This study evaluated effects of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D-conjugate vaccine (PHiDCV) compared with the 7-valent vaccine on nasopharyngeal bacterial colonization, specifically nontypeable Haemophilus influenzae (NTHi). PHiD-CV had no differential effect on nasopharyngeal NTHi colonization.
Keywords: pneumococcal conjugate vaccination, nasopharyngeal bacterial colonization, carriage, nontypeable Haemophilus influenza, Streptococcus pneumonia
Abstract
Background. This study evaluated the effects of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D–conjugate vaccine (PHiD-CV) on nasopharyngeal bacterial colonization compared with the 7-valent pneumococcal conjugate vaccine (7vCRM) in young children.
Methods. A randomized controlled trial in the Netherlands, initiated 2 years after 7vCRM introduction, was conducted between 1 April 2008 and 1 December 2010. Infants (N = 780) received either PHiD-CV or 7vCRM (2:1) at 2, 3, 4, and 11–13 months of age. Nasopharyngeal samples taken at 5, 11, 14, 18, and 24 months of age were cultured to detect Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis, and Staphylococcus aureus. Polymerase chain reaction assays quantified H. influenzae and S. pneumoniae and confirmed H. influenzae as nontypeable (NTHi). Primary outcome measure was vaccine efficacy (VE) against NTHi colonization.
Results. In both groups, NTHi colonization increased with age from 33% in 5-month-olds to 65% in 24-month-olds. Three months postbooster, VE against colonization was 0.5% (95% confidence interval [CI], −21.8% to 18.4%) and VE against acquisition 10.9% (95% CI, −31.3% to 38.9%). At each sampling moment, no differences between groups in either NTHi prevalence or H. influenzae density were detected. Streptococcus pneumoniae (range, 39%–57%), M. catarrhalis (range, 63%–69%), and S. aureus (range, 9%–30%) colonization patterns were similar between groups.
Conclusions. PHiD-CV had no differential effect on nasopharyngeal NTHi colonization or H. influenzae density in healthy Dutch children up to 2 years of age, implying that herd effects for NTHi are not to be expected. Other bacterial colonization patterns were also similar.
Clinical Trials Registration NCT00652951.
Pneumococcal conjugate vaccination is highly effective in preventing serotype-specific invasive pneumococcal disease such as meningitis, sepsis, and invasive pneumonia [1]. In addition, Streptococcus pneumoniae is frequently involved in common mucosal bacterial infections in childhood, such as pneumonia and acute otitis media (AOM) [1, 2]. Unencapsulated or nontypeable Haemophilus influenzae (NTHi) is the second most common bacterial respiratory pathogen [2, 3]. This otopathogen is particularly associated with recurrent otitis and its sequelae, presumably through biofilm formation [3–5].
The human nasopharynx is the reservoir for both pneumococcus and NTHi, and the source of transmission between individuals [6]. The great success of protein-polysaccharide conjugate vaccines, targeting encapsulated bacteria such as pneumococcus, group C meningococcus, and H. influenzae type b (Hib), is based not only on inducing protective antibodies in immunized individuals, but also on reducing nasopharyngeal colonization and preventing transmission of the vaccine-targeted bacteria [7–9]. The consequent indirect (herd) effects following pneumococcal conjugate vaccination resulted in protection of the unimmunized population, which had a major impact on the overall effectiveness [10]. Assessment of nasopharyngeal colonization of vaccine-targeted bacteria in immunized individuals is therefore important for understanding the mechanism and to predict effects after widespread implementation [11].
An experimental protein-polysaccharide conjugate vaccine, in which each of 11 pneumococcal capsular saccharides was conjugated to NTHi-derived protein D (PD) as a carrier protein (11Pn-PD, GlaxoSmithKline Vaccines), was found to not only protect against vaccine serotype-specific pneumococcal AOM, but also to prevent 35% of NTHi AOM episodes [12]. The licensed 10-valent pneumococcal nontypeable H. influenzae protein D–conjugate vaccine (PHiD-CV; GlaxoSmithKline Vaccines) is based on its 11-valent predecessor and uses PD as a carrier for 8 of 10 pneumococcal serotypes [13]. Data regarding the effects of these PD conjugate vaccines on nasopharyngeal bacterial colonization are limited [12, 14, 15]. Therefore, irrefutable evidence that PD conjugate vaccines affect NTHi colonization and thus prevent NTHi transmission is still lacking. Biological effects of the carrier protein may differ from those of conjugated polysaccharides and may not affect nasopharyngeal colonization.
Additionally, pneumococcal conjugate vaccination shifts colonization from vaccine to nonvaccine pneumococcal serotypes [16] and may affect colonization with nonpneumococcal species, such as Staphylococcus aureus or NTHi [17, 18]. In a randomized controlled trial, we compared nasopharyngeal bacterial colonization in children immunized with PHiD-CV with children receiving the 7-valent pneumococcal conjugate vaccine, in which 7 serotypes are covalently bound to the carrier protein CRM197, a nontoxic diphtheria mutant (7vCRM; Pfizer, Inc). This trial was initiated approximately 2 years after introduction of 7vCRM in the Dutch national immunization program.
METHODS
The study protocol and CONSORT checklist are provided as Supplementary Data (see Supplementary Text 1 and Text 2, respectively).
Study Design and Population
We conducted this trial between 1 April 2008 and 1 December 2010. The study area covered the western and central parts of the Netherlands [19]. Healthy infants born after a gestation period of at least 36 weeks and aged 6–12 weeks at the time of the first primary vaccination were eligible. Exclusion criteria have been detailed previously [19]. Infants were visited at home for all study procedures. Participants received no financial compensation. An independent ethics committee (Centrale Commissie Mensgebonden Onderzoek, http://www.ccmo-online.nl) approved the study protocol. The study was undertaken in accordance with the European guidelines for Good Clinical Practice, which incorporate provisions of the Declaration of Helsinki. Written informed consent was obtained from each infant's parent(s)/guardian(s) before enrollment. In the Dutch national immunization program, 7vCRM was introduced for all infants born after 31 March 2006 with no catch-up campaign. PHiD-CV replaced 7vCRM in the Dutch national immunization program for all infants born after 1 March 2011.
Randomization and Masking
Infants were randomly assigned (1:1:1) to receive either (1) PHiD-CV + diphtheria, tetanus, acellular pertussis [DTPa]–hepatitis B virus [HBV]–inactivated poliovirus and Hib vaccine [IPV/Hib], (2) PHiD-CV + DTPa-IPV-Hib (Sanofi Pasteur MSD), or (3) 7vCRM + DTPa-IPV-Hib at 2, 3, 4, and 11–13 months of age, resulting in a 2:1 ratio for immunization with either PHiD-CV or 7vCRM. A randomization list used to number the vaccines was generated using a standard SAS (Statistical Analysis System) program (version 9.2) with a block size of 6 (blocking scheme of 2:2:2). Each participant was assigned to a group via a web-based central randomization system that, on receipt of the infant's birth date and scheduled date for the first home visit, determined the vaccine number to be used. Parents and study site staff were aware of the treatment assignment, but outcome assessors were not.
Procedures
Vaccines were administered intramuscularly in the left and right anterolateral thigh. The composition of PHiD-CV (Synflorix, GlaxoSmithKline Vaccines), 7vCRM (Prevenar/Prevnar, Pfizer, Inc), DTPa-HBV-IPV/Hib (Infanrix hexa, GlaxoSmithKline Vaccines), and DTPa-IPV-Hib (Pediacel, Sanofi Pasteur MSD) has been previously described [19].
Nasopharyngeal samples were obtained transnasally using a flexible, sterile swab with a flocked nylon tip (Eswab 482CE, Copan, Brescia, Italy) by trained study personnel according to World Health Organization procedures [20] at 5, 11, 14, 18, and 24 months of age. With each nasopharyngeal swab, a questionnaire addressing risk factors for bacterial colonization was completed. After sampling, swabs were immediately inoculated in 1 mL of modified liquid Amies transport medium and transferred at room temperature to the Regional Laboratory of Public Health (Haarlem, the Netherlands). Samples were plated as soon as possible (<8 hours), but at the latest within 24 hours after sampling. Swabs were used to inoculate the plates. Identification of H. influenzae, S. pneumoniae, Moraxella catarrhalis, and S. aureus was based on colony morphology and conventional methods of determination, as previously described [9, 17, 21]. To differentiate H. influenzae from Haemophilus haemolyticus, a closely related but nonpathogenic species [22], all H. influenzae isolates underwent polymerase chain reaction (PCR), targeting the glycosyltransferase (lgtC) and outer membrane protein P6 (P6) genes.
One S. pneumoniae colony per plate was subcultured and pneumococcal isolates were serotyped by Quellung reaction. Likewise, 1 H. influenzae colony per plate was subcultured and typed by slide agglutination serotyping. After plating, the remaining medium was stored at −70°C until being transferred to GlaxoSmithKline Vaccines’ laboratories.
Here, the presence of H. influenzae and S. pneumoniae was quantified in all available, properly stored original samples using 2 real-time quantitative PCR (qPCR) assays, targeting the lgtC gene and autolysin (lytA) gene, respectively. DNA was extracted using the NucliSENS easyMAG (bioMérieux), and DNA fragments were amplified using the 7900HT Real-Time PCR System (Applied Biosystems). Bacterial densities were expressed as genome equivalent per milliliter (GE/mL). A serotype-specific PCR for 19A assay was also developed.
Statistical Analysis
This trial was primarily designed to assess immunogenicity [19]. The study was adequately powered to assess effects of PHiD-CV on NTHi colonization using 7vCRM as a nonactive control. Given an estimated 55% prevalence for H. influenzae in the 7vCRM group based on previous experience [21], a sample size of 520 subjects in the PHiD-CV group and 260 subjects in the 7vCRM group would allow demonstration of a positive vaccine efficacy of 19.8% with 80% power at a 1-sided alpha level of 2.5% (calculated with PASS2005).
Statistical analysis was performed on the total vaccinated cohort, meaning that all available data from all participants allocated to a treatment group were analyzed according to the assigned intervention.
The percentage of subjects with a positive culture for the considered bacterium (or serotype) was calculated per group at each sampling moment and across visits, as was the frequency of acquisition of new bacteria (or serotypes). Acquisition was defined as a subject whose nasopharyngeal swab became positive for a bacterium (or serotype) after a previously negative swab for that bacterium (or serotype) [15]. In case of missing data, the occurrence of bacteria (or serotypes) was not considered as a “new acquisition.” Vaccine efficacy of PHiD-CV against bacterial colonization (VEcol) and acquisition (VEacq) was estimated as [(1 – relative risk) × 100] and calculated with 95% confidence intervals (CIs) at each time point and across visits. Statistical significance of vaccine efficacy was based on a lower limit of the 95% CI greater than zero. No adjustment for multiple comparisons was made and conclusions on statistical significance should therefore be interpreted with caution. The statistical analyses were performed using SAS version 9.2 and SDD (SAS Drug and Development) web portal version 3.5.
RESULTS
A total of 780 children were enrolled and allocated to 1 of 3 groups (Figure 1). Recruitment started on 1 April 2008 and was completed on 30 January 2009. Follow-up ended on 1 December 2010. Characteristics of the children did not differ between groups (Table 1). A total of 3863 (99% of planned) nasopharyngeal swabs were collected.
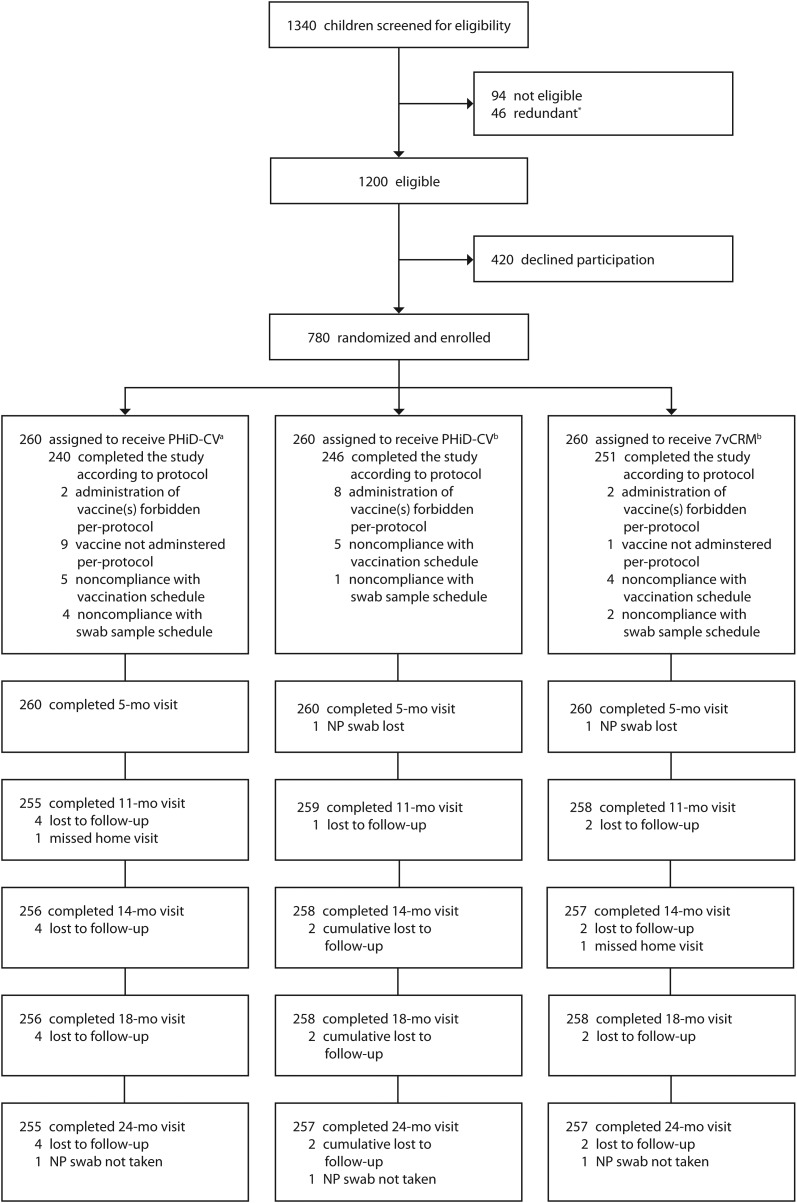
Figure 1.
Trial profile. *Parents of children interested in participating in the study were “redundant” when they were still in the information process after target enrollment had already been achieved. Consequently, the informed consent procedure was cancelled. aCoadministered with diphtheria, tetanus, acellular pertussis, hepatitis B virus, inactivated poliovirus and Hib vaccine (DTPa-HBV-IPV/Hib [GlaxoSmithKline Vaccines]). bCoadministered with DTPa-IPV-Hib. Abbreviations: 7vCRM, 7-valent pneumococcal conjugate vaccine; NP, nasopharyngeal; PHiD-CV, pneumococcal nontypeable Haemophilus influenzae protein D–conjugate vaccine.
Table 1.
Characteristics of Participating Children
| PHiD-CV |
PHiD-CV |
7vCRM |
|
|---|---|---|---|
| DTPa-HBV-IPV/Hib | DTPa-IPV-Hib | DTPa-IPV-Hib | |
| Participants | 260 | 260 | 260 |
| Male sex | 142 (55%) | 130 (50%) | 124 (48%) |
| Feeding from birtha | |||
| Breastfed (partially) >3 months | 125 (49%) | 131 (51%) | 157 (61%) |
| Breastfed (partially) >6 months | 78 (31%) | 78 (30%) | 99 (39%) |
| Presence of siblingsb | 147 (57%) | 131 (50%) | 116 (45%) |
| Daycare attendancec | |||
| At 11–13 monthsd | 172 (67%) | 171 (66%) | 172 (67%) |
| At 23–25 monthse | 189 (74%) | 186 (72%) | 180 (70%) |
| Use of oral or intravenous antibiotics during 1 month before sampling | |||
| At 11–13 monthsd | 26 (10%) | 21 (8%) | 25 (10%) |
| At 23–25 monthse | 15 (6%) | 11(4%) | 18 (7%) |
| Passive tobacco smoke exposure indoors | |||
| At 11–13 monthsd | 16 (6%) | 17 (7%) | 13 (5%) |
| At 23–25 monthse | 16 (6%) | 19 (7%) | 13 (5%) |
| Age at time of vaccination, mean (SD) | |||
| Dose 1 (weeks) | 7.4 (1.2) | 7.6 (1.3) | 7.6 (1.3) |
| Dose 2 (weeks) | 12.0 (1.4) | 12.1 (1.5) | 12.1 (1.4) |
| Dose 3 (weeks) | 16.4 (1.6) | 16.6 (1.6) | 16.6 (1.6) |
| Booster dose (months) | 11.0 (0.2) | 11.1 (0.3) | 11.0 (0.2) |
| Age at time of nasopharyngeal sampling, mean (SD) | |||
| Postprimary | 4.4 (0.5) | 4.5 (0.5) | 4.5 (0.5) |
| Prebooster | 11.0 (0.1) | 11.0 (0.2) | 11.0 (0.1) |
| 3 months postbooster | 14.1 (0.4) | 14.1 (0.4) | 14.1 (0.3) |
| 7 months postbooster | 18.1 (0.3) | 18.1 (0.3) | 18.1 (0.3) |
| 12 months postbooster | 23.2 (0.4) | 23.2 (0.4) | 23.1 (0.4) |
Data are No. (%) unless otherwise specified.
Abbreviations: 7vCRM, 7-valent pneumococcal conjugate vaccine; DTPa, diphtheria, tetanus, acellular pertussis; HBV, hepatitis B virus; Hib, Haemophilus influenzae type b; IPV, inactivated poliovirus; PHiD-CV, pneumococcal nontypeable Haemophilus influenzae protein D–conjugate vaccine; SD, standard deviation.
a Information was asked at 11 months of age.
b Data represent presence of siblings (yes/no) at 5 months of age.
c Defined as at least 4 continuous hours per week with at least 1 child <5 years of age from a different family.
d For PHiD-CV + DTPa-HBV-IPV/Hib group, n = 256; PHiD-CV + DTPa-IPV-Hib group, n = 259; 7vCRM + DTPa-IPV-Hib group, n = 257.
e For PHiD-CV + DTPa-HBV-IPV/Hib group, n = 256; PHiD-CV + DTPa-IPV-Hib group, n = 258; 7vCRM + DTPa-IPV-Hib group, n = 258.
Nasopharyngeal NTHi Colonization, Acquisition, and Density
Based on conventional culture methods, 65% of cultured swabs were positive for H. influenzae. After differentiation from H. haemolyticus, 59% of all samples were confirmed H. influenzae. Of these, 92% were NTHi, while the remainder was encapsulated. NTHi colonization increased with age from approximately 33% at 5 months to 65% at 24 months (Table 2). Prevalence rates were similar in both groups at each sampling moment (Table 2), as were the frequencies of acquisition (Supplementary Table 1). At 3 months postbooster, VEcol was 0.5% (95% CI, −21.8% to 18.4%) and VEacq was 10.9% (95% CI, −31.3% to 38.9%).
Table 2.
Nasopharyngeal Haemophilus influenzae Colonization (Total Vaccinated Cohort)
| PHiD-CV Group |
7vCRM Group |
Vaccine Efficacy | ||||||
|---|---|---|---|---|---|---|---|---|
| n | N | % (95% CI) | n | N | % (95% CI) | % (95% CI) | P Valueb | |
| Any H. influenzaea | ||||||||
| Age 5 months | 185 | 517 | 35.8 (31.6 to 40.1) | 85 | 258 | 32.9 (27.2 to 39.0) | −8.6 (−42.1 to 16.4) | .5747 |
| Age 11–13 months | 307 | 512 | 60.0 (55.6 to 64.2) | 144 | 256 | 56.3 (49.9 to 62.4) | −6.6 (−30.9 to 12.8) | .5626 |
| Age 14–16 months | 319 | 514 | 62.1 (57.7 to 66.3) | 165 | 257 | 64.2 (58.0 to 70.1) | 3.3 (−17.4 to 20.1) | .7564 |
| Age 18–20 months | 354 | 510 | 69.4 (65.2 to 73.4) | 162 | 256 | 63.3 (57.1 to 69.2) | −9.7 (−32.9 to 9.2) | .3535 |
| Age 23–25 months | 384 | 509 | 75.4 (71.5 to 79.1) | 177 | 254 | 69.7 (63.6 to 75.3) | −8.3 (−30.1 to 9.6) | .4079 |
| Across all visits | 486 | 520 | 93.5 (91.0 to 95.4) | 238 | 260 | 91.5 (87.5 to 94.6) | −2.1 (−19.7 to 12.7) | .8265 |
| Nontypeable H. influenzaea | ||||||||
| Age 5 months | 177 | 517 | 34.2 (30.1 to 38.5) | 83 | 258 | 32.2 (26.5 to 38.2) | −6.4 (−39.8 to 18.5) | .6923 |
| Age 11–13 months | 296 | 512 | 57.8 (53.4 to 62.1) | 138 | 255 | 54.1 (47.8 to 60.4) | −6.8 (−31.7 to 13.0) | .5578 |
| Age 14–16 months | 302 | 513 | 58.9 (54.5 to 63.2) | 152 | 257 | 59.1 (52.9 to 65.2) | 0.5 (−21.8 to 18.4) | .9980 |
| Age 18–20 months | 314 | 509 | 61.7 (57.3 to 65.9) | 141 | 254 | 55.5 (49.2 to 61.7) | −11.1 (−36.5 to 9.2) | .3214 |
| Age 23–25 months | 335 | 505 | 66.3 (62.0 to 70.5) | 165 | 254 | 65.0 (58.7 to 70.8) | −2.1 (−23.8 to 15.5) | .8667 |
| Across all visits | 480 | 520 | 92.3 (89.7 to 94.4) | 234 | 260 | 90.0 (85.7 to 93.4) | −2.6 (−20.4 to 12.5) | .7843 |
For each visit, N indicates the number of subjects with swabs cultured and n (%) the number (percentage) of swabs positive for the specified bacterium. For across all visits, N indicates the number of subjects with swabs cultured after at least 1 visit and n (%) the number (percentage) of swabs positive for the specified bacterium after at least 1 visit. Vaccine efficacy of PHiD-CV compared to 7vCRM was estimated as [(1–relative risk) × 100].
Abbreviations: 7vCRM, 7-valent pneumococcal conjugate vaccine; CI, confidence interval; PHiD-CV, pneumococcal nontypeable Haemophilus influenzae protein D–conjugate vaccine.
a Data include only results from samples confirmed by polymerase chain reaction as positive for Haemophilus influenzae after discrimination from Haemophilus haemolyticus. Samples with invalid test results were excluded from the analysis.
b Two-sided conditional exact test.
The presence of H. influenzae detected by qPCR was 44% in 5-month-olds in both groups and increased with age to 84% (95% CI, 80%–87%) and 80% (95% CI, 75%–85%) in 24-month-olds vaccinated with PHiD-CV and 7vCRM, respectively. Of all culture-positive samples (confirmed as true H. influenzae by PCR), 96% was found to be also positive by qPCR, while qPCR detected H. influenzae in 30% of culture-negative samples (Supplementary Table 2). Haemophilus influenzae density increased with age, reaching a plateau in the second year of life. Densities were comparable at each sampling moment, irrespective of the administered vaccine (Figure 2).
Figure 2.
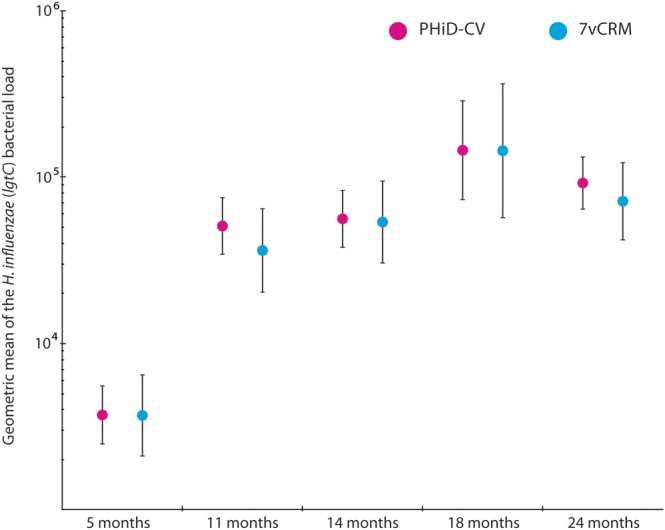
Density of Haemophilus influenzae in nasopharyngeal samples of children (total vaccinated cohort). Density of H. influenzae was measured in original swab media by quantitative polymerase chain reaction targeting the glycosyltransferase gene with values of ≥200 genomic equivalents (GEs) per milliliter defined as positive for the presence of H. influenzae. Point estimates of the geometric means of GEs per milliliter are shown with their 95% confidence intervals (error bars). Abbreviations: 7vCRM, 7-valent pneumococcal conjugate vaccine; lgtC, glycosyltransferase gene; PHiD-CV, pneumococcal nontypeable Haemophilus influenzae protein D–conjugate vaccine.
Nasopharyngeal Pneumococcal Colonization, Acquisition, and Density
Based on conventional culture, 50% of all swabs were positive for S. pneumoniae. Overall pneumococcal colonization rates ranged in both groups from around 39% at 5 months of age to 57% in the second year of life (Table 3). Prevalence of the 7 serotypes included in both vaccines was comparable between groups and declined from approximately 8% in 5-month-olds to 3% in 24-month-olds. Serotypes 1, 5, and 7F were rarely detected (n = 17 [0.4%] of all samples). Acquisition of pneumococci was within the same range in both groups (Supplementary Table 3). Throughout follow-up, 19A was the predominant colonizing serotype, ranging similarly in both groups from 6% to 11% (Table 3), followed by 11A (2%–6%) and 16F (2%–4%; Supplementary Table 4).
Table 3.
Nasopharyngeal Streptococcus pneumoniae Colonization (Total Vaccinated Cohort)
| PHiD-CV Group |
7vCRM Group |
|||||
|---|---|---|---|---|---|---|
| n | N | % (95% CI) | n | N | % (95% CI) | |
| Any S. pneumoniaea | ||||||
| Age 5 months | 211 | 519 | 40.7 (36.4 to 45.0) | 100 | 259 | 38.6 (32.6 to 44.8) |
| Age 11–13 months | 249 | 514 | 48.4 (44.0 to 52.9) | 119 | 258 | 46.1 (39.9 to 52.4) |
| Age 14–16 months | 252 | 514 | 49.0 (44.6 to 53.4) | 126 | 257 | 49.0 (42.8 to 55.3) |
| Age 18–20 months | 286 | 514 | 55.6 (51.2 to 60.0) | 148 | 258 | 57.4 (51.1 to 63.5) |
| Age 23–25 months | 293 | 512 | 57.2 (52.8 to 61.6) | 131 | 257 | 51.0 (44.7 to 57.2) |
| Across all visits | 477 | 520 | 91.7 (89.0 to 94.0) | 233 | 260 | 89.6 (85.3 to 93.0) |
| Serotypes common to both vaccinesb | ||||||
| Age 5 months | 42 | 519 | 8.1 (5.9 to 10.8) | 18 | 259 | 6.9 (4.2 to 10.8) |
| Age 11–13 months | 36 | 514 | 7.0 (5.0 to 9.6) | 15 | 257 | 5.8 (3.3 to 9.4) |
| Age 14–16 months | 27 | 514 | 5.3 (3.5 to 7.6) | 12 | 257 | 4.7 (2.4 to 8.0) |
| Age 18–20 months | 26 | 514 | 5.1 (3.3 to 7.3) | 9 | 258 | 3.5 (1.6 to 6.5) |
| Age 23–25 months | 14 | 512 | 2.7 (1.5 to 4.5) | 7 | 257 | 2.7 (1.1 to 5.5) |
| Across all visits | 90 | 520 | 17.3 (14.2 to 20.8) | 43 | 260 | 16.5 (12.2 to 21.6) |
| Serotype 19A | ||||||
| Age 5 months | 41 | 519 | 7.9 (5.7 to 10.6) | 17 | 259 | 6.6 (3.9 to 10.3) |
| Age 11–13 months | 33 | 514 | 6.4 (4.5 to 8.9) | 19 | 257 | 7.4 (4.5 to 11.3) |
| Age 14–16 months | 42 | 514 | 8.2 (6.0 to 10.9) | 22 | 257 | 8.6 (5.4 to 12.7) |
| Age 18–20 months | 56 | 514 | 10.9 (8.3 to 13.9) | 28 | 258 | 10.9 (7.3 to 15.3) |
| Age 23–25 months | 31 | 512 | 6.1 (4.2 to 8.5) | 20 | 257 | 7.8 (4.8 to 11.8) |
| Across all visits | 146 | 520 | 28.1 (24.3 to 32.2) | 80 | 260 | 30.8 (25.2 to 36.8) |
For each visit, N indicates the number of subjects with swabs cultured and n (%) the number (percentage) of swabs positive for the specified bacterium. For across all visits, N indicates the number of subjects with swabs cultured after at least 1 visit and n (%) the number (percentage) of swabs positive for the specified bacterium after at least 1 visit.
Abbreviations: 7vCRM, 7-valent pneumococcal conjugate vaccine; CI, confidence interval; PHiD-CV, pneumococcal nontypeable Haemophilus influenzae protein D–conjugate vaccine.
a Data include any Streptococcus pneumoniae as identified by conventional culture methods, excluding nontypeable strains.
b Pneumococcal serotypes common to both vaccines are serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F.
Using qPCR, the detection of pneumococci ranged from 57% (95% CI, 52%–61%) and 52% (95% CI, 46%–58%) at 5 months to 80% (95% CI, 76%–84%) and 77% (95% CI, 66%–85%) in the second year of life in children immunized with PHiD-CV and 7vCRM, respectively. Of all culture-positive samples, 99% were found to be also positive by qPCR, while qPCR detected pneumococci in 43% of culture-negative samples (Supplementary Table 2). Of the tested samples across all visits, serotype-specific PCR increased the detection of 19A to an average of 20% and 19% in PHiD-CV and 7vCRM vaccinees, respectively (Supplementary Table 2). Detection of 19A using PCR remained constant during follow-up. In both groups alike, S. pneumoniae density increased with age, reaching a plateau in the second year of life (Figure 3).
Figure 3.
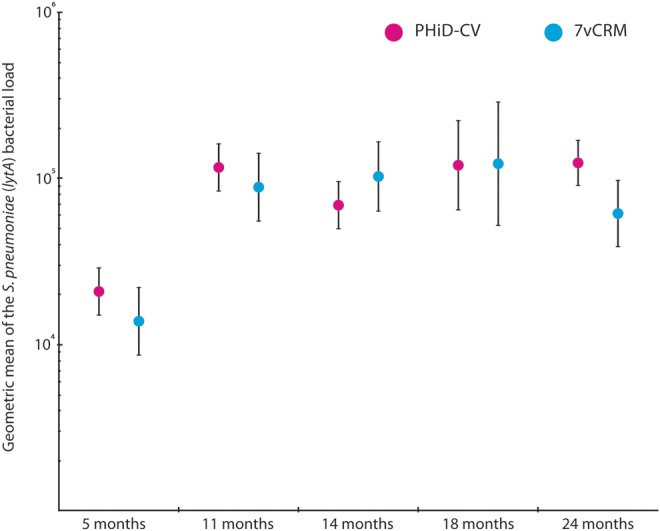
Density of Streptococcus pneumoniae in nasopharyngeal samples of children (total vaccinated cohort). Density of S. pneumoniae was measured in original swab media by quantitative polymerase chain reaction targeting the autolysin gene with values of ≥900 genomic equivalents (GEs) per milliliter defined as positive for the presence of S. pneumoniae. Point estimates of the geometric means of GEs per milliliter are shown with their 95% confidence intervals (error bars). Abbreviations: 7vCRM, 7-valent pneumococcal conjugate vaccine; lytA, autolysin gene; PHiD-CV, pneumococcal nontypeable Haemophilus influenzae protein D–conjugate vaccine.
Nasopharyngeal Colonization With Other Bacterial Species
M. catarrhalis colonization rates ranged from 63% to 69% during follow-up, with no differences between groups (data not shown). Nasopharyngeal colonization with S. aureus decreased with age from approximately 30% at age 5 months to 11% at age 24 months (data not shown). Colonization rates remained within the same ranges between groups, although a trend for a difference was found at age 11–13 months (15% [7vCRM] vs 10% [PHiD-CV]).
DISCUSSION
It has been well described that nasopharyngeal colonization precedes disease [6]. The effect of pneumococcal conjugate vaccination, in particular on prevention of mucosal diseases like AOM and pneumonia, is presumed to be associated with the reduction of nasopharyngeal colonization and density of vaccine pneumococcal serotypes [23]. As mentioned, subsequent group protection has contributed greatly to the success of pneumococcal conjugate vaccination. In our study, we found no differential effect of PHiD-CV immunization on NTHi colonization, acquisition, or density of H. influenzae compared with 7vCRM in healthy Dutch children up to 2 years of age. Therefore, we expect no indirect effects of PHiD-CV on NTHi through prevention of transmission.
The unlicensed 11Pn-PD vaccine reduced 35% of NTHi-caused AOM episodes in the Pneumococcal Otitis Efficacy Trial (POET) with a trend of reduced NTHi colonization [12, 14]. However, the group difference was only observed approximately 3 months postbooster [12], and disappeared at 24 months of age [14]. A study investigating the effects of the licensed PHiD-CV, using a naive, age-matched nonrandomized control group, showed no consistent effect on NTHi colonization [15]. Importantly, carriage rates were low in both studies [14, 15]. In the present study, we found high colonization rates and applied molecular methods to quantify the presence of H. influenzae. We observed no efficacy of PHiD-CV immunization against NTHi colonization or acquisition, at 3 months postbooster or at any other time point. It is unknown if anti-PD antibodies correlate with protection against NTHi-AOM or colonization. Anti-PD antibody concentrations in the present study [19] were lower as those measured in POET [12], but comparable with other PHiD-CV trials. Our use of 7vCRM as nonactive control contrasts with previous studies in which controls did not receive a pneumococcal conjugate vaccine [12, 15]. It has been shown that multiple strains of S. pneumoniae and H. influenzae can coexist in the upper respiratory tract [24]. Therefore, immunization with a pneumococcal conjugate vaccine could exert a bystander effect on the presence of H. influenzae. However, overall and serotype-specific pneumococcal colonization patterns were similar in both vaccine groups. Additionally, no effect on H. influenzae colonization was observed in a recent study evaluating reduced-dose schedules with 7vCRM [21]. Therefore, any possible indirect effects of PHiD-CV on NTHi through effects on S. pneumoniae are unlikely to have influenced our results.
Our study raises the question of how a PD conjugate vaccine induces protection against NTHi-caused AOM, while not affecting presence of NTHi in the nasopharynx—generally regarded to be the point from which respiratory tract infections originate. We propose several possible explanations.
First, although PD may play a role in pathogenesis of NTHi-caused respiratory infections in animal models [25, 26], intervening with its function may not fully prevent a complex biological process such as colonization [26]. Virulence factors other than PD may contribute to colonization of this unencapsulated pathogen. For instance, H. influenzae is highly adaptive and selection of certain phase variants occurs during colonization [27]. Also, specific properties could differ between colonizing H. influenzae strains and those found in disease, such as previously shown for the expression of immunoglobulin A protease [28].
Second, in a chinchilla model for AOM, Johnson and colleagues found that abrogation of protein D's activity reduced NTHi adherence in the middle ear, but not in the nasopharynx—suggesting a compartment-specific effect [26]. Third, the efficacy of the 11Pn-PD vaccine against NTHi AOM could merely reflect prevention of the first pneumococcal AOM. In general, the first otitis episode is more frequently caused by pneumococcus, whereas recurrent episodes are more frequently associated with NTHi etiology [4, 5]. Therefore, preventing the first pneumococcal AOM episode can be hypothesized to prevent sequelae involving NTHi. This hypothesis seems to contradict results of the Finnish otitis media trial (FinOM), in which a statistically nonsignificant increase in H. influenzae AOM was observed after a 3 + 1-dose schedule of 7vCRM [29]. However, although differences in case definitions did not explain the different study results [30], comparing POET with FinOM is fraught with difficulties, owing to dissimilarities in study design and case ascertainment—further complicated by differences in the distribution of AOM-causing pathogens. Nonetheless, surveillance is warranted to monitor bacterial colonization patterns and pathogens involved in AOM following nationwide initiation of pneumococcal conjugate vaccination. Clinical trials assessing efficacy of PHiD-CV against AOM (NCT00839254 and NCT00466947) are currently ongoing.
It is important to emphasize that this study was undertaken at the time of herd immunity with respect to vaccine pneumococcal serotypes [16]. As expected, no major differences were observed between groups with respect to pneumococcal acquisition, colonization, and density. Consistent with previous reports [31, 32], serotypes 1, 5, and 7F, known for their high invasiveness or case-to-carrier ratio, were found to be rarely carried. Also, colonization with potentially cross-reactive serotypes like 6A and 19A and other, non–PHiD-CV pneumococcal serotypes was similar in both groups. Regardless of the administered vaccine and detection method, serotype 19A was the predominant colonizer throughout follow-up. Finally, no major differences were observed between groups in the prevalence of M. catarrhalis and S. aureus in nasopharyngeal samples.
Some limitations need to be addressed. First, we have focused in this study on NTHi colonization in healthy children. Therefore, we cannot speculate on effects of PHiD-CV during NTHi disease or, for example, viral infections, when overgrowth of NTHi could be somewhat contained in children immunized with PHiD-CV. In addition, this study was not powered to detect pneumococcal serotype-specific differences.
Strengths of our study include the longitudinal randomized controlled study design with an adequate sample size to assess the efficacy of PHiD-CV on NTHi colonization, virtual absence of loss to follow-up, and a low rate of protocol deviations. In addition, we had relatively high nasopharyngeal bacterial colonization rates compared with other countries. Finally, we applied molecular methods to measure the densities of H. influenzae and S. pneumoniae in the nasopharynx.
In conclusion, PHiD-CV immunization had no differential effect on nasopharyngeal NTHi colonization, acquisition, or density compared with 7vCRM in healthy children up to 2 years of age in the Netherlands. This implies that herd effects on NTHi are not to be expected following introduction of PHiD-CV. Similar nasopharyngeal pneumococcal, M. catarrhalis, and S. aureus colonization rates were also observed in children vaccinated with either PHiD-CV or 7vCRM.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://www.oxfordjournals.org/our_journals/cid/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We are indebted to all of the participating children and their families who made this study possible. We thank all collaborating institutes, the study staff at the Spaarne Hospital (Hoofddorp, the Netherlands), and laboratory staff at the Regional Laboratory (Haarlem, the Netherlands) for their dedication to this project. We also thank the clinical and laboratory teams at GlaxoSmithKline Biologicals who worked on this study. In particular, we thank A. Fanic for support with statistical analysis; S. Schoonbroodt for laboratory coordination; E. Balian and V. Lella for development, validation, and testing of PCR assays; M. de Gier for study coordination; K. Vercauteren (XPE Pharma & Science c/o GlaxoSmithKline Vaccines) for clinical report writing; and N. Denef (XPE Pharma & Science c/o GlaxoSmithKline Vaccines) for manuscript coordination. Synflorix and Infanrix hexa are trademarks of the GlaxoSmithKline group of companies. Prevenar/Prevnar is a trademark of Pfizer, Inc. Pediacel is a trademark of Sanofi Pasteur MSD. NucliSENS and easyMAG are trademarks of bioMérieux.
Author contributions. Study concept and design: E. A M. S., R. H. V., D. B., and L. S. Acquisition of data: M. R. vdB., J. S., E. P. F. IJ., J. P. B., A. E., and T. G. P. Statistical analysis: N. A. F. Analysis and interpretation of the data: M. R. vdB., J. S., R. H. V., E. A. M. S., K. M. S., N. A. F., T. G. P., D. B., and L. S. Drafting of the manuscript: M. R. vdB., J. S., R. H. V., and E. A. M. S.; Critical revision of the manuscript for important intellectual content: M. R. vdB., J. S., R. H. V., E. P. F. IJ., J. P. B., A. E., E. A. M. S., K. M. S., N. A. F., T. G. P., D. B., and L. S.
Financial support. This study was sponsored by GlaxoSmithKline Biologicals SA. The sponsor was involved in all stages of this study, including the final analysis. M. R. vdB., J. S., R. H. V., and E. A. M. S. drafted the manuscript. GlaxoSmithKline Biologicals SA collaborated with the investigators in the reviewing process of the manuscript before being finalized for submission.
Potential conflicts of interests. E. A. M. S. has received unrestricted research support from Pfizer (Wyeth) and Baxter, consulting fees from Pfizer (Wyeth) and GlaxoSmithKline group of companies, lecturing fees from Pfizer (Wyeth), and grant support for vaccine studies from Pfizer (Wyeth) and GlaxoSmithKline group of companies. R. H. V. has received research support from Pfizer (Wyeth) and GlaxoSmithKline group of companies for vaccine studies, and consulting fees from GlaxoSmithKline group of companies. A. E. has received unrestricted research support from Pfizer (Wyeth), consulting fees from Pfizer (Wyeth) and GlaxoSmithKline group of companies, and grant support for vaccine studies from Pfizer (Wyeth) and GlaxoSmithKline group of companies. N. A. F., T. G. P., D. B., and L. S. are employed by GlaxoSmithKline group of companies. K. M. S. works as a consultant for GlaxoSmithKline group of companies. T. G. P., D. B., and L. S. have stock ownership in GlaxoSmithKline. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.O'Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 2.Rovers MM, Schilder AGM, Zielhuis GA, Rosenfeld RM. Otitis media. Lancet. 2004;363:465–73. doi: 10.1016/S0140-6736(04)15495-0. [DOI] [PubMed] [Google Scholar]
- 3.Murphy TF, Faden H, Bakaletz LO, et al. Nontypeable Haemophilus influenzae as a pathogen in children. Pediatr Infect Dis J. 2009;28:43–8. doi: 10.1097/INF.0b013e318184dba2. [DOI] [PubMed] [Google Scholar]
- 4.Leibovitz E, Jacobs MR, Dagan R. Haemophilus influenzae: a significant pathogen in acute otitis media. Pediatr Infect Dis J. 2004;23:1142–52. [PubMed] [Google Scholar]
- 5.Barkai G, Leibovitz E, Givon Lavi N, Dagan R. Potential contribution by nontypable Haemophilus influenzae in protracted and recurrent acute otitis media. Pediatr Infect Dis J. 2009;28:466–71. doi: 10.1097/inf.0b013e3181950c74. [DOI] [PubMed] [Google Scholar]
- 6.Bogaert D, de Groot R, Hermans P. Streptococcus pneumoniae colonization: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–54. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 7.Segal S, Pollard AJ. Vaccines against bacterial meningitis. Br Med Bull. 2004;72:65–81. doi: 10.1093/bmb/ldh041. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien KL, Millar EV, Zell ER, et al. Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J Infect Dis. 2007;196:1211–20. doi: 10.1086/521833. [DOI] [PubMed] [Google Scholar]
- 9.van Gils EJM, Veenhoven RH, Hak E, et al. Effect of reduced-dose schedules with 7-valent pneumococcal conjugate vaccine on nasopharyngeal pneumococcal carriage in children: a randomized controlled trial. JAMA. 2009;302:159–67. doi: 10.1001/jama.2009.975. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease—United States, 1998–2003. MMWR Morb Mortal Wkly Rep. 2005;54:893–7. [PubMed] [Google Scholar]
- 11.Weinberger DM, Malley R, Lipsitch M. Serotype replacement in disease after pneumococcal vaccination. Lancet. 2011;378:1962–73. doi: 10.1016/S0140-6736(10)62225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prymula R, Peeters P, Chrobok V, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomized double-blind efficacy study. Lancet. 2006;367:740–8. doi: 10.1016/S0140-6736(06)68304-9. [DOI] [PubMed] [Google Scholar]
- 13.Prymula R, Schuerman L. 10-valent pneumococcal nontypeable Haemophilus influenzae PD conjugate vaccine: Synflorix. Expert Rev Vaccines. 2009;8:1479–500. doi: 10.1586/erv.09.113. [DOI] [PubMed] [Google Scholar]
- 14.Prymula R, Kriz P, Kaliskova E, Pascal T, Poolman J, Schuerman L. Effect of vaccination with pneumococcal capsular polysaccharides conjugated to Haemophilus influenzae-derived protein D on nasopharyngeal carriage of Streptococcus pneumoniae and H. influenzae in children under 2 years of age. Vaccine. 2009;28:71–8. doi: 10.1016/j.vaccine.2009.09.113. [DOI] [PubMed] [Google Scholar]
- 15.Prymula R, Hanovcova I, Splino M, Kriz P, Motlova J, et al. Impact of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) on bacterial nasopharyngeal carriage. Vaccine. 2011;29:1959–67. doi: 10.1016/j.vaccine.2010.12.086. [DOI] [PubMed] [Google Scholar]
- 16.Spijkerman J, van Gils EJM, Veenhoven RH, et al. Carriage of Streptococcus pneumoniae 3 years after start of vaccination program, the Netherlands. Emerging Infect Dis. 2011;17:584–91. doi: 10.3201/eid1704101115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Gils EJM, Hak E, Veenhoven RH, et al. Effect of seven-valent pneumococcal conjugate vaccine on Staphylococcus aureus colonization in a randomized controlled trial. PLoS One. 2011;6:e20229. doi: 10.1371/journal.pone.0020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spijkerman J, Prevaes SMPJ, van Gils EJM, et al. Long-term effects of pneumococcal conjugate vaccine on nasopharyngeal carriage of S. pneumoniae, S. aureus, H. influenzae and M. catarrhalis. PLoS One. 2012;7:e39760. doi: 10.1371/journal.pone.0039730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Bergh MR, Spijkerman J, François N, et al. Immunogenicity, safety, and reactogenicity of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine and DTPa-IPV-Hib when coadministered as a 3-dose primary vaccination schedule in the Netherlands: a randomized controlled trial. Pediatr Infect Dis J. 2011;30:e170–8. doi: 10.1097/INF.0b013e31821a0614. [DOI] [PubMed] [Google Scholar]
- 20.O'Brien KL, Nohynek H World Health Organization Pneumococcal Vaccine Trials Carriage Working Group. Report from a WHO Working Group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Pediatr Infect Dis J. 2003;22:e1–11. doi: 10.1097/01.inf.0000049347.42983.77. [DOI] [PubMed] [Google Scholar]
- 21.van Gils EJM, Veenhoven RH, Rodenburg GD, Hak E, Sanders EAM. Effect of 7-valent pneumococcal conjugate vaccine on nasopharyngeal carriage with Haemophilus influenzae and Moraxella catarrhalis in a randomized controlled trial. Vaccine. 2011;29:7595–8. doi: 10.1016/j.vaccine.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 22.Murphy TF, Brauer AL, Sethi S, Kilian M, Cai X, Lesse AJ. Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae. J Infect Dis. 2007;195:81–9. doi: 10.1086/509824. [DOI] [PubMed] [Google Scholar]
- 23.Klugman KP. Contribution of vaccines to our understanding of pneumococcal disease. Philos Trans R Soc Lond B Biol Sci. 2011;366:2790–8. doi: 10.1098/rstb.2011.0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Margolis E, Yates A, Levin BR. The ecology of nasal colonization of Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus: the role of competition and interactions with host's immune response. BMC Microbiol. 2010;10:59. doi: 10.1186/1471-2180-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forsgren A, Riesbeck K. Protein D of Haemophilus influenzae: a protective nontypeable H. influenzae antigen and a carrier for pneumococcal conjugate vaccines. Clin Infect Dis. 2008;46:726–31. doi: 10.1086/527396. [DOI] [PubMed] [Google Scholar]
- 26.Johnson RW, McGillivary G, Denoël P, Poolman J, Bakaletz LO. Abrogation of nontypeable Haemophilus influenzae protein D function reduces phosphorylcholine decoration, adherence to airway epithelial cells, and fitness in a chinchilla model of otitis media. Vaccine. 2011;29:1211–21. doi: 10.1016/j.vaccine.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Clark SE, Snow J, Li J, Zola TA, Weiser JN. Phosphorylcholine allows for evasion of bactericidal antibody by Haemophilus influenzae. PLoS Pathog. 2012;8:e1002521. doi: 10.1371/journal.ppat.1002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vitovski S, Dunkin KT, Howard AJ, Sayers JR. Nontypeable Haemophilus influenzae in carriage and disease: a difference in IgA1 protease activity levels. JAMA. 2002;287:1699–1705. doi: 10.1001/jama.287.13.1699. [DOI] [PubMed] [Google Scholar]
- 29.Eskola J, Kilpi T, Palmu A, et al. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344:403–9. doi: 10.1056/NEJM200102083440602. [DOI] [PubMed] [Google Scholar]
- 30.Palmu A, Jokinen J, Kilpi T Finnish Otitis Media Study Group. Impact of different case definitions for acute otitis media on the efficacy estimates of a pneumococcal conjugate vaccine. Vaccine. 2008;26:2466–70. doi: 10.1016/j.vaccine.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Yildirim I, Hanage WP, Lipsitch M, et al. Serotype-specific invasive capacity and persistent reduction in invasive pneumococcal disease. Vaccine. 2010;29:283–8. doi: 10.1016/j.vaccine.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flasche S, Van Hoek AJ, Sheasby E, et al. Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoS Med. 2011;8:e1001017. doi: 10.1371/journal.pmed.1001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.