Abstract
The renin angiotensin system (RAS) plays an important role in wound repair; however, little is known pertaining to RAS expression in response to thermal and the combination of radiation plus burn injury (CRBI). The purpose of this study was to test the hypothesis that thermal injury modifies expression of RAS components and CRBI delayed this up-regulation of RAS. Skin from uninjured mice was compared to mice receiving local thermal injury or CRBI (injury site). Skin was analyzed for gene and protein expression of RAS components. There was an initial increase in the expression of various components of RAS following thermal injury. However, in the higher CRBI group there is an initial decrease in AT1b (vasoconstriction, pro-proliferative) AT2 (vasodilation, differentiation) and Mas (vasodilation, anti-inflammatory) gene expression. This corresponded with a delay and decrease in AT1, AT2 and MAS protein expression in fibroblasts and keratinocytes. The reduction in RAS receptor positive fibroblasts and keratinocytes correlated with a reduction in collagen deposition and keratinocyte infiltration into the wounded area resulting in a delay of re-epithelialization following CRBI. These data support the hypothesis that delayed wound healing observed in subjects following radiation exposure may be in part due to decreased expression of RAS.
Keywords: angiotensin, combined radiation and burn injury, wound healing
INTRODUCTION
In a nuclear disaster, individuals proximal to the source will be exposed not only to varying amounts of radiation but also thermal injury from the initial explosion and/or subsequent fires. The combination of total body irradiation (TBI) with thermal injury is more commonly referred to as combined radiation and burn injury (CRBI). When a sublethal dose of TBI is combined with a non-lethal dermal injury, increased mortality may occur as compared to either injury alone (1). Currently there is very little data with regards to the mechanism(s) associated with the pathophysiology of CRBI.
The renin angiotensin system (RAS) is best recognized for its role in regulating blood pressure and fluid balance. Physiologic properties associated with angiotensin II (A-II) mediated activity include vasoconstriction, fluid balance, and enhanced aldosterone secretion (2). A-II acts through two main receptors, AT1, which is constitutively expressed and AT2 which is a fetal receptor that is upregulated under conditions of stress such as heart failure and wound healing (3). More recently, it was found that cell growth and proliferation could also be mediated through AT1 activation (4, 5), whereas A-II binding onto AT2 receptors appears to be anti-proliferative, anti-inflammatory, stimulate differentiation (6, 7) and induce vasodilation (3, 8). Most physiological activities of A-II in the adult are ascribed to AT1-mediated activities. These activities include wound healing (4, 9–11), where A-II activation of AT1 regulates the expression of various cytokines and elaboration as well as synthesis of extracellular matrix proteins (12). Takeda and colleagues showed that AT1 receptor signaling blockade using a specific receptor antagonist suppresses angiogenesis during wound healing and that the repair process regulated by AT2 includes the balance of opposing signals between AT1 and AT2 (4). Similarly, Kurosaka et al reported that AT1a receptor knockout mice with full-thickness excisional skin wounds healed much slower and showed reduced angiogenesis as compared to wild type mice (13).
Angiotensin (1–7) (A(1–7)) is a seven amino acid and is a member of the RAS. Unlike A-II, A(1–7) does not have vasoconstrictive activity; in fact it may decrease blood pressure secondary to nitric oxide (NO) release (14). Recent studies have shown this peptide has significant activity in several biological systems including renal function (15) and wound repair (16–18). The main receptor for A(1–7) is Mas, a G protein coupled receptor (19), where its activation leads to the elaboration of NO (20). Some of the effects of A(1–7) however have been attributed to its ability to either directly or indirectly act via the AT1 (21) and AT2 (22) receptors as well. A(1–7) promotes wound healing by increasing the re-epithlialization of wounds and by increasing the proliferation of stem cells at the base of the hair follicles (17). Further, studies by Rodgers and colleagues have shown that antagonism of the receptor for A(1–7) delays healing in a diabetic mouse model (23).
This study focuses on CRBI in mice and its impact on the expression of RAS components. The delay in the upregulation of RAS components in animals receiving CRBI provides evidence for the hypothesis that the RAS is involved in skin healing and that modification of this system delays healing.
Materials and methods
Animal Study Design
This study was approved by the IACUC of the Keck School of Medicine of the University of Southern California. To evaluate impact of thermal injury alone, radiation alone (1 and 6 Gy) and the combination of the two types of injury, C57Bl/6 female mice, 6–8 weeks old (n=5 per group and time point) were purchased from Jackson Laboratories and quarantined at least 1 week prior to injury. The mice were randomized into various injury groups the day prior to injury.
Thermal injury
One day prior to thermal injury induction, the hair from the back of the mice was removed by shaving. On the day of the injury, the mice were anesthetized with intramuscular ketamine (80mg/kg) and xylazine (10mg/kg), and then were placed on a silicone mold, which exposed 10% of the body surface area, and immersed into 75°C water for 10 seconds which results in a partial thickness burn. Immediately after the thermal injury, 3 ml of sterile lactated Ringer’s were administered subcutaneously to provide fluids and nutrition. Buprenex (0.25 mg/kg) was administered twice daily for 3 days after the injury as an analgesic.
Radiation injury
Mice in radiation only or CRBI groups received either 1 or 6 Gy. The TBI was administered at 0.94 Gy/minute using a single aerated chamber irradiator (Atomic Energy, Canada) outfitted with a dual 137Cs source. In the groups to receive CRBI, the mice were irradiated first and then received the thermal injury an hour later.
Necropsy
At specific time points post injury (0.2h (immediate response to injury), Day1 (acute inflammation phase), Day2 (beginning of resolution of inflammation), Day5 (beginning of epithelialization), Day7 (keratinocyte proliferation and migration) and Day10 (initiation of remodeling phase)), groups of animals were euthanized and the skin from the back of the mice (burned area only or a comparable site in the TBI only group) was harvested and placed in formalin for tissue processing to immunohistochemistry or RNALater for measures of gene expression.
Reverse-transcriptase polymerase chain reaction
The gene expression for RAS receptors was quantified in wounded skin using quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) (24). Total RNA was isolated from tissues using RNeasy kit (Qiagen, Valencia, CA) and RNA concentration was measured using a ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE) at 260 and 280 nm. Following RNA quantification, cDNA was synthesized by using SuperScript TM III First strand Synthesis SuperMix (Invitrogen, Foster City, CA). The mRNA expression for AT1a, AT1b, AT2, Mas, ACE, ACE2, renin and angiotensinogen was determined by qRT-PCR using SYBR Green ER (Invitrogen, Foster City, CA). 28S rRNA and GAPDH were used as the control housekeeping primer for each sample and skin from uninjured mice were used to normalize the results. The fold changes are compared between non-injured mice with tissues from injured animals.
Immunohistochemical staining (IHC)
The formalin fixed skin was embedded in paraffin and thin sections (5 µm) were prepared. The slides were rehydrated and were subjected to heat induced epitope retrieval (HIER) in Antigen Retrieval Citra Plus (Biogenex, San Ramon, CA). In order to prevent nonspecific binding the sections were incubated for 10 minutes each in a 5% milk solution followed by 2% horse serum. Goat anti-mouse monoclonal antibodies directed against AT1, AT2 and MAS (Santa Cruz Biotechnology, Santa Cruz, CA) were used at 1:250 dilutions. Avidin-biotin complex method of detection was used where the secondary antibody used was biotinylated horse anti-goat antibody (1/300) which was then attached to streptavidin linked horseradish peroxidase (1/300) (Vector Laboratories, Burlingame, CA). The sections were then incubated in diamino benzidine (DAB) for 8–10 minutes and counterstained using hematoxylin. After mounting they were observed using light microscopy.
Measurement of keratinocyte infiltration and size of hair follicles
Keratinocyte infiltration was measured by using a 10 × 10 optical grid which was calibrated against a micrometer at 100X magnification. The total wound area and re-epithelialized wound area were measured using the optical grid and percentage healed was calculated as described in Rodgers et al (25). In order to measure hair follicle size at the healing edge of the wound photomicrographs were taken of all the slides at 40× magnification. Then using NIS-Elements D v3.2 (Nikon Instruments Inc, Melville, NY) software the hair follicles were traced and their sizes were compared.
Collagen staining
To evaluate the changes in collagen slides were stained with modified Trichrome Masson and imaged under 400× magnification. The total area of newly formed collagen at the healing edge was measured using NIS-Elements D v3.2 (Nikon Instruments Inc, Melville, NY) software. Maturity of deposited collagen was also measured based on the complexity of the collagen network as described in Rodgers et al (25).
Maturity of the collagen deposited was assessed with tissue sections stained with Trichrome stain on a 4-point scale:
Very short, fine, fibrils; very lightly stained; no to minimal deposition.
Fine, long and thin, slightly greater density (disorganized).
Fine fibrils undergoing condensation into slightly thicker, more dense fibres, disorganized in arrangement.
Thicker bands of fibres lying randomly in a basket-weave like pattern, tending to align across the injured area, organized in arrangement.
Statistical analysis
All the data was analyzed using GraphPad 5.0 (GraphPad Software, San Diego, CA, USA). Analysis of variance (ANOVA) along with Tukey posttest was used, where appropriate, to compare data from more than two groups. Mann-Whitney test was used to analyze collagen maturity. An alpha of 0.05 was set a priorii as the threshold for statistical significance. Data are expressed as mean value ± standard error of the mean (SEM).
Results
Gene Expression of RAS Receptors in Relations to Types of Injury
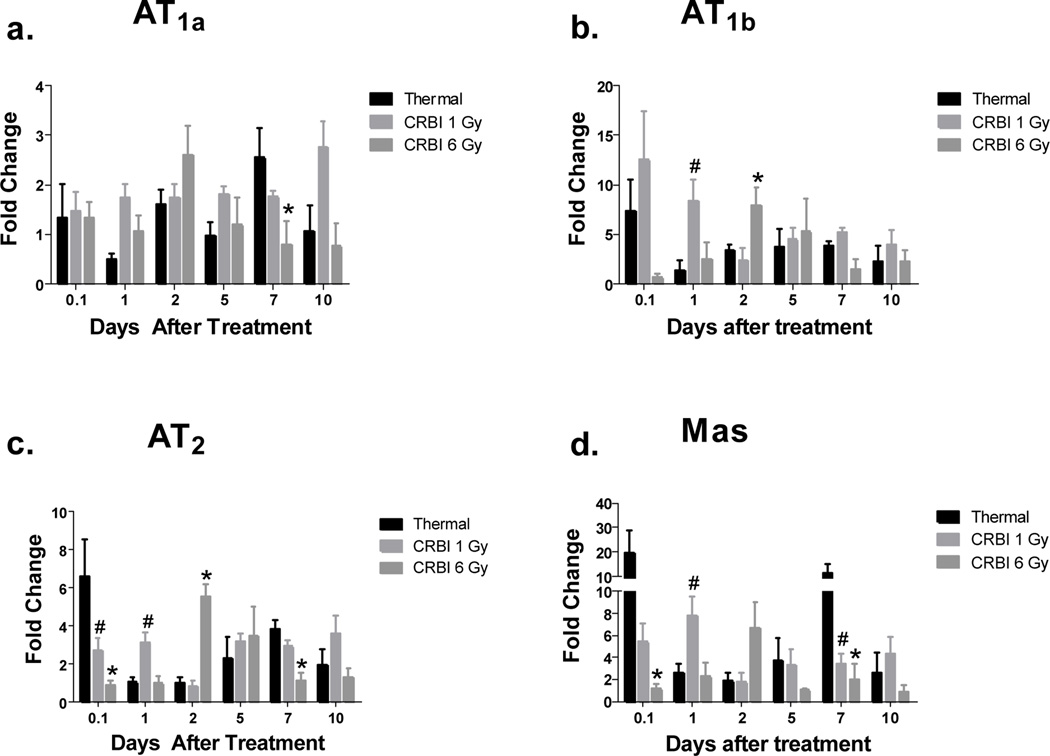
The various types of injuries were compared with tissues derived from uninjured animals. AT1a expression was significantly upregulated at day 7 in the thermal injury group as compared to CRBI 6Gy (Figure 1a). Thermal injury alone caused a marked and immediate (within 2 hours) increase in other RAS receptor gene expression (Figure 1b-d), particularly Mas, which revealed a 20-fold mRNA expression increase (Figure 1d). Mice receiving 1 Gy TBI only also showed an increase in RAS receptors expression however maximal changes appeared at later time points, where Mas expression was increased 5-fold from day 5 onwards (data not shown). In contrast tissue from mice receiving 6 Gy TBI, had a 2–5 fold increase of AT1b and AT2 from day 5 onwards until the end of the study on Day 10, where a 12-fold Mas expression was observed (data not shown). The CRBI groups followed a different pattern of RAS receptor expression compared to thermal injury only. With CRBI 1Gy the expression of AT1b and Mas are markedly increased on the day of the injury as well as the next day as compared to thermal injury alone (Figure 1b and 1d). In contrast, the group receiving CRBI 6 Gy demonstrated a delay in RAS receptor expression as compared to thermal injury by itself until Day 2 where mRNA expression was increased up to 6-fold for AT1b, AT2 and Mas. (Figure 1b-d).
Figure 1.
RAS Receptor Gene Expression: Skin from mice receiving thermal injury, CRBI 1Gy and CRBI 6Gy were collected and mRNA expression for AT1a (a), AT1b (b), AT2 (c) and Mas (d) was measured using qRT-PCR where target gene expression was normalized using GAPDH as the housekeeping gene and then compared to uninjured controls. Error bars are expressed as mean +/− SEM where n=5. #p< 0.05 for CRBI 1Gy as compared to thermal injury and *p<0.05 for CRBI 6Gy as compared to thermal injury
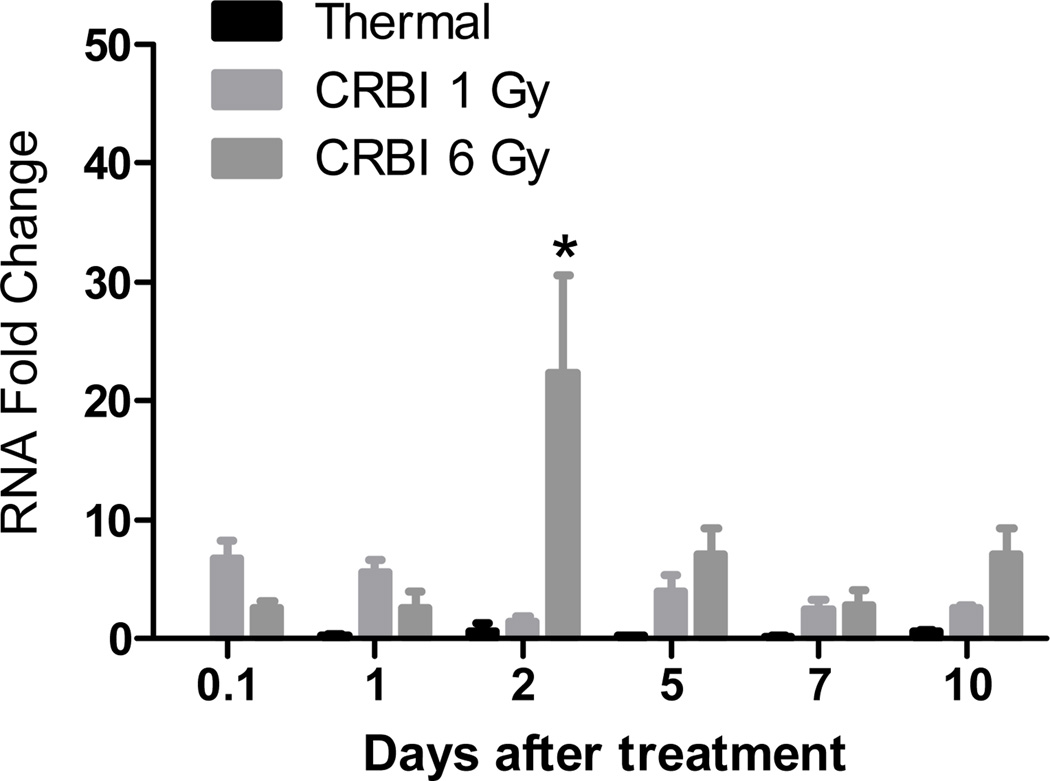
We also examined gene expression of angiotensinogen and the metabolic enzymes regulating the biosynthesis of RAS peptides. In dermal tissue, the expression of angiotensinogen, the precursor of bioactive RAS peptides, ACE or ACE2, enzymes involved in metabolism of angiotensin peptides, genes were not significantly different throughout the observation period for all of the treatment groups (data not shown). The expression of renin was not altered in the thermal injury or 1Gy TBI only groups, however a significant increase in renin gene expression was seen in the group receiving 6 Gy TBI alone, where a 30-fold increase was seen on Day 5 that persisted till Day 10 (data not shown). In animals receiving CRBI 1 Gy, an 8-fold increase in renin gene expression was seen 2 hours after injury (Figure 2). Whereas, in mice receiving 6 Gy CRBI, renin expression was delayed until Day 2 after injury where mRNA expression reached a 20-fold increase (Figure 2).
Figure 2.
Renin gene expression: Skin from mice receiving thermal injury, CRBI 1Gy and CRBI 6Gy were collected and mRNA expression for Renin was measured using qRT-PCR where target gene expression was normalized using GAPDH as the housekeeping gene and then compared to uninjured controls. Error bars are expressed as mean +/− SEM where n=5. *p<0.05 for CRBI 6Gy as compared to thermal injury
RAS receptor protein expression
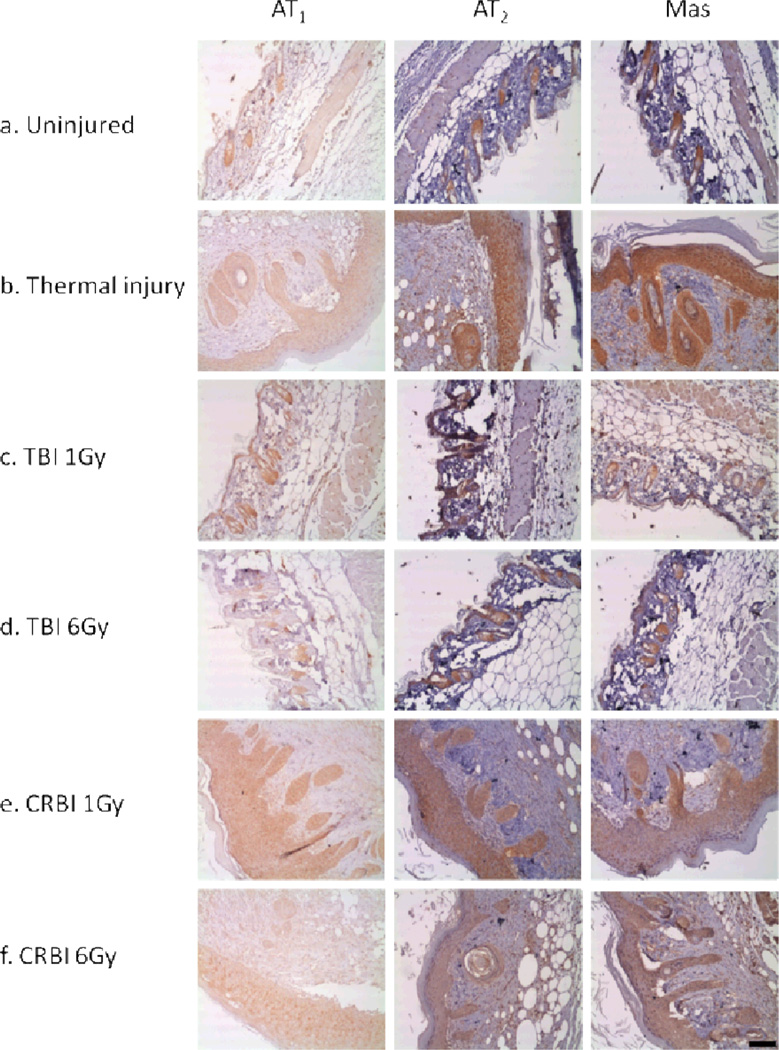
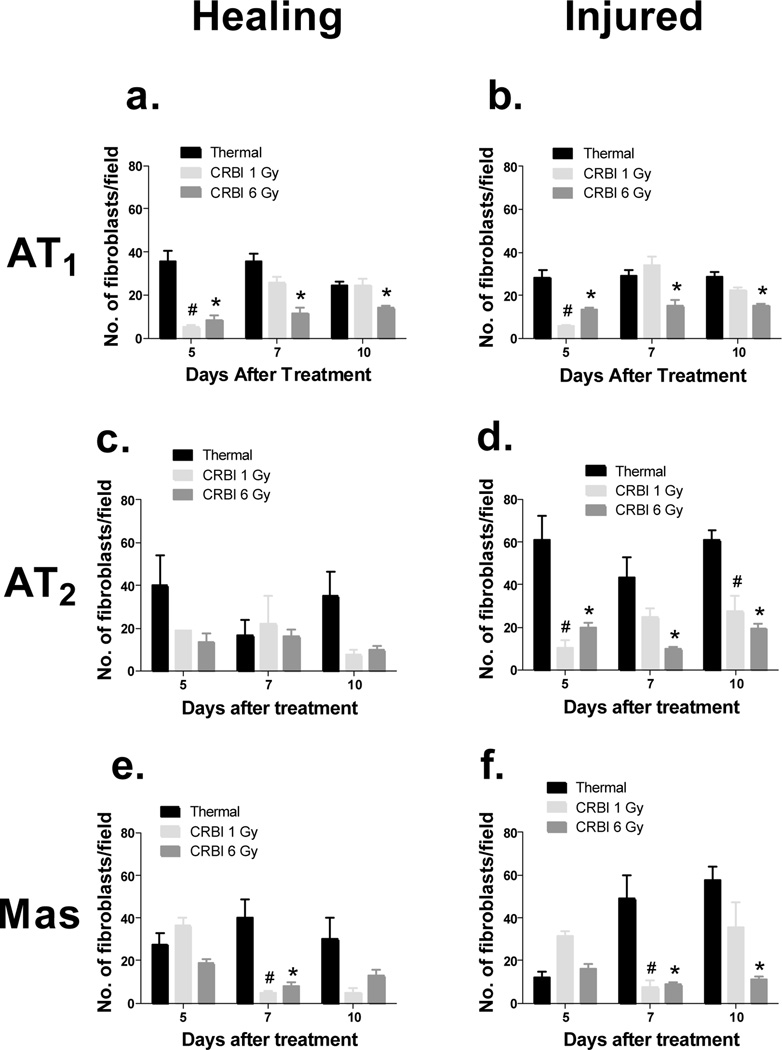
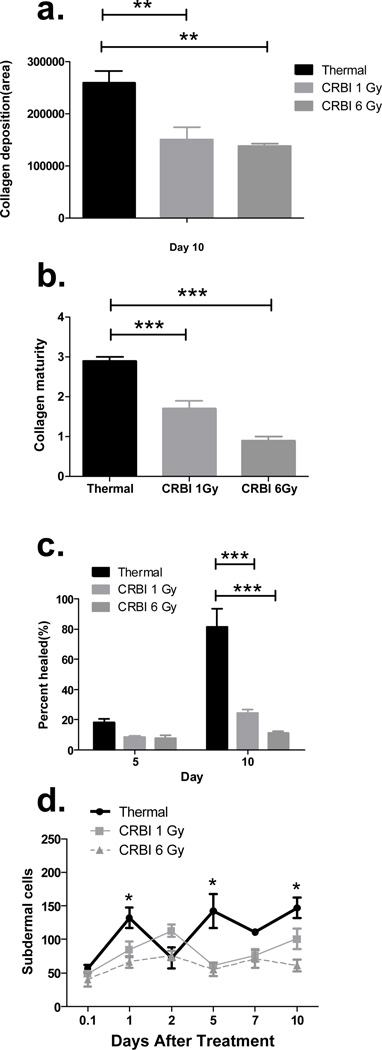
RAS receptor proteins expression was evaluated at the site of injury and compared to uninjured skin using IHC. The expression of RAS receptors was limited and confined to the bulge of the hair follicle in uninjured skin (Figure 3a). The various types of injuries induced RAS receptor expression in keratinocytes, fibroblasts and subdermal cells as summarized in Figure 3. The number of AT1, AT2 and Mas positive fibroblasts were decreased in animals receiving CRBI as compared to animals receiving thermal injury alone (Figure 4[a-f]). Fibroblasts are responsible for the deposition of collagen, thus we evaluated the level of collagen deposition in various injury models using trichrome staining. A reduction in total area of collagen deposition was observed in the CRBI groups as compared to thermal injury by itself at the healing edge of the wound (Figure 5a). In addition, there was a reduction in the maturity of the collagen deposited as well, with the CRBI groups having more immature collagen at Day 10 as compared to thermal injury only (Figure 5b).
Figure 3.
Protein expression of RAS receptors: Skin sections were stained immunohistochemically for AT1, AT2 and Mas in a. Uninjured, b. Thermal injury, c. TBI 1Gy, d. TBI 6Gy, e. CRBI 1Gy, f. CRBI 6Gy. Bar = 100µm
Figure 4.
a. Fibroblast Infiltration into Wound Site: Sections stained immunohistochemically for AT1, AT2 and Mas were enumerated for the total number of fibroblasts stained positively for these receptors, where ‘healing’ stands for the healing edge of the wound and ‘injured’ is the area of the wound that has yet to be re-epithelialized(i-vi). b. IHC for subdermal cells: The number of subdermal cells positively stained for RAS components were counted at the injury site and compared between the different groups. Error bars are expressed as mean +/− SEM where n=5. #p< 0.05 for CRBI 1Gy as compared to thermal injury and *p<0.05 for CRBI 6Gy as compared to thermal injury
Figure 5.
Collagen deposition and keratinocyte infiltration: The above data compares the thermal and CRBI injuries with regards to the total area of collagen deposited at the healing edge of the wound at day 10 (a), the maturity of the collagen deposited at day 10 (b), the percentage of keratinocyte re-epithelialization at day 5 and 10 (c) and the number of subdermal cells positively stained for RAS components at the injury site (d). Error bars are expressed as mean +/− SEM where n=5. p< 0.005 for (a) and (d); p<0.0001 (b) and (c).
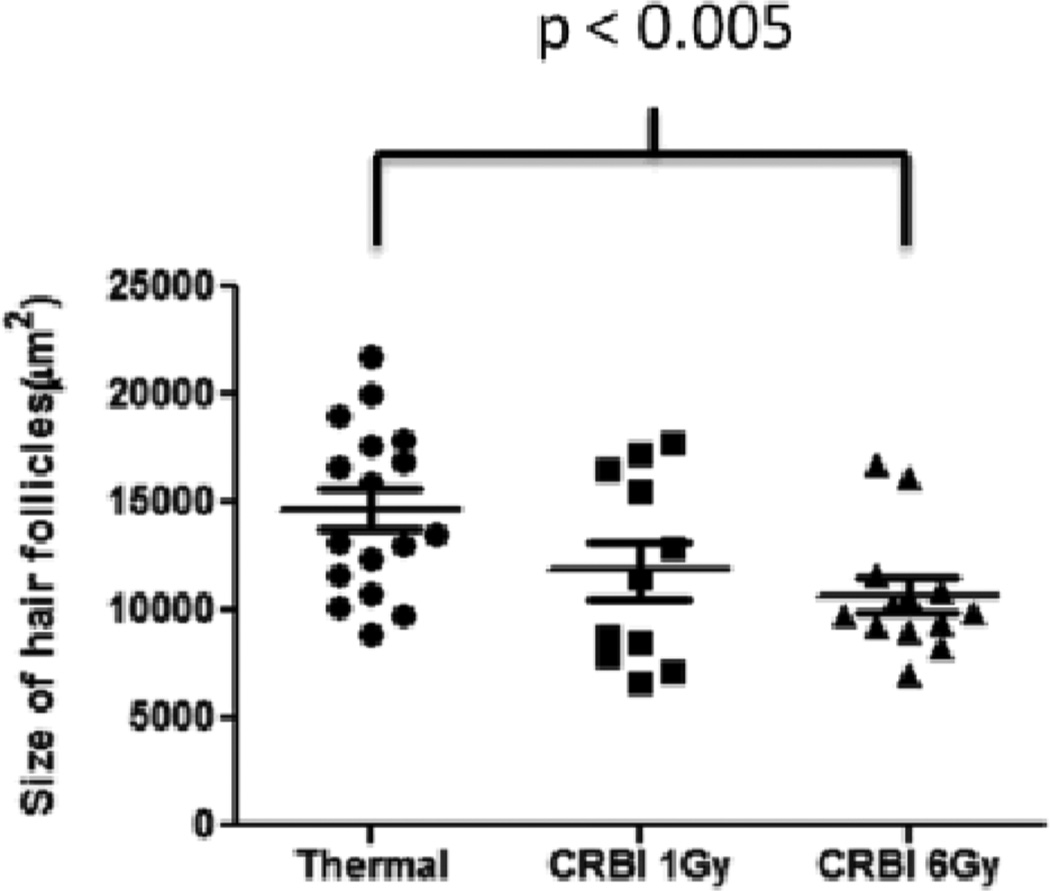
With injury, the keratinocytes that infiltrated the wound stained positive for AT1, AT2 and Mas receptors. However, there was significant reduction in the volume of hyperplastic epithelium and epithelialization at the wound sites of animals with CRBI compared to those from mice receiving only thermal injury (Figure 5c). In addition, the number of subdermal cells staining for AT1, AT2 and Mas receptors was determined. AT2 and Mas positive subdermal cells were relatively lower in number and did not show any significant difference between the injury groups (data not shown), whereas, the number of AT1 positive subdermal cells in the 6 Gy CRBI groups was significantly lower when compared to thermal injury only (Figure 5d). The size of hair follicles at the healing edge of the wound at Day 2 after injury was also measured. A decrease in the size of the hair follicles was found in the 6 Gy CRBI group as compared to thermal injury by itself (Figure 6).
Figure 6.
Hair follicle size: The above graph compares the size of the hair follicles between the injury groups at the healing edge of the wound at day 2. Error bars are expressed as mean +/− SEM where n=5. p< 0.005 for CRBI 6Gy as compared to thermal injury.
After a wound, angiogenesis plays an important role in wound healing. To assess the level of angiogenesis, we quantified the number of blood vessels seen at the wound site. No discernible difference was observed between the different injury groups (data not shown).
DISCUSSION
Delaying of the dermal wound healing process after exposure to radiation is well documented (26). Radiation injury results in decreased production of various pro-proliferative and angiogenic factors, such as basic fibroblast growth factor (FGF) and VEGF, and decreases in progenitors expressing CD31 and CD34. Exposure to radiation also increases MMP2 and MM9 levels in the skin resulting in increased proteolytic cleavage of extracellular matrix. It also reduces the proliferation of fibroblasts and transforms keratinocytes into a less differentiated state (27). Finally, radiation increases and prolongs the production of inflammatory cytokines, such as IL1β, IL6 and TNFα in dermal tissue thus delaying the dermal wound healing response (28). These effects have been associated with delayed dermal wound healing (27, 29, 30). Although the effects of radiation exposure alone have been studied in detail, there is a dearth of knowledge as to the mechanism as how CRBI affects cells. Individuals exposed to high levels of radiation will develop radiation sickness as well as thermal-induced injuries. Thus, it is important to evaluate the impact of the combination of these two types of injuries as compared to these injuries alone on wound healing.
The gene expression data from this study shows that, as compared to mice receiving thermal injury alone, the groups receiving 6 Gy CRBI had delayed expression of RAS receptors AT1b, AT2 and Mas. In addition to the delay in receptor expression, there is also a decrease in the intensity i.e. fold change, of expression in the 6Gy CRBI groups as compared to thermal injury by itself. Previously we have shown that the A-II receptor levels in rat skin are altered in response to full thickness excision and sutured wounds (10, 11). Stecklings et al showed that A-II as well as the receptors AT1 and AT2 is present in the human skin and that they play an important role in cutaneous wound healing (8, 31). Takeda et al found that AT1 inhibition blocks keratinocyte re-epithelialization and myofibroblast recovery, whereas inhibition of AT2 has the opposite effect. These findings suggest that dermal wound healing is regulated by the opposing signals involving AT1 and AT2 (4).
When we evaluated the expression of enzymes important for the biosynthetic enzymes found in the RAS pathway, no notable changes were detected except for renin. Reduction in renin expression was observed in CRBI 6 Gy group when compared to the CRBI 1 Gy group. As renin mediates the formation of A-I, the substrate from which AII and A(1–7) can be formed, this reduction in renin expression may result in a local reduction in the generation of these bioactive peptides. A recent study has shown that local levels of AII are increased through day 7 after dermal injury (32). In contrast, the absence of change in renin expression in the thermal injury group might be due to sufficient endogenous level of angiotensin peptides in the healing skin; however, this hypothesis still needs to be validated. A second hypothesis to explain absence of change in renin expression at the site of injury is the possibility that, like the cardiac system, systemic renin is responsible for the production of A-I and the local RAS then further processes it (33).
The IHC data further confirmed qRT-PCR, where a notable decrease in number of AT1, AT2 and MAS positive fibroblasts was seen in CRBI treated animals at different time points for each of the RAS receptors. These observations reaffirm the fact that even though these receptors may be involved in the wound healing process, their roles may be different. The complexities of keratinocyte-fibroblast interactions have been explained in detail by Werner et al along with the crucial role these interactions play in the wound healing process (34). The delay and decrease in both the number of fibroblasts and the collagen deposition at the site of the injury along with the decrease in keratinocyte re-epithelialization in the CRBI groups as compared to thermal injury by itself could be due to these complex interactions. A-II is known to promote the secretion of collagen (35) and fibronectin (36) in cardiac fibroblasts as well as growth factors such as TGF-β (37, 38) and PDGF (39, 40) through the AT1 receptor. The decreased RAS receptor staining in the CRBI treated groups might explain the delayed wound healing response observed as compared to thermal injury by itself. In order to explore this further it would be interesting to study the effect of CRBI on inflammation as well as factors such as TGF-β and KGF in skin which would give us a better insight into the effect of this type of injury on wound healing.
Hair follicles play an important role in the re-epithelialization process after an injury (41, 42) and the reduced size of hair follicles in the CRBI group as compared to thermal injury by itself points to a delayed healing response. The decrease in number of AT1 positively stained cells in the subdermis of CRBI groups as compared to thermal injury by itself may correlate with a delayed inflammatory response in the CRBI group. Angiotensin II is known to induce inflammation via the AT1 receptor (43) whereas, both AT2 and Mas have been shown to have an anti-inflammatory effect (44–46). This would explain why the subdermal cells, which are predominantly inflammatory cells, stain positively for AT1 whereas AT2 and Mas staining is significantly reduced. Since angiogenesis at the site of injury plays an important role in the wound healing process the number of blood vessels were quantitated. No observable difference was seen between the groups. However, in this respect our study has temporal constraints and it is possible that with a longer study duration that we might see some effects on angiogenesis.
Several studies have shown that progenitor cells from the bone marrow are mobilized to the site of dermal injury and participate in the healing process (47). Radiation has been shown to affect mesenchymal stem cells (MSC’s) by reducing their proliferative and differentiation capacities (48) as well as endothelial progenitor cells (EPC’s) by diminishing their functional capabilities resulting in vascular damage (49, 50). Since progenitor cells are known to enhance the wound healing process both MSC’s and EPC’s have been used successfully to treat chronic wound injuries (51, 52). Moreover, CRBI is also known to severely suppress the bone marrow, which may contribute to the delay in healing. We have previously shown that irradiated mice treated with A-II and A(1–7) accelerated hematopoietic recovery, increased number of hematopoietic progenitors in bone marrow, increased white blood cell count in the peripheral circulation. Further, these two peptides were shown to accelerate wound healing in both normal and delayed wound-healing models (18, 25, 53, 54). Changes in the bone marrow mediated by these peptides might affect wound healing at a number of levels
The data presented above are consistent with our initial hypothesis that the delay and blunting of RAS receptor expression in the combined injury group may in part be responsible for the decreased and delayed wound healing following CRBI. Ongoing work in our lab is evaluating the treatment of dermal wounds in animals exposed to CRBI with angiotensin peptides to further therapeutic options.
CONCLUSION
CRBI is a complex injury and mechanisms by which it damages cells are poorly understood. Our findings show that reduced expression of renin and RAS receptors as a result of CRBI might be one of the possible explanations for delayed wound healing observed in this type of injury. These findings open up new strategies as how to mitigate CRBI-induced effects.
ACKNOWLEDGMENT
This study was funded by the National Institutes of Health (RC1AI080976). Rachel Livingston is thanked for her help with the necropsy. Matthew De Los Santos and Stephanie Camano are thanked for their help with immunohistochemistry.
Footnotes
Conflict of interest: The authors have no conflicts of interest to disclose
REFERENCES
- 1.Ledney GD, Elliott TB, MM M. Modulations of mortality by tissue trauma and sepsis in mice after radiation injury. In: Mossman KL, Mills WA, editors. The biological basis of radiation protection practices. Baltimore: Williams & Wilkins; 1992. pp. 202–217. [Google Scholar]
- 2.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59(3):251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 3.Dinh DT, Frauman AG, Johnston CI, Fabiani ME. Angiotensin receptors: distribution, signalling and function. Clin Sci. 2001;100(5):481–492. [PubMed] [Google Scholar]
- 4.Takeda H, Katagata Y, Hozumi Y, Kondo S. Effects of Angiotensin II receptor signaling during skin wound healing. Am J Pathol. 2004;165(5):1653–1662. doi: 10.1016/S0002-9440(10)63422-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ray PE, Bruggeman LA, Horikoshi S, Aguilera G, Klotman PE. Angiotensin II stimulates human fetal mesangial cell proliferation and fibronectin biosynthesis by binding to AT1 receptors. Kidney Int. 1994;45(1):177–184. doi: 10.1038/ki.1994.21. [DOI] [PubMed] [Google Scholar]
- 6.Laflamme L, de Gasparo M, Gallo J-M, Payet MD, Gallo-Payet N. Angiotensin II induction of neurite outgrowth by AT2 receptors in NG108-15 Cells. J Biol Chem. 1996;271(37):22729–22735. doi: 10.1074/jbc.271.37.22729. [DOI] [PubMed] [Google Scholar]
- 7.Meffert S, Stoll M, Steckelings UM, Bottari SP, Unger T. The angiotensin II AT2 receptor inhibits proliferation and promotes differentiation in PC12W cells. Mol Cell Endocrinol. 1996;122(1):59–67. doi: 10.1016/0303-7207(96)03873-7. [DOI] [PubMed] [Google Scholar]
- 8.Steckelings UM, Henz BM, Wiehstutz S, Unger T, Artuc M. Differential expression of angiotensin receptors in human cutaneous wound healing. Br J Dermatol. 2005;153(5):887–893. doi: 10.1111/j.1365-2133.2005.06806.x. [DOI] [PubMed] [Google Scholar]
- 9.Yahata Y, Shirakata Y, Tokumaru S, et al. A Novel Function of Angiotensin II in Skin Wound Healing. J Biol Chem. 2006;281(19):13209–13216. doi: 10.1074/jbc.M509771200. [DOI] [PubMed] [Google Scholar]
- 10.Abiko M, Rodgers KE, Campeau JD, Nakamura RM, Dizerega GS. Alterations of angiotensin II Receptor levels in sutured wounds in rat skin. J Invest Surg. 1996;9(6):447–453. doi: 10.3109/08941939609025862. [DOI] [PubMed] [Google Scholar]
- 11.Abiko M, Rodgers KE, Campeau JD, Nakamura RM, Dizerega GS. Alterations of angiotensin II receptor levels in full-thickness excisional wounds in rat skin. Wound Repair Regen. 1996;4(3):363–367. doi: 10.1046/j.1524-475X.1996.40313.x. [DOI] [PubMed] [Google Scholar]
- 12.Eto H, Biro S, Miyata M, et al. Angiotensin II type 1 receptor participates in extracellular matrix production in the late stage of remodeling after vascular injury. Cardiovasc Res. 2003;59:200–211. doi: 10.1016/s0008-6363(03)00356-0. [DOI] [PubMed] [Google Scholar]
- 13.Kurosaka M, Suzuki T, Hosono K, et al. Reduced angiogenesis and delay in wound healing in angiotensin II type 1a receptor-deficient mice. Biomed Pharmacother. 2009;63(9):627–634. doi: 10.1016/j.biopha.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Lima AM, Xavier CH, Santos RAS, Fontes MAP. Central administration of angiotensin-(1-7) markedly reduces the tachycardia evoked by acute psychological stress exposure. FASEB J. 2009;23(1_MeetingAbstracts):609–615. [Google Scholar]
- 15.Lambert DW, Hooper NM, Turner AJ. Angiotensin-converting enzyme 2 and new insights into the renin-angiotensin system. Biochem Pharmacol. 2008;75(4):781–786. doi: 10.1016/j.bcp.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodgers KE, Roda N, Felix JC, Espinoza T, Maldonado S, diZerega G. Histological evaluation of the effects of angiotensin peptides on wound repair in diabetic mice. Exp Dermatol. 2003;12(6):784–790. doi: 10.1111/j.0906-6705.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 17.Rodgers K, Xiong S, Felix J, et al. Development of angiotensin (1-7) as an agent to accelerate dermal repair. Wound Repair Regen. 2001;9(3):238–247. doi: 10.1046/j.1524-475x.2001.00238.x. [DOI] [PubMed] [Google Scholar]
- 18.Rodgers KE, Xiong S, diZerega GS. Accelerated recovery from irradiation injury by angiotensin peptides. Cancer Chemother Pharmacol. 2002;49(5):403–411. doi: 10.1007/s00280-002-0434-6. [DOI] [PubMed] [Google Scholar]
- 19.Santos RAS, e Silva ACS, Maric C, et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(14):8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heitsch H, Brovkovych S, Malinski T, Wiemer G. Angiotensin-(1-7)-Stimulated Nitric Oxide and Superoxide Release From Endothelial Cells. Hypertension. 2001;37(1):72–76. doi: 10.1161/01.hyp.37.1.72. [DOI] [PubMed] [Google Scholar]
- 21.Kostenis E, Milligan G, Christopoulos A, et al. G-Protein�coupled receptor mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation. 2005;111(14):1806–1813. doi: 10.1161/01.CIR.0000160867.23556.7D. [DOI] [PubMed] [Google Scholar]
- 22.Lopez Verrilli MA, Pirola CJ, Pascual MM, Dominici FP, Turyn D, Gironacci MM. Angiotensin-(1-7) through AT2 receptors mediates tyrosine hydroxylase degradation via the ubiquitin�proteasome pathway. J Neurochem. 2009;109(2):326–335. doi: 10.1111/j.1471-4159.2009.05912.x. [DOI] [PubMed] [Google Scholar]
- 23.Rodgers KE, Espinoza T, Felix J, Roda N, Maldonado S, diZerega G. Acceleration of healing, reduction of fibrotic scar, and normalization of tissue architecture by an angiotensin analogue, NorLeu3-A(1-7) Plast Reconstr Surg. 2003;111(3):1195–1206. doi: 10.1097/01.PRS.0000047403.23105.66. [DOI] [PubMed] [Google Scholar]
- 24.Strawn WB, Richmond RS, Tallant EA, Gallagher PE, Ferrario CM. Renin-angiotensin system expression in rat bone marrow haematopoietic and stromal cells. Br J Haematol. 2004;126(1):120–126. doi: 10.1111/j.1365-2141.2004.04998.x. [DOI] [PubMed] [Google Scholar]
- 25.Rodgers KE, Roda N, Felix JE, Espinoza T, Maldonado S, diZerega G. Histological evaluation of the effects of angiotensin peptides on wound repair in diabetic mice. Exp Dermatol. 2003;12(6):784–790. doi: 10.1111/j.0906-6705.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 26.Vegesna V, Withers HR, Holly FE, McBride WH. The effect of local and systemic irradiation on impairment of wound healing in mice. Radiat Res. 1993;135(3):431–433. [PubMed] [Google Scholar]
- 27.Goessler UR, Bugert P, Kassner S, et al. In vitro analysis of radiation-induced dermal wounds. Otolaryngology - Head Neck Surg. 2010;142(6):845–850. doi: 10.1016/j.otohns.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 28.M�ller K, Meineke V. Radiation-induced alterations in cytokine production by skin cells. Exp Hematol. 2007;35(4):96–104. doi: 10.1016/j.exphem.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 29.Riedel F, Philipp K, Sadick H, Goessler U, Hörmann K, Verse T. Immunohistochemical analysis of radiation-induced non-healing dermal wounds of the head and neck. In Vivo. 2005;19(2):343–350. [PubMed] [Google Scholar]
- 30.Kinoshita N, Tsuda M, Hamuy R, et al. The usefulness of basic fibroblast growth factor for radiation-exposed tissue. Wound Repair Regen. 2012;20(1):91–102. doi: 10.1111/j.1524-475X.2011.00758.x. [DOI] [PubMed] [Google Scholar]
- 31.Steckelings UM, Wollschläger T, Peters J, Henz BM, Hermes B, Artuc M. Human skin: source of and target organ for angiotensin II. Exp Dermatol. 2004;13(3):148–154. doi: 10.1111/j.0906-6705.2004.0139.x. [DOI] [PubMed] [Google Scholar]
- 32.Wu HJ, Liu HW, Cheng B, et al. The change of angiotensin II production and its receptor expression during wound healing: possible role of angiotensin II in wound healing. Zhonghua Zheng Xing Wai Ke Za Zhi. 2011;27(2):124–128. [PubMed] [Google Scholar]
- 33.Alderman MH. Renin angiotensin system and the heart. Circulation. 2004;110(19):e496–e497. [Google Scholar]
- 34.Werner S, Krieg T, Smola H. Keratinocyte-Fibroblast Interactions in Wound Healing. J Invest Dermatol. 2007;127(5):998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- 35.Lijnen PJ, Petrov VV, Fagard RH. Angiotensin II-induced stimulation of collagen secretion and production in cardiac fibroblasts is mediated via angiotensin II subtype 1 receptors. J Renin-Angiotensin-Aldosterone Syst. 2001;2(2):117–122. doi: 10.3317/jraas.2001.012. [DOI] [PubMed] [Google Scholar]
- 36.Iwami K, Ashizawa N, Do YS, Graf K, Hsueh WA. Comparison of ANG II with other growth factors on Egr-1 and matrix gene expression in cardiac fibroblasts. Am J Physiol. 1996;270(6):H2100–H2107. doi: 10.1152/ajpheart.1996.270.6.H2100. [DOI] [PubMed] [Google Scholar]
- 37.Anderson PW, Zhang XY, Tian J, et al. Insulin and angiotensin II are additive in stimulating TGF-[beta]1 and matrix mRNAs in mesangial cells. Kidney Int. 1996;50(3):745–753. doi: 10.1038/ki.1996.372. [DOI] [PubMed] [Google Scholar]
- 38.Gibbons GH, Pratt RE, Dzau VJ. Vascular smooth muscle cell hypertrophy vs. hyperplasia. Autocrine transforming growth factor-beta 1 expression determines growth response to angiotensin II. J Clin Invest. 1992;90(2):456–461. doi: 10.1172/JCI115881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naftilan AJ, Pratt RE, Dzau VJ. Induction of platelet-derived growth factor A-chain and c-myc gene expressions by angiotensin II in cultured rat vascular smooth muscle cells. J Clin Invest. 1989;83(4):1419–1424. doi: 10.1172/JCI114032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakahara K-i, Nishimura H, Kuro-o M, et al. Identification of three types of PDGF-A chain gene transcripts in rabbit vascular smooth muscle and their regulated expression during development and by angiotensin II. Biochem Biophys Res Communications. 1992;184(2):811–818. doi: 10.1016/0006-291x(92)90662-5. [DOI] [PubMed] [Google Scholar]
- 41.Ito M, Cotsarelis G. Is the hair follicle necessary for normal wound healing? J Invest Dermatol. 2008;128(5):1059–1061. doi: 10.1038/jid.2008.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 2007;21(7):1358–1366. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35(6):881–900. doi: 10.1016/s1357-2725(02)00271-6. [DOI] [PubMed] [Google Scholar]
- 44.Rompe F, Artuc M, Hallberg A, et al. Direct angiotensin II type 2 receptor stimulation acts anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor κB. Hypertension. 2010;55(4):924–931. doi: 10.1161/HYPERTENSIONAHA.109.147843. [DOI] [PubMed] [Google Scholar]
- 45.da Silveira KD, Coelho FM, Vieira AT, et al. Anti-Inflammatory effects of the activation of the angiotensin-(1-7) receptor, Mas, in Experimental Models of Arthritis. J Immunol. 2010;185(9):5569–5576. doi: 10.4049/jimmunol.1000314. [DOI] [PubMed] [Google Scholar]
- 46.Abadir PM, Walston JD, Carey RM, Siragy HM. Angiotensin II Type-2 Receptors Modulate Inflammation Through Signal Transducer and Activator of Transcription Proteins 3 Phosphorylation and TNFα Production. J Interferon Cytokine Res. 2011;31(6):471–474. doi: 10.1089/jir.2010.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cha J, Falanga V. Stem cells in cutaneous wound healing. Clin Dermatol. 2007;25(1):73–78. doi: 10.1016/j.clindermatol.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 48.Schönmeyr BH, Wong AK, Soares M, Fernandez J, Clavin N, Mehrara BJ. Ionizing radiation of mesenchymal stem cells results in diminution of the precursor pool and limits potential for multilineage differentiation. Plast Reconstr Surg. 2008;122(1):64–76. doi: 10.1097/PRS.0b013e31817743cd. [DOI] [PubMed] [Google Scholar]
- 49.Mao XW. A quantitative study of the effects of ionizing radiation on endothelial cells and capillary-like network formation. Technol Cancer Res Treat. 2006;5(2):127–134. doi: 10.1177/153303460600500206. [DOI] [PubMed] [Google Scholar]
- 50.Lee M-O, Song S-H, Jung S, et al. Effect of Ionizing radiation induced damage of endothelial progenitor cells in vascular regeneration. Arterioscler Thromb Vasc Biol. 2012;32(2):343–352. doi: 10.1161/ATVBAHA.111.237651. [DOI] [PubMed] [Google Scholar]
- 51.Hu KX, Sun QY, Guo M, Ai HS. The radiation protection and therapy effects of mesenchymal stem cells in mice with acute radiation injury. Br J Radiol. 2010;83(985):52–58. doi: 10.1259/bjr/61042310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Asai J, Takenaka H, Ii M, et al. Topical application of ex vivo expanded endothelial progenitor cells promotes vascularisation and wound healing in diabetic mice. Int Wound J. 2012 doi: 10.1111/j.1742-481X.2012.01010.x. no-no. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodgers KE, Xiong S, Steer R, diZerega GS. Effect of angiotensin II on hematopoietic progenitor cell proliferation. Stem Cells. 2000;18(4):287–294. doi: 10.1634/stemcells.18-4-287. [DOI] [PubMed] [Google Scholar]
- 54.Rodgers K, Abiko M, Girgis W, St. Amand K, Campeau J, Dizerega G. Acceleration of dermal tissue repair by angiotensin II. Wound Repair Regen. 1997;5(2):175–183. doi: 10.1046/j.1524-475X.1997.50210.x. [DOI] [PubMed] [Google Scholar]