Abstract
Background
Biliary obstruction and cholestasis are serious complications of many liver diseases. While resident hepatic macrophages (Kupffer cells) are frequently implicated in disease progression, most studies fail to differentiate the contribution of Kupffer cells and inflammatory mononuclear phagocytes (iMNPs) that infiltrate the liver subsequent to obstruction.
Aim
This study was undertaken to examine the roles and potential interactions of these two disparate mononuclear phagocyte populations in hepatic injury attending cholestasis.
Methods
Female, C57Bl/6 mice were injected with magnetic beads on day three prior to sham operation or bile duct ligation (BDL) in order to facilitate subsequent Kupffer cell isolation. Three days post surgery, animals were euthanized, and bead-containing Kupffer cells and iMNPs were separated, purified, and analyzed. To examine the ability of Kupffer cells to modulate iMNP activity, iMNPs were isolated from the livers of intact and Kupffer cell-depleted mice on day 3 post-surgery and compared.
Results
Purified Kupffer cells and iMNP populations obtained from BDL mice exhibited heterogeneous morphologies rendering them visually indistinguishable. iMNPs, however, were characterized by the increased expression of Ly-6C and CD11b and the elevated production of chemokines/cytokines characteristic of inflammatory cells. In the absence of Kupffer cells, iMNPs immigrating to the liver following BDL exhibited significant decreases in CD11b and Ly-6C expression, and in pro-inflammatory chemokine/cytokine production.
Conclusions
Kupffer cells and iMNPs exhibit disparate biological responses to biliary obstruction and cholestasis. Kupffer cells play a key role in regulating iMNP influx and activity.
Keywords: liver, bile duct, macrophages, inflammation, biliary obstruction
Introduction
Kupffer cells, resident liver macrophages, are located within the lumen of the sinusoids where they continually survey and clear the hepatic blood flow of bacterial endotoxin, microbial debris, toxic substances, and dead cells (1,2). They are characterized by the cell surface expression of F4/80+CD68+CD115+CD11b−/loLy-6Clo in mice, ED1+ED2+ (CD68+CD163+) in rats and CD14+CD16+ in humans, and their ability to produce a variety of cytokines, e.g., platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α), and interleukin (IL)-6, IL-1β, IL-10, and IL-12 (3–9). In addition to playing a central role in hepatic homeostasis, Kupffer cells demonstrate critical and divergent functions in a variety of diseases ranging from steatohepatitis, alcoholic liver disease and viral hepatitis to biliary obstruction and cholestasis (1,2,10).
Cholestasis is a defect in bile flow that develops for varying reasons: bile synthesis defects, biliary atresia, neonatal hepatitis, and genetic defects in bile secretion genes (11,12). It is a common severe neonatal disorder that often progresses to fibrosis and generally requires liver transplantation (11,12). Liver fibrosis, the deposition of scar tissue in replacement of dead hepatocytes, is a complex process that involves a number of different cell types within the liver including, ostensibly, Kupffer cells. It has been suggested that, upon activation, Kupffer cells rapidly produce TGF-β and PDGF that stimulate the deposition of fibrotic extracellular matrix material by hepatic stellate cells and other hepatic fibroblasts (7). In this regard, Kupffer cells isolated from rats treated with carbon tetrachloride (CCl4), a classic model of hepatotoxicity and liver fibrosis, produced increased amounts of IL-6, IL-1β, TGF-β, and IL-10 for up to 6 weeks of treatment (13). Moreover, CCl4-treated, Kupffer cell-depleted rats exhibited decreased lipid peroxidation, alanine transaminase, alkaline phosphatase, bilirubin levels and collagen accumulation suggesting that Kupffer cells play a key, detrimental role in fibrogenesis and liver injury (14).
In contrast to the preceding reports, several studies found that Kupffer cells attenuated liver injury (15–17). In a mouse model of bile duct obstruction, Kupffer cells suppressed hepatocyte necrosis in an IL-6-dependent manner (16). Osawa and colleagues reported similar findings: Kupffer cell depletion increased hepatocyte apoptosis and decreased hepatocyte regeneration in mice following partial-bile duct ligation (17). Kupffer cells also contributed to the resolution of fibrosis in several animal models of liver injury and repair (18,19). In a hepatotoxicity model, the depletion of Kupffer cells inhibited the decrease in collagen associated with tissue repair (18). Kupffer cell depletion also lead to the increased presence of necrotic hepatocytes in a transgenic mouse model of viral hepatitis (19). Lastly, Tracy and colleagues, studying the role of Kupffer cells in a rat model of biliary obstruction and repair, established that Kupffer cells regulated the influx of neutrophils necessary for the resolution of fibrosis (15,20).
It is crucial to note that the aforementioned studies often failed to distinguish between activities expressed by Kupffer cells and those of inflammatory mononuclear phagocytes (iMNPs) that immigrate to the liver during periods of injury and repair. Rather, these studies often referred to all macrophages found in the liver as Kupffer cells. iMNPs, which immigrate to the liver in response to injurious stimuli, can be differentiated from Kupffer cells by their cell-surface expression of F4/80+CD11b+Ly-6Chi in mice, ED1−ED2+ (CD68−CD163+) in rats, and CD14+CD16− in humans (3–6). In addition to differences in these markers, iMNPs exhibit decreased phagocytic activity in vivo, but an increased capacity to produce cytokines and reactive oxygen species (3,21–23). Efforts undertaken in a number of studies to differentiate between the activities expressed by Kupffer cells and iMNPs revealed the detrimental role of iMNPs in liver injury. In one study, hepatotoxicity and liver fibrosis induced by chronic CCl4 administration was reduced significantly in CCR2−/− and CCR2−/−CCR6−/− mice in which the accumulation of iMNPs in the liver was sharply reduced (24). Similarly, fibrogenesis induced by dimethylnitrosamine was reduced significantly in rats administered a dominant-negative mutant form of monocyte chemoattractant protein-1 (MCP-1), which suppressed iMNP accumulation (25). Recently, Miura and colleagues demonstrated that iMNPs contributed to the increase in steatosis found in mice fed a choline-deficient amino acid-defined diet. Notably, they also reported that Kupffer cells regulated the infiltration of iMNPs at the onset of liver injury (26).
The role of iMNPs and the factors that regulate their immigration to the liver in response to cholestasis have not been clearly delineated. The present study was designed to define and differentiate the contributions of Kupffer cells and iMNPs to the liver damage that occurs in a mouse model of biliary obstruction. While Kupffer cells and iMNPs derived from BDL mice were visibly indistinguishable, they expressed disparate cell surface markers. In general, iMNPs also produced significantly more proinflammatory cytokines and chemokines [e.g., IL-6 and keratinocyte derived chemokine (KC)] while Kupffer cells produce higher levels of TGF-β. Kupffer cells played a critical role in regulating the influx and biological activity of iMNPs. Proinflammatory cytokine/chemokine production and the immigration of F4/80+CD11b+Ly-6Chi iMNPs were reduced markedly in the livers of BDL mice rendered Kupffer cell depleted prior to surgery.
Materials and Methods
Animals
Specific pathogen-free, female C57Bl/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME) and bred in-house. All animals used were 6–12 weeks old and received food and water ad libitum. Animals were treated in accordance to the National Research Council publication entitled “Guide for the Care and Use of Laboratory Animals” (8th Ed.) and an animal welfare protocol approved by Rhode Island Hospital’s Animal Care and Use Committee.
Magnetic and fluorescent beads
Three days prior to surgery, animals were injected intravenously with a 200 μL suspension of magnetic beads (1.3 μm diameter; Calbiochem/EMD Gibbstown, NJ) diluted 1:100 in PBS. Similarly, fluorescein-labeled tracer beads (1 μm diameter; Sigma Aldrich Co., St. Louis, MO) were diluted 1:250 in PBS and a 200 μl suspension was injected i.v. Animals were euthanized at either 2 hours or 3 days post-injection as indicated in the text.
Kupffer cell depletion
Kupffer cells were depleted three days prior to surgery by intravenous administration of multilamellar liposomes containing dichloromethylene diphosphonate (clodronate, a gift obtained from Roche Diagnostics GmbH, Mannheim, Germany) as previously described (16,27).
Surgery
Bile duct ligation (BDL) or sham operations were performed as previously described (16).
Cell isolation
Three days post-surgery, mice were anesthetized. The hepatic nonparenchymal liver cell population (NPC) was isolated following perfusion through the portal vein with 20 ml of a 100 U/ml collagenase A solution (Roche, San Francisco, CA) and purified by centrifugation on a histodenz gradient as previously described (9,16,28). To isolate Kupffer cells and iMNPs, the purified NPCs were resuspended in 500 μl of degassed buffer and passed through a magnetic separation column (Miltenyi Biotech, Auburn, CA) per the manufacturer’s protocol. To recover the magnetic bead-containing Kupffer cells that remained attached, the magnetic column was removed from the magnet and flushed with 2.5 ml of degassed buffer. Both the NPC flow-through and retained cells were enriched by a second passage through new columns. Finally, to isolate infiltrating iMNP, the NPC flow-through was labeled with biotin-conjugated rat anti-mouse F4/80 (clone BM8) and biotin-conjugated rat anti-mouse CD115 (clone AFS98) antibodies (eBioscience San Diego, CA). Antibody labeled cells were incubated with streptavidin coated microbeads (Miltenyi) followed by PE-conjugated streptavidin (when indicated in the text) to label the cells then isolated by passage twice through magnetic columns as described above.
Quantitative real-time quantitative real-time polymerase chain reaction (qtPCR)
Isolated cells were lysed in 800 μl of TRIzol (Roche, Indianapolis, Indiana) and stored at −20°C. RNA extraction and purification were performed according to the manufacture’s protocol. cDNA was then synthesized using the QuantiTect Kit for reverse transcription (Qiagen, Valencia, CA). Kupffer Cell Receptor (KCR) and CSF-1 receptor messages (expressed by resident hepatic macrophages), and 18S ribosomal RNA were quantified by qtPCR as previously described (16).
Cytospin smears
Cells (2×104/200 μl) suspended in PBS and 10% FBS were centrifuged at 600 rpm for 5 min in a Shandon Cytospin3 (Thermo Fisher Scientific, Waltham, MA) onto glass slides. The slides were then stained with Wright-Giemsa stain for observation at 60× magnification with a Nikon Eclipse 50i microscope (Melville, NY).
Flow cytometry
Flow cytometry was conducted in accordance with methods previously described (9,28). Dye-conjugated mAb specific for the following determinants were purchased and use: rat anti-mouse Ly-6C (HK1.4, Biolegends, San Diego, CA), rat anti-mouse CD11b (M1/70, eBioscience), rat anti-mouse Ly-6G (1A8, eBioscience), and rat anti-mouse F4/80 (BM8, eBioscience). PE-conjugated streptavidin was purchased from BD Bioscience, San Diego, CA. Cells were analyzed using a FACS Aria (BD Bioscience) and results were interpreted using FlowJo software (Tree Star, Inc., Ashland, OR.).
Cell culture and protein quantification
Freshly isolated cells (5×104) were cultured in half-area 96-well plates with 100 μL of sterile HEPES-buffered RPMI 1640 medium, supplemented with 1 mM sodium pyruvate, 10% heat-inactivated FBS, 1 mM L-glutamine, 1% essential and nonessential amino acids, 5×10−5 M 2-mercaptoethanol, 100 U/ml penicillin, 100 μg/ml streptomycin and 5 μg/ml bacterial endotoxin (Escherichia coli 0114:B4, Sigma-Aldrich Co.). Forty hours later, supernatants were collected and stored at −80°C until analysis. Concentrations of IL-1β, IL-6, IL-10, KC, MCP-1, macrophage inflammatory protein (MIP)-2, TNF-α, and TGF-β were subsequently quantified using the Bio-Plex 200 system (BioRad Laboratories GmbH, Munich, Germany) and two MILLIPLEX®MAP kits purchased from Millipore (Billerica, MA).
Statistical analysis
Statistical analysis was performed using Prism Software (GraphPad Software Inc., La Jolla, CA). Student’s unpaired, two-tailed t-tests was employed to compare two groups. A P value less than 0.05 was considered significant.
Results
iMNPs accumulate in bile duct obstructed livers
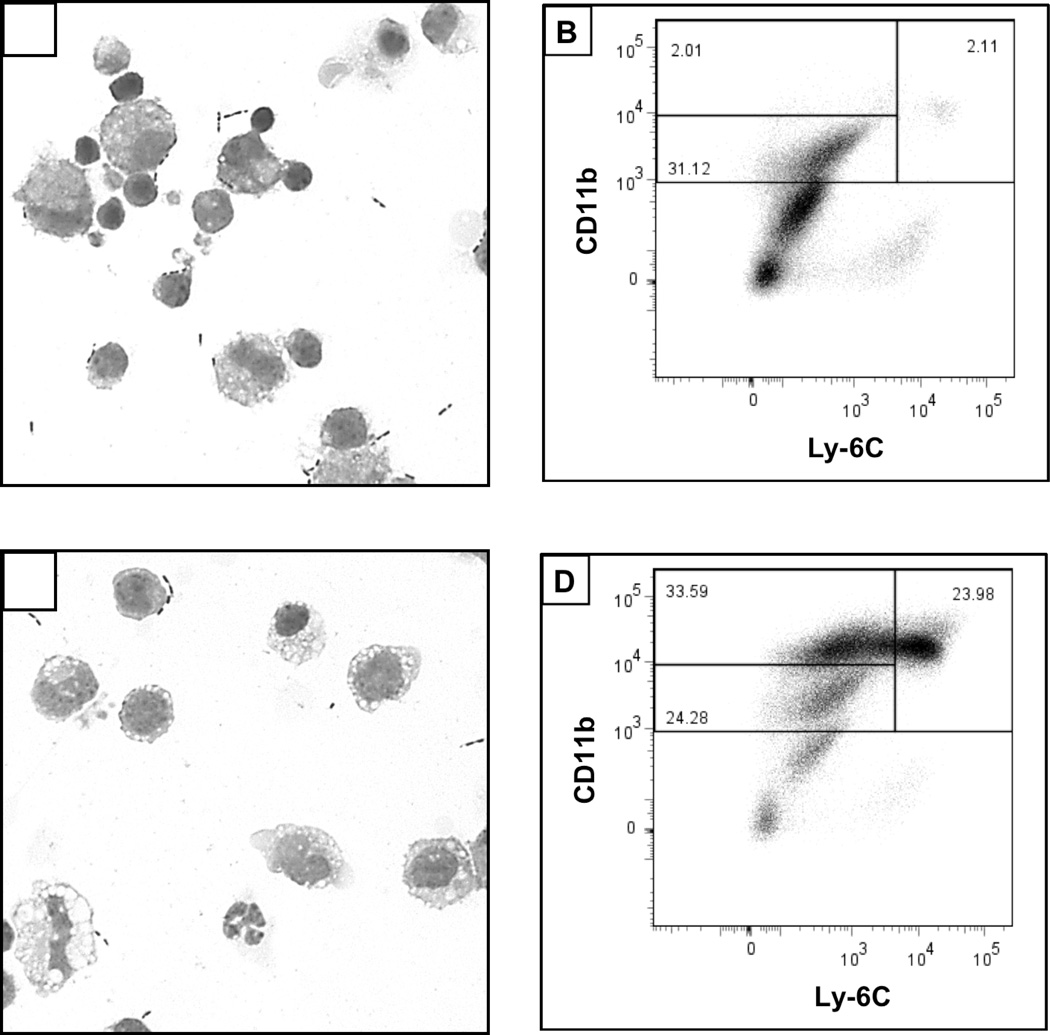
A substantial difference in the general appearance of the hepatic NPC population was observed following biliary obstruction. Particularly, a marked increase in vacuolated cells and neutrophils were seen in cytospin smears of NPC derived from BDL, relative to control (sham-operated), mice on day 3 post-surgery (Figure 1, A vs. C). Moreover, significant increases in the percentages of cells that expressed CD11bhiLy-6Chi (characteristic of iMNPs) were found within the NPC population obtained from the BDL animals (Figure 1, B vs. D).
Figure 1.
BDL promotes vacuolation and the accumulation of CD11b+Ly6C+ cells in the liver. Cytospin smears were prepared and flow cytometry performed on NPC obtained from sham-operated (A, B) and BDL (C, D) mice on day 3 post-surgery. Data shown are representative of 2–4 experiments; cells were pooled from 3–6 animals for each analysis.
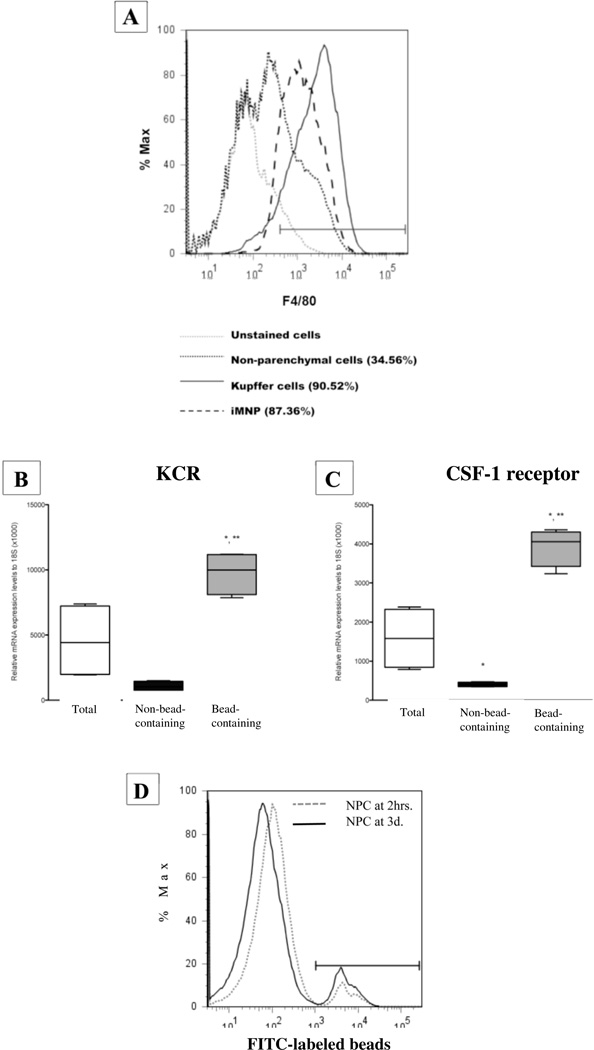
Carbon particles (India ink) injected i.v. into mice are rapidly phagocytosed by Kupffer cells lining the hepatic sinusoids enabling their identification and visual differentiation from other cell types (16,29). Using a similar approach, i.e., magnetic beads injected prior to experimentation, Kupffer cells were readily separate from other cells including immigrating iMNPs. As shown in Figure 2A, F4/80+ cells (Kupffer cells and iMNPs combined) comprised 34% of the total NPC population derived from mice on day 3 post-BDL. A highly enriched, bead-containing Kupffer cell population was isolated by passage of the NPC twice over a magnetic column. Subsequently, iMNPs that accumulated in the liver were purified (~90%) by incubating sequentially with biotin-conjugated macrophage-specific monoclonal antibodies, streptavidin-conjugated magnetic beads and PE-conjugated streptavidin (for flow cytometric analysis). Finally, the labeled cells were passage twice and eluted from a magnetic column.
Figure 2.
Quantitation and characterization of Kupffer cell- and iMNP-enriched populations obtained from the livers of mice using bead technology. The total NPC, enriched Kupffer cell and enriched iMNP populations derived from the livers of mice on day 3 post-BDL, were stained with PE-conjugated anti-F4/80+ (pan-macrophage) mAb and analyzed by flow cytometry. Data derive from a single experiment representative of 3 experiments in which the cells were pooled from 3–6 animals and analyzed (A). Total, bead-containing, and non-bead-containing NPC populations were isolated at 2 hours post-magnetic bead injection. KCR (B) and CSF-1 receptor (C) message levels in each population were quantified by qtPCR. Data derive from the average of two experiments and a total of 11 animals. *Significantly different than total NPCs, P <0.05; **significantly greater than NPCs depleted of bead-containing cells, P <0.05. FITC-labeled tracer beads were injected i.v. and the mice were euthanized at 2 hours and 3 days later. Histograms of the FITC-labeled, bead-containing cells within the total NPC populations are shown (D). Data are representative of 2 experiments in which cells pooled from 3–6 animals were analyzed.
To confirm the nature and purity of the enriched Kupffer cell population, the total, bead-containing and non-bead-containing NPC populations were isolated and the expression level of KCR and CSF-1 receptor messages was quantified by qtPCR (Figures 2B and C). The enriched, bead-containing cell population expressed significantly higher message levels for both receptors than did either the total NPC or the non-bead-containing NPC populations. In particular, the elevated expression of KCR message by the bead-containing cell population and the absence of expression by the non-bead-containing population document the ingestion of magnetic beads and the subsequent enrichment of essentially all Kupffer cells.
To ensure that Kupffer cells do not proliferate following bead ingestion, mice were injected with fluorescein-labeled tracer beads, and euthanized at 2 hours and 3 days post-injection. The total NPC population was isolated and analyzed by flow cytometry, gating on the FITC-labeled bead-containing population (Figure 2D). A single peak was found with no significant shift toward less mean fluorescence intensity over the 3-day period evidencing the absence of cell division. Moreover, ~100% of the bead-containing cells at both 2 hours and 3 days post-inoculation were F4/80+ documenting the exclusive ingestion of beads by macrophages (data not shown).
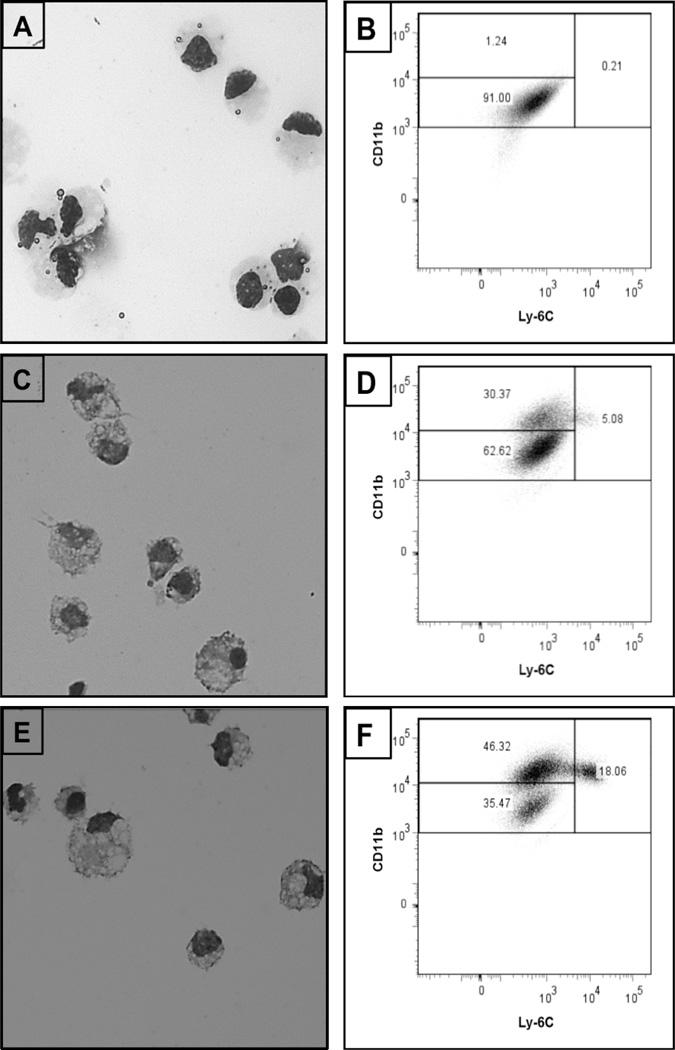
Finally, a visual examination of the purified Kupffer cells isolated from sham-operated animals revealed a generally homogenous population of cells with large nuclei (Figure 3A). The Kupffer cell and iMNP populations purified derived from BDL animals were indistinguishable, and morphologically heterogeneous populations (Figures 3C and E). These populations were further characterized by flow cytometry (Figures 3B, D and F). The Kupffer cells isolated from sham-operated mice composed a single large CD11bintLy-6Clo population; Kupffer cells isolated from BDL mice, on the other hand, were distributed among two large subsets: CD11bintLy-6Clo and CD11bhiLy-6Clo. By comparison, the iMNP population derived from BDL animals contained a much smaller percentage of CD11bintLy-6Clo, but larger percentages of CD11bhiLy-6Clo and CD11bhiLy-6Chi cells.
Figure 3.
Enriched Kupffer cell and iMNP populations obtained from BDL mice exhibit disparate cell-surface markers. Kupffer cells were isolated from sham-operated (A, B) or BDL (C, D) mice on day 3 post-surgery. iMNP were isolated from BDL (E, F) mice on day 3 post-surgery. Cytospin smears were prepared and the purified cells were analyzed by flow cytometry. Data shown were obtained from a single experiment representative of 4 experiments in which the cells derived from 3–6 animals were pooled and analyzed.
iMNPs synthesize pro-inflammatory cytokine and chemokine
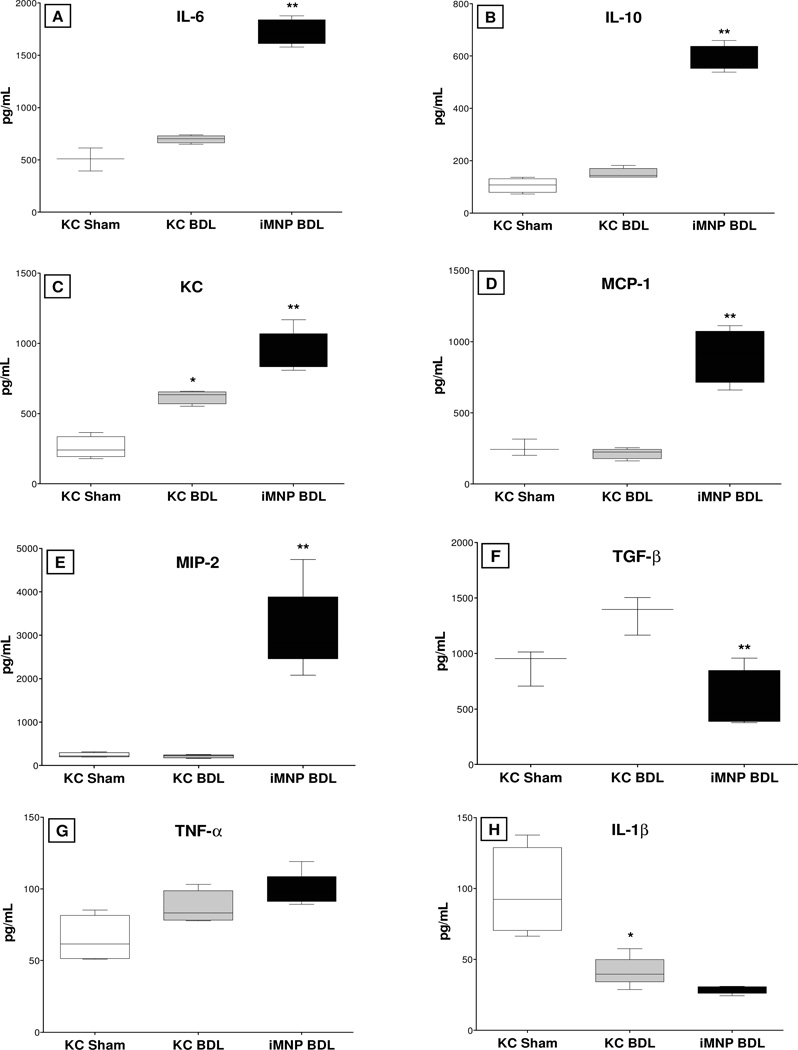
Kupffer cells and iMNPs purified from the livers of sham-operated and/or BDL mice were cultured, the supernates were collected, and the production of cytokines and chemokines was quantified by cytokine bead array analysis. With the exception of IL-1β, cytokine and chemokine levels were slightly (albeit insignificantly) higher in the supernates collected from the culture of Kupffer cells derived from BDL, relatively to sham-operated, mice (Figure 4). In comparison, hepatic iMNPs derived from BDL mice produced significantly more IL-6, IL-10, MIP-2, KC, and MCP-1 than did Kupffer cells derived from the same animals. Notably, Kupffer cells produced higher TGF-β levels; no difference in TNF-α or IL-1β synthesis by these two cell populations was determined.
Figure 4.
iMNP cells produce increased cytokine and chemokine levels relative to Kupffer cells following biliary obstruction. Kupffer cells (KC) and iMNP were purified on day 3 following bile duct ligation (BDL) or sham operation, and cultured. The supernates were collected after 40 hours incubation; the cytokines and chemokines listed were quantified using multiplex bead-array kits. Data are representative of 3 experiments in which cells were pooled from 3–6 animals for each analysis. *Significantly different from Kupffer cells isolated from sham-operated mice: P < 0.05; **Significantly different from Kupffer cells isolated from BDL mice: P <0.05.
Kupffer cells regulate the response of iMNPs to cholestasis
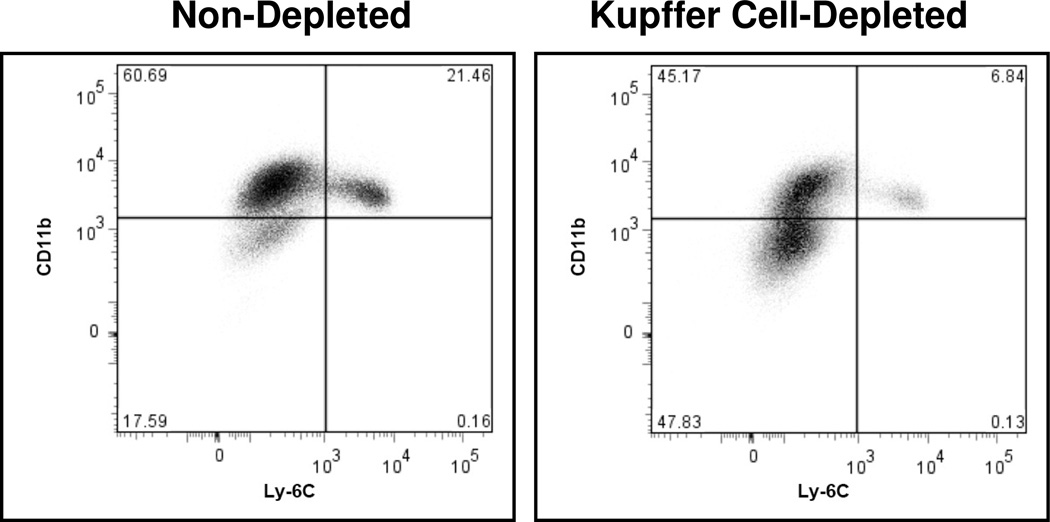
Kupffer cells exert a significant influence on the physiologic function of other hepatic cell types residing or accumulating in the liver (1,2). To determine the effect of Kupffer cells on the response of iMNPs to cholestasis; mice were administered liposome-encapsulated clodronate and rendered Kupffer cell-depleted prior to BDL. In the absence of Kupffer cells, the percentage of Ly-6Chi iMNPs infiltrating the livers of BDL mice was reduced substantially relative to animals that possessed an intact Kupffer cell population (Figure 5). This did not occur as a consequence of the general effect of clodronate on mononuclear phagocytes. When bone marrow cells obtained from clodronate-treated and non-treated animals on day three post-treatment were analyzed by flow cytometry, only slight differences in the percentages of cells expressing Ly-6Chi and Ly-6Cint were observed: 9.94% (clodronate-treated animals) versus 6.11% (non-treated controls) and 29.56% (treated) versus 26.68% (non-treated), respectively. This finding correlates with the published results of other investigators who demonstrated equivalent numbers of monocytes in the peripheral blood of non-treated mice and mice at 3–4 days following clodronate administration (30).
Figure 5.
Accumulation of Ly-6Chi iMNP is suppressed in the livers of BDL, Kupffer cell-depleted mice. iMNP were isolated from Kupffer cell-depleted and non-depleted animals on day 3 post-BDL. Data shown were obtained from a single experiment representative of 4 experiments in which the cells derived from 3–6 animals were pooled and analyzed.
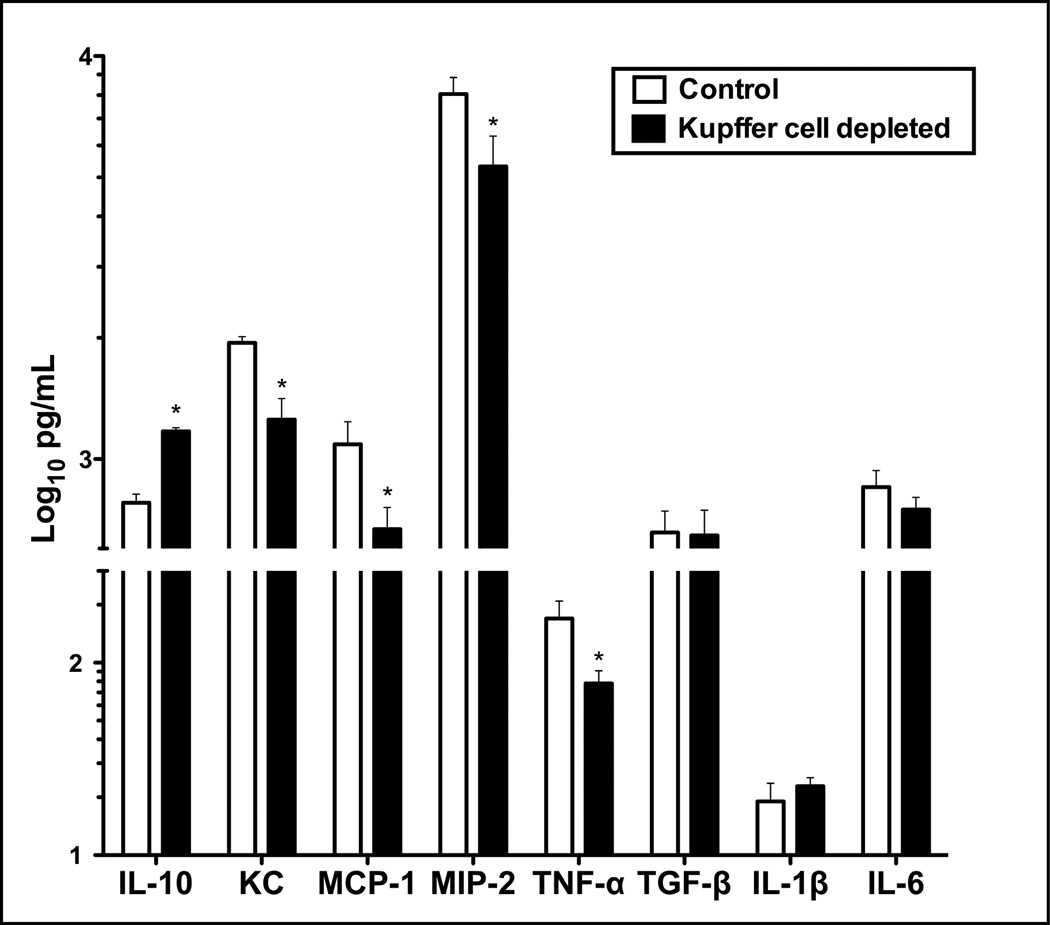
To examine further the effect of Kupffer cells on the response of iMNPs to biliary obstruction, iMNPs were purified from livers of non-treated and Kupffer cell-depleted mice on day 3 post-BDL and cultured. The culture supernates were subsequently collected and the cytokine/chemokine levels quantified. Relative to iMNPs derived from the livers of intact mice, those obtained from Kupffer cell-depleted mice exhibited a marked reduction in pro-inflammatory cytokine/chemokine production. Specifically, the synthesis of TNF-α, KC, MIP-2 and MCP-1 was significantly reduced; IL-10 production, on the other hand, was elevated; IL-1β and IL-6 were unchanged (Figure 6).
Figure 6.
Pro-inflammatory cytokine and chemokine production by infiltrating iMNP is reduced in BDL, Kupffer cell-depleted mice. iMNP were isolated from non-depleted or Kupffer cell-depleted mice on day 3 post-BDL and cultured. Cytokine and chemokine levels in the culture supernates collected after 40 hours incubation were quantified. Data derive from a single experiment representative of 3 experiments; cells pooled from 3–6 animals/group were analyzed. *Significantly different from the control: P< 0.05.
Discussion
Cholestasis occurs as a consequence of a complex series of events that culminates in liver fibrosis and transplantation if left untreated. It can be mimicked experimentally in animals by ligating the common bile duct, resulting in entrapment of hydrophobic bile acids in the liver followed rapidly by Kupffer cell activation, leukocyte immigration, fibrosis and/or hepatocyte necrosis (15,16,20,31). Indeed, the detrimental contributions of Kupffer cells to liver injury are often reported (7,32). Moreover, it has been suggested that distinct Kupffer cell subsets serve disparate functions in animal models of liver injury (3,4). Our data indicate, instead, that iMNPs infiltrating the liver account for the detrimental effects often attributed to Kupffer cells.
The mononuclear phagocyte system consists of a family of cells widely distributed throughout the body. It includes monocytes, which represent 5–10% of leukocytes circulating in the bloodstream, and fixed macrophages in the tissues, e.g., Kupffer cells (33). It is widely held in the literature that the bulk of Kupffer cells derive from progenitor cells in the bone marrow (1,34). In this regard, a recent study reported the existence of two Kupffer cell subsets: a long-lived, radio-resistant"sessile” population; and a larger, radio-sensitive population that is rapidly recruited from the periphery to inflamatory sites (4). Ostensibly, sessile Kupffer cells ultimately derive from the bone marrow acquiring properties dependent upon the hepatic environment and distinct from those expressed by cells recruited in respone to inflamation (4). More recent experiments suggest, however, that the sessile subset represents an entirely different population of myeloid cells derived from the yolk sac during embryogenesis and persistent in adult livers independent of bone marrow progenitors (35).
Egression from the bone marrow is stimulated by MCP-1 and MCP-3, and requires the cell surface expression of chemokine receptor (CCR)-2 (36,37). CCR2−/− mice exhibit a marked reduction in circulating, peripheral blood monocytes (36). Mouse monocytes can be divided into two major subsets dependent upon migration patterns and expression of the cell-surface marker, Ly-6C (30,38). While Ly-6Chi monocytes immigrate into sites of inflammation, Ly-6Clo monocytes traffic to non-inflamed sites and give rise to resident tissue macrophages and monocyte-derived dendritic cells (38,39). It is generally believed that Ly-6Chi monocytes give rise to Ly-6Clo monocytes in the bone marrow (38).
Previously, we reported that India ink injected i.v. into mice enabled the identification and subsequent laser capture microdissection of carbon particle-labeled Kupffer cells in the liver (16). Kupffer cell-associated mRNAs (i.e., Kupffer cell receptor and CSF-1 receptor messages), extracted and purified from the captured material, were enriched 20- to 30-fold relative to the total liver. While this approach permitted analysis of the genes expressed by resident hepatic macrophages, it failed to provide a means of assessing protein (i.e., cytokine) production or characterizing the iMNPs that infiltrate the liver subsequent to biliary obstruction. Injection of magnetic beads, rather than ink, prior to surgery in the experiments reported herein circumvented these shortcomings, readily enabling the separation, purification and analysis of bead-containing Kupffer cells and iMNPs.
In accordance with the experimental results of other investigators (30,38), Kupffer cells derived from sham-operated mice were CD11bloLy-6Clo (Figure 3). CD11b expression, on the other hand, was up-regulated significantly and expressed by ~31% of bead-containing Kupffer cells isolated following BDL demarcating, conceivably, Kupffer cell activation. CD11b expression is often used as a marker to identify iMNPs recently immigrating into tissues (24,40). This finding indicates that CD11b expression does not definitively differentiate iMNPs and resident hepatic macrophages. Notably, highly-enriched Kupffer cell population derived from BDL mice contained only 5% contaminating Ly-6Chi iMNPs. Recently, Bleier and co-workers reported the increased accumulation of CD11b+CD11c+CD8α−CD45+ myeloid DCs in the livers of mice at 3, 7, or 14 days post-BDL (41). We found that enriched iMNPs obtained on day 3 post-BDL expressed little or no CD11c, a marker often associated with monocyte-derived dendritic cells (data not shown). While it is conceivable that iMNPs differentiate into CD11c+ dendritic cells at some later time point, it is pertinent to note that CD11c is a cell-surface adhesion receptor expressed by a variety of cell types including activated neutrophils, which are frequently implicated in animal models of liver injury (31,42,43). Indeed, we previously reported the increased accumulation of neutrophils in the livers of mice following BDL, albeit these neutrophils were not characterized in terms of CD11c expression (31).
With the notable exception of TGF-β, iMNPs purified from the livers of mice on day 3 post-BDL produced higher cytokine (e.g., IL-6), and chemokine (i.e., KC, MIP-2 and MCP-1) levels than did Kupffer cells derived from the same animals. Previously, we reported that Kupffer cell-dependent IL-6 produced shortly after BDL suppressed liver injury (16). It is interesting to note, however, that IL-6 is a pro-inflammatory cytokine capable of exerting a wide variety of beneficial, as well as detrimental, biological effects (44). In a mouse model of liver injury, for example, short-term treatment with IL-6 accelerated hepatic regeneration and repair; chronic IL-6 exposure, on the other hand, promoted liver damage (45). Thus, while the production of IL-6 by Kupffer cells immediately after ligation is beneficial, we speculate that long-term production by iMNPs is detrimental. Furthermore, in accordance with previous reports, we speculate that the elevated production of MIP2 and KC by iMNPs and the resultant accumulation of neutrophils in the livers of BDL mice is also detrimental (31,46).
The factors that regulate the disparate capacities of Kupffer cells and iMNPs to produce cytokines/chemokines following BDL remain to be clarified. It is pertinent to note, however, that the accumulation of apoptotic neutrophils and subsequent ingestion by Kupffer cells suppressed cytokine/chemokine production by Kupffer cells in a mouse model of listeriosis (9). Other investigators reported that TGF-β produced by macrophages following the ingestion of apoptotic neutrophils in vitro suppressed proinflammatory cytokine and chemokine production in an autocrine fashion (47). Conceivably, the enhanced production of TGF-β by Kupffer cells (relative to iMNPs) suppresses their capacity to produce cytokine/chemokine following BDL.
Monocytes circulating in the peripheral blood have a relatively short half-life (~1 day in mice) prior to extravasation and differentiation into tissue macrophages (48). Approximately 8 days is required to replace the hepatic macrophage population depleted by Cl2MDP-L administration; replacement is accelerated 2-fold by inoculating i.v. a heat-killed suspension of Brucella abortus B44 (49). Similarly, BDL promoted the immigration of iMNPs into the livers of Kupffer cell-depleted mice in experiments described herein. Notably, the presence of Kupffer cells exerted a significant influence on the character of the iMNPs recovered from the bile duct ligated livers. Relative to an intact animal, the iMNP population trafficking into the livers of Kupffer cell-depleted, BDL mice exhibited a marked decrease in percentage of cells expressing Ly-6Chi (associated with inflammation) and a concomitant increase in Ly-6Clo expressing cells destined to replace tissue (i.e., hepatic) macrophages. Similarly, Kupffer cell depletion resulted in the decreased accumulation of Ly-6C+ iMNP in a mouse model of hepatic steatohepatitis (26). These data correlate with a significant reduction in a number of proinflammatory cytokines produced by iMNPs immigrating into Kupffer cell-depleted, versus non-depleted, liver following BDL. Thus, the iMNPs recovered from the livers of the BDL, Kupffer cell-depleted mice tended to resemble a population destined to replace tissue macrophages more than the iMNP population characterized in Figures 3 and 4. The Kupffer cell-dependent factors that regulate the character of the iMNPs immigrating to the liver subsequent to biliary obstruction are matters of on-going investigation.
In conclusion, Kupffer cells comprise a resident tissue macrophage population that differs significantly from the mononuclear phagocyte (iMNP) population that infiltrates the liver in response to inflammatory stimuli such as BDL. Notably, Kupffer cells play a critical role in regulating the character and biological activity of iMNPs that immigrate to the liver subsequent to biliary obstruction. Studies undertaken to clarify the role of Kupffer cells in liver disease should make a clear distinction between the functions of these resident macrophages and those expressed by iMNPs, which appear more proinflammatory.
Acknowledgments
The authors would like to thank Stephanie Terrizzi (Brown University’s Flow cytometry and sorting facility) for performing flow cytometric analyses of the samples. This study was supported by National Institutes of Health Research Grants DK068097 and funds provided by Rhode Island Hospital. Dr. CW Cheng was also supported in part by the NSC grant 99-2320-B-038-015. Purchase and operation of FACSAria was supported by NCCR SIG grant #1S10RR021051-01A2.
Abbreviations used
- ALT
alanine aminotransferase
- BDL
bile duct ligation
- CCR
chemokine receptor
- CSF-1
colony-stimulating factor-1
- IL
interleukin
- iMNPs
inflammatory mononuclear phagocytes
- KC
keratinocyte derived chemokine
- KCR
Kupffer cell receptor
- MCP
monocyte chemoattractant protein
- MIP
macrophage inflammatory protein
- NPC
nonparenchymal liver cell
- PDGF
platelet-derived growth factor
- qtPCR
quantitative real-time polymerase chain reaction
- TGF-β
transforming growth factor-β
- TNF-α
tumor necrosis factor-α
References
- 1.Naito M, Hasegawa G, Ebe Y, Yamamoto T. Differentiation and function of Kupffer cells. Med Electron Microsc. 2004;37:16–28. doi: 10.1007/s00795-003-0228-x. [DOI] [PubMed] [Google Scholar]
- 2.Bilzer M, Roggel F, Gerbes AL. Role of Kupffer cells in host defense and liver disease. Liver Int. 2006;26:1175–1186. doi: 10.1111/j.1478-3231.2006.01342.x. [DOI] [PubMed] [Google Scholar]
- 3.Kinoshita M, Uchida T, Sato A, et al. Characterization of two F4/80-positive Kupffer cell subsets by their function and phenotype in mice. J Hepatol. 2010;53:903–910. doi: 10.1016/j.jhep.2010.04.037. [DOI] [PubMed] [Google Scholar]
- 4.Klein I, Cornejo JC, Polakos NK, et al. Kupffer cell heterogeneity: functional properties of bone marrow derived and sessile hepatic macrophages. Blood. 2007;110:4077–4085. doi: 10.1182/blood-2007-02-073841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roggin KK, Kim JC, Kurkchubasche AG, et al. Macrophage phenotype during cholestatic injury and repair: the persistent inflammatory response. J Pediatr Surg. 2001;36:220–228. doi: 10.1053/jpsu.2001.20059. [DOI] [PubMed] [Google Scholar]
- 6.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 7.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salkowski CA, Neta R, Wynn TA, et al. Effect of liposome-mediated macrophage depletion on LPS-induced cytokine expression and radioprotection. J Immunol. 1995;155:3168–3179. [PubMed] [Google Scholar]
- 9.Holub M, Cheng CW, Mott S, et al. Neutrophils sequestered in the liver suppress the proinflammatory response of Kupffer cells to systemic bacterial infection. J Immunol. 2009;183:3309–3316. doi: 10.4049/jimmunol.0803041. [DOI] [PubMed] [Google Scholar]
- 10.Kolios G, Valatas V, Kouroumalis E. Role of Kupffer cells in the pathogenesis of liver disease. World J Gastroenterol. 2006;12:7413–7420. doi: 10.3748/wjg.v12.i46.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sokol RJ, Devereaux M, Dahl R, Gumpricht E. "Let there be bile"--understanding hepatic injury in cholestasis. J Pediatr Gastroenterol Nutr. 2006;43(Suppl 1):S4–S9. doi: 10.1097/01.mpg.0000226384.71859.16. [DOI] [PubMed] [Google Scholar]
- 12.Zollner G, Trauner M. Mechanisms of cholestasis. Clin Liver Dis. 2008;12:1–26. doi: 10.1016/j.cld.2007.11.010. vii. [DOI] [PubMed] [Google Scholar]
- 13.Luckey SW, Petersen DR. Activation of Kupffer cells during the course of carbon tetrachloride-induced liver injury and fibrosis in rats. Exp Mol Pathol. 2001;71:226–240. doi: 10.1006/exmp.2001.2399. [DOI] [PubMed] [Google Scholar]
- 14.Muriel P, Escobar Y. Kupffer cells are responsible for liver cirrhosis induced by carbon tetrachloride. J Appl Toxicol. 2003;23:103–108. doi: 10.1002/jat.892. [DOI] [PubMed] [Google Scholar]
- 15.Roggin KK, Papa EF, Kurkchubasche AG, Tracy TF., Jr Kupffer cell inactivation delays repair in a rat model of reversible biliary obstruction. J Surg Res. 2000;90:166–173. doi: 10.1006/jsre.2000.5879. [DOI] [PubMed] [Google Scholar]
- 16.Gehring S, Dickson EM, San Martin ME, et al. Kupffer cells abrogate cholestatic liver injury in mice. Gastroenterology. 2006;130:810–822. doi: 10.1053/j.gastro.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Osawa Y, Seki E, Adachi M, et al. Role of acid sphingomyelinase of Kupffer cells in cholestatic liver injury in mice. Hepatology. 2010;51:237–245. doi: 10.1002/hep.23262. [DOI] [PubMed] [Google Scholar]
- 18.Chavez E, Alcantar LK, Moreno MG, Muriel P. Gadolinium Chloride Inhibits the Spontaneous Resolution of Fibrosis in CCL(4)-Induced Cirrhosis. Toxicol Mech Methods. 2006;16:507–513. doi: 10.1080/15376510600773446. [DOI] [PubMed] [Google Scholar]
- 19.Sitia G, Iannacone M, Aiolfi R, et al. Kupffer cells hasten resolution of liver immunopathology in mouse models of viral hepatitis. PLoS Pathog. 2011;7:e1002061. doi: 10.1371/journal.ppat.1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harty MW, Papa EF, Huddleston HM, et al. Hepatic macrophages promote the neutrophil-dependent resolution of fibrosis in repairing cholestatic rat livers. Surgery. 2008;143:667–678. doi: 10.1016/j.surg.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Lepay DA, Nathan CF, Steinman RM, Murray HW, Cohn ZA. Murine Kupffer cells. Mononuclear phagocytes deficient in the generation of reactive oxygen intermediates. J Exp Med. 1985;161:1079–1096. doi: 10.1084/jem.161.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lepay DA, Steinman RM, Nathan CF, Murray HW, Cohn ZA. Liver macrophages in murine listeriosis. Cell-mediated immunity is correlated with an influx of macrophages capable of generating reactive oxygen intermediates. J Exp Med. 1985;161:1503–1512. doi: 10.1084/jem.161.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holt MP, Cheng L, Ju C. Identification and characterization of infiltrating macrophages in acetaminophen-induced liver injury. J Leukoc Biol. 2008;84:1410–1421. doi: 10.1189/jlb.0308173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karlmark KR, Weiskirchen R, Zimmermann HW, et al. Hepatic recruitment of the inflammatory Gr1+ monocyte subset upon liver injury promotes hepatic fibrosis. Hepatology. 2009;50:261–274. doi: 10.1002/hep.22950. [DOI] [PubMed] [Google Scholar]
- 25.Imamura M, Ogawa T, Sasaguri Y, Chayama K, Ueno H. Suppression of macrophage infiltration inhibits activation of hepatic stellate cells and liver fibrogenesis in rats. Gastroenterology. 2005;128:138–146. doi: 10.1053/j.gastro.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Miura K, Yang L, van Rooijen N, Ohnishi H, Seki E. Hepatic recruitment of macrophages promotes non-alcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012 doi: 10.1152/ajpgi.00365.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 28.Cheng CW, Duwaerts CC, Rooijen NV, et al. NK cells suppress experimental cholestatic liver injury by an interleukin-6-mediated, Kupffer cell-dependent mechanism. J Hepatol. 2010;54:746–752. doi: 10.1016/j.jhep.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salvidio E, Crosby WH. Thrombocytopenia after intravenous injection of India ink. J Lab Clin Med. 1960;56:711–716. [PubMed] [Google Scholar]
- 30.Sunderkotter C, Nikolic T, Dillon MJ, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 31.Wintermeyer P, Cheng CW, Gehring S, et al. Invariant natural killer T cells suppress the neutrophil inflammatory response in a mouse model of cholestatic liver damage. Gastroenterology. 2009;136:1048–1059. doi: 10.1053/j.gastro.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaeschke H, Gores GJ, Cederbaum AI, et al. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 33.Hume DA, Ross IL, Himes SR, et al. The mononuclear phagocyte system revisited. J Leukoc Biol. 2002;72:621–627. [PubMed] [Google Scholar]
- 34.Crofton RW, Diesselhoff-den Dulk MMC, van Furth R. The origin, kinetics, and characteristics of the Kupffer cells in the normal steady state. J Exp Med. 1978;148:1–17. doi: 10.1084/jem.148.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulz C, Gomez PE, Chorro L, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 36.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 37.Jia T, Serbina NV, Brandl K, et al. Additive roles for MCP-1 and MCP-3 in CCR2-mediated recruitment of inflammatory monocytes during Listeria monocytogenes infection. J Immunol. 2008;180:6846–6853. doi: 10.4049/jimmunol.180.10.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 39.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 40.Shi C, Velazquez P, Hohl TM, et al. Monocyte trafficking to hepatic sites of bacterial infection is chemokine independent and directed by focal intercellular adhesion molecule-1 expression. J Immunol. 2010;184:6266–6274. doi: 10.4049/jimmunol.0904160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bleier JI, Katz SC, Chaudhry UI, et al. Biliary obstruction selectively expands and activates liver myeloid dendritic cells. J Immunol. 2006;176:7189–7195. doi: 10.4049/jimmunol.176.12.7189. [DOI] [PubMed] [Google Scholar]
- 42.Adams DH, Ju C, Ramaiah SK, Uetrecht J, Jaeschke H. Mechanisms of immune-mediated liver injury. Toxicol Sci. 2010;115:307–321. doi: 10.1093/toxsci/kfq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loike JD, Sodeik B, Cao L, et al. CD11c/CD18 on neutrophils recognizes a domain at the N terminus of the A alpha chain of fibrinogen. Proc Natl Acad Sci U S A. 1991;88:1044–1048. doi: 10.1073/pnas.88.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naugler WE, Karin M. The wolf in sheep's clothing: the role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14:109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 45.Jin X, Zimmers TA, Perez EA, et al. Paradoxical effects of short- and long-term interleukin-6 exposure on liver injury and repair. Hepatology. 2006;43:474–484. doi: 10.1002/hep.21087. [DOI] [PubMed] [Google Scholar]
- 46.Ramaiah SK, Jaeschke H. Role of neutrophils in the pathogenesis of acute inflammatory liver injury. Toxicol Pathol. 2007;35:757–766. doi: 10.1080/01926230701584163. [DOI] [PubMed] [Google Scholar]
- 47.Fadok VA, Bratton DL, Konowal A, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Furth R, Cohn ZA. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buiting AMJ, De Rover Z, van Rooijen N. Brucella abortis causes an accelerated repopulation of the spleen and liver of mice by macrophages after their lipopsome-mediated depletion. J Med Microbiol. 1995;42:133–140. doi: 10.1099/00222615-42-2-133. [DOI] [PubMed] [Google Scholar]