Abstract
Background and Objective
Antimicrobial agents provide valuable adjunctive therapy for prevention and control of oral diseases. Limitations in their prolonged use have stimulated the search for new natural occurring agents with more specific activity and fewer adverse effects. Here we sought to determine the anti-bacterial properties of blackberry extract (BBE) in vitro against oral bacterial commensals and periodontopathogens.
Material and Methods
Effects of whole and fractionated BBE on the metabolism of 10 different oral bacteria were evaluated by colorimetric water-soluble tetrazolium-1 (WST-1) assay. Bactericidal effects of whole BBE against F. nucleatum were determined by quantitating colony forming units (CFUs). Cytotoxicity was determined in oral epithelial (OKF6) cells.
Results
BBE at 350-1,400 μg/mL reduced the metabolic activity of P. gingivalis, F. nucleatum and S. mutans. The reduced metabolic activity observed for F. nucleatum corresponded to a reduction in CFUs following exposure to BBE for as little as 1 hour, indicative of its bactericidal properties. An anthocyanin-enriched fraction of BBE reduced the metabolic activity of F. nucleatum but not P. gingivalis or S. mutans, suggesting the contribution of species specific agents in the whole BBE. Oral epithelial cell viability was not reduced following ≤ 6 h exposures to whole BBE (2.24-1400 μg/mL).
Conclusion
BBE alters the metabolic activity of oral periodontopathogens while demonstrating minimal effect on commensals. The specific antibacterial properties of BBE shown in this study along with its anti-inflammatory and antiviral properties previously demonstrated make this natural extract a promising target as an adjunct for prevention and/or complementary therapy of periodontal infections.
Keywords: Blackberry, oral bacteria, antibacterial effect, periodontitis, periodontopathogens, Fusobacterium nucleatum, topical antimicrobial
Periodontal disease results from chronic infection and inflammation of the tissues that support the teeth (1). This oral inflammatory disease affects about 50% of the U.S. population (2), and is driven by pathogenic succession characterized by a shift in microbial species in the gingival sulcus from Gram-positive, facultative, and fermentative microorganisms to predominantly Gram-negative, anaerobic, chemo-organotrophic, and proteolytic microorganisms (1). Bacteria such as Actinomyces viscosus, Actinomyces naeslundii, and Streptococcus spp., are associated with gingivitis and dental caries, whereas Porphyromonas gingivalis, Tannarella forsythia, Treponema denticola, Prevotella intemedia, and Fusobacterium nucleatum are later colonizers associated with periodontopathic biofilms (3). Periodontal disease also is implicated in several systemic diseases including preterm/low birth weight deliveries, cardiovascular events, diabetes, and others systemic conditions (4, 5). Thus, development of new cost-effective measures to prevent and control periodontitis is important for improving oral and systemic health.
Successful treatment of periodontal disease involves elimination of the microbial burden and associated clinical signs of inflammation through mechanical disruption of biofilms, as well as the use of antimicrobial agents in aggressive and unresponsive forms of the disease (6). Use of mouth rinses with antimicrobial activity (e.g. chlorhexidine [CHX]) has been effective for controlling the colonization of oral bacteria including periodontopathogens and reducing gingival inflammation (7-9). Nevertheless, topical antimicrobial rinses such as CHX have restrictions due to potential side effects including, staining, erosive and abrasive effects, as well as limitations on dosage scheduling (10, 11). Since bacterial growth as an oral biofilm is a continuous event important for biological equilibrium within the oral cavity, and few commercial products exist that inhibit this process, it is important to develop new and more efficient therapeutic strategies to control the constant emergence of bacterial pathogens related to oral disease.
Use of natural agents in chewing gums is an important approach for addressing this issue. Previous observations have suggested that blackberry extract (BBE) exhibits anti-inflammatory, anticancer, and antiviral properties (12-15), and a limited number of reports have demonstrated antibacterial activity of blackberries and raspberries against skin and enteric pathogens (14, 16). However, the antimicrobial effect of BBE as a topical agent against periodontal pathogens has not yet been demonstrated. In this study we sought to determine the antibacterial properties of blackberry extract in vitro against oral bacteria associated with gingivitis and periodontal disease, with the potential for this material to be used as a topical agent for treating these infections.
Materials and methods
Plant material, preparation and fractionation of blackberry extracts
Hull blackberries (Rubus eubatus cv. “Hull”) were grown at WindStone Farms (Paris, KY, USA). Seeds and skin were removed using a Langsenkamp type 161 Colossal Pulper and the resultant puree was stored at -20°C. Extracts were obtained from the puree (12, 13, 17). Briefly, blackberry puree (10 g) was treated under sonication for 30 min with 25 mL of extraction solvent of ethanol containing 0.01% HCl (v/v). The supernatants were collected after filtration and dried by rotary evaporation at 40°C. The dried extract was resuspended in deionized water and filtered through a 20-25 μm filter paper and lyophilized to obtain dried BBE. Dried BBE was dissolved in deionized water as a stock solution (140 mg/mL) and stored at -80°C until use, and is referred to as “whole BBE”. The whole BBE was further fractionated by solid phase extraction modified from Skrede et al. (18). Whole BBE was applied to a preconditioned Discovery DSC-18 tube (Supelco, Bellefonte, PA, USA) and eluted sequentially with water, ethyl acetate and finally 50% aqueous methanol. As described previously in Murapa et al. (22), the water fraction had > 98% of total substances and was comprised of < 0.01% phenolic compounds. In contrast, > 99% of the total phenolics in the extract were concentrated in the ethyl acetate and methanol fractions with > 97.3% of the anthocyanins obtained in the methanol fraction. The methanol fraction (anthocyanin-enriched) was dried by a rotary evaporator and reconstituted in DMSO as stock solution and stored at -80°C until use, and is referred to as “anthocyanin-enriched fraction BBE”. Note that the anthocyanin-enriched fraction BBE does not contain methanol. As reported in Dai et al. (2009), the polyphenols and anthocyanins are very stable when stored at -80°C and with no loss of activity for at least 90 days (12). Once thawed, the thawed sample was used once and discarded.
Bacterial strains and growth conditions
Streptococcus mutans (ATCC 25175), Streptococcus oralis (ATCC 10557), Actinomyces naeslundii (ATCC 49340), Actinomyces viscosus (ATCC 43146), Veillonella parvula (ATCC 17745), Prevotella intermedia (ATCC 25611), Fusobacterium nucleatum (ATCC 25586), Porphyromonas gingivalis (ATCC 381), Aggregatibacter actinomycetemcomitans (JP2), and Streptococcus gordonii (ATCC 10558) were used. All the bacteria were grown in Brain Heart Infusion broth supplemented with 5 μg/mL Hemin and 1 μg/mL Menadione (BHI with supplements), at 37°C under anaerobic conditions (80% N2, 10% H2, and 10 % CO2).
Effect of blackberry extract on bacterial metabolism
A bacterial density of 1 × 107 cells/well was seeded into 96-wells plates with 135 μL BHI with appropriate growth supplements. Fifteen μL of test reagent (i.e., different concentrations of whole BBE or anthocyanin-enriched fraction diluted in sterile water) were added to each well. As controls, 15 μL of media, water (BBE vehicle), or 1% CHX gluconate freshly prepared from 20% stock solution (Sigma Chemical Co, St Louis, MO, USA) were added to select wells. The final concentration of CHX was selected based on its known antibacterial properties in vitro and in vivo (19, 20), and its utility in the metabolic assays without adversely affecting optical density readings. In these experiments, the WST-1 assay was used as the metabolic assay, and it served as a surrogate marker of cell proliferation and viability. Here, metabolic activity is defined as the ability of the viable cells to convert the stable tetrazolium salt to a visible dye (formazan) in the WST-1 assay. The assay was performed by incubating plates under anaerobic conditions for 24 h, then 15 μL of cell proliferation reagent WST-1 (Roche, Mannheim, Germany) was added to each well similar to previous reports using this type of metabolic indicator (21). Finally, plates were incubated 1-2 h under the same conditions, and absorbance measured at 420-480 nm, with a reference wavelength of 600 nm using a SpectraMax M2 plate reader (Molecular Devices, Sunnyvale, CA, USA). The percentage inhibition was calculated using the optical densities (OD) of bacterial metabolic activity under different conditions with the following formula: 100 × (OD [Control] – OD [experimental] / OD [Control], where control cultures were treated with media alone and experimental cultures are cultures treated with BBE, vehicle or CHX, as experimental, negative and positive controls, respectively).
Bactericidal effect of blackberry extract
Bacteria were seeded at 1 × 107 into 96-well plates in a total volume of 135 μl of BHI with supplements, and incubated with 15 μL of different concentrations of whole BBE or water (vehicle control) for 1 h or 24 h, at 37°C under anaerobic conditions. After treatment 100 μL of bacterial culture dilutions (2 to 4 log range) were spread on blood agar plates using glass micro-beads, and after 48-72 h of incubation under the same conditions, colony forming units (CFUs) were enumerated.
Cytotoxic effect of BBE in oral epithelial cells
The immortalized keratinocyte cell line OKF6/hTERT-2 (OKF6), established by ectopic expression of the telomerase catalytic subunit (hTERT) in cells from normal oral mucosal epithelium, was obtained from Dr. James Rheinwald, Harvard Medical School, and used for viability assays similarly as previously shown (22, 23). Cells were cultured in Keratinocyte-SFM medium supplemented with bovine pituitary extract (25 μg/mL), recombinant epidermal growth factor (0.2 ng/mL) and penicillin-streptomycin (Ker-SFM) and were maintained at 37°C in a humidified incubator with 5% CO2. For viability assays, cells were plated in 96-well plates at 9,400 cells/well in Ker-SFM. The following day, cells were treated with vehicle control (water) or varying concentrations of BBE for 3, 6 or 24 hours. Cells were exposed to BBE at dilutions ranging from 2.24-1,400 μg/mL final concentration. OKF6 cell viability was determined by quantifying the conversion of resazurin to resorufin using the CellTiter-Blue viability assay (Promega, Madison, WI, USA) (23).
Statistical Analysis
Significant differences between the means for the experimental groups were determined by Student’s t-test (SigmaStat 3.5; Systat Software, Point Richmond, CA). In cell viability studies, differences among treatment groups were determined by ANOVA and Fisher’s least significance test, using Starview software (SAS Institute, Cary, NC). P values were considered significant at < 0.05.
Results
Effects of the whole and an anthocyanin-enriched fraction of BBE on bacterial metabolic activity were evaluated against several oral commensal and pathogenic periodontal bacteria. In these assays, 24 h exposure of BBE significantly reduced metabolic activity of a small group of oral bacteria particularly associated with periodontal disease and dental caries but not that of other pathogens or commensal microorganisms (Table 1). We observed that 700 μg/mL of whole BBE inhibited the metabolic activity of F. nucleatum and P. gingivalis by about 40%. The same concentrations reduced the metabolic activity of S. mutans approximately 30%. Higher whole BBE concentrations (1,400 μg/mL) showed a greater effect only against F. nucleatum, reducing its metabolic activity by about 84%. A similar range of metabolic inhibition (30-57%) was observed for F. nucleatum and P. gingivalis after 24 h of exposure to 0.1% CHX.
Table 1.
Effect of whole BBE on metabolic activity of common oral bacteria.
| Strains | Range of BBE concentrations | ||||
|---|---|---|---|---|---|
| Water | 175 μg/mL | 350 μg/mL | 700 μg/mL | 1400 μg/mL | |
| S. oralis | -3.1 (8.6) | 3.6 (2.7) | -0.5 (9.3) | -7.9 (10.5) | 8.6 (7.8) |
| S. gordonii | 2.3 (3.6) | -1.0 (10.2) | -1.6 (4.5) | -11.1 (13.3) | -11.6 (5.1) |
| S. mutans | 5.2 (9.6) | 16.1 (5.9) | 23.9 (4.0)* | 31.4 (3.4)* | 27.5 (1.7)* |
| A. naeslundii | 3.3 (12.2) | 6.5 (13.3) | 12.9 (7.0) | 11.7 (10.8) | 14.0 (8.7) |
| A. viscosus | -7.1 (7.0) | 0.4 (5.2) | 3.8 (4.9) | -3.4 (8.1) | 10.3 (7.3) |
| V. parvula | -0.2 (2.1) | -0.4 (2.4) | -0.4 (2.6) | -0.03 (2.6) | -0.003 (2.1) |
| P. intermedia | 0.3 (2.3) | 0.1 (2.3) | 0.4 (2.1) | 0.2 (2.6) | 0.3 (2.2) |
| A. actinomycetecomintans | 7.6 (20.0) | -7.2 (17.8) | -14.2 (14.3) | -19.5 (10.3) | -9.6 (10.4) |
| P. gingivalis | 1.6 (6.9) | 13.8 (5.5) | 27.0 (5.3)* | 40.7 (2.0)* | 43.6 (2.5)* |
| F. nucleatum | 1.7 (5.7) | 0.0 (7.0) | 4.5 (9.4) | 40.9 (18.5)* | 83.5 (5.2)* |
Data are mean percentage reductions of metabolic activity following treatment with a range of BBE concentrations. Results from at least two independent experiments performed in triplicate for each strain of bacteria. Standard deviations shown in parenthesis. Values significantly different (p ≤ 0.05) between water treatment vs. BBE extract are identified with an asterisk.
Percentage of metabolic activity inhibition for F. nucleatum and P. gingivalis exposed to 0.1% chlorhexidine for 24 h were 29.1(13.7) and 57(5.9), respectively.
To better understand the pharmacologic properties of BBE, the anthocyanin-enriched fraction of BBE with known antiproliferative and anti-inflammatory properties (13), was next investigated. This enriched fraction exhibited antimicrobial effects similar to that seen with whole BBE against F. nucleatum, reducing metabolic activity about 70% after 24 h exposure (Fig. 1). Of note, the enriched BBE fraction did not significantly inhibit P. gingivalis or S. mutans. These findings suggest that the antibacterial property against F. nucleatum was retained in the anthocyanin-enriched fraction, whereas the specific inhibitory component(s) against P. gingivalis and S. mutans were eliminated from BBE during the fractionation process.
Figure 1. Effect of anthocyanin-enriched fraction of BBE on bacterial metabolic activity of oral pathogens.
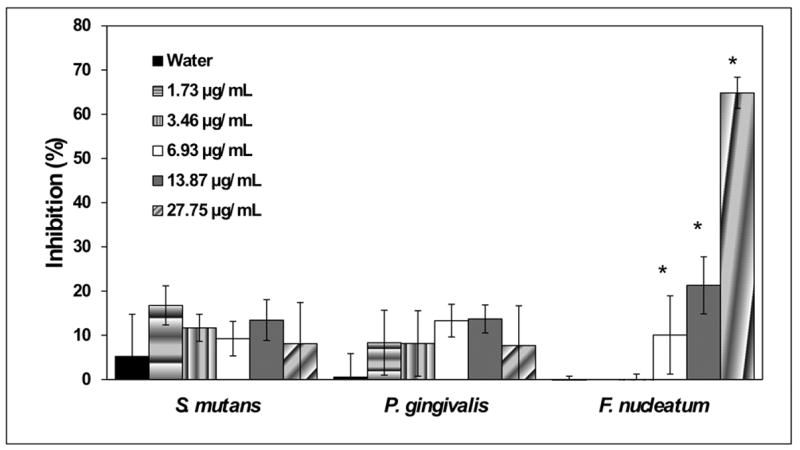
The bars denote the mean percentage inhibition of different fraction concentrations and the standard deviation of at least two independent experiments performed in triplicate for each species of bacteria. Values significantly different (p ≤ 0.05) between water treatment vs. BBE anthocyanin-enriched fraction are identified with an asterisk (*).
Lastly we determined whether the reduction in bacterial metabolic activity observed with F. nucleatum from treatment with whole BBE was due to a reduction in cell viability. This periodontopathogen was selected for these experiments because F. nucleatum was most susceptible to inhibition by BBE. Consistent with the metabolic-based assay findings, the number of CFUs of F. nucleatum was significantly reduced (~4 to 13.6-fold) by BBE concentrations ≥ 350μg/mL (Fig. 2A). Shorter exposure of F. nucleatum (1 h) to whole BBE also induced a similar significant reduction in CFUs (~3-fold) (Fig. 2B). Of note, oral epithelial (OKF6) cell viability was not reduced following ≤ 6 h exposures to whole BBE (2.24-1400 μg/mL), suggesting that at these concentrations and exposure times whole BBE was not harmful to oral epithelial cells. In contrast, viability was reduced 15% and 43% following 24 h exposure to 280 and 1,400 μg/mL whole BBE, respectively (Fig. 3).
Figure 2. Bactericidal effect of the whole BBE against F. nucleatum.

Bacteria were seeded into 96-well plates in BHI broth either in presence or absence of several concentrations of whole BBE extract diluted in water for (A) 24 h or (B) 1 h. Colony forming units (CFUs) were enumerated after bacteria were spread into agar plates as described in Methods. The bars are representative of at least two independent experiments performed in triplicate. Data are expressed as means ± standard deviation. Values significantly different (p ≤ 0.05) between untreated bacteria (control) vs. bacteria exposed to BBE or water are identified with an asterisk (*).
Figure 3. Cytotoxic effect of BBE in oral epithelial (OKF6) cells.
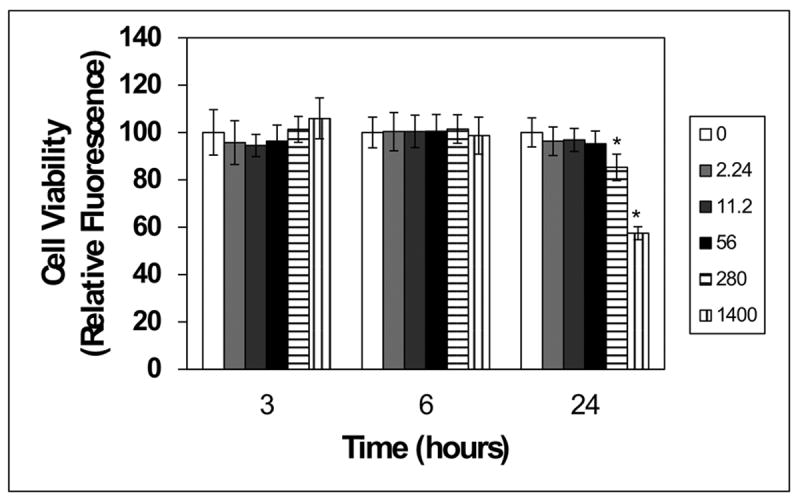
OKF6 cells were plated in 96-well plates (9,400 cells/well) in Ker-SFM for 24 h as described in the methods. Cells were treated with vehicle control (water/white bar) or varying concentrations of BBE for 3, 6 or 24 hours. Cell viability was evaluated using the CellTiter-Blue viability assay. Data for BBE-treated cultures were normalized to the mean for water-treated cultures. Values significantly different (p ≤ 0.05) between water-treated cells vs. OKF6 cells exposed to BBE are identified with an asterisk (*).
Discussion
Overgrowth of specific bacterial species within oral biofilms is associated with periodontal disease (24, 25). Thus, the infectious nature of periodontitis makes the use of topical antimicrobial agents that interfere with bacterial replication and co-aggregation an important strategy for preventing periodontal disease (26-28). To date, CHX has been one of the most effective antimicrobial agents for the adjunctive control of gingivitis and periodontitis. However, its broad spectrum of activity and adverse effect profile limits its prolonged use as a topical oral antimicrobial agent by the general population (10). Therefore, there is a need to develop additional active agents with higher specificity and limited adverse effects that can control the growth of oral pathogens on dental and mucosal surfaces while limiting the effect commensals.
The anti-inflammatory and antimicrobial properties of BBE as well as its biochemical characteristics make it a promising candidate for the development of topical therapeutic strategies focused on controlling bacterial growth in relation to periodontal diseases (13, 15). Accordingly, we tested the antimicrobial properties of BBE against a group of oral bacteria including commensals and periodontopathogens. Using the whole extract from Hull blackberries, we found that the antimicrobial effect was specific, with significant inhibition of metabolic activity occurring in a select group of microorganisms associated with periodontitis (F. nucleatum and P. gingivalis) as well as dental caries (S. mutans), but not the oral commensal microorganisms as occurs with CHX (29). Although the mechanism for the antibacterial specificity shown by BBE needs to be elucidated, it could involve a critical metabolite of anthocyanins, i.e., gallic acid, which is an iron chelator that forms stable complexes with these metal ions and decreases their availability to bacteria species (31). Thus, the differential iron requirements for sustaining bacterial normal growth and/or ability to persist under iron-deficient conditions exhibited by oral bacterial species could make BBE-induced iron starvation a selective process to control pathogenic oral strains. Selective targeting of BBE is important because growing evidence suggests a critical role of commensal bacteria colonizing the mucosal surfaces in maintaining the homeostasis and modulating the innate immune response, which results in a protective effect from pathogens (32). Therefore, the specificity exhibited by the BBE in this study, would be an important characteristic to consider for controlling oral pathogens in the context of polymicrobial communities.
In addition, our studies show that BBE is not only impacting bacterial metabolism, but also has the ability to kill oral bacteria as was observed against F. nucleatum at concentrations ≥ 350 μg/mL. Although the cellular and molecular mechanisms associated with the antimicrobial effects are not fully understood, evidence suggests that berry-derived polyphenols (e.g. anthocyanins) could be involved (33-36). This is consistent with recent evidence that polyphenolic beverages such as red wine, cistus tea and black tea can reduce the total count of adherent oral bacteria (37).
It is noteworthy in our studies that short exposures (1 h) of F. nucleatum to BBE had significant bactericidal effect, and the same range of BBE concentrations did not affect oral epithelial cell viability within the first 6 h. Thus, these antimicrobial properties would be consistent with the short-term topical use of BBE in oral products for bacterial oral diseases. Also, a significant change in F. nucleatum metabolic activity was not observed following 24 h exposure to 350 μg/mL BBE, yet this concentration conferred bactericidal activity as determined by the significant reduction of CFUs. Although this finding may seem counterintuitive, we speculate that at this concentration BBE-derived constituents may interfere more with bacterial metabolism necessary for survival than with events required for bacterial replication as shown by others (38). Thus, the metabolic activity of the “surviving” cells could have increased proportionally such that the overall metabolic activity of the cultures remained unchanged.
It is important to mention that the efficiency in recovering anthocyanins from a natural source depends on several factors, such as the culture, growth conditions, and storage of raw material (39). Similarly, the solvent system used for extraction plays a crucial role since it has been shown that anthocyanins recovery can be substantially improved by select enrichment processes (40). In this research, the antimicrobial effect of previously titrated concentrations of the enriched portion of BBE (13) was evaluated against three oral pathogens. Interestingly, when the anthocyanin-enriched fraction was tested, only the bacterial metabolism of F. nucleatum, not P. gingivalis and S. mutans was affected in a manner similar to that observed with whole BBE. These results suggest that the components within the whole BBE that have antimicrobial activity against P. gingivalis and S. mutans could be different (i.e., non-phenolic components) and would be present in the unused portion of the fraction, in contrast to what was observed for F. nucleatum. Thus, future studies are being directed towards the development of additional chemical isolation strategies that will identify those specific antimicrobial BBE components.
In summary, this study showed that non-cytotoxic BBE concentrations exhibits antimicrobial properties against important periodontal pathogens as well as S. mutans. The minimal effect of BBE on oral earlier colonizers considered commensal bacteria (i.e., Streptococcus and Actinomyces) is also a promising observation for its potential utility as a product in controlling oral disease. Although more studies are clearly needed, including further characterization of BBE, the assessment of BBE against oral biofilms formation and maintenance, its effect on other pathogenic strains from the same species, as well as its potential for tooth staining, these findings suggest that this natural extract has the potential to be used as an antibacterial topical agent for the prevention and control of periodontitis as well as dental caries. Chewing gums containing a variety of agents (e.g. sorbitol, xylitol and CHX) are recognized to be effective adjunctive approaches for maintaining oral health (41-43). Incorporation of BBE in oral release devices such as chewing gum is a long-term goal.
Acknowledgments
We thank Olivia Perkins Mackey and Chunmei Wang for their assistance in the laboratory. This study was supported by a grant 1R41DE018839 01 from the National Institutes of Health, Bethesda, Maryland.
Footnotes
Conflict of interest: The authors report no conflicts of interest related to this study.
References
- 1.Feng Z, Weinberg A. Role of bacteria in health and disease of periodontal tissues. Periodontol 2000. 2006;40:50–76. doi: 10.1111/j.1600-0757.2005.00148.x. [DOI] [PubMed] [Google Scholar]
- 2.Eke PI, Thornton-Evans GO, Wei L, Borgnakke WS, Dye BA. Accuracy of NHANES periodontal examination protocols. J Dent Res. 2010;89:1208–1213. doi: 10.1177/0022034510377793. [DOI] [PubMed] [Google Scholar]
- 3.Socransky SS, Haffajee AD. Periodontal microbial ecology. Periodontol 2000. 2005;38:135–187. doi: 10.1111/j.1600-0757.2005.00107.x. [DOI] [PubMed] [Google Scholar]
- 4.Kuo LC, Polson AM, Kang T. Associations between periodontal diseases and systemic diseases: a review of the inter-relationships and interactions with diabetes, respiratory diseases, cardiovascular diseases and osteoporosis. Public Health. 2008;122:417–433. doi: 10.1016/j.puhe.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Teng YT, Taylor GW, Scannapieco F, et al. Periodontal health and systemic disorders. J Can Dent Assoc. 2002;68:188–192. [PubMed] [Google Scholar]
- 6.Krayer JW, Leite RS, Kirkwood KL. Non-surgical chemotherapeutic treatment strategies for the management of periodontal diseases. Dent Clin North Am. 54:13–33. doi: 10.1016/j.cden.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paolantonio M, D’Ercole S, Pilloni A, et al. Clinical, microbiologic, and biochemical effects of subgingival administration of a Xanthan-based chlorhexidine gel in the treatment of periodontitis: a randomized multicenter trial. J Periodontol. 2009;80:1479–1492. doi: 10.1902/jop.2009.090050. [DOI] [PubMed] [Google Scholar]
- 8.Tomas I, Cousido MC, Tomas M, Limeres J, Garcia-Caballero L, Diz P. In vivo bactericidal effect of 0.2% chlorhexidine but not 0.12% on salivary obligate anaerobes. Arch Oral Biol. 2008;53:1186–1191. doi: 10.1016/j.archoralbio.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Wennstrom J, Lindhe J. The effect of mouthrinses on parameters characterizing human periodontal disease. J Clin Periodontol. 1986;13:86–93. doi: 10.1111/j.1600-051x.1986.tb01419.x. [DOI] [PubMed] [Google Scholar]
- 10.Hepso HU, Bjornland T, Skoglund LA. Side-effects and patient acceptance of 0.2% versus 0.1% chlorhexidine used as post-operative prophylactic mouthwash. Int J Oral Maxillofac Surg. 1988;17:17–20. doi: 10.1016/s0901-5027(88)80222-4. [DOI] [PubMed] [Google Scholar]
- 11.Pontefract H, Hughes J, Kemp K, Yates R, Newcombe RG, Addy M. The erosive effects of some mouthrinses on enamel. A study in situ. J Clin Periodontol. 2001;28:319–324. doi: 10.1034/j.1600-051x.2001.028004319.x. [DOI] [PubMed] [Google Scholar]
- 12.Dai J, Gupte A, Gates L, Mumper RJ. A comprehensive study of anthocyanin-containing extracts from selected blackberry cultivars: extraction methods, stability, anticancer properties and mechanisms. Food Chem Toxicol. 2009;47:837–847. doi: 10.1016/j.fct.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Dai J, Patel JD, Mumper RJ. Characterization of blackberry extract and its antiproliferative and anti-inflammatory properties. J Med Food. 2007;10:258–265. doi: 10.1089/jmf.2006.238. [DOI] [PubMed] [Google Scholar]
- 14.Cavanagh HM, Hipwell M, Wilkinson JM. Antibacterial activity of berry fruits used for culinary purposes. J Med Food. 2003;6:57–61. doi: 10.1089/109662003765184750. [DOI] [PubMed] [Google Scholar]
- 15.Danaher RJ, Wang C, Dai J, Mumper RJ, Miller CS. Antiviral effects of blackberry extract against herpes simplex virus type 1. Oral Surg Oral Med, Oral Pathol Oral Radiol Endod. 2011;112:e31–35. doi: 10.1016/j.tripleo.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martini S, D’Addario C, Colacevich A, et al. Antimicrobial activity against Helicobacter pylori strains and antioxidant properties of blackberry leaves (Rubus ulmifolius) and isolated compounds. Int J Antimicrob Agents. 2009;34:50–59. doi: 10.1016/j.ijantimicag.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Murapa P, Dai J, Chung M, Mumper RJ, D’Orazio J. Anthocyanin-rich fractions of blackberry extracts reduce UV-induced free radicals and oxidative damage in keratinocytes. Phytother Research : PTR. 2012;26:106–112. doi: 10.1002/ptr.3510. [DOI] [PubMed] [Google Scholar]
- 18.Skrede G, Wrolstad RE, Durst RW. Changes in anthocyanins and polyphenolics during juice processing of highbush blueberries (Vaccinium corymbosum L) J Food Sci. 2000;65:357–364. [Google Scholar]
- 19.Eick S, Goltz S, Nietzsche S, Jentsch H, Pfister W. Efficacy of chlorhexidine digluconate-containing formulations and other mouthrinses against periodontopathogenic microorganisms. Quintessence Int. 2011;42:687–700. [PubMed] [Google Scholar]
- 20.Gusberti FA, Sampathkumar P, Siegrist BE, Lang NP. Microbiological and clinical effects of chlorhexidine digluconate and hydrogen peroxide mouthrinses on developing plaque and gingivitis. J ClinPeriodontol. 1988;15:60–67. doi: 10.1111/j.1600-051x.1988.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 21.Tsukatani T, Suenaga H, Higuchi T, et al. Colorimetric cell proliferation assay for microorganisms in microtiter plate using water-soluble tetrazolium salts. J Microbiol Methods. 2008;75:109–116. doi: 10.1016/j.mimet.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Kashleva H, Dongari-Bagtzoglou A. Cytotoxic and cytokine-inducing properties of Candida glabrata in single and mixed oral infection models. Microb Pathog. 2007;42:138–147. doi: 10.1016/j.micpath.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danaher RJ, Wang C, Roland AT, Kaetzel CS, Greenberg RN, Miller CS. HIV protease inhibitors block oral epithelial cell DNA synthesis. Arch Oral Biol. 2010;55:95–100. doi: 10.1016/j.archoralbio.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosan B, Lamont RJ. Dental plaque formation. Microbes Infect. 2000;2:1599–1607. doi: 10.1016/s1286-4579(00)01316-2. [DOI] [PubMed] [Google Scholar]
- 25.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 26.Mandel ID. Chemotherapeutic agents for controlling plaque and gingivitis. J Clin Periodontol. 1988;15:488–498. doi: 10.1111/j.1600-051x.1988.tb01020.x. [DOI] [PubMed] [Google Scholar]
- 27.Loe H, Schiott CR. The effect of mouthrinses and topical application of chlorhexidine on the development of dental plaque and gingivitis in man. J Periodontal Res. 1970;5:79–83. doi: 10.1111/j.1600-0765.1970.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 28.Loe H, Von der Fehr FR, Schiott CR. Inhibition of experimental caries by plaque prevention. The effect of chlorhexidine mouthrinses. Scand J Dent Res. 1972;80:1–9. doi: 10.1111/j.1600-0722.1972.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 29.Walker CB. Microbiological effects of mouthrinses containing antimicrobials. J Clin Periodontol. 1988;15:499–505. doi: 10.1111/j.1600-051x.1988.tb01021.x. [DOI] [PubMed] [Google Scholar]
- 30.Hidalgo M, Oruna-Concha MJ, Kolida S, et al. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J Agric Food Chem. 2012;60:3882–3890. doi: 10.1021/jf3002153. [DOI] [PubMed] [Google Scholar]
- 31.Wang R, Kaplan A, Guo L, et al. The Influence of Iron Availability on Human Salivary Microbial Community Composition. Microb Ecol. 2012 doi: 10.1007/s00248-012-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly D, Conway S. Bacterial modulation of mucosal innate immunity. Mol Immunol. 2005;42:895–901. doi: 10.1016/j.molimm.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Puupponen-Pimia R, Nohynek L, Meier C, et al. Antimicrobial properties of phenolic compounds from berries. J Appl Microbiol. 2001;90:494–507. doi: 10.1046/j.1365-2672.2001.01271.x. [DOI] [PubMed] [Google Scholar]
- 34.Puupponen-Pimia R, Nohynek L, Ammann S, Oksman-Caldentey KM, Buchert J. Enzyme-assisted processing increases antimicrobial and antioxidant activity of bilberry. J Agric Food Chem. 2008;56:681–688. doi: 10.1021/jf072001h. [DOI] [PubMed] [Google Scholar]
- 35.Nohynek LJ, Alakomi HL, Kahkonen MP, et al. Berry phenolics: antimicrobial properties and mechanisms of action against severe human pathogens. Nutr Cancer. 2006;54:18–32. doi: 10.1207/s15327914nc5401_4. [DOI] [PubMed] [Google Scholar]
- 36.Cesoniene L, Jasutiene I, Sarkinas A. Phenolics and anthocyanins in berries of European cranberry and their antimicrobial activity. Medicina (Kaunas) 2009;45:992–999. [PubMed] [Google Scholar]
- 37.Hannig C, Sorg J, Spitzmuller B, Hannig M, Al-Ahmad A. Polyphenolic beverages reduce initial bacterial adherence to enamel in situ. J Dent. 2009;37:560–566. doi: 10.1016/j.jdent.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Riihinen K, Ryynanen A, Toivanentoivanen M, Kononen E, Torronen R, Tikkanen-Kaukanen C. Antiaggregation potential of berry fractions against pairs of Streptococcus mutans with Fusobacterium nucleatum or Actinomyces naeslundii. Phytother Res. 2010 doi: 10.1002/ptr.3228. [DOI] [PubMed] [Google Scholar]
- 39.Viskelis P, Rubinskiene M, Jasutiene I, Sarkinas A, Daubaras R, Cesoniene L. Anthocyanins, antioxidative, and antimicrobial properties of American cranberry (Vaccinium macrocarpon Ait.) and their press cakes. J Food Sci. 2009;74:C157–161. doi: 10.1111/j.1750-3841.2009.01066.x. [DOI] [PubMed] [Google Scholar]
- 40.Leusink GJ, Kitts DD, Yaghmaee P, Durance T. Retention of antioxidant capacity of vacuum microwave dried cranberry. J Food Sci. 2010;75:C311–316. doi: 10.1111/j.1750-3841.2010.01563.x. [DOI] [PubMed] [Google Scholar]
- 41.Ainamo J, Asikainen S, Ainamo A, Lahtinen A, Sjoblom M. Plaque growth while chewing sorbitol and xylitol simultaneously with sucrose flavored gum. J ClinPeriodontol. 1979;6:397–406. doi: 10.1111/j.1600-051x.1979.tb01938.x. [DOI] [PubMed] [Google Scholar]
- 42.Hildebrandt GH, Sparks BS. Maintaining mutans streptococci suppression with xylitol chewing gum. J Am Dent Assoc. 2000;131:909–916. doi: 10.14219/jada.archive.2000.0309. [DOI] [PubMed] [Google Scholar]
- 43.Ly KA, Milgrom P, Rothen M. The potential of dental-protective chewing gum in oral health interventions. J Am Dent Assoc. 2008;139:553–563. doi: 10.14219/jada.archive.2008.0215. [DOI] [PubMed] [Google Scholar]


