Abstract
Growth of the plasma membrane is as fundamental to cell reproduction as DNA replication, chromosome segregation and ribosome biogenesis, yet little is known about the underlying mechanisms. Membrane growth during the cell cycle requires mechanisms that control the initiation, location, and extent of membrane growth, as well as mechanisms that coordinate membrane growth with cell cycle progression. Recent experiments have established links between membrane growth and core cell cycle regulators. Further analysis of these links will yield insights into conserved and fundamental mechanisms of cell growth. A better understanding of the post-Golgi pathways by which membrane growth occurs will be essential for future progress.
Introduction
Intensive analysis of the secretory pathway over the last three decades has led to extraordinary advances in our understanding of the underlying molecular mechanisms. Yet the secretory pathway is also responsible for growth of the plasma membrane. Indeed, it is likely that the most ancient and conserved function of the secretory pathway is to transport lipids and proteins for membrane growth. Yet surprisingly little is known about membrane growth. A PubMed search for the term “secretory pathway” yields thousands of articles. In contrast, a search for “membrane growth” or “membrane addition” yields only a handful. One reason for this disparity is technical: it is much easier to assay secretion than it is to assay membrane growth. This is especially true for vertebrate cells grown in culture, which do not have a uniform shape that lends itself to rapid measurements of membrane growth. Yet there are many interesting and important unanswered questions regarding membrane growth. Here, we consider key unanswered questions and recent progress regarding plasma membrane growth. We focus on membrane growth that occurs during the cell cycle. In addition, we focus on mechanisms that act at the level of membrane trafficking. Others have recently reviewed mechanisms by which the cytoskeleton is polarized to direct membrane growth to specific locations [1,2].
Where and when does membrane growth occur during the cell cycle?
In budding yeast cells, the location of membrane growth is regulated during the cell cycle [3,4]. In early G1, the membrane of the unbudded mother cell undergoes isotropic growth, which occurs uniformly over the cell surface. In late G1, growth becomes polarized to initiate formation of a daughter cell. Growth of the daughter cell remains polarized until early mitosis, when mitotic Cdk1 induces a switch back to isotropic growth [5]. At the end of mitosis, membrane growth is polarized to the site of cytokinesis to drive addition of membrane necessary to complete cell separation. Thus, membrane growth occurs throughout the cell cycle and the location of growth is regulated.
Membrane growth also occurs throughout much of the cell cycle in animal cells. For example, in mouse lymphoblasts an initial rapid burst of growth occurs in G1 followed by constant exponential growth until the beginning of cell division [6]. At least in some situations, membrane growth can also occur during mitosis. For example, in syncytial Drosophila embryos, membrane furrows form during mitosis to surround metaphase spindles. Formation of these furrows is driven by membrane growth that occurs during prophase and metaphase [7,8]. Little is known about the location of plasma membrane growth during the cell cycle in animal cells. Does membrane growth occur uniformly and randomly over the cell surface, or are there specialized sites of growth? In epithelial cells undergoing polarization, membrane growth appears to occur at adhesion sites, but it is unknown whether growth that occurs during the cell cycle also occurs at adhesion sites [9,10].
Studies of membrane growth are limited by a lack of good assays for measuring the location and extent of membrane growth. In cells that are nearly spherical, such as budding yeast and lympoblasts, overall membrane growth can be accurately measured using a variety of techniques [6,11,12]. However, these approaches cannot be used for cells with more complex shapes. There are few methods for analyzing the extent of membrane growth at specific locations. In yeast, growing cells can be coated with a fluorescent lectin that binds to the cell wall [3,13]. After washing away the lectin, zones of cell wall growth can be seen as unlabelled areas of the cell wall. This assay assumes that cell wall growth reflects underlying membrane growth, which may not be the case.
What is the role of endocytosis in membrane growth?
The surface area of the plasma membrane is determined by the relative rates of exocytosis and endocytosis. Rates of endocytosis can be very high - it has been estimated that in non-dividing vertebrate cells endocytosis can internalize the equivalent of the entire plasma membrane in 1 hour [14]. In budding yeast, sites of polar membrane growth are surrounded by endocytic sites, which indicates that endocytosis is constantly occurring during plasma membrane growth [4,15,16]. This is likely to be necessary for recycling of components needed for membrane growth and may also play a critical role in maintaining polarized sites of growth [17]. Together, these examples illustrate that net plasma membrane growth requires precise coordination of exocytosis and endocytosis. In this sense, the plasma membrane is similar to membrane trafficking organelles, such as the Golgi and the endoplasmic reticulum, which must precisely balance membrane coming in and membrane going out to maintain their size. Little is known about how the rates and location of endocytosis are controlled to achieve net plasma membrane growth.
What is the relationship between secretion and membrane growth?
Secretion and membrane growth are both mediated by vesicle trafficking to the plasma membrane. To what extent do these processes overlap? A classic genetic screen in budding yeast identified key components of the secretory pathway and virtually all were found to be required for polarized bud growth, which indicates that there must be considerable overlap [18]. However, the screen was designed to identify mutants that cause defects in secretion of periplasmic enzymes, so it may have missed gene products that mediate membrane growth independently of secretion.
Understanding the relationship between secretion and membrane growth is made difficult by the fact that membrane traffic from the Golgi to the plasma membrane is poorly understood. Traffic from the Golgi can be mediated by classical vesicles, but the molecular composition and diversity of these vesicles is not well-understood. In addition, there is good evidence that transport from the Golgi can also occur via maturation of trans Golgi stacks into diverse tubular carriers [1,19,20]. The relative contributions of vesicles or tubular carriers to growth of the plasma membrane during the cell cycle are not known.
In budding yeast, at least three classes of vesicles are transported from the Golgi to the plasma membrane. One class reaches the plasma membrane via an endosomal compartment, requiring clathrin and the dynamin-related GTPase Vps1 for their generation [21,22]. These vesicles transport secreted periplasmic enzymes such as invertase, and can be distinguished by density from a second class of vesicles that transport the cell wall-degrading enzyme Bgl2 and play a role in membrane growth [23]. A third class transports enzymes needed for cell wall synthesis and is associated with a putative coat protein complex called exomer [24,25].
Key questions regarding these three classes of vesicles remain unanswered. Are Bgl2-containing vesicles responsible for membrane growth? What are the coat proteins that drive formation of vesicles that contain Bgl2? Do exomer-coated vesicles play a role in membrane growth? How is formation of each class of vesicle controlled? An interesting experiment would be to block formation of specific classes of vesicles and observe the effects on polar and isotropic membrane growth during the cell cycle. However, blocking one trafficking pathway can cause cargoes to be redirected to another pathway [22]. Another difficulty is that there may be additional classes of vesicles that play a role in membrane growth. Newly developed methods for tracking specific classes of vesicles in a synchronous manner may help define the contributions of each of the known classes of vesicles [26].
At least some classes of post-Golgi vesicles destined for sites of cell growth in yeast are associated with a multi-protein complex called the exocyst, which plays a role in docking vesicles at the plasma membrane [27]. The exocyst is localized to sites of membrane growth during polar bud growth and during cytokinesis [28]. Proteins called Sec3 and Exo70 appear to serve as docking sites for the exocyst at these locations [28–31]. The exocyst is required for membrane growth that occurs during polar bud growth and cytokinesis, but it is unknown whether it is also required for the isotropic membrane growth that occurs before bud emergence and after the switch from polar to isotropic growth [27,32]. Loss of Exo70 causes defects in membrane growth primarily in early stages of bud growth, and it causes accumulation of Bgl2-containing vesicles, but not invertase containing vesicles [33]. Similar defects are caused by mutations in the GTPase Cdc42, which interacts with Exo70 [33–35]. Thus, there is some evidence for mechanisms that target distinct classes of vesicles to distinct locations during different stages of membrane growth.
Little is known about the nature of post-Golgi vesicles or tubular carriers that mediate plasma membrane growth during the cell cycle in vertebrate cells. The exocyst is conserved in vertebrate cells and is associated with sites of polar membrane growth in non-dividing cells. For example, during polarization of non-dividing epithelial cells in culture, the exocyst is localized to adherens junctions, which may be sites of apical membrane growth that drive cell shape changes [3,9,10,36,37]. The exocyst is also required for late stages of cytokinesis in animal cells [38,39]. There is no evidence that the exocyst is involved in non-polar growth or growth during the cell cycle. It is thus possible that the exocyst facilitates polar membrane growth in both yeast and vertebrates, and that non-polar growth is mediated by different mechanisms.
Given our poor understanding of the molecular mechanisms of post-Golgi traffic, it is entirely possible that distinct classes of vesicles, each with their own unique targeting signals and cargos, mediate general secretion and membrane growth. This is an important question because the existence of distinct classes of vesicles that mediate specific modes of membrane growth would suggest that key aspects of membrane growth are controlled at the level of vesicle formation. An alternative model is that there is a constant flux of identical or similar vesicles to the cell surface, and membrane growth is regulated by modulating the rate of endocytosis.
What are the signals that govern initiation of membrane growth?
In budding yeast, different modes of membrane growth must be initiated at different times during the cell cycle. For example, consider a yeast cell arrested in G1 due to nutrient starvation. When the cell is shifted to rich nutrient conditions, a signaling pathway originating from nutrients will initiate isotropic membrane growth. Once the cell reaches a critical size, another signaling pathway will trigger entry into the cell cycle, leading to initiation of polarized growth of a daughter cell. Additional signals will initiate a switch from polar to isotropic membrane growth. What are the signals that initiate specific kinds of membrane growth at different times during the cell cycle? Also, once membrane growth has been initiated, how are transitions between different modes of membrane growth controlled? At one extreme, one could imagine that initiation of membrane growth generates a steady stream of identical vesicles containing components for membrane growth, and these vesicles are targeted to different growth sites during the cell cycle solely via cytoskeletal rearrangements that track the vesicles to specific locations. At another extreme, each mode of membrane growth could involve generation of a class of vesicles with distinct cargoes and sorting signals that help direct them to the correct location. In non-dividing cells, such as polarized epithelial cells, vesicles with distinct sorting signals are targeted to different locations in the cell, which demonstrates that the capability exists to generate distinct classes of vesicles [40].
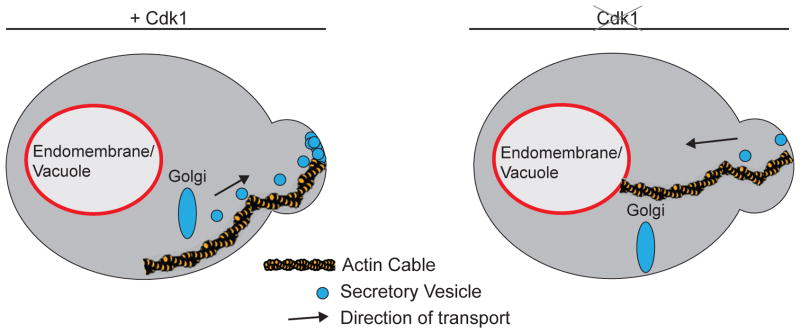
Classic genetic analysis showed that inactivation of temperature sensitive alleles of Cdk1, or inactivation of G1 cyclins that activate Cdk1, leads to a failure to initiate polar bud growth [41,42]. More recent analysis discovered that specific inhibition of Cdk1 after bud emergence causes rapid cessation of polar growth [11]. Thus, Cdk1 is required for both initiation and maintenance of polar growth. How does Cdk1 contribute to polar growth? At least part of the story is that Cdk1 polarizes the actin cytoskeleton to direct membrane traffic to the site of polar growth [5,11,41,43]. However, recent studies suggest that Cdk1 may also control membrane trafficking events that play a role in membrane growth (Figure 1). In these studies, it was discovered that although inhibition of Cdk1 blocks membrane growth, it does not lead to cytoplasmic accumulation of vesicles [15]. This is in contrast to the effects of mutants that depolarize the actin cytoskeleton or mutants that block vesicle fusion at growth sites, which cause extensive accumulation of vesicles [15,18,44,45]. In vivo tracking of post-Golgi vesicles after inhibition of Cdk1 suggested that vesicles may be inappropriately transported to the vacuole, although it was not possible to rule out other fates [15]. Inhibition of Cdk1 did not appear to cause defects in trafficking of Bgl2 or invertase, nor did it cause defects in targeting of GFP-Sec4 to the site of cytokinesis. Together, these observations suggest that Cdk1 controls membrane trafficking events that mediate membrane growth. It could do this by initiating events that mark a special class of vesicles for delivery to the site of membrane growth. In the absence of this mark, the vesicles could be delivered to the vacuole via a default pathway. The mechanisms by which Cdk1 controls membrane traffic are unknown, although a proteomic screen for targets of Cdk1 identified numerous proteins involved in membrane traffic [46]. Little is known about the signals that initiate the isotropic membrane growth that occurs before bud emergence in G1 phase, which are likely to be Cdk1-independent.
Figure 1.
Cdk1 is required for normal membrane trafficking dynamics during bud growth in budding yeast. In normal cells, a steady stream of vesicles can be seen moving along actin filaments into the growing daughter bud (left panel). Inhibition of an analog-sensitive allele of CDK1 causes rapid cessation of bud growth and movement of vesicles away from the bud.
Withdrawal of glucose from growing yeast cells causes rapid cessation of both membrane traffic and polar membrane growth ([47] and D. Kellogg, unpublished data). Thus, membrane growth is regulated by nutrient availability. Glucose depletion results in a rapid drop in intracellular pH. Recently, the protonation state of phosphatidic acid was shown to regulate its interaction with a transcription factor involved in control of phospholipid-responsive genes. These observations suggest a model in which a drop in cytosolic pH after glucose depletion shuts down a metabolic pathway involved in membrane biogenesis [48] It seems likely that cells also require mechanisms that can modulate the rate of membrane growth to ensure that membrane growth is coordinated with overall cell growth when growth is slowed by limited nutrient availability.
What factors govern the extent of membrane growth?
In budding yeast, isotropic growth of the mother cell in G1 is terminated when polar bud growth begins. Similarly, polar membrane growth is terminated when the switch to isotropic growth occurs. How are these transitions controlled? This is an interesting question because the timing of the transitions could determine the extent of membrane growth at specific locations, which could influence cell size and shape.
Recent analysis in budding yeast suggested a mechanism that could control the extent of membrane growth. The analysis focused on the switch from polar to isotropic membrane growth. This switch is induced by Cdk1 in early mitosis, so the mechanism that determines the timing of mitotic Cdk1 activation also determines the extent of polar membrane growth [5,49]. Analysis of signals that control the timing of Cdk1 activation revealed a link between polar membrane growth and Cdk1 activation. Taken together, these and other studies led to a growth-dependent signaling hypothesis for how the extent of polar growth is controlled. In this hypothesis, a signal is generated at the site of polar membrane growth that is proportional to the extent of growth [50]. Downstream mechanisms then read the strength of the signal and trigger activation of Cdk1 when it reaches a threshold level.
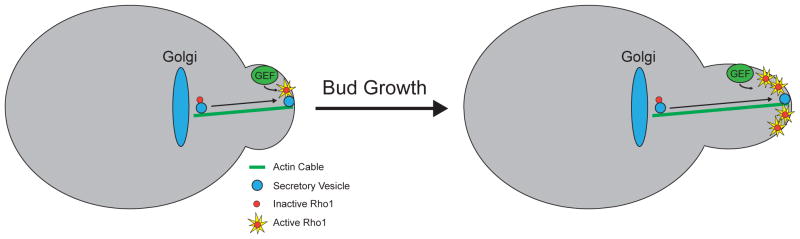
A growth-dependent signal could be generated by delivery of the Rho1 GTPase to the site of membrane growth (Figure 2). Rho1 is delivered by vesicles that mediate membrane growth and is delivered in an inactive form [51]. Rho1 may be kept inactive on vesicles by vesicle-associated GTPase activating proteins [52]. Rho1 becomes activated at the growth site by a guanine nucleotide exchange factor that is localized independently of membrane traffic [51]. Thus, as more and more vesicles arrive at the growth site the strength of the Rho1 signal could increase proportionally, thereby generating a signal that is proportional to the extent of membrane growth. Active Rho1 binds an atypical protein kinase C called Pkc1 and can induce it to undergo autophosphorylation [53]. Phosphorylation of Pkc1 is dependent upon and proportional to membrane growth, consistent with the idea that it relays a growth-dependent signal [50]. Pkc1 activates a form of protein phosphatase 2A (PP2ACdc55) that coordinately controls Wee1 and Cdc25, which control entry into mitosis via Cdk1 inhibitory phosphorylation [50,54–56]. Rho1, Pkc1 and PP2ACdc55 are localized at the site of membrane growth and physically interact [52,53,57–62]. Inactivation of Rho1 or Pkc1 causes cell lysis, which may reflect a defect in normal control of membrane growth.
Figure 2.
A mechanism that could generate a signal that is dependent upon and proportional to the extent of polar membrane growt in budding yeast. The Rho1 GTPase is delivered to the site of membrane growth on vesicles. The Rho1 on vesicles is inactive and it becomes activated by a guanine nucleotide exchange factor when vesicles fuse with the plasma membrane at the site of polar growth. As more and more vesicles arrive, the Rho1 signal could increase in strength in a manner that is proportional to the number of vesicles that fuse with the membrane.
Growth-dependent signaling is attractive because it could determine the extent of membrane growth at specific locations, thereby controlling both cell size and shape. Ribosome biogenesis is linked to membrane growth via Pkc1, so it is possible that diverse aspects of cell growth are linked to membrane growth [63,64]. Key proteins in the pathway are conserved and it appears that the hypothesized mechanism could be readily adapted to cells of diverse size and shape. However, there are major gaps in our understanding of the pathway and it is unknown whether a system thus constituted has the mechanistic capacity to generate a signal that is proportional to growth and flip a switch when the signal reaches a threshold level.
What are the mechanisms that control membrane growth during mitosis and cytokinesis?
Vertebrate cells undergo dramatic changes in architecture as they enter mitosis, often rounding up from an extended flat shape. These global changes require extensive membrane reorganization that are linked to cell cycle progression. The Golgi fragments during mitosis and there is a concomitant cessation of secretion, while fluid-phase endocytic rates do not appear to diminish [65]. However, a drastic reduction in recycling back to the plasma membrane has been reported in mitotic cells compared to interphase cells [65]. Reduced membrane recycling in mitotic cells creates an internal pool of plasma membrane-derived lipid, accounting for the reduced surface area of round mitotic cells versus spread interphase cells. Recycling to the plasma membrane resumes during anaphase and telophase, facilitating membrane growth required for cytokinesis [65]. During cytokinesis, cleavage furrow ingression bisects the segregated chromosomes, enabling the two daughter cells to physically separate during abscission. In eukaryotes, local membrane addition is required for cytokinesis, either for furrow ingression or for abscission [66]. As in polarized membrane growth, the exocyst plays an important role in localized membrane delivery during cytokinesis in many organisms including yeast, flies and humans [32,38,39,67]. The conserved role of the exocyst underscores its importance in regulating membrane growth during diverse biological processes.
Conclusions
The most basic questions regarding plasma membrane growth during the cell cycle remain unanswered. What are the signals that control membrane growth? Also, what are the targets of these signals? It is difficult to imagine genetic screens that could specifically identify membrane trafficking components that mediate membrane growth. A more fruitful approach may be to follow signals known to control membrane growth to identify their targets, which may be key membrane trafficking proteins involved in the formation or targeting of vesicles responsible for membrane growth. Proteins that mediate endocytosis may also be targets of these pathways. Recent work has established that Cdk1 and nutrients send signals that control membrane growth, so these are good starting points and proteome-wide mass spectrometry could identify the targets of these signals that control membrane growth [11,15,47]. There may be considerable rewards in discovery of mechanisms of membrane growth. For example, identification of signals or membrane trafficking mechanisms that specifically control membrane growth could represent an entirely new class of targets for drugs aimed at blocking the proliferation or migration of cancer cells.
Highlights.
Growth of the plasma membrane during the cell cycle is essential for cell reproduction.
Initiation, location, and extent of membrane growth must be precisely controlled.
Recent experiments have linked membrane growth to core cell cycle regulators.
Analysis of these links will yield insights into fundamental mechanisms of cell growth.
Understanding post-Golgi pathways of membrane growth will be essential for future progress.
Acknowledgments
We thank Ben Glick and Wei Guo for critical reading of the manunscript and helpful suggestions. Space constraints limited the number of citations; we apologize to those whose work we were unable to cite. DK is funded by National Institutes of Health Grant GM053959-10. DM is funded by FP7 Marie Curie Grant IRG249298/Growth and Division, Agence Nationale de la Recherche Grant 2010 JCJC 1210 01, Fondation pour la Recherche Medicale Grant INE20100518678, the Centre National de la Recherche Scientifique, Université de Bordeaux 2 and Conseil Régional d’Aquitaine Volet Recherche 20091301015.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anitei M, Hoflack B. Exit from the trans-Golgi network: from molecules to mechanisms. Curr Opin Cell Biol. 2011;23:443–451. doi: 10.1016/j.ceb.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Bi E, Park HO. Cell polarization and cytokinesis in budding yeast. Genetics. 2012;191:347–387. doi: 10.1534/genetics.111.132886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tkacz JS, Lampen JO. Wall replication in saccharomyces species: use of fluorescein-conjugated concanavalin A to reveal the site of mannan insertion. J Gen Microbiol. 1972;72:243–247. doi: 10.1099/00221287-72-2-243. [DOI] [PubMed] [Google Scholar]
- 4.Adams AEM, Pringle JR. Relationship of actin and tubulin distribution in wild-type and morphogenetic mutant Saccharomyces cerevisiae. J Cell Biol. 1984;98:934–945. doi: 10.1083/jcb.98.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *5.Lew DJ, Reed SI. Morphogenesis in the yeast cell cycle: regulation by Cdc28 and cyclins. J Cell Biol. 1993;120:1305–1320. doi: 10.1083/jcb.120.6.1305. An early study that established a role for Cdk’s in controlling the pattern of cell growth, which suggests that Cdk’s also control the pattern of membrane growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tzur A, Kafri R, LeBleu VS, Lahav G, Kirschner MW. Cell growth and size homeostasis in proliferating animal cells. Science. 2009;325:167–171. doi: 10.1126/science.1174294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *7.Lecuit T, Wieschaus E. Polarized insertion of new membrane from a cytoplasmic reservoir during cleavage of the Drosophila embryo. J Cell Biol. 2000;150:849–860. doi: 10.1083/jcb.150.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *8.Sisson JC, Field C, Ventura R, Royou A, Sullivan W. Lava lamp, a novel peripheral golgi protein, is required for Drosophila melanogaster cellularization. J Cell Biol. 2000;151:905–918. doi: 10.1083/jcb.151.4.905. Formation of furrows that surround nuclei in the Drosophila syncytial embryo is a form of membrane growth. These two papers show that membrane growth during furrow formation can occur during mitosis and likely occurs via post-Golgi trafficking mechanisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Scheller RH, Nelson WJ. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell. 1998;93:731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- 10.Yeaman C, Grindstaff KK, Nelson WJ. Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells. J Cell Sci. 2004;117:559–570. doi: 10.1242/jcs.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCusker D, Denison C, Anderson S, Egelhofer TA, Yates JR, 3rd, Gygi SP, Kellogg DR. Cdk1 coordinates cell-surface growth with the cell cycle. Nat Cell Biol. 2007;9:506–515. doi: 10.1038/ncb1568. An adenine analog sensitive version of Cdk1 was used to show that inhibition of Cdk1 leads to rapid cessation of bud growth, suggesting that Cdk1controls membrane growth. [DOI] [PubMed] [Google Scholar]
- 12.Ferrezuelo F, Colomina N, Palmisano A, Gari E, Gallego C, Csikasz-Nagy A, Aldea M. The critical size is set at a single-cell level by growth rate to attain homeostasis and adaptation. Nat Commun. 2012;3:1012. doi: 10.1038/ncomms2015. [DOI] [PubMed] [Google Scholar]
- 13.Nern A, Arkowitz RA. G proteins mediate changes in cell shape by stabilizing the axis of polarity. Mol Cell. 2000;5:853–864. doi: 10.1016/s1097-2765(00)80325-1. [DOI] [PubMed] [Google Scholar]
- 14.Hansen SH, Sandvig K, van Deurs B. Internalization efficiency of the transferrin receptor. Exp Cell Res. 1992;199:19–28. doi: 10.1016/0014-4827(92)90457-j. [DOI] [PubMed] [Google Scholar]
- 15.McCusker D, Royou A, Velours C, Kellogg D. Cdk1-dependent control of membrane trafficking dynamics. Mol Biol Cell. 2012 doi: 10.1091/mbc.E11-10-0834. Previous work suggested that the primary role of Cdk1 in membrane growth was to polarize the cytoskeleton for delivery of new membrane to the site of membrane growth. In this study, analysis of membrane traffic after Cdk1 inhibition in budding yeast suggests that Cdk1 may directly modulate membrane trafficking events involved in membrane growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Layton AT, Savage NS, Howell AS, Carroll SY, Drubin DG, Lew DJ. Modeling vesicle traffic reveals unexpected consequences for Cdc42p-mediated polarity establishment. Curr Biol. 2011;21:184–194. doi: 10.1016/j.cub.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marco E, Wedlich-Soldner R, Li R, Altschuler SJ, Wu LF. Endocytosis optimizes the dynamic localization of membrane proteins that regulate cortical polarity. Cell. 2007;129:411–422. doi: 10.1016/j.cell.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novick P, Field C, Schekman R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell. 1980;21:205–215. doi: 10.1016/0092-8674(80)90128-2. [DOI] [PubMed] [Google Scholar]
- 19.De Matteis MA, Luini A. Exiting the Golgi complex. Nat Rev Mol Cell Biol. 2008;9:273–284. doi: 10.1038/nrm2378. [DOI] [PubMed] [Google Scholar]
- 20.Polishchuk RS, Polishchuk EV, Marra P, Alberti S, Buccione R, Luini A, Mironov AA. Correlative light-electron microscopy reveals the tubular-saccular ultrastructure of carriers operating between Golgi apparatus and plasma membrane. J Cell Biol. 2000;148:45–58. doi: 10.1083/jcb.148.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurunathan S, David D, Gerst JE. Dynamin and clathrin are required for the biogenesis of a distinct class of secretory vesicles in yeast. EMBO J. 2002;21:602–614. doi: 10.1093/emboj/21.4.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harsay E, Schekman R. A subset of yeast vacuolar protein sorting mutants is blocked in one branch of the exocytic pathway. J Cell Biol. 2002;156:271–285. doi: 10.1083/jcb.200109077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harsay E, Bretscher A. Parallel secretory pathways to the cell surface in yeast. J Cell Biol. 1995;131:297–310. doi: 10.1083/jcb.131.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barfield RM, Fromme JC, Schekman R. The exomer coat complex transports Fus1p to the plasma membrane via a novel plasma membrane sorting signal in yeast. Mol Biol Cell. 2009;20:4985–4996. doi: 10.1091/mbc.E09-04-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang CW, Hamamoto S, Orci L, Schekman R. Exomer A coat complex for transport of select membrane proteins from the trans-Golgi network to the plasma membrane in yeast. J Cell Biol. 2006;174:973–983. doi: 10.1083/jcb.200605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boncompain G, Divoux S, Gareil N, de Forges H, Lescure A, Latreche L, Mercanti V, Jollivet F, Raposo G, Perez F. Synchronization of secretory protein traffic in populations of cells. Nat Methods. 2012;9:493–498. doi: 10.1038/nmeth.1928. [DOI] [PubMed] [Google Scholar]
- 27.TerBush DR, Maurice T, Roth D, Novick P. The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. Embo J. 1996;15:6483–6494. [PMC free article] [PubMed] [Google Scholar]
- 28.Finger FP, Hughes TE, Novick P. Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell. 1998;92:559–571. doi: 10.1016/s0092-8674(00)80948-4. [DOI] [PubMed] [Google Scholar]
- 29.Boyd C, Hughes T, Pypaert M, Novick P. Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p. J Cell Biol. 2004;167:889–901. doi: 10.1083/jcb.200408124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He B, Xi F, Zhang X, Zhang J, Guo W. Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. Embo J. 2007;26:4053–4065. doi: 10.1038/sj.emboj.7601834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Orlando K, He B, Xi F, Zhang J, Zajac A, Guo W. Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J Cell Biol. 2008;180:145–158. doi: 10.1083/jcb.200704128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dobbelaere J, Barral Y. Spatial coordination of cytokinetic events by compartmentalization of the cell cortex. Science. 2004;305:393–396. doi: 10.1126/science.1099892. [DOI] [PubMed] [Google Scholar]
- 33.He B, Xi F, Zhang J, TerBush D, Zhang X, Guo W. Exo70p mediates the secretion of specific exocytic vesicles at early stages of the cell cycle for polarized cell growth. J Cell Biol. 2007;176:771–777. doi: 10.1083/jcb.200606134. Analysis of Exo70 mutants leads to the surprising conclusion that membrane growth at different stages of the cell cycle could be mediated by different membrane trafficking mechanisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adamo JE, Moskow JJ, Gladfelter AS, Viterbo D, Lew DJ, Brennwald PJ. Yeast Cdc42 functions at a late step in exocytosis, specifically during polarized growth of the emerging bud. J Cell Biol. 2001;155:581–592. doi: 10.1083/jcb.200106065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu H, Turner C, Gardner J, Temple B, Brennwald P. The Exo70 subunit of the exocyst is an effector for both Cdc42 and Rho3 function in polarized exocytosis. Mol Biol Cell. 2010;21:430–442. doi: 10.1091/mbc.E09-06-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matern HT, Yeaman C, Nelson WJ, Scheller RH. The Sec6/8 complex in mammalian cells: characterization of mammalian Sec3, subunit interactions, and expression of subunits in polarized cells. Proc Natl Acad Sci U S A. 2001;98:9648–9653. doi: 10.1073/pnas.171317898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yeaman C, Grindstaff KK, Wright JR, Nelson WJ. Sec6/8 complexes on trans-Golgi network and plasma membrane regulate late stages of exocytosis in mammalian cells. J Cell Biol. 2001;155:593–604. doi: 10.1083/jcb.200107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fielding AB, Schonteich E, Matheson J, Wilson G, Yu X, Hickson GR, Srivastava S, Baldwin SA, Prekeris R, Gould GW. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J. 2005;24:3389–3399. doi: 10.1038/sj.emboj.7600803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gromley A, Yeaman C, Rosa J, Redick S, Chen CT, Mirabelle S, Guha M, Sillibourne J, Doxsey SJ. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 40.Mellman I, Nelson WJ. Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol. 2008;9:833–845. doi: 10.1038/nrm2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Culotti J, Hartwell LH. Genetic control of the cell division cycle in yeast. 3. Seven genes controlling nuclear division. Exp Cell Res. 1971;67:389–401. doi: 10.1016/0014-4827(71)90424-1. [DOI] [PubMed] [Google Scholar]
- 42.Cross FR. Cell cycle arrest caused by CLN gene deficiency in Saccharomyces cerevisiae resembles START-I arrest and is independent of the mating-pheromone signalling pathway. Mol Cell Biol. 1990;10:6482–6490. doi: 10.1128/mcb.10.12.6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moffat J, Andrews B. Late-G1 cyclin-CDK activity is essential for control of cell morphogenesis in budding yeast. Nat Cell Biol. 2004;6:59–66. doi: 10.1038/ncb1078. [DOI] [PubMed] [Google Scholar]
- 44.Novick P, Botstein D. Phenotypic analysis of temperature-sensitive yeast actin mutants. Cell. 1985;40:405–416. doi: 10.1016/0092-8674(85)90154-0. [DOI] [PubMed] [Google Scholar]
- 45.Osman MA, Cerione RA. Iqg1p, a yeast homologue of the mammalian IQGAPs, mediates cdc42p effects on the actin cytoskeleton. J Cell Biol. 1998;142:443–455. doi: 10.1083/jcb.142.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Holt LJ, Tuch BB, Villen J, Johnson AD, Gygi SP, Morgan DO. Global analysis of Cdk1 substrate phosphorylation sites provides insights into evolution. Science. 2009;325:1682–1686. doi: 10.1126/science.1172867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levi SK, Bhattacharyya D, Strack RL, Austin JR, 2nd, Glick BS. The yeast GRASP Grh1 colocalizes with COPII and is dispensable for organizing the secretory pathway. Traffic. 2010;11:1168–1179. doi: 10.1111/j.1600-0854.2010.01089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young BP, Shin JJ, Orij R, Chao JT, Li SC, Guan XL, Khong A, Jan E, Wenk MR, Prinz WA, et al. Phosphatidic acid is a pH biosensor that links membrane biogenesis to metabolism. Science. 2010;329:1085–1088. doi: 10.1126/science.1191026. [DOI] [PubMed] [Google Scholar]
- 49.Amon A, Tyers M, Futcher B, Nasmyth K. Mechanisms that help the yeast cell cycle clock tick: G2 cyclins transcriptionally activate G2 cyclins and repress G1 cyclins. Cell. 1993;74:993–1007. doi: 10.1016/0092-8674(93)90722-3. [DOI] [PubMed] [Google Scholar]
- 50.Anastasia SD, Nguyen DL, Thai V, Meloy M, MacDonough T, Kellogg DR. A link between mitotic entry and membrane growth suggests a novel model for cell size control. J Cell Biol. 2012;197:89–104. doi: 10.1083/jcb.201108108. Analysis of signals that control entry into mitosis led to a “growth-dependent signaling” hypothesis for how the extent of polar membrane growth could be controlled in budding yeast. The hypothesis suggests that membrane growth generates a signal at the site of membrane growth that is proportional to the extent of growth. Downstream mechanisms read the strength of the signal and trigger cell cycle progression when it reaches a threshold level. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abe M, Qadota H, Hirata A, Ohya Y. Lack of GTP-bound Rho1p in secretory vesicles of Saccharomyces cerevisiae. J Cell Biol. 2003;162:85–97. doi: 10.1083/jcb.200301022. This study found that Rho1 bound to secretory vesicles is inactive and becomes activated at the site of membrane growth, which suggests a mechanism for how a signal could be generated at the site of membrane growth that is proportional to the extent of growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamochi W, Tanaka K, Nonaka H, Maeda A, Musha T, Takai Y. Growth site localization of Rho1 small GTP-binding protein and its involvement in bud formation in Saccharomyces cerevisiae. J Cell Biol. 1994;125:1077–1093. doi: 10.1083/jcb.125.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kamada Y, Qadota H, Python CP, Anraku Y, Ohya Y, Levin DE. Activation of yeast protein kinase C by Rho1 GTPase. J Biol Chem. 1996;271:9193–9196. doi: 10.1074/jbc.271.16.9193. [DOI] [PubMed] [Google Scholar]
- 54.Harvey SL, Charlet A, Haas W, Gygi SP, Kellogg DR. Cdk1-dependent regulation of the mitotic inhibitor Wee1. Cell. 2005;122:407–420. doi: 10.1016/j.cell.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 55.Harvey SL, Kellogg DR. Conservation of mechanisms controlling entry into mitosis: budding yeast wee1 delays entry into mitosis and is required for cell size control. Curr Biol. 2003;13:264–275. doi: 10.1016/s0960-9822(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 56.Pal G, Paraz MT, Kellogg DR. Regulation of Mih1/Cdc25 by protein phosphatase 2A and casein kinase 1. J Cell Biol. 2008;180:931–945. doi: 10.1083/jcb.200711014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andrews PD, Stark MJ. Dynamic, Rho1p-dependent localization of Pkc1p to sites of polarized growth. J Cell Sci. 2000;113 (Pt 15):2685–2693. doi: 10.1242/jcs.113.15.2685. [DOI] [PubMed] [Google Scholar]
- 58.Drees BL, Sundin B, Brazeau E, Caviston JP, Chen GC, Guo W, Kozminski KG, Lau MW, Moskow JJ, Tong A, et al. A protein interaction map for cell polarity development. J Cell Biol. 2001;154:549–571. doi: 10.1083/jcb.200104057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uetz P, Giot L, Cagney G, Mansfield TA, Judson RS, Knight JR, Lockshon D, Narayan V, Srinivasan M, Pochart P, et al. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature. 2000;403:623–627. doi: 10.1038/35001009. [DOI] [PubMed] [Google Scholar]
- 60.Wicky S, Tjandra H, Schieltz D, Yates J, 3rd, Kellogg DR. The Zds proteins control entry into mitosis and target protein phosphatase 2A to the Cdc25 phosphatase. Molecular biology of the cell. 2011;22:20–32. doi: 10.1091/mbc.E10-06-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yasutis K, Vignali M, Ryder M, Tameire F, Dighe SA, Fields S, Kozminski KG. Zds2p regulates Swe1p-dependent polarized cell growth in Saccharomyces cerevisiae via a novel Cdc55p interaction domain. Mol Biol Cell. 2010;21:4373–4386. doi: 10.1091/mbc.E10-04-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rossio V, Yoshida S. Spatial regulation of Cdc55-PP2A by Zds1/Zds2 controls mitotic entry and mitotic exit in budding yeast. J Cell Biol. 2011;193:445–454. doi: 10.1083/jcb.201101134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y, Moir RD, Sethy-Coraci IK, Warner JR, Willis IM. Repression of ribosome and tRNA synthesis in secretion-defective cells is signaled by a novel branch of the cell integrity pathway. Mol Cell Biol. 2000;20:3843–3851. doi: 10.1128/mcb.20.11.3843-3851.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nanduri J, Tartakoff AM. The arrest of secretion response in yeast: signaling from the secretory path to the nucleus via Wsc proteins and Pkc1p. Mol Cell. 2001;8:281–289. doi: 10.1016/s1097-2765(01)00312-4. [DOI] [PubMed] [Google Scholar]
- 65.Boucrot E, Kirchhausen T. Endosomal recycling controls plasma membrane area during mitosis. Proc Natl Acad Sci U S A. 2007;104:7939–7944. doi: 10.1073/pnas.0702511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Albertson R, Riggs B, Sullivan W. Membrane traffic: a driving force in cytokinesis. Trends Cell Biol. 2005;15:92–101. doi: 10.1016/j.tcb.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 67.Murthy M, Teodoro RO, Miller TP, Schwarz TL. Sec5, a member of the exocyst complex, mediates Drosophila embryo cellularization. Development. 2010;137:2773–2783. doi: 10.1242/dev.048330. [DOI] [PMC free article] [PubMed] [Google Scholar]