Abstract
Purpose
Acute Hemorrhagic Leukoencephalitis (AHLE) is a rare demyelinating disorder of acute onset, rapid deterioration and significant morbidity and mortality. Most often described as a post-infectious complication of an upper respiratory illness, its precise pathophysiology remains unclear. We describe two pediatric patients with AHLE with partial complement factor I (FI) deficiency whose successful treatment included the interleukin-1 (IL-1) receptor antagonist, anakinra, implicating a role for FI and IL-1 in this disorder.
Methods
Extensive clinical workup of two patients presenting with AHLE revealed complement abnormalities, specifically related to the alternative pathway and its regulator, FI. Aggressive management with steroids, immunoglobulin, and anakinra ultimately led to improvement of clinical status and near return to neurologic baseline in both patients. Genetic sequencing of the FI coding regions of the patients and their families was performed. In vitro protein expression studies and immunohistochemistry of fixed brain tissue was used to investigate pathogenic mechanisms.
Results
Two novel mutations in FI were identified in our patients, which result in failure to secrete FI. Immunohistochemical evaluation of brain tissue demonstrated positive staining for C3, membrane attack complex (MAC) and IL-1.
Conclusions
We propose AHLE is an unreported, rare phenotype for partial FI deficiency. The upregulation of C3, MAC and IL-1 with subsequent demyelination support a pathologic role for complement activation in AHLE, and suggest anakinra as an important adjunctive therapy in this disease.
Keywords: Acute Hemorrhagic Leukoencephalitis, Complement Factor I, IL-1, IL-1 receptor antagonist
INTRODUCTION
Acute Hemorrhagic Leukoencephalitis (AHLE) is a rare disorder characterized by rapid neurologic decline, peripheral and cerebrospinal neutrophilia, with demyelinating disease evident on neuroimaging. Pathologic features of perivascular neutrophilic and macrophage inflammation and focal demyelination are observed on brain biopsy or postmortem examination [1]. Currently, the etiology of AHLE remains unknown, although it has been associated with upper respiratory infections, mumps, and Mycoplasma pneumoniae. Untreated disease is uniformly fatal. Survival has been reported in rare pediatric cases of AHLE in which early treatment was implemented, including decompressive craniotomy and immunomodulatory therapies such as intravenous immunoglobulin (IVIG) and high dose intravenous corticosteroids [1, 2].
In this report, we describe two pediatric patients with AHLE, in whom partial FI deficiency was identified. Both patients were treated with corticosteroids, IVIG, and hypertonic saline [3] with limited clinical response, but demonstrated dramatic improvement when the interleukin-1 (IL-1) receptor antagonist, anakinra, was added to the treatment regimen. These cases suggest a role for complement and IL-1 in the pathogenesis of AHLE.
MATERIALS & METHODS
Patient Samples and Genetic Sequencing
Blood samples were obtained from the two patients described as well as their immediate family members. All participants, or parents or legal guardians if the patient was a minor child, provided written informed consent under protocols approved by the UCSD institutional review board and have been performed in accordance with the 1964 Declaration of Helsinki and its later amendments. The thirteen exons of CFI were PCR amplified and sequenced to identify coding and splice site mutations using previously described primers [4].
Measurement of serum complement Factor I
Factor I was measured by radial immunodiffusion using an in-house assay developed at National Jewish Health, Denver, CO. The 2 standard deviation range is 29.3 – 58.5 µg/mL based on 30 healthy adults. The data for the range has normal distribution and the interassay variability is 7.7% for 65 specimens.
Expression of recombinant Factor I proteins
A pcDNA3-based expression vector for full-length human FI with a six-histidine tag at the N-terminus of the mature protein [5] was obtained as a generous gift from Dr. Sara C. Nilsson and Dr. Anna M. Blom (Lund University, Malmo, Sweden). Patient mutations in CFI, G212T and G587C, were introduced using a QuikChange protocol as per manufacturer’s instructions with the following primers, CTA CCG TAT CAG TGC CCA AAG AAT GTC ACT GCA GTG TGT GCA ACT AAC AGG and GGA TTA GAG ACC AGT TTG GCT GAA TCT ACT TTT ACT AAG AGA AGA ACT ATG, and their complements respectively. The CFI coding sequence in each of the mutated plasmids was verified by sequencing (Retrogen, Inc., San Diego, CA). Plasmids containing either wild-type CFI or one of the mutations were separately transiently transfected in HEK293 cells [6]. Protein synthesis and transfection efficiency were evaluated by flow cytometry. In these experiments, transfected cells were incubated for 4 hours with Brefeldin A (10 µg/mL) to maximize detection of FI intracellularly. Cells were permeabilized and stained with a murine monoclonal antibody to the six-histidine tag (Roche, Indianapolis, IN) followed by FITC-labeled polyclonal rat anti-mouse antibody (eBioscience, San Diego, CA). A second plasmid containing the IL-6 gene was used as a control for intracellular analysis of a secreted protein. Events were collected using the BD LSR II and FACSDiva software (BD Biosciences) with data analysis using Flow-Jo software. For measurement of protein secretion, supernatants from transiently transfected cells were collected after 24 hours, concentrated over Amicon Centrifugal Filters 10K (Millipore), and bound to Ni-NTA beads (Qiagen, Valencia, CA). Protein was eluted from the beads by serial transfers to buffers containing 0.5M NaCl, 20mM Tris, 10% glycerol, 2mM DTT, 0.1% NP40, EDTA-free mini-complete protease inhibitors (Roche, Indianapolis, IN) and imidazole ranging from 0 mM to 600 mM. Protein expression was determined by Western blots using a mouse monoclonal antibody to the six-histidine tag (Roche Applied Science, Indianapolis, IN). The volume of each fraction loaded was normalized to the absorbance at 280 nm.
Immunohistochemistry
Immunohistochemistry on formalin-fixed, paraffin specimens was performed with rabbit polyclonal anti-human C3c (1:200, DAKO, Carpinteria, CA), murine monoclonal anti-human MAC (1:100, Quidel, San Diego, CA) and murine anti-human IL-1 (1:100, R and D Systems, Minneapolis, MN) using the Biocare Medical Mach 4 Detection System (Biocare Medical, Concord, CA) by modifications of previously published protocols as follows [7, 8]. Briefly, antigen retrieval was performed with Biocare Medical Borg Decloaker (Biocare Medical, Concord, CA). Protease digestion with Proteinase K (Biocare Medical, Concord, CA) for 5 minutes following antigen retrieval was required to completely uncover the C3c epitope [8, 9]. Sections from renal biopsies of patients with lupus glomerulonephritis and sections of normal brain biopsies were used as positive and negative controls, respectively.
RESULTS
Patients
Patient A is a 17 year-old female of Filipino descent, who at 10 years of age developed diffuse cerebral vasogenic edema and cerebrospinal fluid (CSF) pleocytosis of neutrophils and red blood cells [2]. She had been previously healthy, and immunizations were up to date. She had an unremarkable course of community-acquired varicella zoster infection several years prior to admission, and there were no other illnesses to suggest an underlying immunodeficiency. Partial hemicraniectomy for an evolving herniation syndrome was performed and brain biopsy obtained. Histopathology revealed perivascular hemorrhagic necrosis, subacute inflammation in the subcortical white matter, patchy demyelination and an inflammatory infiltrate with a neutrophilic and histiocytic predominance [2]. After an infectious etiology was ruled out, she was treated with high-dose corticosteroids and IVIG, as previously described. She recovered with only a mild learning disability, and remained healthy for seven years.
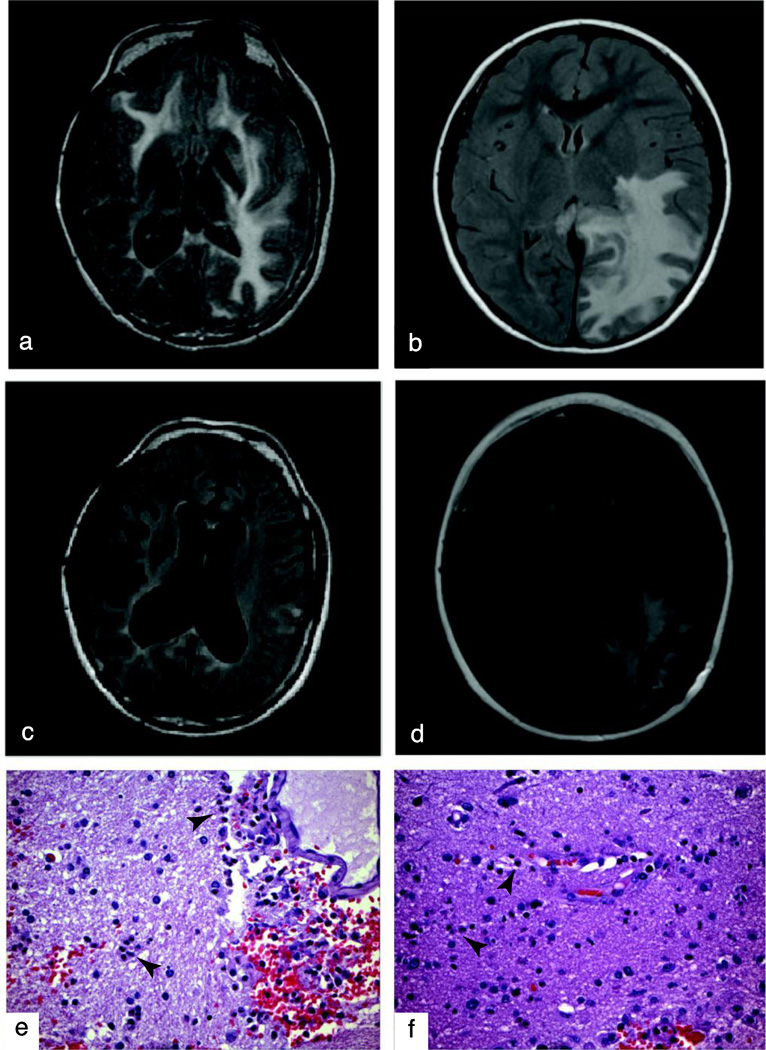
At the age of 17, she suffered a relapse with a similar presentation of headache and hallucinations and was re-admitted to Rady Children’s Hospital, San Diego. In the interim, there were no additional infections, aside from mild upper respiratory infections. On admission, she was afebrile, and her physical exam was notable for nystagmus, reduced pupillary reaction to light and an unsteady gait. MRI of her brain showed an interval development of diffuse white matter edema of the cerebrum and cerebellum (Figure 1a). CSF revealed pleocytosis with 392 WBC/µL of which 73% were neutrophils, and 665 RBC/µL. The concentration of myelin basic protein was >800 ng/mL (normal <1.5 ng/mL). In the CSF, no oligoclonal bands were seen and a source of acute infection could not be identified (Supplemental Table 1).
FIG. 1. Brain imaging and biopsy.
a,c, Brain MRI, Axial T2, on admission for Patient A (a) and Patient B (c). b,d, Brain MRI, Axial T2, Post-discharge. e,f, White matter with multiple foci of perivascular myelin degradation, hemorrhage, edema, and scattered infiltrating neutrophils (arrowheads), foamy macrophages and hemosiderin-laden macrophages in brain biopsies from Patient A (e) and Patient B (f). (H&E, X100)
Despite immediate treatment with high-dose corticosteroids, IVIG, and hypertonic saline for elevated intracranial pressure [3], she developed declining mental status with status epilepticus. In the absence of detectable infection, CSF neutrophilia and neutrophilic brain infiltration suggested an innate immune basis for the injury. Our previous experience with IL-1 receptor antagonist (IL-1Ra) responsive neutrophilic inflammation in autoinflammatory diseases [10] and evidence that the recombinant form of IL-1Ra, anakinra, is effective in reducing CNS-inflammation in patients with neonatal onset multisystem inflammatory disease (NOMID) [11], suggested that this might be an effective adjunctive therapy. Treatment with anakinra was initiated at 100 mg given daily subcutaneously, concurrent with corticosteroids. Within weeks, the patient recovered her pre-admission neurologic status with marked improvement of her MRI findings (Figure 1b). Prednisone and anakinra were tapered over six and eight months, respectively, with no emergence of relapse in 21 months of follow-up.
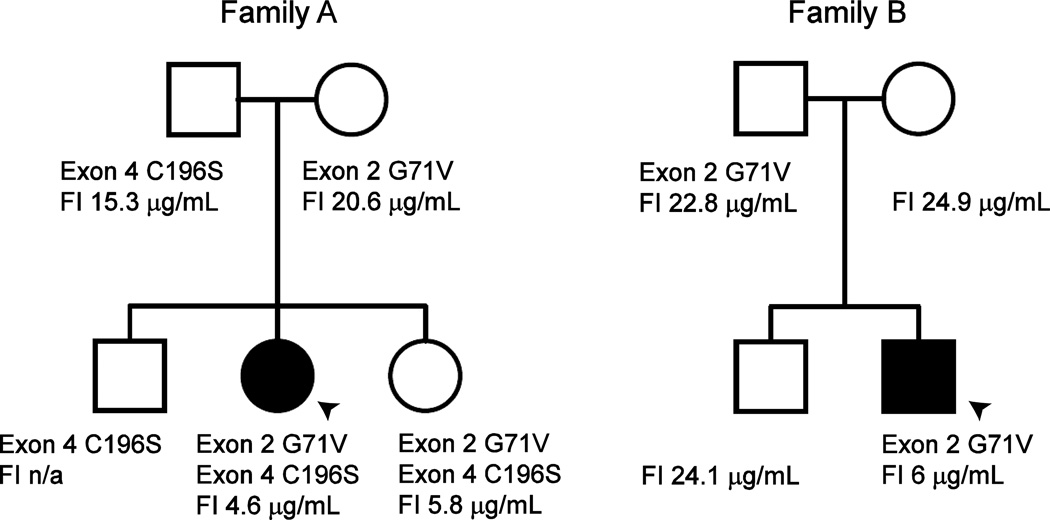
Extensive laboratory evaluation of autoimmune and inflammatory parameters was pursued upon her second AHLE presentation, demonstrating a negative ANA, and absence of C3 nephritic factor, phospholipid antibodies, anti-dsDNA, anti-SSA and anti-SSB antibodies (Supplemental Table 1). Her only significant findings were complement abnormalities with a C3 of 41 mg/dL (normal range 74–127 mg/dL), a C4 of 30 mg/dL (18–47 mg/dL), CH50 of 21 U/mL (30–75 U/mL) and AH50 of <10% of normal (75–170). Further evaluation of the alternative pathway demonstrated Factor B of 43.8 µg/mL (127.6–278.5 µg/mL), Factor D of 1.63 µg/mL (1.69–3.08 µg/mL), properdin 20.2 µg/mL (22.3–67.6 µg/mL), Factor I of 4.6 µg/mL (29–59 µg/mL), and Factor H of 107 µg/mL (160–412 µg/mL) consistent with rapid turnover of the alternative pathway. Complement studies on immediate family members revealed low FI levels in the healthy sister. Her mother, father and brother, also healthy, were found to have mildly reduced FI levels (Figure 2).
FIG. 2. FI mutations and serum levels.
Pedigrees identifying mutations in CFI and their respective serum complement factor I (FI) levels (normal 29–59 µg/mL). Probands with AHLE are indicated by arrowheads. Left, Family A. Two novel mutations identified in the CFI gene. Patient and her sister are compound heterozygotes. Right, Family B. Patient and father share the G71V mutation also found in Family A. A second coding mutation was not identified, but a noncoding or regulatory variant could also explain the markedly decreased FI serum levels
Patient B is a 10 year-old, unrelated male of Filipino descent who presented to Rady Children’s Hospital, San Diego with headache and fatigue, with rapid neurologic decline over several hours. He was previously healthy, and had received all recommended immunizations for his age. His past medical history, as related to infections, was unremarkable except for mild upper respiratory infections and there were no prior illnesses suggestive of immunodeficiency. There was a history of an influenza-like illness three weeks prior to presentation. On admission to the ICU, he was afebrile, and physical exam was notable for difficulties with expressive language and decreased strength. Primary evaluation demonstrated a peripheral leukocytosis of 20.9 ×103 cells/µL, with 91% segmented neutrophils and elevated inflammatory markers, including CRP and ESR (Supplemental Table 1). Examination of the CSF demonstrated a significant pleocytosis (607 cells/µL) of predominantly neutrophils (91%) and myelin basic protein >900 ng/mL. Initial CT imaging of the brain demonstrated vasogenic edema requiring a left posterior craniotomy and MRI revealed the extensive white matter signal abnormalities in the corpus callosum and left temporoparietooccipital lobe consistent with encephalitis (Figure 1c).
Histopathologic examination of brain biopsy tissue revealed neutrophilic inflammation with multiple foci of myelin degradation, predominantly surrounding the vascular structures, with reactive endothelial cell changes and no distinct evidence of vasculitis (Figure 1f). Tests for acute infection with influenza, Epstein-Barr virus, cytomegalovirus, herpes simplex virus, HHV-6, West Nile virus, mycobacteria, cryptococcus and coccidioides were negative. Taken together, these studies support the diagnosis of AHLE.
Due to the clinical improvement in our prior patient, treatment with high-dose intravenous corticosteroids, hypertonic saline, IVIG, and anakinra 100 mg subcutaneously daily was initiated early in his course. Six months after the initiation of treatment, MRI revealed improvement of edema and evolving necrosis at the corpus collosum, without evidence of new white matter lesions (Figure 1d). While on anakinra therapy, the CSF myelin basic protein decreased (16.5 ng/mL) and leukocytosis resolved. His CRP and ESR have remained less than 0.5 mg/dL and 7 mm/hr, respectively. He participated in an intensive inpatient rehabilitation program leading to gradual improvement in fine and gross motor skills, as well as speech, and was discharged after 2 months, an encouraging outcome in this severe disease. Attempts to wean him from anakinra have led to relapse of symptoms, including decreased balance, speech difficulty, and increased spasticity, all of which improve with re-instating anakinra therapy. Two years after his initial presentation, he continues on anakinra 100mg administered subcutaneously daily.
As with Patient A, we identified abnormalities in the alternative complement pathway with a FI level of 6 µg/mL, and Factor H of 136 µg/mL. Consistent with these levels was C3 of <40 mg/dL, C4 of 52 mg/dL, CH50 of 40 U/mL, and AH50 that was reduced to 29% of normal. Complement studies on his immediate family members revealed mildly reduced FI levels in the mother, father and brother.
Molecular findings
As reduced FI levels were the most significant abnormality detected in our patients, we focused on the CFI gene. Sequencing the 13 exons of CFI in our two patients revealed two novel missense mutations. Patient A and her sister are compound heterozygotes for the glycine 71 to valine (G71V) amino acid substitution encoded by exon 2 and the cysteine 196 to serine (C196S) amino acid substitution encoded by exon 4, while her mother, father and brother are heterozygotes with single CFI mutations, consistent with their reduced serum FI levels (Figure 2, Left). Although his FI serum levels are consistent with 2 affected alleles, Patient B is only heterozygous for the exon 2 mutation and lacks any other CFI coding or splice site mutations. His father is also heterozygous for the exon 2 mutation and no mutations were identified in his brother or mother, despite the mildly reduced FI levels similar to the heterozygotes from Family A (Figure 2, Right). Neither mutation was found in one hundred Filipino control alleles.
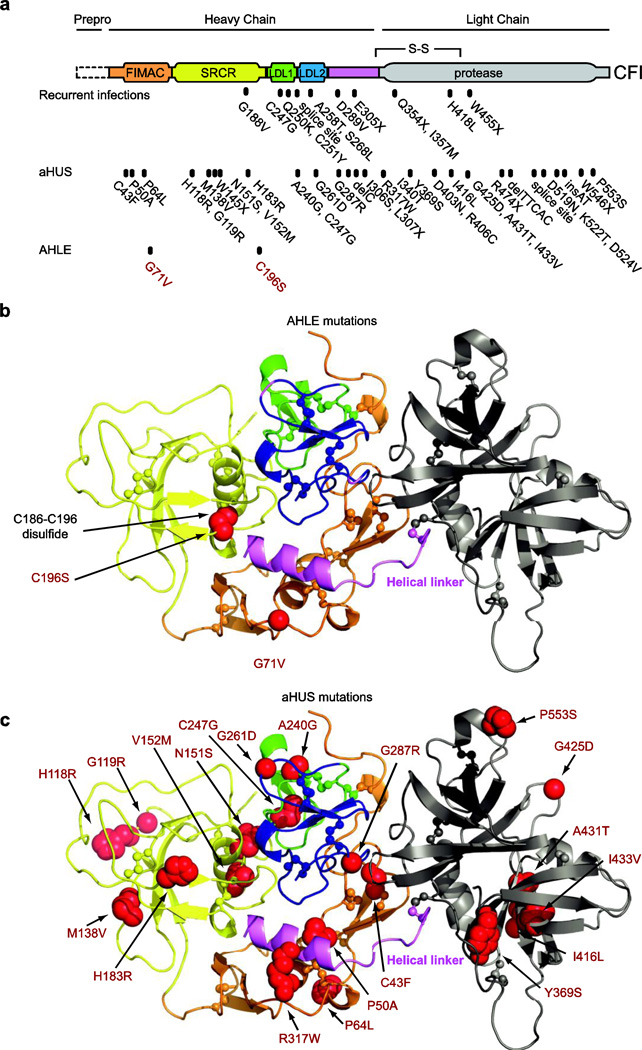
We modeled these mutations and more than 40 identified mutations in CFI [4, 12–16] on the FI structure [17] (Figure 3). The G71V substitution clashed with the core of the Factor I/membrane attack complex (FIMAC) domain. The C196S substitution disrupted a disulfide bond in the scavenger receptor cysteine-rich domain (SRCR). Remarkably, both substitutions could change the packing of the helical linker, thereby disrupting the disulfide bond between the helical linker and the light chain (Figure 3, violet). Thus, these mutations may cause folding problems, and/or early clearing of FI through protein aggregation or degradation, thereby reducing the FI levels.
FIG. 3. Molecular modeling of CFI mutations.
a, Domain structure with the positions of mutations associated with recurrent infections, aHUS, and AHLE [4–6, 12–16]. Mutations are numbered inclusive of the signal peptide (+18 relative to position numbers when the signal peptide is excluded) [13]. b, Structure of human FI [17] with AHLE mutations (red spheres) c. Structure with aHUS mutations (red spheres). Molecular images generated by PyMol [41]. FIMAC, Factor I/membrane attack complex; SRCR, scavenger receptor cysteine-rich
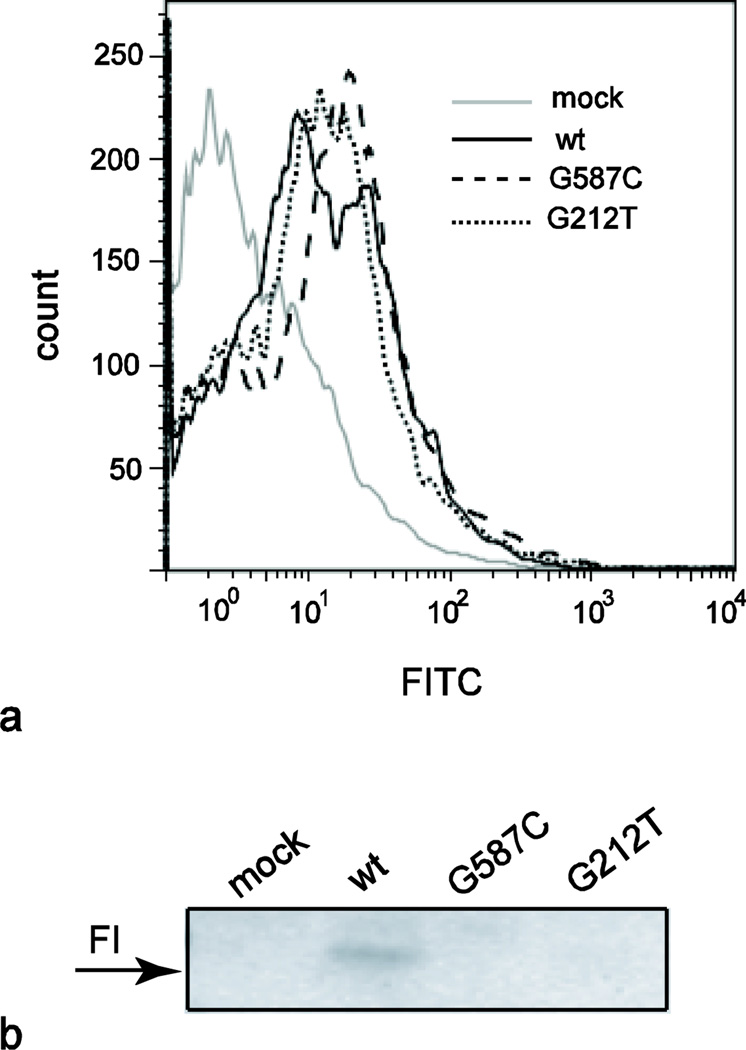
Previously described mutations in CFI have been shown to affect the secretion and/or function of FI [13]. To further evaluate the effect of these mutations on the FI protein, we utilized an in vitro transfection system to express wild-type and mutant FI. Intracellular FI was detected by flow cytometry similarly in the wild-type and mutant CFI transfected cells suggesting no effect of the mutation on the rate of protein translation (Figure 4a). However, subsequent analyses of the cell culture supernatants demonstrated that no FI protein could be detected from cells transfected with constructs containing either of our patients’ mutations (Figure 4b). In contrast, cells transfected with plasmid containing wild-type CFI produce detectable protein that was maintained in the supernatant for at least 24 hours, consistent with previous reports of FI half-life (not shown) [18].
FIG. 4. Recombinant FI protein expression.
a, Flow cytometry demonstrates similar intracellular expression of wild-type FI and mutants in transiently transfected HEK293 cells following treatment with Brefeldin A. b, Western blot of purified cell culture supernatants following transient transfection with wild-type (wt) or mutant CFI His-tagged plasmids. Images are representative of five independent experiments
Complement activation is increased in the brain parenchyma of patients with AHLE
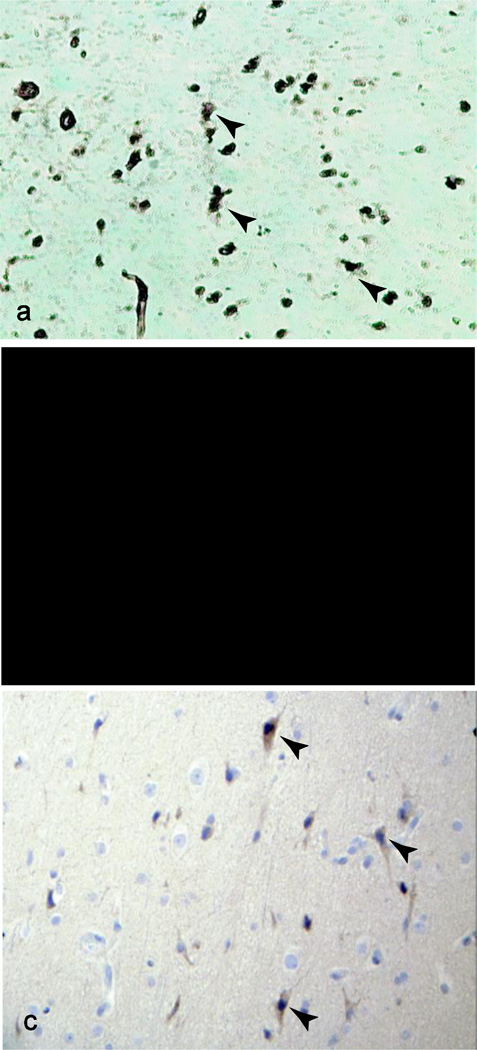
We suspect that in our patients, similar to patients with other CFI mutations [12, 19], uncontrolled activation via C3 occurs, leading to increased C3b production and a relative decrease in circulating C3 levels [8], consistent with our patients’ laboratory studies. Immunohistochemical evaluation of brain biopsy tissue from Patient B, revealed such an increase in C3, as well as increased membrane attack complex (MAC) deposition on neuronal cell surfaces compared to normal brain tissue (Figure 5a,b, Supplemental Figures S1,S2). In addition, immunohistochemical interrogation of the brain biopsy tissue of Patient B demonstrated IL-1β positivity (Figure 5c) that was not observed in normal brain tissue (Supplemental Figure S1), supporting the use of anakinra as a specific treatment of AHLE.
FIG. 5. Histologic analysis of brain tissue.
a–c, Immunohistochemistry of brain biopsy sections from Patient B demonstrating positive staining for C3 (a, X100), C5b-9 (MAC, b, X400) and IL-1 (c, X100). Arrowheads indicate positively staining cells.
DISCUSSION
FI is a plasma phase, serine protease responsible for inactivation of C3b and C4b, thereby preventing the progression to C5 convertases in both the alternative and classical pathways (summarized in Supplemental Figure S3). FI is produced primarily by the liver, but can also be produced by monocytes, keratinocytes, and astrocytes [20]. Although there are very few patients identified with immunodeficiency due to FI deficiency, there is a growing list of disorders associated with similar uncontrolled MAC activation, including atypical Hemolytic Uremic Syndrome (aHUS) [21], macular degeneration [22], and now AHLE. Patients with complete FI deficiency typically have increased susceptibility to pyogenic infections with encapsulated bacteria. Partial FI deficiency has been associated with immune complex-mediated diseases and aHUS (Reviewed in [12]), but a risk for demyelinating disease in these patients, has not been previously described. The clinical challenges of these cases reflect the intricate play of complement within the specialized neuromicroenvironment.
The role of complement in the neuromicroenvironment has been shown to be a double-edged sword, implicated in both cytolytic control of infection and neurogenerative recovery [23]. Although it has been shown that neuronal cells are capable of producing complement factors for control of local inflammatory reactions, they are also exquisitely sensitive to their cytotoxic effects and lysis via the MAC [7, 24]. Complement may be activated by intrinsic CNS proteins, including myelin [25, 26], and dysregulation of complement has been implicated in numerous neurologic diseases [27]. Additionally, there is evidence that complement plays a significant role in murine experimental autoimmune encephalitis (EAE), a model of acute demyelination [23, 28]. Thus, an inflammatory insult may result in amplification of complement-mediated cell death within the brain parenchyma by both a passive transfer of cytokines from the peripheral circulation, and by a secondary central increase in other complement proteins in response to these mediators. We propose that this feedback loop (Supplemental Figure S3) may be the mechanism behind AHLE in the context of partial FI deficiency.
The spectrum of medical phenotypes for FI deficiency, ranging from asymptomatic to severe, is reflected in the literature and it is interesting to note that many of the described patients have only one CFI mutant allele [14–16]. Her requirement for additional aggressive therapy, including anakinra, during relapse of her disease suggests that the cascading effects of complement may also divide patients into differing response groups. Earlier reports described families in which the proband with FI deficiency had recurrent infections from childhood, but their respective siblings, with similar serum complement studies, remained healthy [29, 30], reminiscent of the affected, yet asymptomatic sister of Patient A. The mechanism of this phenotypic variation is unknown and suggests that the siblings may not have been exposed to an environmental trigger or may express an additional protective immune mechanism that modulates the effect of the FI deficiency.
We identified two novel mutations in CFI that were not present in ethnically matched controls, and result in failure to secrete protein in an in vitro transfection system, similar to nearby mutations described in aHUS (C43F, FIMAC domain, and N151S, SRCR domain) [13]. Interestingly, there does not appear to be a correlation between the site of the mutation and subsequent phenotype. In our patients, the primary site of inflammation was in the CNS, whereas the structurally adjacent mutations such as P50A, R317W, and particularly P64L, which could have similar steric effects on FI protein, have only been described in aHUS [12, 14–16]. It is currently unclear why some point mutations led to AHLE and nearby mutations are associated with aHUS. Subsequent clinical laboratory studies in our patients have consistently demonstrated normal renal function, and clinically there has been no increase in frequency of infections. As both of our patients are of Filipino descent, their phenotype may be the result of these mutations on a certain genetic background. Clearly additional patients are required to resolve these questions.
The success of therapy with anakinra is suggested by the improved clinical courses for both of our patients. Interleukin-1β (IL-1β) is a pro-inflammatory cytokine prominent in many inflammatory diseases [31]. The activity of IL-1β is specifically regulated by IL-1Ra, which competes with IL-1β for receptor binding without inducing signal transduction. An imbalance between IL-1β and native IL-1Ra has correlated significantly with disease severity in acute and chronic neuroinflammatory disorders such as brain ischemia, Alzheimer’s disease, and multiple sclerosis [32–35]. Furthermore, in rat models of EAE, administration of recombinant IL-1Ra led to reduced disease severity compared to controls [36]. The identification of IL-1β in the brain biopsy tissue from our patient, which was not seen in normal brain tissue, further supports the use of anakinra as a specific treatment of AHLE. The similarities between the effects of IL-1Ra on slowing the development of EAE and in ameliorating the neurologic findings of AHLE in our patients, suggest that the pro-inflammatory state induced by CFI deficiency is amenable to more targeted immunomodulatory therapies.
We propose that IL-1 inhibition slows neutrophil recruitment and complement protein production [37], reduces MAC formation [38], and prevents further neuronal cell lysis (Supplemental Figure S3). We suspect that in Patient A, anakinra served to control inflammation, including complement-mediated effects by reducing IL-1β signaling [27, 37]. Ultimately, she was weaned from therapy. Patient B, however, has an ongoing requirement for anakinra and complement studies performed while receiving therapy suggest persistent complement consumption with low C3 (31 mg/dL). Thus, despite the similar presentation and serum FI levels in our two patients, anakinra may not be sufficient for full recovery in all cases of AHLE associated with partial FI deficiency. These cases may be amenable to other complement inhibitors, but this approach requires further investigation [39].
Our report has several limitations. Due to the rarity and high mortality rate of AHLE, we are limited to our experiences with two pediatric patients who were treated at a single institution. Similar to descriptions of patients with aHUS, we have identified an association between partial FI deficiency due to mutations in CFI and AHLE, however an exact causal relationship cannot be proven. Although FI was detected at low levels in both patients’ serum and our data indicates that the mutated FI protein is translated in vitro similar to wild-type FI, there was undetectable mutant FI in the transfected cell culture supernatants. Flow cytometry of transfected cells without the protein transport inhibitor Brefeldin A demonstrates that low amounts of mutant FI protein are not retained in the cytosol (data not shown). Therefore, it is not clear if degradation of the mutant protein occurs intracellularly prior to secretion or extracellularly. While Nilsson et al have successfully used this six-histidine tag to identify secreted mutant FI [6], it is possible that the combination of our patients’ mutations and the tag affects secretion. It is also possible that these mutations affect function, but we were unable to perform these assays. Complement is activated in response to numerous stimuli [24, 40] and may be increased in the brain in response to ischemic-reperfusion injury, infection and traumatic lesions, as well as in AHLE. Thus, the deposition of C3 and MAC in our brain biopsy samples may not be unique to AHLE or FI deficiency, but suggests an opportunity for complement-targeted therapeutic intervention that may not have been previously appreciated in neuroinflammatory diseases.
CONCLUSIONS
We have described, for the first time, two individuals with partial FI deficiency and AHLE, a previously unreported phenotype for FI deficiency, supporting a role for complement in the pathogenesis of this disease. We propose that in these predisposed individuals, an infectious insult may trigger uncontrolled activation of complement in the brain parenchyma leading to profound neuroinflammation and demyelination. This is the first evidence of a role for IL-1 and complement in AHLE, and the success of treatment with anakinra observed in our patients may offer both an important adjunctive therapy for this disease and insight into the pathophysiology of demyelinating syndromes.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Dr. Sara C. Nilsson and Dr. Anna M. Blom (Lund University, Malmo, Sweden) for generously providing the FI cDNA, Nikki Bowen (San Diego Branch, Ludwig Institute for Cancer Research, La Jolla, CA and University of California-San Diego School of Medicine, La Jolla, CA) for guidance with protein purification, the Ludwig Institute for Cancer Research (San Diego, CA) for sequencing support, Jeff Murray (University of Iowa, Iowa City, IA) for providing Filipino DNA control samples, and Keith Rapp, Justin Breisch, Barbara Carter and Gordon Pendergrass (Rady Children’s Hospital, San Diego, CA) for assistance with immunohistochemistry. Funding was provided by National Institutes of Health Training Grant T32 AI107469 (CG, LB).
Abbreviations
- AHLE
Acute Hemorrhagic Leukoencephalitis
- aHUS
atypical Hemolytic Uremic Syndrome
- CFI
Complement factor I gene
- CNS
central nervous system
- CSF
cerebrospinal fluid
- EAE
experimental autoimmune encephalitis
- FI
Complement Factor I
- FIMAC
Factor I/membrane attack complex
- IL-1β
Interleukin-1β
- IL-1Ra
IL-1 receptor antagonist
- MAC
membrane attack complex
- SRCR
scavenger receptor cysteine-rich
Footnotes
Conflict of interest: Dr. Hoffman is a consultant for Sobi Biovitrum.
Contributor Information
Lori Broderick, Division of Allergy and Immunology, University of California-San Diego, La Jolla, CA, USA.
Chhavi Gandhi, Division of Allergy and Immunology, University of California-San Diego, La Jolla, CA, USA.
James L. Mueller, San Diego Branch, Ludwig Institute for Cancer Research, La Jolla, CA and Department of Medicine, University of California-San Diego School of Medicine, La Jolla, CA, USA.
Christopher D. Putnam, San Diego Branch, Ludwig Institute for Cancer Research, La Jolla, CA and Department of Medicine, University of California-San Diego School of Medicine, La Jolla, CA, USA.
Katayoon Shayan, Division of Pathology, University of California-San Diego, La Jolla, CA and Rady Children’s Hospital, San Diego, CA, USA.
Patricia C. Giclas, ADx Complement Laboratory and Department of Pediatrics, Allergy and Immunology Division, National Jewish Health, Denver, CO, USA.
Karin S. Peterson, Division of Allergy and Immunology, Division of Rheumatology, University of California-San Diego, La Jolla, CA and Rady Children’s Hospital, San Diego, CA, USA.
Seema S. Aceves, Division of Allergy and Immunology, University of California-San Diego, and Rady Children’s Hospital, San Diego, CA, USA.
Robert M. Sheets, Division of Rheumatology, University of California-San Diego, La Jolla, CA and Rady Children’s Hospital, San Diego, CA, USA.
Bradley M. Peterson, Division of Critical Care, University of California-San Diego, La Jolla, CA and Rady Children’s Hospital, San Diego, CA, USA.
Robert O. Newbury, Division of Pathology, University of California-San Diego, La Jolla, CA and Rady Children’s Hospital, San Diego, CA, USA.
Hal M. Hoffman, Division of Allergy and Immunology, University of California-San Diego, La Jolla, CA and Rady Children’s Hospital, San Diego, CA, USA.
John F. Bastian, Division of Allergy and Immunology, University of California-San Diego, La Jolla, CA and Rady Children’s Hospital, San Diego, CA, USA.
REFERENCES
- 1.Payne ET, Rutka JT, Ho TK, Halliday WC, Banwell BL. Treatment leading to dramatic recovery in acute hemorrhagic leukoencephalitis. J Child Neurol. 2007;22:109–113. doi: 10.1177/0883073807299971. [DOI] [PubMed] [Google Scholar]
- 2.Leake JA, Billman GF, Nespeca MP, Duthie SE, Dory CE, Meltzer HS, et al. Pediatric acute hemorrhagic leukoencephalitis: report of a surviving patient and review. Clin Infect Dis. 2002;34:699–703. doi: 10.1086/338718. [DOI] [PubMed] [Google Scholar]
- 3.Peterson B, Khanna S, Fisher B, Marshall L. Prolonged hypernatremia controls elevated intracranial pressure in head-injured pediatric patients. Crit Care Med. 2000;28:1136–1143. doi: 10.1097/00003246-200004000-00037. [DOI] [PubMed] [Google Scholar]
- 4.Ponce-Castro IM, Gonzalez-Rubio C, Delgado-Cervino EM, Abarrategui-Garrido C, Fontan G, Sanchez-Corral P, et al. Molecular characterization of Complement Factor I deficiency in two Spanish families. Mol Immunol. 2008;45:2764–2771. doi: 10.1016/j.molimm.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Nilsson SC, Karpman D, Vaziri-Sani F, Kristoffersson AC, Salomon R, Provot F, et al. A mutation in factor I that is associated with atypical hemolytic uremic syndrome does not affect the function of factor I in complement regulation. Mol Immunol. 2007;44:1835–1844. doi: 10.1016/j.molimm.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson SC, Trouw LA, Renault N, Miteva MA, Genel F, Zelazko M, et al. Genetic, molecular and functional analyses of complement factor I deficiency. Eur J Immunol. 2009;39:310–323. doi: 10.1002/eji.200838702. [DOI] [PubMed] [Google Scholar]
- 7.Kovacs GG, Gasque P, Strobel T, Lindeck-Pozza E, Strohschneider M, Ironside JW, et al. Complement activation in human prion disease. Neurobiol Dis. 2004;15:21–28. doi: 10.1016/j.nbd.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Chowdhury AR, Ehara T, Higuchi M, Hora K, Shigematsu H. Immunohistochemical detection of immunoglobulins and complements in formaldehyde-fixed and paraffin-embedded renal biopsy tissues; an adjunct for diagnosis of glomerulonephritis. Nephrology (Carlton) 2005;10:298–304. doi: 10.1111/j.1440-1797.2005.00396.x. [DOI] [PubMed] [Google Scholar]
- 9.Rambach G, Maier H, Vago G, Mohsenipour I, Lass-Florl C, Defant A, et al. Complement induction and complement evasion in patients with cerebral aspergillosis. Microbes Infect. 2008;10:1567–1576. doi: 10.1016/j.micinf.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman HM, Rosengren S, Boyle DL, Cho JY, Nayar J, Mueller JL, et al. Prevention of cold-associated acute inflammation in familial cold autoinflammatory syndrome by interleukin-1 receptor antagonist. Lancet. 2004;364:1779–1785. doi: 10.1016/S0140-6736(04)17401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355:581–592. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nilsson SC, Sim RB, Lea SM, Fremeaux-Bacchi V, Blom AM. Complement factor I in health and disease. Mol Immunol. 2011;48:1611–1620. doi: 10.1016/j.molimm.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Nilsson SC, Kalchishkova N, Trouw LA, Fremeaux-Bacchi V, Villoutreix BO, Blom AM. Mutations in complement factor I as found in atypical hemolytic uremic syndrome lead to either altered secretion or altered function of factor I. Eur J Immunol. 2010;40:172–185. doi: 10.1002/eji.200939280. [DOI] [PubMed] [Google Scholar]
- 14.Maga TK, Nishimura CJ, Weaver AE, Frees KL, Smith RJ. Mutations in alternative pathway complement proteins in American patients with atypical hemolytic uremic syndrome. Hum Mutat. 2010;31:E1445–E1460. doi: 10.1002/humu.21256. [DOI] [PubMed] [Google Scholar]
- 15.Kavanagh D, Richards A, Noris M, Hauhart R, Liszewski MK, Karpman D, et al. Characterization of mutations in complement factor I (CFI) associated with hemolytic uremic syndrome. Mol Immunol. 2008;45:95–105. doi: 10.1016/j.molimm.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Bienaime F, Dragon-Durey MA, Regnier CH, Nilsson SC, Kwan WH, Blouin J, et al. Mutations in components of complement influence the outcome of Factor I-associated atypical hemolytic uremic syndrome. Kidney Int. 2010;77:339–349. doi: 10.1038/ki.2009.472. [DOI] [PubMed] [Google Scholar]
- 17.Roversi P, Johnson S, Caesar JJ, McLean F, Leath KJ, Tsiftsoglou SA, et al. Structural basis for complement factor I control and its disease-associated sequence polymorphisms. Proc Natl Acad Sci U S A. 2011;108:12839–12844. doi: 10.1073/pnas.1102167108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moller Rasmussen J, Teisner B, Jepsen HH, Svehag SE, Knudsen F, Kirstein H, et al. Three cases of factor I deficiency: the effect of treatment with plasma. Clin Exp Immunol. 1988;74:131–136. [PMC free article] [PubMed] [Google Scholar]
- 19.Naked GM, Florido MP, Ferreira de Paula P, Vinet AM, Inostroza JS, Isaac L. Deficiency of human complement factor I associated with lowered factor H. Clin Immunol. 2000;96:162–167. doi: 10.1006/clim.2000.4878. [DOI] [PubMed] [Google Scholar]
- 20.Thomas A, Gasque P, Vaudry D, Gonzalez B, Fontaine M. Expression of a complete and functional complement system by human neuronal cells in vitro. Int Immunol. 2000;12:1015–1023. doi: 10.1093/intimm/12.7.1015. [DOI] [PubMed] [Google Scholar]
- 21.Atkinson JP, Liszewski MK, Richards A, Kavanagh D, Moulton EA. Hemolytic uremic syndrome: an example of insufficient complement regulation on self-tissue. Ann N Y Acad Sci. 2005;1056:144–152. doi: 10.1196/annals.1352.032. [DOI] [PubMed] [Google Scholar]
- 22.Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Francis K, van Beek J, Canova C, Neal JW, Gasque P. Innate immunity and brain inflammation: the key role of complement. Expert Rev Mol Med. 2003;5:1–19. doi: 10.1017/S1462399403006252. [DOI] [PubMed] [Google Scholar]
- 24.van Beek J, Elward K, Gasque P. Activation of complement in the central nervous system: roles in neurodegeneration and neuroprotection. Ann N Y Acad Sci. 2003;992:56–71. doi: 10.1111/j.1749-6632.2003.tb03138.x. [DOI] [PubMed] [Google Scholar]
- 25.Silverman BA, Carney DF, Johnston CA, Vanguri P, Shin ML. Isolation of membrane attack complex of complement from myelin membranes treated with serum complement. J Neurochem. 1984;42:1024–1029. doi: 10.1111/j.1471-4159.1984.tb12706.x. [DOI] [PubMed] [Google Scholar]
- 26.Vanguri P, Koski CL, Silverman B, Shin ML. Complement activation by isolated myelin: activation of the classical pathway in the absence of myelin-specific antibodies. Proc Natl Acad Sci U S A. 1982;79:3290–3294. doi: 10.1073/pnas.79.10.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gasque P, Dean YD, McGreal EP, VanBeek J, Morgan BP. Complement components of the innate immune system in health and disease in the CNS. Immunopharmacology. 2000;49:171–186. doi: 10.1016/s0162-3109(00)80302-1. [DOI] [PubMed] [Google Scholar]
- 28.Griffiths MR, Neal JW, Fontaine M, Das T, Gasque P. Complement factor H, a marker of self protects against experimental autoimmune encephalomyelitis. J Immunol. 2009;182:4368–4377. doi: 10.4049/jimmunol.0800205. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Rubio C, Ferreira-Cerdan A, Ponce IM, Arpa J, Fontan G, Lopez-Trascasa M. Complement factor I deficiency associated with recurrent meningitis coinciding with menstruation. Arch Neurol. 2001;58:1923–1928. doi: 10.1001/archneur.58.11.1923. [DOI] [PubMed] [Google Scholar]
- 31.Vyse TJ, Spath PJ, Davies KA, Morley BJ, Philippe P, Athanassiou P, et al. Hereditary complement factor I deficiency. Qjm. 1994;87:385–401. [PubMed] [Google Scholar]
- 32.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basu A, Krady JK, Levison SW. Interleukin-1: a master regulator of neuroinflammation. J Neurosci Res. 2004;78:151–156. doi: 10.1002/jnr.20266. [DOI] [PubMed] [Google Scholar]
- 34.Jauneau AC, Ischenko A, Chan P, Fontaine M. Complement component anaphylatoxins upregulate chemokine expression by human astrocytes. FEBS Lett. 2003;537:17–22. doi: 10.1016/s0014-5793(03)00060-7. [DOI] [PubMed] [Google Scholar]
- 35.Palin K, Verrier D, Tridon V, Hurst J, Perry VH, Dantzer R, et al. Influence of the course of brain inflammation on the endogenous IL-1beta/IL-1Ra balance in the model of brain delayed-type hypersensitivity response to bacillus Calmette-Guerin in Lewis rats. J Neuroimmunol. 2004;149:22–30. doi: 10.1016/j.jneuroim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- 37.Badovinac V, Mostarica-Stojkovic M, Dinarello CA, Stosic-Grujicic S. Interleukin-1 receptor antagonist suppresses experimental autoimmune encephalomyelitis (EAE) in rats by influencing the activation and proliferation of encephalitogenic cells. J Neuroimmunol. 1998;85:87–95. doi: 10.1016/s0165-5728(98)00020-4. [DOI] [PubMed] [Google Scholar]
- 38.Barnum SR, Jones JL. Differential regulation of C3 gene expression in human astroglioma cells by interferon-gamma and interleukin-1 beta. Neurosci Lett. 1995;197:121–124. doi: 10.1016/0304-3940(95)11923-k. [DOI] [PubMed] [Google Scholar]
- 39.Hansch GM, Seitz M, Betz M. Effect of the late complement components C5b-9 on human monocytes: release of prostanoids, oxygen radicals and of a factor inducing cell proliferation. Int Arch Allergy Appl Immunol. 1987;82:317–320. doi: 10.1159/000234216. [DOI] [PubMed] [Google Scholar]
- 40.Holland MC, Morikis D, Lambris JD. Synthetic small-molecule complement inhibitors. Curr Opin Investig Drugs. 2004;5:1164–1173. [PubMed] [Google Scholar]
- 41.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeLano W. The PyMOL Molecular Graphics System. San Carlos, CA, USA: DeLano Scientific; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.