Abstract
Regulation of energy balance in female rats is known to differ along a number of dimensions compared to male rats. Previous work from our lab has demonstrated that in female rats fed dietary supplements containing high-intensity sweeteners that may disrupt a predictive relation between sweet tastes and calories, excess weight gain is demonstrated only when females are also fed a diet high in fat and sugar, and is evidenced primarily in animals already prone to gain excess weight. In contrast, male rats show excess weight gain when fed saccharin-sweetened yogurt supplements when fed both standard chow diets and diets high in fat and sugar, and regardless of their proneness to excess weight gain. The goal of the present experiments was to determine whether ovarian, or other sources of estrogens, contributes to the resistance to excess weight gain in female rats fed standard chow diets along with dietary supplements sweetened with yogurt. Results of the first experiment indicated that when the ovaries were removed surgically in adult female rats, patterns of weight gain were similar in animals fed saccharin-sweetened compared to glucose-sweetened yogurt supplements. In the second experiment, when the ovaries were surgically removed in adult female rats, and local production of estrogens was suppressed with the aromatase inhibitor anastrozole, females fed the saccharin-sweetened yogurt consumed more energy and gained more weight than females fed the glucose-sweetened yogurt. However, when the ovaries were surgically removed prior to the onset of puberty (at 24 – 25 days of age), females given saccharin-sweetened yogurt along with vehicle gained excess weight. In contrast, weight gain was similar in those given saccharin-sweetened and glucose-sweetened yogurt along with anastrozole. The results suggest that behavioral differences between males and females in response to disruption of sweet→calorie relations may result from differences in patterns of local estrogen production. These differences may be established developmentally during the pubertal period in females.
Keywords: Energy balance, learning, estrogens, sweeteners, puberty
Introduction
Overweight and obesity represent increasingly pressing health problems in the United States and throughout the world (e.g., Flegal et al., 2012; James, 2008; Ogden et al., 2012). Efforts to reduce the spread of obesity have focused on a variety of factors, including changing the food environment to reduce energy intake. One method promoted for reducing energy intake is to replace ingredients in the diet that provide energy, such as sugars and fats, with substitutes, such as the high intensity sweeteners saccharin, acesulfame potassium (AceK) and sucralose and the fat substitute olestra, which mimic the sensory properties of sugars and fats (American Dietetic Association, 2004). The rationale behind such substitution approaches is that these replacers will result in reduced energy intake. However, whether such reductions in energy intake actually occur remains an open question. For example, a number of epidemiological studies link consumption of beverages sweetened with high-intensity sweeteners to increased risk of overweight, obesity, diabetes, cardiovascular disease and metabolic syndrome (e.g., Dhingra et al., 2007; Duffey et al., 2012; Fowler et al., 2008; Gardener et al., 2012; Laska et al., 2012; Ludwig, 2009; Lutsey et al., 2008; Nettleton et al., 2009; Yang, 2010) while other studies do not appear to demonstrate such a link (e.g., Bellisle and Drewnowski, 2007; Schulze et al., 2004).
Previously we reported that male rats given high intensity sweeteners (i.e., saccharin, acesulfame potassium, and Stevia extracts) exhibit increased energy intake and body weight gain (for review, see Swithers et al., 2010). We have provided evidence that these deficits in regulating energy balance are based on the disruption of the learned signaling relationship between sweet tastes and caloric or energetic outcomes (Davidson et al., 2011). This relationship is formed early in life (Swithers et al., 2012a) presumably as a result of normal experience with consuming sweet-tasting, caloric foods and fluids.
This learned relation between taste and calories may provide one mechanism that animals use to regulate energy intake. Based on principles of Pavlovian conditioning, as a consequence of experience with sweet tastes in foods that provide energy in the form of calories, animals may come to show conditioned, anticipatory responses that prepare them to effectively utilize that energy (e.g., Woods and Ramsay, 2000). Within this framework, consuming high-intensity sweeteners that taste sweet but deliver few or no calories, should interfere with energy regulation by weakening this predictive relationship (e.g., Davidson and Swithers, 2004; Swithers and Davidson, 2008). This mechanism may contribute to some of the associations observed in humans between consumption of foods and beverages sweetened with high-intensity sweeteners and increased risk of negative health outcomes including overweight, obesity, and metabolic syndrome.
To date, most of the work examining the consequences of disrupting sweet taste-calorie associations on long-term food intake and body weight gain has focused on lean male rats, although data suggest that the most likely consumers of such “diet” foods and beverages are women and those already overweight or obese (e.g., Duffey et al., 2012; Fowler et al., 2008). Recent data from our lab suggest that negative effects of consuming high-intensity sweeteners on weight control may be greatest for these overweight or likely to become overweight. We found that female rats prone to excess weight gain and fed a diet high in fat and sugar, show increased body weight when given dietary supplements sweetened with saccharin compared to those given supplements sweetened with glucose (Swithers et al., 2012b). However, compared to male rats, female rats consuming a standard low-fat lab chow diet appear to be relatively resistant to the additional body weight gain related to consumption of high-intensity sweeteners; that is female rats given saccharin-sweetened diets gain similar amounts as female rats given glucose-sweetened diets (Swithers et al., 2012b). Further, the responses of female rats to high-intensity sweeteners when maintained on diets high in fat and sugar are modulated by their phenotype; females identified as prone to diet-induced obesity (DIO) prior to introduction of the sweeteners show excess weight gain compared to those given a caloric sweetener, while females identified as resistant to diet-induced obesity (DR) gain similar amounts of weight when given caloric and non-caloric sweeteners. In contrast, both DIO and DR males show excess weight gain when provided with high-intensity sweeteners compared to caloric sweeteners (Swithers et al., 2012b).
The mechanisms that might underlie differences in patterns of responding to high-intensity sweeteners, and the resistance to weight gain on a standard chow diet, in female compared to male rats are unknown. However, short-term data from rats tested during the pre-pubertal period suggest that prior to the onset of puberty (e.g. 15 – 25 days of age), responses to high-intensity sweeteners are similar in male and female rats fed a standard chow diet (e.g., Davidson and Swithers, 2004; Swithers et al., 2012a), suggesting that changes occurring during puberty may alter responses of female rats to high-intensity sweeteners. One implication of this finding is that ovarian estrogens might play a role in differences in responses of female rats before puberty compared to following puberty. Alternatively, it is becoming increasingly recognized that physiological and neural function can be influenced not only by circulating estrogens derived from the ovaries, but also by estrogens synthesized locally from precursors such as cholesterol and testosterone through activity of the CYP450 enzyme aromatase. In the rat, tissues expressing aromatase that may be particularly critical for energy regulation include the liver, adipose tissue, the hypothalamus, and even taste buds of the circumvallate papillae (Azcoitia et al., 2011; Belanger et al., 2002; Charlier et al., 2010; Cornil, 2009; Cornil et al., 2006; Cornil and Charlier, 2010; Sanghera et al., 1991; Shibuya et al., 2003; Simpson et al., 2000; Toyoshima et al., 2007). Thus, differences in responding in female rats could also reflect changes in local estrogen synthesis through aromatase activity that follow puberty.
The goal of the present studies was to examine whether ovarian or other estrogens contribute to the observed resistance to weight gain in adult female rats given high-intensity sweeteners. In Experiment 1, the ovaries of adult female rats were removed surgically and the effects of consuming dietary supplements sweetened with the high-intensity sweetener saccharin were compared to supplements sweetened with glucose in female rats. In Experiment 2, the goal was to examine whether blocking the activity of aromatase, thereby inhibiting local estrogen synthesis, affected responding to saccharin-versus glucose-sweetened yogurt diet supplements and whether the consequences of inhibiting local estrogen synthesis by surgical removal of the ovaries were different prior to compared to after puberty. The aromatase inhibitor anastrozole was used because it is designed for oral delivery, and has been previously been demonstrated to significantly suppress estrogen levels in OVX female rats at the dose employed (0.1 mg/kg; Dukes, 1997; Wang et al., 2009). In rats, anastrozole is typically delivered by gavage or provided in drinking water as the sole source of fluids, which can diminish the amount of fluid consumed and thereby impact food intake. Preliminary work demonstrated that when anastrozole or its vehicle (ethanol) was mixed into the dietary supplement we typically use, low-fat yogurt, animals reliably consumed the entire portion of yogurt, and therefore the entire dose of the drug. We took advantage of the willingness of animals to consume the drug voluntarily by dissolving it in the yogurt supplements, allowing us to avoid stress associated with repeated gavage or suppression of fluid intake associated with providing it in drinking water.
Materials and Methods
Experiment 1
Subjects were 25 adult female Sprague-Dawley rats approximately 70 days of age (Harlan, Indianapolis) that received ad libitum access to a standard laboratory chow (Lab Diets 5012) for one week after arrival in the lab. Then, all rats were anesthetized with ketamine and xylazine prior to bilateral ovariectomy (OVX), which was performed as described previously (Swithers et al., 2008). Butorphanol (2.5 mg/kg s.c.) was used for post-operative analgesia. Animals were allowed to recover for 3 weeks prior to being assigned to Glucose or Saccharin groups matched on body weight (Means = 260.1 ± 4.04 and 260.8 ± 4.2 g, glucose [n=12] and saccharin [n=13] groups, respectively). Animals in both groups were given access to 30 g yogurt (Dannon low-fat yogurt ~ 0.6 kcal/g) daily 6 days per week for 4 weeks. Yogurt was provided unsweetened 3 days per week and was sweetened with 0.3% saccharin (Saccharin group) or 20% glucose (Glucose group) on the remaining 3 days per week. Chow and water alone were available on the 7th day of each week. Yogurt intake, food intake and body weight were measured daily. Weight gain was analyzed by a two-way, repeated measures ANOVA (Sweetener X Day) with sweetener as a between-subjects factor and day as a within-subjects factor. Total energy intake (chow plus yogurt) was analyzed with a separate one-way (Sweetener) ANOVA.
Experiment 2
Experiment 2 differed in several ways from Experiment 1 due to differences in the time at which the studies were conducted in the lab. Subjects in this experiment were 48 adult female Sprague-Dawley rats approximately 70 days of age (Harlan, Indianapolis) and weighing 175–200 g upon arrival and 34 adolescent female Sprague-Dawley rats, identified by litter of origin, received from Harlan (Indianapolis) at 21 days of age. In the adolescent group, 1 or 2 animals per litter were assigned to each of the experimental groups, and sample sizes were 8–9 per group due to the availability of animals identified by litter at the specified age. Sample sizes were 12 per group in the animals OVX as adults as in Experiment 1; litter of origin information was not available for adult animals in this Experiment or in Experiment 1. In this experiment, all animals also underwent bilateral OVX, but the anesthetic used was isoflurane in place of ketamine/xylazine; butorphanol (2.5 mg/kg s.c.) was used for post-operative analgesia as in Experiment 1. Pre-pubertal adolescents were OVX at 24 or 25 days of age and were then given ad lib access to a standard laboratory chow (Harlan 2018) and water until 70 days of age. Adults were given 3 weeks to recover from OVX as in Experiment 1. Prior to the introduction of yogurt, body composition was assessed by NMR (EchoMRI). Animals within each surgical age group were assigned to one of four groups, with groups within each surgical age matched on body weight (n= 8 – 9 animals per group for pre-pubertal OVX animals and 12 per group for adult OVX). Within each surgical age group, one set of animals received the aromatase inhibitor anastrozole in their yogurt ration while the other group received the ethanol vehicle.
Anastrozole was first dissolved in 5 ml ethanol, which was then mixed into 1000 g yogurt; vehicle animals received yogurt into which 5 ml ethanol/1000 g yogurt had been mixed. Animals were given 30 g yogurt daily; animals that consumed all 30 g received a dose of approximately 0.1 mg/kg. The concentration of the anastrozole was increased after 10 days of yogurt to accommodate increases in body weight to maintain doses at approximately 0.1 mg/kg.
Half of the animals in the anastrozole group and half of the animals in the vehicle group in each age of OVX group received yogurt sweetened with 0.3% saccharin while the remaining half of the animals received yogurt sweetened with 20% glucose. Yogurt diets were provided daily for 20 days (10 plain and 10 sweetened). In this experiment, no intervening chow alone days were employed since the yogurt was the method by which the anastrozole was delivered to the animals and the goal was to achieve relatively constant inhibition of aromatase activity. The order of presentation of yogurts was semi-randomized such that neither plain nor sweetened yogurt was provided more than 3 days in a row.
Body weight, yogurt intake and food intake were measured daily. Body composition was again assessed at the end of the 20 day yogurt exposure by NMR. Animals OVX as adults and adolescents were tested at separate times; however since procedures were similar, data were analyzed together. Weight gain was analyzed with a 3-way repeated measures ANOVA with Age of OVX and Sweetener as between-subjects factors, Day as a within-subjects factor. Post-hoc testing with one-way repeated measures ANOVAs and LSD tests were conducted where indicated.
In addition, for animals OVX as adolescents, the role of litter of origin on body weight gain was assessed with a 3-Way repeated measures ANOVA with Litter and Sweetener as between-subjects factors and Day as a within-subjects factor. The role of litter of origin on energy intake, fat mass and lean mass was assessed with separate 2-Way (Sweetener X Litter) ANOVA (energy intake), or ANCOVAs (fat mass and lean mass). No significant main effects or interactions of litter of origin were observed for any measures.
Changes in body composition measures (Fat mass and Lean mass) were assessed using separate Two-Way (Age of OVX X Sweetener) ANCOVAs with starting Fat mass and starting Lean mass used as the Covariate. Total caloric intake (yogurt intake plus chow intake) was analyzed using separate One-Way (sweetener) ANOVAs at each surgical age.
All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Purdue University Animal Care and Use Committee.
Results
Experiment 1
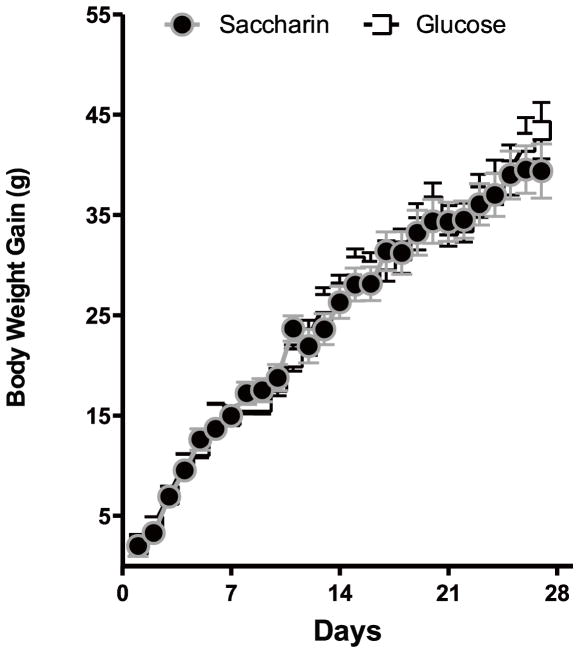
Weight gain in OVX female rats maintained on a standard laboratory chow diet (Figure 1) was affected by the day of testing (Main effect of Day, F 26, 598 = 273.7.4, p < .0000001), but not by the sweetener provided in the yogurt (Fs <1). Total energy intake (yogurt plus chow) across the 28 days of yogurt exposure was also unaffected by the sweetener provided in the yogurt (F 1, 23 = 1.36, p = 0.25; Means = 2254 ± 66 kcal for Glucose and 2148 ± 63 kcal for Saccharin groups).
Figure 1.
Body weight gain in female rats OVX as adults was not affected by the type of sweetener provided.
Experiment 2
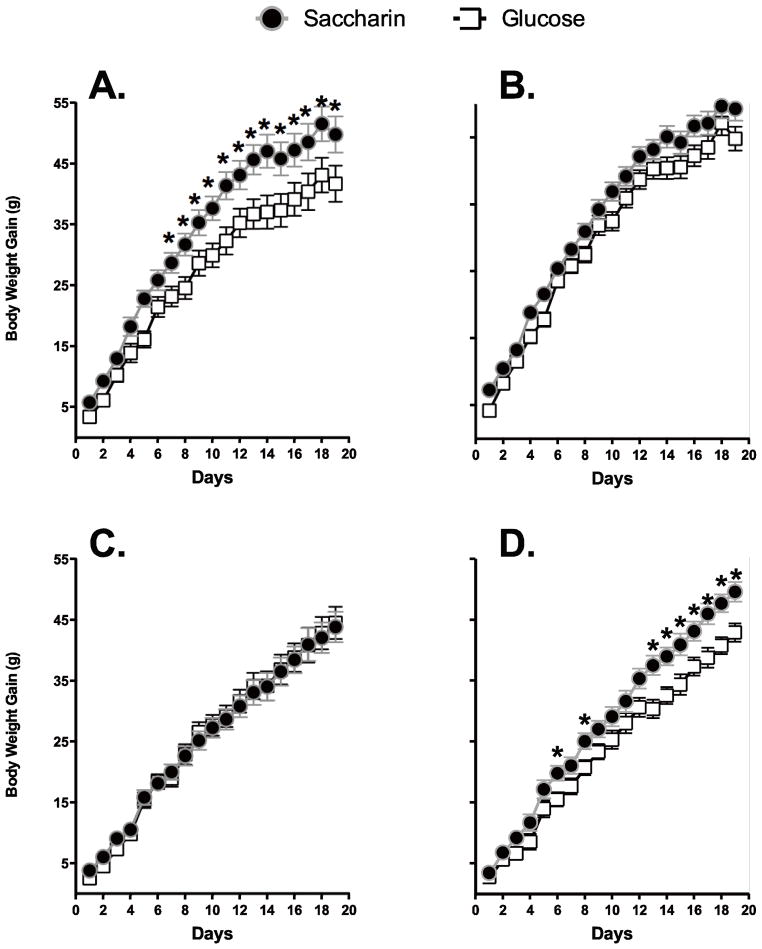
Body weight at the start of the experiment was significantly higher in females OVX prior to puberty than females OVX as adults (Main effect of Age of OVX; F 1, 74 = 10.37, p = .0019; Means = 268.9 ± 2.2 g and 259.6 ±1.9 g, for pre-pubertal and adult OVX, respectively), but there were no differences in body weight across sweetener or drug groups (all other Fs < 1). Body weight gain during yogurt consumption was significantly affected by the Age of OVX, Sweetener provided and Drug (Figure 2; Main effect of Age of OVX, F 1, 74 = 22.5, p = .00001; Main effect of Sweetener, F 1, 74 = 10.3, p = .0020; Main effect of Day, F 18, 1332 = 1934.6, p < .0000001; Day X Age of OVX interaction, F 18, 1332 = 11.6, p < .0000001; Day X Sweetener interaction, F 18, 1332 = 2.8, p = .000064; Age of OVX X Sweetener X Drug X Day interaction, F 18, 1332 = 3.3, p = .000004). Post-hoc analyses revealed that in females OVX as adults, body weight gain in animals given the aromatase inhibitor was significantly higher when animals consumed saccharin-sweetened yogurt compared to glucose-sweetened yogurt beginning on the 7th day of yogurt consumption (Figure 2A; Main effect of Sweetener, F 1, 22 = 6.0, p =.023; Main effect of Day, F 18, 396 = 401.0, p < .0000001; Sweetener X Day interaction, F 18, 396 = 3.2, p = .000083).
Figure 2.
Body weight gain in female rats OVX as adults and given the drug anastrozole to block local estrogen production was significantly higher in females consuming saccharin-sweetened yogurt compared to those consuming glucose-sweetened yogurt (A), while no differences in weight gain were observed in female rats OVX as adults and given the vehicle based on the sweetener consumed (B). In females OVX prior to puberty, weight gain was not affected by the sweetener used when anastrozole was provided (C), but was significantly higher in females given the vehicle and saccharin-sweetened yogurt compared to the vehicle and glucose-sweetened yogurt (D).
* p< 0.05 compared to Glucose-sweetened yogurt
In contrast, body weight gain in females OVX as adults and given the vehicle, was not affected by the type of sweetener consumed (Figure 2B; Main effect of Day, F 18, 396 = 780.3, p < .0000001). In females OVX prior to puberty, weight gain was not affected by the sweetener in animals given anastrozole (Figure 2C; Main effect of Day, F 18, 270 = 354.1, p < .0000001), but was significantly greater in females given the vehicle along with saccharin-sweetened yogurt compared to vehicle and glucose-sweetened yogurt (Figure 2D; Main effect of Sweetener, F 1, 15 = 6.4, p = .023; Main effect of Day, F 18, 270 = 844.0, p < .0000001; Day X Sweetener interaction, F 18, 270 = 4.52, p < .0000001).
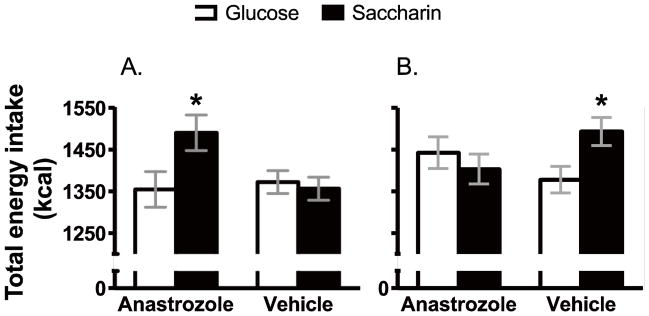
Analysis of energy intake indicated that total energy consumed was affected by the age at which animals were OVX, the sweetener provided as well as the drug condition (Main effect of Sweetener, F 1, 74 = 4.8, p = .031; Age of OVX X Sweetener X Drug interaction, F 1, 74 = 7.0, p = 0.01). Post-hoc analyses indicated energy intake was significantly greater in females OVX as adults and given anastrozole when consuming saccharin-sweetened yogurt compared to those consuming glucose-sweetened yogurt (Figure 3; Main effect of Sweetener, F 1, 22 = 5.0, p = 0.035), while no differences in energy intake were seen based on sweetener in females OVX as adults and given the vehicle (Figure 3; F 1, 22 < 1). In females OVX prior to puberty, energy intake did not differ based on sweetener in animals given the anastrozole (Figure 3; F 1, 15 <1), while energy intake was greater in females given the saccharin-sweetened yogurt and vehicle compared to those given the glucose-sweetened yogurt and vehicle (Figure 3; F 1, 15 = 6.2, p = .025).
Figure 3.
Total energy intake (yogurt plus chow) in females OVX as adults was significantly greater in females given the aromatase inhibitor anastrozole plus saccharin-sweetened yogurt compared to glucose-sweetened yogurt plus drug (A). In females OVX prior to puberty (B) total energy intake was significantly greater in females given saccharin-sweetened yogurt plus the vehicle compared to glucose-sweetened yogurt plus vehicle.
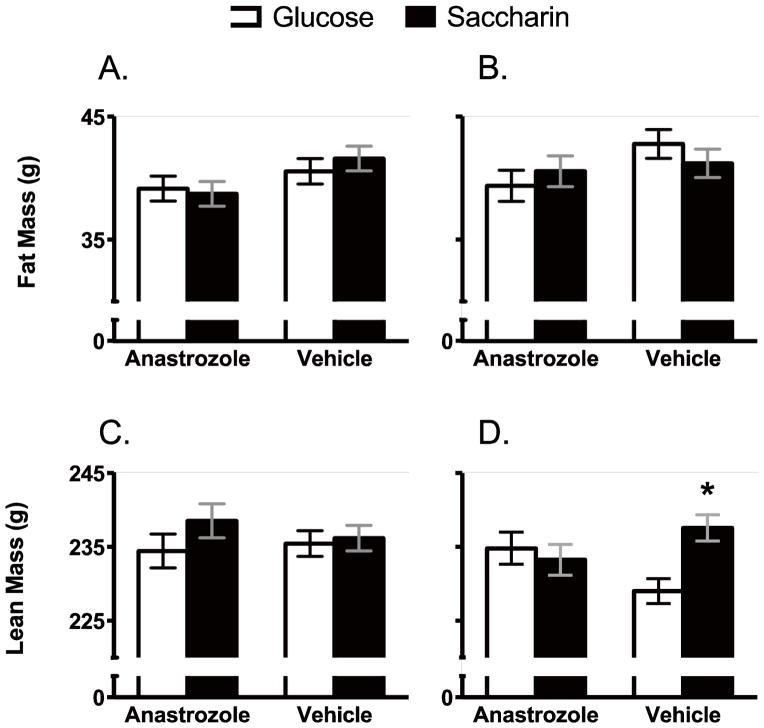
Analysis of body composition indicated that Fat Mass at the end of yogurt consumption was affected by the starting Fat Mass and Drug (Figure 4; Main effect of Starting fat mass, F 1, 73 = 257.1, p < .0000001; Main effect of Drug, F 1, 73 = 5.1, p = 0.027). There was also a trend for the Age of OVX and Sweetener to affect Fat Mass, but it did not reach statistical significance (Age of OVX X Sweetener X Drug interaction, F 1, 73 = 3.08, p = 0.083). Post-hoc analyses indicated that animals given anastrozole had significantly lower fat mass than animals given the vehicle (Means + SEM = 39.6 ± 0.6 and 41.4 ± 0.6 for drug and vehicle animals, respectively). Lean Mass was affected by the age of OVX, the sweetener provided and the drug (Figure 4; Main effect of Starting lean mass, F 1, 73 = 194.9, p < .0000001; Main effect of Sweetener, F 1, 73 = 4.8, p = .032, Age of OVX X Sweetener X Drug interaction, F 1, 73 = 4.7, p = .034). Post-hoc analyses indicated that in females OVX prior to puberty and given the vehicle, lean mass was significantly greater in females consuming the saccharin-sweetened yogurt compared to the glucose-sweetened yogurt (Figure 4D; Main effect of Starting lean mass, F 1, 14 = 32.0, p = .00006; Main effect of Sweetener, F 1, 14 = 12.1, p = .004), but there were no differences in lean mass based on sweetener in any other group (Figures 4C and 4D)..
Figure 4.
Fat mass (A) and (B) was not affected by the age of OVX, the sweetener provided or the drug condition. Lean mass was not affected by sweetener or drug condition in females OVX as adults (C), but was significantly greater in females OVX prior to puberty (D) and given saccharin-sweetened yogurt with the vehicle compared to glucose-sweetened yogurt with the vehicle.
* p< 0.05 compared to Glucose-sweetened yogurt
Discussion
Previous work has demonstrated that when given access to a standard laboratory chow diet, adult female rats, unlike adult male rats, show minimal differences in body weight gain when provided with sweetened dietary supplements that do not reliably predict the delivery of calories (Swithers et al., 2012b). Results of the present study indicate that these differences in responding between males and females are not directly related to changes in ovarian hormones during adulthood, despite the fact that ingestive responding to sweet tastes has previously been reported to respond to changes in ovarian hormones. For example, female rats appear to have heightened preferences for sweet taste compared to males, and responding for sweet tasting solutions is modulated by the stage of the estrus cycle in gonadally-intact females, with increased estrogens linked to increased preference (e.g., Asarian and Geary, 2006; Atchley et al., 2005; Curtis et al., 2005; Kenney and Redick, 1980; Wade and Zucker, 1969). In addition, surgical removal of the ovaries (OVX) in adult female rats results in diminished preferences for sweet solutions, perhaps due to increased taste thresholds for sweet; these effects can be reversed by the administration of exogenous estrogens (Curtis and Contreras, 2006; Curtis et al., 2004; Hrupka et al., 1997; Kenney and Redick, 1980). Further, sex differences in preferences for sweet solutions do not appear to emerge developmentally until around the age of the onset of first estrus (Wade and Zucker, 1969), consistent with the hypothesis that the cyclic fluctuations in ovarian hormones beginning at the onset of puberty may contribute to altered sweet taste preferences in adult female rats compared to male rats.
Thus, it is possible that females may typically experience more variability in the perception of sweet taste compared to males due to hormonal modulation by estrogens. If the perception of the sweetness of the diet does vary based on cyclic changes in estrogens, while the actual caloric content of the diet does not vary, the predictive relation between sweet taste and calories may be less strongly established in females compared to males. If females already enter the experiment with a relatively weak predictive relation between sweet tastes and caloric outcomes, then exposure to saccharin-sweetened diet would be of little consequence in further weakening the relation, even if the variability in sweet tastes has already been eliminated by removal of the ovaries prior to introduction of the high-intensity sweetener.
An alternative possibility is that differences between males and females result not from variance in taste perception, but instead that learning about the relation between the taste and the energetic consequences differs in females compared to males. Performance on a number of Pavlovian learning tasks has been demonstrated to be sexually diergic in rats (see Dalla and Shors, 2009; van Haaren et al., 1990 for review) although the pattern of differences is complex. For example, female rats acquire conditioned eyeblink responses more rapidly than males (Dalla et al., 2009; Shors and Leuner, 2003; Shors et al., 1998; Shors and Miesegaes, 2002; Wood et al., 2001; Wood and Shors, 1998), and show enhanced performance in a fear-potentiated startle paradigms compared to males (de Jongh et al., 2005). In other fear conditioning tasks, male rats appear to acquire conditioned responding more rapidly than females (e.g., Maren et al., 1994; Pryce et al., 1999) and male rats appear to be both more likely to acquire a conditioned taste aversion (Chambers et al., 1981) and slower to extinguish that aversion once acquired (Chambers and Sengstake, 1976), although these sex differences may depend on the experimental conditions used (Chambers et al., 1981; Weinberg et al., 1982).
A role of ovarian estrogens has also been suggested in differences in Pavlovian learning, although the effects again are complex. For example, sex differences in conditioned eyeblink learning tasks are enhanced in gonadally-intact females when training begins during periods of high estrogen levels, while removal of ovarian hormones eliminates sex differences (Bangasser and Shors, 2007; Hodes and Shors, 2005; Leuner et al., 2004; Wood and Shors, 1998). Retention of fear-potentiated startle performance is also enhanced by estrogen in OVX female (Hiroi and Neumaier, 2006).
In contextual fear conditioning tasks performance is also modulated by estrogen, with OVX rats performing at levels similar to males, and estrogen replacement in OVX females impairing performance (Gupta et al., 2001). Taste aversions also appear to be affected by estrogens with administration of estradiol to OVX rats accelerating extinction of learned aversions (Chambers, 1976, 1985; Yuan and Chambers, 1999). Further, sex differences in these classical conditioning tasks appear to emerge only after the onset of puberty (Costanzo et al., 1995; Hodes and Shors, 2005).
Less is known about sex differences in conditioning in tasks that involve appetitive responses. However, male and female rats have been reported to show differences during conditioned flavor preference procedures in which flavor cues are explicitly paired with solutions that provide calories (Ackroff and Sclafani, 2004). In those studies, both male and female rats showed similar strong preferences for a flavor that predicted the delivery of fructose intragastrically, but only if the flavor was delivered orally along with a sweet taste. In contrast, an unsweetened flavor that predicted delivery of fructose intragastrically was subsequently avoided by males, while females neither preferred nor avoided the flavor. However, the study was not designed to assess whether the rate at which learning about sweet-tasting flavors differed between males and females, nor was information regarding the stage of the estrus cycle during training or testing provided. Thus, while data from the present experiments indicate that females are less responsive to disruptions in a predictive relation between sweet tastes and calories, it is not currently clear whether this resistance to disruption is related to differences in the acquisition of relations between sweet tastes and energy and/or differences in extinction between males and females. Additional studies will be required to discriminate among these possibilities.
Thus, perception of sweet tastes and/or learning about consequences of consuming sweetened diets may be affected by cyclic fluctuations in ovarian-derived estrogens, and could contribute to differences in responding to high intensity sweeteners in gonadally-intact female rats relative to males. Such effects would be consistent with other well-documented effects of circulating ovarian hormones on energy balance in female rats, such as the increases in food intake, body weight gain, and adiposity associated with surgical OVX, and the reversal associated with subsequent administration of exogenous estradiol (e.g., Asarian and Geary, 2006; Brown and Clegg, 2010; Butera, 2010; Eckel, 2011; Wade, 1972; Wade and Gray, 1979; Woods et al., 2003). However, these results indicated that differences in the lack of effects of high-intensity sweeteners on weight gain in females cannot be solely explained by activational effects of ovarian hormones. OVX during adulthood failed to affect responses to saccharin compared to glucose in Experiment 1 or in vehicle-treated animals in Experiment 2. In addition, the similarity in the patterns of responding across these two experiments also suggests that the procedural differences between the two studies (e.g. anesthestic, pattern of yogurt presentation, laboratory chow fed) had minimal consequences on the effects of the sweeteners on energy balance.
The current data also suggest that ovarian estrogens during adulthood do not directly explain the differences in the patterns of responding between males and females. Instead, inhibition of aromatase, an enzyme involved in local production of estrogens in a variety of tissues including parts of the brain, adipose tissue, liver and taste cells results in alterations in the pattern of responding to sweet tastes that do not predict the delivery of calories. At present, it is not possible to determine which sites of aromatase activity are critical to this pattern; adipose tissue and/or taste receptors could therefore potentially contribute to how female rats perceive sweet tastes and/or learn relations between sweet tastes and calories.
The data are consistent with the possibility that ovarian sources of circulating estrogens in adulthood play a minimal role in females’ responses to high intensity sweeteners, and that instead, localized production of estrogens by aromatase do play a significant role in females OVX as adults. As described above, aromatase is expressed in a variety of tissues associated with energy balance and food intake, including adipose tissue, taste buds, liver and the brain (Azcoitia et al., 2011; Belanger et al., 2002; Charlier et al., 2010; Cornil, 2009; Cornil et al., 2006; Cornil and Charlier, 2010; Sanghera et al., 1991; Shibuya et al., 2003; Simpson et al., 2000; Toyoshima et al., 2007). At present, it is not clear whether the consequences of inhibiting aromatase activity depend on effects operating centrally and/or peripherally. While previous data have shown that female rats OVX as adults and given a different aromatase inhibitor, letrozole, showed increased body weight gain and improved performance in the Morris water maze, a test of hippocampal-dependent learning (Aydin et al., 2008), that study did not provide direct evidence that either effect depended on central as compared to peripheral effects. However, some evidence is consistent with a central locus of aromatase-dependent effects on learning-related tasks, in particular for a role of hippocampal aromatase activity. For example, in a study in male rats, microinjection of anastrozole (the same aromatase inhibitor employed in the present work) directly into the hippocampus of adult male rats has been demonstrated to reverse impairments in the water maze induced by administration of testosterone, while not affecting impairments produced by estrogen (Moradpour et al., 2006). Further, in female rats, hippocampal synaptic plasticity is modulated by estrogens in vivo, effects that have been attributed to local synthesis of estrogens through aromatase (Fester et al., 2012; Fester et al., 2009; Kretz et al., 2004; Prange-Kiel et al., 2006; Prange-Kiel and Rune, 2006; Rune et al., 2006). In addition, estrogens produced locally in the brain of songbirds (e.g. neuroestrogens) have been clearly linked to behavioral changes in auditory processing in a sex-dependent fashion (e.g., Maney and Pinaud, 2011; Oberlander et al., 2004; Remage-Healey et al., 2010; Remage-Healey et al., 2012; Remage-Healey and Joshi, 2012; Yoder and Vicario, 2012). Nevertheless, identifying the specific sites of activity that contribute to altered responding to sweet tastes following inhibition of aromatase will require additional studies.
Experiment 2 indicates that patterns of responding to high-intensity sweeteners were dramatically altered by the timing of the OVX surgery. Females OVX prior to the onset of puberty, but tested as adults, showed patterns that were dramatically different compared with both females OVX as adults (Experiments 1 and 2), and gonadally-intact females (Swithers et al., 2012b). First, females OVX prior to the onset of puberty, and given the vehicle treatment along with saccharin-sweetened yogurt, ate more and gained more weight than vehicle females given glucose-sweetened yogurt, a pattern similar to that typically observed in adult male rats. Second, administration of anastrozole to females OVX prior to puberty abolished differences in weight gain between animals fed saccharin-sweetened and glucose-sweetened yogurt. Currently, it is not entirely clear why females that are not exposed to ovarian hormones through puberty would respond to inhibition of aromatase activity with a pattern that is reminiscent of adult female rats not given an inhibitor. However, as described above, data indicate that sex differences in sweet preferences as well as in a number of kinds of learning appear to emerge around the time of puberty in rats (e.g., Costanzo et al., 1995; Hodes and Shors, 2005; Wade and Zucker, 1969), suggesting that puberty represents a period during which behavioral and, presumably, structural changes relevant to feeding and learning occur. Further, sex differences in organization of central nervous system structures have been documented to appear following the adolescent/pubertal period in rodents (e.g., Gonzales et al., 2012; Konkle and McCarthy, 2011; Koshibu et al., 2005; Nugent et al., 2011; Schwarz et al., 2010; Wilson et al., 2011).
While much of the work on the consequences of puberty has focused on changes in reproductive structures and behaviors, ingestive behaviors in female rats may undergo changes during the pubertal period. For example, previous work from our lab has demonstrated that administration of the drug mercaptoacetate (MA), which interferes with fatty acid oxidation, produces different effects in male rats compared to both gonadally-intact female rats and females OVX as adults (Swithers et al., 2008). However, females OVX as adolescents prior to puberty (at 23–25 days of age) show responses to MA that are similar to those observed in males; these effects can be reversed by administration of exogenous estradiol, and appear to reflect organizational, rather than activational, changes, consistent with organizational changes in reproductive responses in female mice that appear to depend on exposure to estradiol after postnatal day 15 (e.g., Brock et al., 2011).
While the pattern of data is most consistent with an organizational role of exposure to ovarian hormones during the pubertal period, what specifically is organized remains unknown. However, studies of neural structures implicated in sexual behavior in rats have documented developmental and hormonal effects across the lifespan, including for example, sexually dimorphic patterns in epigenetic changes in estrogen receptors in hypothalamic structures (e.g., Gonzales et al., 2012; Konkle and McCarthy, 2011; Nugent et al., 2011; Schwarz et al., 2010). Thus, the consequences of exposure to ovarian hormones during puberty for regulation of energy balance could include epigenetic alterations in sites related to expression of aromatase activity and/or receptors responsive to estrogens. Delineating these mechanisms will require additional work.
Finally, while the data indicate that differences in the pattern of responding to high-intensity sweeteners in adult females compared to adult males cannot be explained by activational effects of ovarian hormones in adulthood alone, it is possible that the organizational changes that occur during puberty may interact with activational effects that occur during adulthood. At this time we cannot completely eliminate the possibility that circulating estrogens play some role in observed differences in males compared to females (even OVX females) since neither the chow diets nor the yogurts used were specifically estrogen-free. Few data regarding specific amounts of estrogens in commercial yogurt products are available, but previous data has indicated that measurable levels of estrogens are present in commercial dairy products (e.g. (Pape-Zambito et al., 2010) as well as commercial laboratory chows (e.g., Brown and Setchell, 2001). Thus, females OVX as adults may show different responses to the sweeteners because the organizational changes that occurred during puberty have primed them to respond differently to dietary sources of estrogens. Again, additional studies will be required to examine this possibility.
Overall, these data suggest that differences between males and females in response to high-intensity sweeteners may be driven by organizational changes in patterns of local estrogen production, or responses to such local estrogen production, that typically occur during puberty. These data, along with previous work from our lab, underscore the importance of non-ovarian hormones as potential sources of sex differences in the regulation of food intake and energy balance.
Highlights.
Female rats ovariectomized (OVX) as adults do not gain excess weight when given saccharin-sweetened foods.
Females OVX as adolescents show excess weight gain when given saccharin.
Females OVX as adults given saccharin show excess weight gain when local production of estrogen is suppressed with the aromatase inhibitor anastrozole
Local production of estrogens may affect energy regulation in female rats.
Organizational changes in energy balance may occur during puberty in females.
Acknowledgments
We thank Melissa McCurley, Natalie Rappaport, and Ethan Flint for technical assistance, Dr. Terry Davidson for his helpful comments and discussion and Dr. Robert Meisel for his comments on a previous version of the manuscript. Supported by NIH grants R01 DK076078 and P01 HD052112. The funding source had no role in study design; collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ackroff K, Sclafani A. Fructose-conditioned flavor preferences in male and female rats: effects of sweet taste and sugar concentration. Appetite. 2004;42:287–297. doi: 10.1016/j.appet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- American Dietetic A. Position of the American Dietetic Association: use of nutritive and nonnutritive sweeteners.[Erratum appears in J Am Diet Assoc. 2004 Jun;104(6):1013] Journal of the American Dietetic Association. 2004;104:255–275. doi: 10.1016/j.jada.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N. Modulation of appetite by gonadal steroid hormones. Philos Trans R Soc Lond B Biol Sci. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchley DP, Weaver KL, Eckel LA. Taste responses to dilute sucrose solutions are modulated by stage of the estrous cycle and fenfluramine treatment in female rats. Physiol Behav. 2005;86:265–271. doi: 10.1016/j.physbeh.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Aydin M, Yilmaz B, Alcin E, Nedzvetsky VS, Sahin Z, Tuzcu M. Effects of letrozole on hippocampal and cortical catecholaminergic neurotransmitter levels, neural cell adhesion molecule expression and spatial learning and memory in female rats. Neuroscience. 2008;151:186–194. doi: 10.1016/j.neuroscience.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Azcoitia I, Yague JG, Garcia-Segura LM. Estradiol synthesis within the human brain. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Shors TJ. The hippocampus is necessary for enhancements and impairments of learning following stress. Nature neuroscience. 2007;10:1401–1403. doi: 10.1038/nn1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger C, Luu-The V, Dupont P, Tchernof A. Adipose tissue intracrinology: potential importance of local androgen/estrogen metabolism in the regulation of adiposity. Horm Metab Res. 2002;34:737–745. doi: 10.1055/s-2002-38265. [DOI] [PubMed] [Google Scholar]
- Bellisle F, Drewnowski A. Intense sweeteners, energy intake and the control of body weight. Eur J Clin Nutr. 2007;61:691–700. doi: 10.1038/sj.ejcn.1602649. [DOI] [PubMed] [Google Scholar]
- Brock O, Baum MJ, Bakker J. The development of female sexual behavior requires prepubertal estradiol. J Neurosci. 2011;31:5574–5578. doi: 10.1523/JNEUROSCI.0209-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LM, Clegg DJ. Central effects of estradiol in the regulation of food intake, body weight, and adiposity. J Steroid Biochem Mol Biol. 2010;122:65–73. doi: 10.1016/j.jsbmb.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NM, Setchell KD. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Laboratory investigation; a journal of technical methods and pathology. 2001;81:735–747. doi: 10.1038/labinvest.3780282. [DOI] [PubMed] [Google Scholar]
- Butera PC. Estradiol and the control of food intake. Physiol Behav. 2010;99:175–180. doi: 10.1016/j.physbeh.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers KC. Hormonal influences on sexual dimorphism in rate of extinction of a conditioned taste aversion in rats. J Comp Physiol Psychol. 1976;90:851–856. doi: 10.1037/h0077270. [DOI] [PubMed] [Google Scholar]
- Chambers KC. Sexual dimorphisms as an index of hormonal influences on conditioned food aversions. Ann N Y Acad Sci. 1985;443:110–125. doi: 10.1111/j.1749-6632.1985.tb27067.x. [DOI] [PubMed] [Google Scholar]
- Chambers KC, Sengstake CB. Sexually dimorphic extinction of a conditioned taste aversion in rats. Animal learning & behavior. 1976;4:181–185. doi: 10.3758/bf03214032. [DOI] [PubMed] [Google Scholar]
- Chambers KC, Sengstake CB, Yoder RL, Thornton JE. Sexually dimorphic acquisition of a conditioned taste aversion in rats: effects of gonadectomy, testosterone replacement and water deprivation. Physiol Behav. 1981;27:83–88. doi: 10.1016/0031-9384(81)90303-6. [DOI] [PubMed] [Google Scholar]
- Charlier TD, Cornil CA, Ball GF, Balthazart J. Diversity of mechanisms involved in aromatase regulation and estrogen action in the brain. Biochim Biophys Acta. 2010;1800:1094–1105. doi: 10.1016/j.bbagen.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA. Rapid regulation of brain oestrogen synthesis: the behavioural roles of oestrogens and their fates. J Neuroendocrinol. 2009;21:217–226. doi: 10.1111/j.1365-2826.2009.01822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Ball GF, Balthazart J. Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res. 2006;1126:2–26. doi: 10.1016/j.brainres.2006.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Charlier TD. Rapid behavioural effects of oestrogens and fast regulation of their local synthesis by brain aromatase. J Neuroendocrinol. 2010;22:664–673. doi: 10.1111/j.1365-2826.2010.02023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo DJ, Riccio DC, Kissinger S. State-dependent retention produced with estrus in rats. Physiol Behav. 1995;57:1009–1011. doi: 10.1016/0031-9384(94)00007-r. [DOI] [PubMed] [Google Scholar]
- Curtis KS, Contreras RJ. Sex differences in electrophysiological and behavioral responses to NaCl taste. Behav Neurosci. 2006;120:917–924. doi: 10.1037/0735-7044.120.4.917. [DOI] [PubMed] [Google Scholar]
- Curtis KS, Davis LM, Johnson AL, Therrien KL, Contreras RJ. Sex differences in behavioral taste responses to and ingestion of sucrose and NaCl solutions by rats. Physiol Behav. 2004;80:657–664. doi: 10.1016/j.physbeh.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Curtis KS, Stratford JM, Contreras RJ. Estrogen increases the taste threshold for sucrose in rats. Physiol Behav. 2005;86:281–286. doi: 10.1016/j.physbeh.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Dalla C, Papachristos EB, Whetstone AS, Shors TJ. Female rats learn trace memories better than male rats and consequently retain a greater proportion of new neurons in their hippocampi. Proc Natl Acad Sci U S A. 2009;106:2927–2932. doi: 10.1073/pnas.0809650106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Shors TJ. Sex differences in learning processes of classical and operant conditioning. Physiol Behav. 2009;97:229–238. doi: 10.1016/j.physbeh.2009.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Martin AA, Clark K, Swithers SE. Intake of high-intensity sweeteners alters the ability of sweet taste to signal caloric consequences: implications for the learned control of energy and body weight regulation. Q J Exp Psychol (Hove) 2011;64:1430–1441. doi: 10.1080/17470218.2011.552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TL, Swithers SE. A Pavlovian approach to the problem of obesity. International Journal of Obesity & Related Metabolic Disorders. 2004;28:933–935. doi: 10.1038/sj.ijo.0802660. [DOI] [PubMed] [Google Scholar]
- de Jongh R, Geyer MA, Olivier B, Groenink L. The effects of sex and neonatal maternal separation on fear-potentiated and light-enhanced startle. Behav Brain Res. 2005;161:190–196. doi: 10.1016/j.bbr.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D’Agostino RB, Gaziano JM, Vasan RS. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116:480–488. doi: 10.1161/CIRCULATIONAHA.107.689935. [DOI] [PubMed] [Google Scholar]
- Duffey KJ, Steffen LM, Van Horn L, Jacobs DR, Jr, Popkin BM. Dietary patterns matter: diet beverages and cardiometabolic risks in the longitudinal Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr. 2012;95:909–915. doi: 10.3945/ajcn.111.026682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukes M. The relevance of preclinical models to the treatment of postmenopausal breast cancer. Oncology. 1997;54(Suppl 2):6–10. doi: 10.1159/000227748. [DOI] [PubMed] [Google Scholar]
- Eckel LA. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol Behav. 2011;104:517–524. doi: 10.1016/j.physbeh.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fester L, Prange-Kiel J, Zhou L, Blittersdorf BV, Bohm J, Jarry H, Schumacher M, Rune GM. Estrogen-regulated synaptogenesis in the hippocampus: sexual dimorphism in vivo but not in vitro. J Steroid Biochem Mol Biol. 2012;131:24–29. doi: 10.1016/j.jsbmb.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Fester L, Zhou L, Butow A, Huber C, von Lossow R, Prange-Kiel J, Jarry H, Rune GM. Cholesterol-promoted synaptogenesis requires the conversion of cholesterol to estradiol in the hippocampus. Hippocampus. 2009;19:692–705. doi: 10.1002/hipo.20548. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- Fowler SP, Williams K, Resendez RG, Hunt KJ, Hazuda HP, Stern MP. Fueling the obesity epidemic? Artificially sweetened beverage use and long-term weight gain. Obesity (Silver Spring) 2008;16:1894–1900. doi: 10.1038/oby.2008.284. [DOI] [PubMed] [Google Scholar]
- Gardener H, Rundek T, Markert M, Wright CB, Elkind MS, Sacco RL. Diet Soft Drink Consumption is Associated with an Increased Risk of Vascular Events in the Northern Manhattan Study. J Gen Intern Med. 2012 doi: 10.1007/s11606-011-1968-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales KL, Quadros-Mennella P, Tetel MJ, Wagner CK. Anatomically-specific actions of oestrogen receptor in the developing female rat brain: effects of oestradiol and selective oestrogen receptor modulators on progestin receptor expression. J Neuroendocrinol. 2012;24:285–291. doi: 10.1111/j.1365-2826.2011.02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats(1) Brain Res. 2001;888:356–365. doi: 10.1016/s0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- Hiroi R, Neumaier JF. Differential effects of ovarian steroids on anxiety versus fear as measured by open field test and fear-potentiated startle. Behav Brain Res. 2006;166:93–100. doi: 10.1016/j.bbr.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Hodes GE, Shors TJ. Distinctive stress effects on learning during puberty. Horm Behav. 2005;48:163–171. doi: 10.1016/j.yhbeh.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrupka BJ, Smith GP, Geary N. Ovariectomy and estradiol affect postingestive controls of sucrose licking. Physiol Behav. 1997;61:243–247. doi: 10.1016/s0031-9384(96)00360-5. [DOI] [PubMed] [Google Scholar]
- James WP. WHO recognition of the global obesity epidemic. Int J Obes (Lond) 2008;32(Suppl 7):S120–126. doi: 10.1038/ijo.2008.247. [DOI] [PubMed] [Google Scholar]
- Kenney NJ, Redick JH. Effects of ovariectomy and subsequent estradiol replacement on intake of sweet solutions. Physiol Behav. 1980;24:807–809. doi: 10.1016/0031-9384(80)90418-7. [DOI] [PubMed] [Google Scholar]
- Konkle AT, McCarthy MM. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology. 2011;152:223–235. doi: 10.1210/en.2010-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshibu K, Ahrens ET, Levitt P. Postpubertal sex differentiation of forebrain structures and functions depend on transforming growth factor-alpha. J Neurosci. 2005;25:3870–3880. doi: 10.1523/JNEUROSCI.0175-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretz O, Fester L, Wehrenberg U, Zhou L, Brauckmann S, Zhao S, Prange-Kiel J, Naumann T, Jarry H, Frotscher M, Rune GM. Hippocampal synapses depend on hippocampal estrogen synthesis. J Neurosci. 2004;24:5913–5921. doi: 10.1523/JNEUROSCI.5186-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laska MN, Murray DM, Lytle LA, Harnack LJ. Longitudinal associations between key dietary behaviors and weight gain over time: transitions through the adolescent years. Obesity (Silver Spring) 2012;20:118–125. doi: 10.1038/oby.2011.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Shors TJ. High levels of estrogen enhance associative memory formation in ovariectomized females. Psychoneuroendocrinology. 2004;29:883–890. doi: 10.1016/j.psyneuen.2003.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig DS. Artificially sweetened beverages: cause for concern. JAMA. 2009;302:2477–2478. doi: 10.1001/jama.2009.1822. [DOI] [PubMed] [Google Scholar]
- Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome: the Atherosclerosis Risk in Communities study. Circulation. 2008;117:754–761. doi: 10.1161/CIRCULATIONAHA.107.716159. [DOI] [PubMed] [Google Scholar]
- Maney DL, Pinaud R. Estradiol-dependent modulation of auditory processing and selectivity in songbirds. Front Neuroendocrinol. 2011;32:287–302. doi: 10.1016/j.yfrne.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, De Oca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and Pavlovian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Res. 1994;661:25–34. doi: 10.1016/0006-8993(94)91176-2. [DOI] [PubMed] [Google Scholar]
- Moradpour F, Naghdi N, Fathollahi Y. Anastrozole improved testosterone-induced impairment acquisition of spatial learning and memory in the hippocampal CA1 region in adult male rats. Behav Brain Res. 2006;175:223–232. doi: 10.1016/j.bbr.2006.08.037. [DOI] [PubMed] [Google Scholar]
- Nettleton JA, Polak JF, Tracy R, Burke GL, Jacobs DR., Jr Dietary patterns and incident cardiovascular disease in the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2009;90:647–654. doi: 10.3945/ajcn.2009.27597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent BM, Schwarz JM, McCarthy MM. Hormonally mediated epigenetic changes to steroid receptors in the developing brain: implications for sexual differentiation. Horm Behav. 2011;59:338–344. doi: 10.1016/j.yhbeh.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander JG, Schlinger BA, Clayton NS, Saldanha CJ. Neural aromatization accelerates the acquisition of spatial memory via an influence on the songbird hippocampus. Horm Behav. 2004;45:250–258. doi: 10.1016/j.yhbeh.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009–2010. NCHS Data Brief. 2012:1–8. [PubMed] [Google Scholar]
- Pape-Zambito DA, Roberts RF, Kensinger RS. Estrone and 17beta-estradiol concentrations in pasteurized-homogenized milk and commercial dairy products. Journal of dairy science. 2010;93:2533–2540. doi: 10.3168/jds.2009-2947. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Fester L, Zhou L, Lauke H, Carretero J, Rune GM. Inhibition of hippocampal estrogen synthesis causes region-specific downregulation of synaptic protein expression in hippocampal neurons. Hippocampus. 2006;16:464–471. doi: 10.1002/hipo.20173. [DOI] [PubMed] [Google Scholar]
- Prange-Kiel J, Rune GM. Direct and indirect effects of estrogen on rat hippocampus. Neuroscience. 2006;138:765–772. doi: 10.1016/j.neuroscience.2005.05.061. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Lehmann J, Feldon J. Effect of sex on fear conditioning is similar for context and discrete CS in Wistar, Lewis and Fischer rat strains. Pharmacology, biochemistry, and behavior. 1999;64:753–759. doi: 10.1016/s0091-3057(99)00147-1. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci U S A. 2010;107:3852–3857. doi: 10.1073/pnas.0906572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Dong SM, Chao A, Schlinger BA. Sex-specific, rapid neuroestrogen fluctuations and neurophysiological actions in the songbird auditory forebrain. Journal of neurophysiology. 2012;107:1621–1631. doi: 10.1152/jn.00749.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Joshi NR. Changing neuroestrogens within the auditory forebrain rapidly transform stimulus selectivity in a downstream sensorimotor nucleus. J Neurosci. 2012;32:8231–8241. doi: 10.1523/JNEUROSCI.1114-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rune GM, Lohse C, Prange-Kiel J, Fester L, Frotscher M. Synaptic plasticity in the hippocampus: effects of estrogen from the gonads or hippocampus? Neurochem Res. 2006;31:145–155. doi: 10.1007/s11064-005-9004-8. [DOI] [PubMed] [Google Scholar]
- Sanghera MK, Simpson ER, McPhaul MJ, Kozlowski G, Conley AJ, Lephart ED. Immunocytochemical distribution of aromatase cytochrome P450 in the rat brain using peptide-generated polyclonal antibodies. Endocrinology. 1991;129:2834–2844. doi: 10.1210/endo-129-6-2834. [DOI] [PubMed] [Google Scholar]
- Schulze MB, Manson JE, Ludwig DS, Colditz GA, Stampfer MJ, Willett WC, Hu FB. Sugar-sweetened beverages, weight gain, and incidence of type 2 diabetes in young and middle-aged women. JAMA. 2004;292:927–934. doi: 10.1001/jama.292.8.927. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Nugent BM, McCarthy MM. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology. 2010;151:4871–4881. doi: 10.1210/en.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya K, Takata N, Hojo Y, Furukawa A, Yasumatsu N, Kimoto T, Enami T, Suzuki K, Tanabe N, Ishii H, Mukai H, Takahashi T, Hattori TA, Kawato S. Hippocampal cytochrome P450s synthesize brain neurosteroids which are paracrine neuromodulators of synaptic signal transduction. Biochim Biophys Acta. 2003;1619:301–316. doi: 10.1016/s0304-4165(02)00489-0. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Leuner B. Estrogen-mediated effects on depression and memory formation in females. Journal of affective disorders. 2003;74:85–96. doi: 10.1016/s0165-0327(02)00428-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Lewczyk C, Pacynski M, Mathew PR, Pickett J. Stages of estrous mediate the stress-induced impairment of associative learning in the female rat. Neuroreport. 1998;9:419–423. doi: 10.1097/00001756-199802160-00012. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G. Testosterone in utero and at birth dictates how stressful experience will affect learning in adulthood. Proc Natl Acad Sci U S A. 2002;99:13955–13960. doi: 10.1073/pnas.202199999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E, Rubin G, Clyne C, Robertson K, O’Donnell L, Jones M, Davis S. The role of local estrogen biosynthesis in males and females. Trends Endocrinol Metab. 2000;11:184–188. doi: 10.1016/s1043-2760(00)00254-x. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Davidson TL. A role for sweet taste: calorie predictive relations in energy regulation by rats. Behav Neurosci. 2008;122:161–173. doi: 10.1037/0735-7044.122.1.161. [DOI] [PubMed] [Google Scholar]
- Swithers SE, Martin AA, Davidson TL. High-intensity sweeteners and energy balance. Physiol Behav. 2010;100:55–62. doi: 10.1016/j.physbeh.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swithers SE, McCurley M, Hamilton E, Doerflinger A. Influence of ovarian hormones on development of ingestive responding to alterations in fatty acid oxidation in female rats. Horm Behav. 2008;54:471–477. doi: 10.1016/j.yhbeh.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swithers SE, Ogden SB, Laboy AF, Davidson TL. Saccharin pre-exposure enhances appetitive flavor learning in pre-weanling rats. Dev Psychobiol. 2012a doi: 10.1002/dev.21047. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swithers SE, Sample CS, Davidson TL. Adverse effects of high-intensity sweeteners on energy intake and weight control in male and obesity-prone female rats. 2012b. Submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima K, Seta Y, Toyono T, Kataoka S. Immunohistochemical identification of cells expressing steroidogenic enzymes cytochrome P450scc and P450 aromatase in taste buds of rat circumvallate papillae. Arch Histol Cytol. 2007;70:215–224. doi: 10.1679/aohc.70.215. [DOI] [PubMed] [Google Scholar]
- van Haaren F, van Hest A, Heinsbroek RP. Behavioral differences between male and female rats: effects of gonadal hormones on learning and memory. Neurosci Biobehav Rev. 1990;14:23–33. doi: 10.1016/s0149-7634(05)80157-5. [DOI] [PubMed] [Google Scholar]
- Wade GN. Gonadal hormones and behavioral regulation of body weight. Physiol Behav. 1972;8:523–534. doi: 10.1016/0031-9384(72)90340-x. [DOI] [PubMed] [Google Scholar]
- Wade GN, Gray JM. Gonadal effects on food intake and adiposity: a metabolic hypothesis. Physiol Behav. 1979;22:583–593. doi: 10.1016/0031-9384(79)90028-3. [DOI] [PubMed] [Google Scholar]
- Wade GN, Zucker I. Hormonal and developmental influences on rat saccharin preferences. J Comp Physiol Psychol. 1969;69:291–300. doi: 10.1037/h0028208. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang Q, Jin S, Feng M, Kang X, Zhao S, Liu S, Zhao W. Immoderate inhibition of estrogen by anastrozole enhances the severity of experimental polyarthritis. Exp Gerontol. 2009;44:398–405. doi: 10.1016/j.exger.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Gunnar MR, Brett LP, Gonzalez CA, Levine S. Sex differences in biobehavioral responses to conflict in a taste aversion paradigm. Physiol Behav. 1982;29:201–210. doi: 10.1016/0031-9384(82)90004-x. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Westberry JM, Trout AL. Estrogen receptor-alpha gene expression in the cortex: sex differences during development and in adulthood. Horm Behav. 2011;59:353–357. doi: 10.1016/j.yhbeh.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood GE, Beylin AV, Shors TJ. The contribution of adrenal and reproductive hormones to the opposing effects of stress on trace conditioning in males versus females. Behav Neurosci. 2001;115:175–187. doi: 10.1037/0735-7044.115.1.175. [DOI] [PubMed] [Google Scholar]
- Wood GE, Shors TJ. Stress facilitates classical conditioning in males, but impairs classical conditioning in females through activational effects of ovarian hormones. Proc Natl Acad Sci U S A. 1998;95:4066–4071. doi: 10.1073/pnas.95.7.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC, Gotoh K, Clegg DJ. Gender differences in the control of energy homeostasis. Exp Biol Med (Maywood) 2003;228:1175–1180. doi: 10.1177/153537020322801012. [DOI] [PubMed] [Google Scholar]
- Woods SC, Ramsay DS. Pavlovian influences over food and drug intake. Behav Brain Res. 2000;110:175–182. doi: 10.1016/s0166-4328(99)00194-1. [DOI] [PubMed] [Google Scholar]
- Yang Q. Gain weight by “going diet?” Artificial sweeteners and the neurobiology of sugar cravings: Neuroscience 2010. Yale J Biol Med. 2010;83:101–108. [PMC free article] [PubMed] [Google Scholar]
- Yoder KM, Vicario DS. To modulate and be modulated: estrogenic influences on auditory processing of communication signals within a socio-neuro-endocrine framework. Behav Neurosci. 2012;126:17–28. doi: 10.1037/a0026673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan DL, Chambers KC. Estradiol accelerates extinction of a conditioned taste aversion in female and male rats. Horm Behav. 1999;36:1–16. doi: 10.1006/hbeh.1999.1520. [DOI] [PubMed] [Google Scholar]