Abstract
Endogenous opioid peptides enkephalin and dynorphin are major co-transmitters of striatofugal pathways of the basal ganglia. They are involved in the genesis of levodopa-induced dyskinesia and in the modulation of direct and indirect striatal output pathways that are disrupted in Parkinson’s disease. One pharmacologic approach is to develop synthetic glycopeptides closely resembling endogenous peptides to restore their normal functions. Glycosylation promotes penetration of the blood-brain barrier. We investigated CNS penetration of the opioid glycopeptide MMP-2200, a mixed δ/μ-agonist based on leu-enkephalin, as measured by in vivo microdialysis and subsequent mass spectrometric analysis in awake, freely moving rats. The glycopeptide (10 mg/kg) reaches the dorsolateral striatum (DLS) rapidly after systemic (i.p.) administration and is stably detectable for the duration of the experiment (80 min). The detected level at the end of the experiment (around 250 pM) is about 10-fold higher than the level of the endogenous leu-enkephalin, measured simultaneously. This is one of the first studies to directly prove that glycosylation of an endogenous opioid peptide leads to excellent blood-brain barrier penetration after systemic injection, and explains robust behavioral effects seen in previous studies by measuring how much glycopeptide reaches the target structure, in this case the DLS.
Keywords: striatum, leu-enkephalin, glycopeptide, blood-brain barrier penetration, mass spectrometry
Introduction
Parkinson’s disease (PD) is the 2nd most common neurodegenerative disorder. The cardinal motor symptoms are tremor, rigidity, and bradykinesia [38]. The underlying pathology is the loss of dopaminergic neurons with cell bodies located in the substantia nigra and axonal projections to the striatum. The gold standard treatment for PD is dopamine replacement therapy utilizing levodopa, the dopamine precursor, or utilizing dopamine receptor agonists. These therapies become unsatisfactory as the disease progresses due to a variety of effects that occur with dose escalation, including levodopa-induced dyskinesias (LID). Therefore, there is an urgent need to develop non-dopaminergic therapies, especially adjunct therapies that may extend the use of levodopa [38].
Although classic opioids have not found utility in the treatment of PD, they have a profound effect on locomotion and reward behavior mediated by the basal ganglia by acting as modulators of glutamate, GABA and dopamine neurotransmission [17]. High levels of μ- and δ-opioid receptors are present in the striatum [35] and striatal levels of the endogenous opioid peptides dynorphin and met-enkephalin are greatly altered in PD [42]. Striatopallidal projection neurons of the indirect striatal output pathway express enkephalins derived from the precursor preproenkephalin A (PPE-A, Penk), while the striatonigral neurons of the direct striatal output pathway express opioids derived from preproenkephalin B (PPE-B, Pdyn) [10,20]. Following long-term levodopa therapy that eventually produces dyskinesia, both the levels of opioid peptides and the mRNA encoding their precursors are elevated in PD animal models [16,13,7,22]. Postmortem studies in PD patients who have had motor fluctuations due to long-term levodopa use confirm increased striatal Penk and Pdyn expression [23,37,6]. These alterations suggest that the increased expression of enkephalin may be a compensatory mechanism for dopamine depletion in PD. Studies of opioid antagonists in MPTP-lesioned primates with dyskinesia have produced contradictory results [21,24,27,41]. In phase IIa clinical trials low-dose oral naltrexone failed to show any effect [40], whereas high-dose naltrexone had a minimal effect [34]. A trial using naloxone at a dose known to block central opioid receptors also failed to demonstrate reduction in dyskinesia, but did show an extension in duration of action of levodopa [18]. This disparity has led to conflicting concepts that opioids represent either a cause of dyskinesia or a compensatory mechanism. In our view, μ and δ-opioid systems have differing, complex, and opposing effects in PD so that non-selective opioid antagonists may have no net effect. Given this background, novel opioid compounds could provide an important non-dopaminergic therapy for PD and other movement disorders. Selective μ-opioid antagonists have shown promise in preclinical trials of LID [28] and δ-agonists have potent effects in vertebrate models of dopamine denervation [25,26,8,33]. These cyclic alkaloidal drugs may have side effects and toxicities independent of their opioid pathway activity that are not present in their peptide counterparts [1]. The probability of side effects due to the production of active metabolites is less with glycopeptide-based drugs versus alkaloids, since the opioid glycopeptides are degraded to inactive di- and tri-peptides and sugars [19].
We previously showed that systemic administration of MMP-2200, a glycosylated leu-enkephalin analog, Tyr-D-Thr-Gly-Phe-Leu-Ser(β-O-Glc)-CONH2 [30], has potent behavioral effects in 2 rodent models of striatal dopamine depletion [44]. It is also worth noting that when compared to morphine, MMP-2200 shows a better side effect profile as shown in several pain and dependence models, including naloxone precipitated withdrawal [30]. In the current study we expand these findings by measuring the amount of MMP-2200 that reaches the DLS with in vivo microdialysis and mass spectrometry after systemic injection in rats to quantify the amount of glycopeptide in the target structure, the DLS in this case.
Materials and Methods
Glycopeptide Agonist
MMP-2200 was GMP-compliant material synthesized by PolyPeptide Labs in Torrence, CA, and has been described in detail previously [30,44], leu-ENK for standard preparation was purchased from Phoenix Pharmaceuticals (Burlingame, CA). Water and methanol for mobile phases are Burdick & Jackson HPLC grade purchased from VWR (Radnor, PA). All other salts and chemicals were from Sigma Aldrich (St. Louis, MO) unless otherwise noted.
Microdialysis
Adult male Sprague–Dawley rats (Harlan, Indianapolis, IN) weighing between 250 and 350 g were used for all experiments (n=7). Rats were housed in a temperature and humidity controlled room with 12 h light/dark cycles with food and water available ad libitum. All animals were treated as approved by the University of Michigan Unit for Laboratory Animal Medicine (ULAM) and in accordance with the NIH Guidelines for the Care and Use of Laboratory Animals.
Prior to surgery, rats were anesthetized with an intraperitoneal (i.p.) injection of a ketamine (65 mg/kg) and dexdormitor (0.25 mg/kg) mixture prepared in an isotonic salt solution. Concentric microdialysis probes with 2 mm long (AN69 polyacrylonitrile membrane,Hospal, Bologna, Italy) and 0.3 mm diameter active membranes were implanted using an ultra precise model 963 stereotaxic instrument (David Kopf Instruments, Tujunga, CA), into the DLS according to the following coordinates from bregma: AP +1.0, ML ±3.5 DV −5.5 [40]. Although in vitro recoveries were not performed for every probe as is sometimes done with microdialysis studies, we generally achieve ~30–40% in vitro recovery of enkephalin peptides with 2 mm long polyacrylonitrile probes at a flow-rate of 0.9 μl/min. Thus, it is concluded that concentrations of actual peptide content in the brain is ~3 X the reported values for analyzed dialysates. Probes were secured to the skull by acrylic dental cement and metallic screws. Following surgery, rats were allowed to recover and experiments were run 24 h after probe implantation. Microdialysis probes were flushed at a flow rate of 1.5 μL/min with a modified Ringer solution (composition in mM: CaCl2 1.2; KCl 2.7, NaCl 148 and MgCl2 0.85) for 2 h using a Chemyx Fusion 400 syringe pump (Chemyx, Stafford, TX). Perfusion flow rate was then reduced to 0.9 μL/min and samples were collected every 10 min into tubes containing 0.5 μL acetic acid to preserve peptide stability as previously described [29,32]. Basal samples were collected for 40 min and then subjects were injected with MMP-2200 (10 mg/kg i.p.). Samples were then collected for an additional 80 min. Samples were immediately injected on the LC-MS system following collection.
MMP-2200 and leu-ENK detection with capillary LC-MS3
MMP-2200 and Leu-ENK were measured using a modified version of a method previously used for enkephalins in vivo [29,32]. Chromatography columns were 50 μm inner diameter (i.d.) fused silica capillary packed in-house to 4 cm length with 5 μm Alltima C18 reversed-phase particles (Alltech, Deerfield, IL) using a high pressure reservoir. ESI emitter tips were also prepared in-house from a 3 cm length of 40 μm i.d. fused silica capillary using a laser puller (P-2000, Sutter Instruments, Novatao, CA). Columns and tips were joined using a 2 cm PTFE 1/16″ x .010″ sleeve adapter. An air driven fluid pump (DHSF-151, Haskel Inc., Burbank, CA) was used for sample loading and desalting (4000 psi), and a micro HPLC pump (MicroPro, Eldex Laboratories, Napa, CA) for gradient elution (700 psi). Samples were injected using a WPS-3000TPL autosampler (Dionex, Sunnyvale, CA), in partial loop injection mode (5 μL loop).
The 5 μL samples were loaded over 8 min followed by 2 min rinse with H2O to desalt the column at 2.5 μL/min. Following loading and desalting, the injector valve was switched to the gradient pump to elute the peptides at 300 nL/min. Mobile phase A consisted of LC-MS grade water with 0.5% acetic acid and mobile phase B consisted of LC-MS grade methanol with 0.5% acetic acid. The gradient program began with an isocratic step of 10% B then a linear increase to 80% B over 4 min, followed by a linear increase to 100% B in 1 min. An isocratic step at 100% B for 2 min was followed by a linear decrease down to 10% B over 0.5 min. Finally, 10% B was maintained isocratically for 2 min to re-equilibrate the LC system before the next injection. All valve switching and runs were controlled automatically with Xcalibur software (Thermo Fisher Scientific).
The column and emitter tip were coupled to a PV-550 nanospray ESI source (New Objective, Woburn, MA) interfaced to an LTQ XL linear ion trap (LIT) MS (Thermo Fisher Scientific, Waltham, MA). A +2.5 kV potential was applied to a liquid junction prior to the column for electrospray. The MS3 ion transition pathways set on the LIT for MMP-2200 and leu-ENK (both singly charged) were: 1010→686→582,651,689 and, 556→397→278,323,380, respectively.
Detection limits for both leu-ENK and MMP-2200 were around 1 pM. This is in accordance with previous detection limits of leu-ENK using variations of this LC-MS method from our group [29,32]. Detection limit was determined by visual inspection of peak area at a range of concentrations when performing calibration curves. Generally, we consider the limit of detection to be equal to 3 X the signal-to-noise ratio of a blank (aCSF) injection. This can be interpreted as the standard deviation of the blank multiplied by 3, divided by the slope of the calibration curve. Leu-ENK, and to a greater extent MMP-2200, were measured at levels considerably higher than detection limits.
Statistical analysis of the data
Repeated measures ANOVAs were used to evaluate statistical differences over the course of the experiment for both MMP-2200 and leu-ENK measurements using GraphPad Prism 5 (GraphPad, La Jolla, CA). One tail Fisher LSD post hoc tests were used to evaluate the statistical difference between baseline and post-injection of MMP-2200 at different time points. In two animals one data point each in the leu-ENK experiment was more than 3 standard deviations greater than the mean and with Cook’s distance above Di = 4/n. These two animals were excluded from analysis in both measurements of the experiment. The null hypothesis was rejected when p < 0.05.
Results and Discussion
LC-MS measurements of leu-ENK and MMP-2200
Both MMP-2200 and leu-ENK were readily detectable by the current LC-MS method (Figure 1). Following a 10 min loading and desalting phase, both peptides chromatographically eluted in about 5 min. Total run time was 17 min per sample injection.
Figure 1. LC-MS analysis of dialysate peptide content.
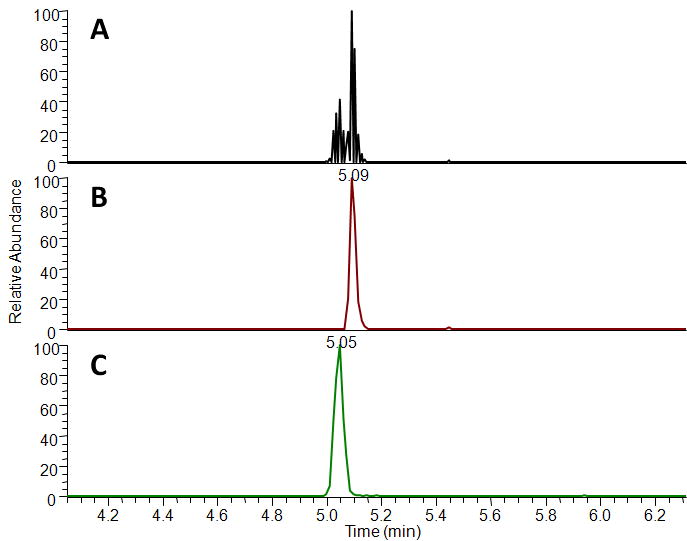
Total ion chromatogram from MS3 analysis of a 10 pM standard including both leu-ENK and MMP-2200 (A). Reconstructed ion chromatograms for MMP-2200 (B) and leu-ENK (C). Run time for each sample was 17 min including 10 min for sample loading and desalting and 7 min for MS analysis.
CNS penetration of MMP-2200
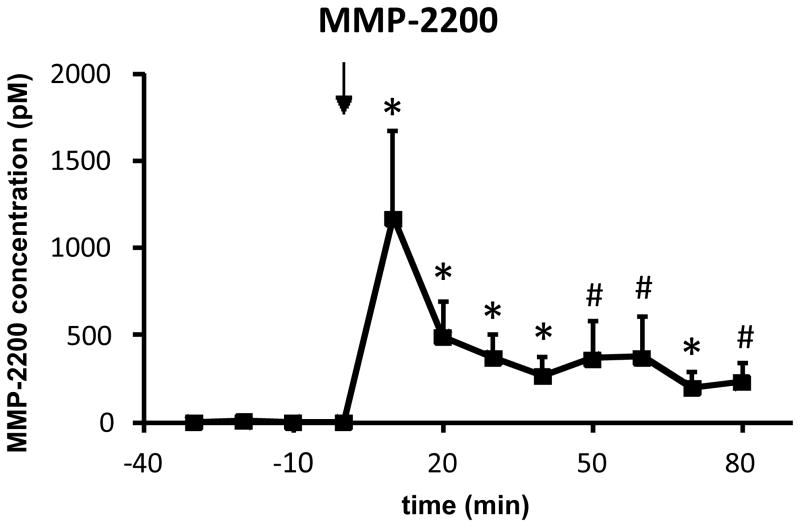
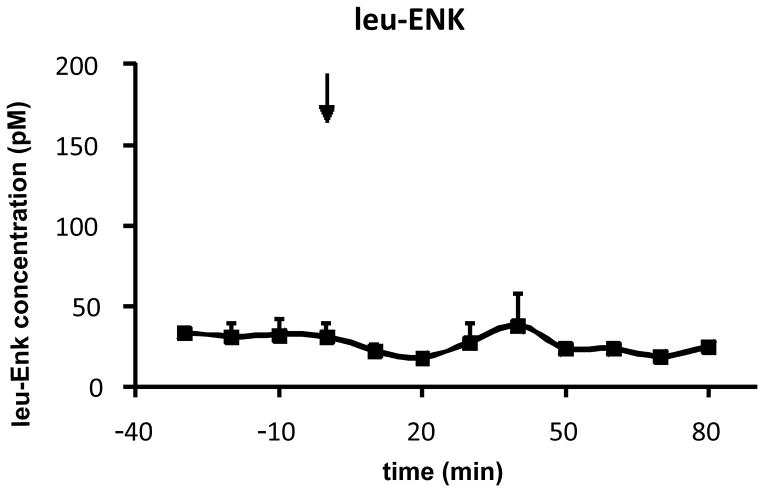
MMP-2200 was systemically injected (10 mg/kg, i.p.) and the uptake into the DLS was measured by in vivo microdialysis and LC-MS analysis. These experiments were performed in awake, freely moving rats, implanted with microdialysis probes in the DLS 24 hr prior to the experiments. It has been demonstrated that the blood-brain barrier (BBB) integrity is established after such a short period following probe implantation [4,43,2,11]. Although some controversy remains regarding the integrity of the BBB following microdialysis surgeries [36], performing experiments 24 h after probe implantation is likely the most suitable method for maintaining BBB integrity. Other factors such as lowering the probe slowly, using narrow (~300 μm) concentric probes and a low flow rate (0.9 μl/min) likely prevented BBB disruption [11]. The animals were fully recovered, their wounds healed, and the probes were fixed in place with dental cement. A highly significant (repeated measures ANOVA: p < 0.01; n = 5) level of MMP-2200 (post hoc tests at single time points: *p < 0.05; #p < 0.09) can be measured from dialysate of the DLS at the first time point (10 min) after injection (arrow) at approximately 1200 pM and remains at active levels (around 250 pM) until the end of the experiment at 80 min, as shown in Figure 2. It must be noted that dialysate concentrations are reported here, and not actual brain peptide levels. Since in-vitro recoveries do not necessarily reflect true in vivo conditions such as active transport of peptide, temperature, and enzymatic degradation, we choose to omit this step and estimate concentrations instead based on past experience (data not shown). Thus, we estimate that our reported values are ~1/3 less than actual concentrations reaching the brain. For comparative reasons, the endogenous leu-ENK level was measured at the same time as MMP-2200 and is depicted in Figure 3. The basal dialysate concentration of leu-ENK is around 25 pM in concordance with the literature [3,32], and remains unchanged during the experiment (repeated measures ANOVA: p = 0.8), further supporting that the BBB was not compromised by the experimental procedure. No quantitative evaluation of brain concentrations of other opioid peptide analogs has been published to date. The rapid rise and fall of MMP-2200 levels between 10 and 20 min in the brain post i.p administration is noteworthy. The pharmakodynamics of this class of drug is not well studied, but membranes may be regarded as an additional compartment that complicates distribution [9].
Figure 2. Proof of blood-brain barrier penetration of the opioid glycopeptide MMP-2200 after systemic administration.
MMP-2200 (10 mg/kg, i.p.) rapidly reaches the DLS as measured by in vivo microdialysis and subsequent mass spectrometric analysis in awake, freely moving rats, implanted 24 hr prior to the experiments (fully recovered, wounds healed, covered by a stage that holds the probe in place; n = 5). A highly significant (repeated measures ANOVA: p < 0.01) level of MMP-2200 (mean±SEM; post hoc tests at each time point post-injection vs. time point 0: *p < 0.05, #p < 0.09) can be measured in the dialysate from the first time point after injection (arrow) and remains at active levels (around 250 pM) until the end of the experiment.
Figure 3. Level of endogenous leu-enkephalin in DLS is unchanged.
The endogenous leu-ENK level (mean±SEM), measured by in vivo microdialysis and subsequent mass spectrometric analysis at the same time as in Figure 2 (n = 5; repeated measures ANOVA: p = 0.8), is unchanged before and after the MMP-2200 (10 mg/kg, i.p.) injection (arrow). The average leu-ENK level remains stable at around 25 pM at both baseline and post-injection.
Centrally-mediated behavioral effects of MMP-2200 and other glycosylated opioid peptides
The high level of MMP-2200 that reaches the DLS after systemic injection explains the strong behavioral effects seen in preclinical models of striatal dopamine depletion [44]. Amphetamine-induced rotations were reduced by 50%, and the apomorphine-induced overshoot in movement of reserpine-treated akinesia rats was reversed by MMP-2200 in that study, both indicators that MMP-2200 reduces downstream effects of dopamine depletion. Additionally, strong behavioral effects in preclinical rodent models of pain that are centrally-mediated were reported by systemically administered (i.v., i.p., s.c.) glycopeptides [5,14,30,31]. Like its unglycosylated parent peptide, MMP-2200 displays similar nanomolar affinities for both δ- and μ-opioid receptors [15]. Combined, these data indicate that glycosylation of endogenous peptides is a valid strategy to achieve BBB-penetration. One previous study in rhesus monkeys suggested that CNS distribution of MMP-2200 was very limited after i.m. injection [12]. The glycopeptide did produce anti-allodynic effects, comparable to morphine, but was not very effective in a tail flick model of thermal nociception. This may reflect differences in route of administration (i.m. vs s.c. or i.v.), differences in pharmacokinetics and brain penetration due to species (rhesus monkey vs rodent), or may even reflect ambiguity in interpretation of the tail flick response in rhesus monkey (antinociception vs euphoria).
Conclusion
This is the first study to directly show that glycosylation of an acyclic endogenous opioid peptide leads to excellent BBB penetration after systemic injection and explains the robust behavioral effects seen in our previous studies in models of dopamine depletion by quantifying the amount of glycopeptide in the target structure, the DLS in this case. We believe that further development of opioid glycopeptides with more receptor specificity, particularly δ-agonists and μ-antagonists could lead to novel therapeutic options for the treatment of LID. This proof-of-principle study substantiates that glycopeptides based on endogenous peptides are valid targets for drug development.
Highlights.
Novel glycosylated opioid peptide MMP-2200 is opioid mu and delta receptor agonist.
MMP-2200 reached dorsolateral striatum (DLS) after systemic administration (i.p.).
MMP-2200 concentration in DLS reached nM range within 10 minutes.
Endogenous leu-enkephalin levels were unchanged after MMP-2200 administration.
Acknowledgments
This work was supported by the American Parkinson’s Disease Association (T.F. and S.J.S.), the Office of Naval Research (grants 14-02-01-0471, 14-05-1-0807), the NSF (CHE-607917), the NIH (NINDS-NS-052727) (R.P.), R37 EB003320 (R.T.K.) and NIDA T32 training grant DA07268 (O.S.M.).
Footnotes
The authors have no conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aldrich JV, Patkar KA, McLaughlin JP. Zyklophin, a systemically active selective kappa opioid receptor peptide antagonist with short duration of action. Proc Natl Acad Sci USA. 2009;106:18396–18401. doi: 10.1073/pnas.0910180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen DD, Crooks PA, Yokel RA. 4-Trimethylammonium antipyrine: a quaternary ammonium nonradionuclide marker for blood-brain barrier integrity during in vivo microdialysis. J Pharmacol Toxicol Meth. 1992;28(3):129–135. doi: 10.1016/1056-8719(92)90074-b. [DOI] [PubMed] [Google Scholar]
- 3.Baseski HM, Watson CJ, Cellar NA, Shackman JG, Kennedy RT. Capillary liquid chromatography with MS3 for the determination of enkephalins in microdialysis samples from the striatum of anesthetized and freely-moving rats. J Mass Spectrom. 2005;40(2):146–153. doi: 10.1002/jms.733. [DOI] [PubMed] [Google Scholar]
- 4.Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem. 1984;43(5):1369–1374. doi: 10.1111/j.1471-4159.1984.tb05396.x. [DOI] [PubMed] [Google Scholar]
- 5.Bilsky EJ, Egleton RD, Mitchell SA, Palian MM, Davis P, Huber JD, Jones H, Yamamura HI, Janders J, Davis TP, Porreca F, Hruby VJ, Polt R. Enkephalin glycopeptide analogues produce analgesia with reduced dependence liability. J Med Chem. 2000;43(13):2586–2590. doi: 10.1021/jm000077y. [DOI] [PubMed] [Google Scholar]
- 6.Calon F, Birdi S, Rajput AH, Hornykiewicz O, Bedard PJ, Di PT. Increase of preproenkephalin mRNA levels in the putamen of Parkinson disease patients with levodopa-induced dyskinesias. J Neuropathol Exp Neurol. 2002;61:186–196. doi: 10.1093/jnen/61.2.186. [DOI] [PubMed] [Google Scholar]
- 7.Cenci MA, Lee CS, Bjorklund A. L-dopa–induced dyskinesia in the rat is associated with striatal overexpression of prodynorphinand glutamic acid decarboxylase mRNA. Eur J Neurosci. 1998;10:2694–2706. [PubMed] [Google Scholar]
- 8.Childers SR, Fleming LM, Selley DE, McNutt RW, Chang KJ. BW373U86: a nonpeptidic delta-opioid agonist with novel receptor-G protein-mediated actions in rat brain membranes and neuroblastoma cells. Mol Pharmacol. 1993;44(4):827–834. [PubMed] [Google Scholar]
- 9.Cox EH, Kerbusch T, Van Der Graaf PH, Danhof M. Pharmacokinetic-Pharmacodynamic Modeling of the Electroencephalogram Effect of Synthetic Opioids in the Rat: Correlation with the Interaction at the Mu-Opioid Receptor. JPharmExpTherap. 1998;284:1095–1103. [PubMed] [Google Scholar]
- 10.Cuello AC, Paxinos G. Evidence for a long Leu-enkephalin striopallidal pathway in rat brain. Nature. 1978;271:178–180. doi: 10.1038/271178a0. [DOI] [PubMed] [Google Scholar]
- 11.de Lange EC, Danhof M, de Boer AG, Breimer DD. Methodological considerations of intracerebral microdialysis in pharmacokinetic studies on drug transport across the blood-brain barrier. Brain Res Brain Res Rev. 1997;25(1):27–49. doi: 10.1016/s0165-0173(97)00014-3. [DOI] [PubMed] [Google Scholar]
- 12.Do Carmo GP, Polt R, Bilsky EJ, Rice KC, Negus SS. Behavioral pharmacology of the mu/delta opioid glycopeptide MMP2200 in rhesus monkeys. J Pharmacol Exp Ther. 2008;326(3):939–948. doi: 10.1124/jpet.108.138180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duty S, Brotchie JM. Enhancement of the behavioral response to apomorphine administration following repeated treatment in the 6-hydroxydopamine-lesioned rat is temporally correlated with a rise in striatal preproenkephalin-B, but not preproenkephalin-A, gene expression. Exp Neurol. 1997;144:423–432. doi: 10.1006/exnr.1997.6431. [DOI] [PubMed] [Google Scholar]
- 14.Egleton RD, Mitchell SA, Huber JD, Palian MM, Polt R, Davis TP. Improved blood-brain barrier penetration and enhanced analgesia of an opioid peptide by glycosylation. J Pharm Exp Ther. 2001;299:967–972. [PubMed] [Google Scholar]
- 15.Elmagbari NO, Egleton RD, Palian MM, Lowery JJ, Schmid WR, Davis P, Navratilova E, Dhanasekaran M, Keyari CM, Yamamura HI, Porreca F, Hruby VJ, Polt R, Bilsky EJ. Antinociceptive structure-activity studies with enkephalin-based opioid glycopeptides. J Pharm Exp Therap. 2004;311:290–297. doi: 10.1124/jpet.104.069393. [DOI] [PubMed] [Google Scholar]
- 16.Engber TM, Susel Z, Kuo S, Gerfen CR, Chase TN. Levodopa replacement therapy alters enzyme activities in striatum and neuropeptide content in striatal output regions of 6-hydroxydopamine lesioned rats. Brain Res. 1991;552:113–118. doi: 10.1016/0006-8993(91)90667-k. [DOI] [PubMed] [Google Scholar]
- 17.Fox SH, Lang AE, Brotchie JM. Translation of Nondopaminergic Treatments for Levodopa-Induced Dyskinesia From MPTP-Lesioned Nonhuman Primates to Phase IIa Clinical Studies: Keys to Success and Roads to Failure Mov. Disord. 2006;21(10):1578–1594. doi: 10.1002/mds.20936. [DOI] [PubMed] [Google Scholar]
- 18.Fox SH, Silverdale M, Kellett M, Davies R, Steiger M, Fletcher N, Crossman A, Brotchie J. Non–subtype-selective opioid receptor antagonism in treatment of levodopa-induced motor complications in Parkinson’s disease. Mov Disord. 2004;19:554–560. doi: 10.1002/mds.10693. [DOI] [PubMed] [Google Scholar]
- 19.Geary LE, Wiley KS, Scott WL, Cohen ML. Degradation of Exogenous Enkephalin in the Guinea-Pig Ileum - Relative Importance of Aminopeptidase, Enkephalinase and Angiotensin Converting Enzyme-Activity. JPharmExpTherap. 1982;221:104–111. [PubMed] [Google Scholar]
- 20.Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992;15:133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Mancilla B, Bedard PJ. Effect of nondopaminergic drugs on L-dopa–induced dyskinesias in MPTP-treated monkeys. Clin Neuropharmacol. 1993;16:418–427. doi: 10.1097/00002826-199310000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Henry B, Crossman AR, Brotchie JM. Effect of repeated L-dopa, bromocriptine, or lisuride administration on preproenkephalin-A and preproenkephalin-B mRNA levels in the striatum of the 6-hydroxydopamine-lesioned rat. Exp Neurol. 1999;155:204–220. doi: 10.1006/exnr.1998.6996. [DOI] [PubMed] [Google Scholar]
- 23.Henry B, Duty S, Fox SH, Crossman AR, Brotchie JM. Increased striatal pre-proenkephalin B expression is associated with dyskinesia in Parkinson’s disease. Exp Neurol. 2003;183:458–468. doi: 10.1016/s0014-4886(03)00064-5. [DOI] [PubMed] [Google Scholar]
- 24.Henry B, Fox SH, Crossman AR, Brotchie JM. Mu- and delta opioid receptor antagonists reduce levodopa-induced dyskinesia in the MPTP-lesioned primate model of Parkinson’s disease. Exp Neurol. 2001;171:139–146. doi: 10.1006/exnr.2001.7727. [DOI] [PubMed] [Google Scholar]
- 25.Hille CJ, Fox SH, Maneuf YP, Crossman AR, Brotchie JM. Antiparkinsonian action of a delta opioid agonist in rodent and primate models of Parkinson’s disease. Exp Neurol. 2001;172(1):189–198. doi: 10.1006/exnr.2001.7763. [DOI] [PubMed] [Google Scholar]
- 26.Hudzik TJ, Howell A, Payza K, Cross AJ. Antiparkinson potential of delta-opioid receptor agonists. Eur J Pharmacol. 2000;396(2–3):101–107. doi: 10.1016/s0014-2999(00)00209-0. [DOI] [PubMed] [Google Scholar]
- 27.Klintenberg R, Svenningsson P, Gunne L, Andren PE. Naloxone reduces levodopa-induced dyskinesias and apomorphine-induced rotations in primate models of parkinsonism. J Neural Transm. 2002;109:1295–1307. doi: 10.1007/s00702-002-0715-6. [DOI] [PubMed] [Google Scholar]
- 28.Koprich JB, Fox SH, Johnston TH, Goodman A, Le Bourdonnec B, Dolle RE, DeHaven RN, DeHaven-Hudkins DL, Little PJ, Brotchie JM. The selective mu-opioid receptor antagonist ADL5510 reduces levodopa-induced dyskinesia without affecting antiparkinsonian action in MPTP-lesioned macaque model of Parkinson’s disease. Mov Disord. 2011;26(7):1225–1233. doi: 10.1002/mds.23631. [DOI] [PubMed] [Google Scholar]
- 29.Li Q, Zubieta JK, Kennedy RT. Practical aspects of in vivo detection of neuropeptides by microdialysis coupled off-line to capillary LC with multistage MS. Anal Chem. 2009;81(6):2242–2250. doi: 10.1021/ac802391b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lowery JJ, Raymond TJ, Giuvelis D, Bidlack JM, Polt R, Bilsky EJ. In Vivo Characterization of MMP-2200, a Mixed δ/μ Opioid Agonist, in Mice. J Pharmacol Exp Ther. 2011;336(3):767–778. doi: 10.1124/jpet.110.172866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lowery JJ, Yeomans L, Keyari CM, Davis P, Porreca F, Knapp BI, Bidlack JM, Bilsky EJ, Polt R. Glycosylation improves the central effects of DAMGO. Chem Biol Drug Dis. 2007;69:41–47. doi: 10.1111/j.1747-0285.2007.00462.x. [DOI] [PubMed] [Google Scholar]
- 32.Mabrouk OS, Li Q, Song P, Kennedy RT. Microdialysis and mass spectrometric monitoring of dopamine and enkephalins in the globus pallidus reveal reciprocal interactions that regulate movement. J Neurochem. 2011;118(1):24–33. doi: 10.1111/j.1471-4159.2011.07293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma brouk OS, Volta M, Marti M, Morari M. Stimulation of delta opioid receptors located in substantia nigra reticulata but not globus pallidus or striatum restores motor activity in 6-hydroxydopamine lesioned rats: new insights into the role of delta receptors in parkinsonism. J Neurochem. 2008;107(6):1647–59. doi: 10.1111/j.1471-4159.2008.05727.x. [DOI] [PubMed] [Google Scholar]
- 34.Manson AJ, Katzenschlager R, Hobart J, Lees AJ. High dose naltrexone for dyskinesias induced by levodopa. J Neurol Neurosurg Psychiatry. 2001;70:554–556. doi: 10.1136/jnnp.70.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350(3):412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- 36.Morgan ME, Singhal D, Anderson BD. Quantitative assessment of blood-brain barrier damage during microdialysis. J Pharmacol Exp Ther. 1996;277(2):1167–1176. [PubMed] [Google Scholar]
- 37.Nisbet AP, Foster OJ, Kingsbury A, Eve DJ, Daniel SE, Marsden CD, Lees AJ. Preproenkephalin and preprotachykinin messenger RNA expression in normal human basal ganglia and in Parkinson’s disease. Neuroscience. 1995;66:361–376. doi: 10.1016/0306-4522(94)00606-6. [DOI] [PubMed] [Google Scholar]
- 38.Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson’s disease. Neurology. 2009;72(21 Suppl 4):S1–136. doi: 10.1212/WNL.0b013e3181a1d44c. [DOI] [PubMed] [Google Scholar]
- 39.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 2007. [Google Scholar]
- 40.Rascol O, Fabre N, Blin O, Poulik J, Sabatini U, Senard JM, Ané M, Montastruc JL, Rascol A. Naltrexone, an opiate antagonist, fails to modify motor symptoms in patients with Parkinson’s disease. Mov Disord. 1994;9:437–440. doi: 10.1002/mds.870090410. [DOI] [PubMed] [Google Scholar]
- 41.Samadi P, Gregoire L, Bedard PJ. Opioid antagonists increase the dyskinetic response to dopaminergic agents in parkinsonian monkeys: interaction between dopamine and opioid systems. Neuropharmacol. 2003;45:954–963. doi: 10.1016/s0028-3908(03)00249-1. [DOI] [PubMed] [Google Scholar]
- 42.Samadi P, Bedard PJ, Rouillard C. Opioids and motor complications in Parkinson’s disease. Trends Pharmacol Sci. 2006;27:512–517. doi: 10.1016/j.tips.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Tetsuya T, Yoshiharu D, Yuko K, Pardridge WM, Akira T. Determination of in vivo steady-state unbound drug concentration in the brain interstitial fluid by microdialysis. Int J Pharma. 1992;81(2–3):143–152. [Google Scholar]
- 44.Yue X, Falk T, Zuniga LA, Szabo L, Porreca F, Polt R, Sherman SJ. Effects of the novel glycopeptide opioid agonist MMP-2200 in preclinical models of Parkinson’s disease. Brain Res. 2011;1413:72–83. doi: 10.1016/j.brainres.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]