Abstract
Patients with schizophrenia may have altered pain perception, as suggested by clinical reports of pain insensitivity, and recent neuroimaging findings. Here, we examined neural responses to an aversive electrical stimulus and the immediate anticipation of such a stimulus using fMRI and a classical conditioning paradigm, which involved pairing an electrical shock with a neutral photograph. Fifteen men with schizophrenia and 13 healthy men, matched for demographic characteristics, electrical stimulation level and scan movement, were studied. The shock induced robust responses in midbrain, thalamus, cingulate gyrus, insula and somatosensory cortex in both groups. However, compared to controls, the schizophrenic patients displayed significantly lower activation of the middle insula (pFWE = 0.002, T=5.72, cluster size =24 voxels). Moreover, the lack of insula reactivity in the schizophrenia group was predicted by the magnitude of positive symptoms (r = −0.46, p=0.04). In contrast, there were no significant differences between the two groups in the magnitude of neural responses during anticipation of the shock. These findings provide support for the existence of a basic deficit in interoceptive perception in schizophrenia, which could play a role in the generation and/or maintenance of psychotic states.
Keywords: Classical Conditioning, Unconditioned Response, Pain, Insula
1 Introduction
It has long been observed that some patients with schizophrenia are relatively insensitive to pain. Kraepelin reported that Dementia Praecox patients could burn themselves with cigarettes and experience needle pricks or injuries without showing adaptive withdrawal reactions (Bonnot et al., 2009; Kraepelin and Robertson, 1919). More recently, a meta-analysis of experimental pain studies indicated that schizophrenic patients show a blunted response to experimental pain (Potvin and Marchand, 2008), a finding confirmed in a detailed review of cases, clinical and experimental studies (Bonnot et al., 2009). Pain insensitivity in schizophrenia is associated with increased morbidity and mortality, but the underlying pathophysiology is poorly understood (Singh et al., 2006). Although antipsychotic medications may have an analgesic effect (Seidel et al., 2010), alterations in pain perception in schizophrenia cannot be solely explained by medication effects (Potvin and Marchand, 2008). Recent neuroimaging studies in schizophrenia found greater somatosensory activation, but diminished insula, posterior cingulate cortex and brainstem responses to thermal pain (de la Fuente-Sandoval et al., 2012; de la Fuente- Sandoval et al., 2010). These reports provide initial evidence that painful stimuli are processed differently in schizophrenia. But pain is a highly subjective experience, and emotional, anticipatory and/or sensory aspects of noxious processing may drive alterations in sensitivity. Considering the communicative and social impairments associated with schizophrenia, a more detailed dissection of aversive experiences in the disorder is warranted.
Here we examined fMRI responses evoked by an aversive electrical shock stimulus in schizophrenia patients and healthy controls. Using a Pavlovian fear-conditioning protocol (Holt et al., 2009; Milad et al., 2007) we examined neural and autonomic responses to conditioned (CS+) and unconditioned stimulus (US, an electrical shock) presentations in a partial reinforcement paradigm. The US was delivered at a 62.5% reinforcement rate, allowing us to compare neural responses to the US with responses to the immediate anticipation of the US (the moment just prior to the offset of unreinforced CS+ trials). In this way, the sensory component of a US response may be isolated, in part, from its expectancy related components (Dunsmoor and Labar, 2012; Linnman et al., 2011a; Linnman et al., 2011b). Responses to an electrical (Linnman et al., 2011a) or auditory (Dunsmoor et al., 2007, 2008; Dunsmoor and Labar, 2012; Knight et al., 2010) US in healthy subjects are accompanied by increased activity in the brainstem and thalamus, as well as in the cingulate, sensory and insular cortices, structures also known to respond to noxious stimuli (Apkarian et al., 2005). In the current investigation, we hypothesized, based on previous evidence (de la Fuente-Sandoval et al., 2012; de la Fuente-Sandoval et al., 2010), that schizophrenic patients would display impaired responses of the insula and brainstem and elevated responses of somatosensory cortex, compared to controls. We further sought to disentangle the sensory and anticipatory aspects of this response, and relate any observed alterations to the symptoms of schizophrenia.
2 Methods
2.1 Subjects
For all subjects, exclusion criteria included severe medical illness, significant head trauma, neurologic illness, substance abuse during the past six months and contraindications for MRI scanning (e.g., implanted metal objects, claustrophobia). Twenty male patients with DSM-IV diagnosed schizophrenia were recruited and characterized by the Massachusetts General Hospital Schizophrenia Program. 17 healthy male subjects were recruited from the community via advertisements. The healthy subjects were without psychiatric disorders as determined by a structured clinical interview (SCID) (First et al., 1995). All subjects gave informed consent, in accordance with the procedures of the Partners Healthcare System Human Research Committee. In the patients, levels of positive and negative symptoms of schizophrenia were evaluated in each patient by one trained rater using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) on the first day of the experimental protocol. Also, symptoms of anxiety and depression were measured on Day 1 of the protocol in all subjects using the Spielberger State and Trait Anxiety Inventory (Spielberger, 1988) and the Beck Depression Inventory (Beck et al., 1961), respectively.
An analysis of the neural correlates of fear and extinction learning and memory in these participants has been published (Holt et al., 2012); however the responses to the US during the fear conditioning part of the study have not previously been reported. After matching for subject (scan-to-scan) movement (Friston et al., 1996; Van Dijk et al., 2012; Weinberger et al., 1996) and individual US shock levels, the final sample here consisted of 15 patients (9 antipsychotic-treated and 6 antipsychotic-free) and 13 controls. Mean age, parental education and handedness did not differ between these two groups. See Table 1 for demographic information and symptom levels for this cohort.
Table 1.
Demographic, clinical and experimental information about the subjects.
| Patients (n=15 ♂) | Controls (n=13 ♂) | |
|---|---|---|
| Age (±SD) | 32 (±10) | 36 (±10) |
| Race/Ethnicity | 10 Caucasian | 8 Caucasian |
| 1 Latino | 2 Latino | |
| 2 African Am. | 1 African Am. | |
| 1 Asian | 1 Asian | |
| 1 Mixed origin | 1 No report | |
| Handedness a | 85 (±24) | 72 (±47) |
| Premorbid IQ b | 107 (±10) | 110 (±6) |
| Mean parental education c | 14 (±4) | 14 (±2) |
| Beck Depression Inventory* | 9.7 (±9) | 1.4 (±2) |
| Spielberger Trait Anxiety*^ | 42 (±14) | 27 (±7) |
| Spielberger State Anxiety* | 38 (±11) | 25 (±4) |
| Age of illness onset | 21 (±5) | n.a. |
| Duration of illness (years) | 10 (±9) | n.a. |
| Current antipsychotic dose (in CPZ) | 341 (±384) | n.a. |
| PANSS Positive Subscale | 14 (±6) | n.a. |
| PANSS Negative Subscale | 13 (±6) | n.a. |
| PANSS General Subscale | 24 (±6) | n.a. |
| Experimental details: | ||
| Shock level (mA@500V) | 1.3 (±0.46) | 1.6 (±0.46) |
| Scan-scan motion (mm) d | 0.09 (±0.03) | 0.07(±0.03) |
| Maximum movement (mm) | 1.20 (±1.06) | 0.74 (±0.86) |
Measured using the Edinburgh Handedness Inventory;
Measured using the American National Adult Reading Test;
Mean years of education for mother and father; CPZ, chlorpromazine equivalents; PANSS, Positive and Negative Syndrome Scale.
Significantly higher in the schizophrenia group than in the control group, p < .005.
Significantly correlated with shock level in the patient group (r = −0.46, p = 0.04) but not in the control group (r = 0.09, p = 0.39).
Calculated as per Van Dijk et al (2012).
2.2 Fear conditioning procedure
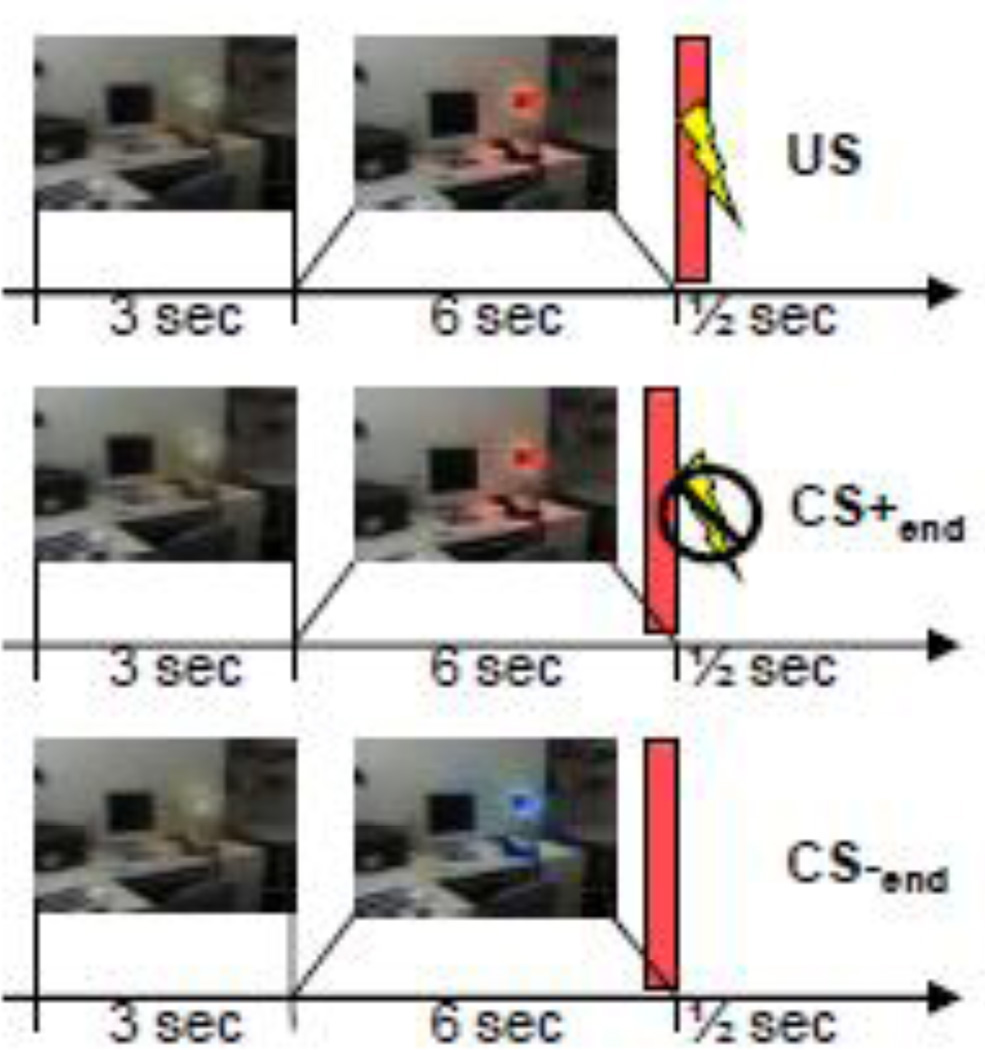
While functional MRI (fMRI) data were simultaneously collected, subjects participated in a partial reinforcement classical conditioning paradigm that has been described in detail previously (Holt et al., 2012; Milad et al., 2007), see Figure 1 for an overview. Briefly, each trial began with an image of a room (the “context”) containing a lamp presented for 3 seconds in the “off” state. The lamp then “turns on” to one of three colors (blue, red, or yellow) for 6 seconds. Two of the colors (CS+) were followed by a 500ms electric shock (US) in 62.5% of the trials, and the third color was never followed by a shock (CS−). The illuminated lamp was presented 32 times for a total of 16 safe trials (CS−), 10 CS+ trials followed by the shock, and six CS+ trials in which the shock was omitted. Between trials, a black screen was displayed for 12 to 18 seconds. The lamp color sequence was counterbalanced across subjects in pseudo-random order. The total length of the run was 13 minutes and 34 seconds.
Figure 1.
Fear conditioning paradigm and time points of interest. The first row indicates a reinforced CS+ event, starting with an unlit lamp for 3 seconds, the lamp then turns on for six seconds (the CS+, a red colored lamp in this example), and is followed by the US (a shock). The second row indicates a non-reinforced CS+ event, and the third row indicates a CS− event (a blue colored lamp in this example). The red rectangle indicates the time point of interest: US, CS+end and CS−end.
2.2.1 Electric shock
The US consisted of a 500 ms train of 1 ms spikes at 50Hz delivered to the second and third fingers of the right hand with currents ranging from 0.2 to 4.0 mA. Prior to the experiment, the level of the shock current was adjusted so that the subject perceived it as “highly annoying but not painful”.
2.2.2 Skin conductance
Skin conductance responses (SCRs) were measured on the palm of the left hand. SCR responses during the interval following the US, the omitted (non-delivered) US, and the CS− offset were calculated by subtracting the mean skin conductance level recorded during the first 2 seconds of this interval from the highest skin conductance level during the ensuing 3 seconds.
2.3 Image Acquisition
MRI data were collected using a Trio 3.0 Tesla whole body, MRI system (Siemens Medical Systems, Iselin, New Jersey) equipped for echo planar imaging with a 12- channel head coil. Subjects were instructed to lie as still as possible and head movement was restricted with foam cushions. After an automated scout image was obtained and automated shimming procedures performed, a high-resolution, T1- weighted, three-dimensional, magnetization prepared rapid acquisition gradient echo (MPRAGE) volume was collected. Functional MRI images, sensitive to blood oxygenation level dependent (BOLD) contrast, were acquired with an interleaved gradient echo T2*-weighted sequence (TR= 3000 ms, TE= 30, Flip angle = 90°), collected in 45 slices. The voxel size was 3.1×3.1×3 mm.
2.4 fMRI Data analysis
2.4.1 Preprocessing
SPM8 (Wellcome Trust Center for Neuroimaging, www.fil.ion.ucl.ac.uk) was used to process the fMRI data. Structural images were segmented and spatially normalized to the Montreal Neurological Institute (MNI305) T1 template. Functional images were realigned, corrected for slice timing, co-registered with the structural volume, resampled to 2×2×2 mm, normalized into MNI space using parameters obtained from the structural normalization process, and smoothed with an 8 mm full with half maximum Gaussian kernel to reduce spatial noise and to compensate for residual misregistration in the spatial normalization process. High-pass temporal filtering with a cutoff of 128 seconds was included in the first-level statistical model to remove the effects of low frequency physiological noise. Serial correlations in the fMRI time series due to aliased biorhythms were estimated using an autoregressive AR(1) model.
2.4.2 First-level model
After preprocessing, each subject’s functional time series was modeled using a general linear model with six regressors signifying the condition onsets and durations, including the context, onsets of the CS+ and the CS−, the US, the offset of the CS+ prior to the omitted US (henceforth called CS+end), and the offset of the CS− (CS−end). Six movement parameters derived from the realignment were included in the model to reduce the effects of residual motion-related noise. The experimental effects of interest were identified using a statistical model containing boxcar functions representing each of the six experimental conditions, convolved with the SPM8 canonical hemodynamic response function.
We focused our analysis on the conditioned response expressed at the CS+end (i.e. the last 500 milliseconds of CS+ presentations that were not followed by a shock) and the US response (500 milliseconds). The last 500 milliseconds of the CS− presentations (CS− end) were used as the baseline condition for the US and CS+end. In a secondary analysis, the two conditions of interest (the US and CS+end) were compared directly. See Figure 1 for an overview. This design allowed us to investigate brain responses associated with the actual delivery of the US and brain responses associated with the immediate expectancy of the US (Linnman et al., 2011a; Linnman et al., 2011b). Multi-collinearity among regressors may lead to instability of parameter estimation and a consequent reduction in sensitivity. In the present design the inherent multiple collinearity of the classical conditioning design (a cue followed by a shock) was not problematic for the present contrasts, as the correlations among regressors were low (r2 < 0.01).
2.4.3 Second-level model
First-level contrast images representing the effects of CS+end, CS−end and US were obtained for each subject, and modeled at the second level using a mixed effects linear model with subject, group and task factors. At the second level, voxel-wise contrasts signifying the US (versus CS−end) response and the CS+end (versus CS−end) response in both controls and schizophrenic patients, and comparing controls versus schizophrenic patients (USControls vs. USSchizophrenia) and (CS+end Controls vs. CS+end Schizophrenia).
Whole-brain regional activations encompassing more than 10 voxels and surviving p<0.05 family wise error correction for multiple comparisons (FWE) for the whole brain are reported.
2.4.4 Post-hoc analyses
In order to further interrogate the data, we evaluated BOLD response differences between medicated and non-medicated patients. Also, to further elucidate possible driving factors behind the observed BOLD signal differences between controls and schizophrenia patients, we performed exploratory post-hoc correlations between BOLD activation and 1) antipsychotic medication dose (in chlorpromazine equivalents) and 2) symptom levels. For these correlations, the mean individual cluster BOLD signal beta estimate of regions showing between-group differences was extracted from the patient group and correlated with scores on the three subscales of the PANSS (measuring negative, positive and general symptoms), with Spielberger State and Trait Anxiety Inventory scores and with Beck Depression Inventory scores.
3 Results
3.1 Skin conductance responses
None of the skin conductance measures of the fear conditioning procedure differed between patients and controls.
3.2 Brain responses to the US
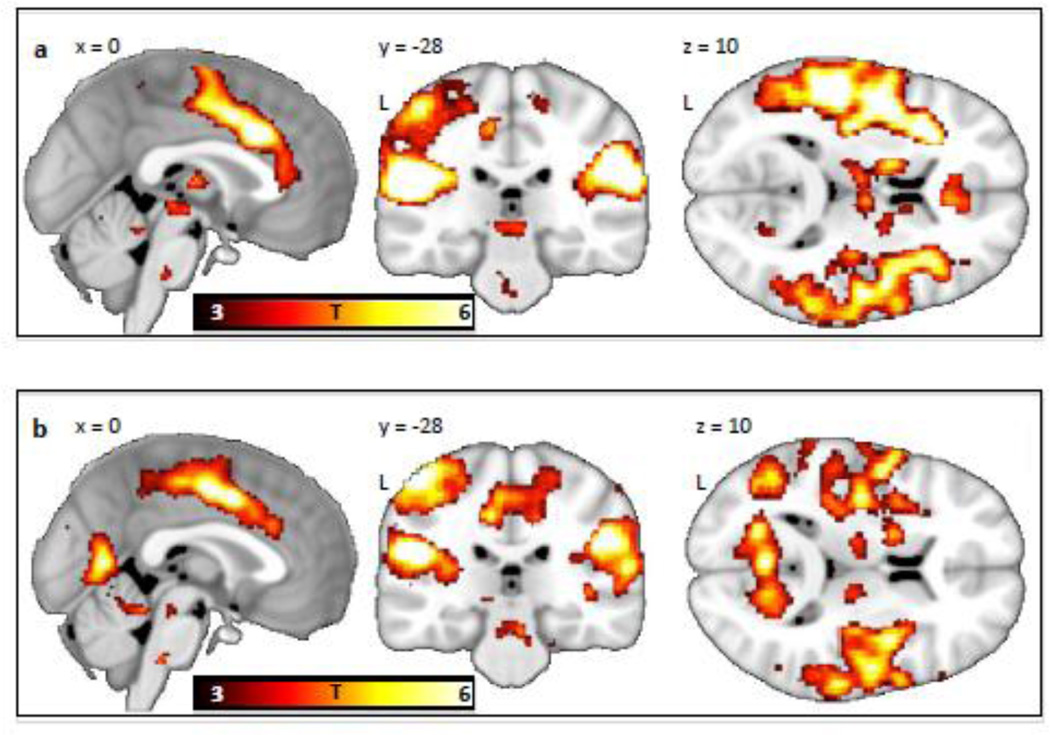
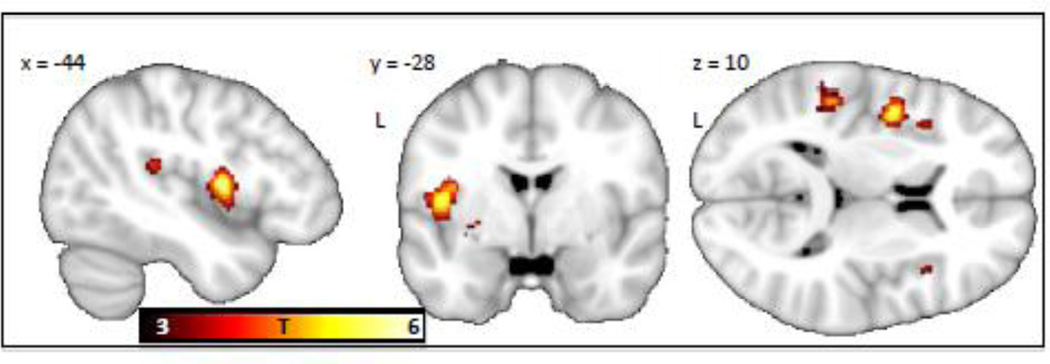
The US elicited responses in midbrain, thalamus, cingulate gyrus, insula and somatosensory regions in both the controls and schizophrenic patients (see Figure 2a&b, and Table 2a&b). The whole brain between-group comparison revealed that the schizophrenic patients exhibited lower BOLD reactivity to the US than the controls in a cluster encompassing the left mid insula (54% of voxels) and the left precentral gyrus (46% of voxels), with the peak at MNIXYZ −44, −2, 10 (pFWE = 0.002, T=5.72, cluster size =24 voxels) (see Figure 3). There were no regions showing greater activation in the patients compared to the controls. In the contrast [controls (US > CS+end] > [patients (US > CS+end)], i.e. controlling for anticipatory components of the shock response, the insula showed a similar trend towards hypoactivation in the schizophrenia group, but did not reach a whole brain-corrected level of significance (puncorrected = 0.004, T=2.62 at MNI (−44, −2, −10)).
Figure 2.
Shock (US versus CS− offset) responses in (a) healthy controls (n=13) and (b) schizophrenic patients (n=15). The contrast maps are displayed at a threshold of T > 3 and overlaid on a template structural MRI image. The color bar denotes T-values.
Table 2.
| a. BOLD responses in healthy controls. | |||||||
|---|---|---|---|---|---|---|---|
| Contrast | cluster | cluster | peak | MNI | Peak region | ||
| p(FWE-cor) | size (voxels) | T | X | y | z | ||
| US – CS−end | |||||||
| <0.001 | 2985 | 10.51 | −50 | −28 | 16 | L. Postcentral Gyrus | |
| 9.91 | −58 | −18 | 22 | L. Postcentral Gyrus | |||
| 8.05 | −40 | −34 | 20 | L. Insula | |||
| <0.001 | 1706 | 7.96 | 60 | −32 | 18 | R. Postcentral Gyrus | |
| 7.28 | 54 | −28 | 30 | R. Inferior Parietal | |||
| Lobule | |||||||
| 7.12 | 56 | −16 | 16 | R. Postcentral Gyrus | |||
| <0.001 | 863 | 7.06 | −2 | 16 | 32 | L. Cingulate Gyrus | |
| 6.72 | −6 | 4 | 40 | L. Cingulate Gyrus | |||
| 6.35 | 4 | 6 | 40 | R. Cingulate Gyrus | |||
| <0.001 | 420 | 6.65 | −52 | −22 | 54 | L. Postcentral Gyrus | |
| 6.16 | −42 | −22 | 48 | L. Postcentral Gyrus | |||
| 5.98 | −44 | −14 | 50 | L. Precentral Gyrus | |||
| <0.001 | 85 | 6.48 | −14 | 8 | 2 | L. Putamen | |
| 5.51 | −14 | 0 | 8 | L. Putamen | |||
| <0.001 | 51 | 6.24 | 10 | −14 | 0 | R. Thalamus | |
| 5.08 | 8 | −22 | −6 | R. Red Nucleus | |||
| <0.001 | 69 | 5.88 | 36 | 0 | −4 | R. Claustrum | |
| 5.21 | 36 | −12 | −4 | R. Claustrum | |||
| 0.007 | 11 | 5.55 | −6 | −22 | −6 | L. Red Nucleus | |
| 0.005 | 13 | 5.33 | −40 | −14 | 62 | L. Precentral Gyrus | |
| CS+end -CS−end | |||||||
| <0.001 | 305 | 7.96 | −50 | −34 | 30 | L. Inferior Parietal | |
| Lobule | |||||||
| 6.22 | −58 | −18 | 22 | L. Postcentral Gyrus | |||
| 5.47 | −60 | −24 | 28 | L. Inferior Parietal | |||
| Lobule | |||||||
| <0.001 | 217 | 7.16 | 36 | 18 | 8 | R. Insula | |
| 6.45 | 40 | 26 | 2 | R. Inferior Frontal | |||
| Gyrus | |||||||
| <0.001 | 119 | 7.12 | 54 | −28 | 32 | R. Inferior Parietal | |
| Lobule | |||||||
| <0.001 | 58 | 5.82 | −32 | 20 | 8 | L. Insula | |
| <0.001 | 45 | 5.63 | 0 | 20 | 32 | L. Cingulate Gyrus | |
| 0.005 | 13 | 5.37 | −4 | 6 | 40 | L. Cingulate Gyrus | |
| b. BOLD responses in schizophrenic patients. | |||||||
|---|---|---|---|---|---|---|---|
| Contrast | cluster | cluster | peak | MNI | Peak region | ||
| p(FWE-cor) | size (voxels) | T | x | y | z | ||
| US – CS−end | |||||||
| <0.001 | 1088 | 9.84 | −46 | −20 | 20 | L. Insula | |
| 7.89 | −50 | −30 | 24 | L. Inferior Parietal | |||
| Lobule | |||||||
| 6.39 | −62 | −22 | 20 | L. Postcentral Gyrus | |||
| <0.001 | 678 | 7.67 | −10 | 12 | 36 | L. Cingulate Gyrus | |
| 6.97 | −6 | 2 | 42 | L. Cingulate Gyrus | |||
| 6.58 | −2 | −8 | 46 | L. Cingulate Gyrus | |||
| <0.001 | 418 | 7.33 | −52 | −24 | 60 | L. Postcentral Gyrus | |
| 5.49 | −38 | −18 | 68 | L. Precentral Gyrus | |||
| <0.001 | 844 | 6.92 | 50 | −28 | 30 | R. Postcentral Gyrus | |
| 6.06 | 64 | −34 | 20 | R. Superior Temporal | |||
| Gyrus | |||||||
| 6.05 | 38 | −20 | 18 | R. Insula | |||
| <0.001 | 240 | 6.82 | −58 | 2 | 0 | L. Superior Temporal | |
| Gyrus | |||||||
| 6.25 | −52 | −2 | 8 | L. Precentral Gyrus | |||
| <0.001 | 77 | 6.63 | −34 | 6 | −6 | L. Claustrum | |
| <0.001 | 111 | 6.2 | −2 | −64 | 8 | L. Posterior Cingulate | |
| 5.3 | −12 | −56 | 0 | L. Occipital Lobe, | |||
| Lingual Gyrus | |||||||
| <0.001 | 60 | 6.03 | 38 | 0 | 8 | R. Claustrum | |
| <0.001 | 51 | 5.97 | 18 | 0 | −10 | R. Lateral Globus | |
| Pallidus | |||||||
| <0.001 | 39 | 5.91 | −46 | −68 | 6 | L. Middle Temporal | |
| Gyrus | |||||||
| 5.28 | −48 | −58 | 10 | L. Middle Temporal | |||
| Gyrus | |||||||
| 0.001 | 28 | 5.83 | 58 | 6 | 6 | R. Precentral Gyrus | |
| <0.001 | 53 | 5.78 | −20 | −68 | 10 | L. Posterior Cingulate | |
| 5.57 | −16 | −70 | 18 | L. Precuneus | |||
| 0.001 | 33 | 5.71 | 36 | −18 | 4 | R. Claustrum | |
| CS+end – CS-end | |||||||
| <0.001 | 89 | 6.15 | −62 | 4 | −4 | L. Superior Temporal | |
| Gyrus | |||||||
| 5.75 | −52 | −2 | 6 | L. Superior Temporal | |||
| Gyrus | |||||||
Figure 3.
Greater shock related activation of the left middle insula was found in controls as compared to schizophrenic patients. The insula cluster was significant at p=0.002, corrected for multiple comparisons across the whole brain. The contrast map is displayed at a threshold of T > 3 and overlaid on a template structural MRI image. The color bar denotes T-values.
3.3 Brain responses to the CS+end
At the time point immediately prior to the offset of the CS+ (CS+end derived from non-reinforced trials), healthy subjects displayed activation in the bilateral inferior parietal lobe, bilateral insula, the left postcentral gyrus and the left cingulate gyrus, (Table 2a). Schizophrenic subjects appeared to display less activation, with a significant response in the left superior temporal gyrus only (Table 2b). However, there were no significant differences between the two groups, consistent with the observation that the responses to CS+end were qualitatively similar between control and schizophrenic subjects at a lower threshold.
3.4 Post-hoc analyses
Within the schizophrenia cohort, we observed no significant differences in BOLD responses between medicated and non-medicated patients, and no significant correlation between antipsychotic dose (in chlorpromazine equivalents) and responses of the insula to the US (parameter estimates at peak between-group difference: r=− 0.04, p=0.44), as well as across the whole brain.
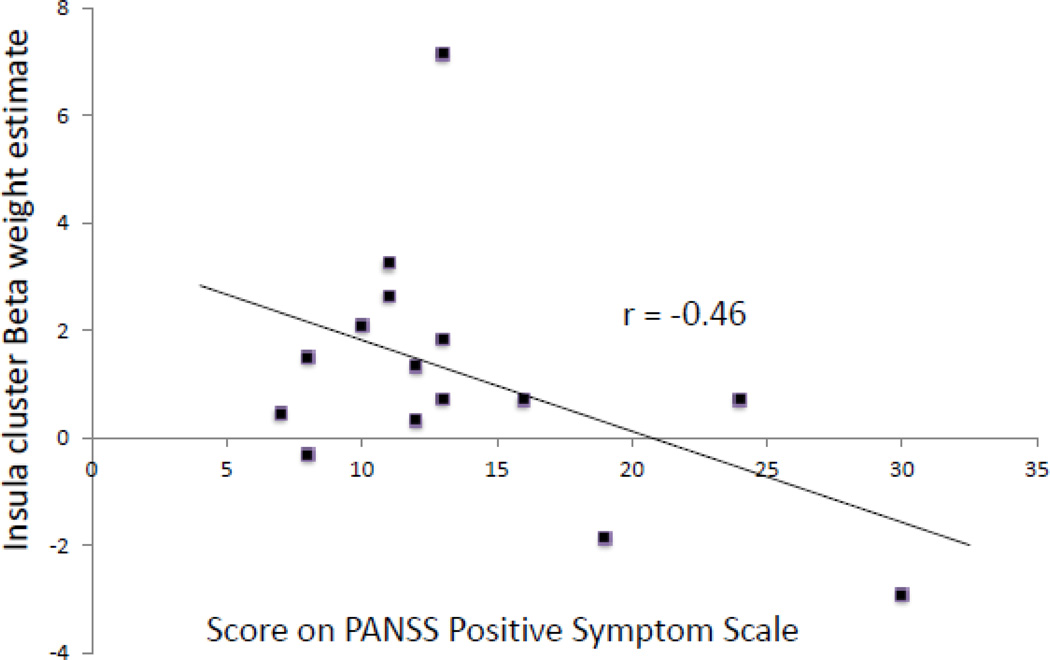
We observed a significant negative correlation within the patient group between responses of the insula to the US and positive symptom levels (r = −0.46, p=0.04) (see Figure 4). There were no correlations between insula response to the US and levels of negative (r=0.32, p=0.24) or general symptoms (r=−0.04, p=0.44), trait anxiety (r=- 0.16, p=0.28) state anxiety (r=0.1, p=0.36) or depressive symptoms (r=−0.26, p=0.17).
Figure 4.
A scatter plot displaying the negative correlation (r=−0.46, p=0.04) between individual BOLD signal (mean beta weight across all voxels in cluster showing between-group difference, at MNIXYZ −44, −2, 10) in the insula and scores on the PANSS Positive Symptom Subscale in the schizophrenia cohort is shown. PANSS, Positive and Negative Syndrome Scale.
4 Discussion
In this study, it is important to note that the predictable and “annoying” shock (the unconditioned stimuli (US)) elicited activation in several brain regions associated with pain processing (Apkarian et al., 2005) in both groups, suggesting that stimuli may not need to be perceived as overtly painful to activate the so-called “pain matrix.” Thus, this suggests that an aversive but not overtly painful electrical shock can be used to test for alterations in pain processing in psychiatric populations, providing the advantages of using experimental stimuli that are not overtly painful (i.e. greater experimental feasibility, subject comfort). However, as we did not perform quantitative sensory testing (QST) in the subjects, the standard approach used to measure pain sensitivity (Rolke et al., 2006), we cannot determine whether patients had alterations in pain sensitivity.
At matched shock intensity, we found a reduction in schizophrenic patients in reactivity of the insula region to an aversive stimulus. The lack of insula reactivity could not be explained by treatment with antipsychotic medications, and is in line with previous studies showing impaired pain reactivity in unmedicated patients with schizophrenia (de la Fuente-Sandoval et al., 2010). The present results thus build on the extensive literature on altered pain processing in schizophrenia (Bonnot et al., 2009; Potvin and Marchand, 2008) by adding two pieces of information: (i) schizophrenic patients display insula hyporeactivity to an aversive electric shock but with no evidence for insula hyporeactivity in anticipation of such a shock, and (ii) insula hyporeactivity may be specifically related to psychosis, as the less reactive the insula, the higher the levels of positive (but not negative) symptoms. A further analysis, contrasting responses to the US minus responses at the end of the CS+, provided some additional evidence, at a trend level (puncorrected=0.004), that the observed insula deficit in schizophrenia may be driven by sensory, rather than immediate anticipatory processes.
Abnormalities of the insula have been found in schizophrenia in a number of previous studies (reviewed in (Wylie and Tregellas, 2010)). For instance, cellular and molecular abnormalities of the insula, such as decreased neuronal somal size (Pennington et al., 2008b), and reduced density of dopamine and cAMP-regulated phosphoprotein 32 kD (DARPP-32) immunoreactive neurons (Nishiura et al., 2011), have been observed in schizophrenia. Also, a proteomic analysis revealed 57 significantly differentially expressed proteins in insular layer 2 in schizophrenic patients (Pennington et al., 2008a). In addition to these molecular abnormalities, macroscopic changes of the insula have been found in patients with schizophrenia using neuroimaging methods. For example, the insula has been identified as one of the regions with the greatest amount of gray matter volume reduction in schizophrenia (Glahn et al., 2008). Also, using functional imaging, insula hyporeactivity to aversive odors (Crespo-Facorro et al., 2001) and to emotional faces (Li et al., 2012; Seiferth et al., 2009; Williams et al., 2007), and altered insular reactivity during emotional social perception (Ebisch et al., 2012) in schizophrenia have been reported. Thus, a variety of convergent evidence suggests that the pathophysiological process associated with schizophrenia may produce alterations in the cellular integrity and function of the insula region (Wylie and Tregellas, 2010).
Interestingly, a number of studies have found negative correlations between the severity of positive symptoms in schizophrenic patients and bilateral (Crespo-Facorro et al., 2000; Duggal et al., 2005) or right (Pressler et al., 2005) insula gray matter volume, with some studies detecting a specific association between hallucination severity and insula gray matter loss in schizophrenia (Glahn et al., 2008; Modinos et al., 2009; Shapleske et al., 2002). The finding of the present study of a negative correlation between positive symptom severity and insular responses to an electrical shock stimulus extends this existing body of work, demonstrating that changes in the insula associated with psychosis relate to impaired processing of aversive information.
The insula has been conceptualized as an interoceptive integration region, where ascending sensory pathways conveying proprioceptive and somatosensory information about the body’s internal state, as well as visual and auditory information about the external world, terminate in the posterior insula. This activity is then re-represented in the mid, and finally in the anterior insula, where polysensory integration produces a global representation of the internal feeling state of the individual (Craig, 2009). Classical conditioning studies examining fear-induced activity commonly report anterior insula activations (reviewed in Sehlmeyer et al., 2009). Our previous studies indicate that the anterior insula increases its activity during both shock presentation and shock expectancy (Linnman et al., 2011a; Linnman et al., 2011b), and these activations may reflect the anticipation and preparation for an impending aversive event (Craig, 2009). In the present study, the peak difference between the two groups in shock response was located in the short insular gyrus of the middle insula, a region that is on the border between a posterior sensory-motor network and an anterior attention network (Cauda et al., 2011). Meta-analyses of functional neuroimaging studies indicate the mid-insula region to be involved in pain, interoception, tactile sensation, motion perception and the discrimination between internally and externally generated stimuli (Kurth et al., 2010). Insular lesions may lead to altered pain thresholds and central pain (Garcia-Larrea et al., 2010), and have been associated with a failure to withdraw from and /or absent or inadequate emotional responses to painful stimuli, a syndrome known as pain asymbolia (Berthier et al., 1988). Stroke patients with posterior insula lesions and limb dysfunction may be convinced that their limbs function normally (anosognosia), that their limb does not belong to them (asomatognosia) or attribute their own body parts to other persons (somatoparaphrenia) (Baier and Karnath, 2008; Karnath et al., 2005). A previous fMRI study on responses to thermal pain in unmedicated patients with schizophrenia demonstrated insula hypo-reactivity to pain (de la Fuente-Sandoval et al., 2010). As we did not use overtly painful stimuli in the current study, it is possible that the observed insula deficit seen here and by de la Fuente-Sandoval and colleagues may not be specifically linked to pain but reflect a different mode of percept self-attribution and interoception (Bonnot et al., 2009), i.e., an impaired ability to determine “is this sensation relevant to me?”. Indeed, while the insula in healthy subjects is preferentially activated by nociceptive stimuli, other salient events, as described above, also lead to its activation (Lotsch et al., 2012). Thus, further studies exploring how overall salience and self-relevance modulate insula function in schizophrenia are warranted.
Since common symptoms of schizophrenia, including poor illness-related insight and the feeling that inner thoughts or experiences are alien or not belonging to the self, roughly resemble symptoms of the insular stroke syndromes described above, it is possible that insula dysfunction in schizophrenia contributes to the psychotic symptoms and poor insight characteristic of the disorder.
Given that the insula is involved in labeling internal sensations as emanating from the body or “self,” abnormalities of the insula may interfere with the ability to distinguish internally generated from externally generated information. These types of deficits have been well-documented in schizophrenia using a wide variety of experimental modalities, including behavioral, electrophysiological and functional imaging techniques (Ford et al., 2012). The frequently reported association between psychosis and insular abnormalities in schizophrenia, including the present finding, provide additional empirical support for this model of psychosis.
We found no differences between patients and controls in responses at the offset of the CS+ cue. However in our previous analysis of these data (Holt et al., 2012), in which we compared overall responses to the CS+ and CS− cues (not to the offset of those cues), we found greater BOLD responses in the control group than in the schizophrenic patients in the thalamus, brainstem, posterior cingulate gyrus, hippocampus and amygdala. This difference between the results of these two analyses is not surprising given that responding to a conditioned cue (the CS+) and the immediate anticipation of an aversive experience (the US) are distinct processes, with different time courses. Supporting this are data showing that following the acquisition of conditioned fear responses, neurons within the amygdala respond immediately at the detection of a conditioned cue, not at the offset of the cue (Quirk et al., 1997). Future studies with higher temporal resolution could further examine these two processes in schizophrenic patients.
It is important to also note that impairment in associative learning or multi-sensory integration may have contributed to our main finding, a reduction in insular responses to the US in schizophrenic patients. Since this insula deficit occurred following the US, not the CS+, it could reflect some degree of impaired initial encoding of the CS+/US association, whereas an abnormality in an amygdala-hippocampal-centered network in schizophrenia may disrupt retrieval of this learned association immediately following the CS+ (Holt et al, 2012).
4.2 Limitations
This study has several limitations and interpretive caveats. First, the patients chose, on average, a slightly lower (not significantly) shock level than did the healthy controls, which seems inconsistent with the prior evidence for heightened pain thresholds in schizophrenia (Bonnot et al., 2009; de la Fuente-Sandoval et al., 2010; Potvin and Marchand, 2008). However, these shock levels do not represent pain thresholds as they are typically determined (Rolke et al., 2006). Rather, the instructions were to choose a shock level that was “highly annoying, but not painful”, so the shock levels here may reflect pain sensitivity and/or anxiety about the procedure. Indeed, the schizophrenic patients had significantly higher levels of anxiety than the controls (Table 1), and, in the patient group, trait anxiety was negatively correlated with shock levels (r=−0.46, p=0.04), indicating that anticipatory anxiety about the procedure, rather than lower pain thresholds, may have led the patients to choose slightly lower shock levels. Future studies would benefit from the use of full quantitative sensory testing to measure pain sensitivity and the inclusion of explicit pain ratings to fully resolve this issue.
Second, our sample size was relatively small. Despite this, we found a strong between-group difference selectively in the insula (p =0.002, corrected for multiple comparisons across the whole brain) accompanied by intact responses of other regions of the pain network in the schizophrenic patients. This pattern of findings (a highly specific change in the patient group that was predicted a priori) is less likely to result from Type 1 error than a group of unpredicted findings. However, because of the small sample size, the absence of a significant difference between patients and controls in brain responses to the CS+end (anticipatory processing) is not conclusive and requires further study in a larger cohort.
Third, although we found no significant differences in responses between medicated and unmedicated patients, and no correlations between antipsychotic dose and insula BOLD signal, we cannot entirely rule out medication effects; studies conducted in a larger number of unmedicated patients are needed to confirm these findings. Nonetheless, it has been well documented that antipsychotic-free schizophrenic patients (Potvin and Marchand, 2008) and first-degree relatives of patients with schizophrenia (Hooley and Delgado, 2001) show pain insensitivity, suggesting that the likely related insula abnormalities in schizophrenia are not antipsychotic-induced. Finally, we did not study female schizophrenic patients or controls. As there are gender differences in pain processing (Greenspan et al., 2007; Linnman et al., 2012), and insula volumes may be particularly diminished in female schizophrenic patients (Duggal et al., 2005), additional studies are needed to determine if women with schizophrenia show functional alterations of the insula.
4.3 Conclusions
This study found evidence for a lack of insula reactivity to an aversive electric shock in schizophrenic patients as compared to controls. The lack of insula reactivity was correlated with levels of positive symptoms. As the mid insula is thought to participate in the labeling of bodily signals as “self,” a compromised insula function in schizophrenia may lead to a dissociation from bodily pain and other internal sensations and/or impaired multi-sensory integration. In light of this, pain insensitivity in schizophrenia, as well as the positive symptoms of the disorder, may be regarded as a possible consequence of dysfunctional sensory perception and interoception.
Acknowledgements
We thank Dr. Mohammed R. Milad for contributing to the study design and data collection methods.
Role of funding sources: This study was supported by the National Institute of Mental Health K23MH076054 and RO1MH095904 (Dr. Holt) and the National Alliance for Research on Depression and Schizophrenia with the Sidney R. Baer, Jr Foundation (Dr. Holt). Dr. Linnman received support from the Swedish Society for Medical Research (SSMF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures
Contributors: Dr. Linnman analyzed the data and wrote the first draft of the manuscript. Mr. Combs collected the data and contributed to data analysis. Dr. Goff contributed to the design of the study and data interpretation. Dr. Holt designed the study, collected data and contributed to data analysis/interpretation and the writing of the manuscript. All authors contributed to and have approved the final manuscript.
Conflicts of Interest: Over the past 3 years, Dr. Goff has served as a consultant to Eli Lilly, Bristol-Meyer-Squibb, Roche, Endo Pharmaceuticals, Genentech, Cypress Bioscience, Dianippon Sumitomo, Solvay, Biovail, and Takeda Pharmaceuticals. He served on a DMC for Otsuka. He received research funding from Janssen, Pfizer, GSK, PamLab and Novartis and he has applied for patents regarding genetic predictors of response to glutamatergic agents and folate. The remaining authors do not have any potential financial interests to disclose.
References
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Baier B, Karnath HO. Tight link between our sense of limb ownership and self-awareness of actions. Stroke. 2008;39(2):486–488. doi: 10.1161/STROKEAHA.107.495606. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of general psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berthier M, Starkstein S, Leiguarda R. Asymbolia for pain: a sensorylimbic disconnection syndrome. Ann Neurol. 1988;24(1):41–49. doi: 10.1002/ana.410240109. [DOI] [PubMed] [Google Scholar]
- Bonnot O, Anderson GM, Cohen D, Willer JC, Tordjman S. Are patients with schizophrenia insensitive to pain? A reconsideration of the question. Clin J Pain. 2009;25(3):244–252. doi: 10.1097/AJP.0b013e318192be97. [DOI] [PubMed] [Google Scholar]
- Cauda F, D'Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. NeuroImage. 2011;55(1):8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Kim J, Andreasen NC, O'Leary DS, Bockholt HJ, Magnotta V. Insular cortex abnormalities in schizophrenia: a structural magnetic resonance imaging study of first-episode patients. Schizophrenia research. 2000;46(1):35–43. doi: 10.1016/s0920-9964(00)00028-1. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Paradiso S, Andreasen NC, O'Leary DS, Watkins GL, Ponto LL, Hichwa RD. Neural mechanisms of anhedonia in schizophrenia: a PET study of response to unpleasant and pleasant odors. JAMA : the journal of the American Medical Association. 2001;286(4):427–435. doi: 10.1001/jama.286.4.427. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Favila R, Gomez-Martin D, Leon-Ortiz P, Graff-Guerrero A. Neural response to experimental heat pain in stable patients with schizophrenia. Journal of psychiatric research. 2012;46(1):128–134. doi: 10.1016/j.jpsychires.2011.09.008. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Sandoval C, Favila R, Gomez-Martin D, Pellicer F, Graff-Guerrero A. Functional magnetic resonance imaging response to experimental pain in drug-free patients with schizophrenia. Psychiatry research. 2010;183(2):99–104. doi: 10.1016/j.pscychresns.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Duggal HS, Muddasani S, Keshavan MS. Insular volumes in first-episode schizophrenia: gender effect. Schizophrenia research. 2005;73(1):113–120. doi: 10.1016/j.schres.2004.08.027. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC. Impact of continuous versus intermittent CS-UCS pairing on human brain activation during Pavlovian fear conditioning. Behavioral neuroscience. 2007;121(4):635–642. doi: 10.1037/0735-7044.121.4.635. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC. Neural correlates of unconditioned response diminution during Pavlovian conditioning. Neuroimage. 2008;40(2):811–817. doi: 10.1016/j.neuroimage.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Labar KS. Brain activity associated with omission of an aversive event reveals the effects of fear learning and generalization. Neurobiology of learning and memory. 2012;97(3):301–312. doi: 10.1016/j.nlm.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisch SJ, Salone A, Ferri F, De Berardis D, Romani GL, Ferro FM, Gallese V. Out of touch with reality? Social perception in first-episode schizophrenia. Social cognitive and affective neuroscience. 2012 doi: 10.1093/scan/nss012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-Iv Axis I Disorders - Patient Edition (SCID-I/P, Version 2.0) New York: Biometrics Reserach Depatment New York State Psychiatric Institute; 1995. [Google Scholar]
- Ford JM, Perez VB, Mathalon DH. Neurophysiology of a possible fundamental deficit in schizophrenia. World Psychiatry. 2012;11(1):58–60. doi: 10.1016/j.wpsyc.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1996;35(3):346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Garcia-Larrea L, Perchet C, Creac'h C, Convers P, Peyron R, Laurent B, Mauguiere F, Magnin M. Operculo-insular pain (parasylvian pain): a distinct central pain syndrome. Brain : a journal of neurology. 2010;133(9):2528–2539. doi: 10.1093/brain/awq220. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, Bullmore E, Fox PT. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biological Psychiatry. 2008;64(9):774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, Gold MS, Holdcroft A, Lautenbacher S, Mayer EA, Mogil JS, Murphy AZ, Traub RJ. Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132(Suppl 1):S26–S45. doi: 10.1016/j.pain.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Coombs G, Zeidan MA, Goff DC, Milad MR. Failure of neural responses to safety cues in schizophrenia. Archives of general psychiatry. 2012;69(9):893–903. doi: 10.1001/archgenpsychiatry.2011.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Lebron-Milad K, Milad MR, Rauch SL, Pitman RK, Orr SP, Cassidy BS, Walsh JP, Goff DC. Extinction memory is impaired in schizophrenia. Biol Psychiatry. 2009;65(6):455–463. doi: 10.1016/j.biopsych.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooley JM, Delgado ML. Pain insensitivity in the relatives of schizophrenia patients. Schizophrenia research. 2001;47(2–3):265–273. doi: 10.1016/s0920-9964(00)00064-5. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Baier B, Nagele T. Awareness of the functioning of one's own limbs mediated by the insular cortex? The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(31):7134–7138. doi: 10.1523/JNEUROSCI.1590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Knight DC, Waters NS, King MK, Bandettini PA. Learning-related diminution of unconditioned SCR and fMRI signal responses. Neuroimage. 2010;49(1):843–848. doi: 10.1016/j.neuroimage.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraepelin E, Robertson GM. Dementia praecox and paraphrenia. Edinburgh: Livingstone; 1919. [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214(5–6):519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Chan RC, Gong QY, Liu Y, Liu SM, Shum D, Ma ZL. Facial emotion processing in patients with schizophrenia and their non-psychotic siblings: a functional magnetic resonance imaging study. Schizophrenia research. 2012;134(2–3):143–150. doi: 10.1016/j.schres.2011.10.019. [DOI] [PubMed] [Google Scholar]
- Linnman C, Beucke JC, Jensen KB, Gollub RL, Kong J. Sex similarities and differences in pain-related periaqueductal gray connectivity. Pain. 2012;153(2):444–454. doi: 10.1016/j.pain.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Rougemont-Bucking A, Beucke JC, Zeffiro TA, Milad MR. Unconditioned responses and functional fear networks in human classical conditioning. Behavioural brain research. 2011a;221(1):237–245. doi: 10.1016/j.bbr.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Zeffiro TA, Pitman RK, Milad MR. An fMRI study of unconditioned responses in post-traumatic stress disorder. Biology of Mood & Anxiety Disorders. 2011b;1(8) doi: 10.1186/2045-5380-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62(5):446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Modinos G, Vercammen A, Mechelli A, Knegtering H, McGuire PK, Aleman A. Structural covariance in the hallucinating brain: a voxel-based morphometry study. Journal of psychiatry & neuroscience : JPN. 2009;34(6):465–469. [PMC free article] [PubMed] [Google Scholar]
- Nishiura K, Kunii Y, Wada A, Matsumoto J, Yang Q, Ikemoto K, Niwa S. Profiles of DARPP-32 in the insular cortex with schizophrenia: a postmortem brain study. Progress in neuro-psychopharmacology & biological psychiatry. 2011;35(8):1901–1907. doi: 10.1016/j.pnpbp.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Pennington K, Dicker P, Dunn MJ, Cotter DR. Proteomic analysis reveals protein changes within layer 2 of the insular cortex in schizophrenia. Proteomics. 2008a;8(23–24):5097–5107. doi: 10.1002/pmic.200800415. [DOI] [PubMed] [Google Scholar]
- Pennington K, Dicker P, Hudson L, Cotter DR. Evidence for reduced neuronal somal size within the insular cortex in schizophrenia, but not in affective disorders. Schizophrenia research. 2008b;106(2–3):164–171. doi: 10.1016/j.schres.2008.08.022. [DOI] [PubMed] [Google Scholar]
- Potvin S, Marchand S. Hypoalgesia in schizophrenia is independent of antipsychotic drugs: a systematic quantitative review of experimental studies. Pain. 2008;138(1):70–78. doi: 10.1016/j.pain.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Pressler M, Nopoulos P, Ho BC, Andreasen NC. Insular cortex abnormalities in schizophrenia: Relationship to symptoms and typical neuroleptic exposure. Biological Psychiatry. 2005;57(4):394–398. doi: 10.1016/j.biopsych.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Armony JL, LeDoux JE. Fear conditioning enhances different temporal components of tone-evoked spike trains in auditory cortex and lateral amygdala. Neuron. 1997;19(3):613–624. doi: 10.1016/s0896-6273(00)80375-x. [DOI] [PubMed] [Google Scholar]
- Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 2006;123(3):231–243. doi: 10.1016/j.pain.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Schoning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, Konrad C. Human fear conditioning and extinction in neuroimaging: a systematic review. PloS one. 2009;4(6):e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel S, Aigner M, Ossege M, Pernicka E, Wildner B, Sycha T. Antipsychotics for acute and chronic pain in adults. J Pain Symptom Manage. 2010;39(4):768–778. doi: 10.1016/j.jpainsymman.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Seiferth NY, Pauly K, Kellermann T, Shah NJ, Ott G, Herpertz-Dahlmann B, Kircher T, Schneider F, Habel U. Neuronal correlates of facial emotion discrimination in early onset schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34(2):477–487. doi: 10.1038/npp.2008.93. [DOI] [PubMed] [Google Scholar]
- Shapleske J, Rossell SL, Chitnis XA, Suckling J, Simmons A, Bullmore ET, Woodruff PW, David AS. A computational morphometric MRI study of schizophrenia: effects of hallucinations. Cerebral cortex. 2002;12(12):1331–1341. doi: 10.1093/cercor/12.12.1331. [DOI] [PubMed] [Google Scholar]
- Singh MK, Giles LL, Nasrallah HA. Pain insensitivity in schizophrenia: trait or state marker? Journal of psychiatric practice. 2006;12(2):90–102. doi: 10.1097/00131746-200603000-00004. [DOI] [PubMed] [Google Scholar]
- Spielberger C. State-Trait Anxiety Inventory. In: Hersen M, Bellack AS, editors. Dictionary of behavioral assessment techniques. New York ; Oxford: Pergamon; 1988. pp. 448–450. [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Mattay V, Callicott J, Kotrla K, Santha A, van Gelderen P, Duyn J, Moonen C, Frank J. fMRI applications in schizophrenia research. NeuroImage. 1996;4(3 Pt 3):S118–S126. doi: 10.1006/nimg.1996.0062. [DOI] [PubMed] [Google Scholar]
- Williams LM, Das P, Liddell BJ, Olivieri G, Peduto AS, David AS, Gordon E, Harris AW. Fronto-limbic and autonomic disjunctions to negative emotion distinguish schizophrenia subtypes. Psychiatry research. 2007;155(1):29–44. doi: 10.1016/j.pscychresns.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Wylie KP, Tregellas JR. The role of the insula in schizophrenia. Schizophrenia research. 2010;123(2–3):93–104. doi: 10.1016/j.schres.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]