Abstract
The present study was carried out to investigate the protective effects of tempol on renal function and the underlying mechanism in streptozotocin-induced diabetic rats. The diabetic rats were randomly divided into Model group (without tempol) and Tempol group. Nondiabetic rats were served as Control group. The mRNA expression of canonical transient receptor potential 6 (TRPC6), transforming growth factor (TGF)-β1 and type IV collagen (Col IV) were examined. The malondialdehyde (MDA) level, activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) in renal tissues were measured to assess redox status in kidneys. We found that tempol significantly reduced 24-h urine output and urine albuminuria excretion in the diabetic rats. Compared with the model group, the concentration of MDA was significantly lower in tempol group. In addition, diabetes decreased activities of SOD and GSH-Px and these responses were prevented by tempol treatment. Moreover, in diabetic rats, the mRNA expression levels of TGF-β1 and Col IV were upregulated. TRPC6 mRNA expression level was downregulated in diabetic kidneys. However, all of these diabetic effects were significantly suppressed by tempol treatment. These results suggest that chronic treatment of diabetic rats with tempol can protect kidneys, possibly by reducing expression of TGF-β1, Col IV, and TRPC6.
Keywords: Diabetic nephropathy, Tempol, TRPC6, TGF-β1, Collagen IV
Introduction
Diabetes mellitus is increasingly prevalent worldwide. Diabetic nephropathy (DN) is one of the most common complications in diabetes mellitus, which is the leading cause of end-stage renal disease (1). The characteristic features of this disease are persistent albuminuria and structural alterations, such as thickened glomerular basement membrane and progressive accumulation of extracellular matrix proteins in the glomerular mesangium(2). Progressive loss of renal function, which ultimately results in end-stage renal disease requiring dialysis or transplantation, is an increasing health problem around the world (3).
The early stage of diabetic mellitus is characterized by renal hyperfiltration, which promotes the eventual development of DN. Although preglomerular afferent arteriolar dilation and diminished responsiveness of this vascular segment to a variety of vasoconstrictors have been proposed to be a contributing factor (4), increasing evidence suggests that dysfunction of glomerular mesangial cells (MCs) is also involved in the diabetic hyperfiltration (5–7). MCs are important targets of metabolic abnormalities in diabetes and contribute to the functional and structural abnormalities of DN. MCs are located within glomerular capillary networks, regulating effective filtration surface area. MC contractility is impaired in diabetes and this impairment disrupt normal glomerular hemodynamics (8). Decreased Ca2+ influx contributes to the impaired contractile function of MCs, presumably due to altered calcium channel number and/or activity (9).
Recently, canonical transient receptor potential (TRPC) proteins have been proposed as Ca2+-permeable cation channels that are activated in response to stimulation of G-protein coupled receptors (10–12). The TRPC family includes seven related members, designated as TRPC1-7 (13). TRPC6 in this family is expressed in MCs and participates in Ang II-induced MC contraction (14–16). Our previous study showed that in glomeruli isolated from streptozotocin (STZ)-induced diabetic rats, TRPC6 was markedly reduced compared with the glomeruli of control rats (15, 17). Furthermore, TRPC6 mRNA in MCs was also significantly decreased by high glucose (17). Accumulating evidence implies that downregulation of TRPC6 leads to the impaired Ca2+ signaling and hypocontractility of diabetic MCs. Thus, the reduced mesangial responsiveness to vasoactive agents seen in diabetes may be partially due to reduced TRPC6 expression (17). Multiple pathogenic mechanisms are now believed to contribute to DN, including inflammatory cytokines, autacoids and oxidative stress. Among them, oxidative stress has been suggested to play an important role in the pathogenesis of DN (18–20). An increased production of reactive oxygen species (ROS), including superoxide anion, contributes to the pathogenesis of renal injury in diabetes mellitus (21). Superoxide dismutase (SOD) is the major antioxidant enzyme for superoxide removal, which converts superoxide into hydrogen peroxide and molecular oxygen. The hydrogen peroxide is further detoxified to water by catalase or glutathione peroxidase(22). As SOD and catalase activities are decreased in diabetes, the improvement of the antioxidant system including SOD could be a potential therapeutic target in diabetic nephropathy (23).
Tempol (4-hydroxy-2, 2, 6, 6-tetramethyl-piperidine-1-oxyl) is a stable, metal-independent low-molecular weight SOD mimetic with an excellent cell-permeability. The reaction of tempol with superoxide anion to form hydrogen peroxide accounts for its “SOD mimetic” action. As reported, tempol normalized blood pressure in hypertensive rats and dilated afferent arterioles of diabetic rabbits (24, 25). And also, tempol protected the kidney from ischemic damage and inhibited hypertensive renal damage in the Dahl salt-sensitive model (26, 27). However, few studies have investigated the preventative effect of tempol on DN and its possible mechanisms of action. Thus, the present study was carried out to examine the effect of tempol on progression of DN and the expressions of several diabetes-associated proteins in a streptozotocin rat model of diabetes. The findings in this study may provide a theoretical basis for clinical application of tempol.
Materials and methods
Animals and experimental protocols
Male Sprague-Dawley rats (~8 wk) were purchased from the Experimental Animal Center of Anhui (Certificate No. SCXK 2005-001). The animals were housed individually in cages at constant humidity (55±5%) and temperature (25±2 °C) with an electrically controlled 12-h light–dark cycle. Food and water were provided ad libitum. This study was approved by the Animal Care and Use Committee of Anhui Medical University, and all studies were conducted in accordance with “Guiding for the Care and Use of Laboratory Animals” adopted by the Committee on the Care and Use of Laboratory Animals of Anhui Medical University. Diabetes was induced by a single injection of STZ (Sigma Chemical Co., St. Louis, MO) into the rat tail vein at 60 mg/kg in sodium citrate buffer (0.01 M, pH 4.5) after fasting overnight. Age- and weight-matched control rats were given an equivalent volume of the vehicle (citrate buffer) alone. The fasting blood glucose (FBG) levels in tail vein blood were determined 72 h later and periodically thereafter with a glucometer (Life Scan One Touch glucometer, Johnson & Johnson). STZ-injected rats with sustained elevation of blood glucose above 16.7mmol/L were designated as diabetic rats (28). The diabetic rats were randomly divided into 2 groups matched for body weight and blood glucose: untreated DM group (Model group, n=10) and a diabetic group given tempol (Sigma Chemical Co., St. Louis, MO) in their drinking water (1 mM) for 6 weeks (Tempol group, n=10), beginning 24 h after the induction of diabetes. Age- and weight-matched rats with sodium citrate buffer injection alone were served as controls (Control group, n=10).
Sample preparation
Body weight, daily food intake, daily water intake and daily urine output of all rats were measured at Pre-therapy, 4 and 6 weeks after tempol treatment. Urine was collected via metabolic cage. After 6 weeks of tempol treatment, the rats after fasting overnight were anesthetized with 10% chloral hydrate (30 mg/kg body weight intraperitoneally), and then blood was drawn from the abdominal aorta for measuring blood chemical parameters. After blood sampling, kidneys were then processed for biochemical, histologic, and immunochemical examinations as described below.
Measurement of urinary parameters
After 24-h urine collection, the samples were centrifuged (3000 rev/min, 4°C, 10 min) and the supernatant was stored at −20°C. The urine samples were used to calculate urine volume and urinary albumin excretion (UAE). Urine protein was measured by the Bradford method (Bio-Rad, Richmond, VA). Meanwhile Urinary TGF-β1 excretion in daily urine was determined by Enzyme-linked Immunosorbent Assay (ELISA) (Senxiong Bio-Tech, Shanghai, China).
Measurement of blood parameters
The FBG levels in tail vein blood were determined with a glucometer at Pre-therapy, 4 and 6 weeks after tempol treatment. Serum samples from rats were collected for measurement of glycosylated serum protein (GSP), fasting serum insulin (FINS), creatinine (Cr) and blood urea nitrogen (BUN). The detection was carried out using diagnostic kits (Jiancheng Institute of Bioengineering Company, Nanjing, China). GSP was determined with Fructosamine assay. BUN was reacted with diacetyl to produce a red compound and measured by spectrophotometer. Serum Cr was measured by the modified picric acid method. FINS was assayed by ELISA (Yuanye Bio-Tech, Shanghai, China). Insulin sensitivity index [ISI = 100/ (FBG×FINS)] was applied to assess the status of insulin resistance.
Measurement of MDA level, activities of SOD and GSH-Px in the kidney
After blood sampling, the kidneys were quickly removed from the animals and the surrounding fat was cleaned. They were washed in a sterile saline solution and weighed. The renal tissue was homogenized in a nine-time volume of ice-cold normal saline, and then the homogenates were centrifuged (4000 rev/min, 4°C, 10 min). The level of malondialdehyde (MDA) and activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) in the kidney were measured following manufacturer’s instruction (Jiancheng Institute of Bioengineering Company, Nanjing, China).
Histological examination
The renal samples were fixed in 4% paraformaldehyde, embedded with paraffin and cut into sections of about 4μm thickness, and stained with periodic acid-Schiff (PAS) staining. The slides were examined under light microscopy with magnification of 400× by a pathologist blind to the experimental profile. The purple color in glomeruli was defined as “positive” and the positive area of each glomeruli was measured using JD801 morphological microscope image analysis system (JEDA Science-Technology Development Co., Ltd, Jiangsu, China). The positive score (PS) of glomeruli in one rat was calculated by averaging positive scores of 30 glomeruli from the same rat using the formula: , where AP represents the positive area and AG represents the area of glomerulus. Six rats were examined in each group.
RT-PCR
Total kidney RNA was extracted using TRIzol (Invitrogen, Germany) according to the instructions from the manufacturer. The extracts wer resuspended in 20 μl of DEPC-treated water. RNA concentration was determined using a biophotometer (Shanghai Scientific China). Four micrograms of RNA underwent reverse transcription to generate cDNA using random hexamer primers and Primescript™ RTase (TaKaRa BIO). PCR was conducted using a RT-PCR kit (Promega, USA). Primer sequences (Shanghai Sangon Bio-Tech) and annealing temperatures for TGF-β1, Col IV, TRPC6 and β-actin PCR are listed in Tab 1. PCR products and a 1 kb DNA molecular weight marker (Promega, USA) were then electrophoresed on a 1% agarose gel, and the gel was visualized and photographed using a gel imaging system (Biosens SC810X, Shanghai Bio-Tech). The gel imaging software was used for quantitative analyses. Relative quantities of target gene expression were compared after normalization to the value of β-actin, a housekeeping gene.
Tab 1.
Oligonucleotide primers used for cDNA amplification.
| mRNA | Sense primer | Antisense primer | Anneal temperature (°C) | PCR target (bp) | Cycles |
|---|---|---|---|---|---|
| TGF-β1 | 5′-CTG TCC AAA CTA AGG CTC GC-3′ | 5′-AG A CAG CCA CTC AGG CGT A-3′ | 55 | 432 | 38 |
| Col IV | 5′-ATT CCT TTG TGA TGC ACA CCA G-3′ | 5′-AAG CTG TAA GCA TTC GCG TAG TA-3′ | 56 | 151 | 38 |
| TRPC6 | 5′-AAA GAT ATC TTC AAA TTC ATG GTC-3′ | 5′-CAC GTC CGC ATC ATC CTC AAT TTC-3′ | 54 | 327 | 38 |
| β-actin | 5′-AGC ATT TGC GGT GCA CGA TGG AGG G-3′ | 5′-ATG CCA TCC TGC GTC TGG ACC TGG C-3′ | 53 | 606 | 38 |
Statistical analysis
Data were expressed as means ±S.D. One-way analysis of variance (ANOVA) was used to test differencea among groups and Student-Newman-Keuls(SNK) test was adopted to assess which two groups have significant difference. Value of P < 0.05 was considered to be significant.
Results
Physical and Metabolic Parameters
Data were obtained at pre-therapy, 4 and 6 weeks after tempol treatment. It was observed that the body weight increased in control group. The body weights in model group were significantly lower in comparison with the control group (P < 0.01) at the end of the experiment. In contrast, the weights in diabetic rats after tempol treatment were higher than the model group. As shown in Tab 2, daily food and water intake (normalized to rat body weight) of control group were unchanged throughout the experiment. However, the daily food and water intake in the model group were significantly higher than control rats. And also, daily food and water intake significantly decreased (P < 0.01) in tempol group. Similarly, urine output (UO, normalized to rat body weight) in the control group did not change throughout the experiment. In contrast, UO were significantly higher in model group than in the control group (P < 0.01). After tempol treatment, UO were lower than that in the model group (P < 0.01). An increase in UAE amount is the earliest manifestation of renal disorder in diabetes. The time course of daily UAE was shown in Tab 2. UAE in the model group increased significantly compared with that in the control group (P < 0.01). Tempol treatment decreased UAE of diabetic rats significantly (P < 0.01) in comparison with the model group at the end of the experiment. Likewise, Urinary TGF-β1 excretion in daily urine in model group increased significantly compared with the control group (P < 0.01). Tempol administration decreased this parameter of diabetic rats significantly (P < 0.01). These results indicate that hypermetabolism in diabetic rats was persistent.
Tab 2.
Effect of Tempol on the physiological parameters in diabetic rats induced by STZ.
| Parameter | Pre-therapy | 4 weeks | 6 weeks | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Control | Model | Tempol | Control | Model | Tempol | Control | Model | Tempol | |
| BW, g | 250±18 | 228±19# | 234±25 | 293±20 | 179±16## | 220±43* | 307±17 | 171±16## | 235±39** |
| Daily food intake, g/100g BW | 9±2 | 12±3# | 14±3## | 10±3 | 25±5## | 17±6** | 10±2 | 25±6## | 13±6** |
| Daily water intake, g/100g BW | 15±3 | 59±8## | 62±18## | 14±2 | 91±12## | 54±13** | 14±2 | 87±9## | 32±12** |
| UO, ml/100g BW | 8±3 | 49±10## | 52±13## | 6±2 | 92±17## | 53±17** | 5±1 | 94±27## | 43±14** |
| Urinary albumin excretion, mg/d | 6.47±0.89 | 24.88±4.97## | 23.35±5.02## | 6.27±0.86 | 50.00±8.62## | 17.04±3.47* | 6.26±0.68 | 48.12±7.73## | 15.82±2.72** |
| Urinary TGF-β1 excretion, ng/d | 3.55±0.71 | 18.80±4.27## | 20.03±4.56## | 3.66±0.78 | 44.91±9.74## | 19.15±5.58** | 3.74±0.59 | 41.12±8.43## | 17.30±3.90** |
| GSP, mmol•L−1 | ND | ND | ND | ND | ND | ND | 1.13±0.09 | 1.78±0.20## | 1.30±0.25** |
| FBG, mmol•L−1 | 4.9±0.5 | 20.0±2.3## | 20.5±2.0## | 4.8±0.9 | 26.3±3.0## | 19. 7±5.4** | 4. 8±0.3 | 30.7±3.5## | 22.1±4.4 ** |
| FINS, mU L−1 | ND | ND | ND | ND | ND | ND | 9.88±1.37 | 17.31±0.76## | 7.91±2.43** |
| ISI | ND | ND | ND | ND | ND | ND | 2.17±0.39 | 0.19±0.03## | 0.62±0.14** |
| KW/BW, mg/g | ND | ND | ND | ND | ND | ND | 7.36±1.10 | 14.45±2.74## | 9.49±2.33** |
BW, body weight; UO, urine output(was normalized to rat body weight); ND, not determined;GSP, glycosylated serum protein; FBG, fasting blood glucose; FINS, fasting serum insulin; ISI, insulin sensitivity index=100/(FBGxFINS); KW, kidney weight(both kidneys); KW/BW, ratio of kidney weight to body weight; Control, nondiabetic rats; Model, diabetic rats; Tempol, diabetic rats treated with tempol(was supplemented to the rat’s drinking water at a concentration of 1 mM); Data are presented as means ± SD. n=10 rats in each group;
p < 0.05,
p < 0.01 vs. control rats;
p < 0.05,
p < 0.01 vs. model rats.
As shown in Tab 2, FBG and GSP were significantly higher in model group than those in control group (P < 0.01). Tempol treatment showed a decreased tendency in FBG and GSP and the difference was significant (P < 0.01). However, blood glucose did not return to normal level at the end of the experiment.
At the 6th week after tempol treatment, FINS levels in model group increased markedly compared with control group, but it was decreased by tempol treatment. Meanwhile, ISI of model group was lower than control group (P < 0.01). Increased ISI of diabetic rats were observed in the tempol group compared with model group. Therefore, tempol treatment could elevate ISI of diabetic rats. Furthermore, the kidney weight/body weight ratio (KW/BW) was significantly increased in model group in comparison with the control group (P <0.01). After tempol administration, KW/BW was decreased significantly compared with model group (P<0.01). In addition, the serum levels of BUN and Cr in model group were markedly higher than the control group (P < 0.01). The creatinine clearance rate of model group decreased obviously compared with control group. Tempol significantly affected the elevated BUN and Cr concentration of diabetic rats (P <0.01). Meanwhile, tempol treatment significantly improved the creatinine clearance rate of diabetic group (P <0.01) (Tab 3).
Tab 3.
Effect of Tempol on serum Creatinine, Urea nitrogen and Creatinine clearance rate of diabetic rats induced by STZ.
| Group | Creatinine (umol/L) | Blood urea nitrogen (mmol/L) | Creatinine clearance rate (ml/min·kg) |
|---|---|---|---|
| Control | 49.80±3.65 | 5.78±0.22 | 3.14±0.52 |
| Model | 123.62±18.33## | 15.95±2.61## | 1.88±0.17## |
| Tempol | 79.03±7.68** | 8.98±0.89** | 3.00±0.53** |
The abbreviations are the same as in the legend to Tab 2. Data are presented as means ± SD. n=10 rats in each group.
p < 0.01 vs. control rats;
p < 0.01 vs. model rats.
MDA level, activities of SOD and GSH-Px in renal tissue
The MDA level, activities of SOD and GSH-Px in renal tissue are shown in Tab 4. The MDA level was increased in model group compared with that in the control group (P <0.01). Tempol treatment reduced MDA level compared to the model group (P <0.01). The activities of SOD and GSH-Px in the control group was significantly higher than that in other two groups (P <0.01). The activities of SOD and GSH-Px were markedly reduced in the model group compared with the control group. The tempol treatment relieved these alterations (P<0.01) (Tab 4).
Tab 4.
Effect of Tempol on MDA level and activities of T-SOD and GSH-Px in the renal tissue of diabetic rats induced by STZ.
| Group | T-SOD (U/mg protein) | MDA (nmol/ mg protein) | GSH-Px (U/ mg protein) |
|---|---|---|---|
| Control | 153.88±29.82 | 3.80±1.25 | 159.30±33.34 |
| Model | 65.68±20.15## | 7.89±1.72## | 82.58±20.18## |
| Tempol | 95.86±32.09* | 4.56±1.51** | 124.51±35.07** |
The abbreviations are the same as in the legend to Tab 2. Data are presented as means ± SD. n=10 rats in each group.
p < 0.01 vs. control rats;
p < 0.05,
p < 0.01 vs. model rats.
Renal histology and morphometric analysis
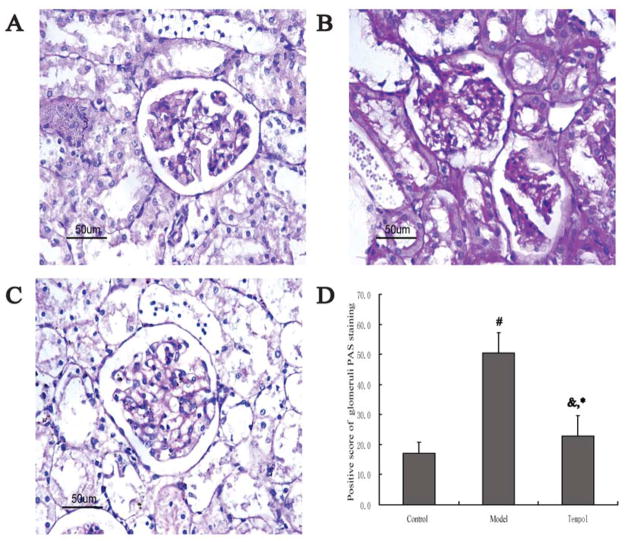
Glomeruli were easily distinguished by their characteristic circular morphological aspect bordered by the peripheral lumen. Fig 1 shows representative histopathological changes in the kidney with PAS staining and morphometric analysis in each group at the 6th week after tempol treatment. The model group showed extensive mesangial matrix expansion (Fig 1B), whereas no change was observed in the control rats (Fig 1A). PAS-positive areas increased significantly in the model group compared with the control group (50.51±6.67 vs. 17.12±3.67, p < 0.01) (Fig 1D). The positive score of glomeruli PAS staining increased markedly in model group compared with control group. However, the positive score of glomeruli PAS staining was reduced significantly (22.79±6.88 vs. 50.51±6.67, p < 0.01) by tempol treatment (Fig 1D).
Fig. 1.
Morphological changes of glomeruli in renal cortex in the different groups (PAS staining×400). A: Control group. B: Model group. C: Tempol group. D: Positive score of glomerular PAS staining in the different groups. Each bar indicates 50 μm. n=6 rats in each group. Data are expressed as the mean ± S.D. &p < 0.05, #p < 0.01 compared with Control group using ANOVA followed by the Student-Newman-Keuls (SNK) test. *p < 0.01 compared with the Model group by SNK test.
The mRNA expression of TGF-β1, Col IV and TRPC6 in the kidney
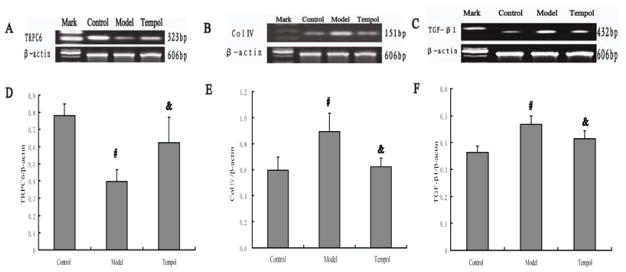
Consistent with the results of immunohistochemistry, expression of TRPC6mRNA in diabetic kidneys was decreased dramatically compared with the control group (P < 0.01). However, expression of TGF-β1 mRNA and Col IV mRNA in diabetic kidneys was increased dramatically relative to the control (P < 0.01). Up-regulation of TGF-β1mRNA and Col IV mRNA expression was obviously decreased by administration of tempol (P < 0.05) (Fig 2). Compared with the model group, tempol treatment increased the expression of TRPC6mRNA significantly (P < 0.05) (Fig 2).
Fig. 2.
Effect of Tempol on mRNA levels of TRPC6, TGF-β1 and Col IV in kidney of STZ-induced diabetic rats. A: TRPC6 mRNA expression. B: Col IV mRNA expression. C: TGF-β1 mRNA expression. D: Quantification of TRPC6 mRNA expression from three groups indicated in A. E: Quantification of Col IV mRNA expression from three groups indicated in B. F: Quantification of TGF-β1 mRNA expression from three groups indicated in C. In all groups, β-actin was used for loading controls. n=4 rats in each group. Data are expressed as the mean ± S.D. #p<0.01 compared with the Control group using ANOVA followed by Student-newman-Keuls (SNK) test; &p<0.05 compared with the Model group by SNK test.
Discussion
As an important complication of diabetes mellitus, DN has become the most common cause of end-stage renal failure among patients undergoing chronic hemodialysis therapy (3). DN is characterized by a series of changes, including renal hypertrophy, oxidative stress, basement membrane thickening and extracellular matrix accumulation, which eventually results in the loss of renal function (29). Although hyperglycemia is clearly a prerequisite for the development of DN, hyperglycemia itself is insufficient for the progression of the diabetic complication. A variety of derangements associated with diabetes contribute to the development of DN. Among them, oxidative stress has been suggested to play an important role in the pathogenesis of DN (30). Enhanced oxidative stress is widely recognized as a key pathogenic factor and contributed to pathological development and progression of DN (31–34). Markers of oxidative stress such as ROS and reduced levels of antioxidants have been found in the blood and renal tissues in both human and experimental diabetes (35–36).
Tempol is a stable and cell membrane-permeable SOD mimetic and has been successfully used to investigate the role of ROS in renal function in intact animals (15,37). In the present study, we used an STZ-induced diabetes rat model to demonstrate the therapeutic efficacy of tempol in early DN. Hyperglycemia, albuminuria, renal hypertrophy, and expansion of mesangial area, which are the hallmarks of DN, were confirmed in STZ-induced diabetic rats. Tsuchida et al (38) reported that high degree of oxidative stress in the renal tissue coincides with biochemical signs typical of DN, such as high level of UAE, which is the classical glomerular injury marker of DN. In our study, the 24-h UAE was markedly higher in diabetic rats than in control rats. Furthermore, the kidney weight/body weight ratio was significantly increased in diabetic rats in comparison with the control rats. These results indicate that the renal disorder in STZ-induced diabetic rats, which is early stage DN, is similar to that seen in humans (39).
Antioxidant enzyme expression in DN patients is decreased. SOD and GSH-Px constitute the principal components of antioxidants and their deficiencies can cause oxidative stress. The superoxide dismutase enzyme system is a primary determinant of superoxide removal (40). The content of MDA is a good index of intensified oxidative stress in the tissues, showing enhanced peroxidation processes (41). It has been reported that an imbalance between the production of ROS and antioxidants is believed to be involved in diabetes-induced renal failure (42). In this study, the production of MDA was enhanced in diabetic rats, indicating an oxidative stress state in experimental diabetic rats. We have demonstrated a significant diminution of SOD and GSH-Px activities in the kidney of rats after 6 weeks of STZ-induced diabetes. The findings are consistent with those previously reported in this model of diabetes (43). After tempol treatment, a significant decrease in MDA level and increase in activities of SOD and GSH-Px were observed in this study. These results suggest that tempol has a renal protective role against oxidative damage in diabetic nephropathy. Reduction of oxidative stress markers was associated with reduction in renal damage parameters. Our study demonstrated that BUN and Cr levels in serum were higher in DN rats than those in control rats. The creatinine clearance rate in diabetic rats was also obviously decreased compared with non-diabetic rats. The results of our experiment showed that continuous administration of tempol could retrieve renal dysfunction and ameliorate hyperglycemia induced by STZ. Tempol significantly affected the elevated FBG and GSP concentrations of diabetic rats. Accordingly, tempol could correct insulin resistance and the associated renal damage in STZ-induced diabetes. Meanwhile, it was also found that tempol markedly suppressed glomerular hypertrophy, the expansion of mesangial matrix and the accumulation of ECM.
The globally increasing number of patients with end-stage renal disease urges the identification of molecular pathways involved in renal pathophysiology, to serve as targets for intervention. TGF-β1 is the key regulator of extracellular matrix remodeling in the mesangium leading to mesangial expansion (44), and induction of TGF-β1 is a well-documented molecular event during the development of diabetic nephropathy both in vitro and in vivo(45–48). Moreover, collagen accumulation is the hallmark of glomerular sclerosis, and increase of Col IV plays a central role in this process. Excessive deposition of Col IV is an established feature associated with diabetic glomerulopathy (49). Studies have demonstrated that several stimuli increase TGF-β1 expression, such as hyperglycemia and oxidative stress (50). In particular, several investigators stated that ROS participates in TGF-β1 expression and that TGF-β1 promotes renal fibrous change that leads to renal damage (51, 52). Up-regulation of TGF-β1 by ROS will lead to excessive production of ECM, resulting in glomerular fibrosis, and ultimately loss of renal function (53). Interestingly, TGF-β1 can also up-regulate Col IV in all glomerular cell types (48). As we expected, the mRNA expression of Col IV and TGF-β1 was dramatically increased in diabetic rats in comparison with the control rats. The results of RT-PCR showed that significant up-regulation of Col IV and TGF-β1 expression was concomitant with deterioration of renal function and contributed to the excessive deposition of glomerular extracellular matrix in DN. The accumulation of TGF-β1 and Col IV in glomeruli in diabetic rats was also inhibited by tempol at the 6th week after starting treatment. As reported, tempol prevented renal injury in the Dahl salt-sensitive hypertensive rat via reduction of oxidative stress and TGF-β1 (54). High glucose induces ROS and up-regulates TGF-β1 and Col IV expression in the glomerular mesangial cells (18, 55). Taken together, these protective effects of tempol are thought to be mediated through the down-regulation of suppression of TGF-β1, Col IV and reduction of excessive ROS production. It is our finding that tempol improved renal function of DN rats and this beneficial effect was associated with the inhibitory effect of tempol on kidney TGF-β1 over-expression and Col IV over-production. Also, renal histological examination revealed that tempol significantly ameliorated diabetic-induced mesangial expansion. These findings imply potential efficacy of tempol in preventing the DN progression.
The early stage of diabetic mellitus is characterized by renal hyperfiltration, which promotes the eventual development of DN. Increasing evidence suggests that the dysfunction of glomerular MCs is involved in diabetic hyperfiltration. Impaired Ca2+ signaling has been inferred to be a major contributing factor to the dysfunction of diabetic MCs (7, 20). TRPC6 has been known to function as receptor operated Ca2+ channel in a variety of cell types (56). For instance, TRPC6 was detected and characterized as a component of the slit diaphragm multiprotein complex of glomerular podocytes, suggesting that it functions as a critical regulator of normal renal function (57). We have revealed that knockdown of endogenous TRPC6 in MCs reduced the Angiotonin (Ang) II-induced channel activity, implying that TRPC6 protein might be the channel or a critical component of the channel complexes mediating the Ang II-stimulated membrane response (17). Thus it is not surprising that TRPC6 participates in the contractile function of MCs and deficiency of this channel protein leads to impairment of agonist-induced mesangial contraction that subsequently results in supernormal glomerular filtration rate. In our study, the mRNA expression level of TRPC6 in the kindney was evaluated by RT-PCR and compared between nondiabetic rats and diabetic rats with and without tempol treatment. TRPC6 mRNA was strongly expressed in the healthy control rats. Comparing to the model group, there was a great improvement of expression of TRPC6 in the tempol group. In our previous article (15), we have found that expression of TRPC6 protein in glomeruli isolated from STZ-diabetic rats was dramatically decreased compared to the glomeruli from non-diabetic control rats. And also, tempol treatment significantly increased expression of TRPC6 protein, suggesting that antioxidant treatment efficiently suppressed the effect of diabetes on TRPC6. These results indicate that diabetes decreased the TRPC6 protein expression by repression of TRPC6 gene by ROS, suggesting a potential implication of TRPC6 in the development of DN.
In summary, the results from this study showed that chronic treatment of diabetic rats with tempol ameliorated the pathological changes in glomeruli, possibly by reducing expression levels of TGF-β1, Col IV, and increasing abundance of TRPC6. Our findings provide a rationale for treating DN with antioxidants.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81173624, Li), the National Institutes of Health, the United States of America (RO1 DK079968-01A2, Ma), the Nature Science Foundation of Anhui Province (11040606M201, Li) and the Nature Science Foundation of Anhui Province Education (KJ2009A81, Li; KJ2012A192, Li).
References
- 1.Ibrahim HN, Hostetter TH. Diabetic nephropathy. J Am Soc Nephrol. 1997;8:487–493. doi: 10.1681/ASN.V83487. [DOI] [PubMed] [Google Scholar]
- 2.Osterby R, Gall MA, Schmitz A, Nielsen FS, Nyberg G, Parving HH. Glomerular structure and function in proteinuric type 2 (non-insulin dependent) diabetic patients. Diabetologia. 1993;36:1064–1070. doi: 10.1007/BF02374500. [DOI] [PubMed] [Google Scholar]
- 3.Choudhury D, Tuncel M, Levi M. Diabetic nephropathy -- a multifaceted target of new therapies. Discov Med. 2010;10:406–415. [PubMed] [Google Scholar]
- 4.Brindeiro CMT, Fallet RW, Lane PH, Carmines PK. Potassium channel contributions to afferent arteriolar tone in normal and diabetic rat kidney. Am J Physiol Renal Physiol. 2008;295:F171–178. doi: 10.1152/ajprenal.00563.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kikkawa R, Kitamura E, Fujiwara Y, Arimura T, Haneda M, Shigeta Y. Impaired contractile responsiveness of diabetic glomeruli to angiotensin II: a possible indication of mesangial dysfunction in diabetes mellitus. Biochem Biophysic Res Commun. 1986;136:1185–1190. doi: 10.1016/0006-291x(86)90459-6. [DOI] [PubMed] [Google Scholar]
- 6.Menè P, Pascale C, Teti A, Bernardini SV, Cinotti GA, Pugliese F. Effects of advanced glycation end products on cytosolic Ca2+ signaling of cultured human mesangial cells. J Am Soc Nephrol. 1999;10:1478–1486. doi: 10.1681/ASN.V1071478. [DOI] [PubMed] [Google Scholar]
- 7.Nutt LK, O’Neil RG. Effect of elevated glucose on endothelin-induced store-operated and non-store-operated calcium influx in renal mesangial cells. J Am Soc Nephrol. 2000;11:1225–1235. doi: 10.1681/ASN.V1171225. [DOI] [PubMed] [Google Scholar]
- 8.Stockand JD, Sansom SC. Glomerular mesangial cells: electrophysiology and regulation of contraction. Physiol Rev. 1998;78:723–744. doi: 10.1152/physrev.1998.78.3.723. [DOI] [PubMed] [Google Scholar]
- 9.Whiteside CI, Hurst RD, Stevanovic ZS. Calcium signaling and contractile response of diabetic glomerular mesangial cells. Kidney Int Suppl. 1995;51:S28–S33. [PubMed] [Google Scholar]
- 10.Horinouchi T, Terada K, Higa T, Aoyagi H, Nishiya T, Suzuki H, et al. Function and regulation of endothelin type A receptor-operated transient receptor potential canonical channels. J Pharmacol Sci. 2011;117:295–306. doi: 10.1254/jphs.11162fp. [DOI] [PubMed] [Google Scholar]
- 11.Bush EW, Hood DB, Papst PJ, Chapo JA, Minobe W, Bristow MR, Olson EN, McKinsey TA. Canonical transient receptor potential channels promote cardiomyocyte hypertrophy through activation of calcineurin signaling. J Biol Chem. 2006;281:33487–33496. doi: 10.1074/jbc.M605536200. [DOI] [PubMed] [Google Scholar]
- 12.Facemire CS, Mohler PJ, Arendshorst WJ. Expression and relative abundance of short transient receptor potential channels in the rat renal microcirculation. Am J Physiol Renal Physiol. 2004;286:F546–F551. doi: 10.1152/ajprenal.00338.2003. [DOI] [PubMed] [Google Scholar]
- 13.Du J, Sours-Brothers S, Coleman R, Ding M, Graham S, Kong D, Ma R. TRPC1 channel is involved in contractile function of glomerular mesangial cells. J Am Soc Nephrol. 2007;18:1437–1445. doi: 10.1681/ASN.2006091067. [DOI] [PubMed] [Google Scholar]
- 14.Sours S, Du J, Chu S, Ding M, Zhou XJ, Ma R. Expression of canonical transient receptor potential (TRPC) proteins in human glomerular mesangial cells. Am J Physiol Renal Physiol. 2006;290:F1507–1515. doi: 10.1152/ajprenal.00268.2005. [DOI] [PubMed] [Google Scholar]
- 15.Graham S, Gorin Y, Abboud HE, Ding M, Lee DY, Shi H, et al. Abundance of TRPC6 protein in glomerular mesangial cells is decreased by ROS and PKC in diabetes. Am J Physiol Cell Physiol. 2011;301:C304–315. doi: 10.1152/ajpcell.00014.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du J, Ding M, Sours-Brothers S, Graham S, Ma R. Mediation of angiotensin II-induced Ca2+ signaling by polycystin 2 in glomerular mesangial cells. Am J Physiol Renal Physiol. 2008;294:F909–918. doi: 10.1152/ajprenal.00606.2007. [DOI] [PubMed] [Google Scholar]
- 17.Graham S, Ding M, Sours-Brothers S, Yorio T, Ma JX, Ma R. Downregulation of TRPC6 protein expression by high glucose, a possible mechanism for the impaired Ca2+ signaling in glomerular mesangial cells in diabetes. Am J Physiol Renal Physiol. 2007;293:1381–1390. doi: 10.1152/ajprenal.00185.2007. [DOI] [PubMed] [Google Scholar]
- 18.Yin X, Zhang Y, Yu J, Zhang P, Shen J, Qiu J, et al. The antioxidative effects of astragalus saponin I protect against development of early diabetic nephropathy. J Pharmacol Sci. 2006;101:166–173. doi: 10.1254/jphs.fp0050041. [DOI] [PubMed] [Google Scholar]
- 19.Yin X, Zhang Y, Wu H, Zhu X, Zheng X, Jiang S, et al. Protective effects of Astragalus saponin I on early stage of diabetic nephropathy in rats. J Pharmacol Sci. 2004;95:256–266. doi: 10.1254/jphs.fp0030597. [DOI] [PubMed] [Google Scholar]
- 20.Vinik AI, Mehrabyan A. Diabetic neuropathies. Med Clin North Am. 2004;88:947–999. xi. doi: 10.1016/j.mcna.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 22.Fridovich I. Superoxide anion radical (O2−), superoxide dismutases, and related matters. J Biol Chem. 1997;272:18515–18517. doi: 10.1074/jbc.272.30.18515. [DOI] [PubMed] [Google Scholar]
- 23.Sindhu RK, Koo JR, Roberts CK, Vaziri ND. Dysregulation of hepatic superoxide dismutase, catalase and glutathione peroxidase in diabetes: response to insulin and antioxidant therapies. Clin Exp Hypertens. 2004;26:43–53. doi: 10.1081/ceh-120027330. [DOI] [PubMed] [Google Scholar]
- 24.Schnackenberg CG, Welch WJ, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membranepermeable superoxide dismutase mimetic: role of nitric oxide. Hypertension. 1998;32:59–64. doi: 10.1161/01.hyp.32.1.59. [DOI] [PubMed] [Google Scholar]
- 25.Schnackenberg CG, Wilcox CS. The SOD mimetic tempol restores vasodilation in afferent arterioles of experimental diabetes. Kidney Int. 2001;59:1859–1864. doi: 10.1046/j.1523-1755.2001.0590051859.x. [DOI] [PubMed] [Google Scholar]
- 26.Patel NS, Chatterjee PK, Chatterjee BE, Cuzzocrea S, Serraino I, Brown PA, Stewart KN, Mota-Filipe H, Thiemermann C. TEMPONE reduces renal dysfunction and injury mediated by oxidative stress of the rat kidney. Free Radic Biol Med. 2002;33:1575–1589. doi: 10.1016/s0891-5849(02)01116-4. [DOI] [PubMed] [Google Scholar]
- 27.Nishiyama A, Yoshizumi M, Hitomi H, Kagami S, Kondo S, Miyatake A, Fukunaga M, Tamaki T, Kiyomoto H, Kohno M, Shokoji T, Kimura S, Abe Y. The SOD mimetic tempol ameliorates glomerular injury and reduces mitogen-activated protein kinase activity in Dahl salt-sensitive rats. J Am Soc Nephrol. 2004;15:306–315. doi: 10.1097/01.asn.0000108523.02100.e0. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto T, Ono Y, Kurono M, Kuromiya A, Nakamura K, Bril V. Improvement of motor nerve conduction velocity in diabetic rats requires normalization of the polyol pathway metabolites flux. J Pharmacol Sci. 2009;109:203–210. doi: 10.1254/jphs.08177fp. [DOI] [PubMed] [Google Scholar]
- 29.Kashihara N, Haruna Y, Kondeti VK, Kanwar YS. Oxidative stress in diabetic nephropathy. Curr Med Chem. 2010;17:4256–69. doi: 10.2174/092986710793348581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Y, Ren K, Liang C, Yuan L, Qi X, Dong J, et al. Renoprotective effect of total glucosides of paeony (TGP) and its mechanism in experimental diabetes. J Pharmacol Sci. 2009;109:78–87. doi: 10.1254/jphs.08112fp. [DOI] [PubMed] [Google Scholar]
- 31.Busch M, Franke S, Rüster C, Wolf G. Advanced glycation end-products and the kidney. Eur J Clin Invest. 2010;40:742–755. doi: 10.1111/j.1365-2362.2010.02317.x. [DOI] [PubMed] [Google Scholar]
- 32.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans JL, Goldfine D, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 34.Li J-M, Shah AM. ROS generation by nonphagocytic NADPH oxidase: potential relevance in diabetic nephropathy. J Am Soc Nephrol. 2003;14:S221–226. doi: 10.1097/01.asn.0000077406.67663.e7. [DOI] [PubMed] [Google Scholar]
- 35.Lee HB, Yu MR, Yang Y, Jiang Z, Ha H. Reactive oxygen species–regulated signaling pathways in diabetic nephropathy. J Am Soc Nephrol. 2003;14:S241–245. doi: 10.1097/01.asn.0000077410.66390.0f. [DOI] [PubMed] [Google Scholar]
- 36.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 37.Majid DS, Nishiyama A, Jackson KE, Castillo A. Superoxide scavenging attenuates renal responses to ANG II during nitric oxide synthase inhibition in anesthetized dogs. Am J Physiol Renal Physiol. 2005;288:F412–F419. doi: 10.1152/ajprenal.00294.2004. [DOI] [PubMed] [Google Scholar]
- 38.Tsuchida H, Imai G, Shima Y, Satoh T, Owada S. Mechanism of salt load-induced hypertension in non-insulin dependent diabetes mellitus model rats: defective dopaminergic system to inhibit Na–K-ATPase activity in renal epithelial cells. Hypertens Res. 2001;24:127–135. doi: 10.1291/hypres.24.127. [DOI] [PubMed] [Google Scholar]
- 39.Hodgkinson AD, Bartlett T, Oates PJ, Millward BA, Demaine AG. The response of antioxidant genes to hyperglycemia is abnormal in patients with type 1 diabetes and diabetic nephropathy. Diabetes. 2003;52:846–851. doi: 10.2337/diabetes.52.3.846. [DOI] [PubMed] [Google Scholar]
- 40.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 41.Kedziora-Kornatowska K, Szram S, Kornatowski T, Szadujkis-Szadurski L, Kedziora J, Bartosz G. The effect of verapamil on the antioxidant defence system in diabetic kidney. Clin Chim Acta. 2002;322:105–112. doi: 10.1016/s0009-8981(02)00167-5. [DOI] [PubMed] [Google Scholar]
- 42.Koya D, Hayashi K, Kitada M, Kashiwagi A, Kikkawa R, Haneda M. Effects of antioxidants in diabetes-induced oxidative stress in the glomeruli of diabetic rats. J Am Soc Nephrol. 2003;14:S250–253. doi: 10.1097/01.asn.0000077412.07578.44. [DOI] [PubMed] [Google Scholar]
- 43.Chaudhry J, Ghosh NN, Roy K, Chandra R. Antihyperglycemic effect of a new thiazolidinedione analogue and its role in ameliorating oxidative stress in alloxan-induced diabetic rats. Life Sci. 2007;80:1135–1142. doi: 10.1016/j.lfs.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 44.Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, et al. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-β antibody in db/db diabetic mice. Proc Natl Acad Sci USA. 2000;97:8015–8020. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Isono M, Mogyorósi A, Han DC, Hoffman BB, Ziyadeh FN. Stimulation of TGF-beta type II receptor by high glucose in mouse mesangial cells and in diabetic kidney. Am J Physiol Renal Physiol. 2000;278:F830–838. doi: 10.1152/ajprenal.2000.278.5.F830. [DOI] [PubMed] [Google Scholar]
- 46.Ziyadeh FN, Sharma K, Ericksen M, Wolf G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-β. J Clin Invest. 1994;93:536–542. doi: 10.1172/JCI117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor β is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci U S A. 1993;90:1814–1818. doi: 10.1073/pnas.90.5.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang W, Zhang Y, Wu H, Zhang X, Gan H, Sun J, Chen Q, Guo M, Zhang Z. Role of cross-talk between the Smad 2 and MAPK pathways in TGF-beta1-induced collagen IV expression in mesangial cells. International Journal of Molecular Medicine. 2010;26:571–576. doi: 10.3892/ijmm_00000501. [DOI] [PubMed] [Google Scholar]
- 49.Pugliese G, Pricci F, Pugliese F, Mene P, Lenti L, Andreani D, Galli G, Casini A, Bianchi S, Rotella CM. Mechanisms of glucose-enhanced extracellular matrix accumulation in rat glomerular mesangial cells. Diabetes. 1994;43:478–490. doi: 10.2337/diab.43.3.478. [DOI] [PubMed] [Google Scholar]
- 50.Forbes JM, Fukami K, Cooper ME. Diabetic nephropathy: where hemodynamics meets metabolism. Exp Clin Endocrinol Diabetes. 2007;115:69–84. doi: 10.1055/s-2007-949721. [DOI] [PubMed] [Google Scholar]
- 51.Iglesias-De La Cruz MC, Ruiz-Torres P, Alcami J, Diez-Marques L, Ortega-Velazquez R, Chen S, Rodriguez-Puyol M, Ziyadeh FN, Rodriguez-Puyol D. Hydrogen peroxide increases extracellular matrix mRNA through TGF-beta in human mesangial cells. Kidney Int. 2001;59:87–95. doi: 10.1046/j.1523-1755.2001.00469.x. [DOI] [PubMed] [Google Scholar]
- 52.Jiang Z, Seo JY, Ha H, Lee EA, Kim YS, Han DC, Uh ST, Park CS, Lee HB. Reactive oxygen species mediate TGF-beta1-induced plasminogen activator inhibitor-1 upregulation in mesangial cells. Biochem Biophys Res Commun. 2003;309:961–966. doi: 10.1016/j.bbrc.2003.08.102. [DOI] [PubMed] [Google Scholar]
- 53.Nishibayashi S, Hattori K, Hirano T, Uehara K, Nakano Y, Aihara M, Yamada Y, Muraguchi M, Iwata F, Takiguchi Y. Functional and structural changes in end-stage kidney disease due to glomerulonephritis induced by the recombinant alpha3(IV)NC1 domain. Exp Anim. 2010;59:157–170. doi: 10.1538/expanim.59.157. [DOI] [PubMed] [Google Scholar]
- 54.Hisaki R, Fujita H, Saito F, Kushiro T. Tempol attenuates the development of hypertensive renal injury in Dahl salt-sensitive rats. Am J Hypertens. 2005;18:707–713. doi: 10.1016/j.amjhyper.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 55.Yuan P, Xue H, Zhou L, Qu L, Li C, Wang Z, et al. Rescue of mesangial cells from high glucose-induced over-proliferation and extracellular matrix secretion by hydrogen sulfide. Nephrol Dial Transplant. 2011;26:2119–2126. doi: 10.1093/ndt/gfq749. [DOI] [PubMed] [Google Scholar]
- 56.Dietrich A, Gudermann T. TRPC6. Handb Exp Pharmacol. 2007;179:125–141. doi: 10.1007/978-3-540-34891-7_7. [DOI] [PubMed] [Google Scholar]
- 57.Reiser J, Polu KR, Moller CC, Kenlan P, Altintas MM, Wei C, et al. TRPC6 is a glomerular slit diaphram-associated channel required for normal renal function. Nat Genet. 2005;37:739–744. doi: 10.1038/ng1592. [DOI] [PMC free article] [PubMed] [Google Scholar]