Abstract
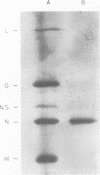
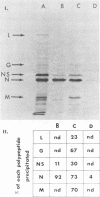
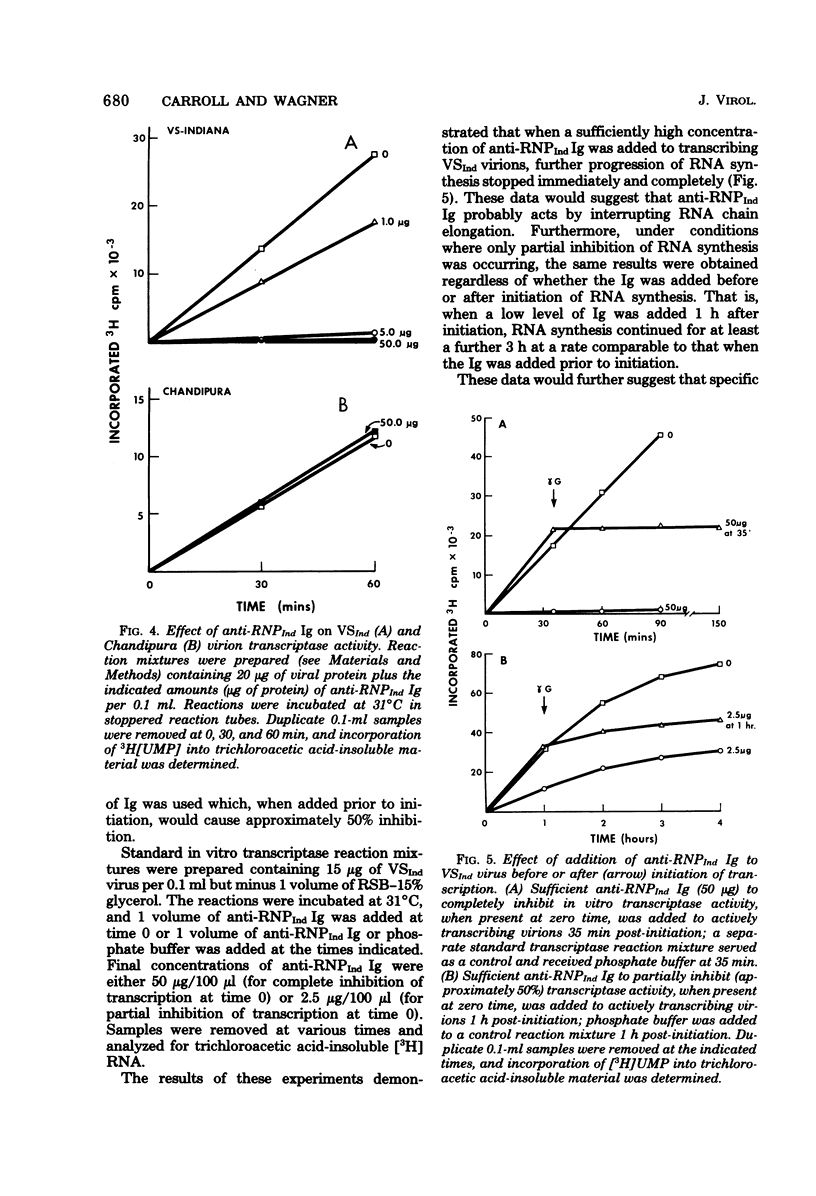
Specific antisera were raised by immunization of rabbits with purified nucleocapsids containing only RNA and N protein (ribonucleoprotein, RNP) obtained from vesicular stomatitis (VS) virions of the Indiana (VSInd) and the New Jersey (VSNJ) serotypes. The specificity of anti-RNPInd serum was demonstrated by selective precipitation of homotypic RNPInd devoid of L and NS proteins; anti-RNPInd serum also selectively precipitated soluble N protein present in cytoplasm of infected cells, but co-precipitated a limited amount of contaminating soluble NS protein. Immunoglobulins prepared from each homotypic antiserum markedly inhibited in vitro transcription of VSInd and VSNJ virions. Anti-RNPInd and anti-RNPNJ immunoglobulins also exhibited cross-reactivity by inhibiting transcription of heterotypic virions, but only to a much lesser degree than in the homotypic reaction. Anti-RNPInd immunoglobulin did not inhibit transcription of the antigenically unrelated Chandipura rhabdovirus, but anti-RNPNJ immunoglobulin did to a very limited extent. The transcription inhibitory activity of anti-RNPInd immunoglobulin was not dependent on RNP immunoprecipitation activity, which could be diluted out well before loss of antitranscriptase activity. Anti-RNPind immunoglobulin appeared to exert its effect on transcription by blocking elongation rather than initiation or reinitiation of RNA transcripts.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D., Huang A. S., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus, II. An RNA polymerase in the virion. Proc Natl Acad Sci U S A. 1970 Jun;66(2):572–576. doi: 10.1073/pnas.66.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A. D., Abraham G., Colonno R. J. Vesicular stomatitis virus: mode of transcription. J Gen Virol. 1977 Jan;34(1):1–8. doi: 10.1099/0022-1317-34-1-1. [DOI] [PubMed] [Google Scholar]
- Barenholz Y., Moore N. F., Wagner R. R. Enveloped viruses as model membrane systems: microviscosity of vesicular stomatitis virus and host cell membranes. Biochemistry. 1976 Aug 10;15(16):3563–3570. doi: 10.1021/bi00661a026. [DOI] [PubMed] [Google Scholar]
- Bishop D. H., Emerson S. U., Flamand A. Reconstitution of infectivity and transcriptase activity of homologous and heterologous viruses: vesicular stomatitis (Indiana serotype), Chandipura, vesicular stomatitis (New Jersey serotype), and Cocal viruses. J Virol. 1974 Jul;14(1):139–144. doi: 10.1128/jvi.14.1.139-144.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Roy P. Dissociation of vesicular stomatitis virus and relation of the virion proteins to the viral transcriptase. J Virol. 1972 Aug;10(2):234–243. doi: 10.1128/jvi.10.2.234-243.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Both G. W., Moyer S. A., Banerjee A. K. Translation and identification of the mRNA species synthesized in vitro by the virion-associated RNA polymerase of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1975 Jan;72(1):274–278. doi: 10.1073/pnas.72.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Brown F. Serological relationships between different strains of vesicular stomatis virus. J Gen Virol. 1972 Sep;16(3):391–398. doi: 10.1099/0022-1317-16-3-391. [DOI] [PubMed] [Google Scholar]
- Cullen S. E., Schwartz B. D. An improved method for isolation of H-2 and Ia alloantigens with immunoprecipitation induced by protein A-bearing staphylococci. J Immunol. 1976 Jul;117(1):136–142. [PubMed] [Google Scholar]
- Dubovi E. J., Wagner R. R. Spatial relationships of the proteins of vesicular stomatitis virus: induction of reversible oligomers by cleavable protein cross-linkers and oxidation. J Virol. 1977 May;22(2):500–509. doi: 10.1128/jvi.22.2.500-509.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Wagner R. R. Dissociation and reconstitution of the transcriptase and template activities of vesicular stomatitis B and T virions. J Virol. 1972 Aug;10(2):297–309. doi: 10.1128/jvi.10.2.297-309.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. M., Wagner R. R. Inhibition by aurintricarboxylic acid and polyethylene sulfonate of RNA transcription of vesicular stomatitis virus. J Virol. 1975 Nov;16(5):1146–1153. doi: 10.1128/jvi.16.5.1146-1153.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imblum R. L., Wagner R. R. Inhibition of viral transcriptase by immunoglobulin directed against the nucleocapsid NS protein of vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1357–1366. doi: 10.1128/jvi.15.6.1357-1366.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Y., Prevec L. Proteins of vesicular stomatitis virus. II. Immunological comparisons of viral antigens. J Virol. 1970 Jul;6(1):20–27. doi: 10.1128/jvi.6.1.20-27.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Naito S., Ishihama A. Function and structure of RNA polymerase from vesicular stomatitis virus. J Biol Chem. 1976 Jul 25;251(14):4307–4314. [PubMed] [Google Scholar]
- Rhodes D. P., Moyer S. A., Banerjee A. K. In vitro synthesis of methylated messenger RNA by the virion-associated RNA polymerase of vesicular stomatitis virus. Cell. 1974 Dec;3(4):327–333. doi: 10.1016/0092-8674(74)90046-4. [DOI] [PubMed] [Google Scholar]
- Villarreal L. P., Holland J. J. Synthesis of poly(A) in vitro by purified virions of vesicular stomatitis virus. Nat New Biol. 1973 Nov 7;246(149):17–19. doi: 10.1038/newbio246017a0. [DOI] [PubMed] [Google Scholar]