Summary
A major public health challenge today is the problem of congenital cytomegalovirus (CMV) transmission. Maternal-fetal CMV infections are common, occurring in 0.5-2% of pregnancies, and these infections often lead to long-term injury of the newborn infant. In spite of the well-recognized burden that these infections place on society, there are as yet no clearly established interventions available to prevent transmission of CMV. In order to study potential interventions, such as vaccines or antiviral therapies, an animal model of congenital CMV transmission is required. The best small animal model of CMV transmission is the guinea pig cytomegalovirus (GPCMV) model. This article summarizes the GPCMV model, putting it into the larger context of how studies in this system have relevance to human health. An emphasis is placed on how the vertical transmission of GPCMV recapitulates the pathogenesis of congenital CMV in infants, making this a uniquely well-suited model for the study of potential CMV vaccines.
Keywords: Guinea pig cytomegalovirus, cytomegalovirus vaccines, congenital cytomegalovirus infection, antiviral therapy, glycoprotein B vaccine, UL83 (pp65) vaccine, ganciclovir, maribavir, cytomegalovirus immune globulin
Congenital infection with CMV is a major cause of disability in newborn infants. CMV transmission occurs in 0.15-2% of pregnancies [1], and leads to significant neurologic disabilities including mental retardation, cerebral palsy, seizure disorders, and developmental delay [2]. Congenital HCMV infection is also the most common infectious disease responsible for sensorineural hearing loss (SNHL) in children [3-5]. The likelihood that CMV will be transmitted from mother to fetus appears to depend upon the presence and duration of preconception immunity. Preconception maternal immunity, particularly long-term immunity characterized by high-avidity antibody to CMV [6], significantly reduces the incidence of infection in the newborn and possibly the severity of CMV disease in the infant that is infected [7, 8]. This observation has fostered considerable interest in the development of CMV vaccines for prevention of congenital transmission [9-12]. Recently, an important study demonstrated that a subunit vaccine directed against a viral envelope glycoprotein, glycoprotein B (gB), expressed as a recombinant protein in cell culture and administered with a novel adjuvant, MF59, demonstrated modest efficacy in prevention of CMV infection in a cohort of young women at high risk for primary infection [13]. Other vaccine approaches in various stages of preclinical and clinical development include DNA vaccines, vectored vaccines based on attenuated poxviruses and virus-like particles, live attenuated vaccines, and peptide-based vaccines [14].
Although the need for a CMV vaccine is compelling and the results from recent clinical trials encouraging, there are many barriers remaining before a vaccine can be licensed. First and foremost, there is an incomplete understanding of those features of the immune response to CMV that provide protection against congenital transmission. CMV, a member of the herpesvirus family, is a large and complex virus, encoding approximately 200 gene products, many of which remain incompletely characterized [15]. This is a key issue in vaccine development, insofar as it is unclear at this time whether vaccination against a limited number of immunogenic CMV proteins would be sufficient in conferring an immune response that protected the fetus. The broad-based immunity conferred by a live, attenuated CMV vaccine might, in principle, be preferable to the subunit vaccine approach. However, there are theoretical concerns about the potential risks of a live, attenuated vaccine with respect to the potential for such a vaccine to result in latent CMV infection, which in turn could be theoretically associated with long-term risks such as atherosclerosis and malignancy [16].
Given the uncertainties about the optimal strategy for vaccination against congenital CMV, additional preclinical study comparing immunization strategies is warranted. Ideally, the safety and potential efficacy of vaccines against human CMV would be evaluated in a preclinical model prior to large-scale clinical trials. Unfortunately, the strict species-specificity of CMVs precludes the study of human CMV vaccines in animal models [17]. Consequently, animal models that utilize species-specific CMVs are employed for the study of antiviral intervention strategies. Of the small animal models for the study of congenital CMV infection, the guinea pig cytomegalovirus (GPCMV) model is uniquely valuable, and has been employed by a number of investigators as a model not only for the study of prevention of congenital transmission of infection, but also for the study of disease pathogenesis in the newborn. Although the major driving force behind continued study of the GPCMV model is for the insights that might be gleaned regarding vaccine strategies, there are intriguing similarities in the manifestations of disease in the vertically infected pup that recapitulate the pathology of disease in infants. This brief review summarizes those aspects of the molecular biology of GPCMV and reproductive biology of guinea pigs that make this model useful; the progress that has been made in the study of vaccines in the GPCMV model; and high-priority areas for future research that can optimize the value of this system for gleaning insights into the pathogenesis of congenital CMV disease in infants.
Virology and molecular biology of guinea pig cytomegalovirus
Virtually all information about GPCMV is derived from studies performed with the strain originally isolated by Hartley in 1957, from infected guinea pig salivary glands, which was provided to the American Type Culture Collection (ATCC) as strain 22122 [18]. Ultrastructural analysis of the GPCMV, both in salivary gland and tissue culture, was initially performed by electron microscopic (EM) in the late 1960s [19], and confirmed that GPCMV had the typical morphological features of a herpesvirus. These studies were later extended to the in vivo analysis of virus morphology in guinea pig salivary glands and visceral organs, and confirmed the morophological similarities of GPCMV to human CMV, including the presence of enveloped ‘dense bodies’ without capsids in the cytoplasm of infected cells [20, 21]. An initial description of the molecular biology of GPCMV came in the early 1980s, when GPCMV DNA was subjected to density gradient centrifugation, and restriction endonuclease analysis of the viral genome generated [22]. A subsequent comprehensive analysis of GPCMV DNA purified from tissue culture-adapted virus resulted in the generation of restriction endonuclease maps of the viral genome, and cross-hybridization studies revealed some limited homology to human CMV DNA [23, 24]. In the early 1990s, specific GPCMV genes were cloned and subjected to sequence analysis, establishing the orientation of the viral genome and the presence of well-characterized homologs of other CMV proteins [25]. A number of these proteins have now been characterized, including homologs of the dominant humoral immune target, the envelope glycoprotein, gB; the dominant cellular immune target, pp65 (UL83); and other glycoproteins, including the gH and gN homologs. The characterization of these GPCMV gene products has in turn been valuable in experimental testing of vaccine strategies designed to prevent congenital GPCMV transmission (described below). Fig. 1 demonstrates a western blot analysis of several of the better-characterized GPCMV virion proteins recognized by anti-GPCMV antibodies.
Fig. 1.
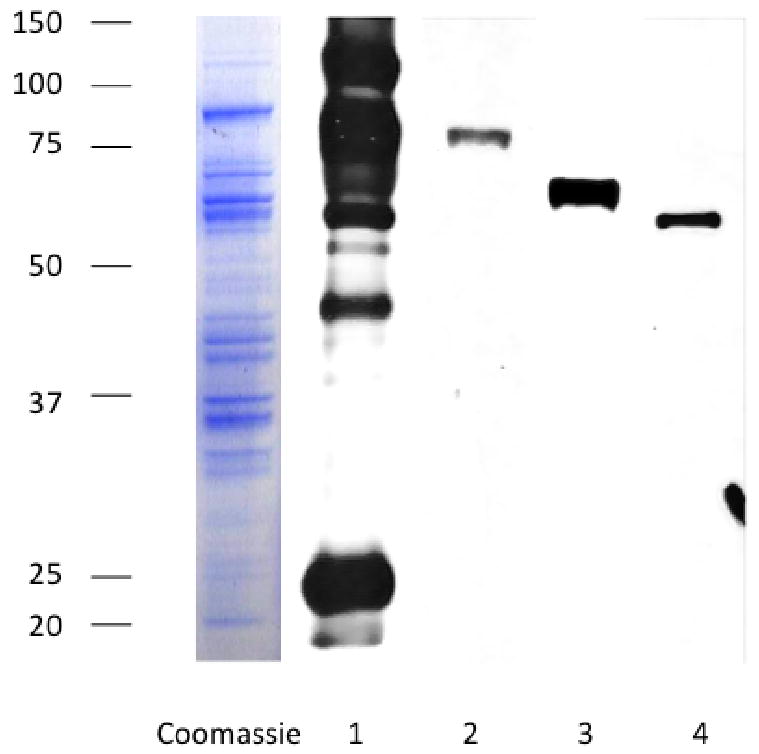
Key constituents of the GPCMV virion. Left panel, Coomassie stain of SDS-PAGE analysis of sucrose gradient-purified GPCMV virus particles. Right panel, western blot analysis of virus particles following hybridization with a polyclonal anti-GPCMV antibody (lane 1); antibody specific for gB (GP55) homolog (lane 2); gH (GP75) homolog (lane 3); gN (GP73) homolog (lane 4); and pp65 (GP83) homolog. These proteins are highly conserved between GPCMV and human CMV, and represent potential vaccine targets.
Recently, an initial draft of the GPCMV genome sequence was published [26]. This draft was assembled from a tissue culture-derived bacterial artificial chromosome (BAC) clone [27], plasmid clones of viral restriction fragments, and direct PCR sequencing of viral DNA. This analysis indicated that the GPCMV genome is approximately 233 kb in length, excluding the terminal repeat sequences. In total, 105 open reading frames (ORFs) of > 100 amino acids with sequence and/or positional homology to other CMV ORFs were annotated in this analysis, including positional and sequence homologs of human CMV ORFs UL23 through UL122. A number of lines of evidence suggest that this sequence (GenBank Accession number, FJ355434) does not represent the bona fide, wild-type sequence of GPCMV. First of all, the original GPCMV BAC construct [27] contains deletions in several regions of the genome, presumably introduced during the cloning process in E. coli. Although these deletions were partially repaired by a second generation GPCMV BAC construct [28, 29], detailed sequence analysis of viral DNA purified directly from salivary glands of infected animals has revealed several interesting observations [30, 31]. These studies demonstrated.
In summary, GPCMV shares many morphological and molecular similarities with human CMV that underscore the value of studying this virus as a model of human CMV disease. These include: a virion morphology similar to human CMV; a genome organization that includes homologs of human CMV that play roles in immune response and viral pathogenesis; and an assemblage of virion proteins that includes key proteins that have emerged as subunit vaccine targets. Additional work is required to determine the full-length, wild-type sequence of the viral genome, but the information available to date has advanced the understanding of GPCMV molecular biology, information that in turn has enabled vaccine and pathogenesis studies that are described below.
The pathogenesis of GPCMV and its usefulness as an animal model of human CMV infection
Pathogenesis studies with GPCMV are typically performed using salivary gland-adapted stocks of virus, used to inoculate either outbred (Hartley) or inbred (JY-9 or strain 2) strains of guinea pigs via subcutaneous, intravascular, or intraperitoneal route. Experimental details of the model have been recently reviewed [32]. Irrespective of route of inoculation, viremia occurs, both in non-pregnant and pregnant animals, and this appears to be the mechanism by which end-organ disease (including of the placental-fetal unit) occurs. Viremia persists for approximately 10 days following the inoculation of nonpregnant animals, and generalized infection and disease involving the lungs, spleen, liver, kidney, thymus, pancreas, and brain is observed for approximately 3 weeks following acute infection [33]. Salivary gland viral titers reach maximal levels at 3 weeks post-infection, and virus persists at high level for at least 10 weeks.
A number of models of GPCMV-induced disease have been described in non-pregnant animals. A mononucleosis-like syndrome has been described in association with acute infection, including atypical lymphocytosis in peripheral blood, splenomegaly, lymphadenopathy, anemia, and neutopenia [34]. Inbred strains of guinea pigs, in particular strain 2, are, in general, more prone to GPCMV disease than are outbred animals, and in particular are at risk for the development of pneumonitis [35]. In guinea pigs that are pharmacologically immunosuppressed, with agents such as cyclophosphamide or cyclosporine, severe GPCMV-induced disease is observed, including pneumonitis, in a model of disease that mimics CMV disease in immunosuppressed patients. This immunosuppression model provides a useful system in which to evaluate antiviral therapies [33-38]. Other models of GPCMV-induced disease that have been described include a neuropathogenesis model, induced by direct inoculation of virus, and characterized by a glial nodule encephalitis [39], and a labyrinthitis model, induced by direct viral inoculation into the cochlea [40]. Since these two studies required direct inoculation of GPCMV into the brain and cochlea, respectively, they may be less relevant to the issue of in utero acquisition of CMV, with its attendant pathology and sequelae, in infants. However, the labyrinthitis model is particularly intriguing, in light of the fact that one of the most important sequelae of congenital CMV infection is SNHL [3-5], and this feature of the pathogenesis of GPCMV is considered in more detail below. A neonatal infection model also been described, and is characterized by a generalized, viremic infection, including hepatitis, pneumonitis, and central nervous system involvement [41-44].
Though these disease models are quite useful in the study of the pathogenesis of CMV infection, clearly the greatest strength of the GPCMV model lies in its ability to cross the guinea pig placenta, leading to fetal infection and disease. A number of aspects of guinea pig reproductive biology are relevant to this model. Guinea pig gestational periods are lengthy compared to other rodents, ranging from 65-70 days [45]. In addition, the only continuous cellular layers separating the maternal and fetal blood circulations in the hemomonochorial placenta of the guinea pig are the syncytiotrophoblast and the endothelium of the fetal capillaries [46], a histology similar to that of the human placenta [47], but quite distinct from the mouse placenta [48]. The ability of GPCMV to produce viremic placental infection following maternal inoculation was recognized in the late 1970s [49-51]. Since these initial reports, a number of experimental endpoints for the GPCMV congenital infection model have been refined, including mortality in dams, pup infection rate, pup mortality, maternal and pup weight, fetal resorption rate, viral load in dams and pups by viral culture and PCR, and magnitude of placental infection. Of particular relevance to human health, it has been observed that congenitally infected pups exhibit end-organ disease, including brain and visceral involvement, and inner ear pathology; thus, direct intracerebral or intracochlear inoculation of GPCMV is not required to recapitulate this important form of pathology [52-54].
In addition to maternal and fetal outcomes, placental pathology has also been described as an experimental endpoint in the GPCMV model [47]. Histopathological and ultrastructural analyses of GPCMV-infected placentas revealed a variety of forms of pathology, depending upon the timing of placental examination relative to maternal inoculation. At early time points post-infection (days 14 and 21), placentas exhibit ischemic injury changes, predictive of pup mortality. In contrast, typical CMV-specific histopathology, consisting of multiple areas of necrosis associated with acute and chronic inflammation, was frequently seen at later time points, on days 21 and 28 postinoculation, localized at the transitional zone between the capillarized labyrinth and the noncapillarized interlobium, a zone containing maternal venous lacunae and fetal arterioles. Given the key role that the placenta plays in transmission of human CMV to the fetus, and the placental pathology that is observed in the context of severe symptomatic congenital infection, these similarities in the guinea pig system can be exploited in future studies of interventional strategies.
Vaccine and antiviral studies in the GPCMV model: past successes, future priorities
The GPCMV model was first studied as a model for vaccination against congenital infection and disease in the early 1980s. In the first reported study [55], a live, attenuated GPCMV vaccine, and a partially purified, soluble envelope vaccine, enriched for envelope antigens and administered with Freund's adjuvant, were compared. Dams previously inoculated with live virus vaccine were protected against acute viremia and death following challenge with virulent salivary gland virus, and a reduced incidence of generalized maternal and fetal infection was observed. Although envelope antigen-vaccinated animals showed acute viremia after similar challenge with virulent virus, infection was less generalized than that in control animals, and CMV was not isolated from the fetuses of these vaccinated mothers. Thus, both strategies were successful in protecting against fetal GPCMV disease. In a later vaccine studies uusing immunoaffinity-purified glycoproteins administered with Freund's adjuvant, and newborn pups were protected against congenital mortality and disease, and immunization was found to significantly reduce in utero transmission [56, 57].
More recently, these studies have taken advantage of the availability of sequence data on specific GPCMV gene products [25, 26] to perform analysis of subunit vaccine approaches. These studies have focused on the GPCMV homologs of gB and pp65 (UL83), two major vaccine candidates in human CMV vaccine trials that represent the dominant targets of humoral and cellular immune responses, respectfully. One expression technique that has been investigated in the GPCMV model is that of DNA vaccines. These studies [58, 59] indicated that: (i) anti-gB responses were augmented by truncation of the gB protein immediately upstream of the transmembrane domain; (ii) immune responses were improved when vaccine was given intradermally by gene gun rather than intramuscular administration; (iii) efficacy study in the guinea pig model of congenital infection demonstrated efficacy against fetal infection using gB, but not pp65, vaccine. A pp65 vaccine based on the GPCMV homolog of the UL83 was shown to be effective in prevention of maternal and fetal GPCMV disease, however, when an alternative expression strategy, VRPs based on an alphavirus platform, was employed [60]. Subunit vaccine studies have also been conducted in the GPCMV model using adjuvanted, purified gB protein expressed by recombinant baculovirus. For these studies, a truncated, secreted form of gB was engineered [61]. In a study of protection against congenital GPCMV infection and disease [62], gB which was coadministered with either Freund's adjuvant or alum. All gB-immunized dams had ELISA and neutralizing-antibody responses, with higher titers noted in the gB/Freund's group. Following challenge of pregnant dams with salivary gland-adapted GPCMV subcutaneously during the 3rd trimester, pregnancy outcomes were monitored. Vaccination resulted in a highly significant reduction in pup mortality and maternal viral load. These studies are of particular interest in light of the recent report of efficacy of a human CMV vaccine for prevention of primary infection in women of child-bearing age [13], and provide support for future studies of gB vaccine for prevention of congenital infection in infants.
The successful cloning of the GPCMV genome as a BAC construct [27, 28] has created the possibility of engineering novel live, attenuated vaccine candidates for study in the guinea pig model. Such vaccines could be designed with targeted deletion of viral immune modulation genes, using the powerful mutagenesis techniques available in E. coli, toward the goal of improving vaccine safety and/or immunogenicity. In one recently reported study, three genes encoding MHC class I homologs (presumed to be involved in evasion of natural killer cell responses) were deleted from GPCMV and the resulting mutant was evaluated as a live attenuated vaccine [63]. Vaccine virus produced elevated cytokine levels and higher antibody titers than challenge with wild-type virus, while immune responses were similar. Vaccine-mediated protection, assessed by maternal DNAemia and pup mortality following pathogenic viral challenge during pregnancy, was comparable between deletion virus and wild-type virus. The GPCMV data suggest that the safety and perhaps efficacy of live attenuated human CMV vaccines could be enhanced by deletion of viral immunomodulatory genes. Studies of other live, attenuated vaccine candidates in the GPCMV model may help direct the preclinical development of such vaccines. One such vaccine candidate is a deletion mutant of GPCMV in which a functional chemokine, GPCMV-MIP [64, 65], was removed from the viral genome. The deletion virus was highly attenuated in its ability to establish labyrinthitis following cochlear inoculation [66, 67], and is currently being evaluated as a live, attenuated vaccine in the GPCMV model.
In addition to studies of preconception immunization, the GPCMV model has also been used for study of therapeutic intervention during pregnancy. This intervention has taken two forms: passive immunization studies, based on administration of neutralizing antibody to the pregnant dam [68, 69], and antiviral therapy of agents active against GPCMV [70, 71]. Both strategies have been successful in interrupting transmission of GPCMV from dam to fetus, and were shown to improve the outcome of pregnancy. Human clinical trials of passive immunoglobulin transfer have been performed, and suggest that CMV immune globulin can favorably impact the outcome of pregnancy in the setting of maternal-fetal transmission in utero [72]. Continued study of immune globulin and antiviral therapies in the pregnant guinea pig can help provide important clues about the potential toxicities and benefits of candidate interventional strategies prior to human clinical trials.
Future perspective
In summary, GPCMV has served as a useful model for human disease for several decades, and has been exploited for vaccine, antiviral, vaccine, and pathogenesis. Moreover, the GPCMV has experienced a renaissance in interest in recent years, as more laboratories, both in academia and in industry, are investing resources into studying this uniquely useful model CMV infection. This increased interest has been driven by several factors. First of all, there is increasingly awareness of the urgent need to find a solution for congenital CMV infection [73-75]. Congenital CMV infection produces considerable morbidity in newborns, and increased awareness has in turn driven interest in basic and translational research aimed at exploring pathogenesis and developing therapeutic interventions: in this context, the guinea pig model is of great interest. Secondly, there has been success reported in a clinical trial of a CMV vaccine [13]. This represents the first report of efficacy of any CMV vaccine for prevention of infection, and this positive result will drive interest in future vaccine studies for congenital CMV. The guinea pig model can serve as a useful screening system for vaccine and antiviral strategies, and may be useful in prioritizing what approaches should move forward in clinical trials. Third, the GPCMV genome has been recently characterized in detail, and tools and reagents are now available to move this model forward. The convergence of these events should result in an increased use of the GPCMV model in the years ahead, until such time as the development of a vaccine or antiviral intervention has solved the problem of congenital CMV infection. Other directions that can be anticipated in the next 5-10 years include an expanded panel of guinea pig-specific immunological reagents for studying the immune response to infection and vaccination, and the development of additional recombinant GPCMVs for use as live, attenuated vaccine candidates. The GPCMV model may ultimately prove to be the pivotal model for determining whether novel antiviral interventions should move forward in phase I human clinical trials for prevention of the important clinical problem of maternal-fetal CMV infection.
Executive Summary.
Congenital cytomegalovirus (CMV) infection Is a major public health issue
CMV is transmitted in utero to up to 2% of all newborns.
Congenital CMV is the most common infectious disease associated with sensorineural hearing loss (SNHL) in children.
Congenital CMV infection leads to other neurodevelopmental disabilities, such as mental retardation, cerebral palsy, and seizure disorders.
Although preconception maternal immunity to CMV helps protect the fetus, there is no licensed vaccine for prevention of CMV infection.
The guinea pig provides a uniquely useful model for study of congenital CMV
Human CMV cannot infect animals due to the species-specificity of CMVs.
Among the small animal CMVs, the guinea pig CMV (GPCMV) is the only CMV that crosses the placenta and infects the fetus, analogous to the biology of human CMV.
The guinea pig has a long gestational period and a hemochorial placenta, structurally and histologically similar to human placenta but distinct from rodent placentas.
Congenitally infected guinea pigs demonstrate intrauterine growth retardation, brain involvement, and sensorineural hearing loss, similar to CMV disease in infants.
Intervention strategies prevent fetal and neonatal disease in the GPCMV model
Subunit vaccine strategies based on the glycoprotein B and pp65 homologs of human CMV provide protection against congenital GPCMV infection and disease.
The cloning of the GPCMV genome as an infectious BAC has enabled generation of novel live, attenuated vaccines with immunogenicity and safety.
Other clinically relevant interventions, such as immune globulin and antiviral therapies, are successful in interrupting GPCMV transmission and preventing disease.
Future strategies for improving the usefulness of the GPCMV model
Refining endpoints for vaccine studies to focus on neuropathogenesis and SNHL.
Expand available immunological tools for a more detailed assessment of guinea pig vaccine immune responses (cytokine responses, T cell responses, NK responses).
Resolve the question of the relative merits of subunit vaccination vs. live, attenuated vaccination for prevention of congenital CMV infection and disease.
Explore novel strategies for preventing congenital CMV transmission using the GPCMV model as a preclinical screening model prior to phase I studies.
Acknowledgments
The contribution of Alistair McGregor and Xiao Cui in the development of the GPCMV model is acknowledged. The authors' research is supported by grants R01HD044864, R01HD038416, and R03AI083919-0109 from the National Institutes of Health.
Contributor Information
Mark R. Schleiss, Email: schleiss@umn.edu.
Michael A. McVoy, Email: mmcvoy@vcu.edu.
Bibliography
- 1.Malm G, Engman ML. Congenital cytomegalovirus infections. Semin Fetal Neonatal Med. 2007;12:154–9. doi: 10.1016/j.siny.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 2.Cheeran MCJ, Lokensgard JR, Schleiss MR. Neuropathogenesis of congenital cytomegalovirus infection: Disease mechanisms and prospects for intervention. Clin Microbiol Rev. 2009;22:1–28. doi: 10.1128/CMR.00023-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pass RF. Congenital cytomegalovirus infection and hearing loss. Herpes. 2005;12:50–5. [PubMed] [Google Scholar]
- 4.Fowler KB, Boppana SB. Congenital cytomegalovirus (CMV) infection and hearing deficit. J Clin Virol. 2006;35:226–31. doi: 10.1016/j.jcv.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Nance WE, Lim BG, Dodson KM. Importance of congenital cytomegalovirus infections as a cause for pre-lingual hearing loss. J Clin Virol. 2006;35:221–5. doi: 10.1016/j.jcv.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Lazzarotto T, Varani S, Spezzacatena P, Gabrielli L, Pradelli P, Guerra B, Landini MP. Maternal IgG avidity and IgM detected by blot as diagnostic tools to identify pregnant women at risk of transmitting cytomegalovirus. Viral Immunol. 2000;13:137–41. doi: 10.1089/vim.2000.13.137. [DOI] [PubMed] [Google Scholar]
- 7.Fowler KB, Stagno S, Pass RF, Britt WJ, Boll TJ, Alford CA. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med. 1992;326:663–7. doi: 10.1056/NEJM199203053261003. [DOI] [PubMed] [Google Scholar]
- 8.Fowler KB, Stagno S, Pass RF. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA. 2003;289:1008–11. doi: 10.1001/jama.289.8.1008. [DOI] [PubMed] [Google Scholar]
- 9.Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R, National Vaccine Advisory Committee Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin Infect Dis. 2004;39:233–9. doi: 10.1086/421999. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths PD, McLean A, Emery VC. Encouraging prospects for immunisation against primary cytomegalovirus infection. Vaccine. 2001;19:1356–62. doi: 10.1016/s0264-410x(00)00377-7. [DOI] [PubMed] [Google Scholar]
- 11.Zhong J, Khanna R. Vaccine strategies against human cytomegalovirus infection. Expert Rev Anti Infect Ther. 2007;5:449–59. doi: 10.1586/14787210.5.3.449. [DOI] [PubMed] [Google Scholar]
- 12.Adler SP. Human CMV vaccine trials: what if CMV caused a rash? J Clin Virol. 2008;41:231–6. doi: 10.1016/j.jcv.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Pass RF, Zhang C, Evans A, Simpson T, Andrews W, Huang ML, Corey L, Hill J, Davis E, Flanigan C, Cloud G. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009;360:1191–9. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schleiss MR. Cytomegalovirus vaccine development. Curr Top Microbiol Immunol. 2008;325:361–82. doi: 10.1007/978-3-540-77349-8_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy E, Shenk T. Human cytomegalovirus genome. Curr Top Microbiol Immunol. 2008;325:1–19. doi: 10.1007/978-3-540-77349-8_1. [DOI] [PubMed] [Google Scholar]
- 16.Söderberg-Nauclér C. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? J Intern Med. 2006;259:219–46. doi: 10.1111/j.1365-2796.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- 17.Kern ER. Pivotal role of animal models in the development of new therapies for cytomegalovirus infections. Antiviral Res. 2006;71:164–71. doi: 10.1016/j.antiviral.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Hartley JW, Rowe WP, Huebner RJ. Serial propagation of the guinea pig salivary gland virus in tissue culture. Proc Soc Exp Biol Med. 1957;96:281–285. doi: 10.3181/00379727-96-23455. [DOI] [PubMed] [Google Scholar]
- 19.Middelkamp JN, Patrizi G, Reed CA. Light and electron microscopy studies of the guinea pig cytomegalovirus. J Ultrastruct Res. 1967;18:85–101. doi: 10.1016/s0022-5320(67)80233-8. [DOI] [PubMed] [Google Scholar]
- 20.Fong CK, Bia F, Hsiung GD. Ultrastructural development and persistence of guinea pig cytomegalovirus in duet cells of guinea pig submaxillary gland. Arch Virol. 1980;64:97–108. doi: 10.1007/BF01318013. [DOI] [PubMed] [Google Scholar]
- 21.Fong CK, Lucia H, Bia FJ, Hsiung GD. Histopathologic and ultrastructural studies of disseminated cytomegalovirus infection in strain 2 guinea pigs. Lab Invest. 1983;49:183–94. [PubMed] [Google Scholar]
- 22.Bia FJ, Summers WC, Fong CK, Hsuing GD. New endogenous herpesvirus of guinea pigs, biological and molecular characterization. J Virol. 1980;36:245–253. doi: 10.1128/jvi.36.1.245-253.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isom HC, Gao M, Wigdahl B. Characterization of guinea pig cytomegalovirus DNA. J Virol. 1984;49:426–436. doi: 10.1128/jvi.49.2.426-436.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao M, Isom HC. Characterization of the guinea pig cytomegalovirus genome by molecular cloning and physical mapping. J Virol. 1984;52:436–447. doi: 10.1128/jvi.52.2.436-447.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schleiss MR. Animal models of congenital cytomegalovirus infection: an overview of progress in the characterization of guinea pig cytomegalovirus (GPCMV) J Clin Virol. 2002;25(2):S37–49. doi: 10.1016/s1386-6532(02)00100-2. [DOI] [PubMed] [Google Scholar]
- 26.Schleiss MR, McGregor A, Choi KY, Date SV, Cui X, McVoy MA. Analysis of the nucleotide sequence of the guinea pig cytomegalovirus (GPCMV) genome. Virol J. 2008;5:139. doi: 10.1186/1743-422X-5-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGregor A, Schleiss MR. Molecular cloning of the guinea pig cytomegalovirus (GPCMV) genome as an infectious bacterial artificial chromosome (BAC) in Escherichia coli. Mol Genet Metab. 2001;72:15–26. doi: 10.1006/mgme.2000.3102. [DOI] [PubMed] [Google Scholar]
- 28.Cui X, McGregor A, Schleiss MR, McVoy MA. Cloning the complete guinea pig cytomegalovirus genome as an infectious bacterial artificial chromosome with excisable origin of replication. J Virol Methods. 2008;149:231–9. doi: 10.1016/j.jviromet.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cui X, McGregor A, Schleiss MR, McVoy MA. The impact of genome length on replication and genome stability of the herpesvirus guinea pig cytomegalovirus. Virology. 2009;386:132–8. doi: 10.1016/j.virol.2008.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nozawa N, Yamamoto Y, Fukui Y, Katano H, Tsutsui Y, Sato Y, Yamada S, Inami Y, Nakamura K, Yokoi M, Kurane I, Inoue N. Identification of a 1.6 kb genome locus of guinea pig cytomegalovirus required for efficient viral growth in animals but not in cell culture. Virology. 2008;379:45–54. doi: 10.1016/j.virol.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 31.Yamada S, Nozawa N, Katano H, Fukui Y, Tsuda M, Tsutsui Y, Kurane I, Inoue N. Characterization of the guinea pig cytomegalovirus genome locus that encodes homologs of human cytomegalovirus major immediate-early genes, UL128, and UL130. Virology. 2009;391:99–106. doi: 10.1016/j.virol.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 32.Schleiss MR. Nonprimate models of congenital cytomegalovirus (CMV) infection: gaining insight into pathogenesis and prevention of disease in newborns. ILAR J. 2006;47:65–72. doi: 10.1093/ilar.47.1.65. [DOI] [PubMed] [Google Scholar]
- 33.Hsuing GD, Choi YC, Bia F. Cytomegalovirus infection in guinea pigs. I. Viremia during acute primary and chronic persistent infection. J Infect Dis. 1978;138:191–196. doi: 10.1093/infdis/138.2.191. [DOI] [PubMed] [Google Scholar]
- 34.Griffith BP, Lucia HL, Bia FJ, Hsuing GD. Cytomegalovirus-induced mononucleosis in guinea pigs. Infect Immun. 1981;32:857–863. doi: 10.1128/iai.32.2.857-863.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bia FJ, Lucia HL, Fong CK, Tarsio M, Hsuing GD. Effects of vaccination on cytomegalovirus-associated interstitial pneumonia in strain 2 guinea pigs. J Infect Dis. 1982;145:742–747. doi: 10.1093/infdis/145.2.742. [DOI] [PubMed] [Google Scholar]
- 36.Aquino-de Jesus MJ, Griffith BP. Cytomegalovirus infection in immunocompromised guinea pigs, a model for testing antiviral agents in vivo. Antiviral Res. 1989;12:181–193. doi: 10.1016/0166-3542(89)90028-4. [DOI] [PubMed] [Google Scholar]
- 37.Bourne N, Bravo FJ, Bernstein DI. Cyclic HPMPC is safe and effective against systemic guinea pig cytomegalovirus infection in immune compromised animals. Antiviral Res. 2000;47:103–109. doi: 10.1016/s0166-3542(00)00100-5. [DOI] [PubMed] [Google Scholar]
- 38.Schleiss MR, Bernstein DI, McVoy MA, Stroup G, Bravo F, Creasy B, McGregor A, Henninger K, Hallenberger S. The non-nucleoside antiviral, BAY 38-4766, protects against cytomegalovirus (CMV) disease and mortality in immunocompromised guinea pigs. Antiviral Res. 2005;65:35–43. doi: 10.1016/j.antiviral.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Booss J, Dann PR, Griffith BP, Kim JH. Glial nodule encephalitis in the guinea pig, serial observations following cytomegalovirus infection. Acta Neuropathol. 1988;75:465–473. doi: 10.1007/BF00687133. [DOI] [PubMed] [Google Scholar]
- 40.Keithley EM, Sharp P, Woolf NK, Harris JP. Temporal sequence of viral antigen expression in the cochlea induced by cytomegalovirus. Acta Otolaryngol. 1988;106:46–54. doi: 10.3109/00016488809107370. [DOI] [PubMed] [Google Scholar]
- 41.Griffith BP, Lavallee JT, Jennings TA, Hsuing GD. Transmission of maternal cytomegalovirus-specific immunity in the guinea pig. Clin Immunol Immunopathol. 1985;35:169–181. doi: 10.1016/0090-1229(85)90063-7. [DOI] [PubMed] [Google Scholar]
- 42.Zheng ZM, Lavallee JT, Bia FJ, Griffith BP. Thymic hypoplasia, splenomegaly and immune depression in guinea pigs with neonatal cytomegalovirus infection. Dev Comp Immunol. 1987;11:407–418. doi: 10.1016/0145-305x(87)90084-x. [DOI] [PubMed] [Google Scholar]
- 43.Booss J, Dann PR, Griffith BP, Kim JH. Host defense response to cytomegalovirus in the central nervous system. Predominance of the monocyte. Am J Pathol. 1989;134:71–78. [PMC free article] [PubMed] [Google Scholar]
- 44.Bravo FJ, Bourne N, Schleiss MR, Bernstein DI. An animal model of neonatal cytomegalovirus infection. Antiviral Res. 2003;60:41–9. doi: 10.1016/s0166-3542(03)00151-7. [DOI] [PubMed] [Google Scholar]
- 45.Bia FJ, Griffith BP, Fong CKY, Hsuing GD. Cytomegaloviral infections in the guinea pig, experimental models for human disease. Rev Infect Dis. 1983;5:177–195. doi: 10.1093/clinids/5.2.177. [DOI] [PubMed] [Google Scholar]
- 46.Enders AC. A comparative study of the fine structure of the trophoblast in several haemochorial placentas. Am J Anat. 1965;116:29–67. doi: 10.1002/aja.1001160103. [DOI] [PubMed] [Google Scholar]
- 47.Griffith BP, McCormick SR, Fong CKY, Lavallee JT, Lucia HL, Goff E. The placenta as a site of cytomegalovirus infection in guinea pigs. J Virol. 1985;55:402–409. doi: 10.1128/jvi.55.2.402-409.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Medearis DN. Mouse cytomegalovirus infection. III. Attempts to produce intrauterine infections. Am J Hyg. 1964;80:113–120. [PubMed] [Google Scholar]
- 49.Choi YC, Hsuing GD. Cytomegalovirus infection in guinea pigs. II. Transplacental and horizontal transmission. J Infect Dis. 1978;138:197–202. doi: 10.1093/infdis/138.2.197. [DOI] [PubMed] [Google Scholar]
- 50.Kumar ML, Nankervis GA. Experimental congenital infection with cytomegalovirus, a guinea pig model. J Infect Dis. 1978;138:650–654. doi: 10.1093/infdis/138.5.650. [DOI] [PubMed] [Google Scholar]
- 51.Johnson KP, Connor WS. Guinea pig cytomegalovirus, transplacental transmission. J Exp Med. 1979;59:263–267. doi: 10.1007/BF01317422. [DOI] [PubMed] [Google Scholar]
- 52.Griffith BP, Lucia HL, Hsuing GD. Brain and visceral involvement during congenital cytomegalovirus infection of guinea pigs. Pediatr Res. 1982;16:455–459. doi: 10.1203/00006450-198206000-00010. [DOI] [PubMed] [Google Scholar]
- 53.Woolf NK, Koehrn FJ, Harris JP, Richman DD. Congenital cytomegalovirus labyrinthitis and sensorineural hearing loss in guinea pigs. J Infect Dis. 1989;160:929–37. doi: 10.1093/infdis/160.6.929. [DOI] [PubMed] [Google Scholar]
- 54.Katano H, Sato Y, Tsutsui Y, Sata T, Maeda A, Nozawa N, Inoue N, Nomura Y, Kurata T. Pathogenesis of cytomegalovirus-associated labyrinthitis in a guinea pig model. Microbes Infect. 2007;9:183–91. doi: 10.1016/j.micinf.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 55.Bia FJ, Griffith BP, Tarsio M, Hsuing GD. Vaccination for the prevention of maternal and fetal infection with guinea pig cytomegalovirus. J Infect Dis. 1980;142:732–738. doi: 10.1093/infdis/142.5.732. [DOI] [PubMed] [Google Scholar]
- 56.Harrison CJ, Britt WJ, Chapman NM, Mullican J, Tracy S. Reduced congenital cytomegalovirus (CMV) infection after maternal immunization with a guinea pig CMV glycoprotein before gestational primary CMV infection in the guinea pig model. J Infect Dis. 1995;172:1212–1220. doi: 10.1093/infdis/172.5.1212. [DOI] [PubMed] [Google Scholar]
- 57.Bourne N, Schleiss MR, Bravo FJ, Bernstein DI. Preconception immunization with a cytomegalovirus (CMV) glycoprotein vaccine improves pregnancy outcome in a guinea pig model of congenital CMV infection. J Infect Dis. 2001;183:59–64. doi: 10.1086/317654. [DOI] [PubMed] [Google Scholar]
- 58.Schleiss MR, Bourne N, Jensen NJ, Bravo F, Bernstein DI. Immunogenicity evaluation of DNA vaccines that target guinea pig cytomegalovirus proteins glycoprotein B and UL83. Virol Immunol. 2000;13:155–167. doi: 10.1089/vim.2000.13.155. [DOI] [PubMed] [Google Scholar]
- 59.Schleiss MR, Bourne N, Bernstein DI. Preconception vaccination with a glycoprotein B (gB) DNA vaccine protects against cytomegalovirus (CMV) transmission in the guinea pig model of congenital CMV infection. J Infect Dis. 2003;188:1868–1874. doi: 10.1086/379839. [DOI] [PubMed] [Google Scholar]
- 60.Schleiss MR, Lacayo JC, Belkaid Y, McGregor A, Stroup G, Rayner J, Alterson K, Chulay JD, Smith JF. Preconceptual administration of an alphavirus replicon UL83 (pp65 homolog) vaccine induces humoral and cellular immunity and improves pregnancy outcome in the guinea pig model of congenital cytomegalovirus infection. J Infect Dis. 2007;195:789–9. doi: 10.1086/511982. [DOI] [PubMed] [Google Scholar]
- 61.Schleiss MR, Jensen NJ. Cloning and expression of the guinea pig cytomegalovirus glycoprotein B (gB) in a recombinant baculovirus: utility for vaccine studies for the prevention of experimental infection. J Virol Methods. 2003;108:59–65. doi: 10.1016/s0166-0934(02)00258-6. [DOI] [PubMed] [Google Scholar]
- 62.Schleiss MR, Bourne N, Stroup G, Bravo FJ, Jensen NJ, Bernstein DI. Protection against congenital cytomegalovirus infection and disease in guinea pigs, conferred by a purified recombinant glycoprotein B vaccine. J Infect Dis. 2004;189:1374–1381. doi: 10.1086/382751. [DOI] [PubMed] [Google Scholar]
- 63.Crumpler MM, Choi KY, McVoy MA, Schleiss MR. A live guinea pig cytomegalovirus vaccine deleted of three putative immune evasion genes is highly attenuated but remains immunogenic in a vaccine/challenge model of congenital cytomegalovirus infection. Vaccine. 2009;27:4209–18. doi: 10.1016/j.vaccine.2009.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haggerty SM, Schleiss MR. A novel CC-chemokine homolog encoded by guinea pig cytomegalovirus. Virus Genes. 2002;25:271–9. doi: 10.1023/a:1020923924471. [DOI] [PubMed] [Google Scholar]
- 65.Penfold M, Miao Z, Wang Y, Haggerty S, Schleiss MR. A macrophage inflammatory protein homolog encoded by guinea pig cytomegalovirus signals via CC chemokine receptor 1. Virology. 2003;316:202–12. doi: 10.1016/s0042-6822(03)00581-6. [DOI] [PubMed] [Google Scholar]
- 66.Schraff SA, Schleiss MR, Brown DK, Meinzen-Derr J, Choi KY, Greinwald JH, Choo DI. Macrophage inflammatory proteins in cytomegalovirus-related inner ear injury. Otolaryngol Head Neck Surg. 2007;137:612–8. doi: 10.1016/j.otohns.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 67.Schraff SA, Brown DK, Schleiss MR, Meinzen-Derr J, Greinwald JH, Choo DI. The role of CMV inflammatory genes in hearing loss. Otol Neurotol. 2007;28:964–9. doi: 10.1097/MAO.0b013e318067bd42. [DOI] [PubMed] [Google Scholar]
- 68.Bratcher DF, Bourne N, Bravo FJ, Schleiss MR, Slaoui M, Myers MG, Bernstein DI. Effect of passive antibody on congenital cytomegalovirus infection in guinea pigs. J Infect Dis. 1995;172:944–50. doi: 10.1093/infdis/172.4.944. [DOI] [PubMed] [Google Scholar]
- 69.Chatterjee A, Harrison CJ, Britt WJ, Bewtra C. Modification of maternal and congenital cytomegalovirus infection by anti-glycoprotein b antibody transfer in guinea pigs. J Infect Dis. 2001;183:1547–53. doi: 10.1086/320714. [DOI] [PubMed] [Google Scholar]
- 70.Schleiss MR, Anderson JL, McGregor A. Cyclic cidofovir (cHPMPC) prevents congenital cytomegalovirus infection in a guinea pig model. Virol J. 2006;1:3–9. doi: 10.1186/1743-422X-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bravo FJ, Cardin RD, Bernstein DI. Effect of maternal treatment with cyclic HPMPC in the guinea pig model of congenital cytomegalovirus infection. J Infect Dis. 2006;193:591–7. doi: 10.1086/499603. [DOI] [PubMed] [Google Scholar]
- 72.Nigro G, Adler SP, La Torre R, Best AM, Congenital Cytomegalovirus Collaborating Group Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353:1350–62. doi: 10.1056/NEJMoa043337. [DOI] [PubMed] [Google Scholar]
- 73.Griffiths PD. Strategies to prevent CMV infection in the neonate. Semin Neonatol. 2002;7:293–9. doi: 10.1016/s1084-2756(02)90123-5. [DOI] [PubMed] [Google Scholar]
- 74.Jeon J, Victor M, Adler SP, Arwady A, Demmler G, Fowler K, Goldfarb J, Keyserling H, Massoudi M, Richards K, Staras SA, Cannon MJ. Knowledge and awareness of congenital cytomegalovirus among women. Infect Dis Obstet Gynecol. 2006;2006:80383. doi: 10.1155/IDOG/2006/80383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ross DS, Dollard SC, Victor M, Sumartojo E, Cannon MJ. The epidemiology and prevention of congenital cytomegalovirus infection and disease: activities of the Centers for Disease Control and Prevention Workgroup. J Womens Health (Larchmt) 2006;15:224–9. doi: 10.1089/jwh.2006.15.224. [DOI] [PubMed] [Google Scholar]


