Abstract
Testosterone has been hypothesized to modulate the trade-off between mating and parenting effort in males. Indeed, evidence from humans and other pair-bonded species suggests that fathers and men in committed relationships have lower testosterone levels than single men and men with no children. To date, only one published study has examined testosterone in relation to motherhood, finding that mothers of young children have lower testosterone than non-mothers. Here, we examine this question in 195 reproductive-age Norwegian women. Testosterone was measured in morning serum samples taken during the early follicular phase of the menstrual cycle, and marital and maternal status were assessed by questionnaire. Mothers of young children (age ≤3) had 14% lower testosterone than childless women and 19% lower testosterone than women who only had children over age 3. Among mothers, age of the youngest child strongly predicted testosterone levels. There was a trend towards lower testosterone among married women compared to unmarried women. All analyses controlled for body mass index (BMI), age, type of testosterone assay, and time of serum sample collection. This is the first study to look at testosterone concentrations in relation to marriage and motherhood in Western women, and it suggests that testosterone may differ with marital and maternal status in women, providing further corroboration of previous findings in both sexes.
Keywords: marriage, motherhood, testosterone, endocrinology
Introduction
In males, testosterone plays a key role in modulating both mating effort and paternal investment. Physiologically, testosterone facilitates reproductive effort by promoting spermatogenesis and supporting the development of sexually dimorphic traits such as upper body muscle mass and increased stature (Bribiescas, 2001). Testosterone also contributes to reproductive effort behaviorally by promoting male-male competition and mate-seeking behaviors (Archer, 2006). The “challenge hypothesis”, which emerged from avian research, posits that in species with biparental care, testosterone levels rise in contexts of male-male competition (particularly related to mating) and then decrease to facilitate care of young, dependent offspring (De Ridder et al., 2000; Nunes et al., 2000; Peters et al., 2002; Reburn and Wynne-Edwards, 1999; Wingfield et al., 1990; Ziegler, 2000). In humans, cross-sectional data suggests that testosterone levels are higher in: (1) unmarried men versus married men (Booth and Dabbs, 1993; Gray et al., 2002; Kuzawa et al., 2009); (2) uncommitted single men versus single men in committed relationships (Burnham et al., 2003; McIntyre et al., 2006; Sakaguchi et al., 2006; van Anders and Watson, 2006); and (3) non-fathers versus fathers (Gray et al., 2006b; Kuzawa et al., 2009; Muller et al., 2009). Longitudinal work supports this hypothesis as well. One cohort study found that testosterone levels were highest prior to marriage, declined during marriage, and then rose again if divorce occurred (Mazur and Michalek, 1998). Similarly, among single men without children, those who partnered and became fathers during a 4.5 year follow-up period had greater testosterone declines than those who remained single and childless (Gettler et al., 2011a).
Despite great interest in testosterone, marriage, and fatherhood in males, virtually no research has investigated this question in females and the role of testosterone in modulating female mating effort and maternal behaviors is poorly understood. Although testosterone clearly plays a critical role in some components of female mating behavior, such as libido (Braunstein et al., 2005; van Anders et al., 2007), its contribution to other aspects of female mating and parenting effort are less apparent. Traditional evolutionary theory holds that intra-sexual mating competition is weak in human females and thus if testosterone primarily modulates intra-sexual mating competition, there should be little association with mating and maternal behavior (Bateman, 1948; Trivers, 1972). Nevertheless, research suggests that testosterone may be associated with mating behaviors in females of some species. In numerous avian species, female testosterone levels vary across the breeding cycle, typically peaking during ovulation and/or prior to the egg laying period, concurrent with the acquisition of mates and territory (Cristol and Johnsen, 1994 ; Gill et al., 2007; Ketterson et al., 2005; Osorno et al., 2010). In humans, several studies have found positive associations between testosterone and female aggression or competition, however it is unclear whether these findings are relevant within a mating context (Edwards and Kurlander, 2010; Harris et al., 1996). In fact, the limited research on female testosterone levels and mating status to date has been inconsistent. While some studies have found that among heterosexual women, being in a committed, monogamous relationship is associated with lower testosterone concentrations (Edelstein et al., 2011; van Anders and Watson, 2006), others have failed to find a relationship (Hooper et al., 2011).
Similarly, the research on testosterone and motherhood is in its infancy. Although there is some evidence that in marmosets, elevated testosterone is associated with decreased care-giving among mothers (Fite et al., 2005), to our knowledge, only one published study has examined testosterone, pair-bonding, and motherhood in humans (Kuzawa et al., 2010). In a cohort of Filipino women, waking salivary testosterone levels were lower in pair-bonded versus single women, and in mothers versus non-mothers. Mothers of young children, moreover, had significantly lower testosterone levels than mothers of older children, and only motherhood remained a significant predictor of testosterone levels in multiple regression models. While the results are suggestive, the sample size (n=67) was small and population demographics skewed heavily towards very young mothers and included few married women without children. Furthermore, the study included women who were breast-feeding and/or using hormonal contraception, and testosterone levels were assayed from sample taken at different points in the menstrual cycle. Given these limitations, we attempted to replicate their study using stricter inclusion criteria in a second, demographically different population.
To this end, we used data from a Norwegian cohort study to test the following primary predictions: (1) testosterone levels are lower in married women than unmarried women; and (2) testosterone levels are lower in women with at least one child under age 3 than in women who do not have a child under 3 (i.e. are either nulliparous or have only older children). Among the mothers, furthermore, we predicted that the age of the youngest child and total parity would both be negatively associated with testosterone levels.
Materials and methods
Study Population
206 women participated in the Energy Balance and Breast Cancer Aspects (EBBA-I) study, based in Tromsø, Norway from 2000–2002. Participants were age 25–35 with self-described regular menstrual cycles and no use of hormonal contraceptives within the past six months. Women who had been pregnant or had breast-fed within the previous six months were excluded from participating in EBBA-I, as were women with known histories of infertility, gynecological disorders, or chronic illness. Women participated for one menstrual cycle and received 1000 Norwegian kroner (approximately $160 USD) on average to cover expenses associated with participation. Human subject approvals were obtained from Institutional Review Boards at all participating institutions. The study subjects and protocol have been previously described in greater detail (Furberg et al., 2005). For the current primary analyses, any woman who had complete data on testosterone level, marital status, motherhood, age, height, and weight was included in the analysis (n=195). The 11 excluded women lacked serum data (n=6), questionnaire data (n=5), or both (n=2). Only mothers were included in secondary analyses (n=84).
Testosterone concentrations
Fasting morning serum samples were collected during the early follicular phase (day 1–2) of the menstrual cycle and sample collection time was recorded to control for diurnal variation (Table 1). Fresh serum was allowed to rest for an hour after which it was centrifuged and immediately assayed at the University of Northern Norway (UNN) Department of Clinical Chemistry laboratory. Samples collected in the first few months of the study were assayed using Immuno1 from Bayer Diagnostics, after which the laboratory switched to the Elecsys 2010 assay from Roche Diagnostics. For both assays, the limit of detection was 1.0 nmol/L, which is high compared to those afforded by more recent female testosterone assay technologies. Average intra-assay variability was less than 5% for Immuno1, and inter-assay variability ranged from 4.0% for low pools to 3.7% for high pools. Average inter-assay variability was 6.5% for Elecsys 2010 and inter-assay variability ranged from 5.7% for low pools to 2.6% for high pools.
Table 1.
Characteristics of the study population (mean±S.D.).
| variable | non- mothers (n=111) |
mothers with a child age 3 or under (n=31) |
mothers with no child age 3 or under (n=53) |
p- value1 |
|---|---|---|---|---|
| Anthropometrics | ||||
| Age (years) | 29.9±3.0 | 31.9±2.4 | 32.5±2.5 | <0.0012 |
| Height (cm) | 166.9±6.6 | 168.3±7.1 | 166.4±6.0 | 0.44 |
| Weight (kg) | 66.8±11.2 | 69.8±11.2 | 69.8±12.6 | 0.21 |
| BMI (kg/m2) | 23.9±3.6 | 24.6±3.5 | 25.1 ±4.0 | 0.15 |
| Reproductive measures | ||||
| Age at menarche (years) | 13.2±1.3 | 13.3±1.2 | 12.8±1.5 | 0.19 |
| Parity | --- | 1.7±0.6 | 1.9±0.9 | 0.383 |
| % married | 48% | 84% | 89% | <0.0012 |
| Serum testosterone concentration (nmol/L) | 1.5±0.5 | 1.2±0.4 | 1.5±0.5 | 0.0064 |
| Time of sample collection (hours since midnight) | 9.1±1.1 | 9.0±0.9 | 9.1±0.9 | 0.92 |
| Energetic variables | ||||
| Average daily energy intake (kJ) | 8.11±0.18 | 8.70±1.96 | 7.79±1.92 | 0.105 |
| Average activity level (scale of 1–3) | 2.2±0.8 | 2.0±0.4 | 2.1±0.7 | 0.57 |
| Average hours of sleep per night | 8.0±1.1 | 7.2±0.7 | 7.6±0.9 | 0.00036 |
Significance level for differences across groups based on univariate ANOVAs, unless otherwise noted. Pair-wise differences as noted below.
Non-mothers differed significantly from mothers of children age≤3 and mothers of children age>3.
P-value based on two-sided, unpaired-t-test.
Mothers with a child age≤3 differed significantly from non-mothers and mothers with no child age≤3.
Mothers of children age≤3 differed significantly from mothers of children age>3.
Non-mothers differed significantly from mothers of children age≤3.
Motherhood and marriage
At baseline, women completed a questionnaire which included marital status (single, married/living as married, widowed, divorced/separated, or other). Women who were divorced or separated (n=6) were grouped with single women for these analyses. No women reported being widowed or “other”. For brevity, in this paper, we refer to the women who reported being “married/living as married” as simply, “married”. Subjects also reported whether they had children or not. The birthdates of those children were collected during follow-up interviews in fall 2004 and using that information, motherhood status, parity, and age of youngest child at baseline were calculated. From this data, two additional variables were derived: (1) youngest child ≤age 3 at baseline; (2) youngest child >age 3 at baseline. Age three was chosen as a cutoff for two reasons: (1) it tends to mark a transition away from complete physical dependence on parental care and increased self-sufficiency; and (2) the sample sizes of the two resulting groups were sufficient to make inferences about the relationship between maternal testosterone and motherhood.
Assessment of additional covariates
Based on previous work in other populations suggesting that energetic factors such as energy intake (Hainer et al., 2001), physical activity (Enea et al., 2011), and sleep (Andersen et al., 2011) may affect testosterone levels in women in some contexts, we included several additional variables in our secondary analyses. In the questionnaires, subjects reported typical hours of sleep per night as well as typical physical activity level in the past year (on a scale of 1–4; 1= sedentary or low activity, 2=moderate activities at least 4 hours per week, 3= hard activities to keep fit for at least 4 hours per week; 4=hard training or exercise for competition several times per week). Assessment of physical activity levels in the EBBA-I subjects is described in greater detail elsewhere (Emaus et al., 2008). Because very few subjects reported high activity, groups 3 and 4 were combined in the analyses. In addition to the questionnaires and clinical visits, subjects complete detailed food diaries for seven days across the cycle (days 3–6 and days 21– 23), which were then scored by nutritionists at the University of Oslo to determine typical energy intake. Height and weight at baseline were recorded using standard techniques (Furberg et al., 2005) and body mass index (BMI) was then calculated as weight (kg)/ height (m2).
Statistical analysis
All analyses were performed first with SAS Version 9.2 (SAS Institute Inc., Cary, NC, USA) and repeated in R by a second, independent analyst to confirm results (R Version 2.9.0). Because testosterone values are typically non-normally distributed, we fit our models using both the raw and log-transformed testosterone values. The log-transformed data fitted better the linear regression model assumptions which included normality and homoscedasticity of the errors and consequently was used for the current analyses. Our primary model (Model 1) was a linear regression model of log(testosterone) as a function of maternal age, BMI, time of testosterone sample collection, marital status (married or not), testosterone assay type (Immuno1 vs. Elecsys), and age of the youngest child. Age of youngest child was coded with two dummy variables to distinguish three groups: women with no children, women with a child ≤ 3 years and women with children all > 3 years. In a separate model (not shown), we also considered the interaction between marital status and age of youngest child. Because the limit of detection (LOD) of the assay was 1.0 nmol/L, all samples with testosterone levels below the LOD were assigned a value of 1.0 nmol/L for analysis. Sensitivity analyses were subsequently conducted to test whether the results differed when we instead assigned a value of LOD/ √2 or LOD/2 to testosterone concentrations below the sensitivity limit.
A secondary linear regression model (Model 2) was fit which was similar to Model 1 but with adjustment for three additional energetic variables: average daily energy intake, typical physical activity level, and typical hours of sleep per night. Finally a pair of models (Models 3 and 4) was fit for mothers only, analyzing testosterone levels in relation to parity and age of youngest child (treated continuously). Parity was modeled both as strictly ordinal (1–5 children) and as groups (1, 2, or 3+ children) and the second of the two models included the energetic variables. All variables were chosen a priori and included in the final models, even if not significant. Model assumptions of linearity between covariates and outcome and normally distributed error with constant variance were checked. For each model, we identified outliers and influential points and reran the models excluding those subjects. All p-values reported are two-tailed, with an alpha level of p=0.05.
Results
Demographic measures
Table 1 displays demographic data stratified into three groups based on motherhood status: women with no children, women with a child age ≤3 years and women with children all age >3 years. The mean age of the women was 30.9 years (range 25.0–35.9), however, non-mothers were significantly younger than both groups of mothers. Fewer than half of non-mothers were married, moreover, compared to over 80% of mothers. Mothers of young children reported significantly higher average daily energy intake than mothers of older children, but neither group differed significantly from non-mothers. Mothers of young children reported getting significantly fewer hours of sleep on a typical night than non-mothers. The three groups showed no significant differences in height, weight, BMI, age at menarche, physical activity level, or time of serum sample collection.
Association of testosterone with marriage and motherhood
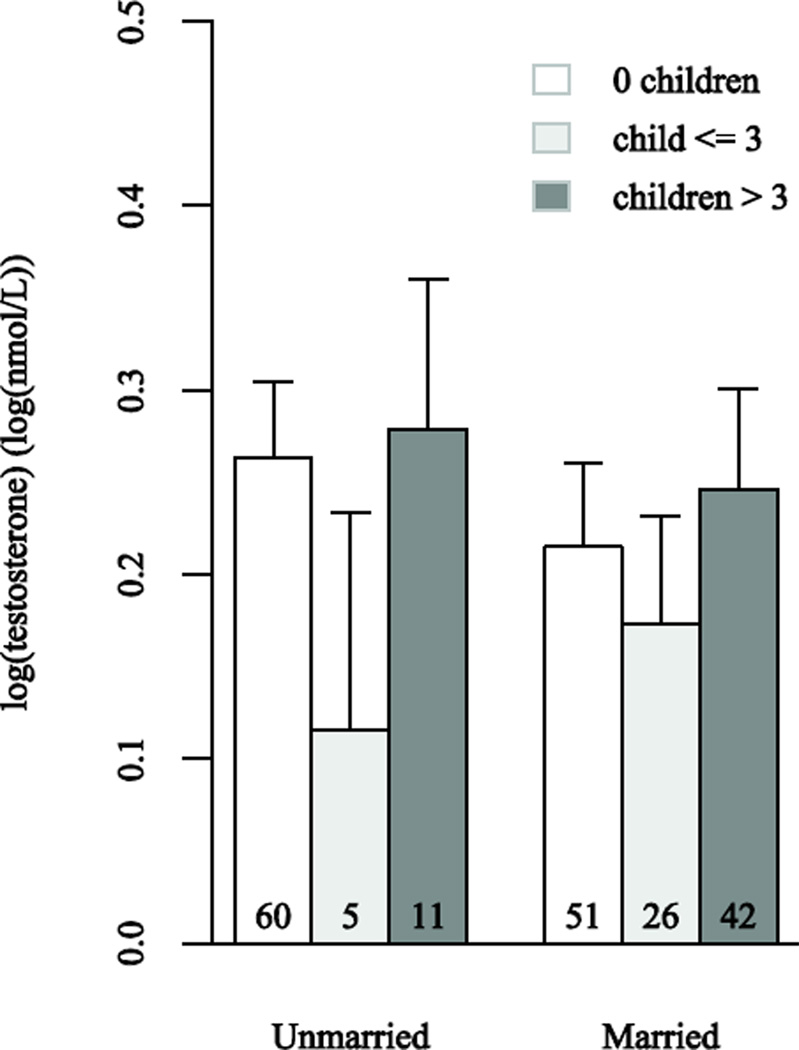
Results from our primary model (Model 1) show that after adjusting for covariates, having a child age ≤3 is associated with lower testosterone levels and there is a trend towards marriage being associated with lower testosterone levels as well (p=0.10) (Table 2). Mothers with a child age ≤3 had lower testosterone levels than both women without children (p=0.02) and mothers of older children (p=0.002; not shown). Reflecting well-documented diurnal rhythms, time of serum sample collection was also a significant predictor of testosterone levels (p=0.01). The type of testosterone assay used was a highly significant predictor of testosterone levels, but neither BMI nor age was associated with testosterone levels. In a separate model, the interaction between marital status and age of youngest child (no children, child age ≤3, and child age >3) was not significant (p=0.28 for two degree of freedom test). Figure 1 shows mean (log) testosterone values and 95% confidence limits by marital and motherhood status from this interaction model, after adjustment for age, BMI, type of testosterone assay, and time of sample collection. In our larger model (Model 2), we further adjusted for average daily energy intake, physical activity level, and hours of sleep per night, however none of these variables was a significant predictor of testosterone levels (Table 2).
Table 2.
Multiple regression models predicting testosterone levels. Testosterone data were log-transformed for the regression, thus units are expressed as log(nmol/L) with 95% CI indicated. Results in bold indicate significance at α=0.05.
| Model 1 p<0.0001; R2=0.25 (n=195) |
Model 2 P<0.0001; R2=0.25 (n=195) |
Model 3(mothers only) p=0.48; R2=0.11 (n=84) |
||||
|---|---|---|---|---|---|---|
| Regression coefficient |
p- value |
Regression coefficient |
p- value |
Regression coefficient |
p- value |
|
| Married1 | −0.033 (−0.072, 0.007) |
0.10 | −0.033 (−0.073, 0.007) |
0.10 | 0.005 (−0.077, 0.088) |
0.90 |
| Mother of child age 3 or under2 | −0.065 (−0.118,−0.011) |
0.02 | −0.065 (−0.112,−0.007) |
0.03 | — | — |
| Mother of older child(ren) only2 | 0.024 (−0.023, 0.070) |
0.32 | 0.026 (−0.022, 0.074) |
0.28 | — | — |
| BMI | −0.002 (−0.003, 0.007) |
0.44 | 0.002 (−0.003, 0.007) |
0.47 | −0.0008 (−0.009, 0.007) |
0.84 |
| Age | −0.002 (−0.008, 0.005) |
0.58 | −0.002 (−0.008, 0.005) |
0.62 | −0.003 (−0.016, 0.010) |
0.65 |
| Time of serum collection | −0.023 (−0.040, −0.005) |
0.01 | −0.023 (−0.041,−0.005) |
.01 | 0.002 (−0.032, 0.035) |
0.93 |
| Testosterone assay type3 | 0.122 (0.080,0.164) |
<0.001 | 0.124 (0.082, 0.168) |
<0.001 | 0.083 (−0.006,0.171) |
0.07 |
| Average energy intake | — | — | 0.002 (−0.008, 0.012) |
0.71 | — | — |
| Physical activity level (medium)4 | — | — | −0.002 (−0.054, 0.050) |
0.94 | — | — |
| Physical activity level (high)4 | — | — | −0.013 (−0.072, 0.046) |
0.67 | — | — |
| Typical hours of sleep per night | — | — | 0.003 (−0.016, 0.022) |
0.73 | — | — |
| Age of youngest child (years) | — | — | — | — | 0.014 (0.004, 0.025) |
0.009 |
| Parity (1, 2, 3+) | — | — | — | — | 0.018 (−0.031,0.068) |
0.46 |
Compared to unmarried.
Compared to women without children.
Immuno1 compared to Elecsys testosterone assay type.
Compared to low physical activity level.
Figure 1.
Comparison of mean log(testosterone) levels in log(nmol/L) by marital and motherhood status. The error bars show the upper 95% confidence interval for the predicted response among subjects with the Immuno1 testosterone assay and the mean values of BMI, age, and time of serum sample collection.
Predictors of testosterone levels among mothers
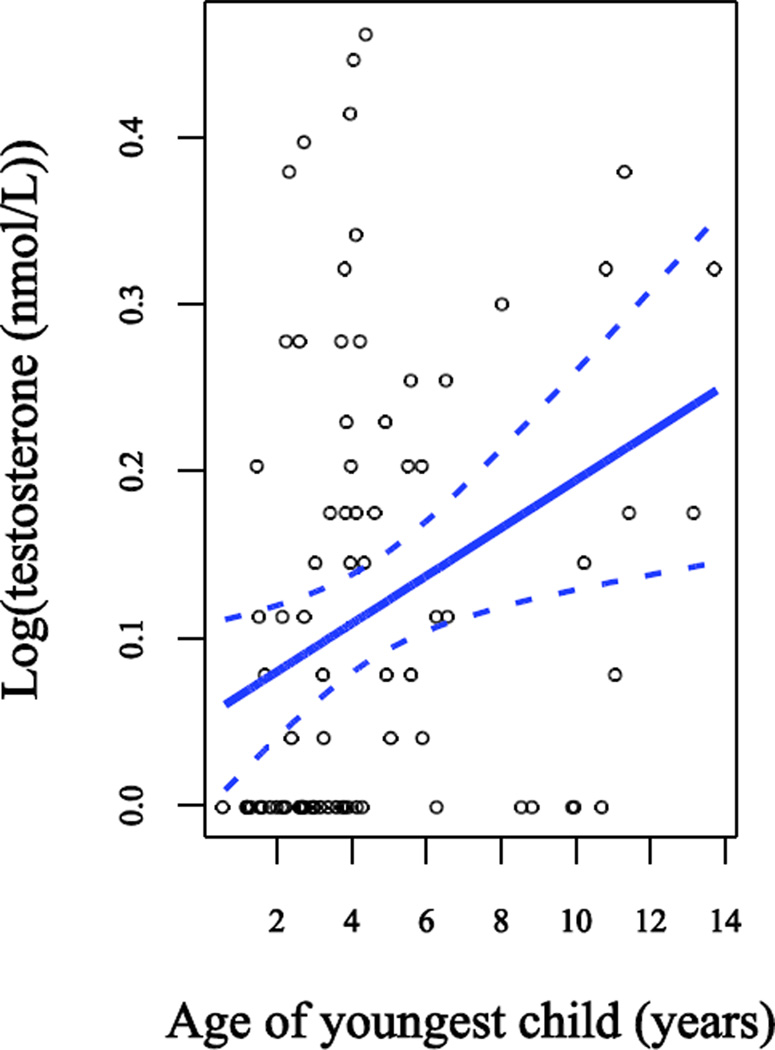
In Model 3 we investigated the influence of parity and age of youngest child (entered as a continuous variable) on testosterone concentrations in mothers, further adjusting for marital status, age, BMI, type of testosterone assay, and time of serum sample collection (Table 2). Age of youngest child was highly significantly and positively associated with testosterone levels (p=0.009) among the 84 mothers in the sample. Time of sample collection was no longer a significant predictor of testosterone levels. The results were unchanged regardless of whether we considered parity continuously (1–5 children) or by group (1, 2, or 3+ children), and the latter is presented in Table 2. When we further adjusted for the energetic variables (caloric intake, activity level, and total sleep), results were very similar and none of the energetic variables was a significant predictor (Model 4, not shown).
Model assumptions were reasonable when using testosterone on the logarithmic scale. The exclusion of outliers and influential points did not change the estimates for our covariates by more than 5%, therefore all subjects were retained in the models. In sensitivity analyses assigning a testosterone value of LOD/√2 (rather than LOD) to samples below the sensitivity limit (not shown), compared to the original models, two differences emerged. First, our estimates for the association between marriage and testosterone concentrations became larger (i.e. less conservative), however due to increased standard errors, our p-values became larger as well. Second, our estimates for the association between having a young child and testosterone concentrations became larger and the p-values became smaller, suggesting that our original estimate and p-values were a conservative assessment of that relationship. These findings were even more pronounced when we reran the sensitivity analyses instead assigning a testosterone value of LOD/2 to samples at the sensitivity limit (not shown).
Discussion
We hypothesized that marriage and motherhood would be associated with lower testosterone levels, and that testosterone levels would be particularly low among mothers of young children. In our 195 reproductive-age Norwegian women, we found differences in the predicted direction, namely married women showed a trend towards lower testosterone levels than unmarried women and mothers of children age ≤3 had significantly lower testosterone concentrations than women with older children. To our knowledge, the results of the current study are the first of their kind in Western women of older reproductive age and they confirm and extend Kuzawa et al. (2010)’s previous work. Both studies found lower testosterone levels (32% lower testosterone in the Filipino study vs. 14% in the current study) among mothers of young children compared to women who did not have young children, despite considerable differences in culture and sociodemographics. In general, the Filipino women were younger (age 20–22 vs 25–35 for our study population), many did not head their own households, and many were still in school, compared to our older, more established population.
Our findings also agree with similar research in males, in which fatherhood is frequently, though not always, associated with lower testosterone levels (Gettler et al., 2011a; Gray et al., 2004; Gray et al., 2002; Gray et al., 2006b; Kuzawa et al., 2009; Muller et al., 2009). In males, lower testosterone levels among fathers may be related to direct involvement with the daily care of children and/or the larger societal context. For instance, in two neighboring, traditional Tanzanian populations, one with high levels of direct paternal care (the Hadza), and the other with low levels of direct paternal care (the Datoga), found that only the high-investing Hadza experienced testosterone changes related to paternal status (Muller et al., 2009). Indeed, cross-cultural differences in child-rearing norms may account for mixed findings on the relationship between paternal testosterone levels and the age of the youngest offspring (Gray, 2003; Gray et al., 2002; Gray et al., 2006a; Muller et al., 2009) or the amount of direct paternal investment (Gettler et al., 2011a; Gray et al., 2002). Future research on this question in women should more closely examine quantity and quality of direct parental care in relation to maternal testosterone levels, particularly longitudinally.
Evolutionarily, the low testosterone levels found in parents of young children may represent a trade-off from investment in mating effort to investment in parenting effort. While testosterone facilitates intra-sexual competition for mates, libido, and aggression, those behaviors may be unnecessary for, or even incompatible with, effective parenting, and thus there may have been natural selection for lowered testosterone levels coincident with increased parental investment (Gray and Campbell, 2009). Alternatively, low testosterone levels in parents of young children could be a by-product of the high energetic demands of caring for a small child, given that some types of acute or chronic stress may be associated with reduced testosterone levels (Bribiescas, 2001). To address this possibility, we included average energy intake, physical activity level, and hours of sleep per night in a larger model, however none of these variables predicted testosterone levels. Other studies have similarly failed to identify energetic variables as important mediators in the relationship between marriage, parenting, and testosterone (Gettler et al., 2011b; Gray et al., 2002; Kuzawa et al., 2010), thus we suggest that the low testosterone levels seen in mothers of young children are unlikely to be attributable to energetic stressors.
One strength of our study is that unlike previous work, we used multivariable modeling to examine how the interaction between marital and parental status was associated with testosterone levels. Among childless women and mothers of older children, marriage tended to be associated with lower testosterone levels. Interestingly, among mothers of young children, the opposite pattern was present, whereby marriage was associated with higher testosterone levels. Although the sample size for that sub-population was small and the results were not statistically significant, it suggests that in those women, there may be an extreme trade-off between intense investment in parental care and minimal investment in mating effort. Because human children are so altricial and our species’ evolved family structures tend to facilitate biparental care, single mothers of young children represent an evolutionarily novel situation. Notably, single mothers of older children did not have lower testosterone levels, possibly indicating that once dependent care becomes less demanding, single mothers can again invest in mating effort. It is also possible that elevated psychosocial stress commonly reported among single mothers (Avison et al., 2007; Franz et al., 2003) may manifest itself in physiological changes that reduce testosterone and other markers of mating effort, though we know of no research that directly addresses this question.
Another notable strength of our study was our inclusion criteria. In Kuzawa et al. (2010), there was cross-subject variation as to when, during the menstrual cycle, testosterone concentrations were measured, and some subjects were using oral contraceptives or breast-feeding at the time of participation. Although the authors considered these important covariates during analysis, our study design reduced or entirely eliminated these potential sources of variation in testosterone levels. Because the original EBBA-I study was designed to look at natural variation in hormone levels across the cycle, women were excluded if they had been pregnant or breast-feeding within the last six months. Similarly, because subjects were ineligible if they had used hormonal contraception within the previous six months and all serum samples were taken within a small window of the follicular phase of the menstrual cycle, thus we can rule out those variables as potential confounders in our analyses.
Issues with the testosterone assays used present several limitations for our study, unfortunately. First, our testosterone level were measured in serum, rather than saliva, as in many related studies (Gray et al., 2002; Kuzawa et al., 2010) and thus reflect free (biologically active) hormone levels as well as the bound, inactive fraction. Ideally, we would prefer to examine both the free and the total levels, as can be done with current assay technologies, however it is worth noting that we found the same relationships with total serum testosterone that have previously been found in studies analyzing salivary (free) testosterone and previous work has found similar relationships between testosterone and fatherhood regardless of whether testosterone was measured in saliva or serum (Kuzawa et al., 2009).
Second, the high LOD of the testosterone assay (1.0 nmol/L) meant that the range of values below 1.0 nmol/L was truncated and all women with testosterone levels at or below that level were assigned a value of 1.0 nmol/L (which actually represents the highest possible concentration that they could have). Because women with young children were disproportionately represented among subjects with testosterone levels at the LOD, it is likely that their group mean is even lower, suggesting that our findings may actually be an underestimate of the true relationship between having a young child and testosterone levels. When we conducted sensitivity analyses assigning LOD//√2 or LOD/2 (rather than simply the LOD, 1.0 nmol/L) to samples with values below the LOD, we found that the estimate of the association between marriage and testosterone grew stronger, but the p-value increased due to increased standard errors. The association between having a young child and testosterone levels similarly strengthened and became more significant, suggesting that we are likely underestimating the strength of the relationship. Therefore, we predict that with the more sensitive assays available today, we would see an even greater effect size between testosterone levels and having a young child. Finally, the interpretation of our results is complicated by the fact that the clinical laboratory switched from the use of one testosterone assay to a second one partway through the study. Although the correlation of testosterone levels measured in samples run in parallel on the two assays was high (particularly among high testosterone samples), it still represents an additional source of error and variation. Nevertheless, as shown, there were still associations between testosterone and motherhood after adjusting for testosterone assay type in our models.
In our study, one potential source of misclassification bias is based on marital status. First, our questionnaire did not differentiate between married and samboer, a legally recognized cohabitation status which is best translated as “living as married”. Unlike in some Western nations, in Norway, there is no stigma to this designation and it is common for samboer couples to have children and never formally wed. Based on that, we considered women who were married or samboer as a single group, indicative of a highly committed relationship. If women who were samboer were actually more similar to unmarried women, their exposure misclassification would likely have been non-differential and biased our results towards the null. In addition, we do not have data on sexual activity or sexual fidelity in any of the women studied (married or single), although results from other studies suggest they may be useful to consider in relation to testosterone concentrations in women (van Anders et al., 2007). Additional research is needed to disentangle the associations between testosterone, relationship status, and sexual activity in women.
Because our study was cross-sectional, we cannot determine the direction of causality or reject the possibility that women with high testosterone levels are less likely to marry and become mothers. Certainly, some clinical conditions characterized by supranormal testosterone levels, such as polycystic ovary syndrome, may be associated with lower fecundity (Mellembakken et al., 2011), however our inclusion criteria excluded women likely to have a hormonal or serious medical condition. To our knowledge, no study has examined whether testosterone levels in women within the normal range are related to their likelihood of marrying or conceiving. Our finding, furthermore, that testosterone levels were only lower in mothers of young children suggests that the suppression is temporary (“state”, not “trait”) and that as children age beyond their peak dependent years, a mother’s testosterone levels may increase.
Our findings agree with and extend previous research in females and the larger body of work in males. Nevertheless, it remains uncertain as to whether the associations between testosterone, marriage, and parenting in females represent adaptations shaped by natural selection (as has been argued persuasively in males) or whether there are alternative explanations. Limited research in other species has found that high testosterone levels may be associated with territorial defense by females, but reduced care of offspring, supporting an adaptive explanation (Fite et al., 2005; Moller et al., 2005). It is also possible that this relationship in females emerged as a by-product of selection for testosterone-mediated modulation of mating and parenting effort in males, although testosterone levels co-vary in males and females in some, but not all, species (Ketterson et al., 2005).
The fact that testosterone production in the two sexes differs further complicates the comparison. In males, the testes produce the majority of testosterone with negligible adrenal contributions, whereas in females, the ovaries and adrenals contribute approximately equally (Longcope, 1986). Thus in men, differences in testosterone associated with pair-bonding and fatherhood are presumed to be related to down-regulation of testosterone production by the testes (Kuzawa et al., 2009). While it may be most obvious to attribute differences in circulating testosterone levels among females to homologous differences in ovarian function, differences in adrenal androgen production and activity may also be implicated. A comparison of luteinizing hormone (LH) and adrenocortiocotropic hormone (ACTH) concentrations in relation to marriage and motherhood might help to resolve this question. If levels of LH, but not ACTH, differ by marital or motherhood status, it would provide evidence for differences in gonadal testosterone production, possibly as a by-product of selection in males. Conversely, differences in ACTH would implicate differences in adrenal androgen production, possibly indicating an adaptive neurobiological response to pair-bonding and parenting. Acute variation in ACTH concentrations in relation to stress and the pulsatile nature of ACTH release may make such a comparison difficult or impractical, however (Carnes et al., 1988; Lennartsson et al., 2012; Van Cauter et al., 1981). It may be similarly important to consider the role that psychological mechanisms play in modulating the relationship between testosterone, marriage, and motherhood in females. Several studies have examined maternal behaviors in relation to testosterone, finding that among new mothers, higher testosterone is associated with less affectionate behavior towards their infants (Fleming et al., 1997), more mood disturbances (Buckwalter et al., 1999), and higher scores on depression and anger inventories (Hohlagschwandtner et al., 2001). In young women, furthermore testosterone administration increased neural response to (non-related) infant crying (Bos et al., 2010).
Finally, we cannot rule out the role that other hormones involved in the co-regulation of female reproductive function may play in driving this relationship. It will be important for future research to consider not only the associations between testosterone, marriage, and motherhood, but also the role of other hormones implicated in mating and nurturing behavior, such as oxytocin (Feldman, 2012; Feldman et al., 2007; Gordon et al., 2010; van Anders et al., 2011), cortisol (Fleming et al., 1997; van Bakel and Riksen-Walraven, 2008), and the ovarian steroids, estradiol and progesterone (Fleming et al., 1997; Rosenblatt, 1994). Indeed, given that much ovarian testosterone is aromatized to estrogen, it is possible that the upregulation of testosterone production associated with mating effort is, in fact, due to selection for increased estrogen production. Similarly, the lower testosterone we have found among women with young children could represent selection for temporary reproductive suppression through decreased estrogen levels. This possibility may be further explored by examining estrogen levels in relation to maternal status.
In conclusion, our study showed that among Norwegian women, both marriage and motherhood were associated with lower testosterone levels and that having a child age ≤3 was a particularly strong predictor of low testosterone levels. Future work should address longitudinal changes in female testosterone levels over time, the biological mechanisms underlying the relationships, and the association between testosterone and quantity and quality of direct maternal care.
Figure 2.
Relationship between log(testosterone) and age of youngest child. Data points are observed values. The lines show the slope and a 95% confidence interval for the slope from the regression model adjusted for marital status, time of serum sample collection, body mass index, age, parity, and testosterone assay type.
Highlights.
We examine testosterone concentrations in relation to marriage and motherhood.
Mothers have lower testosterone than non-mothers.
Among mothers, age of youngest child is negatively associated with testosterone.
Married women show a trend towards lower testosterone than unmarried women.
Acknowledgments
We thank the Norwegian EBBA-I study subjects, as well as our research staff, Gunn Knudsen, Anna Kirsti Jenssen and Sissel Andersen. The EBBA study was supported by a grant from the Norwegian Cancer Society (49 258, 05087); Foundation for the Norwegian Health and Rehabilitation Organizations (59010-2000/2001/2002); Aakre Foundation (5695-2000, 5754-2002), and Health Region East. The current analyses were completed under funding from the National Institutes of Health (K12 ES019852) with additional Biostatistical support from the University of Rochester CTSA (UL1 RR024160). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen ML, Alvarenga TF, Mazaro-Costa R, Hachul HC, Tufik S. The association of testosterone, sleep, and sexual function in men and women. Brain Res. 2011;1416:80–104. doi: 10.1016/j.brainres.2011.07.060. [DOI] [PubMed] [Google Scholar]
- Archer J. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neurosci. Biobehav. Rev. 2006;30:319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Avison WR, Ali J, Walters D. Family structure, stress, and psychological distress: a demonstration of the impact of differential exposure. J. Health Soc. Behav. 2007;48:301–317. doi: 10.1177/002214650704800307. [DOI] [PubMed] [Google Scholar]
- Bateman AJ. Intrasexual selection in Drosophila. Heredity. 1948;2:349–368. doi: 10.1038/hdy.1948.21. [DOI] [PubMed] [Google Scholar]
- Booth A, Dabbs JM., Jr Testosterone and men's marriages. Soc. Forces. 1993;72:463–477. [Google Scholar]
- Bos PA, Hermans EJ, Montoya ER, Ramsey NF, van Honk J. Testosterone administration modulates neural responses to crying infants in young females. Psychoneuroendocrinology. 2010;35:114–121. doi: 10.1016/j.psyneuen.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Braunstein GD, Sundwall DA, Katz M, Shifren JL, Buster JE, Simon JA, Bachman G, Aguirre OA, Lucas JD, Rodenberg C, Buch A, Watts NB. Safety and efficacy of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Arch. Intern. Med. 2005;165:1582–1589. doi: 10.1001/archinte.165.14.1582. [DOI] [PubMed] [Google Scholar]
- Bribiescas RG. Reproductive ecology and life history of the human male. Am. J. Phys. Anthropol. Suppl. 2001;33:148–176. doi: 10.1002/ajpa.10025.abs. [DOI] [PubMed] [Google Scholar]
- Buckwalter JG, Stanczyk FZ, McCleary CA, Bluestein BW, Buckwalter DK, Rankin KP, Chang L, Goodwin TM. Pregnancy, the postpartum, and steroid hormones: effects on cognition and mood. Psychoneuroendocrinology. 1999;24:69–84. doi: 10.1016/s0306-4530(98)00044-4. [DOI] [PubMed] [Google Scholar]
- Burnham TC, Chapman JF, Gray PB, McIntyre MH, Lipson SF, Ellison PT. Men in committed, romantic relationships have lower testosterone. Horm. Behav. 2003;44:119–122. doi: 10.1016/s0018-506x(03)00125-9. [DOI] [PubMed] [Google Scholar]
- Carnes M, Kalin NH, Lent SJ, Barksdale CM, Brownfield MS. Pulsatile ACTH secretion: variation with time of day and relationship to cortisol. Peptides. 1988;9:325–331. doi: 10.1016/0196-9781(88)90268-9. [DOI] [PubMed] [Google Scholar]
- Cristol DA, Johnsen TS. Spring arrival, aggression and testosterone in female red-winged blackbirds (Agelaius phoeniceus) Auk. 1994;111:210–214. [Google Scholar]
- De Ridder E, Pinxten R, Eens M. Experimental evidence of a testosterone-induced shift from paternal to mating behaviour in a facultatively polygynous songbird. Behavioral Ecology and Sociobiology. 2000;49:24–30. [Google Scholar]
- Edelstein RS, Chopik WJ, Kean EL. Sociosexuality moderates the association between testosterone and relationship status in men and women. Horm. Behav. 2011;60:248–255. doi: 10.1016/j.yhbeh.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Kurlander LS. Women's intercollegiate volleyball and tennis: effects of warm-up, competition, and practice on saliva levels of cortisol and testosterone. Horm. Behav. 2010;58:606–613. doi: 10.1016/j.yhbeh.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Emaus A, Veierod MB, Furberg AS, Espetvedt S, Friedenreich C, Ellison PT, Jasienska G, Andersen LB, Thune I. Physical activity, heart rate, metabolic profile, and estradiol in premenopausal women. Med. Sci. Sports Exerc. 2008;40:1022–1030. doi: 10.1249/MSS.0b013e318167411f. [DOI] [PubMed] [Google Scholar]
- Enea C, Boisseau N, Fargeas-Gluck MA, Diaz V, Dugue B. Circulating androgens in women: exercise-induced changes. Sports Med. 2011;41:1–15. doi: 10.2165/11536920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Feldman R. Oxytocin and social affiliation in humans. Horm. Behav. 2012;61:380–391. doi: 10.1016/j.yhbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol Sci. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Fite JE, French JA, Patera KJ, Hopkins EC, Rukstalis M, Ross CN. Elevated urinary testosterone excretion and decreased maternal caregiving effort in marmosets when conception occurs during the period of infant dependence. Horm. Behav. 2005;47:39–48. doi: 10.1016/j.yhbeh.2004.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, Ruble D, Krieger H, Wong PY. Hormonal and experiential correlates of maternal responsiveness during pregnancy and the puerperium in human mothers. Horm. Behav. 1997;31:145–158. doi: 10.1006/hbeh.1997.1376. [DOI] [PubMed] [Google Scholar]
- Franz M, Lensche H, Schmitz N. Psychological distress and socioeconomic status in single mothers and their children in a German city. Soc. Psychiatry Psychiatr. Epidemiol. 2003;38:59–68. doi: 10.1007/s00127-003-0605-8. [DOI] [PubMed] [Google Scholar]
- Furberg AS, Jasienska G, Bjurstam N, Torjesen PA, Emaus A, Lipson SF, Ellison PT, Thune I. Metabolic and hormonal profiles: HDL cholesterol as a plausible biomarker of breast cancer risk. The Norwegian EBBA Study. Cancer Epidemiol. Biomarkers Prev. 2005;14:33–40. [PubMed] [Google Scholar]
- Gettler LT, McDade TW, Feranil AB, Kuzawa CW. Longitudinal evidence that fatherhood decreases testosterone in human males. Proc. Natl. Acad. Sci. U.S.A. 2011a;108:16194–16199. doi: 10.1073/pnas.1105403108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettler LT, McDade TW, Kuzawa CW. Cortisol and testosterone in Filipino young adult men: evidence for co-regulation of both hormones by fatherhood and relationship status. Am J Hum Biol. 2011b;23:609–620. doi: 10.1002/ajhb.21187. [DOI] [PubMed] [Google Scholar]
- Gill SA, Alfson ED, Hau M. Context matters: female aggression and testosterone in a year-round territorial neotropical songbird (Thryothorus leucotis) Proceedings. Biological sciences / The Royal Society. 2007;274:2187–2194. doi: 10.1098/rspb.2007.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin, cortisol, and triadic family interactions. Physiol. Behav. 2010;101:679–684. doi: 10.1016/j.physbeh.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Gray PB. Marriage, parenting, and testosterone variation among Kenyan Swahili men. Am. J. Phys. Anthropol. 2003;122:279–286. doi: 10.1002/ajpa.10293. [DOI] [PubMed] [Google Scholar]
- Gray PB, Campbell BC. Human Male Testosterone, Pair Bonding and Fatherhood. In: Ellison PT, editor. Endocrinology of Social Relationships. Cambridge, MA: Harvard University Press; 2009. pp. 270–293. [Google Scholar]
- Gray PB, Campbell BC, Marlowe FW, Lipson SF, Ellison PT. Social variables predict between-subject but not day-to-day variation in the testosterone of US men. Psychoneuroendocrinology. 2004;29:1153–1162. doi: 10.1016/j.psyneuen.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Gray PB, Kahlenberg SM, Barrett ES, Lipson SF, Ellison PT. Marriage and fatherhood are associated with lower testosterone in males. Evolution and Human Behavior. 2002;23:193–201. [Google Scholar]
- Gray PB, Kruger A, Huisman HW, Wissing MP, Vorster HH. Predictors of South African male testosterone levels: the THUSA study. American Journal of Human Biology. 2006a;18:123–132. doi: 10.1002/ajhb.20471. [DOI] [PubMed] [Google Scholar]
- Gray PB, Yang C-FJ, Pope HG., Jr Fathers have lower salivary testosterone levels than unmarried men and married non-fathers in Beijing, China. Proceedings of the Royal Society of London - Series B: Biological Sciences. 2006b;273:333–339. doi: 10.1098/rspb.2005.3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainer V, Kunesova M, Stunkard AJ, Parizkova J, Stich V, Mikulova R, Starka L. The within-pair resemblance in serum levels of androgens, sex-hormone binding globulin and cortisol in female obese identical twins - effect of negative energy balance induced by very low-calorie diet. Horm. Metab. Res. 2001;33:417–422. doi: 10.1055/s-2001-16233. [DOI] [PubMed] [Google Scholar]
- Harris JA, Rushton JP, Hampson E, Jackson DN. Salivary testosterone and self-report aggressive and pro-social personality characteristics in men and women. Aggressive Behavior. 1996;22:321–331. [Google Scholar]
- Hohlagschwandtner M, Husslein P, Klier C, Ulm B. Correlation between serum testosterone levels and peripartal mood states. Acta Obstet Gynecol Scand. 2001;80:326–330. doi: 10.1034/j.1600-0412.2001.080004326.x. [DOI] [PubMed] [Google Scholar]
- Hooper AE, Gangestad SW, Thompson ME, Bryan AD. Testosterone and romance: the association of testosterone with relationship commitment and satisfaction in heterosexual men and women. Am J Hum Biol. 2011;23:553–555. doi: 10.1002/ajhb.21188. [DOI] [PubMed] [Google Scholar]
- Ketterson ED, Nolan V, Jr, Sandell M. Testosterone in females: mediator of adaptive traits, constraint on sexual dimorphism, or both? Am Nat. 2005;166(Suppl 4):S85–S98. doi: 10.1086/444602. [DOI] [PubMed] [Google Scholar]
- Kuzawa CW, Gettler LT, Huang Y-y, McDade TW. Mothers have lower testosterone than non-mothers: evidence from the Philippines. Horm. Behav. 2010;57:441–447. doi: 10.1016/j.yhbeh.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzawa CW, Gettler LT, Muller MN, McDade TW, Feranil AB. Fatherhood, pairbonding and testosterone in the Philippines. Horm. Behav. 2009;56:429–435. doi: 10.1016/j.yhbeh.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennartsson AK, Kushnir MM, Bergquist J, Jonsdottir IH. DHEA and DHEA-S response to acute psychosocial stress in healthy men and women. Biol. Psychol. 2012;90:143–149. doi: 10.1016/j.biopsycho.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Longcope C. Adrenal and gonadal androgen secretion in normal females. Clin. Endocrinol. Metab. 1986;15:213–228. doi: 10.1016/s0300-595x(86)80021-4. [DOI] [PubMed] [Google Scholar]
- Mazur A, Michalek J. Marriage, divorce, male testosterone. Soc. Forces. 1998;77:315–330. [Google Scholar]
- McIntyre M, Gangestad SW, Gray PB, Chapman JF, Burnham TC, O'Rourke MT, Thornhill R. Romantic involvement often reduces men's testosterone levels--but not always: the moderating role of extrapair sexual interest. J. Pers. Soc. Psychol. 2006;91:642–651. doi: 10.1037/0022-3514.91.4.642. [DOI] [PubMed] [Google Scholar]
- Mellembakken JR, Berga SL, Kilen M, Tanbo TG, Abyholm T, Fedorcsak P. Sustained fertility from 22 to 41 years of age in women with polycystic ovarian syndrome. Hum. Reprod. 2011;26:2499–2504. doi: 10.1093/humrep/der214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller AP, Garamszegi LZ, Gil D, Hurtrez-Bousses S, Eens M. Correlated evolution of male and female testosterone profiles in birds and its consequences. Behavioral Ecology and Sociobiology. 2005;58:534–544. [Google Scholar]
- Muller MN, Marlowe FW, Bugumba R, Ellison PT. Testosterone and paternal care in East African foragers and pastoralists. Proceedings of the Royal Society of London - Series B: Biological Sciences. 2009;276:347–354. doi: 10.1098/rspb.2008.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes S, Fite JE, French JA. Variation in steroid hormones associated with infant care behaviour and experience in male marmosets (Callithrix kuhlii) Anim. Behav. 2000;60:857–865. doi: 10.1006/anbe.2000.1524. [DOI] [PubMed] [Google Scholar]
- Osorno JL, Nunez-de la-Mora A, D'Alba L, Wingfield JC. Hormonal correlates of breeding behavior and pouch color in the Magnificent Frigatebird, Fregata magnificens. Gen. Comp. Endocrinol. 2010;169:18–22. doi: 10.1016/j.ygcen.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Peters A, Cockburn A, Cunningham R. Testosterone treatment suppresses paternal care in superb fairy-wrens, Malurus cyaneus, despite their concurrent investment in courtship. Behavioral Ecology and Sociobiology. 2002;51:538–547. [Google Scholar]
- Reburn CJ, Wynne-Edwards KE. Hormonal changes in males of a naturally biparental and a uniparental mammal. Horm. Behav. 1999;35:163–176. doi: 10.1006/hbeh.1998.1509. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS. Psychobiology of maternal behavior: contribution to the clinical understanding of maternal behavior among humans. Acta Paediatr. Suppl. 1994;397:3–8. doi: 10.1111/j.1651-2227.1994.tb13259.x. [DOI] [PubMed] [Google Scholar]
- Sakaguchi K, Oki M, Hasegawa T, Homma S. Influence of relationship status and personality traits on salivary testosterone among Japanese men. Personality and Individual Differences. 2006;41:1077–1087. [Google Scholar]
- Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man, 1871–1971. London: Heinemann; 1972. pp. 136–179. [Google Scholar]
- van Anders SM, Goldey KL, Kuo PX. The Steroid/Peptide Theory of Social Bonds: integrating testosterone and peptide responses for classifying social behavioral contexts. Psychoneuroendocrinology. 2011;36:1265–1275. doi: 10.1016/j.psyneuen.2011.06.001. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Hamilton LD, Schmidt N, Watson NV. Associations between testosterone secretion and sexual activity in women. Horm. Behav. 2007;51:477–482. doi: 10.1016/j.yhbeh.2007.01.003. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Watson NV. Relationship status and testosterone in North American heterosexual and non-heterosexual men and women: cross-sectional and longitudinal data. Psychoneuroendocrinology. 2006;31:715–723. doi: 10.1016/j.psyneuen.2006.01.008. [DOI] [PubMed] [Google Scholar]
- van Bakel HJ, Riksen-Walraven JM. Adrenocortical and behavioral attunement in parents with 1-year-old infants. Dev. Psychobiol. 2008;50:196–201. doi: 10.1002/dev.20281. [DOI] [PubMed] [Google Scholar]
- Van Cauter EW, Virasoro E, Leclercq R, Copinschi G. Seasonal, circadian and episodic variations of human immunoreactive beta-MSH, ACTH and cortisol. IntJPept. Protein Res. 1981;17:3–13. doi: 10.1111/j.1399-3011.1981.tb01962.x. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, Ball GF. The challenge hypothesistheoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. American Naturalist. 1990:136. [Google Scholar]
- Ziegler TE. Hormones associated with non-maternal infant care: a review of mammalian and avian studies. Folia Primatol. (Basel) 2000;71:6–21. doi: 10.1159/000021726. [DOI] [PubMed] [Google Scholar]