Abstract
During development, a flat neural plate rolls up and closes to form a neural tube. This process, called neural tube closure, is complex and requires morphogenetic events to occur along multiple axes of the neural plate. Recent studies suggest that cell and tissue polarity play a major role in neural tube morphogenesis. While the planar cell polarity pathway is known to be involved in this process, a role for the apicobasal polarity pathway has only recently begun to be elucidated. These studies show that Bone Morphogenetic Proteins (BMPs) can regulate the apicobasal polarity pathway in the neural plate in a cell cycle dependent manner. This dynamically modulates apical junctions in the neural plate, resulting in cell and tissue shape changes that help bend, shape and close the neural tube.
Keywords: neurulation, hinge point, apical constriction, PAR, LGL, tight junctions, adherens junctions, interkinetic nuclear migration, planar cell polarity, organogenesis, tubulogenesis
The central nervous system of vertebrates consists of a hollow neural tube. In higher vertebrates, the neural tube emerges from two distinct types of events called primary and secondary neurulation. Primary neurulation or neural tube closure (NTC) involves the transformation of a flat neural plate into a hollow tube and forms the brain and much of spinal cord. Secondary neurulation hollows a solid nerve cord to form the caudal-most portion of the spinal cord (Schoenwolf, 1985). The focus of this review is NTC, a process which involves the orchestration of dynamic molecular and cellular events regulated by > 240 genes (Harris and Juriloff, 2007; Harris and Juriloff, 2010). Dysregulation of these genes produces NTC defects (NTDs), which are a leading cause of human congenital abnormalities affecting 1–5/1000 pregnancies (Copp and Greene, 2010).
The Biomechanics of Neural Tube Closure
Primary neurulation involves a series of shape changes in the neural plate, beginning with apicobasal thickening or cell elongation along the apicobasal axis of the neural plate (Fig. 1A; (Smith and Schoenwolf, 1997; Colas and Schoenwolf, 2001). This is followed by the formation of a wedge-shaped tissue at the future ventral midline, the median hinge point (MHP; Fig. 1B). Here, polarized cell behaviors and MHP’s association with the subjacent notochord, jointly generate forces, which buckle the ventral midline and elevate the neural folds on either side (Schoenwolf and Franks, 1984; Schoenwolf and Alvarez, 1989; Davidson and Keller, 1999; Colas and Schoenwolf, 2001).
Figure 1. Neural tube closure and convergent extension in the chick.
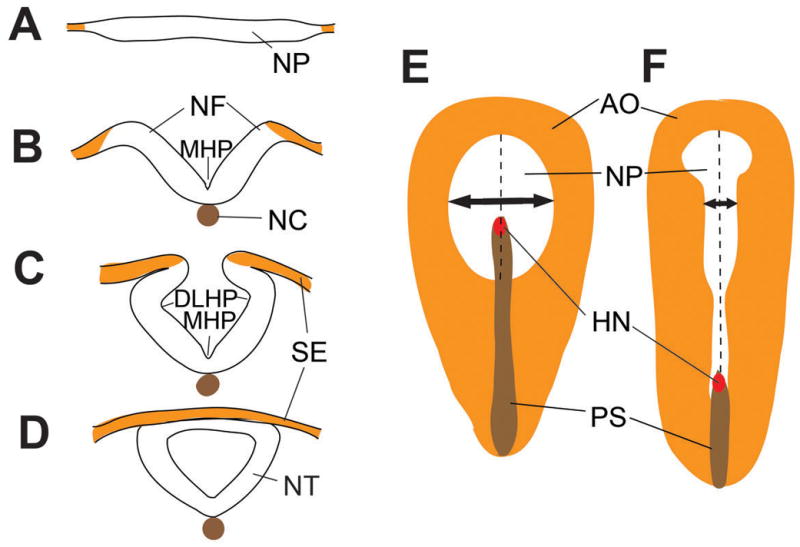
(A–D) Neural tube closure events shown in cross-sectional view. (A) Apicobasal thickening of the neural plate prior to neural tube closure (NTC). (B) Median hinge point (MHP) formation at the ventral midline of the neural plate. Note the association of the notochord (NC) with the MHP and the elevation of the neural folds (NF) above the ventral midline. (C) Formation of the dorsolateral hinge point (DLHP) and the association of the NP with the surface ectoderm (SE). (D) Dorsal midline fusion of the neural plate and SE. (E, F) Top down views of the chick neural plate, with rostral to the top and the dorsal surface facing the viewer. Convergent extension narrows and elongates the neural tube (white tissue; double headed arrow). Area opaca (AO, orange), primitive streak (PS, brown) and Hensen’s node (HN, red) are shown for orientation.
Abbreviations: AJ: adherens junctions; AO: area opaca; DLHP: dorsolateral hinge point; HN: Hensen’s node; LGL: lethal giant larva; MHP: median hinge point; NC: notochord; NF: neural fold; NP: neural plate; NT: neural tube; NTC: neural tube closure; PS: primitive streak; SE: surface ectoderm; TJ: tight junctions.
A pair of dorsolateral hinge points (DLHP) forms at some axial levels of the neural plate (Fig. 1C)(Schoenwolf, 1985; Smith et al., 1994; Ybot-Gonzalez et al., 2002; Ybot-Gonzalez et al., 2007). Cell behaviors similar to MHP occur at the DLHP and help bend the neural folds so that they pivot toward each other. Like the MHP, the DLHP also associates with adjacent tissues, in this case, the surface ectoderm (Fig. 1C). The intrinsic forces generated by polarized cell behaviors at the DLHP, and the extrinsic forces applied by the surface ectoderm, together help appose the neural folds so that dorsal midline fusion can be accomplished (Fig. 1D) (Jacobson and Moury, 1995; Ybot-Gonzalez et al., 2002).
The final steps in NTC involve the dorsal midline fusion of the neural folds and the surface ectoderm (Fig. 1D) (Pyrgaki et al., 2010; Pai et al., 2012; Ray and Niswander, 2012). In the mouse, fusion is thought to occur at three distinct closure sites at different axial levels of the neural plate. This is followed by a rostral and caudal zippering of the dorsal midline (Jaskoll et al., 1991; Fleming et al., 1997; Copp and Greene, 2010). Recent studies have also described a novel “buttoning up” process in the midbrain, where multiple secondary closure sites help accomplish NTC (Van Straaten et al., 1996; Pyrgaki et al., 2010; Teng and Toyama, 2011). These processes are associated with cell death (although this is controversial), changes in cell-shape and adhesion, and most recently, the extension of cytoplasmic processes across the neural folds (Massa et al., 2009; Yamaguchi et al., 2011; Pai et al., 2012; Ray and Niswander, 2012).
Extensive epithelial remodeling is required to accomplish NTC. The planar cell polarity (PCP) pathway, which maintains cell polarity in the plane of the epithelium, is a major regulator of epithelial remodeling during NTC (Wallingford, 2006; Copp and Greene, 2010; Juriloff and Harris, 2012). In vertebrates, the PCP pathway is driven by WNT ligands and regulates convergent extension movements during gastrulation and neurulation. During this process, polarized cell intercalation narrows and elongates the neural tube sufficiently so that the neural folds are juxtaposed and can fuse dorsally (Fig. 1C–F) (Wallingford and Harland, 2002; Zohn et al., 2003; Wallingford, 2012). A number of excellent reviews have focused on the PCP pathway and the regulation of NTC (Zohn et al., 2003; Wallingford, 2006; Kibar et al., 2007a; Kibar et al., 2007b; Wada and Okamoto, 2009). This review will instead focus on the newly emerging role of epithelial remodeling that occurs along the apicobasal axis during NTC. It will also briefly address the question of how polarized events along the three major axes of the neural plate may be coordinated to produce a closed neural tube with correct 3-dimensional morphology.
Cell cycle progression and Interkinetic Nuclear Migration in the neural plate
The neural tube is composed of bipolar progenitor cells that exhibit apical and basal processes and a large cell soma occupied mainly by the cell nucleus (Fig. 2A). Cell cycle progression in the neural tube and other pseudostratified epithelia involves interkinetic nuclear migration. During this process, cell nuclei move along their apical and basal processes, which remain tethered to the apical and basal surfaces of the neural tube for nearly the entire duration of the cell cycle (Fig. 2A) (Sauer, 1935; Baye and Link, 2007; Meyer et al., 2011). At early developmental stages, mitosis almost always occurs at or near the apical surface, while other phases of the cell cycle (G1, G2, S) occur variably throughout the thickness of the neural epithelium (Fig. 2A) (Kosodo and Huttner, 2009).
Figure 2. Epithelial organization and tissue morphogenesis.
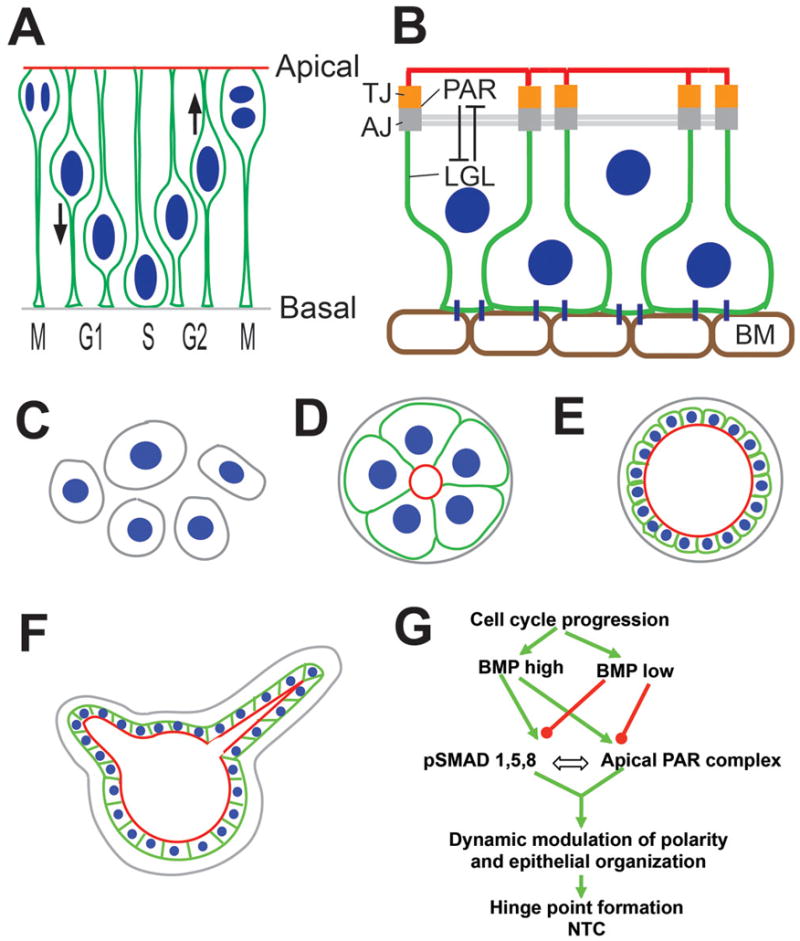
(A) Cross-sectional/apicobasal view of the neural plate showing its pseudostratified epithelial organization and interkinetic nuclear migration. Cell nuclei are shown in blue, and the cell outline, including apical and basal processes, are shown in green. Based on Sauer et al., 1935; Baye and Link, 2007. (B) Neural epithelial cell organization with the apical (red) and basolateral (green) compartments separated by tight junctions (TJ). Adherens junctions (AJ) are located basal to the TJ and associate with the adherens/actin belt (grey). TJ and AJ are associated with the apical PAR complex, which antagonizes basolateral proteins, e.g., lethal giant larva (LGL). BM: basement membrane. (C–F) MDCK cells in 3D cultures (C) can self-organize to form cysts with central lumens (D). The cyst can expand by cell division (E), display dynamic variability in apicobasal polarity, leading to the formation of hollow tubes (F). C-F adapted from Mostov et al., 2003 and Zegers et al., 2003. (G) Cartoon depicting the role of BMP-apicobasal polarity interactions during hinge point formation. For details, see text. Adapted from Eom et al., 2012.
Abbreviations: AJ: adherens junctions; AO: area opaca; DLHP: dorsolateral hinge point; HN: Hensen’s node; LGL: lethal giant larva; MHP: median hinge point; NC: notochord; NF: neural fold; NP: neural plate; NT: neural tube; NTC: neural tube closure; PS: primitive streak; SE: surface ectoderm; TJ: tight junctions.
In addition to interkinetic nuclear migration, other temporally dynamic and polarized behaviors occur along the apicobasal axis of the neural plate. These include apicobasal thickening of the neural plate and the DLHP, apicobasal shortening at the MHP, apical constriction and the basal retention of cell nuclei at both sets of hinge points (Fig. 1; 3) (Sauer, 1935; Colas and Schoenwolf, 2001; Wallingford, 2005; Baye and Link, 2007; Nishimura and Takeichi, 2008; Eom et al., 2011). Basally located nuclei give hinge point cells their “wedge-shaped” appearance in scanning electron micrographs (Fig. 3A, 4C, D) (Smith and Schoenwolf, 1987; Smith and Schoenwolf, 1988). To understand how these polarized cell behaviors are generated and dynamically modulated during NTC, it is first necessary to understand how the apicobasal polarity pathway functions to establish, maintain and modulate epithelial organization.
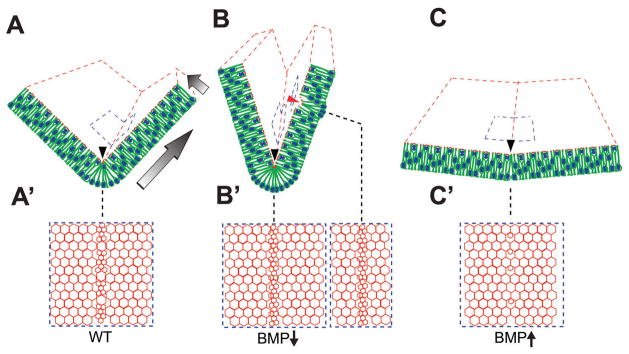
Figure 3. BMP signaling and hinge point formation.
(A, A′) Wild-type neural plate showing the random distribution of nuclei in lateral neural plate and basally located nuclei at the MHP (arrowhead). The blue box along the apical surface of A is magnified in A′ and demonstrates that the basally located nuclei shown at the MHP in A display reduced apical surfaces at the MHP. Graded arrows depict the pSMAD 1,5,8 gradients along the medial-lateral and the apicobasal axes. (B, B′) BMP blockade exaggerates the endogenous MHP (black arrowhead) and can induce ectopic hinge points in lateral neural plate (red arrowhead, B). This is accompanied by increased apical constriction and basal nuclear migration (B, B′). (C, C′f) Increased BMP signaling results in a flattened neural plate. The neural plates shown in B and C do not close correctly. Note that the DLHPs are not shown this figure, but are affected similarly by BMP signaling. Based on data from Eom et al., 2011; 2012.
Abbreviations: AJ: adherens junctions; AO: area opaca; DLHP: dorsolateral hinge point; HN: Hensen’s node; LGL: lethal giant larva; MHP: median hinge point; NC: notochord; NF: neural fold; NP: neural plate; NT: neural tube; NTC: neural tube closure; PS: primitive streak; SE: surface ectoderm; TJ: tight junctions.
Figure 4. Convergent extension and cell cycle kinetics in neural tube closure.
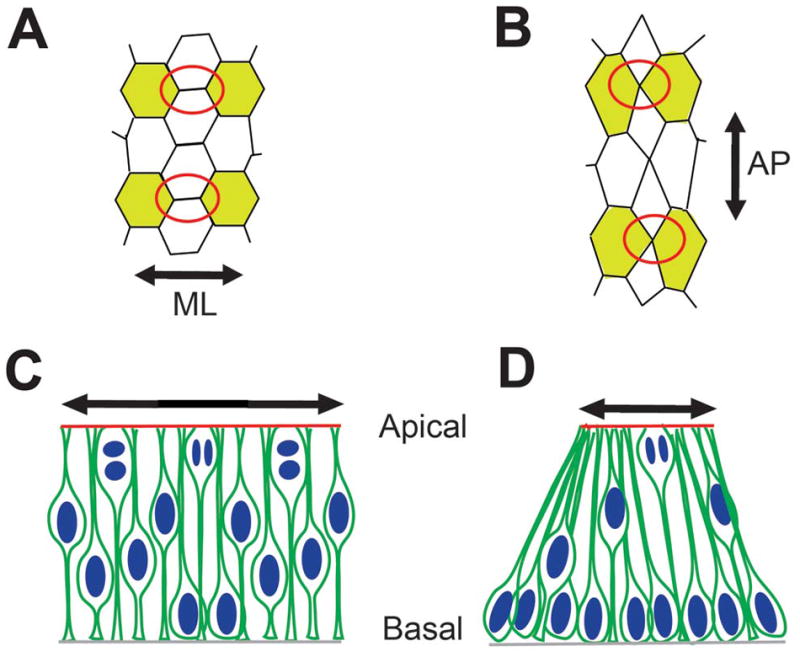
(A, B) Neural plate cells in apical view showing progressive convergent extension. Apical junctional remodeling (red circles in A and B) results in mediolateral convergence and anteroposterior extension (double headed arrows in A and B, respectively). Note that such remodeling can produce apical constriction (Based on data from Nishimura et al., 2012). (C, D) Lateral neural plate cells display asynchronous cell cycle progression, with neighboring cells in different cell cycle phases (C). By contrast, a majority of nuclei at the MHP are basally located (D). Although the same number of cells is shown in C and D, the apical surface area in D is reduced compared to C (compare double headed arrows in C and D). Based on data from Schoenwolf et al., 1987; Eom et al., unpublished observations.
Abbreviations: AJ: adherens junctions; AO: area opaca; DLHP: dorsolateral hinge point; HN: Hensen’s node; LGL: lethal giant larva; MHP: median hinge point; NC: notochord; NF: neural fold; NP: neural plate; NT: neural tube; NTC: neural tube closure; PS: primitive streak; SE: surface ectoderm; TJ: tight junctions.
Dynamic modulations of apicobasal polarity and organogenesis
The cell membranes of epithelial cells are partitioned into apical and basolateral compartments segregated by tight junctions (Fig. 2B) (Margolis and Borg, 2005). This segregation prevents the paracellular transfer of protein and lipids between the two compartments, permitting their functional specialization (Martin-Belmonte et al., 2008). The segregation of epithelial membranes into apical and basolateral compartments depends upon interactions among multiple sets of asymmetrically distributed polarity protein complexes (Suzuki et al., 2001; Bilder, 2004). These include a tight junction-associated PAR-complex composed of PAR3, PAR6 and atypical protein kinase C (aPKC) proteins, and a more apically located Crumbs complex (which includes Crumbs, PATJ and Stardust, among other proteins; (Margolis and Borg, 2005)). These complexes help establish and maintain apical polarity and tight junction integrity in part by excluding basolateral proteins, e.g., Scribble, Disks Large, Lethal Giant Larva (LGL) from the apical compartment (Fig. 2B) (Plant et al., 2003; Yamanaka et al., 2003; Bilder, 2004).
A second type of junction, the adherens junctions, is situated immediately subjacent to tight junctions of epithelial cells (Margolis and Borg, 2005). These junctions are made up of cadherins and are critical for cell adhesion and for the ability of epithelial cells to form three-dimensional tissue assemblies (Harris and Tepass, 2010). This is mainly accomplished by adherens junction interactions with the apical actin/adherens belt, whose remodeling plays a major role in NTC and other morphogenetic processes (Fig. 2B) (Takeichi, 2011). Adherens junctions can also engage in tissue remodeling by interacting with microtubules and the cell’s endocytotic machinery (Harris and Tepass, 2010).
How does epithelial remodeling influence morphogenetic processes? To understand this, it is useful to look at the remarkable ability of epithelial cells to self-organize into complex 3-dimensional structures (Trinkhaus, 1969). This process has been studied in Madin-Darby Canine kidney (MDCK) cells grown in 3-dimensional tissue cultures. These cells display epithelial characteristics in tissue culture and can be induced to lose and then re-acquire epithelial polarity using a low-high Ca2+ switch paradigm (Suzuki et al., 2001). Once depolarized, MDCK cells display a remarkable ability to self-organize into complex 3-dimensional structures, (e.g., cysts and tubes), when grown on solid support such as matrigel or collagen (Mostov et al., 2003; Zegers et al., 2003). These cells derive their initial polarity cues from the solid support, along which they form a basal surface (Fig. 2C, D). This is followed by E-cadherin expression at cell-cell interfaces and the polarized targeting of vacuoles and apical proteins to the future apical surface, leading to the formation of a central lumen lined by an apical surface (Fig. 2D, E). Cyst formation in vitro thus resembles the process of progressive epithelialization and hollowing seen in zebrafish neural tube and in amniote secondary neurulation (Schoenwolf and Delongo, 1980; Lowery and Sive, 2004; Hong and Brewster, 2006).
Interestingly, when treated with appropriate factors, (e.g., Hepatocyte Growth Factor), cyst cells can transiently alter their polarity (become partially polarized or unpolarized), extend into tubes, divide and then re-acquire polarity by the polarized secretion of proteins and vacuoles. Subsequent fusion of apical vacuoles can lead to the formation of a central lumen (Mostov et al., 2003; Zegers et al., 2003). Continuation of this process can produce simple and branched tubules associated with the generation of many types of organs (Fig. 2E, F) (Ewald et al., 2008).
BMP Signaling, apicobasal polarity and neural tube closure
Apicobasal polarity modulations have also been implicated in primary neurulation where a flat epithelial sheet rolls up into a neural tube. A key event in this type of tube formation is the induction of a hinge point, which helps buckle the epithelial plate as described above for primary neurulation (Pilot and Lecuit, 2005). As with the tissue remodeling seen during convergent extension, this process requires the polarized modifications of cell junctions, cell cytoskeleton, endocytosis and cell cycle progression (Colas and Schoenwolf, 2001; Eom et al., 2011; Nishimura et al., 2012; Suzuki et al., 2012). Interestingly, many of these processes are regulated by Bone Morphogenetic Proteins (BMPs).
The basic signaling cascade triggered by BMP ligands is well characterized (Massague and Wotton, 2000). BMP ligands initiate signaling by binding type I and type II serine threonine kinases receptors. Upon ligand binding, type II receptors phosphorylate type 1 receptors. Type I receptors phosphorylate receptor-SMADs (SMAD1, 5 or 8) which bind to the co-SMAD, SMAD4. The Smads are then translocated to the nucleus and regulate BMP-dependent gene transcription (Miyazono et al., 2010).
Although BMP signals have been previously implicated in vertebrate neural cell-fate specification and neural induction, a novel role for BMP signaling in tissue morphogenesis, for example in gastrulating zebrafish, Drosophila wing and amniote NTC, has recently emerged (Teleman et al., 2001; Martin-Castellanos and Edgar, 2002; Gibson and Perrimon, 2005; Liu and Niswander, 2005; Shen and Dahmann, 2005; von der Hardt et al., 2007; Eom et al., 2011; Eom et al., 2012). Multiple BMP pathway mutants (Bmp2−/−, Bmp2+/−; BmpR1A conditional knockouts, Noggin−/−, Bmp5−/−; Bmp7−/− and Smad5−/−) display NTDs, although the underlying cellular reasons are only now beginning to be elucidated (McMahon et al., 1998; Chang et al., 1999; Solloway and Robertson, 1999; Stottmann et al., 2006; Ybot-Gonzalez et al., 2007; Castranio and Mishina, 2009; Stottmann and Klingensmith, 2011).
Based on pSMAD 1,5,8 expression, BMP signaling occurs in the neural plate, surface ectoderm and the underlying head mesenchyme. Accordingly, mutant analyses suggest that this pathway is likely to make a multifaceted contribution to NTC, although its role in hinge point formation is the most extensively studied (Stottmann et al., 2006; Ybot-Gonzalez et al., 2007; Castranio and Mishina, 2009; Eom et al., 2011; Eom et al., 2012). These studies show that BMP attenuation is critically required in the neural plate for MHP and DLHP formation in the bird as well as the mouse (Fig. 3A–C).
Increased BMP signaling in the Noggin−/− mouse correlates with the absence of the DLHP in upper spinal cord (Stottmann et al., 2006; Ybot-Gonzalez et al., 2007). By contrast, reduced BMP signaling in the Bmp2−/− knockout results in premature and exaggerated bending in dorsal neural tube (Ybot-Gonzalez et al., 2007). Focal in vivo BMP manipulations in the chick neural plate confirm these results, with BMP attenuation deepening the endogenous MHP and inducing ectopic hinge points in lateral neural plate (Fig. 3A, B). Focal BMP upregulation prevents MHP formation, resulting in a flat neural plate, where the folds do not elevate or fuse across the dorsal midline (Fig. 3C, C′) (Eom et al., 2011; Eom et al., 2012).
An examination of the cell behaviors at ectopic hinge points reveals that BMP attenuation can induce apical constriction and result in nuclei that are more basally located, precisely what is seen at the endogenous hinge points (Fig. 3A–C′) (Eom et al., 2011; Eom et al., 2012). These studies show that BMP signaling controls polarized MHP behaviors by interacting with apicobasal polarity proteins. They provide the first evidence for ligand-dependent interactions between the phosphorylated (p) versions of SMAD 1,5,8 proteins and the PAR complex (Fig. 3G) (Eom et al., 2011).
The principal function of the PAR complex-pSMAD 1,5,8 association appears to be the stabilization of apicobasal epithelial organization in the neural plate. As a result, low BMP levels, such as those seen at the MHP during NTC, result in compromised apical junctions. Under low BMP conditions, junctional proteins (e.g., PAR complex, NCAD) are removed from the apical compartment via endocytosis into the cytoplasm, while basolateral proteins, like LGL, move into the apical compartment. This is clearly an important component of hinge point formation because direct apical misexpression of LGL is sufficient to induce ectopic hinge points in lateral neural plate. These hinge points are indistinguishable from those induced by BMP attenuation and suggest that BMPs regulate hinge point formation via the apicobasal polarity pathway. Interestingly, the endocytotic removal of apical membranes by BMP attenuation may partially account for apical constriction seen at the hinge point, as has been described in Xenopus bottle cells during gastrulation (Lee and Harland, 2010).
Since BMP signaling maintains neural epithelial organization by stabilizing apical junctions, it is not surprising that a sustained BMP blockade results in a disorganized neural epithelium and frequently in epithelial-mesenchymal transitions. Thus BMP blockade can induce neural cells to either delaminate into the lumen or spontaneously reorganize to form rosettes or cysts containing a central lumen, lined by PAR3 and mitotic cells (Eom et al., 2012). How then is the integrity of the neural epithelium maintained during hinge point formation? The answer to this question lies in the unusual two-dimensional, cell cycle dependent pSMAD 1,5,8 gradient expressed in the neural plate (Fig. 3A). A pSMAD 1,5,8 gradient runs along the mediolateral axis of the neural plate, producing low levels of BMP signaling at the MHP. An orthogonal, spatiotemporal pSMAD 1,5,8 gradient occurs along the apicobasal axis and is modulated in tandem with cell cycle progression. Mitotic cells along the apical surface express high levels of pSMAD 1,5,8, while low levels of BMP signaling are expressed during interphase (Eom et al., 2011). Thus cells experience pSMAD 1,5,8 modulation as they progress through the cell cycle. Since cell cycle progression in the neural plate is asynchronous, cells experiencing high levels of pSMAD 1,5,8 are juxtaposed to those experiencing low levels. This type of pSMAD 1,5,8 modulation can dynamically shunt cells through variable polarity states, inducing transient, cell cycle dependent cell-shape changes in subsets of cells, while their neighbors retain epithelial integrity. Low pSMAD 1,5,8 levels at the MHP resulting from an orthogonal, latero-medial gradient ensure that MHP cells display greater epithelial dynamism than more lateral neural plate cells. This facilitates the formation of a pivot or a hinge around which the neural plate can be buckled and lifted (Fig. 3A).
Cell-shape changes occur throughout the neural plate during NTC and not just the hinge points. The 2-dimensional, pSMAD 1,5,8 gradient may serve as a molecular substrate for coordinating dynamic polarity modulations and cell behaviors across the width and depth of the folding neural plate. This is reflected by the presence of partially polarized cells that occur throughout the mediolateral axis of the neural plate. These cells display apical LGL, reduced apical PAR3 and increased PAR3 in the cytoplasm and have been characterized as partially polarized because they retain their apical and basal process and undergo interkinetic nuclear migration. Interestingly, the numbers of partially polarized cells are the highest at the ventral midline, where BMP signaling is lowest and where the MHP forms. Their numbers go down medial to lateral, in tandem with a progressive, medio-lateral increase in BMP signaling (Fig. 3A). These results suggest that progressive epithelialization during NTC may not just occur in the fish and frog, but is also likely to be a feature of amniote neural epithelium during NTC (Aaku-Saraste et al., 1996; Papan and Campos-Ortega, 1999; Geldmacher-Voss et al., 2003; Hong and Brewster, 2006; Yang et al., 2009; Eom et al., 2011). The flexibility conferred by a partially polarized neural epithelium can accommodate the dual needs of the neural plate, to be sufficiently rigid to execute cohesive morphogenetic movements, while simultaneously being flexible enough to change shapes and position so that the neural plate can be transformed to a neural tube.
The coordination of planar cell polarity and apicobasal polarity mechanisms in NTC
While endocytosis appears to be involved in apical constriction of hinge point cells, other studies have implicated polarized cytoskeletal modulations, which constrict the adherens belt (Suzuki et al., 2012). The role of actin remodeling in cell-wedging has been controversial, with alternative mechanisms being proposed by several studies ((Schoenwolf et al., 1988); reviewed in Schoenwolf and Smith, 1990; Wallingford, 2005). However, a large body of evidence has emerged to suggest a critical role for polarized actomyosin contraction in apical constriction (Karfunkel, 1972; Linville and Shepard, 1972; Schoenwolf et al., 1988; van Straaten et al., 2002; Suzuki et al., 2012). These studies suggest that non-muscle myosin II complexes with the apical actin belt (Ferreira and Hilfer, 1993; Nishimura and Takeichi, 2008). The phosphorylation of the mysoin regulatory light chains (pMLC) by apical Rho Kinase (ROCK), leads to myosin activation and contraction of the adherens belt. Interestingly, an adherens junction scaffolding protein, Shroom3, recruits ROCK to the adherens junctions (Hildebrand, 2005; Nishimura and Takeichi, 2008). As a result, Shroom3 or myosin perturbations prevent apical constriction and result in a failure to close the neural tube (Hildebrand and Soriano, 1999; Haigo et al., 2003; Lee et al., 2007; Kinoshita et al., 2008; Nishimura and Takeichi, 2008; Rolo et al., 2009).
Interestingly, recent studies have shown that pMLC and F-actin are distributed asymmetrically in the adherens junctions of neural plate cells, so that thick ropes of actomyosin run mediolaterally along the apical surface of the neural plate (Nishimura et al., 2012). Celsr1, a PCP pathway atypical cadherin is also heavily concentrated along these actomyosin belts. Celsr1 cooperates with other components of the PCP pathway to activate RhoA in a polarized manner causing planar polarizied actomyosin contraction. This ensures that apical constriction and convergent extension occur in a coordinated manner to promote planar polarized bending of the neural plate (Fig. 4A, B) (Nishimura et al., 2012; Sullivan-Brown and Goldstein, 2012).
Cell Cycle Progression and Neural Tube Closure
One reason for questioning the importance of actin remodeling in cell wedging at hinge points concerns the bipolar nature of neural progenitors and their interkinetic nuclear migration (Sauer, 1935; Schoenwolf and Smith, 1990). How is a bipolar cell apically constricted? Does this involve constricting its already slender apical process, constricting the apical surface of apically located mitotic cells, or cell cycle dynamics? Since the nucleus/cell body is the largest part of a bipolar neural cell, a reduction of the apical surface of the neural plate may simply involve cell cycle-dependent nuclear or somal exclusion from the apical surface (Fig. 4C, D). Indeed, scanning EM images show that the so called wedge-shaped cells, which comprise ~70% of all MHP cells at any given time, have cell bodies abutting the basal edge of the neural plate (Fig. 4D) (Smith and Schoenwolf, 1987).
In the above view, cell wedging at the MHP is a mere consequence of cell cycle dynamics, where any mechanism that prolongs the cell cycle, increases G1, G2 or S phases or reduces mitotic duration, would de facto result in a reduction of the apical surface area. Indeed, cells at the MHP display a longer cell cycle length compared to lateral neural plate cells, spending a disproportionate part of their cell cycle at the base of the neural plate, either in the S or G2 phases (Smith and Schoenwolf, 1987; Smith and Schoenwolf, 1988). Our time-lapse observations suggest that BMP blockade can prolong the cell cycle at ectopic hinge points and increase the duration of time spent at the base of the neural plate (Eom et al., unpublished observations). Thus BMP signaling may be involved in regulating apicobasal junctional remodeling and cell cycle progression in the folding neural plate.
These data suggest that in addition to PCP induced junctional remodeling, interkinetic nuclear migration and cell cycle dynamics may also be critical contributers to apical constriction and NTC (Norden et al., 2009; Kosodo et al., 2011; Spear and Erickson, 2012a, b). Thus any future model that accounts for the coordination between planar and apicobasal events during NTC would need to incorporate the dynamic arrival and departure of mitotic cells to and from the apical surface. This would occur at a slower rate at the MHP, where ~2/3rd of all cell nuclei are basally located and only a few mitotic cells are seen at the apical surface at any given time. Thus PCP-dependent epithelial remodeling at the MHP would have to occur mainly among the apical processes of neural progenitors and not mitotic cells.
Acknowledgments
We thank Charmaine Brown for critically reading the manuscript. This work was supported by an NIH-NINDS (R01 NS049091) grant to SA.
Footnotes
Author Contribution: Dae Seok Eom contributed to the resarch summarized in this manuscript, critically read the manuscript and conceptualized and executed the figures.
Smita Amarnath contributed to the resarch summarized in this manuscript and critically read the manuscript.
Seema Agarwala contributed to the research summarized in this manuscript, wrote the manuscript and conceptualized the summary figures.
References
- Aaku-Saraste E, Hellwig A, Huttner WB. Loss of occludin and functional tight junctions, but not ZO-1, during neural tube closure--remodeling of the neuroepithelium prior to neurogenesis. Dev Biol. 1996;180:664–679. doi: 10.1006/dbio.1996.0336. [DOI] [PubMed] [Google Scholar]
- Baye LM, Link BA. Interkinetic nuclear migration and the selection of neurogenic cell divisions during vertebrate retinogenesis. J Neurosci. 2007;27:10143–10152. doi: 10.1523/JNEUROSCI.2754-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–1925. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- Castranio T, Mishina Y. Bmp2 is required for cephalic neural tube closure in the mouse. Dev Dyn. 2009;238:110–122. doi: 10.1002/dvdy.21829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H, Huylebroeck D, Verschueren K, Guo Q, Matzuk MM, Zwijsen A. Smad5 knockout mice die at mid-gestation due to multiple embryonic and extraembryonic defects. Development. 1999;126:1631–1642. doi: 10.1242/dev.126.8.1631. [DOI] [PubMed] [Google Scholar]
- Colas JF, Schoenwolf GC. Towards a cellular and molecular understanding of neurulation. Dev Dyn. 2001;221:117–145. doi: 10.1002/dvdy.1144. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Greene ND. Genetics and development of neural tube defects. J Pathol. 2010;220:217–230. doi: 10.1002/path.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LA, Keller RE. Neural tube closure in Xenopus laevis involves medial migration, directed protrusive activity, cell intercalation and convergent extension. Development. 1999;126:4547–4556. doi: 10.1242/dev.126.20.4547. [DOI] [PubMed] [Google Scholar]
- Eom DS, Amarnath S, Fogel JL, Agarwala S. Bone morphogenetic proteins regulate neural tube closure by interacting with the apicobasal polarity pathway. Development. 2011;138:3179–3188. doi: 10.1242/dev.058602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom DS, Amarnath S, Fogel JL, Agarwala S. Bone morphogenetic proteins regulate hinge point formation during neural tube closure by dynamic modulation of apicobasal polarity. Birth Defects Res A Clin Mol Teratol. 2012;94:804–816. doi: 10.1002/bdra.23052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewald AJ, Brenot A, Duong M, Chan BS, Werb Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev Cell. 2008;14:570–581. doi: 10.1016/j.devcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MC, Hilfer SR. Calcium regulation of neural fold formation: visualization of the actin cytoskeleton in living chick embryos. Dev Biol. 1993;159:427–440. doi: 10.1006/dbio.1993.1253. [DOI] [PubMed] [Google Scholar]
- Fleming A, Gerrelli D, Greene ND, Copp AJ. Mechanisms of normal and abnormal neurulation: evidence from embryo culture studies. Int J Dev Biol. 1997;41:199–212. [PubMed] [Google Scholar]
- Geldmacher-Voss B, Reugels AM, Pauls S, Campos-Ortega JA. A 90-degree rotation of the mitotic spindle changes the orientation of mitoses of zebrafish neuroepithelial cells. Development. 2003;130:3767–3780. doi: 10.1242/dev.00603. [DOI] [PubMed] [Google Scholar]
- Gibson MC, Perrimon N. Extrusion and death of DPP/BMP-compromised epithelial cells in the developing Drosophila wing. Science. 2005;307:1785–1789. doi: 10.1126/science.1104751. [DOI] [PubMed] [Google Scholar]
- Haigo SL, Hildebrand JD, Harland RM, Wallingford JB. Shroom induces apical constriction and is required for hingepoint formation during neural tube closure. Curr Biol. 2003;13:2125–2137. doi: 10.1016/j.cub.2003.11.054. [DOI] [PubMed] [Google Scholar]
- Harris MJ, Juriloff DM. Mouse mutants with neural tube closure defects and their role in understanding human neural tube defects. Birth Defects Res A Clin Mol Teratol. 2007;79:187–210. doi: 10.1002/bdra.20333. [DOI] [PubMed] [Google Scholar]
- Harris MJ, Juriloff DM. An update to the list of mouse mutants with neural tube closure defects and advances toward a complete genetic perspective of neural tube closure. Birth Defects Res A Clin Mol Teratol. 2010;88:653–669. doi: 10.1002/bdra.20676. [DOI] [PubMed] [Google Scholar]
- Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat Rev Mol Cell Biol. 2010;11:502–514. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD. Shroom regulates epithelial cell shape via the apical positioning of an actomyosin network. J Cell Sci. 2005;118:5191–5203. doi: 10.1242/jcs.02626. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD, Soriano P. Shroom, a PDZ domain-containing actin-binding protein, is required for neural tube morphogenesis in mice. Cell. 1999;99:485–497. doi: 10.1016/s0092-8674(00)81537-8. [DOI] [PubMed] [Google Scholar]
- Hong E, Brewster R. N-cadherin is required for the polarized cell behaviors that drive neurulation in the zebrafish. Development. 2006;133:3895–3905. doi: 10.1242/dev.02560. [DOI] [PubMed] [Google Scholar]
- Jacobson AG, Moury JD. Tissue boundaries and cell behavior during neurulation. Dev Biol. 1995;171:98–110. doi: 10.1006/dbio.1995.1263. [DOI] [PubMed] [Google Scholar]
- Jaskoll T, Greenberg G, Melnick M. Neural tube and neural crest: a new view with time-lapse high-definition photomicroscopy. Am J Med Genet. 1991;41:333–345. doi: 10.1002/ajmg.1320410315. [DOI] [PubMed] [Google Scholar]
- Juriloff DM, Harris MJ. A consideration of the evidence that genetic defects in planar cell polarity contribute to the etiology of human neural tube defects. Birth Defects Res A Clin Mol Teratol. 2012;94:824–840. doi: 10.1002/bdra.23079. [DOI] [PubMed] [Google Scholar]
- Karfunkel P. The activity of microtubules and microfilaments in neurulation in the chick. J Exp Zool. 1972;181:289–301. doi: 10.1002/jez.1401810302. [DOI] [PubMed] [Google Scholar]
- Kibar Z, Capra V, Gros P. Toward understanding the genetic basis of neural tube defects. Clin Genet. 2007a;71:295–310. doi: 10.1111/j.1399-0004.2007.00793.x. [DOI] [PubMed] [Google Scholar]
- Kibar Z, Torban E, McDearmid JR, Reynolds A, Berghout J, Mathieu M, Kirillova I, De Marco P, Merello E, Hayes JM, Wallingford JB, Drapeau P, Capra V, Gros P. Mutations in VANGL1 associated with neural-tube defects. N Engl J Med. 2007b;356:1432–1437. doi: 10.1056/NEJMoa060651. [DOI] [PubMed] [Google Scholar]
- Kinoshita N, Sasai N, Misaki K, Yonemura S. Apical accumulation of Rho in the neural plate is important for neural plate cell shape change and neural tube formation. Mol Biol Cell. 2008;19:2289–2299. doi: 10.1091/mbc.E07-12-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosodo Y, Huttner WB. Basal process and cell divisions of neural progenitors in the developing brain. Dev Growth Differ. 2009;51:251–261. doi: 10.1111/j.1440-169X.2009.01101.x. [DOI] [PubMed] [Google Scholar]
- Kosodo Y, Suetsugu T, Suda M, Mimori-Kiyosue Y, Toida K, Baba SA, Kimura A, Matsuzaki F. Regulation of interkinetic nuclear migration by cell cycle-coupled active and passive mechanisms in the developing brain. EMBO J. 2011;30:1690–1704. doi: 10.1038/emboj.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Scherr HM, Wallingford JB. Shroom family proteins regulate gamma-tubulin distribution and microtubule architecture during epithelial cell shape change. Development. 2007;134:1431–1441. doi: 10.1242/dev.02828. [DOI] [PubMed] [Google Scholar]
- Lee JY, Harland RM. Endocytosis is required for efficient apical constriction during Xenopus gastrulation. Curr Biol. 2010;20:253–258. doi: 10.1016/j.cub.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linville GP, Shepard TH. Neural tube closure defects caused by cytochalasin B. Nat New Biol. 1972;236:246–247. [Google Scholar]
- Liu A, Niswander LA. Bone morphogenetic protein signalling and vertebrate nervous system development. Nat Rev Neurosci. 2005;6:945–954. doi: 10.1038/nrn1805. [DOI] [PubMed] [Google Scholar]
- Lowery LA, Sive H. Strategies of vertebrate neurulation and a re-evaluation of teleost neural tube formation. Mech Dev. 2004;121:1189–1197. doi: 10.1016/j.mod.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Margolis B, Borg JP. Apicobasal polarity complexes. J Cell Sci. 2005;118:5157–5159. doi: 10.1242/jcs.02597. [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, Yu W, Rodriguez-Fraticelli AE, Ewald AJ, Werb Z, Alonso MA, Mostov K. Cell-polarity dynamics controls the mechanism of lumen formation in epithelial morphogenesis. Curr Biol. 2008;18:507–513. doi: 10.1016/j.cub.2008.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Castellanos C, Edgar BA. A characterization of the effects of Dpp signaling on cell growth and proliferation in the Drosophila wing. Development. 2002;129:1003–1013. doi: 10.1242/dev.129.4.1003. [DOI] [PubMed] [Google Scholar]
- Massa V, Greene ND, Copp AJ. Do cells become homeless during neural tube closure? Cell Cycle. 2009;8:2479–2480. doi: 10.4161/cc.8.16.9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12:1438–1452. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer EJ, Ikmi A, Gibson MC. Interkinetic nuclear migration is a broadly conserved feature of cell division in pseudostratified epithelia. Curr Biol. 2011;21:485–491. doi: 10.1016/j.cub.2011.02.002. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- Mostov K, Su T, ter Beest M. Polarized epithelial membrane traffic: conservation and plasticity. Nat Cell Biol. 2003;5:287–293. doi: 10.1038/ncb0403-287. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Honda H, Takeichi M. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell. 2012;149:1084–1097. doi: 10.1016/j.cell.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Takeichi M. Shroom3-mediated recruitment of Rho kinases to the apical cell junctions regulates epithelial and neuroepithelial planar remodeling. Development. 2008;135:1493–1502. doi: 10.1242/dev.019646. [DOI] [PubMed] [Google Scholar]
- Norden C, Young S, Link BA, Harris WA. Actomyosin is the main driver of interkinetic nuclear migration in the retina. Cell. 2009;138:1195–1208. doi: 10.1016/j.cell.2009.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai YJ, Abdullah NL, Mohd-Zin SW, Mohammed RS, Rolo A, Greene ND, Abdul-Aziz NM, Copp AJ. Epithelial fusion during neural tube morphogenesis. Birth Defects Res A Clin Mol Teratol. 2012;94:817–823. doi: 10.1002/bdra.23072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papan C, Campos-Ortega JA. Region-specific cell clones in the developing spinal cord of the zebrafish. Dev Genes Evol. 1999;209:135–144. doi: 10.1007/s004270050237. [DOI] [PubMed] [Google Scholar]
- Pilot F, Lecuit T. Compartmentalized morphogenesis in epithelia: from cell to tissue shape. Dev Dyn. 2005;232:685–694. doi: 10.1002/dvdy.20334. [DOI] [PubMed] [Google Scholar]
- Plant PJ, Fawcett JP, Lin DC, Holdorf AD, Binns K, Kulkarni S, Pawson T. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat Cell Biol. 2003;5:301–308. doi: 10.1038/ncb948. [DOI] [PubMed] [Google Scholar]
- Pyrgaki C, Trainor P, Hadjantonakis AK, Niswander L. Dynamic imaging of mammalian neural tube closure. Dev Biol. 2010;344:941–947. doi: 10.1016/j.ydbio.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray HJ, Niswander L. Mechanisms of tissue fusion during development. Development. 2012;139:1701–1711. doi: 10.1242/dev.068338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolo A, Skoglund P, Keller R. Morphogenetic movements driving neural tube closure in Xenopus require myosin IIB. Dev Biol. 2009;327:327–338. doi: 10.1016/j.ydbio.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer L. Mitosis in the neural tube. J Comp Neurol. 1935;62:377–397. [Google Scholar]
- Schoenwolf GC. Shaping and bending of the avian neuroepithelium: morphometric analyses. Dev Biol. 1985;109:127–139. doi: 10.1016/0012-1606(85)90353-7. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Alvarez IS. Roles of neuroepithelial cell rearrangement and division in shaping of the avian neural plate. Development. 1989;106:427–439. doi: 10.1242/dev.106.3.427. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Delongo J. Ultrastructure of secondary neurulation in the chick embryo. Am J Anat. 1980;158:43–63. doi: 10.1002/aja.1001580106. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Folsom D, Moe A. A reexamination of the role of microfilaments in neurulation in the chick embryo. Anat Rec. 1988;220:87–102. doi: 10.1002/ar.1092200111. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Franks MV. Quantitative analyses of changes in cell shapes during bending of the avian neural plate. Dev Biol. 1984;105:257–272. doi: 10.1016/0012-1606(84)90284-7. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Smith JL. Mechanisms of neurulation: traditional viewpoint and recent advances. Development. 1990;109:243–270. doi: 10.1242/dev.109.2.243. [DOI] [PubMed] [Google Scholar]
- Shen J, Dahmann C. Extrusion of cells with inappropriate Dpp signaling from Drosophila wing disc epithelia. Science. 2005;307:1789–1790. doi: 10.1126/science.1104784. [DOI] [PubMed] [Google Scholar]
- Smith JL, Schoenwolf GC. Cell cycle and neuroepithelial cell shape during bending of the chick neural plate. Anat Rec. 1987;218:196–206. doi: 10.1002/ar.1092180215. [DOI] [PubMed] [Google Scholar]
- Smith JL, Schoenwolf GC. Role of cell-cycle in regulating neuroepithelial cell shape during bending of the chick neural plate. Cell Tissue Res. 1988;252:491–500. doi: 10.1007/BF00216636. [DOI] [PubMed] [Google Scholar]
- Smith JL, Schoenwolf GC. Neurulation: coming to closure. Trends Neurosci. 1997;20:510–517. doi: 10.1016/s0166-2236(97)01121-1. [DOI] [PubMed] [Google Scholar]
- Smith JL, Schoenwolf GC, Quan J. Quantitative analyses of neuroepithelial cell shapes during bending of the mouse neural plate. J Comp Neurol. 1994;342:144–151. doi: 10.1002/cne.903420113. [DOI] [PubMed] [Google Scholar]
- Solloway MJ, Robertson EJ. Early embryonic lethality in Bmp5; Bmp7 double mutant mice suggests functional redundancy within the 60A subgroup. Development. 1999;126:1753–1768. doi: 10.1242/dev.126.8.1753. [DOI] [PubMed] [Google Scholar]
- Spear PC, Erickson CA. Interkinetic nuclear migration: A mysterious process in search of a function. Dev Growth Differ. 2012a;54:300–316. doi: 10.1111/j.1440-169X.2012.01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear PC, Erickson CA. Apical movement during interkinetic nuclear migration is a two-step process. Dev Biol. 2012b;370:33–41. doi: 10.1016/j.ydbio.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stottmann RW, Berrong M, Matta K, Choi M, Klingensmith J. The BMP antagonist Noggin promotes cranial and spinal neurulation by distinct mechanisms. Dev Biol. 2006;295:647–663. doi: 10.1016/j.ydbio.2006.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stottmann RW, Klingensmith J. Bone morphogenetic protein signaling is required in the dorsal neural folds before neurulation for the induction of spinal neural crest cells and dorsal neurons. Dev Dyn. 2011;240:755–765. doi: 10.1002/dvdy.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan-Brown J, Goldstein B. Neural tube closure: the curious case of shrinking junctions. Curr Biol. 2012;22:R574–576. doi: 10.1016/j.cub.2012.06.048. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Yamanaka T, Hirose T, Manabe N, Mizuno K, Shimizu M, Akimoto K, Izumi Y, Ohnishi T, Ohno S. Atypical protein kinase C is involved in the evolutionarily conserved par protein complex and plays a critical role in establishing epithelia-specific junctional structures. J Cell Biol. 2001;152:1183–1196. doi: 10.1083/jcb.152.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Morita H, Ueno N. Molecular mechanisms of cell shape changes that contribute to vertebrate neural tube closure. Dev Growth Differ. 2012;54:266–276. doi: 10.1111/j.1440-169X.2012.01346.x. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Self-organization of animal tissues: cadherin-mediated processes. Dev Cell. 2011;21:24–26. doi: 10.1016/j.devcel.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Teng X, Toyama Y. Apoptotic force: active mechanical of cell death during morphogenesis. Dev Growth Differ. 2011;53:269–276. doi: 10.1111/j.1440-169X.2011.01251.x. [DOI] [PubMed] [Google Scholar]
- Teleman AA, Strigini M, Cohen SM. Shaping morphogen gradients. Cell. 2001;105:559–562. doi: 10.1016/s0092-8674(01)00377-4. [DOI] [PubMed] [Google Scholar]
- Trinkhaus JP. Cells into organs: the forces that shape the embryo. Englewood Cliffs, NJ: Prentice-Hall; 1969. [Google Scholar]
- Van Straaten HW, Janssen HC, Peeters MC, Copp AJ, Hekking JW. Neural tube closure in the chick embryo is multiphasic. Dev Dyn. 1996;207:309–318. doi: 10.1002/(SICI)1097-0177(199611)207:3<309::AID-AJA8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- van Straaten HW, Sieben I, Hekking JW. Multistep role for actin in initial closure of the mesencephalic neural groove in the chick embryo. Dev Dyn. 2002;224:103–108. doi: 10.1002/dvdy.10078. [DOI] [PubMed] [Google Scholar]
- von der Hardt S, Bakkers J, Inbal A, Carvalho L, Solnica-Krezel L, Heisenberg CP, Hammerschmidt M. The Bmp gradient of the zebrafish gastrula guides migrating lateral cells by regulating cell-cell adhesion. Curr Biol. 2007;17:475–487. doi: 10.1016/j.cub.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Wada H, Okamoto H. Roles of planar cell polarity pathway genes for neural migration and differentiation. Dev Growth Differ. 2009;51:233–240. doi: 10.1111/j.1440-169X.2009.01092.x. [DOI] [PubMed] [Google Scholar]
- Wallingford JB. Vertebrate gastrulation: polarity genes control the matrix. Curr Biol. 2005;15:R414–416. doi: 10.1016/j.cub.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Wallingford JB. Planar cell polarity, ciliogenesis and neural tube defects. Hum Mol Genet. 2006;15(Spec No 2):R227–234. doi: 10.1093/hmg/ddl216. [DOI] [PubMed] [Google Scholar]
- Wallingford JB. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu Rev Cell Dev Biol. 2012;28:627–653. doi: 10.1146/annurev-cellbio-092910-154208. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM. Neural tube closure requires Dishevelled-dependent convergent extension of the midline. Development. 2002;129:5815–5825. doi: 10.1242/dev.00123. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Shinotsuka N, Nonomura K, Takemoto K, Kuida K, Yosida H, Miura M. Live imaging of apoptosis in a novel transgenic mouse highlights its role in neural tube closure. J Cell Biol. 2011;195:1047–1060. doi: 10.1083/jcb.201104057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka T, Horikoshi Y, Sugiyama Y, Ishiyama C, Suzuki A, Hirose T, Iwamatsu A, Shinohara A, Ohno S. Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr Biol. 2003;13:734–743. doi: 10.1016/s0960-9822(03)00244-6. [DOI] [PubMed] [Google Scholar]
- Yang X, Zou J, Hyde DR, Davidson LA, Wei X. Stepwise maturation of apicobasal polarity of the neuroepithelium is essential for vertebrate neurulation. J Neurosci. 2009;29:11426–11440. doi: 10.1523/JNEUROSCI.1880-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Cogram P, Gerrelli D, Copp AJ. Sonic hedgehog and the molecular regulation of mouse neural tube closure. Development. 2002;129:2507–2517. doi: 10.1242/dev.129.10.2507. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Gaston-Massuet C, Girdler G, Klingensmith J, Arkell R, Greene ND, Copp AJ. Neural plate morphogenesis during mouse neurulation is regulated by antagonism of Bmp signalling. Development. 2007;134:3203–3211. doi: 10.1242/dev.008177. [DOI] [PubMed] [Google Scholar]
- Zegers MM, O’Brien LE, Yu W, Datta A, Mostov KE. Epithelial polarity and tubulogenesis in vitro. Trends Cell Biol. 2003;13:169–176. doi: 10.1016/s0962-8924(03)00036-9. [DOI] [PubMed] [Google Scholar]
- Zohn IE, Chesnutt CR, Niswander L. Cell polarity pathways converge and extend to regulate neural tube closure. Trends Cell Biol. 2003;13:451–454. doi: 10.1016/s0962-8924(03)00173-9. [DOI] [PubMed] [Google Scholar]