Abstract
A simple synthesis of the dopamine transporter ligand [18F]FECNT with high radiochemical yield and short synthesis time, suitable for routine production is reported. Reaction of 2β-carbomethoxy-3β-(4-chlorophenyl)nortropane with [18F]2-fluoroethyl triflate ([18F]FEtOTf) at room temperature for 4 min. provided [18F]FECNT in 84% decay corrected radiochemical yield. Since [18F]FEtOTf was prepared from [18F]2-fluoroethyl bromide that was isolated from its starting material, formation of unwanted side products and the amount of expensive precursor used could be greatly reduced. The overall radiochemical yields of [18F]FECNT were 40% (n=29) and the total synthesis time was ca. 100 min. The average specific activity of [18F]FECNT was 377.4 GBq/µmol (10.2 Ci/µmol).
Keywords: [18F]FECNT, PET tracer, DAT ligands, Dopamine transporter, Positron emission tomography
1. Introduction
Dopamine (DA) is a neurotransmitter involved in motor control, cognition and reward mechanisms. DA is mainly produced in the cell bodies of neurons found in midbrain. The axonal projections of these dopaminergic neurons form synapses with other regions of brain. Dopamine transporter (DAT), an integral membrane protein present in the terminals of dopaminergic cells, mediates the reuptake of dopamine from the synaptic cleft, thus terminating the signal of the neurotransmitter. DAT transports the dopamine back into dopaminergic cells, where it is metabolized or stored for future use. It has been reported that in the dendritic release of DA leading to auto-inhibition the role of DAT is reversed (Blakely, 2001; Falkenburger et al., 2001). DAT facilitates regulation of DA signal that underlies several aspects of cognition and reward. DAT is a major target for various psychologically active drugs and environmental toxins.
Though DAT is selective for transporting DA compared to other monoamine neurotransmitters, it can also transport some structural analogs of DA into neurons. It is reported (Storch et al., 2004) that the neurotoxicity of 1-methyl-4-phenylpyridinium (MPP+), the active metabolite of 1-methyl-4-phenyl-1,2,3,4-tetrahydropyridine (MPTP), involves transportation of MPP+ into the DA cells by DAT.
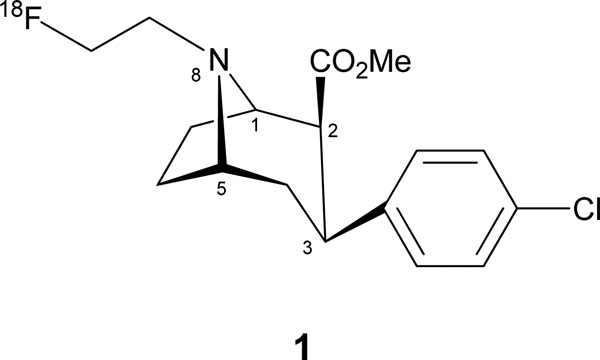
PET imaging using positron emitter labeled DAT ligands provides an important tool to quantitatively assess DAT availability in various pathological and psychological conditions. Since cocaine exhibits high-affinity binding towards DAT, [11C]cocaine (Volkow et al., 1995) and several other 11C and 18F labeled nortropane cocaine analogs have been reported for PET imaging of DAT (Varrone and Halldin, 2010). 2β-Carbomethoxy-3β-(4-chlorophenyl)-8-(2-[18F]fluoroethyl)nortropane ([18F]FECNT, 1; Fig. 1), reported by Goodman et al. (2000) is a highly selective low nanomolar affinity DAT ligand with low specificity for serotonin transporter (SERT) and norepinephrine transporter (NET). The affinity of FECNT for SERT and NET is 25- and 156-fold lower, respectively, than for DAT (Goodman et al., 2000).
Figure 1.
[18F]FECNT structure
Because of its high selectivity towards DAT and shorter equilibration time [18F]FECNT (1) makes an excellent tracer for PET imaging of DAT. Davis et al. (2003) have reported human studies using 1, involving normal and Parkinson's disease subjects.
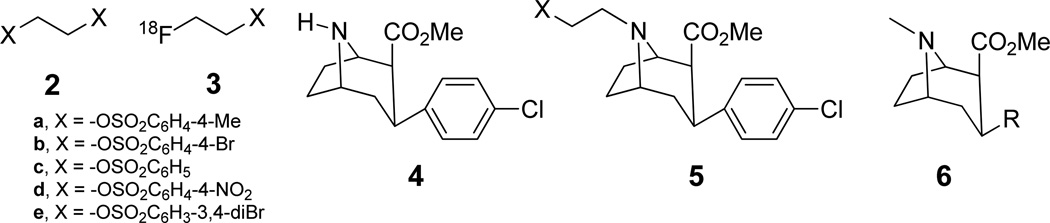
[18F]FECNT (1) has been previously synthesized by coupling nortropane precursor 4 (Fig. 2 with either [18F]2-fluoroethyl tosylate (3a) (Goodman et al., 2000) or brosylate (3b) (Voll et al., 2005), in overall decay corrected radiochemical yields of 20 and 17% respectively and with a synthesis time of about 122 and 150 min. respectively. In these syntheses, 18F-synthons 3a and 3b were prepared by nucleophilic 18F-fluorination of the corresponding disulfonates 2a and 2b. The product mixture containing both the 18F-synthon and the starting material was then subjected to coupling with the precursor 4, producing, in addition to the required 1, large amounts of side products formed by the reaction of 2a–b with 4. Also, since the 18F-synthon has to compete with more reactive disulfonate esters 2a or 2b present in several fold excess, large amounts of the expensive ($80–120 per mg) precursor 4 (3–4 mg) have to be used. In order to reduce the amount of precursor used and also to reduce the side products formed, Musachio et al. (2005) isolated the 18F-synthons 3a–e using HPLC separation and solid-phase extraction (SPE) procedure before reacting them with 4. However, the yields were affected by considerable loss of synthon in the coupling step. Chen et al. (2008) reported that 1 could be prepared by the nucleophilic fluorination of 5 (X = −OSO2CH3). However, in this method there is a high probability of epimerization of chiral center at 2 position next to the carboxylic ester group catalyzed by the base present in the reaction mixture. Base catalyzed epimerization of similar cocaine analogs (6, R= −OC(O)Ph, −OH, −Ph) has been reported in literature (Casale et al., 1992). The epimerization products, 2α-carbomethoxy nortropane derivatives have been shown to be biologically inactive (Madras et al., 1989). Nevertheless, formation of labeled epimerization products reduces the yield and also requires their separation from the final product.
Figure 2.
Previous syntheses of 1
Thus, the reported syntheses of 1 are of low radiochemical yields, time consuming and not cost-effective to be carried out on a routine basis. We herein report a simple, practical and cost-efficient synthesis of 1 that is suitable for routine production.
2. Materials and Methods
2.1 Materials
2β-Carbomethoxy-3β-(4-chlorophenyl)nortropane (4), and the cold standard 2β-carbomethoxy-3β-(4-chlorophenyl)-8-(2-fluoroethyl)nortropane (FECNT) were purchased from ABX Advanced Biochemical Compounds, Germany. Graphpac-GC 80/100 was purchased from Alltech Associates (Deerfield, IL). All other chemicals, reagents and solvents were purchased from either Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA). Thin-layer chromatography (TLC) was carried out using plastic backed silica gel plates containing fluorescent indicator and the spots were made visible using UV light. Melting points were determined using an Electrothermal® melting point apparatus and are uncorrected. 1H NMR spectra were recorded on a Bruker 400 MHz instrument at NMRFAM, University of Wisconsin, Madison, WI. Chemical shift values are reported using TMS as external standard.
2.2 Preparative HPLC
The preparative HPLC system consisted of a Rainin HPX or a Gilson 302 pump, a Gilson 116 or an Applied Biosystems 785A UV detector and a NaI(Tl) single event radioactivity detector. For preparative HPLC purification of the reaction mixture a Waters XTerra Prep RP18 19×100mm HPLC column (Waters Corporation, Milford, MA) and a mobile phase consisting of ethanol-water-triethylamine (50:50:0.1) at a flow rate of 6 mL/min and UV detection at 254nm were used. The retention time of 1 was 26.5 min under these conditions.
2.3 Analytical HPLC
The analytical HPLC system consisted of a Shimadzu LC-10AS pump, an Applied Biosystems 785A UV detector and a radioactivity detector. For analysis of the final product to determine specific activity and radiochemical purity, a Waters Xterra MS C18 5µ 4.6×20mm IS column and a mobile phase consisting of methanol-water-triethylamine 60:40:0.1 at a flow rate of 1 mL/min and UV detection at 220nm were used.
2.4 [18F]Fluoride production
The 18F fluoride was prepared via the 18O(p,n)18F reaction on enriched [18O]H2O using a GE PETtrace 16MeV proton or a CTI RDS 112 11MeV proton medical cyclotron. After irradiation, the [18F] fluoride was recovered by the trap and release method using a Waters Accell Plus QMA Plus light cartridge pre-rinsed with 1N KHCO3 (1 mL) and water (10 mL) and elution with 0.6 mL of a solution of Kryptofix® (8 mg/mL), K2CO3 (2.4 mg/mL) in 80% aq. CH3CN, followed by 0.6 mL of CH3CN. The mixture was azeotropically dried by repeated addition of CH3CN (3 × 0.5 mL) followed by evaporation using argon flow at 85–90 °C.
2.5 Chemistry
2.5.1 1,2-Ethanediyl 4-bromobenzenesulfonate (2b)
Compound 2b has been previously prepared from ethylene glycol and 4-bromobenznesulfonyl chloride using different experimental conditions (Musachio et al., 2005; Voll et al., 2005). To a mixture of anhydrous ethylene glycol (1.55 g, 0.025 mol) and anhydrous pyridine (80 mL), cooled to −5 °C using ice-salt bath, was dropwise added 4-bromobenzenesulfonyl chloride (14.05 g, 0.055 mol) in anhydrous pyridine (20 mL) pre-cooled with ice bath, under argon atmosphere. The temperature was kept below 0 °C during the addition. After stirring at 0–5 °C for 2 h, the reaction was quenched by slowly adding ice and water (100 mL). The mixture was taken up in ethyl acetate (300 mL), washed with 0.5N HCl (3 × 150 mL), water (200 mL) and saturated aqueous bicarbonate (2 × 150 mL) and dried (MgSO4). Evaporation of ethyl acetate under reduced pressure gave crude product as a white solid residue (7 g). Precipitation from CH2Cl2 (20 mL) gave almost pure 2b as a white solid (3 g, 24%). The product was further purified using column chromatography (silica gel, hexane-CH2Cl2) to give pure product (2.6 g). The mother liquor gave a solid (4 g) that was a mixture of mono and dibrosylate.
2.5.2 2-Bromoethyl p-nitrobenzenesulfonate (8)
Preparation of 8 has been reported earlier without experimental details (Smolina et al., 1974). To a stirred mixture of 2-bromoethanol (2.5 g, 0.02 mol) and p-nitrobenzenesulfonyl (nosyl) chloride (4.88 g, 0.022 mol) in anhydrous CH2Cl2 (50 mL) cooled using an ice-bath, was dropwise added 2,6-lutidine (5.2 mL) under argon atmosphere. The yellowish mixture was stirred for 10 min. at ice-bath temperature and overnight at room temperature. The reaction mixture was cooled using an ice-bath and 50 mL of ice-water mixture added under stirring. Organic phase separated and washed with 0.1M HCl (2 × 50 mL), brine (50 mL) and dried (MgSO4). Evaporation of the solvent under reduced pressure gave 8 as a crystalline solid (4.6 g, 74%), which was almost a single spot on TLC (silica gel, EtOAc-hexane, 20:80). The product was further purified using column chromatography (silica gel, hexane-ethyl acetate). M.pt. 101–102 °C (lit. 98–99 °C (Smolina et al., 1974)). 1H NMR (CDCl3): δ 3.53 (2H, t, J = 6.2 Hz, ArCH2), 4.42 (2H, t, J = 6.5 Hz, −CH2Br), 8.15 (2H, d, J = 8.8 Hz, 2 × ArH) and 8.43 ppm (2H, d, J = 8.8 Hz, 2 × ArH).
2.6 Radiochemistry
2.6.1 Reaction of dibrosylate 2b with [18F]fluoride
To the azeotropically dried [18F]fluoride a solution of dibrosylate 2b (2 mg) in CH3CN (0.2 mL) was added and the mixture heated to 85–100 °C for 10 min. The mixture was transferred to a short silica gel column, eluted with CH2Cl2 and analyzed by HPLC.
2.6.2 Synthesis of [18F]FECNT (1)
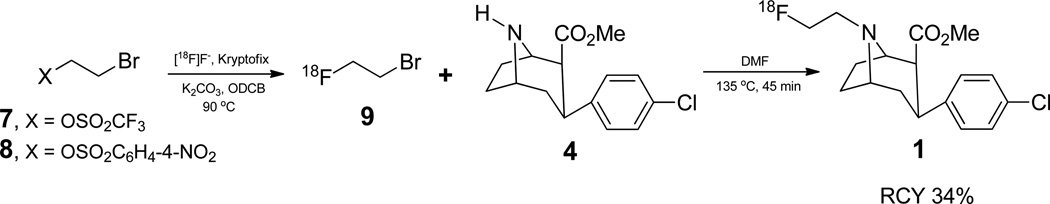
2.6.2.1 Using [18F]2-fluoroethyl bromide (9) prepared from 2-bromoethyl triflate (7)
To the azeotropically dried [18F]fluoride was added 2-bromoethyl triflate (Chi et al., 1987) (10 µL) in 1,2-dichlorobenzene (0.3 mL). The mixture was heated at 85 °C in a closed vessel for 15 min. [18F]2-Fluoroethyl bromide was then distilled off by bubbling argon through the reaction mixture at 25 mL/min. After passing through a short column of P2O5, [18F]2-fluoroethyl bromide was trapped in solution of precursor (4, 3 mg) in N,N-dimethylformamide (DMF) (0.3 mL). After no additional [18F]2-fluoroethyl bromide was trapped (monitored by radioactivity detector), the vial containing the precursor solution was tightly capped and heated at 135 °C for 45 min. The vial was flushed with argon to remove unreacted [18F]2-fluoroethyl bromide. The mixture was diluted with 20% ethanol (1.5 mL) and injected onto semi-prep HPLC. The fraction containing 1 was collected, evaporated to dryness in vacuo and taken-up in saline (3 mL) and filtered through a 0.2 micron filter (Millex-LG, 13 mm) into a sterile vial. For radiochemical purity and specific activity determination, a small amount (20–50 µL) of the final product was analyzed by analytical HPLC.
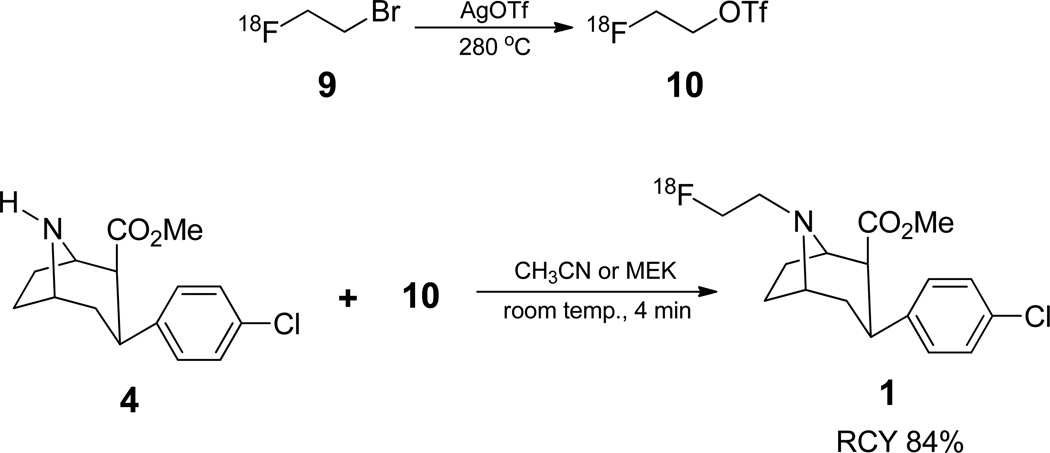
2.6.2.2 Using [18F]2-fluoroethyl triflate (10) prepared from 2-bromoethyl p-nitrobenzenesulfonate (8)
To the azeotropically dried [18F]fluoride was added a solution of 2-bromoethyl p-nitrobenzenesulfonate (8, 5 mg) in 1,2-dichlorobenzene (0.3 mL) and the mixture was heated at 90 °C while argon was bubbled through the reaction mixture at a rate of 25 mL/min. The formed [18F]2-fluoroethyl bromide was passed through a short column of Sicapent® and converted to [18F]2-fluoroethyl triflate (10) (Zhang et al., 2003) by passing through a AgOTf/Graphpac column heated at 280 °C and bubbled into a solution of precursor (4, 0.4 mg) in acetonitrile (0.2 mL) kept at room temperature. The bubbling stopped after about 10 min when no additional activity was trapped. The mixture was left at room temperature for another 4 min and then diluted with 1 mL of 10% aqueous acetonitrile and injected on to semi-prep HPLC.
2.6.2.3 Using [18F]2-fluoroethyl triflate (10) prepared from 1,2-ethanediyl triflate
To the azeotropically dried [18F]fluoride was added a solution of 1,2-ethanediyl triflate (10 mg) in acetonitrile (0.3 mL). The mixture was heated at 90 °C for 10 min. The formed [18F]2-fluoroethyl triflate was distilled using argon flow into another vial containing the precursor (0.5 mg) in acetonitrile (0.2 mL), cooled using an ice-water bath, after passing through a short column of P2O5. The mixture was left at room temperature for 10 min. and taken up in mobile phase (1.5 mL) and injected onto semi-prep HPLC.
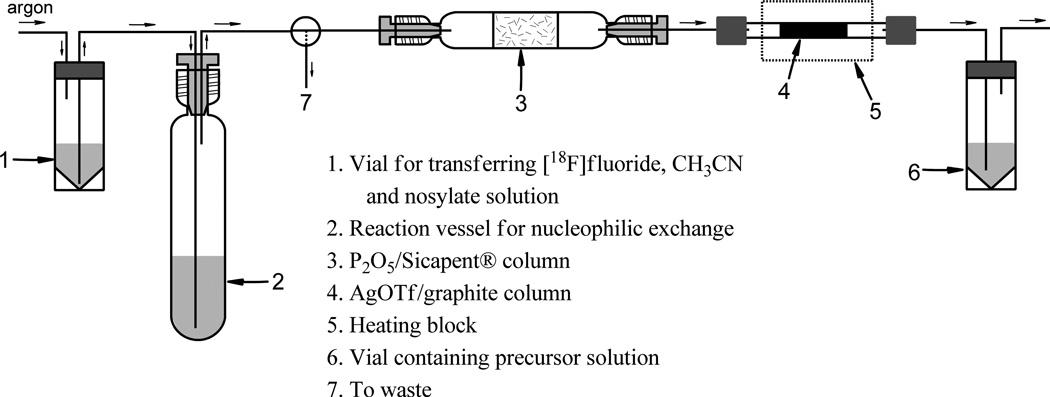
2.7 Experimental setup for the preparation [18F]FECNT using 10
The setup is shown in fig. 3. Vials 1 and 6 are 4 mL v-vials closed with caps containing Teflon lined septum. Vial 1 is used for transferring [18F]fluoride, acetonitrile and nosylate solution. Reaction vessel 2 is a 4 mL Ace® vessel with #7 thread. Vessel 2 is closed with solid Teflon plug with tight holes made for the inlet and outlet. A front-seal O-ring is used for tight seal. The transfer lines are made of Teflon tubing (ID = 0.04 in.), with stainless steel needles for vials 1 and 6 septa. [18F]Fluoride dry-down and the nucleophilic exchange were carried out in vessel 2. During azeotropic distillation the outlet of vessel 2 was directed to waste. Immediately after adding nosylate solution the outlet is connected to the Sicapent® column. The heating block is kept at 280 °C. The AgOTf/Graphpac-GC 80/100 column (Jewett, 1992) is conditioned for at least 2 days at 100 °C and 1 hr at 200 °C under a gentle flow of argon just before the synthesis. The flow rate was ca. 25 mL/min during bubbling. The activity trapped in vial 6 was monitored using a small radioactivity detector. Vial 6 was removed after trapping the activity.
Figure 3.
Experimental setup
3. Results and Discussion
One of the problems with the earlier syntheses of FECNT (1) by coupling the nortropane precursor 4 with a 18F-fluoroethylation synthon, was the difficulty in isolating the 18F-synthon from its starting materials before coupling with precursor 4, We thought that use of a volatile 18F-synthon such as [18F]2-fluoroethyl bromide (9) (Wadsak et al., 2003) would be better as the synthon could be separated from its starting material by a simple distillation instead of a difficult HPLC purification process. Initially, we used 2-bromoethyl triflate (7) as the starting material for 9. Nucleophilic fluorination of 7 with 18F-fluoride in acetonitrile, produced synthon 9 (Scheme 1) in good yields (53% decay corrected, n=31). However, triflate 7 is a moisture-sensitive liquid and it decomposes slowly when exposed to air by repeated handling leading to lower nucleophilic exchange yields over a period of time. We found that 2-bromoethyl nosylate (8) is a better starting material than 7, since 8 is a less moisture-sensitive crystalline solid that is easier to handle and also produced 9 in comparable yields. Nosylate 8 is readily prepared from 2-bromoethanol by reaction with nosyl chloride. After the nucleophilic substitution, synthon 9 was distilled from the reaction mixture using argon flow at 90 °C and was trapped in a solution of nortropane precursor 4 (3 mg) in N,N-dimethylformamide (DMF) (0.2 mL) at −15 °C. After trapping was complete, the coupling reaction was carried out at 135 °C for 45 min.
Scheme 1.
Synthesis of 1 using 9 as coupling agent
Although the distillation process greatly simplified the isolation of synthon from the starting material, the radiochemical yields of the coupling step were still only low to moderate (34% decay corrected) and most of the 9 remained unreacted even after heating at 135 °C for 45 min. And because of slow coupling reaction the total synthesis time still remained high (148 min). Two things could be considered to speed up the coupling reaction and to increase the yields. First one is to use a base. However, base catalyzed epimerization of chiral center α to a carboxylic ester group in similar tropane systems has been reported literature (Casale et al., 1992). The second possibility is to replace the less reactive bromo group in 9 with a more reactive leaving group. Triflate 10 Scheme 2) seemed to be an ideal choice since not only triflate group is more reactive but also bromide can be readily converted to triflate by heating with silver triflate (Jewett, 1992). Thus, synthon 9 could be converted to triflate 10 by passing through a short column of AgOTf/ Graphpac at 280 °C under argon flow, before bubbling through the precursor solution. Our experimental setup is shown in figure 3 (see Materials and Methods section 2.7 for details).
Scheme 2.
Synthesis of 1 using 10 as coupling agent
After the azeotropic drying of 18F-fluoride by repeated addition/evaporation of acetonitrile, a solution of nosylate 8 (5 mg) in 1,2-dichlorobenzene (ODCB) was added to vial 1 and transferred to the reaction vessel 2 while argon was continuously bubbled through the reaction mixture. We found that it was not necessary to wait for the nucleophilic exchange reaction to be complete before starting to distill 9. By bubbling argon through the reaction mixture during the nucleophilic fluorination, 9 could be isolated as it was formed, eliminating the need for a separate isolation step. Bromide 9 after passing through the Sicapent® column was passed through the AgOTf/Graphpac column at 280 °C, where bromide 9 was converted to triflate 10. The formed 10 was trapped in vial 6 in a solution of precursor 4 (0.4 mg) in acetonitrile or methyl ethyl ketone (MEK) (0.2 mL) at room temperature. Trapping was usually complete within 10 min. The mixture was then allowed to stand at room temperature for additional 4 minutes for completion of the reaction. Conversion of 9 to 10 appeared to be almost quantitative since nearly all of the trapped activity remained after flushing the reaction mixture with argon. Usually, when 9 was used as the coupling agent any unreacted 9 was removed during the argon flush.
The crude reaction mixture was diluted with 10% acetonitrile in water (1 mL) and purified by reverse phase HPLC. The HPLC fraction containing [18F]FECNT was evaporated to dryness and taken up in saline (3 mL) and passed through a 0.2 micron filter (Millex-LG, 13mm) into a sterile vial. The time taken for the isolation of HPLC pure material since the start of nucleophilic fluorination was 84 min. The total synthesis time including formulation was 96 min. The final product also can be isolated by passing the diluted HPLC fraction through a C18-SepPak and eluting the product with 1 mL ethanol as reported in literature. The decay corrected (EOB) yields using 10 were; synthon 49%, coupling reaction 84% and overall 40% (n=29%). The average specific activity of the [18F]FECNT was 377.4 GBq/µmol (10.2 Ci/µmol).
Thus, by using a volatile and more reactive 18F-synthon, we were able to carry out all 4 individual steps, nucleophilic fluorination of 8, isolation of bromide 9, conversion to 10 and reaction with precursor 4, concurrently as the first step proceeded. So immediately after the completion of nucleophilic exchange step, the other 3 steps were also essentially complete, thereby drastically reducing the synthesis time. In the previous syntheses of 1, the above mentioned steps have to be done sequentially making the synthesis lengthy. Also by using more reactive triflate 10, the amount of precursor used could be reduced to 0.4 mg compared to 3–4 mg used in reported syntheses. Since the precursor costs about $80–120 per mg, the cost per synthesis is also greatly reduced. In the current synthesis of 1 the radiochemical yields were also much higher (coupling, 84% and overall 40%). We also made 10 by the nucleophilic fluorination of 1,2-ethanediyl triflate. Although this method avoids the bromide to triflate conversion, the ditriflate is very moisture-sensitive and slowly decomposes during storage even at −20 °C, leading to lower yields of 10. Kiesewetter et al. (1989) reported formation of [18F]2-fluoroethanol when 1,2-ethanediyl triflate was used for the preparation of 10.
4. Conclusion
By using more reactive and volatile triflate 10 as the 18F-synthon and separating the synthon from its starting material, better radiochemical yields were achieved while total synthesis time and cost per synthesis were considerably reduced. These advantages make the current procedure suitable for routine production of [18F]FECNT.
Highlights.
An efficient synthesis of dopamine transporter tracer [18F]FECNT is presented.
Coupling using isolated labeling synthon reduces side products formation
Higher radiochemical yields and mild reaction conditons achieved
Very low amounts of expensive precursor used reducing the cost per synthesis
Suitable for routine production of [18F]FECNT
Acknowledgment
The financial support by NIH through grants R01 AA012277-12, R01 AA010079-14 and R01 AA010079-13S1 (to Mary L. Schneider) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A preliminary account was presented at Society of Nuclear Medicine 55th Annual Meeting, New Orleans, LA, June 14–18, 2008, Abstract No. 1229
References
- Blakely RD. Dopamine's Reversal of Fortune. Science. 2001;293:2407–2409. doi: 10.1126/science.1065931. [DOI] [PubMed] [Google Scholar]
- Casale JF, Lewin AH, Bowen JP, Carroll FI. Base-catalyzed C-2 exchange and epimerization of cocaine analogs: methyl 3β-substituted 8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylates. J. Org. Chem. 1992;57:4906–4912. [Google Scholar]
- Chen ZP, Wang SP, Li XM, Liu CY, Tang J, Cao GX, Luo SN, Zhang LF, Jin J. A one-step automated high-radiochemical-yield synthesis of 18F-FECNT from mesylate precursor. Appl. Radiat. Isot. 2008;66:1881–1885. doi: 10.1016/j.apradiso.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Chi DY, Kilbourn MR, Katzenellenbogen JA, Welch MJ. A rapid and efficient method for the fluoroalkylation of amines and amides. Development of a method suitable for incorporation of the short-lived positron emitting radionuclide fluorine-18. J. Org. Chem. 1987;52:658–664. [Google Scholar]
- Davis MR, Votaw JR, Bremner JD, Byas-Smith MG, Faber TL, Voll RJ, Hoffman JM, Grafton ST, Kilts CD, Goodman MM. Initial human PET imaging studies with the dopamine transporter ligand 18F-FECNT. J. Nucl. Med. 2003;44:855–861. [PubMed] [Google Scholar]
- Falkenburger BH, Barstow KL, Mintz IM. Dendrodendritic Inhibition Through Reversal of Dopamine Transport. Science. 2001;293:2465–2470. doi: 10.1126/science.1060645. [DOI] [PubMed] [Google Scholar]
- Goodman MM, Kilts CD, Keil R, Shi B, Martarello L, Xing D, Votaw J, Ely TD, Lambert P, Owens MJ, Camp VM, Malveaux E, Hoffman JM. 18F-labeled FECNT: a selective radioligand for PET imaging of brain dopamine transporters. Nucl. Med. Biol. 2000;27:1–12. doi: 10.1016/s0969-8051(99)00080-3. [DOI] [PubMed] [Google Scholar]
- Jewett DM. A simple synthesis of [11C]methyl triflate. Int. J. Radiat. Appl. Instrum. [A] 1992;43:1383–1385. doi: 10.1016/0883-2889(92)90012-4. [DOI] [PubMed] [Google Scholar]
- Kiesewetter DO, Brucke T, Finn RD. Radiochemical synthesis of [18F]fluororaclopride. Appl. Radiat. Isot. 1989;40:455–460. doi: 10.1016/0883-2889(89)90126-3. [DOI] [PubMed] [Google Scholar]
- Madras BK, Fahey MA, Bergman J, Canfield DR, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. I. [3H]cocaine binding sites in caudate-putamen. J. Pharmacol. Exp. Ther. 1989;251:131–141. [PubMed] [Google Scholar]
- Musachio JL, Shah J, Pike VW. Radiosyntheses and reactivities of novel [18F]2-fluoroethyl arylsulfonates. J. Labelled Compd. Radiopharm. 2005;48:735–747. [Google Scholar]
- Smolina TA, Gopius ED, Us LI, Shchekut'eva LF, Reutov OA. Solvolysis of 2-haloethyl-1-14C-arylsulfonates. Doklady Akademii Nauk SSSR. 1974;216:1070–1072. [Google Scholar]
- Storch A, Ludolph AC, Schwarz J. Dopamine transporter: involvement in selective dopaminergic neurotoxicity and degeneration. J. Neural Transm. 2004;111:1267–1286. doi: 10.1007/s00702-004-0203-2. [DOI] [PubMed] [Google Scholar]
- Varrone A, Halldin C. Molecular Imaging of the Dopamine Transporter. J. Nucl. Med. 2010;51:1331–1334. doi: 10.2967/jnumed.109.065656. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Logan J, Gatley SJ, Dewey SL, MacGregor RR, Schlyer DJ, Pappas N, King P, Wang G-J, Wolf AP. Carbon-11-Cocaine Binding Compared at Subpharmacological and Pharmacological Doses: A PET Study. J. Nucl. Med. 1995;36:1289–1297. [PubMed] [Google Scholar]
- Voll RJ, McConathy J, Waldrep MS, Crowe RJ, Goodman MM. Semi-automated preparation of the dopamine transporter ligand [18F]FECNT for human PET imaging studies. Appl. Radiat. Isot. 2005;63:353–361. doi: 10.1016/j.apradiso.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Wadsak W, Mitterhauser M, Mien L-k, Toegel S, Keppler B, Dudczak R, Kletter K. Radiosynthesis of 3-(2'-[18F]fluoro)-flumazenil ([18F]FFMZ) J. Labelled Compd. Radiopharm. 2003;46:1229–1240. [Google Scholar]
- Zhang M-R, Furutsuka K, Yoshida Y, Suzuki K. How to increase the reactivity of [18F]fluoroethyl bromide: [18F]fluoroethylation of amine, phenol and amide functional groups with [18F]FEtBr, [18F]FEtBr/NaI and [18F]FEtOTf. J. Labelled Compd. Radiopharm. 2003;46:587–598. [Google Scholar]