We projected outcomes for mothers and infants following World Health Organization–recommended regimens to prevent mother-to-child human immunodeficiency virus (HIV) transmission. Compared with Option A, Option B improves life expectancy and saves money; compared with Option B, lifelong maternal therapy is of comparable value to common HIV-related interventions.
Keywords: HIV, mother-to-child transmission, PMTCT, pediatric HIV, cost-effectiveness
Abstract
Background. In 2010, the World Health Organization (WHO) released revised guidelines for prevention of mother-to-child human immunodeficiency virus (HIV) transmission (PMTCT). We projected clinical impacts, costs, and cost-effectiveness of WHO-recommended PMTCT strategies in Zimbabwe.
Methods. We used Zimbabwean data in a validated computer model to simulate a cohort of pregnant, HIV-infected women (mean age, 24 years; mean CD4 count, 451 cells/µL; subsequent 18 months of breastfeeding). We simulated guideline-concordant care for 4 PMTCT regimens: single-dose nevirapine (sdNVP); WHO-recommended Option A, WHO-recommended Option B, and Option B+ (lifelong maternal 3-drug antiretroviral therapy regardless of CD4). Outcomes included maternal and infant life expectancy (LE) and lifetime healthcare costs (2008 US dollars [USD]). Incremental cost-effectiveness ratios (ICERs, in USD per year of life saved [YLS]) were calculated from combined (maternal + infant) discounted costs and LE.
Results. Replacing sdNVP with Option A increased combined maternal and infant LE from 36.97 to 37.89 years and would reduce lifetime costs from $5760 to $5710 per mother–infant pair. Compared with Option A, Option B further improved LE (38.32 years), and saved money within 4 years after delivery ($5630 per mother–infant pair). Option B+ (LE, 39.04 years; lifetime cost, $6620 per mother–infant pair) improved maternal and infant health, with an ICER of $1370 per YLS compared with Option B.
Conclusions. Replacing sdNVP with Option A or Option B will improve maternal and infant outcomes and save money; Option B increases health benefits and decreases costs compared with Option A. Option B+ further improves maternal outcomes, with an ICER (compared with Option B) similar to many current HIV-related healthcare interventions.
(See the Editorial Commentary by Sawe and Lockman on pages 447–9.)
Effective medications for the prevention of mother-to-child human immunodeficiency virus (HIV) transmission (PMTCT) can reduce perinatal HIV transmission to <2% in the absence of breastfeeding and to <5% by 6 months of age among breastfeeding infants [1–3]. As a result, the World Health Organization (WHO) has called for the “virtual elimination” of pediatric HIV [1–3]. Access to antiretroviral medications (ARVs) for PMTCT remains limited, however; only 59% of HIV-infected pregnant women received ARVs for PMTCT in 2010 [4]. As a result, nearly 400 000 new infant HIV infections occur annually, and HIV-infected women experience high postpartum morbidity and mortality [4–6].
In 2010, WHO issued revised guidelines for PMTCT [1]. The guidelines included a renewed emphasis on identification of pregnant, HIV-infected women with CD4 count ≤350 cells/µL or WHO stage 3–4 disease, who require lifelong 3-drug antiretroviral therapy (ART) for treatment of their own HIV infections and for PMTCT. For women with less-advanced disease, WHO recommends a country- or program-level choice between Option A (maternal zidovudine in pregnancy; infant nevirapine [NVP] throughout breastfeeding), and Option B (maternal 3-drug ARV regimens throughout pregnancy and breastfeeding, with interruption after weaning). Select programs are considering Option B+, in which maternal 3-drug regimens are initiated in pregnancy (regardless of maternal CD4) and continued throughout life, including throughout breastfeeding and subsequent pregnancies [3, 7].
HIV prevalence in antenatal care (ANC) is estimated at 16% in Zimbabwe, leading to approximately 61 000 births per year to HIV-infected women [8, 9]. Through 2009, the Zimbabwe National PMTCT Program provided single-dose NVP (sdNVP) to all HIV-infected women, with ART for women identified clinically as ART eligible [8]. Like most countries in sub–Saharan Africa, Zimbabwe initially implemented the revised WHO guidelines with Option A (with antenatal coverage of 46% in 2010) and will soon be examining the feasibility of Options B and B+ [4]. We used validated computer models of HIV disease and PMTCT [10–12] to project the clinical outcomes and cost effectiveness of implementing WHO-recommended PMTCT regimens in Zimbabwe.
METHODS
Analytic Overview
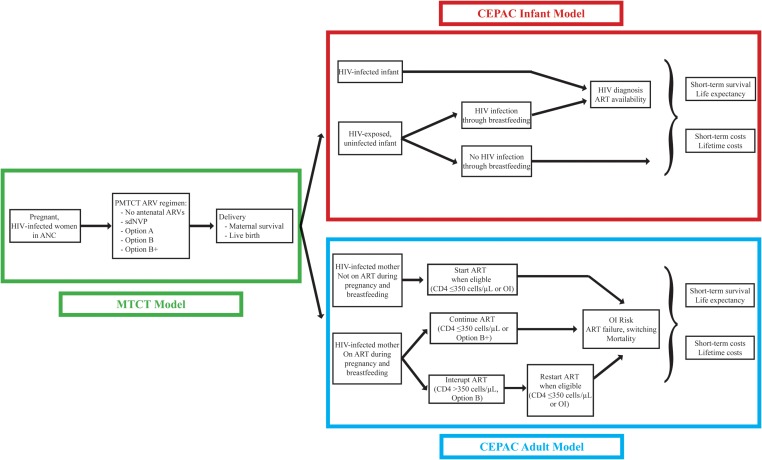
We used 3 validated, linked computer models for this analysis (Figure 1): (1) a model of a single pregnancy and delivery (the mother-to-child HIV transmission [MTCT] model [10]); (2) the Cost-effectiveness of Preventing AIDS Complications (CEPAC) model of HIV infection and mortality among breastfed infants (the CEPAC infant model [13, 14]); and (3) the CEPAC-International model of HIV disease progression among postpartum women (the CEPAC adult model [11, 12, 15]). Clinical outcomes of the linked models included infant HIV infection risk at weaning, maternal life expectancy (LE) from delivery, and infant LE from birth. Economic outcomes, from the healthcare system perspective, included ANC costs (through delivery), maternal HIV-related healthcare costs, and infant healthcare costs.
Figure 1.
Model structure. Three linked models were used for this analysis, as described in the Methods, as well as in the Supplementary Appendix and previous work [10, 14, 15]. The mother-to-child human immunodeficiency virus transmission model includes events during pregnancy and delivery (left panel; Supplementary Figure 1). The Cost-effectiveness of Preventing AIDS Complications (CEPAC) adult model includes events occurring among mothers after delivery (bottom right panel; Supplementary Figure 2A), and the CEPAC infant model includes events for infants after birth (top right panel; Supplementary Figure 2B). Linkages between the models allow a combined analysis in which each woman–infant pair is simulated together from the time of first presentation at antenatal care through pregnancy and delivery, and then each woman and infant are simulated separately throughout their lifetimes. Abbreviations: ANC, antenatal care; ART, 2-drug antiretroviral therapy; ARVs, antiretroviral medications; HIV, human immunodeficiency virus; OI, opportunistic infection; PMTCT, prevention of mother-to-child HIV transmission; sdNVP, single-dose nevirapine.
Incremental cost-effectiveness ratios (ICERs), in US dollars per year of life saved (YLS), were calculated from combined projected lifetime healthcare costs (antenatal + maternal + infant) and combined projected life expectancy (maternal + infant) [16], discounted at 3% per year. We used 2 criteria to interpret cost-effectiveness. First, following WHO guidance, an intervention was considered cost-effective if its ICER compared with the next least-expensive alternative was <3 times the 2008 Zimbabwe per capita gross domestic product, or 3 × $400 = $1200 per YLS [17, 18]. Second, we compared results with the recently reported range of ICERs for ART-related interventions in developing countries ($550–$5200 per YLS) [19]. This work was approved by the Partners Healthcare Institutional Review Board, Boston, Massachusetts.
Modeled Population, PMTCT Regimens, and Uptake of PMTCT Services
The linked models were used to simulate a cohort of pregnant, HIV-infected women in Zimbabwe and their infants. We examined 5 PMTCT regimens: (1) no antenatal ARVs (comparator), (2) sdNVP, (3) WHO Option A, (4) WHO Option B, and (5) Option B+ (Supplementary Table 1). Women were modeled to present to care at 24–28 weeks’ gestation and to breastfeed for 18 months, based on Zimbabwean data [20, 21], with ARV prophylaxis (Options A, B, and B+ ) continued throughout breastfeeding.
To demonstrate the impact of guideline-concordant care, all women in the base-case analyses were assumed to be identified as HIV-infected at their first ANC visit. With no ARVs, women received no antiretroviral medications during pregnancy. With sdNVP, women initiated ART in pregnancy if clinical assessment indicated WHO stage 3–4 disease; CD4 testing was not included, reflecting its limited availability in the sdNVP-based National PMTCT Program in 2009. With Options A and B, women received ART during pregnancy if eligible by either CD4 or clinical criteria, and with Option B+, all women received lifelong ART. With all modeled regimens, women who linked to postnatal HIV care were assumed to undergo clinical and CD4 assessment at 6 weeks postpartum and to initiate ART if eligible, regardless of antenatal regimen received. In the base case, we assumed 100% adherence to PMTCT regimens (initiated at 30 weeks’ gestational age), 100% linkage to postnatal care for mothers and infants, and 100% retention in care and ART availability for women and infants meeting WHO ART initiation criteria [22, 23]. In sensitivity analyses, to reflect real-world programs, we examined reduced access to antenatal and postnatal care.
Model Structure
The 3 simulation models are described in detail in the Supplementary Appendix and in previous publications [10, 14, 15]. The models were linked so that each mother–infant pair was simulated together from the time of first presentation at ANC through delivery (the MTCT model), and then each woman and infant were simulated separately over their lifetimes after delivery (the CEPAC adult and infant models), as in Figure 1 and Supplementary Figures 1 and 2.
Model Input Parameters
Maternal Characteristics, Disease Progression, and ART
Based on Zimbabwean data, mean age at first ANC visit was 24 years [21]; mean CD4 count was 451 cells/µL (36% of women with CD4 count ≤350 cells/µL) [24]. Because detailed data to inform monthly risks of opportunistic infections (OIs) and HIV-related death in the absence of ART were not available from Zimbabwe, we derived these data from a cohort in South Africa (Supplementary Table 2) [25]. Details of ART initiation and switching, as well as CD4 and HIV RNA changes on ART, are provided in the Supplementary Appendix.
MTCT Risks, Infant Mortality Rates, and Infant Life Expectancy Estimates
Risks of MTCT during pregnancy and breastfeeding were calculated from PMTCT studies among breastfeeding populations in Africa, leading to estimates similar to those derived by the Joint United Nations Programme on HIV/AIDS (Supplementary Appendix) [10, 26]. Data and assumptions to inform infant mortality rates and LE values are shown in Table 1 and detailed in the Supplementary Appendix.
Table 1.
Selected Model Input Parameters
| Variable | Value | Data Sources | |||
|---|---|---|---|---|---|
| Clinical Model Input Parameters | |||||
| Baseline Maternal Cohort Characteristics | |||||
| Age, mean, y (SD) | 24 (5) | MOHCW [21] | |||
| Mortality during pregnancy | 0.7% | MOHCW [8] | |||
| Proportion ART eligiblea | 36% | ZVITAMBO trial [24] | |||
| CD4 count, cells/µL (SD) | |||||
| Total cohort | 451 (50) | ZVITAMBO trial [24] | |||
| ART-eligible women | 275 (50) | ZVITAMBO trial [24] | |||
| Non-ART-eligible women | 550 (50) | ZVITAMBO trial [24] | |||
| Uptake of PMTCT services and postnatal care | |||||
| PMTCT uptakeb | 100% (sensitivity analyses: 56%, 80%, 95%) | WHO [1] | |||
| Sensitivity of clinical assessment of ART eligibility | 36% | MTCT-Plus Cohort [47] | |||
| Probability of linking to pediatric HIV diagnosis, care, and ART | 100% (sensitivity analysis: 36%) | WHO/UNICEF [48] | |||
| Probability of linking to postnatal maternal HIV-related care | 100% (sensitivity analyses: 87% if ANC received, 43% if no ANC received) | After ANC: Mean of published values [49–54] No ANC: assumption | |||
| Loss to follow-up from postnatal maternal care | 0% per year (sensitivity analyses: 16% [year 1]; 6% per year [years ≥2]) | [30–32] | |||
| Base Case Value (range for sensitivity analysis) |
|||||
| Maternal HIV Status | |||||
| Mother-to-Child Transmission Risks | PMTCT Regimen Received | ||||
| Intrauterine/intrapartum period (one-time risks) | |||||
| No ARVs | sdNVP | Antenatal ZDVc | 3-Drug Regimen | Data Sources | |
| ART eligible at conception | 0.273 (0.199–0.322) | 0.176 (0.082–0.264) | 0.136 (0.091–0.157) | 0.033 (0.011–0.041) | [24, 55–69] |
| Non-ART eligible at conception | 0.175 (0.127–0.206) | 0.073 (0.033–0.109) | 0.036 (0.024–0.041) | 0.01 (0.004–0.028) | [24, 55–64] [66, 67, 69–71] |
| Postnatal period (rate per 100 person-years among HIV-uninfected infants aged 4-6 weeks) | |||||
| No ARVs | Extended Infant NVP | 3-Drug Regimen | Data Sources | ||
| ART eligible | 9.1 (EBF); 15.4 (MBF) (5.7–28.4) | NA | 4.0 (0–6.4) | [24, 57, 59, 65, 67, 69–72] | |
| Non-ART eligible | 2.9 (EBF); 4.8 (MBF) (1.8–8.8) | 2.7 (1.4–3.7) | 2.2 (0–6.4) | [24, 52, 59, 67, 70–77] | |
| Infant Mortality and Life Expectancy | |||||
| Probability of live birth | 95.7%–98.0% | MOHCW [21] | |||
| Relative increase in infant mortality if maternal death occurs | 2-fold increase | [78–81] | |||
| Short-term mortality risks, % | 1-year risk | 2-year cumulative risk | |||
| HIV-exposed, uninfected children | 7.4 [82] | 9.2 [82] | |||
| HIV-infected children, no ART | |||||
| Intrauterine/intrapartum infection | 51.0 [83] | 65.0 [83] | |||
| Postpartum infection | 24.0 [83] | 38.0 [83] | |||
| HIV-infected children, on ART | 9.5 [84] | 12.0 [85] | |||
| Life-expectancy estimates, y | Base Case Value | Range for Sensitivity Analyses | |||
| HIV-exposed, uninfected children (from weaning) | 50.0 (assumption) | 43.0–67.0 [86, 87] | |||
| HIV-infected children, no ART | |||||
| Intrauterine/intrapartum infection (from birth) | 1.1 [83] | 1.1–2.0 (assumption) | |||
| Postpartum infection (from time of infection) | 9.4 [83] | 5.0–10.0 (assumption) | |||
| HIV-infected children, on ART | |||||
| Intrauterine/intrapartum infection (from birth) | 20.0 (assumption) | 10.0–25.0 (assumption) | |||
| Postpartum infection (from time of infection) | 20.0 (assumption) | 10.0–25.0 (assumption) | |||
| Maternal Disease Progression Parameters | Value | Data Source | |||
| Impact of antiretroviral therapy | |||||
| Efficacy, % HIV RNA suppression at 24 wk | |||||
| First-line ART, TDF/FTC + (NVP or EFV) | |||||
| Initiated during pregnancy | 90% | [88] | |||
| Initiated postpartum, no sdNVP exposure | 90% | OCTANE trial [89] Difference: [90–92] | |||
| Initiated postpartum, with sdNVP exposure | 85% (difference assumed vs no sdNVP, 5% [88]) | ||||
| Second-line ART (ZDV/3TC/LPV/r) | 72% | [93] | |||
| CD4 cell decline over 6 mo following ART interruption | 139 cells/µL | [36–38] | |||
| Laboratory and medication costs | 2008 US Dollars | Data Sources | |||
| Economic Model Input Parameters | |||||
| CD4 assay, performed once in ANC for Options A, B, and B+ | 9.42 | [33] | |||
| Full blood count, performed once in ANC for Options B and B+ | 9.27 | [94] | |||
| Single-dose NVP, 1 maternal and 1 infant dose | 0.06 | ||||
| Antenatal ZDV, Option Ac | 7.67 per month | [27] | |||
| Antenatal TDF/FTC/NVP, Options B and B+, CD4 count ≤350 cells/µLc | 12.12 per month | [27] | |||
| Antenatal TDF/FTC/EFV, Options B and B+, CD4 count >350 cells/µLc | 16.50 per month | [27] | |||
| Postnatal maternal ART | |||||
| First-line TDF/FTC/NVP; TDF/FTC/EFV | 12.12 per month; 16.50 per month | [27] | |||
| Second line, ZDV/3TC/LPV/r | 45.36 per month | [27] | |||
| Pediatric ART, d4T/3TC/NVP | 4.54 per month | [27] | |||
| Healthcare Resource Utilization and Costs | |||||
| Antenatal care | 2008 US Dollars | Data Sources | |||
| Routine antenatal care, 4 visits | 45.77 | Average of: [95, 96] | |||
| Delivery costs, healthcare facility | 54.50 | [96] | |||
| Routine and urgent health care costs: Children | No. of Inpatient Days per Year | No. of Outpatient Visits per Year | Total Cost per Monthd | Data Sources | |
| HIV-infected children, on ART | 2.14 | 6 | 3.32 | [97] | |
| Intrauterine/intrapartum infection, no ART | 18 | 6 | 16.48 | [98] | |
| Postpartum infection, no ART, aged 0–18 mo | 18 | 6 | 16.48 | [98] | |
| Postpartum infection, no ART, aged >18 mo | 11 | 6 | 10.67 | [98] | |
| HIV-exposed, uninfected children, aged 0–18 mo | 1 | 3.5 | 1.73 | Assumptione | |
| HIV-exposed, uninfected infants aged >18 mo | 0 | 1 | 0.26 | Assumptione | |
| Terminal care, last month of life | 5 | 0 | 49.80 | Assumptione | |
| Routine and urgent health care costs: Mothers | No. of Inpatient Days per Event | No. of Outpatient Visits per Event | Total Cost per Eventd | Data Sources | |
| Care for acute opportunistic infections | Cape Town AIDS Cohort [99] | ||||
| WHO stage 3–4 HIV disease, range by specific disease | 1.3–2.9 | 2.7–3.4 | 21.88–39.36 | ||
| Bacterial infection | 2.8 | 2.4 | 32.28 | ||
| Mild fungal infection | 1.2 | 2.3 | 19.04 | ||
| Tuberculosis | 2.9 | 2.2 | 35.66 | ||
| Terminal care, last month of life | 2.39 | 0.77 | 26.18 | ||
| Routine HIV care costs per month | 1.22–7.18 (range by CD4) | ||||
See Supplementary Table 2 for complete list of parameters.
Abbreviations: 3TC, lamivudine; ANC, antenatal care; ART, antiretroviral therapy; ARV, antiretroviral medications; d4T, stavudine; EBF, exclusive breastfeeding (in first 6 months of life, followed by MBF); EFV, efavirenz; FTC, emtricabine; HIV, human immunodeficiency virus; LPV/r, lopinavir/ritonavir; MACS, Multicenter AIDS Cohort Study; MBF, mixed breastfeeding; MOHCW, Zimbabwe Ministry of Health and Child Welfare; NA, not applicable; NVP, nevirapine; PTMCT, prevention of mother-to-child HIV transmission; SD, standard deviation; sdNVP, single-dose nevirapine; TDF, tenofovir; WHO, World Health Organization; ZDV, zidovudine.
a ART eligibility was defined as CD4 count of ≤350 cells/µL or WHO stage 3–4 disease.
b PMTCT uptake was defined as proportion of HIV-infected, pregnant women accessing PMTCT services by the time of delivery. See Supplementary Appendix text and Supplementary Table 2 for details.
c Two months of antentatal drug are assumed in all regimens for the base-case analysis, based on median gestational age at booking in Zimbabwe of 30 weeks.
d Total care costs for mothers and infants were calculated by multiplying resource utilization (number of outpatient visits and inpatient days) by an average of WHO-CHOICE estimates of costs for these encounters in 7 sub–Saharan African countries [28]. See Supplementary Appendix for details.
e See Supplementary Table 2 for description of assumptions of outpatient healthcare resource utilization.
Cost Inputs
Monthly medication costs were from the Clinton Healthcare Access Initiative [27]. Costs of clinical care were determined by estimating resource utilization (number of inpatient days and outpatient visits) for specified health conditions, then multiplying by the estimated costs of these healthcare encounters in Zimbabwe (Table 1 and Supplementary Appendix) [28]. For children aged >18 months, monthly utilization estimates (stratified by HIV and ART status) were multiplied by LE to estimate lifetime healthcare costs.
Model Validation and Sensitivity Analyses
Model-derived risks of MTCT, infant mortality, and postpartum maternal OIs were validated against published data, reported previously with extensive sensitivity analyses [10, 14]. For this study, we conducted univariate and multivariate sensitivity analyses on key PMTCT, pediatric, maternal, and cost parameters.
Access to Care Parameters
We examined the impact of reported rates of PMTCT uptake, defined as the proportion of HIV-infected women receiving PMTCT services and ARVs by delivery (56%, estimated for Zimbabwe in 2009; 80%, the 2009 WHO target goal; 90%, the 2011 WHO target goal; and 95%, reported in neighboring Botswana in 2011) [5, 8, 29]. We varied the availability of CD4 assays from 25% to 100% in Options A, B, and B+; when CD4 count was unavailable in Option A, women were assumed to initiate ART only for WHO stage 3–4 disease. We also examined the impact of reduced pediatric ART availability (36%, estimated for Zimbabwe in 2009) [5] and of reported rates of maternal loss to follow-up (LTFU) from postnatal HIV care (Table 1) [30–32].
Clinical Health Parameters
We defined a lowest-MTCT risk scenario, using the lowest published risks (best reported effectiveness/efficacy) for each modeled regimen (Table 1); a highest-MTCT risk scenario, combining the highest published risks for each regimen; and a scenario assuming equal MTCT risks with Options A and B. We also used 4 assumptions about LE for HIV-exposed and HIV-infected infants: (1) a high pediatric LE scenario, using the upper bound estimates shown in Table 1, (2) a low pediatric LE scenario, using the lower bound estimates, (3) a largest difference scenario (lowest estimates for HIV-infected children; highest estimates for HIV-uninfected children), and (4) a smallest difference scenario (highest estimates for HIV-infected children; lowest estimates for uninfected children).
Finally, we investigated potential maternal health impacts of Option B and B+ in 2 ways. First, we varied the efficacy of first-line ART when resumed after ART interruption, reflecting potential interruption-associated drug resistance. Next, we examined the impact of “treatment fatigue” for women who begin ART with CD4 count >350 cells/µL solely for PMTCT, modeled as (1) an increased risk of virologic failure >6 months after ART initiation or (2) a reduction in second-line ART efficacy.
Cost Parameters
Because estimated costs of healthcare in Zimbabwe are markedly lower than in surrounding countries [28], we repeated the analysis using costs from South Africa (Supplementary Table 2) [33]. In the base case, we conservatively assigned lifelong costs of NVP-based ART to HIV-infected infants; in sensitivity analyses, as an upper bound on pediatric ART costs, we assigned the costs of lifelong lopinavir/ritonavir-based ART to sdNVP-exposed, HIV-infected children. Finally, the nondrug costs of providing 3-drug ARV regimens instead of zidovudine alone (e.g., personnel, laboratory costs) have not been reported; we also examined the impact of such implementation costs in the antenatal period.
RESULTS
Base-Case Results
Pediatric HIV Risk and LE
Among infants born to HIV-infected women, projected 18-month HIV infection rates were 24.8% (no antenatal ARVs), 14.2% (sdNVP), 7.5% (Option A), and 5.7% (Options B and B+) (Table 2). The resulting projected undiscounted LE (including both HIV-infected and HIV-uninfected infants) ranged from 38.35 years (no antenatal ARVs) to 44.18 years (Options B and B+).
Table 2.
Base-Case Results: Projected Maternal and Pediatric Outcomes of the Zimbabwe National Prevention of Mother-to-Child HIV Transmission Program
| Pediatric Life Expectancy, Years From Birth |
Maternal Life Expectancy, Years From Delivery |
||||
|---|---|---|---|---|---|
| 18-Month Infant HIV Infection Risk | Undiscounted | Discounted | Undiscounted | Discounted | |
| Projected Clinical Outcomesa | |||||
| No antenatal ARVsb | 24.8% | 38.35 | 21.34 | 21.25 | 14.69 |
| sdNVP | 14.2% | 41.30 | 22.45 | 20.94 | 14.53 |
| Option A | 7.5% | 43.27 | 23.19 | 21.26 | 14.70 |
| Option B | 5.7% | 44.18 | 23.59 | 21.30 | 14.74 |
| Option B+ | 5.7% | 44.18 | 23.59 | 22.42 | 15.45 |
| Antenatal Care Costs, Through Delivery | Pediatric Lifetime Healthcare Costs, From Birth |
Maternal Lifetime HIV-Related Healthcare Costs, From Delivery |
|||
| Undiscounted | Discounted | Undiscounted | Discounted | ||
| Projected costs, 2008 US Dollarsa | |||||
| No antenatal ARVsb | 85 | 730 | 520 | 8490 | 5280 |
| sdNVP | 92 | 530 | 360 | 8460 | 5300 |
| Option A | 118 | 490 | 310 | 8500 | 5280 |
| Option B | 134 | 370 | 240 | 8450 | 5260 |
| Option B+ | 134 | 370 | 240 | 9820 | 6240 |
Abbreviations: ARVs, antiretroviral medications; HIV, human immunodeficiency virus; sdNVP, single-dose nevirapine.
a Base-case projections assume 100% uptake of PMTCT services by the time of delivery, 100% linkage to HIV care during breastfeeding, no maternal loss to follow-up after delivery, and 100% availability of pediatric antiretroviral therapy (ART) for HIV-infected infants (see Methods).
b No antenatal ARVs refers to receipt of no ARVs or antiretroviral therapy prior to delivery. In all modeled strategies, ART-eligible women who linked to HIV-related healthcare after delivery were assumed to receive ART for their own health in all strategies (Supplementary Table 1).
Pediatric Costs
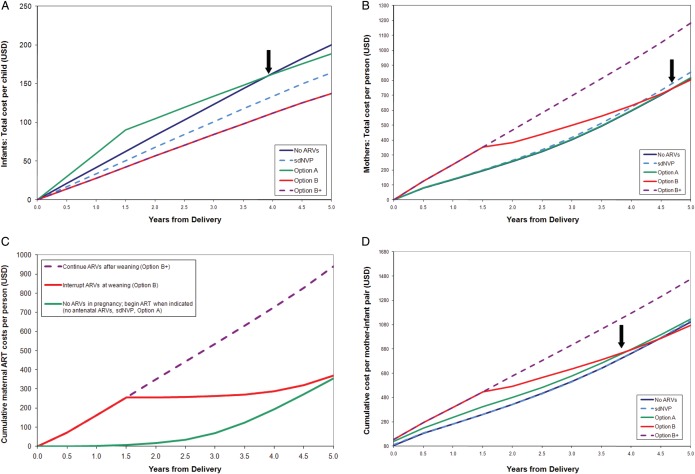
PMTCT regimens that prevented more infant infections resulted in lower pediatric healthcare costs over time. After the early cost of infant NVP during breastfeeding, the pediatric healthcare costs of Option A became less than those of no antenatal ARVs by 4 years after delivery (Figure 2A). This finding persisted over longer horizons; undiscounted lifetime costs per infant ranged from $730 (no antenatal ARVs) to $370 (Options B and B+) (Table 2).
Figure 2.
Projected costs (in US dollars [USD]) over the first 5 years after delivery for modeled prevention of mother-to-child human immunodeficiency virus (HIV) transmission (PMTCT) regimens in Zimbabwe. A–D, Undiscounted costs are shown on the vertical axis, and time from delivery is shown on the horizontal access. A, Total healthcare costs for infants (with 100% pediatric antiretroviral therapy [ART] availability). The costs of daily infant nevirapine (NVP) prophylaxis (Option A) are included in pediatric healthcare costs. Because infant NVP is modeled as a pediatric cost, Option A is more expensive than the others during the first 18 months (while breastfeeding continues). PMTCT regimens that are more effective in preventing infant infections result in slower increases in costs (flatter slopes) as time progresses because pediatric HIV care costs are averted, and the pediatric care costs following Option A become less than those following no antenatal antiretroviral medications (ARVs) by 4 years after delivery (arrow). B, HIV-related healthcare costs for women after delivery (with 100% retention in care). The costs of maternal ART and 3-drug ARV prophylaxis (Options B and B+) are included in maternal HIV-related healthcare costs. Postnatal care costs are similar following the no antenatal ARVs, single-dose NVP (sdNVP), and Option A strategies: women enrolled in HIV-related care following all 3 of these strategies are assumed to begin ART when CD4 count falls to ≤350 cells/µL or stage 3–4 disease develops. Small cost differences result from assumptions regarding NNRTI resistance following sdNVP, but the slopes of these 3lines are similar. In Option B+, all women continue their 3-drug regimens. In Option B, women who did not have advanced disease before pregnancy interrupt their ARVs but remain in care and re-initiate ART once CD4 count falls to ≤350 cells/µL or stage 3–4 disease develops. As a result, maternal costs after weaning are greater with Option B+ than with the other regimens, and costs for Option B (due to delayed ART use) are much lower after weaning (becoming less than the costs after Option A by 5 years after delivery) (arrow). Antenatal costs are not included in (A and B). C, HIV-infected women with CD4 count >350 cells/µL, post-delivery. ART costs for women not eligible for ART during pregnancy (CD4 count >350 cells/µL, no stage 3–4 disease), from the Cost-effectiveness of Preventing AIDS Complications (CEPAC) adult model. Three postnatal scenarios are shown: (1) initiate 3-drug ARVs in pregnancy and continue ARVs after weaning (as in Option B+); (2) initiate 3-drug ARVs in pregnancy and interrupt ARVs after weaning (Option B); and (3) do not initiate ARVs in pregnancy but remain in care and initiate ART when needed (CD4 count ≤350 cells/µL or stage 3–4 disease, as in the no antenatal ARVs, sdNVP, and Option A strategies). Interrupting ART at weaning saves money compared with continuing ART; however, this ART interruption may be associated with negative health impacts for HIV-infected mothers if retention in care is less than 100% (Table 2). D, Total cohort costs over the first 5 years after delivery. These include antenatal care costs (through delivery), maternal HIV-related healthcare costs, and pediatric healthcare costs. Option B becomes cost-saving compared with Option A within 4 years after delivery (arrow). Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral medication; sdNVP, single-dose nevirapine.
Maternal LE
Among HIV-infected women, projected undiscounted maternal LE from delivery was 21.25 years (no antenatal ARVs), 21.26 years (Option A), and 22.42 years (Option B+). Projected maternal LE was lowest in the sdNVP strategy (20.94 years, due to the modeled impact of nonnucleoside reverse transcriptase inhibitor resistance on subsequent first-line ART) and intermediate in the Option B strategy (21.30 years, reflecting benefits from ART during pregnancy and breastfeeding but interruption after weaning).
Maternal Costs
Although small differences in short-term maternal costs resulted from modeled drug resistance following sdNVP, 5-year costs were similar for no antenatal ARVs, sdNVP, and Option A (Figure 2B). Options B and B+, requiring 3-drug regimens during pregnancy and breastfeeding, conferred the greatest initial maternal healthcare costs. Option B conferred lower maternal costs than Option B+ after weaning because of deferred ART costs when women without advanced disease interrupted ART, and maternal costs with Option B were less than with Option A by 5 years after delivery (Figures 2B and 2C). Undiscounted lifetime maternal HIV-related costs per woman ranged from $8450 (Option B) to $9820 (Option B+ (Table 2).
Cost-effectiveness Analysis
Option B was projected to result in a discounted combined lifetime cost (ANC + mother + infant) of $5630 per mother–infant pair and a discounted combined LE (mother + infant) of 38.32 years (Table 3). Compared with Option B, the sdNVP, Option A, and no antenatal ARVs strategies all resulted in lower combined LE (36.03–37.89 years) at greater discounted lifetime costs ($5710–5880 per mother–infant pair) and were therefore “dominated.” Replacing Option B with Option B+ would increase costs ($6620 per mother–infant pair) and LE (39.04 years), with an ICER of $1370 per YLS. Considering total combined costs (ANC + mother + infant), Option B became cost saving compared with Option A by 4 years after delivery (Figure 2D; Supplementary Table 7).
Table 3.
Cost-effectiveness of World Health Organization 2010 Prevention of Mother-to-Child HIV Transmission Guidelines in Zimbabwe
| Modeled Scenario and PMTCT Regimen | Combined Costs per Mother–Infant Pair, Discounted, 2008 US Dollarsa | Combined Life Expectancy per Mother–Infant Pair, Discounted, Years From Deliveryb | ICER, US Dollars per YLS |
|---|---|---|---|
| Base-Case Projectionsc | |||
| Base-case projections (100% PMTCT uptake, retention in postnatal maternal care, pediatric ART availability) | |||
| Option B | 5630 | 38.32 | |
| Option A | 5710 | 37.89 | Dominatedd |
| sdNVP | 5760 | 36.97 | Dominated |
| No antenatal ARVs | 5880 | 36.03 | Dominated |
| Option B+ | 6620 | 39.04 | 1370 |
| Sensitivity Analysese | |||
| Access to care parameters: | |||
| Reduced PMTCT uptake (56% of HIV-infected women receiving ARVs by delivery; 87% linkage to postnatal care) | |||
| Option B | 4930 | 35.69 | |
| Option A | 4980 | 35.44 | Dominated |
| sdNVP | 5000 | 34.92 | Dominated |
| No antenatal ARVs | 5060 | 34.39 | Dominated |
| Option B+ | 5600 | 36.18 | 1370 |
| Increased maternal loss to follow-up after delivery (16% in year 1, 6% per year thereafter) | |||
| Option B | 3420 | 35.23 | |
| Option A | 3560 | 34.90 | Dominated |
| sdNVP | 3620 | 34.06 | Dominated |
| No antenatal ARVs | 3730 | 33.05 | Dominated |
| Option B+ | 3910 | 35.81 | 850 |
| Reduced pediatric ART availability (36% of infected children; 2009 Zimbabwe estimate) | |||
| Option B | 5610 | 38.00 | |
| sdNVP | 5670 | 35.96 | Dominated |
| Option A | 5670 | 37.41 | Dominated |
| No antenatal ARVs | 5690 | 34.06 | Dominated |
| Option B+ | 6590 | 38.71 | 1370 |
| Current access to care (56% PMTCT uptake, 87% linkage to postnatal maternal care, increased maternal LTFU, 36% pediatric ART availability) | |||
| Option B | 3010 | 31.99 | |
| sdNVP | 3090 | 30.94 | Dominated |
| Option A | 3090 | 31.72 | Dominated |
| No antenatal ARVs | 3100 | 29.83 | Dominated |
| Option B+ | 3340 | 32.38 | 850 |
| Clinical health parameters: | |||
| “Treatment fatigue”: monthly risk of virologic failure after 6 mo on first-line NNRTI-based ART = 2.39% for women starting ART with CD4 count >350 cells/µL (Options B/B+) (1.5 × base-case risk) | |||
| Option B | 5700 | 37.82 | |
| Option A | 5710 | 37.89 | 190 |
| sdNVP | 5760 | 36.97 | Dominated |
| No antenatal ARVs | 5880 | 36.03 | Dominated |
| Option B+ | 6700 | 38.67 | 1260 |
| Resource utilization parameters: | |||
| South Africa healthcare costs | |||
| Option B | 14 040 | 38.33 | |
| Option A | 14 260 | 37.89 | Dominated |
| sdNVP | 14 730 | 36.97 | Dominated |
| Option B+ | 15 070 | 39.05 | 1410 |
| No antenatal ARVs | 15 520 | 36.04 | Dominated |
| Additional $150 antenatal implementation cost for 3-drug regimens compared with ZDV alone | |||
| Option A | 5760 | 37.89 | |
| Option B | 5760 | 38.32 | 2 |
| sdNVP | 5770 | 36.97 | Dominated |
| No ARVs | 5880 | 36.03 | Dominated |
| Option B+ | 6750 | 39.04 | 1370 |
Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral medications; HIV, human immunodeficiency virus; ICER, incremental cost-effectiveness ratio; LTFU, lost to follow-up; NNRTI, nonnucleoside reverse transcriptase inhibitor; PMTCT, prevention of mother-to-child transmission; sdNVP, single-dose nevirapine; YLS, year of life saved; ZDV, zidovudine.
a Combined costs = PMTCT program costs + maternal lifetime HIV-related healthcare costs + infant lifetime healthcare cost (per mother–infant pair).
b Combined life expectancy = maternal life expectancy from delivery + infant life expectancy from birth.
c Base-case results. Base-case projections assume 100% uptake of PMTCT services by the time of delivery, 100% linkage to HIV care during breastfeeding, no maternal loss to follow-up after delivery, and 100% availability of pediatric ART for HIV-infected infants.
d Dominated refers to an intervention that is more expensive and less effective than an alternative intervention.
e Sensitivity analyses. Please see Supplementary Table 5 for additional details regarding all sensitivity analyses, including the distribution of costs and life expectancy between mothers and infants.
Sensitivity Analyses
Access-to-Care Parameters
The finding that no antenatal ARVs, sdNVP, and Option A were more costly but less effective than Option B was robust with reduced uptake of PMTCT services or access to CD4 testing, as well as with current availability of pediatric ART, and the ICER of Option B+ compared with Option B in these scenarios remained $1370 per YLS (Table 3, Supplementary Table 5). With reported rates of LTFU from maternal postnatal HIV care, the ICER of Option B+ compared with Option B decreased to $850 per YLS. This ICER remained $850 per YLS when current overall access to care in Zimbabwe was simulated (PMTCT uptake, 56%; pediatric ART availability, 36%; maternal LTFU, 16% in year 1, 6% per year thereafter).
Clinical Health Parameters
Base-case policy conclusions were unchanged in all modeled pediatric LE and MTCT risk scenarios, including when MTCT risks were equal with Options A and B, as well as throughout a variety of “treatment fatigue” scenarios for women initiating 3-drug regimens with CD4 count >350 cells/µL (Supplementary Table 5). Results were sensitive, however, to the risk of virologic failure after 6 months on ART. When this risk was increased 1.5-fold from the base case (to >2.4% per month), Option B no longer dominated Option A; when it was increased 2-fold (to 3.2% per month), Option A dominated Option B (Table 3; Supplementary Table 5).
Cost Parameters
Policy conclusions were unchanged when lifelong lopinavir/ritonavir costs were assigned to sdNVP-exposed, HIV-infected infants (Supplementary Table 5). In sensitivity analyses using South Africa healthcare costs, the ICER of Option B+ compared with Option B was $1410 per YLS (Table 3). The difference in antenatal implementation costs between 3-drug regimens and zidovudine alone needed to be ≥$150 per person to change the comparison between Options A and B (Table 3); at $150 per person, Option B was no longer cost saving but remained very cost-effective ($2 per YLS), compared with Option A. Even with implementation costs as high as $400 per person, the ICER of Option B compared with Option A remained <$400 per YLS (Supplementary Table 6).
DISCUSSION
There are 4 key findings from this work. First, a strategy of providing no antenatal ARVs for PMTCT is more expensive and less effective over a lifetime horizon than strategies based on sdNVP, Option A, or Option B. This result, which occurs because the upfront costs of these PMTCT regimens are greatly outweighed by the downstream costs of caring for HIV-infected infants, lends strong economic support to the well-recognized clinical impact of expanding access to PMTCT programs, regardless of the specific drug regimen provided [5]. Second, in settings where 3-drug ARV regimens are not available for PMTCT [5, 34], replacing sdNVP with Option A benefits infants and mothers and saves money over a lifetime horizon.
Third, healthcare programs would decrease costs and improve outcomes further by implementing Option B instead of Option A. Although short-term drug costs are greater with Option B, the incorporation of healthcare costs for both mothers and infants leads Option B to cost less than Option A within 4 years after delivery, primarily because of averted pediatric HIV costs (Figure 2D). Notably, however, if women with high CD4 counts develop poor adherence after Option B (increasing the monthly risk of late virologic failure by ≥25%) (Supplementary Table 5) or if mothers are lost to follow-up after delivery, Option B leads to shorter projected maternal LE than Option A.
Finally, these results strongly support lifelong ART for all pregnant, HIV-infected women (Option B+) [3, 7]. The interruption of effective ART in Option B may have deleterious effects on maternal health. Randomized trial data comparing maternal health outcomes of Options B and B+ are anticipated soon [35]. In the interim, we assume a rapid rate of CD4 decline after ART interruption based on other trials [36–38], with an associated increased risk of OIs. As a result, Option B+ is projected to increase undiscounted maternal LE by 1.12 years compared with Option B (consistent with modeled impacts of other HIV-related interventions [12, 39]), with an ICER of $1370 per YLS. Although this ICER exceeds the 2008 gross domestic product–based threshold for cost-effectiveness in Zimbabwe ($1200 per YLS) [17, 18], it falls in the lower range of ICERs reported for ART-related interventions in developing countries ($550–$5200 per YLS) [19] and thus represents a return on investment comparable with many current HIV programs in Zimbabwe and other resource-limited settings.
Option B+ may represent an even better healthcare investment compared with Option B under specific conditions. First, ART interruption (Option B) may cause greater detriment to maternal health under real-world programmatic conditions than in our guideline-concordant simulations. When women are lost to follow-up after weaning, disease progression is unobserved and cannot lead to prompt ART reinitiation. Such disease progression is more rapid when ART was interrupted months before LTFU (Option B) than at the time of LTFU (Option B+) because of lower CD4 counts at LTFU in Option B. As a result, Option B leads to a projected discounted LE (11.64 years) even lower than no antenatal ARVs (11.71 years) [10], and Option B+ becomes more cost-effective compared with Option B ($850 per YLS). Second, analyses using cost data from South Africa (ICER, $1410 per YLS; 2008 gross domestic product, $5700) [18] suggest that Option B+ may be very cost-effective compared with Option B in higher-income settings where healthcare costs are greater. Third, this analysis excludes several additional benefits of Option B+ that may render it even more effective and cost-effective, including prevention of maternal tuberculosis (also reducing infection risk in infants) [40], HIV transmission to male partners [40], hepatitis B flares due to ARV interruption [7], and MTCT during subsequent pregnancies when women are already on ART at conception [7].
There are several limitations to this analysis. First, all models necessarily simplify complex processes; for example, assumptions about infant LE involved uncertainties about healthcare in the distant future. However, LE assumptions, cost assumptions, and projected clinical and economic results were similar to those previously reported [41, 42], and we tested the impact of biologic and operational assumptions in extensive sensitivity analyses [10, 14]. Except where noted, the impact on policy conclusions was minimal, primarily because assumptions were consistent across PMTCT strategies. Second, we excluded the potential impact of drug-related viral resistance in infants who become infected despite exposure to modeled ARV regimens, because of limited data about acquisition of such resistance [43, 44] and its impact on later ART effectiveness. If resistant HIV is a greater concern for infants who become infected while exposed to maternal ARVs through breastmilk than to extended NVP monoprophylaxis, the benefits of Options B and B+ vs Option A will be attenuated. Finally, our analysis assumed a healthcare system perspective. If a societal perspective were assumed, interventions that avert HIV infections in infants and prevent morbidity and mortality in women would be even more cost-effective, avoiding transportation costs and lost wages for medical care and permitting the productivity gains of healthy women and of children who will become healthy adults.
As in other studies, we find that PMTCT programs based on sdNVP are cost saving, compared with no PMTCT interventions [45]. This is the first analysis to compare sdNVP and Options A, B, and B+ and to consider both short- and long-term maternal and infant outcomes after PMTCT [16, 41, 42, 45]. We find that, with guideline-concordant care, Option A is cost saving compared with sdNVP; Option B becomes more effective and less expensive than Option A within 4 years of delivery; and Option B+ offers additional clinical benefits and economic value comparable with other widely used HIV interventions. We anticipate that the clinical results of these analyses will be generalizable to many African settings where prolonged breastfeeding is the norm and that the base-case economic results may also be applicable in low-income African countries with healthcare costs similar to Zimbabwe. Although specific policies will depend on available resources as well as important considerations of fairness, feasibility, and priority populations [15, 46], PMTCT programs should move rapidly toward these more effective and economically efficient strategies.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors are indebted to Sue J. Goldie, Steven Sweet, and Stephen Resch for derivation of healthcare cost estimates for Zimbabwe appropriate to the recent period of hyperinflation. We also gratefully acknowledge Jennifer Chu, Katie Doherty, and Kathleen Kelly for assistance with modeling analyses and manuscript preparation, and Batsirai Chikwinya, Agnes Mahomva, Stanley Mashumba, Rumbidzai Mugwagwa, and Charity Zvandaziva for critical interpretation of early model results. We also thank the CEPAC-International team and investigators for their contribution. A. L. C. had full access to all of the data and results of this study and has final responsibility for the decision to submit for publication.
Financial support. This work was supported by the Elizabeth Glaser Pediatric AIDS Foundation; the National Institute of Allergy and Infectious Diseases and the National Institute of Child Health and Human Development, National Institutes of Health (K01 AI078754 to A. L. C.; K24 AI062476 to K. A. F.; R01 AI058736 to R. P. W., A. R., J.-E. P., K. A. F; IMPAACT network to R. P. W., J.-E. P., K. A. F.); and Harvard University Center for AIDS Research (to K. A. F., R. P. W.). The funders had no role in study design, interpretation of results, or decision to publish.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed
References
- 1.World Health Organization. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: towards universal access. Available at: http://whqlibdoc.who.int/publications/2010/9789241599818_eng.pdf. Accessed 30 January 2012. [PubMed] [Google Scholar]
- 2.UNAIDS. Global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. Available at: http://www.unaidsrstesa.org/sites/default/files/global-plan-elimination-hiv-children_en-1.pdf. Accessed 30 May 2012. [Google Scholar]
- 3.World Health Organization. Programmatic update: use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Available at: http://www.who.int/hiv/PMTCT_update.pdf. Accessed 6 June 2012. [PubMed] [Google Scholar]
- 4.World Health Organization. Progress report 2011: global HIV/AIDS response: epidemic update and health sector progress towards universal access. Available at: http://www.who.int/hiv/pub/progress_report2011/en/index.html. Accessed 26 January 2012. [Google Scholar]
- 5.World Health Organization. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Progress report. Available at: http://www.who.int/hiv/pub/2010progressreport/report/en/index.html. Accessed 30 January 2012. [Google Scholar]
- 6.Hargrove JW, Humphrey JH. Mortality among HIV-positive postpartum women with high CD4 cell counts in Zimbabwe. AIDS. 2010;24:F11–14. doi: 10.1097/qad.0b013e328335749d. [DOI] [PubMed] [Google Scholar]
- 7.Schouten EJ, Jahn A, Midiani D, et al. Prevention of mother-to-child transmission of HIV and the health-related Millennium Development Goals: time for a public health approach. Lancet. 2011;378:282–4. doi: 10.1016/S0140-6736(10)62303-3. [DOI] [PubMed] [Google Scholar]
- 8.Zimbabwe Ministry of Health. National HIV estimates. Harare: Zimbabwe Ministry of Health and Child Welfare; 2009. [Google Scholar]
- 9.Index Mundi. Zimbabwe birth rate. Available at: http://www.indexmundi.com/zimbabwe/birth_rate.html. Accessed 30 May 2012. [Google Scholar]
- 10.Ciaranello AL, Perez F, Maruva M, et al. WHO 2010 guidelines for prevention of mother-to-child HIV transmission in Zimbabwe: modeling clinical outcomes in infants and mothers. PLoS One. 2011;6:e20224. doi: 10.1371/journal.pone.0020224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciaranello A, Lockman S, Freedberg KA, et al. First-line antiretroviral therapy after single-dose nevirapine exposure in South Africa: a cost-effectiveness analysis of the OCTANE trial. AIDS. 2011;25:479–92. doi: 10.1097/QAD.0b013e3283428cbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldie SJ, Yazdanpanah Y, Losina E, et al. Cost-effectiveness of HIV treatment in resource-poor settings—the case of Côte d'Ivoire. N Engl J Med. 2006;355:1141–53. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 13.Ciaranello A, Perez F, Engelsmann B, et al. Clinical and economic impact of scaling-up World Health Organization (WHO) 2010 PMTCT guidelines in Zimbabwe [abstract TuPE284]. In: Program and abstracts of the 6th International AIDS Society Conference on HIV pathogenesis, treatment and prevention. (Rome, Italy). Available at: http://pag.ias2011.org/Abstracts.aspx?AID=862. Accessed 22 February 2012. [Google Scholar]
- 14.Ciaranello AL, Perez F, Keatinge J, et al. What will it take to eliminate pediatric HIV? Reaching “virtual elimination" targets for prevention of mother-to-child HIV transmission (PMTCT) in Zimbabwe. PLoS Medicine. 2012;9:e1001156. doi: 10.1371/journal.pmed.1001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walensky RP, Wood R, Ciaranello AL, et al. Scaling up the 2010 World Health Organization HIV Treatment Guidelines in resource-limited settings: a model-based analysis. PLoS Med. 2010;7:e1000382. doi: 10.1371/journal.pmed.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beigi RH, Wiringa AE, Bailey RR, Assi TM, Lee BY. Economic value of seasonal and pandemic influenza vaccination during pregnancy. Clin Infect Dis. 2009;49:1784–92. doi: 10.1086/649013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. WHO-CHOICE: cost-effectiveness thresholds. Available at: http://www.who.int/choice/costs/CER_thresholds/en/index.html. Accessed 30 January 2012. [Google Scholar]
- 18. World Bank. GDP per capita (current US$). Available at: http://worldbank.org/indicator/NY.GDP.PCAP.CD . Accessed 11 October 2012. [Google Scholar]
- 19.Kahn JG, Marseille EA, Bennett R, Williams BG, Granich R. Cost-effectiveness of antiretroviral therapy for prevention. Curr HIV Res. 2011;9:405–15. doi: 10.2174/157016211798038542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iliff P, Ntozini R, Nathoo K, Piwoz E, Moulton L, Humphrey J. Making a working clinical diagnosis of HIV infection in infants in Zimbabwe. Trop Med Int Health. 2008;13:1459–69. doi: 10.1111/j.1365-3156.2008.02178.x. [DOI] [PubMed] [Google Scholar]
- 21.Ministry of Health and Child Welfare Zimbabwe. Maternal and Perinatal Mortality Study 2007. Harare, Zimbabwe: 2008. Author. [Google Scholar]
- 22.World Health Organization. Antiretroviral therapy for HIV infection in infants and children: recommendations for a public health approach (2010 version) Available at: http://www.who.int/hiv/pub/paediatric/infants2010/en/index.html. Accessed 30 January 2012. [PubMed] [Google Scholar]
- 23.World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents—recommendations for a public health approach (2010 version) Available at: http://www.who.int/hiv/pub/arv/adult2010/en/index.html. Accessed 30 January 2012. [PubMed] [Google Scholar]
- 24.Iliff PJ, Piwoz EG, Tavengwa NV, et al. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS. 2005;19:699–708. doi: 10.1097/01.aids.0000166093.16446.c9. [DOI] [PubMed] [Google Scholar]
- 25.Lawn SD, Myer L, Orrell C, Bekker LG, Wood R. Early mortality among adults accessing a community-based antiretroviral service in South Africa: implications for programme design. AIDS. 2005;19:2141–8. doi: 10.1097/01.aids.0000194802.89540.e1. [DOI] [PubMed] [Google Scholar]
- 26.UNAIDS Reference Group on Estimates MaP. Working paper on mother-to-child HIV transmission rates for use in Spectrum. Available at: http://www.epidem.org/Publications/MTCTratesworkingpaper.pdf. Accessed 8 June 2012. [Google Scholar]
- 27.William J. Clinton Foundation. Clinton Foundation HIV/AIDS Initiative (CHAI) antiretroviral price list. 2009. August Available at: http://www.clintonfoundation.org/files/chaiarvpricelistaugust2009english.pdf. Accessed 22 February 2012. [Google Scholar]
- 28.World Health Organization (WHO-CHOICE) Prices for hospitals and health centers. Available at: http://www.who.int/choice/en/ Accessed 30 January 2012. [Google Scholar]
- 29.World Health Organization. Global plan on elimination of new HIV infections among children and keeping their mothers alive. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/pcb/2011/12/20111215_Global%20Plan.pdf. Accessed 30 May 2012. [Google Scholar]
- 30.Brinkhof MW, Dabis F, Myer L, et al. Early loss of HIV-infected patients on potent antiretroviral therapy programmes in lower-income countries. Bull World Health Organ. 2008;86:559–67. doi: 10.2471/BLT.07.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geng EH, Bangsberg DR, Musinguzi N, et al. Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. J Acquir Immune Defic Syndr. 2010;53:405–11. doi: 10.1097/QAI.0b013e3181b843f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toro PL, Katyal M, Carter RJ, et al. Initiation of antiretroviral therapy among pregnant women in resource-limited countries: CD4 + cell count response and program retention. AIDS. 2010;24:515–24. doi: 10.1097/QAD.0b013e3283350ecd. [DOI] [PubMed] [Google Scholar]
- 33.Cleary S, Chitha W, Jikwana S, Okorafor OA, Boulle A. Health Systems Trust: South African health review. Available at: http://www.healthlink.org.za/publications/682. Accessed 22 February 2012. [Google Scholar]
- 34.Dabis F, Stringer J. Debate: HAART should be used for PMTCT in all pregnant women (3rd International Workshop on HIV Pediatrics) Available at: http://regist2.virology-education.com/2011/3hivped/16_July.html ; http://regist2.virology-education.com/supplement/supplement_2011_3.pdf. Accessed 22 February 2012. [Google Scholar]
- 35.National Institutes of Health: IMPAACT Trial Network. P1077: The PROMISE Study (Promoting Maternal and Infant Survival Everywhere) 2010 Synopsis Available at: https://inpaactgroup.org/promise-featured-study . Accessed 8 November 2012. [Google Scholar]
- 36.Kesho Bora Study Group. Impact of triple-ARV prophylaxis during pregnancy and breastfeeding compared with short-ARV prophylaxis for MTCT prevention on maternal disease progression [abstract ThLBB105] 2010 Programs and abstracts of XVII International AIDS Society (Vienna, Austria), Geneva, Switzerland: International AIDS Society. [Google Scholar]
- 37.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4 + count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–96. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 38.Danel C, Moh R, Minga A, et al. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in west Africa (Trivacan ANRS 1269 trial): a randomised trial. Lancet. 2006;367:1981–9. doi: 10.1016/S0140-6736(06)68887-9. [DOI] [PubMed] [Google Scholar]
- 39.Walensky RP, Wood R, Fofana MO, et al. The clinical impact and cost-effectiveness of routine, voluntary HIV screening in South Africa. J Acquir Immune Defic Syndr. 2011;56:26–35. doi: 10.1097/QAI.0b013e3181fb8f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orlando S, Marazzi M, Mancinelli S, et al. Cost-effectiveness of using HAART in prevention of mother-to-child transmission in the DREAM-Project Malawi. J Acquir Immune Defic Syndr. 2010;55:631–4. doi: 10.1097/QAI.0b013e3181f9f9f5. [DOI] [PubMed] [Google Scholar]
- 42.Shah M, Johns B, Abimiku A, Walker DG. Cost-effectiveness of new WHO recommendations for prevention of mother-to-child transmission of HIV in a resource-limited setting. AIDS. 2011;25:1093–102. doi: 10.1097/QAD.0b013e32834670b9. [DOI] [PubMed] [Google Scholar]
- 43.Persaud D, Bedri A, Ziemniak C, et al. Slower clearance of nevirapine resistant virus in infants failing extended nevirapine prophylaxis for prevention of mother-to-child HIV transmission. AIDS Res Hum Retroviruses. 2011;27:823–9. doi: 10.1089/aid.2010.0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeh C, Weidle PJ, Nafisa L, et al. HIV-1 drug resistance emergence among breastfeeding infants born to HIV-infected mothers during a single-arm trial of triple-antiretroviral prophylaxis for prevention of mother-to-child transmission: a secondary analysis. PLoS Med. 2011;8:e1000430. doi: 10.1371/journal.pmed.1000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johri M, Ako-Arrey D. The cost-effectiveness of preventing mother-to-child transmission of HIV in low- and middle-income countries: systematic review. Cost Eff Resour Alloc. 2011;9:3.. doi: 10.1186/1478-7547-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–8. [PubMed] [Google Scholar]
- 47.Carter RJ, Dugan K, El-Sadr WM, et al. CD4 + cell count testing more effective than HIV disease clinical staging in identifying pregnant and postpartum women eligible for antiretroviral therapy in resource-limited settings. J Acquir Immune Defic Syndr. 2010;55:404–10. doi: 10.1097/QAI.0b013e3181e73f4b. [DOI] [PubMed] [Google Scholar]
- 48.UNAIDS/UNICEF/WHO. Children and AIDS: fourth stocktaking report, actions and progress. Available at: http://www.unicef.org/publications/index_51902.html. Accessed 2 February 2012. [Google Scholar]
- 49.Ahoua L, Ayikoru H, Gnauck K, et al. Evaluation of a 5-year programme to prevent mother-to-child transmission of HIV infection in Northern Uganda. J Trop Pediatr. 2010;56:43–52. doi: 10.1093/tropej/fmp054. [DOI] [PubMed] [Google Scholar]
- 50.Manzi M, Zachariah R, Teck R, et al. High acceptability of voluntary counselling and HIV-testing but unacceptable loss to follow up in a prevention of mother-to-child HIV transmission programme in rural Malawi: scaling-up requires a different way of acting. Trop Med Int Health. 2005;10:1242–50. doi: 10.1111/j.1365-3156.2005.01526.x. [DOI] [PubMed] [Google Scholar]
- 51.Kaplan R, Orrell C, Zwane E, Bekker LG, Wood R. Loss to follow-up and mortality among pregnant women referred to a community clinic for antiretroviral treatment. AIDS. 2008;22:1679–81. doi: 10.1097/QAD.0b013e32830ebcee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peltier CA, Ndayisaba GF, Lepage P, et al. Breastfeeding with maternal antiretroviral therapy or formula feeding to prevent HIV postnatal mother-to-child transmission in Rwanda. AIDS. 2009;23:2415–23. doi: 10.1097/QAD.0b013e32832ec20d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stinson K, Boulle A, Coetzee D, Abrams EJ, Myer L. Initiation of highly active antiretroviral therapy among pregnant women in Cape Town, South Africa. Trop Med Int Health. 2010;15:825–32. doi: 10.1111/j.1365-3156.2010.02538.x. [DOI] [PubMed] [Google Scholar]
- 54.Kumwenda J, Mataya R, Kumwenda N, Kafulafula G, Li Q, Taha T. Coverage of highly active antiretroviral therapy (HAART) among postpartum women in Malawi (Abstract WEPDD106) 2009 doi: 10.1258/ijsa.2011.010359. Program and abstracts of 5th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention (Cape Town, South Africa). Geneva, Switzerland: International AIDS Society. [DOI] [PubMed] [Google Scholar]
- 55.Chigwedere P, Seage GR, Lee TH, Essex M. Efficacy of antiretroviral drugs in reducing mother-to-child transmission of HIV in Africa: a meta-analysis of published clinical trials. AIDS Res Hum Retroviruses. 2008;24:827–37. doi: 10.1089/aid.2007.0291. [DOI] [PubMed] [Google Scholar]
- 56.Fawzi WW, Msamanga GI, Hunter D, et al. Randomized trial of vitamin supplements in relation to transmission of HIV-1 through breastfeeding and early child mortality. AIDS. 2002;16:1935–44. doi: 10.1097/00002030-200209270-00011. [DOI] [PubMed] [Google Scholar]
- 57.Leroy V, Karon JM, Alioum A, et al. Twenty-four month efficacy of a maternal short-course zidovudine regimen to prevent mother-to-child transmission of HIV-1 in West Africa. AIDS. 2002;16:631–41. doi: 10.1097/00002030-200203080-00016. [DOI] [PubMed] [Google Scholar]
- 58.Petra Study Team. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): a randomised, double-blind, placebo-controlled trial. Lancet. 2002;359:1178–86. doi: 10.1016/S0140-6736(02)08214-4. [DOI] [PubMed] [Google Scholar]
- 59.Kuhn L, Aldrovandi GM, Sinkala M, Kankasa C, Mwiya M, Thea DM. Potential impact of new WHO criteria for antiretroviral treatment for prevention of mother-to- child HIV transmission. AIDS. 2010;24:1374–7. additional data at http://www.hivpresentation.com/index.cfm?vId=5BB28A44-423A-F6F7-C70DC6EC268CFF57&cID=5C3B824B-423A-F6F7-C2E8CE9BBF9B2D92&show=slide. Accessed 3 October 2012. [PMC free article] [PubMed] [Google Scholar]
- 60.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 61.Thistle P, Gottesman M, Pilon R, et al. A randomized control trial of an ultra-short zidovudine regimen in the prevention of perinatal HIV transmission in rural Zimbabwe. Cent Afr J Med. 2004;50:79–84. [PubMed] [Google Scholar]
- 62.Mofenson L, Taha T, Li Q, et al. Infant extended antiretroviral (ARV) prophylaxis is effective in preventing postnatal mother-to-child HIV transmission (MTCT) at all maternal CD4 counts [abstract TUPEC053] 2009 In: Program and abstracts of 5th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention (Cape Town, South Africa). Geneva, Switzerland: International AIDS Society Available at: http://www.ias2009.org/pag/Abstracts.aspx?AID=1251. Accessed 3 October 2012. [Google Scholar]
- 63.Kumwenda NI, Hoover DR, Mofenson LM, et al. Extended antiretroviral prophylaxis to reduce breast-milk HIV-1 transmission. N Engl J Med. 2008;359:119–29. doi: 10.1056/NEJMoa0801941. [DOI] [PubMed] [Google Scholar]
- 64.Dabis F, Bequet L, Ekouevi DK, et al. Field efficacy of zidovudine, lamivudine and single-dose nevirapine to prevent peripartum HIV transmission. AIDS. 2005;19:309–18. [PMC free article] [PubMed] [Google Scholar]
- 65.Kesho Bora Study Group. Eighteen-month follow-up of HIV-1-infected mothers and their children enrolled in the Kesho Bora study observational cohorts. J Acquir Immune Defic Syndr. 2010;54:533–41. doi: 10.1097/QAI.0b013e3181e36634. [DOI] [PubMed] [Google Scholar]
- 66.Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–80. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 67.Shapiro RL, Hughes MD, Ogwu A, et al. Antiretroviral regimens in pregnancy and breast-feeding in Botswana. N Engl J Med. 2010;362:2282–94. doi: 10.1056/NEJMoa0907736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kilewo C, Karlsson K, Ngarina M, et al. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr. 2009;52:406–16. doi: 10.1097/QAI.0b013e3181b323ff. [DOI] [PubMed] [Google Scholar]
- 69.Tonwe-Gold B, Ekouevi DK, Viho I, et al. Antiretroviral treatment and prevention of peripartum and postnatal HIV transmission in West Africa: evaluation of a two-tiered approach. PLoS Med. 2007;4:e257. doi: 10.1371/journal.pmed.0040257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kesho Bora Study Group. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;1:159. doi: 10.1016/S1473-3099(10)70288-7. [DOI] [PubMed] [Google Scholar]
- 71.Thior I, Lockman S, Smeaton LM, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: the Mashi Study. JAMA. 2006;296:794–805. doi: 10.1001/jama.296.7.794. [DOI] [PubMed] [Google Scholar]
- 72.Coutsoudis A, Pillay K, Kuhn L, Spooner E, Tsai WY, Coovadia HM. Method of feeding and transmission of HIV-1 from mothers to children by 15 months of age: prospective cohort study from Durban, South Africa. AIDS. 2001;15:379–87. doi: 10.1097/00002030-200102160-00011. [DOI] [PubMed] [Google Scholar]
- 73.Leroy V, Newell ML, Dabis F, et al. International multicentre pooled analysis of late postnatal mother-to-child transmission of HIV-1 infection. Ghent International Working Group on Mother-to-Child Transmission of HIV. Lancet. 1998;352:597–600. doi: 10.1016/s0140-6736(98)01419-6. [DOI] [PubMed] [Google Scholar]
- 74.Chasela CS, Hudgens MG, Jamieson DJ, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–81. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vyankandondera J, Luchters S, Hassink E. Reducing risk of HIV-1 transmission from mother to infant through breastfeeding using antiretroviral prophylaxis in infants (SIMBA-study) [abstract LB07) In: Program and abstracts of 2nd International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention. (Paris, France). Geneva, Switzerland: International AIDS Society, 2003. Available at: http://www.iasociety.org/Default.aspx?pageId=11&abstractId=11061. Accessed 26 June 2012. [Google Scholar]
- 76.Palombi L, Marazzi MC, Voetberg A, Magid NA. Treatment acceleration program and the experience of the DREAM program in prevention of mother-to-child transmission of HIV. AIDS. 2007;21(Suppl 4):S65–71. doi: 10.1097/01.aids.0000279708.09180.f5. [DOI] [PubMed] [Google Scholar]
- 77.Thomas TK, Masaba R, Borkowf CB, et al. Triple-antiretroviral prophylaxis to prevent mother-to-child HIV transmission through breastfeeding—the Kisumu Breastfeeding Study, Kenya: a clinical trial. PLoS Med. 2011;8:e1001015. doi: 10.1371/journal.pmed.1001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–43. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 79.Crampin AC, Floyd S, Glynn JR, et al. The long-term impact of HIV and orphanhood on the mortality and physical well-being of children in rural Malawi. AIDS. 2003;17:389–97. doi: 10.1097/00002030-200302140-00013. [DOI] [PubMed] [Google Scholar]
- 80.Nakiyingi JS, Bracher M, Whitworth JA, et al. Child survival in relation to mother's HIV infection and survival: evidence from a Ugandan cohort study. AIDS. 2003;17:1827–34. doi: 10.1097/00002030-200308150-00012. [DOI] [PubMed] [Google Scholar]
- 81.Zaba B, Whitworth J, Marston M, et al. HIV and mortality of mothers and children: evidence from cohort studies in Uganda, Tanzania, and Malawi. Epidemiology. 2005;16:275–80. doi: 10.1097/01.ede.0000155507.47884.43. [DOI] [PubMed] [Google Scholar]
- 82.Marinda E, Humphrey JH, Iliff PJ, et al. Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J. 2007;26:519–26. doi: 10.1097/01.inf.0000264527.69954.4c. [DOI] [PubMed] [Google Scholar]
- 83.Marston M, Becquet R, Zaba B, et al. Net survival of perinatally and postnatally HIV-infected children: a pooled analysis of individual data from sub–Saharan Africa. Int J Epidemiol. 2011;40:385–96. doi: 10.1093/ije/dyq255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8:477–89. doi: 10.1016/S1473-3099(08)70180-4. [DOI] [PubMed] [Google Scholar]
- 85.Fassinou P, Elenga N, Rouet F, et al. Highly active antiretroviral therapies among HIV-1-infected children in Abidjan, Côte d'Ivoire. AIDS. 2004;18:1905–13. doi: 10.1097/00002030-200409240-00006. [DOI] [PubMed] [Google Scholar]
- 86.World Bank. World development indicators. Available at: http://data.worldbank.org/data-catalog/world-development-indicators. Accessed 30 January 2012. [Google Scholar]
- 87.United Nations. World population prospects: the 2008 revision. Available at: http://www.un.org/esa/population/publications/wpp2008/wpp2008_highlights.pdf. Accessed 30 January 2012. [Google Scholar]
- 88.Coetzee D, Hildrebrand K, Boulle A, et al. Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS. 2004;18:887–95. doi: 10.1097/00002030-200404090-00006. [DOI] [PubMed] [Google Scholar]
- 89.Lockman S, Hughes MD, McIntyre J, et al. Antiretroviral therapies in women after single-dose nevirapine exposure. N Engl J Med. 2010;363:1499–509. doi: 10.1056/NEJMoa0906626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stringer JS, McConnell MS, Kiarie J, et al. Effectiveness of non-nucleoside reverse-transcriptase inhibitor-based antiretroviral therapy in women previously exposed to a single intrapartum dose of nevirapine: a multi-country, prospective cohort study. PLoS Med. 2010;7:e1000233. doi: 10.1371/journal.pmed.1000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lockman S, Shapiro RL, Smeaton LM, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;356:135–47. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- 92.Coovadia A, Hunt G, Abrams EJ, et al. Persistent minority K103N mutations among women exposed to single-dose nevirapine and virologic response to nonnucleoside reverse-transcriptase inhibitor–based therapy. Clin Infect Dis. 2009;48:462–72. doi: 10.1086/596486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murphy RA, Sunpath H, Lu Z, et al. Outcomes after virologic failure of first-line ART in South Africa. AIDS. 2010;24:1007–12. doi: 10.1097/QAD.0b013e3283333639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kombe G, Galaty D, Gadhia R, Decker C. Human and financial resource requirements for scaling up HIV/AIDS services in Ethiopia. Available at: http://www.healthsystems2020.org/content/resource/detail/1521. Accessed 22 February 2012. [Google Scholar]
- 95.Bratt JH, Torpey K, Kabaso M, Gondwe Y. Costs of HIV/AIDS outpatient services delivered through Zambian public health facilities. Trop Med Int Health. 2011;16:110–8. doi: 10.1111/j.1365-3156.2010.02640.x. [DOI] [PubMed] [Google Scholar]
- 96.Vander Plaetse B, Hlatiwayo G, Van Eygen L, Meessen B, Criel B. Costs and revenue of health care in a rural Zimbabwean district. Health Policy Plan. 2005;20:243–51. doi: 10.1093/heapol/czi028. [DOI] [PubMed] [Google Scholar]
- 97.Zar HJ, Workman L, le Roux SM, et al. A randomized controlled trial of intermittent compared with daily cotrimoxazole preventive therapy in HIV-infected children. AIDS. 2010;24:2225–32. doi: 10.1097/QAD.0b013e32833d4533. [DOI] [PubMed] [Google Scholar]
- 98.Soderlund N, Zwi K, Kinghorn A. Prevention of vertical transmission of HIV: analysis of cost effectiveness of options available in South Africa. BMJ. 1999;318:1650–6. doi: 10.1136/bmj.318.7199.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Holmes CB, Wood R, Badri M, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defic Syndr. 2006;42:464–9. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.