Abstract
Childhood trauma is associated with smaller gray matter volume, similar to the pattern seen in psychotic disorders. We explored the relationship between childhood abuse, psychosis, and brain volume in a group of 60 individuals with a psychotic disorder and 26 healthy control subjects. We used voxel-based morphometry (VBM) to quantify gray and white matter volume and the Childhood Trauma Questionnaire (CTQ) to measure childhood abuse. Within the psychotic disorders group, total gray matter volume was inversely correlated with the severity of childhood sexual abuse (r=−.34, p=.008), but not other types of abuse. When the 24 patients with sexual abuse were compared with demographically matched samples of 23 patients without sexual abuse and 26 control subjects, only patients with a history of sexual abuse had reduced total gray matter volume (t(48) = 2.3, p = .03; Cohen’s d = .63). Voxel-based analysis revealed a cluster in the prefrontal cortex where volume was negatively correlated with sexual abuse severity. Voxel based comparison of the three matched groups revealed a similar pattern of results, with widespread reductions in psychosis patients with sexual abuse relative to controls that were not found in psychosis patients without sexual abuse. These findings indicate that some of the variance of gray matter volume in psychotic disorders can be explained by a history of sexual abuse.
Keywords: Childhood trauma, psychotic disorders, risk factors, gray matter, brain volume, Voxel-based morphometry, sexual abuse
1. Introduction
Reduced gray matter volume across multiple brain regions is a consistent finding in studies of schizophrenia and bipolar disorder (Ellison-Wright and Bullmore, 2010; Glahn et al., 2008; Hirayasu et al., 2001; Shepherd et al., 2012 ; Smieskova et al., 2010). Global gray matter loss is found in both chronic (Lim et al., 1996; Zipursky et al., 1992) and neuroleptic-naïve patients (Gur et al., 1999), with volume loss ranging from 2–6%. Despite the strong evidence for reduced gray matter volume in psychotic disorders, the etiology and time course of this process remain unclear.
The diathesis-stress model of mental illness suggests that a major life stressor such as childhood trauma could have a serious impact on the trajectory of brain development and contribute to the onset of a psychiatric disorder (Cicchetti, 1995; Mirsky and Duncan, 1986; Rosenthal, 1970). Consistent with this notion, multiple studies have found a higher prevalence of psychotic disorders in individuals who have experienced childhood abuse (Cutajar et al., 2010; Mullen et al., 1993; Spauwen et al., 2006). While abuse early in life is a major risk factor for mental illness, childhood trauma is known to impact brain structure, even in individuals without a psychiatric diagnosis (Twardosz, 2010). Specifically, childhood trauma has been linked to reduced total brain volume (De Bellis, 1999), and selective gray matter volume loss in the hippocampus (Vythilingam et al., 2002), prefrontal cortex (De Bellis, 2002), amygdala (Aas et al., 2012), and visual cortex (Tomoda et al., 2009). Interestingly, many of the brain abnormalities seen in abused individuals, in particular gray matter volume loss, are similar to those found in psychotic disorder patients (Hart and Rubia, 2012; Honea et al., 2005). This overlap led us to explore the relationship between gray matter volume, childhood trauma, and the diagnosis of a psychotic disorder.
We used voxel-based morphometry (VBM) to quantify gray and white matter volume and the Childhood Trauma Questionnaire (CTQ) to measure childhood abuse in a group of patients with a primary psychotic disorder and healthy control subjects with no history of mental illness. With this data we tested the following hypotheses: 1) psychotic disorder patients experience more childhood trauma than healthy control subjects, 2) severity of childhood abuse is negatively correlated with gray matter volume and 3) gray matter volume loss is most pronounced in psychotic disorder patients with a history of childhood abuse.
2. Methods
2.1 Subjects
Participants included 60 psychotic disorder patients (26 schizophrenia, 17 schizoaffective disorder, 17 bipolar disorder type I with psychotic features) and 26 healthy control subjects. Psychotic disorder patients were recruited from the inpatient and outpatient clinic of the Vanderbilt Psychiatric Hospital, and healthy controls were recruited via advertisements within the community. All participants completed written informed consent after approval of the study protocol by the Vanderbilt University Institutional Review Board, Nashville, Tennessee. All participants were administered the Structured Clinical Interview of the DSM-IV-TR (SCID) to confirm diagnoses in patients and rule out current or past psychiatric illness in healthy controls. An estimate of pre-morbid IQ was collected from all participants using the Wechsler Test of Adult Reading (Weschler, 2001). In addition, we assessed all patients with the Hamilton Rating Scale for Depression (HAMD), Young Mania Rating Scale (YMRS) and Positive and Negative Syndrome Scale (PANSS) (Hamilton, 1960; Kay et al., 1987; Young et al., 1978). Exclusion criteria included age less than 16 or greater than 65, estimated pre-morbid IQ less than 70, presence of a systemic medical illness (e.g. diabetes, cardiovascular disease) or central nervous system disorder (e.g. multiple sclerosis, epilepsy) that would affect study results, reported pregnancy or lactation, history of significant head trauma, psychotropic drug use (healthy subjects only), substance abuse within last three months (patients) or lifetime history of substance abuse/dependence (healthy subjects), and MRI contra-indicators (e.g. metal implants, claustrophobia) (for subject demographics, see Table 1).
Table 1.
Demographic and Clinical Characteristics of Participantsa
| Healthy Control Subjects (n = 26) |
All Patients (n = 60) |
Sub-group: patients with sexual abuse (n = 24) |
Sub-group: patients without sexual abuse (n = 23) |
|
|---|---|---|---|---|
| Sex (male/female) | 13/13 | 32/28 | 8/16 | 11/12 |
| Race | 13/12/1 | 40/18/2 | 18/5/1 | 12/10/1 |
| (White/Black/Other) | ||||
| Age | 38.2 (10.8) | 35.5 (13.3) | 41.7 (11.9) | 36.3 (13.5) |
| Participant's Education, | 15.3 (2.0) | 13.0 (2.4) | 12.8 (2.3) | 12.6 (2.5) |
| Yearsb | ||||
| Parental Education, | 13.4 (1.9) | 13.5 (2.6) | 13.1 (2.6) | 13.1 (2.7) |
| Years | ||||
| Pre-morbid IQ, WTARc | 103.7 (13.6) | 98.1 (16.9) | 94.7 (16.7) | 95.8 (17.5) |
| HAM-D | - | 13.4 (10.7) | 16.4 (9.9) | 12.3 (12.6) |
| YMRS | - | 9.0 (8.0) | 8.6 (8.1) | 9.2 (8.9) |
| PANSS Total | - | 63.4 (15.4) | 60.7 (13.9) | 66.5 (17.5) |
HAM-D, Hamilton Depression Rating Scale; PANSS, Positive and Negative Syndrome Scale; WTAR, Wechsler Test of Adult Reading; YMRS, Young Mania Rating Scale
Mean (SD) unless indicated otherwise
Significantly different between Healthy Controls and All Psychotic Disorder Patients, p<.001
Significantly different between Healthy Controls and psychosis patients with sexual abuse, p<.05
2.2 Measures
2.2.1 Childhood Trauma Questionnaire (CTQ)
All participants completed the short version of the Childhood Trauma Questionnaires (CTQ, (Bernstein et al., 1997)), a self-report questionnaire that measures the experience and severity of five different types of childhood trauma: physical abuse, sexual abuse, emotional abuse, emotional neglect, and physical neglect (Bernstein et al., 2003). Each category of trauma is assessed with five questions (e.g. someone tried to touch me in a sexual way or tried to make me touch them) for which the subject had to select a level of frequency: never true, rarely true, sometimes true, often true, or very often true. These responses were then coded on a 5-point Likert scale. Total CTQ scores range from 25–125, with each individual abuse sub-scale ranging from 5–25 and higher scores indicating more severe abuse. The validity and reliability of the CTQ has been verified in independent studies (Paivio and Cramer, 2004; Scher et al., 2001; Wright et al., 2001). Additionally, the CTQ has been previously used to assess childhood trauma in psychotic disorder patients (Compton et al., 2004; Holowka et al., 2003; Schafer et al., 2006) and prior research has demonstrated the validity of self-report measures in individuals with serious mental illness (Goldberg et al., 2002; Meyer et al., 1996; Niv et al., 2007) and of retrospective reports of abuse in patients with psychosis (Dill et al., 1991; Fisher et al., 2011). Individuals were defined as “abused” for each abuse sub-type if their score corresponded with the lowest threshold for “Moderate-Severe Abuse” as defined by the CTQ manual (for rates of abuse in both groups, see Table 2).
Table 2.
Number of participants who reported each type of abuse on the CTQ
| Abuse Sub-Scalea | Healthy Control Subjects n = 26 |
Psychotic Disorder Patients n = 60 |
|---|---|---|
| Physical Abuse | 3 | 22 |
| Sexual Abuse | 1 | 18 |
| Emotional Abuse | 1 | 33 |
| Emotional Neglect | 3 | 36 |
| Physical Neglect | 1 | 16 |
Abuse was defined as a score of ≥ 10 for physical abuse; ≥ 8 for sexual abuse; ≥ 13 for emotional abuse; ≥ 15 for emotional neglect; ≥ 10 for physical neglect
2.2.2 MRI Acquisition
Imaging data for all participants was collected on a 3T Philips Intera Achieva scanner located at the Vanderbilt University Institute of Imaging Science (VUIIS). We acquired a high-resolution T1-weighted fast field echo (FFE) structural scan (170 sagittal slices, matrix = 256×256, 1.0 mm isovoxel resolution, TR/TE = 8.0/3.7 ms) on each subject. Foam padding was used to stabilize the head, and earplugs and headphones were provided for each subject to minimize scanner noise. Each subjects’ anatomical T1-weighted image was visually inspected and images with obvious artifact related to movement (i.e. ringing) or significant signal inhomogeneity were not included in the analysis.
2.2.3 Voxel-based Morphometry
T1-weighted structural brain images were pre-processed and quantitatively analyzed using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/download/) for SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Following bias-correction, the T1-weighted images were segmented and affine (i.e. linear) normalized to MNI space. Following linear normalization, tissue class images were non-linearly normalized using the high-dimensional DARTEL algorithm to predefined templates provided with the VBM8 toolbox. The gray matter tissue class images were then modulated using the non-linear components derived from the high-dimensional DARTEL normalization step, thereby preserving the absolute amount of tissue after correcting for variation in individual brain sizes. The non-linear modulated DARTEL normalized gray matter images were smoothed with an 8 mm kernel and used in the subsequent voxel-based statistical analyses described below. In addition to the non-linear modulated gray matter images derived high-dimensional DARTEL normalization procedure, the native space tissue class images were also output from the VBM8 toolbox from which total gray, white, CSF, and intracranial volume (i.e. GM+WM+CSF) was calculated.
2.3 Statistical Analysis
2.3.1 Childhood Trauma and Brain Volume: Global Effects
The relationship between childhood trauma and brain volumes was analyzed with a partial correlation controlling for age and gender, testing the hypothesis that severity of childhood abuse is correlated with total gray matter volume. A repeated measures analysis of variance (ANOVA) was then used to compare average brain volume between three demographically-similar groups of subjects: psychotic disorder patients who were sexually abused (n = 24; sexual abuse score ≥ 8), psychotic disorder patients who were not sexually abused (n = 23; sexual abuse score ≤ 7), and healthy control subjects (n = 26). Main effects and interactions were tested with planned one-way ANOVAs and independent-samples t-tests.
2.3.2 Childhood Trauma and Brain Structure: Regional Effects
Follow-up voxel-based analyses were performed for all types of abuse that were significantly correlated with global gray matter volume. To examine the relationship between regional brain volumes and childhood trauma in patients with psychosis, the modulated gray matter images derived from VBM8 were entered into a voxel-wise multiple regression analysis with severity of abuse entered as a predictor, and age and gender entered as nuisance covariates. The effect of abuse was examined across the whole brain and in 3 a priori regions of interest (ROIs) based on previous studies: hippocampus, amygdala, and prefrontal cortex. The hippocampus and amygdala masks were taken from the WFU pickatlas (Version 3.0 (Maldjian et al., 2003)). The prefrontal cortex mask was created from the LONI probabilistic atlas of cortical brain structures (Shattuck et al., 2008) and consisted of the superior, middle, and inferior frontal gyri, the middle and lateral orbitofrontal gyri, the gyrus rectus, and the anterior cingulate gyrus (Woodward, 2012).
2.3.3 Childhood Trauma and Brain Structure: Diagnosis Effects
Total gray matter volume was compared between groups using a one-way ANOVA with 3 levels: psychosis with abuse, psychosis without abuse, and control and post-hoc pair-wise t tests. A series of voxel-based t-tests were used to compare regional differences between groups. For voxel-based analyses a threshold adjustment method based on Monte-Carlo simulations with our whole brain and ROI masks (AlphaSim, http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf) was used to protect against Type I errors. Using a voxel p-value of 0.001 for the whole brain, a minimum cluster size of 136 voxels provides a corrected family wise error rate of α = 0.05. For ROI analyses, the following cluster sizes using voxel a p-value of 0.05 provide a corrected family wise error rate of α = 0.05: hippocampus k = 384, amygdala k = 219, pre-frontal cortex k = 231.
3. Results
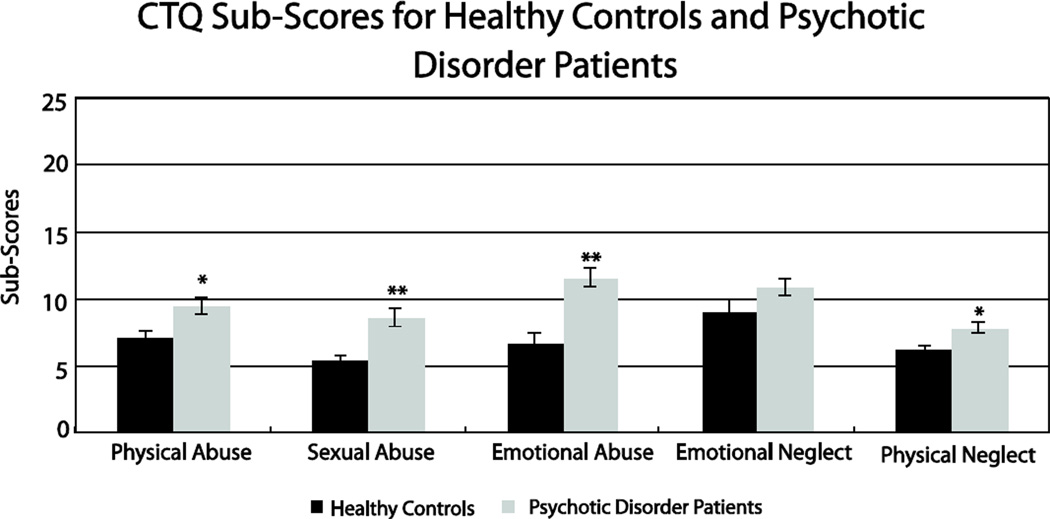
Psychotic disorder patients reported significantly more childhood abuse than healthy control subjects for overall abuse (F(1, 84) = 13.2, p<.001) and all five sub-scales of the CTQ, with the exclusion of emotional neglect which trended in the same direction (physical abuse: F(1, 84) = 5.3, p = .024; sexual abuse: F(1, 84) = 9.4, p = .003; emotional abuse: F(1, 84) = 17.0, p<.001; emotional neglect: F(1, 84) = 2.9, p = .09; physical neglect: F(1, 84) = 5.5, p = .02); Figure 1).
Figure 1.
Psychotic disorder patients reported significantly more childhood abuse than healthy control subjects for all five sub-scales of the Childhood Trauma Questionnaire (CTQ), except for emotional neglect. Independent samples t-tests revealed differences of p< .03 for all other subscales, as indicated by *p<.05, **p<.01.
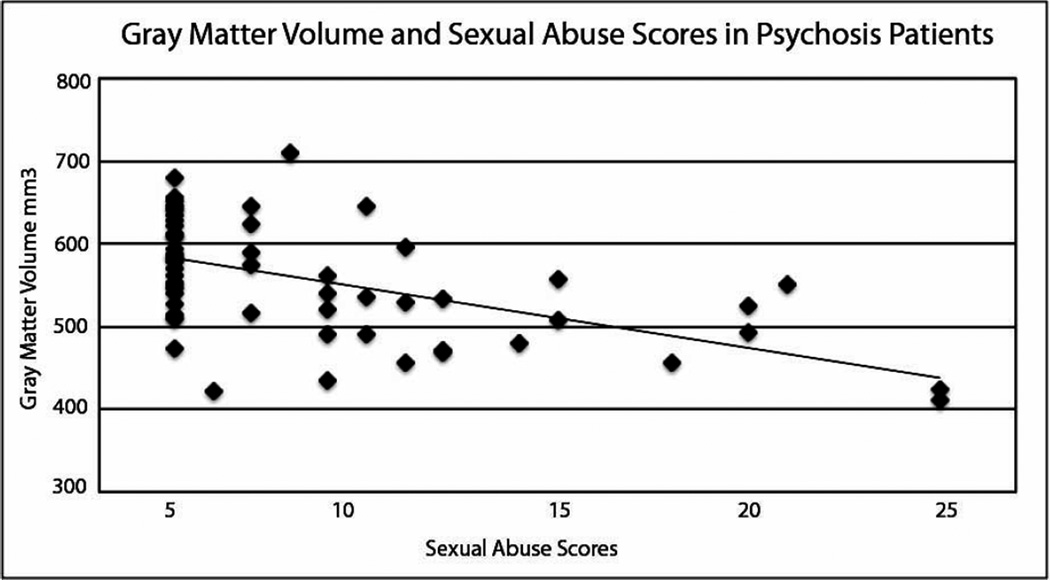
Within the psychotic disorder sample, there was a negative correlation between total CTQ score and gray matter volume (r = −.27, p = .04), but no significant correlation between CTQ score and white matter (r = −.02, p = .87) or CSF volume (r = −.19, p = .15), controlling for age and gender. To test if this relationship was driven by any particular CTQ subscale, similar correlation analyses were performed. We found a significant negative correlation between sexual abuse and gray matter volume, such that more severe sexual abuse was related to smaller global gray matter volume (r = −.34, p = .008; Figure 2). This relationship was specific to sexual abuse, as gray matter volume was not correlated with any other abuse sub-scale (physical abuse: r = −.12, p = .38; emotional abuse: r = −.18, p = .18; emotional neglect: r = −.16, p = .23; physical neglect: r = −.20, p = .13). No relationship was found between sexual abuse score and white matter volume (r = −.11, p = .42) or CSF volume (−.17, p = .20).
Figure 2.
CTQ sub-scale scores for childhood sexual abuse were negatively correlated with gray matter volume for all psychotic disorder patients, when controlling for age and gender (r = −.34, p = .008).
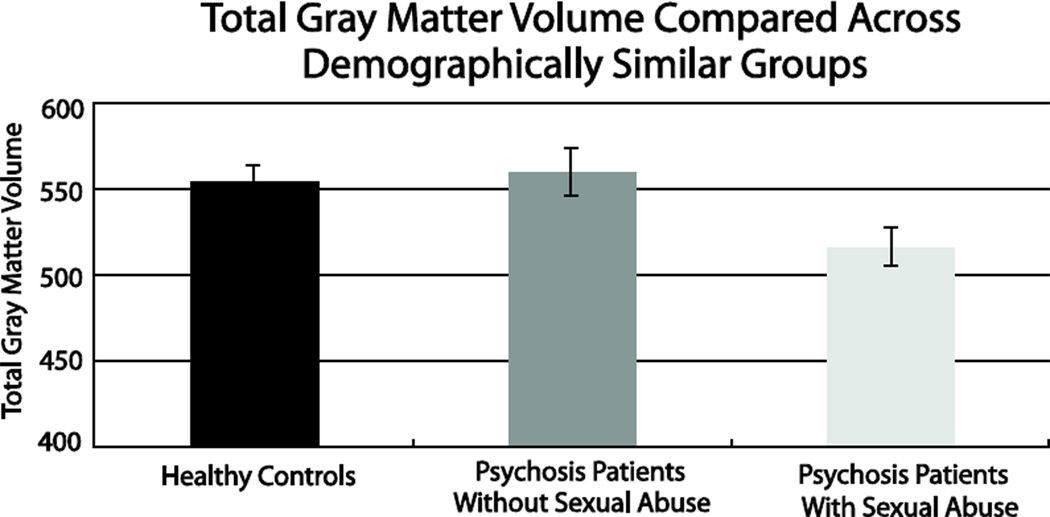
Since both a history of sexual abuse and a psychotic disorder diagnosis have been linked to gray matter volume loss, we attempted to disambiguate the two effects by comparing global gray matter volume between the 24 psychotic disorder patients with sexual abuse to demographically matched samples of psychotic patients without abuse (n = 23) and control subjects (n = 26). All three groups did not significantly differ on age, gender, race, or parental education (Table 1). A 3×3 ANOVA with group and tissue type entered as between and within subjects factors, respectively, revealed a significant group by tissue type interaction (F(4, 140) = 4.93, p = .001). Follow-up ANOVAs indicated that the interaction was due to a significant group effect for gray matter (F(2, 70) = 4.03, p = .02; Figure 3), but not white matter and CSF volumes (F(2, 70) = 1.4, p = .25; F(2, 70) = .45, p = .64, respectively). Specifically, as shown in Figure 3, psychotic patients with a history of sexual abuse had significantly less gray matter volume compared to both healthy control subjects (t(48) = 2.3, p = .03; Cohen’s d = .63) and psychotic disorder patients without a history of sexual abuse (t(45) = 2.4, p = .02; Cohen’s d = .71). Interestingly, psychotic disorder patients without a history of sexual abuse did not differ from healthy control subjects on overall gray matter volume (t(47) = .40, p = .69).
Figure 3.
In demographically matched samples, psychotic disorder patients with childhood sexual abuse (n=24) had significantly decreased gray matter volume compared to healthy controls (n=26) (t(48) = 2.3, p = .03; Cohen’s d = .63) and psychotic disorder patients without sexual abuse (n=23) (t(45) = 2.4, p = .02; Cohen’s d = .71). Psychotic disorder patients without sexual abuse did not differ in gray matter volume from healthy control subjects (t(47) = .40, p = .69).
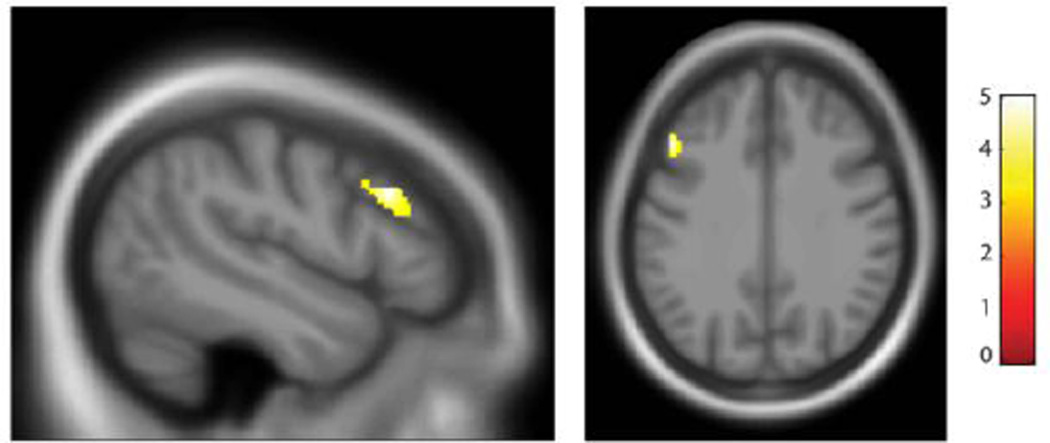
To test whether the reduced total gray matter volume associated with sexual abuse severity in psychosis patients was localized to any specific brain regions, we performed a voxel-based regression analysis using sexual abuse severity as a predictor, controlling for age and gender. At the whole brain level there was one significant cluster in the left middle frontal gyrus, which was negatively correlated with sexual abuse severity (Figure 4). This cluster fell within our a priori defined pre-frontal cortex (PFC) ROI. Sexual abuse severity was not correlated with gray matter volume in the hippocampus or the amygdala.
Figure 4.
The severity of sexual abuse was correlated negatively with gray matter volume in the left middle frontal gyrus (k = 265, -48, 23, 31). Statistical parametric maps thresholded at a p < .001 (cluster-corrected p < .05).
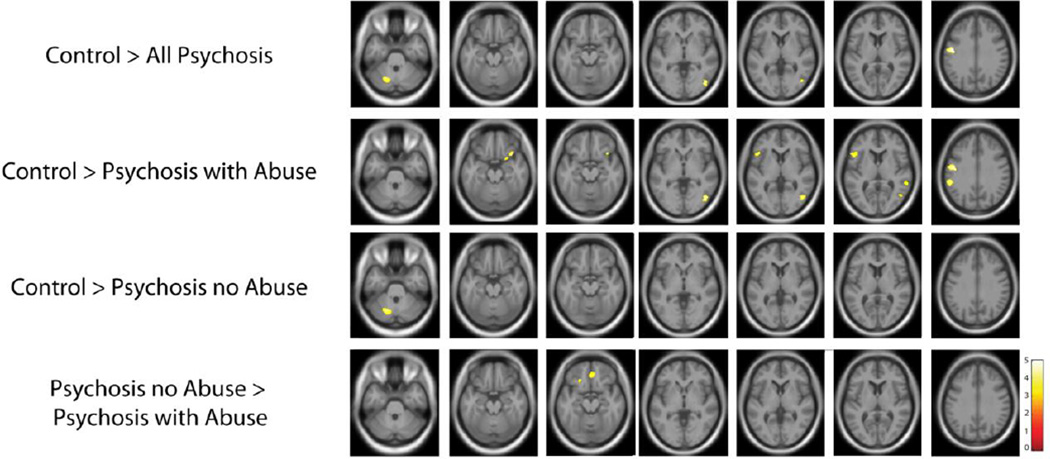
A series of t-tests was used to compare regional gray matter volume between groups (Figure 5, Table 3). Compared to controls, patients with a psychotic disorder demonstrated reduced gray matter volume in the frontal lobes, occipital lobes, and cerebellum. More widespread differences were observed between controls and psychosis patients with a history of sexual abuse, with clusters of gray matter decreases in the frontal, parietal, temporal and occipital lobes in the patient group. In contrast, psychotic patients without a history of abuse had gray matter decreases in only a single cluster in the left cerebellum relative to healthy controls. Comparing the two patient groups revealed reduced gray matter volume in bilateral frontal regions in psychotic patients with a history of sexual abuse. However, at a less stringent threshold, we detected more widely distributed changes in gray matter volume in both psychosis groups (Supplemental Figure 1)
Figure 5.
Gray matter volume loss in psychotic disorder patients with and without a history of sexual abuse. Compared to healthy subjects, psychotic disorder patients demonstrated gray matter volume loss in regions consistently implicated in the disorder, including the frontal lobe, occipital lobe, and the cerebellum. Gray matter volume loss was more pronounced in psychotic disorder patients with a history of sexual abuse, with reductions across the frontal, parietal, and temporal lobes. In contrast, gray matter volume loss in psychotic disorder patients without a history of sexual abuse was significant only in the cerebellum. Comparing the two patient groups revealed bilateral frontal regions with decreased gray matter volume in patients with a history of abuse. Statistical parametric maps thresholded at a p < .001 (cluster-corrected p < .05).
Table 3.
Significant clusters for group comparison
| Hemisphere | No. of Voxels | Z-Score | MNI Peak Voxel | ||
|---|---|---|---|---|---|
| Control > All Psychosis | |||||
| Pre-central Gyrus | Left | 583 | 4.53 | −50, −10, 30 | |
| Cerebellum | Left | 164 | 4.10 | 44, −73, −2 | |
| Inferior Occipital Gyrus | Right | 272 | 4.02 | −20, −66, −33 | |
| Control > Psychosis with Abuse | |||||
| Medial Frontal Gyrus | Left | 881 | 4.48 | −11, 14, 52 | |
| Inferior Temporal Gyrus | Right | 344 | 4.27 | 45, −72, −2 | |
| Precentral Gyrus | Left | 605 | 4.27 | −50, −10, 28 | |
| Inferior Parietal Lobule | Left | 397 | 4.42 | −57, −39, 28 | |
| Inferior Frontal Gyrus | Right | 229 | 3.9 | 36, 18, −20 | |
| Left | 290 | 3.68 | −50, 18, 4 | ||
| Superior Temporal Gyrus | Right | 158 | 3.53 | 60, −42, 7 | |
| Control > Psychosis No Abuse | |||||
| Cerebellum | Left | 348 | 3.91 | −20, −67, −32 | |
| Psychosis No Abuse > | |||||
| Psychosis with Abuse | |||||
| Anterior Cingulate Cortex | Bilateral | 559 | 3.84 | 3, 41, −14 | |
| Inferior Frontal Gyrus | Left | 269 | 3.45 | −18, 24, −18 | |
4. Discussion
In a sample of psychotic disorder patients we found a significant negative correlation between total gray matter volume and severity of childhood sexual abuse, similar to a previous study of healthy subjects (Dannlowski et al., 2012). Psychotic patients with a history of sexual abuse had significantly smaller total gray matter volume than both healthy control subjects and psychotic disorder patients who were not sexually abused, suggesting that a history of sexual abuse contributes to the well-known reduction in overall gray matter volume in psychotic disorder patients (Young et al., 1978).
In addition to these differences in total gray matter volume, we found a pattern of frontal lobe, occipital lobe and cerebellum gray matter reduction in the psychotic disorder patients, consistent with previous studies (Honea et al., 2008). When comparing each psychotic disorder patient group to healthy controls, patients with a history of sexual abuse exhibited a more widespread pattern of gray matter volume reduction, consistent with previous findings of individuals who experienced abuse (Treadway et al., 2009). A direct comparison between the two psychosis groups showed that psychotic disorder patients with sexual abuse had significantly reduced gray matter volume in bilateral prefrontal cortex (PFC), an area previously found to be reduced in individuals who have experienced trauma (Carrion et al., 2001). Importantly, this area covaried linearly with severity of abuse, providing further evidence that the observed gray matter loss is related to the experience of abuse. These data suggest that a specific environmental stressor, such as childhood sexual abuse, may contribute to findings of gray matter reduction often found in psychotic disorder patients (Glahn et al., 2008; Lim et al., 1996).
Relative to healthy controls, psychotic disorder patients without a history of sexual abuse showed significantly reduced gray matter volume only in the cerebellum. The cerebellum has been implicated as a region of neural dysfunction in schizophrenia, which contributes to cognitive impairments via disrupted monitoring of mental events within the context of time (for review, see (Andreasen and Pierson, 2008)). Additionally, other studies have found reduced cerebellar volume in schizophrenia (Ichimiya et al., 2001; Loeber et al., 2001), providing further evidence that this structural difference is likely due to the diagnosis of a psychotic disorder.
In contrast, psychotic disorder patients who experienced sexual abuse displayed a global reduction in gray matter volume, with widespread regions showing significant reduction compared to healthy controls. The pattern of gray matter reduction in this comparison is similar to many previous studies of gray matter volume loss in psychosis (Asami et al., 2012; Bora et al., 2011; Shepherd et al., 2012; Zipursky et al., 1992), and our data point to sexual abuse as a possible mechanism for this decrease in gray matter volume. Multiple theories attempt to explain the link between childhood maltreatment and abnormal neural development (for review see Glaser (Glaser, 2000)). Most focus on an abnormal stress response mediated by the hypothalamic-pituitary-adrenal (HPA) axis (for review, see Lupien (Lupien et al., 2009)). Increased levels of stress hormones have been linked to reductions in brain volume in abused individuals and cortisol levels have been found to correlate with duration of abuse (De Bellis, 1999). Studies on early stress or trauma have found gray matter reductions in the hippocampus (Bremner et al., 1997) and amygdala (Weniger et al., 2009), two structures that are thought to be directly involved in the HPA-axis (for review, see (Herman et al., 2005)). However, we did not find significant reductions in hippocampal or amygdala gray matter volume related to sexual abuse severity in the psychotic disorder subjects. While this was unexpected, previous studies of post-traumatic stress disorder (PTSD) have failed to find reduced hippocampal volume in patients who have experienced trauma (Bonne et al., 2001; De Bellis, 2002), and a recent study of psychosis patients also failed to replicate hippocampal volume reduction associated with childhood trauma (Aas et al., 2012). It is possible that brain changes associated with psychosis, which include volume reduction of hippocampus and amygdala (Heckers and Konradi, 2010), render the hippocampus less likely to exhibit the changes seen in healthy subjects after trauma (Hoy et al., 2011).
We did, however, find that bilateral reduction of frontal cortex gray matter volume in psychotic disorders is associated with sexual abuse, supporting previous findings of selective reductions in the frontal cortex following acute stress (De Bellis, 2002). The PFC is an important component of the HPA-axis, namely in response to stress and in regulating negative feedback (for review, see (Sullivan and Gratton, 2002)). There is also evidence for reduced activation in the prefrontal cortex to fearful faces in patients with PTSD, indicating both structural and functional abnormalities in this region related to trauma (Shin et al., 2005). Together with our findings of total and widespread regional gray matter volume reduction in psychotic disorder patients with sexual abuse, this suggests that the PFC is particularly vulnerable to the stress of childhood abuse.
It is difficult to disambiguate the effects of childhood trauma on brain development from the abnormal brain development associated with psychotic disorders (Borgwardt et al., 2010). Based on the results of this study, we suggest that this is an important distinction to make. While we found smaller gray matter volume in several brain regions in all psychosis patients, regardless of abuse history, the experience of abuse appears to be an additional risk factor for gray matter volume loss.
The relatively small sample sizes, especially after dividing patients on the basis of abuse history, results in less statistical power and is a limitation of the study. For example, at the more liberal uncorrected statistical threshold, patients with psychosis demonstrated widespread gray matter volume loss in regions consistently implicated in VBM studies of psychosis, including insula, superior temporal gyrus, and thalamus (Bora et al., 2011; Shepherd et al., 2012). This was true for both psychosis groups, regardless of abuse history. Consequently, while our data clearly indicate that childhood abuse is associated with distinct effects on brain structure in psychotic disorder subjects, it is also apparent that there is a consistent pattern of grey matter volume loss in psychotic disorder subjects with and without a history of childhood abuse. Studies with larger samples sizes will be required to comprehensively disentangle the effects of childhood abuse on brain morphology from those associated with psychosis.
In addition, the patients who were sexually abused differed significantly on many demographic variables from patients who were not sexually abused, again a well-known pattern (Finkelhor et al., 1990; Polusny, 1995). Despite these constraints, we were able to disambiguate the effects of trauma and diagnosis by comparing three demographically matched sub-groups to isolate a selective effect of sexual abuse on gray matter volume in psychotic disorder patients.
Our results show that classifying individuals by diagnosis alone may not be enough to uncover brain abnormalities in psychotic disorders. Instead, it may be necessary to explore environmental risk factors, such as childhood trauma, in order to better understand individual differences in brain morphology, particularly gray matter loss. Since psychotic disorder patients report more abuse than healthy controls, stratification of patient samples by trauma history appears to be a fruitful approach for future studies of brain structure and function in psychosis.
Supplementary Material
Acknowledgements
Role of Funding Source
The R01 MH70560 granted to Stephan Heckers provided funding for enrollment of subjects into a study protocol that included an MRI, full SCID interview, administration of the SCIP, CTQ and other psychiatric rating scales.
Supported by NIMH grant R01-MH70560 (Dr. Heckers), the Vanderbilt Psychiatric Genotype/Phenotype Project, and the Vanderbilt Institute for Clinical and Translational Research (through grant 1-UL-1-RR024975 from the National Center for Research Resources/NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
JMS, NDW, LEW and SH conceptualized the study. NDW and LEW set up and performed all VBM analyses. JMS and LEW performed all data analyses. JMS wrote the original draft of the manuscript and prepared the manuscript. NDW, LEW and SH edited the manuscript and helped finalize all analyses.
Conflict of Interest
None
References
- Aas M, Navari S, Gibbs A, Mondelli V, Fisher HL, Morgan C, Morgan K, MacCabe J, Reichenberg A, Zanelli J, Fearon P, Jones PB, Murray RM, Pariante CM, Dazzan P. Is there a link between childhood trauma, cognition, and amygdala and hippocampus volume in firstepisode psychosis? Schizophrenia research. 2012;137(1-3):73–79. doi: 10.1016/j.schres.2012.01.035. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biological psychiatry. 2008;64(2):81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asami T, Bouix S, Whitford TJ, Shenton ME, Salisbury DF, McCarley RW. Longitudinal loss of gray matter volume in patients with first-episode schizophrenia: DARTEL automated analysis and ROI validation. NeuroImage. 2012;59(2):986–996. doi: 10.1016/j.neuroimage.2011.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Ahluvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. J Am Acad Child Adolesc Psychiatry. 1997;36(3):340–348. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27(2):169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Bonne O, Brandes D, Gilboa A, Gomori JM, Shenton ME, Pitman RK, Shalev AY. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. The American journal of psychiatry. 2001;158(8):1248–1251. doi: 10.1176/appi.ajp.158.8.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ, Yucel M, Velakoulis D, Pantelis C. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and metaregression analysis. Schizophrenia research. 2011;127(1-3):46–57. doi: 10.1016/j.schres.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Borgwardt SJ, Picchioni MM, Ettinger U, Toulopoulou T, Murray R, McGuire PK. Regional gray matter volume in monozygotic twins concordant and discordant for schizophrenia. Biological psychiatry. 2010;67(10):956–964. doi: 10.1016/j.biopsych.2009.10.026. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis RB, Charney DS. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse--a preliminary report. Biological psychiatry. 1997;41(1):23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, Reiss AL. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biological psychiatry. 2001;50(12):943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Lynch M. Failures in the expectable environment and their impact on individual development: The case of child maltreatment. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology: Risk, disorder, and adaptation. New York: Wiley; 1995. pp. 32–71. [Google Scholar]
- Compton MT, Furman AC, Kaslow NJ. Preliminary evidence of an association between childhood abuse and cannabis dependence among African American first-episode schizophreniaspectrum disorder patients. Drug Alcohol Depend. 2004;76(3):311–316. doi: 10.1016/j.drugalcdep.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Cutajar MC, Mullen PE, Ogloff JR, Thomas SD, Wells DL, Spataro J. Schizophrenia and other psychotic disorders in a cohort of sexually abused children. Archives of general psychiatry. 2010;67(11):1114–1119. doi: 10.1001/archgenpsychiatry.2010.147. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D, Domschke K, Hohoff C, Ohrmann P, Bauer J, Lindner C, Postert C, Konrad C, Arolt V, Heindel W, Suslow T, Kugel H. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological psychiatry. 2012;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, Frustaci K, Ryan ND. Developmental traumatology Part II: Brain development. Biological psychiatry. 1999;45(10):1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, Moritz G. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biological psychiatry. 2002;52(11):1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- Dill DL, Chu JA, Grob MC, Eisen SV. The reliability of abuse history reports: a comparison of two inquiry formats. Compr Psychiatry. 1991;32(2):166–169. doi: 10.1016/0010-440x(91)90009-2. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophrenia research. 2010;117(1):1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Finkelhor D, Hotaling G, Lewis IA, Smith C. Sexual abuse in a national survey of adult men and women: prevalence, characteristics, and risk factors. Child Abuse Negl. 1990;14(1):19–28. doi: 10.1016/0145-2134(90)90077-7. [DOI] [PubMed] [Google Scholar]
- Fisher HL, Craig TK, Fearon P, Morgan K, Dazzan P, Lappin J, Hutchinson G, Doody GA, Jones PB, McGuffin P, Murray RM, Leff J, Morgan C. Reliability and comparability of psychosis patients' retrospective reports of childhood abuse. Schizophr Bull. 2011;37(3):546–553. doi: 10.1093/schbul/sbp103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, Bullmore E, Fox PT. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biological psychiatry. 2008;64(9):774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser D. Child abuse and neglect and the brain--a review. J Child Psychol Psychiatry. 2000;41(1):97–116. [PubMed] [Google Scholar]
- Goldberg RW, Seybolt DC, Lehman A. Reliable self-report of health service use by individuals with serious mental illness. Psychiatr Serv. 2002;53(7):879–881. doi: 10.1176/appi.ps.53.7.879. [DOI] [PubMed] [Google Scholar]
- Gur RE, Turetsky BI, Bilker WB, Gur RC. Reduced gray matter volume in schizophrenia. Archives of general psychiatry. 1999;56(10):905–911. doi: 10.1001/archpsyc.56.10.905. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Rubia K. Neuroimaging of child abuse: a critical review. Front Hum Neurosci. 2012;6:52. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Konradi C. Hippocampal pathology in schizophrenia. Current topics in behavioral neurosciences. 2010;4:529–553. doi: 10.1007/7854_2010_43. [DOI] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(8):1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hirayasu Y, Tanaka S, Shenton ME, Salisbury DF, DeSantis MA, Levitt JJ, Wible C, Yurgelun- Todd D, Kikinis R, Jolesz FA, McCarley RW. Prefrontal gray matter volume reduction in first episode schizophrenia. Cereb Cortex. 2001;11(4):374–381. doi: 10.1093/cercor/11.4.374. [DOI] [PubMed] [Google Scholar]
- Holowka DW, King S, Saheb D, Pukall M, Brunet A. Childhood abuse and dissociative symptoms in adult schizophrenia. Schizophrenia research. 2003;60(1):87–90. doi: 10.1016/s0920-9964(02)00296-7. [DOI] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. The American journal of psychiatry. 2005;162(12):2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Honea RA, Meyer-Lindenberg A, Hobbs KB, Pezawas L, Mattay VS, Egan MF, Verchinski B, Passingham RE, Weinberger DR, Callicott JH. Is gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biological psychiatry. 2008;63(5):465–474. doi: 10.1016/j.biopsych.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy K, Barrett S, Shannon C, Campbell C, Watson D, Rushe T, Shevlin M, Bai F, Cooper S, Mulholland C. Childhood Trauma and Hippocampal and Amygdalar Volumes in First-Episode Psychosis. Schizophr Bull. 2011 doi: 10.1093/schbul/sbr085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimiya T, Okubo Y, Suhara T, Sudo Y. Reduced volume of the cerebellar vermis in neuroleptic-naive schizophrenia. Biological psychiatry. 2001;49(1):20–27. doi: 10.1016/s0006-3223(00)01081-7. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Lim KO, Sullivan EV, Zipursky RB, Pfefferbaum A. Cortical gray matter volume deficits in schizophrenia: a replication. Schizophrenia research. 1996;20(1-2):157–164. doi: 10.1016/0920-9964(95)00081-x. [DOI] [PubMed] [Google Scholar]
- Loeber RT, Cintron CM, Yurgelun-Todd DA. Morphometry of individual cerebellar lobules in schizophrenia. The American journal of psychiatry. 2001;158(6):952–954. doi: 10.1176/appi.ajp.158.6.952. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Meyer IH, Muenzenmaier K, Cancienne J, Struening E. Reliability and validity of a measure of sexual and physical abuse histories among women with serious mental illness. Child Abuse Negl. 1996;20(3):213–219. doi: 10.1016/s0145-2134(95)00137-9. [DOI] [PubMed] [Google Scholar]
- Mirsky AF, Duncan CC. Etiology and expression of schizophrenia: neurobiological and psychosocial factors. Annu Rev Psychol. 1986;37:291–319. doi: 10.1146/annurev.ps.37.020186.001451. [DOI] [PubMed] [Google Scholar]
- Mullen PE, Martin JL, Anderson JC, Romans SE, Herbison GP. Childhood sexual abuse and mental health in adult life. Br J Psychiatry. 1993;163:721–732. doi: 10.1192/bjp.163.6.721. [DOI] [PubMed] [Google Scholar]
- Niv N, Cohen AN, Mintz J, Ventura J, Young AS. The validity of using patient self-report to assess psychotic symptoms in schizophrenia. Schizophrenia research. 2007;90(1-3):245–250. doi: 10.1016/j.schres.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Paivio SC, Cramer KM. Factor structure and reliability of the Childhood Trauma Questionnaire in a Canadian undergraduate student sample. Child Abuse Negl. 2004;28(8):889–904. doi: 10.1016/j.chiabu.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Polusny MA, Foullette VM. Long-term correlates of child sexual abuse: Theory and review of the empirical literature. Applied & Preventive Psychology. 1995;4:143–166. [Google Scholar]
- Rosenthal D. Genetic Theory and Abnormal Behavior. New York: McGraw-Hill; 1970. [Google Scholar]
- Schafer I, Harfst T, Aderhold V, Briken P, Lehmann M, Moritz S, Read J, Naber D. Childhood trauma and dissociation in female patients with schizophrenia spectrum disorders: an exploratory study. J Nerv Ment Dis. 2006;194(2):135–138. doi: 10.1097/01.nmd.0000198199.57512.84. [DOI] [PubMed] [Google Scholar]
- Scher CD, Stein MB, Asmundson GJ, McCreary DR, Forde DR. The childhood trauma questionnaire in a community sample: psychometric properties and normative data. J Trauma Stress. 2001;14(4):843–857. doi: 10.1023/A:1013058625719. [DOI] [PubMed] [Google Scholar]
- Shattuck DW, Mirza M, Adisetiyo V, Hojatkashani C, Salamon G, Narr KL, Poldrack RA, Bilder RM, Toga AW. Construction of a 3D probabilistic atlas of human cortical structures. NeuroImage. 2008;39(3):1064–1080. doi: 10.1016/j.neuroimage.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev. 2012;36(4):1342–1356. doi: 10.1016/j.neubiorev.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of general psychiatry. 2005;62(3):273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Smieskova R, Fusar-Poli P, Allen P, Bendfeldt K, Stieglitz RD, Drewe J, Radue EW, McGuire PK, Riecher-Rossler A, Borgwardt SJ. Neuroimaging predictors of transition to psychosis--a systematic review and meta-analysis. Neurosci Biobehav Rev. 2010;34(8):1207–1222. doi: 10.1016/j.neubiorev.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Spauwen J, Krabbendam L, Lieb R, Wittchen HU, van Os J. Impact of psychological trauma on the development of psychotic symptoms: relationship with psychosis proneness. Br J Psychiatry. 2006;188:527–533. doi: 10.1192/bjp.bp.105.011346. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: side matters. Psychoneuroendocrinology. 2002;27(1-2):99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- Tomoda A, Navalta CP, Polcari A, Sadato N, Teicher MH. Childhood sexual abuse is associated with reduced gray matter volume in visual cortex of young women. Biological psychiatry. 2009;66(7):642–648. doi: 10.1016/j.biopsych.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Grant MM, Ding Z, Hollon SD, Gore JC, Shelton RC. Early adverse events, HPA activity and rostral anterior cingulate volume in MDD. PLoS One. 2009;4(3):e4887. doi: 10.1371/journal.pone.0004887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twardosz S, Lutzker JR. Child maltreatment and the developing brain: A review of neuroscience perspectives. Aggression and Violent Behavior. 2010;15:59–68. [Google Scholar]
- Vythilingam M, Heim C, Newport J, Miller AH, Anderson E, Bronen R, Brummer M, Staib L, Vermetten E, Charney DS, Nemeroff CB, Bremner JD. Childhood trauma associated with smaller hippocampal volume in women with major depression. The American journal of psychiatry. 2002;159(12):2072–2080. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weniger G, Lange C, Sachsse U, Irle E. Reduced amygdala and hippocampus size in traumaexposed women with borderline personality disorder and without posttraumatic stress disorder. J Psychiatry Neurosci. 2009;34(5):383–388. [PMC free article] [PubMed] [Google Scholar]
- Weschler D. Weschler Test of Adult Reading (WTAR) San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. American J Psychiatry. 2012;169(10):1092–1099. doi: 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright KD, Asmundson GJ, McCreary DR, Scher C, Hami S, Stein MB. Factorial validity of the Childhood Trauma Questionnaire in men and women. Depress Anxiety. 2001;13(4):179–183. [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Zipursky RB, Lim KO, Sullivan EV, Brown BW, Pfefferbaum A. Widespread cerebral gray matter volume deficits in schizophrenia. Archives of general psychiatry. 1992;49(3):195–205. doi: 10.1001/archpsyc.1992.01820030027004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.