Abstract
Development of effective new mucosal vaccine adjuvants has become a priority with the increase in emerging viral and bacterial pathogens. We previously reported that cationic liposomes complexed with non-coding plasmid DNA (CLDC) were effective parenteral vaccine adjuvants. However, little is known regarding the ability of liposome-nucleic acid complexes to function as mucosal vaccine adjuvants, or the nature of the mucosal immune responses elicited by mucosal liposome-nucleic acid adjuvants. To address these questions, antibody and T cell responses were assessed in mice following intranasal immunization with CLDC-adjuvanted vaccines. The effects of CLDC adjuvant on antigen uptake, trafficking, and cytokine responses in the airways and draining lymph nodes were also assessed. We found that mucosal immunization with CLDC-adjuvanted vaccines effectively generated potent mucosal IgA antibody responses, as well as systemic IgG responses. Notably, mucosal immunization with CLDC adjuvant was very effective in generating strong and sustained antigen-specific CD8+ T cell responses in the airways of mice. Mucosal administration of CLDC vaccines also induced efficient uptake of antigen by DCs within the mediastinal lymph nodes. Finally, a killed bacterial vaccine adjuvanted with CLDC induced significant protection from lethal pulmonary challenge with Burkholderia pseudomallei. These findings suggest that liposome-nucleic acid adjuvants represent a promising new class of mucosal adjuvants for non-replicating vaccines, with notable efficiency at eliciting both humoral and cellular immune responses following intranasal administration.
Keywords: innate immunity, T cell, pulmonary, dendritic cell, vaccine adjuvant
1. Introduction
Many pathogens attach to or invade mucosal surfaces and mucosal immunity is often the key to controlling initial infections with such pathogens. Mucosal immune responses are typically generated most efficiently when vaccines are administered mucosally, though the majority of vaccines today are administered parenterally [1–4]. Indeed, only a few mucosal vaccines have been approved for human use, including poliovirus, influenza, rotavirus, Salmonella typhi, and Vibrio cholera vaccines [1, 5].
Currently, most mucosal vaccines are prepared using live, attenuated organisms [6–7]. Though effective, such vaccines are costly to prepare, require careful attention to storage conditions, and pose some potential risk to immunosuppressed individuals. Therefore, there is continued interest in the development of effective, non-replicating mucosal vaccines. However, most mucosal antigens are poorly immunogenic and require the use of potent mucosal vaccine adjuvants.
At present, several adjuvants have been used with non-replicating mucosal vaccines, including mutated cholera toxin and E. coli labile toxins, as well as synthetic TLR agonist, such as CpG oligodeoxynucleotides (ODN). [4–5, 8–11]. Cholera toxin (CT) adjuvants elicit strong humoral immunity following mucosal administration, though the risk of systemic toxicity and especially neurotoxicity renders current CT adjuvants generally unsuitable for use in human vaccines. A modified cholera toxin subunit B (CTB) adjuvant is relatively effective as a mucosal adjuvant and eliminates the risk of systemic toxicity. CpG ODN have been widely used as parenteral vaccine adjuvants and as effective mucosal vaccine adjuvants [5, 12–20]. Studies have shown that CpG ODN adjuvants potently activate innate immune responses by stimulating innate immune signaling via TLR9 [21–23]. While each of these adjuvants has certain desirable properties, there are also some characteristics about CTB and CpG that raise efficacy and safety concerns [24–28]. Therefore, there remains a need for more potent, more quickly acting, and potentially safer mucosal adjuvants.
Liposome-based mucosal adjuvants been thoroughly investigated, using a variety of different antigens [29–34]. The impact of mode of antigen association with the liposome (encapsulation, conjugation, and absorptions) and the physiochemical properties of the liposome (size, charge, lipid composition) on immune responses have also been studied [35]. At present, cationic liposomes are particularly advantageous as mucosal adjuvants due their ability to enhance the uptake of the vaccine by antigen presenting cells (APC) and to induce APC activation [36–38]. Indeed, numerous studies have shown that liposomes are essential to achieve efficient immune responses [34, 39–40]. Many liposome-based adjuvants can induce mucosal production of IgA, and some also induce systemic IgG production, but few have been shown to induce effective CD8+ T cell responses. Therefore, there is still a need of broadly effective mucosal vaccine adjuvants, capable of eliciting both humoral and cellular immune responses.
We previously reported that a vaccine adjuvant consisting of cationic liposome-DNA complexes (CLDC) effectively elicited balanced cellular and humoral immunity following parenteral administration [41]. We attribute a majority of the success of the CLDC adjuvanted parenteral vaccines to the combination of the liposome (carrier) and the plasmid DNA (immunostimulant). Combination vaccine adjuvants have recently become area of interest due to the synergistic effect of combining antigen delivery with potent stimulation of the innate immune system [42–43]. CLDC can be classified as a combination adjuvant, and the need for physical association of all three of the components of the CLDC-based vaccines has recently been shown in our laboratory. Mice immunized with Ova plus liposome alone or Ova plus plasmid DNA alone failed to generate significant immune responses [41]. The efficacy of CLDC-based vaccines for immunization against a variety of different antigens in several different species has also been reported, including studies in guinea pigs, woodchucks, and non-human primates, and more recently in normal human volunteers [44–49]. Moreover, recent studies in our laboratory have also revealed that intranasal administration of CLDC alone as an immune therapeutic could generate rapid, non-specific, innate immune protection against inhalational challenge with rapidly lethal bacterial pathogens including Burkholderia and Francisella [50–51].
Therefore, we wondered whether CLDC could also be used effectively as a mucosal vaccine adjuvant. To address this question, we investigated the mucosal adjuvant properties of CLDC combined with soluble protein antigens, delivered by the intranasal (i.n.) route. The ability of CLDC adjuvant to elicit humoral and cellular immune responses was investigated, and experiments were conducted to identify mucosal antigen presenting cells (APCs) responsible for antigen uptake and trafficking to regional lymph nodes. Finally, the ability of CLDC-adjuvanted vaccines to elicit protective immunity against serious pathogens was assessed in a model of lethal pulmonary Burkholderia pseudomallei challenge. In the course of these studies, we identified properties shared by CLDC adjuvants and other mucosal adjuvants, as well as properties unique to CLDC-based mucosal adjuvants.
2. Materials and Methods
2.1 Mice
Specific pathogen-free 6–8-week-old female C57BL/6, BALB/c, and ICR mice were purchased from the Jackson Laboratories (Bar Harbor, ME) or Harlan Laboratories (Indianapolis, IN). All protocols involving animal experiments described in this study were approved by Institutional Animal Care and Use Committee at Colorado State University.
2.2 Reagents and biochemicals
Ovalbumin was purchased from Sigma-Aldrich (St Louis, MO) and was prepared as a 1 mg/ml solution in diH2O. Fluorescent Alexa Fluor 647 ovalbumin was purchased from Invitrogen (San Diego, CA) and was resuspended in PBS at a concentration of 1mg/ml prior to use. All cell preparations were resuspended in complete RPMI (Invitrogen, Carlsbad, CA) containing 10% FBS (Gemini Bio-Products, West Sacramento, CA), 2mM L-glutamine (Invitrogen), 1X non-essential amino acids (Invitrogen), 0.075% sodium bicarbonate (Fisher Scientific, Pittsburgh, PA), 100 U/ml penicillin, and 100 µg/ml streptomycin (Invitrogen).
2.3 Preparation of cationic liposomes-DNA complexes and vaccines
Liposomes were prepared by combining cationic liposome DOTIM octadecenoyloxy(ethyl-2-heptadecenyl-3-hydroxyethyl) imidazolinium chloride and cholesterol in equimolar concentrations as described previously [52]. Cationic liposome-DNA complexes (CLDC) were freshly prepared at room temperature and administered within 30 min. Non-coding plasmid DNA (0.2 mg/ml, Juvaris Biotheraputics) was diluted in sterile Tris-buffered 5% dextrose water. The cationic liposomes were then added with gentle pipetting at a concentration of 100 µl of liposomes per 1 ml of solution, resulting in the spontaneous formation of CLDC. To formulate the CLDC-adjuvanted vaccines, the protein antigen was added to the diluted plasmid DNA solution prior to the addition of the cationic liposomes.
2.4 Intranasal immunizations
Prior to immunization, mice were anesthetized by intraperitoneal (i.p.) injection of ketamine (100mg/kg) with xylazine (10mg/kg). Each mouse was immunized with a total of 20 µl vaccine, which was administered by an equal amount in each nares and allowing the mice to inhale the vaccine. For most experiments, mice were immunized with a total of 2 µg ovalbumin (Ova). Mice were immunized once and boosted 10 days later. Serum was collected 5 – 7 days after the boost for analysis of cellular and humoral immune responses. Saliva was collected following i.p. injection of 10 ug pilocarpine (Sigma-Aldrich) in PBS.
2.5 Antibody response in serum, saliva, and BAL fluid
Antibody responses to Ova were assessed as described previously [53–54]. Briefly, ELISA plates were coated with Ova, blocked to reduce non-specific binding, then incubated with serial dilutions of serum from vaccinated and control mice. Antibody titers were determined using endpoint dilution assay and were expressed as the log reciprocal of the highest dilution of a sample with an OD reading of 0.1 above background.
2.6 Cell collection
Bronchoalveolar lavage (BAL) cells were obtained by airway lavage, as previously described [55]. Cells from the 3–4 washes per mouse were pooled, centrifuged at 1,200 rpm for 5 min at 4°C. The cells were further purified by NH4Cl lysis of the RBC. Lymph node cells were prepared by mechanical disruption and screening through a 70-µm nylon mesh screen (BD Biosciences), followed by NH4Cl lysis. Lung cells were prepared by first mincing the tissues, then digesting in a solution of 5 mg/ml collagenase (type 1A, Sigma-Aldrich) plus DNAase (50 U/ml) and soybean trypsin inhibitor (10 mg/ml) for 20 min at 37°C, as described previously [55]. The cells were then mechanically disrupted through an 18-gauge needle as previously described [56] and further purified by NH4Cl lysis. Cells from each organ source were counted and resuspended in complete medium on ice prior to immunostaining and analysis.
2.8 Antibodies and flow cytometric analysis
Directly conjugated antibodies used for these analyses were purchased from eBioscience (San Diego, CA) or BD Pharmingen (San Diego, CA). The following antibodies were used in various combinations: anti-CD8b (APC, FITC; clone H35-17.2), anti-I-A/I-E (MHC class II, FITC; clone NIMR-4), anti-CD11c (PE-Cy7; clone N418), anti-CD11b (Pacific Blue, biotin; clone M1/70), anti-Ly6G (PE; clone 1A8), anti-Ly6C (Biotin, FITC; clone AL-21), anti-PDCA (PE; clone ebio927), anti-CD45R (B220, Pacific Blue; clone RA3-6B2). Immunostaining was done as described previously [55]. In most cases, cells were fixed in 1% paraformaldehyde for 20 min and stored in FACS buffer at 4°C for 1–2 days prior to analysis. Analysis was carried out with a Cyan ADP flow cytometer (Beckman Coulter, Fort Collins, CO). Data was analyzed using FlowJo software (Tree Star, Ashland, OR).
2.9 MHC-peptide tetramers
Soluble H-2Kb MHC class I tetramers containing the ova8 peptide, SIINFEKL, were produced as described previously [57]. The CD8+ T cell response in mice vaccinated against ovalbumin was assessed in C57BL/6 mice. Single cell suspensions (typically 5×105 to 1×106 cells suspended in 100 µl of complete media) from the lung, peripheral bone marrow, and mediastinal lymph node were incubated with tetramer at 37°C for 90 min. Splenocytes from OT-1 mice (Ova8-specific TCR transgenic mice, provided by T. Potter, National Jewish Medical and Research Center, Denver, CO) were used as positive controls for tetramer staining. Staining and analysis of tetramer-labeled cells was done as described previously [41].
2.10 Cytokine analysis
Cytokine production in lung and BAL samples were assessed using a cytometric bead array (CBA; Becton Dickinson). Lung homogenates were prepped as described previously [58] and the lavage was performed using 1.5 ml of a PBS with EDTA (1mM) solution and processed as previously described [59]. The assay was performed according to the manufacturer’s instructions. Analysis was carried out using a Cyan ADP flow cytometer and data was analyzed using Flowjo software. The limit of detection for this assay for each cytokine was reported by the manufacturer to be 5 pg/ml.
2.11 Uptake of labeled Ovalbumin in the draining lymph node
Uptake and trafficking of Ova by cells in the airways and distribution to the draining lymph node was assessed using Alexa647-labeled Ova (Invitrogen). Alexa647-ova alone, or Alexa647-ova complexed to CLDC, were administered intranasally to mice. Six hours after administration, the mediastinal lymphnode was collected for immunostaining and analysis by flow cytometry.
2.12 Vaccination with heat-killed bacteria for protection from Burkholderia pulmonary challenge
Heat killing of Burkholderia pseudomallei was performed as described previously [60]. Briefly, bacteria were washed and resuspended in PBS, then heated to 80°C for 1 hour. Complete bacterial killing was confirmed by agar plating on LB agar plates. To assess the ability of CLDC adjuvanted vaccines to elicit protection from a lethal infectious challenge, BALB/c mice were vaccinated i.n. with CLDC adjuvant alone, 1 × 105 heat-killed Burkholderia pseudomallei organisms alone, or heat-killed bacteria mixed with 10 µl CLDC in a total volume of 20 µl. Mice were boosted in the same manner 10 days later, and then subjected to lethal i.n. challenge with 7,500 CFU live B. pseudomallei 1026b (8 × LD50) 14 days after the boost, using a bacterial challenge protocol described previously [50]. Mice were monitored for disease symptoms twice daily and were euthanized according to pre-determined humane endpoints.
2.13 Statistical analyses
Statistical analysis was performed using Prism 5.0 software (Graph Pad, La Jolla, CA). For comparisons between two groups, two-tailed non-parametric (Mann-Whitney) t-tests were performed. For comparison of more than two groups, a non-parametric ANOVA (Kruskal-Wallis test) was done, followed by Dunn’s multiple means comparison test. Survival times were determined using Kaplan-Meier curves, followed by the log-rank test. The Bonferroni correction was applied for comparison of more than 2 survival curves. For all comparisons, differences were considered statistically significant for p < 0.05.
3. Results
3.1 Mucosal immunization with CLDC adjuvant elicits systemic and local antibody responses
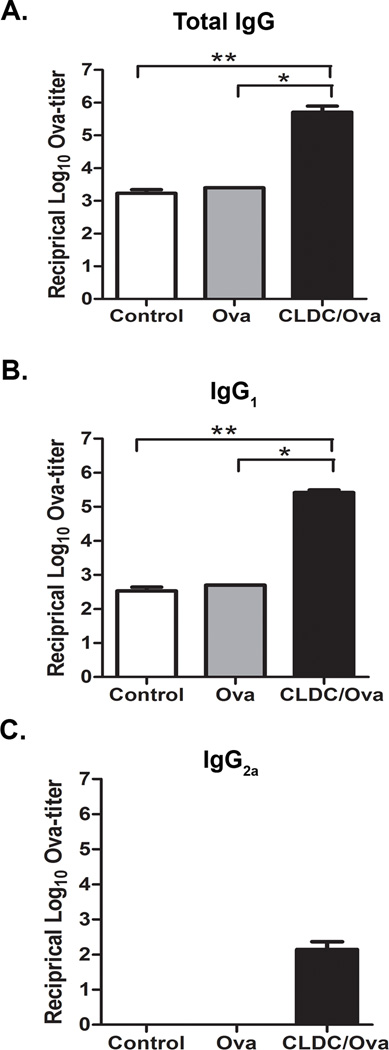
To assess the mucosal adjuvant properties of CLDC, we first investigated the ability of vaccines delivered intranasally (i.n.) with the CLDC adjuvant to generate systemic humoral immune responses, using the model antigen ovalbumin (Ova). Mice were typically immunized twice, 10 days apart. Mice immunized i.n. with a CLDC/Ova vaccine developed significant increases in total serum ova-specific IgG titers, compared to mice vaccinated with Ova alone (Fig. 1A). CLDC adjuvanted vaccines also elicited significant increases in serum ova-specific IgG1 titers (Fig. 1B and 1C).
Fig. 1.
Mucosal immunization with CLDC adjuvant elicits systemic IgG. (A–C) C57BL/6 mice (5/group) were intranasally vaccinated twice with 2 µg ovalbumin protein alone or in conjunction with a CLDC adjuvant as described in Materials and Methods. At three weeks post-vaccination serum was collected. An ELISA for ova-specific antibodies was performed on serial dilutions using secondary antibodies to (A) total IgG, (B) IgG1, and (C) IgG2a. The antibody titers are expressed as the reciprocal of the highest dilution of serum with an OD reading of 0.1 above background. Similar results were seen in one additional experiment. Significant differences (*p<0.05, **p<0.01) were determined by non-parametric ANOVA followed by Dunn’s multiple means comparison.
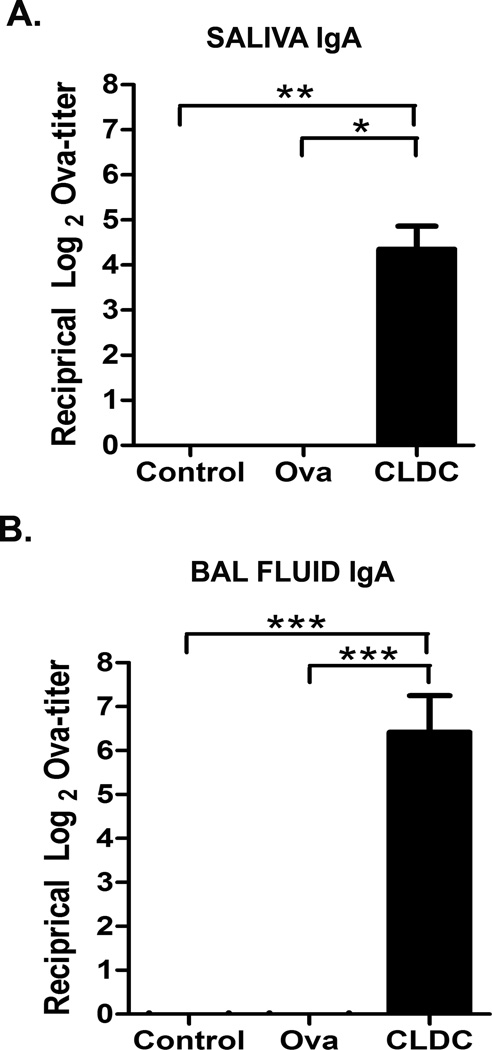
The ability of CLDC-adjuvanted vaccines to induce local IgA responses was assessed next. Intranasal immunization resulted in a significant increase in ova-specific IgA titers in saliva of CLDC/Ova vaccinated mice, compared to mice vaccinated with Ova alone (Fig. 2A). CLDC/Ova also induced significant ova-specific IgA titers in the airways of mice, as assessed in the BAL fluid (Fig. 2B). Thus, it was apparent that the mucosal administration of CLDC adjuvanted vaccines was capable of eliciting significant mucosal IgA responses, as well as significant systemic IgG responses.
Fig. 2.
Mucosal immunization with CLDC adjuvant elicits local IgA responses. (A,B) C57BL/6 mice (10/group) were intranasally vaccinated twice with 2 µg ovalbumin protein alone or in conjunction with a CLDC adjuvant as described in Materials and Methods. At three weeks post-vaccination saliva and BAL fluid were collected. Results were pooled from two independent experiments. An IgA ELISA for ova-specific antibodies was performed on serial dilutions. The antibody titers are expressed as the reciprocal of the highest dilution of a sample with an OD reading of 0.1 above background. Significant differences (*p<0.05, **p<0.01, ***p<0.001) were determined by non-parametric ANOVA followed by Dunn’s multiple means comparison.
3.2 Mucosal immunization with CLDC adjuvant induces antigen-specific CD8 T cell responses
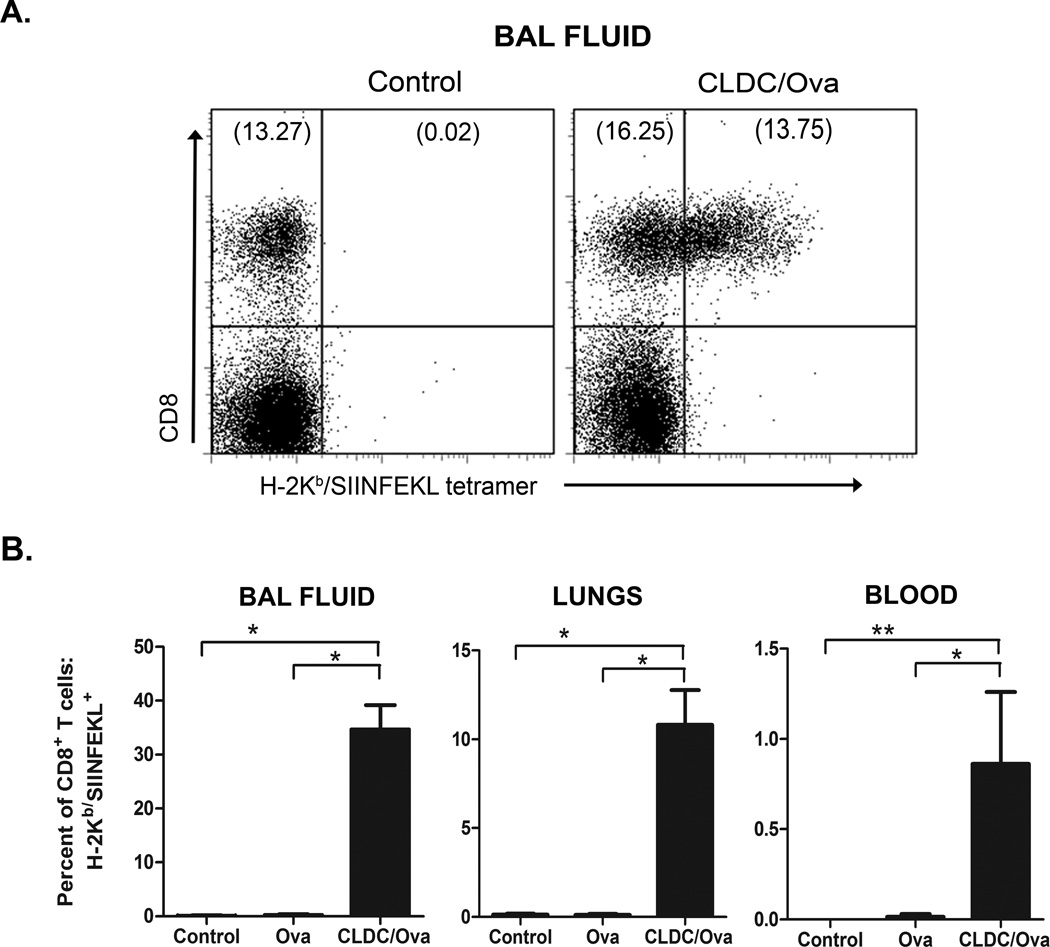
We reported previously that CLDC-adjuvanted vaccines administered parenterally produced strong antigen specific T cell responses, and were particularly effective in stimulating cross-priming and generation of antigen-specific CD8+ T cell responses [41]. Thus, it was of interest to determine whether the CLDC adjuvant could elicit similar responses following mucosal administration. For these experiments, numbers of Ova-specific CD8+ T cells in blood, BAL fluid, and lung tissues were enumerated using H-2Kb-ova8 tetramers and flow cytometry, as noted previously [41]. Following i.n. immunizations, a significant increase in numbers of CD8+ T cells was noted in all three sites evaluated (blood, lung parenchyma, and airways) (Fig. 3B). The expansion of Ova-specific CD8+ T cells was particularly dramatic in the airways of vaccinated mice, with 34.7% of all airway CD8+ T cells being Ova-specific. It was clear therefore that CLDC-adjuvanted vaccines were quite effective in generating CD8+ T cell responses in pulmonary mucosal tissues of vaccinated animals. The presence of antigen specific CD8+ T cells in the airways could be very beneficial for inducing protection against inhaled viral and bacterial pathogens.
Fig. 3.
Mucosal immunization with CLDC-based vaccines results in the cross-priming of CD8+ T cells. C57BL/6 mice (5/group) were intranasally vaccinated twice with 2 µg ovalbumin protein alone or in conjunction with a CLDC adjuvant as described in Materials and Methods. One week after the second immunization, CD8+ T cell responses were measured using H-2Kb/SIINFEKL tetramers as described in Materials and Methods. (A) Representative FACS plot of SIINFEKL-specific CD8+ T cells elicited by vaccination with ova peptide in CLDC adjuvant in the BAL fluid. (B) Total CD8+ T cells were gated for analysis (after excluding MHC class II+ cells), and the percentage of the total CD8+ T cells that were H-2Kb/SIINFEKL+ was plotted for the BAL fluid, lungs, and peripheral blood mononuclear cells. Similar results were seen in one additional experiment, significant differences (*p<0.05, **p<0.01) were determined by non-parametric ANOVA followed by Dunn’s multiple means comparison.
3.3 Mucosal immunization with a CLDC adjuvanted vaccine induces the production of IL-6 in the airways
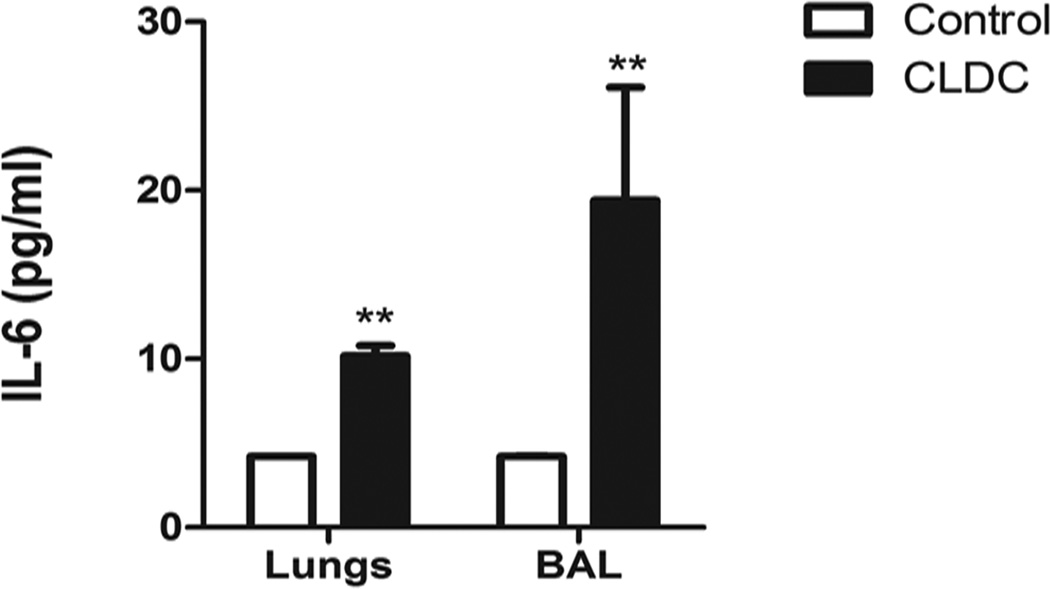
Experiments were conducted next to assess the effects of CLDC on local induction of innate immune responses in the airways and in lung tissues. In prior studies from our lab it was reported that i.n. administration of CLDC stimulated pulmonary production of pro-inflammatory cytokines, including IL-12, IFN-γ, and MCP-1[51, 58, 61–62]. In the present study, we were interested in examining CLDC induction of cytokines known to be involved in IgA antibody class switching, including IL-6, IL-10, and TGF-β [63–66]. While i.n. administration of CLDC did not induce significant increases in IL-10 or TGF-β in the lungs (data not shown), we found that administration of CLDC induced significant increases in IL-6 production in both the airways and lung tissues (Fig. 4). For example, IL-6 concentrations in the airways of mice treated with CLDC increased to 19.40 (pg/ml) ± 6.7, compared to 4.21 (pg/ml) ± 0.01 in sham-treated control animals. Thus, the ability of CLDC to elicit high levels of local IL-6 production may account in part for the ability of the CLDC adjuvant to induce efficient IgA production.
Fig. 4.
Mucosal immunization with CLDC adjuvanted vaccines induce the production of IL-6 in the airways and lung tissues. C57BL/6 mice (5/group) were given intranasal CLDC 24 hours prior to collecting the BAL fluid and lung tissue. The lung supernatant was collected after tissue homogenization, as described in the Materials and Methods. The mouse inflammatory cytometric bead array was used to determine the concentration of IL-6 produced following stimulation with CLDC, as described in the Materials and Methods. Similar results were seen in one additional experiment, the asterisks denote significant differences (** p<0.01) determined by non-parametric Mann-Whitney t Test.
3.4 Antigens complexed to CLDC are delivered efficiently to the mediastinal lymph nodes
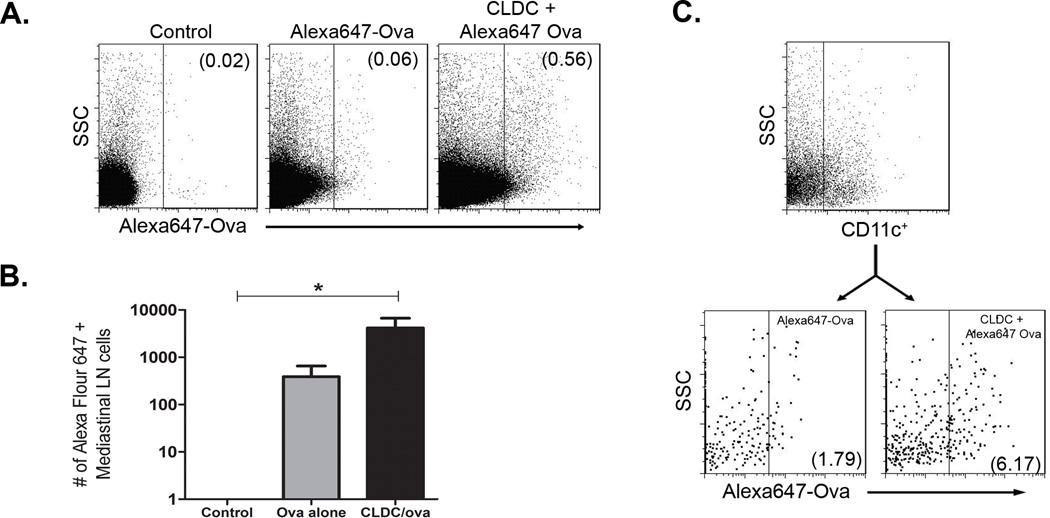
Given that the CLDC adjuvant could generate efficient humoral and cellular immune responses, it was important to try and understand how the adjuvant affected antigen presentation in the lungs. Therefore, experiments were conducted to directly assess the ability of CLDC to enhance delivery of soluble antigens to draining lymph nodes. For these experiments Ova labeled with AlexaFluor 647 was used to facilitate uptake and trafficking studies. Mice were immunized i.n. with Alexa647-Ova alone or Alexa647-Ova complexed to CLDC. Six hours later, antigen uptake in the mediastinal lymph nodes (MLN) was assessed using flow cytometry. We found that administration of Alexa647-Ova complexed to CLDC resulted in significantly greater antigen delivery to the MLN, compared to administration of Alexa647-Ova alone (Fig. 5A and 5B).
Fig. 5.
Antigens complexed to CLDC were delivered to the mediastinal lymph node following mucosal immunization and were taken up efficiently by dendritic cells. Mice (5/group) were given intranasal Alexa647 ovalbumin (5 ug) in association with CLDC 6 hours prior to the collection of the draining mediastinal lymph node. (A) Representative FACS plots of Alexa647+ cells found in the lymphonode, the numbers represent the percent of Alexa647+ cells. (B) Quantification of Alexa647+ cells in the lymphnode. Significant differences (*p<0.05) were determined by non-parametric ANOVA followed by Dunn’s multiple means comparison. (C) Representative FACS plot of CD11c+ dendritic cells found in the lymphnode. There was no significant difference in total number of CD11c+ DC found in the MLN between the mice given Alexa647-ova alone and the mice given Alexa647-ova in conjunction with CLDC. Following the staining of CD11c, the cells were analyzed for the uptake of Alexa647 ovalbumin, and a significant difference was found between the Alexa647-ova alone group and the Alexa647-ova+ CLDC group (**p=0.007). Similar results were seen in one additional experiment.
Next, the impact of CLDC on antigen uptake by pulmonary APCs was assessed. We found that the uptake of labeled Ova by CD11c+ DC in the MLN was significantly greater when the antigen was complexed to CLDC then when it was administered alone (Fig. 5C). The effect of CLDC on uptake of Ova by other APCs in the lung was also investigated. We found that administering Ova complexed to CLDC did not enhance antigen uptake by B cells or macrophages (data not shown). Thus, these results show that CLDC are effective vaccine adjuvants in the lungs because they enhance antigen uptake by pulmonary DC.
3.5 Mucosal immunization with heat killed bacteria and CLDC adjuvant generates effective protective immunity against lethal pulmonary challenge with Burkholderia pseudomallei
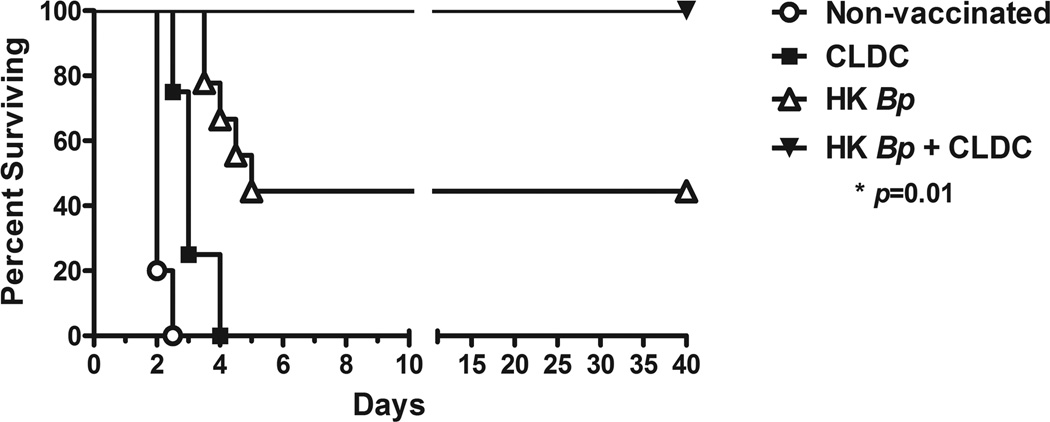
Experiments were conducted next to assess the potential for CLDC-adjuvanted mucosal vaccines to generate robust, protective immunity against an inhaled pathogen. For these experiments, we used a mouse model of lethal Burkholderia pseudomallei pneumonia, based on recent studies conducted by our laboratory [50, 62]. BALB/c mice were vaccinated and boosted i.n. with CLDC adjuvant alone, 1 × 105 heat-killed Burkholderia pseudomallei organisms alone, or heat-killed bacteria mixed with 10 µl CLDC in a total volume of 20 µl. Control mice were not vaccinated. All mice were then subjected to i.n. challenge with 8 × LD50 (7.5 × 103) CFU B. pseudomallei 2 weeks after the last immunization and survival times were determined by the Animal Care and Use Committee at Colorado State University.
All unvaccinated control mice reached end-point prior to day 3 after challenge, and the CLDC alone mice succumbed to disease by day 4. In contrast, 4 of the 9 mice vaccinated with heat-killed bacteria alone survived for > 40 days (Fig. 6). However, it is important to note that all of the surviving mice vaccinated with heat-killed B. pseudomallei only eventually succumbed to chronic disease by day 60 post-challenge (data not shown). In contrast, 100% of mice vaccinated with heat-killed bacteria plus CLDC survived bacterial challenge for > 40 days (Fig. 6). Five of these 9 mice survived past day 60 post-challenge and were considered long-term survivors. Long-term survival tends to correlate with clearing of the organism, but cultures were not performed to confirm this fact. These results indicate that mucosal vaccination using a CLDC-adjuvanted vaccine elicited significant protective local and systemic immunity against a lethal challenge with a very virulent bacterial pathogen.
Fig. 6.
Mucosal immunization with heat killed bacteria and CLDC adjuvant generates effective protective immunity against lethal pulmonary challenge with Burkholderia pseudomallei. BALB/c mice (n = 4–5 mice per non-vaccinated control and CLDC groups, and 9 mice per HK Bp and HK Bp + CLDC groups) were primed intranasally with 1 × 105 CFU heat-killed B. pseudomallei 1026b suspended in D5W buffer or with heat-killed bacteria complexed to the CLDC adjuvant. Mice were boosted in the same manner 10 days later. Mice in the CLDC alone group were primed and boosted with this adjuvant alone. All animals were then challenged intranasally with 7500 CFU live B. pseudomallei 1026b 14 days following the boost, and survival was monitored. Statistical differences in survival times were determined by Kaplan-Meier curves followed by log-rank test. The Bonferroni corrected threshold was applied and comparisons with p < 0.013 were considered significant. (*p = 0.01 for mice vaccinated with heat-killed bacteria alone vs. those vaccinated with heat-killed bacteria complexed to CLDC). Data shown are representative of 2 combined independent experiments.
3.6 Potency of CLDC adjuvant equivalent or superior to that of conventional mucosal vaccine adjuvants
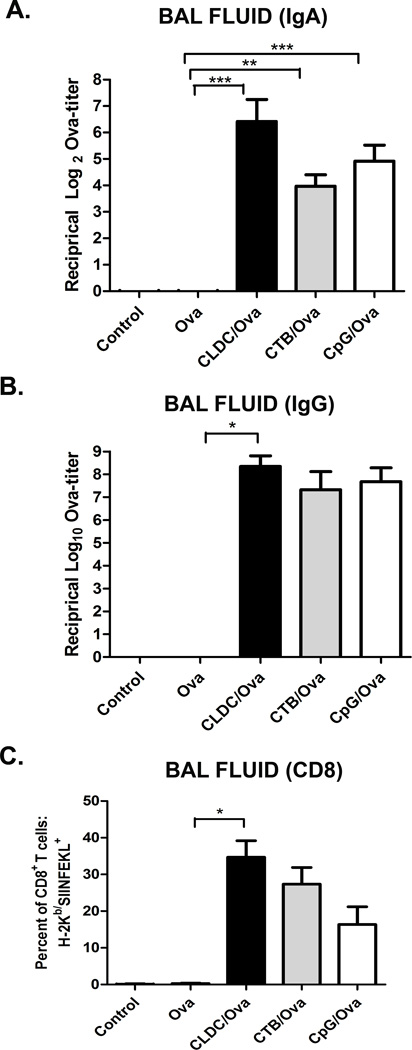
Lastly, to place the potency of CLDC mucosal adjuvant properties in context, CLDC-elicited vaccine responses were compared to those generated by the conventional mucosal adjuvants cholera toxin B (CTB) and CpG oligonucleotides (CpG ODN) [9–10, 12]. Mice were therefore vaccinated i.n. with 2 ug Ova admixed with CLDC, CTB (5µg), or CpG (10µg) [19, 67–70]. Intranasal immunization using each of the three different adjuvants elicited significant increases in ova-specific IgA titers in the BAL fluid of vaccinated mice (Fig. 7A). Of the three adjuvants only the CLDC adjuvant generated significant increases in ova-specific IgG titers in the BAL fluid (Fig 7B). However, it should also be noted that only the CpG ODN adjuvant elicited significant increases in ova-specific IgG2a titers (data not shown). Mucosal adjuvants were also compared for their ability to generate CD8 T cell responses in the lungs. Intranasal immunization with CLDC adjuvant appeared particularly effective in generating antigen-specific CD8 T cell responses, especially in the airways of vaccinated mice (Fig 7C). Overall, these results suggested that at least for soluble protein antigens, CLDC based adjuvants were as effective as current mucosal vaccine adjuvants.
Fig. 7.
Mucosal immunization with CLDC adjuvant elicits potent immune responses equivalent to leading mucosal vaccine adjuvants. (A–C) C57BL/6 mice (5/group) were intranasally vaccinated twice with 2 µg ovalbumin protein alone or in conjunction with a CLDC adjuvant, a CTB adjuvant (5 µg), or a CpG adjuvant (10 µg), as described in Materials and Methods. One week after the second immunization the BAL fluid was collected. (A,B) An ELISA for ova-specific antibodies was performed on serial dilutions of the BAL fluid using secondary antibodies to (A) IgA and (B) Total IgG. The antibody titers are expressed as the reciprocal of the highest dilution of serum with an OD reading of 0.1 above background. (C) Total CD8+ T cells were gated for analysis (after excluding MHC class II+ cells), and the percentage of the total CD8+ T cells that were H-2Kb/SIINFEKL+ was plotted for BAL fluid. Similar results were seen in one additional experiment. Significant differences (*p<0.05, **p<0.01, ***p<0.001) were determined by non-parametric ANOVA followed by Dunn’s multiple means comparison.
4. Discussion
After assessing both humoral and cellular immune responses to soluble antigens delivered intranasally using the CLDC adjuvant, we concluded that CLDC was indeed an effective mucosal vaccine adjuvant. CLDC adjuvanted vaccines were found to be particularly effective at generating mucosal IgA responses, as well as intrapulmonary T cell responses. The ability of the CLDC adjuvant to increase the immunogenicity of a complex particulate antigen (ie, heat-killed bacteria) was also demonstrated.
A variety of immunological properties that have been attributed to cationic liposomes are likely to have contributed to the effectiveness of CLDC as a mucosal vaccine adjuvant. For one, positively charged liposomes rapidly adhere to negatively charged surfaces of cells such as APCs and epithelial cells, increasing their uptake [38, 71]. In addition, cationic liposomes have been shown to directly activate APCs such as DC [38, 72–73]. Finally, the size of the CLDC particles used in this study (approximately 250 nm diameter) is ideal for uptake by DC, including pulmonary DC [72, 74]. Thus, the mucosal adjuvant properties of CLDC are likely dependent to a large degree on cationic liposomes.
The adjuvant activity of cationic liposome-nucleic acid-based adjuvants is also importantly influenced by the nucleic acid component of the vaccine [75]. In the current study, the non-coding plasmid DNA used in the preparation of CLDC contains many CpG motifs and is known to activate innate immunity via TLR9 signaling. Indeed, in our studies we found that cellular immune responses to vaccination with CLDC adjuvanted vaccines were nearly completely abolished in MyD88−/− mice (data not shown), indicating that TLR signaling in the lungs was critical to the activity of CLDC adjuvants. While it is currently not known how well other TLR agonists might function as mucosal adjuvants when complexed to cationic liposomes, it is known that TLR-3 agonists, such as polyI:C, are effective at stimulating immune responses with cationic liposomes [41].
The capacity of CLDC to modulate the airway cytokine environment is another critical feature to consider when assessing the adjuvant activity. Intranasal administration of CLDC has previously been shown to induce the production of IL-12, TNF-α, IFN-γ, IFN-α and IFN-β [51, 58, 61–62]. In the current study we found that CLDC administration induced pulmonary expression of IL-6, a cytokine linked to the induction of IgA class-switching [66, 76–80]. IL-6 has also been shown to stimulate T cell proliferation [81–83], and to enhance generation of protective immunity following vaccination against respiratory pathogens [84–85].
The unique ability of CLDC to elicit the cross-priming of CD8 T cells to protein antigens has been explored previously in the context of parenteral vaccines [41] as well in a therapeutic vaccine used to suppress hyperresponsiveness in the airways [86]. In the present study we found that mucosal vaccination using CLDC as an adjuvant was also capable of rapidly generating pulmonary CD8+ T cell responses. While the mechanism of CLDC mediated cross-priming is not fully understood, it is believed that the cationic liposome component of CLDC results in the slight instability of the endosome resulting in the leakage of endosomal contents into the cytoplasm, leading to the processing and presentation of peptide fragments via MHC class I [36].
By using a labeled antigen (Ova), we were able to directly visualize the interaction of the antigen with relevant APCs, as well as assess how CLDC affected that interaction. We found that complexing the antigen to CLDC resulted in the preferential targeting of antigen to resident pulmonary DC. We believe that DCs are the key cells responsible for increased antigen presentation following antigen delivery with CLDC. We also noted the uptake of labeled antigen by B cells and macrophages in the MLN, but the addition of CLDC did not enhance antigen uptake by these cells. Therefore, we believe that the uptake of antigen by B cells and macrophages in the MLN may have resulted from passive transport of soluble or CLDC bound antigen directly through the lymphatics, without cell associated transport.
The ability of the CLDC adjuvant to improve antigen processing and presentation following mucosal administration is not confined to the respiratory tract. For example, we have recently reported that oral administration of CLDC-adjuvanted vaccines is also capable of generating substantial protective immunity against pulmonary challenge with Yersinia pestis[87]. Thus, induction of efficient mucosal immunity, particularly at pulmonary surfaces, appears to be a general property of CLDC-based adjuvants. Moreover, we also found here that CLDC adjuvants performed well when compared to other conventional adjuvants in terms of potency of both humoral and cellular immunity.
In summary, these findings suggest that liposome-nucleic acid based adjuvants are an important new category of mucosal vaccine adjuvant that generates considerable activity when combined with protein antigens. Properties that appear to contribute to the effectiveness of CLDC for mucosal administration include efficient uptake by DC, rapid transit of antigen-CLDC complexes to regional lymph nodes, and potent induction of cytokines involved in IgA class-switching at mucosal surfaces.
Acknowledgements
The authors wish to acknowledge the assistance of Dr. Scott Hafeman for preparation of liposomes. These studies were supported in part by a grant from the NIH NIAID (U54 AI065357) and by a collaborative research agreement from Juvaris Biotherapeutics.
References
- 1.Belyakov IM, Ahlers JD. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J Immunol. 2009;183(11):6883–6892. doi: 10.4049/jimmunol.0901466. [DOI] [PubMed] [Google Scholar]
- 2.Harandi AM, Medaglini D, Shattock RJ. Vaccine adjuvants: a priority for vaccine research. Vaccine. 28(12):2363–2366. doi: 10.1016/j.vaccine.2009.12.084. [DOI] [PubMed] [Google Scholar]
- 3.Lamm ME. Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol. 1997;51:311–340. doi: 10.1146/annurev.micro.51.1.311. [DOI] [PubMed] [Google Scholar]
- 4.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6(2):148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 5.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11(4 Suppl):S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 6.Belshe RB, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007;356(7):685–696. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 7.Viret JF, et al. Mucosal and systemic immune responses in humans after primary and booster immunizations with orally administered invasive and noninvasive live attenuated bacteria. Infect Immun. 1999;67(7):3680–3685. doi: 10.1128/iai.67.7.3680-3685.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cox E, et al. Adjuvants modulating mucosal immune responses or directing systemic responses towards the mucosa. Vet Res. 2006;37(3):511–539. doi: 10.1051/vetres:2006014. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson K, Holmgren J. Recent advances in mucosal vaccines and adjuvants. Curr Opin Immunol. 2002;14(5):666–672. doi: 10.1016/s0952-7915(02)00384-9. [DOI] [PubMed] [Google Scholar]
- 10.Moyle PM, et al. Mucosal immunisation: adjuvants and delivery systems. Curr Drug Deliv. 2004;1(4):385–396. doi: 10.2174/1567201043334588. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira ML, Areas AP, Ho PL. Intranasal vaccines for protection against respiratory and systemic bacterial infections. Expert Rev Vaccines. 2007;6(3):419–429. doi: 10.1586/14760584.6.3.419. [DOI] [PubMed] [Google Scholar]
- 12.Freytag LC, Clements JD. Mucosal adjuvants. Vaccine. 2005;23(15):1804–1813. doi: 10.1016/j.vaccine.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Harandi AM. The potential of immunostimulatory CpG DNA for inducing immunity against genital herpes: opportunities and challenges. J Clin Virol. 2004;30(3):207–210. doi: 10.1016/j.jcv.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa H, et al. Development of a mucosal vaccine for influenza viruses: preparation for a potential influenza pandemic. Expert Rev Vaccines. 2007;6(2):193–201. doi: 10.1586/14760584.6.2.193. [DOI] [PubMed] [Google Scholar]
- 15.Holmgren J, et al. Mucosal adjuvants and anti-infection and anti-immunopathology vaccines based on cholera toxin, cholera toxin B subunit and CpG DNA. Immunol Lett. 2005;97(2):181–188. doi: 10.1016/j.imlet.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Kensil CR, Mo AX, Truneh A. Current vaccine adjuvants: an overview of a diverse class. Front Biosci. 2004;9:2972–2988. doi: 10.2741/1452. [DOI] [PubMed] [Google Scholar]
- 17.Klinman DM, et al. CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases. Adv Drug Deliv Rev. 2009;61(3):248–255. doi: 10.1016/j.addr.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 18.McCluskie MJ, et al. Mucosal immunization of mice using CpG DNA and/or mutants of the heat-labile enterotoxin of Escherichia coli as adjuvants. Vaccine. 2001;19(27):3759–3768. doi: 10.1016/s0264-410x(01)00088-3. [DOI] [PubMed] [Google Scholar]
- 19.McCluskie MJ, Weeratna RD, Davis HL. The potential of oligodeoxynucleotides as mucosal and parenteral adjuvants. Vaccine. 2001;19(17–19):2657–2660. doi: 10.1016/s0264-410x(00)00496-5. [DOI] [PubMed] [Google Scholar]
- 20.Stevceva L, Ferrari MG. Mucosal adjuvants. Curr Pharm Des. 2005;11(6):801–811. doi: 10.2174/1381612053381846. [DOI] [PubMed] [Google Scholar]
- 21.Dittmer U, Olbrich AR. Treatment of infectious diseases with immunostimulatory oligodeoxynucleotides containing CpG motifs. Curr Opin Microbiol. 2003;6(5):472–477. doi: 10.1016/j.mib.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Jahrsdorfer B, Weiner GJ. CpG oligodeoxynucleotides for immune stimulation in cancer immunotherapy. Curr Opin Investig Drugs. 2003;4(6):686–690. [PubMed] [Google Scholar]
- 23.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5(6):471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 24.Fujihashi K, et al. A dilemma for mucosal vaccination: efficacy versus toxicity using enterotoxin-based adjuvants. Vaccine. 2002;20(19–20):2431–2438. doi: 10.1016/s0264-410x(02)00155-x. [DOI] [PubMed] [Google Scholar]
- 25.Heikenwalder M, et al. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat Med. 2004;10(2):187–192. doi: 10.1038/nm987. [DOI] [PubMed] [Google Scholar]
- 26.Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol. 2004;4(4):249–258. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 27.van Ginkel FW, et al. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J Immunol. 2000;165(9):4778–4782. doi: 10.4049/jimmunol.165.9.4778. [DOI] [PubMed] [Google Scholar]
- 28.van Ginkel FW, Nguyen HH, McGhee JR. Vaccines for mucosal immunity to combat emerging infectious diseases. Emerg Infect Dis. 2000;6(2):123–132. doi: 10.3201/eid0602.000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Acevedo R, et al. Intranasal administration of proteoliposome-derived cochleates from Vibrio cholerae O1 induce mucosal and systemic immune responses in mice. Methods. 2009;49(4):309–315. doi: 10.1016/j.ymeth.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 30.Benoit A, et al. Effects of alveolar macrophage depletion on liposomal vaccine protection against respiratory syncytial virus (RSV) Clin Exp Immunol. 2006;145(1):147–154. doi: 10.1111/j.1365-2249.2006.03114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosada RS, et al. Protection against tuberculosis by a single intranasal administration of DNA-hsp65 vaccine complexed with cationic liposomes. BMC Immunol. 2008;9:38. doi: 10.1186/1471-2172-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakaue G, et al. HIV mucosal vaccine: nasal immunization with gp160-encapsulated hemagglutinating virus of Japan-liposome induces antigen-specific CTLs and neutralizing antibody responses. J Immunol. 2003;170(1):495–502. doi: 10.4049/jimmunol.170.1.495. [DOI] [PubMed] [Google Scholar]
- 33.Tseng LP, et al. Effect of lipopolysaccharide on intranasal administration of liposomal Newcastle disease virus vaccine to SPF chickens. Vet Immunol Immunopathol. 2009;131(3–4):285–289. doi: 10.1016/j.vetimm.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Wang D, et al. Intranasal immunization with liposome-encapsulated plasmid DNA encoding influenza virus hemagglutinin elicits mucosal, cellular and humoral immune responses. J Clin Virol. 2004;31 Suppl 1:S99–S106. doi: 10.1016/j.jcv.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Heurtault B, Frisch B, Pons F. Liposomes as delivery systems for nasal vaccination: strategies and outcomes. Expert Opin Drug Deliv. 7(7):829–844. doi: 10.1517/17425247.2010.488687. [DOI] [PubMed] [Google Scholar]
- 36.Chikh G, Schutze-Redelmeier MP. Liposomal delivery of CTL epitopes to dendritic cells. Biosci Rep. 2002;22(2):339–353. doi: 10.1023/a:1020151025412. [DOI] [PubMed] [Google Scholar]
- 37.Copland MJ, et al. Liposomal delivery of antigen to human dendritic cells. Vaccine. 2003;21(9–10):883–890. doi: 10.1016/s0264-410x(02)00536-4. [DOI] [PubMed] [Google Scholar]
- 38.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 39.Baca-Estrada ME, et al. Intranasal immunization with liposome-formulated Yersinia pestis vaccine enhances mucosal immune responses. Vaccine. 2000;18(21):2203–2211. doi: 10.1016/s0264-410x(00)00019-0. [DOI] [PubMed] [Google Scholar]
- 40.Khatri K, et al. Surface modified liposomes for nasal delivery of DNA vaccine. Vaccine. 2008;26(18):2225–2233. doi: 10.1016/j.vaccine.2008.02.058. [DOI] [PubMed] [Google Scholar]
- 41.Zaks K, et al. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006;176(12):7335–7345. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]
- 42.Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5(7):505–517. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- 43.Mutwiri G, et al. Combination adjuvants: the next generation of adjuvants? Expert Rev Vaccines. 2011;10(1):95–107. doi: 10.1586/erv.10.154. [DOI] [PubMed] [Google Scholar]
- 44.Bernstein DI, et al. Potent adjuvant activity of cationic liposome-DNA complexes for genital herpes vaccines. Clin Vaccine Immunol. 2009;16(5):699–705. doi: 10.1128/CVI.00370-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bernstein DI, et al. The adjuvant CLDC increases protection of a herpes simplex type 2 glycoprotein D vaccine in guinea pigs. Vaccine. 2009 doi: 10.1016/j.vaccine.2009.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang S, et al. A novel vaccine adjuvant for recombinant flu antigens. Biologicals. 2009;37(3):141–147. doi: 10.1016/j.biologicals.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cote PJ, et al. Rapid immunity to vaccination with woodchuck hepatitis virus surface antigen using cationic liposome-DNA complexes as adjuvant. J Med Virol. 2009;81(10):1760–1772. doi: 10.1002/jmv.21566. [DOI] [PubMed] [Google Scholar]
- 48.Fairman J, et al. Enhanced in vivo immunogenicity of SIV vaccine candidates with cationic liposome-DNA complexes in a rhesus macaque pilot study. Hum Vaccin. 2009;5(3):141–150. doi: 10.4161/hv.5.3.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lay M, et al. Cationic lipid/DNA complexes (JVRS-100) combined with influenza vaccine (Fluzone) increases antibody response, cellular immunity, and antigenically drifted protection. Vaccine. 2009;27(29):3811–3820. doi: 10.1016/j.vaccine.2009.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goodyear A, et al. Protection from pneumonic infection with burkholderia species by inhalational immunotherapy. Infect Immun. 2009;77(4):1579–1588. doi: 10.1128/IAI.01384-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Troyer RM, et al. Mucosal immunotherapy for protection from pneumonic infection with Francisella tularensis. Vaccine. 2009;27(33):4424–4433. doi: 10.1016/j.vaccine.2009.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Templeton NS, et al. Improved DNA: liposome complexes for increased systemic delivery and gene expression. Nat Biotechnol. 1997;15(7):647–652. doi: 10.1038/nbt0797-647. [DOI] [PubMed] [Google Scholar]
- 53.Frey A, Di Canzio J, Zurakowski D. A statistically defined endpoint titer determination method for immunoassays. J Immunol Methods. 1998;221(1–2):35–41. doi: 10.1016/s0022-1759(98)00170-7. [DOI] [PubMed] [Google Scholar]
- 54.Maloy KJ, et al. Induction of mucosal and systemic immune responses by immunization with ovalbumin entrapped in poly(lactide-co-glycolide) microparticles. Immunology. 1994;81(4):661–667. [PMC free article] [PubMed] [Google Scholar]
- 55.Bosio CM, Dow SW. Francisella tularensis induces aberrant activation of pulmonary dendritic cells. J Immunol. 2005;175(10):6792–6801. doi: 10.4049/jimmunol.175.10.6792. [DOI] [PubMed] [Google Scholar]
- 56.Fisher JH, et al. Lymphocyte activation in the lungs of SP-D null mice. Am J Respir Cell Mol Biol. 2002;27(1):24–33. doi: 10.1165/ajrcmb.27.1.4563. [DOI] [PubMed] [Google Scholar]
- 57.Ahonen CL, et al. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004;199(6):775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goodyear A, et al. Critical protective role for MCP-1 in pneumonic Burkholderia mallei infection. J Immunol. 184(3):1445–1454. doi: 10.4049/jimmunol.0900411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lambrecht BN, et al. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. J Immunol. 1998;160(8):4090–4097. [PubMed] [Google Scholar]
- 60.Lauw FN, et al. The CXC chemokines gamma interferon (IFN-gamma)-inducible protein 10 and monokine induced by IFN-gamma are released during severe melioidosis. Infect Immun. 2000;68(7):3888–3893. doi: 10.1128/iai.68.7.3888-3893.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Freimark BD, et al. Cationic lipids enhance cytokine and cell influx levels in the lung following administration of plasmid: cationic lipid complexes. J Immunol. 1998;160(9):4580–4586. [PubMed] [Google Scholar]
- 62.Propst KL, et al. Immunotherapy Markedly Increases the Effectiveness of Antimicrobial Therapy for Treatment of Burkholderia pseudomallei Infection. Antimicrob Agents Chemother. doi: 10.1128/AAC.01513-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008;8(6):421–434. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fagarasan S, Honjo T. Intestinal IgA synthesis: regulation of front-line body defences. Nat Rev Immunol. 2003;3(1):63–72. doi: 10.1038/nri982. [DOI] [PubMed] [Google Scholar]
- 65.Macpherson AJ, et al. The immune geography of IgA induction and function. Mucosal Immunol. 2008;1(1):11–22. doi: 10.1038/mi.2007.6. [DOI] [PubMed] [Google Scholar]
- 66.Okahashi N, et al. Oral immunization of interleukin-4 (IL-4) knockout mice with a recombinant Salmonella strain or cholera toxin reveals that CD4+ Th2 cells producing IL-6 and IL-10 are associated with mucosal immunoglobulin A responses. Infect Immun. 1996;64(5):1516–1525. doi: 10.1128/iai.64.5.1516-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fukuiwa T, et al. A combination of Flt3 ligand cDNA and CpG ODN as nasal adjuvant elicits NALT dendritic cells for prolonged mucosal immunity. Vaccine. 2008;26(37):4849–4859. doi: 10.1016/j.vaccine.2008.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim HJ, et al. Intranasal vaccination with peptides and cholera toxin subunit B as adjuvant to enhance mucosal and systemic immunity to respiratory syncytial virus. Arch Pharm Res. 2007;30(3):366–371. doi: 10.1007/BF02977620. [DOI] [PubMed] [Google Scholar]
- 69.Porgador A, et al. Intranasal immunization with CTL epitope peptides from HIV-1 or ovalbumin and the mucosal adjuvant cholera toxin induces peptide-specific CTLs and protection against tumor development in vivo. J Immunol. 1997;158(2):834–841. [PubMed] [Google Scholar]
- 70.Wakabayashi A, et al. Suppression of an already established tumor growing through activated mucosal CTLs induced by oral administration of tumor antigen with cholera toxin. J Immunol. 2008;180(6):4000–4010. doi: 10.4049/jimmunol.180.6.4000. [DOI] [PubMed] [Google Scholar]
- 71.Ahsan F, et al. Targeting to macrophages: role of physicochemical properties of particulate carriers--liposomes and microspheres--on the phagocytosis by macrophages. J Control Release. 2002;79(1–3):29–40. doi: 10.1016/s0168-3659(01)00549-1. [DOI] [PubMed] [Google Scholar]
- 72.Foged C, et al. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm. 2005;298(2):315–322. doi: 10.1016/j.ijpharm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 73.Nakanishi T, et al. Positively charged liposome functions as an efficient immunoadjuvant in inducing immune responses to soluble proteins. Biochem Biophys Res Commun. 1997;240(3):793–797. doi: 10.1006/bbrc.1997.7749. [DOI] [PubMed] [Google Scholar]
- 74.Brewer JM, et al. Vesicle size influences the trafficking, processing, and presentation of antigens in lipid vesicles. J Immunol. 2004;173(10):6143–6150. doi: 10.4049/jimmunol.173.10.6143. [DOI] [PubMed] [Google Scholar]
- 75.Dow S. Liposome-nucleic acid immunotherapeutics. Expert Opin Drug Deliv. 2008;5(1):11–24. doi: 10.1517/17425247.5.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beagley KW, et al. Interleukins and IgA synthesis. Human and murine interleukin 6 induce high rate IgA secretion in IgA-committed B cells. J Exp Med. 1989;169(6):2133–2148. doi: 10.1084/jem.169.6.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.DiCosmo BF, et al. Airway epithelial cell expression of interleukin-6 in transgenic mice. Uncoupling of airway inflammation and bronchial hyperreactivity. J Clin Invest. 1994;94(5):2028–2035. doi: 10.1172/JCI117556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McGhee JR, et al. Regulation of IgA synthesis and immune response by T cells and interleukins. J Clin Immunol. 1989;9(3):175–199. doi: 10.1007/BF00916814. [DOI] [PubMed] [Google Scholar]
- 79.Ruedl C, et al. Humoral and cellular immune responses in the murine respiratory tract following oral immunization with cholera toxin or Escherichia coli heat-labile enterotoxin. Vaccine. 1996;14(8):792–798. doi: 10.1016/0264-410x(95)00231-o. [DOI] [PubMed] [Google Scholar]
- 80.Sato A, et al. CD11b+ Peyer's patch dendritic cells secrete IL-6 and induce IgA secretion from naive B cells. J Immunol. 2003;171(7):3684–3690. doi: 10.4049/jimmunol.171.7.3684. [DOI] [PubMed] [Google Scholar]
- 81.DiCosmo B, et al. Expression of interleukin-6 by airway epithelial cells. Effects on airway inflammation and hyperreactivity in transgenic mice. Chest. 1995;107(3 Suppl):131S. doi: 10.1378/chest.107.3_supplement.131s. [DOI] [PubMed] [Google Scholar]
- 82.Jonuleit H, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27(12):3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 83.Rincon M, et al. Interleukin (IL)-6 directs the differentiation of IL-4-producing CD4+ T cells. J Exp Med. 1997;185(3):461–469. doi: 10.1084/jem.185.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leal IS, et al. Interleukin-6 and interleukin-12 participate in induction of a type 1 protective T-cell response during vaccination with a tuberculosis subunit vaccine. Infect Immun. 1999;67(11):5747–5754. doi: 10.1128/iai.67.11.5747-5754.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Picard C, et al. Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science. 2003;299(5615):2076–2079. doi: 10.1126/science.1081902. [DOI] [PubMed] [Google Scholar]
- 86.Takeda K, et al. Vaccine-induced CD8+ T cell-dependent suppression of airway hyperresponsiveness and inflammation. J Immunol. 2009;183(1):181–190. doi: 10.4049/jimmunol.0803967. [DOI] [PubMed] [Google Scholar]
- 87.Jones A, et al. Protection against pneumonic plague following oral immunization with a non-replicating vaccine. Vaccine. 2010;28(36):5924–5929. doi: 10.1016/j.vaccine.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]