Abstract
The release of the serine proteinase tissue-type plasminogen activator (tPA) from cerebral cortical neurons has a neuroprotective effect in the ischemic brain. Because excitotoxicity is a basic mechanism of ischemia-induced cell death here we investigated the effect of tPA on excitotoxin-induced neuronal death. We report that genetic overexpression of neuronal tPA or treatment with recombinant tPA renders neurons resistant to the harmful effects of an excitotoxic injury in vitro and in vivo. We found that at concentrations found in the ischemic brain tPA interacts with synaptic but not extrasynaptic NMDARs. This effect is independent of tPA’s proteolytic properties and leads to a rapid and transient phosphorylation of the extracellular signal regulated kinases 1 / 2 (ERK ½), with ERK ½-mediated activation of the cAMP response element binding protein (CREB) and induction of the neuroprotective CREB-regulated activating transcription factor 3 (Atf3). In line with these observations, Atf3 down-regulation abrogates the protective effect of tPA against excitotoxin-induced neuronal death. Our data indicate that tPA preferentially activates synaptic NMDARs via a plasminogen-independent mechanism turning on a cell signaling pathway that protects neurons from the deleterious effects of excitotoxicity.
Keywords: Excitotoxicity, neuroprotection, plasminogen, tissue-type plasminogen activator
Introduction
Excitotoxicity has been linked to cell death in several pathological conditions of the central nervous system (CNS) including cerebral ischemia, trauma and seizures 1. N-methyl-D-aspartate receptors (NMDARs) are calcium-permeable ion channels that activate several intracellular signaling pathways that mediate not only physiological processes such as neuronal plasticity, learning and memory 2, but also pathological events such as excitotoxin-induced neuronal death 3. NMDARs are assembled by obligatory NR1 sub-units that interact with NR2A-D subunits. Most of the NR2A-containing NMDARs are located in the synapses and their activation has been coupled to neuronal survival 4. In contrast, the majority of NR2B-containing NMDARs are extrasynaptic and linked to the activation of cell-death pathways 5.
Tissue-type plasminogen activator (tPA) is a serine proteinase that is found in the intravascular space and in the CNS where it plays a pivotal role in the development of synaptic plasticity, learning and memory via plasminogen-dependent and -independent mechanisms 6. A link between tPA and NMDARs has been derived from experimental evidence indicating that tPA enhances the effect of NMDA on intracellular calcium concentrations 7. Several studies have reported that treatment with concentrations of tPA greater than 200 nM 7-8 potentiates the harmful effect of an excitotoxic injury, which together with other observations 9 have led to postulate the hypothesis that tPA mediates excitotoxin-induced neuronal death.
The extracellular signal regulated kinases 1 and 2 (ERK ½) are members of the mitogen-activated protein kinase family that regulate cellular responses to a variety of extracellular stimuli, and mediate several effects of tPA in the CNS 10. The role of ERK ½ activation on cell survival is still controversial. Indeed, whereas some studies indicate that sustained ERK ½ activation leads to neuronal death 11, others have shown that transient activation of ERK ½ turns on several neuroprotective signaling pathways in the CNS 12.
The cAMP response element binding protein (CREB) is a multifunctional transcriptional regulator that plays an important role in neuronal survival 13. Recent evidence indicates that CREB-mediated induction of the activating transcription factor 3 (Atf3) protects neurons from the harmful effects of extrasynaptic NMDARs activation. Indeed, CREB-Atf3 signaling is controlled by synaptic NMDARs and is the central component of a neuroprotective response in the brain 14. In the work presented here we show that either the release of neuronal tPA, or treatment of neuronal cultures with 5 nM of tPA, or the intravenous administration of rtPA in an in vivo model of excitotoxic injury, protects the brain from excitotoxin-induced neuronal death, and that this effect is independent of tPA’s ability to cleave plasminogen into plasmin. We report that tPA activates synaptic NR2A-containing NMDARs and turns on the ERK ½-CREB-Atf3 pro-survival pathway. Our data describe a novel neuroprotective pathway for tPA in the CNS with clinical implications for the potential development of a therapeutic strategy to promote cell survival in patients with neurological conditions associated with excitotoxin-induced neuronal death.
Results
Effect of tPA on excitotoxin-induced neuronal death
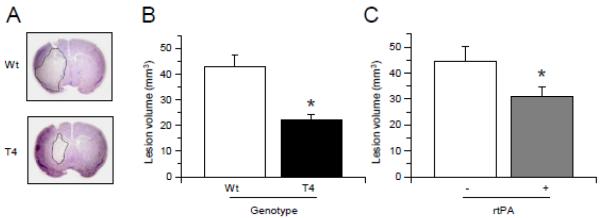
To study the role of tPA on excitotoxin-induced neuronal death T4 mice and their Wt littermate controls were injected with NMDA into the striatum followed by determination of the volume of the lesion as described in the Experimental Methods section. We found that T4 mice have a 48.26 % decrease in the volume of NMDA-induced lesion (42.72 +/−4.8 mm3 in Wt and 22.10 +/−2.3 mm3 in T4 mice; Fig 1, p < 0.05), suggesting that neuronal tPA has a protective effect against excitotoxin-induced cell death. Then we performed similar observations in Wt mice treated with 1 mg/Kg/IV of rtPA or a comparable volume of saline solution immediately after the intrastriatal injection of NMDA. In agreement with our observations in T4 mice, we found that treatment with rtPA induces a 27.62 % decrease in the volume of NMDA-induced lesion (Fig 1, p < 0.05).
Figure 1. tPA protects the brain from excitotoxin-induced cell death.
(A) Representative thionin-stained brain sections of T4 mice and their wild-type (Wt) littermate controls 24 hours after the intrastriatal injection of NMDA. (B) & (C) mean volume of the lesion 24 hours after the intrastriatal injection of NMDA in T4 mice and their wild-type (Wt) littermate controls (B) and in Wt mice treated with rtPA 1 mg/Kg/IV (+) or a comparable volume of saline solution (−) after the injection of NMDA (C). n = 12 per experimental group in B and 11 in C. * in B: p < 0.05 compared to Wt littermate controls. * in C: p < 0.05 compared to Wt mice treated with saline solution. Lines denote SD.
Effect on cell survival of co-treatment with tPA and NMDA
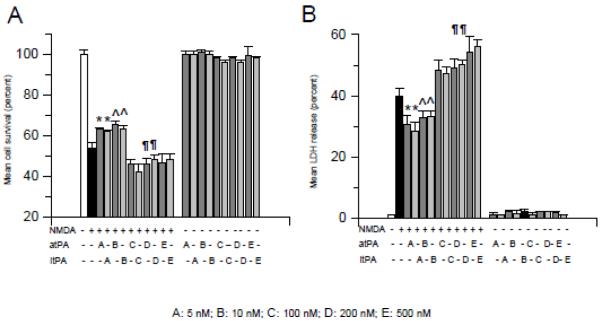
Because it has been reported that tPA potentiates NMDA-induced neuronal death 15, we used the MTT and LDH release assays to study cell survival and death in neurons incubated with either 50 M of NMDA, or 5 - 500 nM of either proteolytically active tPA (atPA), or with tPA with an alanine for serine substitution at the active site Ser481 (proteolytically inactive tPA; itPA), or with a combination of 50 M of NMDA and 5 - 500 nM of either atPA or itPA. Our results indicate that, as previously described 16, treatment with tPA alone does not induce neuronal death. In contrast, neuronal survival decreased from 100 +/−1.9 % in control cells to 53.63 +/− 2.86 % in cells treated with NMDA alone. Surprisingly, co-treatment with 5 or 10 nM of tPA increased cell survival from 53.63 +/− 2.86 % (in neurons treated with NMDA alone) to 63 +/− 0.87 % and 65.17 +/− 2.03, respectively (Fig 2A, p < 0.05). In contrast, co-treatment with either 100 nM, or 200 nM, or 500 nM of tPA decreased neuronal survival from 53.63 +/− 2.86 % (in neurons treated with NMDA alone) to 46.22 +/− 2.25 %, 46 +/− 3.0 % and 46.78 +/− 1.4%, respectively (Fig 2A, p <0.05). In line with these observations, our cell death assay indicated that the release of LDH into the media decreased from 40.75 +/− 2.66 % in neurons incubated with NMDA alone to 30.63 +/− 2.78 % and 32.80 +/− 2.19 % in neurons co-treated with NMDA and 5 or 10 nM of tPA, respectively. In contrast, the release of LDH from neurons co-treated with NMDA and either 100 nM, or 200 nM, or 500 nM of tPA increased from 40.75 +/− 2.66 % (in neurons incubated with NMDA alone) to 48.20 +/− 3.32%, 49.24 +/− 1.86 % and 54.26 +/− 5.23%, respectively. Importantly, co-treatment with proteolytically inactive tPA yielded similar results (Fig 2B, p < 0.05).
Figure 2. Dose-dependent effect of tPA on NMDA-induced neuronal death.
Mean neuronal survival (A) and LDH release (B) in wild-type cerebral cortical neurons incubated with NMDA alone, or with 0 - 500 nM of proteolytically active (atPA; dark gray bars) or inactive (itPA; light gray bars) tPA alone, or with a combination of NMDA and 0 - 500 nM of atPA or itPA. Lines denote SD. n = 22 per experimental group in A and B, respectively. * and ^ in A and B: p < 0.05 compared to neurons incubated with NMDA alone or with neurons co-incubated with NMDA and 100 nM, or 200 nM or 500 nM of tPA. §, ¶ and ± in A and B: p < 0.05 compared to neurons incubated with NMDA alone or with neurons co-incubated with NMDA and 5 or 10 nM of tPA. A-E denotes doses of tPA used in each observation.
Treatment with tPA promotes cell survival in neurons previously exposed to an excitotoxic injury
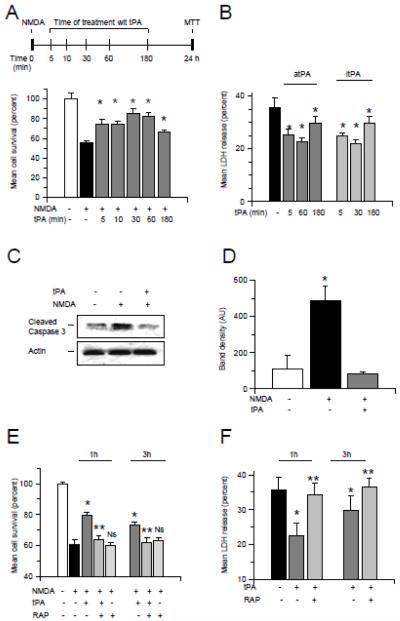
Then we used the experimental paradigm depicted in the upper panel of Fig 3A to investigate whether treatment with tPA after exposure to an excitotoxic injury also has a protective effect. Wt cerebral cortical neurons were incubated with 50 M of NMDA during 55 minutes followed by media change and treatment 5 - 180 minutes later with 5 nM of tPA or a comparable volume of vehicle (control). Cell survival was determined 24 hours later with the MTT assay. We found that cell survival decreased from 100 +/− 3.6 % in controls cells to 55.52 +/− 2.46 % in neurons incubated with NMDA without subsequent treatment with tPA. However, when neurons were treated with 5 nM of tPA either 5, or 10, or 30, or 60 or 180 minutes after the end of exposure to NMDA, cell survival increased to 73.97 +/− 5 %, 74.86 +/− 2.35%, 85.45 +/− 4.8%, 82.07 +/− 4.4% and 66.51 +/− 1.73 %, respectively (Fig 3A, p < 0.05). Importantly, incubation with vehicle (control) did not have an effect on NMDA-induced neuronal death. To study whether the proteolytic activity of tPA is needed for this protective effect, we quantified the release of LDH from Wt cerebral cortical neurons incubated with NMDA during 55 minutes followed 5 - 180 minutes later by treatment with 5 nM of either atPA or itPA, or with plasmin 10 nM. Our results show that the release of LDH into the media decreases from 35.6 +/− 3.8% in neurons incubated with NMDA without subsequent treatment with tPA, to 25.4 +/− 2.2%, 22.60 +/− 5.64 and 29.86 +/− 4.39 % in neurons treated with proteolytically active tPA 5, 60 or 180 minutes after the end of incubation with NMDA, respectively. A similar protective effect was observed with itPA (Fig 3B, p < 0.05). Notably, plasmin failed to protect neurons from NMDA-induced cell death (data not shown). These data indicate that tPA protects neurons from excitotoxin-induced neuronal death via a plasminogen-independent mechanism. To determine whether tPA has an effect of NMDA-induced apoptotic neuronal death, we studied the expression of cleaved caspase-3 in neurons incubated with NMDA followed 1 hour later by treatment with 5 nM of tPA or a comparable volume of vehicle (control). We found that treatment with tPA attenuates NMDA-induced caspase-3 cleavage (Fig 3 C & D).
Figure 3. Cell survival in neurons treated with tPA following exposure to an excitotoxic injury.
A: Upper panel: experimental design to study the effect of treatment with tPA on NMDA-induced neuronal death. Lower panel: mean cell survival in Wt cerebral cortical neurons incubated 55 minutes with NMDA and treated 5 - 180 minutes after the end of the excitotoxic injury with 5 nM of tPA. n = 20 per experimental group. * p < 0.05 compared to neurons incubated with NMDA without ensuing treatment with tPA. Lines denote SD. B. Mean release of LDH into the culture media of Wt neurons incubated 55 minutes with NMDA followed 5 - 180 minutes later by treatment with 5 nM of either active (atPA) or proteolytically inactive tPA (itPA). Lines denote SD. n = 22 per experimental group. * p < 0.05 compared to neurons incubated with NMDA without ensuing treatment with tPA. C & D: Representative Western blot analysis (C) and mean density of the band (D) of cleaved caspase-3 expression in Wt cerebral cortical neurons incubated with 50 μM of NMDA followed 60 minutes later by treatment with 5 nM of tPA or a comparable volume of vehicle (control). Each observation was repeated 4 times. * in D: p < 0.05 when neurons exposed to NMDA without subsequent treatment with tPA are compared to NMDA-treated neurons incubated with tPA 1 hour later. Lines denote SD. E & F. Mean cell survival (C) and release of LDH (D) in Wt cerebral cortical neurons incubated 55 minutes with NMDA followed 60 or 180 minutes after the end of the excitotoxic injury by treatment with 5 nM of tPA alone or in combination with 100 nM of RAP. n = 16 per experimental group. * in (C) and (D) p < 0.05 compared to cells incubated with NMDA without subsequent treatment with tPA. ** p < 0.05 compared to cells incubated with NMDA followed by treatment with tPA. Ns in C: non significant compared to neurons incubated with NMDA alone.
The protective effect of tPA requires the engagement of a member of the LDL receptor family
Because it has been demonstrated that a member of the LDL receptor family is a receptor for tPA in the CNS 17, we investigated whether this receptor also mediates the protective effect of tPA against excitotoxin-induced neuronal death. Cerebral cortical neurons were incubated during 55 minutes with NMDA followed 1 or 3 hours later by treatment with 5 nM of tPA, alone or in combination with 100 nM of the receptor-associated protein (RAP, an inhibitor of binding of members of LDL receptors to their ligands). We found that incubation with NMDA without subsequent treatment with tPA reduces neuronal survival from 100 +/− 0.99 % to 60.83 +/− 2.99% and induces a 32.77 +/− 2.98 % release of LDH into the culture media. However, treatment with 5 nM of tPA 1 or 3 hours after exposure to NMDA increased cell survival to 74.53 +/− 2.01 % and 68.45 +/− 1.81 % and decreased the release of LDH into the media to 22.60 +/− 2.7% and 29.80 +/− 4.30 %, respectively. Importantly, this protective effect of tPA was abrogated by co-treatment with RAP (Fig 3 E & F, p < 0.05).
Extrasynaptic NR2B-containing NMDARs mediate NMDA-induced neuronal death
NMDARs are localized in synaptic and extrasynaptic sites. However, while activation of synaptic NMDARs has a neuroprotective effect, extrasynaptic NMDARs preferentially triggers cell death pathways 14. Thus we decided to investigate if the deleterious effect of NMDA on neuronal survival was mediated by its interaction with extrasynaptic NMDARs. First we blocked synaptic NMDARs by co-application 50 M of bicuculline (blocks GABAA receptor-mediated synaptic inhibition, enhancing the release of glutamate from presynaptic terminals) and 10 M of MK-801 (an irreversible blocker of open NMDA receptor channels), as described elsewhere 14, followed by 55 minutes of incubation with NMDA. We found that NMDA-induced neuronal death is not blocked by inhibition of synaptic NMDARs (Fig 4A). Because approximately 2/3 of synaptic NMDARs contain NR2A sub-units 13 whereas most of the extrasynaptic NMDARs are assembled with NR2B sub-units, we performed similar observations in neurons treated with either 10 nM of the selective NR2A inhibitor TCN-201 or 0.5 M of the NR2B inhibitor Ro 25-6981. We found that NR2A antagonism does not abrogate NMDA-induced neuronal death, confirming our initial observation that synaptic NMDARs do not mediate the harmful effects of NMDA on cell survival. In contrast, the deleterious effect of NMDA was significantly attenuated by NR2B antagonism. Indeed, neuronal survival decreased from 100 +/− 6.66 % in control cells to 64.31 +/− 5.07 % in neurons treated with NMDA alone. In contrast, cell survival was 61.73 +/− 5.07 % and 82.96 +/− 3.80 % in neurons in which either NR2A or NR2B subunits where antagonized, respectively (Fig 4A).
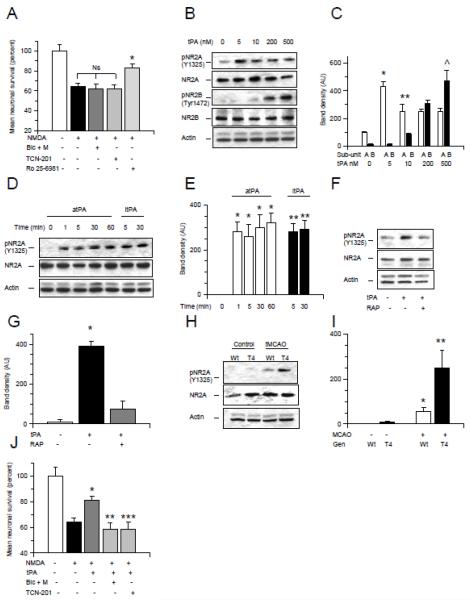
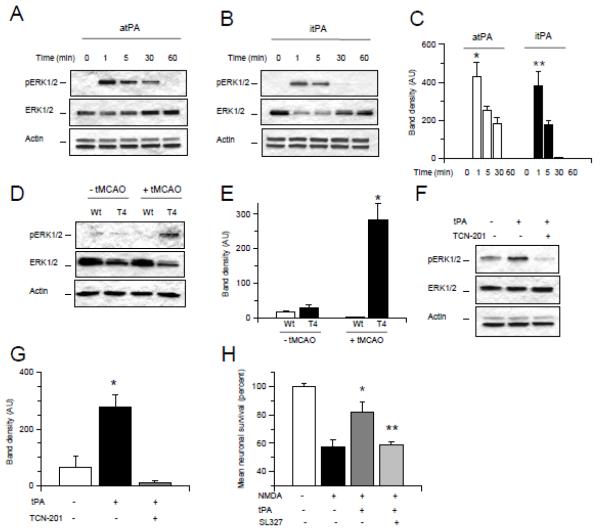
Figure 4. The neuroprotective effect of tPA is mediated by activation of synaptic NR2A-containing NMDA receptors.
A. Mean cell survival in wild-type (Wt) cerebral cortical neurons incubated with 50 M of NMDA alone or in the presence of either a combination of 50 M of bicuculline and 10 M of MK-801 (Bic + M), or 10 M of TCN-201, or 500 nM of Ro 25-6981. n = 20 per experimental group. * p < 0.05 compared to neurons incubated with NMDA alone or in the presence of a combination of bicuculline and MK-801, or TCN-201. Ns = non-significant. Lines represent SD. B & C. Representative Western blot analysis (B) and mean density of the band (C) for pNR2A (Y1325), total NR2A, pNR2B (Tyr1472) and total NR2B expression in Wt cerebral cortical neurons incubated 60 minutes with 0 – 500 nM of tPA. * p < 0.05 compared to untreated neurons or with NR2B phosphorylation in neurons treated with 5 nM of tPA. ** p < 0.05 compared to NR2B phosphorylation in neurons treated with 10 nM of tPA. § p < 0.05 compared to NR2A phosphorylation in neurons treated with 200 nM of tPA. ^ p < 0.05 compared to NR2A phosphorylation in neurons treated with 500 nM of tPA. D & E. Representative Western blot analysis (D) and mean density of the band (E) for pNR2A (Y1325) and total NR2A expression in Wt cerebral cortical neurons incubated 0 – 60 minutes with 5 nM of either active or proteolyticaly inactive tPA (atPA and itPA, respectively). * and ** p < 0.05 compared to untreated neurons. F & G. Representative Western blot analysis (F) and mean density of the band (G) of pNR2A (Y1325) and total NR2A expression in Wt cerebral cortical neurons incubated 5 minutes with 5 nM of tPA alone or in the presence of 100 nM of RAP. * p < 0.05 compared to neurons either untreated or incubated with tPA in the presence of RAP. H & I. Representative Western blot analysis (H) and mean density of the band (I) of pNR2A and total NR2A expression in extracts from the ischemic tissue of T4 mice and their Wt littermate controls one hour after transient occlusion of the middle cerebral artery (tMCAO). * p < 0.05 compared to non-ischemic Wt brains. ** p < 0.05 compared to ischemic Wt brains. F. Mean cell survival in Wt cerebral cortical neurons incubated 55 minutes with NMDA alone or in the presence of either a combination of bicuculline and MK-801 (Bic + M; to block synaptic NMDARs), or TCN-201 (to selectively antagonize NR2A-containing NMDARs) followed 60 minutes later with treatment with 5 nM of tPA. n = 16 per experimental group. *: p < 0.05 compared to neurons incubated with NMDA without ensuing treatment with tPA. ** and ***: p < 0.05 compared to cells incubated with NMDA followed by treatment with tPA alone. Lines depict SD.
NR2A-containing NMDARs mediate the protective effect of tPA against excitotoxin-induced neuronal death
To investigate the effect of tPA on NMDAR activation we studied the expression of NR2A phosphorylated at Y1325 and NR2B phosphorylated at Tyr1472 in Wt cerebral cortical neurons incubated with 0 - 500 nM of tPA. We found that incubation with 5 or 10 nM of tPA induces NR2A but not NR2B phosphorylation. Indeed, we observed NR2B phosphorylation only in cells incubated with 200 or 500 nM of tPA (Fig 4B & C). The effect of tPA on NR2A phosphorylation was already observed at one minute of incubation and was independent of tPA’s ability to cleave plasminogen into plasmin (Fig 4D & E). These data indicate that at concentrations found in vivo after the induction of cerebral ischemia and treatment with rtPA or in vitro after exposure to OGD conditions 16, 18, tPA preferentially activates synaptic NR2A-containing NMDARs. Additionally, our data indicate that the effect of tPA on NR2A phosphorylation requires the engagement of a member of the LDL receptor gene family (Fig 4F & G) suggesting that tPA does not interact directly with the NMDAR but instead that the effect of tPA on NMDARs in mediated by a co-receptor, most likely a member of the LDL receptor family. Because excitotoxicity is a basic mechanism of cell death in the ischemic brain 19, we decided to study the expression of NR2A subunits phosphorylated at Y1325 in brain extracts of T4 mice and their Wt littermate controls one hour after transient occlusion of the middle cerebral artery (tMCAO). We found that compared to Wt littermate controls, overexpression of neuronal tPA is associated with an increase in NR2A phosphorylation in the ischemic tissue (Fig 4H & I). To investigate whether NR2A-containing NMDARs mediate the protective effect of tPA we quantified cell survival in neurons incubated with either bicuculline and MK-801 (to block synaptic NMDARs) or TCN-201 (to selectively block NR2A-containing NMDARs) and exposed during 55 minutes to NMDA followed one hour later by treatment with 5 nM of tPA. We found that either blockade of synaptic NMDARs or selective antagonism of NR2A sub-units abrogates the protective effect of tPA on NMDA-induced neuronal death (Fig 4J, p < 0.05).
TPA induces transient ERK ½ activation via NR2A-containing NMDARs
Because NMDAR activation leads to ERK ½ phosphorylation, we decided to investigate the effect of 0 – 60 minutes of incubation with 5 nM of atPA or itPA on the expression of ERK ½ phosphorylated at Thr202/Tyr204 (pERK ½). We found that tPA induces a rapid and transient activation of ERK ½ and that this effect is independent of tPA’s proteolytic activity (Fig 5A-C). To study the effect of endogenous neuronal tPA on ERK ½ activation, we performed similar observations in brain extracts of T4 mice and their Wt littermate controls under non-ischemic conditions and 1 hour after the induction of experimental cerebral ischemia. Our data show that neuronal overexpression of tPA induces a significant increase in ERK 1/2 activation in the ischemic brain (Fig 5D & E). Additionally, our results indicate that tPA-induced ERK ½ activation is mediated by NR2A-containing synaptic NMDARs (Fig 5F & G) and that the neuroprotective effect of tPA is abrogated by co-treatment with the ERI ½ inhibitor SL327 (Fig 5H, p < 0.05).
Figure 5. ERK ½ activation mediates the protective effect of tPA against excitotoxin-induced neuronal death.
A - C: Representative Western blot analysis for ERK ½ phosphorylated at Thr202/Tyr204 (pERK ½) and total ERK ½ in Wt cerebral cortical neurons incubated 0 – 60 minutes with 5 nM of either proteolytically active or inactive tPA (atPA in (A) and itPA in (B), respectively). C corresponds to mean density of the band in A and B. * and ** p < 0.05 when compared to neurons not treated with tPA or treated with tPA during 5, 30 or 60 minutes. D & E. Representative Western blot analysis (D) and mean density of the band (E) of pERK ½ and total ERK ½ expression in brain extracts of T4 mice and their Wt littermate controls under non-ischemic conditions (- tMCAO) or 1 hour after tMCAO (+ tMCAO). * p < 0.05 compared to Wt brains after tMCAO and to non-ischemic Wt and T4 brains. F & G. Representative Western blot analysis (F) and mean intensity of the band (G) for pERK ½ and total ERK ½ in Wt cerebral cortical neurons following one minute of incubation with 5 nM of tPA alone or in combination with 10 M of TCN-201. * p < 0.05 compared to neurons either left untreated or incubated with tPA in the presence of TCN-201. H. Mean cell survival in Wt cerebral cortical neurons incubated 55 minutes with NMDA followed one hour later by treatment with 5 nM of tPA alone or in combination with the ERK ½ inhibitor SL327 10 M. n =16 per experimental group. * p < 0.05 compared to cells incubated with NMDA without ensuing treatment with tPA. ** p < 0.05 compared to cells incubated with NMDA and treated one hour later with tPA alone. Lines depict SD.
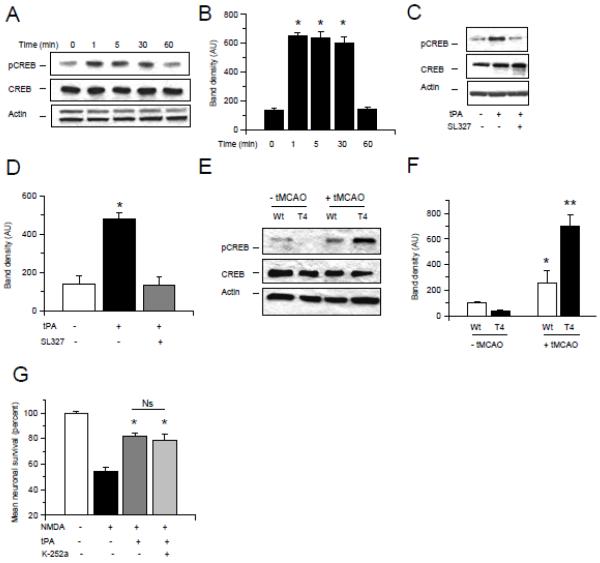
TPA activates the CREB-Atf3 neuroprotective pathway
Because CREB is an ERK ½ target with a pro-survival effect in neurons 20, we studied the expression of CREB phosphorylated at Ser133 (pCREB) in Wt cerebral cortical neurons incubated 0 - 60 minutes with 5 nM of tPA, alone or in combination with the ERK ½ inhibitor SL327. Our results indicate that tPA induces a rapid increase in pCREB expression (Fig 6A & B) and that this effect is mediated by ERK ½ (Fig 6C & D). To investigate the effect of neuronal tPA on CREB phosphorylation in vivo we performed a Western blot analysis for pCREB in brain extracts from Wt and T4 mice 1 hour after tMCAO. We found that neuronal overexpression of tPA is associated with a sustained increase in pCREB expression in the ischemic tissue (Fig 6E & F). Because it has been proposed that the brain derived neurotrophic factor (BDNF) mediates the neuroprotective effect of CREB 21 we investigated whether antagonism of TrkB, the cognate receptor for BDNF, abrogates the protective effect of tPA on neurons previously exposed to NMDA. Our data show that TrkB inhibition does not inhibit the protective effect of tPA (Fig 6G).
Figure 6. TPA activates the ERK ½-CREB-Atf3 pathway.
A - F. Representative Western blot analyses for CREB phosphorylated at Ser133 (pCREB) and total CREB in Wt cerebral cortical neurons incubated 0 – 60 minutes with 5 nM of tPA (A), or treated during 5 minutes with a combination of tPA and the ERK ½ inhibitor SL327 (C), or in extracts prepared from the ischemic tissue from T4 mice and their Wt littermate controls one hour after transient occlusion of the middle cerebral artery (tMCAO; E). B, D & F represent mean density of the band for the experiments showed in A, C and E, respectively. * in B: p < 0.05 compared to neurons treated 0 or 60 minutes with tPA. * in D: p < 0.05 compared to untreated cells or with neurons treated with a combination of tPA and SL327. * in F: p < 0.05 compared to non-ischemic Wt and T4 brains. ** in F: p < 0.05 compared to Wt brains after tMCAO and with non-ischemic Wt and T4 brains. G. Mean cell survival in neurons incubated 55 minutes with NMDA followed 60 minutes later by treatment with 5 nM of tPA alone or in combination with 100 nM of the TrkB inhibitor K-252a. n = 12 per experimental group. * p < 0.05 compared to neurons incubated with NMDA without subsequent treatment with tPA. Ns: non-significant.
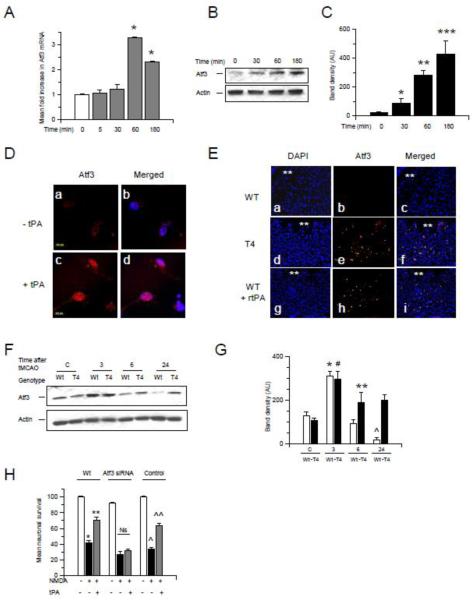
Previous studies indicate that activation of synaptic NMDARs has a neuroprotective effect mediated by induction of the CREB-regulated gene Atf3 14. Thus we decided to investigate the expression of Atf3 following 0 - 180 minutes of incubation with 5 nM of tPA. We found that tPA induces a rapid increase in Atf3 mRNA (3.3 +/− 0.013 fold increase; Fig 7A) and protein (Fig 7B -D) expression. To study the effect of tPA on neuronal Atf3 expression in vivo we performed an immunohistochemical analysis of Atf3 in brain sections of T4 mice and their Wt littermate controls 6 hours after the intracerebral injection of NMDA. A sub-group of Wt animals was treated with 1 mg/Kg/IV of rtPA 1 hour after the injection. We found that Atf3 expression increases in the area surrounding the injection site in T4 but not in Wt mice, and that treatment with rtPA increases Atf3 expression in Wt mice (Fig 7E). Then we performed similar observations in brain extracts of Wt and T4 mice 0 - 24 hours after tMCAO. Our results show that Atf3 expression increases in both Wt and T4 mice 3 hours after tMCAO. However, whereas Atf3 expression decreases at 6 and 24 hours in Wt animals it remains elevated in T4 mice (Fig 7F & G). Then we investigated whether Atf3 mediates the protective effect of tPA. Wt neurons treated with Atf3 siRNA (RT-PCR analysis indicated a 60% decrease in Atf3 mRNA expression) or with a non-targeting control were incubated during 55 minutes with NMDA and treated 60 minutes after the end of exposure to NMDA with 5 nM of tPA. We found that Atf3 down-regulation abrogates the protective effect of tPA against excitotoxin-induced neuronal death (Fig 7H). Importantly, Atf3 down-regulation was associated with a 9.4 +/− 2.6 % decrease in cell survival in neurons not exposed to NMDA. Likewise, the effect of NMDA on neuronal death was enhanced by Atf3 down-regulation (an additional 11.3 +/− 3.3 % decrease in neuronal survival when compared to the effect of NMDA on neurons with Atf3 fully active).
Figure 7. Atf3 mediates the protective effect of tPA against excitotoxin-induced neuronal death. A.
Mean fold increase in Atf3 mRNA expression in Wt cerebral cortical neurons incubated 0 – 180 minutes with 5 nM of tPA. n = 6 per time-point; Lines depict SD. B &C. Representative Western blot analysis (B) and mean density of the band (C) for Atf3 expression in Wt cerebral cortical neurons following 0 - 180 minutes of incubation with 5 nM of tPA. *, **, and ***: p < 0.05 compared to neurons at time 0. n = 6. D. Representative micrographs of Atf3 staining in Wt cerebral cortical neurons treated 6 hours with 5 nM of tPA (+ tPA) or with a comparable volume of vehicle (control; - tPA). Red is Atf3, blue is DAPI. Magnification is 100 X. E. Representative micrographs in the striatum of Wt (a-c) and T4 (d – f) mice 6 hours after the intrastriatal injection of NMDA. A sub-group of Wt mice (g – i) was treated with rtPA 1 mg/Kg/IV 1 hour after the intrastriatal injection of NMDA. Asterisks denote the injected site. Blue is DAPI; red is Atf3. Magnification: 20X. F & G. Representative Western blot analysis (F) and mean density of the band (G) for Atf3 expression in extracts prepared from the ischemic tissue of Wt and T4 mice 0 - 24 hours after tMCAO. * and # p < 0.05 compared to corresponding controls. ** p < 0.05 compared to Wt brains 6 hours after tMCAO. ^: p < 0.05 compared to Wt mice control and 3 and 6 hours after tMCAO. §: p < 0.05 compared to Wt mice 24 hours after tMCAO. n = 6. H. Mean neuronal survival in Wt neurons treated with Atf3 siRNA or with a non-targeting control and incubated with NMDA during 55 minutes followed 1 hour later by treatment with 5 nM of tPA. n = 12 per experimental condition. * p < 0.05 compared to neurons not incubated with NMDA. ** p < 0.05 compared to neurons incubated with NMDA without subsequent treatment with tPA. ^: p < 0.05 compared to neurons treated with a non-targeting control but not incubated with NMDA. ^^ p < 0.05 compared to non-targeting control-treated neurons incubated with NMDA without subsequent treatment with tPA. Ns: non significant. Lines denote SD.
Discussion
The data presented here indicate that tPA protects neurons from excitotoxin-induced death and that this effect is mediated by tPA’s ability to activate synaptic NMDARs. Based on our results here we propose a model where the release of neuronal tPA is the first step of a rapid endogenous neuroprotective response aimed at counteracting the harmful effects of an excessive release of excitotoxins in the synaptic space.
Our findings are in apparent discrepancy with a proposed role of tPA as mediator of excitotoxin-induced neuronal death 22-23. Indeed, we found that tPA potentiates the deleterious effect of NMDA on neuronal survival only at concentrations of tPA several orders of magnitude above those found in neuronal cultures after exposure to hypoxic conditions or in the ischemic brain after treatment with rtPA 16. Thus we believe that our disagreement with previous studies can be explained at least in part by differences in the experimental design, most notably by the use in our work of lower concentrations of tPA.
The observation that either treatment with rtPA or neuronal overexpression of tPA protects against excitotoxin-induced neuronal death indicates that most likely the concentration of tPA in the synaptic space needed to trigger a neuroprotective response is low. Indeed, although our early work shows that treatment with rtPA induces an approximately 25% increase in the concentration of tPA in the ischemic brain, this is still lower compared to the concentration of tPA in the ischemic brain of mice overexpressing neuronal tPA.
Despite the existence of a growing body of evidence indicating that tPA modulates NMDAR function 6, 24, the mechanism underlying this interaction still remains unclear. The data presented here indicate that tPA induces preferential phosphorylation of synaptic NR2A-containing NMDARs and that this interaction does not involve proteolytic cleavage of the receptor. We observed NR2B phosphorylation with doses of tPA greater than 200 nM which, as our previous data indicate 16, 18, results in concentrations unlikely to be found in vivo in the brain or in vitro in neuronal cultures. Additionally, we found that the effect of tPA on NR2A sub-units is independent of tPA’s proteolytic properties.
Calcium entry through synaptic and extrasynaptic NMDARs evokes different transcriptional responses 25, and whereas synaptic NR2A-containing NMDARs are associated with activation of neuroprotective signaling pathways, extrasynaptic NR2B-containing NMDARs have been linked to cell death 14. In agreement with these observations, or data indicate that NMDA-induced neuronal death is mediated by NR2B-but not NR2A-containing NMDARs. Based on our results and the observation that activation of synaptic NMDARs counteracts the deleterious effects of extrasynaptic NMDARs, here we propose a model where the presence of tPA in the synaptic space offsets the harmful effects of NMDA on extrasynaptic NR2B-containing NMDARs by preferentially activating synaptic NR2A-containing NMDARs. This hypothesis is further supported by our observation that either blockade of synaptic NMDARs or selective inhibition of NR2A sub-units abrogates the protective effect of tPA.
Notwithstanding the fact that many components of the NMDAR signaling complex have been identified, it is still unclear what proteins specifically associate with synaptic versus extrasynaptic NMDARs. Our data shows that the effect of tPA on synaptic NR2A sub-units is mediated by a member of the LDL receptor family, most likely LRP1. This finding is of the utmost clinical importance. Indeed, because treatment with direct NMDARs inhibitors have failed clinical trials, the possibility of indirect modulation of NMDAR function via LRP1 may be a therapeutic possibility in patients with neurological conditions such as cerebral ischemia, trauma and seizures.
ERK ½ are members of the mitogen activated protein kinase (MAPK) family that are regulated by a cascade of phosphorylations at Thr/Tyr residues carried out by a MAP kinase kinase ½ which in turn is activated by phosphorylation catalyzed by the Raf family of protein kinases. Our data indicate that tPA activates ERK ½ in neurons via NR2A-containing NMDARs by a mechanism that does not require of tPA’s proteolytic properties. ERK ½ has a dual role in excitotoxin-induced neuronal death. Accordingly, sustained ERK ½ activation following an excitotoxic injury promotes death, while transient ERK ½ activation has a pro-survival effect 12. Our results show that the effect of tPA on ERK ½ activation is rapid and transient and that ERK ½ inhibition abrogates the protective effect of tPA against excitotoxin-induced neuronal death.
CREB is an activator of a neuroprotective genome program induced by the entry of calcium through synaptic NMDARs 26. In contrast, activation of extrasynaptic NR2B-containing NMDARs shuts-off the CREB pathway 13. Although in some experimental systems the protective effect of CREB activation is mediated by BDNF, we found that TrkB antagonism does not abrogate the neuroprotective effect of tPA. Instead, our data indicate that tPA induces the expression of the CREB-regulated activating transcription factor 3 (Atf3) which is known to play a pivotal role in neuroprotection induced by activation of synaptic NMDARs 14. Additionally, we found that ERK ½ mediates tPA-induced neuroprotection and CREB phosphorylation.
Based on our data we propose a model where tPA, released into the synaptic space in response to a stimulus that also induces the excessive release of excitotoxins, activates synaptic NR2A-containing NMDARs. This effect turns on a neuroprotective cell signaling response, mediated by activation of the CREB/Atf3 pathway, that counteracts the harmful consequences of extrasynaptic NR2B-containing NMDARs activation. However, from a clinical perspective, in absence of an effective strategy to induce the release of endogenous neuronal tPA during an acute excitotoxic injury, treatment with rtPA seems to be a more efficient method to induce this neuroprotective response. Our findings bring a novel insight into tPA’s function in the CNS and suggest that the interaction between tPA and NR2A-containing NMDARs may be explored for therapeutic purposes in patients with neurological diseases associated with excitotoxic neuronal death.
Experimental Methods
Animals and reagents
Murine strains were 8-12 weeks old male wild-type (Wt) C57BL/6J mice, and mice with a 10-fold increase in tPA expression in neurons 27 (T4 transgenic mice, kindly provided by Professor JD Vassalli and Doctor R Mandani, from University of Geneva, Switzerland) and their Wt littermate controls. Experiments were approved by the Institutional Animal Care & Use Committee of Emory University, Atlanta GA. Recombinant murine tPA and inactive tPA (itPA) with an alanine for serine substitution at the active site Ser481 (S481A) were purchased from Molecular Innovations (Novi, MI). Other reagents were human recombinant tPA (rtPA; Genentech; San Francisco, CA), the Receptor-Associated Protein (RAP; kindly provided by Dr. Dudley K. Strickland, University of Maryland), the NMDAR antagonist MK-801, the NR2A antagonist TCN-201, the MEK ½ inhibitor SL327, the NR2B antagonist Ro 25 6981 and bicuculline (Tocris Bioscience; Ellisville, MO), NMDA (Sigma-Aldrich; St. Louis, MO), antibodies against NR2A phosphorylated at Y1325, total NR2A and Atf3 (Abcam; Cambridge, MA), NR2B phosphorylated at Tyr1472 and total NR2B (Sigma-Aldrich; St. Louis, MO), CREB phosphorylated at Ser 133, total CREB, ERK ½ phosphorylated at Thr202/Tyr204, total ERK ½ and cleaved caspase-3 (Cell Signaling Technology Inc; Danvers, MA), and anti-actin (Santa Cruz Biotechnology; Santa Cruz, CA); the nuclear marker 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes; Grand Island, NY), and the 3-(4,5 Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Manassas, VA), and lactate dehydrogenase (LDH) release assays (Roche, Indianapolis, IN), the TrkB inhibitor K-252a (Calbiochem, Rockland, Massachusetts), and Atf3 siRNA (E-058604-00-0010) and a non-targeting control (D-001910-10-20;Thermo Scientific Dharmacon; Lafayette, CO), and primers for Gapdh: Mm99999915_g1 and Atf3: Mm00476032_m1 (Applied Biosystems; Carlsbad, CA)
Intracerebral injection of NMDA and animal model of cerebral ischemia
T4 mice and their corresponding Wt littermate controls (n = 12 per experimental group) were anesthetized with 4% chloral hydrate (400 mg/kg/IP) and injected with 1 ml of NMDA 50 mM, using a microsyringe pump (World Precision Instruments; Sarasota, Fl), at an infusion rate of 50 nL/min (total infusion time: 20 minutes), at the following coordinates: bregma: 0.26 mm, mediolateral: 2 mm, dorsoventral: 3 mm 16. To avoid reflow the needle was left in place during 15 minutes after finishing the infusion. A sub-group of Wt mice was treated one hour after NMDA injection with 1 mg/Kg/IV of human recombinant tPA or with a comparable volume of saline solution. Arginin was dialyzed from rtPA with a SnakeSkin Dialysis Tubing (ThermoScientific) prior to its administration. To induce cerebral ischemia the middle cerebral artery (MCA) was exposed in T4 mice and their Wt littermate controls and occluded during 60 minutes with a 6-0 silk suture as described elsewhere 28. Cerebral perfusion (CP) was monitored throughout the surgical procedure with a laser Doppler (Perimed Inc., North Royalton, OH), and only animals with a > 70% decrease in CP after occlusion and complete recovery after suture removal were included in this study. Heart rate, systolic, diastolic and mean arterial blood pressure were controlled with an IITC 229 System (IITC-Lice Science; Woodland Hills, CA). Twenty-four hours after the intrastriatal injection of NMDA animals were transcardially perfused with 4% paraformaldehyde. To measure the volume of brain lesioned by the injection of NMDA, twenty 20 μM cuts were obtained every 100 μ M from bregma: + 1.34 mm through bregma − 0.6 mm and stained with the modified wisconsin thionin staining (1%). The area of lesioned brain was measured in each cut with the NIH-Image analyzer system and the volume of lesioned brain was calculated by: Vlesioned brain = Σ(Areas lesioned brain) × distance between sections. A sub-group of sections was obtained 6 hours after the intracerebral injection of NMDA and stained with antibodies against Atf3.
Neuronal cultures, determination of cell survival/death and immunohistochemical detection of Atf3
Wild-type cerebral cortical neurons were cultured from E19 Wt mice as described elsewhere 29 and incubated during 55 minutes with 50 M of NMDA alone, or 5 - 500 nM of proteolytically active or inactive tPA alone, or with a combination of NMDA 50 M and 5 - 500 nM of proteolytically active or inactive tPA. A sub-group of neurons was treated 5 - 180 minutes after incubation with NMDA with 5 nM of either proteolytically active or inactive tPA, alone or in combination with either 100 nM of RAP, or 10 M of the ERK ½ inhibitor SL327, or 100 nM of the TrkB antagonist K-252a. To block the synaptic or extrasynaptic NMDARs, neurons were incubated 10 minutes before exposure to NMDA with either a combination of 50 M of bicuculline and 10 M of MK-801, or with 0.5 M of the NR2B inhibitor Ro 25-6981, respectively. To selectively antagonize NR2A subunits, neurons were incubated with 10 M of the NR2A antagonist TCN-201 in the presence of 3 M of glycine. To investigate the role of Atf3 on the neuroprotective effect of tPA, Wt cerebral cortical neurons were treated with 1 μM of Atf3 siRNA or with a non-targeting control during 72 hours. RT-PCR analysis for Atf3 showed a 69% reduction in Atf3 mRNA expression. Following Atf3 siRNA- and non-targeting control-treatment neurons were incubated with NMDA during 55 minutes followed 60 minutes later by treatment with 5 nM of tPA. Cell survival/death were determined in all experimental groups 24 later with the MTT and LDH release assays, respectively, following manufacturer’s instructions. For the MTT assay, results are expressed as a percentage of cell survival per experimental group compared to cell survival in neurons not exposed to NMDA. For the LDH release assay results are given as percentage of LDH released into the media in each experimental group compared to LDH released from neurons incubated 24 hours with triton 1%. Each experiment was performed in cultures from three different animals and each observation was repeated 16-22 times. A sub-group of Wt neurons incubated with either 5 nM of tPA or vehicle (control) was fixed 6 hours later and immunostained with an anti-Atf3 antibody (1:1000) and the nuclear marker DAPI. Each observation was repeated 4 times.
Western blot analysis
Wt cerebral cortical neurons were incubated 60 minutes with 0 - 500 nM of tPA, or 0 – 60 minutes with 5 nM of proteolytically active or inactive tPA, or 1 minute with a combination of 5 nM of tPA and either 100 nM of RAP or 10 M of SL327. The brains of T4 mice and their Wt littermate controls were harvested 0 - 24 hours after tMCAO. Extracts were prepared as described elsewhere 29 and 50 g of protein were loaded per sample and blotted with antibodies against pNR2A (Y1325), total NR2A, pNR2B (Tyr1472), total NR2B, pCREB (Ser133), total CREB, pERK ½ (Thr202/Tyr204), total ERK ½, Atf3 and actin. Each observation was repeated 4-6 times. To investigate the effect of tPA on NMDA-induced apoptotic cell death, Wt cerebral cortical neurons were incubated with 50 μM of NMDA followed 1 hour later by treatment with either 5 nM of tPA or a comparable volume of vehicle (control). Twenty-four hours later the expression of cleaved caspase-3 was studied by Western blot analysis. Each observation was repeated 4 times. To quantify the Western blot data, gels were scanned and the density of the band was measured with the NIH-Image analyzer system.
Quantitative RT-PCR analysis
Wt neurons were incubated 0 – 180 minutes with 5 nM of tPA followed by quantification of Atf3 mRNA by fast real-time PCR with fluorescent-tagged probes Gapdh: Mm99999915_g1 and Atf3: Mm00476032_m1 (Applied Biosystems). GAPDH was used as the endogenous reference gene. The relative quantification method (ΔΔCt) was used, with the ratio of the mRNA level for the gene of interest normalized to the level of internal control. Each observation was repeated 6 times.
Statistical analysis
Values are expressed as percentage or mean ± SD when appropriate. Statistical analysis was performed with 1-way ANOVA. p values of less than 0.05 were considered significant.
Acknowledgements
This work was supported in part by National Institutes of Health Grants NS-062073 and P30NS055077 and VA MERIT Award BX000474
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1(8):623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- 2.Mori H, Mishina M. Structure and function of the NMDA receptor channel. Neuropharmacology. 1995;34(10):1219–1237. doi: 10.1016/0028-3908(95)00109-j. [DOI] [PubMed] [Google Scholar]
- 3.Choi DW. Ionic dependence of glutamate neurotoxicity. J.Neurosci. 1987;7(2):369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, et al. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J.Neurosci. 2007;27(11):2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat.Rev.Neurosci. 2010;11(10):682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samson AL, Medcalf RL. Tissue-type plasminogen activator: a multifaceted modulator of neurotransmission and synaptic plasticity. Neuron. 2006;50(5):673–678. doi: 10.1016/j.neuron.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, et al. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat.Med. 2001;7(1):59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- 8.Guo H, Barrett TM, Zhong Z, Fernandez JA, Griffin JH, Freeman RS, et al. Protein S blocks the extrinsic apoptotic cascade in tissue plasminogen activator/N-methyl D-aspartate-treated neurons via Tyro3-Akt-FKHRL1 signaling pathway. Mol.Neurodegener. 2011;6:13. doi: 10.1186/1750-1326-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsirka SE, Rogove AD, Bugge TH, Degen JL, Strickland S. An extracellular proteolytic cascade promotes neuronal degeneration in the mouse hippocampus. J.Neurosci. 1997;17(2):543–552. doi: 10.1523/JNEUROSCI.17-02-00543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pawlak R, Magarinos AM, Melchor J, McEwen B, Strickland S. Tissue plasminogen activator in the amygdala is critical for stress-induced anxiety-like behavior. Nat.Neurosci. 2003;6(2):168–174. doi: 10.1038/nn998. [DOI] [PubMed] [Google Scholar]
- 11.Lesuisse C, Martin LJ. Immature and mature cortical neurons engage different apoptotic mechanisms involving caspase-3 and the mitogen-activated protein kinase pathway. J.Cereb.Blood Flow Metab. 2002;22(8):935–950. doi: 10.1097/00004647-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Luo Y, DeFranco DB. Opposing roles for ERK1/2 in neuronal oxidative toxicity: distinct mechanisms of ERK1/2 action at early versus late phases of oxidative stress. J.Biol.Chem. 2006;281(24):16436–16442. doi: 10.1074/jbc.M512430200. [DOI] [PubMed] [Google Scholar]
- 13.Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat.Neurosci. 2002;5(5):405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- 14.Zhang SJ, Buchthal B, Lau D, Hayer S, Dick O, Schwaninger M, et al. A signaling cascade of nuclear calcium-CREB-ATF3 activated by synaptic NMDA receptors defines a gene repression module that protects against extrasynaptic NMDA receptor-induced neuronal cell death and ischemic brain damage. J.Neurosci. 2011;31(13):4978–4990. doi: 10.1523/JNEUROSCI.2672-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddrop C, Moldrich RX, Beart PM, Farso M, Liberatore GT, Howells DW, et al. Vampire bat salivary plasminogen activator (desmoteplase) inhibits tissue-type plasminogen activator-induced potentiation of excitotoxic injury. Stroke. 2005;36(6):1241–1246. doi: 10.1161/01.STR.0000166050.84056.48. [DOI] [PubMed] [Google Scholar]
- 16.Haile WB, Wu J, Echeverry R, Wu F, An J, Yepes M. Tissue-type plasminogen activator has a neuroprotective effect in the ischemic brain mediated by neuronal TNF-alpha. J.Cereb.Blood Flow Metab. 2012;32(1):57–69. doi: 10.1038/jcbfm.2011.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bu G, Morton PA, Schwartz AL. Identification and partial characterization by chemical cross-linking of a binding protein for tissue-type plasminogen activator (t-PA) on rat hepatoma cells. A plasminogen activator inhibitor type 1-independent t-PA receptor. J.Biol.Chem. 1992;267(22):15595–15602. [PubMed] [Google Scholar]
- 18.Echeverry R, Wu J, Haile WB, Guzman J, Yepes M. Tissue-type plasminogen activator is a neuroprotectant in the mouse hippocampus. J.Clin.Invest. 2010;120(6):2194–2205. doi: 10.1172/JCI41722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothman SM, Olney JW. Glutamate and the pathophysiology of hypoxic--ischemic brain damage. Ann.Neurol. 1986;19(2):105–111. doi: 10.1002/ana.410190202. [DOI] [PubMed] [Google Scholar]
- 20.Arthur JS, Fong AL, Dwyer JM, Davare M, Reese E, Obrietan K, et al. Mitogen- and stress-activated protein kinase 1 mediates cAMP response element-binding protein phosphorylation and activation by neurotrophins. J.Neurosci. 2004;24(18):4324–4332. doi: 10.1523/JNEUROSCI.5227-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walton MR, Dragunow I. Is CREB a key to neuronal survival? Trends Neurosci. 2000;23(2):48–53. doi: 10.1016/s0166-2236(99)01500-3. [DOI] [PubMed] [Google Scholar]
- 22.Tsirka SE, Gualandris A, Amaral DG, Strickland S. Excitotoxin-induced neuronal degeneration and seizure are mediated by tissue plasminogen activator. Nature. 1995;377(6547):340–344. doi: 10.1038/377340a0. [DOI] [PubMed] [Google Scholar]
- 23.Lopez-Atalaya JP, Roussel BD, Levrat D, Parcq J, Nicole O, Hommet Y, et al. Toward safer thrombolytic agents in stroke: molecular requirements for NMDA receptor-mediated neurotoxicity. J.Cereb.Blood Flow Metab. 2008;28(6):1212–1221. doi: 10.1038/jcbfm.2008.14. [DOI] [PubMed] [Google Scholar]
- 24.Samson AL, Nevin ST, Croucher D, Niego B, Daniel PB, Weiss TW, et al. Tissue-type plasminogen activator requires a co-receptor to enhance NMDA receptor function. J.Neurochem. 2008;107(4):1091–1101. doi: 10.1111/j.1471-4159.2008.05687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang SJ, Steijaert MN, Lau D, Schutz G, ucinge-Vivier C, Descombes P, et al. Decoding NMDA receptor signaling: identification of genomic programs specifying neuronal survival and death. Neuron. 2007;53(4):549–562. doi: 10.1016/j.neuron.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 26.Hardingham GE, Arnold FJ, Bading H. Nuclear calcium signaling controls CREB-mediated gene expression triggered by synaptic activity. Nat.Neurosci. 2001;4(3):261–267. doi: 10.1038/85109. [DOI] [PubMed] [Google Scholar]
- 27.Madani R, Hulo S, Toni N, Madani H, Steimer T, Muller D, et al. Enhanced hippocampal long-term potentiation and learning by increased neuronal expression of tissue-type plasminogen activator in transgenic mice. EMBO J. 1999;18(11):3007–3012. doi: 10.1093/emboj/18.11.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belayev L, Busto R, Zhao W, Fernandez G, Ginsberg MD. Middle cerebral artery occlusion in the mouse by intraluminal suture coated with poly-L-lysine: neurological and histological validation. Brain Res. 1999;833(2):181–190. doi: 10.1016/s0006-8993(99)01528-0. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Polavarapu R, She H, Mao Z, Yepes M. Tissue-type plasminogen activator and the low-density lipoprotein receptor-related protein mediate cerebral ischemia-induced nuclear factor-kappaB pathway activation. Am J Pathol. 2007;171(4):1281–1290. doi: 10.2353/ajpath.2007.070472. [DOI] [PMC free article] [PubMed] [Google Scholar]