation and oversight, data interpretation, and preparation of the manuscript. TC, PM, and KM (principal site investigators) contributed to study design, implementation, and oversight and interpretation of the data. LS-C, VC, CN, LM, and RK (site investigators) contributed to data collection and study implementation. WF (protocol co-chair) contributed to study design and implementation. LG provided scientific leadership on study design and contributed to study implementation and data interpretation. AM (study statistician) undertook the analyses and contributed to review and interpretation of the data and preparation of the manuscript. DJL (central data operations coordinator) contributed to site training, study operations and implementation, and data review. SHE (protocol virologist) provided scientific and operational direction for the laboratory aspects of the study and contributed to manuscript preparation. PR (central laboratory quality assurance coordinator) contributed to study development and implementation, and data review and reporting. PA and KG (central protocol specialists) contributed to site management and training, study operations and implementation, and assisted with manuscript preparation. SZ (National Institute of Allergy and Infectious Diseases medical officer), contributed to study design and implementation and data interpretation. LMM (Eunice Kennedy Shriver National Institute of Child Health and Human Development medical officer) contributed to study design and implementation, data interpretation, and manuscript preparation.
Conflicts of interest: We declare that we have no conflicts of interest.
Contributor Information
Prof Hoosen M Coovadia, Maternal Adolescent and Child Health (MatCH), University of the Witwatersrand, Johannesburg, South Africa and Nelson Mandela School of Medicine, University of Kwazulu-Natal, Durban; South Africa.
Elizabeth R Brown, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Mary Glenn Fowler, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Tsungai Chipato, University of Zimbabwe College of Medicine, Harare, Zimbabwe.
Dhayendre Moodley, Centre for the AIDS Programme of Research in South Africa (CAPRISA), Nelson R Mandela School of Medicine, University of KwaZulu Natal, Durban, South Africa.
Karim Manji, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania.
Philippa Musoke, Makerere University-Johns Hopkins University Research Collaboration, Kampala, Uganda.
Lynda Stranix-Chibanda, University of Zimbabwe College of Medicine, Harare, Zimbabwe.
Vani Chetty, Centre for the AIDS Programme of Research in South Africa (CAPRISA), Nelson R Mandela School of Medicine, University of KwaZulu Natal, Durban, South Africa.
Wafaie Fawzi, Harvard University School of Public Health, Boston, MA, USA.
Clemensia Nakabiito, Makerere University-Johns Hopkins University Research Collaboration, Kampala, Uganda.
Lindiwe Msweli, Centre for the AIDS Programme of Research in South Africa (CAPRISA), Nelson R Mandela School of Medicine, University of KwaZulu Natal, Durban, South Africa.
Roderick Kisenge, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania.
Laura Guay, George Washington University School of Public Health and Health Services, Washington DC, USA.
Anthony Mwatha, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Diana J Lynn, Fred Hutchinson Cancer Research Center, Seattle, WA, USA.
Susan H Eshleman, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Paul Richardson, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Kathleen George, Family Health International, Research Triangle Park, NC, USA.
Philip Andrew, Family Health International, Research Triangle Park, NC, USA.
Lynne M Mofenson, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Rockville, MD, USA.
Sheryl Zwerski, National Institute of Allergy and Infectious Diseases, US National Institutes of Health, Bethesda, MD, USA.
Yvonne Maldonado, Stanford University School of Medicine, Stanford, CA, USA.
References
- 1.Coutsoudis A, Dabis F, Fawzi W, et al. and the Breastfeeding and HIV International Transmission Study Group. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154–66. doi: 10.1086/420834. [DOI] [PubMed] [Google Scholar]
- 2.Leroy V, Karon JM, Alioum A, et al. and the West Africa PMTCT Study Group. Twenty-four month efficacy of a maternal short-course zidovudine regimen to prevent mother-to-child transmission of HIV-1 in West Africa. AIDS. 2002;16:631–41. doi: 10.1097/00002030-200203080-00016. [DOI] [PubMed] [Google Scholar]
- 3.Petra Study Team. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa and Uganda (Petra study): a randomised, double-blind, placebo-controlled trial. Lancet. 2002;359:1178–86. doi: 10.1016/S0140-6736(02)08214-4. [DOI] [PubMed] [Google Scholar]
- 4.WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. Lancet. 2000;355:451–55. [PubMed] [Google Scholar]
- 5.Bedri A, Gudetta B, Isehak A, et al. and the Six Week Extended-Dose Nevirapine (SWEN) Study Team. Extended-dose nevirapine to 6 weeks of age for infants to prevent HIV transmission via breastfeeding in Ethiopia, India, and Uganda: an analysis of three randomised controlled trials. Lancet. 2008;372:300–13. doi: 10.1016/S0140-6736(08)61114-9. [DOI] [PubMed] [Google Scholar]
- 6.Kumwenda NI, Hoover DR, Mofenson LM, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359:119–29. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 7.Chasela CS, Hudgens MG, Jamieson DJ, et al. and the BAN Study Group. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–81. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro RL, Hughes MD, Ogwu A, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362:2282–94. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciaranello AL, Park JE, Ramirez-Avila L, Freedberg KA, Walensky RP, Leroy V. Early infant HIV-1 diagnosis programs in resource-limited settings: opportunities for improved outcomes and more cost-effective interventions. BMC Med. 2011;9:59. doi: 10.1186/1741-7015-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Guidelines on HIV and infant feeding 2010. [accessed May 22, 2011];Principles and recommendations for infant feeding in the context of HIV and a summary of evidence. http://whqlibdoc.who.int/publications/2010/9789241599535_eng.pdf. [PubMed]
- 11.WHO. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. [accessed May 21, 2011];Recommendations for a public health approach 2010 version. http://whqlibdoc.who.int/publications/2010/9789241599818_eng.pdf. [PubMed]
- 12.Barker PM, Mphatswe W, Rollins N. Antiretroviral drugs in the cupboard are not enough: the impact of health systems' performance on mother-to-child transmission of HIV. J Acquir Immune Defic Syndr. 2011;56:e45–48. doi: 10.1097/QAI.0b013e3181fdbf20. [DOI] [PubMed] [Google Scholar]
- 13.de Vincenzi I and the Kesho Bora Study Group. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomized controlled trial. Lancet Infect Dis. 2011;11:171–80. doi: 10.1016/S1473-3099(10)70288-7. [DOI] [PubMed] [Google Scholar]
- 14.Nduati RW, John GC, Mbori-Ngacha D, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1175–82. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 15.Moodley D, Moodley J, Coovadia H, et al. and the South African Intrapartum Nevirapine Trial (SAINT) Investigators. A multicenter randomized controlled trial of nevirapine versus a combination of zidovudine and lamivudine to reduce intrapartum and early postpartum mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 2003;187:725–35. doi: 10.1086/367898. [DOI] [PubMed] [Google Scholar]
- 16.Fowler MG, Newell ML. Breast-feeding and HIV-1 transmission in resource-limited settings. J Acquir Immune Defic Syndr. 2002;30:230–39. doi: 10.1097/00042560-200206010-00012. [DOI] [PubMed] [Google Scholar]
- 17.Taha TE, Li Q, Hoover DR, et al. Post-exposure prophylaxis of breastfeeding HIV-exposed infants with antiretroviral drugs to age 14 weeks: updated efficacy results of the PEPI-Malawi Trial. J Acquir Immune Defic Syndr. 2011;57:319–25. doi: 10.1097/QAI.0b013e318217877a. [DOI] [PubMed] [Google Scholar]
- 18.Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Mwiya M, Thea DM. Potential impact of new WHO criteria for antiretroviral treatment for prevention of mother-to- child HIV transmission. AIDS. 2010;24:1374–77. [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhn L, Aldrovandi GM, Sinkala M et al. and the Zambia Exclusive Breastfeeding Study. Effects of early, abrupt weaning on HIV-free survival of children in Zambia. N Engl J Med. 2008;359:130–41. doi: 10.1056/NEJMoa073788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhn L, Aldrovandi GM, Sinkala M, et al. and the Zambia Exclusive Breastfeeding Study (ZEBS) Differential effects of early weaning for HIV-free survival of children born to HIV-infected mothers by severity of maternal disease. PLoS One. 2009;4:e6059. doi: 10.1371/journal.pone.0006059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kafulafula G, Hoover DR, Taha TE, et al. Frequency of gastroenteritis and gastroenteritis-associated mortality with early weaning in HIV-1-uninfected children born to HIV-infected women in Malawi. J Acquir Immune Defic Syndr. 2010;53:6–13. doi: 10.1097/QAI.0b013e3181bd5a47. [DOI] [PubMed] [Google Scholar]
- 22.Medley A, Garcia-Moreno C, McGill S, Maman S. Rates, barriers and outcomes of HIV serostatus disclosure among women in developing countries: implications for prevention of mother-to-child transmission programmes. Bull World Health Organ. 2004;82:299–307. [PMC free article] [PubMed] [Google Scholar]
- 23.Auld AF, Bolu O, Creek T, et al. Potential impact and cost-effectiveness of the 2009 “rapid advice” PMTCT guidelines—15 resource-limited countries, 2010. XVIII International AIDS Conference; 18–23; Vienna, Austria. Jul, 2010. Abstr WEAE0205. [Google Scholar]