Abstract
Second messenger responses rely on where and when the enzymes that propagate these signals become active. Spatial and temporal organization of certain signaling enzymes is controlled in part by A-kinase anchoring proteins (AKAPs). This family of regulatory proteins was originally classified on the basis of their ability to compartmentalize the cyclic adenosine monophosphate (cAMP)-dependent protein kinase (also known as protein kinase A, or PKA). However, it is now recognized that AKAPs position G protein–coupled receptors, adenylyl cyclases, G proteins, and their effector proteins in relation to protein kinases and signal termination enzymes such as phosphodiesterases and protein phosphatases. This arrangement offers a simple and efficient means to limit the scope, duration, and directional flow of information to sites deep within the cell. This review focuses on the pros and cons of reagents that define the biological role of kinase anchoring inside cells and discusses recent advances in our understanding of anchored second messenger signaling in the cardiovascular and immune systems.
Keywords: cell signaling, compartmentalization, cAMP, A-kinase anchoring proteins, protein phosphorylation
FOUNDING THIS FIELD
In the 1950s, Earl Sutherland defined how hormones stimulate the production of the second messenger 3′–5′-cyclic adenosine monophosphate (cAMP) by adenylyl cyclase (AC) (1, 2), and Edwin Krebs and Edmond Fischer demonstrated protein phosphorylation as a principal task for cAMP (3). The first defined recipient of this chemical message was protein kinase A (PKA, also known as the cAMP-dependent protein kinase), a heterotetrameric holoenzyme consisting of two regulatory (R) subunits that maintain two catalytic (C) subunits in an inhibited state (4, 5). When cAMP levels are low, PKA is dormant; however, when cAMP levels are elevated, two molecules of cAMP bind to each R subunit, thereby releasing the active C subunits. The C subunits phosphorylate serine (S) or threonine (T) residues, typically within the sequence -R-R-X-S/T-X (6). The first evidence for localized cAMP signaling was shown in the late 1970s: In heart, both prostaglandin E1 and epinephrine increased cAMP, yet only epinephrine increased glycogen phosphorylase activity and cardiac contraction (7–9).
At approximately the same time, the PKA holoenzyme was discovered to exist in two forms: a cytoplasmic type I PKA and an exclusively particulate type II PKA (10). Thus, it was postulated that activation of PKA was differentially regulated at organelles and on intracellular membranes. In 1982, Theurkauf & Vallee (11) provided evidence for the targeting of PKA subtypes when they showed that type II PKA is anchored to microtubules via its regulatory RII subunit interaction with microtubule-associated protein MAP2. Slightly later, a second neuronal anchoring protein was identified as a protein contaminant that copurifies with RII subunits on cAMP-agarose (12).
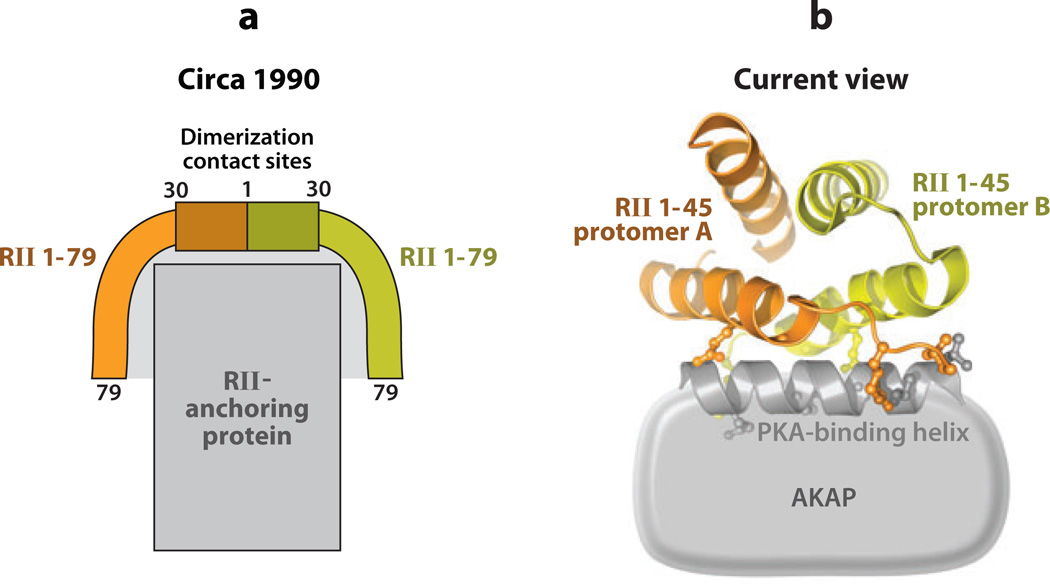
Subsequent technological advances, including protein-protein blotting using RII as the probe (RII overlay) and expression-cloning strategies, have uncovered many more of these anchoring proteins, which are now known as A-kinase anchoring proteins (AKAPs) (13–15). Since then, more than 50 human genes that encode AKAPs have been identified (16). Additionally, the advent of sophisticated molecular biology and genetic screening approaches permitted dramatic breakthroughs in our understanding of the RII-AKAP interface and AKAP action (17). A survey of RII deletion mutants revealed that the first 79 residues were necessary and sufficient for AKAP binding (18), whereas analysis of the reciprocal binding surface on several anchoring proteins identified common helical regions (19). Mutagenesis of such a binding helix in human thyroid clone 31 (Ht31, also known as AKAP-Lbc) dramatically reduced association with RII (20), providing the first evidence that AKAPs could interact with PKA via an amphipathic helix-binding motif (19). Proof of principle was provided when Ht31 peptides were used to uncouple PKA proximity to AMPA-type glutamate receptor ion channels in hippocampal neurons with concomitant effects on synaptic transmission (21). A simple model of this protein-protein interface put forward in the early 1990s (Figure 1a) bears a striking resemblance to the three-dimensional structure of the RII-AKAP interface (Figure 1b) that was solved more than a decade later.
Figure 1.
The evolution of protein kinase A (PKA) anchoring models. (a) Schematic diagram (circa 1990) (18) presenting a model of the anchored RII complex. (b) Contemporary model based on the crystal structure of RIIα1–45 dimer in complex with the A-kinase anchoring protein (AKAP)–in silico binding helix (36, 48).
Much of our progress in understanding AKAP action can be traced through studies performed on a single molecule, the human anchoring protein AKAP79. Using this anchoring protein as the bait in a two-hybrid screen for additional neuronal binding partners identified the phosphatase calcineurin, also known as protein phosphatase 2B (PP2B) (22), suggesting that AKAP79 associates with multiple second messenger–regulated kinases and phosphatases. AKAP79 also interacts with isoforms of protein kinase C (PKC), thus creating macromolecular signaling complexes that can integrate cAMP-, calcium-, phospholipid-, and calmodulin-dependent signals at defined intracellular loci (23). Subsequent studies have shown that AKAP79 and other anchoring proteins interact with a plethora of other signaling proteins, such as G protein–coupled receptors, GTPases, phosphatases, phosphodiesterase, and other kinases (24). Not surprisingly, as the scope of this field has exploded in the past decade, investigators have written many excellent reviews about AKAPs, in particular about their roles in dendrites of the central nervous system and in spatially organized cAMP-responsive events near the plasma membrane (25–27). This article focuses on the pros and cons of reagents that define the biological role of kinase anchoring inside cells and on recent advances in our understanding of anchored second messenger signaling in the cardiovascular and immune systems.
THE PROTEIN KINASE A BINDING DOMAIN IN A-KINASE ANCHORING PROTEINS
A defining characteristic of AKAPs is their binding to the R subunits of PKA through a 14– to 18–amino acid sequence that forms an amphipathic α-helix with hydrophobic residues aligned on the side that contacts PKA (20). These binding domains have been identified in all PKA anchoring proteins, with the exception of pericentrin (19, 20, 28). RII subunits bind to AKAPs with low-nanomolar affinity, but generally the affinity of RI subunits for AKAPs is in the high-nanomolar to submicromolar range (29, 30). However, some dual-specificity anchoring proteins and RI-selective AKAPs bind their cognate R subunits with low-nanomolar affinity (31–33).
Detailed structural analyses of the RII-AKAP complex by nuclear magnetic resonance and X-ray crystallography reveal that the RII dimerization and docking (D/D) domain, which encompasses the first 45 residues of the protein, is organized as an antiparallel dimer, forming an X-type, four-helix bundle. This dimeric protein module constrains a hydrophobic docking groove that associates with the AKAP’s amphipathic helix (34–37). RI subunits dimerize in a similar way, although their D/D domain is larger, incorporating an additional 16-residue helix-turn-helix segment (38, 39). Molecular modeling suggests that the longer N termini of the RI dimer fold back on the four-helix bundle to alter the shape and presentation of the AKAP binding determinants on the RI dimer. More complete structural analysis is necessary to elucidate if there are biologically significant differences between RI and RII anchoring interactions.
PROBING FUNCTION WITH PKA ANCHORING DISRUPTOR PEPTIDES
AKAP-derived peptides that bind to the D/D domains of PKA compete with AKAPs, thereby perturbing the subcellular location of the enzyme. These peptides have been important tools in the study of the functional implications of PKA anchoring in cells. A 24–amino acid peptide derived from AKAP-Lbc (originally named Ht31) was the first anchoring disruptor to be characterized (19). Validation of Ht31 action in cells was provided by studies in which the peptide was perfused into neurons or pancreatic β cells to uncouple aspects of synaptic transmission and insulin secretion coupling, respectively (21, 40). Ht31 adducts have subsequently been used to demonstrate a role for PKA anchoring in numerous important signaling events, such as the modulation of L-type Ca2+ channels and excitation-contraction coupling in the heart, of sperm motility, and of fluid movement in the lens of the eye (41–45). An advantage is that Ht31 has low-nanomolar affinity for the type II PKA (Kd = 2.2 ± 0.03 nM) (19); however, a drawback is that this peptide also perturbs interactions between type I PKA and AKAPs (30).
Second-generation anchoring disruptor peptides were developed with the goal of discriminating between RI and RII interactions with AKAPs. Bioinformatic scrutiny of RII binding domains and peptide array–based optimization were combined to design AKAP–in silico (AKAP-IS), an anchoring disruptor with subnanomolar binding affinity for RII (46). In parallel, peptides patterned after the PKA binding region of D-AKAP2 were developed to interfere with RI or RII interactions (47). Anchoring disruptors that are highly selective for either the anchored type I (the RI anchoring disruptor, also known as RIAD) or the type II (super-AKAP-IS)PKA holoenzymes were subsequently developed (36, 48). These reagents have helped delineate effects regulated by type I or type II PKA, such as cAMP-mediated immune regulation and steroidogenesis (49–52). The recognition that dual-specificity AKAPs have additional determinants that secure the interaction with the RI subunit led to the discovery of an RI specifier region (RISR) (53). A peptide derived from an RISR disrupts type I PKA interaction with the AKAP ezrin independently of peptides that mimic the amphipathic helix (53). In summary, the toolbox of PKA anchoring disruptors is considerable and is frequently used as the first approach to define biological effects mediated by anchored PKA enzymes in cells.
Disruption of PKA anchoring has been used to assess the functional significance of localized pools of PKA but is less useful in a therapeutic context. Furthermore, there would be merit in developing reagents that selectively target an individual AKAP. Another promising approach may be to displace AKAP signaling complexes from their proximity to a particular PKA substrate. For example, peptides that block interaction between AKAP18δ and the PKA substrate phospholamban are equally as effective as the Ht31 peptide that disrupts PKA from the AKAP complex in inhibiting β-adrenergic receptor (βAR)-mediated stimulation of calcium reuptake by SERCA2 (sarcoplasmic/endoplasmic reticulum Ca2+ pump 2) (54). Similarly, peptide-based disruption of the adapter protein EBP50 from ezrin disconnects PKA from Csk to reverse cAMP-mediated inhibition of immune function (55).
LOCALIZED CAMP SIGNALING IN THE HEART
Efficient contraction of the heart requires coordinated handling of cAMP and Ca2+ signaling events in cardiomyocytes. This process, known as excitation-contraction coupling, occurs when an action potential triggers a transient rise in intracellular Ca2+ that drives contraction of cardiomyocytes (56). This process takes place in three phases. Phase 1 is initiated by the brief opening of voltage-gated L-type Ca2+ channels. Small amounts of Ca2+ enter regions of the myocyte where the junctional sarcoplasmic reticulum (SR) is nearby. In phase 2, this localized Ca2+ influx triggers the synchronous activation of ryanodine-sensitive Ca2+ channels (i.e., ryanodine receptors, or RyRs) in the SR to produce a global Ca2+ transient. The concomitant activation of Ca2+-responsive contractile proteins, such as cardiac troponin C, initiates contraction. Phase 3 requires termination of SR Ca2+ release and the transport of Ca2+ back into the SR through the ATP-dependent Ca2+ pump SERCA2, which decreases [Ca2+]i and begins myocyte relaxation.
Distinct AKAP complexes contribute to each phase of excitation-contraction coupling by optimizing the phosphorylation of ion channels and contractile proteins. These anchoring proteins organize cAMP effectors such as PKA, guanine exchange proteins activated by cAMP (EPACs), and cAMP-gated channels (HCN) in relation to ACs, the aforementioned enzymes that synthesize cAMP (57, 58). Although most AC isoforms are expressed in cardiac fibroblasts (59), the major isoforms present in myocytes are AC5 and AC6 (59–61). Interestingly, these enzymes can exert certain opposite functional effects on the heart. Overexpression of AC6 appears to be cardioprotective (62–64), whereas AC5 has been implicated in cAMP production arising from cardiac stress (65, 66). Moreover, deletion of AC6 reveals unique biological functions that are not duplicated by AC5, including reductions in PKA and Akt activity, phospholamban phosphorylation, and altered left ventricular contractile function (67). The distinctions between AC5 and AC6 action may, at least in part, reflect their differential recruitment into macromolecular complexes.
In isolated cardiacmyocytes, disruption of PKA anchoring results in impaired cAMP regulation of L-type Ca2+ channels (41, 68, 69), CFTR (cystic fibrosis transmembrane conductance regulator) chloride channels (70), and IK potassium currents (71). Similar approaches have implicated a role for anchored PKA phosphorylation of numerous targets in myocytes (72–74). At least 15 AKAPs are expressed in the heart, roles for which are discussed below. However, most of these anchoring proteins are not found solely in the heart (16, 25, 75, 76).
AKAPs IN CALCIUM-INDUCED CALCIUM RELEASE
Sympathetic control of the heart through βAR stimulation increases the rate and force of contraction and relaxation of cardiac muscle. As depicted in Figure 2, AKAP18 and AKAP79/150 signaling complexes principally contribute to this vital process (77).
Figure 2.
Combinatorial assembly of cardiac A-kinase anchoring protein (AKAP) signaling complexes. The common name (first column) and alternate name(s) (second column) of each anchoring protein are indicated. Schematic representations of cardiac AKAPs highlight enzyme-binding sites and functional domains (third column). Binding partners are indicated (fourth column). Abbreviations: AC, adenylyl cyclase; AMP, adenosine monophosphate; βAR, β-adrenergic receptor; DH, Dbl homology domain; ERK, extracellular signal-regulated kinase; HIF-1α, hypoxia-inducible factor 1α; IP3-R, inositol 3,4,5-phosphate receptor; KCNQ, KvLQT potassium channel subunit; KSR-1, kinase suppressor of Ras1; Lfc, Lbc first cousin; MEK, mitogen-activated protein kinase kinase; MT, mitochondrial transit peptide; NCX1, sodium-calcium exchanger 1; NFATc, nuclear factor of activated T cells; NMDA-R, N-methyl-d-aspartate receptor; PDE, phosphodiesterase; PDK1, phosphoinositide-dependent kinase-1; PH, pleckstrin homology domain; PKA/C/D/N, protein kinase A/C/D/N; PP1/2A/2B, protein phosphatase 1/2A/2B; PTPD1, protein tyrosine phosphatase D1; RGS, regulator of G protein signaling; RSK, ribosomal S6 kinase; RyR2, ryanodine receptor 2; SAP97, synapse-associated protein 97; Siah2, seven in absentia homolog 2; Trek-1, two pore-domain potassium channel; TrpV1, transient receptor potential cation channel V1; VHL, von Hippel–Lindau protein.
AKAP18α
Of the four AKAP7 gene transcripts, AKAP18α is the smallest and encodes an 81–amino acid protein (42, 78–80) (Figure 2). Myristoylation and palmitoylation of N-terminal residues help tether AKAP18 (also known as AKAP15) to the inner face of the plasma membrane to facilitate βARregulation of the L-type calcium channel CaV1.2 (42, 81).Physical association withAKAP18α channels proceeds through a leucine zipper motif, thereby positioning PKA in proximity to its phosphorylation target (82–84). Peptide-mediated disruption of the AKAP15/18-PKA-channel complex reduces stimulation of CaV1.2 currents by this kinase, a well-known modulator of Ca2+ channels (42, 82).
The AKAP18δ variant, a longer transcript of the AKAP7 gene, acts as a scaffold to coordinate a crucial step for cardiac muscle relaxation, the βAR-promoted reuptake of calcium into the SR by SERCA2 (54) (Figures 2 and 3). Phosphorylation of membrane-bound phospholamban by PKA and/or CamKII (calmodulin-dependent protein kinase II) removes its inhibitory effect on SERCA2, thereby accelerating Ca2+ reuptake and myocyte relaxation. Curiously, AKAP18δ contains a central phosphoesterase domain that binds 5′ AMP (36). Although AKAP18δ has no detectable ligase or hydrolase activity, it may be a sensor of 5′ AMP, an important molecule in defining the metabolic state of a cell and the product of cAMP metabolism by phosphodiesterases (PDEs) (36). Polymorphisms at key residues in this region, which occur at low incidence in the population (> 1%), have been linked to cardiac abnormalities and febrile seizures (85).
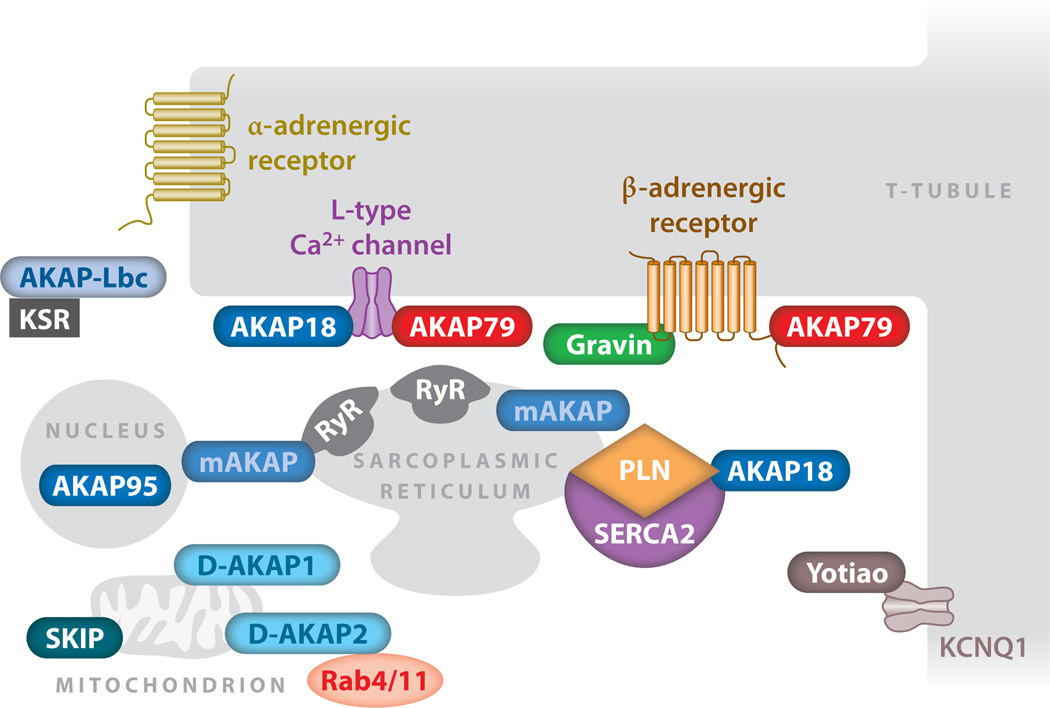
Figure 3.
The A-kinase anchoring protein (AKAP) terrain of cardiomyocytes. The subcellular distribution of AKAPs in cardiomyocytes is depicted. Subcellular organelles, anchoring proteins, and key effector proteins are indicated. Abbreviations: KCNQ, IKs potassium channel subunit; KSR, kinase suppressor of Ras; PLN, phospholamban; RyR, ryanodine receptor; SERCA, sarcoplasmic/endoplasmic reticulum Ca2+ pump; SKIP, sphingosine kinase interacting protein.
AKAP79/150
Products of the AKAP5 gene encode a family of anchoring proteins that include human AKAP79 and the murine and bovine orthologs AKAP150 and AKAP75 (Figure 2). AKAP79/150 is one of the best characterized AKAPs. It has the capacity to interact with a range of important regulatory proteins and a diverse set of ion channels (Figure 2). For example, AKAP79/150 also clusters its cohort of anchored signaling enzymes in proximity to Ca2+ channel subunits via amodified leucine zipper motif located at the extreme C terminus (86) (Figure 3). Localization of AKAP79/150 to the plasma membrane involves three polybasic regions near the N terminus that electrostatically interact with phosphoinositol lipids (87). More recently, it has been shown that this AKAP is palmitoylated on cysteines 36 and 129; this palmitoylation further facilitates association with lipid raft regions in the plasma membrane (88).
A principal function of AKAP79/150 is to integrate cAMP and Ca2+ signaling by tethering PKC and PP2B (22, 23, 89). AKAP79/150-anchored PKC has been implicated in the induction of persistent Ca2+ signals (sparklets) that are produced by recurrent openings of the L-type Ca2+ channel and that increase vascular tone. AKAP150-knockout mice lack persistent Ca2+ sparklets in isolated arterial myocytes and fail to develop angiotensin II–induced hypertension (90, 91). The probability of coupled gating by CaV1.2 is increased by AKAP150, presumably by the facilitation of interactions between the C termini of CaV1.2 (77). Elegant mass spectrometry studies that measured the stoichiometry of AKAP79 complexes show that AKAP79 can form stable homodimers (92). Subsequent evidence points to possible tetrameric assemblies or even hetero-oligomers with AKAP12 [e.g., gravin (93, 94)]. In ventricular cardiac myocytes, AKAP79/150 has also been postulated to target β1/2ARs, AC5/6, PKA, and PP2B to a caveolin 3–associated complex containing CaV1.2 (95, 96). AKAP79/150 also interacts with several classes of the channel-associated MAGUK (membrane-associated guanylate kinase) scaffolding proteins (97).
Coupled gating of CaV1.2 channels occurs in this manner in smooth muscle from hypertensive animals and may increase myogenic tone and blood pressure. Moreover, AKAP79/150-anchored PP2B modulates gene expression during hypertension by dephosphorylation of the transcription factor NFATc3 (98, 99), thus expanding the number of potential binding partners that can be recruited to cardiac AKAP79/150-ion channel complexes.
AKAPs IN CARDIAC REPOLARIZATION
Yotiao
In humans, the sympathetic regulation of the cardiac action potential requires PKA- mediated phosphorylation of the KCNQ1 subunit of the slowly activating delayed rectifier potassium channel IKs (Figures 2 and 3). Yotiao is the smallest (250-kDa) transcript of AKAP9 gene. Yotiao localizes to the plasma membrane, whereas longer splice variants (AKAP350 and AKAP450) localize to the centrosome and the Golgi apparatus (100–102). Yotiao directs PKA, PP1, PDE4D3, and various ACs toward the α subunit (KCNQ1) of IKs (100, 103–105). PKA phosphorylates Ser27 on KCNQ1 to modulate IKs and phosphorylates Ser43 of Yotiao to further enhance regulation by the sympathetic nervous system, whereas dephosphorylation of these sites and suppression of IKs currents are facilitated by anchored PP1 (105–107). Mutations in KCNQ1 or Yotiao that disrupt this complex give rise to type 1 long-QT syndrome (LQT1), an inheritable, potentially lethal arrhythmia syndrome (108, 109). These mutants eliminate cAMP-induced phosphorylation of the channel subunit and the functional response of the IKs current to cAMP. Yotiao anchors certain AC isoforms but surprisingly not AC5 or AC6 (104). Yotiao thus sets up several feedback loops by bringing together enzymes that participate in the control of cAMP availability and the reversible phosphorylation of IKs to modulate membrane repolarization and heart rate.
D-AKAP2
D-AKAP2 also contributes to the regulation of cardiac action potentials (Figure 2). A product of the AKAP10 gene, D-AKAP2 was so named because it can interact with type I or type II PKA subunits. Genetic screens of more than 1,000 European-American individuals identified a single-nucleotide polymorphism (SNP) in D-AKAP2 that correlated with a decrease of the PR interval in the electrocardiogram (110). This nonsynonymous SNP replaces Ile646 with Val in the PKA binding domain of D-AKAP2. Biochemical analyses reveal that this amino acid change results in a threefold increased affinity of cAMP for the RIα subunit of PKA. Another study identified the same Ile646Val substitution in a smaller cohort of patients at risk for sudden cardiac death because of tachycardia and abnormal heart rate variability (111). Mouse models that phenocopy these cardiac abnormalities have deletion of the PKA-anchoring region of D-AKAP2 (111). Although the role for D-AKAP2 in cardiac rhythm is unknown, possible mechanisms include the localization of RI to the outer mitochondrial membrane and the association of the GTPases Rab4 and Rab11 with the RGS domains of the anchoring protein to perturb trafficking of endocytic vesicles (110, 112, 113).
AKAPs IN CARDIAC STRESS RESPONSES
The heart undergoes pathological remodeling in response to different types of cardiac stress. Cardiomyocyte hypertrophy in response to increased catecholamines is associated with the transcription of genes that help increase myocyte size (114). The anchoring proteins AKAP-Lbc and mAKAP (muscle AKAP) organize signaling elements that drive these transcriptional reprogramming events, whereas signaling events coordinated by D-AKAP1 and sphingosine kinase interacting protein (SKIP) have been implicated in other aspects of cardiac stress.
AKAP-Lbc
AKAP-Lbc is a long splice variant of the AKAP13 gene that functions as a PKA anchoring protein and encodes a guanine nucleotide exchange protein (GEF) to activate Rho (19, 115, 116) (Figure 2). In response to α1-adrenergic receptor (α1AR) activation, Rho GTPase activity can facilitate development of cardiac hypertrophy (117). Considerable evidence points to a critical role for AKAP-Lbc in this hypertrophic response. First, occupancy of α1ARs or endothelin-1 receptors increases RhoGEF activity (118). This GEF activity is repressed by anchored PKA–mediated phosphorylation of Ser-1665 via recruitment of 14-3-3 (119, 120). Second, infusion of the adrenergic agonist phenylephrine induces transcription of the AKAP-Lbc gene 3.5-fold (118). Similarly, a twofold increase in AKAP-Lbc transcripts has been noted in patients with hypertrophic cardiomyopathy (121). Elevating the level of this anchoring protein and strengthening the signaling pathways it organizes can enhance pathological cardiac remodeling.
Live-cell imaging, fluorescent kinase AKAP-Lbc is a long splice variant of the AKAP13 gene that functions as a PKA anchoring protein and encodes a guanine nucleotide exchange protein (GEF) to activate Rho (19, 115, 116) (Figure 2). In response to α1-adrenergic receptor (α1AR) activation, Rho GTPase activity can facilitate development of cardiac hypertrophy (117). Considerable evidence points to a critical role for AKAP-Lbc in this hypertrophic response. First, occupancy of α1ARs or endothelin-1 receptors increases RhoGEF activity (118). This GEF activity is repressed by anchored PKA–mediated phosphorylation of Ser-1665 via recruitment of 14-3-3 (119, 120). Second, infusion of the adrenergic agonist phenylephrine induces transcription of the AKAP-Lbc gene 3.5-fold (118). Similarly, a twofold increase in AKAP-Lbc transcripts has been noted in patients with hypertrophic cardiomyopathy (121). Elevating the level of this anchoring protein and strengthening the signaling pathways it organizes can enhance pathological cardiac remodeling.
Live-cell imaging, fluorescent kinase activity reporters, and RNA interference techniques showed that AKAP-Lbc couples the activation of protein kinase D (PKD) with the phosphorylation-dependent nuclear export of the class II histone deacetylase HDAC5 (121, 122) (Figure 3). Increased expression of AKAP-Lbc thereby amplifies a mitogenic signaling pathway that promotes a pathophysiological outcome in cardiacmyocytes. Follow-up studies have revealed that AKAP-Lbc and the scaffolding protein kinase suppressor of Ras1 (KSR-1) form the core of a signaling network that efficiently relays mitogenic signals through a RAF/MEK/ERK1/2 kinase cascade (123). AKAP-Lbc can also organize p38 to stimulate the RhoA effector PKNα (124). Thus, AKAP-Lbc may provide cross talk between cAMP and PKD, Rho, and ERK. Furthermore, indirect support for this concept is provided by evidence that AKAP-Lbc-knockout mice are embryonic lethal owing to cardiac developmental defects (125).
mAKAP
Low levels of cAMP are cardioprotective, whereas chronically elevated cAMP is linked to heart disease. The anchoring protein mAKAP responds to these signals by coordinating a variety of cAMP-responsive enzymes. In the basal state, mAKAP localizes AC5 to organize cAMP synthesis (126). Under pathophysiological conditions, however, changes in the association of PDE with mAKAP alter cAMP levels near RyRs (127); this change in cAMP levels has been linked to arrhythmias (128). Anchored PKA can control both processes as phosphorylation of AC5 provides a feedback mechanism to inhibit synthesis of cAMP (126), whereas phosphorylation-dependent activation of PDE4D3 promotes the degradation of cAMP (129). In addition, mAKAP anchors PKA (130) to control the phosphorylation state of the RyR and anchors EPAC1 to facilitate the Rap1 GTPase-mediated relay of mitogenic signals through an ERK5 kinase cascade (131). mAKAP clusters additional signaling molecules that contribute to cardiac stress responses, including calcineurin, NFATc3 (nuclear factor of activated T cells), protein phosphatase 2A (PP2A), and phospholipase Cε (132–134). These macromolecular assemblies are tethered on the outer membrane of the nuclear envelope via direct binding to nesprin-1α (130, 135).
mAKAP also interfaces with the protein ubiquitination machinery to coordinate signals that occur in response to ischemic insult. When oxygen (O2) levels are reduced, hypoxia-inducible factor 1α (HIF-1α) increases, thereby facilitating nuclear accumulation and initiating a transcriptional pathway (136). HIF-1α levels are kept low under normoxic conditions via its proline hydroxylation by prolyl hydroxylase domain protein (PHD). HIF-1α is then recognized and ubiquitinated by the von Hippel–Lindau protein (pVHL) and targeted for proteasomal degradation, a process facilitated by the anchoring of HIF-1α, PHD, and pVHL to the mAKAP complex. This anchoring ensures HIF-1α degradation under normoxic conditions (137). During hypoxia, PHD activity is reduced by two mechanisms: First, lower O2 content inhibits PHD activity; second, the E3 ligase designated seven in absentia homolog 2 (Siah2) ubiquitinates PHD and targets it for degradation. By anchoring Siah2 to the complex containing PHD, mAKAP facilitates an increase in HIF-1α levels and the initiation of the hypoxia transcriptional program that regulates energy metabolism, O2 transport, and ultimately cell survival (137).
D-AKAP1
D-AKAP1 (also known as s-AKAP84, AKAP121, and AKAP149) is a member of the AKAP1 gene and is found in the outer mitochondrial membrane (138–140) (Figure 3). Overexpression of D-AKAP1 in myocytes reduces cell size and blocks hypertrophy induced by the βAR agonist isoproterenol (141). These changes correlate with a loss of D-AKAP1 protein in aortic banding studies that induce cardiac hypertrophy (142). D-AKAP1 nucleates a signaling complex of PKA, the protein tyrosine phosphatase, and the Src tyrosine kinase on the outer mitochondrial membrane (Figure 2). This constellation of enzymes has been implicated in the control of oxidative metabolism and ATP synthesis (143, 144). Ubiquitin-mediated proteasomal degradation of D-AKAP1 under hypoxic conditions results in lowered mitochondrial respiration (145). D-AKAP1 also inhibits mitochondrial fission by regulating the PKA-dependent interaction of two key proteins, Drp1 and Fis1, necessary for the division of mitochondria (146). One unresolved question is, What is the source of cAMP that activates PKA at the mitochondria? This source may derive from the bicarbonate- and calcium-regulated soluble AC present at mitochondria (147) rather than from the classical ACs regulated by G protein–coupled receptors at the plasma membrane.
Sphingosine Kinase Interacting Protein
Sphingosine-1-phosphate (S1P) is a cardioprotective, antiapoptotic lysophospholipid produced in response to hypoxia and acute ischemia/reperfusion injury (148) (Figure 3). When S1P binds to its receptors on the plasma membrane of smooth muscle endothelial cells, it engages second messenger and ERK signaling pathways that increase vascular tone (149). Production of this important phospholipid requires sphingosine kinases, which catalyze the ATP-dependent phosphorylation of sphingosine to produce S1P (150). Two-hybrid screening identified a sphingosine kinase interacting protein (SKIP), which was also designated a RII anchoring protein on the basis of a region of homology to AKAP110 and AKAP220 (151). More conclusive evidence of its PKA anchoring role was provided when the protein was detected in amass spectrometry screen for cAMP-binding cardiac proteins (152, 153). Other studies detected higher levels of SKIP in patients with heart disease (154). Subsequent biochemical and genetic approaches established that SKIP is a type I–specific AKAP (33, 155). SKIP contains two RI binding sites, and SKIP complexes exist in different states of RI occupancy. Single-molecule pull-down photobleaching experiments show that 41% ± 10% of SKIP sequesters two RI dimers, whereas 59% of the anchoring protein binds a single RI dimer (33). Proteomic and subcellular fractionation experiments show that the SKIP complex is enriched in cardiomyocytes. In these cells, SKIP associates with and facilitates the phosphorylation of a prominent PKA substrate, the coiled-coil helix protein ChChd3 (33, 156).
Other Cardiac AKAPs
Other AKAPs that have been identified in the heart include gravin, ezrin, AKAP95, BIG2, AKAP220, pericentrin, Rab32, and PI3K p110γ (15, 28, 157–162). Additional AKAPs such as troponin T, myospryn, synemin, and myomegalin are localized to sarcomeric structures in cardiac myocytes and are believed to play roles in cardiac contraction (124). However, physiological roles for these AKAPs in the heart and the signaling complexes that they assemble are currently unknown.
ANCHORED cAMP SIGNALS AND MODULATION OF T CELL FUNCTION
cAMP serves as a second messenger for a variety of immunoregulatory and inflammatory mediators—such as prostaglandin E2 (PGE2), catecholamines, serotonin, adenosine, and histamine—that signal to effector T cells from monocytes, macrophages, and naturally occurring and peripherally induced regulatory T cells (Tregs) in settings such as inflammation, chronic infections, asthma, and cancer (Figure 4a,b). However, the spatial and temporal parameters that underlie such events are just beginning to be understood (163, 164). cAMP levels in effector T cells are controlled in part by Tregs that suppress them. Naturally occurring Tregs have high cAMP levels in part due to FOXP3-dependent transcriptional downregulation of PDE3B (165). Cell-to-cell transmission of cAMP to suppress effector T cells proceeds via transfer through gap junctions (166).
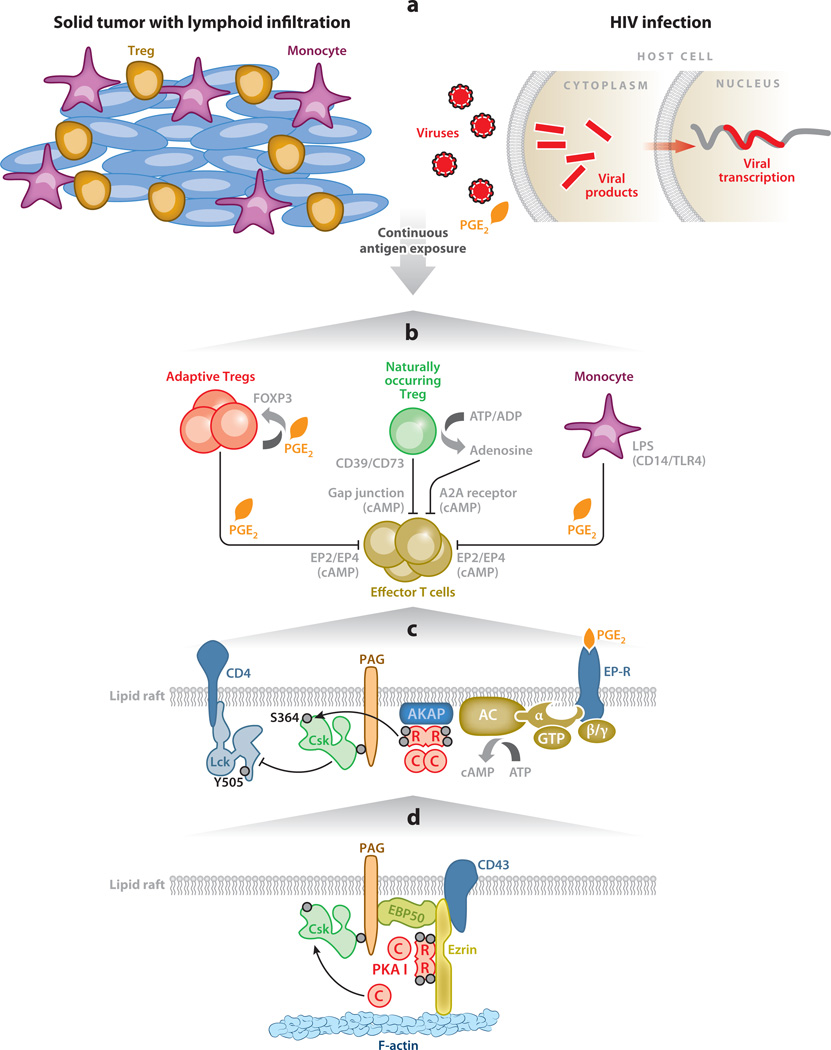
Figure 4.
The immunoregulatory role of anchored cAMP signaling in T cells. (a) Tumor-infiltrating lymphocytes are inhibited by peripherally induced Tregs (orange cells) exposed to chronic antigenic stimulation. Persistent infections, such as HIV, lead to chronic inflammation and immunosuppression, both of which involve production of PGE2 and other inflammatory mediators. (b) Generation of peripherally induced or adaptive Tregs. These cells express COX-2, produce PGE2, and stimulate FOXP3 expression. In contrast, naturally occurring Tregs can transfer cAMP to responder T cells through gap junctions. Pericellular accumulation of adenosine also elicits immunosuppressive responses through a pathway whereby CD39 and CD73 ectoenzymes metabolize ATP to generate adenosine. T cell inhibition and PGE2-responsive induction of FOXP3 can occur as a result of the secretion of PGE2 by LPS-activated monocytes. (c) cAMP inhibits TCR-mediated immunoregulatory functions at membranes. This occurs in lipid rafts through a receptor–G protein–AC–cAMP–PKA type I–Csk pathway that acts on the Src family tyrosine kinase Lck. (d) Ezrin links transmembrane receptors such as CD43 to the actin cytoskeleton via its N-terminal FERM domain and to F-actin via its C terminus. Ezrin functions as an AKAP, bringing type I PKA in proximity to its substrate Csk via a supramolecular signaling complex consisting of PKA, ezrin, EBP50, Cbp/PAG, and Csk. Abbreviations: AC, adenylyl cyclase; AKAP, A-kinase anchoring protein; cAMP, cyclic adenosine monophosphate; COX-2, cyclooxygenase 2; Csk, C-terminal Src kinase; EPB50, ERM binding protein 50; EP-R, receptor for E series of prostaglandins; FERM, band 4.1 protein and ezrin, radixin, and moesin (ERM) protein domain; Lck, lymphocyte-specific protein tyrosine kinase; LPS, lipopolysaccharide; PAG, phosphoprotein associated with glycosphingolipid-enriched microdomains; PGE2, prostaglandin E2; PKA, protein kinase A; TCR, T cell receptor; Treg, regulatory T cell.
Pericellular accumulation of adenosine also elicits immunosuppressive responses. This is a pathway whereby CD39 and CD73 ectonucleotidases on the surface of Tregs metabolize ATP to generate adenosine (167), which activates A2A receptors to enhance intracellular cAMP synthesis and suppress effector T cells (167). In addition, continuous exposure of T cells to an antigen promotes adaptive Tregs that express COX-2 (cyclooxygenase 2), leading to secretion of PGE2 (168–171). PGE2 stimulates FOXP3 expression in Tregs and inhibits effector T cell function through mobilization of a PKA-Csk (C-terminal Src kinase) cascade (172) (Figure 4c). Type I PKA and PDE4 seem to be important for T cell receptor (TCR)-induced signaling and T cell function. Whereas TCR stimulation and concomitant PKA activation in lipid rafts inhibit proximal T cell signaling (173), CD28 costimulation favors the recruitment of a PDE4/β-arrestin signaling unit that derepresses cAMP inhibition as a prelude to the onset of the full T cell response. These findings underscore the importance of enzyme anchoring and how the proximity of signaling enzymes with broad specificity can be used to drive specific, local cellular events.
In effector T cells, the cAMP pathway is involved in the regulation and modulation of immune responses that include antigen-induced proliferation and cytokine production (174, 175). cAMP also induces a Treg response and suppression of T cells via several mechanisms (176). Numerous PKA targets intersect with mitogenic signaling pathways (164). Use of site-selective cAMP agonists with a preference for PKA isotypes and use of peptides that produce isoform-selective anchoring disruption have revealed (a) that an anchored pool of type I PKA in T, B, and natural killer cells plays a dominant immunoregulatory role and (b) that PKA phosphorylation of S364 in Csk is the predominant inhibitory mechanism (31, 48, 50, 53, 175, 177–180). This phosphorylation increases Csk activity fourfold, and docking to its binding protein, Cbp/PAG (Csk-binding protein/phosphoprotein associated with glycosphingolipid-enriched microdomains), further increases its activity two- to threefold (180). Csk, in turn, phosphorylates Lck (lymphocyte-specific protein tyrosine kinase) to suppress its tyrosine kinase activity, thereby reducing ζ-chain phosphorylation of the TCR (181, 182). These events are organized by the Cbp/PAG adapter protein that recruits Csk to the site of action in lipid rafts (172, 180, 183–185). Thus, PKA phosphorylation of Csk and its interaction with Cbp/PAG provide a powerful mechanism for locally terminating antigen receptor–induced Src family tyrosine kinases (Figure 4c,d).
SCAFFOLDING THE PKA-Csk INHIBITORY PATHWAY
The anchoring protein responsible for the compartmentalization of type I PKA in T cells is ezrin. This modular, multifunctional protein is in the ezrin/radixin/moesin (ERM) family of proteins that contribute to the organization of plasma membrane domains by linking microfilaments to the membrane and aligning with membrane adaptor proteins via linker proteins, such as EBP50 (ERM binding protein 50). Ezrin interacts with EBP50 and PAGin a complex to scaffold the PKA-Csk inhibitory pathway in T cells (31) (Figure 4d).
Functional evidence that regulation of T cell function by type I PKA depends on anchoring by ezrin derives from the observations that disruption of type I PKA binding to ezrin via anchoring disruptor peptides displaces PKA from lipid rafts and releases the suppression of T cells. The latter suppression occurs via cAMP-mediated inhibition of proliferation and interleukin-2 (IL-2) production (31, 48, 53). Knockdown of ezrin abrogates regulation by cAMP of IL-2 secretion, whereas reconstitution with an siRNA-resistant wild-type ezrin, but not with a binding-domain mutant ezrin8, restores cAMP regulation of IL-2 secretion (31, 53). In addition, disruption of the ezrin-EBP50-PAG scaffold at the level of the ezrin-EBP50 interaction inhibits the regulation of IL-2 secretion by cAMP (55) (Figure 4d). Expression of the RIAD peptide in transgenic mice also perturbs the ability of the cAMP–type I PKA–Csk pathway to suppress PGE2-mediated inhibition of effector T cells (50). Related studies demonstrate that global displacement of RI enhances IL-2 secretion by T cells and produces resistance to murine AIDS (164). These findings have suggested that hyperactivation of the type I PKA pathway is involved in the T cell dysfunction of HIV infection and common variable immunodeficiency. The cAMP–type I PKA pathway in T cells is thus a putative target for treating immunodeficiency diseases, chronic infections, and cancer (170, 186–189) (Figure 4a). Furthermore, T cells from transgenic mice that are protected from suppression by Tregs through cAMP show improved antitumor responses compared with mice that have normal RI anchoring (I. Cornez & K. Taskén, unpublished data); this is consistent with observations in colorectal cancer patient samples in which PGE2 and cAMP suppress antitumor immunity (176, 189) (Figure 4a). Thus, the cAMP–type I PKA–Csk pathway is a putative therapeutic target in the inflammation that occurs in cancer and some chronic viral diseases.
CONCLUSIONS
The study of enzyme anchoring via AKAPs has provided insight into the elaborate and elegant organization of cellular signaling cascades. In this final section, we highlight three future directions for AKAP research. First, although this field arose from the need to explain hormone action at a molecular level, surprisingly little is known about the architecture of the macromolecular complexes that AKAPs hold together. More structural information regarding AKAP signaling complexes should soon be forthcoming. This is an exciting but daunting objective because AKAPs’ flexibility makes them challenging targets for X-ray crystallography (36, 37, 190). Furthermore, most anchored signaling complexes consist of many proteins; for example, a recent study that used mass spectrometry assigned 16 polypeptide chains within a single (AKAP79-2PP2B-RII-CaM)2 macromolecular assembly (92). Cutting-edge approaches of protein mass spectrometry, single-particle fluorescence imaging, and cryo-electron microcopy will likely be needed to answer key structural questions regarding the stoichiometry and molecular architecture of higher-order AKAP complexes (191–194).
Second, AKAP79/150 can protect PKC from certain pharmacological inhibitors, indicating that the anchoring protein locks this kinase in an active conformation (195, 196). A broader interpretation of this result is that AKAPs are allosteric modifiers that shape the activity of the kinase or phosphatase that they regulate (197). A more far-reaching implication is that the intracellular pharmacology of kinase inhibitor drugs cannot be reliably inferred from in vitro enzyme assays. The allosteric nature of some AKAP-enzyme interactions complicates studies that rely on the analysis of genetically modified mice. Thus, we propose that any comprehensive investigation of AKAP function in a whole-animal context cannot be limited to simply knocking out a gene of interest; instead, it should also include analysis of modified AKAP forms that are unable to interact with a given enzyme-binding partner (56, 96).
Third, research on AKAPs is moving toward understanding second messenger signaling in a pathophysiological context. For such efforts, approaches other than global disruption of intracellular anchoring events will be needed. Small-molecule inhibitors show some promise, although their mechanism of action is unknown, and they have substantial potential for off-target effects (198). Another approach to consider is peptide-mediated disruption of AKAP-associated substrates. Such an approach has been used to displace AKAP18δ from the PKA substrate phospholamban to alter cardiac excitation-contraction coupling (54), and to disconnect PKA from Csk in order to reverse cAMP-mediated inhibition of immune function (55). These methods are akin to others in which native peptides/proteins or peptidomimetics are delivered into cells as a means to overcome some of the limitations associated with the displacement of a full-length enzyme from its intracellular location. Thus, a new generation of AKAP-derived reagents that retrieve a small measure of order from chaos may offer a therapeutic advantage.
ACKNOWLEDGMENTS
The authors wish to thank Melanie Milnes for proofreading and editing this article and Lorene Langeberg and Kristoffer Watten Brudvik for helping prepare the figures. J.D.S. is supported in part by National Institutes of Health (NIH) grant GM48231; C.W.D., by NIH grant GM60419; and K.T., by grants from the Research Council of Norway and the Norwegian Cancer Society.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Rall TW, Sutherland EW, Berthet J. The relationships of epinephrine and glucagon to liver phosphorylase. J. Biol. Chem. 1957;224:463–475. [PubMed] [Google Scholar]
- 2.Sutherland EW. Studies on the mechanism of hormone action. Science. 1972;171:401–408. doi: 10.1126/science.177.4047.401. [DOI] [PubMed] [Google Scholar]
- 3.Fischer EH, Krebs EG. Conversion of phosphorylase b to phosphorylase a in muscle extracts. J. Biol. Chem. 1955;216:121–132. [PubMed] [Google Scholar]
- 4.Corbin JD, Soderling TR, Park CR. Regulation of adenosine 3′,5′-monophosphate-dependent protein kinase. J. Biol. Chem. 1973;248:1813–1821. [PubMed] [Google Scholar]
- 5.Potter RL, Stafford PH, Taylor S. Regulatory subunit of cyclic AMP-dependent protein kinase I from porcine skeletal muscle: purification and proteolysis. Arch. Biochem. Biophys. 1978;190:174–180. doi: 10.1016/0003-9861(78)90265-5. [DOI] [PubMed] [Google Scholar]
- 6.Kemp BE, Benjamini E, Krebs EG. Synthetic hexapeptide substrates and inhibitors of 3′:5′-cyclic AMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA. 1976;73:1038–1042. doi: 10.1073/pnas.73.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keely SL. Activation of cAMP-dependent protein kinase without a corresponding increase in phosphorylase activity. Res. Commun. Chem. Pathol. Pharmacol. 1977;18:283–290. [PubMed] [Google Scholar]
- 8.Hayes JS, Brunton LL, Brown JH, Reese JB, Mayer SE. Hormonally specific expression of cardiac protein kinase activity. Proc. Natl. Acad. Sci. USA. 1979;76:1570–1574. doi: 10.1073/pnas.76.4.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunton LL, Hayes JS, Mayer SE. Hormonally specific phosphorylation of cardiac troponin I and activation of glycogen phosphorylase. Nature. 1979;280:78–80. doi: 10.1038/280078a0. [DOI] [PubMed] [Google Scholar]
- 10.Corbin JD, Sugden PH, Lincoln TM, Keely SL. Compartmentalization of adenosine 3′:5′-monophosphate and adenosine 3′:5′-monophosphate-dependent protein kinase in heart tissue. J. Biol. Chem. 1977;252:3854–3861. [PubMed] [Google Scholar]
- 11.Theurkauf WE, Vallee RB. Molecular characterization of the cAMP-dependent protein kinase bound to microtubule-associated protein 2. J. Biol. Chem. 1982;257:3284–3290. [PubMed] [Google Scholar]
- 12.Sarkar D, Erlichman J, Rubin CS. Identification of a calmodulin-binding protein that co-purifies with the regulatory subunit of brain protein kinase II. J. Biol. Chem. 1984;259:9840–9846. [PubMed] [Google Scholar]
- 13.Lohmann SM, DeCamili P, Enig I, Walter U. High-affinity binding of the regulatory subunit (RII) of cAMP-dependent protein kinase to microtubule-associated and other cellular proteins. Proc. Natl. Acad. Sci. USA. 1984;81:6723–6727. doi: 10.1073/pnas.81.21.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carr DW, Scott JD. Blotting and band-shifting: techniques for studying protein-protein interactions. Trends Biochem. Sci. 1992;17:246–249. doi: 10.1016/0968-0004(92)90402-u. [DOI] [PubMed] [Google Scholar]
- 15.Alto NM, Soderling J, Scott JD. Rab32 is an A-kinase anchoring protein and participates in mitochondrial dynamics. J. Cell Biol. 2002;158:659–668. doi: 10.1083/jcb.200204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pidoux G, Tasken K. Specificity and spatial dynamics of protein kinase A signaling organized by A-kinase-anchoring proteins. J. Mol. Endocrinol. 2010;44:271–284. doi: 10.1677/JME-10-0010. [DOI] [PubMed] [Google Scholar]
- 17.Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat. Rev. Mol. Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 18.Scott JD, Stofko RE, McDonald JR, Comer JD, Vitalis EA, Mangeli J. Type II regulatory subunit dimerization determines the subcellular localization of the cAMP-dependent protein kinase. J. Biol. Chem. 1990;265:21561–21566. [PubMed] [Google Scholar]
- 19.Carr DW, Hausken ZE, Fraser IDC, Stofko-Hahn RE, Scott JD. Association of the type II cAMP-dependent protein kinase with a human thyroid RII-anchoring protein: cloning and characterization of the RII-binding domain. J. Biol. Chem. 1992;267:13376–13382. [PubMed] [Google Scholar]
- 20.Carr DW, Stofko-Hahn RE, Fraser IDC, Bishop SM, Acott TS, et al. Interaction of the regulatory subunit (RII) of cAMP-dependent protein kinase with RII-anchoring proteins occurs through an amphipathic helix binding motif. J. Biol. Chem. 1991;266:14188–14192. [PubMed] [Google Scholar]
- 21.Rosenmund C, Carr DW, Bergeson SE, Nilaver G, Scott JD, Westbrook GL. Anchoring of protein kinase A is required for modulation of AMPA/kainate receptors on hippocampal neurons. Nature. 1994;368:853–856. doi: 10.1038/368853a0. [DOI] [PubMed] [Google Scholar]
- 22.Coghlan VM, Perrino BA, Howard M, Langeberg LK, Hicks JB, et al. Association of protein kinase A and protein phosphatase 2B with a common anchoring protein. Science. 1995;267:108–111. doi: 10.1126/science.7528941. [DOI] [PubMed] [Google Scholar]
- 23.Klauck TM, Faux MC, Labudda K, Langeberg LK, Jaken S, Scott JD. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science. 1996;271:1589–1592. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- 24.Scott JD, Pawson T. Cell signaling in space and time: where proteins come together and when they’re apart. Science. 2009;326:1220–1224. doi: 10.1126/science.1175668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanderson JL, Dell’Acqua ML. AKAP signaling complexes in regulation of excitatory synaptic plasticity. Neuroscientist. 2011;17:321–336. doi: 10.1177/1073858410384740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kritzer MD, Li J, Dodge-Kafka K, Kapiloff MS. AKAPs: the architectural underpinnings of local cAMP signaling. J. Mol. Cell. Cardiol. 2012;52:351–358. doi: 10.1016/j.yjmcc.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenwald EC, Saucerman JJ. Bigger, better, faster: principles and models of AKAP anchoring protein signaling. J. Cardiovasc. Pharmacol. 2011;58:462–469. doi: 10.1097/FJC.0b013e31822001e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diviani D, Langeberg LK, Doxsey SJ, Scott JD. Pericentrin anchors protein kinase A at the centrosome through a newly identified RII-binding domain. Curr. Biol. 2000;10:417–420. doi: 10.1016/s0960-9822(00)00422-x. [DOI] [PubMed] [Google Scholar]
- 29.Hausken ZE, Coghlan VM, Hasting CAS, Reimann EM, Scott JD. Type II regulatory subunit (RII) of the cAMP-dependent protein kinase interaction with A-kinase anchor proteins requires isoleucines 3 and 5. J. Biol. Chem. 1994;269:24245–24251. [PubMed] [Google Scholar]
- 30.Herberg FW, Maleszka A, Eide T, Vossebein L, Tasken K. Analysis of A-kinase anchoring protein (AKAP) interaction with protein kinase A (PKA) regulatory subunits: PKA isoform specificity in AKAP binding. J. Mol. Biol. 2000;298:329–339. doi: 10.1006/jmbi.2000.3662. [DOI] [PubMed] [Google Scholar]
- 31.Ruppelt A, Mosenden R, Gronholm M, Aandahl EM, Tobin D, et al. Inhibition of T cell activation by cyclic adenosine 5′-monophosphate requires lipid raft targeting of protein kinase A type I by the A-kinase anchoring protein ezrin. J. Immunol. 2007;179:5159–5168. doi: 10.4049/jimmunol.179.8.5159. [DOI] [PubMed] [Google Scholar]
- 32.Pidoux G, Witczak O, Jarnaess E, Myrvold L, Urlaub H, et al. Optic atrophy 1 is an A-kinase anchoring protein on lipid droplets that mediates adrenergic control of lipolysis. EMBO J. 2011;30:4371–4386. doi: 10.1038/emboj.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Means CK, Lygren B, Langeberg LK, Jain A, Dixon RE, et al. An entirely specific type I A-kinase anchoring protein that can sequester two molecules of protein kinase A at mitochondria. Proc. Natl. Acad. Sci. USA. 2011;108:E1227–E1235. doi: 10.1073/pnas.1107182108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newlon MG, Roy M, Morikis D, Carr DW, Westphal R, et al. A novel mechanism of PKA anchoring revealed by solution structures of anchoring complexes. EMBO J. 2001;20:1651–1662. doi: 10.1093/emboj/20.7.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newlon MG, Roy M, Morikis D, Hausken ZE, Coghlan V, et al. The molecular basis for protein kinase A anchoring revealed by solution NMR. Nat. Struct. Biol. 1999;6:222–227. doi: 10.1038/6663. [DOI] [PubMed] [Google Scholar]
- 36.Gold MG, Lygren B, Dokurno P, Hoshi N, McConnachie G, et al. Molecular basis of AKAP specificity for PKA regulatory subunits. Mol. Cell. 2006;24:383–395. doi: 10.1016/j.molcel.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Kinderman FS, Kim C, von Daake S, Ma Y, Pham BQ, et al. A dynamic mechanism for AKAP binding to RII isoforms of cAMP-dependent protein kinase. Mol. Cell. 2006;24:397–408. doi: 10.1016/j.molcel.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banky P, Huang LJ, Taylor SS. Dimerization/docking domain of the type Iα regulatory subunit of cAMP-dependent protein kinase: Requirements for dimerization and docking are distinct but overlapping. J. Biol. Chem. 1998;273:35048–35055. doi: 10.1074/jbc.273.52.35048. [DOI] [PubMed] [Google Scholar]
- 39.Banky P, Newlon MG, Roy M, Garrod S, Taylor SS, Jennings PA. Isoform-specific differences between the type Iα and IIα cyclic AMP-dependent protein kinase anchoring domains revealed by solution NMR. J. Biol. Chem. 2000;275:35146–35152. doi: 10.1074/jbc.M003961200. [DOI] [PubMed] [Google Scholar]
- 40.Lester LB, Langeberg LK, Scott JD. Anchoring of protein kinase A facilitates hormone-mediated insulin secretion. Proc. Natl. Acad. Sci. USA. 1997;94:14942–14947. doi: 10.1073/pnas.94.26.14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson BD, Scheuer T, Catterall WA. Voltage-dependent potentiation of L-type Ca2+ channels in skeletal muscle cells requires anchored cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA. 1994;91:11492–11496. doi: 10.1073/pnas.91.24.11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fraser IDC, Tavalin SJ, Lester LB, Langeberg LK, Westphal AM, et al. A novel lipid-anchored A-kinase anchoring protein facilitates cAMP-responsive membrane events. EMBO J. 1998;17:2261–2272. doi: 10.1093/emboj/17.8.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vijayaraghavan S, Goueli SA, Davey MP, Carr DW. Protein kinase A-anchoring inhibitor peptides arrest mammalian sperm motility. J. Biol. Chem. 1997;272:4747–4752. doi: 10.1074/jbc.272.8.4747. [DOI] [PubMed] [Google Scholar]
- 44.Fink MA, Zakhary DR, Mackey JA, Desnoyer RW, Apperson-Hansen C, et al. AKAP-mediated targeting of protein kinase A regulates contractility in cardiac myocytes. Circ. Res. 2001;88:291–297. doi: 10.1161/01.res.88.3.291. [DOI] [PubMed] [Google Scholar]
- 45.Gold MG, Reichow SL, O’Neill SE, Weisbrod CR, Langeberg LK, et al. AKAP2 anchors PKA with aquaporin-0 to support ocular lens transparency. EMBO Mol. Med. 2012;4:15–26. doi: 10.1002/emmm.201100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alto NM, Soderling SH, Hoshi N, Langeberg LK, Fayos R, et al. Bioinformatic design of A-kinase anchoring protein-in silico: a potent and selective peptide antagonist of type II protein kinase A anchoring. Proc. Natl. Acad. Sci. USA. 2003;100:4445–4450. doi: 10.1073/pnas.0330734100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burns-Hamuro LL, Ma Y, Kammerer S, Reineke U, Self C, et al. Designing isoform-specific peptide disruptors of protein kinase A localization. Proc. Natl. Acad. Sci. USA. 2003;100:4072–4077. doi: 10.1073/pnas.2628038100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carlson CR, Lygren B, Berge T, Hoshi N, Wong W, et al. Delineation of type I protein kinase A-selective signaling events using an RI anchoring disruptor. J. Biol. Chem. 2006;281:21535–21545. doi: 10.1074/jbc.M603223200. [DOI] [PubMed] [Google Scholar]
- 49.Torheim EA, Ndhlovu LC, Pettersen FO, Larsen TL, Jha AR, et al. Interleukin-10-secreting T cells define a suppressive subset within the HIV-1-specific T-cell population. Eur. J. Immunol. 2009;39:1280–1287. doi: 10.1002/eji.200839002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mosenden R, Singh P, Cornez I, Heglind M, Ruppelt A, et al. Mice with disrupted type I protein kinase A anchoring in T cells resist retrovirus-induced immunodeficiency. J. Immunol. 2011;186:5119–5130. doi: 10.4049/jimmunol.1100003. [DOI] [PubMed] [Google Scholar]
- 51.McKenzie AJ, Campbell SL, Howe AK. Protein kinase A activity and anchoring are required for ovarian cancer cell migration and invasion. PLoS ONE. 2011;6:e26552. doi: 10.1371/journal.pone.0026552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Di Benedetto G, Zoccarato A, Lissandron V, Terrin A, Li X, et al. Protein kinase A type I and type II define distinct intracellular signaling compartments. Circ. Res. 2008;103:836–844. doi: 10.1161/CIRCRESAHA.108.174813. [DOI] [PubMed] [Google Scholar]
- 53.Jarnaess E, Ruppelt A, Stokka AJ, Lygren B, Scott JD, Tasken K. Dual specificity A-kinase anchoring proteins (AKAPs) contain an additional binding region that enhances targeting of protein kinase A type I. J. Biol. Chem. 2008;283:33708–33718. doi: 10.1074/jbc.M804807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lygren B, Carlson CR, Santamaria K, Lissandron V, McSorley T, et al. AKAP complex regulates Ca2+ re-uptake into heart sarcoplasmic reticulum. EMBO Rep. 2007;8:1061–1067. doi: 10.1038/sj.embor.7401081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stokka AJ, Mosenden R, Ruppelt A, Lygren B, Tasken K. The adaptor protein EBP50 is important for localization of the protein kinase A-Ezrin complex in T-cells and the immunomodulating effect of cAMP. Biochem. J. 2010;425:381–388. doi: 10.1042/BJ20091136. [DOI] [PubMed] [Google Scholar]
- 56.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 57.Sadana R, Dessauer CW. Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. NeuroSignals. 2009;17:5–22. doi: 10.1159/000166277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Willoughby D, Cooper DMF. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol. Rev. 2007;87:965–1010. doi: 10.1152/physrev.00049.2006. [DOI] [PubMed] [Google Scholar]
- 59.Ostrom RS, Naugle JE, Hase M, Gregorian C, Swaney JS, et al. Angiotensin II enhances adenylyl cyclase signaling via Ca2+/calmodulin: Gq–Gs cross-talk regulates collagen production in cardiac fibroblasts. J. Biol. Chem. 2003;278:24461–24468. doi: 10.1074/jbc.M212659200. [DOI] [PubMed] [Google Scholar]
- 60.Iwatsubo K, Minamisawa S, Tsunematsu T, Nakagome M, Toya Y, et al. Direct inhibition of type 5 adenylyl cyclase prevents myocardial apoptosis without functional deterioration. J. Biol. Chem. 2004;279:40938–40945. doi: 10.1074/jbc.M314238200. [DOI] [PubMed] [Google Scholar]
- 61.Okumura S, Kawabe J, Yatani A, Takagi G, Lee MC, et al. Type 5 adenylyl cyclase disruption alters not only sympathetic but also parasympathetic and calcium-mediated cardiac regulation. Circ. Res. 2003;93:364–371. doi: 10.1161/01.RES.0000086986.35568.63. [DOI] [PubMed] [Google Scholar]
- 62.Guellich A, Gao S, Hong C, Yan L, Wagner TE, et al. Effects of cardiac overexpression of type 6 adenylyl cyclase affects on the response to chronic pressure overload. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H707–H712. doi: 10.1152/ajpheart.00148.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takahashi T, Tang T, Lai NC, Roth DM, Rebolledo B, et al. Increased cardiac adenylyl cyclase expression is associated with increased survival after myocardial infarction. Circulation. 2006;114:388–396. doi: 10.1161/CIRCULATIONAHA.106.632513. [DOI] [PubMed] [Google Scholar]
- 64.Gao MH, Tang T, Guo T, Miyanohara A, Yajima T, et al. Adenylyl cyclase type VI increases Akt activity and phospholamban phosphorylation in cardiac myocytes. J. Biol. Chem. 2008;283:33527–33535. doi: 10.1074/jbc.M805825200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yan L, Vatner DE, O’Connor JP, Ivessa A, Ge H, et al. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007;130:247–258. doi: 10.1016/j.cell.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 66.Hu CL, Chandra R, Ge H, Pain J, Yan L, et al. Adenylyl cyclase type 5 protein expression during cardiac development and stress. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1776–H1782. doi: 10.1152/ajpheart.00050.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang T, Gao MH, Lai NC, Firth AL, Takahashi T, et al. Adenylyl cyclase type 6 deletion decreases left ventricular function via impaired calcium handling. Circulation. 2008;117:61–69. doi: 10.1161/CIRCULATIONAHA.107.730069. [DOI] [PubMed] [Google Scholar]
- 68.Burton KA, Johnson BD, Hausken ZE, Westenbroek RE, Idzerda RL, et al. Type II regulatory subunits are not required for the anchoring-dependent modulation of Ca2+ channel activity by cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. USA. 1997;94:11067–11072. doi: 10.1073/pnas.94.20.11067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao TY, Yatani A, Dell’Acqua ML, Sako H, Green SA, et al. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 70.Kockskamper J, Sendhoff K, Erlenkamp S, Bordusa F, Cerovsky V, Glitsch HG. Differences in the protein-kinase-A-dependent regulation of CFTR Cl− channels and Na+-K+ pumps in guinea-pig ventricular myocytes. Pflüg. Arch. 2001;441:807–815. doi: 10.1007/s004240000485. [DOI] [PubMed] [Google Scholar]
- 71.Potet F, Scott JD, Mohammad-Panah R, Escande D, Baro I. AKAP proteins anchor cAMP-dependent protein kinase to KvLQT1/IsK channel complex. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2038–H2045. doi: 10.1152/ajpheart.2001.280.5.H2038. [DOI] [PubMed] [Google Scholar]
- 72.Fink MA, Zakhary DR, Mackey JA, Desnoyer RW, Apperson-Hansen C, et al. AKAP-mediated targeting of protein kinase A regulates contractility in cardiac myocytes. Circ. Res. 2001;88:291–297. doi: 10.1161/01.res.88.3.291. [DOI] [PubMed] [Google Scholar]
- 73.McConnell BK, Popovic Z, Mal N, Lee K, Bautista J, et al. Disruption of protein kinase A interaction with A-kinase-anchoring proteins in the heart in vivo: effects on cardiac contractility, protein kinase A phosphorylation, and troponin I proteolysis. J. Biol. Chem. 2009;284:1583–1592. doi: 10.1074/jbc.M806321200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Patel HH, Hamuro LL, Chun BJ, Kawaraguchi Y, Quick A, et al. Disruption of protein kinase A localization using a trans-activator of transcription (TAT)-conjugated A-kinase-anchoring peptide reduces cardiac function. J. Biol. Chem. 2010;285:27632–27640. doi: 10.1074/jbc.M110.146589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Diviani D, Scott JD. AKAP signaling complexes at the cytoskeleton. J. Cell Sci. 2001;114:1431–1437. doi: 10.1242/jcs.114.8.1431. [DOI] [PubMed] [Google Scholar]
- 76.Malbon CC, Tao J, Wang HY. AKAPs (A-kinase anchoring proteins) and molecules that compose their G-protein-coupled receptor signalling complexes. Biochem. J. 2004;379:1–9. doi: 10.1042/BJ20031648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Navedo MF, Cheng EP, Yuan C, Votaw S, Molkentin JD, et al. Increased coupled gating of L-type Ca2+ channels during hypertension and Timothy syndrome. Circ. Res. 2010;106:748–756. doi: 10.1161/CIRCRESAHA.109.213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gray PC, Johnson BD, Westenbroek RE, Hays LG, Yates JR, III, et al. Primary structure and function of an A kinase anchoring protein associated with calcium channels. Neuron. 1998;20:1017–1026. doi: 10.1016/s0896-6273(00)80482-1. [DOI] [PubMed] [Google Scholar]
- 79.Trotter KW, Fraser IDC, Scott GK, Stutts MJ, Scott JD, Milgram SL. Alternative splicing regulates the subcellular localization of A-kinase anchoring protein 18 isoforms. J. Cell Biol. 1999;147:1481–1492. doi: 10.1083/jcb.147.7.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Henn V, Edemir B, Stefan E, Wiesner B, Lorenz D, et al. Identification of a novel A-kinase anchoring protein 18 isoform and evidence for its role in the vasopressin-induced aquaporin-2 shuttle in renal principal cells. J. Biol. Chem. 2004;279:26654–26665. doi: 10.1074/jbc.M312835200. [DOI] [PubMed] [Google Scholar]
- 81.Hulme JT, Lin TW, Westenbroek RE, Scheuer T, Catterall WA. β-Adrenergic regulation requires direct anchoring of PKA to cardiac CaV1.2 channels via a leucine zipper interaction with A kinase-anchoring protein 15. Proc. Natl. Acad. Sci. USA. 2003;100:13093–13098. doi: 10.1073/pnas.2135335100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hulme JT, Ahn M, Hauschka SD, Scheuer T, Catterall WA. A novel leucine zipper targets AKAP15 and cyclic AMP-dependent protein kinase to the C terminus of the skeletal muscle Ca2+ channel and modulates its function. J. Biol. Chem. 2002;277:4079–4087. doi: 10.1074/jbc.M109814200. [DOI] [PubMed] [Google Scholar]
- 83.Hulme JT, Westenbroek RE, Scheuer T, Catterall WA. Phosphorylation of serine 1928 in the distal C-terminal domain of cardiac CaV1.2 channels during β1-adrenergic regulation. Proc. Natl. Acad. Sci. USA. 2006;103:16574–16579. doi: 10.1073/pnas.0607294103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fuller MD, Emrick MA, Sadilek M, Scheuer T, Catterall WA. Molecular mechanism of calcium channel regulation in the fight-or-flight response. Sci. Signal. 2010;3:ra70. doi: 10.1126/scisignal.2001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gretarsdottir S, Thorleifsson G, Reynisdottir ST, Manolescu A, Jonsdottir S, et al. The gene encoding phosphodiesterase 4D confers risk of ischemic stroke. Nat. Genet. 2003;35:131–138. doi: 10.1038/ng1245. [DOI] [PubMed] [Google Scholar]
- 86.Oliveria SF, Dell’Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–275. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dell’Acqua ML, Faux MC, Thorburn J, Thorburn A, Scott JD. Membrane-targeting sequences on AKAP79 bind phosphatidylinositol-4, 5-bisphosphate. EMBO J. 1998;17:2246–2260. doi: 10.1093/emboj/17.8.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Delint-Ramirez I, Willoughby D, Hammond GVR, Ayling LJ, Cooper DMF. Palmitoylation targets AKAP79 protein to lipid rafts and promotes its regulation of calcium-sensitive adenylyl cyclase type 8. J. Biol. Chem. 2011;286:32962–32975. doi: 10.1074/jbc.M111.243899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bauman AL, Soughayer J, Nguyen BT, Willoughby D, Carnegie GK, et al. Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol. Cell. 2006;23:925–931. doi: 10.1016/j.molcel.2006.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tunquist BJ, Hoshi N, Guire ES, Zhang F, Mullendorff K, et al. Loss of AKAP150 perturbs distinct neuronal processes in mice. Proc. Natl. Acad. Sci. USA. 2008;105:12557–12562. doi: 10.1073/pnas.0805922105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Navedo MF, Nieves-Cintrón M, Amberg GC, Yuan C, Votaw VS, et al. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II–induced hypertension. Circ. Res. 2008;102:e1–e11. doi: 10.1161/CIRCRESAHA.107.167809. [DOI] [PubMed] [Google Scholar]
- 92.Gold MG, Stengel F, Nygren PJ, Weisbrod CR, Bruce JE, et al. Architecture and dynamics of an A-kinase anchoring protein 79 (AKAP79) signaling complex. Proc. Natl. Acad. Sci. USA. 2011;108:6426–6431. doi: 10.1073/pnas.1014400108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gao S, Wang H-y, Malbon C. AKAP5 and AKAP12 form homo-oligomers. J. Mol. Signal. 2011;6:3. doi: 10.1186/1750-2187-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gao S, Wang H-y, Malbon C. AKAP12 and AKAP5 form higher-order hetero-oligomers. J. Mol. Signal. 2011;6:8. doi: 10.1186/1750-2187-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fraser IDC, Cong M, Kim J, Rollins EN, Daaka Y, et al. Assembly of an A kinase-anchoring protein–β2-adrenergic receptor complex facilitates receptor phosphorylation and signaling. Curr. Biol. 2000;10:409–412. doi: 10.1016/s0960-9822(00)00419-x. [DOI] [PubMed] [Google Scholar]
- 96.Nichols CB, Rossow CF, Navedo MF, Westenbroek RE, Catterall WA, et al. Sympathetic stimulation of adult cardiomyocytes requires association of AKAP5 with a subpopulation of L-type calcium channels. Circ. Res. 2010;107:747–756. doi: 10.1161/CIRCRESAHA.109.216127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron. 2000;27:107–119. doi: 10.1016/s0896-6273(00)00013-1. [DOI] [PubMed] [Google Scholar]
- 98.Nieves-Cintrón M, Amberg GC, Navedo MF, Molkentin JD, Santana LF. The control of Ca2+ influx and NFATc3 signaling in arterial smooth muscle during hypertension. Proc. Natl. Acad. Sci. USA. 2008;105:15623–15628. doi: 10.1073/pnas.0808759105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nieves-Cintrón M, Amberg GC, Nichols CB, Molkentin JD, Santana LF. Activation of NFATc3 down-regulates the β1 subunit of large conductance, calcium-activated K+ channels in arterial smooth muscle and contributes to hypertension. J. Biol. Chem. 2007;282:3231–3240. doi: 10.1074/jbc.M608822200. [DOI] [PubMed] [Google Scholar]
- 100.Westphal RS, Tavalin SJ, Lin JW, Alto NM, Fraser IDC, et al. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science. 1999;285:93–96. doi: 10.1126/science.285.5424.93. [DOI] [PubMed] [Google Scholar]
- 101.Schmidt PH, Dransfield DT, Claudio JO, Hawley RG, Trotter KW, et al. AKAP350, a multiply spliced protein kinase A-anchoring protein associated with centrosomes. J. Biol. Chem. 1999;274:3055–3066. doi: 10.1074/jbc.274.5.3055. [DOI] [PubMed] [Google Scholar]
- 102.Witczak O, Skalhegg BS, Keryer G, Bornens M, Tasken K, et al. Cloning and characterization of a cDNA encoding an A-kinase anchoring protein located in the centrosome, AKAP450. EMBO J. 1999;18:1858–1868. doi: 10.1093/emboj/18.7.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Terrenoire C, Houslay MD, Baillie GS, Kass RS. The cardiac IKs potassium channel macromolecular complex includes the phosphodiesterase PDE4D3. J. Biol. Chem. 2009;284:9140–9146. doi: 10.1074/jbc.M805366200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Piggott LA, Bauman AL, Scott JD, Dessauer CW. The A-kinase anchoring protein Yotiao binds and regulates adenylyl cyclase in brain. Proc. Natl. Acad. Sci. USA. 2008;105:13835–13840. doi: 10.1073/pnas.0712100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marx SO, Kurokawa J, Reiken S, Motoike H, D’Armiento J, et al. Requirement of a macromolecular signaling complex for β adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295:496–499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 106.Kurokawa J, Motoike HK, Rao J, Kass RS. Regulatory actions of the A-kinase anchoring protein Yotiao on a heart potassium channel downstream of PKA phosphorylation. Proc. Natl. Acad. Sci. USA. 2004;101:16374–16378. doi: 10.1073/pnas.0405583101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen L, Kurokawa J, Kass RS. Phosphorylation of the A-kinase-anchoring protein Yotiao contributes to protein kinase A regulation of a heart potassium channel. J. Biol. Chem. 2005;280:31347–31352. doi: 10.1074/jbc.M505191200. [DOI] [PubMed] [Google Scholar]
- 108.Chen L, Marquardt ML, Tester DJ, Sampson KJ, Ackerman MJ, Kass RS. Mutation of an A kinase-anchoring protein causes long-QT syndrome. Proc. Natl. Acad. Sci. USA. 2007;104:20990–20995. doi: 10.1073/pnas.0710527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lu JT, Kass RS. Recent progress in congenital long QT syndrome. Curr. Opin. Cardiol. 2010;25:216–221. doi: 10.1097/HCO.0b013e32833846b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kammerer S, Burns-Hamuro LL, Ma Y, Hamon SC, Canaves JM, et al. Amino acid variant in the kinase binding domain of dual-specific A kinase-anchoring protein 2: a disease susceptibility polymorphism. Proc. Natl. Acad. Sci. USA. 2003;100:4066–4071. doi: 10.1073/pnas.2628028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tingley WG, Pawlikowska L, Zaroff JG, Kim T, Nguyen T, et al. Gene-trapped mouse embryonic stem cell-derived cardiac myocytes and human genetics implicate AKAP10 in heart rhythm regulation. Proc. Natl. Acad. Sci. USA. 2007;104:8461–8466. doi: 10.1073/pnas.0610393104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang L, Sunahara RK, Krumins A, Perkins G, Crochiere ML, et al. Cloning and mitochondrial localization of full-length D-AKAP2, a protein kinase A anchoring protein. Proc. Natl. Acad. Sci. USA. 2001;98:3220–3225. doi: 10.1073/pnas.051633398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eggers CT, Schafer JC, Goldenring JR, Taylor SS. D-AKAP2 interacts with Rab4 and Rab11 through its RGS domains and regulates transferrin receptor recycling. J. Biol. Chem. 2009;284:32869–32880. doi: 10.1074/jbc.M109.022582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu. Rev. Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 115.Zheng Y, Olson MF, Hall A, Cerione RA, Toksoz D. Direct involvement of the small GTP-binding protein Rho in lbc oncogene function. J. Biol. Chem. 1995;270:9031–9034. doi: 10.1074/jbc.270.16.9031. [DOI] [PubMed] [Google Scholar]
- 116.Diviani D, Soderling J, Scott JD. AKAP-Lbc anchors protein kinase A and nucleates Gα12-selective Rho-mediated stress fiber formation. J. Biol. Chem. 2001;276:44247–44257. doi: 10.1074/jbc.M106629200. [DOI] [PubMed] [Google Scholar]
- 117.Sah VP, Hoshijima M, Chien KR, Brown JH. Rho is required for Gαq and α1-adrenergic receptor signaling in cardiomyocytes: dissociation of Ras and Rho pathways. J. Biol. Chem. 1996;271:31185–31190. doi: 10.1074/jbc.271.49.31185. [DOI] [PubMed] [Google Scholar]
- 118.Appert-Collin A, Cotecchia S, Nenniger-Tosato M, Pedrazzini T, Diviani D. The A-kinase anchoring protein (AKAP)-Lbc-signaling complex mediates α1 adrenergic receptor-induced cardiomyocyte hypertrophy. Proc. Natl. Acad. Sci. USA. 2007;104:10140–10145. doi: 10.1073/pnas.0701099104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Diviani D, Abuin L, Cotecchia S, Pansier L. Anchoring of both PKA and 14-3-3 inhibits the Rho-GEF activity of the AKAP-Lbc signaling complex. EMBO J. 2004;23:2811–2820. doi: 10.1038/sj.emboj.7600287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jin J, Smith FD, Stark C, Wells CD, Fawcett JP, et al. Proteomic, functional, and domain-based analysis of in vivo 14-3-3 binding proteins involved in cytoskeletal regulation and cellular organization. Curr. Biol. 2004;14:1436–1450. doi: 10.1016/j.cub.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 121.Carnegie GK, Soughayer J, Smith FD, Pedroja BS, Zhang F, et al. AKAP-Lbc mobilizes a cardiac hypertrophy signaling pathway. Mol. Cell. 2008;32:169–179. doi: 10.1016/j.molcel.2008.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carnegie GK, Smith FD, McConnachie G, Langeberg LK, Scott JD. AKAP-Lbc nucleates a protein kinase D activation scaffold. Mol. Cell. 2004;15:889–899. doi: 10.1016/j.molcel.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 123.Smith FD, Langeberg LK, Cellurale C, Pawson T, Morrison DK, et al. AKAP-Lbc enhances cyclic AMP control of the ERK1/2 cascade. Nat. Cell Biol. 2010;12:1242–1249. doi: 10.1038/ncb2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cariolato L, Cavin S, Diviani D. A-kinase anchoring protein (AKAP)-Lbc anchors a PKN-based signaling complex involved in α1-adrenergic receptor-induced p38 activation. J. Biol. Chem. 2011;286:7925–7937. doi: 10.1074/jbc.M110.185645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mayers CM, Wadell J, McLean K, Venere M, Malik M, et al. The Rho guanine nucleotide exchange factor AKAP13 (BRX) is essential for cardiac development in mice. J. Biol. Chem. 2010;285:12344–12354. doi: 10.1074/jbc.M110.106856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kapiloff MS, Piggott LA, Sadana R, Li J, Heredia LA, et al. An adenylyl cyclase-mAKAP β signaling complex regulates cAMP levels in cardiac myocytes. J. Biol. Chem. 2009;284:23540–23546. doi: 10.1074/jbc.M109.030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dodge KL, Khouangsathiene S, Kapiloff MS, Mouton R, Hill EV, et al. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. EMBO J. 2001;20:1921–1930. doi: 10.1093/emboj/20.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 129.Carlisle Michel JJ, Dodge KL, Wong W, Mayer NC, Langeberg LK, Scott JD. PKA-phosphorylation of PDE4D3 facilitates recruitment of the mAKAP signalling complex. Biochem. J. 2004;381:587–592. doi: 10.1042/BJ20040846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kapiloff MS, Schillace RV, Westphal AM, Scott JD. mAKAP: an A-kinase anchoring protein targeted to the nuclear membrane of differentiated myocytes. J. Cell Sci. 1999;112:2725–2736. doi: 10.1242/jcs.112.16.2725. [DOI] [PubMed] [Google Scholar]
- 131.Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, et al. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005;437:574–578. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang L, Malik S, Kelley GG, Kapiloff MS, Smrcka AV. Phospholipase Cε scaffolds to muscle-specific A kinase anchoring protein (mAKAPβ) and integrates multiple hypertrophic stimuli in cardiac myocytes. J. Biol. Chem. 2011;286:23012–23021. doi: 10.1074/jbc.M111.231993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pare GC, Bauman AL, McHenry M, Carlisle Michel JJ, Dodge-Kafka KL, Kapiloff MS. The mAKAP complex participates in the induction of cardiac myocyte hypertrophy by adrenergic receptor signaling. J. Cell Sci. 2005;118:5637–5646. doi: 10.1242/jcs.02675. [DOI] [PubMed] [Google Scholar]
- 134.Dodge-Kafka KL, Bauman A, Mayer N, Henson E, Heredia L, et al. cAMP-stimulated protein phosphatase 2A activity associated with muscle A kinase-anchoring protein (mAKAP) signaling complexes inhibits the phosphorylation and activity of the cAMP-specific phosphodiesterase PDE4D3. J. Biol. Chem. 2010;285:11078–11086. doi: 10.1074/jbc.M109.034868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pare GC, Easlick JL, Mislow JM, McNally EM, Kapiloff MS. Nesprin-1α contributes to the targeting of mAKAP to the cardiac myocyte nuclear envelope. Exp. Cell Res. 2005;303:388–399. doi: 10.1016/j.yexcr.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 136.Heather LC, Clarke K. Metabolism, hypoxia and the diabetic heart. J. Mol. Cell. Cardiol. 2011;50:598–605. doi: 10.1016/j.yjmcc.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 137.Wong W, Goehring AS, Kapiloff MS, Langeberg LK, Scott JD. mAKAP compartmentalizes oxygen-dependent control of HIF-1α. Sci. Signal. 2008;1:ra18. doi: 10.1126/scisignal.2000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Feliciello A, Rubin CS, Avvedimento EV, Gottesman ME. Expression of a kinase anchor protein 121 is regulated by hormones in thyroid and testicular germ cells. J. Biol. Chem. 1998;273:23361–23366. doi: 10.1074/jbc.273.36.23361. [DOI] [PubMed] [Google Scholar]
- 139.Huang LJ, Wang L, Ma Y, Durick K, Perkins G, et al. NH2-Terminal targeting motifs direct dual specificity A-kinase–anchoring protein 1 (D-AKAP1) to either mitochondria or endoplasmic reticulum. J. Cell Biol. 1999;145:951–959. doi: 10.1083/jcb.145.5.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chen Q, Lin RY, Rubin CS. Organelle-specific targeting of protein kinase AII (PKAII): molecular and in situ characterization of murine A kinase anchor proteins that recruit regulatory subunits of PKAII to the cytoplasmic surface of mitochondria. J. Biol. Chem. 1997;272:15247–15257. doi: 10.1074/jbc.272.24.15247. [DOI] [PubMed] [Google Scholar]
- 141.Abrenica B, AlShaaban M, Czubryt MP. The A-kinase anchor protein AKAP121 is a negative regulator of cardiomyocyte hypertrophy. J. Mol. Cell. Cardiol. 2009;46:674–681. doi: 10.1016/j.yjmcc.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 142.Perrino C, Feliciello A, Schiattarella GG, Esposito G, Guerriero R, et al. AKAP121 downregulation impairs protective cAMP signals, promotes mitochondrial dysfunction, and increases oxidative stress. Cardiovasc. Res. 2010;88:101–110. doi: 10.1093/cvr/cvq155. [DOI] [PubMed] [Google Scholar]
- 143.Cardone L, Carlucci A, Affaitati A, Livigni A, DeCristofaro T, et al. Mitochondrial AKAP121 binds and targets protein tyrosine phosphatase D1, a novel positive regulator of src signaling. Mol. Cell. Biol. 2004;24:4613–4626. doi: 10.1128/MCB.24.11.4613-4626.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Livigni A, Scorziello A, Agnese S, Adornetto A, Carlucci A, et al. Mitochondrial AKAP121 links cAMP and src signaling to oxidative metabolism. Mol. Biol. Cell. 2006;17:263–271. doi: 10.1091/mbc.E05-09-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Carlucci A, Adornetto A, Scorziello A, Viggiano D, Foca M, et al. Proteolysis of AKAP121 regulates mitochondrial activity during cellular hypoxia and brain ischaemia. EMBO J. 2008;27:1073–1084. doi: 10.1038/emboj.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim H, Scimia MC, Wilkinson D, Trelles RD, Wood MR, et al. Fine-tuning of Drp1/Fis1 availability by AKAP121/Siah2 regulates mitochondrial adaptation to hypoxia. Mol. Cell. 2011;44:532–544. doi: 10.1016/j.molcel.2011.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Acin-Perez R, Salazar E, Kamenetsky M, Buck J, Levin LR, Manfredi G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 2009;9:265–276. doi: 10.1016/j.cmet.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Means CK, Xiao CY, Li Z, Zhang T, Omens JH, et al. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H2944–H2951. doi: 10.1152/ajpheart.01331.2006. [DOI] [PubMed] [Google Scholar]
- 149.Pitson SM, Moretti PA, Zebol JR, Lynn HE, Xia P, et al. Activation of sphingosine kinase 1 by ERK1/2-mediated phosphorylation. EMBO J. 2003;22:5491–5500. doi: 10.1093/emboj/cdg540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Johnson KR, Becker KP, Facchinetti MM, Hannun YA, Obeid LM. PKC-dependent activation of sphingosine kinase 1 and translocation to the plasma membrane: extracellular release of sphingosine-1-phosphate induced by phorbol 12-myristate 13-acetate (PMA) J. Biol. Chem. 2002;277:35257–35262. doi: 10.1074/jbc.M203033200. [DOI] [PubMed] [Google Scholar]
- 151.Lacana E, Maceyka M, Milstien S, Spiegel S. Cloning and characterization of a protein kinase A anchoring protein (AKAP)-related protein that interacts with and regulates sphingosine kinase 1 activity. J. Biol. Chem. 2002;277:32947–32953. doi: 10.1074/jbc.M202841200. [DOI] [PubMed] [Google Scholar]
- 152.Scholten A, Poh MK, van Veen TA, van Breukelen B, Vos MA, Heck AJ. Analysis of the cGMP/cAMP interactome using a chemical proteomics approach in mammalian heart tissue validates sphingosine kinase type 1-interacting protein as a genuine and highly abundant AKAP. J. Proteome Res. 2006;5:1435–1447. doi: 10.1021/pr0600529. [DOI] [PubMed] [Google Scholar]
- 153.Scholten A, van Veen TA, Vos MA, Heck AJ. Diversity of cAMP-dependent protein kinase isoforms and their anchoring proteins in mouse ventricular tissue. J. Proteome Res. 2007;6:1705–1717. doi: 10.1021/pr060601a. [DOI] [PubMed] [Google Scholar]
- 154.Aye TT, Soni S, van Veen TA, van der Heyden MA, Cappadona S, et al. Reorganized PKA-AKAP associations in the failing human heart. J. Mol. Cell. Cardiol. 2012;52:511–518. doi: 10.1016/j.yjmcc.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 155.Kovanich D, van der Heyden MA, Aye TT, van Veen TA, Heck AJ, Scholten A. Sphingosine kinase interacting protein is an A-kinase anchoring protein specific for type I cAMP-dependent protein kinase. ChemBioChem. 2010;11:963–971. doi: 10.1002/cbic.201000058. [DOI] [PubMed] [Google Scholar]
- 156.Darshi M, Mendiola VL, Mackey MR, Murphy AN, Koller A, et al. ChChd3, an inner mitochondrial membrane protein, is essential for maintaining crista integrity and mitochondrial function. J. Biol. Chem. 2010;286:2918–2932. doi: 10.1074/jbc.M110.171975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nauert JB, Klauck TM, Langeberg LK, Scott JD. Gravin, an autoantigen recognized by serum from myasthenia gravis patients, is a kinase scaffold protein. Curr. Biol. 1997;7:52–62. doi: 10.1016/s0960-9822(06)00027-3. [DOI] [PubMed] [Google Scholar]
- 158.Dransfield DT, Bradford AJ, Smith J, Martin M, Roy C, et al. Ezrin is a cyclic AMP-dependent protein kinase anchoring protein. EMBO J. 1997;16:35–43. doi: 10.1093/emboj/16.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Coghlan VM, Langeberg LK, Fernandez A, Lamb NJC, Scott JD. Cloning and characterization of AKAP 95, a nuclear protein that associates with the regulatory subunit of type II cAMP-dependent protein kinase. J. Biol. Chem. 1994;269:7658–7665. [PubMed] [Google Scholar]
- 160.Li H, Adamik R, Pacheco-Rodriguez G, Moss J, Vaughan M. Protein kinase A-anchoring (AKAP) domains in brefeldin A-inhibited guanine nucleotide-exchange protein 2 (BIG2) Proc. Natl. Acad. Sci. USA. 2003;100:1627–1632. doi: 10.1073/pnas.0337678100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lester LB, Coghlan VM, Nauert B, Scott JD. Cloning and characterization of a novel A-kinase anchoring protein: AKAP 220, association with testicular peroxisomes. J. Biol. Chem. 1996;271:9460–9465. doi: 10.1074/jbc.271.16.9460. [DOI] [PubMed] [Google Scholar]
- 162.Perino A, Ghigo A, Ferrero E, Morello F, Santulli G, et al. Integrating cardiac PIP3 and cAMP signaling through a PKA anchoring function of p110γ. Mol. Cell. 2011;42:84–95. doi: 10.1016/j.molcel.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annu. Rev. Pharmacol. Toxicol. 2001;41:145–174. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- 164.Mosenden R, Tasken K. Cyclic AMP-mediated immune regulation—overview of mechanisms of action in T cells. Cell. Signal. 2011;23:1009–1016. doi: 10.1016/j.cellsig.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 165.Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, et al. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 166.Bopp T, Becker C, Klein M, Klein-Hessling S, Palmetshofer A, et al. Cyclic adenosine monophosphate is a key component of regulatory T cell–mediated suppression. J. Exp. Med. 2007;204:1303–1310. doi: 10.1084/jem.20062129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Aandahl EM, Michaelsson J, Moretto WJ, Hecht FM, Nixon DF. Human CD4+ CD25+ regulatory T cells control T-cell responses to human immunodeficiency virus and cytomegalovirus antigens. J. Virol. 2004;78:2454–2459. doi: 10.1128/JVI.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Aandahl EM, Quigley MF, Moretto WJ, Moll M, Gonzalez VD, et al. Expansion of CD7low and CD7negative CD8 T-cell effector subsets in HIV-1 infection: correlation with antigenic load and reversion by antiretroviral treatment. Blood. 2004;104:3672–3678. doi: 10.1182/blood-2004-07-2540. [DOI] [PubMed] [Google Scholar]
- 170.Johansson CC, Bryn T, Aandahl EM, Areklett MA, Aukrust P, et al. Treatment with type-2 selective and non-selective cyclooxygenase inhibitors improves T-cell proliferation in HIV-infected patients on highly active antiretroviral therapy. AIDS. 2004;18:951–952. doi: 10.1097/00002030-200404090-00015. [DOI] [PubMed] [Google Scholar]
- 171.Mahic M, Yaqub S, Johansson CC, Tasken K, Aandahl EM. FOXP3+CD4+CD25+ adaptive regulatory T cells express cyclooxygenase-2 and suppress effector T cells by a prostaglandin E2-dependent mechanism. J. Immunol. 2006;177:246–254. doi: 10.4049/jimmunol.177.1.246. [DOI] [PubMed] [Google Scholar]
- 172.Torgersen KM, Vang T, Abrahamsen H, Yaqub S, Horejsi V, et al. Release from tonic inhibition of T cell activation through transient displacement of C-terminal Src kinase (Csk) from lipid rafts. J. Biol. Chem. 2001;276:29313–29318. doi: 10.1074/jbc.C100014200. [DOI] [PubMed] [Google Scholar]
- 173.Abrahamsen H, Baillie G, Ngai J, Vang T, Nika K, et al. TCR- and CD28-mediated recruitment of phosphodiesterase 4 to lipid rafts potentiates TCR signaling. J. Immunol. 2004;173:4847–4858. doi: 10.4049/jimmunol.173.8.4847. [DOI] [PubMed] [Google Scholar]
- 174.Aandahl EM, Moretto WJ, Haslett PA, Vang T, Bryn T, et al. Inhibition of antigen-specific T cell proliferation and cytokine production by protein kinase A type I. J. Immunol. 2002;169:802–808. doi: 10.4049/jimmunol.169.2.802. [DOI] [PubMed] [Google Scholar]
- 175.Skalhegg BS, Tasken K, Hansson V, Huitfeldt HS, Jahnsen T, Lea T. Location of cAMP-dependent protein kinase type I with the TCR-CD3 complex. Science. 1994;263:84–87. doi: 10.1126/science.8272870. [DOI] [PubMed] [Google Scholar]
- 176.Yaqub S, Tasken K. Role for the cAMP-protein kinase A signaling pathway in suppression of antitumor immune responses by regulatory T cells. Crit. Rev. Oncog. 2008;14:57–77. doi: 10.1615/critrevoncog.v14.i1.40. [DOI] [PubMed] [Google Scholar]
- 177.Skalhegg BS, Landmark BF, Doskeland SO, Hansson V, Lea T, Jahnsen T. Cyclic AMP-dependent protein kinase type I mediates the inhibitory effects of 3′,5′-cyclic adenosine monophosphate on cell replication in human T lymphocytes. J. Biol. Chem. 1992;267:15707–15714. [PubMed] [Google Scholar]
- 178.Levy FO, Rasmussen AM, Tasken K, Skalhegg BS, Huitfeldt HS, et al. Cyclic AMP-dependent protein kinase (cAK) in human B cells: co-localization of type I cAK (RIα2C2) with the antigen receptor during anti-immunoglobulin-induced B cell activation. Eur. J. Immunol. 1996;26:1290–1296. doi: 10.1002/eji.1830260617. [DOI] [PubMed] [Google Scholar]
- 179.Torgersen KM, Vaage JT, Levy FO, Hansson V, Rolstad B, Tasken K. Selective activation of cAMP-dependent protein kinase type I inhibits rat natural killer cell cytotoxicity. J. Biol. Chem. 1997;272:5495–5500. doi: 10.1074/jbc.272.9.5495. [DOI] [PubMed] [Google Scholar]
- 180.Vang T, Abrahamsen H, Myklebust S, Horejsi V, Tasken K. Combined spatial and enzymatic regulation of Csk by cAMP and protein kinase A inhibits T cell receptor signaling. J. Biol. Chem. 2003;278:17597–17600. doi: 10.1074/jbc.C300077200. [DOI] [PubMed] [Google Scholar]
- 181.Vang T, Torgersen KM, Sundvold V, Saxena M, Levy FO, et al. Activation of the COOH-terminal Src kinase (Csk) by cAMP-dependent protein kinase inhibits signaling through the T cell receptor. J. Exp. Med. 2001;193:497–507. doi: 10.1084/jem.193.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Torgersen KM, Vang T, Abrahamsen H, Yaqub S, Tasken K. Molecular mechanisms for protein kinase A-mediated modulation of immune function. Cell. Signal. 2002;14:1–9. doi: 10.1016/s0898-6568(01)00214-5. [DOI] [PubMed] [Google Scholar]
- 183.Brdicka T, Pavlistova D, Leo A, Bruyns E, Korinek V, et al. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase Csk and is involved in regulation of T cell activation. J. Exp. Med. 2000;191:1591–1604. doi: 10.1084/jem.191.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kawabuchi M, Satomi Y, Takao T, Shimonishi Y, Nada S, et al. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature. 2000;404:999–1003. doi: 10.1038/35010121. [DOI] [PubMed] [Google Scholar]
- 185.Takeuchi S, Takayama Y, Ogawa A, Tamura K, Okada M. Transmembrane phosphoprotein Cbp positively regulates the activity of the carboxyl-terminal Src kinase, Csk. J. Biol. Chem. 2000;275:29183–29186. doi: 10.1074/jbc.C000326200. [DOI] [PubMed] [Google Scholar]
- 186.Aandahl EM, Aukrust P, Muller F, Hansson V, Tasken K, Froland SS. Additive effects of IL-2 and protein kinase A type I antagonist on function of T cells from HIV-infected patients on HAART. AIDS. 1999;13:F109–F114. doi: 10.1097/00002030-199912030-00001. [DOI] [PubMed] [Google Scholar]
- 187.Aukrust P, Aandahl EM, Skalhegg BS, Nordoy I, Hansson V, et al. Increased activation of protein kinase A type I contributes to the T cell deficiency in common variable immunodeficiency. J. Immunol. 1999;162:1178–1185. [PubMed] [Google Scholar]
- 188.Holm AM, Aukrust P, Aandahl EM, Muller F, Tasken K, Froland SS. Impaired secretion of IL-10 by T cells from patients with common variable immunodeficiency-involvement of protein kinase A type I. J. Immunol. 2003;170:5772–5777. doi: 10.4049/jimmunol.170.11.5772. [DOI] [PubMed] [Google Scholar]
- 189.Brudvik KW, Paulsen JE, Aandahl EM, Roald B, Tasken K. Protein kinase A antagonist inhibits β-catenin nuclear translocation, c-Myc and COX-2 expression and tumor promotion in ApcMin/+ mice. Mol. Cancer. 2011;10:149. doi: 10.1186/1476-4598-10-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gold MG, Smith FD, Scott JD, Barford D. AKAP18 contains a phosphoesterase domain that binds AMP. J. Mol. Biol. 2008;375:1329–1343. doi: 10.1016/j.jmb.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Robinson CV, Sali A, Baumeister W. The molecular sociology of the cell. Nature. 2007;450:973–982. doi: 10.1038/nature06523. [DOI] [PubMed] [Google Scholar]
- 192.Heck AJ. At the interface of interaction proteomics and structural biology. Mol. Cell. Proteomics. 2010;9:1633. doi: 10.1074/mcp.E110.001511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gonen T, Cheng Y, Sliz P, Hiroaki Y, Fujiyoshi Y, et al. Lipid-protein interactions in double-layered two-dimensional AQP0 crystals. Nature. 2005;438:633–638. doi: 10.1038/nature04321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jain A, Liu R, Ramani B, Arauz E, Ishitsuka Y, et al. Probing cellular protein complexes using single-molecule pull-down. Nature. 2011;473:484–488. doi: 10.1038/nature10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hoshi N, Langeberg LK, Gould CM, Newton AC, Scott JD. Interaction with AKAP79 modifies the cellular pharmacology of PKC. Mol. Cell. 2010;37:541–550. doi: 10.1016/j.molcel.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hoshi N, Langeberg LK, Scott JD. Distinct enzyme combinations in AKAP signalling complexes permit functional diversity. Nat. Cell Biol. 2005;7:1066–1073. doi: 10.1038/ncb1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Prince JT, Ahn NG. The case of the disappearing drug target. Mol. Cell. 2010;37:455–456. doi: 10.1016/j.molcel.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 198.Christian F, Szaszak M, Friedl S, Drewianka S, Lorenz D, et al. Small molecule AKAP-protein kinase A (PKA) interaction disruptors that activate PKA interfere with compartmentalized cAMP signaling in cardiac myocytes. J. Biol. Chem. 2011;286:9079–9096. doi: 10.1074/jbc.M110.160614. [DOI] [PMC free article] [PubMed] [Google Scholar]