Abstract
Severe vitamin C deficiency (ascorbic acid; AA) was induced in gulo−/− mice incapable of synthesizing their own AA. A number of behavioral measures were studied before and during the deprivation period, including a scorbutic period, during which weight loss was observed in the mice. Mice were then resuscitated with AA supplements. During the scorbutic period gulo−/−mice showed decreased voluntary locomotor activity, diminished physical strength and increased preference for a highly palatable sucrose reward. These behaviors all returned to control levels following resuscitation. Altered trial times in subordinate mice in the tube test for social dominance in the AA-deprived mice persisted following resuscitation and may signify a depressive-like behavior in these mice. Biochemical analyses were undertaken following a second deprivation period. AA deficiency was accompanied by decreased blood glucose levels, oxidative damage to lipids and proteins in the cortex, and decreases in dopamine and serotonin metabolites in both the cortex and striatum. Given the reasonably high proportions of the population that do not consume sufficient AA in the diet, these data have important implications for physical and psychological function in the general population.
Keywords: Vitamin C, scurvy, dopamine, serotonin, behavior, oxidative stress
Introduction
It is often thought that nutritional deficiencies of common vitamins such as vitamin C (ascorbic acid; AA) are no longer a problem, particularly in developed Western cultures. However, the increase in convenience foods, and higher costs associated with preparing and eating fresh foods, mean that a large percentage of the population exist at sub-optimal levels. Depleted (< 28 μM) or deficient (< 11 μM) AA serum levels have been reported in 4 – 70 % of different populations including community samples (Hampl et al., 2004, Cahill et al., 2009), college students (Johnston et al., 1998), and hospitalized elderly (multiple populations reviewed in (Harrison, 2012)).
Scurvy is rarely seen in modern medicine, although cases are still reported, often concomitant with other disease states (e.g. diabetes). This is in sharp contrast to the reports of vitamin deficiency disease, in particular scurvy, both on land (armies, prisons and hospitals) and sea (naval fleets) that are rife throughout history. AA has a number of vital roles in the brain (reviewed in (Harrison and May, 2009)). These include its role as an antioxidant and as a cofactor for a number of related dioxygenase enzymes involved in the synthesis of collagen, neuropeptides, and neurotransmitters. Chief among the latter are to recycle tetrahydrobiopterin that is required for the hydroxylation of L-tyrosine and to donate electrons directly to dopamine β-hydroxylase in the synthesis of norepinephrine (NE) from dopamine (DA). Altered AA levels have been purported to affect catecholamine levels in guinea pigs and genetically altered mouse models, however, the data are equivocal (Deana et al., 1975, Bornstein et al., 2003). AA levels also affect locomotor responses to pharmacological interventions such as scopolamine (Harrison et al., 2010a) and methamphetamine (Chen et al., 2012), indicating a role for AA in neurotransmitter function. These known roles for AA, combined with existing scientific data, and reports of severe behavioral changes common to historical reports of scurvy, suggest a potential role for AA in several behavioral processes, particularly those linked to voluntary locomotor activity. AA uptake is controlled by specific transporters in the intestines (SVCT1 (Savini et al., 2008, Corpe et al., 2010)) and thus mega doses of AA (>1 gram) are not more effective than several daily doses of 250–500 mg. However, correcting low AA may have a beneficial effect on behaviors such as mood and depression. AA intake, supplementation and deprivation have each been shown to exert a significant effect on depression rating scales and other personality measures including stress, mood, and social introversion, indicating a further relationship between AA status and personality measures (Kinsman and Hood, 1971, Heseker et al., 1995, Brody, 2002, Oishi et al., 2009).
The aims of the present study were to investigate behavioral changes that occurred during periods of extreme AA deficiency in mice unable to synthesize vitamin C, and in addition to evaluating any changes that persisted following recovery. Behaviors investigated were based on reports of altered activity, physical strength and psychological states from both historical (Lind, 1772) and modern medical reports (Kinsman and Hood, 1971, Chang et al., 2007). Further, we sought to document the changes in brain AA levels, oxidative damage, and catecholaminergic and serotonergic function that accompanied the dietary deprivation.
Materials and Methods
Animals
Original gulo+/− mice were obtained from Mutant Mouse Regional Resource Centers (http://www.mmrrc.org, stock #000015-UCD). Both gulo−/− and gulo+/− are maintained in an in-house colony following back-crossing for more than 10 generations with C57Bl/6J mice (Jackson labs, Bar Harbor, Maine, Stock #000664). Wild-type control mice and gulo−/− mice were both maintained as separate colonies on a C57Bl/6J background. Gulo−/− mice lack a functional copy of the gulonolactone oxidase gene responsible for the last step in AA synthesis and are thus dependent on dietary AA intake. Wild-type equivalent levels of AA in tissues are maintained by providing de-ionized drinking water with 0.33 g/L AA, plus 20 μl EDTA per liter to help maintain stability of AA in solution. There were three experimental groups; wild-type control mice (WT-CON), gulo−/− control mice (GULO-CON) and gulo−/− mice that underwent experimentally-induced scurvy (GULO-SCV). Each group was comprised of eight mice with equal numbers of male and female mice. Water (supplemented or not) and standard lab chow containing negligible AA (Laboratory Rodent Diet #5001, Lab Diet) were available ad libitum at all times throughout the experiment except where stated for behavioral testing purposes. All mice were weighed weekly except during the Scurvy phase, and the initial few days of resuscitation when GULO-SCV mice were weighed daily. The critical comparison for the study was between the GULO-SCV and GULO-CON groups. The WT-CON group was included in order to be able to identify changes in the gulo−/− mice line that may exist when compared to wild-type mice from the same background strain despite adequate AA supplementation as adults (Harrison et al., 2008). All procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee and were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Experimental Ascorbic Acid treatments
WT-CON mice received non-supplemented drinking water and GULO-CON mice received AA-supplemented water (0.33 g/L) throughout the experiment. GULO-SCV mice received AA-supplemented water (0.33 g/L) throughout the Pre-Treatment phase, up until the start of the Deprivation period from which time they received non-supplemented water. The Deprivation periods lasted until it was judged that mice had developed scurvy as measured by daily weight loss for 4 to 5 days. Resuscitation was achieved by treating GULO-SCV mice with 250 mg/kg AA by intraperitoneal injection (dissolved in deionized water). A diet gel pack (DietGel 76A, Clear H2O, ME) was also placed in the cage with AA added (20 mg per mouse) to provide an additional source of oral AA, nutrition, and hydration. Throughout the rest of the Resuscitation phase GULO-SCV mice received only the AA in the drinking water (0.33 g/L). Timing of AA supplements is depicted in Figure 1.
Figure 1. Experimental design.
Ascorbic acid (AA) treatment and behavioral schedule for experimental groups. Abbreviations: WT-CON, Wild-type control; GULO-CON, Gulo−/− control; GULO-SCV, Gulo−/− scurvy. EZM, Elevated zero maze.
Behavioral tests
The complete range and timing of tests reported are shown in Figure 1. All behavior testing was undertaken using facilities of the Vanderbilt Murine Neurobehavioral Core.
Locomotor activity
Activity was measured in standard locomotor activity chambers (approx. 30 × 30 cm, ENV-510; MED Associates, Georgia, VT, USA). Activity was recorded automatically for 15 minutes by the breaking of infrared beams. Mice were always tested in the same chamber.
Elevated Zero Maze
Anxiety was measured using a standard Elevated Zero Maze (San Diego Instruments, CA). Five-minute trials were filmed from above, and the exploration paths in open and closed zones were analyzed using AnyMaze (Stoelting Co. IL).
Y-maze
Spontaneous alternation was measured in a standard Y-maze made of clear acrylic tubing, with arms 32 cm long. Mice were permitted to explore the maze for 5 minutes, during which time the experimenter noted the number and sequence of arm entries. Alternation was defined as consecutive entries into three different arms (e.g. ABC, BCA). Percent alternation was calculated as Alternations/(Entries – 2).
Social dominance
Social dominance was measured using the tube test. Two mice from the same cage were placed at either end of a 30 cm clear, acrylic tube, 2.5 cm diam., and allowed to run in toward each other. A “winner” was declared when the opposing mouse backed out (Lindzey et al., 1961, Harrison et al., 2009b). Each trial was repeated with mice entering from opposite ends of the tube to eliminate the potential for bias from the testing environment. Each mouse was tested against every other mouse in the home cage. Trial winner and latency to exit the tube were recorded for each trial. Social dominance hierarchy was determined by calculating the number of wins per mouse.
Grip strength
Forelimb grip strength was measured by dragging the mouse gently by the tail across a wire mesh attached to a force meter that automatically recorded the maximum grip strength measured. Each mouse received 5 trials in a single session to minimize effects of experimenter variability.
Horizontal beam
This task measures the ability of a mouse to balance on a cloth-wrapped, 30 cm pole and escape to a platform at either end of the apparatus (Harrison et al., 2008). Mice were given 2 trials per session and scored according to performance: from 4 - fell from apparatus (poorest performance), to, 0 – escaped to either platform within 30 s (best performance).
Wire hang
The wire hang task measures the ability of the mouse to pull itself up into a stable `four paw plus tail' posture (or three paws for larger male mice) on a non-taught plastic-covered wire after suspension from the wire by their front paws only (Harrison et al., 2008). Mice were given 2 trials per session and scored according to performance: 4 – fell from the apparatus (poorest performance), to 0 – achieved a stable posture within 30 s (best performance).
Tail suspension test
The tail suspension task measures the amount of time spent trying to escape from an inescapable situation. The mouse is suspended from a metal plate by taping the base of the tail to the plate and leaving the mouse hanging down about 10 cm above the chamber floor. The mouse is scored according to the time spent making movements to try to escape throughout the 6 min. trial. Trials were videotaped and scored by two investigators for time spent struggling. Any voluntary movement (paw wiggling or whole body contortion) was regarded as escape attempt and in this way physical weakness was not a biasing factor in the scoring as it could have been with automated measurements of struggle-force.
Pellet retrieval
This task designed to measure the mouse's desire for a highly palatable food reward. Mice were not food deprived before this task and testing was undertaken in the afternoon and it was thus presumed that consuming the pellets would not be governed by hunger. During the first trial (Scurvy 1, week 8) mice were placed in the test chamber with three peanut butter pellets (peanut butter flavor sucrose pellet, 5TUT, 20 mg, Test Diets, Richmond, IN, USA) placed in a shallow cup. Mice were permitted up to 15 minutes to consume the pellets. Latency to eat each pellet was recorded manually. During the second trial (Scurvy 2, week 15) the task was repeated in an identical manner except that in addition to the three peanut butter pellets there were also two fruit punch flavored sucrose pellets available in the same cup (5TUT, 45 mg, Test Diets, Richmond, IN, USA). During pilot testing fruit punch was the least preferred flavor compared to peanut butter and banana.
Olfactory learning and olfactory sensitivity
Methods and results from these tests are provided in supplementary data.
Biochemical analyses
All mice were sacrificed at the end of the second scorbutic period as weights in GULO-SCV approached 80% of their pre-deprivation weight, approximately 7 days following first observable weight loss. Following anesthesia and cervical dislocation, the tip of the tail was cut off and saved for genotyping, and tail blood was used for blood glucose measurement. Mice were then decapitated and trunk blood was removed, kept on wet ice for 1 hour to coagulate and spun for 20 minutes at 4°C for separation of serum. Brains were quickly removed and placed in a brain matrix (Zivic Instruments). Using the optic chiasm as a reference point a 2 mm slice was removed (1 mm either side of optic chiasm) and cortex and striatum were dissected from this slice for measurements of monoamine levels. The remaining brain was dissected into cortex, olfactory bulbs, cerebellum, and remaining midbrain areas. Liver and pancreas were also taken. All samples were frozen on dry ice and stored at −80°C until assays. Tissues for AA and malondialdehyde (MDA) determination and brain glucose were weighed, and homogenized in appropriate buffer using a Next Advance Bullet Blender air-cooled homogenizer. Samples for protein carbonyls were homogenized using handheld Teflon-glass homogenizers. Homogenates were then centrifuged at 4°C and the supernatant removed for analysis.
Ascorbic acid
AA was measured by HPLC with electrochemical detection as described previously (Harrison et al., 2008). Values were calculated per gram tissue wet weight.
Malondialdehyde
MDA was analyzed as thiobarbituric reactive substances (TBARS) as described previously (Harrison et al., 2009a). Values were calculated per gram tissue wet weight.
Protein Carbonyls
Protein carbonyls were measured according to published methods (Hawkins et al., 2009), adapted for use with 96 well plates. Values were calculated relative to protein.
Blood Glucose
Blood glucose levels were measured using Accu-Check Aviva glucometer and sample strips.
Brain Glucose
Glucose in brain samples was measured using Glucose (HK) Assay Kit (Sigma; GAHK-20), according to manufacturers instructions.
Neurotransmitters
5-HT, DA, NE and metabolites were measured by HPLC by the Vanderbilt Neurochemistry core. Tissues were homogenized in 0.1M trichloroacetic acid, which contains 10−2 M sodium acetate, 10−4 M EDTA, 5ng/ml isoproterenol (as internal standard) and 10.5 % methanol (pH 3.8). Samples of the supernatant were then analyzed for biogenic monoamines and analyzed by HPLC. Values were calculated relative to the tissue protein level.
Statistical analyses and group numbers
A small pilot experiment was conducted in five male gulo−/− mice and three male wild-type mice to ascertain time to develop scurvy and suitability of behavioral tests. The behavioral schedule was altered accordingly for the three experimental groups but dietary treatments were kept constant. Therefore, behavior is reported in eight mice per group but tissue samples for biochemical analyses of AA and oxidative stress were available for 11 WT-CON, 8 GULO-CON and 13 GULO-SCV mice. Pancreas samples were not taken from pilot mice. Monoamine levels were only analyzed in the 24 experimental mice.
Behavioral data
Behavioral data were analyzed with SPSS Version 19 for Mac. For single measure tests (e.g. Y-maze, EZM), univariate ANOVA was used. In cases of repeated testing across trials (e.g. locomotor activity, tube-test) Repeated Measures ANOVA was used. Significant omnibus ANOVA was followed with Bonferroni-corrected pairwise comparisons among groups unless otherwise stated. Data were first analyzed with both group (WT-CON; GULO-CON; GULO-SCV) and gender (male; female) as between-groups factors. There were no main effects of gender, similar to results from previous studies (Harrison et al., 2008, Harrison et al., 2010b), so data were collapsed across gender and analyses were repeated with group as the only between groups factor. Data were normally distributed except in two cases of Repeated Measures analysis (locomotor activity and tail suspension). In these cases where the assumption of sphericity was violated, analyses were corrected using Greenhouse–Geisser adjustments and corrected values are identified in the text with `G-G'.
Biochemical data
Biochemical data were analyzed in Prism 5 for Mac by Univariate ANOVA with Bonferroni post-hoc comparisons to detect differences between pairs of groups unless otherwise stated. In cases where variance in data was not equal across groups, non-parametric Kruskal-Wallis test was used followed by Dunn's Multiple Comparison Test.
Results
Mouse weights
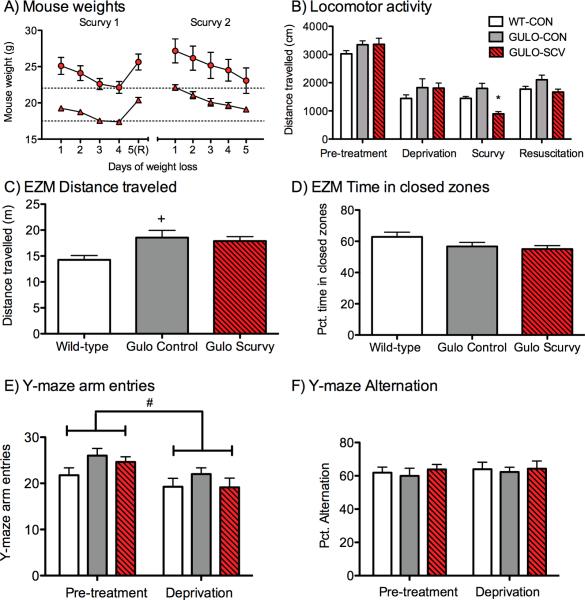
Both male and female mice showed significant weight loss during the two scurvy phases (Fs>27.09, ps<0.001, Fig. 2A). Each daily measurement was significantly lower than the initial `pre-scurvy' weight as determined by Dunnet's post-hoc test (ps<0.05). Resuscitation in the male mice led to weights equivalent to their pre-scurvy weights, whereas in the female mice there was a slight increase in body weight immediately following resuscitation compared to the initial weight (p<0.01). No decreases in body weight were seen in either of the control groups.
Figure 2. Weights, locomotor activity and control tasks.
(A) Mouse weights were recorded daily during the two Scurvy phases in male (circles) and female (triangles) mice. Weight loss was permitted to continue for up to 5 days before resuscitation (marked R on X-axis) or sacrifice. Mice were not permitted to drop below 20% of their pre-deprivation body weight (dashed lines). (B) Locomotor activity before, during and after the initial scurvy phase, GULO-SCV mice only differed from control groups during the Scurvy phase. (C) Elevated zero maze (during pre-treatment phase), distance traveled. WT-CON Mice explored the maze less than the other groups but there were no differences in time spent in open versus closed areas of the maze (D) Elevated zero maze, percent time in closed zone. Experimental groups did not differ on (E) Y-maze arm entries (during pre-treatment and first deprivation phase), or (F) Percent alternation during either test phase. *p<0.05 different from both control groups during the same testing phase, +p<0.05 different from WT-CON only #p<0.05 different from previous test phase (all groups).
Locomotor activity
Activity was measured during the Pre-treatment, Deprivation, Scurvy and Resuscitation phases. There were significant main effects of time (F(3, 63)=97.35, p<0.001, G-G) and group (F(2, 21)=3.74, p<0.05) and a Time × Group interaction (F(6, 63)=2.95, p<0.05, G-G) indicating that changes across testing phases differed among the groups (Fig. 2B). There were no significant differences among groups during the Pre-treatment or Deprivation phases (p values for individual pairwise comparisons >0.47), although all mice showed the expected decreased activity between the first two test sessions due to habituation to the chambers (ps<0.001). During the Scurvy phase, GULO-SCV mice travelled significantly less than WT-CON (p<0.05) and GULO-CON (p<0.001) groups. Furthermore, distance travelled was lower during the scurvy phase for GULO-SCV mice compared to the Deprivation and Resuscitation phases for this group (ps<0.001). Normal activity levels resumed following resuscitation, with no differences among the groups (ps>0.07) indicating that the activity decrease was specific to the Scurvy phase and was not merely additional habituation in the GULO-SCV mice.
EZM
Distance travelled and transitions between zones on the EZM varied among the groups (distance travelled F(2, 21)=4.80, p<0.05, Fig. 2C; transitions F(2, 21)=4.29, p<0.05, data not shown) because WT-CON mice travelled less than GULO-CON mice (ps<0.05). The main anxiety measure of time spent in the closed zones of the maze was not different among groups (F(2, 21)=2.48, p=0.11, Fig. 2D).
Y-maze
All three groups made fewer arm entries on the second trial compared to the first (F(1, 21)=35.06, p<0.001, Fig. 2E), however, there was no difference among the groups at either time point (Group F(2, 21)=1.36, p=0.28). Alternations did not vary among the groups on either trial (F(2, 21)=0.45, p=0.65, Fig. 2F) or between trials (F(1, 21)=0.77, p=0.39).
Grip strength
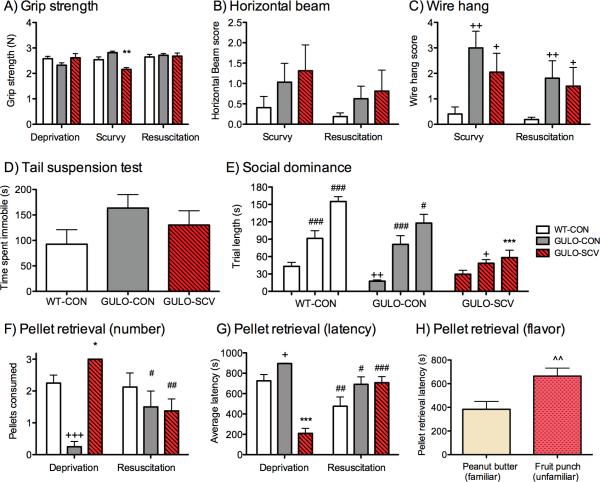
There was no main effect of group on grip strength (F(2, 21)=0.88, p=0.43), but the maximum recorded force varied across the test phases (F(2, 42)=4.20, p<0.05) according to treatment group (Time × Group interaction F(4, 42)=8.53, p<0.001). During the Deprivation and Resuscitation phases there were no differences among the three groups (ps>0.32), however, weaker grip was recorded in the GULO-SCV mice during the Scurvy phase compared to WT-CON (p<0.01) and GULO-CON (p<0.001) groups (Fig. 3A).
Figure 3. Neuromuscular measures, social and emotional tasks and pellet retrieval.
(A) Grip strength was weakened in GULO-SCV mice compared to control groups during the Scurvy phase but not during earlier Deprivation or Resuscitation phases. (B) The Horizontal beam task did not reveal differences in balance among the groups but (C) the Wire Hang task showed that both GULO-SCV and GULO-CON groups were weaker than WT-CON mice. (D) Total time spent immobile in the Tail Suspension task did not vary significantly among groups. (E) Tube-test trial length increased for all groups across the three testing sessions but to a much lesser extent in GULO-SCV mice than in the two control groups. (F) Number of pellets consumed was higher in GULO-SCV mice during Scurvy but not Resuscitation phase. (G) Pellet retrieval latency was also faster in GULO-SCV mice than in control groups during Scurvy phase. (H) Average pellet retrieval time during the second Scurvy phase was slower for fruit punch compared to peanut butter pellets. *p<0.05, **p<0.01, ***p<0.001 different from both control groups during the same testing phase. +p<0.05, ++p<0.01, +++p<0.001 different from WT-CON during the same testing phase. #p<0.05, ##p<0.01, ###p<0.001 different from previous test phase within the same group. ^^p<0.01 Fruit punch pellet retrieval latency different from peanut butter retrieval latency.
Horizontal beam and Wire hang
Horizontal beam and wire hang tests were only conducted during Scurvy and Resuscitation phases because training on these tasks can lead to improved performance which may have masked any neuromuscular deficits in GULO-SCV mice. Slight deficits in performance of the horizontal beam task in the GULO-SCV mice were not significantly different from GULO-CON or WT-CON mice (Group F(2, 21)=1.02, p=0.38). A trend toward lower scores indicating improved performance across groups during the Resuscitation phase was not significant (Trial F(1, 21)=4.10, p=0.056, Fig. 3B). On wire hang measures there was a significant main effect of group (F(2, 21)=6.78, p<0.01) but no main effect of trial (F(1, 21)=2.53, p=0.13) and no interaction between group and trial (F(2, 21)=0.89, p=0.43). Overall, both GULO-CON and GULO-SCV performed more poorly on this task than WT-CON mice (ps<0.05, Fig. 3C).
Tail suspension test
Time spent immobile increased across the six 1 min. time bins (F(5, 105)=5.34, p<0.01, G-G). Although WT-CON mice spent less time immobile over the course of the session than the other two groups, this difference was not significant (F(2, 21)=1.64, p=0.22; Fig. 3D).
Social Dominance Tube-test
The order of social hierarchy within each cage did not alter for the most and least dominant mice across sessions in different treatment phases. There were, however, significant main effects of both test session (F(2, 146)=67.97, p<0.001), and treatment group (F(2, 73)=10.82, p<0.001) on time to complete trials, with a Group by Session interaction (F(4, 146)=1.64, p<0.001; Fig. 3E). WT-CON mice took longer to complete trials than GULO-CON mice during the Pre-treatment phase (p<0.01), and longer than GULO-SCV mice during the Deprivation phase (p<0.05). The greatest differences were seen during the Resuscitation phase when `losing' GULO-SCV mice backed out of the tube significantly faster than either WT-CON or GULO-CON groups (ps<0.01). Further, significant lengthening of trial times was seen at each test session for WT-CON (ps<0.001), and GULO-CON (ps<0.05) but not in GULO-SCV (ps>0.32), although trials during Resuscitation were longer than trials during Pre-treatment in GULO-SCV mice (p<0.05).
Pellet retrieval
There was a significant main effect of group (F(2, 21)=9.83, p<0.001) and a significant Group × Trial interaction (F(2, 21)=9.58, p<0.001) on the number of pellets consumed during the first Scurvy phase and during the Resuscitation phase. GULO-SCV mice consumed more pellets than both GULO-CON (p<0.001) and WT-CON (p<0.05) mice during the Scurvy phase (Fig. 3F). In fact, all GULO-SCV mice consumed all three available pellets, which was not the case for the other two groups. GULO-CON mice also consumed fewer pellets than WT-CON mice (p<0.001). There was no difference among the groups during the Resuscitation phase (ps>0.73). Further, the number of pellets consumed during the Resuscitation phase decreased significantly in GULO-SCV mice (p<0.01), and increased significantly in GULO-CON mice (p<0.05), but did not change in WT-CON mice (p=0.79). Similarly there was a main effect of group (F(2, 21)=13.39, p<0.001) and a Group × Trial interaction (F(2, 21)=24.34, p<0.001) on time to consume the pellets (Fig. 3G). During the Scurvy phase the GULO-SCV mice consumed the pellets significantly faster than both other groups (ps<0.001), but there were no differences among the groups during the Resuscitation phase (ps>0.13).
GULO-SCV mice were re-tested on this trial during the second Deprivation period with the addition of 2 fruit punch flavored pellets. GULO-SCV mice were significantly quicker to the first consumption of peanut butter pellets than fruit punch pellets (F(1, 7)=15.15, p<0.01, Fig. 3H). Once again, all mice consumed all three available peanut butter pellets during the available 15-min. trial. In only one instance did a GULO-SCV mouse consume both fruit punch pellets, and only one mouse consumed none of the fruit punch pellets.
Ascorbic Acid levels
AA measurements were made in cortex, olfactory bulbs, liver, pancreas and serum. In each case the results followed the expected pattern of almost undetectable levels of AA in GULO-SCV mice and equivalent, normal physiological levels of AA in both WT-CON and GULO-CON groups (Table 1; ps<0.001).
Table 1.
Ascorbic acid and Oxidative stress levels in tissues from experimental mice
| WT-CON | GULO-CON | GULO-SCV | ANOVA F or Kruskal-Wallis statistic value | |
|---|---|---|---|---|
| Ascorbic acid | ||||
| Cortex (μMol/g) | 7.10 ± 0.28e | 7.58 ± 0.33e | 0.08 ± 0.01f | K-W 22.61, p<0.001 |
| Olfactory Bulbs (μMol/g) | 6.12 ± 0.82c | 4.72 ± 0.38c | 0.12 ± 0.02d | K-W 22.07, p<0.001 |
| Liver (μMol/g) | 2.73 ± 0.15a | 1.33 ± 0.24a | 0.06 ± 0.03b | K-W 26.38, p<0.001 |
| Pancreas (μMol/g) | 2.07 ± 0.30c | 2.25 ± 0.24c | 0.003 ± 0.002d | K-W 15.18, p<0.001 |
| Serum (μM) | 86.54 ± 10.07a | 52.94 ± 16.86a | 1.75 ± 1.21b | K-W 22.82, p<0.001 |
| MDA (pMol/g) | ||||
| Cortex | 1.88 ± 0.16a | 1.82 ± 0.06a | 2.58 ± 0.11b | K-W 12.70, p<0.01 |
| Liver | 0.28 ± 0.02c | 0.29 ± 0.02c | 1.40 ± 0.17d | K-W 22.65, p<0.01 |
| Pancreas | 0.27 ± 0.02e | 0.26 ± 0.02e | 0.47 ± 0.04f | F(2,21) =19.73, p<0.001 |
| Protein Carbonyls (μMol/g) | ||||
| Cortex | 12.41 ± 0.95a | 14.66 ± 1.06a,b | 18.36 ± 2.11b | K-W 5.97, p<0.05 |
Data reported are group means ± S.E.M. F and K-W values refer to results of initial omnibus analysis of data. Columns with different subscripts show differences between specific groups as revealed in follow-up comparisons
p<0.05;
p<0.01;
p<0.001.
Lipid and protein damage
MDA was measured in cortex, liver and pancreas as a measure of oxidative damage to lipids that arising from prolonged low AA levels. Significant increases in MDA were detected in GUL-SCV mice in each of the three tissues measured (ps<0.01, Table 1). Following the pattern of AA deficiency, significant differences were found between GULO-SCV mice and both control groups (p<0.05) but not between the WT-CON and GULO-CON mice. Protein carbonyls were measured in cortex as a further indication of oxidative damage to proteins. Significant groups differences were found in carbonyl measurements (p<0.05; Table 1). Carbonyls were significantly higher in GULO-SCV than WT-CON mice (p<0.05), but differences were not significant between GULO-CON mice and either other group.
Glucose levels
Blood glucose was normal in WT-CON (130±5.82 mg/dL) and GULO-CON (154.25±8.92 mg/dL), but was significantly lower in GULO-SCV mice (89±2.88 mg/dL, F(2, 28)=36.35, p<0.001); Fig. 6D). To test whether this decrease applied to the brain, glucose was also measured in cortex in 5 WT-CON, 5 GULO-CON, and 4 GULO-SCV mice, but there were no differences among the groups (F(2, 11)=2.83, p=0.10, data not shown).
Monoamine levels
Micro-dissected samples of cortex and striatum were taken for assay of levels of dopamine, norepinephrine and serotonin, plus various metabolites.
Cortex
DA levels varied somewhat among the groups with a small decrease in levels seen in GULO-SCV mice, particularly compared to the GULO-CON mice, which appeared slightly higher than WT-CON. However, these apparent variations were not significant by Univariate ANOVA (F(2, 21)=3.29, p=0.057; Table 2). Levels of three major metabolites of DA were also determined and in each case significant differences were found among the groups (Fs(2, 21)>5.73, ps<0.01) with greatly decreased levels in GULO-SCV compared to control groups (ps<0.05). NE did not differ among the experimental groups in the cortex (F(2, 21)=0.39, p=0.68). Serotonin levels did not vary among the groups (F(2, 21)=1.36, p=0.28), but similar to the dopaminergic system, the major metabolite of serotonin, 5-HIAA, was significantly altered by AA status (F(2, 21)=12.62, p<0.001). GULO-SCV mice had much decreased levels of this metabolite compared to control groups (ps<0.001).
Table 2.
Monoamine levels in tissues from experimental mice
| WT-CON | GULO-CON | GULO-SCV | ANOVA F value | |
|---|---|---|---|---|
| Cortex | ||||
| Dopamine | 14.88 ± 3.60 | 22.56 ± 4.64 | 8.69 ± 3.08 | F =3.29, p=0.057 |
| DOPAC | 3.87 ± 0.61c | 4.00 ± 0.63c | 1.45 ± 0.32d | F =7.07, p<0.01 |
| HVA | 3.81 ± 0.44a | 4.43 ± 0.51a | 2.34 ± 0.38b | F =5.73, p<0.01 |
| 3-MT | 2.36 ± 0.37a | 2.99 ± 0.56a | 0.95 ± 0.24b | F =6.34, p<0.01 |
| Norepinephrine | 4.47 ± 0.23 | 4.79 ± 0.30 | 4.71 ± 0.27 | F =0.39, p=0.68 |
| Serotonin | 4.51 ± 0.39 | 5.29 ± 0.37 | 5.39 ± 0.47 | F =1.36, p=0.28 |
| 5-HIAA | 4.38 ± 0.37e | 4.18 ± 0.34e | 2.40 ± 0.18f | F =12.62, p<0.001 |
| Striatum | ||||
| Dopamine | 95.88 ± 12.90 | 130.16 ± 17.91 | 118.64 ± 14.81 | F =1.29, p=0.30 |
| DOPAC | 12.87 ± 1.88 | 12.62 ± 1.85 | 8.15 ± 0.78 | F =2.80, p=0.083 |
| HVA | 13.80 ± 1.27 | 16.51 ± 1.94 | 12.39 ± 1.04 | F =2.033, p=0.16 |
| 3-MT | 11.63 ± 1.73 | 14.23 ± 1.74 | 11.40 ± 1.38 | F =0.94, p=0.41 |
| Norepinephrine | 2.92 ± 0.41 | 4.25 ± 0.67 | 3.08 ± 0.37 | F =2.093, p=0.15 |
| Serotonin | 8.08 ± 0.43a | 10.41 ± 0.88b | 7.63 ± 0.56a | F =5.29, p<0.05 |
| 5-HIAA | 6.45 ± 0.52c | 7.52 ± 0.48c | 4.20 ± 0.33d | F =14.03, p<0.001 |
Data reported are group mean ± S.E.M. F values refer to results of initial omnibus analysis of data. Columns with different subscripts show differences between specific groups as revealed in follow-up comparisons
p<0.05;
p<0.01.
Striatum
DA levels were approximately 12 to 18-fold higher in striatum than in cortex in GULO-CON and WT-CON groups (Table 2). This difference was about 38-fold in GULO-SCV mice. Nevertheless, there was no main effect of AA status on DA levels (F(2, 21)=1.29, p=0.30) and neither were there any significant changes in the levels of DA metabolites (Fs(2, 21)< 2.80, ps=0.08). Levels of DA metabolites were also greater in striatum than cortex but the average fold change was greater in GULO-SCV mice (DOPAC~8-fold, HVA~6-fold, 3-MT~19-fold) than in the two control groups (DOPAC~4–5-fold, HVA~4-fold, 3-MT~7.5-fold). A significant main effect of group was seen only for striatum/cortex ratios for 3-MT (F(2, 21)=4.18, p<0.05; DA, DOPAC or HVA ps>0.17). NE in striatum was not affected by AA level (F(2, 21)=2.09, p=0.15). There were significant differences in striatal serotonin levels according to AA status (F(2, 21)=5.29, p<0.05). This difference was due to a slight increase in serotonin in the GULO-CON group compared to the other two groups (ps<0.05). As in the cortex 5-HIAA levels were significantly decreased in GULO-SCV compared to the two control groups (F(2, 21)=14.03, p<0.001).
Discussion
The aims of this study were to investigate the behavioral and biochemical changes associated with AA deficiency and scurvy in a mouse model that in many ways recapitulates the human situation. These data are highly relevant today given that a high percentage of the population, even in developed Westernized cultures, exist with depleted or frankly deficient AA levels (Johnston and Thompson, 1998, Cahill et al., 2009). Indeed, cases of scurvy are not unknown in the modern era, particularly in subpopulations with dietary challenges such as diabetes, and in the elderly (Chapman and Marley, 2011, Smith et al., 2011).
In synchrony with the reports of scorbutic sailors from historical reports (Lind, 1772), scorbutic mice showed decreased voluntary locomotor activity and grip strength, and both deficits were rapidly corrected by resuscitation of AA levels. The cause of muscular weakness during early scurvy is not established by these studies, but could be due to diminished catecholamine or neuropeptide synthesis. AA also facilitates carnitine biosynthesis, which is needed for ATP biosynthesis in muscle mitochondria. Although scurvy in guinea pigs is associated with decreased muscle carnitine (Nelson et al., 1981, Rebouche, 1991), similar decreases are not found in mice unable to synthesize AA during AA depletion (Furusawa et al., 2008). It is important to note that although observation of home cage behavior showed less climbing and exploratory activity in the home cage, introduction of a physical stimulus (investigator poking the mouse) did elicit escape behaviors. Thus, mice were still capable of locomotor activity at this point and it was not outright physical impairment that decreased their activity. Decreased activity levels may have been linked to altered dopaminergic and noradrenergic signaling (Viggiano et al., 2003, Viggiano et al., 2004).
The wire hang task requires more physical strength than the horizontal beam task which requires more balance and co-ordination, but GULO-SCV mice did not perform more poorly on this than GULO-CON mice. In fact both groups of gulo−/− mice showed impairment. In contrast, grip strength was affected exclusively in the GULO-SCV group. Similar physical deficits have been reported in gulo−/− previously under physiological and low AA levels (Harrison et al., 2008, Chen et al., 2012) and it is thought that some deficit is present in gulo−/− adult mice as a result of lower AA during prenatal development (Harrison et al., 2008, Harrison et al., 2010b). The decreased ability to pull up the whole body as required for the wire hang makes it difficult to interpret the results from the tail suspension test reported here, and to fully differentiate lower movement due to depressive-like behaviors from lower movement due to physical weakness. AA supplements to wild-type mice potentiated the effects of antidepressant medication in the tail suspension test (Binfare et al., 2009, Moretti et al., 2012), and AA and vitamin E alone or combined decreased anxiety measures in rats in an open field task and also led to a diminished auditory startle response (Hughes et al., 2011). Given the severe changes in neurotransmitter levels reported here, the hypothesis that AA levels may interact with the experience of depression requires further investigation (Smith, 1999, Haenisch and Bonisch, 2011).
Originally it was hypothesized that the profile of social dominance within the cage would change with decreasing AA levels. In fact, the dominance profile did not change across the course of repeated testing. To our knowledge, there are no other reports in the literature of repeated within-cage testing in this task. We found that in both control groups the latency to back out of the maze increased significantly with each test session. The lack of any negative reinforcement from being in the tube (e.g. aggression from dominant mouse) may have served to diminish the speed at which the subordinate mouse backed out with repeated testing. The dominant mouse was not permitted to retain possession of the tube and thus the strength of the reward of winning, and the communicated pressure to the subordinate mouse to back out, may also have diminished with time. In GULO-SCV mice the increase in trial latency was not seen to the same extent, and neither did normal behavior recover following AA resuscitation. Possible reasons for this include a lack of diminishment of the social communication in the GULO-SCV mice, but a more likely explanation is that this task is tapping into a type of depressive behavior. GULO-SCV subordinate mice surrender the tube much more easily and this behavior can be considered analogous to ceasing struggling on the tail suspension task, or other types of tests of behavior despair (e.g. Porsolt forced swim (Porsolt et al., 1977)). Measurements of anxiety using the EZM were only conducted during the pre-treatment phase and not during AA deprivation so we do not have data for how anxiety levels may have altered in the mice. Of particular interest is that changes in this task were apparent during the late AA-Deprivation period but before weight loss was observed in the mice. The mice were not tested during the Scorbutic period because it was thought that lower activity levels might invalidate the task if mice were unwilling to enter or exit the tube. The lower activity levels precluded the inclusion of more tests during the scurvy periods that may have yielded more differences than when mice were tested during the deprivation periods when AA levels were low, but perhaps not low enough to manifest behavioral change. This difference was the only detected behavioral change that persisted through the Resuscitation period, suggesting a permanent change in the depressive behavior and social relationships within the cages. Further work is required to delineate the root cause of this behavioral change.
GULO-SCV mice showed a marked preference for flavored sucrose pellets, which may be an indication of craving for sweets due to hypoglycemia. Causes for hypoglycemia were not investigated, but most likely relate to decreased hepatic glucose production due to decreased food intake. This craving did not extend to unfamiliar flavored pellets in a second test. Novel taste neophobia typically drives mice to taste only small amounts of an unfamiliar food type and may have been a biasing factor in this test. However, given the size of the decrease in blood glucose it seems plausible that the urge to consume sugar could have out-weighed neophobia in this case. Of note is that glucose levels in brain, which could have had a major effect of brain function, did not vary among the groups. Olfactory learning was discontinued because neither gulo−/− group was able to perform at a level equivalent to the WT-CON mice (supplementary data). There were no differences on the olfactory sensitivity test suggesting that this may have been a problem with associative learning. We found no differences in alternation in the Y-maze, reflecting spatial working memory during the early deprivation phase. Previous reports have not found learning and memory differences in gulo−/− mice bred under the same conditions (from gulo−/− dams on 0.33 g/L AA (Harrison et al., 2008)) or bred from gulo+/− dams (Chen et al., 2012), although to our knowledge this is the first time that a test of olfactory based associative learning has been employed with these mice.
Severe deficiency in antioxidants such as AA is known to lead to oxidative stress including changes in alpha-tocopherol and F2-isoprostanes in guinea pigs (Chen et al., 2000), F2-isoprostanes, F4-neuroprostanes and glutathione in gulo−/− mice (Harrison et al., 2008, Harrison et al., 2010b), so the increased MDA in cortex, liver and pancreas, and increased protein carbonyls in cortex were expected in the GULO-SCV mice. What is not known is the extent of oxidative imbalance and how the increase in free radicals and damage could have affected brain function. The major finding of this study was the extensive disruption to dopaminergic and serotonergic systems in the GULO-SCV mice. The measurements were made following prolonged deficiency and it is a reasonable assumption that the changes in neurotransmitter function also followed a progression of severity. If this is the case then these data have important implications for patients with psychiatric illness related to either dopaminergic or serotonergic functions, and for treatment with psychoactive drugs. The implication is that nutritional status, specifically AA levels, could have a major effect on psychological function. It was assumed that 3 days of resuscitation treatments would be sufficient to replete tissues, and indeed mice gained back almost all of the lost weight (which was presumed to be mostly fluids) within 24 hours (Fig. 2a). Nevertheless, we did not directly measure tissue repletion (either by killing additional mice at that time-point, or measuring urine AA output (which can be variable and depends on water consumption).
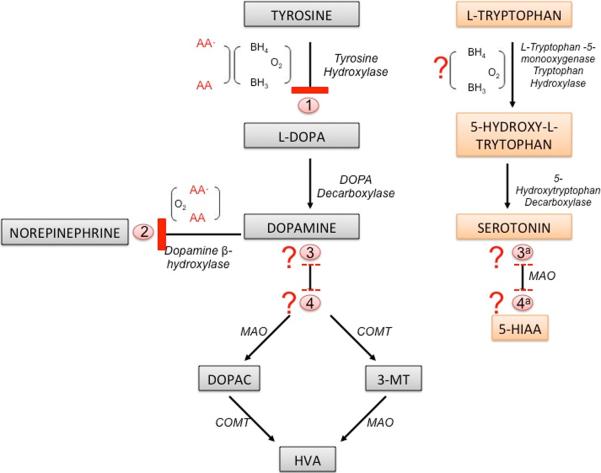
AA is known to increase catecholamine synthesis by two mechanisms. The first is to recycle tetrahydrobiopterin, which is a necessary cofactor for the conversion of tyrosine to L-DOPA by tyrosine hydroxylase (Fig. 4). A breakdown at this step in the synthesis pathway could lead to decreased DA levels (Kobayashi et al., 1995). AA treatment in combination with tyrosine stimulates DOPA, DA and NE in neuroblastoma cells (Seitz et al., 1998). AA is also required directly for the hydroxylation of dopamine by β-hydroxylase in the conversion of DA to NE. Absence of AA for this step would lead to a frank decrease in NE levels, but also potentially increase DA that would otherwise have undergone catabolism (Deana et al., 1975). These reactions require the reductive properties of AA and therefore may be affected both by its availability, and by the overall oxidative conditions in the tissues. Clear changes in DA and in NE levels in low AA conditions were not observed under the present experimental conditions. However, changes in both DA and NE have been observed in heart and adrenal tissue in SVCT2 null fetuses (with non-significant decreases in brain) (Bornstein et al., 2003), and in DA and NE in brain in scorbutic guinea pigs (Deana et al., 1975, Kaufmann et al., 1986). It is possible that the effects of AA level on the catecholamine system depend on developmental stage, tissue type, and length of AA deficiency. The non-significant trend towards decreased DA levels may reflect competition between the various steps, or other regulatory mechanisms to maintain catecholamine levels as long as possible (Kobayashi et al., 1995). The drastic decrease in the three major DA metabolites, DOPAC, HVA and 3-MT suggest one of two possibilities; either there is an as yet unknown direct role for AA in the catabolism of DA into its breakdown products, or, more likely that conservatory processes are in place to prevent breakdown of and preserve levels of the more important molecules, i.e. DA and NE rather than DA metabolites. A further consideration is that levels of each molecule could be further diminished by molecular damage due to localized oxidative stress leading to decreased reuptake. The role of AA to recycle tetrahydrobiopterin, which is also used in the conversion of L-tryptophan to 5-hydroxy-l-tryptophan, could also have accounted for the observed decreases in the serotonin metabolite, 5-HIAA (Stone and Townsley, 1973, Hori, 1975) (Fig. 4).
Figure 4. Potential mechanisms for AA involvement with neurotransmitter levels.
AA deficiency could affect monoamine synthesis and breakdown at several different points in the process: (1) inhibition of tyrosine hydroxylase via lack of recycling of tetrahydrabiopterin may cause a decrease in dopamine, (2) inhibition of dopamine β-hydroxylase would cause a decrease in norepinephrine and a resultant increase in dopamine, and (3) decreased dopamine or need to preserve dopamine levels could lead to inhibition of dopamine catabolism or (4) AA may have an as yet unknown role in synthesis of dopaminergic metabolites. If the decreased dopamine metabolites represent a process for the preservation of dopamine, then it is likely that a similar process is in place for the prevention of serotonin breakdown to 5-HIAA (3a). It is also possible that as with the dopamine metabolites there exists another as yet unknown role for AA in the breakdown of serotonin (4a). Recycling of tetrahydrobiopterin also occurs in serotonin synthesis pathway and represents a further probable role for AA. Abbreviations: BH3 - Trihydrobiopterin, BH4 – Tetrahydrobiopterin, COMT - Catechol-O-methyl-transferase, MAO - Monoamine oxidase aldehyde dehydrogenase, ? – represents unconfirmed role for AA.
Effects of AA deficiency were seen to a far greater extent in cortex than striatum as is evident in the ratios between areas. This may be easily explained by the initial difference between DA levels between the two areas. DA was 12–18 times greater in the striatum than the cortex in the two control groups and this greater pool may explain why DA and metabolite levels were not altered in the striatum of GULO-SCV groups. Nevertheless, the ratio between striatul and cortical levels was much greater in this group suggesting that some disruption had occurred although had not yet reached a critical point. There was no decrease in 5-HT levels in GULO-SCV mice in either cortex or striatum but in both tissues 5-HIAA levels were significantly lower as a result of AA deficiency. 5-HT levels were also higher in striatum compared to cortex but this difference was of a much smaller magnitude (2-fold), which may explain why changes were observable in both brain areas. No specific direct role for AA has been identified in the breakdown of 5-HT to 5-HIAA by monoamine oxidase (MAO) and subsequent steps. Thus, as is the case with the DA metabolites, it may be that the decreased metabolite levels reflect a conservation of 5-HT at the expense of downstream molecules.
NE is also metabolized at least in part through the action of MAO and COMT enzymes, thus it is also possible that breakdown products of NE may also have been affected by low AA. Although we assayed DA and 5-HT metabolites in addition to overall levels of DA, NE and 5-HT, even this combination of data may not be the clearest way to demonstrate global neurotransmitter function. Future studies should therefore also consider levels and functions of neurotransmitter receptors and transporters within the specific areas under investigation. In addition, the activity level of the enzymes themselves may help to account for the changes seen in the metabolites. Perhaps an even more elegant method to access the exact role of AA on neurotransmitter function would be to use microdialysis or voltammetry techniques. AA release from neurons in several brain areas can be evoked by glutamate, despite the lack of a clear explanation of this heteroexchange system and the relationship between the two transporters (reviewed (Rebec and Pierce, 1994)). Thus disruption of AA levels may also have a profound effect on glutamate release and uptake. Given the substantial role that glutamatergic signaling plays in major depressive disorder, alone and in combination with NE, DA and 5-HT systems (Drago et al., 2011, Sanacora et al., 2012), there is even greater reason to investigate the role of each of these neurotransmitter systems in AA-related behavioral change.
In summary, AA deficiency led to decreased voluntary locomotor activity, decreased grip strength, changes in social function and possibly behavioral despair as well as altered preference for a highly palatable sucrose reward pellet in the GULO-SCV mice. These were accompanied by almost undetectable AA levels in brain and other organs, and elevated damage to lipids and proteins indicative of a severe oxidative stress response. These changes were accompanied by a large disruption to catecholaminergic and serotonergic systems. These results have broad reaching significance. First, they offer a new perspective on historical reports of scurvy in sailors and explorers, who often suffered from physical and psychological illnesses that severely compromised their endeavors and achievements. These results offer a physiological explanation for many of these reported behaviors that may help with interpretation of such historical data. Second, and more important for modern psychiatric medicine, is that a simple and easily correctable nutritional deficiency may result in behavioral alterations and neuropathological disruption. Altered neurotransmitter function could also lead to differences in the responsiveness to psychoactive drugs meaning that psychological treatment should also be accompanied by nutritional counseling.
Supplementary Material
Acknowledgements
This work was supported by grant AG038739 NIH/NIA to FEH, NS057674 to JMM and by funds awarded to JL from the Vanderbilt University Department of English.
Abbreviations
- AA
Ascorbic acid
- DA
Dopamine
- NE
Norepinephrine
- 5-HT
Serotonin
- DOPAC
3,4-Dihydroxyphenylacetic acid
- EZM
Elevated Zero Maze
- HVA
Homovanillic acid
- 3-MT
3-methoxytyramine
- 5-HIAA
5-Hydroxyindoleacetic acid
- SVCT2
Sodium Vitamin C Transporter 2
Footnotes
The authors have no conflicts of interest to report.
Cited Literature
- Binfare RW, Rosa AO, Lobato KR, Santos AR, Rodrigues AL. Ascorbic acid administration produces an antidepressant-like effect: evidence for the involvement of monoaminergic neurotransmission. Progress in neuro-psychopharmacology & biological psychiatry. 2009;33:530–540. doi: 10.1016/j.pnpbp.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Bornstein SR, Yoshida-Hiroi M, Sotiriou S, Levine M, Hartwig HG, Nussbaum RL, Eisenhofer G. Impaired adrenal catecholamine system function in mice with deficiency of the ascorbic acid transporter (SVCT2) Faseb J. 2003;17:1928–1930. doi: 10.1096/fj.02-1167fje. [DOI] [PubMed] [Google Scholar]
- Brody S. High-dose ascorbic acid increases intercourse frequency and improves mood: a randomized controlled clinical trial. Biological psychiatry. 2002;52:371–374. doi: 10.1016/s0006-3223(02)01329-x. [DOI] [PubMed] [Google Scholar]
- Cahill L, Corey PN, El-Sohemy A. Vitamin C deficiency in a population of young Canadian adults. American journal of epidemiology. 2009;170:464–471. doi: 10.1093/aje/kwp156. [DOI] [PubMed] [Google Scholar]
- Chang CW, Chen MJ, Wang TE, Chang WH, Lin CC, Liu CY. Scurvy in a patient with depression. Digestive diseases and sciences. 2007;52:1259–1261. doi: 10.1007/s10620-005-9018-8. [DOI] [PubMed] [Google Scholar]
- Chapman JM, Marley JJ. Scurvy and the ageing population. Br Dent J. 2011;211:583–584. doi: 10.1038/sj.bdj.2011.1061. [DOI] [PubMed] [Google Scholar]
- Chen K, Suh J, Carr AC, Morrow JD, Zeind J, Frei B. Vitamin C suppresses oxidative lipid damage in vivo, even in the presence of iron overload. American journal of physiology Endocrinology and metabolism. 2000;279:E1406–1412. doi: 10.1152/ajpendo.2000.279.6.E1406. [DOI] [PubMed] [Google Scholar]
- Chen Y, Curran CP, Nebert DW, Patel KV, Williams MT, Vorhees CV. Effect of vitamin C deficiency during postnatal development on adult behavior: functional phenotype of Gulo(−/−) knockout mice. Genes, brain, and behavior. 2012;11:269–277. doi: 10.1111/j.1601-183X.2011.00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpe CP, Tu H, Eck P, Wang J, Faulhaber-Walter R, Schnermann J, Margolis S, Padayatty S, Sun H, Wang Y, Nussbaum RL, Espey MG, Levine M. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. The Journal of clinical investigation. 2010;120:1069–1083. doi: 10.1172/JCI39191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deana R, Bharaj BS, Verjee ZH, Galzigna L. Changes relevant to catecholamine metabolism in liver and brain of ascorbic acid deficient guinea-pigs. International journal for vitamin and nutrition research Internationale Zeitschrift fur Vitamin-und Ernahrungsforschung. 1975;45:175–182. [PubMed] [Google Scholar]
- Drago A, Crisafulli C, Sidoti A, Serretti A. The molecular interaction between the glutamatergic, noradrenergic, dopaminergic and serotoninergic systems informs a detailed genetic perspective on depressive phenotypes. Progress in neurobiology. 2011;94:418–460. doi: 10.1016/j.pneurobio.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Furusawa H, Sato Y, Tanaka Y, Inai Y, Amano A, Iwama M, Kondo Y, Handa S, Murata A, Nishikimi M, Goto S, Maruyama N, Takahashi R, Ishigami A. Vitamin C is not essential for carnitine biosynthesis in vivo: verification in vitamin C-depleted senescence marker protein-30/gluconolactonase knockout mice. Biological & pharmaceutical bulletin. 2008;31:1673–1679. doi: 10.1248/bpb.31.1673. [DOI] [PubMed] [Google Scholar]
- Haenisch B, Bonisch H. Depression and antidepressants: insights from knockout of dopamine, serotonin or noradrenaline re-uptake transporters. Pharmacology & therapeutics. 2011;129:352–368. doi: 10.1016/j.pharmthera.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Hampl JS, Taylor CA, Johnston CS. Vitamin C deficiency and depletion in the United States: the Third National Health and Nutrition Examination Survey, 1988 to 1994. American journal of public health. 2004;94:870–875. doi: 10.2105/ajph.94.5.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE. A critical review of vitamin C for the prevention of age-related cognitive decline and Alzheimer's disease. Journal of Alzheimer's disease : JAD. 2012;29:711–726. doi: 10.3233/JAD-2012-111853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, Dawes SM, Weaver S, May JM. Ascorbic acid attenuates scopolamine-induced spatial learning deficits in the water maze. Behavioural brain research. 2009a;205:550–558. doi: 10.1016/j.bbr.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Hosseini AH, McDonald MP. Endogenous anxiety and stress responses in water maze and Barnes maze spatial memory tasks. Behavioural brain research. 2009b;198:247–251. doi: 10.1016/j.bbr.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, May JM. Vitamin C function in the brain: vital role of the ascorbate transporter SVCT2. Free radical biology & medicine. 2009;46:719–730. doi: 10.1016/j.freeradbiomed.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, May JM, McDonald MP. Vitamin C deficiency increases basal exploratory activity but decreases scopolamine-induced activity in APP/PSEN1 transgenic mice. Pharmacology, biochemistry, and behavior. 2010a;94:543–552. doi: 10.1016/j.pbb.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Meredith ME, Dawes SM, Saskowski JL, May JM. Low ascorbic acid and increased oxidative stress in gulo(−/−) mice during development. Brain research. 2010b;1349:143–152. doi: 10.1016/j.brainres.2010.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Yu SS, Van Den Bossche KL, Li L, May JM, McDonald MP. Elevated oxidative stress and sensorimotor deficits but normal cognition in mice that cannot synthesize ascorbic acid. Journal of neurochemistry. 2008;106:1198–1208. doi: 10.1111/j.1471-4159.2008.05469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins CL, Morgan PE, Davies MJ. Quantification of protein modification by oxidants. Free radical biology & medicine. 2009;46:965–988. doi: 10.1016/j.freeradbiomed.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Heseker H, Kubler W, Pudel V, Westenhofer J. Interaction of vitamins with mental performance. Bibliotheca nutritio et dieta. 1995:43–55. doi: 10.1159/000424734. [DOI] [PubMed] [Google Scholar]
- Hori S. Effects of sulfhydryl agents on the activation of tryptophan-5- monooxygenase from bovine pineal glands. Biochimica et biophysica acta. 1975;384:58–68. doi: 10.1016/0005-2744(75)90095-9. [DOI] [PubMed] [Google Scholar]
- Hughes RN, Lowther CL, van Nobelen M. Prolonged treatment with vitamins C and E separately and together decreases anxiety-related open-field behavior and acoustic startle in hooded rats. Pharmacology, biochemistry, and behavior. 2011;97:494–499. doi: 10.1016/j.pbb.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Johnston CS, Solomon RE, Corte C. Vitamin C status of a campus population: college students get a C minus. J Am Coll Health. 1998;46:209–213. doi: 10.1080/07448489809600224. [DOI] [PubMed] [Google Scholar]
- Johnston CS, Thompson LL. Vitamin C status of an outpatient population. Journal of the American College of Nutrition. 1998;17:366–370. doi: 10.1080/07315724.1998.10718777. [DOI] [PubMed] [Google Scholar]
- Kaufmann P, Wiens W, Dirks M, Krehbiel D. Changes in social behavior and brain catecholamines during the development of ascorbate deficiency in guinea pigs. Behavioural processes. 1986;13:13–28. doi: 10.1016/0376-6357(86)90013-6. [DOI] [PubMed] [Google Scholar]
- Kinsman RA, Hood J. Some behavioral effects of ascorbic acid deficiency. The American journal of clinical nutrition. 1971;24:455–464. doi: 10.1093/ajcn/24.4.455. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Morita S, Sawada H, Mizuguchi T, Yamada K, Nagatsu I, Hata T, Watanabe Y, Fujita K, Nagatsu T. Targeted disruption of the tyrosine hydroxylase locus results in severe catecholamine depletion and perinatal lethality in mice. The Journal of biological chemistry. 1995;270:27235–27243. doi: 10.1074/jbc.270.45.27235. [DOI] [PubMed] [Google Scholar]
- Lind J. A treatise on the scurvy. S. Crowder etc; London: 1772. [Google Scholar]
- Lindzey G, Winston H, Manosevitz M. Social dominance in inbred mouse strains. Nature. 1961;191:474–476. doi: 10.1038/191474a0. [DOI] [PubMed] [Google Scholar]
- Moretti M, Colla A, de Oliveira Balen G, dos Santos DB, Budni J, de Freitas AE, Farina M, Severo Rodrigues AL. Ascorbic acid treatment, similarly to fluoxetine, reverses depressive-like behavior and brain oxidative damage induced by chronic unpredictable stress. J Psychiatr Res. 2012;46:331–340. doi: 10.1016/j.jpsychires.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Nelson PJ, Pruitt RE, Henderson LL, Jenness R, Henderson LM. Effect of ascorbic acid deficiency on the in vivo synthesis of carnitine. Biochimica et biophysics acta. 1981;672:123–127. doi: 10.1016/0304-4165(81)90286-5. [DOI] [PubMed] [Google Scholar]
- Oishi J, Doi H, Kawakami N. Nutrition and depressive symptoms in community-dwelling elderly persons in Japan. Acta Med Okayama. 2009;63:9–17. doi: 10.18926/AMO/31854. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- Rebec GV, Pierce RC. A vitamin as neuromodulator: ascorbate release into the extracellular fluid of the brain regulates dopaminergic and glutamatergic transmission. Progress in neurobiology. 1994;43:537–565. doi: 10.1016/0301-0082(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Rebouche CJ. Ascorbic acid and carnitine biosynthesis. The American journal of clinical nutrition. 1991;54:1147S–1152S. doi: 10.1093/ajcn/54.6.1147s. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savini I, Rossi A, Pierro C, Avigliano L, Catani MV. SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids. 2008;34:347–355. doi: 10.1007/s00726-007-0555-7. [DOI] [PubMed] [Google Scholar]
- Seitz G, Gebhardt S, Beck JF, Bohm W, Lode HN, Niethammer D, Bruchelt G. Ascorbic acid stimulates DOPA synthesis and tyrosine hydroxylase gene expression in the human neuroblastoma cell line SK-N-SH. Neuroscience letters. 1998;244:33–36. doi: 10.1016/s0304-3940(98)00129-3. [DOI] [PubMed] [Google Scholar]
- Smith A, Di Primio G, Humphrey-Murto S. Scurvy in the developed world. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2011;183:E752–755. doi: 10.1503/cmaj.091938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AP, Clark RE, Nutt DJ, Haller J, Hayward SG, Perry K. Vitamin C, Mood and Cognitive Function in the Elderly. Nutritional neuroscience. 1999;2:249–256. doi: 10.1080/1028415X.1999.11747281. [DOI] [PubMed] [Google Scholar]
- Stone KJ, Townsley BH. The effect of L-ascorbate on catecholamine biosynthesis. The Biochemical journal. 1973;131:611–613. doi: 10.1042/bj1310611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viggiano D, Ruocco LA, Arcieri S, Sadile AG. Involvement of norepinephrine in the control of activity and attentive processes in animal models of attention deficit hyperactivity disorder. Neural Plast. 2004;11:133–149. doi: 10.1155/NP.2004.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viggiano D, Ruocco LA, Sadile AG. Dopamine phenotype and behaviour in animal models: in relation to attention deficit hyperactivity disorder. Neuroscience and biobehavioral reviews. 2003;27:623–637. doi: 10.1016/j.neubiorev.2003.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.