Abstract
While cutaneous wounds of late-gestational fetuses and on through adulthood result in scar formation, wounds incurred early in gestation have been shown to heal scarlessly. Unique properties of fetal fibroblasts are believed to mediate this scarless healing process. In this study, microarray analysis was used to identify differences in the gene expression profiles of cultured fibroblasts from embryonic day 15 (E15; mid-gestation) and embryonic day 18 (E18; late-gestation) skin. Sixty-two genes were differentially expressed and twelve of those genes are associated with inflammation, a process that correlates with scar formation in fetal wounds. One of the differentially expressed inflammatory genes was cyclooxygenase-1 (COX-1). COX-1 was more highly expressed in E18 fibroblasts than in E15 fibroblasts, and these differences were confirmed at the gene and protein level. Differences in COX-1 protein expression were also observed in fetal skin by immunohistochemical and immunofluorescent staining. The baseline differences in gene expression found in mid- and late-gestational fetal fibroblasts suggest that developmental alterations in fibroblasts could be involved in the transition from scarless to fibrotic fetal wound healing. Furthermore, baseline differences in the expression of inflammatory genes by fibroblasts in E15 and E18 skin may contribute to inflammation and scar formation late in gestation.
Keywords: Fetal, scar, scarless, microarray, skin
Introduction
The response to injury is a complicated and highly regulated cascade of events that quickly restores homeostasis after injury; however, this process results in nonfunctional scar tissue that can have detrimental effects. Tissue repair after injury to normal adult skin can be described as having three phases: inflammation, proliferation, and scar formation/remodeling. In contrast to adult wound healing, fetal skin wounds heal without scarring and do not mount an inflammatory response after injury until late in gestation (1, 2)
Many studies have shown that stimulating inflammation enhances the extent of scarring in fetal wounds, and a more substantial inflammatory response to injury is seen in late-gestational fetal skin that heals with a scar. Several pro-inflammatory mediators are produced at higher levels in fibrotic wounds, and exposing wounds that would otherwise heal scarlessly to inflammatory stimuli results in scar formation. These molecules include interleukin-6, interleukin-8, transforming growth factor-β (TGF-β), platelet-derived growth factor, and prostaglandins (3–7). There are also gestational differences in the number and/or activity of several cell types which contribute to inflammation, including endothelial cells which aid in inflammatory cell trafficking, as well as platelets, neutrophils, macrophages, and mast cells (8–12).
In addition to differences in inflammation, changes in the behavior of fibroblasts have been suggested to play a role in regulating scar formation in fetal wounds. Because fibroblasts produce and maintain the extracellular matrix and are responsible for producing collagen, these cells are a key target in understanding the biology of scarring. Studies by Lorenz et al. showed that fetal fibroblasts differ from adult fibroblasts in their ability to mediate scarless repair (13). A model was used in which human fetal skin healed with a scar when grafted cutaneously onto nude mice, but healed scarlessly when grafted subcutaneously. Using species-specific antibodies to detect human and mouse collagen, they showed that adult murine fibroblasts were responsible for producing scar tissue in normal grafts, but fetal human fibroblasts produced normal collagen in the scarless subcutaneous grafts (13). Studies have also demonstrated differences between fibroblasts from skin that heals with or without scarring (14–21). In the current study we cultured fibroblasts from embryonic day 15 (E15) and embryonic day 18 (E18) skin, ages that correspond to scarless and fibrotic healing phenotypes, respectively (7, 22). To better understand the mechanisms involved in scarless repair, microarray analysis was used to compare the baseline transcriptomes of E15 and E18 fibroblasts. Differences in genes associated with proliferation, cell signaling, and cell-cell interactions as well as the inflammatory response were identified.
Materials and Methods
Generation of fetal skin
FVB mice were purchased from Taconic Farms (Germantown, NY) and Col3.6-GFPtpz mice, which express green fluorescent protein (GFP) under the control of the collagen 1a1 (col 1a1) promoter, were obtained from Dr. David Rowe (University of Connecticut). Protocols approved by the Ohio State University Institutional Animal Care and Use Committee were followed for all animal studies. Dorsal fetal skin was collected at E15 or E18 from time-mated mice as described previously (12). These time points were chosen based on earlier studies indicating that wounds made at E15 heal without scarring while those made at E18 heal with a scar (7). Skin samples were used for cell culture as described below or fixed in 10% formalin. Formalin-fixed tissue was embedded in paraffin for standard immunohistochemistry or incubated in phosphate buffered saline (PBS) with 30% sucrose (Fisher Scientific, Fair Lawn, NJ) for 24 hours then frozen in tissue freezing medium (Triangle Biomedical Sciences, Durham, NC) for immunofluorescence.
Cell culture
Freshly harvested E15 or E18 dorsal skin was cut into small pieces and allowed to adhere, dermal side down, to the bottom of a 6-well tissue culture plate. Skin from individual fetuses was cultured in separate wells. Dulbecco’s Modified Eagles Medium (high glucose) supplemented with non-essential amino acids, sodium pyruvate, L-glutamine, penicillin/streptomycin, and 20% fetal bovine serum (all reagents from Invitrogen, Grand Island, NY) was added as described previously (7). The fibroblasts were allowed to grow for 5 days at 37°C and 5% carbon dioxide, during which time the culture medium was changed daily (passage 1). On day 5, the tissue pieces were removed using sterile forceps. Adherent cells were trypsinized and transferred to a T75 flask for 24 hours (passage 2). These cells were then lysed either in RIPA (radioimmunoprecipitation assay) lysis buffer (Thermo Scientific, Rockford, IL) for protein isolation or Trizol (Invitrogen) for RNA isolation. After isolation, RNA from multiple fetuses within the same litter was pooled. A total of three pooled samples from independent litters at each time point (E15 and E18) were used. To confirm that the cultured cells were fibroblasts, Western blotting was performed for the intermediate filament vimentin, a commonly used marker of fibroblasts. All fibroblast samples expressed vimentin. Microarray results confirmed that the cells expressed high levels of vimentin, but keratin expression was below the background threshold level (data not shown).
Microarray gene expression analysis
Pooled RNA isolated from cultured E15 or E18 fetal fibroblasts by Trizol (Invitrogen) extraction was treated with DNase (Qiagen Sciences, Germantown, MD) and purified with the RNeasy kit (Qiagen) according to manufacturers’ instructions. RNA quality and concentration were assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA) and Nanodrop (Nanodrop technologies, Wilmington, DE), respectively. Single stranded complimentary DNA (cDNA) was generated using the Ambion WT expression kit (Affymetrix, Santa Clara, CA). cDNA was then fragmented and labeled with the Ambion WT terminal labeling kit (Affymetrix). Labeled cDNA was hybridized to GeneChip Mouse Gene 1.0 ST Array chips (Affymetrix) for 16 hours at 45° C. Chips were scanned with the GeneChip Scanner 3000 7G system. Signal intensities were quantified using Affymetrix software. Background correction and quantile normalization was performed to adjust for technical bias, and gene expression levels were summarized using the Robust Multichip Average method (23). A filtering method based on percentage of samples above noise cutoff was applied to filter out low expression genes (this included a minimum of two genes with gene expression levels above six). A linear model was employed to detect differentially expressed genes. In order to improve the estimates of expression variability and statistical tests for differential expression, a moderate t-statistic with variance smoothing was employed for this study (24). The significance level was set at 0.05. A volcano plot of log2 fold and log10 p-value and heatmap with hierarchical clustering dendrogram using Euclidean distance and average linkage were prepared to visualize the test results and the expression data.
Real-Time reverse transcription-polymerase chain reaction (RT-PCR) Gene Expression Assays
Taqman (Applied Biosystems Inc, Foster City, CA) gene expression assays were used to confirm gene expression differences for cyclooxygenase-1 (COX-1), cluster of differentiation 109 (CD109), and suppressor of cytokine signaling 2 (SOCS2) by real-time RT-PCR. All reagents, primers, and probes (pre-designed, pre-optimized) were obtained from Applied Biosystems (Applied Biosystems). Three hundred ng of total RNA was used for each reverse transcription reaction. All reverse transcription reactions, including no-template controls and controls without reverse transcriptase, were prepared using the high capacity RNA to cDNA kit (Applied Biosystems) and run in a GeneAmp PCR 9700 Thermocycler (Applied Biosystems). Gene expression levels were quantified using the ABI Prism 7900HT Sequence detection system (Applied Biosystems). Comparative real-time RT-PCR was performed in triplicate, including no-template controls. The housekeeping gene hypoxanthine-guanine phosphoribosyltransferase (HPRT) was used for normalization. Relative expression was calculated using the comparative Ct method.
Western blot analysis
COX-1 protein levels were determined by Western blot using standard methods. Total protein (20 μg) was separated on 12% sodium dodecyl sulfate-polyacrylamide gels, and transferred onto polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA). The membranes were cut at the 50 kilo-Dalton mark, blocked with 5% milk in tris buffered saline with 0.1% tween 20 (TBST), and antibodies were used to probe for COX-1 (Cayman Chemical, Ann Arbor, MI) or β-actin (Cell Signaling Technology, Danvers, MA) overnight at 4°C. The membrane was washed in TBST and incubated for 1 hour with horse radish peroxidase-conjugated goat anti-rabbit antibody (Cell Signaling). Proteins were visualized using Immun-Star Western C chemiluminescence reagents (Bio-Rad) and digital images were captured using the Chemidoc XRS imaging system (Bio-Rad). Densitometry was performed using Quantity One software (Bio-Rad) and data are presented as a ratio of the density of the bands for COX-1 to β-actin.
Immunohistochemical staining for COX-1 in fetal skin
Formalin fixed, paraffin embedded tissue sections (4 μm) were deparaffinized in Clear-rite 3 (Richard-Allan Scientific, Kalamazoo, MI) and rehydrated in a series of ethanol washes ending in phosphate buffered saline (PBS). Antigen unmasking was performed by steaming for 10 minutes in Dako Target Retrieval Solution (DakoCytomation, Carpinteria, CA) followed by 10 minutes of cooling at room temperature. Endogenous peroxidase activity was blocked by incubation for 15 minutes with 0.3% hydrogen peroxide in methanol. The tissue was blocked with 10% normal goat serum for 30 minutes. Anti-COX-1 antibody (Cayman Chemical) was allowed to react with the tissue overnight at 4°C. Sections were washed with PBS and a biotinylated goat anti-rabbit antibody (Vector Laboratories, Burlingame, CA) was added for 30 minutes. After washing in PBS, avidin-biotin-horseradish peroxidase complex (ABC-HRP kit; Vector Laboratories) was allowed to bind for 30 minutes and colorimetric development was performed by reaction with 3,3′-diaminobenzidine for 10 minutes. Samples were counterstained briefly with hematoxylin then dehydrated in a series of ethanol washes ending in Clear-rite 3 (Richard-Allan). Sections were coverslipped with Permount (Fisher Scientific) and digital pictures were taken with a Zeiss Axiocam digital camera (Carl Zeiss, Thornwood, NY) mounted on an Axioscope 40 microscope (Carl Zeiss)
Immunofluorescent staining for COX-1 in dermal fibroblasts
Cryosections (10 μm) of formalin-fixed tissue were placed in PBS. Non-specific antibody binding was blocked with 10% normal goat serum for 30 minutes. Anti-COX-1 antibody (Cayman Chemical) was allowed to react with the tissue overnight at 4°C. Sections were washed with PBS and Alexa fluor 594 conjugated goat anti-rabbit antibodies (Life Technologies, Eugene, OR) was added for 30 minutes. Samples were washed in PBS then incubated for 30 minutes at room temperature in PBS containing 1 mM magnesium chloride (Sigma, St. Louis, MO). Samples were counterstained with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI; Invitrogen) then coverslipped using Prolong Gold (Invitrogen). Digital images were taken on an Olympus FV1000 spectral confocal microscope (Olympus America Inc., Melville, NY).
Results
Microarray analysis of gene expression in cultured fetal fibroblasts
Affymetrix GeneChip mouse gene 1.0 ST arrays were used to analyze the expression profile of 28,853 genes in cultured E15 and E18 dermal fibroblasts. Using a cutoff threshold of a 1.5-fold difference in mean log2 expression or greater and a p-value < 0.05, sixty-two unique genes showed differential expression between E15 and E18 fibroblasts as shown by volcano plot (FIG. 1). Of these genes, twenty-two were up-regulated in E18 fibroblasts compared to E15 fibroblasts (TABLE 1), while forty genes were down-regulated in E18 fibroblasts compared to E15 fibroblasts (TABLE 2). Hierarchical clustering of genes with similar expression levels and clustering of samples can be seen in the heatmap shown (FIG. 2). Clustering distinctly stratified E15 fibroblast samples from E18 fibroblast samples (FIG. 2).
FIG. 1.
Volcano plot of differentially expressed genes between E15 and E18 fibroblasts. Log2 of the fold change in gene expression was plotted against Log10 of the p-value from microarray samples. For each age group, n=3 pooled samples from separate litters of mice. Genes were identified as having a significant change in expression if the fold change was 1.5 or greater and the p-value less than 0.05 (red dots).
TABLE 1.
List of genes with higher expression in E18 fetal fibroblasts compared to E15 fetal fibroblasts.
| Gene Symbol | Gene Description | fold ↑ in E18 | p-value |
|---|---|---|---|
| AY036118 | cDNA sequence AY036118 | 2.46 | <0.001 |
| ND6 | NADH dehydrogenase subunit 6 | 2.38 | <0.001 |
| Serping1 | serine (or cysteine) peptidase inhibitor, clade G, member 1 | 2.04 | 0.001 |
| Slc38a1 | solute carrier family 38, member 1 | 1.94 | 0.008 |
| Cck | cholecystokinin | 1.85 | 0.005 |
| Slc7a11 | solute carrier family 7 (cationic amino acid transporter, y+ system), member 11 | 1.80 | 0.013 |
| Il11 | interleukin 11 | 1.74 | 0.010 |
| Ptgs1 (COX-1) | prostaglandin-endoperoxide synthase 1 | 1.72 | <0.001 |
| Thbd | thrombomodulin | 1.69 | 0.005 |
| Gm5070 | predicted gene 5070 | 1.66 | <0.001 |
| Npr3 | natriuretic peptide receptor 3 | 1.66 | 0.007 |
| Il1rl2 | interleukin 1 receptor-like 2 | 1.66 | 0.015 |
| ND3 | NADH dehydrogenase subunit 3 | 1.65 | 0.015 |
| Gm5921 | predicted gene 5921 | 1.59 | 0.007 |
| Lrrc32 | leucine rich repeat containing 32 | 1.58 | 0.012 |
| LOC100047986 | similar to eukaryotic translation elongation factor 1 gamma | 1.57 | 0.007 |
| Rps6 | ribosomal protein S6 | 1.55 | 0.001 |
| Rpl17 | ribosomal protein L17 | 1.55 | 0.001 |
| ATP6 | ATP synthase F0 subunit 6 | 1.54 | 0.001 |
| ND4L | NADH dehydrogenase subunit 4L | 1.54 | 0.003 |
| Usmg5 | upregulated during skeletal muscle growth 5 | 1.53 | 0.013 |
| 4932438A13Rik | RIKEN cDNA 4932438A13 gene | 1.51 | 0.012 |
TABLE 2.
List of genes with lower expression in E18 fetal fibroblasts compared to E15 fetal fibroblasts.
| Gene Symbol | Gene_Description | fold ↓ in E18 | p-value |
|---|---|---|---|
| Lyz2 | lysozyme 2 | 19.04 | <0.001 |
| Tnn | tenascin N | 6.45 | <0.001 |
| Actc1 | actin, alpha, cardiac muscle 1 | 4.46 | <0.001 |
| Igfbp5 | insulin-like growth factor binding protein 5 | 2.55 | <0.001 |
| Thy1 | thymus cell antigen 1, theta | 2.52 | 0.003 |
| C1qtnf3 | C1q and tumor necrosis factor related protein 3 | 2.45 | <0.001 |
| Crabp1 | cellular retinoic acid binding protein I | 2.24 | 0.001 |
| Itga11 | integrin alpha 11 | 1.94 | 0.012 |
| S100a4 | S100 calcium binding protein A4 | 1.78 | 0.011 |
| Avpr1a | arginine vasopressin receptor 1A | 1.77 | 0.003 |
| Cspg4 | chondroitin sulfate proteoglycan 4 | 1.70 | 0.011 |
| Col8a1 | collagen, type VIII, alpha 1 | 1.70 | 0.001 |
| Cd109 | CD109 antigen | 1.67 | <0.001 |
| Chst2 | carbohydrate sulfotransferase 2 | 1.66 | 0.006 |
| Fxyd5 | FXYD domain-containing ion transport regulator 5 | 1.66 | 0.028 |
| Fbln5 | fibulin 5 | 1.65 | <0.001 |
| Rpl36 | ribosomal protein L36 | 1.65 | 0.032 |
| Gas6 | growth arrest specific 6 | 1.63 | 0.015 |
| Arhgdib | Rho, GDP dissociation inhibitor (GDI) beta | 1.63 | 0.01 |
| Flrt2 | fibronectin leucine rich transmembrane protein 2 | 1.62 | 0.022 |
| Crispld2 | cysteine-rich secretory protein LCCL domain containing 2 | 1.62 | 0.016 |
| Ckb | creatine kinase, brain | 1.59 | 0.049 |
| Socs2 | suppressor of cytokine signaling 2 | 1.58 | 0.049 |
| Ephx1 | epoxide hydrolase 1, microsomal | 1.58 | 0.009 |
| Prrx2 | paired related homeobox 2 | 1.56 | 0.035 |
| Cd59a | CD59a antigen | 1.56 | 0.040 |
| Imp3 | IMP3, U3 small nucleolar ribonucleoprotein, homolog (yeast) | 1.56 | 0.004 |
| Smoc2 | SPARC related modular calcium binding 2 | 1.56 | 0.003 |
| Plxdc2 | plexin domain containing 2 | 1.55 | 0.003 |
| Grem1 | gremlin 1 | 1.55 | 0.019 |
| Cav1 | caveolin 1, caveolae protein | 1.55 | 0.039 |
| Ecm1 | extracellular matrix protein 1 | 1.55 | 0.017 |
| Crip1 | cysteine-rich protein 1 (intestinal) | 1.55 | 0.027 |
| 1110032E23Rik | RIKEN cDNA 1110032E23 gene | 1.55 | 0.005 |
| 2700023E23Rik | RIKEN cDNA 2700023E23 gene | 1.54 | 0.002 |
| Fhl1 | Four and a half LIM domains 1 | 1.53 | 0.043 |
| Ankrd28 | ankyrin repeat domain 28 | 1.53 | 0.004 |
| Rgs4 | regulator of G-protein signaling 4 | 1.51 | 0.021 |
| Lrrc17 | leucine rich repeat containing 17 | 1.51 | 0.032 |
| Rnpep | arginyl aminopeptidase (aminopeptidase B) | 1.50 | 0.036 |
FIG. 2.

Heatmap of gene expression patterns. The expression levels (log2) of E15 fibroblast (left 3 columns) and E18 fibroblast (right 3 columns) genes from each array which had a fold change ≥1.5 and a p-value <0.05 are shown. Red denotes higher expression levels and green denotes lower expression levels. Hierarchical clustering was used to group both the samples and the genes. For each age group, n=3 pooled samples from separate litters of mice.
Functional grouping of differentially expressed genes
The Ingenuity Pathways Knowledge Database was used to categorize differentially expressed genes into functional groups. The top five “molecular and cellular function” groups were: cellular growth and proliferation, cellular movement, cell-to-cell signaling and interaction, cell signaling, and small molecule biochemistry (TABLE 3). Interestingly, many of the genes (twenty-four) were included in two or more of these functional groups. Five of these genes were in all five functional groups. Examination of the “diseases and disorders” functional groups showed that many of the differentially expressed genes fell into the inflammatory response category. The twelve differentially expressed genes involved in the inflammatory response were: Rho GDP dissociation inhibitor (GDI) beta, caveolin 1, cluster of differentiation 59a, gremlin 1, interleukin 11, lysozyme 2, cyclooxygenase-1, S100 calcium binding protein A4, serine peptidase inhibitor clade G member 1, suppressor of cytokine signaling 2, thymus cell antigen 1 theta, and thrombomodulin. The other top “diseases and disorders” functional groups were not as relevant to our model and included: cancer, cardiovascular disease, renal/urological disease, and gastrointestinal disease. Since the inflammatory response was one of the most significant groups highlighted by the Ingenuity Pathways Knowledge Database and inflammation regulates healing outcomes in fetal wounds, further examination focused on inflammatory genes.
TABLE 3.
Functional groups of differentially expressed genes
| Gene Functional Group | |||||
|---|---|---|---|---|---|
| Cellular Growth and Proliferation | Cellular Movement | Signaling and Interaction | Cell Signaling | Small Molecule Biochemistry | |
| CAV1 | ● | ● | ● | ● | ● |
| CCK | ● | ● | ● | ● | ● |
| GAS6 | ● | ● | ● | ● | ● |
| RGS4 | ● | ● | ● | ● | ● |
| THBD | ● | ● | ● | ● | ● |
| CSPG4 | ● | ● | ● | ● | |
| IL11 | ● | ● | ● | ● | |
| S100A4 | ● | ● | ● | ● | |
| THY1 | ● | ● | ● | ● | |
| ARHGDIB | ● | ● | ● | ||
| AVPR1A | ● | ● | ● | ||
| FBLN5 | ● | ● | ● | ||
| GREM1 | ● | ● | ● | ||
| IGFBP5 | ● | ● | ● | ||
| Lyz1/Lyz2 | ● | ● | ● | ||
| COX-1 | ● | ● | ● | ||
| SERPING1 | ● | ● | ● | ||
| SOCS2 | ● | ● | ● | ||
| TNN | ● | ● | ● | ||
| FHL1 | ● | ● | |||
| FXYD5 | ● | ● | |||
| ITGA11 | ● | ● | |||
| PRRX2 | ● | ● | |||
| SLC7A11 | ● | ● | |||
| ANKRD28 | ● | ||||
| AY036118 | ● | ||||
| C1qtnf3 | ● | ||||
| Cd59a | ● | ||||
| CHST2 | ● | ||||
| CKB | ● | ||||
| COL8A1 | ● | ||||
| CRIP1 | ● | ||||
| ECM1 | ● | ||||
| EPHX1 | ● | ||||
| IL1RL2 | ● | ||||
| LRRC32 | ● | ||||
| NPR3 | ● | ||||
| RPS6 | ● | ||||
| SLC38A1 | ● | ||||
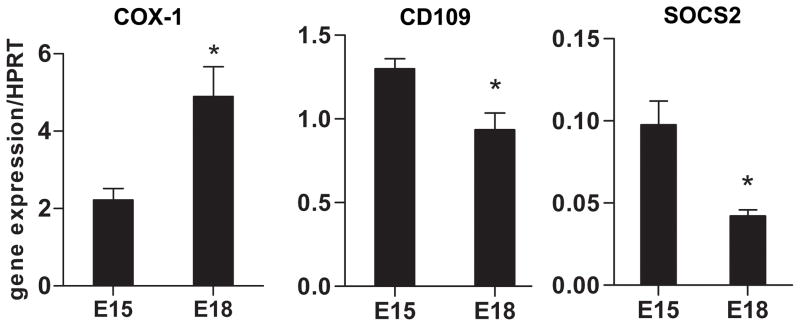
Real-time RT-PCR confirmation of selected genes
Differential gene expression was confirmed by real-time RT-PCR for three genes of interest: COX-1, SOCS2, and CD109 (FIG. 3). All three genes have been implicated in inflammation, which is known to be involved in the transition from scarless (E15 wound) to fibrotic (E18 wound) healing in fetal skin. Significant differences in mRNA expression were detected for all three genes, confirming the differences in gene expression seen by microarray analysis. Both CD109 and SOCS2, which have been shown to have anti-inflammatory roles, were down-regulated in E18 fibroblasts compared to E15 fibroblasts. Conversely, the pro-inflammatory prostaglandin-producing enzyme COX-1 was up-regulated in E18 fibroblasts, consistent with the increased inflammation seen in scar-forming E18 wounds.
FIG. 3.
Real-time RT-PCR confirmation of microarray data. Real-time RT-PCR was performed on RNA isolated from E15 or E18 fibroblast cultures to determine COX-1 (left), CD109 (middle), and SOCS2 (right) mRNA expression. For each age group, n=3 pooled samples from separate litters of mice. The mean ratio of target gene expression compared to the housekeeping gene HPRT +/− standard error of the mean (SEM) is shown. *p-value <0.05, student’s two-tailed t-test.
Analysis of COX-1 protein expression in fetal fibroblasts and fetal skin
Based on the documented importance of the cyclooxygenase enzymes and their prostaglandin products in wound healing and scar formation, further analysis was focused on COX-1. Western blot analysis showed that COX-1 protein levels were higher in cultured E18 fibroblasts compared to E15 fibroblasts (FIG. 4), confirming the differences seen in mRNA levels by microarray and real-time RT-PCR. Immunohistochemical and immunofluorescent staining for COX-1 was also performed in normal E15 and E18 skin (FIG. 5). Overall, more COX-1 staining was seen in E18 skin compared to E15 skin by immunohistochemistry. Interestingly, there were striking differences in COX-1 staining in the epidermal layer. Minimal COX-1 staining was seen in interfollicular epidermal keratinocytes in E15 skin, whereas keratinocytes in E18 skin displayed intense staining for COX-1. Positive staining was apparent in dermal cells and in endothelial cells lining blood vessels in both E15 and E18 skin. Using skin from mice expressing GFP under the control of the Col 1a1 promoter (Col-GFP), immunofluorescent staining and confocal microscopy demonstrated the presence of COX-1-positive/GFP-positive dermal fibroblasts in vivo.
FIG. 4.
Western blot analysis for COX-1 protein in cultured fetal fibroblasts. COX-1 protein levels were compared in cultured E15 and E18 fibroblasts using Western blot. COX-1 and βactin bands from two representative samples are shown (top). For each age group, n=4 fibroblast protein samples from individual fetuses. Densitometry was used to determine the ratio of COX-1/β-actin (bottom). Bars represent the mean ratio +/− SEM. * p-value <0.05, student’s two-tailed t-test.
FIG. 5.
COX-1 protein expression in fetal skin. Immunohistochemistry was used to determine COX-1 protein expression in E15 and E18 skin samples. For each age group, n=5 fetal skin samples from individual fetuses. Representative photomicrographs are shown for E15 (top row left) and E18 (top row right) fetal skin. Enlarged insets (lower left corners) show localization of COX-1 in dermal cells (brown staining). Black scale bars represent 50 μm. Immunofluorescent staining for COX-1 was performed on E15 (middle row) and E18 (bottom row) skin from Col-GFP expressing mice. Images showing dermal fibroblasts (white arrows) staining positive for both COX-1 (left column, red) and Col-GFP (center column, green) are shown, with merged images in the right column. Samples were counterstained with DAPI (blue) to visualize cell nuclei. The white scale bars in the bottom panels represent 10μm.
Discussion
Many studies have shown that inflammation is involved in the transition from scarless to fibrotic healing during skin development (3, 6, 7, 25, 26). In addition to differences in inflammation, changes in intrinsic characteristics of fetal fibroblasts may also be involved in this transition. Important experiments by Lorenz, et al suggested that fetal fibroblasts have a unique ability to mediate scarless healing (13). Several studies have also demonstrated that cultured mid-gestational fibroblasts and fibroblasts from more developed skin display basal differences in proliferation and migration rates, contractile ability and gene expression, and that they respond differently when stimulated by mediators such as TGF-β and prostaglandin E2 (PGE2) (14–21, 27, 28). Despite these observations, global changes in basal dermal fibroblast gene expression at different stages of fetal skin development have not been assessed. While several other studies have used a global approach to study scarless repair, these studies examined normal skin or wound samples (29–31). Because whole tissue was used, the specific contribution of the fibroblasts to any alterations in gene expression detected could not be determined.
In the current study, microarray analysis was used to compare gene expression profiles of cultured fetal fibroblasts generated from explants of E15 (scarless healing) and E18 (fibrotic healing) murine skin. Skin from these ages have been shown to undergo scarless (E15) and fibrotic (E18) healing (7, 22); therefore, microarray analysis of E15 and E18 fibroblasts was used to screen for differences in basal gene expression that might contribute to scar formation. Cells derived from explant cultures are often used to study dermal fibroblast function and this method offers a way to study fibroblasts separately from other cell types within the skin. However, there are limitations to studying fibroblasts in vitro, as they are not likely to behave exactly the same as fibroblasts within the skin in vivo. The cells used here were grown on plastic tissue culture plates in media supplemented with 20% serum. Although these conditions are often used for growing fibroblasts from skin explants, there is evidence that the conditions under which fibroblasts are grown, including the substrate used for fibroblast adherence, can alter cellular behavior and gene expression (32). Murine cells were used in these studies because the gestational ages corresponding to scarless and fibrotic healing are well defined in the mouse and murine fetal skin is relatively easy to generate, but the gene expression differences observed here should be confirmed in other animal models and human cells before drawing broad conclusions about the results.
In the present study, sixty-two genes were found to be differentially expressed between E15 and E18 fibroblasts. Although some studies compare fibroblasts from mid-gestation and adult skin to help understand scarless repair, a comparison of fibroblasts from mid-gestation (E15) and late-gestation (E18) fetal skin was used in the present studies. The narrow developmental timeframe examined here may partially explain why a relatively small number of genes were differentially expressed. However, the advantage of comparing cells from fetal skin at two different gestational ages is that all of the skin samples were obtained in utero. Therefore, the gene expression differences identified are more likely to be involved in the transition from scarless to fibrotic healing rather than simply being a result of comparing skin from two distinct environmental conditions. Several interesting genes were found to be differentially expressed. For example, tenascin showed higher expression in E15 fibroblasts compared to E18 fibroblasts and this extracellular matrix molecule has been shown previously to be elevated during the rapid reepithelialization of early fetal wounds (33). There were also a number of genes that suggested differences in cell growth and differentiation such as NADH proteins and ATP synthases. Analysis of differentially expressed genes using the Ingenuity Knowledge Database showed that the functional group with the most differentially expressed genes was “cell growth and proliferation” and the majority of those genes were up-regulated in E15 fibroblasts. These results are in line with previous studies demonstrating that mid-gestational fibroblasts proliferate rapidly in culture (27). Interestingly, the inflammatory response functional group also contained several differentially regulated genes. Further analysis focused on genes from this group due to the demonstrated importance of inflammation in the transition from scarless to fibrotic healing. SOCS2 (34, 35) and CD109 (36, 37) are two genes with anti-inflammatory functions that were up-regulated in E15 fibroblasts compared to E18 fibroblasts. In addition to limiting inflammation, CD109 can also be anti-fibrotic, which may be explained by its ability to alter TGF-β signaling by facilitating TGF-β receptor degradation (38) and/or sequestering TGF-β1 (39).
Another gene of interest identified by microarray was COX-1, which was up-regulated in E18 fibroblasts. In addition to higher COX-1 levels in cultured E18 fibroblasts and enhanced dermal COX-1 staining in E18 skin, keratinocytes in E18 skin also stained more intensely for COX-1 compared to keratinocytes in E15 skin. Cyclooxygenase enzymes (COX-1 and COX-2) are important during inflammation, as they convert arachidonic acid into pro-inflammatory prostaglandins, such as PGE2. The COX-2 isoform is induced after skin injury and thus has been the focus of most wound healing studies, including studies by our lab showing that COX-2 is expressed at higher levels in scar-forming fetal wounds and that PGE2 levels correlate with inflammation and scar formation in both fetal and adult wound healing models (7, 40). However, several studies have suggested that COX-1 also contributes to PGE2 production during wound healing, as the selective COX-1 inhibitor SC-560 reduces PGE2 levels in healing wounds (41, 42). Because COX-1 present at the time of wounding can quickly convert arachidonic acid to pro-inflammatory prostaglandins, the increased basal levels of COX-1 observed in fibroblasts and other cells in E18 skin could impact inflammation at early stages after injury.
In the current study, novel differences in gene expression between E15 and E18 fibroblasts were identified which may be important to the transition from scarless to fibrotic healing in fetal wounds. Among these genes were those associated with proliferation, cell signaling, and the inflammatory response. Alterations in the expression of inflammatory genes, in particular, may contribute to the transition from non-inflammatory, scarless healing early in development to inflammatory, fibrotic healing at later stages of development in fetal skin.
Acknowledgments
The authors would like to thank Dr. David Rowe (University of Connecticut) for the Col3.6-GFPtzp mouse strain. We would also like to acknowledge the OSUCCC Microarray and Nucleic Acid Shared Resources as well as the Campus Microscopy and Imaging Facility for help in completing microarrays, real-time RT-PCR, and confocal imaging. These studies were supported by funds from the OSU Department of Pathology and the Bruning Foundation. The authors are supported in part by NIH grant CA127109 (TAW) and have no conflicts of interest to disclose.
References
- 1.Wilgus TA. Regenerative healing in fetal skin: a review of the literature. Ostomy Wound Manage. 2007;53(6):16–31. [PubMed] [Google Scholar]
- 2.Satish L, Kathju S. Cellular and Molecular Characteristics of Scarless versus Fibrotic Wound Healing. Dermatol Res Pract. 2010:790234. doi: 10.1155/2010/790234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liechty KW, Adzick NS, Crombleholme TM. Diminished interleukin 6 (IL-6) production during scarless human fetal wound repair. Cytokine. 2000;12(6):671–6. doi: 10.1006/cyto.1999.0598. [DOI] [PubMed] [Google Scholar]
- 4.Liechty KW, Crombleholme TM, Cass DL, Martin B, Adzick NS. Diminished interleukin-8 (IL-8) production in the fetal wound healing response. J Surg Res. 1998;77(1):80–4. doi: 10.1006/jsre.1998.5345. [DOI] [PubMed] [Google Scholar]
- 5.Hsu M, Peled ZM, Chin GS, Liu W, Longaker MT. Ontogeny of expression of transforming growth factor-beta 1 (TGF-beta 1), TGF-beta 3, and TGF-beta receptors I and II in fetal rat fibroblasts and skin. Plast Reconstr Surg. 2001;107(7):1787–94. doi: 10.1097/00006534-200106000-00023. discussion 95–6. [DOI] [PubMed] [Google Scholar]
- 6.Haynes JH, Johnson DE, Mast BA, Diegelmann RF, Salzberg DA, Cohen IK, et al. Platelet-derived growth factor induces fetal wound fibrosis. J Pediatr Surg. 1994;29(11):1405–8. doi: 10.1016/0022-3468(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 7.Wilgus TA, Bergdall VK, Tober KL, Hill KJ, Mitra S, Flavahan NA, et al. The impact of cyclooxygenase-2 mediated inflammation on scarless fetal wound healing. Am J Pathol. 2004;165(3):753–61. doi: 10.1016/S0002-9440(10)63338-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naik-Mathuria B, Gay AN, Zhu X, Yu L, Cass DL, Olutoye OO. Age-dependent recruitment of neutrophils by fetal endothelial cells: implications in scarless wound healing. J Pediatr Surg. 2007;42(1):166–71. doi: 10.1016/j.jpedsurg.2006.09.058. [DOI] [PubMed] [Google Scholar]
- 9.Olutoye OO, Yager DR, Cohen IK, Diegelmann RF. Lower cytokine release by fetal porcine platelets: a possible explanation for reduced inflammation after fetal wounding. J Pediatr Surg. 1996;31(1):91–5. doi: 10.1016/s0022-3468(96)90326-7. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong JR, Ferguson MW. Ontogeny of the skin and the transition from scar-free to scarring phenotype during wound healing in the pouch young of a marsupial, Monodelphis domestica. Dev Biol. 1995;169(1):242–60. doi: 10.1006/dbio.1995.1141. [DOI] [PubMed] [Google Scholar]
- 11.Hopkinson-Woolley J, Hughes D, Gordon S, Martin P. Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. J Cell Sci. 1994;107 ( Pt 5):1159–67. doi: 10.1242/jcs.107.5.1159. [DOI] [PubMed] [Google Scholar]
- 12.Wulff BC, Parent AE, Meleski MA, Dipietro LA, Schrementi ME, Wilgus TA. Mast Cells Contribute to Scar Formation during Fetal Wound Healing. J Invest Dermatol. doi: 10.1038/jid.2011.324. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorenz HP, Lin RY, Longaker MT, Whitby DJ, Adzick NS. The fetal fibroblast: the effector cell of scarless fetal skin repair. Plast Reconstr Surg. 1995;96(6):1251–9. doi: 10.1097/00006534-199511000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Carter R, Jain K, Sykes V, Lanning D. Differential expression of procollagen genes between mid- and late-gestational fetal fibroblasts. J Surg Res. 2009;156(1):90–4. doi: 10.1016/j.jss.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 15.Colwell AS, Krummel TM, Longaker MT, Lorenz HP. Early-gestation fetal scarless wounds have less lysyl oxidase expression. Plast Reconstr Surg. 2006;118(5):1125–9. doi: 10.1097/01.prs.0000221056.27536.db. discussion 30–1. [DOI] [PubMed] [Google Scholar]
- 16.Gosiewska A, Yi CF, Brown LJ, Cullen B, Silcock D, Geesin JC. Differential expression and regulation of extracellular matrix-associated genes in fetal and neonatal fibroblasts. Wound Repair Regen. 2001;9(3):213–22. doi: 10.1046/j.1524-475x.2001.00213.x. [DOI] [PubMed] [Google Scholar]
- 17.Parekh A, Sandulache VC, Singh T, Cetin S, Sacks MS, Dohar JE, et al. Prostaglandin E2 differentially regulates contraction and structural reorganization of anchored collagen gels by human adult and fetal dermal fibroblasts. Wound Repair Regen. 2009;17(1):88–98. doi: 10.1111/j.1524-475X.2008.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirt-Burri N, Scaletta C, Gerber S, Pioletti DP, Applegate LA. Wound-healing gene family expression differences between fetal and foreskin cells used for bioengineered skin substitutes. Artif Organs. 2008;32(7):509–18. doi: 10.1111/j.1525-1594.2008.00578.x. [DOI] [PubMed] [Google Scholar]
- 19.Ramelet AA, Hirt-Burri N, Raffoul W, Scaletta C, Pioletti DP, Offord E, et al. Chronic wound healing by fetal cell therapy may be explained by differential gene profiling observed in fetal versus old skin cells. Exp Gerontol. 2009;44(3):208–18. doi: 10.1016/j.exger.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Rolfe KJ, Richardson J, Vigor C, Irvine LM, Grobbelaar AO, Linge C. A role for TGF-beta1-induced cellular responses during wound healing of the non-scarring early human fetus? J Invest Dermatol. 2007;127(11):2656–67. doi: 10.1038/sj.jid.5700951. [DOI] [PubMed] [Google Scholar]
- 21.Sandulache VC, Parekh A, Dohar JE, Hebda PA. Fetal dermal fibroblasts retain a hyperactive migratory and contractile phenotype under 2-and 3-dimensional constraints compared to normal adult fibroblasts. Tissue Eng. 2007;13(11):2791–801. doi: 10.1089/ten.2006.0412. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg SR, Quirk GL, Sykes VW, Kordula T, Lanning DA. Altered procollagen gene expression in mid-gestational mouse excisional wounds. J Surg Res. 2007;143(1):27–34. doi: 10.1016/j.jss.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4(2):249–64. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 24.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 25.Krummel TM, Michna BA, Thomas BL, Sporn MB, Nelson JM, Salzberg AM, et al. Transforming growth factor beta (TGF-beta) induces fibrosis in a fetal wound model. J Pediatr Surg. 1988;23(7):647–52. doi: 10.1016/s0022-3468(88)80638-9. [DOI] [PubMed] [Google Scholar]
- 26.Lin RY, Sullivan KM, Argenta PA, Meuli M, Lorenz HP, Adzick NS. Exogenous transforming growth factor-beta amplifies its own expression and induces scar formation in a model of human fetal skin repair. Ann Surg. 1995;222(2):146–54. doi: 10.1097/00000658-199508000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brink HE, Bernstein J, Nicoll SB. Fetal dermal fibroblasts exhibit enhanced growth and collagen production in two- and three-dimensional culture in comparison to adult fibroblasts. J Tissue Eng Regen Med. 2009;3(8):623–33. doi: 10.1002/term.204. [DOI] [PubMed] [Google Scholar]
- 28.Schor SL, Schor AM, Rushton G, Smith L. Adult, foetal and transformed fibroblasts display different migratory phenotypes on collagen gels: evidence for an isoformic transition during foetal development. J Cell Sci. 1985;73:221–34. doi: 10.1242/jcs.73.1.221. [DOI] [PubMed] [Google Scholar]
- 29.Chen W, Fu X, Ge S, Sun T, Zhou G, Han B, et al. Profiling of genes differentially expressed in a rat of early and later gestational ages with high-density oligonucleotide DNA array. Wound Repair Regen. 2007;15(1):147–55. doi: 10.1111/j.1524-475X.2006.00195.x. [DOI] [PubMed] [Google Scholar]
- 30.Colwell AS, Longaker MT, Peter Lorenz H. Identification of differentially regulated genes in fetal wounds during regenerative repair. Wound Repair Regen. 2008;16(3):450–9. doi: 10.1111/j.1524-475X.2008.00383.x. [DOI] [PubMed] [Google Scholar]
- 31.Kathju S, Satish L, Rabik C, Rupert T, Oswald D, Johnson S, et al. Identification of differentially expressed genes in scarless wound healing utilizing polymerase chain reaction-suppression subtractive hybridization. Wound Repair Regen. 2006;14(4):413–20. doi: 10.1111/j.1743-6109.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- 32.Cukierman E, Pankov R, Yamada KM. Cell interactions with three-dimensional matrices. Curr Opin Cell Biol. 2002;14(5):633–9. doi: 10.1016/s0955-0674(02)00364-2. [DOI] [PubMed] [Google Scholar]
- 33.Whitby DJ, Longaker MT, Harrison MR, Adzick NS, Ferguson MW. Rapid epithelialisation of fetal wounds is associated with the early deposition of tenascin. J Cell Sci. 1991;99 ( Pt 3):583–6. doi: 10.1242/jcs.99.3.583. [DOI] [PubMed] [Google Scholar]
- 34.Lee JS, Paek NS, Kwon OS, Hahm KB. Anti-inflammatory actions of probiotics through activating suppressor of cytokine signaling (SOCS) expression and signaling in Helicobacter pylori infection: a novel mechanism. J Gastroenterol Hepatol. 2010;25(1):194–202. doi: 10.1111/j.1440-1746.2009.06127.x. [DOI] [PubMed] [Google Scholar]
- 35.Machado FS, Johndrow JE, Esper L, Dias A, Bafica A, Serhan CN, et al. Anti-inflammatory actions of lipoxin A4 and aspirin-triggered lipoxin are SOCS-2 dependent. Nat Med. 2006;12(3):330–4. doi: 10.1038/nm1355. [DOI] [PubMed] [Google Scholar]
- 36.de Andres MC, Imagawa K, Hashimoto K, Gonzalez A, Goldring MB, Roach HI, et al. Suppressors of cytokine signalling (SOCS) are reduced in osteoarthritis. Biochem Biophys Res Commun. 2011;407(1):54–9. doi: 10.1016/j.bbrc.2011.02.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vorstenbosch JG-BC, Trzeciak A, Fang R, Mustoe T, Philip A. Abstract: Overexpression of CD109 in the Epidermis Reduces Inflammation, Improves Wound Healing and Scarring. Plast Reconstr Surg. 2010;125(6 suppl):70. [Google Scholar]
- 38.Bizet AA, Tran-Khanh N, Saksena A, Liu K, Buschmann MD, Philip A. CD109-mediated degradation of TGF-beta receptors and inhibition of TGF-beta responses involve regulation of SMAD7 and Smurf2 localization and function. J Cell Biochem. 2011 doi: 10.1002/jcb.23349. [DOI] [PubMed] [Google Scholar]
- 39.Litvinov IV, Bizet AA, Binamer Y, Jones DA, Sasseville D, Philip A. CD109 release from the cell surface in human keratinocytes regulates TGF-beta receptor expression, TGF-beta signalling and STAT3 activation: relevance to psoriasis. Exp Dermatol. 2011;20(8):627–32. doi: 10.1111/j.1600-0625.2011.01288.x. [DOI] [PubMed] [Google Scholar]
- 40.Wilgus TA, Vodovotz Y, Vittadini E, Clubbs EA, Oberyszyn TM. Reduction of scar formation in full-thickness wounds with topical celecoxib treatment. Wound Repair Regen. 2003;11(1):25–34. doi: 10.1046/j.1524-475x.2003.11106.x. [DOI] [PubMed] [Google Scholar]
- 41.Kampfer H, Brautigam L, Geisslinger G, Pfeilschifter J, Frank S. Cyclooxygenase-1-coupled prostaglandin biosynthesis constitutes an essential prerequisite for skin repair. J Invest Dermatol. 2003;120(5):880–90. doi: 10.1046/j.1523-1747.2003.12140.x. [DOI] [PubMed] [Google Scholar]
- 42.Muller-Decker K, Hirschner W, Marks F, Furstenberger G. The effects of cyclooxygenase isozyme inhibition on incisional wound healing in mouse skin. J Invest Dermatol. 2002;119(5):1189–95. doi: 10.1046/j.1523-1747.2002.19501.x. [DOI] [PubMed] [Google Scholar]