Abstract
Encapsulation complexes are assemblies in which a reversibly formed host more or less completely surrounds guest molecules. Host structures held together by hydrogen bonds have lifetimes in organic solvents of milliseconds to hours, long enough to directly observe the encapsulated guest by NMR spectroscopy. We describe here the action of alkyl ammonium compounds as guests that gather up to six molecules of the host module to form encapsulation complexes. The stoichiometry of the complexes—the largest hydrogen-bonded host capsules to date—is determined by the size and concentration of the guest.
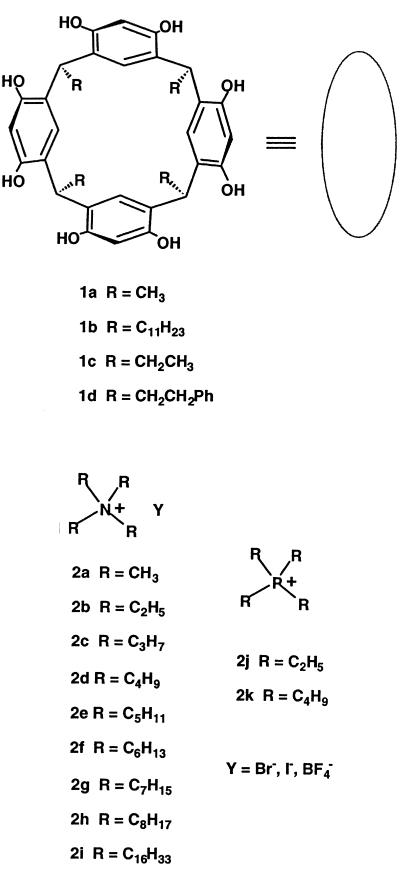
In 1997 Atwood and MacGillivray (1) reported the structure of resorcinarene 1a (Scheme S1) in the solid state and suggested its expanded possibilities for molecular recognition in solution (for a related structure see ref. 2). Such compounds had hardly been ignored before then: As early as 1982, their curvature and functional groups provided the skeleton of the first cavitand (3) and subsequently, from two such units, the carcerands (4, 5) and hydrogen-bonded capsules (6–9) were prepared. The single resorcinarene unit has recognition capabilities in its own right. In 1988 Aoyama (10, 11) described the formation of stoichiometric, 1:1 complexes of 1b with dicarboxylic acids, ribose, and even with terpenes, and steroids in organic solution (12). Later, Aoki (13), Rissanen and coworkers (14), and others (ref. 15; a related structure including disordered solvent was obtained from 1d) found dimeric capsules of 1c with alkyl ammonium guests in the solid state. But when the structure of 1a revealed it to be a spectacular, spherical hexamer, surrounding an enormous cavity, it became apparent that encapsulation of much larger guests and alternate stoichiometries might be possible with this host. To our knowledge, no such complexes have been described in solution, and we introduce them here.
Scheme 1.
Materials and Methods
The resorcinarenes 1b and 1d were prepared by known procedures (16). The quaternary ammonium and phosphonium guests were purchased from Aldrich and Fluka and used without further purification. The stock solutions of 1b in commercial CDCl3 were saturated by shaking with H2O before the complexation studies. All NMR spectra [one-dimensional 1H-, 19F-, 31P-, two-dimensional rotating-frame Overhauser effect spectroscopy (ROESY)] were recorded with Bruker DRX 600-MHz spectrometer by using the solvent signal as internal reference.
Results and Discussion
The earlier finding that quaternary ammonium guests are useful for the detection of other capsular hosts in the gas phase by electrospray ionization-MS (17) was applied to the characterization of assemblies from Scheme S1. These salts act as ionic labels for MS but show high affinity for deep cavitands even in aqueous solution (18). The π bonds that coat the inner surfaces of structures 1 provide a thin layer of negative charge that is complementary to quaternary ammonium salts. It seemed likely that the cation–π interactions could be recruited to generate and stabilize assemblies of Scheme S1. Water-saturated CDCl3, the widely accepted industrial standard for molecular recognition with synthetic receptors (19), was used as the solvent for the NMR studies.
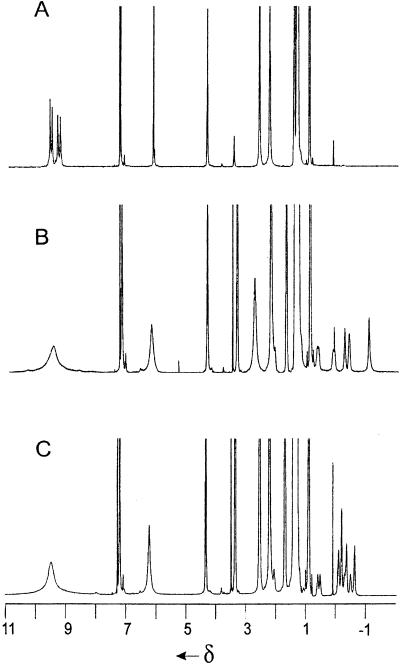
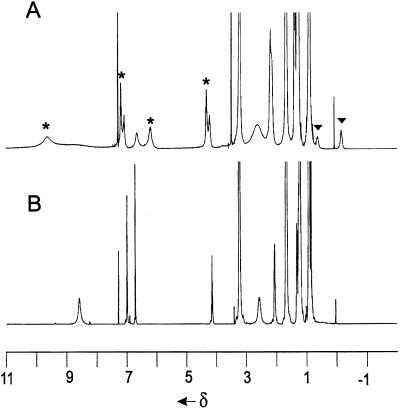
The 1H NMR spectrum of resorcinarene 1b in this solution (see Fig. 2) features the expected set of signals for the protons of resorcinol rings and methine bridges; these are in accord with a C4V-symmetric crown conformation. The multiplet of hydroxyl groups is centered at 9.6 ppm whereas the resonance for water is found at 3.3 ppm (20).
Figure 2.
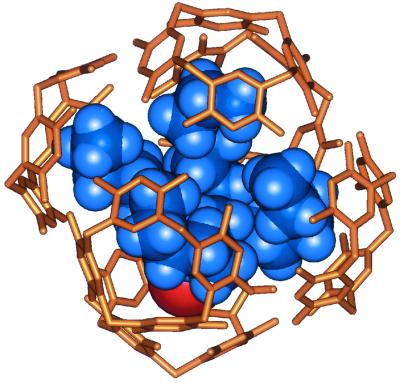
An energy-minimized structure of the ion pair 2g+Br− encapsulated within the hexameric host 1. The heptyl chains of the guest are folded such that their distal methylenes are nested in the resorcinarene cavities of the host. The aliphatic chains of 1 as well as the water molecules involved in the assembly have been deleted for viewing clarity.
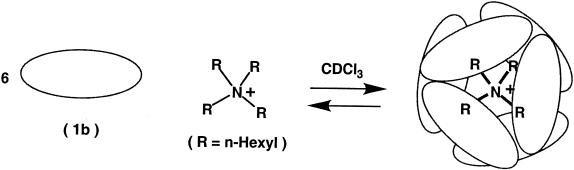
Addition of tetrahexylammonium bromide (2f+ Br−) to the solution of 1b results in the broadening of the signals for the OH groups, water, and the protons in 2-positions of the resorcinol rings (Fig. 1B). The large upfield shifts and the separate signals for bound and free guest are characteristic of encapsulation complexes; the guest exchange rates are slow on the NMR time scale (600 MHz, 295 K). Steric barriers isolate the ammonium salts from the bulk solution and numerous hydrogen bonds must be broken to allow the passage of molecules into and out of the cavity. The spectrum contains five broadened signals corresponding to the protons of complexed 2f+. These sharpen considerably as the temperature is raised to 313 K. The integration of the corresponding signals (the assignments were confirmed by two-dimensional correlated spectroscopy and ROESY experiments) clearly shows the 6:1 ratio between 1b and 2f+ guest. This ratio does not change upon further addition of the salt and is consistent with our formulating the structure of the complex as a self-assembled hexamer (1, 2) of 1b encapsulating one 2f+ (Scheme S2).
Figure 1.
The 1H-NMR spectra in CDCl3 saturated with H2O (600 MHz, 295 oK): 1b (A), 1b + 2f+ Br− (B), and 1b + 2g+ Br− (C).
Scheme 2.
The resonance of water is unchanged at 3.3 ppm and indicates that it, too, participates in the assembly. The ROESY spectrum shows exchange cross peaks between signals of the encapsulated and free cations. The poorly soluble 1d dissolves nicely in the presence of 2f+ Br−, and the NMR spectrum (not shown) again shows the guest in the hexameric host.
Analogous 6:1 complexes are formed between 1b and tetrapentyl, tetrabutyl, and tetrapropyl ammonium bromide as well as tetrabutylphosphonium bromide. In general, the longer aliphatic chains of the guests showed the most upfield shift for the protons of their methyl groups (Δδ = −1.3 to −2.5 ppm). This increased shielding of the methyl groups places them, on average, nearer to the concave surfaces of the resorcinarene faces of the hexameric assembly. The complex of 1b and tetraheptylammonium bromide proved an exception: The spectrum shows a complicated pattern from 0 to −0.8 ppm (Fig. 1C). The two-dimensional ROESY spectra revealed that the methyl protons of the complexed 2g+ emerge at −0.2 ppm (Δδ = −1.1 ppm) whereas the most upfield signal corresponds to the protons of the methylene groups. This feature suggests a hindered motion and folding of the tetraheptyl chains inside the cramped quarters. An energy minimized structure for this ion pair inside the hexameric host is shown in Fig. 2.
A very complex spectrum was observed for the tetraoctylammonium cation. Because the pattern depended on the concentration of guest, it was not possible to assign the signals of the encapsulated guest or accurately determine the host/guest stoichiometry. The tetrahexadecylammonium cation was not complexed under these conditions.
The volume of the cavity found for 1a6⋅8H2O in the crystalline state (1.4 mm3) (1, 2) is several times that of 2d+. This suggested encapsulation of the ion pair 2d+X− occurred in the cases discussed above (21). This was shown to be the case for 2d+. We compared the upfield portion of the NMR spectrum of encapsulated 2d+ I− in 1b with the corresponding BF4− salt. The spectra differ starkly, even though both salts make hexameric complexes. The 19F-NMR spectrum of the BF4− salt contains two signals at −148.5 and −151.5 ppm corresponding to the complexed and free BF4− anions, respectively. No complexes of 2d+ were observed with larger anions such as tosylates, BBu4− and BPh4−. Even with the counterion inside, there appears to be ample room for additional guests or solvent molecules within the capsules, and this proved to be true for the few cases examined. Specifically, addition of para-phenyl toluene to the complex of 2d+ Br− in the hexamer gave rise to a new set of NMR peaks corresponding to encapsulated para-phenyl toluene, as well as a shift of the upfield signals of the original guest (spectrum not shown). Accordingly, the spectra of Figs. 1 and 3A represent not only the encapsulated guest and counterions but also an unknown number of encapsulated solvent molecules.
Figure 3.
The 1H-NMR spectra in CDCl3 saturated with H2O (600 MHz, 295 oK): [1b] = 15 mM, [2c+] = 30 mM) (A) and [1b] = 15 mM, [2c+] = 60 mM (B). The signals of the hexameric capsule are marked with * and those of the guest with ▴.
The smaller salts, tetraethylammonium and phosphonium bromides showed a 1:2 stoichiometry consistent with a dimeric form of 1b surrounding the guest. The signal for the water protons was also shifted downfield (Δδ = 1.5 ppm), indicative of strong hydrogen bonding; no evidence could be obtained for encapsulation of secondary guests in the dimeric capsules. Tetramethylammonium cation was not complexed by 1b under these conditions.
The stability of the 1:6 complex 2c+Br− and 1b (Fig. 3A) depends on the amount of the salt present in solution. At [1b]/[2c+] = 0.5, a second set of signals emerges for the protons of the complex. At [1b]/[2c+] = 0.25, no signals could be detected for the 1:6 complex and the exchange of the guest is no longer slow on the NMR time scale at 313 K (Fig. 3B). Analogous behavior was observed for 2c-g+Br−, a behavior that suggests that the new complex is not a capsule; instead it appears to be the monomeric resorcinarene, probably hydrogen-bonded to the bromide counterion(s) (for the x-ray structures of this type of complexes, see refs. 22 and 23). It appears that bromide is the cause of this disassembly. In accordance with this interpretation it was found that the 1:6 complex with 2d+ iodide is much more stable in the presence of its excess salt than the complex with the corresponding bromide. The 1:6 complex of 1b with 2d+ BF4− also stayed intact in the presence of 10-fold molar excess of the guest.
The size of the cation also plays a role in the stability of the 1:6 complexes: In general, the capsules containing the larger cations are more stable to disassembly by their excess corresponding tetraalkylammonium bromides.
Conclusions
The behavior of the resorcinarene reveals it to be a library of receptor stoichiometries in solution, with the hexameric form the largest hydrogen-bonded assembly reported to date. Its properties are emergent, expressed as a function of guest structure and concentration. In this respect it resembles metal-ligand assemblies (for examples see refs. 24–28) in which the guest frequently determines which of several hosts are formed. For hydrogen-bonded capsules the multiplicity behavior of Scheme S1 is unique (29–32). It shows a diversity that testifies to the plasticity of the weak, intermolecular forces that drive molecular recognition phenomena.
Acknowledgments
We are grateful to the Skaggs Research Foundation and the National Institutes of Health for financial support, Professor Dmitry Rudkevich for advice, and Drs. Paul Wash and Laura Pasternak for NMR assistance. We thank Professors J. Atwood and L. MacGillivary for advice and crystallographic data.
Abbreviation
- ROESY
rotating-frame Overhauser enhancement spectroscopy
References
- 1.MacGillivray L R, Atwood J L. Nature (London) 1997;389:469–472. [Google Scholar]
- 2.Gerkensmeier T, Iwanek W, Agena C, Froelich R, Kotila S, Naether C, Mattay J. Eur. J. Org. Chem. 1999. 2257–2262. [Google Scholar]
- 3.Moran J R, Karbach S, Cram D J. J Am Chem Soc. 1982;104:5826–5828. [Google Scholar]
- 4.Sherman J C, Knobler C C, Cram D J. J Am Chem Soc. 1991;113:2194–2204. [Google Scholar]
- 5.Cram D J, Cram J M. Container Molecules and Their Guests. Cambridge, U.K.: R. Soc. Chem.; 1994. [Google Scholar]
- 6.Conn M M, Rebek J., Jr Chem Rev. 1997;97:1647–1668. doi: 10.1021/cr9603800. [DOI] [PubMed] [Google Scholar]
- 7.Sherman J C. Tetrahedron. 1995;51:3395–3422. [Google Scholar]
- 8.Heinz T, Rudkevich D M, Rebek J., Jr Nature (London) 1998;394:764–766. [Google Scholar]
- 9.Shivanyuk A, Paulus E F, Böhmer V. Angew Chem Int Ed. 1999;38:2906–2909. doi: 10.1002/(sici)1521-3773(19991004)38:19<2906::aid-anie2906>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 10.Aoyama Y, Tanaka Y, Toi H, Ogoshi H. J Am Chem Soc. 1988;110:634–635. [Google Scholar]
- 11.Aoyama Y, Tanaka Y, Sugahara S J. J Am Chem Soc. 1989;111:5397–5404. [Google Scholar]
- 12.Kobayashi K, Asakawa Y, Kikuchi Y, Toi H, Aoyama Y. J Am Chem Soc. 1993;115:2648–2654. [Google Scholar]
- 13.Murayama K, Aoki K. Chem. Commun. 1998. 607–608. [Google Scholar]
- 14.Shivanyuk A, Rissanen K, Kolehmainen E. Chem. Commun. 2000. 1107–1108. [DOI] [PubMed] [Google Scholar]
- 15.Rose K N, Barbour L J, Orr G W, Atwood J L. Chem. Commun. 1998. 407–408. [Google Scholar]
- 16.Tunstad L M, Tucker J A, Dalcanale E, Weiser J, Bryant J A, Sherman J C, Helgeson R C, Knobler C B, Cram D J. J Org Chem. 1989;54:1305–1312. [Google Scholar]
- 17.Schalley C A, Martin T, Obst U, Rebek J., Jr J Am Chem Soc. 1999;121:2133–2138. [Google Scholar]
- 18.Haino T, Rudkevich D M, Rebek J., Jr J Am Chem Soc. 1999;121:11253–11254. [Google Scholar]
- 19.Adrian J C, Jr, Wilcox C S. J Am Chem Soc. 1991;113:678–680. [Google Scholar]
- 20.Tanaka Y, Aoyama Y. Bull Chem Soc Jpn. 1990;63:3343–3344. [Google Scholar]
- 21.Lützen A, Renslo A R, Schalley C A, O'Leary B M, Rebek J., Jr J Am Chem Soc. 1999;121:7455–7456. [Google Scholar]
- 22.Murayama K, Aoki K. Chem. Commun. 1997. 119–120. [Google Scholar]
- 23.Lippmann T, Wilde, Pink A, Schäfer M, Hesse M, Mann G. Angew Chem Int Ed Engl. 1993;32:1195–1197. [Google Scholar]
- 24.Hasenknopf B, Lehn J-M, Kneisel B O, Baum G, Fenske D. Angew Chem Int Ed Engl. 1996;35:1838–1840. [Google Scholar]
- 25.Baxter P N W, Khoury R G, Lehn J-M, Baum G, Fenske D. Chem Eur J. 2000;6:4140–4148. doi: 10.1002/1521-3765(20001117)6:22<4140::aid-chem4140>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Beissel T, Powers R E, Parac T N, Raymond K N. J Am Chem Soc. 1999;121:4200–4206. [Google Scholar]
- 27.Umemoto K, Yamaguchi K, Fujita M. J Am Chem Soc. 2000;122:7150–7151. [Google Scholar]
- 28.Fujita M, Umemoto K, Yoshizawa M, Fujita N, Kusukawa T, Biradha K. Chem. Commun. 2001. 509–518. [Google Scholar]
- 29.Rivera J M, Martín T, Rebek J., Jr J Am Chem Soc. 1998;120:819–820. [Google Scholar]
- 30.Schalley C A, Rivera J M, Martín T, Santamaria J, Siuzdak G, Rebek J., Jr Eur J Org Chem. 1999;6:1325–1331. [Google Scholar]
- 31.Hof F, Nuckolls C, Craig S L, Martin T, Rebek J., Jr J Am Chem Soc. 2000;122:10991–10996. [Google Scholar]
- 32.Rivera J M, Craig S L, Martin T, Rebek J., Jr Angew Chem Int Ed Engl. 2000;39:2130–2132. doi: 10.1002/1521-3773(20000616)39:12<2130::aid-anie2130>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]