Abstract
The curative potential of hematopoietic stem cell transplantation (HSCT) in patients with chronic granulomatous disease (CGD) depends upon availability of a suitable donor, successful donor engraftment and maintenance of long-term donor chimerism. Twelve consecutive children (median age 59.5 months; range, 8–140) with severe CGD (serious bacterial/fungal infections pre-transplant, median 3; range, 2–9) received myeloablative HSCT using sibling bone marrow (SibBM; n=5), unrelated cord blood (UCB; n=6), and sibling cord blood (SibCB; n=1) at our center between 1997–2010. SibBM and SibCB were HLA matched at 6/6 while UCB were 5/6 (n=5) or 6/6 (n=1). Recipients of SibBM were conditioned with Busulfan and Cyclophosphamide (Bu/Cy) + ATG while 6 of 7 CB recipients received Fludarabine/Bu/Cy/ATG. Seven patients received G-CSF-mobilized granulocyte transfusions from directed donors. The first 2 UCB recipients had primary graft failure but were successfully re-transplanted with UCB. Highest acute GvHD was grade III (n=1). Extensive chronic GvHD developed in 3 patients. All patients are alive with median follow-up of 70.5 (range 12–167) months with high donor chimerism (>98%, n=10; 94%, n=1; and 92%, n=1). Myeloablative HSCT led to correction of neutrophil dysfunction, durable donor chimerism, excellent survival, good quality of life, and low incidence of GvHD regardless of graft source.
INTRODUCTION
Chronic Granulomatous Disease (CGD), an inherited disorder of phagocytic function secondary to a loss or deficiency of phagocyte membrane proteins gp91phox, p22phox p47phox or p67phox, occurs in about 1 in 200,000 individuals and often presents with recurrent life threatening bacterial and fungal infections and/or granulomatous manifestations. Additionally, many patients develop aberrant immune-mediated inflammatory responses which can result in colitis, urinary tract obstruction, chorioretinitis, gastric outlet obstruction and chronic dysphagia(1). Almost 3/4th of patients present before age five(2) despite the disease exhibiting a wide spectrum of clinical phenotypes(3, 4). The use of prophylactic and aggressive antimicrobial therapy and interferon gamma has led to significant improvements in survival and reduction in morbidity. However, patients with severe phenotypes will have major lifethreatening infections during childhood leading to significant mortality and morbidity particularly from fungal infections like Aspergillosis which may account for one-third of all deaths. Furthermore, CGD patients with severe functional phenotype (lowest oxidant production) who survive childhood face high mortality after 20 years of age, likely a result of cumulative organ injury from recurrent infection and CGD related inflammatory disease(5). Hematopoietic stem cell transplantation (HSCT) is currently the only curative therapy for patients with CGD whereby engrafted donor cells correct the neutrophil killing defect and immune deficiency by replacing abnormal NADPH oxidase producing phagocytic cells. The long-term benefit of HSCT is dependent on sustained high levels of donor chimerism and continuous production of donor derived cells for the rest of the recipient’s life. Barriers to successful outcomes of HSCT include increased resistance to engraftment, existing comorbidities related to prior infections and granulomas and increased risk of transplant related mortality. Additionally, access to transplant and the availability of a suitable donor are equally important factors. Most of the published data on HSCT for CGD pertains to the use of bone marrow grafts from HLA matched siblings(6, 7). However, matched sibling donors are only available for about 25% of patients. For the remainder, alternative graft sources must be found. A recent paper describes good outcome in 7 patients undergoing unrelated BMT following myeloablative conditioning(7). However, availability of unrelated BM donors is limited for many racial and ethnic minority patients. Unrelated umbilical cord blood donors offer the advantages of faster availability, higher probability of finding a suitable match, lower incidence of GVHD and no risk to the donor(8–10). This report underscores the success of myeloablative HSCT using cord blood and bone marrow grafts from related and unrelated donors to treat pediatric patients with severe CGD and highlights the importance of early transplantation, good infection control, and aggressive supportive care.
MATERIALS AND METHODS
Patients
Twelve consecutive pediatric patients with CGD, with histories of multiple infections and thus considered clinically severe, received HSCT following myeloablative conditioning at Duke University Medical Center between August 1997 and June 2010. All CGD patients who underwent HSCT during this period were included in the study. All patients were enrolled in a Duke University Medical Center Institutional Review Board (IRB) approved protocol or treatment plan for transplantation. Written informed consent was obtained for all patients according to the Declaration of Helsinki. Preliminary data on patients #5 and #7 were previously published(11).
Graft Sources
Sibling donors were genotypic matches at 6/6 or 10/10 HLA loci. Bone marrow was harvested from the iliac crest using standard methods on the day of transplant. Bone Marrow from ABO mismatched donors were depleted of red blood cells and/or plasma as needed before infusion to the recipient. Marrows were not manipulated in any other way. Cord blood donors were typed at low resolution for Human Leucocyte Antigen (HLA) Class I (A and B loci) and high resolution for HLA-DRB1. A minimum match of 4/6 (using low to intermediate resolution matching at HLA class I and high resolution matching at HLA Class II) was required for unrelated cord blood donor/recipient pairs. Cord blood donors were procured through the National Marrow Donor Program (NMDP) “Be the Match” registry. On the day of transplant, cord blood units were thawed and washed(12) before infusion.
Conditioning Regimens
All patients were cytoreduced using fully myeloablative regimens. As summarized in table 2, pre-transplant conditioning regimens were as follows: SibBM patients (n=5) received IV busulfan (1mg/kg/dose or 40m2/dose every 6 hours x 16 doses over four days), cyclophosphamide (50mg/kg/dose) daily for four days +/− ATG; SibCB (n=1) and five of six UCB patients received fludarabine (25mg/m2/dose, daily for 3 doses), IV busulfan (1mg/kg/dose or 40mg/m2/dose every 6 hours for four days), cyclophosphamide (50mg/kg/day for 4 days) and equine ATG (30 mg/kg/dose, daily x 3 days). Busulfan pharmacokinetics was studied after the first dose, and subsequent doses were adjusted to maintain a steady state of 600 to 900 ng/mL. Three patients underwent two transplants each. One (patient #3) had graft failure following a matched, related donor, T-cell depleted graft after reduced intensity conditioning (RIC) with fludarabine and cyclophosphamide at another institution. He received fludarabine, busulfan, cyclophosphamide and ATG for his subsequent transplant. The other two had received myeloablative conditioning and cord blood graft at our institution. Patient #5 had initially received busulfan, cyclophosphamide and ATG as preparation for his first transplant. For the second transplant, he received a single dose of total body irradiation (200 cGy), cyclophosphamide (50mg/kg/dose) for one dose, and fludarabine (40mg/m2/dose, daily for 5 days). Patient #7 had received fludarabine, busulfan, cyclophosphamide and equine ATG as conditioning for his first transplant. His second transplant conditioning regimen consisted of Campath (anti-CD52 antibody) (0.9mg/kg/day for 5 days), fludarabine (25mg/m2/dose, daily for 5 days) and cyclophosphamide (30mg/kg/dose, daily for 2 days).
Table 2.
Donor Source, HLA match, Graft-versus-Host disease (GVH) prophylaxis and Granulocyte Transfusion.
| Patient | Donor Source | HLA Match (of 6) | Total Nuc. Cell Dose (x107/kg) | GvH Prophylaxis | Granulocyte Infusions | Conditioning Regimen |
|---|---|---|---|---|---|---|
| 1 | Sibling Bone Marrow | 6 | 79.4 | Csa/Mtx | No | Busulfan/Cytoxan |
| 2 | Sibling Bone Marrow | 6 | 16.6 | Csa/Mtx | No | Busulfan/Cytoxan |
| 3a* | Sibling Bone Marrow | 6 | NA | Csa/TCD | No | Fludarabine/Cytoxan + eATG |
| 3b | Sibling Bone Marrow | 6 | 40 | Csa/Mtx | No | Fludarabine/Busulfan/Cytoxan + rATG |
| 4 | Sibling Bone Marrow | 6 | 34.8 | Csa/Mtx | No | Busulfan/Cytoxan |
| 5a* | Unrelated Cord Blood | 5 | 4.15 | Csa/Steroid | Yes | Busulfan/Cytoxan + eATG |
| 5b | Unrelated Cord Blood | 5 | 4.14 | Csa/Steroid | Yes | TBI(200cGy)/Fludarabine/Cytoxan |
| 6 | Sibling Bone Marrow | 6 | 55 | Csa/Mtx | No | Busulfan/Cytoxan + eATG |
| 7a* | Unrelated Cord Blood | 5 | 8.5 | Csa/MMF | Yes | Fludarabine/Busulfan/Cytoxan + eATG |
| 7b | Unrelated Cord Blood | 4 | 6.87 | Csa/MMF | Yes | Campath/Fludarabine/Cytoxan |
| 8 | Unrelated Cord Blood | 5 | 3.47 | Csa/MMF | Yes | Fludarabine/Busulfan/Cytoxan + eATG |
| 9 | Unrelated Cord Blood | 5 | 4.8 | Csa/MMF | Yes | Fludarabine/Busulfan/Cytoxan + eATG |
| 10 | Sibling Cord Blood | 6 | 3.2 | Csa/Steroid | Yes | Fludarabine/Busulfan/Cytoxan + eATG |
| 11 | Unrelated Cord Blood | 5 | 12.6 | Csa/MMF | Yes | Fludarabine/Busulfan/Cytoxan + eATG |
| 12 | Unrelated Cord Blood | 6 | 11.2 | Csa/MMF | Yes | Fludarabine/Busulfan/Cytoxan + eATG |
ATG: Anti-thymocyte globulin, eATG: equine ATG, rATG: rabbit ATG. Csa: Cyclosporine, Mtx: Methotrexate, MMF: Mycophenolate Mofetil, TCD: T-cell depleted
GVHD Prophylaxis
GVHD prophylaxis (Table 2) consisted of standard Cyclosporine (CSA) with methotrexate on days 1, 3, 6 and 11 for all SibBM transplants(13); cord blood transplants recipients received CSA with either 45 mg/kg/day mycophenolate (MMF) (n=5) or 1mg/kg/day of methylprednisolone (n=2). Methylprednisolone was preferred in patients who had been on prior steroids due to history of colitis or other immune or inflammatory problems. In cord blood recipients, CSA was administered for 9 months and then tapered over 2–3 months, if there was no active GVHD. In SibBM, cyclosporine was tapered after 6 months. Methylprednisolone or mycophenolate mofetil was continued for 2 to 3 months in patients without ongoing GVHD. Acute GVHD (aGVHD) and chronic GVHD (cGVHD) were scored according to standard criteria(14, 15).
Supportive Care Measures
Patients were admitted to reverse isolation rooms on a dedicated pediatric transplant unit with positive pressure ventilation and high-efficiency particulate air filtration. Standard prophylaxis against viral pathogens and Pneumocystis carinii was used. Fungal prophylaxis was administered with low-dose amphotericin-B until 1999 and with voriconazole thereafter. Empiric broad spectrum antibiotic therapy was started with the first fever. Intravenous immunoglobulin (500mg/kg per dose) was given weekly until day 100 and then ‘as needed’ to maintain IgG levels greater than 500mg/dL until discontinuation of GVHD therapy and documentation of antibody production. Veno-occlusive disease prophylaxis was continuous infusion heparin (100 units/kg/day) from day −10 to day +28. Patients received TPN, transfusions of leukocytedepleted and irradiated packed red cells and platelets as needed. Granulocyte colony stimulating factor (10 microgram/kg/day) was given intravenously daily starting on day 0 and continued until white blood cell (WBC) count of 5 × 109/L or higher was achieved.
Granulocyte Transfusions
GCSF mobilized, irradiated granulocyte transfusions were used during neutropenia to prevent serious infections in patients undergoing cord blood transplants. Granulocyte donors were either parents or other family members who underwent medical and blood product donor clearance followed by placement of central venous line (CVL). G-CSF (10mcg/kg) was given subcutaneously the night before pheresis which was performed twice weekly, 3–4 days apart. Donors were supported with oral iron, vitamin K, and calcium supplements. Each pheresis product was irradiated to prevent engraftment and subsequently divided into 3 aliquots with a maximum dose of 5×109 cells/kg of recipient body weight. Granulocytes were plasma and/or RBC depleted if the blood types of the recipient, granulocyte donor and stem cell donor were not compatible. Twice weekly pheresis allowed daily granulocyte transfusions 6 days per week. Patients received granulocyte infusions until sustained engraftment with a WBC count over 5,000 and no active infections. Engraftment was evaluated based on rising WBC counts beginning the day after the patient did not receive granulocytes.
Follow-up
Patients underwent neutrophil functional assays, blood chimerism testing, immune function studies, and other routine post-transplant multi-system evaluations at day 100, 6 months, 9 months, 12 months and then yearly post-transplant or whenever clinically indicated.
Statistics
Descriptive analyses were conducted using patient data obtained through July 2011. Patient listings are provided as are time to event analyses. The Kaplan-Meier estimator was used to describe overall survival (OS) and event-free survival (EFS)(16). OS considered a patient death as the event and censored on the last date of contact while EFS considered either graft failure or death as the event. The cumulative incidence estimator(17) was used to describe the probability of an event occurrence such as neutrophil engraftment, platelet engraftment or GVHD. Engraftment was defined as the first day of an absolute neutrophil count (ANC) of >0.5×109/L for 3 consecutive days not secondary to granulocyte infusions, and platelet engraftment as platelet counts >20×109/L or >50 × 109/L for 7 consecutive days without platelet transfusions. For patients receiving granulocytes (days 1 to 6 of the week), engraftment was determined on the basis of ANC on day after the “off” day for granulocyte transfusion. Intra and post-transplant complications, respiratory burst assays, and performance status were evaluated using descriptive measures.
RESULTS
Patient Demographics
All patients were diagnosed by respiratory burst assays. Mutation analysis data was available for 9 of 12 patients. Patient characteristics are presented in Table 1. Eight patients had the Xlinked Gp91phox mutation of the CYBB gene; the remaining four had autosomal recessive inheritance. Eleven patients were male and eight were Caucasian. Three patients were CMV seropositive pre-transplant. There were two sets of affected siblings within the cohort. The patients were transplanted at a median age of 59.5 months (range, 8–140), weighed a median of 16.9 kg (range, 9–60). Their weight for age percentile ranged from <5th to the 75th. However, 5 patients were below 5th percentile at the time of transplant. At the time of transplant, all patients had a performance status score of 80 to 100 (Lansky) with cardiac, lung and renal function in the normal range. One patient had a history of urethral polyps and 4 of 12 patients had a history of extensive colitis requiring treatment with systemic corticosteroids prior to transplantation.
Table 1.
Patient Characteristics
| Patient | Age at HCT (months) | Gender | Mutation | Inheritance | Pretransplant Infections*** |
|---|---|---|---|---|---|
| 1 | 8 | F | unknown | AR | Colitis, bronchiolitis, thrush |
| 2 | 25 | M | unknown | AR | Diffuse candida peritonitis, thrush |
| 3 | 56 | M | gp91phox | x-linked | Multiple pneumonias, apthous ulcers |
| 4 | 17 | M | unknown | AR | Burkholderia pneumonia |
| 5 | 122 | M | gp91phox | x-linked | Pneumonias, lung aspergilloma, multiple osteomyelitis, salmonella gastroenteritis |
| 6 | 109 | M | p22phox | AR | Serratia osteomyelitis, mycobacterium skin nodules, resistant lung acid fast bacilli, bladder granulomas |
| 7 | 18 | M | gp91phox | x-linked | Staphylococcal skin infection, lymphadenitis, pulmonary nodules |
| 8 | 100 | M | gp91phox | x-linked | Kidney granulomas, pneumonia, colitis |
| 9 | 140 | M | gp91phox | x-linked | Nocardia pneumonia, gut mucormycosis, sinusitis, colitis |
| 10 | 73 | M | gp91phox | x-linked | Klebsiella abscess, pulmonary nodules, multiple pneumonias, gingivitis, colitis |
| 11 | 14 | M | gp91phox | x-linked | MRSA liver abscess, Staphylococcus aureus Neck abscess, mediastinal cyst |
| 12 | 46 | M | gp91phox | x-linked | Serratia Abscess, apthous ulcers |
Patients had a median of 3 serious infections by the time they underwent stem cell transplantation.
Pre-Transplant Infections
All patients had suffered multiple episodes of serious bacterial and or fungal infections prior to transplant (Table 1). These infections included Candida peritonitis, Klebsiella abscess, hepatic abscess, gut mucormycosis, peritonitis, osteomyelitis, and multiple pneumonias. One patient presented with a large invasive lung and chest wall aspergilloma. This patient required treatment with interferon gamma, voriconazole and caspofungin and granulocyte transfusions for almost a year to successfully control his aspergilloma prior to proceeding to HSCT. One pair of siblings (patient # 8 and 9) had histories of fungal pneumonias partially treated with fluconazole, itraconazole and posaconazole at the time they presented for transplantation. Patient 10 presented with a long history of pulmonary nodules, which had been previously biopsied. At presentation to our institution he had multiple areas of scarring and ground-glass appearance prior to transplant. Infections were adequately treated prior to transplant and no patient had a clinically active infection at the time of starting cytoreduction. All patients had failed prophylaxis with antibiotics and 8 of 12 had also failed prior treatment with interferon.
Graft Characteristics
Graft sources (Table 2) were matched sibling bone marrow (SibBM) in 5, unrelated donor cord blood (UCB) in 6, and sibling cord blood (SibCB) in 1 patient. SibBM and SibCB donors were genotypic HLA matches at 6/6 or 10/10. UCB units for the first transplant matched at 5/6 (n=5) or 6/6 (n=1) loci using low-resolution typing for HLA-A and –B and high-resolution typing for HLA-DRB1. The HLA matching of UCB units used for second transplant were 4/6 (n=1) and 5/6 (n=1). The CB units contained a median pre-cryopreservation total nucleated cell dose of 4.87×107 cells/kg (range, 3.2 – 12.6×107 cells/kg) and a median CD34 dose of 2.0×105 cells/kg (range, 1.1 – 5.0 × 105 cells/kg). SibBM contained a median total nucleated cell dose of 4.03×108 cells/kg (range, 1.66 – 7.94 × 108 cells/kg) and a median CD34 dose of 6.02×106/kg (range, 3.86 – 22.71 × 106 cells/kg). ABO mismatched bone marrows underwent red cell and/or plasma depletion and were washed prior to transplantation.
Survival and quality of life
At the time of the analysis, all patients were alive and disease free with a median follow-up of 70.5 (range, 12–167) months as shown in Table 3. No new infection episodes were seen in any patient after 8 months post-transplant. All, but one patient with chronic GvHD (Lansky 60) have Lansky scores of 80–100. All school age children returned back to full time school within 18 months after transplantation.
Table 3.
Transplant Outcomes
| Patient | Neutophil Engraftment Day | Platelet Engraftment Day | Follow Up Duration (years) | aGvHD Grade | aGVHD Location | cGVHD | Transplant Complications | Donor Chimerism |
|---|---|---|---|---|---|---|---|---|
| 1 | 18 | 40 | 13.9 | 0 | None | None | 98% | |
| 2 | 20 | 59 | 13.5 | 0 | None | Coagulase negative Staphylococcal bacteremia | 98% | |
| 3 | 16 | 33 | 8 | 2 | Gut | Limited skin | None | 94%* |
| 4 | 11 | 39 | 7 | 0 | None | Streptococcus viridans bacteremia, parainfluenza 3 | 98% | |
| 5 | 32 | 74 | 6.33 | 3 | Skin | Extensive gut & skin | Autoengrafted initial transplant, urine polyoma virus, ITP | >98%* |
| 6 | 16 | 40 | 6.5 | 1 | Skin | None | Pancreatitis | >98% |
| 7 | 16 | - | 5.4 | 1 | Skin | None | Autcengrafted initial transplant | >98%* |
| 8 | 29 | 170 | 3.6 | 2 | Skin | Extensive gut & skin | Gram positive coccibacteremia, Stool C.difficile infection, bladder granuloma, pericardialeffusion | >98% |
| 9 | 43 | 36 | 3.6 | 2 | Skin | Extensive skin | CMV viremia, Urine polyoma virus, ITP | >98% |
| 10 | 29 | 72 | 2 | 1 | Skin | None | CMV viremia, Urine polyoma virus infection, Idiopathic Pneumonia Syndrome | 92% |
| 11 | 18 | 70 | 1.4 | 2 | Skin | None | ITP | >98% |
| 12 | 19 | 43 | 1 | 2 | Skin | Limited skin | Parainfluenza 3 | 98% |
Chimerism following second transplant. Patient 7 did not have platelet aplasia during his second transplant
Engraftment
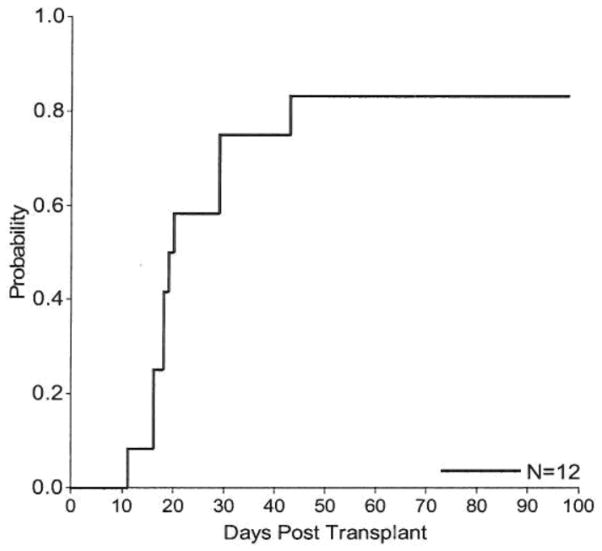
All patients engrafting after their first transplant achieved neutrophil engraftment by day 43 postfirst transplant with a cumulative incidence of 83.3% (95% CI, 55.2–100.0) (Figure 1). Two patients failed to engraft with their first transplant but engrafted after a second transplant which were performed on days 62 and 63 post first-transplant, respectively. In the patients engrafting after the first transplant, neutrophil engraftment occurred in a median of 16 days (range, 11–20) in BM and 29 days (range, 16–43) in CB recipients. Overall, the median time to neutrophil engraftment was 20 days with range 11 to 43 days. When considering graft failure as an event, the 6-month EFS probability was 83.3% (95% CI, 48.2%–95.6%). All patients engrafting after the first transplant achieved platelet engraftment >20,000×109/L (20K) and >50,000 × 109/L (50K) within 180 days. Platelet engraftment >20K occurred in a median of 34 days (range, 26–160) in the whole group; 31.5 days (range, 29–54) in BM and 42.5 days (range, 26–160) in CB recipients, respectively. Platelet engraftment >50K occurred in a median of 43 days (range, 33–170) in the whole group; 40 days (range, 33–59) in BM and 71 days (range, 36–170) in CB recipients, respectively. Two UCBT recipients had graft failure as described earlier but both engrafted successfully after second UCBT(11).
Figure 1.
Cumulative Incidence of neutrophil engraftment to 0.5×109/L after the first transplant.
Granulocyte Transfusions
Seven patients received granulocyte transfusion in the post-transplant period. The median number of transfusions per patient was 24 (range, 12–36) with a median duration of 4 (range, 2–6) weeks.
GVHD
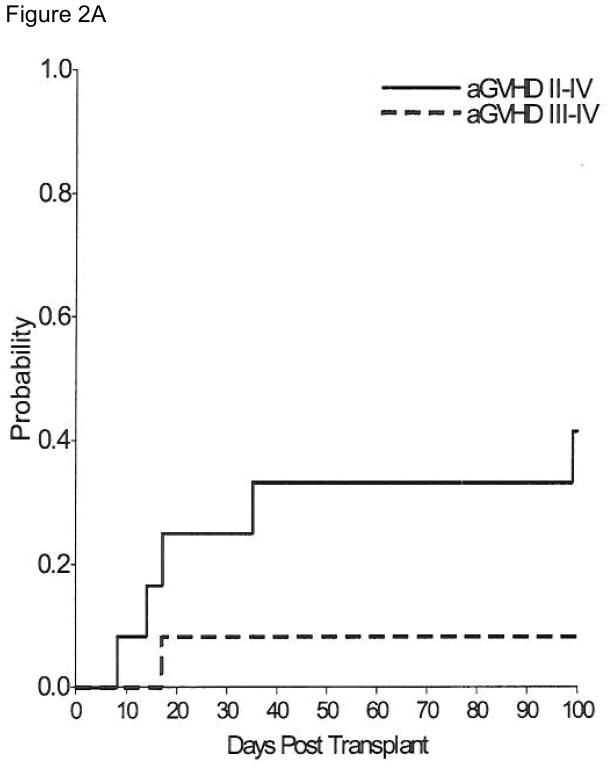
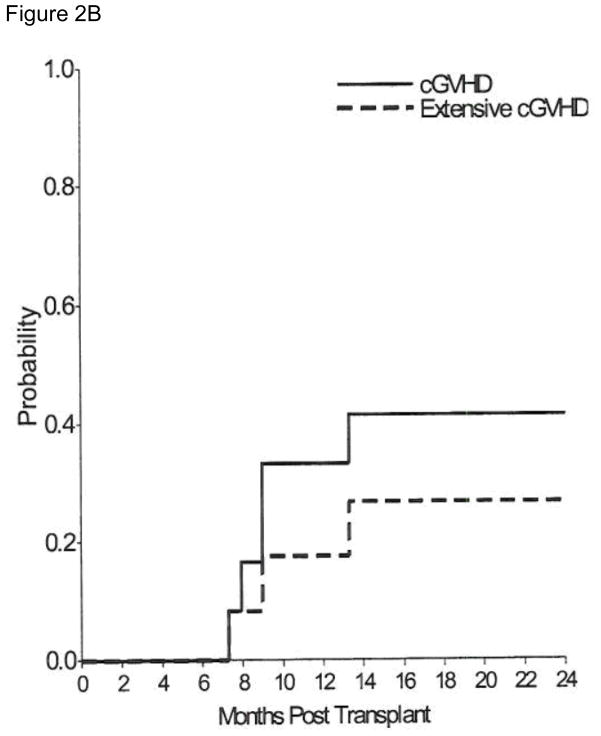
Acute GVHD (Figure 2A) developed in 9 patients and was limited to the skin in 8 (grade 1 in 3 patients and grade 2 in 5 patients). The overall cumulative incidence estimate by day 100 of grade II–IV acute GVHD was 33.3% (95% CI, 7.3–59.3). Grade III acute GvHD of the gut developed in one patient who had received a second transplant. Five patients developed chronic GvHD, 2 limited to skin and 3 with extensive disease. One of these patients initially developed extensive skin and gut chronic GVHD which progressed to a macrophage-activationlike syndrome (MAS) with multifocal arthritis, fevers and cytopenia. He responded to interleukin-1 receptor antagonist therapy. The cumulative incidence estimate of overall chronic GvHD at 2 years was 33.3% (95% CI, 7.3–59.3) and of extensive chronic GVHD was 26.7% (95% CI, 1.8–516) (Figure 2B). Three patients developed immune thrombocytopenia in the post-transplant period. They were successfully treated, two with steroids and Rituximab and one with steroids alone.
Figure 2.
Figure 2A – Cumulative Incidence of grades II–IV and III–IV acute GvHD.
Figure 2B – Cumulative Incidence of overall chronic GvHD and of extensive chronic GvHD.
Infections and Other Post Transplant Complications
Two (patients #9, 10) had CMV reactivation requiring treatment, three (patients #2, 4, 8) had uncomplicated gram positive bacteremia, three (patients #5, 9, 10) had urine polyoma virus infections, one (patient #6) had mild pancreatitis, one (patient #8) had pericardial effusion requiring pericardiocentesis, and one (patient #10) required treatment for idiopathic pneumonia syndrome (IPS). Patient #10 with IPS is currently 18 months post-transplant, physically active with no oxygen requirement and has normal lung function tests. Pericardial fluid in patient #8 was exudative in nature and negative by PCR for HHV-6, EBV, CMV, Adenovirus, Parvovirus B- 19, and AFB smear, bacterial, viral, and fungal cultures. One patient developed MAS, as described in the previous section. No patient developed any new infections after 8 months posttransplant.
Chimerism
By day 100, all patients had >90% donor chimerism and at their most recent follow up, donor chimerism in whole blood was >98% in 10 patients, 94% in one (patient #3) and 92% in another (patient #10). At the last follow-up, all patients had normal respiratory burst assays and/or dihydrorhodamine (DHR) fluorescence cytometry and were free of infection.
DISCUSSION
Results from our cohort of severe CGD patients undergoing HSCT demonstrated that myeloablative HSCT is successful and results in long term survival, low incidence of GVHD, freedom from infections, and improved quality of life if performed early in the course of the disease and with aggressive supportive care. Most of the published data describe the use of related or unrelated bone marrow in patients who were transplanted at multiple centers. Our study involves a consecutive series of patients including seven with cord blood grafts who were transplanted at a single center utilizing uniform supportive care and cytoreduction.
Soncini et al reported outcome in 20 patients transplanted between 1998 and 2007 using matched SibBM (n=9), matched SibCB (n=1), unrelated matched BM (n=6), unrelated matched peripheral blood stem cell (PBSC; n=2), mismatched PBSC (n=1) and mismatched UCB (n=1). Four patients received RIC regimen. Overall survival was 90%, and 2 patients required a subsequent bone marrow boost to maintain adequate neutrophil oxidative burst. The 2 patients that did not survive received RIC conditioning and died from disseminated fungal infections(18). Schuetz et al reported the outcomes of 12 CGD patients, 6 of which were transplanted with matched unrelated bone marrow, 3 with matched PBSC and 3 with matched SibBM, 9 received MA conditioning and 3 received RIC. Overall survival was 75%, with 67% survival in the group receiving MA conditioning. In addition 1 of the 3 patients undergoing transplant with RIC had autologous recovery(19). In a retrospective registry based study of 27 patients Seger et al reported the European experience from 1985 to 2000 from 14 centers. Twenty three had received myeloablative and 4 reduced intensity conditioning. The overall survival at a median follow-up of 24 months was 92% and 75% in the myeloablative and reduced intensity cohorts, respectively. It is crucial to note that only half of the patients receiving RIC had successfully engrafted(20). Martinez et al reported excellent outcomes of 11 children transplanted at a single center. With 7 receiving unrelated matched BM, and 4 receiving matched SibBM, all patients are alive at a median of 2.5 years and 9 of the 11 patients achieved full donor chimerism(7). Gungor et al. reported the outcomes of 11 patients receiving RIC utilizing busulfan at a total dose of 8mg/kg, Fludarabine at a total dose of 180 mg/m2, and 4 days of ATG, undergoing bone marrow (n=10) or PBSC (n=1) transplants. There was one death in that cohort(21). Outcomes of our cohort as measured by overall survival and sustained donor chimerism compare favorably with the published reports of HSCT using matched sibling, unrelated bone marrow as well as unrelated PBSC in CGD patients.
Patients with CGD are prone to graft failure after HSCT which may largely be explained by exaggerated immune responses(22). The first reported transplant for CGD developed graft rejection 2 months post-transplant(23). Prior infections and resultant organ dysfunctions may also increase transplant related complications, particularly if patients are transplanted later in the course of their disease. In recent years, there has been significant interest in RIC as an option to decrease chemotherapy related toxicity and limit transplant related mortality and morbidity. However, there has been limited success with this modality. In a study published in 2001, 10 CGD patients were treated with T-cell depleted, matched sibling peripheral blood stem cell transplants. With a median follow up of 17 months (range, 8–26 months), 1 patient had graft failure, 1 had graft rejection, and there were 3 fatalities. While, 7 of 10 patients in this cohort were alive at the time of publication, the rate of high (>90%) donor chimerism in the myeloid as well as lymphoid fractions was low(24). Various investigators have considered the use of RIC to decrease the short and long term toxicity of transplant. In the European cohort, three of 4 patients who received RIC transplants developed graft failure(20) with one patient recovering donor chimerism following donor leukocyte infusion (DLI). With the success of HSCT in CGD critically dependent on achieving high level of donor chimerism and maintaining it over the long term, limited experience from RIC-HSCT suggests that lower immunosuppression in this context is associated with higher incidence of graft failure.
All patients with severe CGD are at high-risk regimen related toxicity because of previous infections, altered and hyperactive immune system and organ dysfunctions. Despite the young age of our patient cohort, all had developed multiple serious infections prior to being referred for transplantation. These infections required hospitalizations, surgery and prolonged anti-microbial therapy. We elected to support some of these patients with supplemental transfusions of irradiated, directed donor GCSF-mobilized granulocytes to help reduce or control infections during their aplasia during the first month post-transplant. This approach may have contributed to improved outcomes, particularly in recipients of cord blood grafts.
The negative impact of pre-transplant infections on transplant outcomes was highlighted by Segar et al in a registry-based European Group for Blood and Marrow Transplantation (EBMT) retrospective study of 27 BMT performed between 1985 and 2000 at 14 centers(20). Sixteen patients were considered high-risk due to either disseminated infection or significant pulmonary restrictions. Four out the 16 received RIC with all receiving sibling bone marrow. The disease free survival in the high risk group was 69% and the overall survival 75%. No patient died in the low-risk group while in the high-risk group, 4 patients died and 3 had autologous recovery(20). It may be possible to lower regimen related toxicity by performing transplants early in the course of the disease before repeated infections and cumulative effects of an overactive immune system have taken a toll on various organ systems and pushed the patients in the ‘high-risk’ category. The outcomes could also be improved by providing excellent supportive care including optimization of pre-transplant infection control, aggressive management of intratransplant infections and the use of granulocyte transfusion during the neutropenic period for select patients. For example, patient #5, a 10 year old who presented with a visible deformity of his chest wall secondary to a large active aspergilloma was biopsied and then aggressively treated with caspofungin, voriconazole, interferon gamma, followed by granulocyte infusions during the transplant period. He was successfully transplanted and is currently over 6 years post-transplant, and disease free. Additionally, the impact of pulmonary issues on the outcomes is particularly important. Lungs are frequently diseased in CGD patients due to repeated bacterial and fungal infections, non-infectious granulomas and sometimes from the impact of surgeries.
Most of these complications increase with age and it is likely that a younger CGD patient will, in general, have healthier lungs. High rate of survival and low incidence of complications in our patients reflects transplantation at a younger age, high pre-transplant Lansky scores, near normal organ function in most patients and implementation of aggressive supportive care measures. While it is understandable that physicians and parents may be reluctant to refer children who appear well for a complex therapy like HSCT, our data suggests that early transplant when organs are still in a good functional state is optimal and can lead to excellent outcomes.
Most of the published data on HSCT in CGD involves the use of matched-sibling bone marrow (table 4) (18–20, 24–26). The probability of finding a matched sibling donor in most populations is about 25%, and even lower within siblings of patients with genetic diseases. For the remainder of patients, an alternate donor source including unrelated bone marrow, haploidentical related donor or unrelated cord blood units should be found. The reported survival and long-term donor chimerism following unrelated BMT and PBSC is relatively lower(27) and there is no published data on haploidentical transplant in CGD. Published data on the use of cord blood as a graft source in CGD is limited to a total of 7 patients in 5 case reports describing 3 unrelated and 4 related CBT(18, 28–32) (Table 5). It is certainly possible that other cases of CBT with poor outcomes have not been reported in the literature. Various cytoreductive regimens and GVHD prophylaxis were used in the published reports. In contrast, our cohort consists of six unrelated and one related CBT who were transplanted utilizing a uniform regimen and to our knowledge is the largest cohort of cord blood transplants for CGD. It is important to note that all CBT recipients are alive and well with high donor chimerism. Despite small numbers, our study highlights the potential of using this valuable graft source to achieve long term success. We believe that the use of unrelated umbilical cord blood is a feasible option for patients lacking matched sibling donors.
Table 4.
Summary of Current Series and Published Data on Bone Marrow Transplants for CGD.
| Source | Center or Group | Donor Source (# of patients) | Conditioning | Median Follow-up (months) | OS% | Engraftment % | Patients achieving high (>90%) donor chimerism |
|---|---|---|---|---|---|---|---|
| Tewari, 2012 | Duke University Medical Center | R-M-BM (5) | MAC | 97 | 100 | 100 | 100 |
| Gozdzik, 2011 | Univeristy Children’s Hospital, Cracow | R-M-BM (2), U-M-BM (3), U-MM-BM(1) | MAC (5), RIC (1) | Mean: 20 | 100 | 100 | 100 |
| Soncini, 2009 | UK Northern Supra Regional HSCT Unit | R-M-BM (9), U-M-BM (6), U-M-PBSC (2), U-MM-PBSC (1) | MAC(14), RIC(4) | 61 | 88 MAC(100), RIC (50) | MAC: 100 RIC: 75 |
NA |
| Schuetz, 2009 | Universtiy Hospital Ulm | R-M-BM (3), U-M-BM (6), U-M-PBSC(3) | MAC (9), RIC (3) | 53 | 75 MAC(57), RIC (100) | MAC: 100 RIC: 66 |
92 |
| Güngör, 2005 | University Children’s Hospital of Zurich | M-U-BM(1), M-R-BM(2) | RIC | 17 | 100 | 100 | 100 |
| Seger, 2002 | EBMT | R-M-BM(25), U-M-BM (2) | MAC(23), RIC (4) | 24 | 85 MAC(92), RIC (75) | MAC: 95 RIC: 50 |
85 |
| Horwitz, 2001 | National Institutes of Health, Bethesda | R-M-BM(10) | RIC | 17 | 70 | 80 | 60 |
R-M-BM: Related matched bone marrow, U-M-BM: Unrelated matched bone marrow; U MM-BM: Unrelated mismatched bone marrow; U-M-PBSC: Unrelated matched peripheral blood stem cells; U-MM-PBSC: Unrelated mismatched peripheral blood stem cells. MAC: Myeloablative conditioning.
Table 5.
Summary of Current Series and Published Data on Cord Blood Transplant for CGD
| Source | Center or Group | Donor Source (# of patients) | Conditioning | Median Follow-up (months) | OS (%) | Engraftment (%) | Patients achieving high (>90%) donor chimerism |
|---|---|---|---|---|---|---|---|
| Tewari, 2012 | Duke University Medical Center | U-M-CB (5), U-MM-CB (1), R-M-CB (1) | MAC | 44 | 100 | 100 | 100 |
| Jaing, 2010 | Chang Gung Children’s Hospital | U-MM-CB (1) | MAC | 8 | 100 | 100 | 100 |
| Goussetis, 2010 | Aghia Sofia Children’s Hospital | R-M-CB (2) | MAC | 155 | 100 | 100 | 100 |
| Soncini, 2009 | UK Northern Supra Regional HSCT Unit | R-M-CB (1), U-MM-CB (1) | MAC | 61 | 100 | 100 | 50 |
| Mochizuki, 2009 | Fukushima Medical University School of Medicine | U-MM-CB (1) | RIC | 36 | 100 | 100 | 100 |
| Suzuki, 2007 | Sapporo Medical University School of Medicine | U-M-CB (1) | RIC | 14 | 100 | 100 | 100 |
R-M-CB: Related matched cord blood; U-M-CB: Unrelated matched cord blood; U-MM CB: Unrelated mismatched cord blood, MAC: Myeloablative conditioning.
CGD has a wide clinical spectrum ranging from patients with trivial, infrequent infections, to those with repeated life-threatening infections early in life. This clinical variability must be taken into consideration when making therapeutic decisions regarding the use of prophylactic antibiotics, various supportive care measures, interferon, and HSCT. Despite extensive laboratory research, gene therapy is currently not available for clinical use leaving HSCT as the only curative option. Criteria for identifying appropriate transplant candidates, in particular those that are early in the disease course, are yet to be developed. Our data suggests that patients with severe phenotype CGD who develop early immunologic or inflammatory problems or multiple serious infections should be transplanted early. In this report we present evidence that HSCT utilizing a variety of donor and graft sources including unrelated cord blood units following myeloablative conditioning results in durable engraftment and excellent long term survival, regardless of graft source. Correction of aberrant phagocytic function and immune mediated inflammation, prevention of new infections, with an acceptable incidence of transplantrelated morbidity was seen. We conclude that HSCT, including those utilizing unrelated cord blood donors, should be considered early in pediatric patients with severe phenotype CGD early in the course of their disease.
Acknowledgments
PT was involved with conceptualizing the study, collection of clinical data, analysis of the data, writing of the manuscript, and all the coordination efforts; VKP was involved in conceptualizing the study, analysis of the data, collection of clinical data, editing the manuscript and direct contribution in the generation of graft data; JK was involved in conceptualizing the study, collection of clinical data, editing the manuscript and direct contribution in the generation of graft data; SHP, TAD, KP, and PLM were involved with collection of clinical data and preparation of the manuscript; HLM provided critical input in preparation and editing of the manuscript. Partial financial support was provided by the National Heart Lung and Blood Institute of the NIH. The authors thank the staff of the Pediatric Blood and Marrow Transplant Program for providing excellent care to these patients, and the patients and their families. They also thank the staff of the Stem Cell Laboratory who prepared and qualified all grafts and granulocyte transfusions.
References
- 1.Rosenzweig SD. Inflammatory manifestations in chronic granulomatous disease (CGD) J Clin Immunol. 2008;28 (Suppl 1):S67–72. doi: 10.1007/s10875-007-9160-5. [DOI] [PubMed] [Google Scholar]
- 2.Winkelstein JA, Marino MC, Johnston RB, Jr, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–169. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi S, Murayama S, Takanashi S, et al. Clinical features and prognoses of 23 patients with chronic granulomatous disease followed for 21 years by a single hospital in Japan. EurJPediatr. 2008;167:1389–1394. doi: 10.1007/s00431-008-0680-7. [DOI] [PubMed] [Google Scholar]
- 4.Liese J, Kloos S, Jendrossek V, et al. Long-term follow-up and outcome of 39 patients with chronic granulomatous disease. Journal of Pediatrics. 2000;137:687–693. doi: 10.1067/mpd.2000.109112. [DOI] [PubMed] [Google Scholar]
- 5.Kuhns DB, Alvord WG, Heller T, et al. Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med. 2010;363:2600–2610. doi: 10.1056/NEJMoa1007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Giudice I, Iori AP, Mengarelli A, et al. Allogeneic stem cell transplant from HLAidentical sibling for chronic granulomatous disease and review of the literature. Annals of Hematology. 2003;82:189–192. doi: 10.1007/s00277-002-0590-0. [DOI] [PubMed] [Google Scholar]
- 7.Martinez CA, Shah S, Shearer WT, et al. Excellent survival after sibling or unrelated donor stem cell transplantation for chronic granulomatous disease. J Allergy Clin Immunol. 2012;129:176–183. doi: 10.1016/j.jaci.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurtzberg J, Prasad VK, Carter SL, et al. Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008;112:4318–4327. doi: 10.1182/blood-2007-06-098020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasad R, Singh R, Mishra OP, Pandey M. Dapsone induced methemoglobinemia: Intermittent vs continuous intravenous methylene blue therapy. Indian J Pediatr. 2008;75:245–247. doi: 10.1007/s12098-008-0053-2. [DOI] [PubMed] [Google Scholar]
- 10.Rocha V, Wagner JE, Jr, Sobocinski KA, et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. Eurocord and International Bone Marrow Transplant Registry Working Committee on Alternative Donor and Stem Cell Sources. N Engl J Med. 2000;342:1846–1854. doi: 10.1056/NEJM200006223422501. [DOI] [PubMed] [Google Scholar]
- 11.Parikh SH, Szabolcs P, Prasad VK, et al. Correction of chronic granulomatous disease after second unrelated-donor umbilical cord blood transplantation. PediatrBlood Cancer. 2007;49:982–984. doi: 10.1002/pbc.21365. [DOI] [PubMed] [Google Scholar]
- 12.Rubinstein P, Dobrila L, Rosenfield RE, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci U SA. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96:2062–2068. [PubMed] [Google Scholar]
- 14.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 15.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EMP. Non Parametric estimation from incomplete observations. Journal of American Statistics Association. 1958;53:457–481. [Google Scholar]
- 17.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 18.Soncini E, Slatter MA, Jones LB, et al. Unrelated donor and HLA-identical sibling haematopoietic stem cell transplantation cure chronic granulomatous disease with good longterm outcome and growth. BrJHaematol. 2009;145:73–83. doi: 10.1111/j.1365-2141.2009.07614.x. [DOI] [PubMed] [Google Scholar]
- 19.Schuetz C, Hoenig M, Gatz S, et al. Hematopoietic stem cell transplantation from matched unrelated donors in chronic granulomatous disease. ImmunolRes. 2009;44:35–41. doi: 10.1007/s12026-008-8068-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seger RA, Gungor T, Belohradsky BH, et al. Treatment of chronic granulomatous disease with myeloablative conditioning and an unmodified hemopoietic allograft: a survey of the European experience, 1985–2000. Blood. 2002;100:4344–4350. doi: 10.1182/blood-2002-02-0583. [DOI] [PubMed] [Google Scholar]
- 21.Gungor T. Successful Half-Dose Busulfan/Full-Dose Fludaribine Based Reduced Intensity Conditioning In High-Risk Pediatric AND Adult Chronic Granulomatous Disease (CGD) Patients. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2010;16:S181–S182. [Google Scholar]
- 22.Yang SX, Panoskaltsis-Mortari A, Shukla M, Blazar BR, Haddad IY. Exuberant inflammation in nicotinamide adenine dinucleotide phosphate-oxidase-deficient mice after allogeneic marrow transplantation. Journal of Immunology. 2002;168:5840–5847. doi: 10.4049/jimmunol.168.11.5840. [DOI] [PubMed] [Google Scholar]
- 23.Goudemand J, Anssens R, mas-Marsalet Y, Farriaux JP, Fontaine G. Attempt to treat a case of chronic familial granulomatous disease by allogenic bone marrow transplantation. ArchFrPediatr. 1976;33:121–129. [PubMed] [Google Scholar]
- 24.Horwitz ME, Barrett AJ, Brown MR, et al. Treatment of chronic granulomatous disease with nonmyeloablative conditioning and a T-cell-depleted hematopoietic allograft. NEnglJMed. 2001;344:881–888. doi: 10.1056/NEJM200103223441203. [DOI] [PubMed] [Google Scholar]
- 25.Gozdzik J, Pituch-Noworolska A, Skoczen S, et al. Allogeneic Haematopoietic Stem Cell Transplantation as Therapy for Chronic Granulomatous Disease-Single Centre Experience. J Clin Immunol. 2011 doi: 10.1007/s10875-011-9513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gungor T, Halter J, Klink A, et al. Successful low toxicity hematopoietic stem cell transplantation for high-risk adult chronic granulomatous disease patients. Transplantation. 2005;79:1596–1606. doi: 10.1097/01.tp.0000163466.73485.5e. [DOI] [PubMed] [Google Scholar]
- 27.Peters C, Cornish JM, Parikh SH, Kurtzberg J. Stem cell source and outcome after hematopoietic stem cell transplantation (HSCT) in children and adolescents with acute leukemia. Pediatr Clin North Am. 2010;57:27–46. doi: 10.1016/j.pcl.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Jaing TH, Lee WI, Cheng PJ, Chen SH, Huang JL, Soong YK. Successful unrelated donor cord blood transplantation for chronic granulomatous disease. Int J Hematol. 2010;91:670–672. doi: 10.1007/s12185-010-0537-5. [DOI] [PubMed] [Google Scholar]
- 29.Mochizuki K, Kikuta A, Ito M, et al. Successful unrelated cord blood transplantation for chronic granulomatous disease: a case report and review of the literature. Pediatr Transplant. 2009;13:384–389. doi: 10.1111/j.1399-3046.2008.00996.x. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki N, Hatakeyama N, Yamamoto M, et al. Treatment of McLeod phenotype chronic granulomatous disease with reduced-intensity conditioning and unrelated-donor umbilical cord blood transplantation. International Journal of Hematology. 2007;85:70–72. doi: 10.1532/IJH9706129. [DOI] [PubMed] [Google Scholar]
- 31.Goussetis E, Konialis CP, Peristeri I, et al. Successful hematopoietic stem cell transplantation in 2 children with X-linked chronic granulomatous disease from their unaffected HLA-identical siblings selected using preimplantation genetic diagnosis combined with HLA typing. Biol Blood Marrow Transplant. 2010;16:344–349. doi: 10.1016/j.bbmt.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Bhattacharya A, Slatter M, Curtis A, et al. Successful umbilical cord blood stem cell transplantation for chronic granulomatous disease. Bone Marrow Transplant. 2003;31:403–405. doi: 10.1038/sj.bmt.1703863. [DOI] [PubMed] [Google Scholar]