Abstract
Since prostate cancer (CaP) is regulated by androgen receptor (AR) activity, metastatic CaP is treated with androgen deprivation therapy (ADT). Despite initial response, patients on ADT eventually progress to castration-resistant CaP (CRPC), which is currently incurable. We previously showed that cleavage of the 280kDa structural protein Filamin A (FlnA) to a 90kDa fragment, and nuclear localization of the cleaved product, sensitized CRPC cells to ADT. Hence, treatment promoting FlnA nuclear localization would enhance androgen responsiveness. Here, we show that FlnA nuclear localization induced apoptosis in CRPC cells during ADT, identifying it as a treatment tool in advanced CaP. Significantly, the natural product genistein-combined-polysaccharide (GCP) had a similar effect. Investigation of the mechanism of GCP-induced apoptosis showed that GCP induced FlnA cleavage and nuclear localization, and that apoptosis resulting from GCP treatment was mediated by FlnA nuclear localization. Two main components of GCP are genistein and daidzein: the ability of GCP to induce G2 arrest was due to genistein whereas sensitivity to ADT stemmed from daidzein; hence both were needed to mediate GCP's effects. FlnA cleavage is regulated by its phosphorylation; we show that ADT enhanced FlnA phosphorylation, which prevented its cleavage, whereas GCP inhibited FlnA phosphorylation, thereby sensitizing CaP cells to ADT. In a mouse model of CaP recurrence, GCP, but not vehicle, impeded relapse following castration; indicating that GCP, when administered with ADT, interrupted the development of CRPC. These results demonstrate the efficacy of GCP in promoting FlnA nuclear localization and enhancing androgen responsiveness in CaP.
Keywords: Androgen Receptor, Filamin A, prostate cancer, genistein combined polysaccharide
Introduction
Prostate cancer (CaP), both localized and involving distant metastases, depends on the androgen receptor (AR) for growth and survival. Therefore, metastatic CaP is treated with androgen deprivation therapy (ADT) (Catalona 1994); however, patients on this treatment eventually relapse, indicative of the development of castration-resistant CaP (CRPC). Although a number of FDA-approved treatments for CRPC are currently available, the condition remains essentially incurable, with high mortality rates. Response rate to ADT and time-to-progression, however, varies among patients and therefore, the goal of the present project was to conduct preclinical studies to identify therapy that ensure sensitivity to ADT in the majority of patients, in order to increase the survival rate in this population.
Previously, we demonstrated that the structural protein Filamin A (FlnA), which in the cytoplasm promotes actin stress fiber formation, is localized to the nucleus of androgen-dependent cells, where it impeded CRPC development (Bedolla, et al. 2009; Wang, et al. 2007). 88.4% of metastatic deposits from CRPC patients expressed cytoplasmic FlnA, whereas 75% of hormone naïve localized tumors expressed nuclear FlnA (Bedolla et al. 2009). In support of these observations. in vitro studies showed that cytoplasmic localization of FlnA was associated with increased cell motility and invasion (Bedolla et al. 2009) whereas nuclear localization was associated with castration sensitive growth (Wang et al. 2007). Our results indicated that therapies that promoted the induction of FlnA nuclear translocation would enhance the effectiveness of ADT. The overall goal of the present studies was to identify a clinically safe drug that can promote FlnA localization to the nucleus.
Full-length FlnA (280 kDa) is mainly cytoplasmic, and consists of an N-terminal actin-binding domain followed by 24 repeats, each 96-amino acids long, interrupted by two hinge domains H1 and H2 (van der Flier and Sonnenberg 2001). Proteolysis of FlnA by cleavage at H1 between repeats 15 and 16 created a 170 kDa N-terminal fragment and a C-terminal 110 kDa fragment, which was further cleaved at H2 between repeats 23 and 24, to yield a 90 kDa fragment, which can translocate to the nucleus. Surprisingly, FlnA was also found to localize to the nucleolus, where it inhibited rRNA production (Deng, et al. 2012), but the mechanism of transportation to that organelle is currently unknown. The hinge domain of the AR binds to the C-terminal domain of FlnA at repeats 18-19, and co-localizes to the nucleus (Loy, et al. 2003; Ozanne, et al. 2000). Phosphorylation of FlnA at Ser 2152 prevented cleavage of the protein (Gorlin, et al. 1990), whereas dephosphorylation of FlnA induced nuclear localization, and promoted sensitivity to ADT (Bedolla et al. 2009; Wang et al. 2007). Despite the attractiveness of using FlnA to enhance androgen sensitivity, the translational potential of these studies, until now, was impeded by a lack of clinically safe drugs which prevent FlnA phosphorylation and promote its cleavage.
Here, for the first time, we show that Genistein Combined Polysaccharide (GCP), a natural product induces FlnA nuclear localization. GCP consists of 9% genistein, 6% daidzein, 2% glycetin, 3% equol, 15% lipid, 5% protein, and 60% carbohydrate (deVere White, et al. 2010). An initial case study reported that 1.5g GCP daily for 6 weeks caused tumor regression in a 63-year old man presenting with T3, Gleason grade 6 (3+3) CaP and reduced serum levels of prostate specific antigen (PSA) from 19.4 ng/ml to 10.2 ng/ml in 3 weeks, indicative of reduction in AR activity (Ghafar, et al. 2002). Following this report, the effect of GCP in CaP was investigated in a number of laboratories. In vitro and in vivo studies revealed that GCP reduced cell growth in both androgen-dependent and -independent cells (Bemis, et al. 2004; Vinall, et al. 2007). GCP markedly suppressed mTOR-p70S6K signaling and attenuated excessive androgen signaling, which is a hallmark of advanced CaP (Tepper, et al. 2007; Vinall et al. 2007).
Clinical studies from our institute in men with localized CaP demonstrated the safety of GCP and resulted in no change or decline in PSA (deVere White, et al. 2004). In an additional study of GCP alone in men with a diagnosis of CaP but no prior treatment, participants showed no evidence of metastasis; however, serum genistein levels did not correlate with PSA (deVere White et al. 2010). These studies encouraged investigation of GCP in advanced disease. In vitro studies showed that GCP treatment in combination with AR knockdown (Vinall et al. 2007) or co-treatment with the AR antagonist bicalutamide (Burich, et al. 2008) had enhanced efficacy. However, the mechanism of enhanced efficacy of GCP in the absence of androgens was unknown. Although GCP is known to inhibit the PI3K/Akt/mTOR pathway, its effect on AR suppression was independent of this pathway (Tepper et al. 2007). The lack of a mediator of GCP action on the AR severely impaired efforts to understand its role in CaP control, until now.
Collaboration between two independent teams investigating the actions of GCP and Filamin A, as shown here, finally led to the demonstration that GCP targets FlnA and cleaves this molecule to the 90 kDa fragment, which can then translocate to the nucleus and impede relapse following ADT. Our results show that nuclear localization of FlnA promotes androgen-dependence, and that the cooperative effect of GCP and ADT in CaP results from simultaneous effects of these two treatments on this molecule both in vitro and in vivo. Our results therefore identify GCP-induced FlnA nuclear localization as a therapeutic module that enhances the efficacy of ADT.
Methods
Cell culture and materials
LNCaP and CWR22Rv1 (ATCC, Manassas, VA), C4-2 cells (Urocor, Oklahoma City, OK), CWR-R1 cells (Dr Elizabeth Wilson, University of North Carolina) and PC-346C cells (Dr. W.M. van Weerden, Josephine Nefkens Institute, Erasmus MC, Rotterdam, Netherlands) were cultured in fetal bovine serum (FBS) or charcoal stripped serum (CSS) as indicated. All cell line used here was investigated for the presence of contaminants and their cellular origins verified prior to use. The following plasmids were used in the transfections: pCMV-FlnA, FlnA(16-24) and FlnA(1-15) plasmids kindly provided by Dr. E.W. Yong, National University of Singapore, Singapore; PSA-luciferase construct (hPSA-luc) kindly provided by Dr. XuBao Shi, University of California Davis. Cells were transfected using Lipofectamine 2000 reagent (Invitrogen, Grand Island, NY) according to manufacturer's specifications based on established protocols.
Materials used
GCP and bicalutamide were kindly provided by AminoUp, Japan and AstraZeneca, Cheshire, UK, respectively. PKI(14-22) was from Calbiochem, DHT and genistein were from Sigma-Aldrich, and daidzein was from Fisher Scientific. PKI, Genistein, daidzein and bicalutamide were dissolved in DMSO, while DHT was dissolved in ethanol. For in vitro studies, GCP was dissolved in a solution of 50%DMSO and 50% ethanol, whereas in vivo, it was provided as a suspension in peanut oil.
Subcellular fractionation
Cells were collected in 0.5 ml of Buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.5 mM DTT) containing 200 μl of 10% IGEPAL and protease inhibitors (0.1 mM benzamidine, 1 mM PMSF, 10 μg/ml each phenathroline, leupeptin, aprotinin, and pepstatin A). Following 10 min incubation at room temperature, the lysates were transferred to ice and centrifuged at 4°C at 1500 rpm in a benchtop refrigerated centrifuge (Eppendorf 5417R) for 5 min, and the supernatant collected as the cytosolic fraction. The pellet containing the nuclei was resuspended in 150 ml of Buffer B (20 mM HEPES, pH 7.9, 0.4M NaCl, 1 mM EDTA, 10% Glycerol) containing the same protease inhibitors and solubilized by vigorous shaking using a sonicator at 4°C for 2h. The suspension was then centrifuged at 4°C as before for 5 min and the supernatant collected as the nuclear fraction.
Antibodies used
Rabbit polyclonal antibodies against β-actin, AR, cyclin A, cyclin B and cyclin D1 were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antibodies against Akt, GAPDH and α-Tubulin, along with rabbit polyclonal antibodies against Caspase 3, Lamin A, PARP and phospho- Filamin A (S2152) were from Cell Signaling (Beverly, MA). Mouse monoclonal anti-Filamin A antibody (C-terminal) was from Millipore (Billerica, MA), rabbit polyclonal anti-Filamin A antibody (C-terminal) (for immunofluorescence) was from Abcam (Cambridge, MA), and rabbit polyclonal anti-Filamin A (N-terminal) was from Santa Cruz Biotechnology, Santa Cruz, CA.
RNA inhibition
Anti-FlnA siRNA duplex with the following target sequence was purchased from Dharmacon Research Inc., Lafayette, CO: 5′-CAACGTTGGTAGTCATTGT-3′. A pool of 4 duplexes sold as AR siRNA (Santa Cruz Biotechnology, Santa Cruz, CA) with the following sequences: Strand #1: 5′-CAGUCCCACUUGUGUCAAATT-3′, Strand #2: 5′- CUGAUCUGUGGAGAUGAATT-3′, Strand #3: 5′-GUCGUCUUCGGAAAUGUUATT-3′ and Strand #4: 5′-GACAGUGUCACACAUUGAATT-3′. Control was a pool of 4 scrambled non-specific siRNA duplex (siCONTROL Non-Targeting siRNA Pool, Dharmacon Research, Inc.).
Analysis of cell proliferation or apoptosis using flow cytometry
Cells were grown under desired conditions in 60 mm dishes at 1 × 106 cells/dish. Flow cytometry was conducted on FACSCalibur (Becton Dickinson Immunocytometry Systems, San Jose, CA). Cells were illuminated with 200 mW of 488 nm light produced by an argon-ion laser and 635 nm light produced by a red-diode laser. Fluorescence was read through a 630/22 nm band-pass filter (for propidium iodide) or a 661/16 nm band-pass filter (for Annexin V-Alexa Fluor 647). Data was collected on 20,000 cells as determined by forward and right angle light scatter and stored as frequency histograms; data used for cell cycle analysis was further analyzed using MODFIT (Verity software, Topsham, ME).
MTT Assay
Cells were cultured in 24-well plates and treated as indicated. Following treatment, each well was incubated with 25 μl of 5 mg/ml 3-[4,5-Dimethylthiazol-2yl]-2,5-diphenyl-tetrazolium bromide (MTT) (Sigma-Aldrich, St. Louis, MO) for 1 hour in a 5% CO2 incubator at 37°C, which converted the reactants to formazan in actively dividing cells. Proliferation rates were estimated by colorimetric assay reading formazan intensity in a plate reader at 562 nm.
Western blotting
Whole cell extracts were prepared by lysing cells in 300 μl cell lysis buffer (50 mM Tris HCl, pH 7.4, 150 mM NaCl, and 1% NP-40, and protease inhibitors: 0.1 mM benzamidine, 1 mM phenylmethylsulfonyl fluoride, 10 mg/ml each of phenathroline, leupeptin, aprotinin, and pepstatin A) and phosphatase inhibitors: 20 mM β-glycerol phosphate, 1 mM Na-orthovanadate, and 10 mM NaF. Proteins were quantitated by BCA assay (Pierce, Rockford IL) and fractionated on 29:1 acrylamide-bis SDS-PAGE. Electrophoresis was performed at 150 V for 2 h using minivertical electrophoresis cells (Mini-PROTEAN 3 Electrophoresis Cell, Bio-Rad, Hercules, CA). The gels were electroblotted for 2 h at 200 mA using Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad) onto 0.2-μm polyvinylidene difluoride membrane (Osmonics, Westborough, MA). The blots were stained overnight with primary antibodies at 4°C and detected by enhanced chemiluminescence (Pierce) following incubation with a peroxidase-labeled secondary antibody (Donkey anti-mouse IgG or Goat anti-rabbit IgG, Fc specific, Jackson Immunoresearch, West Grove, PA).
Immunofluorescence
Cells were rinsed with PBST (Phosphate Buffered Saline with 0.05% Tween-20) and fixed with ice cold methanol for 15 min at room temperature. Fixed cells were washed three times with PBST and blocked with 10% BSA for 30 min at room temperature. Primary antibody, prepared in 1% BSA, was applied to the cells, which were incubated at 4°C overnight. Cells were then washed three times with PBST and FITC conjugated anti-rabbit secondary antibody (Jackson Immuno Research), diluted 1:1000 in 1% BSA, was added and incubated for 30 min at room temperature in the dark. After washing three times with PBST, slow fade mounting medium with DAPI (Invitrogen, Grand Island, NY) was applied to the slides prior to mounting of the cover slips.
Quantitative Real-time PCR
Total cellular RNA was prepared from cells utilizing RNeasy® mini kit (Qiagen Inc. CA) based on manufacturer's protocol. cDNA was synthesized from 1μg RNA using QuantiTect® reverse transcription kit based on manufacturer's protocol. cDNAs were diluted 1:4 in ddH2O and 2μl of diluted cDNA was added to 5μl of EXPRESS SYBR GreenER qPCR SuperMix (Invitrogen, Grand Island, NY) and 200 nM of each primer. GAPDH was used as the endogenous expression standard. PCR conditions were: 20 sec initial denaturation step at 95°C, 40 cycles at 95°C for 3 sec, 60°C for 30 sec, followed by additional 95 cycles starting at 60°C with 0.5°C increase e per cycle for melt curve analysis. GAPDH forward: 5′-TGCACCACCAACTGCTTA and reverse 5′- AGAGGCAGGGATGATGTTC. Filamin A forward 5′-AAGTGACCGCCAATAACGAC and reverse 5′-GGCGTCACCCTGTGACTTAT.
Evaluation of mouse retrovirus
Cell lysates were prepared using RIPA Buffer containing protease and phosphatase inhibitors and normalized to 2 ug/ul using BCA Protein Assay (Pierce). 50 ug of lysate was loaded per lane, run on a 12% SDS PAGE gel and transferred using semi dry transfer unit (Bio-Rad) at 15 V for 70 minutes. Gag antibody (1:250) and Beta Actin antibody (1:10000, Sigma A-1978) were diluted in 5% Milk Protein TBS Tween and incubated overnight with rocking at 4C. Membranes were washed 4×TBS Tween for 10 mins at room temperature and then incubated with goat anti mouse secondary (1:5000, Santa Cruz SC-2005). Membranes were washed again 4×TBS Tween for 10 mins at room temperature and incubated for 1 minute in SuperSignal West Pico Chemiluminescent Substrate (Pierce 34080). Membranes were drained of excess fluid and images were taken using FluorChem E Imager and analyzed using AlphaView SA software (Protein Simple).
Mouse xenograft studies
4- to 5-week-old athymic nude male mice (n=24, Harlan, Indianapolis, IN) were implanted with 12.5 mg, 90-day release testosterone pellets (Innovative Research of America, Sarasota, FL) prior to injection of CWR22 prostate tumor suspension subcutaneously in the right flank. These experiments were conducted under an IACUC approved protocol. Cells were mixed 1:1 with 50% Matrigel (BD Biosciences) prior to subcutaneous injection (2.5×106 cells per injection). When palpable tumors were observed, their pellets were removed, and the animals were left untreated (n=6) or treated with vehicle (n=8) or GCP (1.2mg/kg/day) (n=10) as a suspension in water dispersed in peanut oil by esophageal gavage for 14 consecutive days, after which drug treatment was discontinued. Control animals were given water droplets dispersed in peanut oil only (n=8). Uniformity of the suspension was maintained by vigorous shaking prior to gavage for each animal. 21 days after start of experiment, the animals were castrated (n=22) and or left intact (sham operated, n=2). Sham operated mice were opened up but then closed again without castration. 2 mice from each group (sham castrated, castrated-untreated, castrated-vehicle treated, castrated-GCP treated) were sacrificed 3 days post-castration for molecular analysis. 4 others were sacrificed due to large tumor size. The remaining 12 mice (6 on GCP and 6 on vehicle) were used as described in results. Tumor dimensions were measured twice a week using calipers. At the end of the study, tumor bearing mice were euthanized using CO2 gas followed by cervical dislocation.
Immunohistochemistry
Mouse tumors were fixed in 10% buffered formalin (Medical Industries, Richmond IL) at room temperature. The tumor was paraffin-embedded and processed based on established protocols. Paraffin-embedded cell blocks were then sectioned and sections were heated to 60°C, cleared and rehydrated in xylene and graded alcohols. Antigen retrieval was performed with 10 mM citrate buffer at pH 6.0 for 10 minutes in a pressure chamber at 121°C and 10 more minutes without pressure. Slides were allowed to cool for another 20 minutes, followed by sequential rinsing in TBS-T (50 mM Tris HCl, pH 7.4, 150 mM NaCl, Tween-20 (0.1%)). Endogenous peroxidase activity was quenched by incubation in TBS-T containing 3% hydrogen peroxide. Slides were then blocked with 10% BSA. Each incubation step was carried out at room temperature and was followed by three sequential washes (5 minutes each) in TBS-T. Sections were incubated in mouse monoclonal anti-Filamin A antibody against the C-terminal (Millipore, Billerica, MA), diluted in TBS-T containing 1% BSA (1 hour), followed by incubations with biotinylated secondary antibody for 15 minutes, peroxidase-labelled streptavidin for 15 minutes (LSAB-2 Dako Corp, Carpenteria, CA) and diaminobenzidine and hydrogen peroxide chromogen substrate (Dako Corp., Carpenteria, CA). Slides were counter-stained with hematoxylin and mounted. Negative controls were incubated with Universal Mouse Negative Control (DAKO Corp., Carpenteria, CA) in place of primary antibody.
Statistical Analysis
Tumor volumes were calculated as V= length × height × π/6 .The change in tumor volume was calculated as percentage of the volume of the tumor at the time of castration. Analyses using an unpaired, two-tailed Student's t-test were used to compare tumor sizes of the two study groups: untreated vs GCP-treated - p < 0.05 was considered statistically significant. All in vitro experiments using MTT or luciferase data were performed in triplicate. Data is presented as relative luciferase activity using means of untreated controls as standards, normalized to the corresponding reading for β-gal.
Results
Nuclear localization of a 90 KDa FlnA fragment induces apoptosis in prostate cancer cells
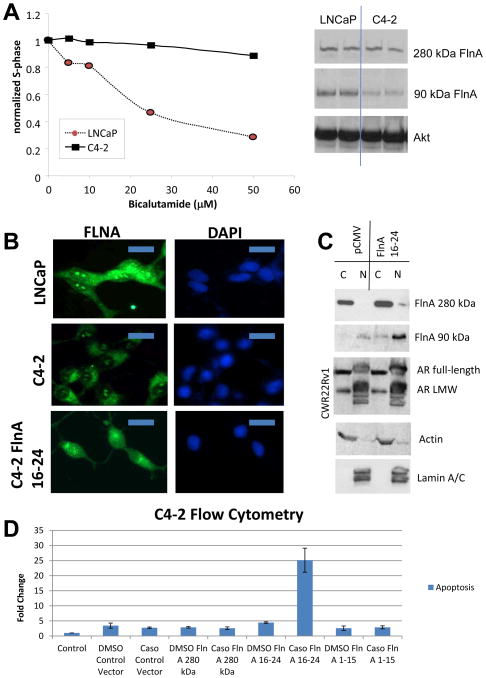
We previously demonstrated that androgen-sensitive and –insensitive cell lines differentially expressed nuclear localization of Filamin A (FlnA) a structural protein (Bedolla et al. 2009; Wang et al. 2007). A castrate resistant subline of LNCaP cells, C4-2, has been developed from tumors obtained by co-implantation of LNCaP cells with bone stromal cells in castrated nude mice (Thalmann, et al. 1994). We previously showed that C4-2 cells express very high AR transcriptional activity compared to LNCaP cells (Ghosh, et al. 2005). Unlike LNCaP, C4-2 cells were not growth arrested by the anti-androgen bicalutamide (Casodex) (Figure 1A, left), a competitive inhibitor of AR ligand binding (Masiello, et al. 2002). C4-2 cells expressed lower levels of 90kDa FlnA compared to LNCaP(Figure 1A, right), and lower levels of FlnA in the nucleoplasm (Figure 1B), but not in the nucleolus. Transfection of a plasmid expressing C-terminal FlnA (FlnA 16-24), which resulted in the appearance of the 90 kDa FlnA fragment (Loy et al. 2003; Wang et al. 2007), restored nuclear expression of FlnA in C4-2 cells (Figure 1B). This effect required the presence of the androgen receptor (AR) because it was not observed in AR null PC-3 cells (Wang et al. 2007), but in C4-2 cells expressing full-length AR as well as CWR22Rv1 cells expressing a truncated form of AR (Figure 1C). Transfection of FlnA 16-24, but not full-length FlnA or FlnA 1-15 (N-terminal) induced apoptosis in cells treated with the AR antagonist bicalutamide (Figure 1D). Taken together, these results demonstrate that nuclear localization of the 90 kDa fragment of FlnA induces apoptosis in CaP cells.
Figure 1. Nuclear localization of a 90 kDa FlnA fragment induces apoptosis in prostate cancer cells.
(A) (left) LNCaP and C4-2 cells were treated with increasing doses of the anti-androgen bicalutamide for 48 hrs and the percentage of cells in S-phase was analyzed by flow cytometry in PI-stained cells. (right) Untreated LNCaP and C4-2 cells. Western blotting revealed that both cell lines expressed equal levels of 280 kDa FlnA, but LNCaP cells expressed higher levels of 90 kDa FlnA compared to C4-2. (B) LNCaP, C4-2 and C4-2 cells stably transfected with FlnA 16-24 (C4-2 16-24) were stained by immunofluorescence for FlnA with an antibody specific to the C-terminus of FlnA. Immunofluorescent staining shows nuclear localization of 90kDa FlnA in LNCaP cells whereas in C4-2 cells FlnA was mainly localized to the cytoplasm but not in the nucleoplasm, although bright staining in the nucleolus was also detected. In contrast, C4-2 16-24 cells expressed this protein equally in the cytoplasm and the nucleus, while nucleolar staining could still be detected. Scale bar: 10 μm. (C) Subcellular fractionation of CWR22Rv1 cells transfected with 1 μg/ml cDNA expressing FlnA 16-24 showing that the 16-24 fragment goes into the nucleus. Some full length FlnA enters the nucleus when transfected with FlnA 16-24 due to nucleolar localization. (D) Flow cytometric analysis in PI-stained, ethanol fixed C4-2 cells to determine the % cells undergoing apoptosis. Apoptosis was considered to be the cells staining with Annexin V, and showed that transfection with FlnA 16-24 induced apoptosis in C4-2 cells.
Treatment with genistein combined polysaccharide (GCP) replicates the effects of FlnA nuclear localization
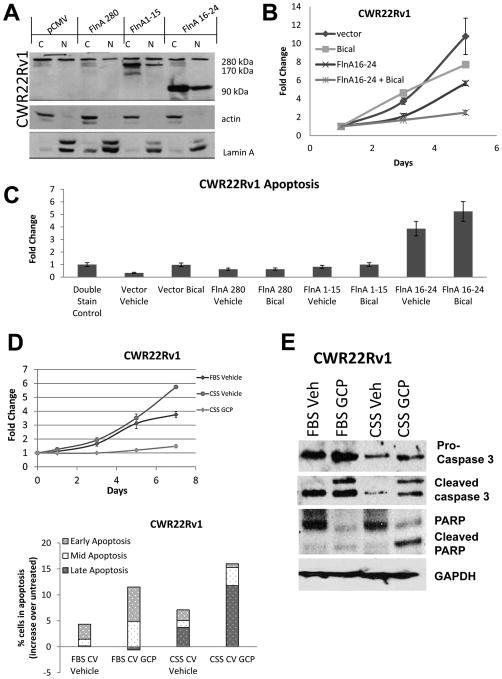
The above results indicated that restoration of FlnA nuclear localization likely restored hormone-sensitive behavior in CaP. Therefore, pharmaceutical approaches to FlnA cleavage were pursued. Castration resistant CWR22Rv1 cells derived from a relapsed CWR22 tumor were chosen for these studies because they express multiple forms of AR – a 114 kDa full-length form containing a H874Y point mutation and an in-frame tandem duplication of exon 3; as well as truncated AR species lacking the ligand binding domain (LBD) (Tepper, et al. 2002).– hence treatment that promotes hormone sensitivity in these cells would be widely applicable. Transfection of FlnA 16-24, but not full-length FlnA or FlnA 1-15, promoted expression of the 90 kDa fragment both in the nucleus and the cytoplasm (Figure 2A), sensitized these cells to bicalutamide (Figure 2B), and induced apoptosis (Figure 2C).
Figure 2. Treatment with genistein combined polysaccharide (GCP) replicates the effects of FlnA nuclear localization.
(A) Expression of the transfected proteins when CWR22Rv1 cells were transiently transfected for 48 hours with 2 μg/ml of an empty vector, full-length FlnA (280 kDa), FlnA 1-15 (170 kDa) or FlnA 16-24 (90 kDa). Actin and Lamin A were used as markers of the cytoplasm and the nucleus, respectively. (B) As estimated by MTT assay, neither 10 μM bicalutamide nor FlnA 16-24 individually had significant effects on the growth of CWR22Rv1 cells, but in combination, they prevented cell growth. Each point on the graph represents mean ± S.D. of 3 independent readings. (C) CWR22Rv1 cells underwent apoptosis when transfected with FlnA 16-24 but not when transfected with an empty vector, FlnA 280 or FlnA 1-15 as determined by flow cytometry after 48 hours in Annexin V and propidium iodide stained cells. (D) (upper) MTT assay showing effect of CWR22Rv1 cells with GCP or vehicle in FBS and CSS containing medium. Each point on the graph represents mean ± S.D. of 3 independent readings. (lower) Analysis of apoptosis by flow cytometry in propidium iodide and Annexin V stained cells demonstrating that CWR22Rv1 cells treated with 100 μg/ml GCP for 48 hours experienced more apoptosis than vehicle treated cells. Early apoptosis: Annexin V staining, Mid-apoptosis: Annexin V+PI staining, Late apoptosis: PI staining. Results presented represent numbers after subtraction from untreated cells. Note the increase in apoptosis in cells cultured in CSS vs FBS containing medium. (E) Western blots demonstrating that 100 μg/ml GCP after 48 hrs increased apoptosis as demonstrated by cleaved Caspase 3 and PARP levels.
The physiological range of testosterone (T) in FBS is 55.1 – 97.5pM whereas that in CSS is 15.6 – 19.0pM (Sedelaar and Isaacs 2009). Hence, culture in CSS result in ADT in vitro. GCP sensitized CWR22Rv1 cells to ADT, resulting in growth arrest and apoptosis (Figure 2D), which was enhanced in low androgen levels. This was accompanied by increases in caspase 3 and Poly (ADP-ribose) polymerase (PARP) cleavage, indicative of apoptosis, especially in low-androgen medium (Figure 2E). These results demonstrated that the effect of GCP was very similar to that induced by FlnA16-24.
FlnA cleavage to the 90 kDa fragment mediates GCP-induced apoptosis
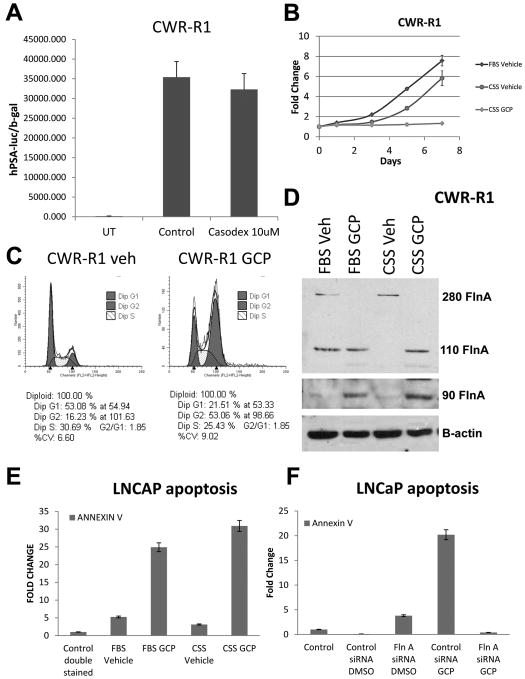
We previously showed that the AR activated similar pathways in CWR22Rv1 and CWR-R1, two cell lines established from two independent relapsed CWR22 tumors (Chen, et al. 2010a). Similar to CWR22Rv1 (Tepper et al. 2002), CWR-R1 cells were refractory to hormonal treatment (Figure 3A); significantly, these cells were also sensitized to cytostatic effects of GCP in low-androgen media (Figure 3B). Investigation of the mechanism of this effect indicated that GCP induced G2/M arrest in prostate cancer cells (Figure 3C), similar to its main component genistein as has been demonstrated by other laboratories in various cell lines (Han, et al. 2010; Lian, et al. 1998; Schmidt, et al. 2008; Zhao, et al. 2009). Further, GCP suppressed the levels of 280 kDa FlnA while causing an increase in the levels of the cleaved FlnA levels (110 kDa and 90 kDa) (Figure 3D) thereby indicating that GCP causes FlnA cleavage.
Figure 3. Treatment with GCP induces FlnA cleavage to the 90 kDa fragment which mediates GCP-induced apoptosis.
(A) Luciferase assay to determine AR transcriptional activity on a PSA promoter to determine the effect of 10 μM bicalutamide or vehicle in CWR-R1 cells. Cells were cultured in FBS and collected after 48 hrs. Each point on the graph represents mean ± S.D. of 3 independent readings. (B) MTT assay showing effect of CWR-R1 cells with GCP or vehicle in FBS and CSS containing medium. Each point on the graph represents mean ± S.D. of 3 independent readings. (C) Cell cycle analysis by flow cytometry in PI stained, ethanol-fixed cells demonstrating that CWR-R1 cells treated with 100 μg/ml GCP for 48 hrs undergo G2/M arrest. (D) Immunoblots to determine levels of various FlnA fragments in CWR-R1 cells cultured in FBS vs. CSS and treated with either vehicle or 100 μg/ml GCP for 48 hrs. An antibody to the C-terminal end of FlnA which recognizes the 280, 110 and 90 kDa bands was used in this study. (E) Apoptosis in LNCaP cells determined by flow cytometry in Annexin V and PI-stained in medium containing FBS or CSS in the presence of vehicle or 100 μg/ml GCP for 48 hrs. (F) Role of FlnA in GCP-induced apoptosis. LNCaP cells were subjected to control or FlnA siRNA and treated with vehicle or 100 μg/ml GCP for 48 hrs. Apoptosis was determined by flow cytometry in cells stained with propidium iodide and Annexin V.
CWR-R1 and CWR22Rv1 lines had been propagated in mice and were contaminated by mouse retrovirus (Paprotka, et al. 2011). On the other hand, LNCaP cells were free from this virus (Sfanos, et al. 2011). Similar to CWR22Rv1, GCP induced apoptosis in LNCaP cells, and this effect was more prominent in CSS-cultured cells (Figure 3E). Therefore, we investigated whether GCP-induced apoptosis was mediated by FlnA. Significantly, FlnA siRNA, but not control siRNA, prevented GCP-induced apoptosis in LNCaP cells (Figure 3F). These results show that GCP induced apoptosis is enhanced with ADT and is mediated by FlnA.
GCP's effects reflect a combination of both genistein and daidzein
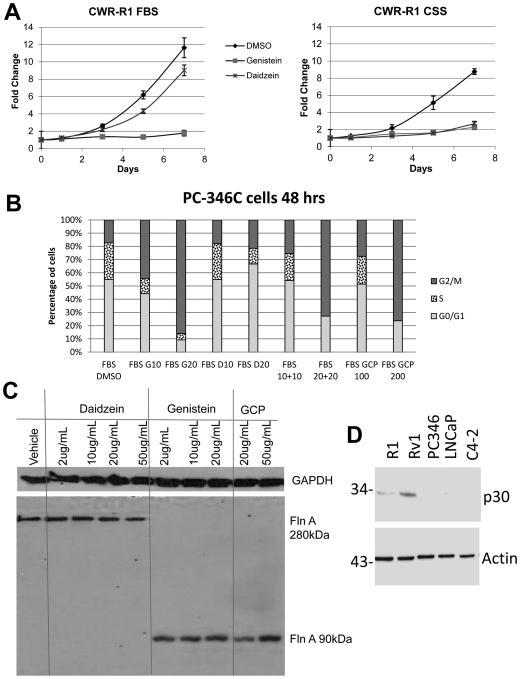
Next, we investigated which components of GCP are responsible for its effects. In human patients, the serum levels of genistein and daidzein were determined to be 43 and 51 μmol/L respectively (about 10 μg/ml each in an average adult male), following 6 months of GCP intake at 5g/day (deVere White et al. 2010). At comparable doses, daidzein reduced proliferation in the absence, rather than the presence, of androgens (22.4% decrease in FBS, p=0.039, vs. 69.5% decrease in CSS, p<0.0001) whereas the effect of genistein remained virtually unchanged in FBS (84.68% decrease, p=0.002) vs CSS (74% decrease, p<0.0001) (Figure 4A). We verified these results in androgen sensitive PC-346C CaP cells (Figure 4B) (Limpens, et al. 2006; van Weerden, et al. 1996) that did not express the mouse retrovirus (Figure 4D). Genistein induced G2 arrest in PC-346C cells, while daidzein growth arrested them at G1 (Figure 4B), while GCP resulted in growth arrest in both G1 and G2, with very few cells in S-phase. Significantly, genistein, but not daidzein, restored the levels of the 90 kDa FlnA fragment (Figure 4C). Therefore, genistein and daidzein are both needed to replicate the effects of GCP.
Figure 4. A combination of genistein and daidzein are required for rendering the effects of GCP.
(A) MTT assay showing the effect of 10 μg/ml genistein or daidezein on CWR-R1 cells grown in either FBS or CSS containing medium. Each point on the graph represents mean ± S.D. of 3 independent readings. (B) Cell cycle analysis by flow cytometry in PI-stained, ethanol-fixed PC-346C cells demonstrating that treatment for 48 hours with GCP and genistein undergo G2/M arrest while cells treated with Daidzein do not. Note that the combination of daidzein (10 μg/ml) and genistein (10 μg/ml) is equivalent in effect to 100 μg/ml GCP, whereas the combination of daidzein (20 μg/ml) and genistein (20 μg/ml) is equivalent in effect to 200 μg/ml GCP. (C) Western blots demonstrating that 48 hour treatment with genistein and GCP, but not daidzein, increases expression of the 90kDa FlnA fragment in C4-2 cells. An antibody to the C-terminal end of FlnA which recognizes the 280, 110 and 90 kDa bands was used in this study. (D) To ensure that the cell lines we used (other than CWR22Rv1 and CWR22R1) do not carry murine retroviruses, we used a Gag antibody (Paprotka et al. 2011) which detect all murine retroviral gag proteins, thus offering a highly sensitive way of detecting viral infection. The cell lysates from the five cell lines were western blotted with Gag and β-actin antibody. As expected, CWR22Rv1 and R1 express murine retroviral gag proteins, whereas others do not.
Castration-sensitive LNCaP cells exhibited greater sensitivity to GCP+ADT compared to CRPC C4-2 cells
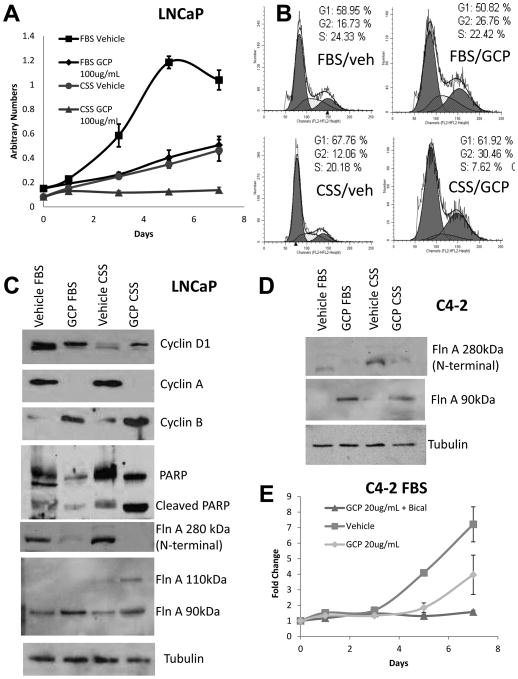
LNCaP cells retain the ability to convert T in FBS to effective levels of dihydrotestosterone (DHT), a stronger ligand for AR, whereas they are unable to do so in CSS. Culture in CSS induced 55% reduction in LNCaP cell growth (p=0.0014) compared to culture in FBS. GCP treatment in FBS also reduced growth by 51% compared to vehicle (p=0.0006); however, the combination of ADT and GCP decreased growth by 87% (p=0.0002) (Figure 5A), demonstrating greater efficacy of GCP in lower levels of androgens. Flow cytometry showed that GCP induced G2 arrest in LNCaP cells in FBS, whereas in CSS, GCP also depleted cells in S-phase (Figure 5B). Western blotting of lysates collected from GCP- and vehicle-treated cells in FBS and CSS showed that GCP treatment decreased cyclin D1 levels, completely suppressed cyclin A levels, but increased cyclin B levels, thereby supporting a G2 arrest (Figure 5C). Further, GCP induced cleavage of PARP, especially in the presence of CSS, indicating the induction of apoptosis (Figure 5C). Western blotting showed that GCP promoted the expression of the 90 kDa fragment of FlnA in LNCaP cells as well, both in FBS and CSS, similar to our observation in CWR22Rv1 (Figure 5C).
Figure 5. GCP treatment sensitized castration-sensitive LNCaP cells to androgen withdrawal induced apoptosis by increasing levels of 90 kDa FlnA.
(A) MTT assay in LNCaP cells showing that GCP reduced cell numbers in low-androgen media. Cells were plated in medium containing either FBS (high-androgen) or CSS (low-androgen) in the presence of vehicle (50% DMSO, 50% ethanol) or 100 μg/ml GCP for up to 7 days. Each point on the graph represents mean ± S.D. of 3 independent readings. (B) Cell cycle analysis by flow cytometry in propidium iodide (PI) stained ethanol-fixed cells demonstrating that 100 μg/ml GCP induced cell cycle arrest in LNCaP cells cultured for 48 hrs in medium with CSS, but not FBS. In the presence of FBS, GCP induced an increase in cells in G2, only, while in CSS additionally, there was a lack of cells in S-phase. (C, D) Immunoblots to determine levels of various proteins in (C) LNCaP and (D) C4-2 cells cultured in FBS vs CSS in vehicle or 100 μg/ml GCP for 48 hrs. Note that an antibody to the N-terminal end of FlnA was used to determine the 280 kDa band while an antibody to the C-terminal end of FlnA was used to determine the 110 and 90 kDa bands. (E) MTT assay showing growth arrest in C4-2 cells treated with 10 μM bicalutamide and 20 μg/ml GCP despite the continued growth in GCP alone. Each point on the graph represents mean ± S.D. of 3 independent readings.
C4-2 cells do not express inherent levels of the 90 kDa fragment, yet GCP induced expression of the 90 kDa fragment in these cells as well (Figure 5D). Culture in CSS did not affect the ability of these cells to grow, despite the addition of 100 μg/ml GCP (not shown), likely due to aberrant DHT production in C4-2 cells even from castrate levels of T (Cai, et al. 2011). In contrast, bicalutamide, a competitive inhibitor of AR ligand binding, induced growth arrest even in the presence of 20 μg/ml GCP in C4-2 cells (Figure 5E). The differential effects in FBS and CSS in LNCaP cells, and CSS vs bicalutamide in C4-2, indicate that GCP is ineffective in the presence of ligands, but in the absence of ligands (with bicalutamide) is able to overcome castrate resistant AR activation.
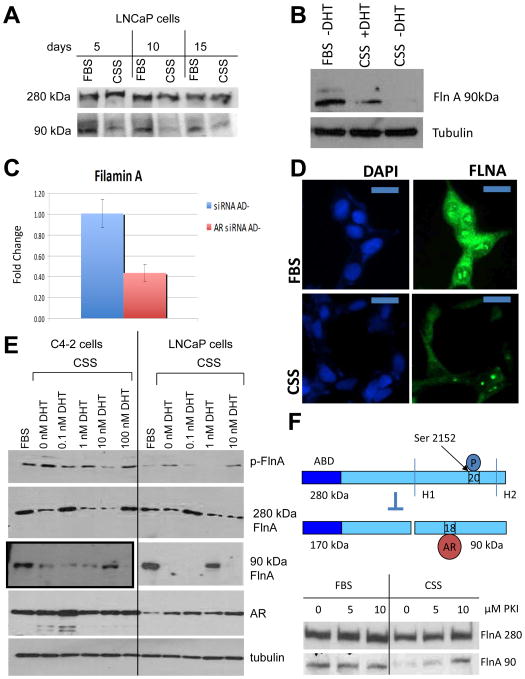
Androgen deprivation phosphorylates FlnA and prevents its cleavage to the 90 kDa fragment
Next, we investigated the molecular mechanism of the process by which GCP sensitized CaP cells to ADT. Prolonged culture in CSS reduced the levels of 90 kDa FlnA (Figure 6A), whereas supplementation of cells in CSS with 1 nM DHT restored its levels (Figure 6B). These results indicated that cleavage of full-length FlnA to the 90 kDa fragment is androgen regulated, consistent with the increase in 280 kDa FlnA with CSS seen in Figures 3D, 5C,D. This increase was not related to transcriptional control; levels of FlnA mRNA were decreased by AR siRNA, but not control siRNA (Figure 6C), implicating the increase in 280 kDa FlnA levels to post-transcriptional modifications. Interestingly, FlnA levels decreased both from the nucleoplasm and the cytoplasm when LNCaP cells were cultured in CSS for prolonged periods (Figure 6D), but in the nucleolus remained unchanged. Further studies are required to explain how nucleolar FlnA is protected.
Figure 6. ADT prevents Filamin A cleavage to the 90 kDa fragment and phosphorylates FlnA at Ser 2152.
(A) LNCaP cells were cultured for up to 15 days in FBS or CSS containing medium, and the levels of 90 and 280 kDa FlnA were determined by western blotting. (B) Western blot showing decreased expression of the 90 kDa FlnA fragment in LNCaP cells in CSS vs FBS, whereas its levels were restored by the addition of 1 nM DHT. (C) FlnA expression in CWR22Rv1 cells is regulated by the AR. AR siRNA decreased FlnA mRNA expression by ∼60% as determined by qPCR in medium with CSS (AD-). (D) LNCaP cells were grown in either FBS or CSS containing medium, and FlnA protein expression was determined by immunofluorescence. Scale bar: 10 μm. Note that cells grown in FBS containing medium show higher expression of FlnA compared to cells grown in CSS containing medium. (E) LNCaP and C4-2 cells were cultured in FBS or CSS with increasing levels of DHT as indicated for 48 hrs. Western blots demonstrate the levels of expression of various proteins. Note that C4-2 cells actually have much lower levels of FlnA 90 kDa, therefore the blot shown here for C4-2 was at a higher exposure. (F) (Upper) Scheme showing FlnA cleavage at H1 is regulated by phosphorylation at Ser 2152. When FlnA is phosphorylated, it does not undergo cleavage and remains in the cytoplasm, whereas upon dephosphorylation, FlnA is cleaved to the 90 kDa fragment which translocates to the nucleus. (Lower) LNCaP cells were cultured in FBS or CSS with increasing levels of the PKA inhibitor PKI 14-22 (PKI) for 48 hrs. Western blotting demonstrated that culture in CSS depleted levels of the 90 kDa FlnA, but treatment with increasing doses of PKA inhibitor in CSS containing medium restored its expression.
C4-2 cells expressed higher levels of FlnA phosphorylation at Ser2152 compared to LNCaP (Figure 6E). In both cell lines, culture in CSS stimulated FlnA phosphorylation, but treatment with low doses, but not high doses, of DHT prevented ADT-induced FlnA phosphorylation (Figure 6E). These results are consistent with the observation that low doses of DHT stimulate whereas high doses inhibit cell growth (Hofman, et al. 2001). Loss of 90 kDa FlnA coincided with an increase in FlnA phosphorylation or with an increase in 280 kDa FlnA, indicating that the level of 90 kDa FlnA results from cleavage of the 280 kDa form or is negatively regulated by FlnA phosphorylation. As a result, FlnA cleavage to the 90 kDa fragment was also regulated by androgens (Figure 6E).
Additionally, we verified that FlnA phosphorylation regulates its cleavage, using a regulator of Protein kinase A (PKA), known to phosphorylate FlnA (Jay, et al. 2004; Jay, et al. 2000). Since we previously showed that a PKA inhibitor increased FlnA cleavage and nuclear translocation (Bedolla et al. 2009), we treated LNCaP cells cultured in FBS or CSS with increasing concentrations of the PKA inhibitor 14-22 (PKI). Significantly, the PKI had no effect in FBS, but in CSS medium, loss of expression of the 90 kDa fragment was prevented by treatment with the PKI (Figure 6F). Taken together, the above data demonstrates that ADT suppresses cleavage to 90 kDa FlnA by enhancing phosphorylation at Ser2152.
GCP dephosphorylates FlnA at ser 2152 and promotes the formation of the 90 kDa fragment and FlnA nuclear localization
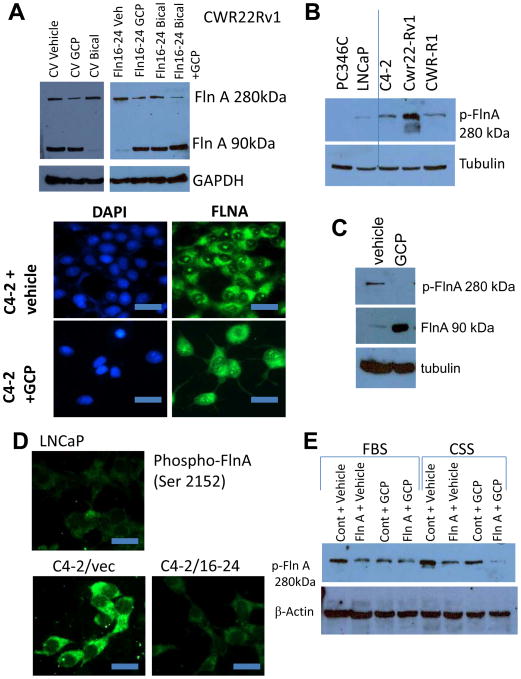
In support of data showing that ADT prevents FlnA cleavage to the 90 kDa fragment, 48 hrs treatment with bicalutamide depleted CWR22Rv1 cells of the 90 kDa FlnA fragment while the level of full-length FlnA increased. In contrast, GCP increased the levels of 90 kDa FlnA while reducing the levels of the full-length protein (Figure 7A, upper). Comparison of LNCaP and C4-2 cells with vehicle or GCP showed that GCP treatment in C4-2 cells restored FlnA localization to the nucleus (Figure 7A, lower). These results prompted us to examine the role of GCP on FlnA phosphorylation.
Figure 7. GCP prevents phosphorylation of full-length FlnA and promotes cleavage of FlnA to its 90 kDa fragment.
(A) (Upper) Western blot showing expression of FlnA in CWR22Rv1 cells. Treatment with 10 μM bicalutamide depleted levels of 90 kDa FlnA while transfection with FlnA 16-24 and further treatment with 100 μg/ml GCP for 48 hrs rescued the expression of the 90 kDa FlnA fragment. Note higher exposure of left panel compared to right. (Lower) LNCaP and C4-2 cells treated with vehicle or 100 μg/ml GCP were stained with an antibody specific to the C-terminus FlnA to determine subcellular localization. LNCaP cells and C4-2 cells treated with GCP showed FlnA expression in the nucleus while C4-2 cells treated with vehicle showed expression only in the cytosol. Scale bar: 10 μm. (B) Western blot comparing levels of phosphorylated full-length FlnA (Ser 2152) in various cell lines. (C) Western blot demonstrating that GCP prevented phosphorylation of full-length FlnA and increased expression of 90 kDa FlnA. (D) LNCaP cells, and C4-2 cells transfected with vector or FlnA 16-24 were stained with an antibody specific for phosphorylated FlnA (Ser 2152) and FlnA phosphorylation levels determined by immunofluorescence. Scale bar: 10 μm. (E) LNCaP Cells were cultured in medium containing either FBS or CSS and treated as indicated. Growth in CSS containing medium increased the phosphorylation of full-length FlnA while treatment with GCP reduced full-length FlnA phosphorylation.
Androgen-dependent PC-346C and LNCaP cells expressed lower levels of phospho-FlnA, compared to the CRPC lines C4-2, CWR22Rv1 and CWR-R1 (Figure 7B). Hence we investigated the effect of GCP in CRPC cells. GCP treatment of C4-2 cells prevented FlnA phosphorylation and cleaved the protein to the 90 kDa fragment (Figure 7C). In support, by immunofluorescence, C4-2 cells expressed higher levels of phospho-FlnA (Ser 2152) compared to LNCaP (Figure 7D). Incidentally, phosphorylated FlnA was localized in the cytoplasm; showing that FlnA phosphorylation prevented its nuclear translocation. Transfection with FlnA 16-24 in C4-2 cells prevented its phosphorylation, in support for a role of its effector GCP in suppressing FlnA phosphorylation. Culture in CSS increased the levels of FlnA phosphorylation at Ser 2152, which was suppressed by GCP treatment (Figure 7E). These results show that GCP sensitizes CaP cells to ADT by preventing ADT-induced FlnA phosphorylation.
GCP treatment prevented relapse in the CWR22 xenograft mouse model following ADT
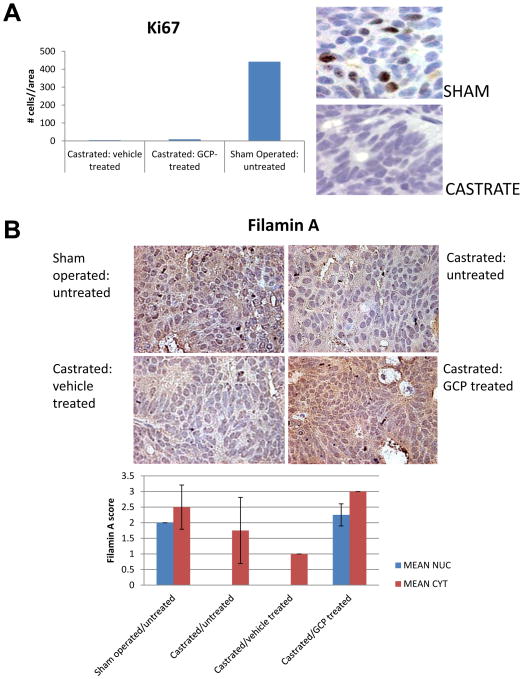
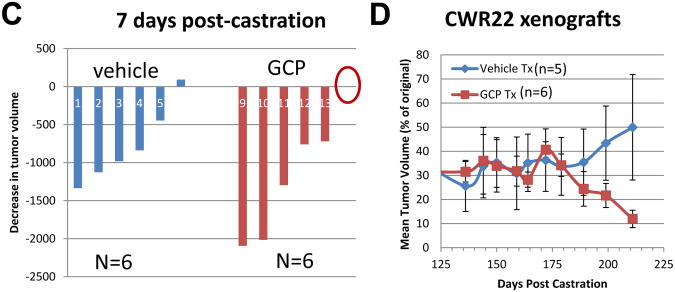
Since GCP sensitized CaP cells to ADT, we investigated whether it impeded development of CRPC following ADT. Athymic nu/nu mice were subcutaneously implanted with CWR22 tumor extracts. When the tumors were palpable, mice were treated with GCP or vehicle as described in Materials and Methods. Following 2 weeks of treatment, the animals underwent sham operation, or were castrated; initial tumors from each group were collected 3 days post-surgery and showed strong Ki67 staining, indicative of proliferation, in sham-operated but not castrated animals (Figure 8A). Sham operated, untreated tumors showed strong nuclear localization of FlnA, while castrated tumors, either untreated, or treated with vehicle only, showed weak cytoplasmic, but no nuclear staining for FlnA (Figure 8B). In contrast, castrated, GCP treated tumors exhibited strong FlnA staining (brown) both in the nucleus and in the cytoplasm (Figure 8B). Within the first 7 days following castration, the tumors in GCP-treated mice regressed (mean reduction 1148 mm3, n=6) compared to vehicle-treated mice (mean reduction 772 mm3, n=6) (Figure 8C). The animals were monitored for up to 7 months (211 days) following castration. During this time, control CWR22 xenografts regressed approximately 38 days post-castration (mean reduction 40%, p=0.0008; n=6), after which the tumors stabilized for several months, and then relapsed after 6 months post-castration (179 days, relapse was defined as two or more consecutive increases in tumor volume >5% each time) (one control animal had to be sacrificed due to large tumor size). There was essentially no significant difference between the control (n=5) and GCP (n=6) groups until 6 months post-castration, after which, tumors in vehicle-treated mice remained steady relapsed (p>0.05), whereas GCP-treated tumors continued to regress (p=0.0056) (Figure 8D). After 211 days (7 months post-castration), tumors in vehicle-treated mice were distinctly larger (mean reduction 50% compared to initial tumor volumes; p=0.25) than the ones in GCP-treated mice (mean reduction 88% compared to initial volumes; p=0.002) (Figure 8D). Taken together, these results indicated that GCP treatment prolonged the effects of castration (ADT) in this model.
Figure 8. GCP treatment prevented relapse in the CWR22 xenograft mouse model.
(A) The tumors were paraffin-embedded, sectioned and stained with anti-Ki67 antibody to determine the effect of GCP and castration on proliferation. Note that tumors in sham-operated animals were positive for Ki67 staining whereas those that were castrated, either in the presence or absence of GCP lacked this staining. Tumors were collected 3 days after the operation. (B) FlnA and AR staining (brown) in tumors from animals who were (i) sham operated, untreated (ii) castrated, untreated (iii) castrated, vehicle treated and (iv) castrated, GCP-treated. Note high FlnA staining in the latter group. (C) Both vehicle and GCP-treated tumors rapidly underwent tumor regression within 7 days following castration. Waterfall plot showing tumor regression in the animals 7-days post castration. Note that GCP-treated tumors underwent greater levels of regression compared to those in vehicle-fed mice. Red circle denotes one tumor that did not undergo significant change in volume. (D) After the initial regression (up to ∼35 days) tumor volume remained essentially steady until 179 days post-castration after which GCP-treated tumors continued to regress while vehicle-treated tumors began to recover, and were distinctly larger than the tumors in GCP-treated mice after 211 days.
Discussion
CaP patients with metastases, treated with ADT, frequently relapse, leading to CRPC, which essentially remains incurable. Studies found that although proliferation indices were consistently suppressed following ADT (Matsushima, et al. 1999; Westin, et al. 1995), apoptosis was only partially affected (Murphy, et al. 1991; Westin et al. 1995). Surviving cells likely undergo growth arrest and lie dormant following ADT, but revive when androgen-independent stimulants release them from growth arrest (Agus, et al. 2002; Agus, et al. 1999). Hence, our goal was to develop treatments that promote apoptosis during ADT in CaP cells. Here we show that proteolysis of FlnA to the 90 kDa fragment and its subsequent nuclear localization induces apoptosis and sensitizes AR-positive CaP cells to ADT.
Full-length FlnA is a well-known mediator of cell migration (Feng and Walsh 2004). FlnA proteolysis has two effects: (1) it prevents cell migration mediated by full-length FlnA scaffolding interaction between actin on its N-terminal end and actin-binding proteins on its C-terminal end (Bedolla et al. 2009), and (2) the C-terminal FlnA in the nucleus suppresses AR activity (Loy et al. 2003). FlnA had no effect in AR-negative PC-3 cells, but inhibited growth of not only LNCaP and C4-2 cells which express a full-length AR with a mutation in its LBD (T877A), but also CWR22Rv1 and CWR-R1 cells which express both full-length and a truncated AR lacking the LBD (Chen et al. 2010a; Chen, et al. 2010b). Hence, FlnA is a likely target that may serve to regulate the ability of CaP cells to respond to androgens, and the objective of these studies was to identify clinically safe pharmaceutical agents that regulate FlnA proteolysis.
We previously showed that FlnA cleavage to the 90 kDa fragment and subsequent nuclear translocation suppresses migration (Bedolla et al. 2009). The repeat structure of FlnA allows it to act as scaffold regulating co-localization of various cell migration regulators such as integrins (D'Addario, et al. 2002; Ott, et al. 1998; Robertson, et al. 2003; Travis, et al. 2004) and migfilin (Das, et al. 2011). Various studies have reported that Filamin A promotes cell migration (Gawecka, et al. 2010; Nagano, et al. 2002), whereas others show that it suppresses migration (Xu, et al. 2010). It is likely that the differential effect is caused by a relative expression of intact vs cleaved FlnA. Surprisingly, a recent study indicated the presence of FlnA in the nucleolus where it inhibits rRNA production (Deng et al. 2012). FlnA expresses a nucleolar localization signal, and the C-terminal end of the molecule (FlnA 16-24) is needed to inhibit rRNA proteins (Deng et al. 2012). Our results reveal that despite the decrease in cytoplasmic and nuclear levels of FlnA with ADT, the expression of nucleolar FlnA remains unchanged, indicating that it is protected from degradation. It is important to note that nucleolar FlnA is not phosphorylated (Figure 7D); hence reflects the cleaved product as well. Further studies are required to determine whether nucleolar FlnA plays a role in AR signaling.
The data presented show that the AR affects both FlnA levels and proteolysis. Androgens promote FlnA cleavage to the 90 kDa fragment; thus, ADT prevents FlnA cleavage and FlnA nuclear (but not nucleolar) localization. Androgens had a biphasic effect on FlnA expression and cleavage. At low androgen levels, there was very little FlnA cleavage whereas at physiologic levels of DHT, FlnA cleavage was resumed, likely caused by decreased FlnA phosphorylation (Ohta and Hartwig 1995). However, at very high levels (100 nM DHT), FlnA was again phosphorylated. Therefore, FlnA likely is cleaved and as a result remains in the nucleus only at physiological levels of androgens and is dispersed when these levels are too high or too low. Loss of 90 kDa FlnA may be one cause for resistance to ADT in advanced CaP.
Interestingly, GCP-induced FlnA dephosphorylation promoted FlnA cleavage and nuclear localization. AR levels regulate FlnA expression, phosphorylation, cleavage and nuclear localization, while FlnA in turn regulates AR transcriptional activity; hence, it is not surprising that the interaction between these factors play a role in the efficacy of ADT. We show that both genistein and daidzein are needed for effective regulation – neither component of GCP, by itself, could achieve this effect. Therefore, GCP-induced G2 arrest is mediated by genistein, while in CSS (but not in FBS), daidzein induced G1 arrest, explaining why GCP caused androgen responsiveness. Other studies had noted combination effects of genistein and daidzein that may contribute to other effects of GCP noted by us. While multiple reports noted that genistein inhibits metastasis in most cancers (Pavese, et al. 2010; Zhang, et al. 2010), including prostate cancer (Lakshman, et al. 2008), some reports noted that genistein promoted metastasis (Nakamura, et al. 2011). Daidzein appears to prevent genistein-induced metastasis in some models (Singh-Gupta, et al. 2010), but not in others (Martinez-Montemayor, et al. 2010). Our data indicate that the effect of dietary isoflavones on tumor promotion and metastasis may be related to the ability of the isoflavones to induce FlnA proteolysis.
The kinase mediating FlnA phosphorylation has not yet been identified. The Ser 2152 site on FlnA is a substrate for p90RSK (Woo, et al. 2004), PKA (Jay et al. 2004; Jay et al. 2000) and PKCα (Tigges, et al. 2003), which are likely candidates. We previously showed that ADT causes the upregulation of ErbB3, a member of the EGFR family (Chen, et al. 2011; Chen, et al. 2010c). This family stimulates not only PI3K, but also the MAPK family, including JNK, which in turn activates p90RSK (Zhang, et al. 2001). On the other hand, p90RSK is inhibited by genistein (Gwin, et al. 2011), and by daidzein (Kang, et al. 2007). Genistein also inhibits EGFR (Aggarwal and Shishodia 2006) and mTOR (Anastasius, et al. 2009) and members of our group had demonstrated earlier that mTOR is one of the primary targets of GCP (Tepper et al. 2007). Previous studies have indicated that MAPK regulates mTOR activity via RSK (Carriere, et al. 2008; Roux, et al. 2007), which may indicate a prominent role for this kinase in GCP-mediated FlnA proteolysis.
Several lines of evidence indicate that GCP-induced FlnA cleavage prevents ligand-independent AR transcriptional activity. First of all, GCP inhibited growth of androgen-dependent LNCaP cells in CSS, but had no effect on C4-2 cells in CSS, whereas in the presence of bicalutamide, GCP induced growth arrest in C4-2 cells. Upon ligand binding, the AR undergoes a conformational change that allows it to enter the nucleus and induce transcriptional activity (Kuil, et al. 1995). In some CRPC cells, the AR can be activated by non-specific ligands which allow the cells to propagate in CSS, and also by ligand-independent AR conformational change, which induces resistance to anti-androgens such as bicalutamide (Feldman and Feldman 2001). Hence, GCP was able to prevent ligand-independent AR activity, and overcome the effects of bicalutamide, but was ineffective when aberrant ligands activated the AR. Secondly, GCP and FlnA 16-24 induced growth arrest in CWR22Rv1 cells expressing a truncated AR lacking the LBD. Our data indicate that this effect can be prevented by the presence of nuclear FlnA but further studies are needed to elucidate the mechanism by which FlnA promotes this effect. In this respect, FlnA is distinct from other AR co-repressors such as NCoR and SMRT and likely explains results showing the inability of the latter to inhibit AR activity in CRPC lines (Laschak, et al. 2011).
Finally, we show that in the CWR22 androgen-dependent xenograft model, relapsed tumors following castration were observed in vehicle-treated, but not GCP-treated mice, although both tumors regressed at similar rates. This was accompanied by induction of FlnA in GCP. Our current and previous results (Wang et al. 2007) reveal that nuclear localization of the 90 kDa FlnA fragment sensitizes CaP cells to the effects of ADT. These results indicate that since increased nuclear FlnA, co-administration of GCP together with ADT may be an important therapeutic strategy to prevent CRPC development.
Acknowledgments
The authors wish to thank Christopher B. Wee and Honglin Chen for their expert technical assistance in mouse xenograft and qPCR studies, respectively. We also thank Dr. E.W. Yong, National University of Singapore, Singapore for pCMV-FlnA, FlnA(16-24) and FlnA(1-15). Human PSA-luciferase construct (hPSA-luc) was kindly provided by Dr. XuBao Shi, University of California Davis, Dept of Urology. CWR-R1 cells were provided by Dr Elizabeth Wilson (University of North Carolina) while PC-346C cells were obtained from Dr. W.M. van Weerden, Josephine Nefkens Institute, Erasmus MC, Rotterdam, Netherlands. Casodex (bicalutamide) was kindly provided by AstraZeneca, Cheshire, UK, while GCP was generously provided by Amino Up, Shin-ei, Kiyota, Sapporo, Japan. The work reported here does not represent the views or opinions of the Department of Veteran Affairs or the United States Government.
Funding: This work was supported by a Biomedical Laboratory Research & Development (BLRD) service Merit Award (I01BX000400) from the Department of Veterans Affairs and by R01CA133209 from the National Cancer Institute.
Footnotes
Declaration of Interest: None
References
- Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Agus DB, Akita RW, Fox WD, Lewis GD, Higgins B, Pisacane PI, Lofgren JA, Tindell C, Evans DP, Maiese K, Scher HI, Sliwkowski MX. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- Agus DB, Cordon-Cardo C, Fox W, Drobnjak M, Koff A, Golde DW, Scher HI. Prostate cancer cell cycle regulators: response to androgen withdrawal and development of androgen independence. Journal of the National Cancer Institute. 1999;91:1869–1876. doi: 10.1093/jnci/91.21.1869. [DOI] [PubMed] [Google Scholar]
- Anastasius N, Boston S, Lacey M, Storing N, Whitehead SA. Evidence that low-dose, long-term genistein treatment inhibits oestradiol-stimulated growth in MCF-7 cells by down-regulation of the PI3-kinase/Akt signalling pathway. J Steroid Biochem Mol Biol. 2009;116:50–55. doi: 10.1016/j.jsbmb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Bedolla RG, Wang Y, Asuncion A, Chamie K, Siddiqui S, Mudryj MM, Prihoda TJ, Siddiqui J, Chinnaiyan AM, Mehra R, de Vere White, Ghosh PM. Nuclear versus cytoplasmic localization of filamin A in prostate cancer: immunohistochemical correlation with metastases. Clin Cancer Res. 2009;15:788–796. doi: 10.1158/1078-0432.CCR-08-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis DL, Capodice JL, Desai M, Buttyan R, Katz AE. A concentrated aglycone isoflavone preparation (GCP) that demonstrates potent anti-prostate cancer activity in vitro and in vivo. Clin Cancer Res. 2004;10:5282–5292. doi: 10.1158/1078-0432.CCR-03-0828. [DOI] [PubMed] [Google Scholar]
- Burich RA, Holland WS, Vinall RL, Tepper C, White RW, Mack PC. Genistein combined polysaccharide enhances activity of docetaxel, bicalutamide and Src kinase inhibition in androgen-dependent and independent prostate cancer cell lines. BJU Int. 2008;102:1458–1466. doi: 10.1111/j.1464-410X.2008.07826.x. [DOI] [PubMed] [Google Scholar]
- Cai C, Chen S, Ng P, Bubley GJ, Nelson PS, Mostaghel EA, Marck B, Matsumoto AM, Simon NI, Wang H, Balk SP. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71:6503–6513. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriere A, Cargnello M, Julien LA, Gao H, Bonneil E, Thibault P, Roux PP. Oncogenic MAPK signaling stimulates mTORC1 activity by promoting RSK-mediated raptor phosphorylation. Curr Biol. 2008;18:1269–1277. doi: 10.1016/j.cub.2008.07.078. [DOI] [PubMed] [Google Scholar]
- Catalona WJ. Management of cancer of the prostate. N Engl J Med. 1994;331:996–1004. doi: 10.1056/NEJM199410133311507. [DOI] [PubMed] [Google Scholar]
- Chen H, Libertini SJ, George M, Dandekar S, Tepper CG, Al-Bataina B, Kung HJ, Ghosh PM, Mudryj M. Genome-wide analysis of androgen receptor binding and gene regulation in two CWR22-derived prostate cancer cell lines. Endocr Relat Cancer. 2010a;17:857–873. doi: 10.1677/ERC-10-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Libertini SJ, Wang Y, Kung HJ, Ghosh P, Mudryj M. ERK regulates calpain 2-induced androgen receptor proteolysis in CWR22 relapsed prostate tumor cell lines. J Biol Chem. 2010b;285:2368–2374. doi: 10.1074/jbc.M109.049379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Mooso BA, Jathal MK, Madhav A, Johnson SD, van Spyk E, Mikhailova M, Zierenberg-Ripoll A, Xue L, Vinall RL, deVere White RW, Ghosh PM. Dual EGFR/HER2 inhibition sensitizes prostate cancer cells to androgen withdrawal by suppressing ErbB3. Clin Cancer Res. 2011;17:6218–6228. doi: 10.1158/1078-0432.CCR-11-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Siddiqui S, Bose S, Mooso B, Asuncion A, Bedolla RG, Vinall R, Tepper CG, Gandour-Edwards R, Shi X, Lu XH, Siddiqui J, Chinnaiyan AM, Mehra R, Devere White RW, Carraway KL, 3rd, Ghosh PM. Nrdp1-mediated regulation of ErbB3 expression by the androgen receptor in androgen-dependent but not castrate-resistant prostate cancer cells. Cancer Res. 2010c;70:5994–6003. doi: 10.1158/0008-5472.CAN-09-4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Addario M, Arora PD, Ellen RP, McCulloch CA. Interaction of p38 and Sp1 in a mechanical force-induced, beta 1 integrin-mediated transcriptional circuit that regulates the actin-binding protein filamin-A. J Biol Chem. 2002;277:47541–47550. doi: 10.1074/jbc.M207681200. [DOI] [PubMed] [Google Scholar]
- Das M, Ithychanda SS, Qin J, Plow EF. Migfilin and filamin as regulators of integrin activation in endothelial cells and neutrophils. PLoS One. 2011;6:e26355. doi: 10.1371/journal.pone.0026355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Lopez-Camacho C, Tang JY, Mendoza-Villanueva D, Maya-Mendoza A, Jackson DA, Shore P. Cytoskeletal protein filamin A is a nucleolar protein that suppresses ribosomal RNA gene transcription. Proc Natl Acad Sci U S A. 2012;109:1524–1529. doi: 10.1073/pnas.1107879109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deVere White RW, Hackman RM, Soares SE, Beckett LA, Li Y, Sun B. Effects of a genistein-rich extract on PSA levels in men with a history of prostate cancer. Urology. 2004;63:259–263. doi: 10.1016/j.urology.2003.09.061. [DOI] [PubMed] [Google Scholar]
- deVere White RW, Tsodikov A, Stapp EC, Soares SE, Fujii H, Hackman RM. Effects of a high dose, aglycone-rich soy extract on prostate-specific antigen and serum isoflavone concentrations in men with localized prostate cancer. Nutr Cancer. 2010;62:1036–1043. doi: 10.1080/01635581.2010.492085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- Feng Y, Walsh CA. The many faces of filamin: a versatile molecular scaffold for cell motility and signalling. Nat Cell Biol. 2004;6:1034–1038. doi: 10.1038/ncb1104-1034. [DOI] [PubMed] [Google Scholar]
- Gawecka JE, Griffiths GS, Ek-Rylander B, Ramos JW, Matter ML. R-Ras regulates migration through an interaction with filamin A in melanoma cells. PLoS One. 2010;5:e11269. doi: 10.1371/journal.pone.0011269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafar MA, Golliday E, Bingham J, Mansukhani MM, Anastasiadis AG, Katz AE. Regression of prostate cancer following administration of Genistein Combined Polysaccharide (GCP), a nutritional supplement: a case report. J Altern Complement Med. 2002;8:493–497. doi: 10.1089/107555302760253694. [DOI] [PubMed] [Google Scholar]
- Ghosh PM, Malik SN, Bedolla RG, Wang Y, Mikhailova M, Prihoda TJ, Troyer DA, Kreisberg JI. Signal transduction pathways in androgen-dependent and -independent prostate cancer cell proliferation. Endocr Relat Cancer. 2005;12:119–134. doi: 10.1677/erc.1.00835. [DOI] [PubMed] [Google Scholar]
- Gorlin JB, Yamin R, Egan S, Stewart M, Stossel TP, Kwiatkowski DJ, Hartwig JH. Human endothelial actin-binding protein (ABP-280, nonmuscle filamin): a molecular leaf spring. J Cell Biol. 1990;111:1089–1105. doi: 10.1083/jcb.111.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwin J, Drews N, Ali S, Stamschror J, Sorenson M, Rajah TT. Effect of genistein on p90RSK phosphorylation and cell proliferation in T47D breast cancer cells. Anticancer Res. 2011;31:209–214. [PubMed] [Google Scholar]
- Han H, Zhong C, Zhang X, Liu R, Pan M, Tan L, Li Y, Wu J, Zhu Y, Huang W. Genistein induces growth inhibition and G2/M arrest in nasopharyngeal carcinoma cells. Nutr Cancer. 2010;62:641–647. doi: 10.1080/01635581003605490. [DOI] [PubMed] [Google Scholar]
- Hofman K, Swinnen JV, Verhoeven G, Heyns W. E2F activity is biphasically regulated by androgens in LNCaP cells. Biochem Biophys Res Commun. 2001;283:97–101. doi: 10.1006/bbrc.2001.4738. [DOI] [PubMed] [Google Scholar]
- Jay D, Garcia EJ, de la Luz Ibarra M. In situ determination of a PKA phosphorylation site in the C-terminal region of filamin. Mol Cell Biochem. 2004;260:49–53. doi: 10.1023/b:mcbi.0000026052.76418.55. [DOI] [PubMed] [Google Scholar]
- Jay D, Gercia EJ, Lara JE, de la Luz Ibarra M. Determination of a cAMP-dependent protein kinase phosphorylation site in the C-terminal region of human endothelial actin-binding protein. Arch Biochem Biophys. 2000;377:80–84. doi: 10.1006/abbi.2000.1762. [DOI] [PubMed] [Google Scholar]
- Kang NJ, Lee KW, Rogozin EA, Cho YY, Heo YS, Bode AM, Lee HJ, Dong Z. Equol, a metabolite of the soybean isoflavone daidzein, inhibits neoplastic cell transformation by targeting the MEK/ERK/p90RSK/activator protein-1 pathway. J Biol Chem. 2007;282:32856–32866. doi: 10.1074/jbc.M701459200. [DOI] [PubMed] [Google Scholar]
- Kuil CW, Berrevoets CA, Mulder E. Ligand-induced conformational alterations of the androgen receptor analyzed by limited trypsinization. Studies on the mechanism of antiandrogen action. J Biol Chem. 1995;270:27569–27576. doi: 10.1074/jbc.270.46.27569. [DOI] [PubMed] [Google Scholar]
- Lakshman M, Xu L, Ananthanarayanan V, Cooper J, Takimoto CH, Helenowski I, Pelling JC, Bergan RC. Dietary genistein inhibits metastasis of human prostate cancer in mice. Cancer Res. 2008;68:2024–2032. doi: 10.1158/0008-5472.CAN-07-1246. [DOI] [PubMed] [Google Scholar]
- Laschak M, Bechtel M, Spindler KD, Hessenauer A. Inability of NCoR/SMRT to repress androgen receptor transcriptional activity in prostate cancer cell lines. Int J Mol Med. 2011;28:645–651. doi: 10.3892/ijmm.2011.735. [DOI] [PubMed] [Google Scholar]
- Lian F, Bhuiyan M, Li YW, Wall N, Kraut M, Sarkar FH. Genistein-induced G2-M arrest, p21WAF1 upregulation, and apoptosis in a non-small-cell lung cancer cell line. Nutr Cancer. 1998;31:184–191. doi: 10.1080/01635589809514701. [DOI] [PubMed] [Google Scholar]
- Limpens J, Schroder FH, de Ridder CM, Bolder CA, Wildhagen MF, Obermuller-Jevic UC, Kramer K, van Weerden WM. Combined lycopene and vitamin E treatment suppresses the growth of PC-346C human prostate cancer cells in nude mice. J Nutr. 2006;136:1287–1293. doi: 10.1093/jn/136.5.1287. [DOI] [PubMed] [Google Scholar]
- Loy CJ, Sim KS, Yong EL. Filamin-A fragment localizes to the nucleus to regulate androgen receptor and coactivator functions. Proc Natl Acad Sci U S A. 2003;100:4562–4567. doi: 10.1073/pnas.0736237100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Montemayor MM, Otero-Franqui E, Martinez J, De La Mota-Peynado A, Cubano LA, Dharmawardhane S. Individual and combined soy isoflavones exert differential effects on metastatic cancer progression. Clin Exp Metastasis. 2010;27:465–480. doi: 10.1007/s10585-010-9336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiello D, Cheng S, Bubley GJ, Lu ML, Balk SP. Bicalutamide functions as an androgen receptor antagonist by assembly of a transcriptionally inactive receptor. J Biol Chem. 2002;277:26321–26326. doi: 10.1074/jbc.M203310200. [DOI] [PubMed] [Google Scholar]
- Matsushima H, Goto T, Hosaka Y, Kitamura T, Kawabe K. Correlation between proliferation, apoptosis, and angiogenesis in prostate carcinoma and their relation to androgen ablation. Cancer. 1999;85:1822–1827. doi: 10.1002/(sici)1097-0142(19990415)85:8<1822::aid-cncr24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Murphy WM, Soloway MS, Barrows GH. Pathologic changes associated with androgen deprivation therapy for prostate cancer. Cancer. 1991;68:821–828. doi: 10.1002/1097-0142(19910815)68:4<821::aid-cncr2820680426>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Nagano T, Yoneda T, Hatanaka Y, Kubota C, Murakami F, Sato M. Filamin A-interacting protein (FILIP) regulates cortical cell migration out of the ventricular zone. Nat Cell Biol. 2002;4:495–501. doi: 10.1038/ncb808. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Wang Y, Kurita T, Adomat H, Cunha GR. Genistein increases epidermal growth factor receptor signaling and promotes tumor progression in advanced human prostate cancer. PLoS One. 2011;6:e20034. doi: 10.1371/journal.pone.0020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y, Hartwig JH. Actin filament cross-linking by chicken gizzard filamin is regulated by phosphorylation in vitro. Biochemistry. 1995;34:6745–6754. doi: 10.1021/bi00020a020. [DOI] [PubMed] [Google Scholar]
- Ott I, Fischer EG, Miyagi Y, Mueller BM, Ruf W. A role for tissue factor in cell adhesion and migration mediated by interaction with actin-binding protein 280. J Cell Biol. 1998;140:1241–1253. doi: 10.1083/jcb.140.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne DM, Brady ME, Cook S, Gaughan L, Neal DE, Robson CN. Androgen receptor nuclear translocation is facilitated by the f-actin cross-linking protein filamin. Mol Endocrinol. 2000;14:1618–1626. doi: 10.1210/mend.14.10.0541. [DOI] [PubMed] [Google Scholar]
- Paprotka T, Delviks-Frankenberry KA, Cingoz O, Martinez A, Kung HJ, Tepper CG, Hu WS, Fivash MJ, Jr, Coffin JM, Pathak VK. Recombinant origin of the retrovirus XMRV. Science. 2011;333:97–101. doi: 10.1126/science.1205292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavese JM, Farmer RL, Bergan RC. Inhibition of cancer cell invasion and metastasis by genistein. Cancer Metastasis Rev. 2010;29:465–482. doi: 10.1007/s10555-010-9238-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson SP, Twigg SR, Sutherland-Smith AJ, Biancalana V, Gorlin RJ, Horn D, Kenwrick SJ, Kim CA, Morava E, Newbury-Ecob R, Orstavik KH, Quarrell OW, Schwartz CE, Shears DJ, Suri M, Kendrick-Jones J, Wilkie AO. Localized mutations in the gene encoding the cytoskeletal protein filamin A cause diverse malformations in humans. Nat Genet. 2003;33:487–491. doi: 10.1038/ng1119. [DOI] [PubMed] [Google Scholar]
- Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, Sonenberg N, Blenis J. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem. 2007;282:14056–14064. doi: 10.1074/jbc.M700906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt F, Knobbe CB, Frank B, Wolburg H, Weller M. The topoisomerase II inhibitor, genistein, induces G2/M arrest and apoptosis in human malignant glioma cell lines. Oncol Rep. 2008;19:1061–1066. [PubMed] [Google Scholar]
- Sedelaar JP, Isaacs JT. Tissue culture media supplemented with 10% fetal calf serum contains a castrate level of testosterone. Prostate. 2009;69:1724–1729. doi: 10.1002/pros.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfanos KS, Aloia AL, Hicks JL, Esopi DM, Steranka JP, Shao W, Sanchez-Martinez S, Yegnasubramanian S, Burns KH, Rein A, De Marzo AM. Identification of replication competent murine gammaretroviruses in commonly used prostate cancer cell lines. PLoS One. 2011;6:e20874. doi: 10.1371/journal.pone.0020874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Gupta V, Zhang H, Yunker CK, Ahmad Z, Zwier D, Sarkar FH, Hillman GG. Daidzein effect on hormone refractory prostate cancer in vitro and in vivo compared to genistein and soy extract: potentiation of radiotherapy. Pharm Res. 2010;27:1115–1127. doi: 10.1007/s11095-010-0107-9. [DOI] [PubMed] [Google Scholar]
- Tepper CG, Boucher DL, Ryan PE, Ma AH, Xia L, Lee LF, Pretlow TG, Kung HJ. Characterization of a novel androgen receptor mutation in a relapsed CWR22 prostate cancer xenograft and cell line. Cancer Res. 2002;62:6606–6614. [PubMed] [Google Scholar]
- Tepper CG, Vinall RL, Wee CB, Xue L, Shi XB, Burich R, Mack PC, de Vere White RW. GCP-mediated growth inhibition and apoptosis of prostate cancer cells via androgen receptor-dependent and -independent mechanisms. Prostate. 2007;67:521–535. doi: 10.1002/pros.20548. [DOI] [PubMed] [Google Scholar]
- Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, Hopwood VL, Pathak S, von Eschenbach AC, Chung LW. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54:2577–2581. [PubMed] [Google Scholar]
- Tigges U, Koch B, Wissing J, Jockusch BM, Ziegler WH. The F-actin cross-linking and focal adhesion protein filamin A is a ligand and in vivo substrate for protein kinase C alpha. J Biol Chem. 2003;278:23561–23569. doi: 10.1074/jbc.M302302200. [DOI] [PubMed] [Google Scholar]
- Travis MA, van der Flier A, Kammerer RA, Mould AP, Sonnenberg A, Humphries MJ. Interaction of filamin A with the integrin beta 7 cytoplasmic domain: role of alternative splicing and phosphorylation. FEBS Lett. 2004;569:185–190. doi: 10.1016/j.febslet.2004.04.099. [DOI] [PubMed] [Google Scholar]
- van der Flier A, Sonnenberg A. Structural and functional aspects of filamins. Biochim Biophys Acta. 2001;1538:99–117. doi: 10.1016/s0167-4889(01)00072-6. [DOI] [PubMed] [Google Scholar]
- van Weerden WM, de Ridder CM, Verdaasdonk CL, Romijn JC, van der Kwast TH, Schroder FH, van Steenbrugge GJ. Development of seven new human prostate tumor xenograft models and their histopathological characterization. Am J Pathol. 1996;149:1055–1062. [PMC free article] [PubMed] [Google Scholar]
- Vinall RL, Hwa K, Ghosh P, Pan CX, Lara PN, Jr, de Vere White RW. Combination treatment of prostate cancer cell lines with bioactive soy isoflavones and perifosine causes increased growth arrest and/or apoptosis. Clin Cancer Res. 2007;13:6204–6216. doi: 10.1158/1078-0432.CCR-07-0600. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kreisberg JI, Bedolla RG, Mikhailova M, deVere White RW, Ghosh PM. A 90 kDa fragment of filamin A promotes Casodex-induced growth inhibition in Casodex-resistant androgen receptor positive C4-2 prostate cancer cells. Oncogene. 2007;26:6061–6070. doi: 10.1038/sj.onc.1210435. [DOI] [PubMed] [Google Scholar]
- Westin P, Stattin P, Damber JE, Bergh A. Castration therapy rapidly induces apoptosis in a minority and decreases cell proliferation in a majority of human prostatic tumors. Am J Pathol. 1995;146:1368–1375. [PMC free article] [PubMed] [Google Scholar]
- Woo MS, Ohta Y, Rabinovitz I, Stossel TP, Blenis J. Ribosomal S6 kinase (RSK) regulates phosphorylation of filamin A on an important regulatory site. Mol Cell Biol. 2004;24:3025–3035. doi: 10.1128/MCB.24.7.3025-3035.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Bismar TA, Su J, Xu B, Kristiansen G, Varga Z, Teng L, Ingber DE, Mammoto A, Kumar R, Alaoui-Jamali MA. Filamin A regulates focal adhesion disassembly and suppresses breast cancer cell migration and invasion. J Exp Med. 2010;207:2421–2437. doi: 10.1084/jem.20100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhong S, Dong Z, Chen N, Bode AM, Ma W. UVA induces Ser381 phosphorylation of p90RSK/MAPKAP-K1 via ERK and JNK pathways. J Biol Chem. 2001;276:14572–14580. doi: 10.1074/jbc.M004615200. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Zhu G, Gu S, Chen X, Hu H, Weng S. Genistein inhibits osteolytic bone metastasis and enhances bone mineral in nude mice. Environ Toxicol Pharmacol. 2010;30:37–44. doi: 10.1016/j.etap.2010.03.016. [DOI] [PubMed] [Google Scholar]
- Zhao R, Xiang N, Domann FE, Zhong W. Effects of selenite and genistein on G2/M cell cycle arrest and apoptosis in human prostate cancer cells. Nutr Cancer. 2009;61:397–407. doi: 10.1080/01635580802582751. [DOI] [PMC free article] [PubMed] [Google Scholar]