Abstract
Purpose
There are limited data on serum 25-hydroxyvitamin D [25(OH)D] and bone measures in men of African ancestry. To better understand racial differences in vitamin D status and bone health a cross-sectional study among 446 Caucasian men in the US and 496 men of African ancestry in Tobago (aged ≥65 years) was conducted.
Methods
Serum 25(OH)D (liquid chromatography and tandem mass spectrometry) was measured and peripheral quantitative computed tomography (pQCT) scans were administered. Bone measures estimated included trabecular and cortical volumetric bone mineral density (vBMD), bone mineral content (BMC), bone geometry (cross-sectional area and cortical thickness), and polar and axial strength strain indices (SSIp and SSIx).
Results
Men of African ancestry had higher 25(OH)D than Caucasians (34.7 vs. 27.6 ng/ml, p<0.01). Among Caucasians, 25(OH)D was positively (p trend <0.05) associated with cortical vBMD, total BMC, cortical thickness, SSIp and SSIx at the distal radius after adjustment for potential confounders. Similar patterns were observed at the distal tibia. In contrast, in men of African ancestry, there was an inverse association (p trend<0.05) between 25(OH)D and cross-sectional area, and SSIx. Race modified (p for interaction<0.05) the association between 25(OH)D and total BMC, cross-sectional area, SSIp, SSIx, and trabecular vBMD of the radius. In men of African ancestry, there was evidence of a threshold effect (at approximately 18 ng/ml) for 25(OH)D on tibial total BMC and cortical thickness.
Conclusions
More studies are needed to better comprehend these race differences for 25(OH)D and bone density, geometry and indices of bone strength.
Keywords: 25(OH)D, pQCT, men, radius, tibia, vBMD
Introduction
Serum 25-hydroxyvitamin D (25(OH)D) is an indicator of vitamin D nutritional status[1] and decreases with advancing age[2]. The possible mechanisms for the age related loss in serum 25 (OH)D include lower vitamin D intake, decreased vitamin D absorption, limited sun exposure and decreased cutaneous synthesis of vitamin D[3]. Vitamin D deficiency has a profound impact on bone growth and bone mineralization in young adults and on bone loss in older adults[4].
Low bone mineral density (BMD) and osteoporosis are growing clinical and public health problems among older men[5]. Positive[2, 3, 6–10] and null[11–13] associations between serum 25(OH)D concentrations and areal bone mineral density (aBMD) have been reported in men. However, limitations of past studies include small sample sizes, inadequate adjustment for potential confounders, reliance on dual X-ray absorptiometry (DXA) assessments of aBMD, and the use of unreliable radioimmunoassay methods to measure serum 25(OH)D levels. For example, Binkley et al. found markedly different serum 25(OH)D values after comparing radioimmunoassay (RIA) and competitive binding protein assays to high performance liquid chromatography (HPLC)[14]. The use of RIA or competitive binding protein assays have been shown to overestimate 25(OH)D levels and have poorer reliability compared to HPLC and liquid chromatography tandem mass spectrometry (LC-MS/MS) methods[15, 16]. Moreover, most studies have focused on Caucasian men; there are limited data on serum 25(OH)D concentrations and BMD and skeletal geometry in men of African ancestry residing in the Caribbean.
In the current study, we determined the associations between serum 25(OH)D levels measured using LC-MS/MS and bone mineral content (BMC), trabecular and cortical volumetric BMD, and bone structural geometry at the radius and tibia among older men of Caucasian and African descent. We hypothesize that associations of serum 25(OH)D status and measures of bone quality will differ by race after adjusting for potential confounders.
Methods
Study Population
The Osteoporotic Fractures in Men Study (MrOS) initially recruited 5995 men ages 65 and older from March 2000 through April 2002 as previously described[17, 18]. Men with a history of bilateral hip replacement and men who were unable to walk without the assistance of another person were excluded from MrOS. 5229 men (95.5% of survivors) completed some portion of the second visit between March 21st, 2005 and May 3rd, 2006. For the current 25(OH)D sub-study, the Pittsburgh center of MrOS was included for this analysis. A sample of 446 Caucasian subjects that had peripheral quantitative computed tomography (pQCT) measurements at visit 2 were selected to have their serum assayed for 25(OH)D.
Between 1997 and 2003, 3,170 men aged 40 years or older were recruited for prostate cancer screening study on the Island of Tobago[19]. Age-eligible men had to be ambulatory, non-institutionalized and not terminally ill. Between 2004 and 2007, 2482 men (70% of survivors) completed a second clinic visit. There were 618 Afro-Caribbean men aged ≥65 years (with all 4 grandparents of African ancestry) at this second visit. A random sample of 496 of these men were chosen for the sub-study 25(OH)D.
Our cross-sectional study compared 446 Caucasian men to 496 men of African ancestry. The Institutional Review Board (IRB) for the University of Pittsburgh and the Tobago Division of Health and Social Services approved the study protocol and written informed consent was obtained from all participants prior to data collection.
Measurement of 25(OH)D
Measures for 25(OH)D2 (derived from ergocalciferol) and 25(OH)D3 (derived from cholecalciferol) in both cohorts were performed simultaneously at the Mayo Clinic by conventional LC-MS/MS as previously described in serum collected after an overnight fast and frozen until assay[20]. Samples from both cohorts were included in each assay run. The minimum detectable limit for 25(OH)D2 was 4 ng/mL and for 25(OH)D3 was 2 ng/mL. The number of men with detectable 25(OH)D2 was 193 (20.5%), similar to a prior report.[3] Total 25(OH)D was quantified as the sum of 25(OH)D2 and 25(OH)D3. Reliability was assessed by measuring 46 duplicate 25(OH)D3 samples and the coefficient of variation (CV) was 5.8% for total 25(OH)D.
Peripheral QCT Measurements
QCT examinations at both sites were performed on the Stratec XCT 2000 scanner with voxel size of 0.5 mm and scan speed of 25 mm/s. Trabecular bone properties were measured by taking an ultra distal slice at 4% of the length of the radius and tibia. Variables measured at this site included total cross-sectional area (mm2), total BMC (mg/mm), trabecular vBMD (mg/cm3), and the polar (SSIp) and axial (SSIx) strength strain index (mm3). Cortical bone properties were measured at a region 33% of the length of the radius and tibia. Measurements at this site included cross-sectional area (mm2), total BMC (mg/mm), cortical vBMD (mg/cm3), cortical thickness (mm), and SSIp and SSIx (mm3). The strength strain indices denote the density-weighted polar or axial section moduli and reflect the torsional and bending rigidity of the long bone shaft. High quality assessment of bone mineral properties by pQCT was assured by minimizing participant movement. A quality assurance (QA) phantom scan was scanned daily and used to monitor the stability of each pQCT scanner. QA insured that density (attenuation) of the phantom did not change by more than +/− 0.5% which would be logged as a failure. A cross-calibration check was also performed between the centers. The European Forearm Phantom (EFP) was scanned 3 times at each site at 200, 100 and 50 mg/cm3, respectively. Coefficients of variation (CV) were determined by repeating pQCT scans on 15 subjects with repositioning (CV ≤ 2.1%)[21].
Potential Confounders
Questionnaires and clinical evaluations were administered by trained clinical staff[17]. Demographic, lifestyle, and medical characteristics were obtained entirely through self-report with the exception of diabetes. Men were classified as having diabetes if they self-reported a diagnosis of diabetes, took insulin, or hypoglycemic medications (baseline or visit 2), or had glucose levels ≥126 mg/dl after a minimum of an 8 hour fast.
All variables for MrOS with the exception of education (attended college vs. never attended college), history of fracture, and alcohol intake (drinks/week), dietary calcium (mg/day), supplemental calcium (yes or no), and health status (Excellent/good vs. fair/poor/very poor) were obtained at visit 2. Season of blood draw was coded as winter: January–March; spring: April–June; summer: July–September; fall: October–December. A calibrated balance beam scale was used to measure body weight in kilograms (kg). Participants wore indoor clothing and removed their shoes when their weight was being measured. Height was measured in centimeters (cm) using a Harpenden Stadiometer (Dyfed, UK) or wall mounted height board. BMI was calculated using weight (kg)/height/(m2). Self-report of current smoking (yes or no), caffeine intake (mg/day), and average time walking per day in previous 7 days (≤1 hour, >1 hour) were also determined. Dietary information on calcium and caffeine intake in Mr. OS was obtained using the modified Block Food Frequency[22]. Caffeine intake was assessed in the Tobago Bone Health Study by assuming that one cup of caffeinated coffee, tea or soda contained 95, 55 and 45mg of caffeine, respectively. Dietary calcium intake in this population was determined by frequency of selected food items including fish, bone chewing, green leafy vegetables, beans, milk, cheese, and cheese dishes that contain high dietary calcium and are frequently consumed in the local diet[21]. Subjects were asked to report if a doctor or health care provider informed them they had certain medical conditions including history of fracture, hypertension, myocardial infarction, osteoporosis, osteoarthritis and rheumatoid arthritis (RA).
Statistical Analysis
We defined 25(OH)D “deficiency” as <20 ng/ml, “insufficiency” as 20–<30 ng/ml, and “sufficiency” as ≥30 ng/ml based on previous reports[1]. Chi-square tests for categorical variables and analysis of variance (ANOVA) for continuous variables were performed to test if baseline characteristics differed by 25(OH)D status. The Kruskal-Wallis test was used if a continuous variable was not normally distributed. Race specific linear regression analysis was used to calculate least square means and examine associations between 25(OH)D and pQCT parameters. A Bonferroni correction was used to adjust for pairwise comparisons between 25(OH)D groups and pQCT parameters. Factors consistently associated with different bone parameters or those associated with 25(OH)D at p<0.05 were considered for inclusion in multivariable models. Interactions between race and vitamin D cutoffs were assessed to evaluate if the association between 25(OH)D and pQCT parameters differed by race. A test of linear trend across categories of 25(OH)D status was also performed based on the ordinal value for each category. To further explore these relationships, we performed spline analysis to test for possible thresholds and linearity for all bone parameters. Restricted cubic spline regression was used with knots at the 5th, 25th, 75th and a reference group at the 95th percentile was set to create the spline plot. Threshold effects were evaluated by identifying potential inflection points on the spline and performing a test of equality to determine if the slopes above and below the cut point were equal. If more than one cutoff was identified (p<0.05) we chose the 25(OH)D value with the lowest p-value as the threshold. Spline plots were displayed if 25(OH)D thresholds were found. Secondary analyses using 25(OH)D tertiles based on the distribution of the entire population were also conducted. Results did not differ when comparing 25(OH)D tertiles and clinical cutoffs (findings not shown to avoid redundancy). All statistical analyses were performed using the Statistical Analysis System (SAS, version 9.1; SAS Institute, Cary, NC).
Results
Men of African descent had greater serum 25(OH)D than Caucasians (34.7 vs. 27.6 ng/ml, p<0.001) (Table 1). Caucasians were older and had greater BMI, caffeine intake, and dietary calcium intake than men of African descent. Caucasian men were also significantly more likely to have a college education, take calcium supplements, report good/excellent health, history of a fracture, and hypertension. Men of African descent had a greater prevalence of diabetes than Caucasians (p<0.001).
Table 1.
Characteristics of men from the MrOs study and the Tobago study by race
| Caucasians (N=446) | African Ancestry (N=496) | P-value | |
|---|---|---|---|
| Serum 25(OH)D level, mean (SD), ng/ml | 27.6 ± 8.3 | 34.7 ± 9.7 | <0.001 |
| Age, mean (SD), yrs | 76.9 ± 4.9 | 71.6 ± 5.9 | <0.001 |
| BMI, mean (SD), kg/m2 | 28.5 ± 4.2 | 27.2 ± 9.1 | 0.009 |
| Season of blood draw, % | 0.006 | ||
| Winter | 24.2 | 31.6 | |
| Spring | 26.7 | 20.0 | |
| Summer | 24.9 | 20.4 | |
| Fall | 24.2 | 28.0 | |
| Attended college, % | 46.9 | 3.8 | <0.001 |
| Average walk time per day,% | |||
| ≤1 hour | 45.7 | 92.7 | |
| >1 hour | 53.3 | 7.3 | |
| Caffeine intake, median (IQR), mg/day | 184.0 (0.0–408.0) | 100.0 (45.0–150.0) | <0.001 |
| Alcohol, median (IQR), drinks/week | 0.5 (0.0–6.0) | 0.0 (0.0–1.0) | <0.001 |
| Smoking currently,% | 3.6 | 6.1 | 0.080 |
| Dietary calcium intake, mean (SD), mg/day | 793.5 ± 356 | 439.0 ± 209.8 | <0.001 |
| Supplemental calcium, % | 64.6 | 28.8 | <0.001 |
| Health good or excellent, % | 89.2 | 82.0 | 0.002 |
| History of any fracture, % | 54.7 | 13.0 | <0.001 |
| Diabetes, % | 19.7 | 34.1 | <0.001 |
| Hypertension, % | 52.9 | 46.2 | 0.041 |
| Myocardial Infarction, % | 16.1 | 1.4 | <0.001 |
| Osteoporosis, % | 3.6 | 3.1 | 0.716 |
| Osteoarthritis, % | 26.7 | 5.6 | <0.001 |
| Rheumatoid arthritis, % | 6.7 | 3.7 | 0.040 |
Table 2 shows characteristics by cutoffs of 25(OH)D. Caucasian men who were 25(OH)D sufficient had lower BMI, higher probability of taking calcium supplements, less likely to have RA and MI, and have their blood drawn in the summer than deficient men. Vitamin D sufficient men of African descent were less likely to have a history of a fracture than deficient men.
Table 2.
Characteristics of the men by clinical cutoffs of serum 25(OH)D (ng/ml)
| Caucasians | African Ancestery | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Deficient (<20) N=70 | Insufficient (20–<30) N=214 | Sufficient (≥30) N=162 | P-value | Deficient (<20) N=22 | Insufficient (20–<30) N=122 | Sufficient (≥30) N=352 | P-value | |
| Age, mean (SD), yrs | 77.5 ± 5.1 | 76.9 ± 4.8 | 76.7 ± 4.8 | 0.581 | 72.8 ± 7.5 | 72.1 ± 6.4 | 71.4 ± 5.7 | 0.339 |
| BMI, mean (SD), kg/m2 | 29.0 ± 4.6 | 28.8 ± 4.1 | 27.8 ± 4.0 | 0.022 | 27.1 ± 4.5 | 28.3 ± 4.9 | 26.9 ± 10.3 | 0.347 |
| Season of blood draw, % | 0.001 | 0.599 | ||||||
| Winter | 38.6 | 25.2 | 16.7 | 40.9 | 36.1 | 29.5 | ||
| Spring | 21.4 | 31.3 | 22.8 | 22.7 | 18.9 | 20.2 | ||
| Summer | 14.3 | 23.4 | 31.5 | 13.7 | 22.1 | 20.2 | ||
| Fall | 25.7 | 20.1 | 29.0 | 22.7 | 22.9 | 30.1 | ||
| Attended college, % | 48.6 | 50.9 | 40.7 | 0.139 | 4.6 | 5.7 | 3.1 | 0.346 |
| Average walk time per day,% | 0.606 | 0.603 | ||||||
| ≤1 hour | 55.7 | 43.9 | 43.8 | 95.5 | 82.0 | 85.0 | ||
| >1 hour | 44.3 | 56.1 | 56.2 | 4.5 | 18.0 | 15.0 | ||
| Caffeine intake, median (IQR), mg/day | 272.0 (136.0–408.0) | 192.0 (0.0–368.0) | 140.0 (0.0–408.0) | 0.164 | 75.0 (45.0–150.0) | 100.0 (45.0–150.0) | 100.0 (45.0–150.0) | 0.682 |
| Alcohol, median (IQR), drinks/week | 0.5 (0.0–4.0) | 0.6 (0.0–6.0) | 0.5 (0.0–6.0) | 0.706 | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.0 (0.0–1.0) | 0.289 |
| Smoking currently, % | 5.7 | 2.3 | 4.3 | 0.326 | 9.1 | 4.9 | 6.3 | 0.609 |
| Dietary calcium, mean (SD), mg/day | 727.0 ± 333.5 | 792.6 ± 360.7 | 823.4 ± 357.3 | 0.167 | 389.2 ± 198.3 | 443.1 ± 201.2 | 440.7 ± 213.5 | 0.520 |
| Supplemental calcium, % | 44.3 | 65.0 | 72.8 | 0.002 | 23.8 | 31.3 | 28.3 | 0.730 |
| Health good or excellent, % | 84.3 | 89.7 | 90.7 | 0.330 | 81.8 | 82.0 | 82.1 | 0.999 |
| History of any fracture, % | 55.7 | 51.4 | 58.6 | 0.371 | 27.3 | 8.4 | 13.7 | 0.042 |
| Diabetes, % | 24.3 | 19.6 | 17.9 | 0.532 | 31.8 | 36.1 | 33.5 | 0.855 |
| Hypertension, % | 61.4 | 51.9 | 50.6 | 0.290 | 47.3 | 42.2 | 47.3 | 0.525 |
| Myocardial Infarction, % | 15.7 | 21.0 | 9.9 | 0.014 | 4.6 | 0.8 | 1.4 | 0.329 |
| Osteoporosis, % | 4.3 | 4.2 | 2.5 | 0.619 | 4.6 | 3.3 | 2.9 | 0.892 |
| Osteoarthritis, % | 27.1 | 25.7 | 27.8 | 0.899 | 18.2 | 4.2 | 5.3 | 0.051 |
| Rheumatoid arthritis, % | 14.3 | 5.1 | 5.6 | 0.023 | 4.6 | 5.8 | 2.9 | 0.204 |
Multivariate Association between 25(OH)D status and pQCT parameters at the radius
At the distal radius, Caucasian men with sufficient 25(OH)D had greater total BMC (132.4 vs. 125.7 mg/mm, p=0.044) and cortical thickness (3.32 vs. 3.11 mm, p=0.016) than deficient men (Table 3). Positive linear trends were observed between 25(OH)D level and cortical vBMD (p=0.042), total BMC content (p trend=0.007), cortical thickness (p trend=0.003) and SSIp (p trend=0.028) and SSIx (p trend=0.012). In men of African descent, 25(OH)D was negatively associated with cross-sectional area (p for trend=0.005) and there was a borderline inverse association for SSIp (p for trend=0.060) and SSIx (p for trend=0.048). At the ultra distal radius, there was no association between 25(OH)D and any skeletal parameters among men of either Caucasian or African descent.
Table 3.
Peripheral QCT skeletal measures by clinical cutoffs of serum 25(OH)D (ng/ml)
| Caucasians | African Descent | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Deficient (<20) N=70 Mean (SE) | Insufficient (20–<30) N=214 Mean (SE) | Sufficient (≥30) N=162 Mean (SE) | P for trend | Deficient (<20) N=22 Mean (SE) | Insufficient (20–<30) N=122 Mean (SE) | Sufficient (≥30) N=352 Mean (SE) | P for trend | P interaction race and serum 25 (OH)D | |
| Distal radius (33%) | |||||||||
| Cortical vBMD (mg/cm3) | 1149.3 (6.7) | 1151.6 (6.1) | 1158.3 (6.3) | 0.042 | 1200.7 (6.3) | 1199.6 (2.8) | 1203.9 (1.6) | 0.229 | 0.359 |
| Total BMC (mg/mm) | 125.7 (3.6) | 128.0 (3.3) | 132.4 (3.4)* | 0.007 | 149.4 (4.1) | 147.2 (1.8) | 144.5 (1.1) | 0.107 | 0.003 |
| Cortical thickness (mm) | 3.11 (0.10) | 3.19 (0.09) | 3.32 (0.09)* | 0.003 | 3.52 (0.09) | 3.52 (0.04) | 3.54 (0.02) | 0.670 | 0.054 |
| Total area (mm2) | 143.2 (4.1) | 143.4 (3.8) | 145.9 (3.9) | 0.291 | 161.6 (5.0) | 155.7 (2.2) | 150.5 (1.3) | 0.005 | 0.006 |
| SSIp (mm3) | 354.0 (14.4) | 363.3 (13.3) | 376.5 (13.7) | 0.028 | 436.4 (18.2) | 426.3 (8.1) | 412.1 (4.7) | 0.060 | 0.005 |
| SSIx (mm3) | 193.5 (7.9) | 200.0 (7.3) | 207.8 (7.5) | 0.012 | 243.2 (10.4) | 239.9 (4.6) | 230.4 (2.7) | 0.048 | 0.002 |
| Ultra distal radius (4%) | |||||||||
| Trabecular vBMD (mg/cm3) | 194.0 (9.0) | 182.5 (8.3) | 181.6 (8.5) | 0.118 | 187.5 (11.9) | 201.2 (5.3) | 204.9 (3.0) | 0.170 | 0.015 |
| Total BMC (mg/mm) | 126.1 (4.8) | 122.6 (4.5) | 127.9 (4.6) | 0.310 | 139.7 (5.4) | 142.1 (2.4) | 140.6 (1.4) | 0.792 | 0.480 |
| Cross-sectional area (mm2) | 361.9 (13.6) | 362.4 (12.6) | 371.8 (13.0) | 0.244 | 349.3 (13.0) | 354.3 (5.8) | 344.6 (3.3) | 0.217 | 0.109 |
| SSIp (mm3) | 493.8 (27.0) | 484.6 (25.0) | 516.6 (25.8) | 0.118 | 596.1 (30.2) | 589.3 (13.4) | 576.2 (7.7) | 0.327 | 0.079 |
| SSIx (mm3) | 267.9 (14.8) | 258.6 (13.7) | 275.1 (14.1) | 0.252 | 316.6 (16.6) | 320.9 (7.4) | 316.9 (4.3) | 0.754 | 0.328 |
| Distal tibia (33%) | |||||||||
| Cortical vBMD (mg/cm3) | 1122.0 (6.6) | 1124.6 (6.1) | 1129.8 (6.2) | 0.090 | 1173.3 (7.5) | 1166.4 (3.2) | 1168.6 (1.8) | 0.975 | 0.232 |
| Total BMC (mg/mm) | 378.4 (9.4) | 388.9 (8.7) | 396.4 (8.8)* | 0.012 | 403.9 (12.6) | 415.7 (5.4) | 413.4 (3.1) | 0.840 | 0.465 |
| Cortical thickness (mm) | 5.31 (0.14) | 5.33 (0.13) | 5.54 (0.13)** | 0.009 | 5.19 (0.18) | 5.37 (0.08) | 5.36 (0.04) | 0.595 | 0.314 |
| Cross-sectional area (mm2) | 461.8 (11.2) | 475.3 (10.3) | 474.4 (10.5) | 0.241 | 506.1 (17.7) | 515.9 (7.6) | 508.0 (4.3) | 0.559 | 0.525 |
| SSIp (mm3) | 2046.7 (71.7) | 2126.3 (66.3) | 2158.5 (67.3) | 0.053 | 2384.6 (107.8) | 2510.7 (46.3) | 2458.8 (26.3) | 0.800 | 0.500 |
| SSIx (mm3) | 1278.5 (46.3) | 1333.7 (42.8) | 1350.9 (43.5) | 0.060 | 1463.0 (73.4) | 1549.2 (31.5) | 1513.8 (17.9) | 0.783 | 0.382 |
| Ultra distal tibia (4%) | |||||||||
| Trabecular vBMD (mg/cm3) | 224.5 (7.8) | 222.3 (7.1) | 228.7 (7.2) | 0.282 | 224.0 (10.4) | 233.4 (4.5) | 226.3 (2.6) | 0.475 | 0.383 |
| Total BMC (mg/mm) | 359.1 (12.2) | 359.9 (11.2) | 369.3 (11.3) | 0.173 | 374.0 (14.0) | 380.5 (6.0) | 370.4 (3.4) | 0.277 | 0.215 |
| Cross-sectional area (mm2) | 1252.8 (34.7) | 1253.5 (31.9) | 1260.2 (32.3) | 0.731 | 1260.6 (40.3) | 1214.1 (17.2) | 1204.1 (9.9) | 0.225 | 0.298 |
| SSIp (mm3) | 2215.6(140.6) | 2273.6(129.3) | 2375.3(131.1) | 0.099 | 2536.4 (154.2) | 2606.1 (65.9) | 2526.7 (37.7) | 0.481 | 0.231 |
| SSIx (mm3) | 1189.7 (77.0) | 1193.3 (70.9) | 1242.0 (71.8) | 0.267 | 1264.3 (82.1) | 1322.8 (35.1) | 1270.1 (20.1) | 0.429 | 0.397 |
p ≤0.050 (Sufficient vs. deficient 25(OH)D, Bonferroni-adjusted )
p ≤0.050 (Sufficient vs. insufficient 25(OH)D, Bonferroni-adjusted)
All models adjusted for age, BMI, season of blood draw, walk time, caffeine intake, alcohol, smoking, dietary calcium, supplementary calcium, health status, history of fracture, diabetes, myocardial infarction, and rheumatoid arthritis
Multivariate Association between 25(OH)D status and pQCT parameters at the tibia
Caucasians with sufficient 25(OH)D had greater total BMC (396.4 vs. 378.4 mm/mg, p=0.037) and cortical thickness (5.54 vs. 5.33 mm, p=0.017) than deficient and insufficient men, respectively (Table 3). There were positive linear trends with total BMC (p=0.012) and cortical thickness (p for trend=0.009) in Caucasians. Serum 25(OH)D was not associated with any of the tibia measures in men of African descent. Also, no significant associations were found at the ultra distal region for men of either race.
Effect of race on the multivariate association between 25(OH)D status and pQCT parameters
Significant interactions were observed between race and 25(OH)D primarily at the distal radius. Race modified the association between 25(OH)D levels and total BMC (p for interaction=0.003), cross-sectional area (p for interaction=0.006), SSIp (p for interaction=0.005) and SSIx (p for interaction=0.002). The associations between 25(OH)D and total BMC, cross-sectional area, and SSIp and SSIx were positive among Caucasian men and null or negative among African ancestry men leading to the statistically significant interaction term. Race also significantly (p=0.015) modified the association between 25(OH)D and trabecular vBMD at the ultra distal radius. Caucasians had lower radial trabecular vBMD in the sufficient (181.6 ± 8.5 mg/cm3) and insufficient (182.5 ± 8.3 mg/cm3) groups when compared to the deficient (194.0 ± 9.0 mg/cm3) group.
Restricted Cubic Spline Analysis
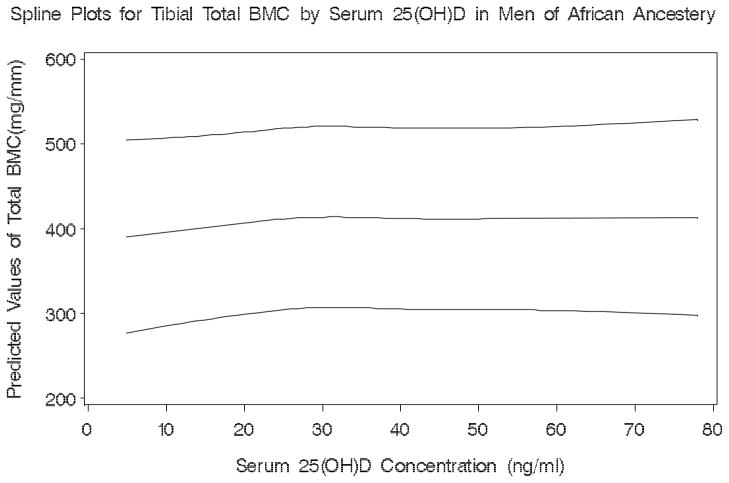
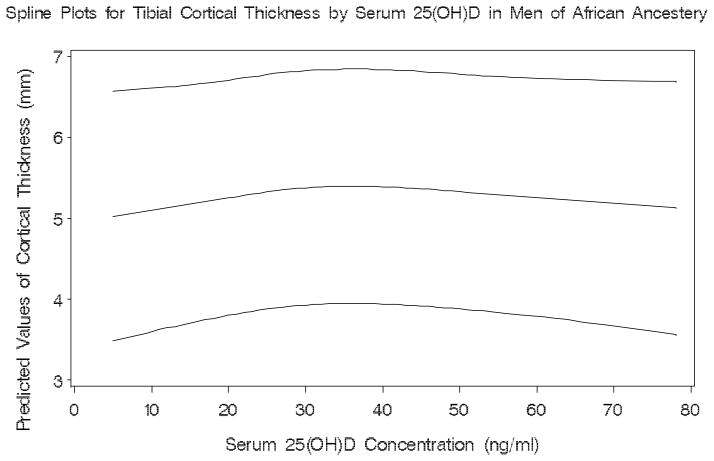
There was evidence of a threshold effect on tibial total BMC (p for test of non-equal slopes=0.026) at 19 ng/ml of serum 25(OH)D among men of African descent (Figure 1). For tibial cortical thickness a threshold (p for test of non-equal slopes=0.011) was also identified for serum 25(OH)D at 18 ng/ml (Figure 2). Among Caucasians, we observed no significant thresholds for any of the bone parameters.
Figure 1.
Plot showing the predicted values and the lower and upper 95% confidence limits of tibial total BMC by serum 25(OH)D in men of African Ancestery
Spline Plots for Tibial Total BMC by Serum 25(OH)D in Men of African Ancestery
Figure 2.
Plot showing the predicted values and the lower and upper 95% confidence limits of tibial cortical thickness by serum 25(OH)D in men of African ancestery
Spline Plots for Tibial Cortical Thickness by Serum 25(OH)D in Men of African Ancestery
Discussion
There are limited data available on the relationship between 25(OH)D and bone quality measures in men of African ancestry. Among community-dwelling older men, we found that the association between serum 25(OH)D levels and pQCT measures differed by race. Among Caucasians residing in the US, there were significant associations of 25(OH)D with cortical vBMD, BMC, cortical thickness, SSIp, and SSIx. However, in men of African descent residing in the Caribbean, associations noted between 25(OH)D and pQCT parameters were primarily null. The distribution of serum 25(OH)D varied substantially by race and few men of African descent were 25(OH)D deficient. Thus, it is imperative to interpret these findings with caution.
The statistically significant interactions that we observed suggest that race may modify the association between 25(OH)D and these skeletal parameters. In Caucasian men, the associations were largely positive between 25(OH)D and total BMC, cross-sectional area, and SSI at the distal radius, while in the African ancestry men these associations were either null or negative. We also found that Caucasian men with 25(OH)D sufficiency had lower trabecular vBMD than deficient men. On the other hand, men of African descent who were 25(OH)D sufficient had greater trabecular vBMD than those who were deficient. A previous study did not find a significant race by 25(OH)D interaction effect on measures of aBMD at the hip, lumbar spine, or radius among Caucasian, African American, and Hispanic men.[6] However, the men in this study were significantly younger (mean age 48 years) than those in our study possibly contributing to the different findings.
The mean serum concentration of 25(OH)D was substantially higher in the African ancestry compared with Caucasian men in our study. Possible explanations for this observation include a higher exposure to sunlight, lower BMI, and lower smoking and alcohol consumption among the men of African descent. African Americans typically have lower levels of serum 25(OH)D than Caucasians across all age groups and both genders due at least in part to differences in skin pigmentation and dietary vitamin D intake[2, 23]. However, mean 25(OH)D concentrations in the African continent are relatively high, but vary considerably according to geography, climate, and other factors[24]. There are limited data in other African ancestry populations, particularly in tropical climates where there is high sunexposure such as on the Caribbean island of Tobago where our subjects were recruited.
Several studies have examined the cross-sectional association of 25(OH)D and bone measures in men[2, 7–10, 25]. Similar to our findings, the MINOS study found that 25(OH)D levels measured with RIA were positively associated with cortical thickness (p<0.05) and BMC (p<0.01) at the femoral neck in older (ages 56–85) Caucasian men after adjusting for age, body weight, testosterone concentration, and season[9]. Similarly, the Rancho Bernardo study reported that 25(OH)D (measured with a competitive binding protein assay) was significantly and positively associated with aBMD at the hip, (p=0.01) and spine, (p=0.001) among 414 Caucasian men who had a mean age of 74 years. Conversely, another study that included 372 men aged 65–96, found that serum 25(OH)D (measured with RIA) was not associated with cortical vBMD in Caucasian men.
Fewer studies have examined the association of 25(OH)D with measures of skeletal health in ethnic minorities[6, 10]. The National Health and Nutritional Examination Survey (NHANES III) found a positive association between 25(OH)D measured by RIA and total hip aBMD in Caucasian, African American and Hispanic American men aged 50 years and older. However, the mean difference in aBMD between the highest and lowest quintile was larger in Caucasians (0.040 g/cm2, p<0.001) than men of African descent (0.024 g/cm2, p=0.03) after adjustment for potential confounders[10]. On the other hand, Hannan et al. found no association between 25(OH)D and hip, spine, and radius aBMD among 331 black men in Massachusetts[6]. We also found no associations between 25(OH)D and volumetric BMD in men of African descent. However, at the distal radius, men of African descent had lower cross-sectional area, SSIp and SSIx with greater 25(OH)D concentrations (although the associations were relatively weak). There is no clear explanation for why men of African descent would have lower bone size and strength with higher 25(OH)D. Men of African descent have been shown to be resistant to the resorptive effects of PTH[28]. Also, intermittent and chronic PTH regimens have been shown to stimulate osteoblastic bone formation at the periosteal surface and postpone osteoblast apoptosis resulting in greater cross-sectional area and cortical thickness and thus bone strength with treatment[26, 27].
The associations between 25(OH)D and bone measures were only observed at the distal (cortical) bone regions of the radius and tibia. Consistent with our findings, Lauretani et al. found that 25(OH)D was positively associated with tibial cortical vBMD, but not trabecular vBMD in women[25]. The mechanisms responsible for the differential associations of 25(OH)D with cortical versus trabecular bone are unclear. PTH excess has been shown to have primarily catabolic effects on cortical bone and maintain or even have anabolic effects on trabecular bone.[29] Several studies have shown that PTH administration and elevated serum PTH levels are associated with preserved trabecular bone structure[30–32]. In cortical bone, excess levels of PTH can result in increased endocortical resorption, cortical thinning and cortical porosity[30, 32–34].
Serum 25(OH)D thresholds were identified at approximately 18 ng/ml for total BMC and cortical thickness at the distal tibia in men of African ancestry. The associations of 25(OH)D with tibial total BMC and tibial cortical thickness appear to be positive and linear at values less than the threshold and null above these values. The biological mechanism is unclear for why these thresholds would be limited to the distal tibia, but not the distal radius in men of African descent. Our data, among men of African ancestry, showed a primarily negative linear association at the distal radius and no trends at the distal tibia. For thresholds to be identified on a plot a strong departure from linearity needs to be observed at a specific point. Among Caucasians, the strong linear positive associations between 25(OH)D and bone negated any prospect of finding significant thresholds. The optimal serum 25(OH)D concentration needed to maintain adequate bone density and geometry has not been established[35]. Optimal concentrations have been defined as those for which PTH levels plateau in the normal range[36]. However, this definition has led to the reporting of a wide range of 25(OH)D thresholds: from 8 to 46 ng/ml. A previous cross-sectional study based on aBMD levels found a threshold at 31 ng/ml with a target of 37 to 41 ng/ml for 25(OH)D for men and women aged ≥20 years[10]. Recent cohort studies in older men and women found that hip fracture risk was reduced among participants with 25(OH)D levels >20 ng/ml[35, 37]. In addition, men with 25(OH)D levels below 20 ng/ml experienced greater hip aBMD loss while rates were similar at higher levels[3]. All of this evidence suggests that a lower 25(OH)D threshold may exist for bone density and geometry than previously recognized
A recent report in the MrOS cohort found that the strongest predictor of non-vertebral fracture was SSIx (HR=2.2 per SD decrease)[38]. There was also a 70% increase in the risk of fracture per SD decrease in total BMC of the radius. The difference between 25(OH)D sufficiency and deficiency (Caucasians) for total BMC and SSIx in our study was approximately 0.4 SDs. A decrease in BMD of 0.5 SD can greatly increase the risk of an osteoporotic fracture in older adults[39].
Numerous potential confounders varied considerably by race. Substantial lifestyle and cultural differences were observed between the two population samples. Men of African descent had markedly lower calcium intake, alcohol intake, physical activity, education level, and caffeine intake compared to Caucasians. Therefore, we are unable to infer whether race modified the association between 25(OH)D and skeletal parameters based on strictly biological differences, lifestyle disparities, or a combination of both factors. In the Tobago cohort, dietary factors were not measured using a standardized questionnaire similar to Block’s FFQ in the MrOS cohort, therefore these measurements may not be as accurate. Our study adjusted for many factors; however residual confounding is an issue in observational studies and cannot be eliminated. Also, this study did not control for PTH (not measured in MrOS) which may mediate the association between 25(OH)D and bone strength. Finally, some of findings may have achieved statistical significance due to chance. However, our study also has several notable strengths including the use of a LC-MS/MS assay to measure serum 25(OH)D, adjustment for many potential confounders, a relatively large sample of men of African descent, and the use of pQCT to measure both cortical and trabecular bone, vBMD, bone geometry, and indices of bone strength. In contrast to DXA, pQCT can estimate vBMD and distinguish trabecular from cortical bone.
In summary, men of Caucasian and African descent differed in the association of serum 25(OH)D levels to several skeletal parameters. Levels of serum 25(OH)D were more likely to be associated with bone measures in Caucasians than men of African descent in our study. Thresholds for 25(OH)D were identified for men of African ancestry at approximately 18 ng/ml indicating that above this level there is minimal relationship with bone measures. The association between 25(OH)D and bone measures was more evident at the regions that primarily consist of cortical bone. Prospective studies that analyze the association of serum 25(OH)D with trabecular and cortical bone loss in men of different ethnicities are needed.
Acknowledgments
Source of Funding The Osteoporotic Fractures in Men (MrOS) Study is supported by National Institutes of Health funding. The following institutes provide support: the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute on Aging (NIA), the National Center for Research Resources (NCRR), and NIH Roadmap for Medical Research under the following grant numbers: U01 AR45580, U01 AR45614, U01 AR45632, U01 AR45647, U01 AR45654, U01 AR45583, U01 AG18197, U01-AG027810, and UL1 RR024140. The Tobago Bone Health Study was supported by NIAMS grant R01-AR049747 and National Cancer Institute grant R01-CA84950. American Diabetes Association (1-04-JF-46, Strotmeyer ES).
Footnotes
Conflict of Interest: All authors have no conflict of interest and nothing to disclose.
Contributor Information
Kamil E. Barbour, Email: barbourk@edc.pitt.edu.
Joseph M. Zmuda, Email: zmudaj@edc.pitt.edu.
Mara J Horwitz, Email: HorwMx@UPMC.EDU.
Elsa S. Strotmeyer, Email: strotmeyere@edc.pitt.edu.
Robert Boudreau, Email: boudreaur@edc.pitt.edu.
Rhobert W. Evans, Email: evansr@edc.pitt.edu.
Kristine E. Ensrud, Email: ensru001@umn.edu.
Christopher L. Gordon, Email: chrisgordon@sympatico.ca.
Moira A. Petit, Email: mpetit@umn.edu.
Alan L. Patrick, Email: apatrick_4127@yahoo.co.uk.
Jane A. Cauley, Email: jcauley@edc.pitt.edu.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Zadshir A, Tareen N, Pan D, Norris K, Martins D. The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis. 2005;15(4 Suppl 5):S5–101. [PubMed] [Google Scholar]
- 3.Ensrud KE, Taylor BC, Paudel ML, Cauley JA, Cawthon PM, Cummings SR, Fink HA, Barrett-Connor E, Zmuda JM, Shikany JM, Orwoll ES. Serum 25-hydroxyvitamin D levels and rate of hip bone loss in older men. J Clin Endocrinol Metab. 2009;94(8):2773–80. doi: 10.1210/jc.2008-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22(4):477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 5.Khosla S, Amin S, Orwoll E. Osteoporosis in men. Endocr Rev. 2008;29(4):441–64. doi: 10.1210/er.2008-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hannan MT, Litman HJ, Araujo AB, McLennan CE, McLean RR, McKinlay JB, Chen TC, Holick MF. Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J Clin Endocrinol Metab. 2008;93(1):40–6. doi: 10.1210/jc.2007-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuchuk NO, Pluijm SM, van Schoor NM, Looman CW, Smit JH, Lips P. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab. 2009;94(4):1244–50. doi: 10.1210/jc.2008-1832. [DOI] [PubMed] [Google Scholar]
- 8.Saquib N, von MD, Garland CF, Barrett-Connor E. Serum 25-hydroxyvitamin D, parathyroid hormone, and bone mineral density in men: the Rancho Bernardo study. Osteoporos Int. 2006;17(12):1734–41. doi: 10.1007/s00198-006-0191-1. [DOI] [PubMed] [Google Scholar]
- 9.Szulc P, Munoz F, Marchand F, Chapuy MC, Delmas PD. Role of vitamin D and parathyroid hormone in the regulation of bone turnover and bone mass in men: the MINOS study. Calcif Tissue Int. 2003;73(6):520–30. doi: 10.1007/s00223-002-2103-5. [DOI] [PubMed] [Google Scholar]
- 10.Bischoff-Ferrari HA, Dietrich T, Orav EJ, wson-Hughes B. Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study of younger and older adults. Am J Med. 2004;116(9):634–9. doi: 10.1016/j.amjmed.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Dennison E, Eastell R, Fall CH, Kellingray S, Wood PJ, Cooper C. Determinants of bone loss in elderly men and women: a prospective population-based study. Osteoporos Int. 1999;10(5):384–91. doi: 10.1007/s001980050244. [DOI] [PubMed] [Google Scholar]
- 12.Melin A, Wilske J, Ringertz H, Saaf M. Seasonal variations in serum levels of 25-hydroxyvitamin D and parathyroid hormone but no detectable change in femoral neck bone density in an older population with regular outdoor exposure. J Am Geriatr Soc. 2001;49(9):1190–6. doi: 10.1046/j.1532-5415.2001.49236.x. [DOI] [PubMed] [Google Scholar]
- 13.Hannan MT, Felson DT, wson-Hughes B, Tucker KL, Cupples LA, Wilson PW, Kiel DP. Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15(4):710–20. doi: 10.1359/jbmr.2000.15.4.710. [DOI] [PubMed] [Google Scholar]
- 14.Binkley N, Krueger D, Cowgill CS, Plum L, Lake E, Hansen KE, DeLuca HF, Drezner MK. Assay variation confounds the diagnosis of hypovitaminosis D: a call for standardization. J Clin Endocrinol Metab. 2004;89(7):3152–7. doi: 10.1210/jc.2003-031979. [DOI] [PubMed] [Google Scholar]
- 15.Lensmeyer GL, Wiebe DA, Binkley N, Drezner MK. HPLC method for 25-hydroxyvitamin D measurement: comparison with contemporary assays. Clin Chem. 2006;52(6):1120–6. doi: 10.1373/clinchem.2005.064956. [DOI] [PubMed] [Google Scholar]
- 16.Lips P, Chapuy MC, wson-Hughes B, Pols HA, Holick MF. An international comparison of serum 25-hydroxyvitamin D measurements. Osteoporos Int. 1999;9(5):394–7. doi: 10.1007/s001980050162. [DOI] [PubMed] [Google Scholar]
- 17.Blank JB, Cawthon PM, Carrion-Petersen ML, Harper L, Johnson JP, Mitson E, Delay RR. Overview of recruitment for the osteoporotic fractures in men study (MrOS) Contemp Clin Trials. 2005;26(5):557–68. doi: 10.1016/j.cct.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Orwoll E, Blank JB, Barrett-Connor E, Cauley J, Cummings S, Ensrud K, Lewis C, Cawthon PM, Marcus R, Marshall LM, McGowan J, Phipps K, Sherman S, Stefanick ML, Stone K. Design and baseline characteristics of the osteoporotic fractures in men (MrOS) study--a large observational study of the determinants of fracture in older men. Contemp Clin Trials. 2005;26(5):569–85. doi: 10.1016/j.cct.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Bunker CH, Patrick AL, Konety BR, Dhir R, Brufsky AM, Vivas CA, Becich MJ, Trump DL, Kuller LH. High prevalence of screening-detected prostate cancer among Afro-Caribbeans: the Tobago Prostate Cancer Survey. Cancer Epidemiol Biomarkers Prev. 2002;11(8):726–9. [PubMed] [Google Scholar]
- 20.Singh RJ, Taylor RL, Reddy GS, Grebe SK. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab. 2006;91(8):3055–61. doi: 10.1210/jc.2006-0710. [DOI] [PubMed] [Google Scholar]
- 21.Sheu Y, Cauley JA, Bunker CH, Wheeler VW, Patrick AL, Gordon CL, Kammerer CM, Zmuda JM. Correlates of trabecular and cortical volumetric BMD in men of African ancestry. J Bone Miner Res. 2009;24(12):1960–8. doi: 10.1359/JBMR.090522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Block G, Subar AF. Estimates of nutrient intake from a food frequency questionnaire: the 1987 National Health Interview Survey. J Am Diet Assoc. 1992;92(8):969–77. [PubMed] [Google Scholar]
- 23.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007;20(7):713–9. doi: 10.1016/j.amjhyper.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 24.Prentice A, Schoenmakers I, Jones K, Jarjou L, Goldberg G. Vitamin D Deficiency and Its Health Consequence in Africa. Clinical Reviews in Bone and Mineral Metabolism. 2009 doi: 10.1007/s12018-009-9038-6. Ref Type: Journal (Full) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lauretani F, Bandinelli S, Russo CR, Maggio M, Di IA, Cherubini A, Maggio D, Ceda GP, Valenti G, Guralnik JM, Ferrucci L. Correlates of bone quality in older persons. Bone. 2006;39(4):915–21. doi: 10.1016/j.bone.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindsay R, Zhou H, Cosman F, Nieves J, Dempster DW, Hodsman AB. Effects of a one-month treatment with PTH(1-34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. J Bone Miner Res. 2007;22(4):495–502. doi: 10.1359/jbmr.070104. [DOI] [PubMed] [Google Scholar]
- 27.Parfitt AM. Parathyroid hormone and periosteal bone expansion. Journal of Bone and Mineral Research. 2002;17(10):1741–3. doi: 10.1359/jbmr.2002.17.10.1741. [DOI] [PubMed] [Google Scholar]
- 28.Cosman F, Morgan DC, Nieves JW, Shen V, Luckey MM, Dempster DW, Lindsay R, Parisien M. Resistance to bone resorbing effects of PTH in black women. J Bone Miner Res. 1997;12(6):958–66. doi: 10.1359/jbmr.1997.12.6.958. [DOI] [PubMed] [Google Scholar]
- 29.Duan Y, De LV, Seeman E. Parathyroid hormone deficiency and excess: similar effects on trabecular bone but differing effects on cortical bone. J Clin Endocrinol Metab. 1999;84(2):718–22. doi: 10.1210/jcem.84.2.5498. [DOI] [PubMed] [Google Scholar]
- 30.Dempster DW, Muller R, Zhou H, Kohler T, Shane E, Parisien M, Silverberg SJ, Bilezikian JP. Preserved three-dimensional cancellous bone structure in mild primary hyperparathyroidism. Bone. 2007;41(1):19–24. doi: 10.1016/j.bone.2007.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone (1-34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res. 2003;18(11):1932–41. doi: 10.1359/jbmr.2003.18.11.1932. [DOI] [PubMed] [Google Scholar]
- 32.Parisien M, Silverberg SJ, Shane E, de la CL, Lindsay R, Bilezikian JP, Dempster DW. The histomorphometry of bone in primary hyperparathyroidism: preservation of cancellous bone structure. J Clin Endocrinol Metab. 1990;70(4):930–8. doi: 10.1210/jcem-70-4-930. [DOI] [PubMed] [Google Scholar]
- 33.Kotowicz MA, Klee GG, Kao PC, O’Fallon WM, Hodgson SF, Cedel SL, Eriksen EF, Gonchoroff DG, Judd HL, Riggs BL. Relationship between serum intact parathyroid hormone concentrations and bone remodeling in type I osteoporosis: evidence that skeletal sensitivity is increased. Osteoporos Int. 1990;1(1):14–22. doi: 10.1007/BF01880411. [DOI] [PubMed] [Google Scholar]
- 34.Silverberg SJ, Shane E, de la CL, Dempster DW, Feldman F, Seldin D, Jacobs TP, Siris ES, Cafferty M, Parisien MV. Skeletal disease in primary hyperparathyroidism. J Bone Miner Res. 1989;4(3):283–91. doi: 10.1002/jbmr.5650040302. [DOI] [PubMed] [Google Scholar]
- 35.Cauley JA, Parimi N, Ensrud KE, Bauer DC, Cawthon PM, Cummings SR, Hoffman AR, Shikany JM, Barrett-Connor E, Orwoll E. Serum 25-hydroxyvitamin D and the risk of hip and nonspine fractures in older men. J Bone Miner Res. 2010;25(3):545–53. doi: 10.1359/jbmr.090826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bischoff-Ferrari HA. The 25-hydroxyvitamin D threshold for better health. J Steroid Biochem Mol Biol. 2007;103(3–5):614–9. doi: 10.1016/j.jsbmb.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 37.Cauley JA, Lacroix AZ, Wu L, Horwitz M, Danielson ME, Bauer DC, Lee JS, Jackson RD, Robbins JA, Wu C, Stanczyk FZ, LeBoff MS, Wactawski-Wende J, Sarto G, Ockene J, Cummings SR. Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med. 2008;149(4):242–50. doi: 10.7326/0003-4819-149-4-200808190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheu Y, Zmuda JM, Boudreau RM, Petit MA, Ensrud KE, Bauer DC, Gordon CL, Orwoll ES, Cauley JA. Bone strength measured by peripheral quantitative computed tomography and the risk of non-vertebral fractures: The osteoporotic fractures in men (MrOS) study. J Bone Miner Res. 2010 doi: 10.1002/jbmr.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001;12(12):989–95. doi: 10.1007/s001980170006. [DOI] [PubMed] [Google Scholar]