Abstract
Translational research for the treatment and prevention of breast cancer depends upon the four Ms: models, molecules, and mechanisms in order to create medicines. The process, to target the estrogen receptor (ER) in estrogen-dependent breast cancer, has yielded significant advances in patient survivorship and the first approved medicines (tamoxifen and raloxifene) to reduce the incidence of any cancer in high- or low-risk women. This review focuses on the critical role of the few ER-positive cell lines (MCF-7, T47D, BT474, ZR-75) that continue to advance our understanding of the estrogen-regulated biology of breast cancer. More importantly, the model cell lines have provided an opportunity to document the development and evolution of acquired antihormone resistance. The description of this evolutionary process that occurs in micrometastatic disease during up to a decade of adjuvant therapy would not be possible in the patient. The use of the MCF-7 breast cancer cell line in particular has been instrumental in discovering a vulnerability of ER-positive breast cancer exhaustively treated with antihormone therapy. Physiologic estradiol acts as an apoptotic trigger to cause tumor regression. These unanticipated findings in the laboratory have translated to clinical advances in our knowledge of the paradoxical role of estrogen in the life and death of breast cancer.
Never in the field of breast cancer research [human conflict] was so much owed by so many to so few.
(With apologies to the late Winston Spencer Churchill, Prime Minister, August 20, 1940 reporting on the successful winning of the Battle of Britain).
Introduction
The past four decades have witnessed the successful evolution of effective breast cancer therapies as scientific research has translated into clinical practice. Breast cancer therapy began its story with combination cytotoxic chemotherapy. Chemotherapy, though able to create complete responses in some cases of breast cancer, works non-specifically, causing harmful and sometimes intolerable, life-threatening side effects. Antiestrogen therapies, by contrast, provide significant therapeutic improvement by focusing on a target, the tumor estrogen receptor (ER) [1]. It is important to point out that the ER was initially used not as a therapeutic target, but as a predictor of response to endocrine ablation, such as oophorectomy [2]. The innovation of targeting the tumor ER specifically using the non-steroidal antiestrogen tamoxifen (Figure 1) [3] ultimately changed the prognosis of women with breast cancer by proposing two new treatment strategies: a new approach to therapy with long-term early adjuvant tamoxifen treatment following surgery and subsequently the possibility of using tamoxifen for chemoprevention [1,2]. In both cases the target would be the ER, to be blocked by tamoxifen.
Figure 1.
Chemical structures of 17β-Estradiol, raloxifene, tamoxifen, and tamoxifen’s metabolies n-desmethyl tamoxifen, 4-hydroxytamoxifen, and endoxifene.
Tamoxifen is approved by the Food and Drug Administration (FDA) to treat node-positive and node-negative breast cancer patients with long-term adjuvant therapy, and is approved to lower the incidence of breast cancer in high-risk pre- and postmenopausal women. In both applications, clinical trials established and confirmed that patients with ER-positive breast cancer are the ones who benefit. Tumors that are ER-negative do not respond to tamoxifen. In addition to blocking estrogen’s binding to its receptor, another means of limiting estrogenic activity in breast tissue is by blocking the synthesis of estrogen. Aromatase inhibitors block estrogen’s conversion from its androgen precursor thereby limiting the production of estrogen [4,5]. This approach has proven beneficial clinically with fewer side effects than tamoxifen and improvements in recurrence rates and survival for postmenopausal patients [6–9].
The benefit of antihormone (data primarily from tamoxifen trials) therapy targeted to the ER is impressive in terms of both recurrence-free survival and decreases in mortality [7,10]. Millions of women now live longer, healthier lives based on the application of translational research [1]. Women of any age with ER-positive tumors experience an approximately 30% mortality reduction when treated with long-term (5 year) adjuvant tamoxifen [7,10]. Postmenopausal women, however, receive greater clinical benefit with aromatase inhibitors rather than tamoxifen, in terms of lower breast cancer recurrence rates and fewer side effects [6]. Aromatase inhibitors can be used instead of tamoxifen for five years, after tamoxifen for five years, or by switching to an aromatase inhibitor after a year or two of tamoxifen. The important principle is to ensure compliance so that at least five years of antihormone treatment is used.
Breast cancer prevention trials built on the previous clinical experience with tamoxifen to demonstrate tamoxifen’s efficacy in preventing ER-positive invasive breast cancer in women at high-risk [11]. However, few high-risk women benefit from population-based chemoprevention with tamoxifen while many are exposed to side effects such as endometrial cancer and thromboembolic events [12]. As a result, a paradigm shift occurred with the finding that non-steroidal antiestrogens are, in fact, selective ER modulators (SERMs). The laboratory discovery that SERMs can maintain bone density but prevent mammary carcinogenesis led to the idea of treating osteoporosis while preventing breast cancer at the same time [13–15]. It is fair to say that the laboratory finding [16] that tamoxifen increases the growth of human endometrial cancer but stops the growth of breast cancer, and its subsequent clinical confirmation [16,17], really stressed the need to find a new chemopreventive medicine. Raloxifene is a drug similar in structure to tamoxifen (Figure 1) which is now prescribed indefinitely as a medicine to prevent osteoporosis, offering a beneficial side effect of breast cancer prevention in postmenopausal women [18,19]. Additionally, raloxifene is FDA-approved as a prevention strategy to reduce the incidence of ER-positive breast cancer in at-risk postmenopausal women without increasing the incidence of endometrial cancer as occurs with tamoxifen [20,21]. Figure 1 illustrates the structures of estradiol, raloxifene, tamoxifen, and related metabolites.
With this brief clinical background of progress in the quality of life and survivorship for women with breast cancer, and the practical progress in reducing the incidence of breast cancer, several principles emerge to focus laboratory efforts to enhance further advances. Long-term therapy is the key to successful increases in survivorship and only ER-positive tumors are responsive to antihormone therapy. However, because of the finding that five or more years of therapy can control recurrences of the growth of micrometastatic primary breast cancer, it is acquired resistance to antihormone therapy that must be addressed. Models must replicate clinical experience with the ER-positive tumor. The surviving cells whose growth is not blocked by antihormones have the plasticity to respond to treatment in a Darwinian model of continued growth and replication.
We will first describe the limited types of ER-positive breast cancer cells available to the scientific community and the strategies used in the laboratory to create models to mimic clinical experience i.e. years of antihormone therapy. Through the creation of reproducible models, mechanisms can be deciphered to apply to new clinical treatment strategies.
Cell lines as platforms for modeling acquired antihormone resistance
The Early Breast Cancer Trialists’ Collaborative Group recently showed that after about 5 years of tamoxifen therapy for women with ER-positive breast tumors (10,645 women), yearly breast cancer mortality rate was reduced by 30% for 15 years after treatment initiation [7]. If we estimate that ER-positive breast cancer, the most prevalent type, accounts for 75% of all breast cancer, it follows that about half of the breast cancers may have or acquire resistance to antihormone therapy. This, combined with the fact that over 200,000 new cases of breast cancer [22] are expected to occur each year, makes acquired resistance a critical issue in breast cancer research and women’s health. Prevention of primary breast cancer or the maintenance of patients to prevent recurrence of the disease is an important advance in translational research that continues to reduce healthcare costs and improve survivorship for millions of patients worldwide. Although it is fair to say that few women at high risk for breast cancer elect the chemoprevention option, there are more than half a million women using raloxifene to prevent osteoporosis and prevent breast cancer at the same time [18]. However, tumors that form during long-term raloxifene treatment [19] have acquired resistance to this SERM.
It is currently impossible to analyze the cell biology of every patient’s individual breast tumor and predict outcomes, both practically and financially. The actual relationship of the cancer cell with supporting stroma of an individual tumor cannot yet be reconstructed under laboratory conditions, but what can be achieved at this stage is the interrogation of available cell lines to focus on a specific group of ER-positive tumors and obtain general principles with which to plan treatments. In other words, laboratory models in vitro and in vivo represent the medium for a conversation between the laboratory and the clinic. These models represent important subgroups of breast tumors in patients.
Breast cancer cell lines that are ER-positive are of specific value to conduct translational research to understand the mechanisms by which hormone-responsive breast tumors may develop acquired antihormone resistance. The ER-positive models to be discussed here are: ZR-75, BT-474, T47D, and MCF-7. Each cell line is available from the American Type Culture Collection (ATCC) but there are individual variants maintained in specific laboratories. The current ER statuses (Figure 2), ER protein regulation (Figure 2), hormone responsiveness to the principal steroidal estrogens estradiol and estrone (Figure 3), and the relative ability of tamoxifen and its metabolites to block combined circulating levels of estrone and estradiol (Figure 4) are illustrated. All cells tested have been confirmed by DNA fingerprinting.
Figure 2.
A. ERα expression levels in different ER positive cells. Cell lysates of MCF-7, T47D, ZR-75-1, BT474, MCF-7:5C, and MCF-7:2A were harvested. MCF-7, T47D, ZR-75-1 and BT474 cells were cultured under conditions with estrogen (10% FBS), while MCF-7:5C and MCF-7:2A cells were cultured under estrogen-free conditions (10%SFS). ERα expression levels were examined by immunoblotting with primary antibody. Immunoblotting for β-actin was determined for loading control. B. Modulation of ERα expression in the absence of estrogen. Wild-type ER positive MCF-7, T47D, ZR-75-1, and BT474 cells were cultured under conditions with estrogen (10% FBS) or without estrogen (10% SFS) for 3 days, respectively. Cell lysates were harvested. ERα expression levels were examined by immunoblotting with primary antibody. Immunoblotting for β-actin was determined for loading control.
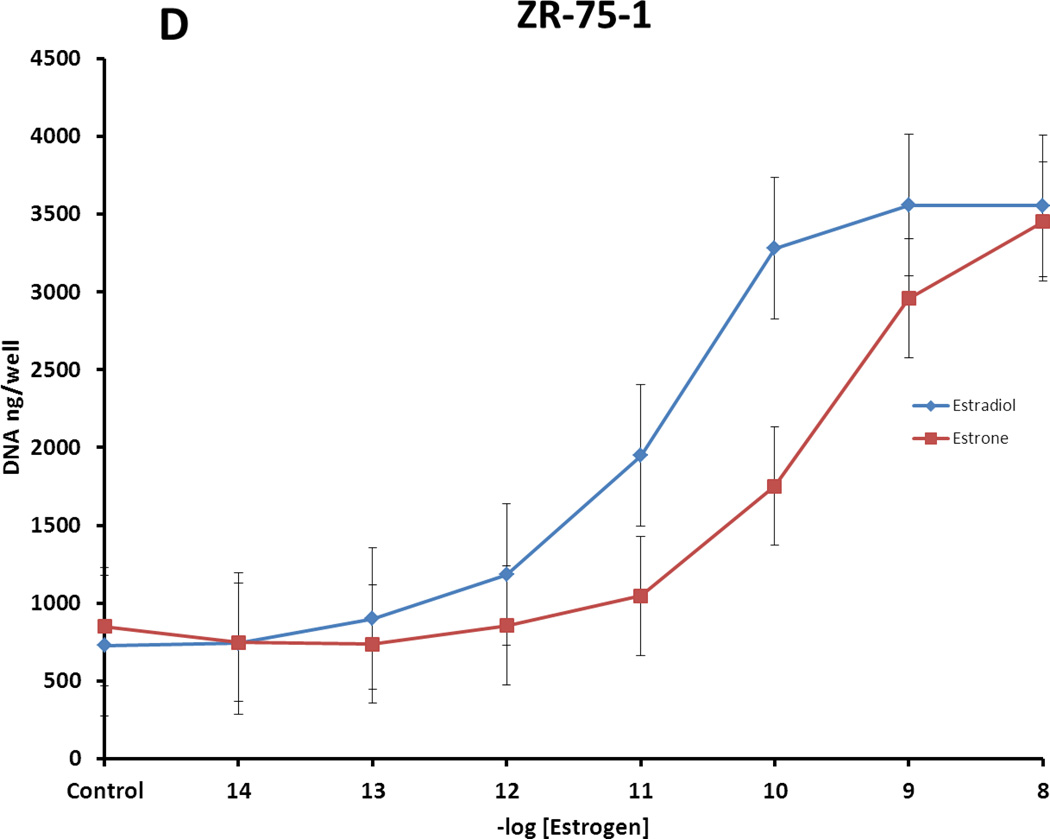
Figure 3.
Proliferative responses of different ER-positive breast cancer cell lines to treatments with estradiol (E2) and estrone (E1). Growth of cells was determined by measuring DNA per well after 7 day treatments. A. MCF-7:WS8 cells, hypersensitive clones of MCF-7 cell line; B. T47D:A18 cells, hypersensitive clone of T47D cell line; C. BT474 ER-positive breast cancer cells (ATCC); D. ZR-75-1 ER-positive breast cancer cells (ATCC). Estradiol is the most potent of the natural estrogens in a woman’s body, and estrone, with the 17β hydroxyl oxidized to a ketone, is less potent. It does, however, significantly contrinue to breast cancer cell growth.
Figure 4.
Biological response of MCF-7 cells after 7 day treatment with premenopausal levels of estrone (E1, 8nM) and estradiol (E2, 4nM) found in plasma of premenopausal women during follicular phase of menstrual cycle [174] and tamoxifen metabolites 4OHT (6.3 nM), N-desmethyl-Tam (558 nM), tamoxifen (386 nM) and endoxifene (35.6 nM) at concentrations found in plasma of extensive metabolizers of tamoxifen [175]. As shown in the figure, combination of E1/E2 induce cell growth and treatment with combination of tamoxifen and its metabolites has minor effect on cells. Combination treatment of E1/E2 and tamoxifen metabolites does not ablate the proliferation of the cells. However, addition of another tamoxifen metabolite endoxifen at concentrations found in plasma of extensive metabolizers of tamoxifen (35.6 nM) produces almost complete inhibitory effect on cell growth. Treatment with combination of all tamoxifen metabolites (including endoxifen) does not have any major biological effect.
The ZR-75 breast cancer cell line
The ZR-75 human breast cancer cell line was derived in the late 1970s from a 63-year-old postmenopausal female patient with metastatic ductal carcinoma of the breast. The cells were taken from the ascites three months after initiation of tamoxifen treatment and exhibit estrogen and insulin responsiveness [23]. As ZR-75 cells are passaged they retain their epithelial morphology, remaining similar in appearance to their original source biopsy, though their chromosome count decreases from approximately 75 to 72 after 38 passages [23]. ZR-75 cells are ER-positive, glucocorticoid receptor (GR)-positive, androgen receptor (AR)-positive, and progesterone receptor (PR)-positive [23]. Tamoxifen (10−6 M) causes growth inhibition and the cells die [24]. Also, the cells are specifically growth-stimulated by insulin, and inhibited by androgens and glucocorticoids [23].
The BT-474 breast cancer cell line
The BT-474 cell line comprises ER-positive, PR-positive epithelial cancer cells derived from invasive ductal breast carcinoma of a 60-year-old female patient [25]. Notably, these cells also express the nuclear receptor human epidermal growth factor receptor 2 (HER2) [26]. With 55 chromosomes, they grow in adherent patches in tissue culture, and are tumorigenic [25]. BT-474 cells grow in response to estradiol, via their ER (see Figure 3).
The T47D breast cancer cell line
The T47D cell line originates from a pleural effusion of a 54-year-old female patient with infiltrating ductal breast carcinoma. The cells have approximately 60 to 70 chromosomes, multiple mitochondria, and irregular nuclei and nucleoli [27]. They maintain their epithelial morphology after several years of passage, can produce casein, and can be grown in a monolayer in vitro [27]. First described as an ER-positive, PR-positive, AR-positive, GR-positive, epithelial cell carcinoma model, it has since been established that the nuclear receptor levels and hormone responsiveness depend on the culture conditions [28]. T47D cells express ER and PR in estrogen-rich media, but lose most PR and ER expression when grown in the absence of estrogen [28].
Classically, estradiol stimulates proliferation of the T47D cell line through the ER, and stimulates estrogen-regulated proteins such as PR, while tamoxifen inhibits this growth [29]. The stimulatory action of physiologic estrogens and the inhibition caused by tamoxifen and its principal metabolites are shown in Figures 3 and 4, respectively. Without the nuclear receptors, however, neither estradiol nor tamoxifen can influence growth since their mechanism of action through ER is eliminated [28].
The MCF-7 breast cancer cell line
The majority of investigations into acquired antiestrogen drug resistance have utilized the MCF-7 cell line so prevalent in breast cancer laboratories. The MCF-7 cell line has been the topic of an earlier review [30]. MCF-7 cells are used ubiquitously in research for ER-positive breast cancer cell experiments and many subclones have been established, representing different classes of ER-positive tumors with varying nuclear receptor expression levels.
The MCF-7 cell line was derived from the pleural effusion of a 69-year-old female patient with a diagnosis of adenocarcinoma of the breast [31]. This particular patient had undergone three years of radiotherapy and hormone therapy, most likely high-dose diethylstilbestrol (DES), a synthetic estrogen (the cell line was created before tamoxifen was available for clinical use). The cells were noted to be ER-positive [32]. In the mid-1970s Lippman [33,34] demonstrated that nonsteroidal antiestrogens in general and tamoxifen in particular could stop the growth of MCF-7 cells in culture, and this could be reversed with the administration of exogenous estradiol.
In the early 1980s, MCF-7 cells were shown to form tumors in vivo [35] with estrogen administration, but estrogen did not significantly stimulate growth of the same cells in vitro [36]. At the time, it was proposed that a factor existing in the animal but not in culture, be it a second messenger system or peptide growth factor, was required for the profound growth influence of estrogen on MCF-7 cells [36]. However, a landmark discovery occurred in 1986 identifying a contaminant of phenol red (phenolsulfonphthalein) (Figure 5), the pH indicator in media, as estrogenic [37,38]. The media was therefore causing cells to grow [37]. All previous studies measuring estrogen’s impact on the cells were undermined since the effects were confounded by additional estrogen in the media. The discovery allowed complete withdrawal of estrogen from the cells and the subsequent ability to document the real impact of estrogen on various cell functions including proliferation and apoptosis of MCF-7 cells [39–43].
Figure 5.
Phenolsulfonphthalein (phenol red), the pH indicator in cell culture media, is structurally similar to the natural estrogen estradiol (Figure 1) and synthetic estrogens. Unlike normal chemical titration analyses that use a pH indicator at very low concentrations, phenol red is incorporated at µM levels in culture media. The estrogenicity was found to vary from batch to batch [176]. However, a potent estrogenic contaminant (right) exerts growth stimulatory effects on breast cancer cells [38].
Being ER-positive, the MCF-7 cell line grows and proliferates with estrogens, in concentrations as low as 10−11 M estradiol (Figure 3) [30]. Tamoxifen competitively inhibits DNA synthesis in MCF-7 cells, binding to the same ER as do estrogens, though with a 1000-fold lower affinity than estradiol [30]. When added to the cells simultaneously, estradiol can reverse this inhibition at a concentration 100-fold lower than tamoxifen (10−7 M vs. 10−8 M) causing cell growth (Figure 4) [30]. The actions of tamoxifen and its metabolites on estrogen-stimulated proliferation are shown in Figure 4. Pure antiestrogens, such as fulvestrant, that destroy ER, also inhibit growth of MCF-7 cells [44].
ER regulation in ER-positive breast cancer cell lines
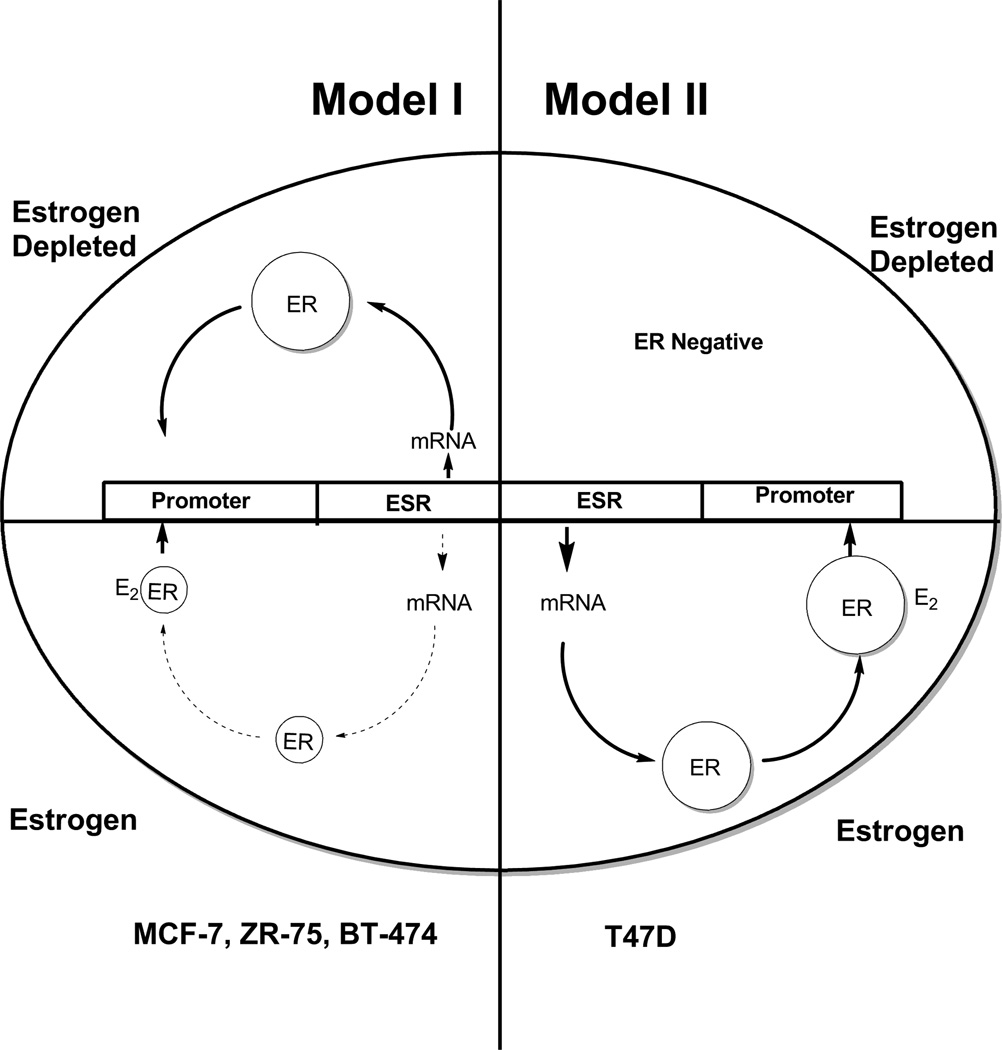
Figure 2 illustrates ER expression in the four described ER-positive breast cancer cell lines in different media conditions. ZR-75, BT-474, and MCF-7 cells increase expression of ER in the absence of estrogens, represented here by phenol red-free media supplemented with charcoal-stripped fetal bovine serum (SFS). Estrogen exposure to these cells causes decreased ER mRNA and protein levels [45]. T47D cells, by contrast, express more ER in an estrogenic environment, shown here as red media with fetal bovine serum (FBS) [45]. As previously stated, T47D ER expression is lost in an estrogen-free environment. Tamoxifen causes increased ER protein levels in MCF-7 and T47D cells, while fulvestrant causes decreased protein levels in both cell lines [45]. The alternate models of ER regulation in the cell lines has previously been summarized [45] and is now updated and illustrated in Figure 6 for convenience. The consistent model (Model I) of ER regulation is an upregulation of ER in the absence of estrogen. However, T47D does not conform and requires estrogen for ER synthesis (Model II).
Figure 6.
The diagrammatic representation of cellular estrogen receptor (ER) regulation in media with or without estradiol (E2). This diagram is based on the general responses to estrogen illustration by Western blotting in Figure 2 and presented in detail in [45]. Model I ER regulation (MCF-7, ZR-75, BT-474) has an upregulation of ER message and protein in an estrogen-depleted environment, but ER is downregulated at the mRNA and protein level in the presence of estrogen. Model II ER regulation (T47D) has upregulation of ER message and protein in an estrogen-containing environment but ER is not produced in an estrogen-depleted environment. Cells lose ER to become ER-negative.
Models of acquired antihormone resistance in vitro
ER-negative breast cancer cells, such as the MDA-MB-231 and SKBr3 cell lines, do not respond to antihormone treatment. There are some ER-positive cell lines that also exhibit intrinsic resistance; that is, antihormones do not create a subpopulation of these cells that are resistant over time. They simply do not respond initially, perhaps via growth factor receptor overexpression allowing other mechanisms of growth stimulation. Osborne’s group showed in 1992 [46] that when ER-positive MCF-7 cells are transfected with HER2, the cells are intrinsically resistant to antihormones such as tamoxifen, presenting HER2 as a potentially important factor for tamoxifen sensitivity and drug resistance.
To investigate the properties of acquired antihormone-resistant breast cancer cells, populations of MCF-7 cells have been created that are adapted to various antihormone environments. MCF-7 cells, more than the other three ER-positive cell lines T47D, BT-474, and ZR-75, are well-suited for antihormone resistance studies since they are easily cultured and retain ER expression when treated with antihormones; they are routinely used in the laboratory and have produced more data of practical knowledge for patient care than any other breast cancer cell line (see final section). Figure 7 illustrates the lineages of different subtypes of MCF-7 cells maintained in the laboratory.
Figure 7.
A flow diagram representation of the defined antihormone-resistant cell lines derived from MCF-7 cells. The Jordan laboratory obtained original “Soule” MCF-7 cells from the Michigan Cancer Foundation as a gift from Dr. Dean Edwards who was then at the University of Texas. The Clark laboratory obtained MCF-7 cells from the ATCC cell collection. All cells are genotyped by DNA fingerprinting.
One such in vitro model illustrating the varied attributes of tamoxifen-resistant cells are the MCF-7 LCC subclones (see Figure 7). The MCF-7:LCC1 variant represents an estrogen-independent breast cancer cell line obtained from in vivo selection in oophorectomized nude mice and re-cultured in vitro to become a stable cell line [47,48]. Though estrogen-independent, the cells are still tamoxifen-sensitive [47]. When this cell line was selected for tamoxifen resistance in vitro, the MCF-7:LCC2 clone was created. MCF-7:LCC2 cells are stable, ER-positive, and respond to the pure antiestrogen, fulvestrant [49]. Along the same lineage, MCF-7:LCC9 cells were derived by selecting in vitro MCF-7:LCC1 cells for fulvestrant resistance, and subsequently, these cells exhibit cross-resistance to tamoxifen [50].
Another early antiestrogen-resistant variant of MCF-7 cells is the LY2 line. MCF-7:LY2 cells are resistant to LY117018, a potent antiestrogen related to raloxifene [51]. The LY2 cells also exhibit cross-resistance to tamoxifen and continue to be responsive to estrogen but with lower ER levels than MCF-7. The cell line was created by selection with increasing the concentration of LY117018 up to 1 µM as MCF-7 cells became resistant [51]. A related MCF-7 raloxifene-resistant line MCF-7/RAL was created by growing MCF-7 cells in estrogen-free culture with 1 µM raloxifene for over a year [52]. These cells grow in response to estradiol and raloxifene, and are growth-inhibited by fulvestrant [53]. Most importantly the cells exhibit an unusual apoptotic response to estradiol in vivo (see next section). The MCF-7/F cell line was established by culturing the parental MCF-7 cells in fulvestrant-containing estrogen-free media for 18 months. ER expression was lost, and the cells became resistant to all antihormone therapies [54].
Short-term estrogen deprivation causes distinct responses of MCF-7 cells in comparison to long-term (over six months) estrogen deprivation. These studies are important to mimic the early response of ER-positive breast cancer to aromatase inhibition. Culture of MCF-7 cells in media that is phenol red-free with charcoal-stripped serum (estrogen-free) causes immediate proliferation inhibition [39,43]. Slowed proliferation continues for about a month after estrogen removal, indicating the cells have not yet found adaptive or compensatory growth mechanisms. When stimulated with estradiol, the proliferation rate of these short-term estrogen-deprived cells increases, and antiestrogens again inhibit growth [39,40]. Over time, MCF-7 cells deprived of estrogen eventually adapt their growth in estrogen-free media, losing their estrogen sensitivity, but antiestrogens continue to inhibit growth [40]. The ER is retained and expanded.
In 1995, Santen’s group hypothesized [55] MCF-7 cells develop hypersensitivity to minute concentrations of estradiol (or indeed any available estrogen) after estradiol deprivation as a means of adapting to estrogen withdrawal and spontaneous growth. They noted that when MCF-7 cells are deprived of estrogen for 1–6 months, a 104-fold lower concentration of estradiol is needed for maximal growth, when compared to normally cultured MCF-7 cells. This model suggests an explanation for spontaneous growth that occurs after estrogen withdrawal; that is, the breast cancer cells are hypersensitive to minute environmental concentrations of estrogen [55]. Indeed this is a valid hypothesis as the estrogen-deprived cell population adapts by selecting any available cell to grow in the environment: a Darwinian model.
Long-term estrogen deprived (LTED) MCF-7 cells form a stable cell line that has been used to investigate estrogen’s effect on breast cancer cells over varied exposures and lengths of time. MCF-7:LTED cells, in contrast to their short-term estrogen-deprived counterparts, are able to grow despite lack of estrogen in the media, and are growth-inhibited by estradiol [40].
MCF-7:5C cells were developed by long-term estrogen withdrawal from the parental wild-type MCF-7 breast cancer cells [56,57]. The ER in MCF-7:5C cells is wild-type, and expression levels are similar to MCF-7 [56] (see Figure 2). This hormone-independent, ER-positive, PR-negative clonal population proved useful in representing the behavior of long-term estrogen-deprived breast cancer cells; that is, those of postmenopausal women decades after menopause, or patients who have undergone long-term antihormone therapy, e.g. 5-year aromatase inhibitor treatment [57]. MCF-7:5C cells are unresponsive to 4-hydroxytamoxifen, and estradiol does not enhance growth [56,57] but triggers estradiol-induced apoptosis [41].
The MCF-7:2A cell line is similar to the MCF-7:5C cell line and was generated from long-term estrogen withdrawal from MCF-7 cells. Uniquely, MCF-7:2A cells express two forms of the ER, a 66 kDa wild-type and a 77 kDa mutant (see Figure 2) [45,58]. The wild-type ER, expressed 4- to 10-fold higher than the mutant, is still functional, whereas the mutant ER, containing a repeat of exons 6 and 7 in the ER gene [59], can no longer bind estrogens nor antiestrogens. MCF-7:2A cells grow in estrogen-free media since they are estrogen-independent. In contrast to its parental cell line, the 2A cells show no response to estradiol during the first seven days of treatment, then begin to die via apoptosis during week two. Both tamoxifen and pure antiestrogens block growth in these cells [45,58].
In search of other in vitro models illustrating antihormone-resistant breast cancer cells, the T47D cell line can offer additional information. T47D cells differ from MCF-7 cells in that their tumor suppressor protein p53 is mutated on one allele of the gene (194 Leu-->Phe) [60]. Also, MCF-7 cells continually express ER whereas T47D lose ER expression when estrogen is withdrawn for extended periods of time [61]. The T47D:A18 variant is ER-positive and PR-positive, derived from culturing the T47D cell line in estrogen-rich media [61]. They grow in response to estrogen and are inhibited by 4-hydroxytamoxifen [61]. T47D:C4 cells, in contrast, were established by culturing T47D cells in estrogen-free media [28,61]. The parental cells are transformed into ER-negative, PR-negative cells which are unresponsive to antihormone therapy [62].
To address mechanistic issues of antihormone resistance, T47D-r cells, also derived from the parental T47D line, were created to be resistant to fulvestrant [63]. Proteomic analysis was used to compare T47D versus T47D-r cells to identify 38 proteins with significantly (2-fold up- or down-regulation) different expression [63]. Furthermore, mRNA expression differed for 11 of the proteins. These data are evidence supporting the molecular and mechanistic changes that occur to T47D breast cancer cells as they become increasingly resistant to antiestrogens [63].
The T47Dco subclone is estrogen- and antiestrogen-resistant, and expresses PR regardless of estrogen stimulation. Progestins inhibit proliferation of T47Dco cells [64]. Initially described as ER-negative [64], it was subsequently shown that the cells express three mutant ERs that have no ability to bind ligand [65]. This cell line allows for extensive study on progestins’ effect on breast cancer independently of estrogen, as well as on ER mutations as a mechanism of hormone resistance.
When ZR-75 cells are treated with tamoxifen for six months, both ER and PR levels decrease, but the antihormone is still able to impede the cancer growth. Tamoxifen resistance occurs after a year of tamoxifen treatment, as evidenced by the tamoxifen-resistant subclone ZR-75-9a1, a distinct ER-negative, PR-negative cell line [66]. Table 1 summarizes the discussed cell lines’ subclones used for modeling ER-positive breast cancer cells in vitro.
Table 1.
Various subclones generated from different ER-positive breast cancer cell lines. To simulate different scenarios of therapy and development of resistance to SERMs, cells were cultured in different environments to create stable cell lines. Fulv: fulvestrant, Tam: tamoxifen, Ral: raloxifene, Ref: reference number
| Parental Line | Subclone | How subclone was generated | Subclone’s resistance | Ref |
|---|---|---|---|---|
| ZR-75 | 9a1 | long-term tam treatment | tam | 66 |
| T47D | ER-negative | estrogen withdrawal | antihormones | 28 |
| T47D | −r | long-term fulv treatment | fulv | 63 |
| T47D | A18 | estrogen-rich culture | - | 61 |
| T47D | C4 | estrogen withdrawal | antihormones | 61 |
| T47D | co | PR expression selection without estrogen | estrogen, antiestrogen | 65 |
| MCF-7 | Ral | long-term ral treatment | ral | 52 |
| MCF-7 | F | long-term fulv treatment without estrogen | fulv | 54 |
| MCF-7 | 5C | estrogen withdrawal | tam | 56, 57 |
| MCF-7 | 2A | estrogen withdrawal | - | 58 |
| MCF-7 | LY2 | LY117018 selection | tam, LY117018 | 51 |
| MCF-7 | LCC1 | estrogen withdrawal | - | 47, 48 |
| MCF-7 | LCC2 | estrogen withdrawal, tam selection | tam | 49 |
Models of acquired antihormone resistance in vivo
Laboratory studies of endometrial cancer in vivo aided in the understanding of acquired resistance to tamoxifen. Estradiol significantly increases the growth rate of human ER-positive endometrial cancer transplanted into ovariectomized nude mice, while the growth rate of ER-negative endometrial cancer in this model is unaffected by estradiol treatment [67]. However, ER-positive endometrial tumors implanted in nude mice also grew more quickly in response to tamoxifen or estradiol treatment than the control-treated mice [68]. When medroxyprogesterone acetate (MPA) (a standard therapy for endometrial cancer) was administered to the tamoxifen-treated animals implanted with endometrial tumors, inhibition of growth was increased in comparison to the tamoxifen-treated tumors alone. In contrast, the growth of ER-negative endometrial cancer injected into athymic mice was unaffected by all treatments [68].
Subsequently, the human endometrial tumor EnCa101 was pivotal in enhancing knowledge of the target site specificity of tamoxifen, as well as by other similar triphenylethylene antiestrogens (e.g. clomiphene, trioxifene, nafoxidine) [69]. Athymic mice transplanted with both MCF-7 breast and EnCa101endometrial tumors, and treated with either estradiol, tamoxifen or the combination, demonstrated that estradiol increases the growth in both tumors. Tamoxifen, however, blocks breast cancer growth while enhancing the growth of endometrial cancer [16]. These data were rapidly translated to patient care [17], with breast cancer patients being given routine gynecological examinations to detect endometrial cancer that was slightly but significantly increased during adjuvant tamoxifen therapy. The target site specific action of tamoxifen in breast and endometrium was hypothesized to be dependent on differential modulation of the estrogenic actions of tamoxifen in different target tissues [70]. The concept was supported by studies of antiestrogens with reduced estrogenic action. Keoxifene (subsequently called raloxifene) and LY117018 are less estrogenic in the rodent uterus and have less of an effect on EnCa101 growth stimulation [69,71]. Further, ICI 164,384, since it is a pure antiestrogen with no intrinsic estrogenicity, did not stimulate EnCa101 tumor growth, and was able to block tamoxifen-induced growth [15]. Clinical studies demonstrate that unlike tamoxifen, raloxifene [18] and fulvestrant [72] have no estrogen-like action in the human uterus.
MCF-7 models in vitro eventually evolved one step further toward clinical practice when they were adapted into models in vivo which mirror more closely clinical care. Models in vivo create a new dimension to assess the importance of a functioning physiologic interaction between cancer cells, the interaction of angiogenesis, cellular metabolism, and respiration that are not created in cell culture. The first studies of MCF-7 cells implanted into nude mice were published in the 1980s. MCF-7 cells implanted into mice with intact ovaries, or simultaneously with estrogen into ovariectomized mice, grew in an estrogen-dependent manner [35].
In the 1980s, transplanted models of MCF-7 human breast cancer into athymic mice were used to investigate the unique aspects of acquired resistance to SERMs. Tamoxifen acts as a competitive inhibitor of estradiol-stimulated growth, i.e. the action of tamoxifen as an antitumor agent is reversed by increasing the dose of estradiol [73]. Similarly, months of tamoxifen therapy do not destroy implanted MCF-7 tumors [74,75], as estrogen can reactivate tumor growth. Eventually acquired resistance to tamoxifen occurred after four months of treatment, wherein neither tamoxifen nor estrogen deprivation could produce significant tumor regression [76]. Breast tumors then grew despite tamoxifen treatment demonstrating that acquired resistance to antihormone therapy had developed.
However, a similar study came to a different conclusion; MCF-7 tumors grew in the athymic mouse not despite tamoxifen therapy but because of tamoxifen therapy [77]. When the MCF-7 tumors resistant to tamoxifen were transplanted into new athymic animals, these ER-positive, PR-positive tumors were found to grow in response to either estradiol or tamoxifen treatment. It is also noteworthy that the tamoxifen-stimulated tumors expressed twice the level of ER when compared to their estradiol-stimulated counterparts [77]. A survey of other steroidal and non-steroidal antiestrogens demonstrated that tamoxifen-stimulated growth is dependent on the estrogen-like actions of tamoxifen. Less estrogenic agents do not increase the growth of acquired tamoxifen resistance in MCF-7 tumors [78]. There is cross-resistance with other antiestrogens e.g. toremifene or raloxifene [79,80] but not fulvestrant. Overall, this model mimics the development of acquired resistance to tamoxifen during the treatment of metastatic breast cancer. The tumors become resistant to therapy in about two years.
Many of the previously discussed MCF-7 subclones have been examined in animal models. When the MCF-7/RAL cells are transplanted into athymic ovariectomized mice, they are able to form tumors when treated with either estradiol or raloxifene. Eventually, after about eight months of re-transplantation, the tumors grow only in response to raloxifene, and are inhibited by estradiol [53].
MCF-7/LCC1 cells are estrogen-responsive and tamoxifen-sensitive in vivo. MCF-7/LCC2 cells, on the other hand, behave estrogen-independently in vivo. They continue to exhibit tamoxifen resistance in vivo as they do in vitro [49]. The MCF-7/LCC9 cell line, consistent with its in vitro action, can form tumors in the athymic ovariectomized mouse, and are unresponsive to fulvestrant [50].
Similarly, MCF-7 cells with acquired resistance to tamoxifen (MCF-7:Tam) in vivo implanted in athymic ovariectomized mice grow in response to tamoxifen or estradiol but the steroidal antiestrogen RU 39,411 or ICI 164,384 inhibit growth [78]. However, long-term transplantation of MCF-7:Tam tumors into athymic mice eventually results in a change in response to physiologic estradiol with rapid tumor regression [81,82]. Similarly, MCF-7:5C cells injected into athymic ovariectomized mice undergo apoptosis when treated with estradiol, causing complete tumor regression [41]. This unusual change in the biology of the tumors will be revisited in the next section.
T47D cells have also been examined in vivo to evaluate the role of SERMs to create acquired antihormone resistance. T47D cells transplanted into athymic ovariectomized mice can generate tumors in response to estradiol, and tamoxifen can inhibit this estrogen-stimulated growth. However, after high-dose (1.5 mg daily) tamoxifen treatment, the tumor cells become tamoxifen-resistant after about eight weeks, wherein tamoxifen begins to stimulate tumor growth [83]. The T47D cells giving rise to tamoxifen-stimulated tumors produce a subtype of T47D cell named T47D:Tam. Other SERMs, Arzoxifene and LY117018, did not increase growth of T47D:Tam tumors in vivo; likewise, Arzoxifene and LY117018 did not increase the growth of estradiol-stimulated T47D tumors either. This indicates a lack of cross-resistance between tamoxifen and the other antiestrogens in T47D cells in vivo [84].
In addition to SERM studies, models in vivo also examined the effect of aromatase inhibition on ER-positive cell lines. In 1994, nude mice were injected with MCF-7 cells transfected with the human aromatase gene to study the action of aromatase inhibitors in vivo for the treatment of breast cancer [85]. In the normal nude mouse, tumors grew in response to ovarian estrogen and were inhibited by aromatase inhibitors and tamoxifen. The aromatase substrate, androstenedione, was administered to the ovariectomized mice in order to model human disease since mice express no androgen precursor. Ovariectomized nude mice injected with aromatase-transfected MCF-7 cells grew tumors utilizing estrogen produced through the aromatization of androstenedione via the aromatase pathway. Aromatase inhibitors (4-hydroxyadrostenedione and CGS 16949A) and tamoxifen were able to block the tumor growth. This latter model represents postmenopausal women whose tumors grow not in response to ovarian estrogen, but estrogen generated through the aromatization of androgens found primarily in the adipose tissue. MCF-7 cells transfected with the aromatase gene and injected into ovariectomized mice were inhibited better with the combination treatment of fulvestrant and anastrozole than either agent alone. This suggests the targeting of both aromatase and the ER for better treatment of postmenopausal breast cancer patients [86]. These studies provide a rationale behind aromatase inhibitors’ efficacy in the clinical setting [85].
Laboratory models set the stage for intense evaluation of antihormone-resistant breast cancer cells. By continuing investigation of mechanisms of resistance, many unique and sometimes paradoxical effects of hormones and antihormones on ER-positive breast tumors have been discovered. The finding that an estrogen and an antiestrogen could eventually stimulate breast cancer growth demonstrated the unique qualities of acquired resistance to SERMs [77]. The aforementioned individual findings now began to form models for the evolution of acquired resistance that can not only be interrogated in the laboratory but applied to clinical care.
Evolution of acquired antihormone resistance
Based on laboratory evidence from both individual reports and studies of up to a decade, the evolution of acquired resistance to SERMs can now be described in distinct phases following long-term SERM treatment and long-term experiments in vitro and in vivo (Figure 8) [87,88]. The evolution (Figure 8) of acquired resistance occurs after an initial period of therapeutic success where antiestrogenic activity predominates and the SERMs are competitive inhibitors of estrogen-stimulated tumor growth in athymic mice [73,74]. The therapeutic phase of SERM action can be maintained for a year or two (at most) but eventually tumors start to grow despite continued tamoxifen [76]. However, these tumors can be re-transplanted into other tamoxifen-treated ovariectomized athymic mice [77]. Paradoxically, both physiologic estradiol and tamoxifen (there is cross-resistance with raloxifene and toremifene) [79] can then cause growth, indicating Phase I resistance. The pure antiestrogens ICI 164,382 and fulvestrant block Phase I growth with either tamoxifen or estradiol. A similar form of acquired resistance to tamoxifen occurs with the T47D breast cancer cell line [83,84]. This type of acquired resistance is characteristic of resistance to tamoxifen during the treatment of metastatic ER-positive breast cancer and is why either fulvestrant or an aromatase inhibitor are effective second-line therapeutic agents in the clinic [89,90]. The laboratory principles are illustrated in Figure 8.
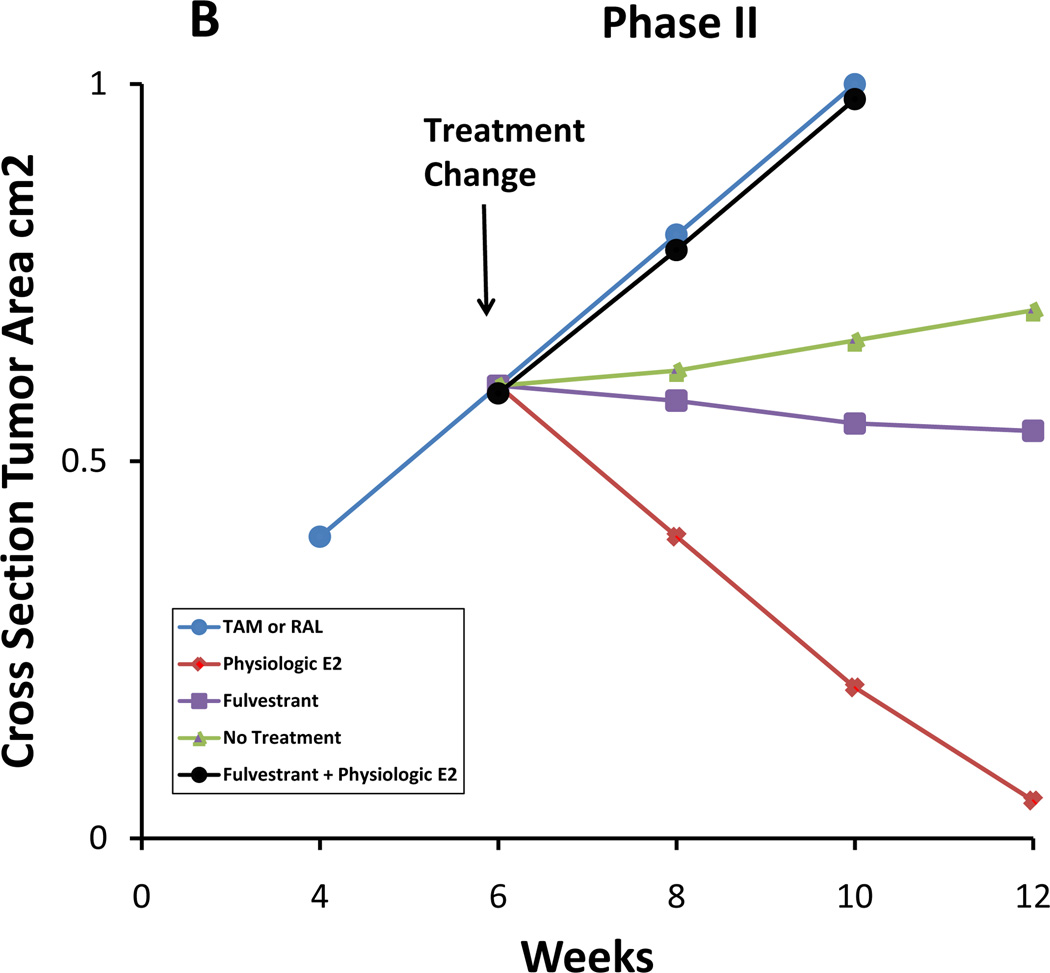
Figure 8.
Evolution of acquired SERM resistance. After long-term treatment with SERMs (1–2 years in vivo), initially responsive ER-positive tumors become resistant to treatment and are stimulated by SERMs (Phase I of resistance) as well as by E2. After long-term transplantation into SERM-treated animal (5+ years), breast tumor growth is inhibited by E2, though still stimulated by SERMs (Phase II of resistance). A stylized representation of MCF-7 tumor growth is illustrated in Figure 9. This process with SERMs in vivo is replicated with estrogen deprivation with MCF-7 breast cancer cells in vitro; cells initially start to grow spontaneously but estrogen still induces growth (hypersensitivity). This is Phase I. Long-term estrogen deprivation causes spontaneous growth in culture but apoptosis with physiologic estrogens both in vitro and in vivo (Phase II).
However, these laboratory data are inconsistent with the successful adjuvant treatment of node-positive and node-negative ER-positive breast cancer with five years of tamoxifen [7]. In fact, not only is tamoxifen effective during adjuvant therapy but it is also effective at maintaining recurrence-free survival and reducing mortality by 30% from the 10 years following tamoxifen being stopped. Laboratory studies have now provided an insight into this clinical advance.
Repeated transplantation of tamoxifen-resistant tumors into subsequent generations of tamoxifen-treated athymic mice results in a change in the clonal selection of tumor cells. Not only do the tumors remain tamoxifen-dependent for growth over a five-year period but the constant exposure to tamoxifen changes the tumor response to estradiol from being a survival signal to an apoptotic trigger. Tumor regression occurs in response to physiologic estrogen and this has been proposed as a mechanism to explain the decreasing mortality of tamoxifen-treated patients following adjuvant tamoxifen [81,82]. In other words, short-term adjuvant tamoxifen only pushes acquired resistance into Phase I resistance where estradiol is still a growth stimulator once tamoxifen is stopped. In contrast, longer tamoxifen forces clonal selection into Phase II resistance where apoptosis occurs upon exposure to a woman’s own estrogen. This is illustrated when a comparison between Figures 9A and 9B is made. Indeed it was proposed that since tumors that regress and subsequently regrow in response to physiological estrogen can again respond to subsequent antihormone treatments, then this could be applied in the clinic [82]. This experiment has recently been reported in a clinical study by Ellis [91].
Figure 9.
Diagram of the growth rates of MCF-7 tumors during the evolution of drug resistance to selective estrogen receptor modulators (SERMs). A. During Phase I SERM resistance, tumors transplanted into athymic mice grow in response to either a SERM, tamoxifen (Tam) or raloxifene (Ral), or estrogen, but no estrogen (equivalent to the use of an aromatase inhibitor used clinically after Tam resistance occurs) or fulvestrant does not support growth (fulvestrant in used in this indication as a second-line therapy). B. During Phase II SERM resistance, tumors transplanted into athymic mice treated with SERMs now grow with a SERM (Tam or Ral). No treatment (equivalent to an aromatase inhibitor clinically) causes growth to slow, as does administering fulvestrant, but physiologic estradiol (E2) causes dramatic apoptosis and tumor regression. Paradoxically, physiologic E2 plus fulvestrant actually causes tumor growth. The low concentration of fulvestrant cancels out the apoptotic effect of E2 thereby redirecting E2 as a growth signal, but higher concentrations of fulvestrant now have effective antitumor effects. This is now noted clinically [92].
The evolution of cell populations to long-term antihormone therapies has been replicated with raloxifene in a 10-year study in vivo [53]. The reason for doing this is because raloxifene will be used indefinitely to prevent osteoporosis [19] and breast cancer [21]. The same evolution of acquired resistance occurs with the development of Phase I and Phase II raloxifene resistance characterized by Phase I resistance with estradiol- or raloxifene-stimulated tumor growth and Phase II resistance characterized with estradiol-induced tumor regression. It is perhaps relevant to point out that MCF-7 cells exposed to both raloxifene and estrogen deprivation in vitro rapidly advance to Phase II resistance with estradiol-induced apoptosis in vivo [52].
Additionally, there are a couple of other clinically relevant points that can be made about acquired SERM resistance in the laboratory. The T47D cell line advances to Phase I tamoxifen resistance but does not progress to Phase II. The fact that T47D cells have mutant p53 may be relevant as estrogen-induced apoptosis does not develop.
The pure antiestrogen fulvestrant is an excellent antiestrogen/antitumor agent in the laboratory but results have been disappointing clinically until the recent successful use of twice the recommended dose [92]. Laboratory studies with Phase II tamoxifen-resistant tumors grown in athymic mice suggest that the second-line use of fulvestrant in an environment of physiologic estrogen is destined to fail and, in fact, cause enhanced tumor growth [93]. The reason for this is unknown.
The fact that aromatase inhibitors are now the adjuvant treatment of choice for postmenopausal patients with ER-positive breast cancer makes an examination of acquired resistance mandatory. Suffice to say that the principles first described for SERMs are true for aromatase inhibitors and the development of acquired resistance to estrogen deprivation in vivo [94–96] and in vitro [30,39–41,97].
Mechanisms of acquired antihormone resistance
Breast cancer can be resistant to antihormones in varied ways. As previously noted, intrinsic resistance can occur de novo wherein antihormone therapy generates no disease regression. This occurs in ER-negative tumors, as well as in some subgroups of ER-positive tumors. However, we will focus on the mechanisms involved in the evolution of acquired antihormone resistance. Acquired resistance to antihormone therapy can be caused by three main mechanisms to be discussed here: loss of ER function, aberrant growth factor signaling, and estrogen-induced apoptosis.
Loss of ER function as a mechanism of acquired antihormone resistance
Experiments in vitro provide an initial platform for studying the mechanisms of acquired antihormone resistance. Firstly, if the ER in breast cancer cells is altered, the effects of antihormones will be altered accordingly. If ER expression is lost, the whole mechanism of endocrine therapy will be undermined; ER-mediated actions will no longer contribute to proliferation or apoptosis. Similarly, if ER is mutated in such a way that no longer binds its ligands, resistance will occur. Nonetheless, ER mutation is not a major factor in drug resistance but one example that has provided insight into ER modulation of antiestrogen action [98–101].
If the promoter regions of ER target genes are hypermethylated during acquired resistance, transcription of ER target genes is again blocked, abrogating antihormone efficacy in vitro [102]. Coupling of ubiquitin conjugation to ER degradation (CUE) domains are approximately 50 amino acids long and bind monoubiquitin molecules used in trafficking and ubiquitylation [103]. CUE domain-containing protein-2 (CUEDC2) is shown to have an inverse correlation with ER protein expression in breast cancer cells in vitro. High levels of CUEDC2 protein expression correlate with tamoxifen resistance, probably due to loss of ER via the ubiquitin/proteosome pathway [104].
If the ER is inactivated because of histone methylation or deacetylation, treating breast cancer cells that have acquired resistance to antihormones with a histone deacetylase (HDAC) inhibitor can re-activate the ER. This concept has been illustrated using ER-negative MDA-MB-231 wherein an HDAC inhibitor generates both ER and aromatase expression. Letrozole can then be used as effective treatment [105], suggesting a potential treatment mechanism for ER-positive cells that have lost ER expression during acquired resistance. Loss or reduction of ER as a primary cell survival pathway can also be replaced by an increase in the mosaic of growth factor signaling pathways. These pathways can modulate and subvert steroid hormone receptor synthesis and action [106,107]
Growth factor signaling as a compensatory mechanism of survival
Growth factor signaling and ER crosstalk are consistent mechanisms by which acquired resistance to antihormones develops. It provides the breast cancer cells a means of escape from suppressive signaling and a way to continue proliferation. Growth factors may be able to contribute enough proliferative signal to drive ER-target gene transcription even without normal ER ligand [108]. Growth factor signaling contributes indirectly to ER function, both genomically and non-genomically [108].
An important mechanism for bypassing antihormone-induced apoptosis is through increased expression of membrane receptor tyrosine kinases, including epidermal growth factor receptor (EGFR), insulin-like growth factor receptor (IGFR), fibroblast growth factor receptor (FGFR) and HER2. These membrane receptors can activate not only the ER signaling pathway [109], but also the MAPK and AKT signal transduction pathways through increased phosphorylation of p42/44. This is demonstrated in vitro using MCF-7:LTED cell growth inhibition by IGFR knockdown [110]. OSI-906, an IGFR tyrosine kinase inhibitor, prevents MCF-7:LTED growth both in vitro and in vivo [110].
When EGFR is transfected into ZR-75 cells, the cells become estrogen-independent. These cells become ER-negative when tamoxifen is introduced and continued to grow using EGF and its receptor, indicating a possible growth mechanism for antihormone resistant breast cancer cells [111]. Further, ZR-75 cells treated with a 5-azacytidine (a DNA methylation inhibitor used to study influence of epigenetic changes on acquired estrogen independence) develop estrogen independence when grown in estrogen-free media, increasing their HER2 and EGFR expression. Growth of these antihormone-resistant cells can be slowed by an anti-EGFR antibody, indicating a crucial role of EGFR and growth factor signaling in the progression of antihormone resistance in ZR-75 cells [112]. When EGF-stimulated growth was measured in MCF-7 cells, it was not able to be blocked by tamoxifen, 4-hydroxytamoxifen, nor ICI 164384, suggesting an important growth factor influence on their proliferation [113]. Further, breast cancer cells with amplified FGFR show increased resistance to 4-hydroxytamoxifen in vitro, reversible with FGFR-targeted siRNA, indicating a mechanism driving endocrine resistance [114].
If cancer cells are using downstream signaling pathways to continue their growth independent of ER, then blocking key signaling molecules could reveal additional mechanisms of escape. Antagonists of downstream ER signaling pathway proteins, such as mammalian target of rapamycin (mTOR) and phosphoinositide 3-kinase (PI3K), provide potential targets to prevent breast cancer growth after antihormone resistance occurs. The combination of tamoxifen and the mTOR inhibitor RAD001 have an additive effect on MCF-7 cells, together blocking tumor growth in vitro better than either agent alone [115], identifying mTOR as an important target to delay the development of antihormone resistance.
Breast cancer cells that have acquired letrozole resistance highly overexpress the growth factor progranulin when compared to their letrozole-sensitive counterparts in vitro [116]. Progranulin is shown in the laboratory to cause breast cancer cells to acquire letrozole resistance, and knocking down this growth factor can confer letrozole-sensitivity to cells that had acquired letrozole resistance, thereby blocking their proliferation [116]. This example again demonstrates the complexity and flexibility of breast cancer cells to utilize growth factor signaling for survival after long-term antihormone therapy [116].
Long-term estrogen-deprived ER-positive breast cancer cells transfected with the human aromatase gene were studied in ovariectomized athymic nude mice to elucidate mechanisms of acquired resistance to aromatase inhibitors in vivo. Similar concepts emerge in vivo as have been described in vitro. Letrozole-resistant tumors express decreased levels of ER compared with letrozole-sensitive tumors in vivo, and an increase in HER2 (6-fold) and IGFR tyrosine kinase receptors and their downstream signaling proteins (e.g. MAPK), suggesting a shift in signaling pathways away from ER [96,117–120]. Inhibiting these tumors with the anti-HER2 trastuzumab restores letrozole sensitivity [120,121] by downregulating HER2 and restoring ER expression [105]. This indicates that letrozole-resistant ER-positive tumors utilize HER2 signaling to survive despite therapy. HER2 and ER expression were shown in vivo to correlate inversely with one another; that is, when HER2 is inactivated by trastuzumab or herceptin, ER expression increases and the cells become re-sensitized to antihormones and aromatase inhibition [96,118]. EGFR inhibitors are also able to restore letrozole sensitivity [119].
Proteins involved in the MAPK signaling pathway, p-Raf, p-Mek1/2, and p-MAPK, are increased in tumors in vivo that have acquired resistance to letrozole [119,120,122], suggesting the activation of aberrant signaling for compensatory proliferation after long-term aromatase inhibition. Blocking ER with fulvestrant simultaneously with the PI3K inhibitor wortmannin is more effective than antihormone alone, suggesting that the pathway involving PI3K provides a means of growth escape to long-term antihormone-treated breast cancers [123].
Growth factors, e.g. the nuclear coactivator Amplified in Breast Cancer-1 (AIB1, also called SRC-3 and NCoA-3) can activate the ER pathway during antihormone treatment. In the clinical setting, high levels of AIB1 expression in tamoxifen-treated tumors is associated with worse disease-free survival for breast cancer patients, illustrating the importance of AIB1 in the resistance pathway [124]. AIB1 exerts control over many of the growth factor signaling pathways relevant to acquired antihormone resistance, such as EGFR, HER2, PI3K, and mTOR, and interacts with many proteins associated with transcription, cell cycle regulation, and protein degradation [125,126].
Estrogen-induced apoptosis mechanisms during acquired Phase II resistance
The most significant aspect of the evolution of antihormone resistance is the drift toward reconfiguring signaling networks to make the cell survive with no estrogen, but this creates a vulnerability to estrogen-induced apoptosis. After five years of treatment with antihormones, the sophisticated growth pathways become sensitive and paradoxically collapsed by estrogen, once a growth and survival signal. Clinically in the past, women with breast cancer have been successfully treated with high-dose estrogen therapy [127,128]. This was the first effective chemical therapy for any cancer and was the standard-of-care before tamoxifen [129]. Investigation has sought to uncover mechanisms by which apoptosis occurs in Phase II acquired resistance, and how estrogen makes this switch in signaling.
B-cell lymphoma 2 (Bcl-2) is a signaling molecule expressed in 40–80% of primary breast cancers that functions to prevent apoptosis [130], thereby contributing to malignancy and resistance. It acts as an anti-apoptotic signal in long-term estrogen-deprived ER-positive breast cancer cells [131] to subvert estrogen-induced apoptosis. Inhibition of Bcl-2 via siRNA in vitro confers caspase-7 and caspase-9 activation and causes the cells to be synergistically sensitive to estrogen-induced apoptosis [131], making Bcl-2 an interesting therapeutic target. Bcl-2-interacting killer (BIK) regulates calcium release from the endoplasmic reticulum that triggers downstream mitochondria-mediated apoptosis, also inhibiting Bcl-2. High levels of BIK’s inhibitory chaperone, GRP78, in ER-positive breast cancer cells, prevents apoptosis and causes endocrine resistance, [132], thereby asserting itself as another potential therapeutic target.
Studies of varied ER-positive breast cancer cells began to investigate the unique properties of physiologic estrogen that causes tumor regression in postmenopausal women [40]. Santen’s group showed in 2001 [40] estrogen-independent growth of MCF-7:LTED cells, and significant reduction of tumor growth when treated with estradiol. Using annexin V staining and Western blot analysis, the experiments demonstrated induction of FasL, a death receptor ligand associated with the apoptosis cascade, when cells were treated with estradiol [40]. This finding established the notion of estrogen-inducing Fas-mediated apoptosis in LTED breast cancer cells. Apoptosis via the Fas/FasL pathway was increased seven-fold in the estradiol-treated LTED breast cancer cells when compared to the vehicle-treated LTED cells [40]. Fas mRNA and protein were also increased in MCF-7:Tam tumors in vivo, correlated with decreases in NF-κB expression. The laboratory experiment showed that increased Fas signaling and simultaneous suppression of NF-κB’s anti-apoptotic signaling may be characteristic of estradiol-induced apoptosis [93].
Estrogen-induced apoptosis can also originate through the intrinsic mitochondrial apoptosis pathway, when cytochrome C is released from the mitochondria [41]. This is shown in the laboratory using MCF-7:5C cells in vivo [41]. MCF-7:5C cells injected into ovariectomized athymic mice exhibited increased apoptotic protein (e.g. Bax, Bim, p53) expression and tumor regression when treated with estradiol [41].
In tamoxifen-stimulated (Phase II resistant) MCF-7 xenografts, fulvestrant can reverse estrogen-induced apoptosis, stimulating growth and expression of phosphorylated HER2, HER3, p-ERK1/2, and p-GSK3α and β proteins [133]. Pertuzumab blocks the interaction of p-HER2 and HER3 and is able to decrease tumor growth in this model in vivo, suggesting that fulvestrant stimulation of antihormone-resistant ER-positive breast cancers depend not on ER or ER target genes, but on the HER2/HER3 signaling pathway [133].
Additionally, AIB1 is required for estrogen-induced apoptosis in MCF-7:5C cells in vitro. The Wellstein group found that AIB1 is involved in signaling pathways that encourage apoptosis in this context, most prominently through associations with G-protein-coupled receptors, PI3K, Wnt, and Notch signaling pathways [126]. MCF-7 gene expression was examined for the WS8 (wild-type), 5C, and 2A derived cell lines to examine differences in gene regulation during Phase II estrogen-induced apoptosis [97]. For the cell line most sensitive to estrogen-induced apoptosis (MCF-7:5C), genes associated with estrogen signaling, endoplasmic reticulum stress, and inflammation were upregulated, along with apoptotic genes such as BIM and caspase-4, in comparison to WS8 and 2A cells. Analysis of the gene regulation and protein expression indicates that estrogen-induced apoptosis is induced through an inflammatory response in the breast cancer cells, inducing proinflammatory genes (e.g. IL, IFM, arachidonic acid) [97]. The aforementioned examples allow translational research to apply laboratory-revealed mechanisms of acquired resistance to antihormones toward treatment strategies for overcoming or preventing such resistance in ER-positive breast cancer.
Clinical translation via cell models of ER-positive breast cancer
Laboratory models in vitro and in vivo are the invaluable link to clinical translation and enhanced patient survivorship. During the past three decades, the ER-positive breast cancer cell line MCF-7 has been indispensable in this process not only to test therapeutic strategies but also to advance our understanding of hormone-dependent cancer growth [30]. The MCF-7 cell line was the first hormone-responsive breast cancer cell line used effectively to decipher hormone action in breast cancer [30]. Additionally, the ER from MCF-7 cells was prepared on an “industrial scale” to prepare the first monoclonal antibodies [134,135]. These antibodies are now used ubiquitously to determine the ER status of a patient’s tumor by immunohistochemistry [136–139] or flow cytometry [140–142]. However, it was the acquisition of monoclonal antibodies that permitted the cloning and sequencing of the human ER [143–145]. This advance has had a major impact on our understanding of the structure-function relationships of ER-mediated cell regulation.
The availability of ER-positive breast cancer cells and the development of models to test therapeutic strategies continues to play an essential part in the development of clinical trials. By way of example, we will close by considering the role of the MCF-7 cell line in patient care. To set the scene we will place the comments in the context of current clinical practice. There are two therapeutic scenarios to consider: disease in the premenopausal patient and disease in the postmenopausal patient.
Premenopausal women who present with ER-positive breast tumors are generally prescribed combination cytotoxic chemotherapy with five years of adjuvant tamoxifen treatment, while postmenopausal women with ER-positive breast cancer are likely to receive an aromatase inhibitor. If these antiestrogenic approaches fail to prevent recurrence, fulvestrant is used as a second-line antihormone treatment [146].
The strategy of targeting the ER in the tumor micrometastases with long-term adjuvant tamoxifen was created using the 7,12-dimethylbenz(a)anthracene (DMBA)-induced rat mammary carcinoma model [1,3,147]. The first specific aromatase inhibitor, 4-hydroxyandrostenedione (formestane) was compared and contrasted to tamoxifen in the DMBA-induced rat mammary carcinoma model [148–150], but with the development of the model of estrogen-simulated MCF-7 tumors grown in athymic mice in the early 1980s [35,36], the DMBA model was discarded. Initial studies in the athymic mouse model [74] only served to confirm the previous results in the DMBA model but the breakthrough with the MCF-7 model really occurred with the discovery of the evolution of drug resistance to either tamoxifen (or indeed any SERMs) or aromatase inhibitors. We will consider several examples of progress using models of resistance in available breast cancer cell lines that are changing patient care.
The discovery that in vivo acquired tamoxifen resistance is unique, as the tumors grow with either tamoxifen or physiologic estrogen [77], recreated a new dimension to consider in therapeutics: the tumor was amplifying the weak estrogen-like properties of tamoxifen by cell selection. An antiestrogenic strategy of no estrogen (an aromatase inhibitor) or an antiestrogen with no estrogen-like properties was required. The genesis and development of fulvestrant, the injectable long-acting pure steroidal antiestrogen is long, dating back to the mid-1970s, but only now is the clinical community able to apply the drug optimally for appropriate patient care [92].
The idea for studying the therapeutic value of 6,7-substituted estradiol analogs was started through a joint research scheme between ICI pharmaceutical division and Leeds University. The idea was to develop a cytotoxic carrier molecule based on the binding of estradiol to ER that would invariably target and destroy ER-positive metastases [151]. The last compound tested in the series was a 7-substituted (-CH2-)10 chain with the alkylating function on the end. This was based on the knowledge from Roussel Uclaf chemists who had made resin columns to extract and purify the ER [152]. The 7-substitution was an appropriate substitution to retain ER binding. The project to discover ER-targeted cytotoxic agents was abandoned but subsequently, and independently, scientists at ICI pharmaceuticals discovered the merits of this class of molecules to create a “pure” antiestrogen [153]. The lead compound, ICI 164,384, first tested successfully in the tamoxifen-stimulated MCF-7 tumor athymic mouse model [78], provided the reassurance necessary for the clinical development of fulvestrant [44] or an aromatase inhibitor as a second-line agent following the failure of tamoxifen [89,90]. The clinical results mimicked the animal data.
Osborne’s group made the important discovery that transfection of the HER2/neu gene would enhance and accelerate the development of resistance in MCF-7 cells to tamoxifen [46]. This has had important implications for the selection of breast cancer patients for tamoxifen treatment. Indeed, it is the important interplay and interaction of the ER and growth factor receptor pathways that is currently a major focus of translational research. The question has become, “what are the mechanisms and changes that occur in breast cancer cell populations that cause acquired resistance?” Once this question is answered, it will be followed by a different question of, “how do we use the knowledge to delay the process and improve survivorship?” A clinical trial was launched in 2009 comparing lapatinib, a HER2 tyrosine kinase inhibitor, with letrozole versus letrozole alone in postmenopausal hormone receptor-positive patients who have acquired tamoxifen resistance [154]. Lapatinib increases progression-free survival in these patients better than the aromatase inhibitor alone, illustrating a compensatory mechanism of antihormone-resistant cells via HER2 after tamoxifen failure [154]. There are ongoing preclinical and clinical trials investigating the EGFR pathway as a growth mechanism after acquired resistance, comparing antihormone treatments, such as tamoxifen and aromatase inhibitors, with and without EGFR inhibitors, such as gefitinib and erlotinib [155,156].
Breast cancer cells that have acquired resistance to antiestrogen therapy are shown to remain sensitive to therapies targeted against the PI3K pathway [157]. Signaling molecules in the PI3K pathway are frequently mutated in antihormone-resistant ER-positive breast cancer, and comprise a targetable pathway to inhibit for effective therapy [157]. Multiple Phase I and Phase II prospective randomized trials focused on combinations of PI3K pathway inhibitors (e.g. everolimus, trastuzumab, lapatinib, gefitinib, enzastaurin, tipifarnib, BMS-754807, IMCA12, AMG479) and antihormone treatments (e.g. letrozole, exemestane, tamoxifen, anastrozole, fulvestrant) are underway [157] and predicted to provide valuable information.
The encouraging study of mTOR inhibitors in antihormone resistance has advanced to a successful Phase II trial comparing the effectiveness of letrozole, an aromatase inhibitor, treatment alone versus letrozole plus the mTOR inhibitor, everolimus, in patients with ER-positive breast cancer. The results [158] demonstrate increased response rates for the combination arm, which has prompted the initiation of a Phase III clinical trial comparing everolimus in combination with exemestane, a different aromatase inhibitor, for postmenopausal women with ER-positive breast cancer resistant to other aromatase inhibitors [159,160].
Brodie’s group has advanced knowledge of the development of acquired resistance to aromatase inhibitors. Fulvestrant (to destroy the ER) plus an aromatase inhibitor is superior to either strategy alone [86] and trastuzumab reverses letrozole resistance and amplifies the sensitivity of breast cancer cells to estrogen [161]. Each of these strategies have been addressed in clinical trials [162–164] recruiting patients with ER-positive tumors in late-stage breast cancer, but it will be in the adjuvant setting that most gains may occur for patient survivorship. Osborne’s group [155,165] has independently pioneered the strategy of using multiple inhibitors of the growth factor receptor family in combination with either estrogen deprivation or tamoxifen therapy and these strategies are moving into clinical trial.
However, it is the laboratory knowledge derived from the evolution of acquired resistance to long-term antihormone therapy that is providing an insight into past clinical research and future opportunities. All MCF-7 or T47D laboratory models for SERM resistance in vivo develop acquired resistance within a year or two. This is consistent with the endocrine treatment of metastatic breast cancer but does not explain the remarkable success of five years adjuvant tamoxifen to create a 30% decrease in mortality, not only during therapy but sustained for ten years after therapy stops [7]. The treatment of micrometastatic disease with tamoxifen is clearly different than treatment of established tumors. A breakthrough occurred in the early 1990s with the finding that three repeated transplantations of small MCF-7 tumor pieces into subsequent generations of tamoxifen-treated athymic mice for more than five years exposes a vulnerability to the tumor cells that rapidly die during physiologic estrogen treatment [81,82]. This phenomenon was originally advanced [81] to explain the sustained anti-tumor action of tamoxifen when adjuvant treatment is stopped. It was suggested that women’s own estrogen causes apoptosis in micrometastases during Phase II of acquired resistance. Subsequent studies in vitro with estrogen-deprived MCF-7 breast cancer cells demonstrated estradiol-induced apoptosis [40,41].
Based on these studies with MCF-7 cells alone, clinical trials have demonstrated the effectiveness of both high- and low-dose estrogen therapy to treat breast cancer following the development of acquired resistance to antihormone therapy in metastatic disease [91,166]. The approach [81,167] is now being applied indirectly to adjuvant clinical trials of long-term adjuvant therapy (Study of Letrozole Extension), where it is anticipated that a three-month drug holiday per year for five years may reduce recurrence rates during letrozole adjuvant therapy. This is the same principle that is now applied to explain [168] the efficacy of low-dose estrogen replacement alone to reduce the incidence of breast cancer in women with a median of 20 years past their menopause (i.e. long-term estrogen deprivation) [169].
For the future of research in cellular models of breast cancer and acquired resistance to antihormone therapy there are four new developments. Firstly, new primary breast cancer cell lines are being developed and tested both in vivo and in vitro for drug sensitivity. Secondly, a huge pool of human breast cancer cell lines has been interrogated for drug sensitivity and pathway analysis completed to procure new clinical strategies for treatment [170,171]. Thirdly, signatures have been created to define acquired drug resistance to tamoxifen in existing breast cancer cell lines [114,172] that can be applied to clinical trial. Finally, new methodologies are now available to enrich for breast cancer stem cells and expanding this populations for drug sensitivity testing [173]. Should the future of the “many” new cell systems from primary tumors deliver the promise achieved by the “few” cell lines in the past then there is every reason to believe that enormous progress will occur in the successful treatment and prevention of breast cancer in the coming decades.
Acknowledgments
This work (VCJ) was supported by the Department of Defense Breast Program under Award number W81XWH-06-1-0590 Center of Excellence; subcontract under the SU2C (AACR) Grant number SU2C-AACR-DT0409; the Susan G Komen For The Cure Foundation under Award number SAC100009 and the Lombardi Comprehensive Cancer Center Support Grant (CCSG) Core Grant NIH P30 CA051008. The views and opinions of the author(s) do not reflect those of the US Army or the Department of Defense.
References
- 1.Jordan VC. Tamoxifen: Catalyst for the change to targeted therapy. Eur J Cancer. 2008;44:30–38. doi: 10.1016/j.ejca.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen EV, Jordan VC. The estrogen receptor: a model for molecular medicine. The Dorothy P. Landon AACR Prize for Translational Research. Clinical Cancer Res. 2003;9:1980–1989. [PubMed] [Google Scholar]
- 3.Jordan VC, Koerner S. Tamoxifen (ICI 46,474) and the human carcinoma 8S oestrogen receptor. Eur J Cancer. 1975;11:205–206. doi: 10.1016/0014-2964(75)90119-x. [DOI] [PubMed] [Google Scholar]
- 4.Brodie A. Aromatase and its inhibitors--an overview. J Steroid Biochem Mol Biol. 1991;40:255–261. doi: 10.1016/0960-0760(91)90190-g. [DOI] [PubMed] [Google Scholar]
- 5.Chumsri S, Howes T, Bao T, Sabnis G, Brodie A. Aromatase, aromatase inhibitors, and breast cancer. J Steroid Biochem Mol Biol. 2011;125:13–22. doi: 10.1016/j.jsbmb.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, Buyse M, Baum M, Buzdar A, Colleoni M, Coombes C, Snowdon C, Gnant M, Jakesz R, Kaufmann M, Boccardo F, Godwin J, Davies C, Peto R. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 7.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van de Velde CJ, Rea D, Seynaeve C, Putter H, Hasenburg A, Vannetzel JM, Paridaens R, Markopoulos C, Hozumi Y, Hille ET, Kieback DG, Asmar L, Smeets J, Nortier JW, Hadji P, Bartlett JM, Jones SE. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trial. Lancet. 2011;377:321–331. doi: 10.1016/S0140-6736(10)62312-4. [DOI] [PubMed] [Google Scholar]
- 9.Regan MM, Neven P, Giobbie-Hurder A, Goldhirsch A, Ejlertsen B, Mauriac L, Forbes JF, Smith I, Lang I, Wardley A, Rabaglio M, Price KN, Gelber RD, Coates AS, Thurlimann B for the members of the BIG 1–98 Collaborative Group and the International Breast Cancer Study Group (IBCSG) Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1–98 randomised clinical trial at 8·1 years median follow-up. Lancet Oncol. 2011;12:1101–1108. doi: 10.1016/S1470-2045(11)70270-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 11.Fisher B, Constantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N other National Surgical Adjuvant Breast and Bowel Project Investigators. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 12.Fisher B, Constantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, Bevers TB, Kavanah MT, Atkins JN, Margolese RG, Runowicz CD, James JM, Ford LG, Wolmark N. Tamoxifen for the prevention of breast cancer: Current status of the national surgical adjuvant breast and bowel project P-1 study. J Natl Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 13.Jordan VC, Phelps E, Lindgren JU. Effects of antiestrogens on bone in castrated and intact female rats. Breast Cancer Res Treat. 1987;10:31–35. doi: 10.1007/BF01806132. [DOI] [PubMed] [Google Scholar]
- 14.Gottardis MM, Jordan VC. Antitumor actions of keoxifene and tamoxifen in the N-nitrosomethylurea-induced rat mammary carcinoma model. Cancer Res. 1987;47:4020–4024. [PubMed] [Google Scholar]
- 15.Lerner LJ, Jordan VC. Development of antiestrogens and their use in breast cancer: eighth Cain memorial award lecture. Cancer Res. 1990;50:4177–4189. [PubMed] [Google Scholar]
- 16.Gottardis MM, Robinson SP, Satyaswaroop PG, Jordan VC. Contrasting actions of tamoxifen on endometrial and breast tumor growth in the athymic mouse. Cancer Res. 1988;48:812–815. [PubMed] [Google Scholar]
- 17.Fornander T, Cedermark B, Mattsson A, Skloog L, Theve T, Askergren J, Rutqvist L, Glas U, Silfversward C, Somell A, Wilking N, Hjalmar ML. Adjuvant tamoxifen in early breast cancer: occurrence of new primary cancers. Lancet. 1989;1(8630):117–120. doi: 10.1016/s0140-6736(89)91141-0. [DOI] [PubMed] [Google Scholar]
- 18.Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, Norton L, Nickelsen T, Bjarnason NH, Morrow M, Lippman ME, Black D, Glusman JE, Costa A, Jordan VC. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- 19.Martino S, Cauley JA, Barrett-Connor E, Powles TJ, Mershon J, Disch D, Secrest RJ, Cummings SR CORE Investigators. Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst. 2004;96:1751–1761. doi: 10.1093/jnci/djh319. [DOI] [PubMed] [Google Scholar]
- 20.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher L, Pajon ER, Jr, Wade JL, 3rd, Robidoux A, Margolese RG, James J, Lippman SM, Runowicz CD, Ganz PA, Reis SE, McCaskill-Stevens W, Ford LG, Jordan VC, Wolmark N National Surgical Adjuvant Breast and Bowel Project (NSABP) Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2727–2741. doi: 10.1001/jama.295.23.joc60074. [DOI] [PubMed] [Google Scholar]
- 21.Vogel VG, Costantino JP, Wickerham DL, Cronin WM, Cecchini RS, Atkins JN, Bevers TB, Fehrenbacher L, Pajon ER, Wade JL, 3rd, Robidoux A, Margolese RG, James J, Runowicz CD, Ganz PA, Reis SE, McCaskill-Stevens W, Ford LG, Jordan VC, Wolmark N National Surgical Adjuvant Breast and Bowel Project. Update of the national surgical adjuvant breast and bowel project study of tamoxifen and raloxifene (STAR) P-2 trial: Preventing breast cancer. Cancer Prev Res. 2010;3:696–706. doi: 10.1158/1940-6207.CAPR-10-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jemal A, Siegel R, Xu J, Ward E. Cancer Statistics, 2010. CA: A Cancer Journal for Clinicians. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 23.Engel LW, Young NA, Tralka TS, Lippman ME, O’Brien SJ, Joyce MJ. Establishment and characterization of three new continuous cell lines derived from human breast carcinomas. Cancer Res. 1978;38:3352–3364. [PubMed] [Google Scholar]
- 24.Allegra JC, Lippman ME. The effects of 17β estradiol and tamoxifen on the ZR-75-1 human breast cancer cell line in defined medium. Eur J Cancer. 1980;16(8):1007–1015. doi: 10.1016/0014-2964(80)90246-7. [DOI] [PubMed] [Google Scholar]
- 25.Lasfargues EY, Coutinho WG, Redfield ES. Isolation of two human tumor epithelial cell lines from solid breast carcinomas. J Natl Cancer Inst. 1978;61:967–978. [PubMed] [Google Scholar]
- 26.Kraus MH, Popescu NC, Amsburgh SC, King CR. Overexpression of the EGF receptor-related proto-oncogene erbB-2 in human mammary tumor cell lines by different molecular mechanisms. EMBO J. 1987;6:605–610. doi: 10.1002/j.1460-2075.1987.tb04797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keydar I, Chen L, Karby S, Weiss FR, Delarea J, Radu M, Chaitcik S, Brenner HJ. Establishment and characterization of a cell line of human breast carcinoma origin. Eur J Cancer. 1979;15:659–670. doi: 10.1016/0014-2964(79)90139-7. [DOI] [PubMed] [Google Scholar]
- 28.Murphy CS, Meisner LF, Wu SQ, Jordan VC. Short- and long-term estrogen deprivation of T47D human breast cancer cells in culture. Eur J Cancer Clin Oncol. 1989;25:1777–1788. doi: 10.1016/0277-5379(89)90348-9. [DOI] [PubMed] [Google Scholar]
- 29.Chalbos D, Vignon F, Keydar I, Rochefort H. Estrogens stimulate cell proliferation and induce secretory proteins in a human breast cancer cell line (T47D) J Clin Endocrinol Metab. 1982;55:276–283. doi: 10.1210/jcem-55-2-276. [DOI] [PubMed] [Google Scholar]
- 30.Levenson AS, Jordan VC. MCF-7: the first hormone-responsive breast cancer cell line. Cancer Res. 1997;57:3071–3078. [PubMed] [Google Scholar]
- 31.Soule MD, Vazquez J, Long A, Albert S, Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973;51:1409–1413. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- 32.Brooks SC, Locke ER, Soule HD. Estrogen receptor in a human cell line (MCF-7) from breast carcinoma. J Biol Chem. 1973;248:6251–6253. [PubMed] [Google Scholar]
- 33.Lippman ME, Bolan G. Oestrogen-responsive human breast cancer in long term tissue culture. Nature. 1975;258:339–341. doi: 10.1038/256592a0. [DOI] [PubMed] [Google Scholar]
- 34.Lippman M, Bolan G, Huff K. The effects of estrogens and antiestrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res. 1976;36:4595–4601. [PubMed] [Google Scholar]
- 35.Soule HD, McGrath CM. Estrogen responsive proliferation of clonal human breast carcinoma cells in athymic mice. Cancer Lett. 1980;10:177–189. doi: 10.1016/0304-3835(80)90042-7. [DOI] [PubMed] [Google Scholar]
- 36.Shafie SM. Estrogen and the growth of breast cancer: new evidence suggests indirect action. Science. 1980;209:701–702. doi: 10.1126/science.6994231. [DOI] [PubMed] [Google Scholar]
- 37.Berthois Y, Katzenellenbogen JA, Katzenellenbogen BS. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986;83:2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bindal RD, Katzenellenbogen JA. Bis(4-hydroxyphenyl)[2 from commercial preparations of phenol red (phenolsulfonphtalein) J Med Chem. 1988;31(10):1978–1983. doi: 10.1021/jm00118a020. [DOI] [PubMed] [Google Scholar]
- 39.Katzenellenbogen BS, Kendra KL, Norman MJ, Berthois Y. Proliferation, hormonal responsiveness, and estrogen receptor content of MCF-7 human breast cancer cells grown in the short-term and long-term absence of estrogens. Cancer Res. 1987;47(16):4355–4360. [PubMed] [Google Scholar]
- 40.Song RX, Mor G, Naftolin F, McPherson RA, Song J, Zhang Z, Yue W, Wang J. Santen -(phenoxysulfonyl)phenyl]methane: isolation and structure elucidation of a novel estrogen RJ. Effect of long-term estrogen deprivation on apoptotic responses of breast cancer cells to 17beta-estradiol. J Natl Cancer Inst. 2001;93:1714–1723. doi: 10.1093/jnci/93.22.1714. [DOI] [PubMed] [Google Scholar]
- 41.Lewis JS, Meeke K, Osipo C, Ross EA, Kidawi N, Li T, Bell E, Chandel NS, Jordan VC. Intrinsic mechanism of estradiol-induced apoptosis in breast cancer cells resistant to estrogen deprivation. J Natl Cancer Inst. 2005;97:1746–1759. doi: 10.1093/jnci/dji400. [DOI] [PubMed] [Google Scholar]
- 42.Cho HS, NG PA, Katzenellenbogen BS. Differential regulation of gene expression by estrogen in estrogen growth-independent and -dependent MCF-7 human breast cancer cell sublines. Mol Endocrinol. 1991;5:1323–1330. doi: 10.1210/mend-5-9-1323. [DOI] [PubMed] [Google Scholar]
- 43.Welshons WV, Jordan VC. Adaptation of estrogen-dependent MCF-7 cells to low estrogen (phenol red-free) culture. Eur J Cancer Clin Oncol. 1987;23:1935–1939. doi: 10.1016/0277-5379(87)90062-9. [DOI] [PubMed] [Google Scholar]
- 44.Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51:3867–3873. [PubMed] [Google Scholar]
- 45.Pink JJ, Jordan VC. Models of estrogen receptor regulation by estrogens and antiestrogens in breast cancer cell lines. Cancer Res. 1996;56:2321–2330. [PubMed] [Google Scholar]
- 46.Benz CC, Scott GK, Sarup JC, Johnson RM, Tripathy D, Coronado E, Shepard HM, Osborne CK. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1992;24(2):85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 47.Clarke R, Brunner N, Katzenellenbogen BS, Thompson EW, Norman MJ, Koppi C, Paik S, Lippman ME, Dickson RB. Progression of human breast cancer cells from hormone-dependent to hormone-independent growth both in vitro and in vivo. Proc. Natl. Acad. Sci. 1989;86:3649–3653. doi: 10.1073/pnas.86.10.3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brunner N, Boulay V, Fojo A, Freter CE, Lippman ME, Clarke R. Acquisition of hormone-independent growth in MCF-7 cells is accompanied by increased expression of estrogen-regulated genes but without detectable DNA amplifications. Cancer Res. 1993;53:283–290. [PubMed] [Google Scholar]
- 49.Brunner N, Frandsen TL, Holst-Hansen C, Bei M, Thompson EW, Wakeling AE, Lippman ME, Clarke R. MCF7/LCC2: a 4-hydroxytamoxifen resistant human breast cancer variant that retains sensitivity to the steroidal antiestrogen ICI 182,780. Cancer Res. 1993;53:3229–3232. [PubMed] [Google Scholar]
- 50.Brunner N, Boysen B, Jirus S, Skaar TC, Holst-Hansen C, Lippman J, Frandsen T, Spang-Thomsen M, Fuqua SA, Clarke R. MCF7/LCC9: an antiestrogen-resistant MCF-7 variant in which acquired resistance to the steroidal antiestrogen ICI 182,780 confers an early cross-resistance to the nonsteroidal antiestrogen tamoxifen. Cancer Res. 1997;57:3486–3493. [PubMed] [Google Scholar]
- 51.Bronzert DA, Greene GL, Lippman ME. Selection and characterization of a breast cancer cell line resistant to the antiestrogen LY117018. Endocrinol. 1985;117:1409–1417. doi: 10.1210/endo-117-4-1409. [DOI] [PubMed] [Google Scholar]
- 52.Liu H, Lee ES, Gajdos C, Pearce ST, Chen B, Osipo C, Loweth J, McKian K, De Los Reyes A, Wing L, Jordan VC. Apoptotic action of 17beta-estradiol in raloxifene-resistant MCF-7 cells in vitro and in vivo. J Natl Cancer Inst. 2003;95:1586–1597. doi: 10.1093/jnci/djg080. [DOI] [PubMed] [Google Scholar]
- 53.Balaburski GM, Dardes RC, Johnson M, Haddad B, Zhu F, Ross EA, Sengupta S, Klein-Szanto A, Liu H, Lee ES, Kim H, Jordan VC. Raloxifene-stimulated experimental breast cancer with the paradoxical actions of estrogen to promote or prevent tumor growth: a unifying concept in anti-hormone resistance. Int J Oncol. 2010;37:387–398. doi: 10.3892/ijo_00000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu H, Cheng D, Weichel AK, Osipo C, Wing LK, Chen B, Louis TE, Jordan VC. Cooperative effect of gefitinib and fumitremorgin c on cell growth and chemosensitivity in estrogen receptor alpha negative fulvestrant-resistant MCF-7 cells. Int J Oncol. 2006;29:1237–1246. [PubMed] [Google Scholar]
- 55.Masamura S, Santner SJ, Heitjan DF, Santen RJ. Estrogen deprivation causes estradiol hypersensitivity in human breast cancer cells. J Clin Endocrinol Metab. 1995;80(10):2918–2925. doi: 10.1210/jcem.80.10.7559875. [DOI] [PubMed] [Google Scholar]
- 56.Jiang SY, Wolf DM, Yingling JM, Chang C, Jordan VC. An estrogen receptor positive MCF-7 clone that is resistant to antiestrogens and estradiol. Mol Cell Endocrinol. 1992;90:77–86. doi: 10.1016/0303-7207(92)90104-e. [DOI] [PubMed] [Google Scholar]
- 57.Lewis JS, Osipo C, Meeke K, Jordan VC. Estrogen-induced apoptosis in a breast cancer model resistant to long-term estrogen withdrawal. J Steroid Biochem Mol Biol. 2005;94:131–141. doi: 10.1016/j.jsbmb.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 58.Pink JJ, Jiang SY, Fritsch M, Jordan VC. An estrogen-independent MCF-7 breast cancer cell line which contains a novel 80-kilodalton estrogen receptor-related protein. Cancer Res. 1995;55:2583–2590. [PubMed] [Google Scholar]
- 59.Pink JJ, Wu SQ, Wolf DM, Bilimoria MM, Jordan VC. A novel 80 kDa human estrogen receptor containing a duplication of exons 6 and 7. Nucleic Acids Res. 1996;24(5):962–969. doi: 10.1093/nar/24.5.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vojtesek B, Lane DP. Regulation of p53 protein expression in human breast cancer cell lines. J Cell Sci. 1993;105:607–612. doi: 10.1242/jcs.105.3.607. [DOI] [PubMed] [Google Scholar]
- 61.Murphy CS, Pink JJ, Jordan VC. Characterization of a receptor-negative, hormone-nonresponsive clone derived from a T47D human breast cancer cell line kept under estrogen-free conditions. Cancer Res. 1990;50:7285–7292. [PubMed] [Google Scholar]
- 62.Pink JJ, Bilimoria MM, Assikis J, Jordan VC. Irreversible loss of the oestrogen receptor in T47D breast cancer cells following prolonged oestrogen deprivation. Br J Cancer. 1996;74:1227–1236. doi: 10.1038/bjc.1996.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huber M, Bahr I, Kratzschmar JR, Becker A, Muller EC, Donner P, Pohlenz HD, Schneider MR, Sommer A. Comparison of proteomic and genomic analyses of the human breast cancer cell line T47D and the antiestrogen-resistant derivative T47D-r. Mol Cell Proteomics. 2004;3:43–55. doi: 10.1074/mcp.M300047-MCP200. [DOI] [PubMed] [Google Scholar]
- 64.Horwitz KB, Freidenberg GR. Growth inhibition and increase of insulin receptors in antiestrogen-resistant T47DCO human breast cancer cells by progestins: implications for endocrine therapies. Cancer Res. 1985;45:167–173. [PubMed] [Google Scholar]
- 65.Graham ML, 2nd, Krett NL, Miller LA, Leslie KK, Gordon DF, Wood WM, Wei LL, Horwitz KB. T47DCO cells, genetically unstable and containing estrogen receptor mutations, are a model for the progression of breast cancers to hormone resistance. Cancer Res. 1990;50:6208–6217. [PubMed] [Google Scholar]
- 66.van ben Berg HW, Lynch M, Martin J, Nelson J, Dickson GR, Crockard AD. Characterisation of a tamoxifen-resistant variant of the ZR-75-1 human breast cancer cell line (ZR-75-9a1) and the ability of the resistant phenotype. Br J Cancer. 1989;59:522–526. doi: 10.1038/bjc.1989.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Satyaswaroop PG, Zaino RJ, Mortel R. Human endometrial adenocarcinoma transplanted into nude mice: growth regulation by estradiol. Science. 1983;219:58–60. doi: 10.1126/science.6849115. [DOI] [PubMed] [Google Scholar]
- 68.Zaino RJ, Satyaswaroop PG, Mortel R. Hormonal therapy of human endometrial adenocarcinoma in a nude mouse model. Cancer Res. 1985;45:539–541. [PubMed] [Google Scholar]
- 69.Jordan VC, Gottardis MM, Satyaswaroop PG. Tamoxifen-stimulated growth of human endometrial carcinoma. Ann N Y Acad Sci. 1991;622:439–446. doi: 10.1111/j.1749-6632.1991.tb37886.x. [DOI] [PubMed] [Google Scholar]
- 70.Jordan VC, Robinson SP. Species-specific pharmacology of antiestrogens: role of metabolism. Fed Proc. 1987;46:1870–1874. [PubMed] [Google Scholar]
- 71.Gottardis MM, Ricchio ME, Satyaswaroop PG, Jordan VC. Effect of steroidal and nonsteroidal antiestrogens on the growth of a tamoxifen-stimulated human endometrial carcinoma (EnCa101) in athymic mice. Cancer Res. 1990;50:3189–3192. [PubMed] [Google Scholar]
- 72.Addo S, Yates RA, Laight A. A phase I trial to assess the pharmacology of the new oestrogen receptor antagonist fulvestrant on the endometrium in healthy postmenopausal volunteers. Br J Cancer. 2002;87:1354–1359. doi: 10.1038/sj.bjc.6600644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ilino Y, Wolf DM, Langan-Fahey SM, Johnson DA, Ricchio M, Thompson ME, Jordan VC. Reversible control of oestradiol-stimulated growth of MCF-7 tumours by tamoxifen in the athymic mouse. Br J Cancer. 1991;64:1019–1024. doi: 10.1038/bjc.1991.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Osborne CK, Hobbs K, Clark GM. Effect of estrogens and antiestrogens on growth of human breast cancer cells in athymic nude mice. Cancer Res. 1985;45:584–590. [PubMed] [Google Scholar]
- 75.Gottardis MM, Robinson SP, Jordan VC. Estradiol-stimulated growth of MCF-7 tumors implanted in athymic mice: a model to study the tumoristatic action of tamoxifen. J Steroid Biochem. 1988;30:311–314. doi: 10.1016/0022-4731(88)90113-6. [DOI] [PubMed] [Google Scholar]
- 76.Osborne CK, Coronado EB, Robinson JP. Human breast cancer in the athymic nude mouse: cytostatic effects of long-term antiestrogen therapy. Eur J Cancer Clin Oncol. 1987;23:1189–1196. doi: 10.1016/0277-5379(87)90154-4. [DOI] [PubMed] [Google Scholar]
- 77.Gottardis MM, Jordan VC. Development of tamoxifen-stimulated growth of MCF-7 tumors in athymic mice after long-term antiestrogen administration. Cancer Res. 1988;48:5183–5187. [PubMed] [Google Scholar]
- 78.Gottardis MM, Jiang SY, Jeng MH, Jordan VC. Inhibition of tamoxifen-stimulated growth of an MCF-7 tumor variant in athymic mice by novel steroidal antiestrogens. Cancer Res. 1989;49:4090–4093. [PubMed] [Google Scholar]
- 79.Lee ES, Schafer JM, Yao K, England G, O’Regan RM, De Los Reyes A, Jordan VC. Cross-resistance of triphenylethylene-type antiestrogens but not ICI 182,780 in tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6:4893–4899. [PubMed] [Google Scholar]
- 80.O'Regan RM, Cisneros A, England GM, MacGregor JI, Muenzner HD, Assikis VJ, Bilimoria MM, Piette M, Dragan YP, Pitot HC, Chatterton R, Jordan VC. Effects of the antiestrogens tamoxifen, toremifene, and ICI 182,780 on endometrial cancer growth. J Natl Cancer Inst. 1998;90:1552–1558. doi: 10.1093/jnci/90.20.1552. [DOI] [PubMed] [Google Scholar]
- 81.Wolf DM, Jordan VC. A laboratory model to explain the survival advantage observed in patients taking adjuvant tamoxifen therapy. Recent Results Cancer Res. 1993;127:23–33. doi: 10.1007/978-3-642-84745-5_4. [DOI] [PubMed] [Google Scholar]
- 82.Yao K, Lee ES, Bentrem DJ, England G, Schafer JI, O’Regan RM, Jordan VC. Antitumor action of physiological estradiol on tamoxifen-stimulated breast tumors grown in athymic mice. Clin Cancer Res. 2000;6:2028–2036. [PubMed] [Google Scholar]
- 83.Schafer JM, Lee ES, O’Regan RM, Yao K, Jordan VC. Rapid development of tamoxifen-stimulated mutant p53 breast tumors (T47D) in athymic mice. Clin Cancer Res. 2000;6:4373–4380. [PubMed] [Google Scholar]
- 84.Schafer JM, Lee ES, Dardes RC, Bentrem D, O'Regan RM, De Los Reyes A, Jordan VC. Analysis of cross-resistance of the selective estrogen receptor modulators arzoxifene (LY353381) and LY117018 in tamoxifen-stimulated breast cancer xenografts. Clin Cancer Res. 2001;7:2505–2512. [PubMed] [Google Scholar]
- 85.Yue W, Zhou D, Chen S, Brodie A. A new nude mouse model for postmenopausal breast cancer using MCF-7 cells transfected with the human aromatase gene. Cancer Res. 1994;54:5092–5095. [PubMed] [Google Scholar]
- 86.Macedo LF, Sabnis GJ, Goloubeva OG, Brodie A. Combination of anastrozole with fulvestrant in the intratumoral aromatase xenograft model. Cancer Res. 2008;68:3516–3522. doi: 10.1158/0008-5472.CAN-07-6807. [DOI] [PubMed] [Google Scholar]
- 87.Jordan VC. Selective estrogen receptor modulation: Concept and consequences in cancer. Cancer Cell. 2004;5:207–213. doi: 10.1016/s1535-6108(04)00059-5. [DOI] [PubMed] [Google Scholar]
- 88.Jordan VC. The 38th David A. Karnofsky Lecture: The Paradoxical Actions of Estrogen in Breast Cancer—Survival or Death? J Clin Oncol. 2008;26:3078–3082. doi: 10.1200/JCO.2008.17.5190. [DOI] [PubMed] [Google Scholar]
- 89.Osborne CK, Pippen J, Jones SE, Parker LM, Ellis M, Come S, Gertler SZ, May JT, Burton G, Dimery I, Webster A, Morris C, Elledge R, Buzdar A. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: results of a North American trial. J Clin Oncol. 2002;20:3386–3395. doi: 10.1200/JCO.2002.10.058. [DOI] [PubMed] [Google Scholar]
- 90.Howell A, Robertson JF, Quaresma Albano J, Aschermannova A, Mauriac L, Kleeberg UR, Vergote I, Erikstein B, Webster A, Morris C. Fulvestrant, formerly ICI 182,780 is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J Clin Oncol. 2002;20:3396–3403. doi: 10.1200/JCO.2002.10.057. [DOI] [PubMed] [Google Scholar]
- 91.Ellis MJ, Gao F, Dehdashti F, Jeffe DB, Marcom PK, Carey LA, Dickler MN, Silverman P, Fleming GF, Kommareddy A, Jamalabadi-Majidi S, Crowder R, Siegel BA. Lower-dose vs high-dose oral estradiol therapy of hormone receptor-positive, aromatase inhibitor-resistant advanced breast cancer: a phase 2 randomized study. JAMA. 2009;302:774–780. doi: 10.1001/jama.2009.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Di Leo A, Jerusalem G, Petruzelka L, Torres R, Bondarenko IN, Khasanov R, Verhoeven D, Pedrini JL, Smirnova I, Lichinitser MR, Pendergrass K, Garnett S, Lindemann JP, Sapunar F, Martin M. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28:4594–4600. doi: 10.1200/JCO.2010.28.8415. [DOI] [PubMed] [Google Scholar]
- 93.Osipo C, Gajdos C, Liu H, Chen B, Jordan VC. Paradoxical action of fulvestrant in estradiol-induced regression of tamoxifen-stimulated breast cancer. J Natl Cancer Inst. 2003;95:1597–1608. doi: 10.1093/jnci/djg079. [DOI] [PubMed] [Google Scholar]
- 94.Long BJ, Jelovac D, Thiantanawat A, Brodie AM. The effect of second-line antiestrogen therapy on breast tumor growth after first-line treatment with aromatase inhibitor letrozole: long-term studies using the intratumoral aromatase postmenopausal breast cancer model. Clin Cancer Res. 2002;8(7):2378–2388. [PubMed] [Google Scholar]
- 95.Brodie A, Jelovac D, Long BJ. Predictions from a preclinical model: studies of aromatase inhibitors and antiestrogens. Clin Cancer Res. 2003;9:455S–459S. [PubMed] [Google Scholar]
- 96.Brodie A, Macedo L, Sabnis G. Aromatase resistance mechanisms in model systems in vivo. J Steroid Biochem Mol Biol. 2010;118:283–287. doi: 10.1016/j.jsbmb.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ariazi EA, Cunliffe HE, Lewis-Wambi JS, Slifker MJ, Willis AL, Ramos P, Tapia C, Kim HR, Yerrum S, Sharma CG, Nicolas E, Balagurunathan Y, Ross EA, Jordan VC. Estrogen induces apoptosis in estrogen deprivation-resistant breast cancer through stress responses as identified by global gene expression across time. Proc Natl Acad Sci U S A. 2011;108:18879–18886. doi: 10.1073/pnas.1115188108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wolf DM, Jordan VC. The estrogen receptor from a tamoxifen stimulated MCF-7 tumor variant contains a point mutation in the ligand binding domain. Breast Cancer Res Treat. 1994;31:129–138. doi: 10.1007/BF00689683. [DOI] [PubMed] [Google Scholar]
- 99.Levenson AS, Jordan VC. The key to the antiestrogenic mechanism of raloxifene is amino acid 351 (aspartate) in the estrogen receptor. Cancer Res. 1998;58:1872–1875. [PubMed] [Google Scholar]
- 100.MacGregor Schafer J, Liu H, Bentrem DJ, Zapf JW, Jordan VC. Allosteric silencing of activating function 1 in the 4-hydroxytamoxifen estrogen receptor complex is induced by substituting glycine for aspartate at amino acid 351. Cancer Res. 2000;60:5097–5105. [PubMed] [Google Scholar]
- 101.Liu H, Lee ES, Deb Los Reyes A, Zapf JW, Jordan VC. Silencing and reactivation of the selective estrogen receptor-estrogen receptor alpha complex. Cancer Res. 2001;61:3632–3639. [PubMed] [Google Scholar]
- 102.Ottaviano YL, Issa JP, Parl FF, Smith HS, Baylin SB, Davidson NE. Methylation of the estrogen receptor gene CpG island marks loss of estrogen receptor expression in human breast cancer cells. Cancer Res. 1994;54:2552–2555. [PubMed] [Google Scholar]
- 103.Prag G, Misra S, Jones EA, Ghirlando R, Davies BA, Horazdovsky BF, Hurley JH. Mechanism of ubiquitin recognition by the CUE domain of Vps9p. Cell. 2003;113:609–620. doi: 10.1016/s0092-8674(03)00364-7. [DOI] [PubMed] [Google Scholar]
- 104.Pan X, Zhou T, Tai YH, Wang C, Zhao J, Cao Y, Chen Y, Zhang PJ, Yu M, Zhen C, Mu R, Bai ZF, Li HY, Li AL, Liang B, Jian Z, Zhang WN, Man JH, Gao YF, Gong WL, Wei LX, Zhang XM. Elevated expression of CUEDC2 protein confers endocrine resistance in breast cancer. Nat Med. 2011;17:708–714. doi: 10.1038/nm.2369. [DOI] [PubMed] [Google Scholar]
- 105.Chumsri S, Sabnis GJ, Howes T, Brodie AM. Aromatase inhibitors and xenograft studies. Steroids. 2011;76:730–735. doi: 10.1016/j.steroids.2011.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cormier EM, Wolf MF, Jordan VC. Decrease in estradiol-stimulated progesterone receptor production in MCF-7 cells by epidermal growth factor and possible clinical implication for paracrine-regulated breast cancer growth. Cancer Res. 1989;49:576–580. [PubMed] [Google Scholar]
- 107.Robinson SP, Jordan VC. The paracrine stimulation of MCF-7 cells by MDA-MB-231 cells: possible role in antiestrogen failure. Eur J Cancer Clin Oncol. 1989;25:493–497. doi: 10.1016/0277-5379(89)90262-9. [DOI] [PubMed] [Google Scholar]
- 108.Arpino G, Wiechmann L, Osborne CK, Schiff R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: Molecular mechanism and clinical implications for endocrine therapy resistance. Endocr. Rev. 2008;29:217–233. doi: 10.1210/er.2006-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–247. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fox EM, Miller TW, Balko JM, Kuba MG, Sanchez V, Smith RA, Liu S, Gonzalez-Angulo AM, Mills GB, Ye F, Shyr Y, Manning HC, Buck E, Arteaga CL. A Kinome-Wide Screen Identifies the Insulin/IGF-I Receptor Pathway as a Mechanism of Escape from Hormone Dependence in Breast Cancer. Cancer Res. 2011;71:6773–6784. doi: 10.1158/0008-5472.CAN-11-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van Agthoven T, van Agthoven TL, Portengen H, Foekens JA, Dorssers LC. Ectopic expression of epidermal growth factor receptors induces hormone independence in ZR-75-1 human breast cancer cells. Cancer Res. 1992;52:5082–5088. [PubMed] [Google Scholar]
- 112.van Agthoven T, van Agthoven TL, Dekker A, Foekens JA, Dorssers LC. Induction of estrogen independence of ZR-75-1 human breast cancer cells by epigenetic alterations. Mol Endocrinol. 1994;8:1474–1483. doi: 10.1210/mend.8.11.7533260. [DOI] [PubMed] [Google Scholar]
- 113.Cormier EM, Jordan VC. Contrasting ability of antiestrogens to inhibit MCF-7 growth stimulated by estradiol or epidermal growth factor. Eur J Cancer Clin Oncol. 1989;25:57–63. doi: 10.1016/0277-5379(89)90051-5. [DOI] [PubMed] [Google Scholar]
- 114.Turner N, Pearson A, Sharpe R, Lambros M, Geyer F, Lopez-Garcia MA, Natrajan R, Marchio C, Iorns E, Mackay A, Gillett C, Grigoriadis A, Tutt A, Reis-Filho JS, Ashworth A. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 2010;70:2085–2094. doi: 10.1158/0008-5472.CAN-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Treeck O, Wackwitz B, Haus U, Ortmann O. Effects of a combined treatment with mTOR inhibitor RAD001 and tamoxifen in vitro on growth and apoptosis of human cancer cells. Gynecol Oncol. 2006;102:292–299. doi: 10.1016/j.ygyno.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 116.Abrhale T, Brodie A, Sabnis G, Macedo L, Tian C, Yue B, Serrero G. GP88 (PC-Cell Derived Growth Factor, progranulin) stimulates proliferation and confers letrozole resistance to aromatase overexpressing breast cancer cells. BMC Cancer. 2011;11:231. doi: 10.1186/1471-2407-11-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brodie A, Sabnis G. Adaptive changes result in activation of alternate signaling pathways and acquisition of resistance to aromatase inhibitors. Clin Cancer Res. 2011;17:4208–4213. doi: 10.1158/1078-0432.CCR-10-2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sabnis G, Brodie A. Adaptive changes results in activation of alternate signaling pathways and resistance to aromatase inhibitor resistance. Mol Cell Endocrinol. 2011;340:142–147. doi: 10.1016/j.mce.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 119.Brodie A, Sabnis G, Macedo L. Xenograft models for aromatase inhibitor studies. J Steroid Biochem Mol Biol. 2007;106:119–124. doi: 10.1016/j.jsbmb.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 120.Macedo LF, Sabnis G, Brodie A. Preclinical modeling of endocrine response and resistance: focus on aromatase inhibitors. Cancer. 2008;112:679–688. doi: 10.1002/cncr.23191. [DOI] [PubMed] [Google Scholar]
- 121.Sabnis G, Brodie A. Understanding resistance to endocrine agents: molecular mechanisms and potential for intervention. Clin Breast Cancer. 2010;10:E6–E15. doi: 10.3816/CBC.2010.n.014. [DOI] [PubMed] [Google Scholar]
- 122.Brodie A, Jelovac D, Sabnis G, Long B, Macedo L, Goloubeva O. Model systems: mechanisms involved in the loss of sensitivity to letrozole. J Steroid Biochem Mol Biol. 2005;95:41–48. doi: 10.1016/j.jsbmb.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 123.Sabnis G, Goloubeva O, Jelovac D, Schayowitz A, Brodie A. Inhibition of the phosphatidylinositol 3-kinase/Akt pathway improves response of long-term estrogen-deprived breast cancer xenografts to antiestrogens. Clin Cancer Res. 2007;13:2751–2757. doi: 10.1158/1078-0432.CCR-06-2466. [DOI] [PubMed] [Google Scholar]
- 124.Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, Wong J, Allred DC, Clark GM, Schiff R. Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst. 2003;95:353–361. doi: 10.1093/jnci/95.5.353. [DOI] [PubMed] [Google Scholar]
- 125.Lahusen T, Henke RT, Kagan BL, Wellstein A, Riegel AT. The role and regulation of the nuclear receptor co-activator AIB1 in breast cancer. Breast Cancer Res Treat. 2009;116:225–237. doi: 10.1007/s10549-009-0405-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hu ZZ, Kagan BL, Ariazi EA, Rosenthal DS, Zhang L, Li JV, Huang H, Wu C, Jordan VC, Riegel AT, Wellstein A. Proteomic analysis of pathways involved in estrogen-induced growth and apoptosis of breast cancer cells. PLoS One. 2011;6:e20410. doi: 10.1371/journal.pone.0020410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Haddow A, Watkinson JM, Paterson E. Influence of synthetic oestrogens upon advanced malignant disease. Br Med J. 1944;2:393–398. doi: 10.1136/bmj.2.4368.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ingle JN, Ahmann DL, Green SJ, Edmonson JH, Bisel HF, Kvols LK, Nichols WC, Creagan ET, Hahn RG, Rubin J, Frytak S. Randomized clinical trial of diethylstilbestrol versus tamoxifen in postmenopausal women with advanced breast cancer. N Engl J Med. 1981;304:16–21. doi: 10.1056/NEJM198101013040104. [DOI] [PubMed] [Google Scholar]
- 129.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 130.Nahta R, Esteva FJ. Bcl-2 antisense oligonucleotides: A potential novel strategy for the treatment of breast cancer. Semin Oncol. 2003;30:143–149. doi: 10.1053/j.seminoncol.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 131.Song RX, Zhang Z, Mor G, Santen RJ. Down-regulation of Bcl-2 enhances estrogen apoptotic action in long-term estradiol-depleted ER(+) breast cancer cells. Apoptosis. 2005;10:667–678. doi: 10.1007/s10495-005-1903-2. [DOI] [PubMed] [Google Scholar]
- 132.Zhou H, Zhang Y, Fu Y, Chan L, Lee AS. Novel mechanism of anti-apoptotic function of 78-kDa glucose-regulated protein (GRP78): endocrine resistance factor in breast cancer, through release of B-cell lymphoma 2 (BCL-2) from BCL-2-interacting killer (BIK) J Biol Chem. 2011;286:25687–25696. doi: 10.1074/jbc.M110.212944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Osipo C, Meeke K, Cheng D, Weichel A, Bertucci A, Liu H, Jordan VC. Role for HER2/neu and HER3 in fulvestrant-resistant breast cancer. Int J Oncol. 2007;30:509–520. [PubMed] [Google Scholar]
- 134.Greene GL, Nolan C, Engler JP, Jensen EV. Monoclonal antibodies to human estrogen receptor. Proc Natl Acad Sci U S A. 1980;77(9):5115–5119. doi: 10.1073/pnas.77.9.5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Greene GL, Fitch FW, Jensen EV. Monoclonal antibodies to estrophilin: probes for the study of estrogen receptors. Proc Natl Acad Sci U S A. 1980;77:157–161. doi: 10.1073/pnas.77.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Poulsen HS, Ozzello L, King WJ, Greene GL. The use of monoclonal antibodies to estrogen receptors (ER) for immunoperoxidase detection of ER in paraffin sections of human breast cancer tissue. J Histochem Cytochem. 1985;33:87–92. doi: 10.1177/33.2.2578501. [DOI] [PubMed] [Google Scholar]
- 137.DeSombre ER, Thorpe SM, Rose C, Blough RR, Andersen KW, Rasmussen BB, King WJ. Prognostic usefullness of estrogen receptor immunocytochemical assays for human breast cancer. Cancer Res. 1986;46:4256s–4264s. [PubMed] [Google Scholar]
- 138.Bevitt DJ, Milton ID, Piggot N, Henry L, Carter MJ, Toms GL, Lennard TW, Westley B, Angus B, Horne CH. New monoclonal antibodies to oestrogen and progesterone receptors effective for paraffin section immunohistochemistry. J Pathol. 1997;183:228–232. doi: 10.1002/(SICI)1096-9896(199710)183:2<228::AID-PATH895>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 139.King WJ, Greene GL. Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature. 1984;307:745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- 140.Ford CH, Al-Bader M, Al-Ayadhi B, Francis I. Reassessment of estrogen receptor expression in human breast cancer cell lines. Anticancer Res. 2011;31:521–527. [PubMed] [Google Scholar]
- 141.Cao S, Hudnall SD, Kohen F, Lu LJ. Measurement of estrogen receptors in intact cells by flow cytometry. Cytometry. 2000;41:109–114. [PubMed] [Google Scholar]
- 142.Girdler F, Browell DA, Cunliffe WJ, Shenton BK, Hemming JD, Scorer P, Young JR, Brotherick I. Use of the monoclonal antibody DAKO-ERbeta (8D5-1) to measure oestrogen receptor beta in breast cancer cells. Cytometry. 2001;45:65–72. doi: 10.1002/1097-0320(20010901)45:1<65::aid-cyto1145>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 143.Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, Staub A, Jensen E, Scrace G, Waterfield M. Cloning of the human estrogen receptor cDNA. Proc Natl Acad Sci U S A. 1985;82:7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986;231(4742):1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- 145.Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 146.Catania C, Ascione G, Adamoli L, De Pas T, Medici M, Franceschelli L, Verri E, Magni E, Sanna G, Torrisi R, Goldhirsch A, Nolè F. Fulvestrant in heavily pre-treated patients with advanced breast cancer: results from a single compassionate use programme centre. Breast Cancer Res Treat. 2007;106:97–103. doi: 10.1007/s10549-006-9481-8. [DOI] [PubMed] [Google Scholar]
- 147.Jordan VC, Allen KE. Evaluation of the antitumor activity of the non-steroidal antioestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma model. Eur J Cancer. 1980;16:239–251. doi: 10.1016/0014-2964(80)90156-5. [DOI] [PubMed] [Google Scholar]
- 148.Brodie AM, Schwarzel WC, Shaikh AA, Brodie HJ. The effect of an aromatase inhibitor, 4-hydroxy-4-androstene-3,17-dione, on estrogen-dependent processes in reproduction and breast cancer. Endocrinol. 1977;100:1684–1695. doi: 10.1210/endo-100-6-1684. [DOI] [PubMed] [Google Scholar]
- 149.Brodie AM, Marsh DA, Wu JT, Brodie HJ. Aromatase inhibitors and their use in controlling oestrogen-dependent processes. J Steroid Biochem. 1979;11:107–112. doi: 10.1016/0022-4731(79)90283-8. [DOI] [PubMed] [Google Scholar]
- 150.Brodie AM, Garrett WM, Hendrickson JR, Tsai-Morris CH. Effects of aromatase inhibitor 4-hydroxyandrostenedione and other compounds in the 7,12-dimethylbenz(a)anthracene-induced breast carcinoma model. Cancer Res. 1982;42:3360s–3364s. [PubMed] [Google Scholar]
- 151.Jordan VC, Fenuik L, Allen KE, Cotton RC, Richardson D, Walpole AL, Bowler J. Structural derivatives of tamoxifen and oestradiol 3-methyl ether as potential alkylating antioestrogens. Eur J Cancer. 1981;17:193–200. doi: 10.1016/0014-2964(81)90036-0. [DOI] [PubMed] [Google Scholar]
- 152.Bucourt R, Vignau M, Torelli V. New biospecific adsorbents for the purification of estradiol receptor. J Biol Chem. 1978;253:8221–8228. [PubMed] [Google Scholar]
- 153.Wakeling AE, Bowler J. Steroidal pure antioestrogens. J Endocrinol. 1987;112(3):R7–R10. doi: 10.1677/joe.0.112r007. [DOI] [PubMed] [Google Scholar]
- 154.Johnston S, Pippen J, Jr, Pivot X, Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A, Kennedy MJ, Press MF, Maltzman J, Florance A, O’Rourke L, Oliva C, Stein S, Pegram M. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J Clin Oncol. 2009;27:5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 155.Osborne CK, Neven P, Dirix LY, Mackey JR, Robert J, Underhill C, Schiff R, Gutierrez C, Migliaccio I, Anagnostou VK, Rimm DL, Magill P, Sellers M. Gefitinib or placebo in combination with tamoxifen in patients with hormone receptor-positive metastatic breast cancer: a randomized phase II study. Clin Cancer Res. 2011;17:1147–1159. doi: 10.1158/1078-0432.CCR-10-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cristofanilli M, Valero V, Mangalik A, Royce M, Rabinowitz I, Arena FP, Kroener JF, Curcio E, Watkins C, Bacus S, Cora EM, Anderson E, Magill PJ. Phase II, randomized trial to compare anastrozole combined with gefitinib or placebo in postmenopausal women with hormone receptor-positive metastatic breast cancer. Clin Cancer Res. 2010;16:1904–1914. doi: 10.1158/1078-0432.CCR-09-2282. [DOI] [PubMed] [Google Scholar]
- 157.Miller TW, Balko JM, Arteaga CL. Phosphatidylinositol 3-kinase and antiestrogen resistance in breast cancer. J Clin Oncol. 2011;29:4452–4461. doi: 10.1200/JCO.2010.34.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, Campone M, Kubista E, Greil R, Bianchi G, Steinseifer J, Molloy B, Tokaji E, Gardner H, Phillips P, Stumm M, Lane HA, Dixon JM, Jonat W, Rugo HS. Phase II Randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2630–2637. doi: 10.1200/JCO.2008.18.8391. [DOI] [PubMed] [Google Scholar]
- 159. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); Novartis Pharmaceuticals. Everolimus in Combination With Exemestane in the Treatment of Postmenopausal Women With Estrogen Receptor Positive Locally Advanced or Metastatic Breast Cancer Who Are Refractory to Letrozole or Anastrozole (BOLERO-2) 2000- [cited 2011 Nov 16]. Available from: http://clinicaltrials.gov/show/NCT00863655 NLM Identifier: NCT00863655. [Google Scholar]
- 160.Baselga J, Campone M, Piccart M, Burris HA, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2011 Dec 7; doi: 10.1056/NEJMoa1109653. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sabnis G, Schayowitz A, Goloubeva O, Macedo L, Brodie A. Trastuzumab reverses letrozole resistance and amplifies the sensitivity of breast cancer cells to estrogen. Cancer Res. 2009;69:1416–1428. doi: 10.1158/0008-5472.CAN-08-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dodwell D, Coombes G, Bliss JM, Kilburn LS, Johnston S SoFEA Trial Management Group. Combining fulvestrant (Faslodex) with continued oestrogen suppression in endocrine-sensitive advanced breast cancer: the SoFEA trial. Clin Oncol (R Coll Radiol) 2008;20:321–324. doi: 10.1016/j.clon.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 163.Swiss Group for Clinical Cancer Research. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); Trastuzumab and Letrozole in Treating Postmenopausal Women With Progressive Advanced Breast Cancer. 2000- [cited 2011 Nov 15]. Available from: http://clinicaltrials.gov/show/NCT00238290 NLM Identifier: NCT00238290. [Google Scholar]
- 164.Koeberle D, Ruhstaller T, Jost L, Pagani O, Zaman K, von Moos R, Oehlschlegel C, Crowe S, Pilop C, Thuerlimann B Swiss Group for Clinical Cancer Research (SAKK) Combination of trastuzumab and letrozole after resistance to sequential trastuzumab and aromatase inhibitor monotherapies in patients with estrogen receptor-positive, HER-2-positive advanced breast cancer: a proof-of-concept trial (SAKK 23/03) Endocr Relat Cancer. 2011;18:257–264. doi: 10.1530/ERC-10-0317. [DOI] [PubMed] [Google Scholar]
- 165.Massarweh S, Osborne CK, Creighton CJ, Qin L, Tsimelzon A, Huang S, Weiss H, Rimawi M, Schiff R. Tamoxifen resistance in breast tumors is driven by growth factor receptor signaling with repression of classic estrogen receptor genomic function. Cancer Res. 2008;68:826–833. doi: 10.1158/0008-5472.CAN-07-2707. [DOI] [PubMed] [Google Scholar]
- 166.Lonning PE, Taylor PD, Anker G, Iddon J, Wie L, Jorgensen LM, Mella O, Howell A. High-dose estrogen treatment in postmenopausal breast cancer patients heavily exposed to endocrine therapy. Breast Cancer Res Treat. 2001;67:111–116. doi: 10.1023/a:1010619225209. [DOI] [PubMed] [Google Scholar]
- 167.Sabnis GJ, Macedo LF, Goloubeva O, Schayowitz A, Brodie AM. Stopping treatment can reverse acquired resistance to letrozole. Cancer Res. 2008;68:4518–4524. doi: 10.1158/0008-5472.CAN-07-5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jordan VC, Ford LG. Paradoxical clinical effect of estrogen on breast cancer risk: a "new" biology of estrogen-induced apoptosis. Cancer Prev Res (Phila) 2011;4:633–637. doi: 10.1158/1940-6207.CAPR-11-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, Margolis KL, Stefanick ML, Brzyski R, Curb JD, Howard BV, Lewis CE, Wactawski-Wende J WHI Investigators. Health outcomes afters stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011;305:1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Heiser LM, Wang NJ, Talcott CL, Laderoute KR, Knapp M, Guan Y, Hu Z, Ziyad S, Weber BL, Laquerre S, Jackson JR, Wooster RF, Kuo WL, Gray JW, Spellman PT. Integrated analysis of breast cancer cell lines reveals unique signaling pathways. Genome Biol. 2009;10:R31. doi: 10.1186/gb-2009-10-3-r31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Heiser LM, Sadanandam A, Kuo WL, Benz SC, Goldstein TC, Ng S, Gibb WJ, Wang NJ, Ziyad S, Tong F, Bayani N, Hu Z, Billig JI, Dueregger A, Lewis S, Jakkula L, Korkola JE, Durinck S, Pepin F, Guan Y, Purdom E, Neuvial P, Bengtsson H, Wood KW, Smith PG, Vassilev LT, Hennessy BT, Greshock J, Bachman KE, Hardwicke MA, Park JW, Marton LJ, Wolf DM, Collisson EA, Neve RM, Mills GB, Speed TP, Feiler HS, Wooster RF, Haussler D, Stuart JM, Gray JW, Spellman PT. Subtype and pathway specific responses to anticancer compounds in breast cancer. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1018854108. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mendes-Pereira AM, Sims D, Dexter T, Fenwick K, Assiotis I, Kozarewa I, Mitsopoulos C, Hakas J, Zvelebil M, Lord CJ, Ashworth A. Genome-wide functional screen identifies a compendium of genes affecting sensitivity to tamoxifen. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1018872108. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu X, Ory V, Chapman S, Yuan H, Timofeeva OA, Albanese C, Haddad B, Rhim JS, Dritschilo A, Riegel A, McBride A, Schlegel R. ROCK inhibitor and feeder cells induce the conditional immortalization of epithelial cells. Am J Path. doi: 10.1016/j.ajpath.2011.10.036. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jordan VC, Fritz NF, Lagan-Fahey S, Thompson M, Tormey DC. Alteration of endocrine parameters in premenopausal women with breast cancer during long-term adjuvant therapy with tamoxifen as the single agent. J Natl Cancer Inst. 1991;83:1488–1491. doi: 10.1093/jnci/83.20.1488. [DOI] [PubMed] [Google Scholar]
- 175.Murdter TE, Schroth W, Bacchus-Gerybadze L, Winter S, Heinkele G, Simon W, Fasching PA, Fehm T, Eichelbaum M, Schwab M, Brauch H German Tamoxifen and AI Clinicians Group. Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther. 2011;89:708–717. doi: 10.1038/clpt.2011.27. [DOI] [PubMed] [Google Scholar]
- 176.Welshons WV, Wolf MF, Murphy CS, Jordan VC. Estrogenic activity of phenol red. Mol Cell Endocrinol. 1988;57:169–178. doi: 10.1016/0303-7207(88)90072-x. [DOI] [PubMed] [Google Scholar]