Abstract
Background
Atherogenic dyslipidemia (AD), defined as low HDL-C plus elevated triglycerides (TG), comorbid to T2DM, increases cardiometabolic risk for CAD even when LDL-C is at target. In T2DM males, AD was shown to correlate with β-cell function loss, yet it is not established whether this applies across gender.
Aim
To establish the prevalence and severity of AD in T2DM females, and to determine how it relates to cardiometabolic phenotype, glucose homeostasis, micro- and macrovascular complications, and 10-year absolute CV risk (UKPDS Risk Engine).
Methods
340 T2DM females were ranked according to quintiles (Q) of the continuous variable log(TG)/HDL-C, with AD prevalence defined as HDL-C <50 mg.dL-1 plus TG ≥150 mg.dL-1, and β-cell function assessed with HOMA.
Results
AD prevalence was 35%; mean HDL-C and TG were 52 (15) and 160 (105) mg.dL-1. AD was significantly related to central fat, metabolic syndrome, sedentarity and skeletal sarcopenia, as well as to hsCRP, fibrinogen, uric acid, cystatin-C, Big ET-1, and 10-year UKPDS CV risk. AD correlated stepwise with lower β-cell function and hyperbolic product, and with accelerated loss of residual insulin secretion, higher HbA1c and prevalent microangiopathy.
Conclusions
log(TG)/HDL-C is a simple means to grade AD and residual macrovascular risk in T2DM females. This ratio associates with major non-LDL cardiometabolic variables and ranks predicted CAD risk. In addition, log(TG)/HDL-C identifies worsening glucose homeostasis, poorer glycemic control, and prevalent microangiopathy.
Keywords: HDL-C, Triglycerides, Cardiovascular risk, Microangiopathy, β-cell function, Hyperbolic product, Gender, Diabetes
Introduction
Current guidelines recommend intensive lowering of low-density lipoprotein cholesterol (LDL-C) in type 2 diabetes mellitus (T2DM) patients [1-5]. The common form of T2DM, associated with insulin resistance (IR) and the metabolic syndrome (MetS) is characterized by a singular, non-LDL dyslipidemia, defined as atherogenic dyslipidemia (AD). The hallmark of AD is decreased levels of high-density lipoprotein cholesterol (HDL-C) together with raised triglycerides (TG). AD substantially contributes to residual vascular risk (RVR), even when LDL-C is at or below targets, both in diabetic and nondiabetic populations. The presence and/or severity of AD can be established either from the combined occurrence of high TG levels and low HDL-C or from the ratio of fasting TG to fasting HDL-C, with prior log transformation of TG levels allowing to compute a broad range of TG values [2-11]. Whereas screening for AD provides clinically relevant information for assessing RVR, it is rarely performed due to lack of agreement or consensual cut-offs to define/grade AD from baseline HDL-C and TG levels prior to lipid-lowering therapies (LLD).
We previously reported that log(TG)/HDL-C is a useful measure of increased cardiometabolic risk in T2DM males, in whom the ratio also relates to poorer metabolic control and β-cell function loss [7]. In diabetic females, there exists a reversed dichotomy in risk factors for CAD vis-à-vis nondiabetic women, the former no longer benefiting from female gender’s risk protection. Thus, diabetic women have more severe dyslipidemia, more prevalent obesity and abdominal fat, higher subclinical inflammatory markers, more severe/prevalent hypertension or MetS. Worryingly, diabetic women are less often at targets for major modifiable micro- and macrovascular risk factors, leaving them exposed to higher RVR than males [12-15]. The aim of the present study was (i) to establish and grade AD prevalence in a cohort of T2DM females using the continuous variable log(TG)/HDL-C; (ii) to determine whether this ratio relates to their cardiometabolic phenotype or glucose homeostasis determinants; and (iii) to assess the association between AD and micro- or macrovascular complications, and its impact on absolute 10-year CV risk from the United Kingdom Prospective Diabetes Study (UKPDS) calculator [16].
Methods
This cross-sectional study included 340 female T2DM outpatients, attending the Diabetes Clinic between January 2008 and June 2011. Patients were divided according to quintiles (Q) of baseline, pre-LLD log(TG)/HDL-C values. The following variables were recorded: age, ethnicity, attained educational level (dichotomised as low vs. high, based on higher achieved education degree [no education, primary or secondary vs. higher education and university]), known diabetes duration, family history (CVD, diabetes mellitus), self-reported leisure-time (LT) weekly exercise duration, and daily-time spent watching screen(s) (television, computers and/or visual numeric media), as surrogate for LT sedentarity [7]. The presence of a MetS (score ≥3/5) was defined according to the joint harmonized IDF/NHLBI/AHA/WHF/IAS/IASO criteria [17,18].
Pre-LLD values were retrieved from patients’ records, and used to assess AD prevalence in LLD-treated patients; otherwise, in non-LLD-treated patients, current lipids and AD prevalence were obtained from last available lipid values from the same specimen. In LLD-treated patients, current lipids values were those from last available, post-LLD lipid values. AD prevalence was defined as the combination of MetS criteria for low HDL-C in females (<50 mg.dL-1) and high fasting TG (≥150 mg.dL-1 for both genders) at baseline. AD severity was assessed from log(fasting TG)/fasting HDL-C. Normal values for log(TG)/HDL-C, from 44 healthy white Caucasian females: 0.036 (mean); 0.034 (median); 0.014 (SD); 0.014 (min.); 0.094 (max.); 0.025 (perc. 25) and 0.042 (perc. 75) [7].
Patients were measured for body weight, height, body mass index (BMI), relative and total body fat (BodyFat Analyzer, Omron BF 500), waist circumference and conicity index. Ultrasonographic evidence for fatty liver was considered in the presence of hyperreflectivity in the absence of etiological factors associated with liver steatosis, including excess ethanol intake [19-25]. Computer-based Homeostasis Model Assessment (HOMA) of insulin sensitivity and β-cell function was previously detailed ( http://www.dtu.ox.ac.uk). Values of HOMA B (%) were plotted as a function of HOMA S (%), defining a hyperbolic product area (β×S) [unit: %2; normal value: 100%, corresponding to 104 %2, which represents the true, underlying β-cell function. (BxS) loss over a subject’s lifetime span was obtained by dividing 100-(BxS) by subjects’ age at the time of HOMA-modeling, providing an estimate of annual (BxS) loss rate (%.year-1) [26-29].
Current use of oral antidiabetic drugs, insulin, blood pressure (BP)-lowering drugs, aspirin, and LLD(s) was recorded. Hypertension was defined as systolic BP ≥140 mmHg and/or diastolic BP ≥90 mmHg, or current treatment with BP-lowering drug(s). Coronary artery disease (CAD) was inferred from medical history (myocardial infarction, angioplasty, stenting, revascularization surgery and/or significant coronary stenosis confirmed by angiography) and systematic review of procedures, screening (exercise testing, echocardiography) or subclinical disease imaging data in the patient’s records. Stroke was defined according to UK Prospective Diabetes Study (UKPDS) criteria: any neurological deficit ≥1 month, without distinction between ischaemic, embolic and haemorrhagic strokes. In patients with multiple CV events, only the first one was considered for prevalence [16]. Peripheral artery disease (PAD) was defined by a medical history of lower-limb(s) claudication and/or clinical or imaging evidence for ischemic diabetic foot, angioplasty, stenting, revascularization surgery and/or significant lower-limb artery stenosis at Doppler ultrasonography and/or angiography. UKPDS Risk Engine’s 10-year absolute CV risk for individuals in primary CV prevention was based on the 10 following variables: known T2DM duration, age, gender, ethnicity, smoking status, chronic atrial fibrillation, HbA1clevel, systolic BP, total C and HDL-C[16].
Diabetic retinopathy (DRP) was diagnosed following dilated fundus examination and fluoangiography [30]. The presence of peripheral neuropathy was established from clinical examination (knee and ankle reflexes, Semmes-Weinstein 5.07 monofilament test) and/or 4-limbs electromyography. Normo, micro- and macro-albuminuria were defined as urinary albumin excretion <30 (normo-), 30–299 (microalbuminuria) and ≥300 μg.mg creatinine-1 (macro-albuminuria) from first-morning urine sample. Glomerular filtration rate (eGFR) was estimated using the Modified Diet in Renal Disease formula [31]. Glycated haemoglobin (HbA1c), total cholesterol (C), HDL-C, TG, LDL-C (from Friedewald’s formula), non-HDL-C (by subtracting HDL-C from total C), apolipoproteins (apo) A1 and B100, high-sensitivity C-reactive protein (hsCRP), Big endothelin-1 [32], uric acid, cystatin C, fibrinogen, leucocytes, total and free testosterone, sex-hormone-binding globulin, ferritin, liver enzymes (aspartate aminotransferase [AST], alanine aminotransferase [ALT], γ-glutamyl transferase [γGT]), homocysteine, folic acid and vitamin B12 were determined by routine laboratory methods. Each patient gave informed consent, the research was carried out in compliance with the declaration of Helsinki, and the protocol was approved by the local Institutional Review Board (Commission d'Ethique Biomédicale Hospitalo-Facultaire de l'UCL, Faculté de Médecine).
Statistical methods
Results are presented as means (± 1 standard deviation (SD)) or proportions. The significance of differences between AD quintiles was assessed by one-way analysis of variance for linear trend between means, and by a Chi-squared test for trend for differences in proportions with modified Bonferroni’s adjustment procedure to minimize type 1 error resulting from multiple testing. Results were considered significant or non-significant (NS) for p< or ≥0.05, respectively.
Results
Patient’s characteristics are described in Table 1. There were 340 T2DM females in the whole cohort, 85% of whom from Caucasian ancestry. AD prevalence was 35%. When patients were divided according to AD quintiles of log(TG)/HDL-C, patients in the 2nd quintile (Q II) had a mean log(TG)/HDL-C similar to that of control, nondiabetic subjects (see Methods), whereas patients from the 3rd quintile (Q III) had a mean log(TG)/HDL-C that corresponded to the 75th centile of controls. In the whole T2DM cohort, mean age (1 SD) and diabetes duration were 67 (13) years and 15 (10) years, respectively, without significant trends across quintiles (Qs). Past- or current smoking prevalence was not different between Qs, whereas ethanol intake showed a significant decreasing trend (p <0.0001). LT physical activity and screen-watching daily duration were significantly different across Qs, with increased sedentarity and lesser exercise. There was a significant stepwise decrease in the proportion of patients with higher educational level across quintiles, from 44% (QI) to 18% (QV), which nevertheless was above Bonferroni’s adjusted level of significance. Hypertension prevalence was 85% in the whole cohort, with increasing values across Qs, and mean systolo-diastolic BP values 139 (21) / 78 (11) mmHg, without trends across Qs (Table 1).
Table 1.
Patient’s characteristics
| all patients | Q I | Q II | Q III | Q IV | Q V | P | ||
|---|---|---|---|---|---|---|---|---|
| n |
|
340 |
68 |
68 |
68 |
68 |
68 |
|
| log (TG).HDL-C-1 |
|
0.045 |
0.026 |
0.035 |
0.042 |
0.050 |
0.071 |
~ |
| age |
years |
67 (13) |
67 (13) |
66 (13) |
67 (13) |
68 (12) |
65 (14) |
NS |
| diabetes duration |
years |
15 (10) |
15 (10) |
14 (9) |
15 (11) |
16 (9) |
17 (9) |
NS |
| smoking § |
% |
72-28 |
75-25 |
77-23 |
72-28 |
68-32 |
68-32 |
NS |
| ethanol |
U.week-1 |
5 (11) |
9 (14) |
8 (13) |
5 (11) |
3 (6) |
2 (3) |
0.0001 |
| education |
% |
71:29 |
56:44 |
69:31 |
73:27 |
75:25 |
82:18 |
0.0007 |
| hypertension |
% |
85 |
78 |
82 |
85 |
88 |
91 |
0.0181 |
| Hb A1c |
% |
7.87 (1.62) |
7.50 (1.82) |
7.50 (1.54) |
7.94 (1.62) |
8.06 (1.50) |
8.34 (1.49) |
0.0073 |
| Hb A1c |
mmol.mol-1 |
63 (13) |
58 (14) |
59 (12) |
63 (13) |
65 (12) |
68 (12) |
<0.0001 |
| microangiopathy |
% |
51 |
40 |
45 |
55 |
58 |
60 |
0.0045 |
| retinopathy |
% |
30 |
24 |
27 |
40 |
31 |
29 |
NS |
| peripheral polyneuropathy |
% |
25 |
19 |
16 |
25 |
41 |
28 |
0.0111 |
| macroangiopathy |
% |
22 |
16 |
18 |
28 |
19 |
26 |
NS |
| Epworth score |
|
6 (4) |
5 (4) |
6 (4) |
5 (3) |
7 (5) |
7 (4) |
0.0066 |
| pathological (>9) |
% |
18 |
9 |
17 |
12 |
25 |
25 |
0.0066 |
| OSAS | % | 6 | 0 | 1 | 9 | 7 | 12 | 0.0011 |
Results are expressed as means (1SD) or proportions (%) §: never vs. former/current; HbA1c:glycated haemoglobin; HDL-C: high density lipoprotein cholesterol; OSAS: obstructive sleep apnea/hyponea syndrome; Q: quintile; TG: triglycerides; NS: not significant.
Current glycemic control, inferred from the average of last-available HbA1c, was above target (>7.0%) in the whole group, and significantly worsened across Qs, with a mean absolute differences of 0.84% between Q I/Q II and Q V. The proportion of patients at HbA1c target (33% in the whole cohort) also significantly decreased across Qs, from 47% (Q I) to 21% (Q V; p <0.0001). Prevalence of any microangiopathy (51% in the whole cohort) significant increased across Qs, from 40% (Q I) to 60% (Q V; p 0.0045). The increase in microvascular complications prevalence across Qs remained significant after adjustment for HbA1c (p 0.0261 [overall microangiopathy]; p 0.0114 [peripheral neuropathy]). When patients were analyzed according to Qs of non-HDL-C, there were no associations between the latter and the frequency of microangiopathy, overall or site-specific. As regards macroangiopathy, there was no trend for higher prevalence across Qs of log(TG)/HDL-C: (overall prevalence (22%); CAD (15%); TIA/stroke (6%) and PAD (5%). Epworth’s daytime sleepiness score and sleep apnoea syndrome prevalence significantly increased across Qs, from 5 (4) and 0% (Q I) to 7 (4) and 12% (Q V; p 0.0066 and 0.0011, respectively; Table 1).
There were highly-significant trends across Qs for progressively higher values in BMI, waist circumference, fat mass, conicity index, waist-to-height ratio, visceral fat, and fat-free mass index. Mean insulin sensitivity, β-cell function, and the hyperbolic product between them [BxS] were lower than the normal (100%) value in the whole cohort: 57% (HOMA S), 65% (HOMA B), and 28.6% [BxS], respectively. There were stepwise decreases across Qs in HOMA S and [BxS], from 82% (HOMA S) and 35.9% [BxS] (Q I) to 39% (HOMA S) and 22.5% (BxS) (Q V). [BxS] loss rate significantly worsened across Qs: 1.17 %.yr-1 (Q I) to 1.52 %.yr-1 (Q V). There was a significant increase across Qs in fasting insulinemia: 77 (Q I) to 141 pmol.l-1 (Q V; p<0.0001). Liver steatosis frequency (64% prevalent in the whole cohort) significantly rose across Qs: 27% (Q I) to 83% (Q V; p<0.0001). A MetS phenotype was present in 86% of the entire cohort; there was a highly-significant stepwise rise in both MetS prevalence and score across Qs: 59% and 2.6 (Q I) to 100% and 4.7 (Q V; both p <0.0001). The contribution of discrete components to the MetS score for each Q showed significant trends for higher prevalence of hypertension (p 0.0181) and enlarged waist circumference (p <0.0001; Table 2).
Table 2.
Cardiometabolic phenotype
| all patients | Q I | Q II | Q III | Q IV | Q V | P | ||
|---|---|---|---|---|---|---|---|---|
| n |
|
340 |
68 |
68 |
68 |
68 |
68 |
|
| body mass index |
kg. m-2 |
30.3 (6.6) |
26.6 (4.9) |
28.9 (6.5) |
31.6 (5.8) |
31.2 (6.8) |
33.2 (6.8) |
<0.0001 |
| weight |
kg |
78.5 (17.4) |
69.4 (14.3) |
75.9 (17.7) |
81.1 (14.5) |
81.3 (17.9) |
84.9 (18.3) |
<0.0001 |
| waist circumference |
cm |
100 (15) |
90 (12) |
99 (13) |
104 (13) |
103 (16) |
107 (14) |
<0.0001 |
| fat mass |
% |
41.0 (6.8) |
38.5 (7.8) |
40.1 (6.7) |
42.9 (5.4) |
41.2 (6.7) |
42.5 (6.2) |
0.0006 |
| conicity index |
m2.kg-1 |
1.32 (0.10) |
1.27 (0.10) |
1.32 (0.08) |
1.34 (0.09) |
1.34 (0.10) |
1.36 (0.10) |
<0.0001 |
| waist. height-1 |
|
0.62 (0.10) |
0.56 (0.08) |
0.61 (0.08) |
0.65 (0.09) |
0.64 (0.11) |
0.67 (0.09) |
<0.0001 |
| visceral fat |
0-30 score |
10 (3) |
8 (3) |
9 (3) |
11 (3) |
11 (3) |
11 (3) |
<0.0001 |
| fat free mass index |
kg.m-2 |
17.8 (3.4) |
17.2 (3.9) |
17.3 (3.3) |
18.1 (2.3) |
17.8 (3.5) |
18.8 (3.5) |
0.0377 |
| HOMA S |
% |
57 (42) |
82 (52) |
67 (45) |
46 (34) |
45 (26) |
39 (30) |
<0.0001 |
| HOMA B |
% |
65 (48) |
55 (40) |
54 (31) |
77 (63) |
70 (45) |
70 (53) |
0.0158 |
| HOMA product [BxS] |
% |
28.6 (18.5) |
35.9 (21.1) |
30.6 (20.3) |
26.8 (18.0) |
25.9 (11.9) |
22.5 (17.1) |
<0.0001 |
| [BxS] loss rate |
%.yr-1 |
1.29 (0.51) |
1.17 (0.53) |
1.22 (0.47) |
1.30 (0.47) |
1.26 (0.29) |
1.52 (0.68) |
0.0008 |
| fasting insulinaemia |
pmol.1-1 |
114 (72) |
77 (48) |
103 (78) |
126 (80) |
127 (69) |
141 (69) |
<0.0001 |
| metabolic syndrome |
% |
86 |
59 |
82 |
94 |
94 |
100 |
<0.0001 |
| metabolic syndrome score |
0/5 to 5/5 |
3.7 (1.1) |
2.6 (1.0) |
3.3 (0.9) |
3.8 (0.8) |
4.3 (0.9) |
4.7 (0.6) |
<0.0001 |
| liver steatosis | % | 64 | 27 | 55 | 76 | 80 | 83 | <0.0001 |
Results are expressed as means (1 SD) or proportions (%). HOMA S and HOMA B: insulin sensitivity and β-cell function determined from Homeostatic Model Assessment. Q: quintile.
As regards glucose-lowering therapies, 58% of patients were treated with metformin; 41% with a β-cell stimulant; and/or 50% with exogenous insulin. There was a significant trend for higher insulin use or higher dosage across quintiles: 35% and 0.42 UI.day-1.kg-1 (Q I) to 58% and 1.13 UI.day-1.kg-1 (Q V; p 0.0058 and <0.0001, respectively). LLD(s) were given to 63% of the whole cohort, with statin; fenofibrate and/or ezetimibe prescribed to 51%; 19% and/or 3%. There was a significant trend for increased fenofibrate use across Qs: 9% (Q I) to 39% (Q V; p <0.0001). None of the patients were treated with niacin.
Mean baseline TG values were 206 (191) mg.dL-1 in the entire cohort. There were no significant differences across Qs in baseline LDL-C and non-HDL-C. As for current lipid values, mean LDL-C was 100 (36) mg.dL-1; HDL-C 52 (15) mg.dL-1; and TG 160 (105) mg.dL-1. Mean non-HDL-C was 132 (42) mg.dL-1. There were significant trends for increasing values across Qs in non-HDL-C, apoB100, and apoB100.apoA1-1 (p 0.0078, <0.0001 and <0.0001, respectively). There were also significant trends for decreasing values across Qs in apoA1 and in LDL size’s surrogate [LDL-C/apoB100] (both p <0.0001). Increasing levels across Qs were also observed for hsCRP, fibrinogen, Big ET-1, uric acid, cystatin C, and magnesium, and for decreasing levels in SHBG (Table 3). In the entire cohort, mean eGFR was 76 (28) ml.min-1.1.73m-2, and albuminuria 85 (273) μg.mg creatinine-1, without significant differences between Qs.
Table 3.
Laboratory values
| all patients | Q I | Q II | Q III | Q IV | Q V | P | ||
|---|---|---|---|---|---|---|---|---|
| n |
|
340 |
68 |
68 |
68 |
68 |
68 |
|
| atherogenic dyslipidemia |
% |
35 |
1 |
12 |
25 |
56 |
82 |
~ |
| pre-LLD lipids |
|
|
|
|
|
|
|
|
| LDL-C |
mg.dl-1 |
150 (35) |
149 (25) |
159 (46) |
155 (28) |
152 (39) |
137 (28) |
NS |
| non-HDL-C |
mg.dl-1 |
187 (41) |
172 (28) |
191 (54) |
193 (36) |
193 (46) |
187 (36) |
NS |
| HDL-C |
mg.dl-1 |
52 (15) |
69 (17) |
54 (8) |
50 (9) |
46 (11) |
42 (8) |
~ |
| triglycerides |
mg.dl-1 |
206 (191) |
120 (66) |
159 (80) |
205 (98) |
225 (204) |
317 (308) |
~ |
| current lipids |
|
|
|
|
|
|
|
|
| total cholesterol |
mg.dl-1 |
185 (43) |
195 (35) |
185 (45) |
182 (43) |
181 (41) |
180 (51) |
NS |
| LDL-C |
mg.dl-1 |
100 (36) |
102 (31) |
101 (38) |
104 (38) |
102 (34) |
90 (36) |
NS |
| non-HDL-C |
mg.dl-1 |
132 (42) |
121 (33) |
126 (42) |
132 (41) |
137 (39) |
145 (49) |
0.0078 |
| apoB100 |
mg.dl-1 |
92 (27) |
80 (22) |
90 (26) |
91 (25) |
96 (26) |
103 (30) |
<0.0001 |
| HDL-C |
mg.dl-1 |
52 (15) |
74 (11) |
59 (6) |
50 (4) |
44 (4) |
35 (5) |
~ |
| apoA1 |
mg.dl-1 |
164 (30) |
195 (24) |
164 (20) |
166 (23) |
152 (22) |
143 (32) |
<0.0001 |
| triglycerides |
mg.dl-1 |
160 (105) |
91 (42) |
121 (55) |
142 (49) |
176 (73) |
272 (152) |
~ |
| apoB100.apoA1-1 |
|
0.54 (0.19) |
0.39 (0.12) |
0.52 (0.16) |
0.52 (0.14) |
0.62 (0.17) |
0.67 (0.22) |
<0.0001 |
| LDL-C.apoB-1 |
|
1.05 (0.30) |
1.23 (0.27) |
1.07 (0.30) |
1.08 (0.26) |
1.00 (0.26) |
0.86 (0.25) |
<0.0001 |
|
hsCRP |
mg.dl-1 |
0.50 (0.68) |
0.28 (0.38) |
0.46 (0.90) |
0.53 (0.69) |
0.50 (0.48) |
0.70 (0.74) |
0.0082 |
| fibrinogen |
mg.dl-1 |
340 (78) |
313 (66) |
340 (76) |
334 (72) |
348 (69) |
365 (97) |
0.0025 |
| Big ET-1 |
pg.ml-1 |
6.29 (2.31) |
5.55 (2.45) |
5.14 (1.21) |
6.31 (2.53) |
6.71 (1.84) |
7.88 (2.46) |
<0.0001 |
| uric acid |
mg.dl-1 |
5.3 (1.7) |
4.8 (1.6) |
4.8 (1.5) |
5.6 (1.4) |
5.4 (1.7) |
5.8 (1.8) |
0.0002 |
| cystatin C |
mg.1-1 |
0.88 (0.30) |
0.75 (0.23) |
0.78 (0.28) |
0.88 (0.26) |
0.91 (0.28) |
1.03 (0.38) |
<0.0001 |
| magnesium |
mEq.dl-1 |
1.63 (0.20) |
1.67 (0.22) |
1.71 (0.16) |
1.61 (0.17) |
1.64 (0.21) |
1.56 (0.19) |
<0.0001 |
| SHBG | nmol.1-1 | 48 (33) | 66 (45) | 45 (28) | 43 (23) | 46 (29) | 40 (30) | <0.0001 |
Results are expressed as means (1SD) or proportions (%). apo: apoliprotein; C: cholesterol; ET: endothelin; HDL: high-density lipoprotein; hsCRP: high sensitivity C-reactive protein; LDL: low-density lipoprotein; LLD: lipid-lowering drug(s); Q: quintile; SHBG: sex hormone-binding globulin; NS: not significant.
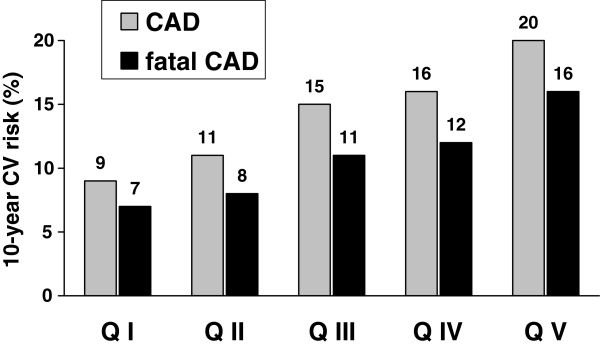
In the entire cohort, 258 patients were in primary CV prevention, and as a result eligible for UKPDS risk estimation. Their mean 10-year absolute CV risk prediction was: 13 (11) % (CAD); 10 (10) % (fatal CAD); 11 (16) % (stroke) and 2 (3) % (fatal stroke). Figure 1 illustrates UKPDS risk for CAD, after adjustment for inter-quintile differences in mean age: there were significant trends for stepwise heightened risk across Qs: 9% (CAD) and 7% (fatal CAD) (Q I) to 20% (CAD) and 16% (fatal CAD) (Q V; both p <0.0001). No increasing trends across Qs were observed for risk of stroke or lethal stroke (not illustrated).
Figure 1.
United Kingdom Prospective Diabetes Study Risk Engine’s 10-year absolute predicted risk of developing non-fatal or fatal coronary artery disease (CAD; grey bars) or fatal CAD (solid bars) in 258 T2DM females according to quintiles of log (TG)/HDL-C ranking distribution. Within each quintile, data were obtained from subsets of patients (in % for each quintile) in primary cardiovascular prevention (Q I: 81%; Q II: 81%; Q III: 69%; Q IV: 78%; and Q V: 71%). HDL-C: high-density lipoprotein cholesterol; TG: triglycerides. Significance of differences between quintiles: both P<0.0001 (CAD and fatal CAD).
Discussion
The main findings of the present study are fourfold. Firstly, we observed a high prevalence of AD in T2DM females, with 35% affected, similar to that observed in T2DM males. Secondly, the AD ratio log(TG)/HDL-C is an easy means to estimate IR, and was strongly related to a cardiometabolic phenotype characterized by IR, MetS, hyperinsulinemia, central fat accretion, sarcopenia, liver steatosis and sedentary lifestyle. Thirdly, this ratio was linked to a stepwise gradient for residual risk of future CAD. Lastly, this ratio was related to decreased insulin secretion, β-cell function loss, poorer glycemic control and higher frequency of microangiopathies. The AD ratio may therefore allow to easily identifying a comprehensive modifiable component of non-LDL, lipid-related residual vascular risk, as well as pinpoint worsening in glucose homeostasis determinants [5-7].
The hallmark of AD is decreased HDL-C level together with raised TG, with LDL-C often only marginally elevated [2-5,33-40]. Computing a ratio from fasting TG (numerator) and fasting HDL-C (denominator) allows not only for grading AD severity as a continuous variable, but also for incorporating the effect of mutually-reinforcing or diverging confounders affecting both fraction’s components and that of one variable on the variance of the other [7]. An analysis of isolated TG or HDL-C, both indispensable determinants to define AD, as standard continuous variables would not be able to incorporate a measure of AD. Using consensual MetS cutoffs for defining decreased HDL-C and elevated TG, we observed a high prevalence of AD in T2DM females (more than one-third). In comparison with log(TG)/HDL-C values from non-diabetic female controls, the mean AD ratio in T2DM females was well above normal values, from the fourth AD quintile onwards. This demonstrates that >40% of this population may present with AD of varying severity. This finding was expected, as raised TG and low HDL-C levels are used to define MetS phenotype, and as the latter is highly prevalent (86%) in T2DM [17,18].
To rule out any confounding effect of LLD, we used baseline TG and HDL-C to compute the AD ratio, prior to anti-dyslipidemic drug(s). This proved appropriate, as the mean difference between baseline and current TG levels in this study averaged 46 mg.dL-1. This approach allowed thus to precisely establishing the true, underlying magnitude and frequency of AD in T2DM females [5,7]. There is a known gender difference in HDL-C and TG levels in the general population, also observed in this diabetic sample, with T2DM females exhibiting higher mean HDL-C and lower TG values than their T2DM males, by an average 6 and 12 mg/dl, respectively (data not shown) [12-14]. As for lifestyle-related confounders of AD prevalence/severity, our data showed a significant decreasing trend across AD quintiles for alcohol intake, the inverse association being expected due to ethanol’s HDL-C-raising effects. On the other hand, we observed a trend for lower educational level. This may promote less healthy behaviours, and result in adverse (i.e. less cardioprotective) anthropometrics and body composition.
The AD ratio was associated, in a stepwise gradation, with higher levels of numerous non-lipid cardiometabolic markers: fasting insulinemia; hsCRP; fibrinogen; Big ET-1; uric acid, and cystatin C. Compensatory hyperinsulinemia is likely to represent a major underlying driver for both AD and for the proatherogenic changes observed in non-LDL lipids and non-lipid CV markers. Endothelin-1 upregulation and/or nitric oxide release imbalance by endothelial cells is another putative pathway linking IR to macroangiopathy. Thus, insulin stimulates endothelin-1 production and action through MAP-kinase-dependent pathways, while ET-1 induces IR and reactive hyperinsulinemia. Such a feed-back loop may contribute to the pathogenesis of macrovascular disease in cardiometabolic states [32]. Expectedly, SHBG levels significantly decreased across quintiles, since IR/portal hyperinsulinemia are associated with decreased liver production and lower levels of this androgen transporter [4,5,24,25,32,39].
Accumulating evidence links AD in T2DM not only to residual macrovascular risk, but also to residual risk for new-onset or progression of microangiopathy [11]. In this study, log(TG)/HDL-C was associated with lesser residual insulin secretion, as well as with more severe hyperbolic product (BxS) loss over time, and heralded poorer glycemic control and earlier or more intensive need for stepping-up glucose-lowering therapies, as we previously reported in T2DM males [7,22,23,29,41,42]. The AD ratio was associated with predicted CAD risk and prevalent overall microangiopathy. When patients were analyzed according to quitiles of non-HDL-C, another variable which rises alongside the severity of AD, we found no association between the latter and the frequency of microangiopathy. This, together with the independence from diabetes control comforts the relevance of resorting to the log(TG)/HDL-C ratio to estimate a modifiable lipid-related component of residual vascular risk linked to AD. Noteworthy, treatment with fenofibrate, a PPAR-α agonist that specifically targets AD, exerts beneficial effects on macrovascular disease in T2DM patients with AD, but also confers vascular protection against retinal, renal and lower-limb microangiopathies [3,4,9-11].
The present study has several limitations. Firstly, as a cross-sectional hypothesis-generating study, it does not allow for inferring causality relationships. Secondly, as we used routine measurement of HDL-C, without HDL subclass assessment, we cannot discriminate between atheroprotective vs. dysfunctional, less atheroprotective or even atherogenic particles. As the latter often concur with decreased HDL-C, a low HDL-C level in the ratio may nevertheless capture to some extent these qualitative defects. Finally, the population under study was mostly of European Caucasian ancestry, and the present findings require replication in other racial/ethnic subgroups. Moreover, the observed associations were documented from females patients with established T2DM, and may not necessarily exist in newly-diagnosed T2DM females, nor in other types of diabetes or in women with normal glucose homeostasis [43-45].
In conclusion, log(TG)/HDL-C allows for grading AD and estimating non-LDL-related residual vascular risk in T2DM females. This AD ratio negatively associates with the two major determinants of glucose homeostasis. This means that patients with more severe AD will have poorer metabolic control in addition to higher predicted CAD risk. The ratio log(TG)/HDL-C appears a simple, cheap noninvasive means to better precise gender-specific assessment and care of the female patient with diabetes. Identifying patients with abnormal AD ratio should allow improving selection and follow-up of those most at risk, more likely to benefit from available or newer therapies targeting hypertriglyceridemia and/or hypo-HDL-cholesterolemia.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
MPH was involved in the design and conduct of the study, as well as in collecting patients’ data; MPH, SAA and MFR were involved in analysis and interpretation; preparation and review of the manuscript, and decision to submit the manuscript for publication. All authors read and approved the final manuscript.
Contributor Information
Michel P Hermans, Email: michel.hermans@diab.ucl.ac.be.
Sylvie A Ahn, Email: michel.rousseau@uclouvain.be.
Michel F Rousseau, Email: rousseau@card.ucl.ac.be.
References
- American Diabetes Association. Standards of medical care in diabetes - 2009. Diabetes Care. 2009;32:S13–S61. doi: 10.2337/dc09-S013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, Witztum JL. Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2008;51:1512–1524. doi: 10.1016/j.jacc.2008.02.034. [DOI] [PubMed] [Google Scholar]
- Fruchart J-C, Sacks F, Hermans MP, Assmann G, Brown WV, Ceska R, Chapman MJ, Dodson PM, Fioretto P, Ginsberg HN, Kadowaki T, Lablanche J-M, Marx N, Plutzky J, Reiner Z, Rosenson RS, Staels B, Stock JK, Sy R, Wanner C, Zambon A. Zimmet P for the Residual Risk Reduction Initiative. The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in patients with dyslipidemia. Am J Cardiol. 2008;102:1–34. doi: 10.1016/j.amjcard.2008.02.089. [DOI] [PubMed] [Google Scholar]
- Hermans MP, Fruchart JC. Reducing residual vascular risk in patients with atherogenic dyslipidaemia: where do we go from here? Clinical Lipidology. 2010;5:811–826. doi: 10.2217/clp.10.65. [DOI] [Google Scholar]
- Hermans MP, Ahn SA, Rousseau MF. Recent Advances in the Pathogenesis, Prevention and Management of Type 2 Diabetes and its Complications. Rijeka (Croatia): Intech; 2011. Residual vascular risk in T2DM: the next frontier. [Google Scholar]
- Davignon J, Cohn JS. Triglycerides: a risk factor for coronary heart disease. Atherosclerosis. 1996;124(Suppl):S57–S64. doi: 10.1016/0021-9150(96)05858-3. [DOI] [PubMed] [Google Scholar]
- Hermans MP, Ahn SA, Rousseau MF. Log(TG)/HDL-C is related to both residual cardiometabolic risk and β-cell function loss in type 2 diabetes males. Cardiovasc Diabetol. 2010;9:88. doi: 10.1186/1475-2840-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar N, Sandhu MS, Ricketts SL, Butterworth AS, Di Angelantonio E, Boekholdt SM, Ouwehand W, Watkins H, Samani NJ, Saleheen D, Lawlor D, Reilly MP, Hingorani AD, Talmud PJ, Danesh J. Triglyceride Coronary Disease Genetics Consortium and Emerging Risk Factors Collaboration. Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010;375:1634–1639. doi: 10.1016/S0140-6736(10)60545-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC Jr, Cushman WC, Simons-Morton DG, Byington RP. ACCORD Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruchart J-C, Sacks FM, Hermans MP. Implications of the ACCORD lipid study: perspective from the Residual Risk Reduction Initiative (R3i) Curr Med Res Opin. 2010;26:1793–1797. doi: 10.1185/03007995.2010.489341. [DOI] [PubMed] [Google Scholar]
- Hermans MP. Prevention of microvascular diabetic complications by fenofibrate: Lessons from FIELD and ACCORD. Diab Vasc Dis Res. 2011;8:180–189. doi: 10.1177/1479164111407783. [DOI] [PubMed] [Google Scholar]
- Singh R, Wiegers SE, Goldstein BJ. Impact of gender on diabetes mellitus and its associated cardiovascular risk factors. J Gend Specif Med. 2001;4:28–36. [PubMed] [Google Scholar]
- Mosca L, Appel LJ, Benjamin EJ. American Heart Association, American College of Cardiology, American College of Nurse Practitioners, American College of Obstetricians and Gynecologists, American College of Physicians, American Medical Women’s Association, Association of Black Cardiologists, Centers for Disease Control and Prevention, National Heart, Lung, and Blood Institute, National Institutes of Health, Office of Research on Women’s Health, Society of Thoracic Surgeons and World Heart Federation et al. Evidence-based guidelines for cardiovascular disease prevention in women. J Am Coll Cardiol. 2004;43:900–921. doi: 10.1016/j.jacc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Legato MJ, Gelzer A, Goland R, Ebner SA, Rajan S, Villagra V, Kosowski M. Writing Group for The Partnership for Gender-Specific Medicine. Gender-specific care of the patient with diabetes: review and recommendations. Gend Med. 2006;3:131–158. doi: 10.1016/S1550-8579(06)80202-0. [DOI] [PubMed] [Google Scholar]
- Hermans MP, Ahn S, Rousseau MF. Neurohormonal biomarkers and UKPDS stroke risk in type 2 diabetic women on primary cardiovascular prevention. Diabetes Metab Syndr Clin Res Rev. 2008;2:93–98. doi: 10.1016/j.dsx.2008.02.001. [DOI] [Google Scholar]
- Stevens RJ, Coleman RL, Adler AI, Stratton IM, Matthews DR, Holman RR. UKPDS 66: risk factors for myocardial infarction case fatality and stroke case fatality in type 2 diabetes. Diabetes Care. 2004;27:201–207. doi: 10.2337/diacare.27.1.201. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr. International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; International Association for the Study of Obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Sadikot S, Hermans MP. Here we go again … The metabolic syndrome revisited! Diab Metab Syndr. 2010;4:111–120. doi: 10.1016/j.dsx.2010.05.011. [DOI] [Google Scholar]
- Valdez R, Seidell JC, Ahn YI, Weiss KM. A new index of abdominal adiposity as an indicator of risk for cardiovascular disease: a cross-population study. Int J Obes. 1992;16:77–82. [PubMed] [Google Scholar]
- Kyle UG, Morabia A, Schutz Y, Pichard C. Sedentarism affects body fat mass index and fat-free mass index in adults aged 18 to 98 years. Nutrition. 2004;20:255–260. doi: 10.1016/j.nut.2003.11.019. [DOI] [PubMed] [Google Scholar]
- Burkhauser RV, Cawley J. Beyond BMI: the value of more accurate measures of fatness and obesity in social science research. J Health Econ. 2008;27:519–529. doi: 10.1016/j.jhealeco.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Dehout F, Haumont S, Gaham N, Amoussou-Guenou KD, Hermans MP. Metabolic syndrome in Bantu subjects with type 2 diabetes from sub-Saharan extraction: prevalence, gender differences and HOMA-hyperbolic product. Diab Metab Syndr. 2008;2:5–11. doi: 10.1016/j.dsx.2007.11.008. [DOI] [Google Scholar]
- Munoko T, Hermans MP. Phenotypic characterization of first generation Maghrebian migrants with type 2 diabetes: a gender-based comparison with a reference North-Caucasian Belgian cohort. Diab Met Syndr. 2008;2:115–124. doi: 10.1016/j.dsx.2008.02.004. [DOI] [Google Scholar]
- Hermans MP, Ahn SA, Rousseau MF. Cardiometabolic phenotype and UKPDS risk in male type 2 diabetic patients with obstructive sleep apnoea syndrome. Diabetes Metab Syndr. 2009;3:50–54. doi: 10.1016/j.dsx.2008.10.011. [DOI] [Google Scholar]
- Hermans MP, Ahn SA, Rousseau MF. Micro- and macrovascular risk and complications in T2DM males with obstructive sleep apnoea. J Clin Metab Diab. 2011;2:64–70. [Google Scholar]
- Levy JC, Matthews DR, Hermans MP. Correct Homeostasis Model Assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21:2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- Hermans MP, Levy J, Morris RJ, Turner RC. Comparison of insulin sensitivity tests across a range of glucose tolerance from normal to diabetes. Diabetologia. 1999;42:678–687. doi: 10.1007/s001250051215. [DOI] [PubMed] [Google Scholar]
- Hermans MP, Levy J, Morris RJ, Turner RC. Comparison of tests of beta-cell function across a range of glucose tolerance from normal to diabetes. Diabetes. 1999;48:1779–1786. doi: 10.2337/diabetes.48.9.1779. [DOI] [PubMed] [Google Scholar]
- Hermans MP. Diabetic macro- and microvascular disease in type 2 diabetes. Diabetes Vasc Dis Res. 2007;4:S7–S11. [Google Scholar]
- Hermans MP, Ahn SA, Rousseau MF. Statin therapy and cataract in Type 2 diabetes. Diabetes Metab. 2011;37:139–143. doi: 10.1016/j.diabet.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- Hermans MP, Ahn SA, Gruson D, Rousseau MF. The metabolic syndrome phenotype is associated with raised circulating Big endothelin-1 independently of coronary artery disease in type 2 diabetes. Diab Metab Syndr. 2007;1:229–237. doi: 10.1016/j.dsx.2007.09.001. [DOI] [Google Scholar]
- Dobiásová M, Frohlich J. The plasma parameter log (TG/HDL-C) as an atherogenic index: correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FERHDL) Clin Biochem. 2001;34:583–588. doi: 10.1016/S0009-9120(01)00263-6. [DOI] [PubMed] [Google Scholar]
- Hermans MP, Ahn SA, Rousseau MF. The non-HDL-C/HDL-C ratio provides cardiovascular risk stratification similar to the ApoB/ApoA1 ratio in diabetics: comparison with reference lipid markers. Diab Metab Syndr. 2007;1:23–28. doi: 10.1016/j.dsx.2006.11.003. [DOI] [Google Scholar]
- da Luz PL, Favarato D, Faria-Neto JR Jr, Lemos P, Chagas AC. High ratio of triglycerides to HDL-cholesterol predicts extensive coronary disease. Clinics (Sao Paulo) 2008;63:427–432. doi: 10.1590/S1807-59322008000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel WB, Vasan RS, Keyes MJ, Sullivan LM, Robins SJ. Usefulness of the triglyceride-high-density lipoprotein versus the cholesterol-high-density lipoprotein ratio for predicting insulin resistance and cardiometabolic risk (from the Framingham Offspring Cohort) Am J Cardiol. 2008;101:497–501. doi: 10.1016/j.amjcard.2007.09.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner V, Johnson BD, Zineh I, Rogers WJ, Vido D, Marroquin OC, Bairey-Merz CN, Sopko G. The triglyceride/high-density lipoprotein cholesterol ratio predicts all-cause mortality in women with suspected myocardial ischemia: a report from the Women’s Ischemia Syndrome Evaluation (WISE) Am Heart J. 2009;157:548–555. doi: 10.1016/j.ahj.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero A, Andrés E, Ordoñez B, León M, Laclaustra M, Grima A, Luengo E, Moreno J, Bes M, Pascual I, Civeira F, Pocoví M, Alegría E, Casasnovas JA. MESYAS (MEtabolic Syndrome Active Subjects) Study Investigators. Usefulness of triglycerides-to-high-density lipoprotein cholesterol ratio for predicting the first coronary event in men. Am J Cardiol. 2009;104:1393–1397. doi: 10.1016/j.amjcard.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Kim-Dorner SJ, Deuster PA, Zeno SA, Remaley AT, Poth M. Should triglycerides and the triglycerides to high-density lipoprotein cholesterol ratio be used as surrogates for insulin resistance? Metabolism. 2010;59:299–304. doi: 10.1016/j.metabol.2009.07.027. [DOI] [PubMed] [Google Scholar]
- Hermans MP, Sacks F, Ahn SA, Rousseau MF. Non-HDL-cholesterol as valid surrogate to apolipoprotein B100 measurement in diabetes: Discriminant Ratio and unbiased equivalence. Cardiovasc Diabetol. 2011;10:20. doi: 10.1186/1475-2840-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J, Atkinson AB, Bell PM, McCance DR, Hadden DR. Beta-cell deterioration determines the onset and rate of progression of secondary dietary failure in type 2 diabetes mellitus: the 10-year follow-up of the Belfast Diet Study. Diabet Med. 1998;15:290–296. doi: 10.1002/(SICI)1096-9136(199804)15:4<290::AID-DIA570>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- Sorrentino SA, Besler C, Rohrer L, Meyer M, Heinrich K, Bahlmann FH, Mueller M, Horváth T, Doerries C, Heinemann M, Flemmer S, Markowski A, Manes C, Bahr MJ, Haller H, von Eckardstein A, Drexler H, Landmesser U. Endothelial-vasoprotective effects of high-density lipoprotein are impaired in patients with type 2 diabetes mellitus but are improved after extended-release niacin therapy. Circulation. 2010;121:110–122. doi: 10.1161/CIRCULATIONAHA.108.836346. [DOI] [PubMed] [Google Scholar]
- Li C, Ford ES, Meng YX, Mokdad AH, Reaven GM. Does the association of the triglyceride to high-density lipoprotein cholesterol ratio with fasting serum insulin differ by race/ethnicity? Cardiovasc Diabetol. 2008;7:4. doi: 10.1186/1475-2840-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans MP, Fruchart JC. Reducing vascular events risk in patients with dyslipidemia: an update for clinicians. Ther Adv Chronic Dis. 2011;2:307–323. doi: 10.1177/2040622311413952. [DOI] [PMC free article] [PubMed] [Google Scholar]