Abstract
Background
The Institute of Medicine (IOM) has recommended that each person with cancer should have access to clinical trials, which have been associated with improving care quality and disparities. With no effective enrollment monitoring system, patterns of trial enrollment remain unclear.
Purpose
We developed a population-based, statewide system designed to facilitate monitoring of cancer trial enrollment and targeting of future interventions to improve it.
Methods
Person-level cancer incidence data from the North Carolina Central Cancer Registry (NCCCR), person-level treatment trial accrual data from the National Cancer Institute (NCI), and county-level Area Resource Files (ARF) measures for 12 years, 1996–2007, were studied. De-identified person-level data necessitated county-level analysis. Enrollment rates were estimated as the ratio of trial enrollment to cancer incidence for each race, gender, year, and county combination. Multivariable analysis examined factors associated with trial accrual. Sensitivity analyses examined spurious fluctuations and temporal discordance of incidence and enrollment.
Results
The NCI treatment trial enrollment rate was 2.39% for whites and 2.20% for minorities from 1996 to 2007, and 2.88% and 2.47%, respectively, for 2005–2007. Numerous counties had no minority enrollment. The 2005–2007 enrollment rates for white and minority females was 4.04% and 3.59%, respectively, and for white and minority males was 1.74% and 1.36%, respectively. Counties with a medical school or NCI Community Clinical Oncology Program (CCOP)-affiliated practice had higher trial enrollment.
Limitations
We examined NCI trial accrual only – industry-sponsored and investigator-initiated trials were excluded; however, NCI studies comprise the majority of all clinical trial participants. Delays in data availability may hinder immediacy of population-based analyses.
Conclusions
Model stability and consistency suggest this system is effective for population-based enrollment surveillance. For North Carolina, it suggests a worsening disparity in minority trial enrollment, though our analyses elucidate targets for intervention. Regional enrollment variation suggests the importance of access to clinical research networks and infrastructure. Substantial gender differences merit further examination.
Keywords: cancer, surveillance, clinical trials enrollment, disparities
Introduction
Randomized controlled trials are a central driver of research discovery and advances in cancer care, and the gold standard in assessing efficacy of new cancer interventions.1 Treatment efficacy increasingly hinges on specific genotypes that may be expressed in only a limited subset of the population; thus, enhanced and expanded trial inclusion of diverse populations is needed to develop broadly-effective treatments.2 At the same time, it is widely believed that clinical trials are a conduit for communicating new developments in high-quality state-of-the-art care, and a vehicle for accelerating the dissemination of evidence-based discoveries into practice.2–5 Academic medical centers (AMCs) have long-served as the major hubs of clinical research; however, less than one percent of Americans seek their care from AMCs, representing both a bottleneck in clinical trial access and an impediment to bridging the discovery-delivery gap.6, 7
For these reasons and others, the Institute of Medicine (IOM) has recommended that each person with cancer should have access to high quality clinical trials. The National Institutes of Health (NIH) has invested heavily in provider-based research networks (PBRNs) through the Roadmap.8–12 PBRNs provide critical research infrastructure opening access and facilitating clinical trial enrollment outside of AMCs in the community, where the majority of cancer patients seek treatment.2, 9 The National Cancer Institute’s Community Clinical Oncology Program (CCOP) and Minority-Based Community Clinical Oncology Program (MBCCOP) are cancer-focused PBRNs.2 The North Carolina Comprehensive Cancer Program (NC-CCP) and many other state cancer control programs have embraced this guidance and are moving to address goals relating to trial enrollment. .13–15 For North Carolina, improving access to clinical trials is part of a multi-part strategy to optimize cancer outcomes for all North Carolinians, with a particular emphasis on resolving profound racial disparities: In North Carolina, African Americans experience cancer-specific mortality that ranges from 25% to an astounding 2.6 times greater than that of whites for the most common cancers.16, 17
Despite such interest, advocacy, and investment, there is exceptionally little empirical information and no practical system for these programs to understand how enrollment may differ by geographic region, race, or other characteristics amenable to interventions to increase enrollment. Meanwhile, it is estimated nationally that fewer than 5% of the adult cancer population receives treatment through an NCI-sponsored clinical trial, and among minorities, enrollment estimates are even lower and appear to be declining18–21. In North Carolina, these percentage estimates are unknown. We describe a novel statewide system for ongoing monitoring and public reporting of NCI clinical trial enrollment developed to extend clinical trials access to broader patient populations. We articulate our methods so they may be replicated in other states.
Methods
Person-level cancer incidence data were obtained from the NC Central Cancer Registry (CCR) for years 1996–2007. Person-level NCI clinical trial accrual data were obtained from the NCI Cancer Therapy Evaluation Program (CTEP) through a Freedom of Information Act request. From both data sets, variables included age at diagnosis/enrollment, gender, race, ethnicity, county of residence, and cancer type. From CTEP, data also included protocol number, protocol name, study phase (e.g., I–III), and NIH administration code. Using descriptive data from CTEP, the Physician PDQ, ClinicalTrials.gov, and caCORE, trials were categorized according to their primary intent: treatment, prevention/early detection, symptom/side-effect control, diagnostic, or "other."22–24 Select socioeconomic and regional health care organization data were obtained from Area Resource Files.25 Locations of NCI Community Clinical Oncology Program (CCOP)-affiliated community practices were obtained from the Southeast Cancer Control Consortium.26, 27
The primary goal was to inform the development of a new trial enrollment monitoring system; thus, analysis was primarily descriptive in nature and focused on variation and stability of treatment trial enrollment measures among adults aged 21 and older. There was insufficient information on personal identifiers, to allow linking the datasets at the person-level; thus, the data were pooled and analyzed at the county-level. Minority status was defined only by race because ethnicity data from CTEP and CCR were incomplete. Trial accrual rate estimates were calculated by dividing the count of annual enrollment by the count of newly incident cases for each race, gender, county, and year combination. Three-year averages were used to mitigate spurious fluctuations resulting from sparse data for several counties and race-gender combinations. Univariate and bivariate trend analysis were conducted for the entire study period and, to understand trend changes, for the most current three years (2005–2007). Extensive sensitivity analyses examined temporal parity between year of incident diagnosis and trial enrollment, and variation in enrollment by trial phase based on the assumptions that (a) enrollment may or may not occur in the same year as diagnosis, and (b) compared to Phase III enrollment, Phase I enrollment may less often occur in the same year as diagnosis, perhaps as patients exhaust mainstream therapies. We used Pearson chi-square tests to examine variable distributions between cancer incidence and trial enrollment and by in-county presence of a CCOP-affiliated practice or a medical school.
A limited analytic model assessed feasibility of multivariable analysis for predictors associated with trial accrual. Because data are structured at the county-level and the dependent variable (accrual rate) is binomially distributed, analysis included a repeated measures approach (Proc Glimmix) using race and gender combinations in SAS (9.2). This modeling approach assumes that each race and gender combination is sampled from the same population and any effects (log-linear odds) over time within this sampling unit are constant. This approach is analogous to having a cohort of 400 (100 counties × 4 race/gender combinations), each of which has a random slope and intercept, with all covariates in the model (AHEC, CCOP, etc) considered to be fixed effects. Model parameters are estimates of log odds associated with each covariate at the county level.
Results
Between 1996 and 2007, 479,123 adults were diagnosed with cancer in NC; 11,362 adults enrolled in NCI-sponsored treatment trials, yielding an estimated overall enrollment rate of 2.37% (Table 1). The average age at diagnosis was 65.0, and the average age at trial enrollment was 57.8. The proportion of women who enrolled in trials was approximately twice that of men (3.20% vs. 1.55%). Minority men had the lowest enrollment rate at 1.33% (OR 0.84 95% CI 0.77–0.92, compared to white men), while the enrollment rate of white women was highest at 3.21% -- 2.4 times higher than minority men. In recent years enrollment rates among all races and genders increased except among minority men (1.36% - data not shown).
Table 1.
Descriptive Characteristics: Cancer incidence and NCI treatment trial enrollment, North Carolina.
| Overall (1996–2007) | Overall (2005–2007) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Incidence (n) |
Number Enrolled (n) |
Est. Enrollment Rate (%) |
OR | 95% Wald CI | Cancer Incidence (n) |
Number Enrolled (n) |
Est. Enrollment Rate (%) |
OR | 95% Wald CI |
|||
| Overall | 479,123 | 11,362 | 2.37% | 142,012 | 4,008 | 2.88% | ||||||
| Gender | ||||||||||||
| Male | 241,983 | 3,752 | 1.55% | 1.00 | 71,158 | 1,199 | 1.68% | 1.00 | ||||
| Female | 237,131 | 7,598 | 3.20% | 2.10 | *** | (2.02 – 2.19) | 70,850 | 2,808 | 3.96% | 2.41 | *** | (2.25 – 2.58) |
| Unknown/not documented | 9 | 12 | - | 4 | 1 | - | ||||||
| Race | ||||||||||||
| White | 385,782 | 9,222 | 2.39% | 1.00 | 112,955 | 3,258 | 2.88% | 1.00 | ||||
| Minority | 93,341 | 2,057 | 2.20% | 0.92 | *** | (0.88 – 0.97) | 29,057 | 719 | 2.47% | 0.85 | *** | (0.79 – 0.93) |
| Unknown/not documented | 0 | 83 | - | 0 | 31 | - | ||||||
| Gender * Race | ||||||||||||
| White Males | 194,020 | 3,068 | 1.58% | 1.00 | 56,625 | 984 | 1.74% | 1.00 | ||||
| White Females | 191,756 | 6,148 | 3.21% | 2.06 | *** | (1.97 – 2.15) | 56,327 | 2,273 | 4.04% | 2.38 | *** | (2.20 – 2.57) |
| Minority Males | 47,963 | 640 | 1.33% | 0.84 | *** | (0.77 – 0.92) | 14,533 | 198 | 1.36% | 0.78 | *** | (0.67 – 0.91) |
| Minority Females | 45,375 | 1,411 | 3.11% | 2.00 | *** | (1.87 – 2.13) | 14,523 | 521 | 3.59% | 2.10 | *** | (1.89 – 2.34) |
| Unknown/not documented | 9 | 95 | - | 4 | 32 | - | ||||||
| Age | ||||||||||||
| Avg age- Diagnosis, Enrollment | 65.0 (14.0) | 57.8 (13.0) | 64.8 (14.1) | 58.4 (12.9) | ||||||||
| AHEC Region (# counties) | ||||||||||||
| AHEC Area L (5) | 18,458 | 369 | 2.00% | 0.74 | *** | (0.66 – 0.82) | 5,314 | 164 | 3.09% | 1.15 | (0.97 – 1.36) | |
| AHEC Charlotte (8) | 73,623 | 1,649 | 2.24% | 0.83 | *** | (0.78 – 0.88) | 22,945 | 578 | 2.52% | 0.93 | (0.83 – 1.04) | |
| AHEC Eastern (23) | 55,089 | 1,398 | 2.54% | 0.94 | * | (0.88 – 1.01) | 15,649 | 527 | 3.37% | 1.26 | *** | (1.12 – 1.41) |
| AHEC Greensboro (8) | 62,305 | 1,595 | 2.56% | 0.95 | (0.89 – 1.01) | 18,330 | 630 | 3.44% | 1.28 | *** | (1.15 – 1.43) | |
| AHEC Mountain (16) | 50,223 | 1,104 | 2.20% | 0.81 | *** | (0.76 – 0.87) | 14,816 | 407 | 2.75% | 1.02 | (0.90 – 1.52) | |
| AHEC Wake (9) | 62,260 | 1,233 | 1.98% | 0.73 | *** | (0.68 – 0.78) | 19,541 | 442 | 2.26% | 0.83 | ** | (0.74 – 0.94) |
| AHEC Southeast (5) | 25,114 | 473 | 1.88% | 0.69 | *** | (0.63 – 0.77) | 7,161 | 158 | 2.21% | 0.81 | * | (0.68 – 0.97) |
| AHEC Southern (9) | 42,109 | 1,001 | 2.38% | 0.88 | *** | (0.82 – 0.95) | 12,140 | 304 | 2.50% | 0.93 | (0.81 – 1.06) | |
| AHEC Northwest (17) | 88,801 | 2,391 | 2.69% | 1.00 | - | 25,861 | 699 | 2.70% | 1.00 | |||
| Unknown/County not documented | 1,141 | 149 | - | 255 | 99 | - | ||||||
| CCOP in County? | ||||||||||||
| No | 309,416 | 6,697 | 2.16% | 1.00 | 88,397 | 2,194 | 2.48% | 1.00 | ||||
| Yes† | 168,566 | 4,516 | 2.68% | 1.24 | *** | (1.20 – 1.29) | 53,360 | 1,715 | 3.21% | 1.31 | *** | (1.22 – 1.39) |
| Medical School in County? | ||||||||||||
| No (n=96) | 431,874 | 9,890 | 2.29% | 1.00 | 128,591 | 3,468 | 2.70% | 1.00 | ||||
| Yes (n=4) | 46,108 | 1,323 | 2.87% | 1.26 | *** | (1.19 – 1.34) | 13,166 | 441 | 3.35% | 1.25 | *** | (1.13 – 1.38) |
| Percentile uninsured (county-level) | ||||||||||||
| Quartile 1 (Fewest uninsured) | 77,579 | 2,105 | 2.71% | 1.00 | 22,176 | 733 | 3.31% | 1.00 | ||||
| Quartile 2 | 111,772 | 2,809 | 2.51% | 0.93 | * | (0.88 – 0.98) | 32,352 | 984 | 3.04% | 0.92 | (0.83 – 1.01) | |
| Quartile 3 | 164,788 | 3,558 | 2.16% | 0.79 | *** | (0.74 – 0.83) | 49,306 | 1,224 | 2.48% | 0.74 | *** | (0.68 – 0.82) |
| Quartile 4 (Most uninsured) | 123,843 | 2,741 | 2.21% | 0.81 | *** | (0.77 – 0.86) | 37,923 | 968 | 2.55% | 0.77 | *** | (0.70 – 0.85) |
| Unknown/County not documented | 1,141 | 149 | - | 255 | 99 | - | ||||||
| Years | ||||||||||||
| 1996–1998 | 99,872 | 1,780 | 1.78% | 1.00 | - | - | - | - | - | - | ||
| 1999–2001 | 113,138 | 2,886 | 2.55% | 1.44 | *** | (1.36 – 1.53) | - | - | - | - | - | - |
| 2002–2004 | 124,101 | 2,688 | 2.17% | 1.22 | *** | (1.15 – 1.30) | - | - | - | - | - | - |
| 2005–2007 | 142,012 | 4,008 | 2.82% | 1.60 | *** | (1.51 – 1.69) | - | - | - | - | - | - |
Number of Counties with a CCOP Practice: 1996–98: 9; 1999–2000: 10; 2001–03: 11; 2004–07: 12
Statistical Significance: 0.001: *** ; 0.01: ** ; 0.1: *
Counties with NCI CCOP-affiliated practices and medical schools typically had greater enrollment (Table 2). The average enrollment rate among counties with a CCOP practice was 2.68% -- 25% greater than non-CCOP/medical school counties. Similarly, average enrollment in counties with a medical school was 2.87% -- 34% greater than non-CCOP/medical school counties. The county-level proportion of uninsured was inversely related to county-level enrollment (OR 0.81, 95% CI 0.77–0.86).
Table 2.
Cancer incidence and NCI treatment trial enrollment, North Carolina, by county presence of an NCI CCOP practice or a medical school.
| Counties with CCOP Practice (1996–2007) | Counties with Medical School (1996–2007) | All Other Counties (1996–2007) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer Incidence (n) |
Number Enrolled (n) |
Estimated Enrollment Rate (%) |
OR | Cancer Incidence (n) |
Number Enrolled (n) |
Estimated Enrollment Rate (%) |
OR | Cancer Incidence (n) |
Number Enrolled (n) |
Estimated Enrollment Rate (%) |
OR | ||||
| Overall† | 168,566 | 4,516 | 2.68% | 1.26 | *** | 46,108 | 1,323 | 2.87% | 1.35 | *** | 284,795 | 6,084 | 2.14% | 1.00 | - |
| Proportion of Popluation that is Minority Race | |||||||||||||||
| General population | 27.20% | 35.24% | 25.58% | ||||||||||||
| Incidence population | 19.02% | 25.98% | 18.73% | ||||||||||||
| Trial population | 18.78% | 24.64% | 17.14% | ||||||||||||
| Gender | |||||||||||||||
| Male | 83,247 | 1,490 | 1.79% | 1.00 | 22,262 | 404 | 1.81% | 1.00 | 146,232 | 2,073 | 1.42% | 1.00 | |||
| Female | 85,314 | 3,020 | 3.54% | 2.01 | *** | 23,846 | 918 | 3.85% | 2.17 | *** | 138,559 | 4,005 | 2.89% | 2.07 | *** |
| Unknown/not documented | 5 | 6 | - | 0 | 1 | - | 4 | 6 | - | ||||||
| Race | |||||||||||||||
| White | 136,498 | 3,634 | 2.66% | 1.00 | 34,127 | 984 | 2.88% | 1.00 | 231,461 | 4,997 | 2.16% | 1.00 | |||
| Minority | 32,068 | 848 | 2.64% | 0.99 | 11,981 | 326 | 2.72% | 0.94 | 53,334 | 1,043 | 1.96% | 0.90 | ** | ||
| Unknown/not documented | 0 | 34 | - | 0 | 13 | - | 0 | 44 | - | ||||||
| Gender * Race | |||||||||||||||
| White Males | 67,239 | 1,187 | 1.77% | 1.00 | 16,262 | 299 | 1.84% | 1.00 | 118,341 | 1,737 | 1.47% | 1.00 | |||
| White Females | 69,255 | 2,443 | 3.53% | 2.04 | *** | 17,865 | 684 | 3.83% | 2.13 | *** | 113,118 | 3,258 | 2.88% | 1.99 | *** |
| Minority Males | 16,008 | 285 | 1.78% | 1.01 | 6,000 | 99 | 1.65% | 0.90 | 27,891 | 313 | 1.12% | 0.76 | *** | ||
| Minority Females | 16,059 | 561 | 3.49% | 2.01 | *** | 5,981 | 227 | 3.80% | 2.11 | *** | 25,441 | 726 | 2.85% | 1.97 | *** |
| Unknown/not documented | 5 | 40 | - | 0 | 14 | - | 4 | 50 | - | ||||||
| Age | |||||||||||||||
| Avg age at Diagnosis, Enrollment | 64.48 (14.29) | 57.5 (13.0) | 64.48 (14.43) | 57.3 (13.9) | 65.42 (13.66) | 58.0 (12.9) | |||||||||
| Percentile uninsured (county-level) | |||||||||||||||
| Quartile 1 (Fewest uninsured) | 6,190 | 556 | 8.98% | 1.00 | 6,952 | 97 | 1.40% | 1.00 | 64,437 | 1,452 | 2.71% | 1.00 | |||
| Quartile 2 | 24,412 | 800 | 3.28% | 0.34 | *** | 33,239 | 985 | 2.96% | 2.16 | *** | 78,159 | 1,811 | 2.52% | 1.03 | |
| Quartile 3 | 70,722 | 1,685 | 2.38% | 0.25 | *** | - | - | - | - | 91,515 | 1,796 | 2.15% | 0.87 | *** | |
| Quartile 4 (Most uninsured) | 67,242 | 1,475 | 2.19% | 0.23 | *** | 5,917 | 241 | 4.07% | 3.00 | *** | 50,684 | 1,025 | 2.21% | 0.90 | ** |
| Unknown/County not documented | 0 | 0 | - | 0 | 0 | - | 0 | 0 | - | ||||||
| Years (Unknown counties not removed) | |||||||||||||||
| 1996–1998 | 32,623 | 647 | 1.98% | 1.00 | 10,012 | 216 | 2.16% | 1.00 | 62,042 | 1,026 | 1.65% | 1.00 | |||
| 1999–2001 | 38,757 | 1,078 | 2.78% | 1.41 | *** | 10,817 | 336 | 3.11% | 1.45 | *** | 68,439 | 1,621 | 2.37% | 1.44 | *** |
| 2002–2004 | 43,826 | 1,076 | 2.46% | 1.24 | *** | 12,113 | 330 | 2.72% | 1.27 | ** | 73,089 | 1,461 | 2.00% | 1.21 | *** |
| 2005–2007 | 53,360 | 1,715 | 3.21% | 1.64 | *** | 13,166 | 441 | 3.35% | 1.57 | *** | 81,225 | 1,976 | 2.43% | 1.48 | *** |
ORs for "Overall" compare CCOP and Medical School counties to "All Other Counties;" for other measures, ORs compare among categories within measures.
Statistical Significance: 0.001: *** ; 0.01: ** ; 0.1: *
Counties with a CCOP or a medical school tended to have a greater proportion of minorities. In CCOP counties, 18.78% of the clinical trial population was composed of minorities, a comparable percentage to the 19.02% of the incident cancer population composed of minorities in CCOP counties (Table 2). The ratio of these percentages reflects the comparable proportionality of minorities in these groups: 0.987. In medical school counties minorities represented 24.64% of trial enrollment and 25.98% of incident cancer (ratio: .948). In all other counties, minorities represented 17.14% of trial enrollment and 18.73% of incident cancer (ratio: .915). Differences in enrollment by gender were comparable among CCOP, medical school, and all other counties.
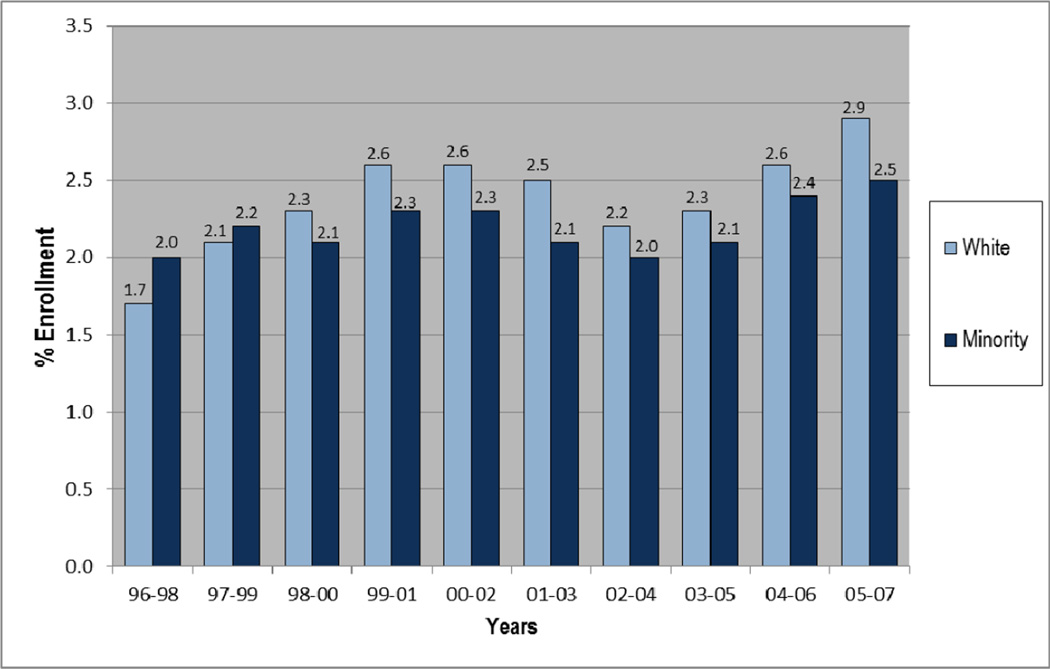
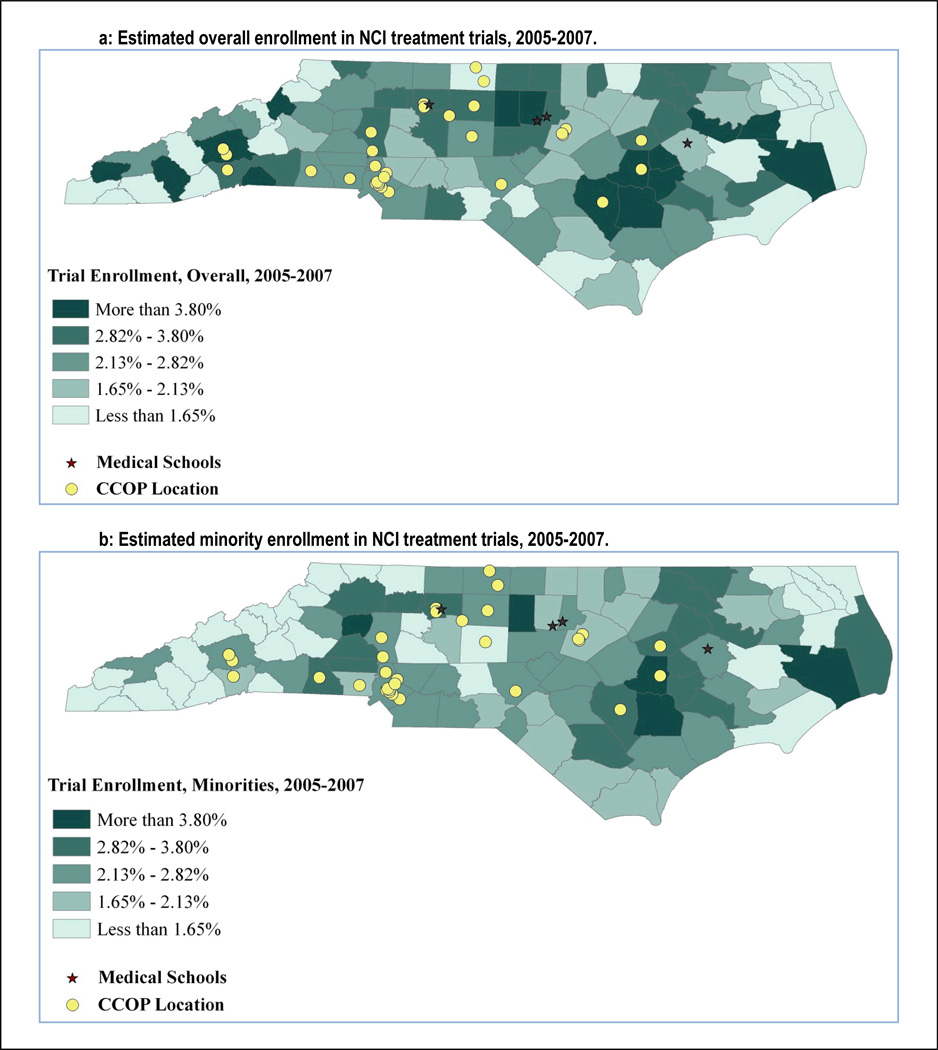
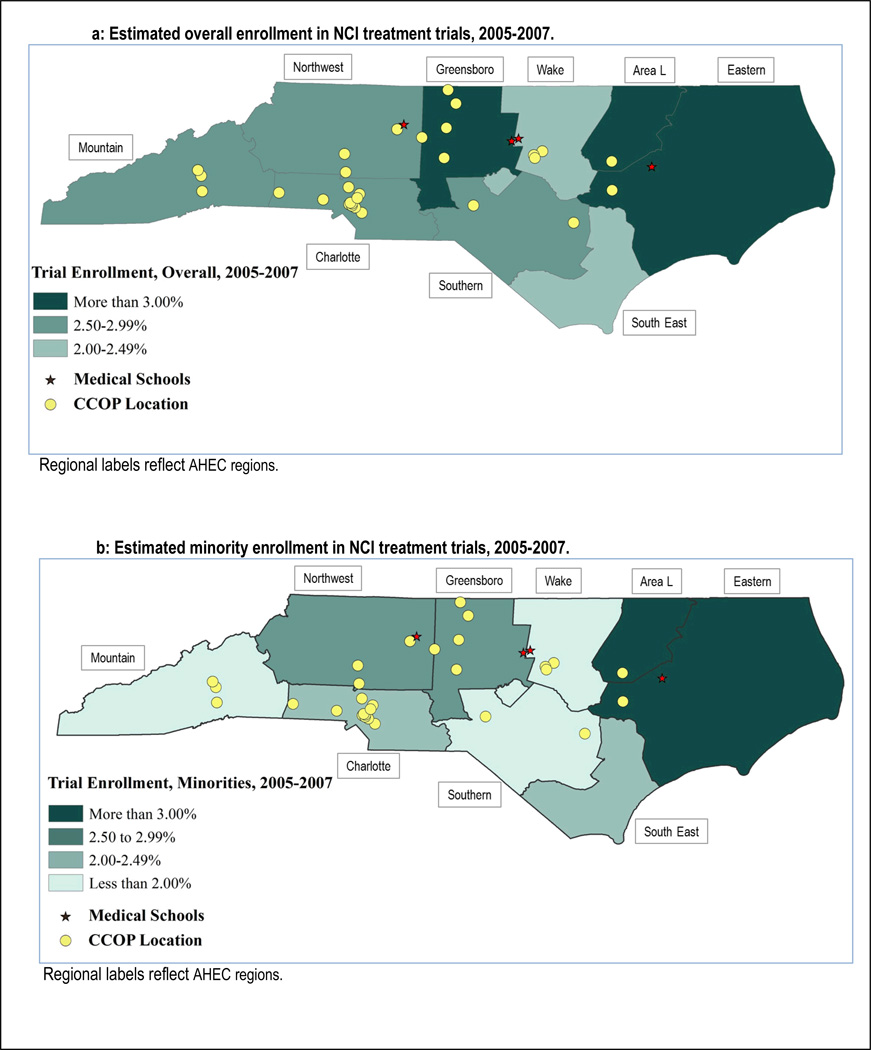
Figure 1 presents enrollment over time represented by 3-year averages. Overall, enrollment increased slightly over time, though more-so and more consistently among whites than among minorities (7.9% vs. 2.9%, respectively). The average enrollment rate was highest in the most recent 3-year period at 2.9% among whites, and 2.5% among minorities. Figure 2 presents county-level estimated enrollment rates by race from 2005 to 2007 and indicates the location of CCOP practices and medical schools. In Figure 2, and in Figure 3 in which counties have been grouped into regions, we see wide geographic variation in enrollment rates, including no enrollment among minorities in multiple counties.
Figure 1.
Trends in NCI Treatment Trial Enrollment Rates in North Carolina, by Race; 3-year averages, 1996–2007
Figure 2.
Geographic and racial variation in NCI treatment trial enrollment in North Carolina, by county, 2005–2007.
Figure 3.
Geographic and racial variation in NCI treatment trial enrollment in North Carolina, by AHEC Region, 2005–2007.
In a limited multivariable analysis, having a CCOP in the county was associated with substantially greater accrual rate (OR 1.51, 95% CI: 1.10–2.08) (Table 3). Having a medical school in the county tended to to increase accrual (OR 1.14, 95% CI: 0.68–1.9). Local insurance coverage was relevant, as counties with the smallest proportion of uninsured tended to have greater clinical trial enrollment (OR 1.53, 95% CI: 1.03–2.27). Enrollment in clinical trials increased over time (OR 1.08, 95% CI: 1.04–1.12), supporting the trend shown in Figure 1.
Table 3.
County-level factors associated with NCI treatment trial enrollment rates.
| County Characteristic | OR | 95% CI |
|---|---|---|
| CCOP practice in county | 1.51 | (1.10 – 2.08) |
| Medical School in county | 1.14 | (0.68 – 1.90) |
| Uninsured population | - | |
| Quartile 1: Fewest Uninsured | 1.53 | (1.03 – 2.27) |
| Quartile 2: Few Uninsured | 1.16 | (0.82 – 1.65) |
| Quartile 3: More Uninsured | 0.93 | (0.68 – 1.28) |
| Quartile 4: Most Uninsured | (ref) | |
| Region of the state (AHEC region) | ||
| AHEC Area L | 1.00 | (0.57 – 1.76) |
| AHEC Charlotte | 0.92 | (0.59 – 1.44) |
| AHEC Eastern | 0.81 | (0.54 – 1.20) |
| AHEC Greensboro | 1.19 | (0.77 – 1.85) |
| AHEC Mountain | 0.97 | (0.66 – 1.42) |
| AHEC Wake | 0.80 | (0.51 – 1.25) |
| AHEC Southeast | 0.77 | (0.43 – 1.39) |
| AHEC Southern | 0.83 | (0.51 – 1.35) |
| AHEC Northwest | (ref) | |
| Time (Year) | 1.08 | (1.04 – 1.12) |
CCOP: Community Clinical Oncology Program; AHEC: Area Health Education Center AHEC Regions are presented in Figure 3.
Based on sensitivity analyses, the enrollment estimates and trends were consistent between Phase III and overall enrollment, with overall enrollment rates slightly greater though consistently in-parallel with trends in Phase III trial enrollment rates. Contemporaneous and time-lagged estimates were similar, as were estimates using two-year and three-year moving averages, though three-year estimates were more smoothed, as expected. (Data not presented).
Discussion
The goal of this study was to develop a statewide system for ongoing monitoring and public reporting of NCI clinical treatment trial enrollment. This system builds upon the work of prior studies, which had many strengths, but also limitations including use of self-report of trial participation,28 estimates of cancer burden,29 data from a single institution,30 or data from a limited number of years.28, 31 This system has used actual state-wide population-based data on clinical trials participation from the NCI, and cancer incidence data from the North Carolina Central Cancer Registry spanning twelve years. It has demonstrated good consistency through rigorous sensitivity analyses, suggesting stability and utility in measuring trial enrollment rates over time. Our analyses revealed a modest increase in enrollment over time, though with substantial variation by gender and race. Men enrolled in trials at less than half the rate of women. Minority men had the lowest enrollment rate, enrolling at a significantly lower rate than white men. Geographic variation was evident, with the highest accrual rates in counties with a medical school or CCOP practice.
The finding that counties with a medical school or CCOP had higher enrollment rates points to the importance of access to these research resources with regard to both overall enrollment and attenuation of racial enrollment disparities. The importance of CCOPs is likely even greater than suggested here, since several CCOP practices serve multiple counties. For example, in the south-central region of the state, one CCOP practice is a referral center for a multi-county area, as demonstrated in the elevated clinical trial enrollment rates in the contiguous non-CCOP counties. This observation highlights the fact that health care is largely a local phenomenon, and without clinical trials networks and outreach programs, a large proportion of the North Carolina population would have no access to NCI clinical trials. It also speaks to the greater issue of health care disparities and differences in access to primary and specialty care. In North Carolina, the areas of low trial enrollment roughly correspond to those areas experiencing the greatest racial health disparities and challenges with health care access. This finding calls for future research to model geographic clustering more extensively than was possible within the scope of this study. The wide variation in enrollment by county also suggests merit in more extensive examination of additional factors that may be associated with enrollment.
Gender differences in enrollment speak to the relevance of not only geographic access, but also access in terms of trials and inclusion criteria relevant for men. The data in this study include accrual for 29 breast cancer trials, but only 17 prostate cancer trials in the year 2007. A current review of ClinicalTrials.gov reflects a similarly disproportionate picture.32 Lower enrollment rates among men thus may be in part due to the relative dearth of prostate cancer studies, restrictive inclusion criteria, and possibly men’s comparative lack of awareness or understanding of cancer trials.33, 34 Further examination of gender disparities in clinical trials enrollment is warranted, and may involve examination of more inherently sociological issues including men’s health care communication, care seeking behaviors, and perceptions of clinical trials.
This preliminary examination empirically has documented racial disparities in trial enrollment at the population-level and points to opportunities for intervention. Specifically, racial disparities appear to be associated with both gender and proximity to clinical research infrastructure. Thus, the perception of health care as a local phenomenon appears to be particularly true for minorities in North Carolina. This finding substantiates the NCI’s significant investment in resolving cancer health disparities by extending clinical trials outside academic centers into the community. Several NCI programs are designed to support research networks facilitating clinical trials access and cutting edge cancer care delivery among medically underserved populations, including the Cancer Disparities Research Partnership (CDRP) Program, the Community Networks Program (CNP), the Community Clinical Oncology Program (CCOP), the Minority-Based CCOP (MB-CCOP) and the Community Cancer Centers Program (NCCCP).2, 23, 35–37 This study lends empirical support to the long anecdotally observed effectiveness of these programs.5, 38
Among design considerations, first, there is not always temporal concordance between cancer incidence and trial enrollment, and such temporal alignment may differ among phase I, phase II, and phase III trials. Extensive sensitivity analyses examined enrollment for each phase of trial individually and collectively, including scenarios of temporal concordance (enrollment in same year as incidence), one-year lagged enrollment, and two-year lagged enrollment. This model was found to be consistently stable. While point estimates varied slightly, three-year averages and time trends were consistent, pointing to reliability of this approach for ongoing surveillance and monitoring. Second, as is standard among cancer registries, the NC CCR delays the release of data to researchers to assure complete case ascertainment before doing so. This delay may impede the timeliness of monitoring interventions designed to increase enrollment. However, the NCI trial data remain more current. Future research may examine the stability of these data alone and their independent utility for reporting to meet basic monitoring needs prior to availability of concurrent CCR data. Third, and very importantly, these data were examined at the county-level due to data use restrictions. Future analyses using person-level linked data and more granular geographic units would be much more informative and yield findings that are much more conducive to intervention. Thus, the goal of obtaining and examining person-level linked data remains significant. Finally, because this study examines North Carolina, its generalizability may be limited; however, these methods can be used by researchers to estimate trial enrollment rates in other states. Replication using national cancer registry data from the National Cancer Institute and Centers for Disease Control and Prevention35, 39 would allow nationwide estimates of county-level clinical trial enrollment.
This system was developed to track participation in NCI-sponsored cancer clinical trials. Comprehensive enrollment data on investigator-initiated and industry trials are exceedingly difficult to obtain, in large part due to their fragmented and often proprietary nature, which explains why enrollment rates in non-NCI trials nationally remains unknown. It is believed that most treatment trial enrollment is through NCI-sponsored trials; however, non-NCI trials tend to be early-phase trials whereas NCI trials are more often large-population Phase III trials.12 Future research should examine non-NCI trials access to and enrollment in smaller, early-phase industry-sponsored and investigator initiated trials remains important.
As cancer care becomes increasingly personalized, the need for heterogeneous enrollment is essential to the generalizability of trial results. Participation by patients from all subpopulations is needed if all are to benefit from advances in cancer research. With no population-based system for tracking trial enrollment, and no registry integrating both incidence and enrollment details, the system described here was developed collaboratively as a part of the NC-CCP, the Carolina Community Network (the NCI CNP in North Carolina), and the University of North Carolina’s University Cancer Research Fund (UCRF).15, 17, 36 A goal of these groups is to optimize cancer outcomes for North Carolinians, and so this system will be integrated into its Integrated Cancer Information and Surveillance System (ICISS)17, 40 for communicating with stakeholders and ongoing reporting of practical information for targeting interventions to increase enrollment, particularly among underserved and low-access populations. It is fully anticipated that this system will provide the same utility for other state systems pursuing these goals.
Acknowledgements
We thank Melissa Green and the Greensboro area Community Research Advocates who assisted in the conduct of the study, including interpretation of results. We thank Wayne Psek, Jinny Kim, Danny Yeh, and Charles Belden for their assistance with data management and earlier versions of this work. We thank Lori Minasian, Troy Budd, and colleagues from the NCI for their support in obtaining the clinical trial enrollment data, and Karen Knight, Chandrika Rao and colleagues at the North Carolina Central Cancer Registry for their support in obtaining the cancer incidence data. This study was reviewed and approved by the Institutional Review Board (IRB) at the University of North Carolina at Chapel Hill (IRB #08-1265).
Funding Acknowledgement
Portions of this work were supported by the Carolina Community Network through a grant from the National Cancer Institute [grant number U01 CA114629]; a contract with the National Cancer Institute [HHSW261200800726P]; training grants from the National Cancer Institute [grants number R25T CA 57726 and 5R25 CA 116339]; and the Integrated Cancer Information and Surveillance System (ICISS), UNC Lineberger Comprehensive Cancer Center with funding provided by the University Cancer Research Fund (UCRF) via the State of North Carolina.
Footnotes
Declaration of Conflicting Interests
The authors declare that there are no conflicts of interest.
References
- 1.Sullivan P, Goldmann D. The promise of comparative effectiveness research. JAMA. 2011;305:400–401. doi: 10.1001/jama.2011.12. [DOI] [PubMed] [Google Scholar]
- 2.Minasian LM, Carpenter WR, Weiner BJ, et al. Translating research into evidence-based practice: the National Cancer Institute Community Clinical Oncology Program. Cancer. 2010;116:4440–4449. doi: 10.1002/cncr.25248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamb S, Greenlick MR, McCarty D. Institute of Medicine. Washington, DC: National Academy Press; 1998. Bridging the Gap Between Practice and Research. [PubMed] [Google Scholar]
- 4.Lanier D. Practice-based research networks: laboratories for improving colorectal cancer screening in primary care practice. Med Care. 2008;46:S147–S152. doi: 10.1097/MLR.0b013e31817f0d00. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter WR, Reeder-Hayes K, Bainbridge J, et al. The role of organizational affiliations and research networks in the diffusion of breast cancer treatment innovation. Med Care. 2011;49:172–179. doi: 10.1097/MLR.0b013e3182028ff2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green LA, Fryer GE, Jr, Yawn BP, Lanier D, Dovey SM. The ecology of medical care revisited. N Engl J Med. 2001;344:2021–2025. doi: 10.1056/NEJM200106283442611. [DOI] [PubMed] [Google Scholar]
- 7.Institute of Medicine. Bridging the Gap Between Practice and Research. Washington DC: National Academies Press; 1998. [PubMed] [Google Scholar]
- 8.Zerhouni EA. Clinical research at a crossroads: the NIH roadmap. J Investig Med. 2006;54:171–173. doi: 10.2310/6650.2006.X0016. [DOI] [PubMed] [Google Scholar]
- 9.Zerhouni E. Medicine. The NIH Roadmap. Science. 2003;302:63–72. doi: 10.1126/science.1091867. [DOI] [PubMed] [Google Scholar]
- 10.Zerhouni EA. Translational and clinical science--time for a new vision. N Engl J Med. 2005;353:1621–1623. doi: 10.1056/NEJMsb053723. [DOI] [PubMed] [Google Scholar]
- 11.Zerhouni EA. US biomedical research: basic, translational, and clinical sciences. JAMA. 2005;294:1352–1358. doi: 10.1001/jama.294.11.1352. [DOI] [PubMed] [Google Scholar]
- 12.Institute of Medicine. Ensuring Quality Cancer Care. Washington, DC: National Academies Press; 1999. [PubMed] [Google Scholar]
- 13.GCC (Georgia Cancer Coalition) Georgia Center for Oncology Research and Education (Georgia CORE) 2011 [Google Scholar]
- 14.Comis RL, Miller JD, Aldige CR, Krebs L, Stoval E. Public attitudes toward participation in cancer clinical trials. J Clin Oncol. 2003;21:830–835. doi: 10.1200/JCO.2003.02.105. [DOI] [PubMed] [Google Scholar]
- 15.Shepherd W, editor. North Carolina Comprehensive Cancer Program. North Carolina's Plan for Comprehensive Cancer Control: A living plan by the people of North Carolina. 2010 ed. Raleigh, NC: NC DHHS; 2010. Mar 25, [Google Scholar]
- 16.North Carolina State Center for Health Statistics. Raleigh, NC: NCSCHS; 2010. 2008 Cancer Mortality Rates by Race. [Google Scholar]
- 17.O'Malley M, Blouin R, Pisano E, Rimer B, Roper W, Earp H. Research for North Carolina: The University Cancer Research Fund. North Carolina Medical Journal. 2008;96:299–302. [PubMed] [Google Scholar]
- 18.Christian MC, Trimble EL. Increasing participation of physicians and patients from underrepresented racial and ethnic groups in National Cancer Institute-sponsored clinical trials. Cancer Epidemiol Biomarkers Prev. 2003;12:277s–283s. [PubMed] [Google Scholar]
- 19.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 20.Stewart JH, Bertoni AG, Staten JL, Levine EA, Gross CP. Participation in surgical oncology clinical trials: gender-, race/ethnicity-, and age-based disparities. Ann Surg Oncol. 2007;14:3328–3334. doi: 10.1245/s10434-007-9500-y. [DOI] [PubMed] [Google Scholar]
- 21.Tejeda HA, Green SB, Trimble EL, et al. Representation of African-Americans, Hispanics, and whites in National Cancer Institute cancer treatment trials. J Natl Cancer Inst. 1996;88:812–816. doi: 10.1093/jnci/88.12.812. [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute. Rockville, MD: Center to Reduce Cancer Health Disparities, Community Networks Program (CNP) [Google Scholar]
- 23.National Cancer Institute. Rockville, MD: NCI; 2011. Center to Reduce Cancer Health Disparities, Community Networks Program (CNP) [Google Scholar]
- 24.National Institutes of Health and National Library of Medicine. US Department of Health and Human Services; 2011. ClinicalTrials.gov. [Google Scholar]
- 25.US Department of Health and Human Services - Health Resources and Services Administration. Area Resource File (ARF) Rockville, MD: 2009–2010. [Google Scholar]
- 26.Southeast Cancer Control Consortium. Southeast Cancer Control Consortium, Inc.; 2011. [Google Scholar]
- 27.National Cancer Institute. Rockville, MD: NIH; 2011. Community Clinical Oncology Program. [Google Scholar]
- 28.Baquet CR, Commiskey P, Daniel Mullins C, Mishra SI. Recruitment and participation in clinical trials: socio-demographic, rural/urban, and health care access predictors. Cancer Detect Prev. 2006;30:24–33. doi: 10.1016/j.cdp.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sateren WB, Trimble EL, Abrams J, et al. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J Clin Oncol. 2002;20:2109–2117. doi: 10.1200/JCO.2002.08.056. [DOI] [PubMed] [Google Scholar]
- 30.Kanarek NF, Tsai HL, Metzger-Gaud S, et al. Geographic proximity and racial disparities in cancer clinical trial participation. J Natl Compr Canc Netw. 2010;8:1343–1351. doi: 10.6004/jnccn.2010.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baquet CR, Ellison GL, Mishra SI. Analysis of Maryland cancer patient participation in national cancer institute-supported cancer treatment clinical trials. J Clin Oncol. 2008;26:3380–3386. doi: 10.1200/JCO.2007.14.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Advanced Search. National Institutes of Health and National Library of Medicine; 2011. ClinicalTrials.gov. [Google Scholar]
- 33.Comis R, Colaizzi D, Miller J. Cancer clinical trials (CCT) awareness and attitudes in cancer survivors. Journal of Clinical Oncology, 2006 ASCO Annual Meeting Proceedings. 2006;24:6061. [Google Scholar]
- 34.Miller JD, Kotowski MR, Comis RL, et al. Measuring cancer clinical trial understanding. Health Commun. 2011;26:82–93. doi: 10.1080/10410236.2011.527624. [DOI] [PubMed] [Google Scholar]
- 35.National Cancer Institute. SEER Data. Rockville, MD: 2011. [Google Scholar]
- 36.Vines AI, Teal R, Meyer C, Manning M, Godley P. Connecting community with campus to address cancer health disparities: a community grants program model. Prog Community Health Partnersh. 2011;5:207–212. doi: 10.1353/cpr.2011.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clauser SB, Johnson MR, O'Brien DM, Beveridge JM, Fennell ML, Kaluzny AD. Improving clinical research and cancer care delivery in community settings: evaluating the NCI community cancer centers program. Implement Sci. 2009;4:63. doi: 10.1186/1748-5908-4-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reeder-Hayes KE, Bainbridge J, Meyer AM, et al. Race and age disparities in receipt of sentinel lymph node biopsy for early-stage breast cancer. Breast Cancer Res Treat. 2011 doi: 10.1007/s10549-011-1398-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention. Atlanta, GA: Centers for Disease Control and Prevention; 2009. State Cancer Profiles. [Google Scholar]
- 40.University Cancer Research Fund. Chapel Hill, NC: 2012. Integrated Cancer Information and Surveillance System. [Google Scholar]