Abstract
Background
Heterogeneity in clinical outcomes may be caused by factors working at multiple levels, e.g., between groups, between subjects, or within subjects over time. A more nuanced assessment of differences in variation among schizophrenia patients and between patients and healthy comparison subjects can clarify etiology and even facilitate the identification of patient subtypes with common neuropathology and clinical course.
Methods
We compared trajectories (mean duration 3.5 years) of cognitive impairments in a sample of 201 community-dwelling schizophrenia (SCZ) patients (aged 40–100 years) with 67 healthy comparison (HC) subjects. We employed growth mixture models to discover subclasses with more homogenous between-subject variation in cognitive trajectories. Post hoc analyses determined factors associated with class membership and class-specific correlates of cognitive trajectories.
Results
Three latent classes were indicated: Class 1 (85% HC and 50% SCZ) exhibited relatively high and stable trajectories of cognition, Class 2 (15% HC and 40% SCZ) exhibited lower, modestly declining trajectories, and Class 3 (10% SCZ) exhibited lower, more rapidly declining trajectories. Within the patient group, membership in Classes 2–3 was associated with worse negative symptoms and living in a board and care facility.
Discussion
These results bridge the gap between schizophrenia studies demonstrating cognitive decline and those demonstrating stability. Moreover, a finer-grained characterization of heterogeneity in cognitive trajectories has practical implications for interventions and for case management of patients who show accelerated cognitive decline. Such a characterization requires study designs and analyses sensitive to between- and within-patient heterogeneity in outcomes.
Keywords: Late-Life Schizophrenia, Cognition, Trajectories, Heterogeneity, Growth Mixture Models
1. Introduction
Despite a century of efforts, understanding the etiology of schizophrenia has been continually complicated by the heterogeneous nature of this syndrome. Indeed, when Eugen Bleuler coined the contemporary term, he used the plural (“the schizophrenias”)(Bleuler, 1911). Identification of meaningful subtypes with greater homogeneity could facilitate efforts to identify common neuropathology. Numerous efforts have been undertaken over the past century to identify subtypes based on clinical features or endophenotypes such as neurophysiological markers, neural substrates, and other neurological “soft signs”(Jablensky, 2006), but no fully valid system has yet emerged(Carpenter, 1979). One such marker, neuropsychological dysfunction, is an important dimension of schizophrenia in that the deficits are at least partially present well before the onset of symptoms(Woodberry, 2008), and neuropsychological deficits are among the best predictors of the level of functional disability associated with this disorder(Green, 1996; Green, 2000). Trajectories reflecting more rapid cognitive decline could also be part of a larger hypothesized pattern of accelerated biological aging among some schizophrenia patients(Kirkpatrick, 2008).
Identifying variation in trajectories of cognitive impairments requires careful choice of study design and statistical analyses. For example, trajectories estimated from cross-sectional designs are confounded by age cohort effects (Thompson, 2011). Even when age cohort effects are minimal, cross-sectional data at best provide estimates of the average trajectory of a sample. An average is an accurate representation of individual trajectories only if the trajectories of all subjects in the sample are roughly parallel, a rare occurrence(Kraemer H.C., 2000). A definitive understanding of variation in cognitive trajectories thus requires longitudinal studies with years of repeated cognitive assessments. Linear mixed effects models (LMEs) have become the standard analytical methodology for assessing trajectories obtained from such longitudinal studies(Laird N.M., 1982). Yet prior employment of LMEs has resulted in a limited understanding of the heterogeneity of cognitive trajectories in late-life schizophrenia because of a reliance on certain modeling assumptions, specifically, that random effects and error terms have the same distribution for every subject in the sample. Allowing for unobserved subgroups among which random effects and error terms have different distributions could lead to a more accurate assessment of between-subject heterogeneity in cognitive trajectories.
Growth mixture models (GMMs) are a generalization of LMEs often providing more realistic estimates of heterogeneity in longitudinal trajectories(Muthen, 1999). This is accomplished by postulating the existence of latent classes, or subgroups of subjects exhibiting similarity with regard to unobserved (latent) variables that underlie the distribution of explicit or observable outcome variables. With GMMs, latent classes are defined as unobserved groups within which the random effects and error terms are normally distributed with constant mean and variance(Muthen, 1999). GMMs offer two potential advantages over LMEs: (i) GMMs enable flexible, data-driven estimates of the random effect and error distributions that can more accurately reflect observed heterogeneity; (ii) GMMs allow for classification of individual subjects into latent classes based on the largest probability of class membership. Consequently, subject-level factors can be directly assessed for association with class membership and hence with different trajectory subtypes.
We applied GMMs in this study to a longitudinal sample of 201 middle-aged and older patients with schizophrenia and 67 demographically matched healthy comparison subjects who were administered clinical and cognitive assessments an average of 3.9 times over 3.5 years (range: 1 to 16 years). We investigated whether latent classes exhibited evidence of different patterns of within-subject change and levels of variation and whether latent class membership in the patient group was predicted by clinical variables and other patient-level characteristics.
2. Methods
2.1 Subjects
The initial pool of participants included 336 clinically stable outpatients with schizophrenia (SCZ) and 67 healthy comparison (HC) subjects, aged ≥ 40 years, collected as part of participation in the University of California, San Diego Advanced Center in Innovation in Services and Interventions Research (ACISIR) on late-life psychoses. The individual studies differed in their use of clinical (chart) diagnosis versus semi-structured diagnostic interviews depending on the primary goals of each study. We have previously used data from an overlapping cohort of these subjects to report on stability of cognitive functioning in schizophrenia(Eyler-Zorrilla, 2000; Heaton, 2001; Nayak-Savla G, 2006; Palmer, 2003). This report, however, examines for the first time the distribution of within-subject variation and the impact of less restrictive modeling assumptions on the assessment of cognitive trajectories.
Criteria for inclusion included: (1) DSM-III-R or DSM-IV(1987; 1994)) diagnosis of schizophrenia or schizoaffective disorder (as established either by the patients treatment provider or via structured interview) or, for the HC group, no history of neuropsychiatric disorders as determined by clinical interview, (2) Mattis Dementia Rating Scale (DRS)(Mattis, 1973) administered on two or more occasions, and (3) no current substance abuse. Participants were also excluded if they had a comorbid diagnosis of dementia or other significant neurological conditions. Due to the goals of the original studies from which this dataset was compiled, patients with schizophrenia were community dwelling, i.e., either living independently (alone or with family or friends), or in community-based assisted living (board and care) facilities. All participants provided written informed consent for secondary analyses at the time of initial enrollment. The study was approved by the UCSD Human Subjects Protections Program.
2.2 Assessments
2.2.1 Demographic information
Demographic information such as age, years of education, gender, ethnicity, living situation, as well as clinical history including age of onset of psychosis and duration of illness was collected.
2.2.2 Cognitive ability
Cognitive ability was evaluated with the Mattis Dementia Rating Scale (DRS)(Jurica, 1991). Although originally developed for evaluating severity of dementia, the DRS has proven a reliable predictor of functional capacity in schizophrenia(Green, 2000). It was selected because it can be administered to lower functioning patients who may not tolerate lengthier neurocognitive testing. Although the DRS is comprised of several subscales, we employed just the total score (range 0–144; higher scores representing better performance) for the present analyses because of its psychometric superiority over the subscales(Smith, 1994). Furthermore, global cognitive functioning is among the best cognitive predictors of the functional impact of schizophrenia (Green, 2000), and global dementia screening scores have been typically used in the few studies that have reported an age-related decline among elderly patients with schizophrenia(Harvey, 2001).
2.2.3 Presence and severity of psychopathology
Presence and severity of psychopathology in SCZ subjects were measured with the positive and negative subscale scores of the Positive and Negative Syndrome Scale (PANSS)(Kay, 1987).
2.2.4 Antipsychotic medication use
Antipsychotic medication use was determined by calculating the Chlorpromazine Equivalent dosage (CPZE) for all antipsychotic medications, based on standardized formulas(Jeste, 982; Vahia, 2010).
2.3 Statistical analyses
We compared HC and SCZ subjects on demographic variables (age, gender, years of education, ethnicity) using t-tests for continuous variables and chi-square tests for categorical variables. Using a propensity score approach(Rosenbaum, 1983), we performed 3-1 matching of SCZ to HC subjects and then compared the matched subjects to ascertain if samples were more evenly balanced on potential confounders. Propensity scores were computed, via logistic regression, as the probability of being in the SCZ group conditional on covariates. Balancing groups on propensity scores will also tend to balance groups on the covariates used to compute the propensity scores(Rosenbaum, 1983).
We fit growth-mixture models (GMMs) to the matched longitudinal DRS trajectory data, allowing for class-specific random effects of intercepts (baseline levels) and slopes (change with age). We also allowed for class-specific error variances. Class-invariant fixed effects included baseline age, diagnostic group (HC vs. SCZ) and years of education. GMMs thus accounted for subject differences in DRS scores by age, education, and schizophrenia status, allowing for latent classes based on unexplained differences (i.e., extra-normal variation) in random intercepts and slopes. Beginning with one class, we fitted models with an increasing number of classes and selected the final model using the Deviance Information Criterion (DIC), a measure of model performance that selects for good model fit while guarding against over-fitting from inclusion of too many classes(Spiegelhalter, 2002); lower values of DIC indicate a better balance between model fit and complexity when comparing models.
We performed post hoc analyses examining factors related to class membership for the best-fitting GMM model. We examined whether SCZ group status was related to class membership using a chi-squared test, and for the SCZ patients we determined differences among classes in demographics and in duration of illness, age of onset, living situation, antipsychotic medication usage, and severity of illness (PANSS negative, positive, and total symptoms) using F-tests and chi-squared tests. Also for SCZ patients, we fit LMEs to each of the latent classes separately, determining class-specific factors related to trajectories of cognitive change. All analyses were performed in R Version 2.13.0(2008).
3. Results
In the initial sample, HC participants were on average older, more educated, and more predominantly male (Table 1) compared to the SCZ patients. Thus, propensity score analyses included these three variables in logistic regressions predicting SCZ status. After 3-1 propensity score matching, we obtained a subsample of 201 SCZ patients and 67 HC subjects. In the matched sample, education and gender were no longer statistically different. The HC subjects remained significantly older, though less so than in the original sample. SCZ subjects had significantly lower DRS scores at baseline; this was unchanged after propensity score matching. Subjects in the matched sample were on study for an average of 3.5 years (sd = 2.8) and were assessed an average of 3.9 times (sd = 2.3), with a median inter-assessment period of 1.3 years.
Table 1.
HC and SCZ Groups Before and After Propensity Scorea
| Initial Sample | Propensity Score-Matched Sample | |||||
|---|---|---|---|---|---|---|
| HC N=67 |
SCZ N=336 |
HC N=67 |
SCZ N=201 |
|||
| Variable | Mean (SD) | Mean (SD) | t-test (df)b; p-value |
Mean (SD) | Mean (SD) | t-test (df)b; p-value |
| Age | 66.5 (12.6) | 53.6(8.6) | 8.0 (79); <0.001 | 67.6(66.5 | 57.7(8.5) | 5.3 (87); <0.001 |
| Education | 13.1 (2.5) | 12.2 (2.7) | 2.8 (98); 0.006 | 13.1(2.5) | 12.6(2.6) | 1.3 (118); 0.205 |
| Dementia | 138.9 (3.7) | 129.0(11.2) | 13.0 (318); <0.001 | 138.9 (3.7) | 129.5(11.5) | 10.3 (266); <0.001 |
|
Rating Scale at Baseline |
||||||
| N (%) | N (%) | χ2 (df); p-value | N (%) | N (%) | χ2 (df); p-value | |
| Gender | ||||||
| Female | 42 (63%) | 119 (35%) | 16.2(1);<0.001 | 42 (63%) | 103 (51%) | 2.2(1); 0.137 |
| Male | 25 (37%) | 217 (65%) | 25 (37%) | 98 (49%) | ||
| Race | ||||||
| Non-Caucasian | 9 (13%) | 71 (21%) | 1.6(1); 0.202 | 9 (13%) | 45 (22%) | 2.0(1); 0.160 |
| Caucasian | 58 (87%) | 265 (79%) | 58 (87%) | 156 (78%) | ||
| Hispanic | ||||||
| Non-Hispanic | 57 (85%) | 294 (88%) | 0.12(1); 0.733 | 57 (85%) | 181 (90%) | 0.8(1); 0.371 |
| Hispanic | 10 (15%) | 42 (12%) | 10 (15%) | 14 (10%) | ||
| Living Situation | ||||||
| Independent | 67 (100%) | 158 (47%) | 61.4(1);<0.001 | 67 (100%) | 106 (53%) | 61.4(1);<0.001 |
| Board and Care | 0 (0%) | 178 (53%) | 0 (0%) | 95 (47%) | ||
Propensity scores computed conditional on age, gender, education, race/ethnicity.
Degrees of freedom are from Saitherthwaite approximations for t-test with unequal variances.
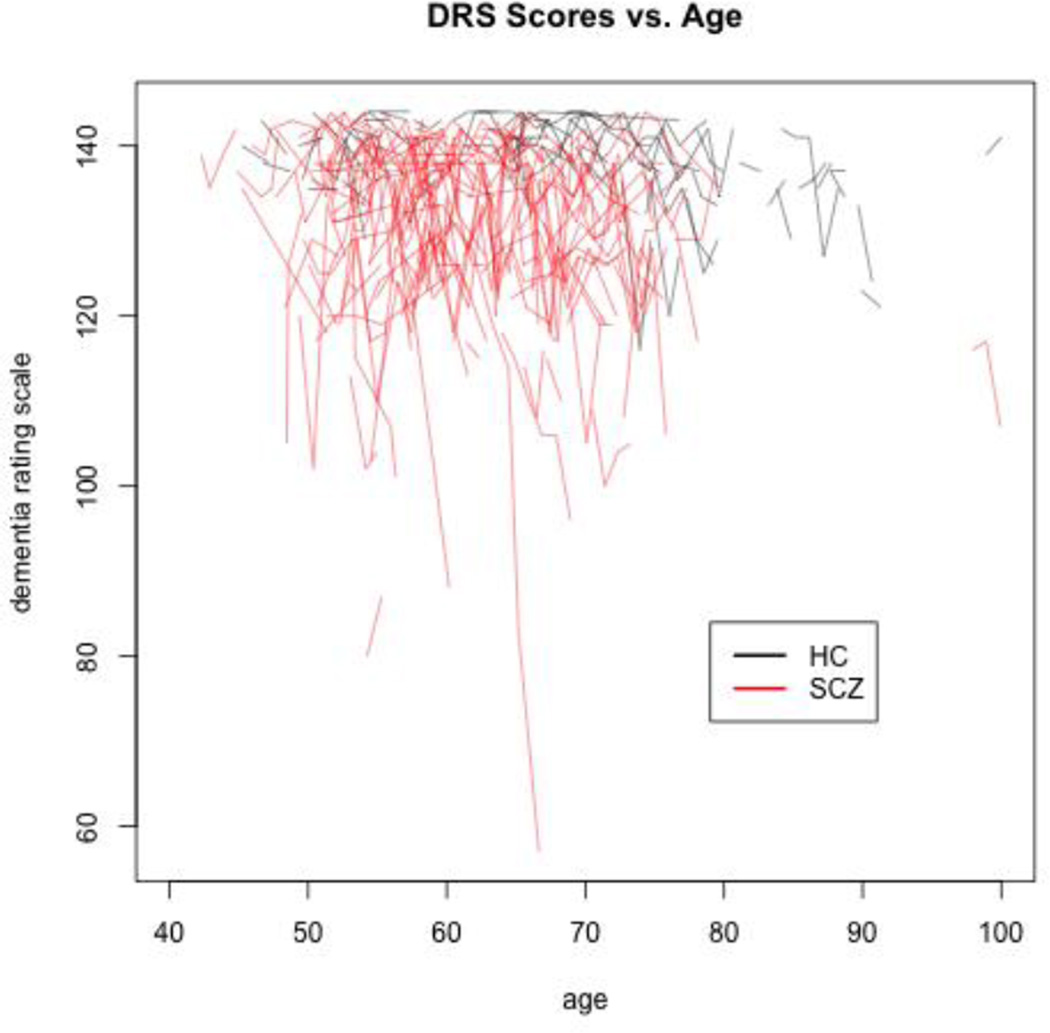
The sample evidenced a high degree of between-subject variation in DRS trajectories, more so within the SCZ group (Figure 1). The DIC analysis for the GMMs showed that the 1-Class model gave the worst trade-off between model fit and complexity and the 3-Class model gave the best (see Table 2; DIC for a 4-Class GMM, not shown in Table 2, was 6,799). The 3-Class model thus provided the most parsimonious model of observed levels of extra-normal variation in random intercepts and slopes.
Figure 1.
Dementia Rating Scale Trajectories for N=67 Healthy Control (HC) and N=134 Schizophrenia (SCZ) Subjects in Propensity Score-Matched Sample.
Table 2.
Latent Growth Curve Model Parameter Estimates
| 1-Class Model | 2-Class Model | 3-Class Model | |
|---|---|---|---|
| Coefficient (Units) | coef (se); p-value | coef (se); p-value | coef (se); p-value |
| Intercept | |||
| Class 1 | 131.1 (.52);<.001 | 136.0 (.42); <.001 | 137.7 (.35);<.001 |
| Class 2 | 124.1 (.91);<.001 | 128.6 (.63); <.001 | |
| Class 3 | 112.5 (1.94);<.001 | ||
| Education* (Yrs) | 1.33 (.20); <.001 | .94(.16);<.001 | .54(.10);<.001 |
| Baseline Age** (Yrs) | −.30 (.05); <.001 | −.11 (.04); .003 | −.07 (.03); .010 |
| SCZ (−.5, .5)*** | −7.52 (1.21);<.001 | −3.89(.86):<.001 | −3.36(.63);<.001 |
| Change in Age (Yrs) | |||
| Class 1 | −.41 (.18); .011 | −.13 (.14); .187 | .03 (.14); .597 |
| Class 2 | −.89 (.41); .015 | −.43 (.25); .042 | |
| Class 3 | −2.11 (1.03); .020 | ||
| Error Standard Deviation | |||
| Class 1 | 5.19 | 3.10 | 2.61 |
| Class 2 | 9.75 | 6.12 | |
| Class 3 | 12.82 | ||
| Number of Subjects | |||
| Class 1 | 268 | 191 | 137 |
| Class 2 | 77 | 102 | |
| Class 3 | 29 | ||
| DIC | 6961 | 6879 | 6730 |
Centered by subtracting the sample mean years of education (13.2 years)
Centered by subtracting the sample mean baseline age (56.1 years)
HC subjects coded as −.5, SCZ patients coded as .5
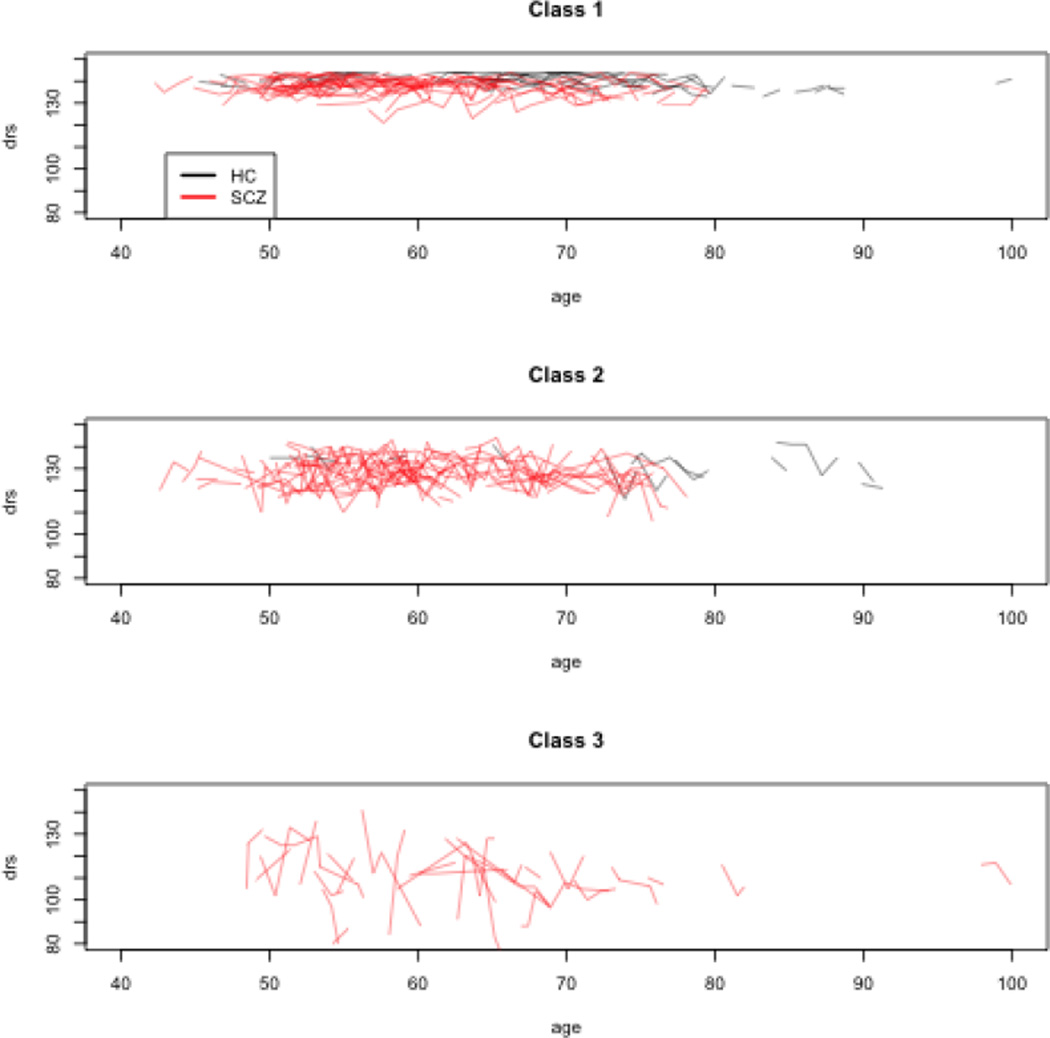
Figure 2 presents the trajectories from the 3-Class model with individual subjects classified by highest posterior probability of class membership. SCZ status and higher baseline age predicted significantly lower baseline DRS scores for all three latent classes. Mean within-subject change in DRS scores was negligible in the Class 1 (.03 points per year) but marginally significantly negative in Class 2 (−.43 points per year) and more so in Class 3 (−2.11 points per year). Baseline DRS scores were highest in Class 1 and lowest in Class 3. Error standard deviations were also smallest in Class 1 (2.61) intermediate in Class 2 (6.12) and highest in Class 3 (12.82).
Figure 2.
Dementia Rating Scale Trajectories for3-Class Model
Of the HC subjects, 57 (85%) were assigned to Class 1, 10 (15%) to Class 2, and zero (0%) to Class 3. Of the SCZ patients, 101 (50%) were assigned to Class 1, 81 (40%) to Class 2, and 19 (10%) to Class 3. The difference in proportion of HC and SCZ subjects assigned to each class was highly significant (χ2=26.2, df=2, p<.0001). Within the SCZ group, classes differed significantly in PANSS Negative and Total Syndrome Scales, with Class 3 having the highest and Class 1 the lowest scores (Table 3). Class 1 had a significantly higher proportion of SCZ subjects residing independently than Class 2, with Class 3 having the lowest proportion (resp. 69%, 42%, and 16%). The only other variable that differed significantly was years of education, with Class 1 the highest and Class 3 the lowest. Education differences do not completely account for class membership: DRS scores at baseline are still related to class membership (p<.001) after controlling for education, as are DRS score slopes (p=0.021). Fitting LMEs to SCZ subjects for each class separately, after controlling for education and demographic variables and baseline levels of negative symptoms, change in negative symptoms was a significant predictor of change in DRS scores within classes, with the smallest effect in Class 1 and the largest effect in Class 3 (Class 1: coef=−.10, se=.06, p=.079; Class 2: coef=−.27, se=.09, p=.005; Class 3: coef=−1.28, se=.33, p=.0003).
Table 3.
Comparison of Schizophrenia Subjects from Three-Class Growth Mixture
| Variable | All SCZ (N=201) |
Class 1 (N=101) |
Class 2 (N=81) |
Class 3 (N=19) |
|
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | F-test (df1/df2); p-value | |
| Baseline Age | 57.7(8.5) | 56.7(7.8) | 58.2 (8.9) | 60.9 (9.0) | 2.2 (2,198); .111 |
| Education | 12.7(2.6) | 13.2(2.3) | 12.4(2.7) | 10.9(3.1) | 47.6 (2,198); <.001 |
| PANSS* | |||||
| Negative | 14.3 (5.7) | 13.1(5.1) | 14.3 (4.6) | 20.6(8.1) | 13.6 (2,153); <.001 |
| Positive | 13.8(5.3) | 13.3 (5.4) | 14.1 (5.5) | 14.6 (3.4) | .6 (2,153); .573 |
| Total | 56.3 (15.3) | 53.4(14.1) | 56.7 (14.4) | 68.1 (18.9) | 6.6 (2,153); .002 |
| Age of Onset | 30.9(13.2) | 31.1(13.8) | 30.1 (11.6) | 33.2(15.9) | .4 (2,177) .661 |
| Duration of | 27.1 (13.5) | 26.0(13.0) | 28.3 (13.5) | 27.6(16.4) | 2.2 (2,177).111 |
| Illness (yrs) | |||||
| CPZE total** | 5.0 (7.9) | 5.3 (8.2) | 4.7 (7.9) | 4.6 (7.3) | .1 (2,140) .917 |
| N (%) | N (%) | N (%) | N (%) | Chi-square test (df); p-value | |
| Race | |||||
| Non-Caucasian | 45 (22%) | 23 (23%) | 14 (17%) | 8 (42%) | 5.5 (2); .065 |
| Caucasian | 156 (78%) | 78 (77%) | 67 (83%) | 11 (58%) | |
| Hispanic | |||||
| Non-Hispanic | 181 (90%) | 95 (94%) | 68 (84%) | 18 (95%) | 5.6 (2); .060 |
| Hispanic | 20 (10%) | 6 (6%) | 13 (16%) | 1 (5%) | |
| Gender | |||||
| Female | 103 (51%) | 51 (50%) | 39 (48%) | 13 (68%) | 2.6 (2); .276 |
| Male | 98 (49%) | 50 (50%) | 42 (52%) | 6 (32%) | |
| Schizoaffective | 57 (28.4%) | 26 (32.1%) | (29) 28.7% | 2 (10.5%) | 3.6 (2),.171 |
| Living Situation | |||||
| Independent | 107 (53%) | 70 (69%) | 34 (42%) | 3 (16%) | 25.3 (2);<.001 |
| Board and Care | 94 (47%) | 31 (31%) | 47 (58%) | 16 (84%) | |
Positive and Negative Syndrome Scale
Chlorpromazine Equivalent dosage
4. Discussion
Our results suggests a middle-ground between studies of community dwelling outpatients indicating stability and studies using data from institutionalized patients indicating accelerated decline. Previous research has demonstrated that people with schizophrenia typically have subtle cognitive deficits relative to demographically similar healthy peers before the onset of acute illness(Woodberry, 2008) and experience an exacerbation of these deficits concurrent with the first episode of psychosis(Seidman, 2006). Thereafter, some researchers have found that throughout the adult lifespan, even among older adults, course of cognitive deficits among the majority of outpatients tends to be stable(Irani, 2011; Kurtz, 2005), while others have found that in subjects aged 65 and over, formerly and currently institutionalized patients experience accelerated cognitive decline relative to controls(Friedman, 2001; Harvey, 2003, 2010). We determined that in the typical older adult with schizophrenia (i.e., the majority of patients, as represented by Class 1), rapid decline in cognitive ability is not evident, but that in a sizable proportion of later-life patients schizophrenia is associated with higher instability and a higher risk of moderately accelerated cognitive decline relative to healthy controls.
This more nuanced assessment of heterogeneity in cognitive trajectories was made possible by employing growth mixture models, allowing for subgroup differences in patterns of variation over time. Specifically, 50% of the schizophrenia patients in our sample demonstrated lower than normal but fairly stable cognitive trajectories, similar to cognitive trajectories in prior studies from other groups(Irani, 2011; Szoke, 2008) and from our group that utilized rANOVAs(Heaton, 2001) or LMEs(Nayak-Savla G, 2006) in schizophrenia outpatients. However, a sizable proportion of patients (40% in Class 2 and 10% in Class 3) display cognitive trajectories suggestive of decline somewhat more rapid than among the HC sample, trajectories more similar to those in prior studies of that have shown, in older samples than ours, that past(Harvey, 2010) and currently(Harvey, 1995, 1999) institutionalized patients experience accelerated cognitive decline relative to controls in subjects aged 65 and over.Smith et al. (1994) found an average decline for older normal subjects of approximately .2 points on the DRS total score per year, whereas a decline of approximately 7.5 points per year occurred in less than 5% of normal subjects and in more than 60% of dementia cases. In our sample, average declines for Class 2 subjects (−.43 points per year) are slightly elevated over older normal subjects and declines in Class 3 subjects (−2.11 points per year) are highly elevated but not reaching levels seen in dementia patients.
Patients living in board and care facilities, perhaps the closest analogue in our study to long-term institutionalization, had a much higher probability of belonging to Classes 2 and 3. Board and care residents with schizophrenia may therefore be considered as having a “poor outcome” similar to those in institutionalized settings(Harvey, 1995, 2010). This finding is concordant with those of an earlier study from our center(Auslander, 2001) which compared two groups of community-dwelling participants: independent living (those living alone or with someone in a house or apartment) versus those living in board and care facilities. Although the two groups were comparable in terms of severity of positive and depressive symptoms, board and care residence was associated with worse cognitive impairment, worse negative symptoms, worse health-related quality of wellbeing, earlier age of onset and longer duration of illness, as well as a lower likelihood of having ever been married.
Patients in Classes 2 and 3 also experienced a greater degree of within-subject variability over time. Because of the high degree of heterogeneity in cognitive trajectories both between and within subjects, the focus of longitudinal research on cognitive change in schizophrenia may shift to identify the determinants and mechanisms of cognitive stability versus decline. In our investigation, latent classes of cognitive trajectories were not differentiated by age of onset, duration of illness, antipsychotic medication use, or positive symptom severity. Patient membership in latent classes characterized by worse cognition, higher levels of variation, and a tendency toward accelerated cognitive decline was, however, associated with more severe negative symptoms at baseline. Moreover, change in negative symptoms was associated with change in cognition within latent classes over the course of the study with much more pronounced effects in Class 2 and especially Class 3 compared with Class 1. While negative symptoms have long been associated with greater cognitive impairment and poor functional outcome in cross-sectional studies of schizophrenia, with patients being typically classified as those with a “deficit syndrome”(Cohen, 2010), to our knowledge they have not previously been associated with risk of accelerated cognitive decline. Determining whether negative symptoms precede cognitive decline would offer an opportunity for gaining increased insight into the underlying mechanisms of this relationship, necessitating further research with longitudinal data of greater duration.
There are several potential clinical implications of our study. Overall our study demonstrates heterogeneity in course of schizophrenia. The focus of past studies has largely been on determining whether and to what extent average change in cognitive ability outpaces that of healthy comparators. Our analyses suggest that schizophrenia may involve subgroups with different trajectories of cognitive ability, some of which involve clinically significant decline. As such, beyond quantifying mean level of change, future work may benefit form identifying risk factors that predict subgroups with declining cognitive trajectories. Our study highlighted two risk factors for apparent accelerated cognitive decline: negative symptoms and living in a board and care. If replicated, preventative cognitive remediation strategies may be particularly valuable for the subset of patients with prominent negative symptoms. Additionally, the finding that residing in a board and care facility was predictive of cognitive decline suggests that environmental factors and demands (or lack thereof) may influence stability of cognitive ability. We speculate that since many of the instrumental activities of daily living, such as medication management, cooking, and financial and leisure activities are managed by staff rather than by the residents themselves, diminished cognitive demands may produce risk for cognitive decline as seen in institutionalized older adults without schizophrenia. Of course, we cannot rule out that individuals who are predisposed to risk of cognitive decline may be more likely to be placed in supportive housing, yet it may be that incorporation of cognitive rehabilitation, exercise, and other types of environmental stimulation in the milieu of board and care homes could mitigate risk for cognitive decline.
One potential limitation of the current study is the use of the DRS rather than a more comprehensive neuropsychological test battery. The DRS has the advantage of being tolerable by patients from a wide range of capabilities, but it is not ideal for evaluating differential patterns among specific cognitive domains or for making fine discriminations within the non-impaired range of performance. The DRS has been consistently shown, however, to differentiate cognitive functioning among inpatients and outpatients with schizophrenia and healthy comparison participants(Evans, 1999), as well as moderate to high correlations on functional capacity measures among outpatients with schizophrenia(Twamley, 2002), capacity to medication adherence(Jeste, 2003), and consent to research or treatment(Palmer, 2004). Employing the present analyses with a more comprehensive test battery in future studies would be ideal to evaluate the reliability of the present findings. A potential caveat in interpreting the longitudinal results is the possibility that older schizophrenia patients may be less prone to practice effects than younger patients or older healthy subjects (Granholm et al., 2010).
Another potential limitation is the use of patients' education as a covariate, since a patient’s formal education may be truncated by onset of schizophrenia. However, perhaps because of the focus of our Research Center on older adults, there were relatively few participants in the present database for whom age-of-illness onset was substantially earlier than the age at which formal schooling is usually completed (mean age of onset=30.9, sd=13.2). Our data on race and ethnicity were quite limited: 80% of the sample was non- Latino Caucasian, and 14% of the sample was African American. We thus had no power to detect differences on a more fine-grained parsing of ethnicity. We were also unable to determine if age-associated medical conditions may have had a compounding impact on cognition in some individuals. The broader issue of the impact of medical illness burden on cognitive outcomes is beyond the scope of this study.
Follow-up periods longer than 3.5 years on average would have provided an improved ability to assess the slope and shape of cognitive trajectories, as well as the antecedents and predictors of accelerated cognitive decline. As a secondary analysis of a dataset combined from larger studies, some aspects of the available data and sampling may have been suboptimal for the goals of the present analyses. This includes the imbalance in the number of controls versus subjects with schizophrenia, as well as differences in specific procedures for establishing diagnosis within the patient group. A priori matching of schizophrenia patients and healthy comparison subjects on baseline age and other relevant covariates would have been preferable.
Limitations notwithstanding, the methods and results of this study bridge the gap between prior studies that demonstrate cognitive decline and those that demonstrate stability. Obtaining a fuller understanding of cognitive trajectories in later-life also has practical implications for intervention and case management of patients who show accelerated cognitive decline. The rule with schizophrenia in any dimension or characteristic is heterogeneity. Methodological approaches that accurately describe heterogeneity in outcome trajectories can help characterize the relationship of schizophrenia to treatment history, treatment response, and imaging patterns that inform neuropathology. To accomplish these goals, study methods, including design and analyses, must be sensitive to heterogeneity in trajectories.
Acknowledgements
We would like to thank Meryl Zwanger, Sandra Dorsey, Rebecca Daley, and Ben Kovachy for their assistance in editing and preparation of the manuscript.
Funding Body Agreements and policies:
We would like to acknowledge funding support from NIH grants P30 MH080002, R01 MH70886, and R01 MH064722.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: All authors contributed to and have approved the final manuscript. In particular the contributions of each author were:
Wesley K. Thompson: Conceptual design; statistical analyses; provided first draft of manuscript.
Gauri N. Savla: Interpretation of results; assisted with writing of manuscript; assisted with contextual overview.
Ipsit V. Vahia: Interpretation of results; assisted editing manuscript.
Colin A. Depp: Interpretation of results; assisted editing manuscript.
Ruth O’Hara: Interpretation of results; assisted editing manuscript.
Dilip V. Jeste: Interpretation of results; assisted with conceptual overview.
Barton W. Palmer: Interpretation of results; assisted with writing of manuscript; provided contextual overview.
Conflict of Interest:
Drs. Thompson, Savla, Vahia, Depp, O’Hara, and Palmer have no financial disclosures or conflicts of interests. AstraZeneca, Bristol-Myers Squibb, Eli Lilly and Janssen donate medication for a NIMH-funded research grant of which Dr. Jeste is the principal investigator.
Contributor Information
Wesley K. Thompson, Email: wkthompson@ucsd.edu.
Gauri N. Savla, Email: gnsavla@gmail.com.
Ipsit V. Vahia, Email: ipsitv@gmail.com.
Colin A. Depp, Email: cdepp@ucsd.edu.
Ruth O’Hara, Email: roh@stanford.edu.
Dilip V. Jeste, Email: djeste@ucsd.edu.
Barton W. Palmer, Email: bpalmer@ucsd.edu.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Third Edition-Revised. Washington D.C.: American Psychiatric Association; 1987. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Auslander LA, Lindamer LL, Delapena J, Harless K, Polichar D, Patterson TL, et al. A comparison of community-dwelling older schizophrenia patients by residential status. Acta Psychiatry Scandinavia. 2001;103(5):380–386. doi: 10.1034/j.1600-0447.2001.00262.x. [DOI] [PubMed] [Google Scholar]
- Bleuler E. In: Dementia Praecox, or the Group of Schizophrenias. Zinkin J, translator. New York, NY: New York: International Universities Press; 1911. 1950. [Google Scholar]
- Carpenter WT, Stephens JH. An attempted integration of information relevant to schizophrenic subtypes. Schizophr Bulletin. 1979;5:490–506. doi: 10.1093/schbul/5.3.490. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Brown LA, Minor KS. The psychiatric symptomatology of deficit schizophrenia: A meta-analysis. Schizophr Research. 2010;118:122–127. doi: 10.1016/j.schres.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Evans JD, Negron AE, Palmer BW, Paulsen JS, Heaton RK, Jeste DV. Cognitive deficits and psychopathology in hospitalized versus community-dwelling elderly schizophrenia patients. J Geriatric Psychiatry and Neurology. 1999;12:11–15. doi: 10.1177/089198879901200104. [DOI] [PubMed] [Google Scholar]
- Eyler-Zorrilla LT, Heaton RK, McAdams LA, Zisook S, Jeste DV. Cross-sectional study of older outpatients with schizophrenia and healthy comparison subjects: No difference in age-related cognitive decline. American Journal of Psychiatry. 2000;157:1324–1326. doi: 10.1176/appi.ajp.157.8.1324. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Harvey PD, Coleman T, Moriarty PJ, Bowie C, Parrella M, White L, Alder D, Davis KL. Six-year follow-up study of cognitive and functional status across the lifespan in schizophrenia: A comparison with Alzheimer's disease and normal aging. American Journal of Psychiatry. 2001;158:1441–1448. doi: 10.1176/appi.ajp.158.9.1441. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the "right stuff"? Schizophrenia Bulletin. 2000;26:119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Harvey PD. Cognitive impairment in elderly patients with schizophrenia: Age related changes. International Journal of Geriatric Psychiatry. 2001;16(Suppl 1):S78–S85. doi: 10.1002/1099-1166(200112)16:1+<::aid-gps565>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Bertisch H, Friedman JI, Marcus S, Parrella M, White L, Davis KL. The course of functional decline in geriatric patients with schizophrenia: Cognitive functional and clinical symptoms as determinants of change. American Journal of Geriatric Psychiatry. 2003;11:610–619. doi: 10.1176/appi.ajgp.11.6.610. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Lombardi J, Kincaid MM, Parrella M, White L, Powchik P, Davidson M. Cognitive functioning in chronically hospitalized schizophrenic patients: Age-related changes and age disorientation as a predictor of impairment. Schizophrenia Research. 1995;17:15–24. doi: 10.1016/0920-9964(95)00026-i. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Reichenberg A, Bowie CR, Patterson TL, Heaton RK. The Course of Neuropsychological Performance and Functional Capacity in Older Patients with Schizophrenia: Influences of Previous History of Long-Term Institutional Stay. Biological Psychiatry. 2010;67:933–939. doi: 10.1016/j.biopsych.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PD, Silverman JM, Mohs RC, Parrella M, White L, Powchik P, Davidson M, Davis KL. Cognitive decline in late-life schizophrenia: A longitudinal study of geriatric chronically hospitalized patients. Biological Psychiatry. 1999;45:32–40. doi: 10.1016/s0006-3223(98)00273-x. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Gladsjo JA, Palmer BW, Kuck J, Marcotte TD, Jeste DV. Stability and course of neuropsychological deficits in schizophrenia. Archives of General Psychiatry. 2001;58:24–32. doi: 10.1001/archpsyc.58.1.24. [DOI] [PubMed] [Google Scholar]
- Irani F, Kalkstein S, Moberg EA, Moberg PJ. Neuropsychological performance in older patients with schizophrenia: A meta-analysis of cross-sectional and longitudinal studies. Schizophrenia Bulletin. 2011;37:1318–1326. doi: 10.1093/schbul/sbq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablensky A. Subtyping schizophrenia: Implications for genetic research. Molecular Psychiatry. 2006;11:815–836. doi: 10.1038/sj.mp.4001857. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Wyatt RJ. Understanding and Treating Tardive Dyskinesia. New York, NY: Guilford Press, Inc; 982. [Google Scholar]
- Jeste SD, Patterson TL, Palmer BW, Dolder CR, Goldman S, Jeste DV. Cognitive predictors of medication adherence among older outpatients with schizophrenia. Schizophrenia Research. 2003;63:49–58. doi: 10.1016/s0920-9964(02)00314-6. [DOI] [PubMed] [Google Scholar]
- Jurica PL, Leitten CL, Mattis S. Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc; 1991. Dementia Rating Scale-2. [Google Scholar]
- Kay S, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Messias E, Harvey PD, Fernandez-Egea E, Bowie CR. Is schizophrenia a syndrome of accelerated aging? Schizophr Bulletin. 2008;34:1024–1032. doi: 10.1093/schbul/sbm140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Y JA, Taylor JL, Kupfer DJ. How can we learn about developmental processes from cross-sectional studies, or can we? American Journal of Psychiatry. 2000;157:163–171. doi: 10.1176/appi.ajp.157.2.163. 157. [DOI] [PubMed] [Google Scholar]
- Kurtz MM. Neurocognitive impairment across the lifespan in schizophrenia: An update. Schizophrenia Research. 2005;74:15–26. doi: 10.1016/j.schres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Laird NM, W JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale. Odessa, FL: Psychological Assessment Resources, Inc; 1973. [Google Scholar]
- Muthen B, Shedden K. Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics. 1999;55:463–469. doi: 10.1111/j.0006-341x.1999.00463.x. [DOI] [PubMed] [Google Scholar]
- Nayak-Savla G MD, Roesch SC, Heaton RK, Jeste DV, Palmer BW. An evaluation of longitudinal neurocognitive performance among middle-aged and older schizophrenia patients: Use of mixed-model analyses. Schizophr Research. 2006;83:215–223. doi: 10.1016/j.schres.2005.12.851. [DOI] [PubMed] [Google Scholar]
- Palmer BW, Bondi MW, Twamley EW, Thal L, Golshan S, Jeste DV. Are late-onset schizophrenia-spectrum disorders a neurodegenerative condition? Annual rates of change on two dementia measures. Journal of Neuropsychiatry Clinical Neuroscience. 2003;15(45–52) doi: 10.1176/jnp.15.1.45. [DOI] [PubMed] [Google Scholar]
- Palmer BW, Dunn LB, Appelbaum PS, Jeste DV. Correlates of treatment-related decision-making capacity among middle-aged and older patients with schizophrenia. Archives of General Psychiatry. 2004;61:230–236. doi: 10.1001/archpsyc.61.3.230. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- Seidman LJ, Buka SL, Goldstein JM, Tsuang MT. Intellectual decline in schizophrenia: Evidence from a prospective birth cohort 28 year follow-up study. Journal of Clinical and Experimental Neuropsychology. 2006;28:225–242. doi: 10.1080/13803390500360471. [DOI] [PubMed] [Google Scholar]
- Smith GE, Ivnik RJ, Malec JF, Kokmen E, Tangalos E, Petersen RC. Psychometric Properties of the Mattis Dementia Rating Scale. Assessment. 1994;1(2):123–131. doi: 10.1177/1073191194001002002. [DOI] [PubMed] [Google Scholar]
- Spiegelhalter DJ, Best NG, Carlin BP, Van der Linde A. Bayesian Measures of Model Complexity and Fit (with Discussion) Journal of the Royal Statistical Society, Series B. 2002;64:583–616. [Google Scholar]
- Szoke A, Trandafir A, Dupont M, Meary A, Schurhoff F, Leboyer M. Longitudinal studies of cognition in schizophrenia: Meta-analysis. British Journal of Psychiatry. 2008;192:248–257. doi: 10.1192/bjp.bp.106.029009. [DOI] [PubMed] [Google Scholar]
- Thompson WK, Hallmayer J, O'Hara R. Design considerations for characterizing psychiatric trajectories across the lifespan. American Journal of Psychiatry. 2011;9(168):894–903. doi: 10.1176/appi.ajp.2011.10111690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Doshi RR, Nayak GV, Palmer BW, Golshan S, Heaton RK, Patterson TL, Jeste DV. Generalized cognitive impairments, everyday functioning ability, and living independence in patients with psychosis. American Journal of Psychiatry. 2002;159:2013–2020. doi: 10.1176/appi.ajp.159.12.2013. [DOI] [PubMed] [Google Scholar]
- Vahia IV, Palmer BW, Depp C, Fellows I, Golshan S, Kraemer HC, Jeste DV. Is late-onset schizophrenia a subtype of schizophrenia? Acta Psychiatry Scandinavia. 2010;122:414–426. doi: 10.1111/j.1600-0447.2010.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: A meta-analytic review. American Journal of Psychiatry. 2008;165:579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]