Abstract
Introduction
Young women with breast cancer face treatments that impair ovarian function, but it is not known if malignancy itself impacts ovarian reserve. As more breast cancer patients consider future fertility, it is important to determine if ovarian reserve is impacted by cancer, prior to any therapeutic intervention.
Methods
A cross-sectional study was conducted comparing if ovarian reserve, as measured by anti-mullerian hormone (AMH), follicle stimulating hormone (FSH) and inhibin B (inhB), differed between 108 women with newly diagnosed breast cancer and 99 healthy women without breast cancer. Breast cancer participants were ages 28–44 and were recruited from two clinical breast programs. Healthy women ages 30–44 without a history of infertility were recruited from gynecology clinics and the community.
Results
The median age (interquartile range) was 40.2(5.5) years for breast cancer participants and 33.0(4.6) years for healthy controls. The unadjusted geometric mean AMH levels (SD) for breast cancer participants and controls were 0.66(3.6) ng/mL and 1.1(2.9) ng/mL, respectively. Adjusting for age, body mass index, gravidity, race, menstrual pattern and smoking, mean AMH levels were not significantly different between breast cancer participants and healthy controls (0.85 vs. 0.76 ng/mL, p=0.60). FSH and inhB levels did not differ by breast cancer status. In exploratory analysis, the association between AMH and breast cancer status differed by age (p-interaction=0.02). AMH may be lower with breast cancer status in women older than 37. In younger women, AMH levels did not differ significantly by breast cancer status.
Conclusions
Among the youngest of breast cancer patients, ovarian reserve as measured by AMH, FSH and inhibin B did not differ significantly from healthy women of similar age. In older breast cancer patients, ovarian reserve may be adversely impacted by cancer status. These findings support the potential success and need for fertility preservation strategies prior to institution of cancer treatment.
Keywords: Breast cancer, AMH, ovarian reserve, fertility preservation
Introduction
Breast cancer is the leading cause of cancer in young women, accounting for 25% of cases diagnosed by age 40 [1]. At diagnosis, young breast cancer patients face a myriad of challenges, including decisions on preserving fertility prior to cancer treatment. It is well-established that chemotherapy used to treat breast cancer incurs damage to the ovarian reserve, or the quantity and quality of remaining oocytes [2–4], resulting in loss of fertility and premature ovarian failure [5, 6]. However, it is not known whether malignancy itself impacts ovarian reserve.
In young men who are newly diagnosed with certain types of cancer, semen parameters may be decreased prior to any gonadotoxic treatment [7, 8], suggesting an independent effect of malignancy on reproductive potential. As more female breast cancer patients consider future fertility, it is important to determine if ovarian reserve is impacted by cancer, prior to any therapeutic intervention. This will allow us to better understand the interplay between cancer and ovarian function as well as guide stimulation for egg or embryo banking.
Ovarian reserve is measured by hormone and ultrasound biomarkers, including anti-Mullerian hormone (AMH), follicle stimulating hormone (FSH), inhibin B (inhB) and antral follicle count (AFC) [9–12]. Among these measures, AMH is a good candidate biomarker because it is relatively stable across the menstrual cycle and has been associated with reproductive outcomes from time to menopause to assisted reproduction (ART) responses in normal and infertile women [13, 14].
In young women with cancer, several studies compared ovarian reserve markers and ovarian stimulation outcomes between cancer patients and infertile controls [15–19]. These studies reported mixed results and are limited by the heterogeneity of cancer diagnoses and use of infertile women as the control population. Therefore, the objective of this study was to test if hormone measures of ovarian reserve differ between young breast cancer patients and healthy women without a history of infertility. We hypothesized that AMH levels in young breast cancer patients would be lower than AMH in healthy women.
Methods
We performed a cross-sectional study to determine if AMH, FSH, and inhB levels differ in young women with and without breast cancer. Participants were ages 28 to 44, premenopausal (defined as having at least one period within the past year), and had no history of gonadotoxic therapy or oophorectomy. Breast cancer participants were recruited from breast oncology clinics at the University of Pennsylvania and the University of California, San Diego, diagnosed with an in situ or invasive cancer between 2008 and 2011, and enrolled within 4 months of cancer diagnosis, prior to any systemic cancer treatment. Healthy controls were participants of an ongoing study on ovarian aging and fecundability and recruited at the University of North Carolina, Chapel Hill, between 2008 and 2011 through general clinics, emails to University faculty and staff, and flyers and informational letters for the community. By screening questionnaires at enrollment, control participants did not have a diagnosis of breast cancer. The cohort study of breast cancer patients was approved by the institutional review boards (IRB) of the University of California San Diego and University of Pennsylvania; the cohort study of healthy participants was approved by the IRB of University of North Carolina.
Breast cancer participants underwent a blood draw prior to start of systemic cancer therapy. With a limited time window prior to start of cancer treatment, blood draws were not timed to occur in the early follicular phase. Healthy participants provided early follicular phase (menstrual cycle day 2, 3, or 4) serum. Sera were extracted from all samples and frozen at −80C. Clinical data were abstracted from medical records and self-reported questionnaires.
Study samples were assayed for AMH, FSH, inhB and estradiol at the University of Southern California Reproductive Endocrine Research Laboratory. AMH was measured by the AMH enzyme-linked immunosorbent assay kits (Beckman Coulter, AMH Gen II assay, Brea, CA, USA). The limit of detectability was 0.17 ng/mL. The inter-assay coefficient of variability (CV) was 4.8%. FSH was measured by a direct immunochemiluminometric assay using the automated Immulite system. The limit of detectability was 1.0 IU/L and inter-assay CV was 5.0%. Estradiol was measured by radioimmunoassay after an organic solvent extraction step. The limit of detectability was 0.49 pg/mL and inter-assay CV was 13.7%. InhB assays used a monoclonal two site enzyme-linked immunosorbent assay. The limit of detectability was 9.4 pg/mL and inter-assay CV was 1.5%. Values below detection thresholds were given half of the threshold value in analyses.
Analyses were conducted using Stata software (Release 12, College Station, TX) and R freeware (Version 2.14.1, R Foundation for Statistical Computing, Vienna, Austria). Graphic displays of continuous variables were explored to determine data distributions. Hormone levels were transformed to natural log values to minimize the impact of skewed distributions. Transformed hormone measures were observed to have a Gaussian distribution. Continuous variables were summarized by means (standard deviation [SD]) or medians (interquartile range [IQR]), and compared by breast cancer status using the Student’s t-test or Wilcoxon ranksum test, as appropriate. Categorical variables were characterized as proportions and compared using chi-squared or exact methods, as appropriate. Linear regression methods were used to model the association between log-transformed hormone levels and cancer status while adjusting for potential confounding by age, cancer status and demographic variables. Variables with p<0.1 based on the Wald test for univariate associations and known confounders from prior data [20] were included in the multivariate models. Analysis of FSH and inhB were restricted to participants with an estradiol of <80 pg/mL to approximate for the early follicular phase. To explore the hypothesis of potential effect modification by age, we examined the interaction between age and breast cancer status by including the product of these 2 variables in the model. Since this interaction was significant, we conducted additional exploratory analysis via the bootstrap procedure, to identify an age-cutpoint at which age-AMH regression lines for cases and controls intersected. Specifically, 200 bootstrap replicates were sampled. For each such sample, the full model with the interaction was fitted, and the age cutpoint was computed. The distribution of the 200 age cutpoints was used to obtain a point-estimate (mean of the estimates across the 200 samples) and bias-corrected (BCa) 95% confidence interval for the age cutpoint [21]. In post-hoc analysis, we stratified the sample by women younger than the age cutpoint estimate and women older than the age cutpoint estimate, and examined the relationship between breast cancer status and AMH within each stratum.
A priori power calculations were performed based on the sample size of the cohorts to determine the detectable difference in AMH, the primary outcome of interest, between the two populations. In healthy women between 30 and 44, the geometric mean (SD) for AMH was observed to be 1.6 (2.3) ng/mL [22]. With 80% power, alpha error of 0.05, the study is powered to detect a difference of at least 0.38 SD in AMH levels between the two populations.
Results
The study included a total of 207 participants, 108 women with breast cancer and 99 healthy women without breast cancer. Table 1 summarizes the characteristics of the participants. The median age of breast cancer participants was 40.2 years (range 28.5 to 44.9). The majority of breast cancer patients had invasive ductal carcinoma and tumors expressing estrogen and/or progesterone receptors. Control women had a median age of 33.9 (range 29.3–42.5). Compared to healthy participants, breast cancer participants were significantly older, less likely to be Caucasian and had higher gravidity. Body size, exposure to cigarette smoking and regularity of menstrual pattern were similar between the two groups.
Table 1.
Baseline characteristics and hormone measures of ovarian reserve of breast cancer and healthy participants
| Breast cancer participants (N=108) | Healthy participants (N=99) | p-value | |
|---|---|---|---|
|
| |||
| Age, Median (IQR) | 40.2 (5.5) | 33.0 (4.6) | <0.001a |
| <35 N(%) | 11 (10) | 63 (63) | |
| 35–39 N(%) | 27 (25) | 29 (29) | |
| >40 N(%) | 70 (65) | 7 (7) | |
|
| |||
| Race and ethnicity (%) | 0.002b | ||
| Caucasian | 77 (72) | 86 (87) | |
| African American | 13 (12) | 5 (5) | |
| Asian | 8 (7) | 4 (4) | |
| Hispanic | 9 (8) | 0 (0) | |
| Other | 0 (0) | 4 (4) | |
|
| |||
| Regular menses (%) | 96 (89) | 88 (88) | 0.96b |
|
| |||
| Prior pregnancy (%) | 72 (67) | 58 (59) | 0.20b |
|
| |||
| Gravidity, Median (IQR) | 2 (3) | 1 (1) | <0.001a |
|
| |||
| BMI, Mean (SD) | 25.3 (5.9) | 25.1 (5.9) | 0.84d |
|
| |||
| Current cigarette smoking (%) | 6 (6) | 1 (1) | 0.12 |
|
| |||
| Breast cancer type (%) | |||
| Ductal | 97 (90) | ||
| Lobular | 4 (4) | - | - |
|
| |||
| Cancer stage (%) | - | - | |
| 0 | 5(5) | ||
| I | 33 (30) | ||
| II | 50 (46) | ||
| III | 19 (18) | ||
|
| |||
| Estrogen or progesterone receptor positive (%) | 72 (66) | - | - |
|
| |||
| BRCA status (%) | |||
| Positive | 10 (9) | - | - |
| Negative | 38 (36) | ||
| Unknown | 59 (55) | ||
|
| |||
| AMH (ng/ml), Geometric mean (SD) | 0.66 (3.5) | 1.1 (2.9) | <0.001d |
|
| |||
| AMH below limit of detectability (<0.17 ng/mL), n (%) | 21 (19) | 7 (7) | 0.009b |
|
| |||
| FSH (mIU/ml), Geometric mean (SD)e | 7.7 (2.4) | 7.3 (1.5) | 0.52d |
|
| |||
| InhB (pg/ml), Geometric mean (SD)e | 27.7 (3.0) | 30.9 (1.8) | 0.45d |
Wilcoxon ranksum test
Chi-square test
Fisher’s exact test
Student’s t-test
Restricted to participants with estradiol levels ≤ 80 pg/mL(45 breast cancer and 91 healthy participants)
Unadjusted AMH, FSH and inhB levels are depicted in Table 1. The geometric mean (SD) AMH levels were 0.66 (3.5) ng/mL for breast cancer participants and 1.1 (2.9) ng/mL for controls. In univariate analysis, AMH was significantly lower in breast cancer participants; more breast cancer participants had AMH levels below assay detection than controls (19% vs. 7%, p=0.009). AMH was also inversely associated with age (p<0.001) and gravidity (p=0.03), but was not significantly associated with race and ethnicity (p=0.61), regularity of menstrual cycles (p=0.33), cigarette smoking (p=0.67), and BMI (p=0.18). Among cancer characteristics, AMH was not associated with stage (p=0.81) or hormone receptor status (p=0.35). FSH and inhB were similar between the two groups among the subset of participants (45 women with breast cancer and 91 healthy women) with estradiol levels less than 80 pg/mL.
A multivariable linear regression model was constructed to examine the association between log-transformed AMH levels and cancer status, while controlling for potential confounding. AMH was not significantly associated with breast cancer status in a model adjusting for age, gravidity, BMI, race/ethnicity, regularity of menstrual cycles, and cigarette smoking. Table 2 depicts the predicted geometric mean AMH levels from this model. FSH and inhB were no different by breast cancer status in separate models adjusting for age, gravidity, race, regularity of menstrual cycles, cigarette smoking and BMI. In the model for FSH, the geometric mean FSH (95% CI) for breast cancer participants and healthy controls were 7.6 (6.6–8.7) and 7.2 (5.7–8.9) mIU/mL, respectively (p=0.70). In the model for inhB, the geometric mean inhB for breast cancer participants and healthy controls were 29.4 (24.0–36.2) and 29.4 (21.5–40.0) pg/mL, respectively (p=0.98).
Table 2.
Adjusted geometric mean values of AMH (ng/mL)a
| Characteristic | AMH (95% CI) | p-value |
|---|---|---|
|
| ||
| Breast Cancer status | 0.60 | |
| Affected | 0.85 (0.66–1.09) | |
| Unaffected | 0.76 (0.58–0.98) | |
|
| ||
| Age | <0.001 | |
| <35 | 1.46 (1.08–1.97) | |
| 35–39 | 1.02 (0.76–1.38) | |
| >40 | 0.37 (0.27–0.50) | |
|
| ||
| BMI | 0.37 | |
| <18.5 | 1.72 (0.64–4.65) | |
| 18.5–24.9 | 0.83 (0.68–1.02) | |
| 25.0–29.9 | 0.72 (0.53–1.0) | |
| >30 | 0.70 (0.46–1.07) | |
|
| ||
| Prior pregnancy | 0.57 | |
| 0 | 0.75 (0.57–0.99) | |
| ≥ 1 | 0.83 (0.68–1.0) | |
|
| ||
| Race/ethnicity | 0.42 | |
| Caucasian | 0.78 (0.65–0.92) | |
| Non-Caucasian | 0.92 (0.64–1.3) | |
|
| ||
| Regular menses | 0.56 | |
| Yes | 0.82 (0.69–0.96) | |
| No | 0.70 (0.43–1.13) | |
|
| ||
| Current cigarette smoking | 0.54 | |
| Yes | 0.59 (0.22–1.58) | |
| No | 0.81 (0.69–0.95) | |
As predicted by linear regression models with log-transformed AMH as the outcome, and breast cancer status, age, gravidity, race, BMI, regularity of menstrual cycles, and cigarette smoking as predictor variables (R2=0.21)
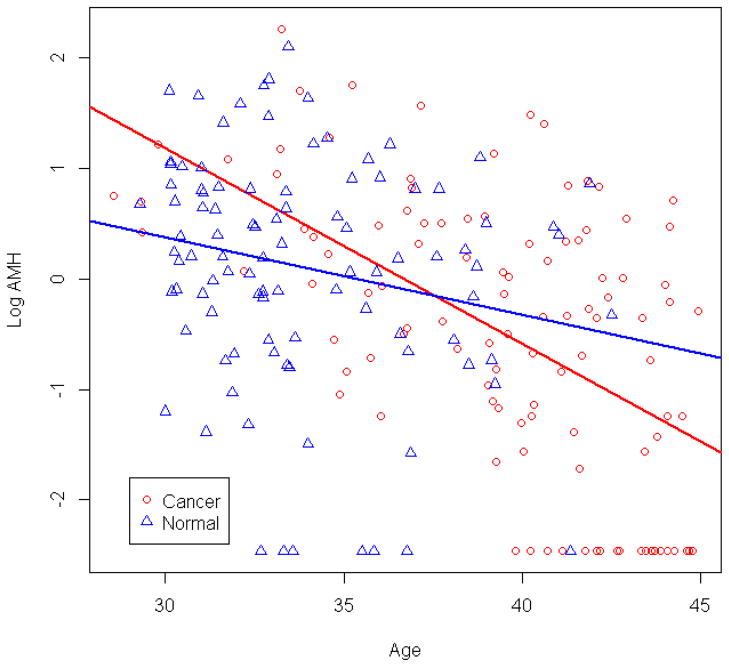
Figure 1 depicts log-transformed AMH levels graphed by age and breast cancer status. The curves were observed to intersect, suggesting potential effect modification. In a model controlling for gravidity, BMI, race, regularity of menstrual cycles and cigarette smoking, the association between breast cancer and AMH levels differed by age (p-valueinteraction=0.017). Via bootstrapping resampling, the age (95% CI) at which the interaction was observed was 37.2 (33.7, 40.6). In post-hoc analysis, AMH levels were not significantly associated with breast cancer status in women younger than 37 (β=0.24, 95% CI −0.24, 0.72, p=0.32). However, in women 37 years and older, AMH was significantly lower in breast cancer patients than in healthy women (β= −0.85, 95% CI −1.48, −0.22, p=0.009). In a model adjusting for age in addition to the interaction between age 37 and breast cancer, the association between breast cancer and AMH levels remained significantly different by age ≥ or < 37 (p-valueinteraction=0.008). In separate models examining FSH and inhB, no significant interactions between breast cancer status and hormone level were observed (data not shown).
Figure 1.
Log-transformed AMH levels (95% CI) by age and cancer status.
Discussion
This study compared AMH, FSH and inhB, three measures of ovarian reserve, between young women with newly diagnosed breast cancer and healthy controls without a history of infertility. The results showed that AMH, FSH and inhB levels did not differ by breast cancer status. For AMH, exploratory analyses suggest that levels may be lower in breast cancer patients older than 37 years of age, compared to healthy controls. These findings support that ovarian reserve, as measured by these biomarkers, is not adversely impacted by the presence of breast cancer in younger women.
The data contribute to very limited literature examining the impact of cancer on ovarian reserve measures. Previously, FSH and antral follicle count (AFC) have been reported to be similar between female patients facing a variety of cancer and autoimmune disease diagnoses and infertile control women [15, 16, 23–25]. A single small study of 26 newly diagnosed breast cancer patients between ages 30 and 40 demonstrated similar AMH levels between cancer patients (0.86 ng/mL, range 0.07–9.1) and women who achieved a successful birth after IVF for male factor infertility (0.94 ng/mL, range 0.2–7.7) [26]. However, the authors reported that 29% of cancer patients had AMH levels less than the lower limit of their healthy controls. Moreover, this study did not study breast cancer patients who are in their early 40s and still of reproductive age. Recently, the European FertiPROTEKT Network published data comparing AMH levels between 38 Hodgkin Lymphoma (HL) and Non-Hodgkin Lymphoma (NHL) women prior to cancer treatment and healthy controls from the community, similar to the design of our study [27]. The mean age of the cancer and control group was 25.5 years (range 18–33), and lower AMH levels (mean ± SD) were observed in lymphoma patients (2.1 ± 1.5 ng/mL) than healthy controls (3.2 ± 2.2 ng/mL). Of note, lymphomas are the primary cancer type associated with lower sperm quality in males [7, 8]. Though bound by small sample sizes, generally less than 50 participants with cancer per study, these early data on biochemical and ultrasound measures of ovarian reserve prior to cancer treatment suggest that cancer may impact ovarian reserve, but the effect may differ by type of cancer.
A complementary approach to testing if ovarian reserve is diminished by cancer is to compare ovarian stimulation outcomes between cancer patients undergoing egg and embryo banking prior to treatment and healthy controls. Several studies have reported that the number of retrieved eggs, proportions of mature eggs, and fertilization rates among those who banked embryos have not been found to be different between fertility preservation cases and infertile controls when all cancers are considered [15–17, 23]. Specifically, when considering breast cancer patients versus other cancer types, the data are mixed. Two studies suggest lower numbers of eggs retrieved and higher requirements for medications used to stimulate the ovaries in breast cancer patients than other cancers [19, 28]. However, in the FertiPROTEKT Network study, the number of retrieved eggs was higher in breast cancer than in lymphoma patients [27]. Taken together, there is some evidence that malignancies may differentially affect ovarian function, but the literature to date has had limited power to test specific malignancies by pooling diagnoses and reporting small sample sizes. Moreover, as most young female cancer patients are not infertile, healthy women without a history of infertility may be a more appropriate comparison group. Choice of infertile controls, even with tubal factor or male factor diagnoses, does not eliminate the possibility of underlying ovarian reserve problems, which could bias comparisons with cancer patients toward the null.
We report the first data to compare ovarian reserve between women with and without breast cancer in the general population, rather than the infertility population. As most women with breast cancer do not have a history of infertility, this design allows for a comparison of cancer patients and the source population from which they arise. Recruitment of control participants included both clinics and the community. With this strategy, the control population of the current study was more representative of the general population and minimized confounding by indication. Furthermore, one cancer type was targeted, with results most generalizable to young women with breast cancer. Overall, we did not find lower AMH levels in breast cancer patients than in healthy controls, suggesting that ovarian reserve as measured by AMH is generally not adversely impacted by breast cancer. A strength of the study was the ability to analyze measures in a sizeable population that was adequately powered to detect smaller differences in AMH, but a type II error cannot be ruled out for differences in AMH that are smaller. As expected, AMH was inversely associated with age in the breast cancer population, similar to data reported for the general population.
In exploratory analyses, we found that the relationship between AMH and breast cancer status may differ by age. In women older than 37 years, AMH was lower than in controls. It is possible that breast cancer diagnosis impacts ovarian reserve, but to a limited extent, such that in younger women with ample ovarian reserve, oocyte loss relative to the remaining cohort is minimal. It is also possible that we did not observe a difference in younger women because of limited power. However, in women with diminished ovarian reserve, e.g. older women, this impact would be more significant. Thus, some women may have a “susceptibility window”. Similar findings have been noted with tobacco use and ovarian reserve [29]. Such a hypothesis would need to be examined in a larger cohort.
There are several limitations to the study. While control women were drawn from the general population, it is important to recognize that controls were recruited from a different geographic region and for studying hormonal predictors of time to pregnancy. As such, while we are able to adjust for known confounders such as age, there may be additional unknown differences between the two populations and possible confounding for which we cannot adjust. It is also possible that higher AMH levels in healthy controls who are over 37 reflect a selection bias in the population of women who attempt natural fecundity at older reproductive age. Third, the timing of blood draws was in the early follicular phase for healthy controls, but throughout the menstrual cycle for breast cancer participants. For this reason, AMH levels were selected as the primary outcome as levels appear to be independent of gonadotropins and are relatively stable through the menstrual cycle and do not follow fluctuations seen in FSH and inhB [30–32]. Because of the variability of FSH and inhB over the menstrual cycle, samples for these comparisons was limited to those with estradiol levels < 80pg/mL to reflect timing in the early follicular phase. In breast cancer participants, blood sampling could occur before or after surgery, and it is not known whether stress related to surgery could impact ovarian reserve. We postulate that this is unlikely to affect AMH levels significantly as they are normal in women with hypothalamic amenorrheic women compared to women with amenorrhea due to ovarian failure [33]. Data on infertility or a history of decreased ovarian reserve prior to cancer diagnosis was not collected. Therefore, it is possible that if some of the breast cancer participants had decreased ovarian reserve prior to diagnosis, this would bias the results away from the null. Breast cancer status in controls was ascertained by self-report only, but as the incidence of breast cancer is low in this age group, case misclassification is less likely. Another limitation of the study was that we were unable to compare stimulation outcomes. It may be possible that cancer impacts ovarian function/response to stimulation and this effect is not reflected in basal measures of ovarian reserve. Finally, a proportion of both cancer and healthy participants self-reported menstrual irregularity and are potential patients with polycystic ovarian syndrome (PCOS). But while PCOS is typically associated with higher AMH levels, menstrual pattern was not related to AMH in this dataset.
Importantly, the current study utilizes the new AMH Gen II assay. Until recently, two ELISA assays (DSL 10–14400 and Immunotech) have been available for commercial use. The assays use different pairs of monoclonal antibodies and are standardized differently. The new AMH Gen II assay has been developed by Beckman Coulter using DSL antibodies but calibrated to the Immunotech assay [34, 35]. Currently, the AMH Gen II assay is the primary test kit for AMH that is available on the market, with Beckman Coulter as the sole manufacturer. The assay is highly specific and reproducible, with inter-assay coefficients of variation between 5.3–7.7% [36]. Several studies have reported conversion factors between the two assays [30, 37–39], but the conversion factors varied widely. In our dataset, the healthy participants had AMH levels measured with the DSL assay as well as the Gen II assay. Using the conversion factors on DSL AMH levels would have resulted in different associations between AMH and cancer status (data not shown). This finding supports the use of the same AMH assay among all samples of a study.
In conclusion, the study presents new data on AMH in young breast cancer patients prior to gonadotoxic treatment. Among the youngest of breast cancer patients, AMH levels did not differ significantly from those in healthy women of similar age. Exploratory analysis suggests AMH levels may be lower in older breast cancer patients compared to controls. Consistent with prior studies, FSH and inhibin B were also not impacted by cancer status. Altogether, these findings suggest that ovarian reserve as measured by these biomarkers is generally not adversely impacted by the presence of breast cancer and provide support for the potential success of fertility preservation strategies prior to institution of cancer treatment.
Acknowledgments
We are grateful to Minya Pu, MS, for her guidance in graph generation. The study was funded by the National Institute of Child Health and Human Development (HD058799 [IS], HD060229 [AS]) and the American Cancer Society (MRSG-08-110-01-CCE [IS]).
Footnotes
Ethical Standards
The study complied with the current laws of the United States.
Conflict of Interest
Anne Steiner has been a consultant for Roche Diagnostics.
Angela DeMichele has been a consultant for Pfizer.
Bibliography
- 1.DevCan: Probability of Developing or Dying of Cancer Software VSRaABNCI. 2012 www.srab.cancer.gov/devcan.
- 2.Warne GL, Fairley KF, Hobbs JB, Martin FI. Cyclophosphamide-induced ovarian failure. N Engl J Med. 1973;289(22):1159–1162. doi: 10.1056/NEJM197311292892202. [DOI] [PubMed] [Google Scholar]
- 3.Anderson RA, Themmen AP, Al-Qahtani A, Groome NP, Cameron DA. The effects of chemotherapy and long-term gonadotrophin suppression on the ovarian reserve in premenopausal women with breast cancer. Hum Reprod. 2006;21(10):2583–2592. doi: 10.1093/humrep/del201. [DOI] [PubMed] [Google Scholar]
- 4.Su HI, Sammel MD, Green J, Velders L, Stankiewicz C, Matro J, Freeman EW, Gracia CR, DeMichele A. Antimullerian hormone and inhibin B are hormone measures of ovarian function in late reproductive-aged breast cancer survivors. Cancer. 2010;116(3):592–599. doi: 10.1002/cncr.24746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Partridge AH, Gelber S, Peppercorn J, Ginsburg E, Sampson E, Rosenberg R, Przypyszny M, Winer EP. Fertility and menopausal outcomes in young breast cancer survivors. Clin Breast Cancer. 2008;8(1):65–69. doi: 10.3816/CBC.2008.n.004. [DOI] [PubMed] [Google Scholar]
- 6.Schover LR. Premature ovarian failure and its consequences: vasomotor symptoms, sexuality, and fertility. J Clin Oncol. 2008;26(5):753–758. doi: 10.1200/JCO.2007.14.1655. [DOI] [PubMed] [Google Scholar]
- 7.Bahadur G, Ozturk O, Muneer A, Wafa R, Ashraf A, Jaman N, Patel S, Oyede AW, Ralph DJ. Semen quality before and after gonadotoxic treatment. Hum Reprod. 2005;20(3):774–781. doi: 10.1093/humrep/deh671. [DOI] [PubMed] [Google Scholar]
- 8.Bahadur G, Ling KL, Hart R, Ralph D, Wafa R, Ashraf A, Jaman N, Mahmud S, Oyede AW. Semen quality and cryopreservation in adolescent cancer patients. Hum Reprod. 2002;17(12):3157–3161. doi: 10.1093/humrep/17.12.3157. [DOI] [PubMed] [Google Scholar]
- 9.Su HI. Measuring ovarian function in young cancer survivors. Minerva Endocrinol. 2010;35(4):259–270. [PubMed] [Google Scholar]
- 10.Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12(6):685–718. doi: 10.1093/humupd/dml034. [DOI] [PubMed] [Google Scholar]
- 11.Hendriks DJ, Kwee J, Mol BW, te Velde ER, Broekmans FJ. Ultrasonography as a tool for the prediction of outcome in IVF patients: a comparative meta-analysis of ovarian volume and antral follicle count. Fertil Steril. 2007;87(4):764–775. doi: 10.1016/j.fertnstert.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 12.La Marca A, Broekmans FJ, Volpe A, Fauser BC, Macklon NS. Anti-Mullerian hormone (AMH): what do we still need to know? Hum Reprod. 2009;24(9):2264–2275. doi: 10.1093/humrep/dep210. [DOI] [PubMed] [Google Scholar]
- 13.Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97(5):1673–1680. doi: 10.1210/jc.2011-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, Stabile G, Volpe A. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART) Hum Reprod Update. 2010;16(2):113–130. doi: 10.1093/humupd/dmp036. [DOI] [PubMed] [Google Scholar]
- 15.Knopman JM, Noyes N, Talebian S, Krey LC, Grifo JA, Licciardi F. Women with cancer undergoing ART for fertility preservation: a cohort study of their response to exogenous gonadotropins. Fertil Steril. 2009;91(4 Suppl):1476–1478. doi: 10.1016/j.fertnstert.2008.07.1727. [DOI] [PubMed] [Google Scholar]
- 16.Quintero RB, Helmer A, Huang JQ, Westphal LM. Ovarian stimulation for fertility preservation in patients with cancer. Fertil Steril. 93(3):865–868. doi: 10.1016/j.fertnstert.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Robertson AD, Missmer SA, Ginsburg ES. Embryo yield after in vitro fertilization in women undergoing embryo banking for fertility preservation before chemotherapy. Fertil Steril. 95(2):588–591. doi: 10.1016/j.fertnstert.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 18.Pal L, Leykin L, Schifren JL, Isaacson KB, Chang YC, Nikruil N, Chen Z, Toth TL. Malignancy may adversely influence the quality and behaviour of oocytes. Hum Reprod. 1998;13(7):1837–1840. doi: 10.1093/humrep/13.7.1837. [DOI] [PubMed] [Google Scholar]
- 19.Domingo J, Guillen V, Ayllon Y, Martinez M, Munoz E, Pellicer A, Garcia-Velasco JA. Ovarian response to controlled ovarian hyperstimulation in cancer patients is diminished even before oncological treatment. Fertil Steril. 2012;97(4):930–934. doi: 10.1016/j.fertnstert.2012.01.093. [DOI] [PubMed] [Google Scholar]
- 20.Freeman EW, Gracia CR, Sammel MD, Lin H, Lim LC, Strauss JF., 3rd Association of anti-mullerian hormone levels with obesity in late reproductive-age women. Fertil Steril. 2007;87(1):101–106. doi: 10.1016/j.fertnstert.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 21.Efron BTRJ. An Introduction to the Bootstrap. Boca Raton: CRC Press; 1994. [Google Scholar]
- 22.Steiner AZ, Herring AH, Kesner JS, Meadows JW, Stanczyk FZ, Hoberman S, Baird DD. Antimullerian hormone as a predictor of natural fecundability in women aged 30–42 years. Obstet Gynecol. 2010;117(4):798–804. doi: 10.1097/AOG.0b013e3182116bc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das M, Shehata F, Moria A, Holzer H, Son WY, Tulandi T. Ovarian reserve, response to gonadotropins, and oocyte maturity in women with malignancy. Fertil Steril. 96(1):122–125. doi: 10.1016/j.fertnstert.2011.04.070. [DOI] [PubMed] [Google Scholar]
- 24.Moria A, Das M, Shehata F, Holzer H, Son WY, Tulandi T. Ovarian reserve and oocyte maturity in women with malignancy undergoing in vitro maturation treatment. Fertil Steril. 95(5):1621–1623. doi: 10.1016/j.fertnstert.2010.12.041. [DOI] [PubMed] [Google Scholar]
- 25.Das M, Shehata F, Moria A, Holzer H, Son WY, Tulandi T. Ovarian reserve, response to gonadotropins, and oocyte maturity in women with malignancy. Fertil Steril. 2011;96(1):122–125. doi: 10.1016/j.fertnstert.2011.04.070. [DOI] [PubMed] [Google Scholar]
- 26.Yu B, Douglas N, Ferin MJ, Nakhuda GS, Crew K, Lobo RA, Hershman DL. Changes in markers of ovarian reserve and endocrine function in young women with breast cancer undergoing adjuvant chemotherapy. Cancer. 2010;116(9):2099–2105. doi: 10.1002/cncr.25037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lawrenz B, Fehm T, von Wolff M, Soekler M, Huebner S, Henes J, Henes M. Reduced pretreatment ovarian reserve in premenopausal female patients with Hodgkin lymphoma or non-Hodgkin-lymphoma-evaluation by using antimullerian hormone and retrieved oocytes. Fertil Steril. 2012 doi: 10.1016/j.fertnstert.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 28.Quintero RB, Helmer A, Huang JQ, Westphal LM. Ovarian stimulation for fertility preservation in patients with cancer. Fertil Steril. 2010;93(3):865–868. doi: 10.1016/j.fertnstert.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Plante BJ, Cooper GS, Baird DD, Steiner AZ. The impact of smoking on antimullerian hormone levels in women aged 38 to 50 years. Menopause. 2010;17(3):571–576. doi: 10.1097/gme.0b013e3181c7deba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hehenkamp WJ, Volkers NA, Broekmans FJ, de Jong FH, Themmen AP, Birnie E, Reekers JA, Ankum WM. Loss of ovarian reserve after uterine artery embolization: a randomized comparison with hysterectomy. Hum Reprod. 2007;22(7):1996–2005. doi: 10.1093/humrep/dem105. [DOI] [PubMed] [Google Scholar]
- 31.La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod. 2006;21(12):3103–3107. doi: 10.1093/humrep/del291. [DOI] [PubMed] [Google Scholar]
- 32.Tsepelidis S, Devreker F, Demeestere I, Flahaut A, Gervy C, Englert Y. Stable serum levels of anti-Mullerian hormone during the menstrual cycle: a prospective study in normo-ovulatory women. Hum Reprod. 2007;22(7):1837–1840. doi: 10.1093/humrep/dem101. [DOI] [PubMed] [Google Scholar]
- 33.Li HW, Anderson RA, Yeung WS, Ho PC, Ng EH. Evaluation of serum antimullerian hormone and inhibin B concentrations in the differential diagnosis of secondary oligoamenorrhea. Fertil Steril. 2011;96(3):774–779. doi: 10.1016/j.fertnstert.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 34.Nelson SM, La Marca A. The journey from the old to the new AMH assay: how to avoid getting lost in the values. Reprod Biomed Online. 23(4):411–420. doi: 10.1016/j.rbmo.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 35.Kumar A, Kalra B, Patel A, McDavid L, Roudebush WE. Development of a second generation anti-Mullerian hormone (AMH) ELISA. J Immunol Methods. 362(1–2):51–59. doi: 10.1016/j.jim.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 36.Kumar A, Kalra B, Patel A, McDavid L, Roudebush WE. Development of a second generation anti-Mullerian hormone (AMH) ELISA. J Immunol Methods. 2010;362(1–2):51–59. doi: 10.1016/j.jim.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 37.Lee JR, Kim SH, Jee BC, Suh CS, Kim KC, Moon SY. Antimullerian hormone as a predictor of controlled ovarian hyperstimulation outcome: comparison of two commercial immunoassay kits. Fertil Steril. 95(8):2602–2604. doi: 10.1016/j.fertnstert.2011.01.126. [DOI] [PubMed] [Google Scholar]
- 38.Streuli I, Fraisse T, Chapron C, Bijaoui G, Bischof P, de Ziegler D. Clinical uses of anti-Mullerian hormone assays: pitfalls and promises. Fertil Steril. 2009;91(1):226–230. doi: 10.1016/j.fertnstert.2007.10.067. [DOI] [PubMed] [Google Scholar]
- 39.Freour T, Mirallie S, Bach-Ngohou K, Denis M, Barriere P, Masson D. Measurement of serum anti-Mullerian hormone by Beckman Coulter ELISA and DSL ELISA: comparison and relevance in assisted reproduction technology (ART) Clin Chim Acta. 2007;375(1–2):162–164. doi: 10.1016/j.cca.2006.06.013. [DOI] [PubMed] [Google Scholar]