Abstract
Bisphenol A (BPA) is a widely produced, endocrine disrupting compound that is pervasive in the environment. Data suggest that developmental exposure to BPA during sexual differentiation of the brain leads to later behavioral consequences in offspring. Outbred deer mice (Peromyscus maniculatus bairdii) are an excellent animal model for such studies as they exhibit well-defined sex- and steroid-dependent behaviors. Here, dams during gestation and lactation were fed a phytoestrogen-free control diet, the same diet supplemented with either ethinyl estradiol (0.1 parts per billion), or one of three doses of BPA (50 mg, 5 mg, 50 μg/kg feed weight). After weaning, pups were maintained on control diet until they reached sexually maturity and then assessed for both spatial learning capabilities and anxiety-like and exploratory behaviors. Relative to controls, males exposed to the two upper but not the lowest dose of BPA demonstrated similar impairments in spatial learning, increased anxiety and reduced exploratory behaviors as ethinyl estradiol-exposed males, while females exposed to ethinyl estradiol, but not to BPA, exhibited masculinized spatial abilities. We also determined whether dams maintained chronically on the upper dose of BPA contained environmentally relevant concentrations of BPA in their blood. While serum concentrations of unconjugated BPA in controls were below the minimum level of detection, those from dams on the BPA diet were comparable (5.48 ± 2.07ng/ml) to concentrations observed that have been observed in humans. Together, these studies demonstrate that developmental exposure to environmentally relevant concentrations of BPA can disrupt adult behaviors in a dose- and sex-dependent manner.
Keywords: diet-exposure, bisphenol A, endocrine disruptor, spatial learning, anxiety-like behavior, exploratory behavior, sexual dimorphism, serum concentrations, Peromyscus
Introduction
Endocrine disrupting compounds (EDCs) are a concern, given their potential to cause reproductive dysfunction and behavioral abnormalities (Vandenberg et al., 2009; Wolstenholme et al., 2011a). Of these chemicals, bisphenol A (BPA) is among the most pervasive (Biedermann et al., 2010; Galloway et al., 2010) and can act either as an estrogen receptor agonist or antagonist, but may also mediate effects through other steroid receptor pathways as well (Charles et al., 2007; Kuiper et al., 1998; Moriyama et al., 2002; Prasanth et al., 2010; Ryan et al., 2010; Stump et al., 2010). Given this broad range of potential molecular targets, developmental exposure to BPA might be expected to influence an assortment of phenotypic features in a sex- and dose-dependent way but not necessarily in a predictable manner.
BPA exposure has been inferred to disrupt reproductive processes,, cognitive abilities, social and emotional behaviors, and the neural networks that support these aspects of phenotype (Frye et al., 2011; Rubin, 2011), although not all authorities agree with these conclusions (Ryan et al., 2010; Stump et al., 2010). Of these studies, only a few have reported whether or not prenatal and early developmental exposure to BPA influences sexually dimorphic adult behaviors (Cox et al., 2010; Jašarević et al., 2011; Xu et al., 2011). Such traits, if under sexual selection and controlled by sex hormones, are expected to be exaggerated (Andersson and Simmons, 2006), but highly sensitive to endocrine disruption (Jašarević et al., 2011).
Sex differences in spatial learning, generally favoring males, have been reported for a variety of species, including humans (Galea and Kimura, 1993; Gaulin, 1992; Williams et al., 1990). In the laboratory, spatial learning is assessed by measuring latency to reach the escape hole, path length, errors (i.e., entering a blind hole), and search strategy. The latter is classified into three categories: 1) random/mixed strategy characterized as unorganized hole searches and multiple crossings of the maze center before locating the escape hole; 2) serial/thigmotaxic strategy, where the animal moves around the periphery of the maze in a clockwise or counter-clockwise direction and enters at least two incorrect (blind) holes prior to locating the escape hole; 3) direct strategy, where the mouse moves directly to the quadrant containing the escape hole and either immediately enters this hole or moves to an adjacent blind hole prior to entering the correct hole. Predictably, animals employing the direct strategy exhibit shorter latencies and path lengths and commit fewer errors than animals using the other two strategies (Harrison et al., 2006b; Jašarević et al., 2011; Mueller and Bale, 2007; Rodriguez et al., 2010).
For many of these laboratory tasks, males exhibit shorter latencies, commit fewer search errors, and adopt the direct search strategies more quickly than females (e.g., Galea et al. 1996; Galea et al. 1994; Jašarević et al. 2011; Jašarević et al. 2012; Rodriguez et al. 2010). However, individual differences in anxiety appear to modulate performance and sex differences on spatial learning tasks (Herrero et al. 2006), with the least anxious animals tending to perform the best (Carr et al., 2003; Cox et al., 2010; Eliam-Stock et al., 2012; Jašarević et al., 2011; Kim et al., 2011; Ryan and Vandenbergh, 2006; Xu et al., 2010; Xu et al., 2011). Although the length and route of exposure to an EDC such as BPA differed widely in these studies, the common outcome has been that exposed males exhibit longer latencies and path lengths, and higher search errors during spatial learning tasks, as well as higher anxiety-like behavior than controls (Carr et al., 2003; Cox et al., 2010; Eliam-Stock et al., 2012; Jašarević et al., 2011; Kim et al., 2011; Ryan and Vandenbergh, 2006; Xu et al., 2010; Xu et al., 2011). Given the interaction between anxiety and spatial learning, it is possible that BPA induces elevated anxiety levels in BPA-exposed animals, with compromised performance on a spatial learning tasks a secondary outcome.
While progress has been made on investigating the effects of BPA exposure on behavior, the dose-dependent effects of developmental exposure to BPA through the maternal diet on sex differences in spatial learning and anxiety-like behaviors remains unclear. Deer mouse males exposed to 50mg/kg feed weight (fw) BPA exhibited increased anxiety-like behavior and deficits in spatial learning compared with unexposed males, with no effect observed in exposed females (Jašarević et al., 2011). However, the possibility remains that BPA exposure exerts sex-dependent effects on anxiety and cognition at lower exposures. In most studies where the effects of developmental BPA exposure has been assessed in rodents after consumption of food by the mother, an upper dose of 50 mg BPA/kg fw has been considered to be still environmentally relevant (Anderson et al., 2012; Cox et al., 2010; Dolinoy et al., 2007; Wolstenholme et al., 2011b) and provides serum concentrations of BPA in laboratory mice that are close to those measured in humans unknowingly exposed to the chemical (Sieli et al., 2011). However, serum concentrations have not been measured in deer mice, which are the focus of our experiments.
Outbred deer mice were chosen as the animal for the present studies, because deer mice have maintained naturally occurring sex differences despite housing in standardized laboratory environments (Layne, 1968). Inbreeding may obscure naturally occurring sex differences (Gray, 1971; Harker and Whishaw, 2002; O’Leary et al., 2011; Thompson, 1953), and, as a result, use of inbred strains may underestimate the effect of BPA exposure on sex-dependent behaviors. The present study sought to address these gaps by measuring levels of circulating BPA in the blood of adult breeder females chronically exposed to the 50mg BPA/kg fw dose and also by determining whether the effects of development BPA exposure on sex differences in spatial learning, exploration, and anxiety-like behaviors are dose-dependent. Two behavioral mazes, Barnes Maze and Elevated Plus Maze (EPM), were used to assess the adult behavioral responses. The Barnes Maze measures spatial learning and memory, while the EPM analyzes exploratory and anxiety-like behaviors that are generally correlated with spatial cognition, as an animal has to demonstrate motivation and confidence to explore and learn about the surrounding environment.
Material and Methods
Animal husbandry
Outbred adult deer mouse females and deer mouse males (50 of each), free of common rodent pathogens, were purchased from the Peromyscus Genetic Stock Center (PGSC) at the University of South Carolina (Columbia, SC), and placed in quarantine for a minimum of 8 weeks to ensure that they did not carry any transmittable and zoonotic diseases. From the time the animals had been captured in 1948 from the wild, P. maniculatus bairdii, captive stocks have been carefully bred by the PGSC to maintain their outbred status. All experiments were approved by University of Missouri Animal Care and Use Committee and performed in accordance with National Institutes of Health Animal Care and Use Guidelines. Sentinel testing for common rodent pathogens was performed on a quarterly basis and no rodent pathogens have been detected in the colony.
Virgin females, 8 to 12 weeks of age, were randomly assigned to receive one of five diets: (i) a low phytoestrogen AIN 93G diet supplemented with 7% corn oil (CTL; Control); (ii) AIN93G supplemented with 50 mg of BPA/kg fw (upper dose; U); (iii) AIN93G supplemented with 5 mg of BPA/kg fw (M dose); (iv) AIN93G supplemented with 50 μg of BPA/kg fw (L dose); or (v) AIN93G diet supplemented with 0.1 parts per billion of ethinyl estradiol (EE), as the FDA required positive control for BPA studies (vom Saal et al., 2005). Diets were administered 2 weeks prior to mating, and dams remained on the diet throughout pregnancy and lactation, as sexual differentiation of the brain extends into the early postnatal period (McCarthy, 2008). Litters with singleton births were excluded from these studies because social isolation during development and adolescence may confound cognitive and social behavior in a sex-dependent manner in rodents (Einon, 1980). To obtain sufficient numbers of offspring some dams were bred more than once. The sample sizes of adult offspring in relation to their sex and diet exposure, plus the number of different litters sampled and the male and female offspring within each litter are summarized in Supplemental Table 1.
After weaning, all offspring were placed on the CTL diet and housed with no more than 3 same-sex siblings per cage until sexual maturity (age ≈60 d). In contrast to common laboratory rodent species, P. maniculatus bairdii does not respond well to handling (Martin et al., 2007), and thus animals were only handled during weekly cage changes and behavioral testing. To minimize background exposure to BPA beyond treatment regimen, deer mice were housed in white polypropylene cages (32cm x 18cm x 24 cm) under standard conditions (25 ± 2 °C and 50% ± 10% humidity), with ad libitum access to BPA-free water provided in glass bottles and diet specific to each treatment group. All animals were maintained on a long day light cycle (16 h light:8 h dark) to induce sexual maturity in males and females.(Layne, 1968). To reduce any potential social housing and accompanying dominance/subordinate effects, adult deer mice were moved into single-housing conditions one week prior to behavioral testing.
As female behaviors are possibly influenced by stage of the reproductive cycle, it would have been desirable to test animals at the same stage of their cycle. However, the estrous cycle of Peromyscus is poorly characterized (Dewsbury et al., 1977), and the animals may even be spontaneous ovulators (Bradley and Terman, 1979).
BPA concentrations in serum of dams chronically fed a BPA-supplemented diet
Serum was collected at 700 CST from a cohort of deer mouse females near the end of their reproductive lifespan that consumed the CTL diet (n=7), the AIN93G diet supplemented with 50mg BPA/kg fw (U dose) (n = 9) or 0.1 ppb EE/kg fw (n = 6) diet continuously for approximately 12 months. The U dose only was employed, as a previous study in which laboratory mice (Mus) received isotopically labeled BPA indicated that the U dose tested here would likely provide measurable concentrations (Sieli et al., 2011), whereas the M and L doses would not. Moreover, other studies have demonstrated that there is an approximately linear dose response curve for BPA (Doerge et al., 2010, 2011; Taylor et al., 2011; Teeguarden et al., 2011) allowing serum concentrations of the M and L lower doses to be extrapolated. Serum concentrations of conjugated and unconjugated BPA were measured as described previously (Padmanabhan et al., 2008; Sieli et al., 2011). Briefly, samples were divided into two aliquots (each of 150–200 μL) and reference standards [5 ng each of deuterated 16-BPA (BPA-d16) and 13C12-bisphenol A] were included in each to serve as an internal control to estimate recovery through the analytical steps. Analyte separation and quantification were carried out by using an Agilent 1100 series HPLC interfaced with an Applied Biosystems API 2000 electrospray tandem mass spectrometer (HPLC-MS/MS; Applied Biosystems, Foster City, CA), as described previously (Padmanabhan et al., 2008; Sieli et al., 2011). The method detection limit was 0.1 ng/mL and quantification was based on isotopic dilution method.
Spatial learning
The Barnes maze (Barnes, 1979) was used to test spatial learning and memory, but modified for Peromyscus as described in detail elsewhere (Jašarević et al., 2011; Jašarević et al., 2012). It consisted of a circular platform with 12 escape-holes, one placed every 30°, only one of which led to the home cage of the animal. Four spatial geometric shapes (triangle, square, circle, and star) were placed at the same height (~10 cm) every 90° inside the maze wall to act as cues for the mice in finding the correct escape hole.
Each animal was assigned an escape-hole number, with hole numbers for consecutively tested mice switched by 90°.However, the exit hole number and the positions of the spatial cues relative to the escape hole remained fixed for any individual animal across all acquisition trials and the subsequent probe trial. At the beginning of each training day, the maze was rotated 90°.s Animals were provided two shaping trials followed by seven days of two-trial evaluations per day for 300 s each, with a 30-min inter-trial interval. A trial consisted of placing the mouse in the center of the maze, but randomly relative to the location of the spatial cues, in an opaque starting box to allow the tracking system (described below) to detect the center body-point. The starting box was lifted, and a trial initiated once the mouse had begun to move in response to a stimulatory light measuring 1200 lux (versus ~400 lux for vivarium room lighting).. If the animal failed to enter the exit hole within the first 30 s of a trial (46% of trials), a recording of a barn owl was played to motivate predator avoidance and thus maze escape (Clarke 1983).
Each trial was recorded with an EthoVision XT video camera (Noldus Technologies, Leesburg, VA). Time to enter the assigned escape-hole (latency) and path length were tracked by using accompanying automated tracking EthoVision XT software. Latency performance was averaged across trials on the same day for each individual. Latency, escape errors and search strategy were quantified from the video recordings and tracking image composites produced by the EthoVision XT software, respectively. Fig. 2A illustrates the three main spatial strategies used by the animals in these experiments. The random search strategy (coded 1) was operationally defined as localized searches of holes separated by the animal repeatedly crossing the center of the maze. Serial search strategy (coded 2) was defined as a systematic search of consecutive holes in a clockwise or counterclockwise direction. Finally, direct search strategy (coded 3) was defined as navigating directly to the target quadrant without crossing the center of the maze more than once and with three or fewer errors (Harrison et al., 2006a).
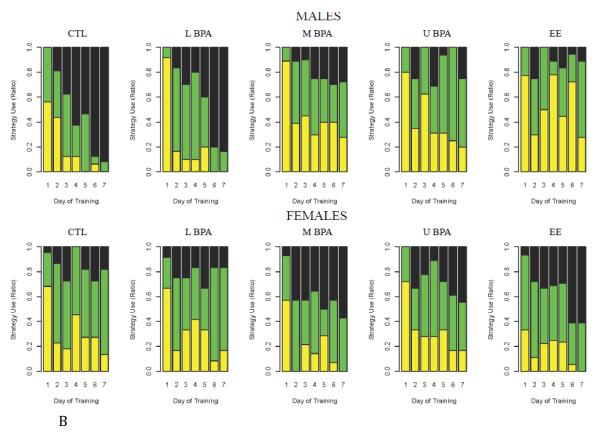
Fig. 2.
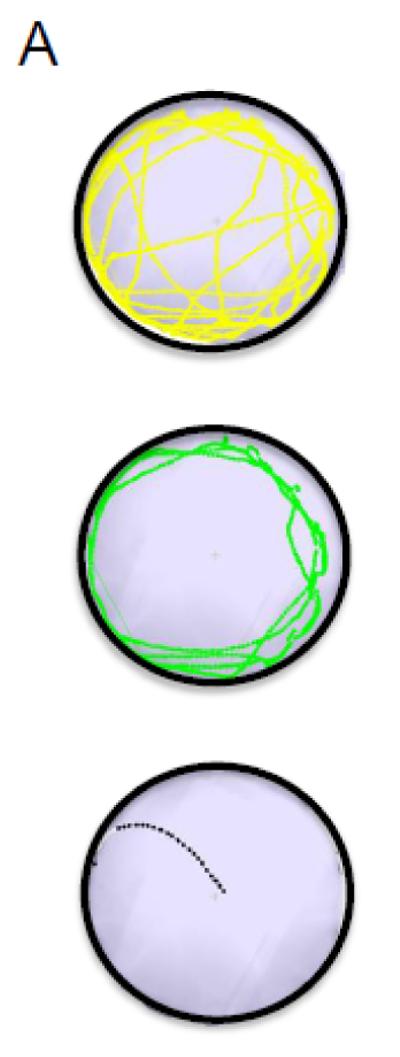
Dose- and sex-dependent effects of early developmental exposure to BPA and EE on search strategy. (A) Schematic diagram illustrating different navigational strategies used to locate correct exit hole: random (top), serial (middle) and direct (bottom). (B) Proportion of BPA, EE and CTL animals utilizing random, serial, or direct search strategies across acquisition training. During acquisition training animals navigate through the maze by using one of three strategies: the random/mixed strategy (yellow) was characterized by unorganized hole searches and crossing the center of maze several times prior to locating the escape hole; the serial/thigmotaxic strategy (green) occurred when the animal moved around the periphery of the maze in a clockwise or counter-clockwise direction and entered at least two incorrect holes prior to locating the escape hole; the direct search strategy (black) was defined by the mouse moving directly to the quadrant containing the escape hole and either immediately entering this hole or moving to an adjacent blind hole prior to entering the correct hole. Control males acquired the direct search strategy more rapidly than all cohorts except low dose BPA males and EE exposed females (Ps < 0.05; see Table 1 and Supplementary Table 2 for further details).
Exploratory and anxiety-like behavior
One week after animals were tested in the Barnes maze, exploratory and anxiety-like behaviors were measured by using the elevated plus maze (EPM), as described previously (Fountain et al., 2008; Jašarević et al., 2011; Jašarević et al., 2012). The EPM was in a plus configuration with two opposite open arms (30 cm), a middle platform (5 × 5 cm), and two opposing closed arms (30 cm). Each animal was placed on the center of the platform and allowed to explore the maze for 300 s. Each trial was recorded with EthoVision XT software (Noldus Technologies, Leesburg, VA, USA), which automatically scores total time spent in open and closed arms and number of closed and open arm entries and center entries.
Statistical analyses
Serum BPA concentration analyses: For the unconjugated and conjugated serum BPA concentrations, a completely randomized design (CRD), in which there were three dam diets (CTL, n=8; EE, n=7, and BPA, n=9), was used. PROC MIX procedure of SAS and Fisher’s protected least significance difference was then used to analyze for differences. Barnes Maze Data Analyses: The data were analyzed as a split plot in space and time (Steel, 1996). The linear statistical model contained the fixed effects of diet, sex, day and all possible interactions with diet, sex and day. In an initial analysis, the individual pup within diet and sex was used as the denominator of F for the main plot effects. Day and interactions of day with main plot effects were tested by using the residual mean square as the denominator of F. In the second analysis, litter was used as the denominator of F for day, sex, and maternal diet, while, the remaining interactions used the residual mean square as the denominator of F. Mean differences were determined by using Fisher’s protected Least Significant Difference (LSD).
Three discrete search strategies for the escape hole were defined as described previously (Jašarević et al., 2011). The first, coded 1, was random, the second (2) was serial, the third (3) (direct). The data were analyzed by using a repeated measurement design with PROC GLIMMIX and SAS version 9.2 software analyses (SAS Institute, Cary, NC). As above, the analyses were done with testing both the individual unit and litter as the denominator of F. Both methods used a cumulative logit link and a multinomial distribution, i.e. all three search strategies were included in this analysis. Since this initial analysis indicated a significant three-way interaction between diet x sex x day, another cumulative logit analysis for each day was performed, where diet, sex, and diet x sex interaction were modeled. To pinpoint the differences further, a third analysis on search strategy was performed on which the two less efficient strategies (1 and 2) were combined and compared against the more efficient search strategy (3), thereby resulting in a binomial distribution. The PROC GLIMMIX was again used. Here the model contrasted diet, sex, diet x sex effects for each day with a logit link. The differences between the least square means were based on average logits. Tabled data were converted to probabilities, which is the probability of the treatment group employing one of the less efficient search strategies compared to the most efficient and direct search strategy.
Elevated Plus Maze (EPM) Data Analyses: The proportion of total EPM time spent in open and closed arms and immobile, as well as total number of arm entries, average velocity, and total distance travelled were analyzed by ANOVA, which included the effects of sex, diet, and sex x diet. SAS version 9.2 Software (SAS Institute, Cary, NC) was also employed for these analyses. For the pairwise comparisons that were significant, a Cohen’s d ( [X̄1 - X̄2]/ pooled standard deviation) was also determined.
Results
Serum BPA concentrations in deer mouse dams on the BPA-supplemented diet
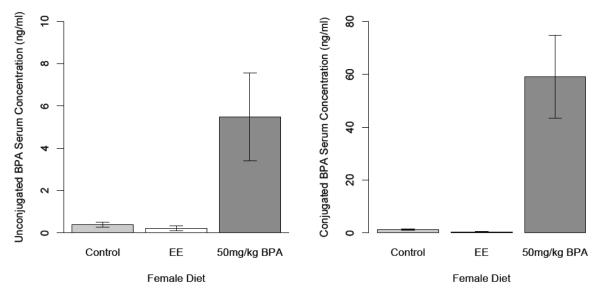
Fig. 1 provides a summary of the serum BPA concentrations in deer mouse dams chronically fed (~12 months a diet supplemented with either 50 mg BPA/kg fw (U), 0.1 ppb EE, or CTL diet. These mice had not been developmentally exposed to BPA themselves, but were members of the cohort of dams fed the U diet and whose offspring were examined previously in the behavioral assessments detailed below. The mean concentration of unconjugated (free) native BPA for females on the U diet was 5.48 ± 2.07 ng/ml (range 0.79 to 19.3 ng/ml) and was significantly different (P<0.05) from that of those dams on the CTL and EE diets, whose serum concentrations ranged from 0.1 to 0.79 ng/ml, and 0.1 to 0.78 ng/ml, respectively, with 0.1 ng/mL being the limit of detection. As anticipated, the concentration of conjugated BPA for females consuming the U diet was at least 10-fold higher than that of the free BPA (Fig. 2B) (range 1.6 ng/ml to 157 ng/ml) and barely above the limit of detection for the majority of animals consuming either the CTL diet or E diets.
Fig 1.
Unconjugated and conjugated serum BPA concentrations for dams chronically fed the CTL, EE (0.1 ppb), and 50 mg BPA/ kg fw (U-dose). Error bars equal SEM. Bars with different superscripts are significantly different P<0.05.
BPA dose and sex-dependent responses for spatial learning
As illustrated in Fig. 2a & b where the individual was used as the denominator of F, animals in all groups predominantly used a random search strategy on the first day of acquisition training, but strategy shifts became evident across training, as indicated by day by sex by diet effects (P < 0.0001]. This three-way interaction was then broken down further to provide contrasts of sex by diet differences in the frequency with which the three search strategies (random, serial, direct) were used on each of the seven training days. Significant diet by sex effects emerged after day 5; P= 0.0476, 0.0001, 0.0001 for days 5 to 7, respectively and were examined further.
A statistical analysis of the search strategy data with litter as the denominator of F is not straightforward, because, for the multinomial and binomial analyses to converge, each search strategy must be used on each day. This condition was only met on days 2, 5, and 7 of the trial, representing the early, mid, last day, but not on the other days. For example, on days 3 and 6 for upper dose (U) males, day 3 for EE-exposed males, and day 4 for CTL females, none of the animals and hence none of the litters within a particular diet grouping employed the direct search strategy, thereby precluding an estimation of P values for those days (Fig. 2b). When the two most inefficient search strategies (random and serial) were combined and compared against the direct search strategy in a binomial analysis, the overall P value for diet x sex interaction on day 2 was 0.5 (data not shown). By day 5, the multinomial analysis for diet x sex interaction was 0.2, but the binomial analysis for this same interaction was 0.09, i.e. tending towards significance. Moreover, when individual treatments and sexes were compared, significant differences based on maternal diet and sex emerged (Table 1). Specifically, by day 5, CTL males outperformed CTL females (P = 0.04). Comparison of the males revealed that U-dose BPA males were more likely to use one of the inefficient search strategies compared to CTL males (P = 0.02), with the latter group also tending to use the direct search strategy more than EE-exposed males (P = 0.06). By day 7, the overall multinomial and binomial analyses were markedly different (P = 0.002 and P < 0.0001, respectively), and there were several significant differences based on maternal diet and sex (Table 1). CTL and L-dose BPA males employed the direct search strategy more than their CTL and L-dose BPA-exposed females (P = 0.001 and 0.02, respectively). Comparing across treatments, CTL males outperformed EE-exposed males, U-dose BPA-exposed males, and M-dose BPA-exposed males (P= 0.0007, 0.003, 0.005, respectively). The three latter groups also used the direct search strategy less than L-dose BPA males (P= 0.006, 0.05, and 0.04, respectively; data not shown). In comparing females across treatment groups, M-dose BPA- and EE-exposed females demonstrated a better ability to use the direct search strategy than CTL females (P= 0.02 and 0.009, respectively). Finally, EE females were much more likely to use the direct search strategy than EE-exposed males (P = 0.005), indicating that EE exposure during development feminized males and masculinized females in terms of spatial navigational skill.
Table 1.
Probability of direct search strategy use compared to combination of random and serial search strategy where the litter was used as the denominator of F for days 5 and 7 of Barnes Maze testing.
| Day of Testing | Maternal Diet and Sex | Probability of Direct Search Strategy (%) |
P Value of Different Sexes Within Same Maternal Diet |
P value Compared to Same Sex Control Group |
|---|---|---|---|---|
|
| ||||
| Day 5 | CTL Males | 53.9 | 0.04 | --- |
| CTL Females | 18.0 | --- | ||
| L BPA Males | 31.9 | 0.78 | 0.48 | |
| L BPA Females | 33.3 | 0.40 | ||
| M BPA Males | 27.3 | 0.25 | 0.17 | |
| M BPA Females | 49.2 | 0.08 | ||
| U BPA Males | 6.4 | 0.18 | 0.02 | |
| U BPA Females | 25.3 | 0.61 | ||
| EE Males | 17.5 | 0.42 | 0.06 | |
| EE Females | 29.9 | 0.43 | ||
|
| ||||
| Day 7 | CTL Males | 91.7 | 0.0013 | --- |
| CTL Females | 18.2 | --- | ||
| L BPA Males | 83.3 | 0.02 | 0.60 | |
| L BPA Females | 16.6 | 0.91 | ||
| M BPA Males | 27.8 | 0.10 | 0.005 | |
| M BPA Females | 57.1 | 0.02 | ||
| U BPA Males | 31.2 | 0.43 | 0.003 | |
| U BPA Females | 44.4 | 0.08 | ||
| EE Males | 11.1 | 0.005 | 0.0007 | |
| EE Females | 61.1 | 0.009 | ||
These data were confirmed with individual pup as the denominator. There are some differences in the estimates and P values when the individual animal (Supplementary Table 2) versus the litter (Table 1) is considered the unit, because the litter considers the effects of pseudoreplicates or individuals of the same sex within the litter and removes the variation due to genetic relatedness. Nonetheless, both methods provide the same overall trends. Another caveat to using the individual as the unit has been to permit the analyses to be performed on days 3, 4 and 6, in addition to days 2, 5, and 7. To accomplish this analysis, one “artificial replicate” was added to any treatment group where the direct search strategy had not been “employed” by any of the individual mice tested in the group. In general, CTL and L-dose BPA males adapted quickly to the direct strategy across days of training, whereas females learned more slowly (Fig. 2b). This direct strategy was severely compromised in males with the EE, U- and M-dose BPA exposures relative to CTL males for days 5-7, respectively (Supplemental Table 2). Again, the use of the direct strategy in these U and M males was not significantly different than that of females developmentally exposed to the same concentrations of BPA, while, as inferred above from Fig. 2b, the L-dose BPA males behaved essentially the same as CTL males (P = 0.51, 0.61, 0.60 on days 5-7, respectively) and learned to use the direct strategy significantly more quickly than L-dose BPA females by day 6 (P = 0.008 and 0.02 on days 6 and 7, respectively).
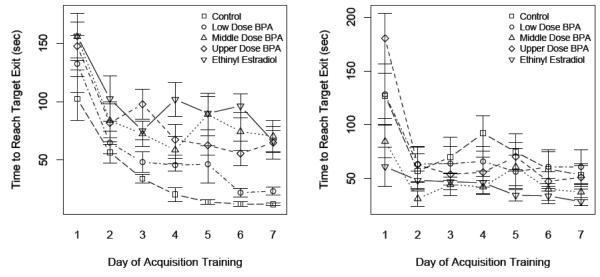
Consistent with shifts in search strategies during acquisition training, latencies, i.e. time for individual animals to reach the target exit hole, decreased across training (Fig. 3; P < 0.0001). When the data were analyzed at the level of the litter (rather than individual animal), CTL males exhibited shorter latencies than CTL females (P = 0.0103), EE-exposed males (P < 0.0008), and U-dose and M-dose males (P = 0.03, P = 0.02, respectively). CTL males did not differ, however, from L-dose males and EE-exposed females (Ps > 0.05). On the other hand, EE-exposed females had shorter latencies across acquisition training than EE-exposed males (P = 0.0013).
Fig. 3.
Sex-by-diet differences in spatial learning and memory in the Barnes maze. Latency to escape maze by day of acquisition training in males (A) and females (B) developmentally exposed to BPA and EE. For information on significant differences between sexes and across diets, see Supplementary Table 3.
Latency P-values for differences between males and females on the same diet and for comparisons of the effects of the different diets on males and females relative to same sex controls for days 2-7 of testing are presented in Supplementary Table 3. These data are consistent with the analyses based on litter described above but provide breakdown of the behavioral differences over time of training. For example, CTL males demonstrated improved latency relative to CTL females, EE-males, and U- and M-dose males by day 4 and this advantage was maintained through the rest of acquisition training. EE, on the other hand, shortened female latency to values no different than those of CTL males in the later days of training. U-dose and M-dose females also were beginning to demonstrate behaviors resembling those of CTL males by day 4 of training, and this effect persisted through day 7.
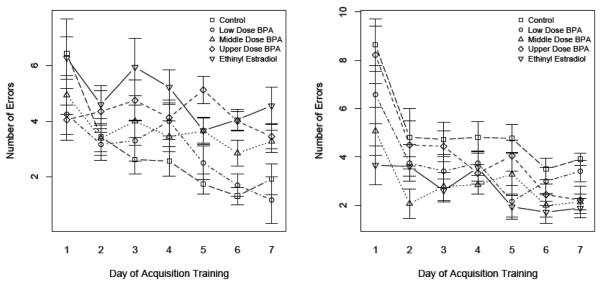
The total numbers of errors that an animal committed prior to entering the target exit hole decreased across acquisition training for all groups (P = 0.001) (Fig. 4). With litter (rather than individual animal) employed for the main plot effect, CTL males committed only about half the number of errors as CTL females (2.8 ± 0.4 versus 5.1 ± 0.3; P = 0.0002) and EE-exposed males (4.9 ± 0.3; P = 0.02). These CTL males also committed fewer errors than U-dose males (4.2 ± 0.4; P = 0.02) but did not differ (P > 0.05) from either M-dose (3.6 ± 0.4 errors) or L-dose (2.9 ± 0.4 errors) males. As with latency values presented above, EE had a masculinization effect, with EE-exposed females (2.7 ± 0.4 errors) committing about the same number of errors as CTL males and significantly fewer errors (P = 0.002) than EE males (4.9 ± 0.3 errors). There was also a decrease in errors in females that had experienced developmental exposure to the M- (2.9 ± 0.4 errors) and L- (3.6 ± 0.4 errors) doses of BPA when they were compared to CTL females (5.1 ± 0.3 errors) (P = 0.0005 and 0.01, respectively). However, differences between CTL females and U-dose females (4.2 ± 0.4) were not significant.
Fig. 4.
Sex-by-diet differences in escape errors by day of acquisition training of males (A) and females (B) exposed to BPA and EE. For information on significant differences between sexes and across diets, see Supplementary Table 4.
A detailed breakdown of P-values for different days of training based on individual animal performance is presented in Supplementary Table 4. Again, the results were generally confirmatory. By as early as day 3, CTL males were committing fewer errors than CTL females and EE males, and, by day 5, they were making fewer errors than the U- and M-dose BPA males.
Dose-response for anxiety-like and exploratory behavior
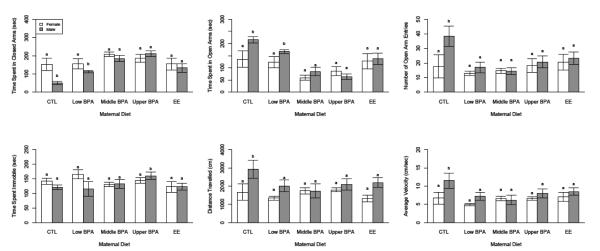
The EPM, which contains open and closed arms separated by an open platform, provides a useful means of assessing exploration (entries into and time spent in the open arms) and anxiety-like behavior (time spent in the closed arms, time spent immobile) (Fig. 5). The major comparisons among all mice assessed in the EPM revealed maternal diet effects for time spent in open arms (P < 0.0001), closed arms (P < 0.0001), immobile (P = 0.0135), and the proportion of time spent in the open arms relative to total time (P < 0.0001).
Fig. 5.
Sex-by-species differences in exploratory (A, open arms) and anxiety-like (B, closed arms) behavior, as well as time spent immobile (C) and total activity (D, total arm entries) in the Elevated Plus Maze (EPM). Values marked by different letters indicate a significant difference (P < 0.05) between the male and female deer mice. For information on significant differences between sexes and across diets, see Supplementary Table 5.
With litter employed as the main plot effect, it was clear that developmental exposure to EE and the U- and M- doses of BPA, led to loss of the enhanced exploratory behavior characteristic of male deer mice (Fig. 5). CTL males spent more time in open arms (215.3 ± 19.6 s) than CTL females (134.1 ± 21.4 s; P=0.01) (Cohen’s d= 1.09), EE-males (135.8 ± 20.5 s; P=0.01) (Cohen’s d= 1.07), U-males (64.6 ± 22.8 s; P<0.0001) (Cohen’s d= 2.02), and M-males (85.1 ± 26.2 s; P<0.0001) (Cohen’s d= 1.75). These data are essentially the reciprocal of accumulated time spent in the closed arms of the maze (Fig. 5), where CTL males (51.7 ± 19.9 s) spent significantly less time than CTL females (153.5 ± 21.8; P = 0.003) (Cohen’s d= −1.37), EE- males (133.6 ± 20.8 s; P = 0.01) (Cohen’s d= −1.10), U-males (217.0 ± 23.1; p < 0.0001) (Cohen’s d= −2.2), and M-males (185.1 ± 26.5 s; P = 0.008) (Cohen’s d= −1.79). CTL males were not significantly different (P > 0.05) than L-males in time spent in either the open or closed arms (171.4 ± 27.7 s and 109.5 ± 28.1 s, respectively).
Responses to EE and to BPA of males noted in the litter-based analysis were confirmed in the statistical analysis based on individual pups (Supplementary Table 5). Sex differences observed in CTL animals for time in the open arms was lost in male mice exposed developmentally to EE and the U- and M-doses of BPA. There was even a significant effect of the L-dose on this parameter (P = 0.0075). One exception to the feminizing influence of these estrogenic compounds was that EE-males travelled further than the EE-exposed females (P = 0.002). The data also indicated that CTL males exhibited more open arm entries (P = 0.0005), moved more quickly (P = 0.0029) and traveled further (P = 0.0024) than female CTL mice, and that each of these male features was diminished as a result of EE-, U-, and M-exposure.
There was one marginal response of BPA-exposed females relative to CTL females in the EPM study. The M-BPA females made more open arm entries (P = 0.0377) than the CTL female mice. There were no other differences (Ps > 0.05) among females across diets.
Discussion
This study is first to characterize serum concentrations of unconjugated and conjugated BPA following chronic exposure (~12 months) of female deer mice to BPA through the diet and to demonstrate that developmental exposure to BPA through the maternal diet has a significant impact on subsequent sex differences in spatial learning, exploration, and anxiety in a dose-dependent manner.
BPA exposure occurs predominantly through consumption of contaminated water and food (Galloway et al., 2010; Vandenberg et al., 2009; Willhite et al., 2008), Therefore, studies that model the effects of dietary intake are most useful for risk assessment. In the present study, chronic exposure of female deer mice dams to 50 mg/kg fw BPA resulted in unconjugated BPA serum concentrations of 5.48 ± 2.07 ng/mL, values quite similar to those noted in pregnant women unknowingly exposed to the chemical (Padmanabhan et al., 2008; Teeguarden et al., 2011; Vandenberg et al., 2010) and in the range anticipated from an acute study performed with deuterated BPA in laboratory mice (Sieli et al., 2011). While the present study has been the first to characterize serum BPA concentrations in deer mice, the fact that the levels observed were comparable to those measured in humans and laboratory mice (Padmanabhan et al., 2008; Teeguarden et al., 2011; Vandenberg et al., 2010) indicates that deer mice are an appropriate animal model for BPA studies. On the basis of the linear response curve observed with BPA (Doerge et al., 2010, 2011; Taylor et al., 2011; Teeguarden et al., 2011), dietary exposure to 5 mg BPA/kg fw, i.e. the M dose, would yield serum concentrations of active chemical close to the limit of detection but still well within human exposures. On the other hand, consumption of the L-dose (50μg/kg fw BPA) could not be expected to lead to measurable serum concentrations of the active form of BPA. Although only a fraction of the circulating chemical would be expected to transfer across the placenta and, in lactating dams, into the milk (Balakrishnan et al., 2010; Ikezuki et al., 2002; Kawamoto et al., 2007; Nishikawa et al., 2010; Vandenberg et al., 2010), fetuses and neonates possess a decreased ability to metabolize BPA compared with adults and may, therefore, experience higher concentrations of unconjugated BPA than the mother (Ikezuki et al., 2002; Kawamoto et al., 2007; Nishikawa et al., 2010).
Our underlying vulnerability hypothesis predicts that sexually selected traits would exhibit heightened sensitivity to developmental exposure to BPA and other EDCs (Geary, 2010; Jašarević et al., 2011). Consistent with this prediction, the results presented here demonstrate that developmental exposure to BPA at the M and U doses disrupts normal spatial learning and exploratory behaviors and produces anxiety-like effects in male but not in female deer mice. Males exposed to either the U or M dose of BPA exhibited defective navigational skills and reduced exploratory behaviors compared to CTL males (Fig. 2 and 3).. Moreover, with one exception, namely improved latency at a single time point (P < 0.03) (Supplementary Table 2), there were no measurable effects on females as a result of either BPA dose exposure. It should be stressed that definitive staging of the estrous cycle in Peromyscus is challenging and that our data do not take into account behavioral changes in females that might be linked to fluctuations in ovarian hormones. For example, the high variance in Barnes Maze performance of females, independent of their diet, might be due to such unaccounted variance in hormones affecting spatial learning. While this shortcoming might be considered a limitation on using the female of this species for behavioral studies, the deer mouse remains a valuable rodent model for examining behavioral traits that are under strong sexual selection and especially vulnerable to endocrine disruption in males.
While the two higher doses of BPA clearly compromised normal male behavior, the lowest (L) dose of BPA had little or no effect on any of the behavioral parameters measured in either male or female deer mice in the Barnes maze and essentially represented a second set of controls. As with our previous findings (Jašarević et al., 2011), developmentally exposed EE females exhibited a complete masculinization of spatial abilities, outperforming EE-exposed males on all criteria of spatial learning. Masculinization of female spatial abilities and demasculinization of male spatial abilities, following developmental exposure to estradiol and its synthetic analog, EE, has been reported previously for other rodent species (Delclos et al., 2009; McCarthy, 2008; Ryan and Vandenbergh, 2006). However, as noted above, such sex-reversed responses were not consistently observed in females exposed to any of the doses of BPA, raising questions as to whether BPA acts comparably to EE in terms of its estrogenic actions in the deer mouse.
The possibility must be considered, therefore, that BPA-induced developmental programming not entirely dependent upon its ability to interfere with the actions of gonadal steroid hormones. The limbic and hypothalamic-pituitary-adrenal (HPA) axis circuits within the hippocampus, a structure abundant in corticosterone, mineralocorticoid and glucocorticoid receptors, are implicated in regulating spatial learning and memory (Han et al., 2005). Maturation of the HPA axis and hippocampus is not complete before sexual maturation in rodents (de Kloet et al., 2005), making this neural network particularly vulnerable to disruption by EDC. BPA exposure, for example, alters the relative abundance of glucocorticoid receptors in the hippocampus and concomitantly decreases spatial recognition memory in rodents (Poimenova et al., 2010). In this regard, BPA may act as a glucocortoid rather than an estrogen receptor agonist (Prasanth et al., 2010). Hence, BPA exposure during early development may lead to permanent alterations in corticosterone-dependent circuits of the hippocampus, thereby, providing an alternative mechanism to explain how it influences developmental programming of the brain. Whatever the specific mechanism involved, our findings reveal a male vulnerability to BPA during early development with consequences in terms of spatial learning deficits that are dose dependent and occur at environmentally relevant exposures.
BPA exposure not only demasculinized male spatial learning, it narrowed the magnitude of sex differences in anxiety-like and exploratory behaviors in a dose dependent manner, as has been recently reported in rats exposed perinatally to BPA (Jones and Watson, 2012). Similarly, sex differences among the deer mice were lost following developmental exposure to the U and M doses of BPA (Fig. 5; Supplementary Table 5). Specifically, males from these two treatment groups spent more time in the closed arms and made fewer open arm entries compared to CTL males, EE-exposed males, and the L dose of BPA-exposed males. This finding is not surprising, as heightened anxiety in male rodents following BPA exposures has been consistently identified across species (Cox et al., 2010; Farabollini et al., 1999; Jašarević et al., 2011; Wolstenholme et al., 2011b). The data again suggest that BPA exposure can perturb early programming of neural circuits modulated by glucocorticoids as well as sex steroids (Poimenova et al., 2010; Prasanth et al., 2010).
Conclusions
Our data demonstrate that typical sex differences in spatial learning, anxiety-like and exploratory behaviors of adult deer mice are diminished following developmental exposure to BPA through the maternal diet. Importantly, a dose-dependent effect of developmental BPA exposure on these adult traits was observed in males but only marginally, if at all, in females. Finally, chronic exposure to a diet supplemented with the upper dose of BPA tested yielded serum concentrations of BPA that were within environmentally relevant range of exposure and similar to those observed in human biomonitoring studies.
Supplementary Material
Highlights.
Deer mice offspring were exposed to three levels of BPA through maternal diet.
Upper dose exposure led to environmentally relevant serum concentrations of BPA in dams.
The two higher doses of BPA feminized exposed males in terms of spatial learning and exploratory behaviors.
Unlike EE, developmental exposure to BPA did not cause a sex reversal in female behaviors.
Acknowledgements
We thank Stephen Cobb and Paizlee T. Sieli for assistance with animal husbandry, and Mr. Wayne Shoemaker for constructing the behavior testing apparatuses. This work was supported by a National Institutes of Health Challenge Grant RC1 ES018195 (to C.S.R.), a Mizzou Advantage grant (to C.S.R., D.C.G. & R.M.R.), and support from Food for the 21th Century Program to RMR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson OS, Nahar MS, Faulk C, Jones TR, Liao C, Kannan K, Weinhouse C, Rozek LS, Dolinoy DC. Epigenetic responses following maternal dietary exposure to physiologically relevant levels of bisphenol A. Environ Mol Mutagen. 2012;53:334–342. doi: 10.1002/em.21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M, Simmons LW. Sexual selection and mate choice. Trends Ecol Evol. 2006;21:296–302. doi: 10.1016/j.tree.2006.03.015. [DOI] [PubMed] [Google Scholar]
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nemeroff CB, Reyes TM, Simerly RB, Susser ES, Nestler EJ. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68:314–319. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. Journal of comparative and physiological psychology. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Biedermann S, Tschudin P, Grob K. Transfer of bisphenol A from thermal printer paper to the skin. Anal Bioanal Chem. 2010;398:571–576. doi: 10.1007/s00216-010-3936-9. [DOI] [PubMed] [Google Scholar]
- Bradley EL, Terman CR. Ovulation in Peromyscus maniculatus bairdii under laboratory conditions. Journal of Mammalogy. 1979;60:543–549. [Google Scholar]
- Carr R, Bertasi F, Betancourt A, Bowers S, Gandy BS, Ryan P, Willard S. Effect of neonatal rat bisphenol a exposure on performance in the Morris water maze. J Toxicol Environ Health A. 2003;66:2077–2088. doi: 10.1080/713853983. [DOI] [PubMed] [Google Scholar]
- Charles GD, Gennings C, Tornesi B, Kan HL, Zacharewski TR, Bhaskar Gollapudi B, Carney EW. Analysis of the interaction of phytoestrogens and synthetic chemicals: an in vitro/in vivo comparison. Toxicol Appl Pharmacol. 2007;218:280–288. doi: 10.1016/j.taap.2006.11.029. [DOI] [PubMed] [Google Scholar]
- Cox KH, Gatewood JD, Howeth C, Rissman EF. Gestational exposure to bisphenol A and cross-fostering affect behaviors in juvenile mice. Hormones and Behavior. 2010;58:754–761. doi: 10.1016/j.yhbeh.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nature reviews. Neuroscience. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Dewsbury DA, Estep DQ, Lanier DL. Estrous Cycles of Nine Species of Muroid Rodents. Journal of Mammalogy. 1977;58:89–92. [PubMed] [Google Scholar]
- Doerge DR, Twaddle NC, Vanlandingham M, Fisher JW. Pharmacokinetics of bisphenol A in neonatal and adult Sprague-Dawley rats. Toxicol Appl Pharmacol. 2010;247:158–165. doi: 10.1016/j.taap.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Doerge DR, Twaddle NC, Vanlandingham M, Fisher JW. Pharmacokinetics of bisphenol A in neonatal and adult CD-1 mice: inter-species comparisons with Sprague-Dawley rats and rhesus monkeys. Toxicol Lett. 2011;207:298–305. doi: 10.1016/j.toxlet.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13056–13061. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einon D. Spatial memory and response strategies in rats: Age, Sex and rearing differences in performance. Quarterly Journal of Experimental Psychology. 1980;32:473–489. doi: 10.1080/14640748008401840. [DOI] [PubMed] [Google Scholar]
- Eliam-Stock T, Serrano P, Frankfurt M, Luine VN. Bisphenol-A Impairs Memory and Reduces Dendritic Spine Density in Adult Male Rats. Behav Neurosci. 2012;126:175–185. doi: 10.1037/a0025959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabollini F, Porrini S, Dessi-Fulgherit F. Perinatal exposure to the estrogenic pollutant bisphenol A affects behavior in male and female rats. Pharmacol Biochem Behav. 1999;64:687–694. doi: 10.1016/s0091-3057(99)00136-7. [DOI] [PubMed] [Google Scholar]
- Fountain ED, Mao J, Whyte JJ, Mueller KE, Ellersieck MR, Will MJ, Roberts RM, Macdonald R, Rosenfeld CS. Effects of diets enriched in omega-3 and omega-6 polyunsaturated fatty acids on offspring sex-ratio and maternal behavior in mice. Biology of reproduction. 2008;78:211–217. doi: 10.1095/biolreprod.107.065003. [DOI] [PubMed] [Google Scholar]
- Frye CA, Calamandrei LC, Dessi-Fulgheri F, Fernandez M, Fusani L, Kah O, Kajta M, Le Page Y, Patisaul HB, Wojtowicz AK, Panzica GC. Endocrine disruptors: A Review of Some Sources, Effects, and Mechanisms of Actions on Behaviour and Neuroendocrine Systems. J Neuroendocrinol. 2011;24:144–159. doi: 10.1111/j.1365-2826.2011.02229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LAM, Kimura D. Sex differences in route-learning. Personality and Individual Differences. 1993;14:53–65. [Google Scholar]
- Galloway T, Cipelli R, Guralnik J, Ferrucci L, Bandinelli S, Corsi AM, Money C, McCormack P, Melzer D. Daily bisphenol A excretion and associations with sex hormone concentrations: results from the InCHIANTI adult population study. Environmental Health Perspectives. 2010;118:1603–1608. doi: 10.1289/ehp.1002367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulin SJC. Evolution of sex differences in spatial ability. Yearbook of Physical Anthropology. 1992;35:125–151. [Google Scholar]
- Geary DC. Male, female : the evolution of human sex differences. 2nd ed American Psychological Association; Washington, DC: 2010. Chapter 10; pp. 291–294. [Google Scholar]
- Gray JA. Sex differences in emotional behaviour in mammals including man: endocrine bases. Acta psychologica. 1971;35:29–46. doi: 10.1016/0001-6918(71)90029-1. [DOI] [PubMed] [Google Scholar]
- Han F, Ozawa H, Matsuda K, Nishi M, Kawata M. Colocalization of mineralocorticoid receptor and glucocorticoid receptor in the hippocampus and hypothalamus. Neuroscience research. 2005;51:371–381. doi: 10.1016/j.neures.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Harker KT, Whishaw IQ. Place and matching-to-place spatial learning affected by rat inbreeding (Dark-Agouti, Fischer 344) and albinism (Wistar, Sprague-Dawley) but not domestication (wild rat vs. Long-Evans, Fischer-Norway) Behavioural brain research. 2002;134:467–477. doi: 10.1016/s0166-4328(02)00083-9. [DOI] [PubMed] [Google Scholar]
- Harrison FE, Reiserer RS, Tomarken AJ, McDonald MP. Spatial and nonspatial escape strategies in the Barnes maze. Learn Mem. 2006a;13:809–819. doi: 10.1101/lm.334306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison FE, Reiserer RS, Tomarken AJ, McDonald MP. Spatial and nonspatial escape strategies in the Barnes maze. Learning & memory. 2006b;13:809–819. doi: 10.1101/lm.334306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero AI, Sani C, Venero C. Individual differences in anxiety trait are related to spatila learning abilities and hippocampal expression of mineral corticoid receptors. Neurobiology of Learning & Memory. 2006;86:150–159. doi: 10.1016/j.nlm.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Jašarević E, Sieli PT, Twellman EE, Welsh TH, Jr., Schachtman TR, Roberts RM, Geary DC, Rosenfeld CS. Disruption of adult expression of sexually selected traits by developmental exposure to bisphenol A. Proc Natl Acad Sci U S A. 2011;108:11715–11720. doi: 10.1073/pnas.1107958108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jašarević E, Williams SA, Roberts RM, Geary DC, Rosenfeld CS. Spatial Navigation Strategies in Peromyscus: a Comparative Study. Animal Behaviour. 2012 doi: 10.1016/j.anbehav.2012.08.015. epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones BA, Watson NV. Perinatal BPA exposure demasculinizes males in measures of affect but has no effect on water maze learning in adulthood. Hormones and Behavior. 2012;61:605–610. doi: 10.1016/j.yhbeh.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- Layne J. Ontogeny. In: King J, editor. Biology of Peromyscus (Rodentia) American Society for Mammologist; Stillwater, OK: 1968. pp. 148–253. [Google Scholar]
- Luine VN. Steroid Hormone Modulation of Hippocampal Dependent Spatial Memory. Stress. 1997;2:21–36. doi: 10.3109/10253899709014735. [DOI] [PubMed] [Google Scholar]
- Martin LB, Trainor BC, Finy MS, Nelson RJ. HPA activity and neotic and anxiety-like behavior vary among Peromyscus species. General and comparative endocrinology. 2007;151:342–350. doi: 10.1016/j.ygcen.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, Hataya Y, Shimatsu A, Kuzuya H, Nakao K. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab. 2002;87:5185–5190. doi: 10.1210/jc.2002-020209. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiology & Behavior. 2007;91:55–65. doi: 10.1016/j.physbeh.2007.01.017. [DOI] [PubMed] [Google Scholar]
- O’Leary TP, Savoie V, Brown RE. Learning, memory and search strategies of inbred mouse strains with different visual abilities in the Barnes maze. Behavioural brain research. 2011;216:531–542. doi: 10.1016/j.bbr.2010.08.030. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Siefert K, Ransom S, Johnson T, Pinkerton J, Anderson L, Tao L, Kannan K. Maternal bisphenol-A levels at delivery: a looming problem? J. Perinatol. 2008;28:258–263. doi: 10.1038/sj.jp.7211913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poimenova A, Markaki E, Rahiotis C, Kitraki E. Corticosterone-regulated actions in the rat brain are affected by perinatal exposure to low dose of Bisphenol A. Neuroscience. 2010;167:741–749. doi: 10.1016/j.neuroscience.2010.02.051. [DOI] [PubMed] [Google Scholar]
- Prasanth GK, Divya LM, Sadavisan C. Bisphenol-A can bind to human gluccocorticoid receptor as an agonist: an in silico study. Journal of Applied Toxicology. 2010;30:769–774. doi: 10.1002/jat.1570. [DOI] [PubMed] [Google Scholar]
- Rodriguez CA, Torres A, Mackintosh NJ, Chamizo VD. Sex differences in the strategies used by rats to solve a navigation task. J Exp Psychol Anim Behav Process. 2010;36:395–401. doi: 10.1037/a0017297. [DOI] [PubMed] [Google Scholar]
- Rubin BS. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol. 2011 doi: 10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Hotchkiss AK, Crofton KM, Gray LE., Jr. In utero and lactational exposure to bisphenol A, in contrast to ethinyl estradiol, does not alter sexually dimorphic behavior, puberty, fertility, and anatomy of female LE rats. Toxicological sciences : an official journal of the Society of Toxicology. 2010;114:133–148. doi: 10.1093/toxsci/kfp266. [DOI] [PubMed] [Google Scholar]
- Ryan BC, Vandenbergh JG. Developmental exposure to estrogens alters anxiety and spatial memory in female mice. Hormones and Behavior. 2006;50:85–93. doi: 10.1016/j.yhbeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Sieli PT, Jašarević E, Warzak DA, Mao J, Ellersieck MR, Liao C, Kannan K, Collet SH, Toutain PL, Vom Saal FS, Rosenfeld CS. Comparison of serum bisphenol A concentrations in mice exposed to bisphenol A through the diet versus oral bolus exposure. Environ. Health Perspect. 2011;119:1260–1265. doi: 10.1289/ehp.1003385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel R. Chapter 16: Analysis of Variance IV: Split-Plot Designs and Analysis. 3rd ed McGraw-Hill Higher Education; 1996. Principles and Procedures of Statistics: A Biometrical Approach; pp. 400–428. [Google Scholar]
- Stump DG, Beck MJ, Radovsky A, Garman RH, Freshwater LL, Sheets LP, Marty MS, Waechter JM, Jr., Dimond SS, Van Miller JP, Shiotsuka RN, Beyer D, Chappelle AH, Hentges SG. Developmental neurotoxicity study of dietary bisphenol A in Sprague-Dawley rats. Toxicol Sci. 2010;115:167–182. doi: 10.1093/toxsci/kfq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Vom Saal FS, Welshons WV, Drury B, Rottinghaus G, Hunt PA, Toutain PL, Laffont CM, VandeVoort CA. Similarity of bisphenol A pharmacokinetics in rhesus monkeys and mice: relevance for human exposure. Environ. Health Perspect. 2011;119:422–430. doi: 10.1289/ehp.1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeguarden JG, Calafat AM, Ye X, Doerge DR, Churchwell MI, Gunawan R, Graham M. 24-Hour Human Urine and Serum Profiles of Bisphenol A During High Dietary Exposure. Toxicol Sci. 2011 doi: 10.1093/toxsci/kfr160. [DOI] [PubMed] [Google Scholar]
- Thompson WR. The inheritance of behaviour: behavioural differences in fifteen mouse strains. Canadian journal of psychology. 1953;7:145–155. doi: 10.1037/h0083586. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environmental Health Perspectives. 2010;118:1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocrine reviews. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Richter CA, Ruhlen RR, Nagel SC, Timms BG, Welshons WV. The importance of appropriate controls, animal feed, and animal models in interpreting results from low-dose studies of bisphenol A. Birth Defects Res A Clin Mol Teratol. 2005;73:140–145. doi: 10.1002/bdra.20120. [DOI] [PubMed] [Google Scholar]
- Willhite CC, Ball GL, McLellan CJ. Derivation of a bisphenol A oral reference dose (RfD) and drinking-water equivalent concentration. J Toxicol Environ Health B Crit Rev. 2008;11:69–146. doi: 10.1080/10937400701724303. [DOI] [PubMed] [Google Scholar]
- Williams CL, Barnett AM, Meck WH. Organizational effects of early gonadal secretions on sexual differentiation in spatial memory. Behav Neurosci. 1990;104:84–97. doi: 10.1037//0735-7044.104.1.84. [DOI] [PubMed] [Google Scholar]
- Wolstenholme JT, Rissman EF, Connelly JJ. The role of Bisphenol A in shaping the brain, epigenome and behavior. Hormones and Behavior. 2011a;59:296–305. doi: 10.1016/j.yhbeh.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Taylor JA, Shetty SR, Edwards M, Connelly JJ, Rissman EF. Gestational exposure to low dose bisphenol A alters social behavior in juvenile mice. PLoS ONE. 2011b;6:e25448. doi: 10.1371/journal.pone.0025448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.-h., Zhang J, Wang Y.-m., Ye Y.-p., Qing-qing L. Perinatal exposure to bisphenol-A impairs leanring-memory by concomitant down-regulation of N-methyl-D-aspartate receptors of hippocampus of male offspring mice. Hormones and Behavior. 2010;58:326–333. doi: 10.1016/j.yhbeh.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Xu X, Tian D, Hong X, Lei C, Lingdan X. Sex-specific influence of exposure to bisphenol-A between adolescence and young adulthood on mouse behaviors. Neuropharmacology. 2011;61:565–573. doi: 10.1016/j.neuropharm.2011.04.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.