Abstract
Chronic administration of mood stabilizers to rats downregulates the brain arachidonic acid (AA) cascade. This downregulation may explain their efficacy against bipolar disorder (BD), in which brain AA cascade markers are elevated. The atypical antipsychotics, olanzapine (OLZ) and clozapine (CLZ), also act against BD. When given to rats, both reduce brain cyclooxygenase activity and prostaglandin E2 concentration; OLZ also reduces rat plasma unesterified and esterified AA concentrations, and AA incorporation and turnover in brain phospholipid. To test whether CLZ produces similar changes, we used our in vivo fatty acid method in rats given 10 mg/kg/day i.p. CLZ, or vehicle, for 30 days; or 1 day after CLZ washout. [1-14C]AA was infused intravenously for 5 min, arterial plasma was collected and microwaved brain was analyzed. CLZ increased incorporation coefficients and rates Jin,i of plasma unesterified AA into brain phospholipids i, while decreasing plasma unesterified but not esterified AA. These effects disappeared after washout. Thus, CLZ and OLZ similarly downregulated kinetics and cyclooxygenase expression of the brain AA cascade, likely by reducing plasma unesterified AA availability. Atypical antipsychotics and mood stabilizers may be therapeutic in BD by downregulating, indirectly or directly respectively, the elevated brain AA cascade of that disease.
Keywords: clozapine, antipsychotic, arachidonic acid, phospholipid, incorporation, bipolar disorder, plasma, brain
Introduction
Bipolar disorder (BD) is a progressive neuropsychiatric illness characterized by recurrent episodes of depression and mania (BD I) or hypomania (BD II) (reviewed in (Basselin et al. 2010)). It is treated with the mood stabilizers lithium, valproate, carbamazepine or lamotrigine (Geddes et al. 2010, Greil et al. 1997, Bowden et al. 2000, Cipriani et al. 2010, Calabrese et al. 2003), or with the atypical antipsychotic olanzapine (OLZ), all of which are FDA approved (Bowden et al. 2000, Scherk et al. 2007). Another atypical antipsychotic CLZ, a tricyclic dibenzodiazepine, is not FDA-approved but has been reported effective in acute BD mania (Calabrese et al. 1996, Scherk et al. 2007), in rapid cycling and in patients with refractory BD (Zarate et al. 1995).
Studies in unanesthetized rats indicate that the chronically administered mood stabilizers selectively downregulate various aspects of the brain arachidonic acid (AA, 20:4n-6) cascade (Shimizu & Wolfe 1990, Basselin et al. 2010). Since the cascade is upregulated in the BD brain, in association with neuroinflammation, excitotoxicity, apoptosis and synaptic loss (Kim et al. 2010, Kim et al. 2011b, Rao et al. 2010, Rao et al. 2012), this downregulation may contribute to the therapeutic efficacy of the mood stabilizers (Basselin et al. 2010). This interpretation is supported by evidence that topiramate, initially thought effective in BD but later shown ineffective in phase III clinical trials (Kushner et al. 2006), did not alter any measured parameter of the brain AA cascade in rats (Bazinet et al. 2005a, Ghelardoni et al. 2005, Bazinet et al. 2006, Chang et al. 2001, Chang et al. 1996, Ghelardoni et al. 2004, Bosetti et al. 2002, Bosetti et al. 2003, Shimshoni et al. 2011, Ramadan et al. 2011), and that lithium pretreatment dampened AA cascade upregulation in animal models of neuroinflammation (Basselin et al. 2007, Basselin et al. 2010).
As OLZ and CLZ also are effective in BD (see above) (Calabrese et al. 1996, Hegerl 2012, Cipriani et al. 2010, Frye et al. 1998), we thought it of interest to test the hypothesis that, like the FDA-approved mood stabilizers, these atypical antipsychotics can downregulate the rat brain AA cascade. Supporting this hypothesis, we reported recently that chronically administration of OLZ to rats, to produce a plasma drug level therapeutically relevant to BD, reduced AA turnover and incorporation in brain phospholipid, total brain cyclooxygenase (COX) activity and PGE2 concentration, markers of the brain AA cascade. These effects of OLZ were ascribed to a concomitant reduction of the plasma concentration of unesterified AA (the form that enters the brain (Washizaki et al. 1994, Purdon et al. 1997)), thus of AA availability to brain (Cheon et al. 2011). We also have reported that chronic CLZ, like OLZ, decreased COX activity and PGE2 concentration in rat brain (Kim et al. 2012).
In the present study, we used our in vivo kinetic method to test whether CLZ like OLZ also would reduce rat brain AA kinetics (turnover and incorporation of AA in phospholipid) and the plasma unesterified AA concentration. Showing this would argue further that the AA cascade is a common target of anti-BD atypical antipsychotics as well as mood stabilizers (Rapoport & Bosetti 2002, Rapoport et al. 2009), and that our in vivo fatty acid model could be used to screen for new drug candidates by measuring AA cascade kinetics in rodents (Robinson et al. 1992).
CLZ was injected i.p. daily in rats for 30 days to produce a therapeutically relevant plasma concentration. Radiolabeled AA was infused intravenously for 5 min in unanesthetized rats after the last CLZ injection, and brain AA kinetics and brain and plasma concentrations were determined (Chang et al. 2001, Robinson et al. 1992). Studies were performed also in a vehicle-treated group, and in a washout group (CLZ-W) that received CLZ for 30 days and was injected with vehicle 24 h later, sufficient time for CLZ to have entirely disappeared from blood and brain, where its half-lives are 1.5 h and 1.6 h, respectively (Baldessarini et al. 1993, Kontkanen et al. 2002). An abstract of part this work has been published (Modi et al. 2011).
METHODS AND MATERIALS
Animals
The study was conducted following the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (Publication no. 80-23) and was approved by the Animal Care and Use Committee of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Chemicals and reagents were purchased from Sigma Chemicals (St. Louis, MO, USA) unless otherwise indicated. Male CDF-344 rats, weighing 180–200 g (Charles River; Wilmington, MA, USA), were acclimatized for one week in an animal facility with controlled temperature, humidity and light cycle, and had ad libitum access to water and NIH-31 diet, which contains 4% crude fat by weight. Dietary fatty acids (% of total fatty acid) consisted of 20.1% saturated, 22.5% monounsaturated, 47.9% linoleic, 5.1% α-linolenic, 0.02% AA, 2.0% eicosapentaenoic and 2.3% DHA (Demar et al. 2005, Igarashi et al. 2006). Rats were divided randomly into three groups, a vehicle control group, a CLZ treatment group and a CLZ washout group (CLZ-W) that was given vehicle 24 h after the last CLZ injection.
CLZ (NIMH Chemical Synthesis and Drug Supply Program, Bethesda, MD, USA) was dissolved in 1% glacial acetic acid to a concentration of 10.0 mg/ml and then neutralized with 0.1 N NaOH to pH 6.0. A drug solution was prepared once weekly and stored at 4°C. Chronic CLZ-treated rats (CLZ) received 10 mg/kg/day CLZ in 0.5 ml vehicle once daily for 30 days intraperitoneally (i.p.) as in our prior study (Kim et al. 2012), and were sacrificed 1 h after the last injection. The dose was chosen on the basis of D2 receptor occupancy by CLZ (Farde & Nordstrom 1992), and is consistent with chronically administered clinically-relevant doses used previously in rats (Tulipano et al. 2007, Cooper et al. 2008, Halim et al. 2004, Levant et al. 2006). A control group received the same volume of vehicle under parallel conditions. A third washout group (CLZ-W) received CLZ for 30 days followed by vehicle injection on the surgery day (24 h washout). On the last day of injection, the rat was injected with its appropriate treatment 1 h before its brain was removed for analysis.
Surgical procedure
A rat was anesthetized with 1–3% halothane, and polyethylene catheters (PE50, Clay Adams, Becton Dickinson, Sparks, MD, USA) filled with heparinized isotonic saline were inserted into the right femoral artery and vein (Cheon et al. 2011, Chang et al. 1996). The rat was allowed to recover from surgery with its hindquarters loosely wrapped and taped to a wooden block for 3 h in a temperature-controlled recovery chamber maintained at 25°C, while body temperature was maintained at 37°C with a rectal probe and a feedback heating element (TACT-2DF Temperature controller, Physitemp Instruments, Clifton, NJ, USA). CLZ or vehicle was injected 1 h before [1-14C]AA infusion. Heart rate and blood pressure were monitored after recovery from surgery using a CyQ BPM02 system (CyQ 103/302; CyberSense, Nicholasville, KY, USA).
[1-14C]AA infusion
[1-14C]AA (50 mCi/mmol, > 98% pure, Moravek Biochemicals, Brea, CA, USA) was dissolved in saline containing 50 mg/ml fatty acid-free bovine serum albumin by sonicating for 10 min (Sigma-Aldrich, St. Louis, MO, USA) (DeGeorge et al. 1989). One h after the last injection, an unanesthetized rat was infused intravenously for 5 min with 1.3 ml containing 170 μCi/kg of AA, at a rate of 0.223(1 + e −0.032t) ml/min (t = sec) with a computer-controlled variable rate infusion pump (No. 22; Harvard Apparatus, South Natick, MA, USA), to achieve a steady-state plasma specific activity within 1 min (Washizaki et al. 1994). Arterial blood samples were collectedat 0, 15, 30, 45, 90, 180, 240 and 300 s during infusion to determine radioactiveand unlabeled concentrations of unesterified AA in plasma. Five min after starting infusion, the rat was anesthetized with sodium pentobarbital (50 mg/kg, i.v.) and subjected to head-focused microwave irradiation to stop brain metabolism (5.5 kW, 4.8s; Cober Electronics, Norwalk, CT, USA) (Deutsch et al. 1997, Bazinet et al. 2005a). The brain was excised, dissected sagittally and stored at −80°C for further analysis.
Plasma and brain lipid extraction and separation
Total lipids were extracted from frozen plasma and from one cerebral hemisphere by the Folch method (Folch et al. 1957). Heptadecanoic acid (17:0) was added as an internal standard prior to extraction. Neutral lipids were separated from the total lipid extracts by thin layer chromatography (TLC) on silica gel plates (Silica Gel 60A TLC plates; Whatman, Clifton, NJ, USA) using a mixture of heptane: diethyl ether: glacial acetic acid (60:40:3 by volume) (Skipski et al. 1968). Authentic standards of cholesteryl ester, triacylglycerol, unesterified fatty acids, cholesterol, and phospholipids were run in separate lanes to identify lipid bands. Phospholipid classes (ChoGpl, choline glycerophospholipid; PtdSer, phosphatidylserine; PtdIns, phosphatidylinositol; EtnGpl, ethanolamine glycerophospholipid) were separated in chloroform: methanol: H2O: glacial acetic acid (60:50:4:1 by volume) (Skipski et al. 1967) and identified with unlabeled standards in separate lanes. The lipid bands were visualized under ultraviolet light after spraying the plates with 0.03% (w/v) 6-p-toluidine-2-naphthalene sulfonic acid (Acros, Fairlawn, NJ, USA) in 50 mM Tris buffer (pH 7.4). Each band was scraped, and the silica gel was used directly to quantify radioactivity by scintillation counting or to prepare fatty acid methyl esters (FAMEs) (see below). Prior to methylation, appropriate quantities of di-17:0-PC was added as an internal standard to quantify brain esterified lipids, and 17:0 (heptadecaenoic acid) was added to quantify brain unesterified fatty acids.
Quantification of radioactivity
Radioactivityin plasma total lipid extracts collected over the course of the 5 min infusion was determined using a liquid scintillation analyzer (2200CA, TRI-CARB®; Packard Instruments, Meriden, CT, USA) following reconstitution in 5 ml Cocktail mix.
FAME preparation and gas chromatography analysis
FAMEs were formed by heating the scrapes in 1% H2SO4 in methanol at 70 °C for 3 h. FAMEs were separated on a SP™-2330 fused silica capillarycolumn (30 m × 0.25 mm inner diameter, 0.25 μm film thickness)(Supelco, Bellefonte, PA, USA), using gas chromatography (GC) with a flame ionization detector (Model 6890N; Agilent Technologies, Palo Alto, CA, USA). Runs were initiated at 80°C, with a temperature gradient to 150°C(10°C/min) and 200°C (6°C/min), and held at 200°C for 10 min, and then increased to 240°C for a total run time of 38 min. Fatty acid concentrations (nmol/g brain or nmol/ml plasma) were calculated by proportional comparison of the GC peak areas to that of the 17:0 internal standard.
Quantification of labeled and unlabeled acyl-CoA
Acyl-CoA species were extracted from the remaining microwaved half-brain usingan affinity chromatography method (Deutsch et al. 1994). After adding 10 nmol heptadecanoyl-CoA (17:0-CoA) as an internal standard to the weighed half brain (~0.8 g), the sample was homogenized in 2 ml of 25 mM potassium phosphate and sonicated for 20 s with a probe sonicator (Model W-225; Misonix, Farmingdale, NY, USA). Isopropanol (2 ml) was added to the homogenate, which was sonicated for another 20 s. Proteins were precipitated with saturated ammonium sulphate and shaking the sample lightly by hand. Then, acetonitrile (4 ml) was added and the sample vortexed for 10 min prior to centrifugation. The supernatant was collected and diluted with 10 ml of 25 mM potassium phosphate. Each sample was passed three times through an activated oligonucleotide purification cartridge (ABI Masterpiece™, OPC®; Applied Biosystems, Foster City, CA), and the cartridge was washed with 10 ml of 25 mM potassium phosphate. Acyl-CoA species were eluted with 500 μl of elution buffer (75% isopropanol/25% 1 mM glacial acetic acid by volume). Samples were dried under nitrogen and reconstituted in 100 μl of elution buffer for HPLC analysis. Extracted acyl-CoA species were separated on a reverse-phase HPLC column (Symmetry C-18, 5-μm particle size, 250 × 4.6 mm; Waters-Millipore, Milford, MA, USA), using HPLC (Beckman, Fullerton, CA, USA) and a pump coupled with a UV/VISdetector (System Gold, Model 168, Beckman). HPLC was performed using a linear gradient system composed of: (A) 75 mM potassium phosphate, pH 4.9 and (B) 100% acetonitrile. The composition of the initial solvent system (44% B, 1 min), was changed to 49% B over 25 min and then to 68% B over 10 min, maintained at 68% B for 4 min, returned to 44% B over 6 min, and held at 44% B for 6 min (52 min total run time). UV detection was set at 260 nm for integration of concentrations and at 280 nm for identification of acyl-CoAs (260/280= 4:1). Peaks were identified from retention times of authentic acyl-CoA standards. Endogenous acyl-CoA concentrations (nmol/g brain) were calculated by direct proportional comparison of the peak areas with the peak area of the 17:0-CoA internal standard. The arachidonoyl-CoA (AA-CoA) peak was collected, its concentration quantified and its radioactivity determined by liquid scintillation counting in order to calculate the specific activity.
Calculations
We used our established in vivo kinetic model for quantifying brain fatty acid kinetic parameters (Robinson et al. 1992). Unidirectional incorporation coefficients, of AA, representing incorporation from plasma into brain lipid i (phospholipid, triacylglycerol or cholesteryl ester), were calculated as follows:
| (Eq. 1) |
represents radioactivity of brain lipid i at time T = 5 min (time of termination of experiment), t is time after starting infusion, and is the plasma concentration of labeled unesterified AA during infusion. Integrals of plasma radioactivity were determined by trapezoidal integration. Since AA synthesis within brain from its dietary precursor linoleic acid (18:2n-6) represents less than 0.5% of the plasma AA flux into brain (DeMar et al. 2006), the rate of incorporation Jin,i (nmol·s−1·g−1) of plasma unesterified AA into brain lipid i, represents the rate of metabolic loss by the brain, and is calculated as follows:
| (Eq. 2) |
Cpl (nmol·ml−1) is the concentration of unlabeled unesterified AA in plasma. The “dilution factor” λ, defined as the steady-state ratio during [1-14C]AA infusion, of specific activity of the brain arachidonoyl-CoA pool to the specific activity of plasma unesterified AA, was determined as follows:
| (Eq. 3) |
Net rates of incorporation of unlabeled unesterified AA from brain arachidonoyl-CoA into brain lipid i, JFA,i (nmol·s−1·g−1) equal:
| (Eq. 4) |
The fractional turnover of AA within phospholipid i, due to deacylation and reacylation, FFA,i (%·h−1) is defined as:
| (Eq. 5) |
Statistical analysis
Data are presented as mean ± SD. Data were analyzed with a one-way analysis of variance (ANOVA) followed by Bonferroni’s post-hoc test to compare differences between: (i) the CLZ and control group, and (ii) the CLZ-W and control group. Statistically significant differences between CLZ with or without washout relative to controls are indicated by asterisks; * p < 0.05, ** p < 0.01, *** p < 0.001. Statistical analysis was performed on GraphPad Prism (version 4.03, GraphPad Software, San Diego, CA, USA).
RESULTS
Body weight and physical parameters
Rats chronically administered CLZ with or without washout weighed 12% and 10% less, respectively, than controls (270.4 ± 10.49, 238.6 ± 21.37, 243.4 ± 13.6 g for control, CLZ and CLZ-W, respectively, p < 0.01) (Table 1). CLZ with or without washout reduced mean arterial blood pressure by 18% and 13%, respectively, compared to control (Table 1). Heart rate did not change significantly. Body temperature did not differ significantly among the three groups, since it was maintained at 37°C by a rectal probe and a heating element.
Table 1.
Physiological parameters
| Control | CLZ | CLZ-W | |
|---|---|---|---|
| Body weight (g) | 270.4 ± 10.49 | 238.6 ± 21.4*** | 243.4 ± 13.6** |
| Arterial blood pressure (mm Hg) | 138.1 ± 5.38 | 113.3 ± 4.9*** | 120.0 ± 8.67** |
| Heart rate (beats/min) | 424.4 ± 28.3 | 389.1 ± 40.6 | 448.2 ± 33.3 |
| Body temperature (°C) | 37.0 ± 0.5 | 37.0 ± 0.4 | 36.8 ± 0.4 |
Chronic clozapine-treated rats (CLZ, n=8) received 10 mg/kg/day clozapine in 0.5 ml vehicle once daily for 30 days intraperitoneally (i.p.) and were killed 1 hour after the last injection. 24 hour washout group (CLZ-W, n=8) received full period of clozapine, and followed by one vehicle injection on the surgery day. A control group (n=10) received the same volume of vehicle under parallel conditions.
Values are means ± SD. Data were analyzed by one-way ANOVA followed by Bonferroni’s post-hoc test, which compared differences between i) control and CLZ, and ii) control and CLZ-W.
p < 0.05,
p < 0.01,
p < 0.001 compared to controls.
Plasma Kinetics
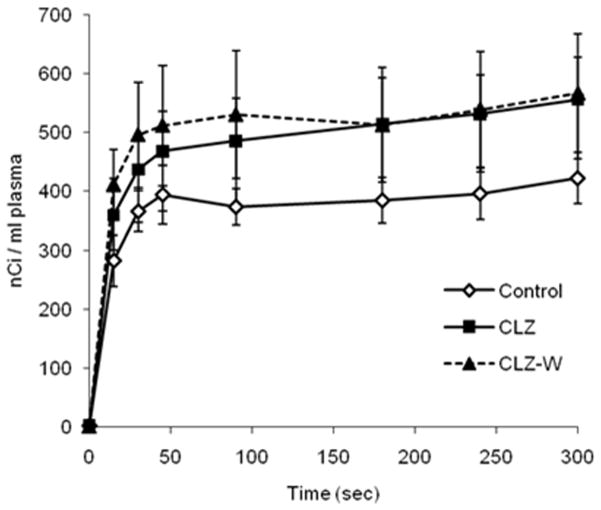
Figure 1 shows steady-state plasma radioactivity during the 5-min [1-14C]AA infusion. Steady-state radioactivity was achieved within one minute in all groups, but was higher at all times for the CLZ and CLZ-W groups than control. Thus, integrated plasma radioactivity was significantly higher in the CLZ (148330 ± 19833 nC.s/mL) and CLZ-W (152507 ± 27312 nCi.s/mL) groups than in the control group (112057 ± 10684 nCi.s/mL) (p < 0.05), suggesting that CLZ prolonged the plasma half-life of unesterified plasma AA (Kapetanovic et al. 1982).
Figure 1.
Plasma and brain fatty acids
As illustrated in Table 2, esterified 18:0 concentration was increased significantly by 2.5-fold by CLZ compared to control in plasma cholesteryl ester, without there being any other significant fatty acid change in either lipid. Cholesteryl ester 18:0 concentration did not differ significantly between the CLZ-W group and control. Esterified fatty acid concentrations in plasma triglycerides and phospholipids did not differ significantly (data not shown).
Table 2.
Esterified fatty acid concentrations in plasma cholesteryl ester
| Fatty Acid | Control (n=10) | CLZ (n=8) | CLZ-W (n=8) |
|---|---|---|---|
|
| |||
| (nmol/ml plasma) | |||
| 16:0 | 45.6 ± 6.3 | 44.4 ± 2.8 | 40.7 ± 7.9 |
| 16:1 | 15.4 ± 4.6 | 13.7 ± 4.1 | 11.0 ± 7.6 |
| 18:0 | 6.9 ± 1.9 | 17.6 ± 12.9* | 7.2 ± 2.2 |
| 18:1n-9 | 21.1 ± 2.0 | 23.4 ± 2.8 | 22.1 ± 4.4 |
| 18:1n-7 | 5.8 ± 0.8 | 5.5 ± 0.7 | 5.5 ± 0.8 |
| 18:2n-6 | 123.6 ± 15.1 | 115.2 ± 5.1 | 110.5 ± 20.6 |
| 18:3n-3 | 3.3 ± 1.9 | 2.9 ± 0.5 | 2.4 ± 0.9 |
| 20:4n-6 | 267.4 ± 46.1 | 251.2 ± 52.5 | 254.8 ± 67.5 |
| 20:5n-3 | 22.6 ± 6.7 | 24.2 ± 5.1 | 21.3 ± 9.1 |
| 22:5n-3 | 8.6 ± 3.7 | 8.1 ± 2.6 | 9.2 ± 1.3 |
| 22:6n-3 | 13.9 ± 3.8 | 12.0 ± 1.6 | 13.1 ± 3.0 |
| Total | 537.0 ± 73.6 | 520.8 ± 56.1 | 501.3 ± 108.6 |
| SFA | 52.5 ± 7.0 | 60.0 ± 14.9 | 47.9 ± 9.5 |
| MUFA | 42.2 ± 5.5 | 42.6 ± 5.3 | 38.5 ± 11.9 |
| PUFA | 442.3 ± 68.5 | 418.2 ± 52.8 | 414.9 ± 93.3 |
| n-6 PUFA | 402.7 ± 60.8 | 379.0 ± 53.4 | 378.1 ± 86.2 |
| n-3 PUFA | 39.5 ± 11.5 | 39.2 ± 5.8 | 36.8 ± 11.9 |
ND, Not detected; SFA, Saturated fatty acid; MUFA, Monounsaturated fatty acid; PUFA, Polyunsaturated fatty acid. SFA, Saturated fatty acid; MUFA, Monounsaturated fatty acid; PUFA, Polyunsaturated fatty acid. Values are means ± SD. Data were analyzed by one-way ANOVA followed by Bonferroni’s post-hoc test, which compared differences between i) control and CLZ, and ii) control and CLZ-W.
p < 0.05 compared to control.
As illustrated in Table 3, chronic CLZ compared to control caused a widespread decrease in the plasma concentrations of the majority of measured unesterified fatty acids, including net n-3 and n-6 PUFA concentrations. Many but not all of the reductions were significant even after the 1 day washout, indicating that they did not depend on the continued presence of CLZ in the body. With regard to unesterified AA, chronic CLZ significantly decreased the plasma concentration by 46 % (p < 0.001) compared to control (13.6 ± 6.0 vs. 25.2 ± 8.7 nmol/ml) (Table 3), but this effect was not significant for the CLZ-W group (20.0 ± 5.4 nmol/ml). Unesterified 18:2n-6 and 18:3n-3 were decreased in both the CLZ and CLZ-W groups.
Table 3.
Unesterified fatty acid concentrations in plasma and esterified fatty acid concentrations in total brain phospholipid
| Fatty acid | Unesterified fatty acid in plasma
|
Esterified fatty acids in brain total phospholipids
|
|||||
|---|---|---|---|---|---|---|---|
| Control (n=10) | CLZ (n=8) | CLZ-W (n=8) | Control (n=10) | CLZ (n=8) | CLZ-W (n=8) | ||
|
| |||||||
| (nmol/ml plasma) | (nmol/g brain) | ||||||
| 16:0 | 451.3 ± 73.8 | 195.3 ± 55.8 *** | 259.9 ± 54.2 *** | 24674 ± 985 | 25013 ± 985 | 25313 ± 1656 | |
| 16:1 | 65.0 ± 20.2 | 21.1 ± 8.3 *** | 33.7 ± 12.6 ** | ND | ND | ND | |
| 18:0 | 89.8 ± 17.4 | 50.7 ± 6.6 *** | 71.3 ± 24.9 | 25258 ± 1489 | 25655 ± 1827 | 26841 ± 2236 | |
| 18:1n-9 | 329.9 ± 56.9 | 139.0 ± 45.3 *** | 209.0 ± 46.0 *** | 22900 ± 1899 | 24033 ± 2169 | 26946 ± 3351 ** | |
| 18:2n-6 | 356.4 ± 74.6 | 141.1 ± 47.1 *** | 217.9 ± 37.0 *** | 724 ± 100 | 750 ± 63 | 927 ± 87 *** | |
| 18:3n-3 | 24.6 ± 5.0 | 8.8 ± 3.0 *** | 14.7 ± 3.5 *** | ND | ND | ND | |
| 20:1n-9 | ND | ND | ND | 2491 ± 541 | 2814 ± 439 | 3496 ± 999 * | |
| 20:4n-6 | 25.2 ± 8.7 | 13.6 ± 6.0 ** | 20.0 ± 5.4 | 10017 ± 822 | 10106 ± 539 | 10310 ± 761 | |
| 20:5n-3 | 14.7 ± 4.9 | 5.1 ± 1.6 *** | 8.3 ± 1.8 ** | ND | ND | ND | |
| 22:4n-6 | ND | ND | ND | 3149 ± 212 | 3292 ± 258 | 3383 ± 368 | |
| 22:5n-6 | ND | ND | ND | 230 ± 21 | 235 ± 25 | 208 ± 24 | |
| 22:5n-3 | 18.2 ± 4.6 | 6.5 ± 2.8 *** | 8.4 ± 4.2 ** | 118 ± 28 | 121 ± 11 | 123 ± 9 | |
| 22:6n-3 | 37.0 ± 10.9 | 14.6 ± 5.5 *** | 23.3 ± 5.4 ** | 13281 ± 975 | 14078 ± 992 | 14181 ± 1157 | |
| Total | 1423.2 ± 296.2 | 600.1 ± 171.4*** | 872.2 ± 146.6*** | 108810 ± 5791 | 112551 ± 7417 | 118868 ± 9579 * | |
| SFA | 552.3 ± 89.4 | 250.2 ± 62.9 *** | 336.9 ± 64.2 *** | 49932 ± 2315 | 50668 ± 2792 | 52153 ± 3727 | |
| MUFA | 394.9 ± 74.3 | 160.2 ± 52.1 *** | 242.7 ± 57.7 *** | 31358 ± 2828 | 33301 ± 3255 | 37584 ± 5150 ** | |
| PUFA | 476.1 ± 107.0 | 189.7 ± 63.6 *** | 292.6 ± 45.9 *** | 27519 ± 1965 | 28582 ± 1735 | 29131 ± 2233 | |
| n-6 PUFA | 381.5 ± 82.5 | 154.7 ± 51.8 *** | 237.9 ± 35.6 *** | 14120 ± 1045 | 14384 ± 772 | 14828 ± 1135 | |
| n-3 PUFA | 94.5 ± 24.9 | 35.1 ± 12.3 *** | 54.7 ± 11.5 *** | 13399 ± 994 | 14198 ± 999 | 14303 ± 1155 | |
ND, Not detected; SFA, Saturated fatty acid; MUFA, Monounsaturated fatty acid; PUFA, Polyunsaturated fatty acid.
SFA, Saturated fatty acid; MUFA, Monounsaturated fatty acid; PUFA, Polyunsaturated fatty acid.
Values are means ± SD. Data were analyzed by one-way ANOVA followed by Bonferroni’s post-hoc test, which compared differences between i) control and CLZ, and ii) control and CLZ-W.
p < 0.05,
p < 0.01,
p < 0.001 compared to control.
Esterified fatty acid concentrations within brain phospholipids were not affected significantly by chronic CLZ treatment (Table 3). After CLZ-W however, esterified concentrations of 18:1n-9, 18:2n-6 and 20:1n-9 and of total monounsaturated fatty acids were significantly higher than in the control group (Table 3).
Chronic CLZ had few significant effects on esterified concentrations of fatty acids in each of four brain phospholipids, but none on esterified AA or DHA, the major PUFAs (Tables 4 and 5). In ChoGpl, oleate (18:1n-9) and 20:1n-9 were increased by CLZ-W but not CLZ compared to control). In EtnGpl, palmitate (16:0), 18:1n-9, 18:1n-7, 18:2n-6, 20:1n-9 adrenate (22:4n-6) and 22:6n-3 were increased by CLZ-W but not CLZ (Table 4). In PtdIns, 16:0, 18:1n-9 and 18:2n-6 were increased by CLZ, whereas 16:0 and 18:1n-7 were increased by CLZ-W (Table 5). In PtdSer, 16:0 was decreased by CLZ whereas 18:1n-9 was increased in CLZ-W (Table 5).
Table 4.
Fatty acids concentrations in brain choline glycerophospholipids and ethanolamine glycerophospholipids
| Fatty Acid | choline glycerophospholipids
|
ethanolamine glycerophospholipids
|
||||
|---|---|---|---|---|---|---|
| Control (n=10) | CLZ (n=8) | CLZ-W (n=8) | Control (n=10) | CLZ (n=8) | CLZ-W (n=8) | |
|
| ||||||
| nmol/g brain | nmol/g brain | |||||
| 16:0 | 21095 ± 856 | 21326 ± 753 | 21311 ± 1288 | 2691 ± 164 | 2759 ± 172 | 2955 ± 284 * |
| 18:0 | 7714 ± 608 | 7819 ± 683 | 8333 ± 993 | 8422 ± 746 | 8821 ± 532 | 8880 ± 552 |
| 18:1n-9 | 11029 ± 648 | 11389 ± 785 | 12054 ± 1031* | 7714 ± 937 | 8193 ± 853 | 9816 ± 1434** |
| 18:1n-7 | 4066 ± 254 | 4184 ± 397 | 4480 ± 508 | 1708 ± 295 | 2004 ± 260 | 2386 ± 435 *** |
| 18:2n-6 | 401 ± 41 | 421 ± 34 | 448 ± 47 | 248 ± 60 | 229 ± 52 | 372 ± 33 *** |
| 20:1n-9 | 593 ± 120 | 646 ± 91 | 771 ± 191 * | 1468 ± 336 | 1714 ± 285 | 2139 ± 588 ** |
| 20:4n-6 | 2582 ± 193 | 2501 ± 181 | 2479 ± 208 | 5398 ± 509 | 5418 ± 323 | 5736 ± 319 |
| 22:4n-6 | 223 ± 37 | 234 ± 26 | 210 ± 40 | 2383 ± 186 | 2523 ± 176 | 2635 ± 242 * |
| 22:6n-3 | 1741 ± 202 | 1894 ± 236 | 1895 ± 252 | 8211 ± 640 | 8650 ± 729 | 9051 ± 556 * |
| Total | 49444 ± 2344 | 50413 ± 2949 | 51980 ± 3988 | 38502 ± 2809 | 40581 ± 2873 | 44222 ± 3412 ** |
Values are means ± SD. Data were analyzed by one-way ANOVA followed by Bonferroni’s post-hoc test, which compared differences between i) control and CLZ, and ii) control and CLZ-W.
p < 0.05,
p < 0.01,
p < 0.001 compared to controls.
Table 5.
Fatty acids concentrations in brain phosphatidylinositol and phosphatidylserine
| Fatty Acid | phosphatidylinositol
|
phosphatidylserine
|
||||
|---|---|---|---|---|---|---|
| Control (n=10) | CLZ (n=8) | CLZ-W (n=8) | Control (n=10) | CLZ (n=8) | CLZ-W (n=8) | |
|
| ||||||
| nmol/g brain | nmol/g brain | |||||
| 16:0 | 470 ± 88 | 616 ± 106 * | 618 ± 111 * | 418 ± 117 | 311 ± 54 * | 428 ± 147 |
| 18:0 | 1746 ± 178 | 1852 ± 267 | 1916 ± 294 | 7376 ± 593 | 7163 ± 516 | 7712 ± 741 |
| 18:1n-9 | 539 ± 198 | 830 ± 225 * | 744 ± 161 | 3618 ± 421 | 3621 ± 458 | 4332 ± 853 * |
| 18:1n-7 | 195 ± 66 | 267 ± 42 | 275 ± 67 * | ND | ND | ND |
| 18:2n-6 | 35 ± 13 | 62 ± 31 * | 50 ± 9 | 40 ± 18 | 39 ± 11 | 37 ± 9 |
| 20:1n-9 | 70 ± 35 | 99 ± 25 | 102 ± 42 | 361 ± 90 | 354 ± 60 | 484 ± 244 |
| 20:4n-6 | 1471 ± 192 | 1607 ± 233 | 1507 ± 233 | 565 ± 72 | 579 ± 124 | 588 ± 122 |
| 22:6n-3 | 134 ± 42 | 167 ± 51 | 153 ± 51 | 3195 ± 226 | 3367 ± 171 | 3082 ± 419 |
| Total | 4658 ± 716 | 5501 ± 911 * | 5366 ± 791 | 16205 ± 1144 | 16055 ± 1314 | 17300 ± 1916 |
Values are means ± SD. Data were analyzed by one-way ANOVA followed by Bonferroni’s post-hoc test, which compared differences between i) control and CLZ, and ii) control and CLZ-W.
p < 0.05, compared to controls.
Brain acyl-CoA concentrations
Chronic CLZ, with or without washout, did not change significantly the brain concentration of unlabeled arachidonoyl-CoA compared to control (Table 6). CLZ alone, however, increased the concentration of labeled AA-CoA compared to control (p < 0.05), which was not significantly different between CLZ-W and control. There was no significant difference in concentrations of the other measured brain acyl-CoA species, except for palmitoyl-CoA, which was significantly reduced in the CLZ-W group compared to control (Table 6).
Table 6.
Brain acyl-CoA concentrations and λ
| Control (n=5) | CLZ (n=5) | CLZ-W (n=4) | |
|---|---|---|---|
|
| |||
| (nmol/g brain) | |||
| Mystearoyl-CoA | 0.26 ± 0.07 | 0.28 ± 0.09 | 0.16 ± 0.11 |
| Palmitoyl-CoA | 9.19 ± 0.35 | 10.08 ± 1.18 | 8.13 ± 0.87* |
| Stearoyl-CoA | 6.29 ± 1.18 | 6.26 ± 0.63 | 6.62 ± 0.77 |
| Oleayl-CoA | 12.10 ± 0.57 | 13.68 ± 1.77 | 12.90 ± 1.45 |
| Linoleoyl-CoA | 0.49 ± 0.12 | 0.45 ± 0.11 | 0.51 ± 0.14 |
| Docosahexaenoyl-CoA | 0.83 ± 0.19 | 0.89 ± 0.13 | 0.70 ± 0.21 |
| Arachidonoyl-CoA (nmol/g brain) | 0.63 ± 0.08 | 0.71 ± 0.11 | 0.61 ± 0.19 |
| [14C]AA-CoA (nCi/g brain) | 0.38 ± 0.09 | 0.78 ± 0.16 * | 0.68 ± 0.44 |
| λa | 0.029 ± 0.013 | 0.020 ± 0.013 | 0.033 ± 0.013 |
Values are means ± SD. Data were analyzed by one-way ANOVA followed by Bonferroni’s post-hoc test, which compared differences between i) control and CLZ, and ii) control and CLZ-W.
p < 0.05, compared to control.
λ (Eq. 3) is the steady-state ratio during [1-14C]AA infusion of specific activity of brain arachidonoyl-CoA pool to specific activity of plasma unesterified AA.
Brain Kinetics
CLZ significantly increased the AA incorporation coefficient, (Eq. 1) into ChoGpl compared with control (p < 0.05; Table 7). CLZ-W significantly increased into total phospholipids (p < 0.01), ChoGpl (p < 0.01) and PtdIns (p < 0.05) compared to control (Table 7). Reflecting the reduction in unesterified plasma AA concentration, CLZ significantly decreased Jin,i (Eq. 2), the incorporation rate of unesterified AA from plasma into brain total phospholipids by 36% compared with control (Table 7). CLZ-W did not significantly affect Jin,i for total or individual brain phospholipids (Table 7).
Table 7.
Incorporation Coefficients ( ) and Incorporation Rates (Jin,i) of AA from Plasma into Brain Phospholipids
|
|
Jin,I (nmol/g/s × 10
−4)
|
||||||
|---|---|---|---|---|---|---|---|
| Control (n=10) | CLZ (n=8) | CLZ-W (n=8) | Control (n=10) | CLZ (n=8) | CLZ-W (n=8) | ||
| Total phospholipid | 28.5 ± 2.6 | 33.5 ± 2.3 | 35.2 ± 6.8** | 71.5 ± 23.7 | 45.7 ± 20.6* | 72.3 ± 30.8 | |
| ChoGpl | 12.3 ± 1.15 | 15.4 ± 1.5* | 15.9 ± 3.1** | 30.7 ± 10.2 | 20.9 ± 9.4 | 32.6 ± 14.1 | |
| PtdSer | 2.7 ± 0.4 | 2.9 ± 0.3 | 3.2 ± 0.8 | 6.9 ± 2.7 | 4.0 ± 1.8 | 6.6 ± 3.3 | |
| PtdIns | 10.1 ± 0.9 | 11.3 ± 0.7 | 12.0 ± 2.3* | 25.2 ± 8.4 | 15.5 ± 7.1 | 24.7 ± 10.1 | |
| EtnGpl | 3.5 ± 0.5 | 3.9 ± 0.5 | 4.1 ± 0.8 | 8.7 ± 2.8 | 5.4 ± 2.6 | 8.4 ± 3.5 | |
ChoGpl, choline glycerophospholipids; PtdSer, phosphatidylserine; PtdIns, phosphatidylinositol; EtnGpl, ethanolamine glycerophospholipids
Values are means ± SD. Data were analyzed by one-way ANOVA followed by Bonferroni’s post-hoc test, which compared differences between i) control and CLZ, and ii) control and CLZ-W.
p < 0.05,
p < 0.01, compared to control.
The dilution factor λ (Eq. 3) was not significantly changed by CLZ (0.020 ± 0.013) or CLZ-W (0.033 ± 0.013) compared with control (0.029 ± 0.013) (Table 6). Inserting λ into Eq. 5 provided rates of incorporation of non-esterified AA from the brain precursor AA-CoA pool into phospholipids, JFA,i (Eq. 4). JFA,i and AA turnover FFA,i due to deacylation-reacylation were not changed significantly in the CLZ or CLZ-W group compared with control group (Table 8).
Table 8.
Net incorporation rate of brain AA-CoA into brain phospholipids (JFA) and AA turnover (FFA)
|
JFA (nmol/g/s × 10 −2)
|
F FA (% per hour)
|
|||||
|---|---|---|---|---|---|---|
| Control (n=5) | CLZ (n=5) | CLZ-W (n=4) | Control (n=5) | CLZ (n=5) | CLZ-W (n=4) | |
| Total Phospholipids | 21.6±8.4 | 18.4±3.9 | 18.5±3.3 | 7.7±2.8 | 6.5±1.5 | 6.3±1.3 |
| ChoGpl | 9.4±3.7 | 8.5±2.1 | 8.2±1.6 | 12.9±4.6 | 11.9±3.2 | 11.5±2.6 |
| PtdSer | 2.0±0.8 | 1.6±0.3 | 1.6±0.4 | 13.2±6.0 | 10.6±2.9 | 9.6±2.2 |
| PtdIns | 7.5±2.8 | 6.2±1.1 | 6.5±1.0 | 17.4±6.2 | 15.0±5.3 | 15.1±2.7 |
| EtnGpl | 2.7±1.1 | 2.1±0.4 | 2.2±0.3 | 1.8±0.7 | 1.3±0.3 | 1.3±0.2 |
Values are means ± SD.
ChoGpl, choline glycerophospholipids; PtdSer, phosphatidylserine; PtdIns, phosphatidylinositol; EtnGpl, ethanolamine glycerophospholipids
DISCUSSION
Baseline concentrations of unesterified and esterified plasma fatty acids, of esterified brain fatty acids in cholesteryl ester and individual phospholipids, and brain acyl-CoA species, and AA incorporation coefficients, rates and turnovers in brain phospholipids, of control (vehicle-treated) rats were comparable to published values in rats fed the NIH-31 diet in this study. This diet has a high content of the n-3 PUFAs, EPA (2.0%) and DHA (2.3%) (see Methods), unlike some other rodent diets (e.g., Teklad 2018 diet, Harlan Laboratories, USA) that lack EPA and DHA (Basselin et al. 2007, Chang et al. 1996, Bazinet et al. 2006, Chang et al. 2001, Bazinet et al. 2005a, Lee et al. 2008, Lee et al. 2010, Cheon et al. 2011). The unesterified plasma AA concentration in control rats on this diet also is within the published range of 16–42 μM, as are concentrations of other unesterified and esterified fatty acids, including palmitate (16:0), stearate (18:0), oleate (18:1n-9), linoleate (18:2n-6) and α-linolenate (18:3n-3) (Bazinet et al. 2006, Bazinet et al. 2005a, Basselin et al. 2007, Lee et al. 2008, Demar et al. 2005). This confirms the validity and reproducibility of our analytical methods, our kinetic model and the unanesthetized rat preparation (Robinson et al. 1992).
The major findings of this study is that chronic CLZ (10 mg/kg/day i.p. 30 days) that produced a therapeutically relevant plasma concentration, compared with vehicle, significantly decreased rates of AA incorporation (Jin,i) from plasma into brain total phospholipid, largely by decreasing the plasma concentration and thus availability to brain of unesterified AA (Washizaki et al. 1994). This effect was statistically insignificant after the 24-h washout period (CLZ-W), reflecting normalization of the plasma unesterified AA concentration. Since AA is a substrate for COX and other oxidative enzymes within the brain AA cascade (Shimizu & Wolfe 1990), its reduced plasma AA availability likely contributes to the reported decreases in brain COX activity and PGE2 concentration following CLZ (Kim et al. 2012). These changes and their 24-h reversibility are similar to those caused by chronic OLZ (Cheon et al. 2011), suggesting that each of the two atypical antipsychotic agents use in BD reduces rat brain AA metabolism and does so by decreasing plasma unesterified AA. This mechanism deserves to be tested with other antipsychotics.
Whereas OLZ significantly reduced AA turnover within total phospholipid and PtdIns in rat brain (Cheon et al. 2011), CLZ’s effect on turnover in total and individual phospholipids was statistically insignificant. The difference may have reflected drug dose effects, or the lower variance of the OLZ data due to more rats having been studied with it. Further, calculated turnover depends on the dilution coefficient λ, which was reduced insignificantly by 31% by CLZ compared to vehicle, which would tend to counterbalance the influence of reduced the Jin,i for AA (Eq. 5).
The rate of AA incorporation into phospholipid i, Jin,i, is the product of the incorporation coefficient and plasma unesterified AA concentration (Eq. 2). It equals the rate of AA metabolic loss from brain, since AA cannot be synthesized de novo or converted significantly from its linoleic acid precursor in brain (Holman 1986, Deutsch et al. 1997, Chang et al. 2001, DeMar et al. 2004, DeMar et al. 2006). Although was increased by chronic CLZ, suggesting greater brain avidity for unesterified AA, Jin,i was reduced due to the decreased plasma unesterified AA concentration. That Jin,i returned to baseline following the 24 h washout suggests that CLZ’s effect on plasma unesterified AA required significant drug in the body, since CLZ half-lives are 1.5 h and 1.6 h, respectively, in rat plasma and brain (Baldessarini et al. 1993, Kontkanen et al. 2002).
The esterified AA concentration within total brain phospholipids did not change despite the reduction in Jin,i, possibly because downstream AA metabolism was reduced proportionately to the reduction in Jin,i, as evidenced by the reduced brain COX activity and PGE2 concentration (Kim et al. 2012). This finding confirms a prior report that measured brain fatty acid concentrations at a daily CLZ dose of 20 mg/kg (Levant et al. 2006) and highlights the importance of measuring fluxes as well as concentrations when testing drug effects on brain fatty acid metabolism. In this regard, despite significant increases in expression of cytosolic phospholipase A2 (cPLA2)-IVA, secretory sPLA2-IIA, and COX-2 in postmortem BD frontal cortex, suggesting disturbed AA kinetics, phospholipid and fatty acid concentrations were minimally different from control values (Kim et al. 2011b, Igarashi et al. 2010). In vivo PET imaging of and Jin,i might be used to further examine antipsychotic drug effects on brain AA kinetics in bipolar patients (Thambisetty et al. 2012).
The widespread reductions in unesterified concentrations of plasma fatty acids following CLZ were not related to reductions in plasma esterified concentrations, which are found following chronic OLZ in rats (Cheon et. al., unpublished observations). They thus may be related to CLZ’s effects on hydrolysis of esterified circulating fatty acids by liver, adipose or other tissue. In humans, CLZ decreased expression of hepatic lipase involved in lipoprotein secretion, and of adipose lipases that regulate lipolysis and secretion of unesterified fatty acids (Duncan et al. 2008, Raclot 2003, Ferno et al. 2009, Gavino & Gavino 1992). Similar peripheral actions have been proposed for OLZ, which also decreases plasma unesterified fatty acid concentrations in rats (Cheon et al. 2011, Albaugh et al. 2011, Albaugh et al. 2012) and in humans (Vidarsdottir et al. 2010, Kaddurah-Daouk et al. 2007, Albaugh et al. 2011).
Chronic CLZ with or without washout increased the AA incorporation coefficient into rat brain ChoGpl, as does OLZ and valproate (Cheon et al. 2011, Chang et al. 2001, Chang et al. 1996, Bazinet et al. 2006). An increased represents increased “affinity” of serial reactions involving diffusion, transport and enzymatic activation leading to entry of plasma AA into the sn-2 position of brain phospholipid (Sun & MacQuarrie 1989, Robinson et al. 1992, Kirkilionis 2010, Rapoport 2008). The increase may involve upregulated expression of brain dopaminergic D2 and D4 receptors or of the NR2B subunit of N-methyl-D aspartate (NMDA) receptors caused by chronic drug (Tarazi et al. 1997, Lidow & Goldman-Rakic 1997, Silvestri et al. 2000, Janowsky et al. 1992, Kabbani & Levenson 2006, Meshul et al. 1996, Ossowska et al. 2002), given that D2-like and NMDA receptors can be coupled to cPLA2 and AA release from membrane phospholipid (Basselin et al. 2006, Bhattacharjee et al. 2005, Piomelli & Di Marzo 1993). It also could represent a compensatory response to reduced plasma unesterified AA, as also found following chronic valproate and OLZ (Bazinet et al. 2005b, Cheon et al. 2011, Ramadan et al. 2011).
Like chronic CLZ, chronic administration of OLZ to rats, to produce a plasma drug level therapeutically relevant to BD, reduced AA turnover and AA incorporation into phospholipid, total brain cyclooxygenase (COX) activity and PGE2 concentration. These effects also were ascribed to a reduced plasma concentration of unesterified AA, thus of AA availability to brain (Cheon et al. 2011). Chronic CLZ, like OLZ, decreased COX activity and PGE2 concentration in rat brain (Kim et al. 2012). These similarities suggest that the AA cascade is a common target of anti-BD atypical antipsychotics as well as mood stabilizers (Rapoport & Bosetti 2002, Rapoport et al. 2009), and that our in vivo kinetic fatty acid method could be used to screen for new drug candidates in rodents (Robinson et al. 1992).
Fatty acid concentrations and for AA remained elevated in some brain phospholipids 24 h following CLZ washout, suggesting a withdrawal effect of CLZ on membrane fatty acid concentrations as reported following OLZ (Cheon et al. 2011). CLZ is an amphiphilic molecule that is positively charged at physiologic pH, and can interact with acidic and neutral phospholipid polar head groups via electrostatic and repulsion forces (Soderlund et al. 1999, Parry et al. 2008, Jutila et al. 2001). This interaction may have caused long-lasting disruption in membrane phospholipid, despite CLZ’s absence from brain after the 24-h washout.
The reductions by CLZ and OLZ of plasma unesterified AA concentration and brain COX activity and PGE2 concentration are similar to effects of chronic dietary n-6 PUFA deprivation in rats (Kim et al. 2011a). This suggests a possible therapeutic advantage of reducing dietary n-6 PUFA content as a stand-alone therapy or in combination with CLZ or OLZ, since each treatment reduces plasma unesterified AA availability.
A paradoxical aspect of atypical antipsychotics is that they cause weight loss in male rats but weight gain in humans, despite inducing similar metabolic disturbances related to insulin resistance and hyperlipidemia (Albaugh et al. 2006, Albaugh et al. 2011, Kaddurah-Daouk et al. 2007, Minet-Ringuet et al. 2006, Vidarsdottir et al. 2010). This discrepancy has not been resolved, although female rats appear more likely to develop obesity than male rats following i.p. OLZ (Fell et al. 2007) but not CLZ (Albaugh et al. 2006), or dietary antipsychotics (Albaugh et al. 2006, Minet-Ringuet et al. 2006). Several effects of atypical antipsychotics relevant to this study have been reported in rats and humans, including reduced unesterified plasma fatty acid concentrations by OLZ in rats (Albaugh et al. 2011, Albaugh et al. 2012) and humans (Vidarsdottir et al. 2010, Kaddurah-Daouk et al. 2007, Albaugh et al. 2011). In this study, CLZ also reduced plasma unesterified fatty acid concentrations. Whether this occurs in humans remains to be tested.
In conclusion, application of our in vivo fatty acid model in unanesthetized rats showed that chronic CLZ reduced AA incorporation rates into brain phospholipids by decreasing the unesterified plasma AA concentration in plasma, despite increasing AA incorporation coefficients. This effect required the presence of CLZ in the body, since it was absent after the 24-h washout. The decreases in AA incorporation rates, brain COX activity and PGE2 concentration overlap with effects of OLZ (Cheon et al. 2011). When related to studies of the direct action of mood stabilizers on the rat brain AA cascade, this paper further supports the overall hypothesis that targeting the brain AA cascade is a reasonable approach for treating BD and can be used to screen for novel drug candidates in rats.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute on Aging, NIH. Clozapine was supplied by National Institute of Mental Health’s Chemical Synthesis and Drug Supply Program. The authors thank Dr. Mireille Basselin for her valuable comments.
Abbreviations
- AA
arachidonic acid
- AA-CoA
arachidonoyl-CoA
- CLZ
clozapine
- CLZ-W
clozapine with washout
- ChoGpl
choline glycerophospholipid
- COX
cyclooxygenase
- cPLA2
cytosolic phospholipase A2
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic
- EtnGpl
ethanolamine glycerophospholipid
- FAME
fatty acid methyl esters
- GC
gas chromatography
- NMDA
N-methyl-D-aspartate
- OLZ
olanzapine
- PG
prostaglandin
- PtdIns
phosphatidylinositol
- PtdSer
phosphatidylserine
- sPLA2
secretory phospholipase A2
- sn
stereospecifically numbered
- PUFA
polyunsaturated fatty acid
- TLC
thin layer chromatography
- DHA
docosahexaenoic acid
Footnotes
The authors have no conflict of interest.
References
- Albaugh VL, Henry CR, Bello NT, Hajnal A, Lynch SL, Halle B, Lynch CJ. Hormonal and metabolic effects of olanzapine and clozapine related to body weight in rodents. Obesity (Silver Spring) 2006;14:36–51. doi: 10.1038/oby.2006.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albaugh VL, Judson JG, She P, Lang CH, Maresca KP, Joyal JL, Lynch CJ. Olanzapine promotes fat accumulation in male rats by decreasing physical activity, repartitioning energy and increasing adipose tissue lipogenesis while impairing lipolysis. Mol Psychiatry. 2011;16:569–581. doi: 10.1038/mp.2010.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albaugh VL, Vary TC, Ilkayeva O, et al. Atypical antipsychotics rapidly and inappropriately switch peripheral fuel utilization to lipids, impairing metabolic flexibility in rodents. Schizophr Bull. 2012;38:153–166. doi: 10.1093/schbul/sbq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldessarini RJ, Centorrino F, Flood JG, Volpicelli SA, Huston-Lyons D, Cohen BM. Tissue concentrations of clozapine and its metabolites in the rat. Neuropsychopharmacology. 1993;9:117–124. doi: 10.1038/npp.1993.50. [DOI] [PubMed] [Google Scholar]
- Basselin M, Chang L, Bell JM, Rapoport SI. Chronic lithium chloride administration attenuates brain NMDA receptor-initiated signaling via arachidonic acid in unanesthetized rats. Neuropsychopharmacology. 2006;31:1659–1674. doi: 10.1038/sj.npp.1300920. [DOI] [PubMed] [Google Scholar]
- Basselin M, Kim HW, Chen M, Ma K, Rapoport SI, Murphy RC, Farias SE. Lithium modifies brain arachidonic and docosahexaenoic metabolism in rat lipopolysaccharide model of neuroinflammation. Journal of lipid research. 2010;51:1049–1056. doi: 10.1194/jlr.M002469. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Basselin M, Ramadan E, Rapoport SI. Imaging brain signal transduction and metabolism via arachidonic and docosahexaenoic acid in animals and humans. Brain research bulletin. 2012;87:154–171. doi: 10.1016/j.brainresbull.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basselin M, Villacreses NE, Lee HJ, Bell JM, Rapoport SI. Chronic lithium administration attenuates up-regulated brain arachidonic acid metabolism in a rat model of neuroinflammation. J Neurochem. 2007;102:761–772. doi: 10.1111/j.1471-4159.2007.04593.x. [DOI] [PubMed] [Google Scholar]
- Bazinet RP, Lee HJ, Felder CC, Porter AC, Rapoport SI, Rosenberger TA. Rapid high-energy microwave fixation is required to determine the anandamide (N-arachidonoylethanolamine) concentration of rat brain. Neurochem Res. 2005a;30:597–601. doi: 10.1007/s11064-005-2746-5. [DOI] [PubMed] [Google Scholar]
- Bazinet RP, Rao JS, Chang L, Rapoport SI, Lee HJ. Chronic valproate does not alter the kinetics of docosahexaenoic acid within brain phospholipids of the unanesthetized rat. Psychopharmacology (Berl) 2005b;182:180–185. doi: 10.1007/s00213-005-0059-7. [DOI] [PubMed] [Google Scholar]
- Bazinet RP, Rao JS, Chang L, Rapoport SI, Lee HJ. Chronic carbamazepine decreases the incorporation rate and turnover of arachidonic acid but not docosahexaenoic acid in brain phospholipids of the unanesthetized rat: relevance to bipolar disorder. Biol Psychiatry. 2006;59:401–407. doi: 10.1016/j.biopsych.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee AK, Chang L, Lee HJ, Bazinet RP, Seemann R, Rapoport SI. D2 but not D1 dopamine receptor stimulation augments brain signaling involving arachidonic acid in unanesthetized rats. Psychopharmacology (Berl) 2005;180:735–742. doi: 10.1007/s00213-005-2208-4. [DOI] [PubMed] [Google Scholar]
- Bosetti F, Rintala J, Seemann R, Rosenberger TA, Contreras MA, Rapoport SI, Chang MC. Chronic lithium downregulates cyclooxygenase-2 activity and prostaglandin E(2) concentration in rat brain. Mol Psychiatry. 2002;7:845–850. doi: 10.1038/sj.mp.4001111. [DOI] [PubMed] [Google Scholar]
- Bosetti F, Weerasinghe GR, Rosenberger TA, Rapoport SI. Valproic acid down-regulates the conversion of arachidonic acid to eicosanoids via cyclooxygenase-1 and -2 in rat brain. J Neurochem. 2003;85:690–696. doi: 10.1046/j.1471-4159.2003.01701.x. [DOI] [PubMed] [Google Scholar]
- Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry. 2000;57:481–489. doi: 10.1001/archpsyc.57.5.481. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Bowden CL, Sachs G, et al. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. J Clin Psychiatry. 2003;64:1013–1024. doi: 10.4088/jcp.v64n0906. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Kimmel SE, Woyshville MJ, Rapport DJ, Faust CJ, Thompson PA, Meltzer HY. Clozapine for treatment-refractory mania. Am J Psychiatry. 1996;153:759–764. doi: 10.1176/ajp.153.6.759. [DOI] [PubMed] [Google Scholar]
- Chang MC, Contreras MA, Rosenberger TA, Rintala JJ, Bell JM, Rapoport SI. Chronic valproate treatment decreases the in vivo turnover of arachidonic acid in brain phospholipids: a possible common effect of mood stabilizers. J Neurochem. 2001;77:796–803. doi: 10.1046/j.1471-4159.2001.00311.x. [DOI] [PubMed] [Google Scholar]
- Chang MC, Grange E, Rabin O, Bell JM, Allen DD, Rapoport SI. Lithium decreases turnover of arachidonate in several brain phospholipids. Neurosci Lett. 1996;220:171–174. doi: 10.1016/s0304-3940(96)13264-x. [DOI] [PubMed] [Google Scholar]
- Cheon Y, Park JY, Modi HR, Kim HW, Lee HJ, Chang L, Rao JS, Rapoport SI. Chronic olanzapine treatment decreases arachidonic acid turnover and prostaglandin E(2) concentration in rat brain. Journal of neurochemistry. 2011;119:364–376. doi: 10.1111/j.1471-4159.2011.07410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipriani A, Rendell J, Geddes JR. Olanzapine in the long-term treatment of bipolar disorder: a systematic review and meta-analysis. Journal of psychopharmacology. 2010;24:1729–1738. doi: 10.1177/0269881109106900. [DOI] [PubMed] [Google Scholar]
- Cooper GD, Harrold JA, Halford JC, Goudie AJ. Chronic clozapine treatment in female rats does not induce weight gain or metabolic abnormalities but enhances adiposity: implications for animal models of antipsychotic-induced weight gain. Progress in neuro-psychopharmacology & biological psychiatry. 2008;32:428–436. doi: 10.1016/j.pnpbp.2007.09.012. [DOI] [PubMed] [Google Scholar]
- DeGeorge JJ, Noronha JG, Bell J, Robinson P, Rapoport SI. Intravenous injection of [1-14C]arachidonate to examine regional brain lipid metabolism in unanesthetized rats. J Neurosci Res. 1989;24:413–423. doi: 10.1002/jnr.490240311. [DOI] [PubMed] [Google Scholar]
- DeMar JC, Jr, Lee HJ, Ma K, Chang L, Bell JM, Rapoport SI, Bazinet RP. Brain elongation of linoleic acid is a negligible source of the arachidonate in brain phospholipids of adult rats. Biochim Biophys Acta. 2006;1761:1050–1059. doi: 10.1016/j.bbalip.2006.06.006. [DOI] [PubMed] [Google Scholar]
- DeMar JC, Jr, Ma K, Bell JM, Rapoport SI. Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids. J Neurochem. 2004;91:1125–1137. doi: 10.1111/j.1471-4159.2004.02789.x. [DOI] [PubMed] [Google Scholar]
- Demar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. alpha-Linolenic acid does not contribute appreciably to docosahexaenoic acid within brain phospholipids of adult rats fed a diet enriched in docosahexaenoic acid. Journal of neurochemistry. 2005;94:1063–1076. doi: 10.1111/j.1471-4159.2005.03258.x. [DOI] [PubMed] [Google Scholar]
- Deutsch J, Grange E, Rapoport SI, Purdon AD. Isolation and quantitation of long-chain acyl-coenzyme A esters in brain tissue by solid-phase extraction. Anal Biochem. 1994;220:321–323. doi: 10.1006/abio.1994.1344. [DOI] [PubMed] [Google Scholar]
- Deutsch J, Rapoport SI, Purdon AD. Relation between free fatty acid and acyl-CoA concentrations in rat brain following decapitation. Neurochem Res. 1997;22:759–765. doi: 10.1023/a:1022030306359. [DOI] [PubMed] [Google Scholar]
- Duncan RE, Sarkadi-Nagy E, Jaworski K, Ahmadian M, Sul HS. Identification and functional characterization of adipose-specific phospholipase A2 (AdPLA) J Biol Chem. 2008;283:25428–25436. doi: 10.1074/jbc.M804146200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farde L, Nordstrom AL. PET analysis indicates atypical central dopamine receptor occupancy in clozapine-treated patients. Br J Psychiatry Suppl. 1992:30–33. [PubMed] [Google Scholar]
- Fell MJ, Anjum N, Dickinson K, Marshall KM, Peltola LM, Vickers S, Cheetham S, Neill JC. The distinct effects of subchronic antipsychotic drug treatment on macronutrient selection, body weight, adiposity, and metabolism in female rats. Psychopharmacology (Berl) 2007;194:221–231. doi: 10.1007/s00213-007-0833-9. [DOI] [PubMed] [Google Scholar]
- Ferno J, Vik-Mo AO, Jassim G, et al. Acute clozapine exposure in vivo induces lipid accumulation and marked sequential changes in the expression of SREBP, PPAR, and LXR target genes in rat liver. Psychopharmacology (Berl) 2009;203:73–84. doi: 10.1007/s00213-008-1370-x. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Frye MA, Ketter TA, Altshuler LL, Denicoff K, Dunn RT, Kimbrell TA, Cora-Locatelli G, Post RM. Clozapine in bipolar disorder: treatment implications for other atypical antipsychotics. Journal of affective disorders. 1998;48:91–104. doi: 10.1016/s0165-0327(97)00160-2. [DOI] [PubMed] [Google Scholar]
- Gavino VC, Gavino GR. Adipose hormone-sensitive lipase preferentially releases polyunsaturated fatty acids from triglycerides. Lipids. 1992;27:950–954. doi: 10.1007/BF02535570. [DOI] [PubMed] [Google Scholar]
- Geddes JR, Goodwin GM, Rendell J, Azorin JM, Cipriani A, Ostacher MJ, Morriss R, Alder N, Juszczak E. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet. 2010;375:385–395. doi: 10.1016/S0140-6736(09)61828-6. [DOI] [PubMed] [Google Scholar]
- Ghelardoni S, Bazinet RP, Rapoport SI, Bosetti F. Topiramate does not alter expression in rat brain of enzymes of arachidonic acid metabolism. Psychopharmacology (Berl) 2005;180:523–529. doi: 10.1007/s00213-005-2189-3. [DOI] [PubMed] [Google Scholar]
- Ghelardoni S, Tomita YA, Bell JM, Rapoport SI, Bosetti F. Chronic carbamazepine selectively downregulates cytosolic phospholipase A2 expression and cyclooxygenase activity in rat brain. Biol Psychiatry. 2004;56:248–254. doi: 10.1016/j.biopsych.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Greil W, Ludwig-Mayerhofer W, Erazo N, et al. Lithium versus carbamazepine in the maintenance treatment of bipolar disorders--a randomised study. J Affect Disord. 1997;43:151–161. doi: 10.1016/s0165-0327(96)01427-9. [DOI] [PubMed] [Google Scholar]
- Halim ND, Weickert CS, McClintock BW, Weinberger DR, Lipska BK. Effects of chronic haloperidol and clozapine treatment on neurogenesis in the adult rat hippocampus. Neuropsychopharmacology. 2004;29:1063–1069. doi: 10.1038/sj.npp.1300422. [DOI] [PubMed] [Google Scholar]
- Hegerl U. Review: risperidone, olanzapine and haloperidol are the most effective drugs for acute mania in adults with bipolar I disorder. Evidence-based mental health. 2012;15:45. doi: 10.1136/ebmental-2011-100476. [DOI] [PubMed] [Google Scholar]
- Holman RT. Control of polyunsaturated acids in tissue lipids. J Am Coll Nutr. 1986;5:183–211. doi: 10.1080/07315724.1986.10720125. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI, DeMar JC., Jr Low liver conversion rate of alpha-linolenic to docosahexaenoic acid in awake rats on a high-docosahexaenoate-containing diet. Journal of lipid research. 2006;47:1812–1822. doi: 10.1194/jlr.M600030-JLR200. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Ma K, Gao F, Kim HW, Greenstein D, Rapoport SI, Rao JS. Brain lipid concentrations in bipolar disorder. Journal of psychiatric research. 2010;44:177–182. doi: 10.1016/j.jpsychires.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowsky A, Neve KA, Kinzie JM, Taylor B, de Paulis T, Belknap JK. Extrastriatal dopamine D2 receptors: distribution, pharmacological characterization and region-specific regulation by clozapine. J Pharmacol Exp Ther. 1992;261:1282–1290. [PubMed] [Google Scholar]
- Jutila A, Soderlund T, Pakkanen AL, Huttunen M, Kinnunen PK. Comparison of the effects of clozapine, chlorpromazine, and haloperidol on membrane lateral heterogeneity. Chem Phys Lipids. 2001;112:151–163. doi: 10.1016/s0009-3084(01)00175-x. [DOI] [PubMed] [Google Scholar]
- Kabbani N, Levenson R. Antipsychotic-induced alterations in D2 dopamine receptor interacting proteins within the cortex. Neuroreport. 2006;17:299–301. doi: 10.1097/01.wnr.0000199460.24412.04. [DOI] [PubMed] [Google Scholar]
- Kaddurah-Daouk R, McEvoy J, Baillie RA, Lee D, Yao JK, Doraiswamy PM, Krishnan KR. Metabolomic mapping of atypical antipsychotic effects in schizophrenia. Mol Psychiatry. 2007;12:934–945. doi: 10.1038/sj.mp.4002000. [DOI] [PubMed] [Google Scholar]
- Kapetanovic IM, Sweeney DJ, Rapoport SI. Age effects on haloperidol pharmacokinetics in male, Fischer-344 rats. J Pharmacol Exp Ther. 1982;221:434–438. [PubMed] [Google Scholar]
- Kim HW, Cheon Y, Modi HR, Rapoport SI, Rao JS. Effects of chronic clozapine administration on markers of arachidonic acid cascade and synaptic integrity in rat brain. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HW, Rao JS, Rapoport SI, Igarashi M. Dietary n-6 PUFA deprivation downregulates arachidonate but upregulates docosahexaenoate metabolizing enzymes in rat brain. Biochim Biophys Acta. 2011a;1811:111–117. doi: 10.1016/j.bbalip.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HW, Rapoport SI, Rao JS. Altered expression of apoptotic factors and synaptic markers in postmortem brain from bipolar disorder patients. Neurobiol Dis. 2010;37:596–603. doi: 10.1016/j.nbd.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HW, Rapoport SI, Rao JS. Altered arachidonic acid cascade enzymes in postmortem brain from bipolar disorder patients. Mol Psychiatry. 2011b;16:419–428. doi: 10.1038/mp.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkilionis M. Exploration of cellular reaction systems. Brief Bioinform. 2010;11:153–178. doi: 10.1093/bib/bbp062. [DOI] [PubMed] [Google Scholar]
- Kontkanen O, Lakso M, Wong G, Castren E. Chronic antipsychotic drug treatment induces long-lasting expression of fos and jun family genes and activator protein 1 complex in the rat prefrontal cortex. Neuropsychopharmacology. 2002;27:152–162. doi: 10.1016/S0893-133X(02)00289-0. [DOI] [PubMed] [Google Scholar]
- Kushner SF, Khan A, Lane R, Olson WH. Topiramate monotherapy in the management of acute mania: results of four double-blind placebo-controlled trials. Bipolar Disord. 2006;8:15–27. doi: 10.1111/j.1399-5618.2006.00276.x. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Rao JS, Chang L, Rapoport SI, Bazinet RP. Chronic N-methyl-D-aspartate administration increases the turnover of arachidonic acid within brain phospholipids of the unanesthetized rat. J Lipid Res. 2008;49:162–168. doi: 10.1194/jlr.M700406-JLR200. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Rao JS, Chang L, Rapoport SI, Kim HW. Chronic imipramine but not bupropion increases arachidonic acid signaling in rat brain: is this related to ‘switching’ in bipolar disorder? Mol Psychiatry. 2010;15:602–614. doi: 10.1038/mp.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levant B, Crane JF, Carlson SE. Sub-chronic antipsychotic drug treatment does not alter brain phospholipid fatty acid composition in rats. Progress in neuro-psychopharmacology & biological psychiatry. 2006;30:728–732. doi: 10.1016/j.pnpbp.2005.11.034. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Goldman-Rakic PS. Differential regulation of D2 and D4 dopamine receptor mRNAs in the primate cerebral cortex vs. neostriatum: effects of chronic treatment with typical and atypical antipsychotic drugs. J Pharmacol Exp Ther. 1997;283:939–946. [PubMed] [Google Scholar]
- Meshul CK, Bunker GL, Mason JN, Allen C, Janowsky A. Effects of subchronic clozapine and haloperidol on striatal glutamatergic synapses. J Neurochem. 1996;67:1965–1973. doi: 10.1046/j.1471-4159.1996.67051965.x. [DOI] [PubMed] [Google Scholar]
- Minet-Ringuet J, Even PC, Lacroix M, Tome D, de Beaurepaire R. A model for antipsychotic-induced obesity in the male rat. Psychopharmacology (Berl) 2006;187:447–454. doi: 10.1007/s00213-006-0433-0. [DOI] [PubMed] [Google Scholar]
- Modi HR, YC, HWK LC, JSR, Rapoport SI. Effect of chronic administration of clozapine on arachidonic acid (AA) turnover. Society of Biological Psychiatry 66th Annual Meeting 2011; 12–14 May, 2011; 2011. p. 740. [Google Scholar]
- Ossowska K, Pietraszek M, Wardas J, Dziedzicka-Wasylewska M, Nowicka D, Wolfarth S. Chronic treatments with haloperidol and clozapine alter the level of NMDA-R1 mRNA in the rat brain: an in situ hybridization study. Pol J Pharmacol. 2002;54:1–9. [PubMed] [Google Scholar]
- Parry MJ, Alakoskela JM, Khandelia H, Kumar SA, Jaattela M, Mahalka AK, Kinnunen PK. High-affinity small molecule-phospholipid complex formation: binding of siramesine to phosphatidic acid. J Am Chem Soc. 2008;130:12953–12960. doi: 10.1021/ja800516w. [DOI] [PubMed] [Google Scholar]
- Piomelli D, Di Marzo V. Dopamine D2 receptor signaling via the arachidonic acid cascade: modulation by cAMP-dependent protein kinase A and prostaglandin E2. J Lipid Mediat. 1993;6:433–443. [PubMed] [Google Scholar]
- Purdon D, Arai T, Rapoport S. No evidence for direct incorporation of esterified palmitic acid from plasma into brain lipids of awake adult rat. J Lipid Res. 1997;38:526–530. [PubMed] [Google Scholar]
- Raclot T. Selective mobilization of fatty acids from adipose tissue triacylglycerols. Prog Lipid Res. 2003;42:257–288. doi: 10.1016/s0163-7827(02)00066-8. [DOI] [PubMed] [Google Scholar]
- Ramadan E, Basselin M, Taha AY, Cheon Y, Chang L, Chen M, Rapoport SI. Chronic valproate treatment blocks D2-like receptor-mediated brain signaling via arachidonic acid in rats. Neuropharmacology. 2011;61:1256–1264. doi: 10.1016/j.neuropharm.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao JS, Harry GJ, Rapoport SI, Kim HW. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry. 2010;15:384–392. doi: 10.1038/mp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao JS, Kellom M, Reese EA, Rapoport SI, Kim HW. Dysregulated glutamate and dopamine transporters in postmortem frontal cortex from bipolar and schizophrenic patients. Journal of affective disorders. 2012;136:63–71. doi: 10.1016/j.jad.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rapoport SI. Brain arachidonic and docosahexaenoic acid cascades are selectively altered by drugs, diet and disease. Prostaglandins Leukot Essent Fatty Acids. 2008;79:153–156. doi: 10.1016/j.plefa.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport SI, Basselin M, Kim HW, Rao JS. Bipolar disorder and mechanisms of action of mood stabilizers. Brain Res Rev. 2009;61:185–209. doi: 10.1016/j.brainresrev.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport SI, Bosetti F. Do lithium and anticonvulsants target the brain arachidonic acid cascade in bipolar disorder? Arch Gen Psychiatry. 2002;59:592–506. doi: 10.1001/archpsyc.59.7.592. [DOI] [PubMed] [Google Scholar]
- Robinson PJ, Noronha J, DeGeorge JJ, Freed LM, Nariai T, Rapoport SI. A quantitative method for measuring regional in vivo fatty-acid incorporation into and turnover within brain phospholipids: review and critical analysis. Brain Res Brain Res Rev. 1992;17:187–214. doi: 10.1016/0165-0173(92)90016-f. [DOI] [PubMed] [Google Scholar]
- Scherk H, Pajonk FG, Leucht S. Second-generation antipsychotic agents in the treatment of acute mania: a systematic review and meta-analysis of randomized controlled trials. Arch Gen Psychiatry. 2007;64:442–455. doi: 10.1001/archpsyc.64.4.442. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Wolfe LS. Arachidonic acid cascade and signal transduction. J Neurochem. 1990;55:1–15. doi: 10.1111/j.1471-4159.1990.tb08813.x. [DOI] [PubMed] [Google Scholar]
- Shimshoni JA, Basselin M, Li LO, Coleman RA, Rapoport SI, Modi HR. Valproate uncompetitively inhibits arachidonic acid acylation by rat acyl-CoA synthetase 4: relevance to valproate’s efficacy against bipolar disorder. Biochim Biophys Acta. 2011;1811:163–169. doi: 10.1016/j.bbalip.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri S, Seeman MV, Negrete JC, et al. Increased dopamine D2 receptor binding after long-term treatment with antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl) 2000;152:174–180. doi: 10.1007/s002130000532. [DOI] [PubMed] [Google Scholar]
- Skipski VP, Barclay M, Reichman ES, Good JJ. Separation of acidic phospholipids by one-dimensional thin-layer chromatography. Biochim Biophys Acta. 1967;137:80–89. doi: 10.1016/0005-2760(67)90010-0. [DOI] [PubMed] [Google Scholar]
- Skipski VP, Good JJ, Barclay M, Reggio RB. Quantitative analysis of simple lipid classes by thin-layer chromatography. Biochim Biophys Acta. 1968;152:10–19. doi: 10.1016/0005-2760(68)90003-9. [DOI] [PubMed] [Google Scholar]
- Soderlund T, Jutila A, Kinnunen PK. Binding of adriamycin to liposomes as a probe for membrane lateral organization. Biophys J. 1999;76:896–907. doi: 10.1016/S0006-3495(99)77253-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun GY, MacQuarrie RA. Deacylation-reacylation of arachidonoyl groups in cerebral phospholipids. Ann N Y Acad Sci. 1989;559:37–55. doi: 10.1111/j.1749-6632.1989.tb22597.x. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Florijn WJ, Creese I. Differential regulation of dopamine receptors after chronic typical and atypical antipsychotic drug treatment. Neuroscience. 1997;78:985–996. doi: 10.1016/s0306-4522(96)00631-8. [DOI] [PubMed] [Google Scholar]
- Thambisetty M, Gallardo KA, Liow JS, et al. The utility of (11)C-arachidonate PET to study in vivo dopaminergic neurotransmission in humans. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32:676–684. doi: 10.1038/jcbfm.2011.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulipano G, Rizzetti C, Bianchi I, Fanzani A, Spano P, Cocchi D. Clozapine-induced alteration of glucose homeostasis in the rat: the contribution of hypothalamic-pituitary-adrenal axis activation. Neuroendocrinology. 2007;85:61–70. doi: 10.1159/000100981. [DOI] [PubMed] [Google Scholar]
- Vidarsdottir S, de Leeuw van Weenen JE, Frolich M, Roelfsema F, Romijn JA, Pijl H. Effects of olanzapine and haloperidol on the metabolic status of healthy men. J Clin Endocrinol Metab. 2010;95:118–125. doi: 10.1210/jc.2008-1815. [DOI] [PubMed] [Google Scholar]
- Washizaki K, Smith QR, Rapoport SI, Purdon AD. Brain arachidonic acid incorporation and precursor pool specific activity during intravenous infusion of unesterified [3H]arachidonate in the anesthetized rat. J Neurochem. 1994;63:727–736. doi: 10.1046/j.1471-4159.1994.63020727.x. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Tohen M, Baldessarini RJ. Clozapine in severe mood disorders. J Clin Psychiatry. 1995;56:411–417. [PubMed] [Google Scholar]