Abstract
XK is a putative transporter of unknown function that is ubiquitously expressed and linked through disulfide bonds to Kell protein, an endothelin-3 (ET-3)-converting enzyme. We generated three knockout (KO) mice that lacked either Xk, Kell or both proteins and characterized erythrocyte cation levels, transport and hematological parameters. Absence of Xk or Kell was accompanied by changes in erythrocyte K+, Mg2+, Na+ and Ca2+ transport that were associated with changes in mean cellular volume and corpuscular hemoglobin concentration mean. Baseline Ca2+-ATPase activity was undetected in erythrocytes from all three mouse types but was restored upon pre-incubation with ET-3. Consistent with these alterations in Ca2+ handling, we observed increased Gardos channel activity in Kel and Xk KO mice. In addition Kel deletion was associated with increased Mg2+ permeability while Xk deletion blocked Na/Mg exchanger activity. Our results provide evidence that cellular divalent cation regulation is functionally coupled to the Kell/XK system in erythrocytes and loss of this complex may contribute to acanthocytosis formation in McLeod syndrome.
Keywords: Kell/Xk complex, Ca2+ ATPase, magnesium, erythrocytes
INTRODUCTION
Human XK, a 50.9 kDa protein, is present as a heterodimer covalently linked through a disulfide bond to the Kell glycoprotein that function as an endothelin-3-converting enzyme (ECE-3) on the erythrocyte cell surface [1]. XK gene (XK) is located on chromosome Xp21.1 [2; 3]. Mouse Xk protein has 82% amino acid similarity with human XK and is also linked to the mouse Kell protein. The mouse Xk gene is organized into three exons with differences in expression pattern when compared to the human gene [4; 5]. The transport substrate for XK protein is still unknown. However, the translated human XK sequence predicts a 444 amino acids protein that has similarities to a membrane transporter with 10 hydrophobic regions and no consensus sequence for N-glycosylation but with amphipathicity in the transmembrane segments that could facilitate pore formation [3]. Of interest, a similar topography has been predicted for the glutamate, dopamine and noradrenaline transporters further supporting the contention that XK protein may function as a membrane transporter protein in erythrocytes.
Human Kell is a highly polymorphic, type II glycoprotein [3; 4; 6] that is mainly expressed in erythroid tissue but is also observed in human non-erythroid tissues including skeletal muscle, heart and brain [1; 7; 8; 9]. Mouse Kell has 74% amino acid similarity with human Kell and has ECE-3 activity [10]. Mouse Kell gene (Kel) is located on chromosome 6 and is organized into 19 exons. It contains 8 N-linked sugars, rather than 5 when compared to the human Kell glycoprotein, and one additional extracellular cysteine and a shorter intracellular N-terminal [10]. Kell glycoprotein is a member of the M13 family of zinc endopeptidases that are involved in regulation of bioactive peptides. However, no overt abnormalities have been documented in Kell null mice [11]. There are reports showing that Kell's endothelin converting enzyme activity has preference for big ET-3 unlike ECE-1 or ECE-2 [1; 12]. Furthermore, it has been shown that a phenotype similar to endothelin receptor B (ETB) or ET-3 deficient mice, is present in ECE-1 deficient mice demonstrating that big ET-3, as well as ET-1, is an important substrate for ECE-1 [12]. In erythrocytes, the function of ET-3 is not clear. However, we have reported that ET-3 activates the Gardos channel (Ca2+-activated K+ channel) via activation of ETB receptors in mouse and human erythrocytes suggesting a potential role in cellular volume regulation [11; 13].
In this report, we investigated the role of the Kell/Xk complex in cellular K+, Na+ Ca2+ and Mg2+ homeostasis and the role of Xk protein in the transport of K+ and Ca2+ in ex vivo erythrocytes from mice that lacked Kel, Xk or Kel/Xk genes. We now provide evidence that the Kell/Xk complex functions as a regulator of erythrocyte volume and suggest a potential role for Xk protein in Ca2+ transport. This information brings new insights into the function of Xk in divalent cation homeostasis and allows the identification of novel mechanisms for erythrocyte volume regulation.
MATERIAL and METHODS
Generation and characterization of Xk-knockout and Kel/Xk-KO mice
Animals received water and chow ad libitum and were housed according to the guidelines and approval from the Institutional Animal Care and Use Committee of Children's Hospital Boston and Harvard Medical School. Generation and characterization of Xk-KO and the double knockout mice, Kel/Xk-KO is described in the online supplementary section. Kel-KO (Kel knockout) has been described previously [11]. Male mice of 4 to 6 months old with genotypes of Xk-KO, Kel-KO, double Xk/Kel-KO, and their wild-type (WT) counterpart were used. All mice were backcrossed into C57BL6, (Charles River Laboratories, Cambridge, MA). The mice received an intraperitoneal injection of pentobarbital to anesthetize and collect blood from cardiac puncture and tissue collection according to the guidelines and approval from the Institutional Animal Care and Use Committee of Children's Hospital Boston and Harvard Medical School.
Drugs and chemicals
Charybdotoxin (ChTX) and ET-3 were purchased from RBI Signal Innovation (Natick, MA). All peptides were prepared as indicated by the manufacturer and stored at −20°C for less than 3 months. A23187 ionophore was purchased from Calbiochem-Novabiochem Corp. (La Jolla, CA). 86Rb was purchased from Du Pont-New England Nuclear. All other reagents were purchased from Sigma Chemical Co. (St. Louis, MO).
Hematological parameters
Blood was collected in the presence of Na-EDTA from penthobarbital-anesthetized animals. Whole blood was used within one hour of blood draw to perform erythrocyte and reticulocyte counts for each sample using an ADVIA 120 hematology analyzer and a software program specific for mouse blood (Bayer Diagnostics, Tarrytown, NY) as previously described [14]. Whole blood was passed through cotton to decrease the number of leukocytes and then centrifuged at 1812 xg for 4 min and 4°C. Erythrocytes were washed 4 times with choline washing solution containing: 172 mM Choline Chloride, 1 mM MgCl2 and 10 mM Tris-MOPS pH 7.4 at 4°C and kept on ice until used.
Erythrocyte cation content
Total erythrocyte Na+, K+, Mg2+ and Ca2+ content were determined in freshly isolated mouse erythrocytes by atomic absorption spectrophotometry as previously described [15]. Briefly, following five washes in choline chloride washing solution (CWS: 172 mM Choline Chloride, 10 mM Tris-MOPS pH 7.4 at 4°C, 1 mM MgCl2) an aliquot of 50% cell suspension in washing solution was used to determine manual hematocrit, cell Na+ and Mg2+ (1:50 dilution in 0.02% acationox), cell Ca2+ (1:25 dilution in 0.02% acationox) and cell K+ (1:500 in double-distilled water).
Gardos Channel activity
Measurement of Gardos Channel activity was performed as described [15]. Briefly, freshly washed erythrocytes were suspended at a hematocrit of 2% in normal influx media containing 10 μCi /ml 86Rb in the presence or absence of 50 nM ChTX. Pre-incubations with ET-3 were carried out for 20 min at 37°C in an isotonic saline. The same concentrations of active peptides or drugs were also added to the influx media. The erythrocyte-associated radioactivity was counted in a gamma counter (model 41600 HE, Isomedic ICN Biomedicals Inc. Costa Mesa, CA). K+ uptake was linear up to 5 min and fluxes were calculated from the slope of the linear regression as previously described [13].
Na/Mg exchanger activity
Erythrocytes were loaded with Mg2+ at 10% hematocrit for 30 min at 37°C in the presence of 6 μM A23187. The Mg2+ loading solution contained: 128 mM KCl, 12 mM MgCl2, 10 mM Glucose, and 10 mM Tris MOPS pH 7.4 (37°C). The ionophore was removed by four consecutive washes using the same loading solution with 0.1% BSA at 37°C. The cells were further washed 4 times with Mg2+-free CWS at 4°C to remove extracellular Mg2+. A 50% cell suspension was made in Mg2+-free CWS. Cellular Na+, K+, Ca2+, and Mg2+ content were determined as described above. Flux started when 0.4 ml of 50% cell suspension was added to either 8 ml of flux media containing: 170 mM NaCl or 170 mM Glucamine Cl, 10 mM Glucose; 10 mM Tris MOPS pH 7.4 (37°C); 1 mM ouabain and 0.01 mM bumetanide. Ouabain was used to inhibit Na+ transport mediated by the pump and bumetanide was used to inhibit Na/K/2Cl co-transport activity. Quinidine was also used to evaluate the activity of the Na/Mg exchanger. Triplicate samples were taken at 5 and 45 min and centrifuged at 4°C. Supernatants were carefully removed and quickly transferred to 4-ml plastic tubes. Total Mg2+ concentration of the supernatants was determined by atomic absorption. Mg2+ efflux was calculated from the slope of the linear regression of total Mg2+ supernatant content versus time and expressed as mmol/L cell × h. The Na/Mg exchanger was calculated as the difference between Mg2+ efflux in the NaCl and GlucamineCl flux media, and values were corrected for mean cellular volume (MCV) and expressed as mmol/1013 cell × h.
Na transport
Erythrocytes were loaded with equal amount of Na+ and K+ and fluxes were measured as described [16]. Briefly, erythrocytes were loaded with equal amount of Na+ and K+ using a nystatin (40 mg/mL) solution: 77 mM NaCl, 77 mM KCl, and 55 mM sucrose. Na/K pump activity was estimated as the ouabain-sensitive fraction (1 mM ouabain) of Na+ efflux into a medium containing 155 mM choline chloride and 10 mM KCl. The Na-K-2Cl cotransport (NKCC) was estimated as bumetanide-sensitive Na+ and K+ efflux into medium containing 174 mM choline chloride and 1 mM ouabain with or without 10 μM bumetanide. Na/H exchange (NHE) activity was estimated as hydroxylmethyl amiloride-sensitive (HMA) Na+ efflux by hypertonic stimulation in a solution containing: 175 mM choline chloride, 1 mM MgCl2, 10 mM glucose, 1 mM ouabain, 0.01 mM bumetanide, 10 mM Tris-MOPS (pH 7.4 at 37°C).
Ca2+ ATPase activity
Enzymatic activities were measured in erythrocyte membranes essentially as described [17]. Freshly isolated erythrocytes were lysed with 10 volumes of hypotonic buffer contained 10 mM Tris-MOPS, pH 7.4, 0.1% β-mercaptoethanol, 10 μM phenylmethylsulfonyl fluoride, and 25 μg/ml each of leupeptin and aprotinin at 4°C. Intact cells were removed by pelleting at low speed (500 g for 3 min). The supernatant was then centrifuged at 30,000 g to separate the membranes from the cytosol. The supernatant was discarded and the membrane pellet was washed four times by centrifugation and resuspension in hypotonic lysis buffer [17; 18]. The membranes were stored at −80°C in aliquots of 50 μL (3mg/mL) until ready to use. The Ca2+-ATPase activity was measured in a buffer containing: 130 mM KCl, 20 mM K-phosphate buffer pH 7.2 at 37°C and 5 mM MgCl2. A master mix cocktail was prepared using 5 μL of 0.5 mM phosphoenolpyruvate, 5 μL of 0.2 mM NADH, 7μl pyruvate kinase (2IU), 5.6 μL lactate dehydrogenase (2IU), 5 μL of 5 mM A23187 in the presence or absence of 5 μL of 15 μM CaCl2 and/or 5 μL 5 μM calmodulin: and 127.4–137.4 μL of assay buffer. The membrane protein content was determined by the BCA method according to manufacturer's instructions (Pierce). An aliquot of 175 μL of master mix was added to 15 μl of membrane preparation and an initial absorbance was taken at 340nM. Then, 5 μl of ATP (7mM) was added to all the conditions at 0 time. A record was taken at 5-10-15-25 min at 37°C. The effect of ETB receptor on the Ca2+-ATPase was determined in the presence or absence of ET-3 in the ATPase assay. The ATPase activity was calculated as μmol (mg protein × min)−1 from the change in absorbance per min.
Statistical analysis
All values are presented as mean ± SD or SE. One-way analysis of variance (ANOVA) was used to detect significant difference between the mouse groups followed by Tukey's HSD analysis.
Results
Disruption of Kell/Xk complex alters mouse erythrocyte parameters
We characterized erythrocytes obtained from Kel-KO, Xk-KO, and Kel/Xk-KO mice. Using these animal models, we first studied the effects of Kell, Xk and Kell/Xk absence on hematological parameters (Table 1). We found that mean cellular volume (MCV) was significantly increased in the erythrocytes from Kel-KO mice, which was accompanied by a slight decrease in corpuscular hemoglobin concentration mean (CHCM), increased reticulocytes (2.17 to 3.15%, n=6, p<0.03) and hemoglobin distribution width (HDW). No other hematological parameters were altered in these animals. In Xk-KO, we observed an increase in CHCM when compared to the wild-type (WT) that was not accompanied by any significant effect on reticulocyte levels or red cell distribution width (RDW). In the double knockout mouse, a further increase in cellular volume was accompanied by a slight decrease in hemoglobin concentration (CHCM). These changes were associated with a modest increase in reticulocytes level (2.71 to 3.6 %, p<0.03).
Table 1.
Hematological parameters in blood from Kel-KO, Xk-KO and wild-type mice
| WT | Kel-KO | Xk-KO | Kel/Xk-KO | |
|---|---|---|---|---|
|
|
||||
| MCV, fL | 46.6±0.3 | 48.3±0.1* | 46.4±0.6 | 49.8±0.7* |
| CHCM, g/dL | 28.9±0.3 | 26.8±0.6* | 29.2±0.2* | 27.7±0.1* |
| Reticulocytes, % | 2.71±0.2 | 3.15±0.1* | 2.9±0.5 | 3.6±0.2* |
| RDW, % | 13.0±0.4 | 13.4±0.2 | 13.0±0.5 | 12.8±0.8 |
| HDW, g/dL | 1.99±0.1 | 2.8±0.1* | 2.08±0.0 | 1.82±0.0 |
Determination of hematological parameters was performed using ADVIA (Bayer, NJ) with mouse software.
MCV, mean cellular volume; CHCM, corpuscular hemoglobin concentration mean; RDW, red cell distribution width; HDW, hemoglobin distribution width. WT, wild-type n=10; Kel-KO, Kel knockout n=10; Xk-KO, Xk knockout n=10; Kel/Xk-KO, double knockout n=5,
p<0.03 compared to WT, ANOVA analysis. Values represent the mean±SD.
To test whether Kel/Xk complex can modulate ion transport activity that may contribute to changes in hematological parameters and cellular volume, we evaluated the total cation content in the three different mouse models and compared them to WT mice (Table 2). We found that cellular levels of K+, the major intracellular cation, were significantly reduced only in the erythrocytes of Kel-KO mice but not in Xk-KO and Kel/Xk mice. A small but significant increase in Na+ was observed in the Kel/Xk-KO. We also observed that Ca2+ content was 2.7 fold higher in erythrocytes from Xk-KO and 3.2 fold increased in cells from Kel/Xk-KO mice when compared to WT mice. However, the absence of Kel did not significantly affect Ca2+ levels. These data suggest that the Xk protein complex plays an important role in cellular Ca2+ homeostasis in mouse erythrocytes. Furthermore, Mg2+ levels measured as total ion content by atomic absorption spectroscopy showed increased Mg2+ levels in the Xk-KO and decreased Mg2+ levels in Kel-KO erythrocytes. Together these results implicate the Kell/Xk complex as an important regulator of cellular cation content in erythrocytes and suggest its potential role in erythrocyte volume regulation.
Table 2.
Cation content in erythrocytes from Kel-KO, Xk-KO and wild-type mice
| WT | Kel-KO | Xk-KO | Kel/Xk-KO | |
|---|---|---|---|---|
|
|
||||
| Na+, mmol/Kg Hb | 21.3±0.7 | 21.0±0.5 | 20.7±0.9 | 22.5±1.3* |
| K+, mmol/Kg Hb | 443.2±39 | 410.8±40* | 444.9±35 | 442.2±44 |
| Mg2+, mmol/Kg Hb | 8.81±0.7 | 7.28±0.8** | 9.32±0.3# | 8.6±1.0β |
| Ca2+, mmol/Kg Hb | 0.14±0.0 | 0.12±0.0 | 0.39±0.2* | 0.45±0.2**, β |
Total ion content was determined by atomic absorption spectrophotometry (Perkin Elmer) using appropriate standard for ion calibration.
WT, wild-type n=10; Kel-KO, Kel knockout n=10; Xk-KO, Xk knockout n=10; Kel/Xk-KO, double knockout,
p<0.04,
p<0.004,
p<0.05,
when compared to wild-type, β p<0.01, when compared to Xk-KO, n=6, One-way ANOVA test followed by Tukey analysis. Values represent the mean±SD.
Disordered K+ transport in Xk-KO and Kel/Xk KO mouse erythrocytes
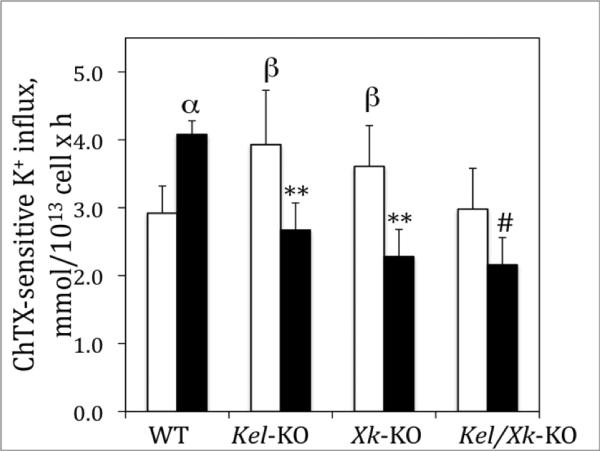
We previously described an increase in Gardos channel activity in Kel-KO [11]. We studied Gardos channel activity in our Xk KO and Kel/Xk KO mice. We observed that the activity of the channel was also elevated in Xk-KO erythrocytes when compared to WT but not in the double KO mice (Figure 1). These results suggest that absence of Xk or Kel, independently, can lead to disordered Gardos channel activity that in turn may lead to alterations in erythrocyte volume. Measurements of the K+ transport mediated by the Na/K pump indicated a slight decrease (20.5 ±0.7 to 17.3±0.5 mmol/L 1013 cell × h, n=4, p<0.02) in its activity in Xk-KO and double knockout but not in the Kel-KO. Furthermore, K+ transport via NKCC cotransport was also slightly elevated in Xk-KO when compared to WT (29.55±0.1 to 27.7±0.3 mmol/1013 cell × h, n=4, p<0.03) but not in Kel-KO or double knockout. When Na+ transport was evaluated, we found that absence of Xk leads to a small increase in NHE activity (23.9±0.7 to 25.9±0.6 mmol/1013 cell × h, n= 4, p<0.03 compared to wild-type, one-way ANOVA test followed by Tukey analysis) that is not observed in the double KO mice suggesting that Kell/Xk complex does not play a significant effect on Na+ homeostasis.
Figure 1. Effects of ET-3 on Ca+2-activated K+ influx (Gardos Channel) in Kel and Xk-KO mouse erythrocytes.
Gardos channel was measured as detailed in Material and Methods section in the presence (▪) or absence of 700 nM ET-3 (□). The bars represent the mean ± SE of three experiments in duplicate determinations. Comparison between baseline and ET-3; α p<0.037; **p<0.011; # p<0.035; WT vs Kel-KO or Xk-KO; β p<0.034).
In circulation, erythrocyte Kell might mediate ET-3 activation, which could then bind to ETB receptors and exert its actions [13; 15]. We and other have shown that ET-3 mediates its effects on Gardos channel via activation of the ETB receptor [13]. We show that K+ transport mediated by the Gardos channel is enhanced by pre-incubation with ET-3 in erythrocytes from WT as previously described [15]. However, deletion of either Kel or Xk gene or both significantly blunted this effect (Figure 1), suggesting that the Kell/Xk protein complex plays a role in the ET-3/ETB receptor mediated regulation of the Gardos channel.
Ca2+-ATPase is blunted in Kel and Xk-gene ablated erythrocytes and rescued by ET-3
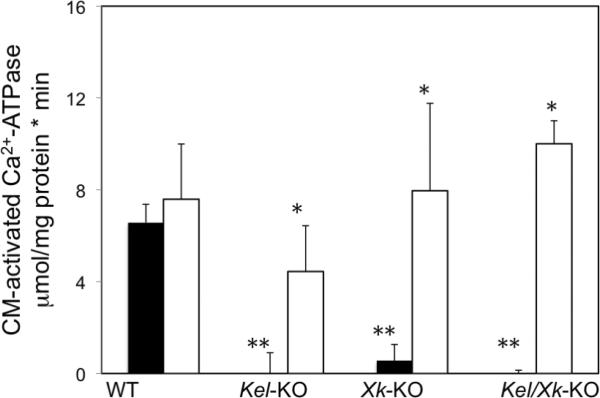
The Ca2+-ATPase in plasma membranes is responsible for calcium extrusion out of the cell and is one of the key systems involved in the control of total and free cytosolic Ca2+ levels in erythrocytes and consequently the Gardos channel. We tested whether the effects of Kel and Xk gene deletion on total calcium content could be responsible for the altered Gardos channel activity. We measured the activity of the calmodulin-dependent Ca2+ ATPase. (Figure 2). We observed that Ca2+ ATPase virtually undetectable in Kel/Xk-KO and that the presence of Kell or Xk independently did not restore Ca2+ ATPase activity. These results are in agreement with our findings of disordered total calcium content observed in these mouse KO erythrocytes. We then investigated the effects of ET-3 on Ca2+ ATPase activity (Figure 2). We observed that ET-3 re-established enzyme activity in Kel-KO erythrocytes but to levels still lower than observed in cells from WT mice. In Xk-KO erythrocytes, the effects of ET-3 were virtually identical to the WT suggesting that ET-3 plays an important role in the regulation of Ca2+ ATPase. Altogether, these results show that Kell/Xk complex regulates Ca2+-ATPase activity and ET-3 may override this effect possibly via activation of ETB receptor signaling. Alternatively, it might suggest that the Kell/Xk complex in mouse erythrocytes might interfere with calmodulin activation of the Ca2+-ATPase. Further studies are under investigation.
Figure 2. Calmodulin-activated Ca2+ ATPase in mouse erythrocytes.
Ca2+ATPase was measured as described in Material and Methods section in the presence (□) or absence (▪) of 700 nM ET-3. CM = calmodulin. The values are the mean±SD of two experiments in triplicate determinations. WT vs Kel-KO or Xk-KO or double KO, ** p<0.001; Comparison in the presence or absence of ET-3, * p<0.04,
Xk deletion blunts Mg2+ transport in mouse erythrocytes
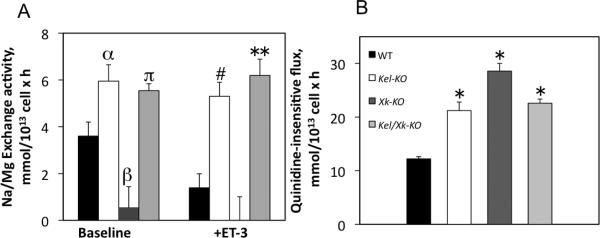
NME is a critical modulator of intracellular Mg2+ and therefore important regulator of cation transport and volume regulatory mechanisms in erythrocytes [19]. We studied the activity of NME in these KO mice. We found that NME activity was significantly reduced by the absence of Xk and increased in Kel-KO compared to WT mice (Figure 3A). However, in Kel/Xk-KO, we found that NME activity resembled Kel-KO NME activity suggesting that Kell may act as an NME regulatory unit. This data is consistent with both reduced Mg2+ content observed in Kel-KO erythrocytes and increased Mg2+ content observed in Xk-KO erythrocytes (Table 2). We also evaluated Na+-independent Mg2+ fluxes in these mice. We found that the absence of the Kell/Xk complex induces a significant increase in the permeability of Mg2+ (Figure 3B) that was resistant to ion transport inhibitors such as ouabain, bumetanide, and quinidine. This effect was significantly greater in Kel-KO than in Xk-KO strongly supporting the contention that the complex might play a novel role in the regulation of Mg2+ permeability. As the Xk transport protein substrate is still unknown, we hypothesized that Kell protein regulates Mg2+ transport via Xk protein. No significant differences were observed in ouabain, bumetanide and quinide resistant Na+ or K+ permeability (data not shown).
Figure 3. Effects of Kell/Xk deletion gene on Na/Mg exchange and quinidine-insensitive Mg2+ fluxes in erythrocytes.
A) NME, Na-dependent Mg2+ flux in Kel-KO, Xk-KO and double KO in the presence or absence of 700 nM ET-3 in vitro as described in Methods. N=3, αp<0.03; βp<0.03; πp<0.02; # p<0.04; **p<0.03. B) Na-independent Mg2+ flux and quinidine–insensitive Mg2+ flux. n=6, WT vs Kel-KO, or Xk-KO or double KO, *p<0.002. One-way ANOVA test followed by Tukey analysis. Values represent mean ± SE.
To evaluate if ET-3 may alter the effects of Kel or Xk gene deletion on Mg2+ flux, transport of Mg2+ in the presence of ET-3 was also evaluated in erythrocytes. The lack of Kell or Kell/Xk complex induced a significant increase in NME flux that was significantly affected by the presence of ET-3 (Figure 3A), suggesting that deletion of Kel/Xk may lead to alterations in the NME signaling pathway. In addition, we observed that the effect of Xk deletion on the NME flux was not affected by ET-3. These observations suggest that Xk is under the regulation of Kell to transport Mg2+ in erythrocytes but ET-3 cannot directly regulate it. Therefore, since Kel deletion increases ouabain/bumetanide/quinide resistant Mg2+ fluxes and Xk deletion completely blocks NME, we speculate that Xk protein might transport Mg2+ in erythrocytes. Further studies will be needed to explore this hypothesis.
DISCUSSION
In this report, we investigated the effects of Kell, Xk or Kell/Xk gene ablation on cellular volume regulation and erythrocyte cation homeostasis. Regulation of cellular volume is a critical physiological event. It is a tightly controlled process whereby cells restore their normal steady-state volume in response to an osmotic challenge. The cell responds to cell swelling by a rapid efflux of K+ followed by Cl− and water. On the other hand, cell shrinkage is corrected by a rapid net influx of Na+ and Cl−, followed by water. Therefore, volume-regulatory ion flux pathways are activated, highly regulated, and coordinated in normal cells. Cellular volume regulatory systems are well described, however, the mechanism for activation, control, and coordination of these pathways are not entirely clear. Our results provide evidence that gene ablation of the Kell/Xk system leads to significant alterations in divalent cation homeostasis. In these three animal models, our results demonstrate that the Kell/XK complex plays an important role in erythrocyte volume regulation as deletion of any part of the complex has important effects on cation levels, transport and cellular volume parameters. We speculate that disruption of Kel or Xk gene contributes to increases in cation permeability at the plasma membrane that lead changes in cellular volume. Our findings suggest that Kell/Xk complex might be directly responsible for regulating ion transport in erythrocytes and these abnormalities might contribute to the formation of acanthocytic erythrocytes. In addition, these findings expand on our current understanding of cation volume regulation and suggest a heretofore unrecognized role for the Kell/Xk complex as a novel and important regulatory component of cellular volume regulation as it simultaneously modulates the activity of several erythrocyte membrane ion transporters.
Xk is part of the large multiprotein 4.1R cytoskeletal membrane complex that binds to the middle lobe (α-lobe, lobe B) of the 30 kDa domain of 4.1R [20], and is a member of the 4.1 family. The 4.1R protein complex is thought to function as an important component of structural and mechanical integrity of the red cell by binding to the junctional node of the spectrin-actin network. 4.1R is also associated with cellular polarity, differentiation, and signal transduction. It seems that the 4.1R complex compartmentalizes many plasma membrane proteins to act in concert to exert important cellular events such as signaling, ion and small molecule transport. Consequently, altered expression of XK protein might lead to significant changes in the cytoskeletal stability and intra-erythrocytic milieu that in turn may affect cellular cation homeostasis.
The absence of Kell/Xk together or independently virtually eliminated the activity of the Ca2+ATPase suggesting a critical role of Kell/Xk in cellular Ca2+ homeostasis. These alterations in calcium homeostasis were associated with significant increases in Ca2+-dependent K+ flux and hematological parameters such as mean cellular volume and red cell distribution width. Thus, we speculate that the reduction in Ca2+-ATPase activity will induce accumulation of intracellular Ca2+ that will lead to increases in K+ flux mediated by Gardos channel activation in mouse erythrocytes. These results are in agreement with the increase in total calcium levels measured in the Xk-KO and Kel/Xk-KO erythrocytes.
It has been reported that activation of ETB receptor will induce a reduction in Ca2+-ATPase activity and increase in Ca2+ accumulation in other cell types [21; 22]. In platelets, ET-3 has been shown to blunt Ca2+ mobilization by blocking Ca2+-ATPase activity [23]. In contrast, in erythrocytes from WT mice we did not observe any significant changes in Ca2+-ATPase activity in the presence of ET-3. However, ET-3 did stimulate a significant increase in Ca2+-ATPase activity in KO erythrocytes, which might explain the reduction of the Gardos channel activity in the presence of ET-3. Thus, increases in Ca2+-ATPase activity are associated with reduced intracellular Ca2+ ions. These data provide strong evidence for a link between the Ca2+-ATPase and the Kell/Xk complex in regulating intracellular Ca2+ levels.
NME has been reported to be an important cellular Mg2+ regulatory system in erythrocytes [24; 25; 26]. Its activity is altered in many pathological diseases such as diabetes [26], sickle cell disease [19], and hypertension [27]. Mg2+ is a well-described regulator of many enzymes and has been shown to play important roles in many cellular functions including cellular volume regulation. We found that NME was blunted in Xk-KO erythrocytes and increased in Kel-KO and double knockout. In addition, we found that Mg2+ permeability, independent of NME activity, in both Xk-KO and Kel-KO was elevated suggesting that the Kell/Xk protein complex plays an important role in Mg2+ homeostasis (Figure 3). Whether Kell/XK protein complex modulates Mg2+ transport or not by transporting Mg2+ through Xk is under investigation. We posit that Xk either transports Mg2+ or is functionally coupled to NME. The molecular identity of NME is emerging and monoclonal antibodies have been used to block the NME in vitro [28; 29]. Further electrophysiological studies are required to investigate if Xk is indeed a Mg2+ transport protein, however, our studies support the hypothesis that Kell functions as a regulatory protein of Mg2+ transport and as such provides a novel target for cellular Mg2+ homeostasis and volume regulation.
We observed that absence of Kell or both Kell/Xk induced a slight increase in Na+ transport via NHE activity. These results are consistent with previous findings since Xk protein has been linked to 4.1R [20] and deficiency of erythrocyte 4.1R protein is associated with a significant elevation of NHE activity leading to abnormal Na+ content and volume changes in these cells [16]. However, Xk deletion did not result in any significant differences in Na+ content in mouse erythrocytes as was observed in 4.1R deficient mice [16]. Our results suggest that these slight alterations on NEH activity are partially mediated by Kell/Xk association with 4.1R protein in mouse erythrocytes.
The McLeod Syndrome is a rare X-linked neuroacanthocytosis caused by various mutations in the XK gene leading to the absence of Kx antigen at the erythrocyte cell surface. The phenotype of patients with McLeod Syndrome is in part characterized by the presence of abnormal acanthocytic circulating erythrocytes described as dense, slightly contracted cells with numerous irregular thorny surface projections and terminal bulbs [30; 31; 32; 33]. These characteristics are common in cells from patients with erythrocyte volume regulatory disorders that are associated with cation transport abnormalities such as observed in patients with sickle cell disease and thalassemia. Acanthocytic erythrocytes from patients with McLeod phenotype are short lived presumably as a consequence of high expression of phosphatidylserine, a signal molecule for erythrocyte removal [34; 35; 36].
In summary, we have demonstrated that Kell and Xk proteins modulate cation transport in mouse erythrocytes. The absence of Kell or Xk protein induces significant changes in intracellular Mg2+ and Ca2+ levels and permeability suggesting that Xk transporter substrate might be a divalent cation transporter. Consequently, further studies are warranted to characterize the XK transport substrate in erythrocytes. Our results provide evidence for a novel role of the Kell/XK complex on ion transport homeostasis that, to the best of our knowledge, has not been previously described. These results may have direct clinical consequences as they contribute to our understanding of the pathophysiology of McLeod syndrome.
Acknowledgments
We thank Ms. Jessica Alves for her excellent technical support. This work was supported in part Advocacy for Neuro-acanthocytosis Patients and the National Institutes of Health grants HL090632 (to AR), HL075716 (to SL), and HL096518 (to JRR).
Abbreviations
- ANOVA
analysis of variance
- BSA
bovine serum albumin
- CHCM
corpuscular hemoglobin concentration mean
- ChTX
charybdotoxin
- CM
calmodulin
- CWS
choline washing solution
- ECE-3 or ECE-2
endothelin-3 or 2-converting enzymes
- ET-1
endothelin-1
- ET-3
endothelin-3
- ETB
endothelin receptor B
- Gardos Channel
Ca2+-activated K+ channel
- HDW
hemoglobin distribution width
- HMA
hydroxylmethyl amiloride
- Kel
mouse Kel gene
- KO
knockout
- LD
lactate dehydrogenase
- MCV
mean cellular volume
- NHE
Na-H exchanger
- NKCC
Na/K/2Cl co-transporter
- NME
Na-Mg exchanger
- RDW
red distribution width
- WT
wild-type
- XK
human XK gene
- Xk
mouse Xk gene.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship Contributions: AR and SL designed all the experiments, analyzed all data, and wrote the manuscripts; MH and SMY develop the KO mice. JRR provided statistical analysis of the data and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
REFERENCES
- [1].Lee S, Lin M, Mele A. Proteolytic processing of big endothelin-3 by the kell blood group protein. Blood. 1999;94:1440–50. [PubMed] [Google Scholar]
- [2].Ho MF, Monaco AP, Blonden LA. Fine mapping of the McLeod locus (XK) to a 150-380-kb region in Xp21. Am. J. Hum. Gen. 1992;50:317–30. [PMC free article] [PubMed] [Google Scholar]
- [3].Ho M, Chelly J, Carter N. Isolation of the gene for McLeod syndrome that encodes a novel membrane transport protein. Cell. 1994;77:869–80. doi: 10.1016/0092-8674(94)90136-8. [DOI] [PubMed] [Google Scholar]
- [4].Collec E, Colin Y, Carbonnet F. Structure and expression of the mouse homologue of the XK gene. Immunogenetics. 1999;50:16–21. doi: 10.1007/s002510050681. [DOI] [PubMed] [Google Scholar]
- [5].Lee S, Russo D, Redman CM. The Kell blood group system: Kell and XK membrane proteins. Sem. Hematol. 2000;37:113–21. doi: 10.1016/s0037-1963(00)90036-2. [DOI] [PubMed] [Google Scholar]
- [6].Khamlichi S, Bailly P, Blanchard D. Purification and partial characterization of the erythrocyte Kx protein deficient in McLeod patients. Eur. J. Biochem. / FEBS. 1995;228:931–4. [PubMed] [Google Scholar]
- [7].Russo D, Wu X, Redman CM, Lee S. Expression of Kell blood group protein in nonerythroid tissues. Blood. 2000;96:340–6. [PubMed] [Google Scholar]
- [8].Jung HH, Russo D, Redman C, Brandner S. Kell and XK immunohistochemistry in McLeod myopathy. Muscle & nerve. 2001;24:1346–51. doi: 10.1002/mus.1154. [DOI] [PubMed] [Google Scholar]
- [9].Claperon A, Rose C, Gane P. The Kell protein of the common K2 phenotype is a catalytically active metalloprotease, whereas the rare Kell K1 antigen is inactive. Identification of novel substrates for the Kell protein. J. Biol. Chem. 2005;280:21272–83. doi: 10.1074/jbc.M500100200. [DOI] [PubMed] [Google Scholar]
- [10].Lee S, Russo DC, Pu J, Ho M, Redman CM. The mouse Kell blood group gene (Kel): cDNA sequence, genomic organization, expression, and enzymatic function. Immunogenetics. 2000;52:53–62. doi: 10.1007/s002510000251. [DOI] [PubMed] [Google Scholar]
- [11].Zhu X, Rivera A, Golub MS. Changes in red cell ion transport, reduced intratumoral neovascularization, and some mild motor function abnormalities accompany targeted disruption of the Mouse Kell gene (Kel) Am. J. Hematol. 2009;84:492–8. doi: 10.1002/ajh.21453. [DOI] [PubMed] [Google Scholar]
- [12].Sha Q, Redman CM, Lee S. Endothelin-3-converting enzyme activity of the KEL1 and KEL6 phenotypes of the Kell blood group system. J. Biol. Chem. 2006;281:7180–2. doi: 10.1074/jbc.M507776200. [DOI] [PubMed] [Google Scholar]
- [13].Rivera A, Jarolim P, Brugnara C. Modulation of Gardos channel activity by cytokines in sickle erythrocytes. Blood. 2002;99:357–603. doi: 10.1182/blood.v99.1.357. [DOI] [PubMed] [Google Scholar]
- [14].Rivera A. Reduced sickle erythrocyte dehydration in vivo by endothelin-1 receptor antagonists. American journal of physiology. Cell Physiol. 2007;293:C960–6. doi: 10.1152/ajpcell.00530.2006. [DOI] [PubMed] [Google Scholar]
- [15].Rivera A, Rotter MA, Brugnara C. Endothelins activate Ca(2+)-gated K(+) channels via endothelin B receptors in CD-1 mouse erythrocytes. Am. J. Physiol. 1999;277:C746–54. doi: 10.1152/ajpcell.1999.277.4.C746. [DOI] [PubMed] [Google Scholar]
- [16].Rivera A, De Franceschi L, Peters LL. Effect of complete protein 4.1R deficiency on ion transport properties of murine erythrocytes. Am. J. Physiol. Cell Physiol. 2006;291:C880–6. doi: 10.1152/ajpcell.00436.2005. [DOI] [PubMed] [Google Scholar]
- [17].Romero JR, Fabry ME, Suzuka S, Nagel RL, Canessa M. Red blood cells of a transgenic mouse expressing high levels of human hemoglobin S exhibit deoxy-stimulated cation flux. J. Membr. Biol. 1997;159:187–96. doi: 10.1007/s002329900282. [DOI] [PubMed] [Google Scholar]
- [18].Bize I, Guvenc B, Robb A, Buchbinder G, Brugnara C. Serine/threonine protein phosphatases and regulation of K-Cl cotransport in human erythrocytes. Am. J. Physiol. 1999;277:C926–36. doi: 10.1152/ajpcell.1999.277.5.C926. [DOI] [PubMed] [Google Scholar]
- [19].Rivera A, Ferreira A, Bertoni D, Romero JR, Brugnara C. Abnormal regulation of Mg2+ transport via Na/Mg exchanger in sickle erythrocytes. Blood. 2005;105:382–6. doi: 10.1182/blood-2003-11-3755. [DOI] [PubMed] [Google Scholar]
- [20].Salomao M, Zhang X, Yang Y. Protein 4.1R-dependent multiprotein complex: new insights into the structural organization of the red blood cell membrane. Proc. Nat. Acad. Sci. USA. 2008;105:8026–31. doi: 10.1073/pnas.0803225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Touyz RM, Deng LY, Schiffrin EL. Endothelin subtype B receptor-mediated calcium and contractile responses in small arteries of hypertensive rats. Hypertension. 1995;26:1041–5. doi: 10.1161/01.hyp.26.6.1041. [DOI] [PubMed] [Google Scholar]
- [22].Jouneaux C, Mallat A, Serradeil-Le Gal C. Coupling of endothelin B receptors to the calcium pump and phospholipase C via Gs and Gq in rat liver. J. Biol. Chem. 1994;269:1845–51. [PubMed] [Google Scholar]
- [23].Gagnet C, Brunet A, Pernollet MG, Devynck MA, Astarie-Dequeker C. Endothelin-3, Ca2+ mobilization and cyclic GMP content in human platelets. Eur. J. Pharm. 1996;310:67–72. doi: 10.1016/0014-2999(96)00367-6. [DOI] [PubMed] [Google Scholar]
- [24].Feray JC, Garay R. An Na+-stimulated Mg2+-transport system in human red blood cells. Bioch. Biophys. Acta. 1986;856:76–84. doi: 10.1016/0005-2736(86)90012-x. [DOI] [PubMed] [Google Scholar]
- [25].Flatman PW. The control of red cell magnesium. Magnes. Res. 1988;1:5–11. [PubMed] [Google Scholar]
- [26].Ferreira A, Rivera A, Romero JR. Na+/Mg2+ exchange is functionally coupled to the insulin receptor. J. Cell. Physiol. 2004;199:434–40. doi: 10.1002/jcp.10463. [DOI] [PubMed] [Google Scholar]
- [27].Touyz RM, Yao G. Inhibitors of Na+/Mg2+ exchange activity attenuate the development of hypertension in angiotensin II-induced hypertensive rats. J. Hyperten. 2003;21:337–44. doi: 10.1097/00004872-200302000-00025. [DOI] [PubMed] [Google Scholar]
- [28].Schweigel M, Park HS, Etschmann B, Martens H. Characterization of the Na+-dependent Mg2+ transport in sheep ruminal epithelial cells. Am. J. Physiol. Gastrointestinal and liver physiology. 2006;290:G56–65. doi: 10.1152/ajpgi.00014.2005. [DOI] [PubMed] [Google Scholar]
- [29].Schweigel M, Kolisek M, Nikolic Z, Kuzinski J. Expression and functional activity of the Na/Mg exchanger, TRPM7 and MagT1 are changed to regulate Mg homeostasis and transport in rumen epithelial cells. Magnes. Res. 2008;21:118–23. [PubMed] [Google Scholar]
- [30].Jung HH, Hergersberg M, Vogt M. McLeod phenotype associated with a XK missense mutation without hematologic, neuromuscular, or cerebral involvement. Transfusion. 2003;43:928–38. doi: 10.1046/j.1537-2995.2003.t01-1-00434.x. [DOI] [PubMed] [Google Scholar]
- [31].Danek A, Rubio JP, Rampoldi L. McLeod neuroacanthocytosis: genotype and phenotype. Ann. Neurol. 2001;50:755–64. doi: 10.1002/ana.10035. [DOI] [PubMed] [Google Scholar]
- [32].Hardie RJ, Pullon HW, Harding AE. Neuroacanthocytosis. A clinical, haematological and pathological study of 19 cases. Brain. 1991;114(Pt 1A):13–49. [PubMed] [Google Scholar]
- [33].Wimer BM, Marsh WL, Taswell HF, Galey WR. Haematological changes associated with the McLeod phenotype of the Kell blood group system. Brit. J. Haematol. 1977;36:219–24. doi: 10.1111/j.1365-2141.1977.tb00642.x. [DOI] [PubMed] [Google Scholar]
- [34].Redman CM, Huima T, Robbins E, Lee S, Marsh WL. Effect of phosphatidylserine on the shape of McLeod red cell acanthocytes. Blood. 1989;74:1826–35. [PubMed] [Google Scholar]
- [35].Schroit AJ, Tanaka Y, Madsen J, Fidler IJ. The recognition of red blood cells by macrophages: role of phosphatidylserine and possible implications of membrane phospholipid asymmetry. Biol. Cell. 1984;51:227–38. doi: 10.1111/j.1768-322x.1984.tb00303.x. [DOI] [PubMed] [Google Scholar]
- [36].Lin S, Yang E, Huestis WH. Relationship of phospholipid distribution to shape change in Ca(2+)-crenated and recovered human erythrocytes. Biochem. 1994;33:7337–44. doi: 10.1021/bi00189a039. [DOI] [PubMed] [Google Scholar]