SUMMARY
Bacterial actins, in contrast to their eukaryotic counterparts, are highly divergent proteins whose wide-ranging functions are thought to correlate with their evolutionary diversity. One clade, represented by the MamK protein of magnetotactic bacteria, is required for the subcellular organization of magnetosomes, membrane-bound organelles that aid in navigation along the earth’s magnetic field. Using a fluorescence recovery after photobleaching assay in Magnetospirillum magneticum AMB-1, we find that, like traditional actins, MamK forms dynamic filaments that require an intact NTPase motif for their turnover in vivo. We also uncover two proteins, MamJ and LimJ, which perform a redundant function to promote the dynamic behavior of MamK filaments in wildtype cells. The absence of both MamJ and LimJ leads to static filaments, a disrupted magnetosome chain, and an anomalous build-up of cytoskeletal filaments between magnetosomes. Our results suggest that MamK filaments, like eukaryotic actins, are intrinsically stable and rely on regulators for their dynamic behavior, a feature that stands in contrast to some classes of bacterial actins characterized to date.
INTRODUCTION
Despite their apparent simplicity, bacterial cells display a stunning degree of subcellular complexity with strict control over protein and DNA localization, cell shape and cell division. Homologs of eukaryotic tubulin, actin and intermediate filaments are at the heart of many of these essential processes (Cabeen and Jacobs-Wagner, 2010). The bacterial actins, in particular, are an intriguing group of proteins whose widespread diversity and varied functions are just beginning to be appreciated (Derman et al., 2009; Shaevitz and Gitai, 2010). Bacterial actins comprise a large family of proteins that share the common “actin fold,” a tertiary structure whose primary function is to bind and hydrolyze nucleotides (Bork et al., 1992; Kabsch and Holmes, 1995). Beyond this core actin fold, the overall sequence similarity of actins is highly conserved in eukaryotes yet extremely divergent in bacteria (Fig. 1A) (Derman et al., 2009). This diversity at the primary sequence level is thought to translate into distinct characteristics for each group of bacterial actins. In fact, research thus far has suggested that features such as polymer morphology, filament dynamics and cellular function may be specific to each of the phylogenetically distinct groups of bacterial actins (Rivera et al., 2011). The bulk of the detailed molecular studies to date have focused on a few select groups of bacterial actins while the function and cell biology of the vast majority of these proteins remain unknown. In this study, we have undertaken a focused examination of one group of bacterial actins defined by MamK, a protein responsible for organization of subcellular organelles in magnetotactic bacteria.
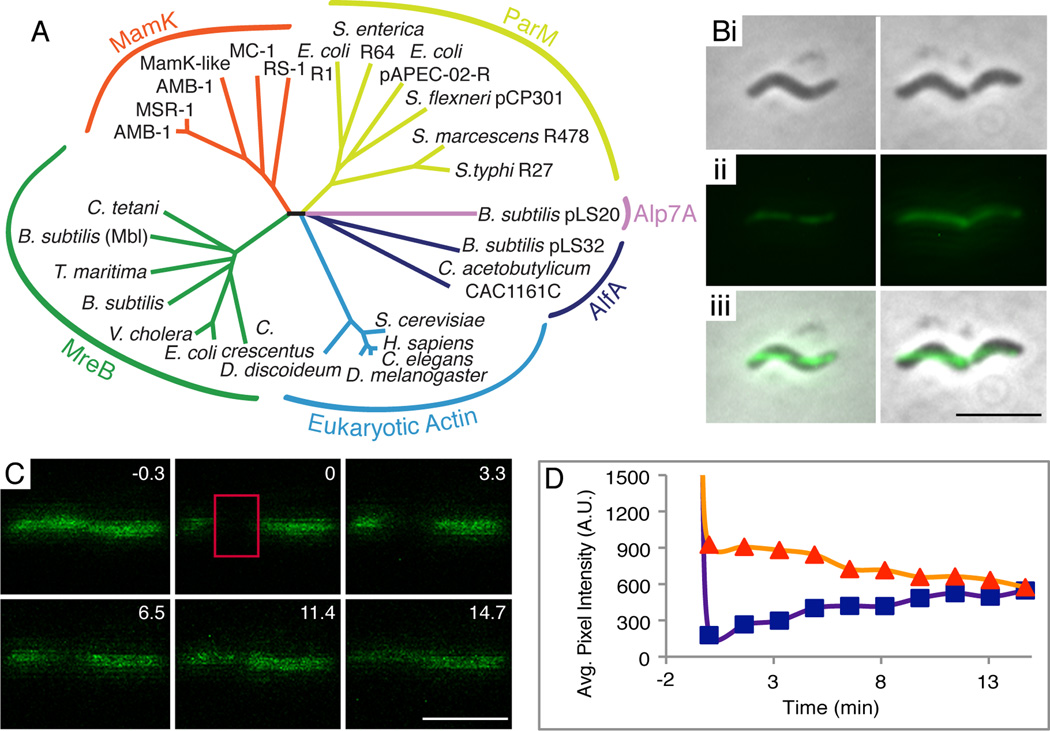
Figure 1.
MamK-GFP forms dynamic filaments in wildtype AMB-1. (A) Phylogenetic tree of representative actin-like proteins. (B) Pole-to-pole linear localization of MamK-GFP: (i) phase contrast images, (ii) fluorescence images, (iii) merge of i and ii (scalebar = 4 µm). (C) FRAP of MamK-GFP filaments in one representative cell. Numbers indicate minutes pre or post photobleaching. Red box demarcates photobleached region (scalebar = 2 µm). (D) FRAP analysis reveals full recovery of filament fluorescence where the orange and purple lines intersect. Orange triangles indicate average pixel intensity of the whole filament and purple squares indicate the average pixel intensity of the bleached region. Average pixel intensity was measured in arbitrary units.
Magnetotactic bacteria form magnetosomes, organelles composed of inner-membrane invaginations that allow for the biomineralization of crystalline magnetic minerals within the compartments. Magnetosomes are organized into chains that function as compass needles, leading to the passive alignment of the aquatic bacteria with the magnetic field of the earth and facilitating their search for low oxygen environments (Frankel and Bazylinski, 2009). Many of the molecular determinants of magnetosomes have been uncovered in recent years, including a large genomic region termed the magnetosome island (MAI), that encodes many of the factors required for formation of magnetosome membranes, biomineralization of magnetic minerals, and magnetosome chain assembly (Jogler and Schüler, 2009; Murat et al., 2010). One of these factors is MamK, an actin homolog that typifies a novel branch of the bacterial actin tree (Komeili et al., 2006). High resolution imaging of cells of Magnetospirillum magneticum AMB-1 (AMB-1) and Magnetospirillum gryphiswaldense MSR-1 (MSR-1) by electron cryotomography (ECT), revealed that magnetosomes are flanked by a series of cytoskeletal filaments (Katzmann et al., 2010; Komeili et al., 2006). In the absence of MamK, functional magnetosomes form but fail to assemble into well-organized chains. Additionally, the cytoskeletal filaments imaged by ECT are no longer visible near magnetosomes. Subsequent work has shown that MamK is associated with magnetosomes and that it is capable of forming filaments in vitro (Pradel et al., 2006; Rioux et al., 2010; Taoka et al., 2007). These observations suggest that MamK is a necessary component of a magnetosome cytoskeleton, with a crucial function in either the establishment or maintenance of the magnetosome chain. Despite these insights into the general function and in vitro assembly properties of MamK, the in vivo dynamic properties of its filaments remain unknown.
In eukaryotes, actin-binding proteins are the predominant determinants of filament dynamics. These proteins nucleate, cap, crosslink, or bundle filaments; they also lead to filament branching, elongation, stabilization, or depolymerization (dos Remedios et al., 2003). To date, the only known bacterial actin regulator is parR-ParC, the DNA-protein complex that interacts with the plasmid segregating ParM protein (Garner et al., 2004). Free ParM filaments undergo catastrophic depolymerization in a manner similar to the dynamic instability of eukaryotic microtubules. Once ParM binds to the parR-ParC complex on duplicated plasmids, its filaments are stabilized resulting in separation of the plasmids to either end of the cell (Garner et al., 2004). A similar pattern of behavior has been noticed for the Alp7 family of bacterial actins where a presumed cognate DNA-protein receptor stabilizes filaments in order to allow for segregation of plasmids (Derman et al., 2009). In contrast, all other bacterial actin-binding proteins identified thus far participate in the downstream effects of bacterial actins or potentially in their localization within the cell. For example, members of the MreB bacterial actin family, which are involved in various functions including cell shape determination, bind proteins that directly or indirectly influence cell shape through remodeling of the peptidoglycan layer (Alyahya et al., 2009; Bendezú et al., 2009; Carballido-López et al., 2006; Defeu Soufo and Graumann, 2006; Divakaruni et al., 2007; Kruse et al., 2005; van den Ent et al., 2010; White et al., 2010). Yet, to date, no regulators of filament dynamics have been identified for this family of bacterial actins.
In this study our goals were to determine if MamK filaments are dynamic in vivo, to ascertain the impact of filament dynamics on magnetosome organization, and to search for proteins that could regulate its behavior. Using fluorescence recovery after photobleaching (FRAP) we show that in AMB-1, GFP-tagged MamK forms dynamic filaments when its nucleotide-binding pocket is intact. We further show that filament turnover depends on redundant functions performed by MamJ, a protein previously known to interact with MamK, and its newly described paralog, LimJ. Finally, we show that the action of MamJ and LimJ is required for the proper assembly of the magnetosome chain. In a broader context, these results argue that MamK filaments are stable in their default state and require the specific action of regulators to become dynamic, a behavior that is similar to that proposed for the AlfA group of bacterial actins and in stark contrast to the inherently unstable ParM and Alp7 protein families (Derman et al., 2009; Garner et al., 2004; Polka et al., 2009).
RESULTS
MamK-GFP forms dynamic filaments
Actin filaments are generally dynamic, meaning that monomers within the filament are replaced during the lifespan of the filament. We first wanted to establish if MamK filaments are static, dynamic (like most actins), or if they display the microtubule-like dynamic instability of ParM (Garner et al., 2004). FRAP was used to determine whether MamK-GFP monomers are exchanged within filaments in AMB-1. In a FRAP experiment, fluorescently tagged proteins are irreversibly photobleached, and fluorescence is regained only when those monomers are replaced. Fluorescent protein movement from the cytoplasmic pool or from the unbleached regions of the filament to the bleached region leads to recovery of fluorescence over time. MamK-GFP expressed at levels similar to endogenous MamK levels (Fig. S1) was used because it restores both magnetosome alignment and filament formation in a mamK deletion strain (Komeili et al., 2006). Cells grown to exponential phase in 10 % oxygen were immobilized on agarose-coated slides in a microaerobic chamber. The agarose pads were sealed to prolong the low oxygen environment preferred by AMB-1 and to reduce evaporation during the course of the experiment.
Wildtype MamK-GFP filaments in AMB-1 form a pole-to-pole line of even fluorescence along the inner curvature of the cell (Fig. 1B). At time zero (ti), a fragment of the MamK-GFP filament and some cytoplasm was exposed to a high-intensity laser (488 nm) until the region was photobleached (red rectangle, Fig. 1C). Dynamic behavior of MamK-GFP filaments is characterized by a steady and time-dependent increase in fluorescence throughout the bleached region (Fig. 1C and 1D, purple line). In contrast, static filament behavior shows no increase of fluorescence. Regardless of recovery, most cells exhibit an overall decrease of fluorescence of the whole filament, likely due to generalized photobleaching upon exposure to the laser during imaging, filament drift out of the plane of focus, or incorporation of bleached MamK-GFP monomers (Fig. 1D; orange line). The time elapsed between ti and the moment the fluorescence intensity of the bleached section reaches 50 % of the whole filament’s fluorescent intensity is defined as the “half-time of recovery” (t1/2).
MamK-GFP filaments in wildtype AMB-1 cells were dynamic, with a fluorescence recovery t1/2 of 11 ± 6 minutes (Table 1). The rate of recovery is much slower than the 10 to 45 second t1/2 of plasmid-segregating bacterial actins (Becker et al., 2006; Derman et al., 2009), but quite similar to the 8.5 ± 1.5 minute t1/2 of Mbl, an MreB family member (Carballido-López and Errington, 2003). Intriguingly, in half of the cells, the bleached region did not show fluorescence recovery (Table 1). This is not due to technical limitations, for certain mutants of MamK fused to GFP show recovery in 100% of imaged cells (data not shown). The mechanism for the lack of recovery in some cells is currently unclear; however, this population may reflect an unknown active block to MamK filament turnover (see Discussion).
Table 1.
Fluorescence recovery after photobleaching
| Construct Cells3 |
Strain | t1/2 1 | % Rec.2 | Tot. # |
|---|---|---|---|---|
| mamK-gfp | wildtype AMB-1 | 11 ± 6 | 47 | 34 |
| mamKE143A-gfp | wildtype AMB-1 | N/A | 0 | 7 |
| mamKD161A-gfp | wildtype AMB-1 | N/A | 0 | 9 |
| mamK-gfp | ΔMAI | N/A | 0 | 9 |
| mamK-gfp | ΔmamJ | 13 ± 9 | 20 | 25 |
| mamK-gfp | ΔlimJ | 12 ± 8 | 35 | 23 |
| mamK-gfp | ΔmamJ ΔlimJ | N/A | 0 | 25 |
| mamK-gfp-rbs-mamJ | ΔmamJ ΔlimJ | 8 ± 4 | 13 | 32 |
| mamK-gfp-rbs-limJ | ΔmamJ ΔlimJ | 6 ± 4 | 47 | 19 |
| mamK-gfp-rbs-limJ | ΔMAI | N/A | 0 | 36 |
| mamK-gfp-rbs-mamJ | ΔMAI | N/A | 0 | 21 |
| mamK-gfp-rbs-limJ | ΔMAI | N/A | 0 | 36 |
Half-time of recovery was measured in minutes. Shown are the average ± one standard deviation.
Percent of cells whose bleached regions regained at least half of the overall filament fluorescence.
Total number of cells observed, composed of recovering and non-recovering cells.
N/A = not applicable because cells do not recover.
Another feature of MamK-GFP filament dynamics was the lack of a clear pattern or directionality to the recovery of fluorescence. Instead, the bulk of the fluorescence recovery is distributed across the bleached region (Fig. 1C). This can be explained by a model where multiple small filaments in the bleached region add new fluorescent monomers to their growing ends until all bleached monomers are replaced. This explanation is consistent with ECT images that show many, short, overlapping filaments flanking magnetosomes when MamK is expressed in AMB-1 (Komeili et al., 2006). Additionally, when expressed in Escherichia coli, MamK appears to form multiple short filaments that can overlap with one another (Pradel et al., 2006). This absence of a discernable pattern to fluorescence recovery was also noted for the MreB-like protein, Mbl, and the plasmid segregation protein AlfA (Becker et al., 2006; Carballido-López and Errington, 2003). In both cases, their behavior was hypothesized to be due to multiple filaments of mixed polarity coming together to form the fluorescent actin “cable” (Becker et al., 2006; Carballido-López and Errington, 2003). This initial characterization of MamK filament dynamics indicates that its properties more closely resemble those of the MreB family of proteins and are distinct from dynamic instability typified by the plasmid segregating ParM family.
MamK-GFP filament dynamics depend on an intact NTP binding site
Because ATP binding and hydrolysis are critical for actin filament dynamics, we wondered if MamK-GFP filament turnover required an intact NTP binding site. Conserved motifs found in the actin fold are involved in binding the NTP and the metal ion necessary for NTP hydrolysis (Bork et al., 1992; Kabsch et al., 1990). The aspartate residue in one of these motifs, the “Phosphate 2” loop, is invariant across domains of life (Fig. 2A) and helps coordinate the Mg2+ required for ATP hydrolysis (Becker et al., 2006; Bork et al., 1992; Kabsch et al., 1990; Vorobiev et al., 2003). Alanine substitutions of the conserved aspartate lead to a loss of ATPase activity (Jensen and Gerdes, 1997), stationary filaments (Becker et al., 2006; Defeu Soufo and Graumann, 2006), and a loss of function of the various bacterial actin mutants, which, when in the presence of wildtype versions, exhibit trans-dominance (Becker et al., 2006; Defeu Soufo and Graumann, 2006; Derman et al., 2009; Jensen and Gerdes, 1997).
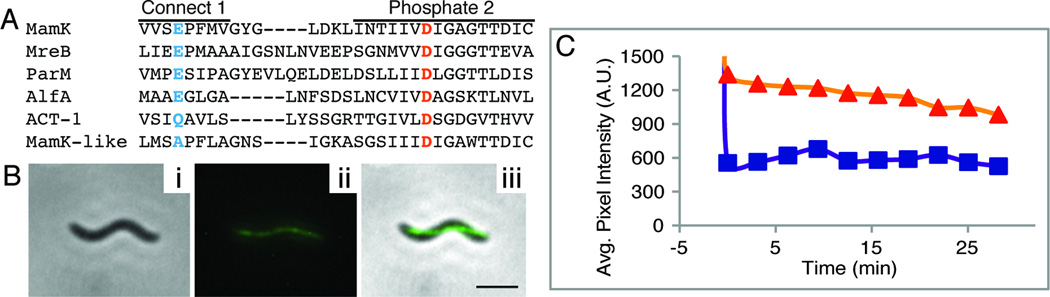
Figure 2.
Dynamic MamK-GFP filaments depend on intact NTP binding motifs. (A) Alignment of the Connect 1 and Phosphate 2 loop motifs in selected bacterial actins. In blue is a conserved glutamate (E143 in AMB-1) and in orange a strongly conserved aspartate (D161 in AMB-1). (B) Pole-to-pole linear localization of MamKD161A-GFP in a wildtype AMB-1 background: (i) phase contrast image, (ii) fluorescence image, (iii) merge of i and ii (scalebar = 2 µm). (C) FRAP analysis reveals a lack of fluorescence recovery. Details as in Fig. 1D. Similar results were obtained with MamKE143A-GFP in wildtype AMB-1.
We made the equivalent mutation, creating MamKD161A-GFP, and assayed for fluorescence recovery of bleached filaments in a wildtype AMB-1 background. The MamKD161A-GFP filament exhibited wildtype, pole-to-pole localization (Fig. 2B), indicating that the likely loss of ATP hydrolysis is not necessary for filament polymerization. Formation of the MamKD161A-GFP filament did not require wildtype MamK, since MamKD161A-GFP filaments were similar to those of MamK-GFP in a mamK deletion strain (Fig. S2A and B). All MamKD161A-GFP filaments in the wildtype AMB-1 background were static as evidenced by the lack of recovery of the bleached region even after 12 to 31 minutes of observation (Table 1, Fig. 2C). The loss of filament turnover in the presence of unlabeled wildtype MamK indicates that the MamKD161A-GFP mutant may exert a dominant negative effect on wildtype filament turnover, like that observed in other bacterial actin aspartate mutants (Becker et al., 2006; Defeu Soufo and Graumann, 2006; Jensen and Gerdes, 1997). Alternatively, it is possible that two kinds of filaments are present; one composed solely of endogenous MamK and the other of MamKD161A-GFP monomers.
To confirm our results, we mutated another conserved residue in the predicted active site of MamK (Fig. 2A) and found that none of the cells with MamKE143A-GFP in a wildtype background showed filament recovery (Table 1). The equivalent ParM and Alp7A point mutants lead to stable, static filaments further supporting the hypothesis that MamK filament turnover is likely an active process (Derman et al., 2009; Garner et al., 2004).
MamK-GFP filament turnover requires element(s) from the magnetosome island
Having established that MamK-GFP forms dynamic filaments, we next asked whether this behavior is an intrinsic property of MamK or if it is stimulated by other factors. Thus, we examined MamK-GFP filament turnover in a mutant strain of AMB-1 lacking the entire magnetosome island (ΔMAI) (Komeili et al., 2006). The MAI-encoded proteins are likely candidates because most genes known to be involved in magnetosome formation and alignment, including mamK, are found within this region. Although MamK-GFP formed filaments in the ΔMAI mutant, their overall morphology was shorter and wider than filaments in a wildtype background (Fig. S2C). Also, in contrast to wildtype cells, none of the filaments in the ΔMAI mutant showed fluorescence recovery after photobleaching (Table 1), which suggests that dynamic turnover is not an intrinsic property of MamK-GFP and requires the action of additional proteins. Furthermore, the abnormal morphology of the filaments in the ΔMAI strain may indicate that other factors are needed for the proper assembly and localization of MamK-GFP filaments.
MamJ, LimJ, and other MAI-encoded proteins promote MamK filament dynamics
We used a candidate approach to look for proteins encoded by the MAI that may be involved in the regulation of MamK filament dynamics. MamJ, an acidic protein unique to magnetotactic bacteria, plays a role in magnetosome chain organization in the closely related species MSR-1 (Scheffel et al., 2006). MamJMSR-1 was also shown to interact with MamKMSR-1 in a bacterial two-hybrid assay, and AMB-1 MamJ and MamK are able to transcomplement the MSR-1 mamJ and mamK deletion strains, respectively (Katzmann et al., 2010; Scheffel and Schüler, 2007). Furthermore, mamJ and mamK are cotranscribed (Scheffel et al., 2006), indicating a potential functional interaction (reviewed in (Osterman and Overbeek, 2003)). For these reasons, we hypothesized that MamJ may be a regulator of MamK polymerization dynamics.
We created an unmarked non-polar mamJ deletion in AMB-1 and found that, in contrast to the clumped magnetosomes observed in the MSR-1 ΔmamJ strain, the magnetosomes of the AMB-1 mamJ deletion strain form a chain that, by whole cell transmission electron microscopy (TEM), is indistinguishable from the chain of the wildtype AMB-1 strain (Fig. S4A). The coefficient of magnetism (Cmag), a measure based on the differential light scattering of cells as they align in a changing magnetic field (Komeili et al., 2004), also shows that the ΔmamJ strain behaves like the wildtype strain (Table S1). Furthermore, MamK-GFP in a mamJ deletion background forms wildtype filaments along the length of the cell (Fig. S2D). Surprisingly, MamK-GFP filaments exhibited similar dynamics in wildtype AMB-1 and the ΔmamJ strain with a t1/2 of 13 ± 9 minutes (Table 1). These results suggest that MamJ cannot be the sole regulator of MamK filament dynamics. Strikingly, a partial and imperfect duplication of the region encompassing mamJ exists about 37 kb downstream of mamJ (Murat et al., 2010). This MAI region contains amb1003, a gene encoding for a moderately conserved MamJ paralog here renamed LimJ (like MamJ) (Fig. S3).
Similar to MamJ, LimJ can be divided into three domains: an N-terminal region that has 38 % identity to MamJ (64 % similarity), a C-terminal region with 26 % identity to MamJ (70 % similarity), and a central region with 40 % identity (64 % similarity) to the CAR (central acidic repetitive) domain of MamJ proteins (Fig. S3) (Scheffel and Schüler, 2007). Although the LimJ central region does not have the repetitive character that MamJ proteins generally have, it is enriched for acidic residues. To address the potential involvement of LimJ in MamK filament dynamics, an unmarked non-polar limJ deletion was created. When imaged by TEM, the ΔlimJ strain, like the ΔmamJ mutant, shows wildtype chain organization (Fig. S4A) and a wildtype response to changing magnetic fields (Table S1). The morphology of MamK-GFP filaments was also unchanged in ΔlimJ cells (Fig. S2E). Moreover, we found that 35 % of these mutant cells displayed MamK-GFP filament recovery, with a t1/2 of 12 ± 8 minutes (Table 1). Because the rate of recovery in the mutant is very similar to that of wildtype cells, we conclude that the loss of LimJ by itself does not alter MamK-GFP filament dynamics.
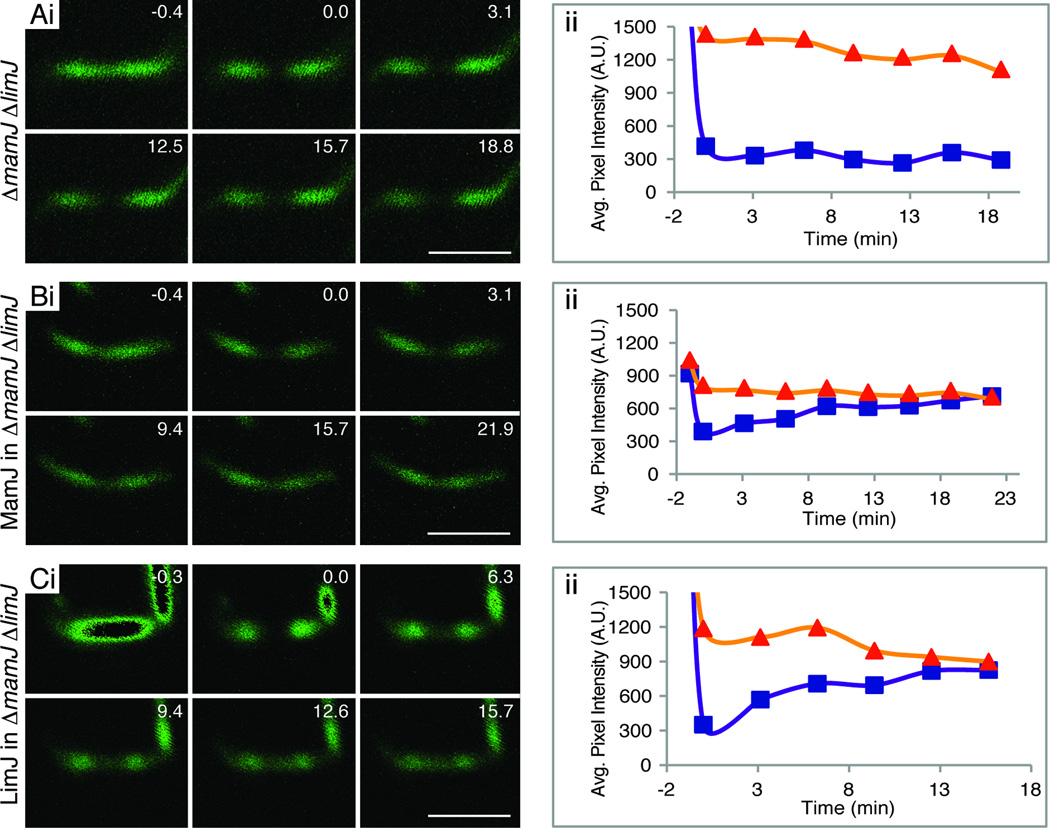
Although the absence of either MamJ or LimJ had no effect on MamK-GFP filament turnover, we addressed the possibility that the two proteins may play redundant roles in this process by constructing a mamJ limJ double deletion strain. Initial phenotypes of this mutant were similar to the wildtype AMB-1 strain: the cells had linear, pole-to-pole MamK-GFP filaments, the magnetosomes chains were similar to the wildtype strain when imaged by TEM, and both cultures turned similarly in a changing magnetic field (Table S1). Although ΔmamJ ΔlimJ cells are similar to wildtype in many aspects, MamK-GFP filaments in this strain were static when assayed by FRAP (Table 1, Fig. 3A). Of 25 bleached filaments, none had any degree of recovery 20 to 40 minutes post bleaching, strongly indicating that MamJ and LimJ have a redundant function that is necessary for MamK-GFP filament dynamics (Table 1).
Figure 3.
MamJ and LimJ share a necessary, redundant function in promoting MamK-GFP filament dynamics. (A) MamK-GFP FRAP assay in one representative ΔmamJ ΔlimJ cell shows a lack of recovery in this strain. (B) MamK-GFP + MamJ FRAP in one representative ΔmamJ ΔlimJ cell. (C) MamK-GFP + LimJ FRAP in one representative ΔmamJ ΔlimJ cell. Fluorescence recovery is seen in (B) and (C) indicating that either MamJ or LimJ can support the dynamic behavior of MamK-GFP filaments. Dark center regions of the first panel represent saturated pixels. Details of (i) as in Fig. 1C; details of (ii) as in Fig. 1D.
To confirm that the loss of MamK-GFP filament turnover was specific to the absence of both proteins, we complemented the ΔmamJ ΔlimJ strain with a plasmid expressing either MamJ or LimJ. In each case, a bicistronic transcript was made such that mamK-GFP is followed by the limJ ribosome-binding site and then by either limJ or mamJ. Cells containing the complementation plasmids had wildtype filament and cellular morphology, but more importantly, either construct was sufficient to restore MamK-GFP filament dynamics in the ΔmamJ ΔlimJ strain (Fig. 3B and C). MamJ expression in the double deletion strain led to 13 % of the filaments recovering with an 8 ± 4 minute t1/2, while LimJ expression in the same background led to 47 % of the filaments recovering with a 6 ± 4 minute t1/2 (Table 1). The lower number of cells that recovered when complemented with the mamJ construct may be due to a difference in MamJ expression between the wildtype and the transcomplemented strain.
MamJMSR-1 had been reported to interact with MamKMSR-1 in a bacterial two-hybrid assay (Scheffel and Schüler, 2007). However, using this and other assays we could not detect any significant interactions between MamK and MamJ, MamK and LimJ, or LimJ and MamJ (Supporting information, Tables S4A–D). Regardless, these results show that MamJ and LimJ independently contribute a redundant function necessary for MamK-GFP filament turnover. Whether this regulatory function is due to either protein directly altering MamK-GFP polymerization dynamics or to an indirect effect is unknown.
If no other magnetosome island proteins mediate MamK filament turnover, and none plays a required accessory role, MamJ or LimJ should be sufficient to promote MamK filament dynamics in a ΔMAI strain. However, when constructs expressing either MamK-GFP and MamJ or MamK-GFP and LimJ were placed in ΔMAI cells, MamK-GFP filaments were still static (Table 1). This implies that MamJ or LimJ require the presence of at least one other MAI protein to allow for the turnover of MamK-GFP filaments.
MamJ and LimJ play a role in magnetosome chain organization
As stated above, TEM images do not reveal significant defects in the organization of the magnetosome chain in the ΔmamJ ΔlimJ strain (Fig. 4A). Like in wildtype, a chain of magnetite crystals separated by several gaps can be seen in the cells. However, whole cell TEM does not allow for the visualization of empty magnetosome compartments. To determine if the gaps in the chain reflect the presence of empty magnetosome compartments, as seen in wildtype AMB-1, or if they hint at a defect in chain organization such as that found in the ΔmamK strain, we used two complementary approaches to detect empty magnetosome compartments in AMB-1.
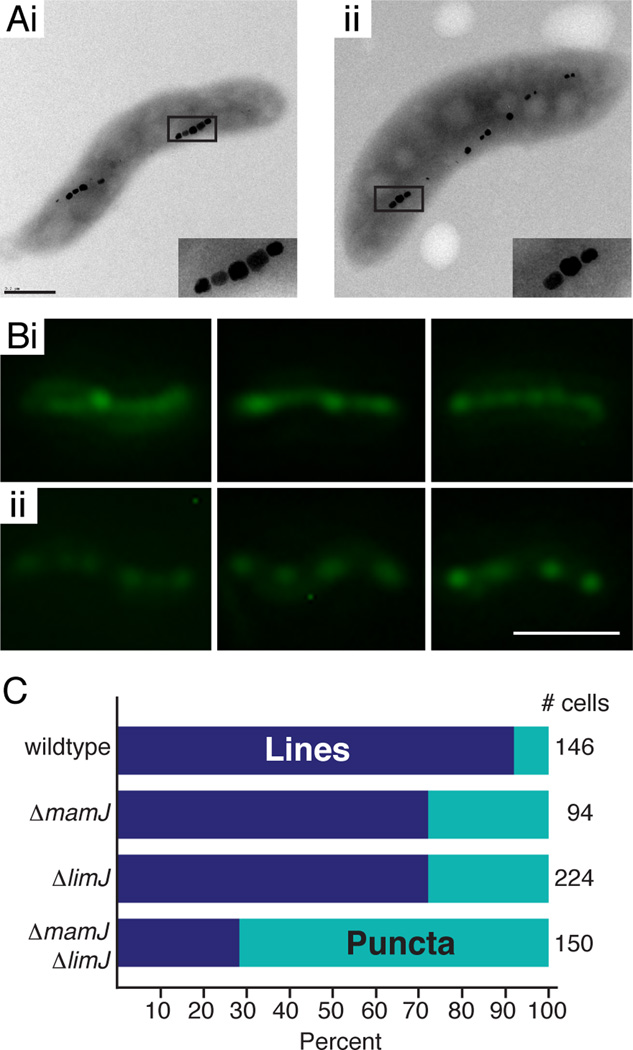
Figure 4.
Characterization of the ΔmamJ ΔlimJ strain by TEM and GFP-MamI localization reveals a potential defect in magnetosome chain organization. (A) A representative wildtype AMB-1 cell (i) or ΔmamJ ΔlimJ cell (ii) as visualized by TEM (scalebar = 400 nm). Insets are a 2.5-fold magnification of the images. (B) Fluorescence microscopy of GFP-MamI in (i) wildtype AMB-1 cells -- the first two panels have lines with puncta; the last panel has puncta predominating -- and (ii) ΔmamJ ΔlimJ cells with puncta (scalebar = 2 µm). (C) Percent of cells with lines (with or without puncta) in dark blue and percent of cells with puncta (sans lines) in teal. Total number of cells tallied in column at right.
Initially, we used a GFP fusion to MamI, a protein implicated in early stages of magnetosome membrane biogenesis and shown to be a promising marker for in vivo visualization of magnetosome localization (Murat et al., 2010; Quinlan et al., 2011). In wildtype AMB-1, GFP-MamI is localized as a coherent line in approximately 92 % of the cells imaged (Fig. 4B and 4C). In contrast, when expressed in the ΔmamJ ΔlimJ mutant strain GFP-MamI localizes as a continuous line in only 28 % of the cells, while in over 70 % of the cells, it appears as a series of dots roughly organized into a line (Fig. 4B and 4C). Furthermore, in either the single ΔmamJ or ΔlimJ strain, the number of cells displaying continuous GFP-MamI localization is slightly decreased (70 % as compared to 92 % in wildtype) (Fig. S4B and C and Fig. 4C).
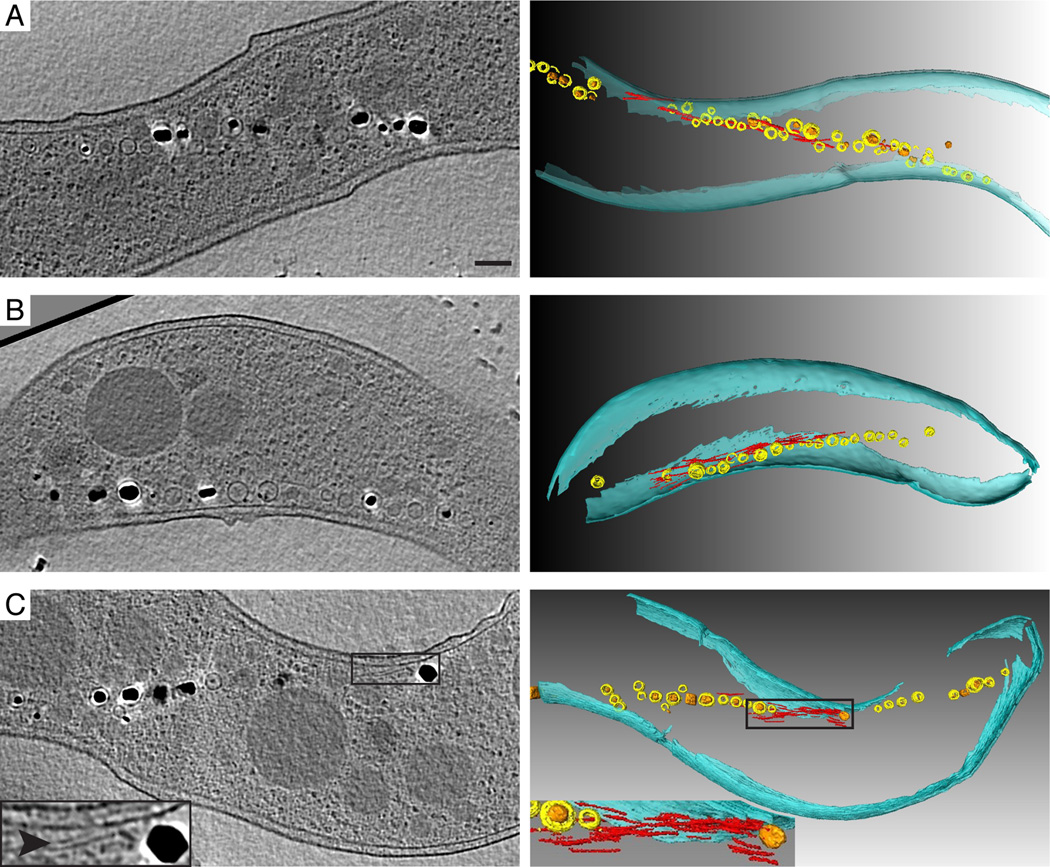
These results suggest that global changes in the organization of the magnetosome chain may occur when MamJ and LimJ are absent. To obtain a closer view of the architecture of the magnetosome chain in these strains, we turned to electron cryotomography (ECT), a high resolution TEM technique capable of generating near-native three-dimensional views of the imaged specimen. ECT confirmed the defects in chain organization of the mutant strains suggested by the GFP-MamI localization study. In some of the 60 imaged ΔmamJ cells, small gaps lacking magnetosome membranes could be seen in the chain, while no significant defects in the localization or distribution of the magnetosome-associated filaments were seen (Fig. 5A). In contrast, none of the 19 ΔlimJ cells had noticeable chain organization defects when compared to the wildtype strain (Fig. 5B). However, major changes in the organization of magnetosomes and the distribution of magnetosome-associated filaments were observed in the ΔmamJ ΔlimJ strain. In each of the 17 cells imaged, the magnetosome chain was interrupted by one or more 100–250 nm gaps devoid of magnetosomes (Fig. 5C). In general, this mutant phenotype bears a strong resemblance to the ΔmamK mutant, where numerous gaps appear in the magnetosome chain (Komeili et al., 2006). Strikingly, the distribution of magnetosome-associated filaments was drastically altered in the majority of the ΔmamJ ΔlimJ cells imaged. In general, few filaments were seen flanking the magnetosome chain, and, in 13 of 17 cells, filaments were found in concentrated bundles within the gaps of the chain, a phenotype not observed in either single deletion strain (Fig. 5A and 5B). In addition to the defects in chain formation, ECT imaging revealed that magnetosome membrane size was more variable in all three mutants than in the wildtype. Furthermore, smaller invaginations appeared throughout the chain, in contrast to the localization of small magnetosomes near the ends of the chain in wildtype cells. This implies that MamJ and LimJ may affect magnetosome invagination size or the sites of new magnetosome synthesis.
Figure 5.
Characterization of ΔmamJ, ΔlimJ, and ΔmamJ ΔlimJ cells by ECT reveals defects in chain organization and placement of magnetosome-associated filaments. ECT of (A) a ΔmamJ cell, (B) a ΔlimJ cell, and (C) a ΔmamJ ΔlimJ cell.Reconstructed slices (left) and segmentation analyses (right) of the same cells are shown. Magnetosome membranes are colored yellow and magnetite crystals are orange. Filaments likely to be MamK are colored red and the cell envelope is in teal. Arrowhead points to filaments highlighted in red in the segmentation panel. Insets are magnifications of the filaments in the gap between magnetosomes. Scalebar = 200 nm for all reconstructed slices.
Taken together, these results show that, similar to MamJMSR-1 in MSR-1, MamJ and its paralog LimJ play an important role in the organization of magnetosomes in AMB-1. In combination with the FRAP data, the unique phenotypes of the ΔmamJ ΔlimJ strain, namely the concentration of bundles of filaments within the gaps of the chain, also suggest that MamK filament dynamics are required for the assembly or maintenance of the magnetosome chain.
DISCUSSION
The use of cytoskeletal filaments for positioning and partitioning of subcellular organelles is emerging as a common theme in bacteria (Savage et al., 2010). MamK from AMB-1, is a member of a phylogenetically distinct branch of bacterial actins with a specific function in the organization of magnetosome organelles into chains (Komeili et al., 2006). Here we show that MamK filaments are dynamic in vivo and that, similar to other actin-like proteins, this behavior depends on an intact ATPase active site. The rate and pattern of recovery of MamK filaments is similar to those seen for the MreB family of bacterial actins and distinct from the ParM, AlfA, and Alp7 families, which function in plasmid segregation (Becker et al., 2006; Carballido-López and Errington, 2003; Derman et al., 2009; Garner et al., 2004). The relatively random patterns of recovery observed for MamK, the pattern of MamK polymerization in E. coli and the arrangement of magnetosome-associated filaments seen with ECT favor a model similar to that proposed for the MreB-like protein, Mbl, and the plasmid segregation protein AlfA where multiple overlapping filaments are undergoing dynamic turnover simultaneously (Becker et al., 2006; Carballido-López and Errington, 2003). For MreB, movement of individual monomers in vivo has been observed and it is possible that a similar mechanism is in place for MamK filaments (Kim et al., 2006). An alternative scenario, also consistent with the results presented here, is that individual MamK filaments undergo catastrophic depolymerization events that are controlled by MamK regulators. These two models are inspired by observations made for other actin-like proteins. However, it is formally possible that more unusual modes of filament dynamics account for the fluorescence recovery observed by FRAP, including the sliding of short, intact unbleached filaments into the photobleached area or filament exchange between the larger bundle of bleached filaments and a cytoplasmic pool of filaments. Such dynamics would be similar to recent reports detailing the movement of short patches of MreB filaments underneath the cell membrane (Dominguez-Escobar et al., 2011; Garner et al., 2011). More sensitive microscopic assays and single molecule experiments are needed to reliably distinguish between these models and establish a clearer picture of the movement of individual MamK monomers within filaments.
The regulation of a bacterial actin by specific proteins has been best characterized in the ParM plasmid segregation system wherein the regulators stabilize inherently unstable polymers (Garner et al., 2004). Other systems where plasmid segregation is mediated by the bacterial actins AlfA and Alp7A show features that are distinct from ParM (Becker et al., 2006; Derman et al., 2009; Garner et al., 2004; Polka et al., 2009). AlfA forms stable filaments in vitro and dynamic ones in vivo, an activity speculated to be mediated by the DNA binding protein AlfB (Becker et al., 2006; Polka et al., 2009; Tanaka, 2010). Under physiologically relevant expression levels, Alp7A, a member of another plasmid segregation system, requires elements from the plasmid to form dynamically unstable filaments in vivo. However, when overexpressed in the absence of the plasmid, AlfA forms filaments that are no longer dynamically unstable (Derman et al., 2009). Our results suggest that MamK filaments are intrinsically stable in native conditions and rely on at least two proteins for their dynamic behavior. MamK-GFP filaments produced in the ΔMAI strain were static in the FRAP assay implicating the involvement of other MAI factors. Through a candidate-based approach, we identified MamJ and its paralog, LimJ, as two MAI constituents that are required for promoting MamK filament dynamics in vivo. However, the bacterial two-hybrid analysis suggests that MamJ and LimJ do not interact directly with MamK. Since they are also not sufficient to restore MamK dynamics in the ΔMAI strain, it is likely that their action on MamK filament turnover is indirect. Perhaps, as seen in MSR-1 and suggested in this study, these proteins mainly act to ensure that MamK filaments are efficiently recruited to magnetosomes where the activity of other regulators can promote their dynamic turnover (Scheffel et al., 2006). However, the possibility that MamJ and LimJ may have a direct role in the dynamics of MamK filaments, perhaps to stimulate its ATPase activity, cannot be dismissed. Additionally, the ability of MamJ and/or LimJ to control MamK dynamics and interact with the filament may depend on the formation of a higher order complex with other unidentified factors. One possibility for an additional MamK regulator is Amb0994, a putative methyl accepting chemotaxis protein encoded within the MAI. In a split-GFP assay, an in vivo interaction between Amb0994 and MamK was observed in AMB-1 (Philippe and Wu, 2010). While the authors propose that this interaction may link the rotation and alignment of cells in external magnetic fields to cellular motility factors, it might be possible that Amb0994 also acts as a regulator of MamK dynamics. Further biochemical and genetic tests are needed to define not only the elements in the MAI that may act to promote MamK filament recovery in vivo, but also the mechanism by which these regulators control the dynamics of MamK filaments.
The current study has mainly focused on instances where MamK filaments showed dynamic behavior in vivo. However, in wildtype cells, we consistently observed that approximately half of the cells failed to show any significant recovery in the FRAP assay. We have ruled out technical issues as the basis of this phenomenon but have not been able to determine its cause or significance to the organization of magnetosome chains. It has recently been shown that a cryptic magnetosome island, termed the magnetosome islet, exists in AMB-1 (Rioux et al., 2010). One of the genes of this region encodes the MamK-like protein, a homolog of MamK with unknown function (Rioux et al., 2010). A striking feature of MamK-like is that its predicted ATPase active site contains a change at position 141 (corresponding to position 143 in MamK) from glutamate to alanine which, based on our results with MamK, should abolish its filament dynamics (Fig. 2). If MamK-like and MamK interact and can exist as mixed polymers within the cell, we would predict that these filaments should be static. Perhaps these mixed filaments arise in a stochastic manner and can account for the lack of recovery seen in approximately half of the cells imaged.
Regardless of the specific mode by which MamJ and LimJ contribute to MamK filament dynamics, their action in the cell has a clear bearing on the organization of the magnetosome chain. Fluorescence microscopy, using GFP-MamI as a marker for magnetosomes, and ECT imaging showed that the absence of MamJ and LimJ led to the appearance of several gaps throughout the magnetosome chain. In general this phenotype is similar to that seen in the ΔmamK mutant implying that the dynamic properties of MamK are central to its function in magnetosome organization (Komeili et al., 2006). In addition, when these two factors were absent, bundles of filaments, similar in dimension to the magnetosome cytoskeleton and presumed to be MamK, were accumulated within the gaps in the chain. Surprisingly, when mamJ is deleted in the closely related MSR-1 species, magnetosomes clump into aggregates within the cell, a phenotype that is distinct from that observed in our studies of AMB-1. Additionally, in the MSR-1 ΔmamJ mutant, no filaments are observed near magnetosomes, whereas magnetosome filaments were readily observed in the ΔmamJ ΔlimJ strain, albeit with a reduced frequency as compared to the wildtype strain. Differences in chain organization phenotypes have also been observed between the AMB-1 ΔmamK and MSR-1 ΔmamK mutants (Katzmann et al., 2010; Komeili et al., 2006). Since AMB-1 mamJ and mamK can transcomplement the deletions of their homologs in MSR-1, the variations seen in these studies highlight the importance of cellular context in the organization of the magnetosome chain (Katzmann et al., 2010; Scheffel et al., 2006). For example, in AMB-1, magnetosome chains can be aligned independent of magnetite biomineralization while in MSR-1 magnetic interactions between adjacent magnetosomes are thought to be an important component of chain organization. Also, the magnetosome islet mentioned above, contains a gene coding for a MamJ-like protein. The extra functionality, if any, provided by these homologs of MamK and MamJ may account for the phenotypic differences between the two species of magnetotactic bacteria.
Finally, it is possible that the observed mamJ and limJ deletion phenotypes are a mix of the loss of MamK filament dynamics and an independent function performed by MamJ and LimJ. This is partially supported by the observation that the ΔmamJ mutant displays wildtype patterns of MamK-GFP recovery but contains occasional gaps in the chain when imaged by ECT. Additionally, GFP-MamI localization in either the ΔmamJ or ΔlimJ mutant was mildly disrupted when compared to the wildtype, although not as severely as that seen in the double deletion strain. However, the bundling of filaments within the gaps in the chain appears to be specific to the loss of both mamJ and limJ, raising the possibility that this phenotype is a consequence of loss of MamK filament dynamics.
Taken together, the results presented here provide a deeper view into the dynamics, regulation and function of one member of the MamK branch of bacterial actin-like proteins. Many of the features of the AMB-1 MamK protein described here are likely to be universal since the protein is ubiquitously present in diverse species of magnetotactic bacteria. More broadly, the similarities between the filament dynamics of MamK and MreB proteins hint at a shared mechanism for regulation of these functionally distinct groups of proteins.
EXPERIMENTAL PROCEDURES
Growth Conditions
Magnetospirillum magneticum sp. AMB-1 (AMB-1) was grown in a modified MG medium as described in (Komeili et al., 2006; Murat et al., 2010). Frozen stocks were streaked out onto MG plates and grown for five to seven days in Oxoid anaerobic jars with 4 % oxygen. Colonies were inoculated into 1.5 ml snap-cap tubes filled with liquid MG and grown for three to five days. These were the starter cultures for all subsequent cultures. Cultures for conjugations were 1/100 inoculations into 15 ml or 50 ml screw-cap tubes filled with MG medium grown for 2 days. Cultures for microscopy and Cmag measurements were 1/100 inoculations into 10 ml of MG medium in either 1) glass culture tubes (with 10 ml of headspace) incubated in a microaerobic glovebox with 10 % oxygen for one or two days, or 2) Balch tubes with nitrogen bubbled through the media to remove all oxygen in the headspace and grown for one or two days. Cultures for FRAP experiments were 1/100 inoculations into 10 ml of MG medium in glass culture tubes (with 10 ml of headspace) incubated in a microaerobic glovebox with 10 % oxygen for one day. Antibiotic concentrations for AMB-1 and growth conditions for Escherichia coli were as described in (Murat et al., 2010). Media for the bacterial two-hybrid strains and assay was prepared according to the BacterioMatch II manual (Agilent).
Molecular Biology
Cloning of constructs was done according to standard molecular biology techniques (Sambrook and Russell, 2001). Polymerase chain reactions with primers by Integrated DNA Technologies (listed in Table S3) and Promega’s GoTaq Green Master Mix were performed on a Biorad MyCycler thermocycler. Plasmids used in this paper are listed in Table S2. Restriction enzymes, Calf Intestinal Phosphatase, and T4 DNA ligase were purchased from New England Biolabs.
Plasmid Construction
MamKE143A-GFP and MamKD161A-GFP were made by inverse PCR of pAK22 with oligos: KE143A forward and reverse, and KD161A forward and reverse, leading to pAK216 and pAK224, respectively. Genomic AMB-1 DNA (gAMB-1) was used as a template to amplify mamJ (oligos od105 and od106) and limJ (oligos od107 and od109). These were cloned into the XbaI, SacII sites of pAK22 and XbaI, SacI sites of pAK22, respectively, creating pAK476 and the mamK-gfp-rbs-limJ construct (pAK477). Inverse PCR of pAK476 with oligos od134 and od135 led to the mamK-gfp-rbs-mamJ construct (pAK478). For the bacterial two-hybrid assay, PCR products (oligos od84 and od2 for mamK and od85 and od4 for mamJ) were cloned out of frame into the EcoRI, BamHI sites of pBT leading to bait vectors expressing either a single glutamine residue (pAK394), or a glycine followed by glutamine (pAK395). Inverse PCR of pAK394 (oligos od155 and od156) and of pAK395 (oligos od157 and od158) led to plasmids expressing bait-MamK (pAK499) and bait-MamJ (pAK500). mamK (oligos od88 and od89) and mamJ (oligos od90 od91) PCR products were cloned into the BamHI, EcoRI sites of pBT, creating pAK399 and pAK400, respectively. limJ PCR products (oligos od137 and od138) were inserted into the NotI, XhoI sites of pBT and pTRG, creating pAK408 and pAK410 respectively.
Strain Construction
The nonpolar mamK deletion and the MAI deletion were described previously (Komeili et al., 2006). The mamJ and limJ unmarked non-polar deletions were constructed by allelic replacement using a counter-selectable marker as previously described (Murat et al., 2010). Oligos used are listed in Table S3. The mamJ, limJ double deletion was made by deleting limJ in a ΔmamJ background.
MamK-GFP and MamK expression in various strains (Fig. S1) were assayed by SDS-PAGE followed by Western blotting. Equivalent numbers of AMB-1 cells, collected from cultures grown to similar cell densities, were lysed by boiling in 6X Sample buffer and loaded on to a 12% polyacrylamide gel. MamK and MamK-GFP were detected with MamK polyclonal rabbit antibodies (ProSci, Inc.) and HRP-conjugated goat anti-rabbit antibodies (Bio-Rad), both diluted 1:5000 in 5% milk in TBST.
Magnetic Response (Cmag)
The maximum and minimum optical densities of cell cultures in a changing magnetic field (achieved by rotating a strong bar magnet) were measured with a Spectronic 20D+ (Thermo Scientific) spectrophotometer at the wavelength of 400 nm. Cmag is the ratio of the highest to the lowest absorbance reading.
Fluorescence and Phase Contrast microscopy
Light microscopy was carried out using a Nikon Eclipse 80i microscope equipped with a Qimaging RETIGA 2000R Fast 1394 camera. GFP-tagged proteins were excited by the X-Cite 120 Fluorescence Illumination System. Images were analyzed with QCapturePro 5.1 software (Media Cybernetics).
Fluorescence Recovery After Photobleaching
Agarose pads were formed by spotting 1 % agarose prepared in MG between two microscope slides. 1.5 ml of AMB-1 cells in exponential phase were spun, and the pellet, resuspended in ~15 µl of MG, was spotted onto an agarose pad. Pads were sealed between the cover slip and glass slide with VALAP (equal amounts petroleum jelly (Vaseline), Lanolin, and Paraffin wax). Slides were prepared in the microaerobic chamber under 10 % oxygen. FRAP experiments were carried out on a Zeiss 510 UV/Vis Meta laser scanning confocal microscope. MamK-GFP filaments were imaged using 488 nm excitation at 4–7 % laser power. The filaments were bleached using 488 nm laser light at 70–80 % laser power for 7–10 iterations. For each strain, we determined the lowest laser power necessary to image and bleach the filaments in order to minimize overall bleaching of the filament. Images were captured through the 100 × oil objective with the LSM510 Imaging Software 3.2 (Zeiss). Images were analyzed using ImageJ (NIH) and iVision (BioVision Technologies).
Bacterial two-hybrid assay
The assay was performed as described in the BacterioMatch II manual, up until the plating of the transformed cells. Transformed cells were pelleted and then resuspended in 200 µl of M9+ His-dropout Broth. 10 µl were plated onto Nonselective Screening Medium (to assay transformation efficiency), and 180 µl were plated onto Selective Screening Medium (to assay for interaction). Colonies of various sizes grew on some of the selective plates after 1–3 days of incubation at 37°C. Colonies were re-patched onto selective plates and onto Dual Selective Screening plates. Tiny colonies consistently did not grow when patched; colonies that were large enough to grow when patched were the only colonies counted as showing interaction. Data is presented as the percent of colonies that showed interaction out of the total number of transformed cells.
ECT
Tilt series of images of AMB-1 cells were collected on a FEG G2 Polara FEI transmission electron microscope operating at 300 keV, cooled to −180°C with liquid nitrogen, using Leginon (Suloway et al., 2009) on a 4k × 4k lens-coupled Gatan UltraCAM. The tilting scheme was −63° to +63° at 1° intervals. The energy slit-width was 20 eV; the defocus was ~15 µm; the total dose for each tilt-series was ~120 e/Å2; and the magnification was set such that each CCD pixel corresponded to 0.95 nm at the specimen level. Images were binned two-fold before tilt series were reconstructed using IMOD (Kremer et al., 1996), or RAPTOR (Amat et al., 2008) distributed in all lab computers by Peach (Leong et al., 2005). Segmentation and 3-D visualization were carried out manually using IMOD and Amira (Mercury Computer Systems). 60 ∆mamJ, 19 ∆limJ, and 17 ∆mamJ ∆limJ cells were imaged.
Sequence analysis
Accession numbers for the bacterial actin-like proteins (Fig. 1A) are as follows: MamK clade: YP_420328.1, CAM78025.1, ZP_00054405.2, YP_002955471.1, YP_866166.1, ACU87671. ParM clade: P11904.1, NP_941097, NP_058228.1, NP_863404.1, AAT37581, AAL72301. AlfA clade: BAA24871.1, AAK79133. Eukaryotic clade: NP_116614.1, NP_001605.1, NP_001014725.1, NP_499809.1, XP_644247.1. MreB clade: NP_420354.1, NP_390681.2, AAA58054, NP_228398.1, AAA67878.1, NP_781022, AAF93588. Alp7A: ACU27363. Sequences for figure 2 are from (Derman et al., 2009) and from sequences used in figure 1A. Alignments were performed by Clustal W 2.1 (Chenna et al., 2003), and the phylogenetic tree was visualized with Unrooted by Manolo Gouy (Gouy).
Imaging software
Figures were prepared using BioVision Technologies iVision (conversion from 12 or 24 bits to 8 bits, adjustment of levels, false coloring), Adobe Photoshop (rotating, cropping, adjustment of levels, resizing), and Adobe Illustrator (figure layout).
Supplementary Material
ACKNOWLEDGEMENTS
We thank the members of the Komeili laboratory for their valuable research and editorial input. O.D., M.B., and S.K. thank Steve Ruzin and Denise Schichnes at the UC Berkeley Biological Imaging Facility. O.D. and J.C. thank Kent McDonald and Reena Zalpuri at the Robert D. Ogg Electron Microscope Lab. Z.L. thanks Alasdair McDowall and Martin Pilhofer for help with data collection. The cryotomography work was supported by NIH grant R01 GM094800B to G.J.J. A.K. was supported through grants from the Packard Foundation and the National Institutes of Health (R01GM084122).
REFERENCES
- Alyahya SA, Alexander R, Costa T, Henriques AO, Emonet T, Jacobs-Wagner C. RodZ, a component of the bacterial core morphogenic apparatus. Proc Natl Acad Sci U S A. 2009;106:1239–1244. doi: 10.1073/pnas.0810794106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat F, Moussavi F, Comolli LR, Elidan G, Downing KH, Horowitz M. Markov random field based automatic image alignment for electron tomography. J Struct Biol. 2008;161:260–275. doi: 10.1016/j.jsb.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Becker E, Herrera NC, Gunderson FQ, Derman AI, Dance AL, Sims J, Larsen RA, Pogliano J. DNA segregation by the bacterial actin AlfA during Bacillus subtilis growth and development. Embo J. 2006;25:5919–5931. doi: 10.1038/sj.emboj.7601443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendezú FO, Hale CA, Bernhardt TG, de Boer PA. RodZ (YfgA) is required for proper assembly of the MreB actin cytoskeleton and cell shape in E. coli. Embo J. 2009;28:193–204. doi: 10.1038/emboj.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork P, Sander C, Valencia A. An ATPase domain common to prokaryotic cell cycle proteins, sugar kinases, actin, and hsp70 heat shock proteins. Proc Natl Acad Sci U S A. 1992;89:7290–7294. doi: 10.1073/pnas.89.16.7290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeen MT, Jacobs-Wagner C. The bacterial cytoskeleton. Annu Rev Genet. 2010;44:365–392. doi: 10.1146/annurev-genet-102108-134845. [DOI] [PubMed] [Google Scholar]
- Carballido-López R, Errington J. The bacterial cytoskeleton: in vivo dynamics of the actin-like protein Mbl of Bacillus subtilis. Dev Cell. 2003;4:19–28. doi: 10.1016/s1534-5807(02)00403-3. [DOI] [PubMed] [Google Scholar]
- Carballido-López R, Formstone A, Li Y, Ehrlich SD, Noirot P, Errington J. Actin homolog MreBH governs cell morphogenesis by localization of the cell wall hydrolase LytE. Dev Cell. 2006;11:399–409. doi: 10.1016/j.devcel.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defeu Soufo HJ, Graumann PL. Dynamic localization and interaction with other Bacillus subtilis actin-like proteins are important for the function of MreB. Mol Microbiol. 2006;62:1340–1356. doi: 10.1111/j.1365-2958.2006.05457.x. [DOI] [PubMed] [Google Scholar]
- Derman AI, Becker EC, Truong BD, Fujioka A, Tucey TM, Erb ML, Patterson PC, Pogliano J. Phylogenetic analysis identifies many uncharacterized actin-like proteins (Alps) in bacteria: regulated polymerization, dynamic instability and treadmilling in Alp7A. Mol Microbiol. 2009;73:534–552. doi: 10.1111/j.1365-2958.2009.06771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divakaruni AV, Baida C, White CL, Gober JW. The cell shape proteins MreB and MreC control cell morphogenesis by positioning cell wall synthetic complexes. Mol Microbiol. 2007;66:174–188. doi: 10.1111/j.1365-2958.2007.05910.x. [DOI] [PubMed] [Google Scholar]
- Dominguez-Escobar J, Chastanet A, Crevenna AH, Fromion V, Wedlich-Soldner R, Carballido-Lopez R. Processive movement of MreB-associated cell wall biosynthetic complexes in bacteria. Science. 2011;333:225–228. doi: 10.1126/science.1203466. [DOI] [PubMed] [Google Scholar]
- dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, Berry DA, Nosworthy NJ. Actin binding proteins: regulation of cytoskeletal microfilaments. Physiol Rev. 2003;83:433–473. doi: 10.1152/physrev.00026.2002. [DOI] [PubMed] [Google Scholar]
- Frankel RB, Bazylinski DA. Magnetosomes and magneto-aerotaxis. Contrib Microbiol. 2009;16:182–193. doi: 10.1159/000219380. [DOI] [PubMed] [Google Scholar]
- Garner EC, Campbell CS, Mullins RD. Dynamic instability in a DNA-segregating prokaryotic actin homolog. Science. 2004;306:1021–1025. doi: 10.1126/science.1101313. [DOI] [PubMed] [Google Scholar]
- Garner EC, Bernard R, Wang W, Zhuang X, Rudner DZ, Mitchison T. Coupled, circumferential motions of the cell wall synthesis machinery and MreB filaments in B. subtilis. Science. 2011;333:222–225. doi: 10.1126/science.1203285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouy M. Pôle Bio-Informatique Lyonnais. Laboratoire de biométrie et biologie évolutive; Unrooted. [Google Scholar]
- Jensen RB, Gerdes K. Partitioning of plasmid R1. The ParM protein exhibits ATPase activity and interacts with the centromere-like ParR-parC complex. J Mol Biol. 1997;269:505–513. doi: 10.1006/jmbi.1997.1061. [DOI] [PubMed] [Google Scholar]
- Jogler C, Schüler D. Genomics, genetics, and cell biology of magnetosome formation. Annu Rev Microbiol. 2009;63:501–521. doi: 10.1146/annurev.micro.62.081307.162908. [DOI] [PubMed] [Google Scholar]
- Kabsch W, Mannherz HG, Suck D, Pai EF, Holmes KC. Atomic structure of the actin:DNase I complex. Nature. 1990;347:37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Kabsch W, Holmes KC. The actin fold. Faseb J. 1995;9:167–174. doi: 10.1096/fasebj.9.2.7781919. [DOI] [PubMed] [Google Scholar]
- Katzmann E, Scheffel A, Gruska M, Plitzko JM, Schüler D. Loss of the actin-like protein MamK has pleiotropic effects on magnetosome formation and chain assembly in Magnetospirillum gryphiswaldense. Mol Microbiol. 2010;77:208–224. doi: 10.1111/j.1365-2958.2010.07202.x. [DOI] [PubMed] [Google Scholar]
- Kim SY, Gitai Z, Kinkhabwala A, Shapiro L, Moerner WE. Single molecules of the bacterial actin MreB undergo directed treadmilling motion in Caulobacter crescentus. Proc Natl Acad Sci U S A. 2006;103:10929–10934. doi: 10.1073/pnas.0604503103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeili A, Vali H, Beveridge TJ, Newman DK. Magnetosome vesicles are present before magnetite formation, and MamA is required for their activation. Proc Natl Acad Sci U S A. 2004;101:3839–3844. doi: 10.1073/pnas.0400391101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komeili A, Li Z, Newman DK, Jensen GJ. Magnetosomes are cell membrane invaginations organized by the actin-like protein MamK. Science. 2006;311:242–245. doi: 10.1126/science.1123231. [DOI] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- Kruse T, Bork-Jensen J, Gerdes K. The morphogenetic MreBCD proteins of Escherichia coli form an essential membrane-bound complex. Mol Microbiol. 2005;55:78–89. doi: 10.1111/j.1365-2958.2004.04367.x. [DOI] [PubMed] [Google Scholar]
- Leong PA, Heymann JB, Jensen GJ. Peach: a simple Perl-based system for distributed computation and its application to cryo-EM data processing. Structure. 2005;13:505–511. doi: 10.1016/j.str.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Murat D, Quinlan A, Vali H, Komeili A. Comprehensive genetic dissection of the magnetosome gene island reveals the step-wise assembly of a prokaryotic organelle. Proc Natl Acad Sci U S A. 2010;107:5593–5598. doi: 10.1073/pnas.0914439107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterman A, Overbeek R. Missing genes in metabolic pathways: a comparative genomics approach. Curr Opin Chem Biol. 2003;7:238–251. doi: 10.1016/s1367-5931(03)00027-9. [DOI] [PubMed] [Google Scholar]
- Philippe N, Wu LF. An MCP-like protein interacts with the MamK cytoskeleton and is involved in magnetotaxis in Magnetospirillum magneticum AMB-1. J Mol Biol. 2010;400:309–322. doi: 10.1016/j.jmb.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Polka JK, Kollman JM, Agard DA, Mullins RD. The structure and assembly dynamics of plasmid actin AlfA imply a novel mechanism of DNA segregation. J Bacteriol. 2009;191:6219–6230. doi: 10.1128/JB.00676-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradel N, Santini CL, Bernadac A, Fukumori Y, Wu LF. Biogenesis of actin-like bacterial cytoskeletal filaments destined for positioning prokaryotic magnetic organelles. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0603760103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan A, Murat D, Vali H, Komeili A. The HtrA/DegP family protease MamE is a bifunctional protein with roles in magnetosome protein localization and magnetite biomineralization. Mol Microbiol. 2011 doi: 10.1111/j.1365-2958.2011.07631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux JB, Philippe N, Pereira S, Pignol D, Wu LF, Ginet N. A second actin-like MamK protein in Magnetospirillum magneticum AMB-1 encoded outside the genomic magnetosome island. PLoS One. 2010;5:e9151. doi: 10.1371/journal.pone.0009151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera CR, Kollman JM, Polka JK, Agard DA, Mullins RD. Architecture and Assembly of a Divergent Member of the ParM Family of Bacterial Actin-like Proteins. J Biol Chem. 2011;286:14282–14290. doi: 10.1074/jbc.M110.203828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Savage DF, Afonso B, Chen AH, Silver PA. Spatially ordered dynamics of the bacterial carbon fixation machinery. Science. 2010;327:1258–1261. doi: 10.1126/science.1186090. [DOI] [PubMed] [Google Scholar]
- Scheffel A, Gruska M, Faivre D, Linaroudis A, Plitzko JM, Schüler D. An acidic protein aligns magnetosomes along a filamentous structure in magnetotactic bacteria. Nature. 2006;440:110–114. doi: 10.1038/nature04382. [DOI] [PubMed] [Google Scholar]
- Scheffel A, Schüler D. The acidic repetitive domain of the Magnetospirillum gryphiswaldense MamJ protein displays hypervariability but is not required for magnetosome chain assembly. J Bacteriol. 2007;189:6437–6446. doi: 10.1128/JB.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaevitz JW, Gitai Z. The structure and function of bacterial actin homologs. Cold Spring Harb Perspect Biol. 2010;2 doi: 10.1101/cshperspect.a000364. a000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suloway C, Shi J, Cheng A, Pulokas J, Carragher B, Potter CS, Zheng SQ, Agard DA, Jensen GJ. Fully automated, sequential tilt-series acquisition with Leginon. J Struct Biol. 2009;167:11–18. doi: 10.1016/j.jsb.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T. Functional analysis of the stability determinant AlfB of pBET131, a miniplasmid derivative of bacillus subtilis (natto) plasmid pLS32. J Bacteriol. 2010;192:1221–1230. doi: 10.1128/JB.01312-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoka A, Asada R, Wu LF, Fukumori Y. Polymerization of the actin-like protein MamK, which is associated with magnetosomes. J Bacteriol. 2007;189:8737–8740. doi: 10.1128/JB.00899-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Ent F, Johnson CM, Persons L, de Boer P, Löwe J. Bacterial actin MreB assembles in complex with cell shape protein RodZ. Embo J. 2010;29:1081–1090. doi: 10.1038/emboj.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobiev S, Strokopytov B, Drubin DG, Frieden C, Ono S, Condeelis J, Rubenstein PA, Almo SC. The structure of nonvertebrate actin: implications for the ATP hydrolytic mechanism. Proc Natl Acad Sci U S A. 2003;100:5760–5765. doi: 10.1073/pnas.0832273100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CL, Kitich A, Gober JW. Positioning Cell Wall Synthetic Complexes by the Bacterial Morphogenetic Proteins MreB and MreD. Mol Microbiol. 2010 doi: 10.1111/j.1365-2958.2010.07108.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.