Abstract
Introduction
Autoantibodies to ribonucleoprotein are associated with a variety of autoimmune diseases, including rheumatoid arthritis (RA). Many studies on associations between human leukocyte antigen (HLA) alleles and RA have been reported, but few have been validated in RA subpopulations with anti-La/SS-B or anti-Ro/SS-A antibodies. Here, we investigated associations of HLA class II alleles with the presence of anti-Ro/SS-A or anti-La/SS-B antibodies in RA.
Methods
An association study was conducted for HLA-DRB1, DQB1, and DPB1 in Japanese RA and systemic lupus erythematosus (SLE) patients that were positive or negative for anti-Ro/SS-A and/or anti-La/SS-B antibodies.
Results
An increased prevalence of certain class II alleles was associated with the presence of anti-Ro/SS-A antibodies as follows: DRB1*08∶03 (Pc = 3.79×10−5, odds ratio [OR] 3.06, 95% confidence interval [CI] 1.98–4.73), DQB1*06∶01 (Pc = 0.0106, OR 1.70, 95%CI 1.26–2.31), and DPB1*05∶01 (Pc = 0.0040, OR 1.55, 95%CI 1.23–1.96). On the other hand, DRB1*15∶01 (Pc = 0.0470, OR 3.14, 95%CI 1.63–6.05), DQB1*06∶02 (Pc = 0.0252, OR 3.14, 95%CI 1.63–6.05), and DPB1*05∶01 (Pc = 0.0069, OR 2.27, 95% CI 1.44–3.57) were associated with anti-La/SS-B antibodies. The DPB1*05∶01 allele was associated with anti-Ro/SS-A (Pc = 0.0408, OR 1.69, 95% CI 1.19–2.41) and anti-La/SS-B antibodies (Pc = 2.48×10−5, OR 3.31, 95%CI 2.02–5.43) in SLE patients.
Conclusion
HLA-DPB1*05∶01 was the only allele associated with the presence of both anti-Ro/SS-A and anti-La/SS-B antibodies in Japanese RA and SLE patients.
Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease susceptibility to which is associated with genetic and environmental factors [1], [2], [3]. Altered frequencies of human leukocyte antigen (HLA) alleles are known to be associated with RA in most ethnic groups studied. Some HLA-DR alleles are reported to be positively associated with RA susceptibility [4]. A conserved amino acid sequence at position 70–74 (QKRAA, RRRAA, or QRRAA) in the HLA-DRβ chain is shared between the RA-associated HLA-DR alleles; this was therefore designated the shared epitope (SE) [4].
The presence of autoantibodies to ribonucleoprotein is associated with a variety of autoimmune diseases, including Sjögren’s Syndrome (SS), systemic lupus erythematosus (SLE), and RA. Anti-La/SS-B antibodies share many features with anti-Ro/SS-A antibodies, and almost all anti-La/SS-B antibody-positive RA patients also have anti-Ro/SS-A antibodies, whereas about one fifth of anti-Ro/SS-A antibody-positive RA patients also have anti-La/SS-B antibodies. HLA-DR2 (DRB1*15 and *16) and DR3 are strongly associated with anti- Ro/SS-A or anti-La/SS-B antibodies in European primary SS populations [5], [6], [7], [8]. On the other hand, DRB1*08∶03 was reported to be associated with anti-La/SS-B antibodies and *15∶01 with anti-Ro/SS-A antibodies in primary SS, SLE, and asymptomatic individuals in the Japanese population [9]. However, few studies have focused on the association of anti-La/SS-B and anti-Ro/SS-A antibodies with HLA alleles in RA [10]. Here, we elucidate HLA class II associations with the presence of autoantibody in Japanese RA patients.
Materials and Methods
Patients and Controls
Nine hundred twenty five RA and 622 SLE patients were recruited at Sagamihara Hospital, Nagasaki Medical Center, Yokohama Minami Kyosai Hospital, Tama Medical Center, Kitasato University, Komagome Hospital, Himeji Medical Center, Morioka Hospital, and Kyushu Medical Center. All patients were native Japanese living in Japan. All patients with RA fulfilled the 1988 American College of Rheumatology Criteria for RA [11] and did not overlap any other collagen diseases. All patients with SLE fulfilled the American College of Rheumatology criteria for SLE [12]. The RA patients with SS also fulfilled the Japanese Ministry of Health Criteria for the diagnosis of SS [13]. This study was reviewed and approved by the research ethics committees of each participating institute, Sagamihara Hospital Research Ethics Committee, Nagasaki Medical Center Research Ethics Committee, Yokohama Minami Kyosai Hospital Research Ethics Committee, Tama Medical Center Research Ethics Committee, University of Tsukuba Research Ethics Committee, Kitasato University Ethics Committee, Komagome Hospital Ethics Committee, Himeji Medical Center Ethics Committee, Morioka Hospital Ethics Committee, and Kyushu Medical Center Ethics Committee. Written informed consent was obtained from all study participants. This study was conducted in accordance with the principles expressed in the Declaration of Helsinki. Anti-Ro/SS-A and anti-La/SS-B antibodies were detected using Mesacup-2 test (Medical & Biological Laboratories, Nagoya, Japan), or Ouchterlony double immunodiffusion method (TFB, Hachioji, Japan). RA patients who visited Sagamihara Hospital (n = 1538) were classified as anti-Ro/SS-A antibodies positive RA (n = 225, 14.6%) and anti-La/SS-B antibodies positive RA (n = 37, 2.4%).
Genotyping
Genotyping of HLA-DRB1, DQB1, and DPB1 was performed by polymerase chain reaction using sequence-specific oligonucleotide probes, WAKFlow HLA typing kits (Wakunaga, Hiroshima, Japan), using a Bio-Plex 200 system (Bio-Rad, Hercules, CA). HLA-DRB1 alleles encoding the SE are as follows: *01∶01, *04∶01, *04∶04, *04∶05, *04∶10, *10∶01, *14∶02, and *14∶06 [14]. One of each DRB1 and DQB1 locus could not be typed in the present study. These were revealed to be novel HLA alleles, DRB1* 08∶36∶02 and DQB1*06∶51, by sequencing of the isolated alleles [15].
Statistical Analysis
Differences of RA characteristics, allele frequencies, or amino acid residue frequencies were analyzed by Student’s t-test or Fisher’s exact test using 2×2 contingency tables. Adjustment for multiple comparisons was performed using the Bonferroni method. Corrected P (Pc) values were calculated by multiplying the P value by the number of alleles or amino acid residues tested.
Results
Characteristics of Anti-Ro/SS-A and/or Anti-La/SS-B Antibody-positive RA and SLE Patients
Characteristics of anti-Ro/SS-A-positive but anti-La/SS-B-negative [Ro(+)La(−)] RA and anti-Ro/SS-A- and anti-La/SS-B-positive [Ro(+)La(+)] RA patients are given in Table 1. Mean age and percentage of males in the Ro(+)La(−)RA and Ro(+)La(+)RA groups were lower than in the anti-Ro/SS-A- and anti-La/SS-B-negative [Ro(−)La(−)] patients. Percentage of secondary SS in the Ro(+)La(−)RA and Ro(+)La(+)RA was higher than in the Ro(−)La(−)RA. There were no significant differences in terms of disease duration, rheumatoid factor or anti-citrullinated peptide antibody positivity, or Steinbrocker stage.
Table 1. Characteristics of RA and SLE patients.
| Ro(+)La(−)RA | Ro(+)La(+)RA | Ro(−)La(−)RA | P [Ro(+)La(−) vs. Ro(−)La(−)] | P [Ro(+)La(+) vs. Ro(−)La(−)] | |
| Number | 181 | 40 | 704 | ||
| Mean age, years (SD) | 59.8 (13.1) | 59.6 (12.2) | 63.9 (11.8) | 0.0002* | 0.0390* |
| Male, n (%) | 15 (8.3) | 0 (0.0) | 143 (20.5) | 0.0001 | 0.0003 |
| Disease duration, years (SD) | 14.3 (10.3) | 17.7 (13.5) | 14.3 (10.9) | 0.9940* | 0.1017* |
| Steinbrocker stage III and IV, n (%) | 75 (51.0) | 18 (62.1) | 311 (57.4) | 0.1897 | 0.7019 |
| Association of secondary SS, n (%) | 34 (18.8) | 12 (30.0) | 26 (3.7) | 1.41×10−10 | 1.07×10−7 |
| Rheumatoid factor positive, n (%) | 146 (89.0) | 30 (88.2) | 545 (87.6) | 0.6878 | 1.0000 |
| ACPA positive, n (%) | 135 (87.1) | 30 (96.8) | 506 (91.8) | 0.0832 | 0.4999 |
| Ro(+)La(−)SLE | Ro(+)La(+)SLE | Ro(−)La(−)SLE | P [Ro(+)La(−) vs. Ro(−)La(−)] | P [Ro(+)La(+) vs. Ro(−)La(−)] | |
| Number | 129 | 45 | 137 | ||
| Mean age, years (SD) | 49.1 (15.3) | 46.0 (15.5) | 50.0 (14.7) | 0.7118* | 0.1676* |
| Male, n (%) | 11 (8.5) | 2 (4.4) | 15 (10.9) | 0.5417 | 0.2483 |
| Disease duration, years (SD) | 13.3 (12.1) | 7.2 (5.8) | 15.6 (12.1) | 0.1656* | 0.0001* |
| Association of secondary SS, n (%) | 6 (4.7) | 3 (6.7) | 1 (0.7) | 0.0598 | 0.0473 |
| Anti-dsDNA antibody positive, n (%) | 104 (80.6) | 31 (68.9) | 104 (75.9) | 0.3948 | 0.2720 |
SS: Sjögren’s syndrome, RA: rheumatoid arthritis, SLE: systemic lupus erythematosus, ACPA: anti-citrullinated peptide antibody, dsDNA: double-stranded-DNA, Ro(+)La(−): anti-Ro/SS-A-positive but anti-La/SS-B-negative, Ro(+)La(+): anti-Ro/SS-A- and anti-La/SS-B-positive, Ro(−)La(−): anti-Ro/SS-A- and anti-La/SS-B-negative. Association was tested by Fisher’s exact test using 2×2 contingency tables or Student’s t-test. *Student’s t-test was employed.
Characteristics of Ro(+)La(−) and Ro(+)La(+) SLE patients are also given in Table 1. Disease duration in the Ro(+)La(+) groups was shorter than in the Ro(−)La(−). Percentage of secondary SS in the Ro(+)La(+)SLE was higher than in the Ro(−)La(−)SLE. There were no significant differences in terms of mean age, percentage of males or anti-double-stranded-DNA antibody positivity.
Association of HLA Class II Allele Frequencies with the Presence of Anti-Ro/SS-A Antibodies
We tested whether HLA class II was associated with the presence of anti-Ro/SS-A antibodies, comparing the Ro(+)La(−)RA and Ro(−)La(−)RA groups. A significant positive association was found for DRB1*08∶03 and anti-Ro/SS-A antibodies (Pc = 3.79×10−5, odds ratio [OR] 3.06, 95% confidence interval [CI] 1.98–4.73, Table 2). The DQB1*06∶01 allele was also associated with the presence of anti-Ro/SS-A antibodies (Pc = 0.0106, OR 1.70, 95% CI 1.26–2.31, Table 2). Further, the HLA-DPB1*05∶01 allele was associated with anti-Ro/SS-A antibodies (Pc = 0.0040, OR 1.55, 95% CI 1.23–1.96). Frequencies of DR4 and SE alleles were lower in Ro(+)La(−)RA than in Ro(−)La(−)RA (P = 0.0146, OR 0.73, 95% CI 0.57–0.94 and P = 0.0089, OR 0.73, 95% CI 0.57–0.92, respectively). Frequencies of DR2 alleles (DRB1*15 and *16) in Ro(+)La(−)RA and Ro(−)La(−)RA were not significantly different (P = 0.1028, OR 1.29). Thus, there were positive associations between certain alleles of HLA-DRB1, DQB1, and DPB1 and the presence of anti-Ro/SS-A antibodies in RA patients.
Table 2. HLA allele frequencies in Ro(+)La(−) RA patients.
| Ro(+)La(−) | Ro(−)La(−) | P | OR | Pc | 95%CI | |
| DRB1*01∶01 | 21 (5.8) | 110 (7.8) | 0.2162 | 0.73 | NS | |
| DRB1*04∶01 | 6 (1.7) | 52 (3.7) | 0.0667 | 0.44 | NS | |
| DRB1*04∶03 | 5 (1.4) | 20 (1.4) | 1.0000 | 0.97 | NS | |
| DRB1*04∶04 | 0 (0.0) | 3 (0.2) | 1.0000 | 0.55 | NS | |
| DRB1*04∶05 | 92 (25.4) | 406 (28.8) | 0.2132 | 0.84 | NS | |
| DRB1*04∶06 | 7 (1.9) | 25 (1.8) | 0.8256 | 1.09 | NS | |
| DRB1*04∶07 | 0 (0.0) | 3 (0.2) | 1.0000 | 0.55 | NS | |
| DRB1*04∶10 | 4 (1.1) | 33 (2.3) | 0.2136 | 0.47 | NS | |
| DRB1*07∶01 | 0 (0.0) | 6 (0.4) | 0.6086 | 0.30 | NS | |
| DRB1*08∶02 | 11 (3.0) | 21 (1.5) | 0.0727 | 2.07 | NS | |
| DRB1*08∶03 | 38 (10.5) | 52 (3.7) | 1.35×10−6 | 3.06 | 3.79×10−5 | (1.98–4.73) |
| DRB1*08∶23 | 1 (0.3) | 0 (0.0) | 0.2045 | 11.69 | NS | |
| DRB1*09∶01 | 42 (11.6) | 243 (17.3) | 0.0082 | 0.63 | 0.2283 | (0.44–0.89) |
| DRB1*10∶01 | 3 (0.8) | 12 (0.9) | 1.0000 | 0.97 | NS | |
| DRB1*11∶01 | 3 (0.8) | 17 (1.2) | 0.7809 | 0.68 | NS | |
| DRB1*12∶01 | 14 (3.9) | 39 (2.8) | 0.2985 | 1.41 | NS | |
| DRB1*12∶02 | 7 (1.9) | 20 (1.4) | 0.4724 | 1.37 | NS | |
| DRB1*13∶01 | 1 (0.3) | 3 (0.2) | 1.0000 | 1.30 | NS | |
| DRB1*13∶02 | 7 (1.9) | 60 (4.3) | 0.0434 | 0.44 | NS | (0.20–0.98) |
| DRB1*14∶02 | 0 (0.0) | 2 (0.1) | 1.0000 | 0.78 | NS | |
| DRB1*14∶03 | 8 (2.2) | 10 (0.7) | 0.0180 | 3.16 | 0.5051 | (1.24–8.06) |
| DRB1*14∶05 | 3 (0.8) | 14 (1.0) | 1.0000 | 0.83 | NS | |
| DRB1*14∶06 | 9 (2.5) | 15 (1.1) | 0.0689 | 2.37 | NS | |
| DRB1*14∶07 | 0 (0.0) | 1 (0.1) | 1.0000 | 1.29 | NS | |
| DRB1*14∶54 | 13 (3.6) | 34 (2.4) | 0.2041 | 1.51 | NS | |
| DRB1*15∶01 | 28 (7.7) | 75 (5.3) | 0.1006 | 1.49 | NS | |
| DRB1*15∶02 | 36 (9.9) | 124 (8.8) | 0.5373 | 1.14 | NS | |
| DRB1*16∶02 | 2 (0.6) | 8 (0.6) | 1.0000 | 0.97 | NS | |
| DQB1*02∶01 | 0 (0.0) | 6 (0.4) | 0.6086 | 0.30 | NS | |
| DQB1*03∶01 | 39 (10.8) | 133 (9.4) | 0.4280 | 1.16 | NS | |
| DQB1*03∶02 | 22 (6.1) | 73 (5.2) | 0.5134 | 1.18 | NS | |
| DQB1*03∶03 | 46 (12.7) | 229 (16.3) | 0.1038 | 0.75 | NS | |
| DQB1*03∶06 | 0 (0.0) | 3 (0.2) | 1.0000 | 0.55 | NS | |
| DQB1*04∶01 | 96 (26.5) | 432 (30.7) | 0.1384 | 0.82 | NS | |
| DQB1*04∶02 | 7 (1.9) | 35 (2.5) | 0.6987 | 0.77 | NS | |
| DQB1*05∶01 | 25 (6.9) | 125 (8.9) | 0.2461 | 0.76 | NS | |
| DQB1*05∶02 | 14 (3.9) | 27 (1.9) | 0.0471 | 2.06 | 0.7071 | (1.07–3.97) |
| DQB1*05∶03 | 6 (1.7) | 29 (2.1) | 0.8322 | 0.80 | NS | |
| DQB1*06∶01 | 72 (19.9) | 179 (12.7) | 0.0007 | 1.70 | 0.0106 | (1.26–2.31) |
| DQB1*06∶02 | 27 (7.5) | 75 (5.3) | 0.1291 | 1.43 | NS | |
| DQB1*06∶03 | 1 (0.3) | 3 (0.2) | 1.0000 | 1.30 | NS | |
| DQB1*06∶04 | 6 (1.7) | 57 (4.0) | 0.0259 | 0.40 | 0.3884 | (0.17–0.93) |
| DQB1*06∶09 | 1 (0.3) | 1 (0.1) | 0.3673 | 3.90 | NS | |
| DPB1*01∶01 | 1 (0.3) | 0 (0.0) | 0.2045 | 11.69 | NS | |
| DPB1*02∶01 | 87 (24.0) | 387 (27.5) | 0.2061 | 0.83 | NS | |
| DPB1*02∶02 | 15 (4.1) | 59 (4.2) | 1.0000 | 0.99 | NS | |
| DPB1*03∶01 | 16 (4.4) | 56 (4.0) | 0.6573 | 1.12 | NS | |
| DPB1*04∶01 | 10 (2.8) | 55 (3.9) | 0.3498 | 0.70 | NS | |
| DPB1*04∶02 | 30 (8.3) | 171 (12.1) | 0.0409 | 0.65 | 0.6540 | (0.44–0.98) |
| DPB1*05∶01 | 165 (45.6) | 493 (35.0) | 0.0002 | 1.55 | 0.0040 | (1.23–1.96) |
| DPB1*06∶01 | 0 (0.0) | 9 (0.6) | 0.2181 | 0.20 | NS | |
| DPB1*09∶01 | 24 (6.6) | 112 (8.0) | 0.4400 | 0.82 | NS | |
| DPB1*13∶01 | 4 (1.1) | 16 (1.1) | 1.0000 | 0.97 | NS | |
| DPB1*14∶01 | 5 (1.4) | 25 (1.8) | 0.8194 | 0.77 | NS | |
| DPB1*17∶01 | 0 (0.0) | 5 (0.4) | 0.5900 | 0.35 | NS | |
| DPB1*19∶01 | 2 (0.6) | 5 (0.4) | 0.6368 | 1.56 | NS | |
| DPB1*38∶01 | 0 (0.0) | 3 (0.2) | 1.0000 | 0.55 | NS | |
| DPB1*41∶01 | 1 (0.3) | 7 (0.5) | 1.0000 | 0.55 | NS | |
| DPB1*47∶01 | 0 (0.0) | 3 (0.2) | 1.0000 | 0.55 | NS |
RA: rheumatoid arthritis, Ro(+)La(−)RA: anti-Ro/SS-A-positive but anti-La/SS-B-negative RA, Ro(−)La(−)RA: ani-Ro/SS-A- and anti-La/SS-B-negative RA. OR: odds ratio, CI: confidence interval, Pc: corrected P value, NS: not significant. Allele frequencies are shown in parenthesis (%). Associations were established by Fisher’s exact test using 2×2 contingency tables.
Association of HLA Class II Allele Frequencies with the Presence of Anti-La/SS-B Antibodies
We then compared Ro(+)La(+)RA and Ro(−)La(−)RA HLA class II allele frequencies to seek associations with anti-La/SS-B antibodies. A significant positive association was found for DRB1*15∶01 and anti-La/SS-B antibodies (Pc = 0.0470, OR 3.14, 95%CI 1.63–6.05, Table 3). The DQB1*06∶02 allele was also associated with the presence of anti-La/SS-B antibodies (Pc = 0.0252, OR 3.14, 95% CI 1.63–6.05, Table 3). Further, the HLA-DPB1*05∶01 allele was also associated with anti-La/SS-B antibodies (Pc = 0.0069, OR 2.27, 95%CI 1.44–3.57, Table 3). Frequencies of SE and DR4 alleles were lower in Ro(+)La(+)RA than Ro(−)La(−)RA (P = 0.0367, OR 0.59, 95%CI 0.36–0.95, P = 0.0324, OR 0.57, 95%CI 0.34–0.95, respectively). Frequencies of DR2 alleles in Ro(+)La(+)RA were higher than Ro(−)La(−)RA patients (P = 0.0364, OR 1.81, 95%CI 1.06–3.09). Thus, there was an association of HLA class II alleles with anti-La/SS-B antibodies in RA patients.
Table 3. HLA allele frequencies in Ro(+)La(+) RA patients.
| Ro(+)La(+) | Ro(−)La(−) | P | OR | Pc | 95%CI | |
| DRB1*01∶01 | 3 (3.8) | 110 (7.8) | 0.2739 | 0.46 | NS | |
| DRB1*03∶01 | 1 (1.3) | 0 (0.0) | 0.0538 | 53.15 | NS | |
| DRB1*04∶01 | 0 (0.0) | 52 (3.7) | 0.1097 | 0.16 | NS | |
| DRB1*04∶03 | 0 (0.0) | 20 (1.4) | 0.6214 | 0.42 | NS | |
| DRB1*04∶04 | 0 (0.0) | 3 (0.2) | 1.0000 | 2.49 | NS | |
| DRB1*04∶05 | 15 (18.8) | 406 (28.8) | 0.0555 | 0.57 | NS | |
| DRB1*04∶06 | 1 (1.3) | 25 (1.8) | 1.0000 | 0.70 | NS | |
| DRB1*04∶07 | 0 (0.0) | 3 (0.2) | 1.0000 | 2.49 | NS | |
| DRB1*04∶10 | 5 (6.3) | 33 (2.3) | 0.0492 | 2.78 | NS | (1.05–7.32) |
| DRB1*07∶01 | 1 (1.3) | 6 (0.4) | 0.3213 | 2.96 | NS | |
| DRB1*08∶02 | 4 (5.0) | 21 (1.5) | 0.0414 | 3.48 | NS | (1.16–10.38) |
| DRB1*08∶03 | 5 (6.3) | 52 (3.7) | 0.2280 | 1.74 | NS | |
| DRB1*09∶01 | 6 (7.5) | 243 (17.3) | 0.0205 | 0.39 | 0.5728 | (0.17–0.90) |
| DRB1*10∶01 | 0 (0.0) | 12 (0.9) | 1.0000 | 0.69 | NS | |
| DRB1*11∶01 | 1 (1.3) | 17 (1.2) | 1.0000 | 1.04 | NS | |
| DRB1*12∶01 | 4 (5.0) | 39 (2.8) | 0.2865 | 1.85 | NS | |
| DRB1*12∶02 | 3 (3.8) | 20 (1.4) | 0.1223 | 2.70 | NS | |
| DRB1*13∶01 | 0 (0.0) | 3 (0.2) | 1.0000 | 2.49 | NS | |
| DRB1*13∶02 | 4 (5.0) | 60 (4.3) | 0.7735 | 1.18 | NS | |
| DRB1*14∶02 | 0 (0.0) | 2 (0.1) | 1.0000 | 3.49 | NS | |
| DRB1*14∶03 | 1 (1.3) | 10 (0.7) | 0.4566 | 1.77 | NS | |
| DRB1*14∶05 | 2 (2.5) | 14 (1.0) | 0.2111 | 2.55 | NS | |
| DRB1*14∶06 | 3 (3.8) | 15 (1.1) | 0.0681 | 3.62 | NS | |
| DRB1*14∶07 | 0 (0.0) | 1 (0.1) | 1.0000 | 5.83 | NS | |
| DRB1*14∶54 | 2 (2.5) | 34 (2.4) | 1.0000 | 1.04 | NS | |
| DRB1*15∶01 | 12 (15.0) | 75 (5.3) | 0.0017 | 3.14 | 0.0470 | (1.63–6.05) |
| DRB1*15∶02 | 7 (8.8) | 124 (8.8) | 1.0000 | 0.99 | NS | |
| DRB1*16∶02 | 0 (0.0) | 8 (0.6) | 1.0000 | 1.02 | NS | |
| DQB1*02∶01 | 2 (2.5) | 6 (0.4) | 0.0647 | 5.99 | 0.9710 | |
| DQB1*03∶01 | 12 (15.0) | 133 (9.4) | 0.1183 | 1.69 | NS | |
| DQB1*03∶02 | 4 (5.0) | 73 (5.2) | 1.0000 | 0.96 | NS | |
| DQB1*03∶03 | 6 (7.5) | 229 (16.3) | 0.0392 | 0.42 | 0.5886 | (0.18–0.97) |
| DQB1*03∶06 | 0 (0.0) | 3 (0.2) | 1.0000 | 2.49 | NS | |
| DQB1*04∶01 | 15 (18.8) | 432 (30.7) | 0.0238 | 0.52 | 0.3573 | (0.29–0.92) |
| DQB1*04∶02 | 6 (7.5) | 35 (2.5) | 0.0198 | 3.18 | 0.2974 | (1.30–7.80) |
| DQB1*05∶01 | 3 (3.8) | 125 (8.9) | 0.1484 | 0.40 | NS | |
| DQB1*05∶02 | 1 (1.3) | 27 (1.9) | 1.0000 | 0.65 | NS | |
| DQB1*05∶03 | 3 (3.8) | 29 (2.1) | 0.2450 | 1.85 | NS | |
| DQB1*06∶01 | 12 (15.0) | 179 (12.7) | 0.4962 | 1.21 | NS | |
| DQB1*06∶02 | 12 (15.0) | 75 (5.3) | 0.0017 | 3.14 | 0.0252 | (1.63–6.05) |
| DQB1*06∶03 | 0 (0.0) | 3 (0.2) | 1.0000 | 2.49 | NS | |
| DQB1*06∶04 | 4 (5.0) | 57 (4.0) | 0.5656 | 1.25 | NS | |
| DQB1*06∶09 | 0 (0.0) | 1 (0.1) | 1.0000 | 5.83 | NS | |
| DPB1*02∶01 | 11 (13.8) | 387 (27.5) | 0.0061 | 0.42 | 0.0913 | (0.22–0.80) |
| DPB1*02∶02 | 1 (1.3) | 59 (4.2) | 0.3713 | 0.29 | NS | |
| DPB1*03∶01 | 5 (6.3) | 56 (4.0) | 0.3746 | 1.61 | NS | |
| DPB1*04∶01 | 2 (2.5) | 55 (3.9) | 0.7656 | 0.63 | NS | |
| DPB1*04∶02 | 4 (5.0) | 171 (12.1) | 0.0506 | 0.38 | 0.7591 | |
| DPB1*05∶01 | 44 (55.0) | 493 (35.0) | 0.0005 | 2.27 | 0.0069 | (1.44–3.57) |
| DPB1*06∶01 | 0 (0.0) | 9 (0.6) | 1.0000 | 0.92 | NS | |
| DPB1*09∶01 | 6 (7.5) | 112 (8.0) | 1.0000 | 0.94 | NS | |
| DPB1*13∶01 | 2 (2.5) | 16 (1.1) | 0.2517 | 2.23 | NS | |
| DPB1*14∶01 | 4 (5.0) | 25 (1.8) | 0.0660 | 2.91 | 0.9907 | |
| DPB1*17∶01 | 0 (0.0) | 5 (0.4) | 1.0000 | 1.58 | NS | |
| DPB1*19∶01 | 0 (0.0) | 5 (0.4) | 1.0000 | 1.58 | NS | |
| DPB1*38∶01 | 0 (0.0) | 3 (0.2) | 1.0000 | 2.49 | NS | |
| DPB1*41∶01 | 0 (0.0) | 7 (0.5) | 1.0000 | 1.16 | NS | |
| DPB1*47∶01 | 1 (1.3) | 3 (0.2) | 0.1985 | 5.93 | NS |
RA: rheumatoid arthritis, Ro(+)La(+)RA: anti-Ro/SS-A- and anti-La/SS-B-positive RA, Ro(−)La(−)RA: anti-Ro/SS-A- and anti-La/SS-B-negative RA. OR: odds ratio, CI: confidence interval, Pc: corrected P value, NS: not significant. Allele frequencies are shown in parenthesis (%). Associations were established by Fisher’s exact test using 2×2 contingency tables.
Independent Associations of DRB1 and DPB1 with the Presence of Anti-Ro/SS-A Antibodies
A two-locus analysis was performed to identify the primary role of DRB1*08∶03, DQB1*06∶01, or DPB1*05∶01 for the production of anti-Ro/SS-A antibodies in RA patients. The OR for DRB1*08∶03 in patients lacking DQB1*06∶01 was 0.91 (not significant), while the OR for DQB1*06∶01 in patients without DRB1*08∶03 was 1.16 (not significant, Table 4). These differences did not reach statistical significance because of the strong linkage disequilibrium (LD) between DRB1*08∶03 and DQB1*06∶01 which results in a low frequency of DRB1*08∶03 in patients without DQB1*06∶01. On the other hand, the OR for DRB1*08∶03 in patients lacking DPB1*05∶01 was 5.59 (Pc = 0.0004), while it was 1.77 for DPB1*05∶01 in patients without DRB1*08∶03(Pc = 0.0002, Table 4). This suggests independent effects of DRB1*08∶03 and DPB1*05∶01 on the production of anti-Ro/SS-A antibodies in RA.
Table 4. HLA class II allele frequencies in RA cases with or without specific class II alleles.
| allele positivity | Ro(+)La(−) | Ro(−)La(−) | P | OR | Pc | 95%CI | ||
| DRB1*08∶03 | (−) | DQB1*06∶01 | 32 (10.9) | 124 (9.5) | 0.4481 | 1.16 | NS | |
| (−) | DPB1*05∶01 | 143 (48.6) | 456 (34.9) | 1.41×10−5 | 1.77 | 0.0002 | (1.37–2.28) | |
| (+) | DQB1*06∶01 | 40 (58.8) | 55 (53.9) | 0.6364 | 1.22 | NS | ||
| (+) | DPB1*05∶01 | 22 (32.4) | 37 (36.3) | 0.6254 | 0.84 | NS | ||
| DQB1*06∶01 | (−) | DRB1*08∶03 | 0 (0.0) | 2 (0.2) | 1.0000 | 0.91 | NS | |
| (−) | DPB1*05∶01 | 119 (50.4) | 421 (39.1) | 0.0016 | 1.59 | 0.0264 | (1.20–2.11) | |
| (+) | DRB1*08∶03 | 38 (30.2) | 50 (15.2) | 0.0005 | 2.42 | 0.0141 | (1.49–3.93) | |
| (+) | DPB1*05∶01 | 46 (36.5) | 72 (21.8) | 0.0018 | 2.06 | 0.0294 | (1.32–3.22) | |
| DPB1*05∶01 | (−) | DRB1*08∶03 | 14 (17.5) | 22 (3.7) | 1.41×10−5 | 5.59 | 0.0004 | (2.73–11.45) |
| (−) | DQB1*06∶01 | 23 (28.8) | 111 (18.4) | 0.0357 | 1.78 | 0.5356 | (1.05–3.02) | |
| (+) | DRB1*08∶03 | 24 (8.5) | 30 (3.7) | 0.0023 | 2.41 | 0.0654 | (1.38–4.19) | |
| (+) | DQB1*06∶01 | 49 (17.4) | 68 (8.4) | 0.0001 | 2.28 | 0.0011 | (1.54–3.39) | |
| allele positivity | Ro(+)La(+) | Ro(−)La(−) | P | OR | Pc | 95%CI | ||
| DRB1*15∶01 | (−) | DQB1*06∶02 | 0 (0.0) | 0 (0.0) | ||||
| (−) | DPB1*05∶01 | 32 (53.3) | 430 (34.0) | 0.0033 | 2.22 | 0.0490 | (1.32–3.74) | |
| (+) | DQB1*06∶02 | 12 (60.0) | 75 (52.8) | 0.6356 | 1.34 | NS | ||
| (+) | DPB1*05∶01 | 12 (60.0) | 63 (44.4) | 0.2337 | 1.88 | NS | ||
| DQB1*06∶02 | (−) | DRB1*15∶01 | 0 (0.0) | 2 (0.2) | 1.0000 | 4.19 | NS | |
| (−) | DPB1*05∶01 | 32 (53.3) | 431 (33.9) | 0.0033 | 2.22 | 0.0488 | (1.32–3.74) | |
| (+) | DRB1*15∶01 | 12 (60.0) | 73 (52.9) | 0.6353 | 1.34 | NS | ||
| (+) | DPB1*05∶01 | 12 (60.0) | 62 (44.9) | 0.2368 | 1.84 | NS | ||
| DPB1*05∶01 | (−) | DRB1*15∶01 | 2 (20.0) | 26 (4.3) | 0.0724 | 5.54 | NS | |
| (−) | DQB1*06∶02 | 2 (20.0) | 27 (4.5) | 0.0771 | 5.32 | NS | ||
| (+) | DRB1*15∶01 | 10 (14.3) | 49 (6.1) | 0.0205 | 2.57 | 0.5739 | (1.24–5.34) | |
| (+) | DQB1*06∶02 | 10 (14.3) | 48 (6.0) | 0.0195 | 2.63 | 0.2918 | (1.27–5.46) |
RA: rheumatoid arthritis, Ro(+)La(−)RA: anti-Ro/SS-A-positive but anti-La/SS-B-negative RA, Ro(−)La(−)RA: anti-Ro/SS-A- and anti-La/SS-B-negative RA, Ro(+)La(+)RA: anti-Ro/SS-A- and anti-La/SS-B-positive RA, OR: odds ratio, CI: confidence interval, Pc: corrected P value, NS: not significant. Allele frequencies are shown in parenthesis (%). Associations were established by Fisher’s exact test using 2×2 contingency tables.
The two-locus analysis was also performed to identify the primary role of DRB1*15∶01, DQB1*06∶02, or DPB1*05∶01 for the production of anti-La/SS-B antibodies in RA patients. OR for DPB1*05∶01 was 2.22 in patients without DRB1*15∶01 (Pc = 0.0490, Table 4) and was 2.22 in patients without DQB1*06∶02 (Pc = 0.0490, Table 4). This might suggests independent effects of DPB1*05∶01 and DRB1*15∶01 or DQB1*06∶02 on the production of anti-La/SS-B antibodies in RA.
Effects of SE on the Association of HLA Class II Alleles
We examined whether the positive association of DRB1*08∶03, DQB1*06∶01, and DPB1*05∶01 alleles is secondary to the decrease of SE in the Ro(+)La(−)RA patients. When the patients with SE were excluded from the analysis, the DRB1*08∶03 and DQB1*06∶01 allele frequencies were significantly higher in Ro(+)La(−)RA than Ro(−)La(−)RA (Pc = 0.0008, OR 3.33, 95%CI 1.91–5.82, and Pc = 0.0191, OR 2.05, 95%CI 1.33–3.15, respectively, Table 5). On the other hand, DPB1*05∶01 frequency was still higher in Ro(+)La(−)RA than Ro(−)La(−)RA, although the effect was not statistically significant (Pc = 0.5887, OR 1.53, 95%CI 1.03–2.28, Table 5).
Table 5. HLA class II allele frequency in the RA cases without SE alleles.
| Ro(+)La(−) | Ro(−)La(−) | P | OR | Pc | 95%CI | |
| DRB1*0803 | 29 (21.0) | 29 (7.4) | 4.60×10−5 | 3.33 | 0.0008 | (1.91–5.82) |
| DQB1*0601 | 47 (34.1) | 79 (20.2) | 0.0016 | 2.05 | 0.0191 | (1.33–3.15) |
| DPB1*0501 | 60 (43.5) | 131 (33.4) | 0.0392 | 1.53 | 0.5887 | (1.03–2.28) |
| Ro(+)La(+) | Ro(−)La(−) | P | OR | Pc | 95%CI | |
| DRB1*1501 | 7 (20.6) | 36 (9.2) | 0.0660 | 2.56 | NS | |
| DQB1*0602 | 7 (20.6) | 36 (9.2) | 0.0660 | 2.56 | 0.7915 | |
| DPB1*0501 | 15 (44.1) | 131 (33.4) | 0.2579 | 1.57 | NS |
SE: shared epitope, OR: odds ratio, CI: confidence interval, Pc: corrected P value, NS: not significant. Allele frequencies are shown in parenthesis (%). Associations were established by Fisher’s exact test using 2×2 contingency tables.
We also examined whether the positive association of DRB1*15∶01, DQB1*06∶02, and DPB1*05∶01 alleles is secondary to the decrease of SE in the Ro(+)La(+)RA patients. When the patients with SE were excluded from the analysis, these allele frequencies were still higher in the Ro(+)La(+)RA than Ro(−)La(−)RA, although the effect was not statistically significant (Table 5).
Effects of Secondary SS on the Association of HLA Class II Alleles
The associations of anti-Ro/SS-A and anti-La/SS-B antibodies with secondary SS were described in Table 1, and the association of secondary SS with HLA class II alleles was investigated. No association was observed between DPB1*05∶01 or anti-Ro/La antibody-associated HLA alleles and secondary SS. The associations of DRB1*08∶03 (Pc = 0.0001, OR 3.13, 95%CI 1.97–4.96), DQB1*06∶01 (Pc = 0.0173, OR 1.75, 95%CI 1.27–2.43), or DPB1*05∶01 (Pc = 0.0064, OR 1.59, 95%CI 1.23–2.05) with anti-Ro/SS-A antibodies remain significant after excluding RA patients with secondary SS. However, the associations of DRB1*15∶01 (Pc = 0.3450, OR 2.93, 95%CI 1.34–6.42), DQB1*06∶02 (Pc = 0.1848, OR 2.93, 95%CI 1.34–6.42), or DPB1*05∶01 (Pc = 0.0941, OR 2.16, 95%CI 1.26–3.70) with anti-La/SS-A antibodies did not reach statistical significance after excluding RA patients with secondary SS, because of reduced sample numbers after the exclusion.
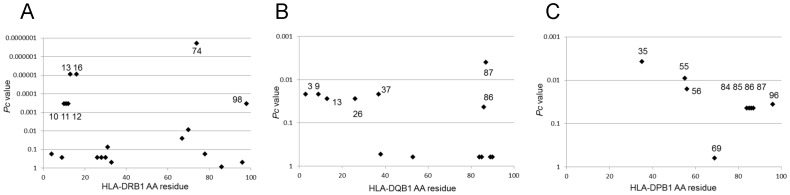
Certain Amino Acid Residues in the DRβ, DQβ, and DPβ Chains are Associated with the Presence of Anti-Ro/SS-A Antibodies
Amino acid residues in HLA-DRβ, DQβ, and DPβ chains were analyzed for their potential associations with anti-Ro/SS-A antibodies. Amino acid positions 13, 16, and 74 in the DRβ chain showed strong associations with the presence of anti-Ro/SS-A antibodies (Figure 1A). The amino acid residue at position 74 associated with anti-Ro/SS-A is different from the SE at that position; these three amino acid residues (13, 16 and 74) are shared by DRB1*08∶02, *08∶03, and *08∶23. The amino acid position 87 in the DQβ chain showed associations with anti-Ro/SS-A antibodies (Figure 1B). Finally, amino acid positions 35, 55, and 56 in the DPβ chain showed associations with anti-Ro/SS-A antibodies (Figure 1C). These three residues are shared by DPB1*05∶01 and *38∶01. Thus, certain amino acid residues in the DRβ, DQβ, and DPβ chains are associated with the presence of anti-Ro/SS-A antibodies.
Figure 1. Associations of amino acid residues in DRβ (A), DQβ (B), and DPβ chains (C) with the presence of anti-Ro/SS-A antibodies.
Corrected P (Pc) values were calculated by multiplying the P value by the number of amino acid residues tested. Associations were established by Fisher’s exact test using 2×2 contingency tables.
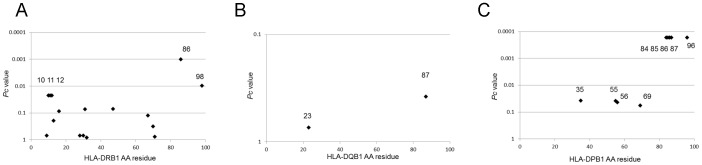
Certain Amino Acid Residues in the DRβ, DQβ, and DPβ Chains are Associated with the Presence of Anti-La/SS-B Antibodies
Amino acid residues in HLA-DRβ, DQβ, and DPβ chains were also analyzed for associations with anti-La/SS-B antibodies. Positions 86 in the DRβ chain showed strong associations with anti-La/SS-B antibodies (Figure 2A). No associations were observed for any residues in the DQβ chain (Figure 2B), whereas positions 84–87 and 96 in the DPβ chain showed associations with anti-La/SS-B antibodies (Figure 2C). Thus, association analysis suggested roles for certain defined amino acid residues in DRβ and DPβ.
Figure 2. Associations of amino acid residues in DRβ (A), DQβ (B), and DPβ chains (C) with the presence of anti-La/SS-B antibodies.
Corrected P (Pc) values were calculated by multiplying the P value by the number of amino acid residues tested. Associations were established by Fisher’s exact test using 2×2 contingency tables.
Association of HLA-DPB1*05∶01 with the Presence of Anti-Ro/SS-A and Anti-La/SS-B Antibodies in SLE Patients
We also tested whether HLA-DPB1 was associated with the presence of anti-Ro/SS-A or anti-La/SS-B antibodies in SLE patients. The DPB1*05∶01 allele was associated with anti-Ro/SS-A (Pc = 0.0408, OR 1.69, 95% CI 1.19–2.41, Table 6) and anti-La/SS-B antibodies (Pc = 2.48×10−5, OR 3.31, 95%CI 2.02–5.43, Table 6). A significant negative association was found for DPB1*02∶01 and anti-La/SS-B antibodies (Pc = 0.0283, OR 0.34, 95%CI 0.17–0.70, Table 6). Thus, there was an association of DPB1*05∶01 allele with anti-Ro/SS-A and anti-La/SS-B antibodies in SLE patients.
Table 6. HLA-DPB1 allele frequency in the SLE patients.
| Ro(+)La(−) vs. Ro(−)La(−) | Ro(+)La(+) vs. Ro(−)La(−) | ||||||||||
| Ro(+)La(−) | Ro(+)La(+) | Ro(−)La(−) | P | OR | Pc | 95%CI | P | OR | Pc | 95%CI | |
| DPB1*01∶01 | 0 (0.0) | 1 (1.1) | 0 (0.0) | 0.2473 | 9.20 | NS | |||||
| DPB1*02∶01 | 49 (19.0) | 10 (11.1) | 73 (26.6) | 0.0393 | 0.65 | 0.4711 | (0.43–0.97) | 0.0022 | 0.34 | 0.0283 | (0.17–0.70) |
| DPB1*02∶02 | 11 (4.3) | 3 (3.3) | 19 (6.9) | 0.1939 | 0.60 | NS | 0.3084 | 0.46 | NS | ||
| DPB1*03∶01 | 13 (5.0) | 3 (3.3) | 10 (3.6) | 0.5239 | 1.40 | NS | 1.0000 | 0.91 | NS | ||
| DPB1*04∶01 | 6 (2.3) | 0 (0.0) | 9 (3.3) | 0.6045 | 0.70 | NS | 0.1198 | 0.15 | NS | ||
| DPB1*04∶02 | 27 (10.5) | 7 (7.8) | 25 (9.1) | 0.6622 | 1.16 | NS | 0.8315 | 0.84 | NS | ||
| DPB1*05∶01 | 118 (45.7) | 56 (62.2) | 91 (33.2) | 0.0034 | 1.69 | 0.0408 | (1.19–2.41) | 1.91×10−6 | 3.31 | 2.48×10−5 | (2.02–5.43) |
| DPB1*06∶01 | 1 (0.4) | 0 (0.0) | 2 (0.7) | 1.0000 | 0.53 | NS | 1.0000 | 0.60 | NS | ||
| DPB1*09∶01 | 18 (7.0) | 4 (4.4) | 23 (8.4) | 0.6264 | 0.82 | NS | 0.2544 | 0.51 | NS | ||
| DPB1*13∶01 | 6 (2.3) | 3 (3.3) | 10 (3.6) | 0.4509 | 0.63 | NS | 1.0000 | 0.91 | NS | ||
| DPB1*14∶01 | 8 (3.1) | 2 (2.2) | 8 (2.9) | 1.0000 | 1.06 | NS | 1.0000 | 0.76 | NS | ||
| DPB1*19∶01 | 1 (0.4) | 0 (0.0) | 2 (0.7) | 1.0000 | 0.53 | NS | 1.0000 | 0.60 | NS | ||
| DPB1*38∶01 | 0 (0.0) | 0 (0.0) | 2 (0.7) | 0.4995 | 0.21 | NS | 1.0000 | 0.60 | NS | ||
SLE: systemic lupus erythematosus, Ro(+)La(−): anti-Ro/SS-A-positive but anti-La/SS-B-negative, Ro(+)La(+): anti-Ro/SS-A- and anti-La/SS-B-positive, Ro(−)La(−): anti-Ro/SS-A- and anti-La/SS-B-negative SLE patients. OR: odds ratio, CI: confidence interval, Pc: corrected P value, NS: not significant. Allele frequencies are shown in parenthesis (%). Associations were established by Fisher’s exact test using 2×2 contingency tables.
Discussion
Several studies have shown that certain HLA-DR alleles are associated with the presence of anti-Ro/SS-A or anti-La/SS-B antibodies in patients with autoimmune diseases. However, few studies have focused on the association of HLA alleles with anti-Ro/SS-A or anti-La/SS-B antibodies in RA. To the best of our knowledge, this is the first report of a positive association of HLA-DPB1*05∶01 with anti-Ro/SS-A and anti-La/SS-B antibodies in RA, although a tendency towards a higher frequency of this allele in Japanese patients with anti-Ro/SS-A or anti-La/SS-B antibodies has been reported before [9]. Recent studies have noted associations of DPB1 alleles with several diseases [16], [17], [18], [19], [20], but here we report an association of DPB1*05∶01 with anti-Ro/SS-A and anti-La/SS-B antibodies in Japanese RA and SLE patients.
It was reported that RA patients with anti-Ro/SS-A antibodies had a more severe disease course, and that they were less frequently DR4-positive than patients without such antibodies [21], [22]. In contrast, the presence of anti-Ro/SS-A antibodies in RA was reported to be positively associated with DR4 in some other studies [10], [23]. Here, we found that frequencies of DR4 and the SE were lower in anti-Ro/SS-A-positive RA patients (Figure 2). Although the implications of this finding are not clear, it might suggest that the role of SE may not be as important in anti-Ro/SS-A-positive RA. Alternatively, the genetic background of anti-Ro/SS-A-positive and -negative RA may be different, and genetic factors other than SE may play a significant role in the former.
It was reported that anti-Ro/SS-A-positive patients were more frequently DR3- or DR2-positive in the context of other autoimmune diseases like primary SS and SLE in European populations [5]. An association of DRB1*15∶01 and anti-Ro/SS-A antibodies has been reported in the Japanese population [9]. Such an association of DR2 with the presence of anti-Ro/SS-A was not confirmed in our study on RA patients, but an association of DR2 with the presence of anti-La/SS-B was observed. Although DRB1*08∶03 was reported to be associated with anti-La/SS-B in Japanese [9], we observed here that it was associated with the presence of anti-Ro/SS-A antibodies in our RA patients. These could be explained by differences in the pathogenesis of RA and SLE.
Amino acid residues 13, 16 and 74 of the HLA-DRβ chain were found to be associated with the presence of anti-Ro/SS-A antibodies (Figure 1). Residues 13 and 74 form the HLA-DR peptide-binding groove [24]. Amino acid residues 84–87 and 96 in the DPβ chain were associated with anti-La/SS-B antibodies. Similarly, amino acid residues 85 and 86 form the peptide-binding grooves of HLA-DP molecules. These data suggest the involvement of peptide antigens bound to specific HLA molecules in controlling the production of anti-Ro/SS-A or anti-La/SS-B antibodies.
It has been determined that patients with anti-Ro/SS-A antibodies are more prone to develop adverse effects when treated with gold salts and other drugs [25], [26], [27]. Recent studies have shown that adverse drug reactions are associated with drug-specific HLA class I alleles, for example A*31∶01 and B*15∶02 with carbamazepine, and A*31∶01 with methotrexate [28], [29], [30]. Furthermore, A*31∶01 is in LD with DRB1*08∶03 and *15∶01 (0.56% and 0.63% of haplotype frequency, respectively, see http://hla.or.jp/haplo/haplonavi.php?type=haplo&lang=en). Frequencies of these class II alleles were higher in anti-Ro/SS-A or anti-La/SS-B antibody- positive RA patients (Tables 2, 3). Large scale association studies for HLA class I and anti-Ro/SS-A or anti-La/SS-B antibodies should be performed. Because of the limited sample size of the present study, the observed significance of the statistical association was modest. The association with HLA-DP needs to be confirmed in future independent studies. Because the allelic distribution of HLA in other ethnic populations is different from that in the Japanese, the role of HLA-DP in anti-Ro/SS-A or anti-La/SS-B antibody production in RA in other populations should be determined.
This is the first identification of an association of HLA-DPB1*05∶01 with positivity for anti-Ro/SS-A or anti-La/SS-B antibodies in RA. Our findings support the role of HLA-DP, as well as DR, in the pathogenesis of autoantibody production.
Acknowledgments
We thank Ms. Mayumi Yokoyama (Sagamihara Hospital) for secretarial assistance.
Funding Statement
This work was supported by Grants-in-Aid for Scientific Research (B, C) (22390199, 22591090) and for Young Scientists (24791018) from the Japan Society for the Promotion of Science, Health and Labour Science Research, Grants from the Ministry of Health, Labour, and Welfare of Japan, Grants-in-Aid for Clinical Research from National Hospital Organization, Research Grants from Daiwa Securities Health Foundation, Research Grants from Japan Research Foundation for Clinical Pharmacology, Research Grants from The Nakatomi Foundation, Research Grants from the Takeda Science Foundation, and research grants from pharmaceutical companies: Abbott Japan Co., Ltd., Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Merck Sharp and Dohme Inc., Pfizer Japan Inc., Takeda Pharmaceutical Company Limited and Teijin Pharma Limited. The funders had no role in study design, data collection and analysis, decision to publish, or preparing the manuscript.
References
- 1.Perricone C, Ceccarelli F, Valesini G (2011) An overview on the genetic of rheumatoid arthritis: a never-ending story. Autoimmun Rev 10: 599–608. Epub 2011 Apr 2022. [DOI] [PubMed]
- 2. Scott IC, Steer S, Lewis CM, Cope AP (2011) Precipitating and perpetuating factors of rheumatoid arthritis immunopathology: linking the triad of genetic predisposition, environmental risk factors and autoimmunity to disease pathogenesis. Best Pract Res Clin Rheumatol 25: 447–468. [DOI] [PubMed] [Google Scholar]
- 3.Lewis SN, Nsoesie E, Weeks C, Qiao D, Zhang L (2011) Prediction of disease and phenotype associations from genome-wide association studies. PLoS ONE 6: e27175. Epub 22011 Nov 27174. [DOI] [PMC free article] [PubMed]
- 4. Reveille JD (1998) The genetic contribution to the pathogenesis of rheumatoid arthritis. Curr Opin Rheumatol 10: 187–200. [DOI] [PubMed] [Google Scholar]
- 5. Hernandez-Molina G, Leal-Alegre G, Michel-Peregrina M (2010) The meaning of anti-Ro and anti-La antibodies in primary Sjogren’s syndrome. Autoimmun Rev 10: 123–125. [DOI] [PubMed] [Google Scholar]
- 6. Rischmueller M, Lester S, Chen Z, Champion G, Van Den Berg R, et al. (1998) HLA class II phenotype controls diversification of the autoantibody response in primary Sjogren’s syndrome (pSS). Clin Exp Immunol 111: 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gottenberg JE, Busson M, Loiseau P, Cohen-Solal J, Lepage V, et al. (2003) In primary Sjogren’s syndrome, HLA class II is associated exclusively with autoantibody production and spreading of the autoimmune response. Arthritis Rheum 48: 2240–2245. [DOI] [PubMed] [Google Scholar]
- 8. Tzioufas AG, Wassmuth R, Dafni UG, Guialis A, Haga HJ, et al. (2002) Clinical, immunological, and immunogenetic aspects of autoantibody production against Ro/SSA, La/SSB and their linear epitopes in primary Sjogren’s syndrome (pSS): a European multicentre study. Ann Rheum Dis 61: 398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miyagawa S, Shinohara K, Nakajima M, Kidoguchi K, Fujita T, et al. (1998) Polymorphisms of HLA class II genes and autoimmune responses to Ro/SS-A-La/SS-B among Japanese subjects. Arthritis Rheum 41: 927–934. [DOI] [PubMed] [Google Scholar]
- 10. Schneeberger E, Citera G, Heredia M, Maldonado Cocco J (2008) Clinical significance of anti-Ro antibodies in rheumatoid arthritis. Clin Rheumatol 27: 517–519. [DOI] [PubMed] [Google Scholar]
- 11. Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, et al. (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31: 315–324. [DOI] [PubMed] [Google Scholar]
- 12. Hochberg M (1997) Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 40: 1725. [DOI] [PubMed] [Google Scholar]
- 13. Tsuboi H, Matsumoto I, Wakamatsu E, Nakamura Y, Iizuka M, et al. (2010) New epitopes and function of anti-M3 muscarinic acetylcholine receptor antibodies in patients with Sjogren’s syndrome. Clin Exp Immunol 162: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Furukawa H, Oka S, Shimada K, Sugii S, Ohashi J, et al. (2012) Association of human leukocyte antigen with interstitial lung disease in rheumatoid arthritis: A protective role for shared epitope. PLoS ONE 7: e33133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oka S, Furukawa H, Kashiwase K, Tsuchiya N, Tohma S (2012) Identification of a novel HLA allele, HLA-DQB1*06:51, in a Japanese rheumatoid arthritis patient. Tissue Antigens 80: 386–387. [DOI] [PubMed] [Google Scholar]
- 16. Kamatani Y, Wattanapokayakit S, Ochi H, Kawaguchi T, Takahashi A, et al. (2009) A genome-wide association study identifies variants in the HLA-DP locus associated with chronic hepatitis B in Asians. Nat Genet 41: 591–595. [DOI] [PubMed] [Google Scholar]
- 17. Noguchi E, Sakamoto H, Hirota T, Ochiai K, Imoto Y, et al. (2011) Genome-wide association study identifies HLA-DP as a susceptibility gene for pediatric asthma in Asian populations. PLoS Genet 7: e1002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kominami S, Tanabe N, Ota M, Naruse TK, Katsuyama Y, et al. (2009) HLA-DPB1 and NFKBIL1 may confer the susceptibility to chronic thromboembolic pulmonary hypertension in the absence of deep vein thrombosis. J Hum Genet 54: 108–114. [DOI] [PubMed] [Google Scholar]
- 19. Raychaudhuri S, Sandor C, Stahl EA, Freudenberg J, Lee HS, et al. (2012) Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat Genet 44: 291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lyons PA, Rayner TF, Trivedi S, Holle JU, Watts RA, et al. (2012) Genetically distinct subsets within ANCA-Associated Vasculitis. N Engl J Med 367: 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boire G, Menard HA (1988) Clinical significance of anti-Ro(SSA) antibody in rheumatoid arthritis. J Rheumatol 15: 391–394. [PubMed] [Google Scholar]
- 22. Boire G, Menard HA, Gendron M, Lussier A, Myhal D (1993) Rheumatoid arthritis: anti-Ro antibodies define a non-HLA-DR4 associated clinicoserological cluster. J Rheumatol 20: 1654–1660. [PubMed] [Google Scholar]
- 23. Tishler M, Moutsopoulos HM, Yaron M (1992) Genetic studies of anti-Ro (SSA) antibodies in families with rheumatoid arthritis. J Rheumatol 19: 234–236. [PubMed] [Google Scholar]
- 24. Jardetzky TS, Brown JH, Gorga JC, Stern LJ, Urban RG, et al. (1994) Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature 368: 711–718. [DOI] [PubMed] [Google Scholar]
- 25. Tishler M, Golbrut B, Shoenfeld Y, Yaron M (1994) Anti-Ro(SSA) antibodies in patients with rheumatoid arthritis–a possible marker for gold induced side effects. J Rheumatol 21: 1040–1042. [PubMed] [Google Scholar]
- 26. Tishler M, Nyman J, Wahren M, Yaron M (1997) Anti-Ro (SSA) antibodies in rheumatoid arthritis patients with gold-induced side effects. Rheumatol Int 17: 133–135. [DOI] [PubMed] [Google Scholar]
- 27. Kamada N, Kinoshita K, Togawa Y, Kobayashi T, Matsubara H, et al. (2006) Chronic pulmonary complications associated with toxic epidermal necrolysis: report of a severe case with anti-Ro/SS-A and a review of the published work. J Dermatol 33: 616–622. [DOI] [PubMed] [Google Scholar]
- 28. Chung WH, Hung SI, Hong HS, Hsih MS, Yang LC, et al. (2004) Medical genetics: a marker for Stevens-Johnson syndrome. Nature 428: 486. [DOI] [PubMed] [Google Scholar]
- 29. McCormack M, Alfirevic A, Bourgeois S, Farrell JJ, Kasperaviciute D, et al. (2011) HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med 364: 1134–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furukawa H, Oka S, Shimada K, Rheumatoid Arthritis-Interstitial Lung Disease Study Consortium, Tsuchiya N, et al.. (2012) HLA-A*31:01 and methotrexate-induced interstitial lung aisease in Japanese rheumatoid arthritis patients: A multi-drug hypersensitivity marker? Ann Rheum Dis in press. [DOI] [PubMed]