Abstract
PEGylated lipopeptide surfactants carrying drug-interactive motifs specific for a peptide-nitroxide antioxidant, JP4-039, were designed and constructed to facilitate the solubilization of this drug candidate as micelles and emulsion nanoparticles. A simple screening process based on the ability that prevents the formation of crystals of JP4-039 in aqueous solution was used to identify agents that have potential drug-interactive activities. Several protected lysine derivatives possessing this activity were identified, of which α-Fmoc-ε-tBoc lysine is the most potent, followed by α-Cbz- and α-iso-butyloxycarbonyl-ε-tBoc-lysine. Using polymer-supported liquid-phase synthesis approach, a series of synthetic lipopeptide surfactants with PEG head group, varied numbers and geometries of α-Fmoc or α-Cbz-lysyl groups located at interfacial region as the drug-interactive domains, and oleoyl chains as the hydrophobic tails were synthesized. All α-Fmoc-lysyl-containing lipopeptide surfactants were able to solubilize JP4-039 as micelles, with enhanced solubilizing activity for surfactants with increased numbers of α-Fmoc groups. The PEGylated lipopeptide surfactants with α-Fmoc-lysyl groups alone tend to form filamentous or worm-like micelles. The presence of JP4-039 transformed α-Fmoc-containing filamentous micelles into dots and bar-like mixed micelles with substantially reduced sizes. Fluorescence quenching and NMR studies revealed that the drug and surfactant molecules were in a close proximity in the complex. JP4-039-loaded emulsion carrying α-Cbz-containing surfactants demonstrated enhanced stability over drug loaded emulsion without lipopeptide surfactants. JP4-039-emulsion showed significant mitigation effect on mice exposed to a lethal dose of radiation. PEGylated lipopeptides with an interfacially located drug-interactive domain are therefore tailor-designed formulation materials potentially useful for drug development.
Keywords: surfactant, Fmoc, interface, micelle, emulsion, nitroxide, JP4-039
1. INTRODUCTION
Drug discovery is a costly and inefficient process. Many promising hits identified from initial screens are often excluded from further investigation due to poor water solubility and/or low bioavailability. Incorporating drug formulation expertise early in the drug discovery process can significantly improve biological, pharmacological and toxicological profiles of many potentially valuable drug candidates, enable an in vivo evaluation, and maximize the value of a compound library [1,2]. Added features such as targeted delivery and slow release can further improve the overall performance of active pharmaceutical ingredients (APIs) [3,4]. Lipid-based formulations such as micelles, liposomes, or emulsions are important carriers for poorly water-soluble APIs [3,5]. However, such systems are often not well suited for many moderately hydrophobic APIs due to a poor mixing of hydrophobic carrier materials with APIs, which leads to gradual exclusion and “leakage” from lipid formulations [5].
Our primary interest is to develop suitable nanoparticle carriers that solubilize JP4-039, a novel peptidomimetic nitroxide antioxidant that showed promising radiation mitigating activities in 32Dcl3 cells and in mouse models [6–11]. JP4-039 (Fig. 3C) is a mitochondria targeted scavenger for reactive oxygen species [6–8]. It has been shown to have protective effects for mucosa [9], skin [10], and bone marrow stems cells against radiation-induced damage. It also ameliorates the irradiation-induced delay of wound healing of bone and improves the survival of mice exposed to large doses of ionizing radiation [11]. Poor water solubility prevented the assessment of the in vivo activity of JP4-039, and we have previously developed biocompatible emulsion- and liposome-based formulations for this agent suitable for either systemic (i.p. and i.v.) or topical such as skin [10] and esophageal [9] deliveries. However, JP4-039 slowly disassociated from the emulsion or liposome particles and started to crystalize. Optimization strategies using existing surfactants and oils were not particularly effective because most commercial formulation materials share extensive structural similarities. Here, we report that new surfactant molecules possessing specific drug interactive functionalities can be tailored to improve the solubility of a poorly water-soluble, yet moderately hydrophobic drug candidate in the form of a micelle complex, and that incorporation of drug-interactive surfactants can significantly improve the formulation stability of drug-loaded emulsion particles.
Figure 3.
Solubilization of JP4-039 facilitated by PEG-lipopeptide 4 micelles. Various amounts of tetra chain PEG5,000-lysyl-[lysyl-(α-Fmoc-ε-oleyol-lysine)2]2 were mixed with JP4-039 in CHCl3 followed by solvent evaporation, the drug-loaded micelles were prepared by hydration in saline. The amounts of solubilized JP4-030 were determined by OD448 measurements from supernatant.
2. MATERIALS AND METHODS
α-Fmoc-ε-Boc-lysine, di-Boc-lysine, DCC, NHS, TFA, TEA were from AAPPTEC; THF anhydrous was from Acros Organic; Monomethoxy PEG with MW of 1,000, 2,000, and 5,000, eosin Y, DMAP, ninhydrin, oleoyl chloride, sesame oil, and other unspecified reagent pure chemicals were from Sigma-Aldrich. 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine and soy phosphatidyl choline (95%) were purchased from Avanti Polar Lipids. JP4-039 was synthesized by Asymchem Inc. according to Frantz et al. [12]
2.1. Screening of protected amino acid derivatives as solubilizers and inhibitor of crystallization for JP4-039
α-acetyl-ε-Boc-lysine, α-iso-butoxycarbamoyl-ε-Boc-lysine were synthesized from α-NH2-ε-Boc-lysine (1 mmole) dissolved in 5 mL of saturated sodium bicarbonate solution by adding a 4-fold excess of acetyl anhydride or isobutyl chloroformate dissolved in 5 mL of THF over a period of 5 minutes, respectively. THF was removed from the reaction mixture, the remaining mixture was diluted with 50 mL of ethyl acetate. The organic phase was washed with 20 mL of saturated NaHCO3, saline, citric acid (0.5 M) and water sequentially. The organic phase was dried over anhydrous sodium sulfate, and the solvent was removed. The solid residues were recrystallized from ethyl acetate/hexanes mixture. These derivatives, together with a panel of commercially available amine protected derivatives of lysine, phenylalanine, and glycine were prepared as 1–100 mM solution or suspension in 0.1 M Na2HPO4 buffer. Meanwhile, 0.447 μg of JP4-039 dissolved in 5 μL of methanol was added to each well of a 96-well polystyrene plate. One hundred μL of amino acid solution/suspension were added to the methanol solution and mixed well. The physical status of the samples in each well was inspected visually for the physical appearance of brown crystals periodically over 2 h. Some samples were photographed under a microscope after 20 min.
2.2 Synthesis of PEG-amino acid or –peptide-lipid conjugates
2.2.1. Monomethoxy PEG2,000-α-Fmoc-ε-Oleoyl Lysine (1)
Monomethoxy PEG2,000 OH (1 mmol) was esterified with α-Fmoc-ε-t-Boc lysine (2 mmol) with DCC (2.2 mmol) and DMAP (0.1 mmol) in CH2Cl2 at room temperature overnight. Solid precipitate was removed by filtration. The filtrate was concentrated by evaporation. The PEG derivative was precipitated with 10 volumes of cold ethyl ether and washed three times with the same solvent. Additional washes with cold ethanol were used to remove DMAP. The PEG-α-Fmoc-ε-t-Boc lysine ester was dissolved in 4 mL of CH2Cl2 to which 4 mL of TFA was added to deprotect the Boc group for 20 min at room temperature. After removal of most of CH2Cl2, the PEG-α-Fmoc-ε-NH2-lysine ester was precipitated with cold ether and washed two more times with the same solvent. The PEG-α-Fmoc-ε-NH2-lysine ester is end-capped with oleoyl chloride (2 mmol) and TEA (2 mmol) for 20 min. PEG-α-Fmoc-ε-Oleoyl lysine ester (1) was purified by ether precipitation for three times and ethanol precipitation twice. The yield was 87% for PEG2,000. 1H NMR (400 MHz) δ 7.69-7.19 (m, 8H), 5.23-5.22 (m, 2H), 5.08 (s, 2H), 4.98-4.95 (m, 2H), 4.12-4.10 (m, 1H), 3.56-3.52 (PEG peaks), 3.26 (s, 3H), 3.21-3.17 (m, 2H), 2.09-1.88 (m, 6H), 1.27-1.16 (m, 28H), 0.77 (t, 3H).
2.2.2. Monomethoxy PEG1,000-α-Fmoc-lysyl-α-Fmoc-ε-(dioleoyl-lysyl) lysine (2)
PEG-α-Fmoc-ε-NH2-lysine ester (1 mmol) was reacted with 4 mmol of TEA, α-Fmoc-ε-t-Boc lysine (1.5 mmol), DCC (1.7 mmol) and NHS (1.5 mmol) in CH2Cl2:THF 1:1 at 0°C for 20 minutes, then at room temperature overnight. The reaction was determined to be completed by negative results with ninhydrin tests. Monomethoxy PEG1,000 α-Fmoc-lysyl-α-Fmoc-ε-t-Boc-lysine ester was purified by cold ether and ethanol precipitations, TFA deprotection, followed by ether precipitation and washes to give PEG1,000-α-Fmoc-lysyl-α-Fmoc-ε-NH2-lysine ester. This ε-NH2-terminated PEG1,000-lysine derivative was end capped with 4 mmol of TEA, N,N′-di-oleoyl lysine (1.5 mmol) pre-activated with DCC (1.7 mmol) and NHS(1.5 mmol) overnight. The resulting 2 was purified similarly with ether and ethanol precipitations. The yield for 2 with methoxy PEG1,000 is around 75%. 1H NMR δ 7.70-7.20 (m, 24H), 5.35-5.34 (m, 2H), 5.14-5.09 (m, 6H), 4.27-4.22 (m, 2H), 3.70-3.61 (PEG peaks), 3.39 (s, 3H), 3.21-3.07 (m, 6H), 2.01-1.97 (m, 6H), 1.49-1.23 (m, 40H), 0.89 (t, 3H).
2.2.3. Monomethoxy PEG2,000-lysyl-(α-Fmoc-ε-oleoyl lysine)2 (3)
Esterification of methoxy PEG2,000-OH (1 mmol) with di-t-Boc lysine (2 mmol), DCC (2.2 mmol) and DMAP (0.1 mmol) in CH2Cl2 overnight, followed by the similar ether and ethanol precipitation steps yielded monomethoxy PEG-di-Boc-lysine ester. After TFA deprotection and ether precipitation and washes, the PEG-lysine ester was conjugated with 4 mmol of TEA, α-Fmoc-ε-t-Boc-lysine (3 mmol) with DCC (3.5 mmol) and NHS (3 mmol) in CH2Cl2:THF 1:1 at 0°C for 20 minutes, then room temperature overnight. The reaction was confirmed to be completed by negative upon ninhydrin tests. Monomethoxy PEG-lysyl-(α-Fmoc-ε-t-Boc-lysine)2 was purified by cold ether and ethanol precipitations, TFA deprotection, followed by ether precipitation and washes to give PEG-lysyl-(α-Fmoc-ε-NH2-lysine)2. This ε-NH2-lysyl-terminated PEG derivative was end capped with 4 mmol of TEA and 4 mmol of oleoyl chloride for 20 minutes. After routine ether and ethanol precipitations and washes, purified 3 was obtained in 72% yield. 1H NMR δ 7.36-7.34 (m, 16H), 5.35-5.30 (m, 4H), 5.14-5.09 (m, 4H), 4.27-4.22 (m, 6H), 3.70-3.61 (PEG peaks), 3.41 (s, 3H), 3.21-3.07 (m, 6H), 2.01-1.97 (m, 6H), 1.49-1.23 (m, 62H), 0.89 (t, 6H).
2.2.4. Monomethoxy PEG5,000-lysyl-[lysyl-(α-Fmoc-ε-oleoyl lysine)2]2(4)
The PEG-lysine ester (1 mmol) derived from methoxy PEG5,000 was conjugated with di-t-Boc-lysine (3 mmol) with DCC (3.5 mmol) and NHS (3 mmol) in CH2Cl2: THF 1:1 at 0 °C for 20 minutes, then room temperature overnight. The reaction was confirmed to be completed by ninhydrin tests. Monomethoxy PEG5,000-lysyl-(di-t-Boc-lysine)2 was purified by cold ether and ethanol precipitations. TFA deprotection, followed by ether precipitations and washes gave PEG-lysyl-(α-NH2-ε-NH2-lysine)2. This tetra ε-NH2-terminated PEG-lysine derivative was conjugated with α-Fmoc-ε-Boc-lysine (5 mmol), DCC (6 mmol) and NHS (5 mmol) in CH2Cl2: THF 1:1 at 0°C for 20 minutes, then room temperature overnight. The reaction was confirmed to be completed by negative upon ninhydrin tests. Monomethoxy PEG5,000-lysyl-[lysyl-(α-Fmoc-ε-t-Boc lysine)2]2 was purified by cold ether and ethanol precipitations, TFA deprotection, followed by ether precipitation and washes to give PEG-lysyl-[lysyl(α-Fmoc-ε-NH2-lysine)2]2. Finally, end capping with oleoyl chloride (8 mmol), TEA (10 mmol) for 20 minute, followed by ether and ethanol precipitations and washes gave 4 in 79% yield. 1H NMR δ 7.36-7.34 (m, 32H), 5.35-5.27 (m, 8H), 5.14-5.09 (m, 7H), 4.27-4.22 (m, 6H), 3.70-3.61 (PEG peaks), 3.41 (s, 3H), 3.21-3.07 (m, 9H), 2.01-1.97 (m, 9H), 1.49-1.23 (m, 130H), 0.89 (t, 6H).
2.2.5. Methoxy PEG2,000-α-Cbz-ε-oleoyl-lysine (5)
PEG2,000-α-Cbz-lysyl-α-Cbz-ε-oleoyl-lysine (6), methoxy PEG2,000-α-Cbz-lysyl-α-Cbz-lysyl-α-Cbz-ε-oleyol-lysine (7), and methoxy PEG2,000-carbamoyl-1-palmitoyl-2-oleoyl-sn-glycero-3-phosphotidylethanolamine (PEG-POPE) (8). Three PEG-lysyl-lipidic conjugates with varying number of α-Cbz-lysine residues and single oleoyl chain were similarly synthesized and purified as 1 except using α-Cbz-ε-Boc-lysine, instead of α-Fmoc-ε-Boc-Lysine for 1 to 3 repeated cycles. Control surfactant PEG2,000-oleate was prepared by reacting methoxy PEG2,000 with oleoyl chloride and TEA; and PEG-phospholipid conjugate was synthesized by reacting methoxy PEG2,000 activated by phosgene with POPE and TEA, followed by ether precipitation according to a published scheme [13].
1H NMR for lipopeptide 5: 1H NMR δ 7.29-7.19 (m, 5H), 5.23-5.22 (m, 2H), 4.98-4.95 (m, 2H), 4.12-4.10 (m, 1H), 3.56-3.52 (PEG peaks), 3.26 (s, 3H), 3.21-3.17 (m, 2H), 2.09-1.88 (m, 6H), 1.27-1.16 (m, 28H), 0.77 (t, 3H).
1H NMR for lipopeptide 6: 1H NMR δ 7.37-7.29 (m, 10H), 5.37-5.35 (m, 2H), 5.12-5.09 (m, 4H), 4.25-4.22 (m, 2H), 3.70-3.64 (PEG peaks), 3.40 (s, 3H), 3.21-3.17 (m, 4H), 2.03-1.89 (m, 6H), 1.40-1.24 (m, 34H), 0.90 (t, 3H).
1H NMR for lipopeptide 7: 1H NMR δ 7.36-7.34 (m, 15H), 5.35-5.34 (m, 2H), 5.14-5.09 (m, 6H), 4.27-4.22 (m, 2H), 3.70-3.61 (PEG peaks), 3.39 (s, 3H), 3.21-3.07 (m, 6H), 2.01-1.97 (m, 6H), 1.49-1.23 (m, 40H), 0.89 (t, 3H).
1H NMR for PEG-POPE, compound 8: 1H NMR δ 5.35-5.34 (m, 2H), 3.69-3.64 (PEG peaks), 3.92–3.96 (m, 4H), 3.57-3.55 (m, 2H), 3.39 (s, 3H), 2.29-2.28 (m, 2H), 1.37-1.27 (m, 50H), 0.89 (t, 3H).
MALDI-TOF mass spectroscopy data for lipopeptide 1–4 are shown in Figure S1
2.3. Critical micelle concentration (CMC)
CMC was determined based on the red shift of the maximal absorption of Eosin Y when incorporated into more hydrophobic microenvironment in the core of micelle [14]. Briefly, Eosin Y solution was added to a final concentration of 1 mM to a series of surfactant solution prepared in distilled water and incubated for 30 minute at room temperature. OD542 nm was measured and plotted against surfactant concentration from which CMC values were estimated.
2.4. Preparation of micelle formulations with or without JP4-039
Typically micelles were prepared by hydration of dried thin films of amino acid derivatives or lipopeptides with suitable aqueous solutions with constant vortex, until clear solution is formed. The final concentration is approximately 100–200 mg/mL. Lipopeptides, amino acid derivatives or nitroxide compounds were first dissolved in chloroform. The solutions were aliquoted to a glass test tube, mixed well, then blown with a constant nitrogen stream to remove the bulk of the solvent. The residual solvent was removed by applying high vacuum for 2 hrs. To determine micelle-facilitated solublization of JP4-039, various molar ratios of surfactant to drug were applied to make surfactant-drug mixture in water (final concentration of JP4-039 was 5 mg/mL). After at least 30 minutes, the samples were briefly centrifuged at 13,000 rpm. One half of the supernatant were recovered and to which an equal volume of ethanol were added to dissolve/disrupt micelle drug complexes. The amounts of drug in the samples were quantitated using OD448nm.
2.5. Particle size measurement
To estimate hydrodynamic sizes of micelle particles, a solution of surfactant, with or without drug incorporated, was prepared in distilled water from a dried film. Samples were further diluted ten times in distilled water and sizes were measured by laser dynamic light scattering using a particle sizer (Zetasizer Nano ZS instrument, Malvern). Size measurement of emulsion particles were conducted using a Coulter N4 particle sizer after a 100-fold dilution in saline.
2.6. Cryo-EM of lipopeptide 4 micelles and lipopeptide 4-JP4-039 complexes
The micelles were prepared by hydration of dried lipopeptide films in distilled water at a final concentration of 100 mg/mL. The samples examined were lipopeptide 4 alone (A) and lipopeptide 4-JP4-039 complex (B) made at a molar ratio of 1.6:1. Four μL of samples, diluted 5-fold in distilled water, were immediately applied onto perforated Quantifoil grid (Quantifoil Micro Tools, Jena, Germany), blotted with a filter paper and plunge-frozen in liquid ethane using an FEI Vitrobot™ Mark III (FEI, Hiilsboro, OR). Low dose (10 ~ 15 e−/Å2) projection images were collected on a 4K × 4K Gatan CCD camera (Gatan, Inc., Warrendale, PA), with an FEI Tecnai TF20 electron microscope at nominal magnification of 29,000 to 50,000 and underfocus values ranging from 1.0 to 2.5 μm.
The diameters of lipopeptide 4 tubular micelles (~100 counted) and the length of bar-shaped JP4-039-lipopeptide 4 mixed micelles (~240 counted) were measured using a density plot tool in Gatan Digital Micrograph software (Gatan, Inc., Warrendale, PA).
2.7. Fluorescence quenching studies
Micelle formulations with or without JP4-039 were prepared with 10 mg of lipopeptide 4 containing 0, 0.25 or 0.5 mg of JP4-039 by hydration method in 300 μl of saline as described. The fluorescence intensity was recorded on a Synergy H1 Hybrid reader (BioTek), using an excitation wave length of 300 nm and varied emission wave length from 350 nm to 500 nm.
2.8. 1H NMR spectroscopies for micelle formulations
Micelles were prepared from lipopeptide 4 or α-Fmoc-ε-tBoc-lysine (with or without JP4-039 or 4-acetamide-TEMPO) in D2O containing 100 mM NaCl (for lipopeptide 4) or 100 mM KHCO3 (for α-Fmoc-ε-Boc-lysine). 1H NMR spectrum was recorded using a Bruker 400 MHz NMR and a recycling pulse delay of 20s was used to ensure the accurate proton integration. For initial trials, d-DMSO was used as the alternative solvent.
2.9. Hemolysis assay
Rat red blood cells (RBCs) were isolated from freshly collected rat blood with added anticoagulant by washing three times with 10 volumes of cold PBS (1500 rpm for 10 min). RBCs were then diluted to 2% w/v with ice cold DPBS and utilized immediately for the hemolysis assay. One mL of diluted RBC suspension was treated with various concentrations (0–5 mM) of PEG-lipopeptides, Tween 80, and Triton X-100, respectively, and then incubated at 37 °C in an incubator shaker for 2 h. The samples were centrifuged at 1500 rpm for 10 min at 4 °C, and 100 μL of supernatant from each sample was transferred into a 96-well plate. The release of hemoglobin was determined by the absorbance at 540 nm using a micro-plate reader. RBCs treated with Triton X-100 and DPBS were considered as the positive and negative controls, respectively. Hemoglobin release was calculated as (ODsample−ODnegative control)/(ODpositive control−ODnegative control) × 100%
2.10. Emulsion formulation for JP4-039 and stability
JP4-039 (4 mg) were formulated in the following emulsion composed of sesame oil (100 mg) and soy phosphatidyl choline (50 mg); or sesame oil (100 mg/mL), soy phosphatidyl choline (40 mg), together with a co-surfactant of either PEG2,000-oleate (29.6 mg/mL, 0.0128 μmole), PEG2,000-α-CBz-ε-oleoyl-lysine (32.5 mg/mL, 0.0128 μmole), PEG2,000-α-CBz-α-CBz-ε-oleoyl-lysine (35.9 mg/mL, 0.0128 μmole), or PEG2,000-α-CBz-lysyl-α-CBz-lysyl-α-CBz-ε-oleoyl-lysine (39.3 mg/mL, 0.0128 μmole). All ingredients were dissolved in chloroform, mixed well, and then the solvent was removed under N2 stream, followed by vacuum desiccation for 2 hrs. The oily residues were suspended in saline, sonicated under an ice bath with a probe sonicator with a maximal output of 20 mW for 60 minutes under a N2 stream, until the sizes were reduced below 150 ~ 250 nm. Initial particle sizes were estimated by laser dynamic light scattering method (Coulter N4 particle sizer). Drug loading rate in the freshly prepared formulations and those stored at 4 °C for 7 days were determined after low speed centrifuge to remove any precipitates in the samples. Organic components were extracted three times with equal volume of chloroform under vortex. The organic phase was pooled and the solvent were removed under a nitrogen stream. The residues were reconstituted to 1 ml with chloroform. The drug contents were determined using OD448nm reading.
2.11. Radiation mitigation activity against whole body irradiation in mice
All mice were irradiated with a total-body dose of 9.5 Gy delivered by a 137Cs J. L. Shepherd Mark 1 irradiator at a dose rate of 0.8 Gy/min. The mice were then divided into two groups (10–15 mice per group). These mice were injected i.p. with JP4-039 formulated in emulsion or control formulations alone 24 h after irradiation. The JP4-039 dosage was 20 mg/kg. Mice were followed until they have lost 20% of their body weight or appear moribund, at which time they are euthanized.
3. RESULTS
3.1. Selection of drug-interactive motifs
Dilution of an alcoholic solution of JP4-039 with saline instantaneously triggers crystal formation due to limited water solubility and high crystalline properties of this compound. We have identified several readily available amino acid derivatives with different N-protecting groups that are capable of inhibiting the crystallization of JP4-039 in aqueous solution. Fig. 1 shows microscopic evidence that one of the lysine derivatives effectively reduced the size as well as the number of JP4-039 crystals in saline in a dose-dependent manner, and eventually completely eliminated the formation of JP4-039 crystals at sufficient quantities. We compared a group of ε-Boc-lysine derivatives bearing various modifying groups on the α-NH2 position. Based on the ability of crystal inhibition at varied molar ratios, the amino acids with the bulkiest Fmoc are ranked the most potent one, followed by the amino acids with midsized iso-butyloxycarbonyl and benzyloxycarbonyl (Cbz) groups, while the amino acids with compact t-Boc and the smallest acetyl group were ineffective (Table 1). We replaced the free carboxyl group of α-Fmoc-ε-Boc-lysyl with a methoxy PEG1000 as an ester and found that it still maintained the full capacity of the free acid derivative (not shown).
Figure 1.
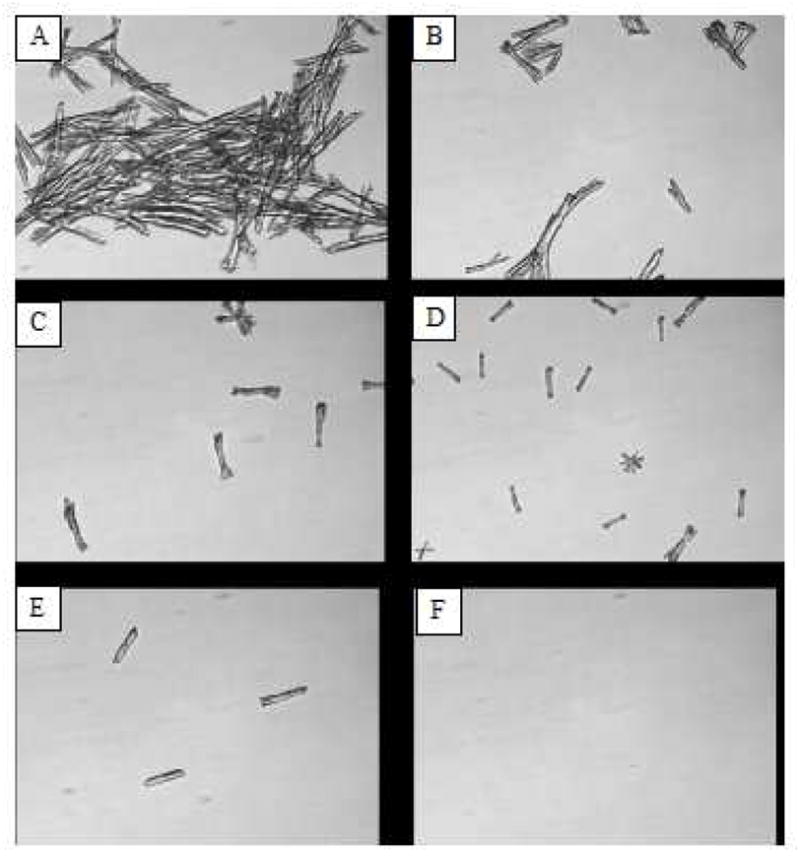
α–Fmoc-ε-t-Boc-lysine prevented crystal formation of JP4-039 in saline. An aliquot of 5 μL of 0.1 M JP4-039 in absolute alcohol was added to 100 μL of saline containing A: 0 mM, B, 5 mM, C: 10 mM, D: 20 mM, E: 37.5 mM, F: 50 mM. Imagies were taken after 20 minute incubation time, under a light microscope.
Table 1.
Screening of Amino Acid Derivatives that Facilitate Solubilization of JP4-039. U: Initially soluble but unstable after 5 min; V: forming vesicles; I: insoluble; S: soluble.
| Molar ratios | 5 | 10 | 20 | 30 | 40 | 50 | |
|---|---|---|---|---|---|---|---|
| Boc-Phe-OH | U | U | U | U | U | V |

|
| Cbz-Tyr-OH | V | U | U | U | U | V | |
| Cbz-(Isb)-Lys-OH | V | V | V | V | V | V | |
| Di-(tBoc)-Lys-OH | V | V | V | V | V | V | |
| Cbz-β-Ala-OH | I | I | I | I | I | I | |
| tBoc-(Cbz)-Lys-OH | I | I | I | I | I | S | |
| Cbz-(tBoc)-Lys-OH | I | I | I | I | S | S | |
| Di-(Cbz)-Lys-OH | I | I | S | S | S | S | |
| Isb-(Cbz)-Lys-OH | I | S | S | S | S | S | |
| Fmoc-(tBoc)-Lys-OH | S | S | S | S | S | S | |
| Ace-(Cbz)-Lys-OH | I | I | I | I | I | S | |
| CBz-(Ace)-Lys-OH | I | I | I | I | I | S | |
| Benz-Phe-OH | I | U | U | U | U | S |
3.2. Synthesis of PEGylated lipopeptides bearing drug-interactive motifs
In the interest of testing if these drug-interactive motifs can be incorporated into lipid-based drug formulations to increase drug-carrier compatibility and drug loading capacity, seven types of PEG-lipopeptides carrying varied numbers of α-Fmoc or α-Cbz lysine residues at the interface region and a control PEG-lipid conjugate were synthesized (Fig. 2). The single chain PEG-lipoamino acid derivative 1 was synthesized by first esterifying monomethoxy PEG-OH with α-Fmoc-ε-Boc-lysine, followed by deprotection of the t-Boc group, then end-capped with oleic chloride. A double chain lipopeptide with two consecutive α-Fmoc-lysine residues was made by end capping of monomethoxy PEG-α-Fmoc-lysyl-α-Fmoc-ε-NH2-lysine with an α, ε-dioleoyl lysine to obtain PEG-lipopeptide 2. PEG-lipopeptide 3 and 4 were prepared by end-capping monomethoxy PEG-lysine conjugates carrying two or four α-Fmoc-lysyl groups attached through one or three lysine bridges with oleic chloride. The long chain lipid tails enable these PEG derivatives to associate tightly with each other in micelles, or anchor to emulsion or liposome formulations with additional lipid components. Additional single lipid chain methoxy-PEG-lipopeptides containing 1–3 consecutive α-Cbz-lysyl groups (PEG-lipopeptide 5–7) were similarly synthesized. Methoxy-PEG2,000-carbamoyl-POPE (8) was synthesized by reacting palmitoyl oleoyl phosphatidyl ethanolamine with methoxy-PEG2,000 activated with phosgene according to a published methodology [13].
Figure 2.
Chemical structures of PEG-lipid and PEG-lipopeptide conjugates used in this study.
3.3. Characterization of self-assemblies of PEGylated lipopeptides
Particle size measurement by dynamic light scattering method for α-Fmoc-ε-tBoc-lysine prepared in 0.1 M KHCO3 revealed that the majority of particles had measured diameters between 2–5 nm, which indicates these are micelles (Figure S2a, Supporting Info). All PEG lipid and lipopeptide conjugates readily formed a transparent dispersion in water, with the suspension made from PEG-lipopeptides containing α-Fmoc-lysyl units showing significantly increased viscosity, suggesting the presence of elongated, worm-like micellar assemblies (filomicelles) that self-entangle with each other. Measured critical micelle concentration (CMC) values of 3.4–6.8 μM are in a range that is comparable to those reported for nonionic surfactants with long aliphatic chains (Table 2).
Table 2.
Drug solubilizing and physical properties of PEG-lipopeptides
| PEG-lipopeptides | Drug:carrier ratio for solubilization (mol:mol) | CMC (μM) | Particle Sizes w/o JP4-039 (nm) | Particle Sizes w/JP4-039 (nm) |
|---|---|---|---|---|
| PEG2000-FmocLys-Oleate | 1:10.7 | 5.0 | Not done | Not done |
| PEG2000-Lys(FmocLys-Oleate)2 | 1:5.0 | 4.2 | 106.7+/−2.5 | 94.2+/−1.7 |
| PEG1000-FmocLys-FmocLys-Lys-di-Oleate | 1:6.3 | Not done | Not done | Not done |
| PEG5000-Lys[Lys(FmocLys –Oleate)2]2 | 1:1.5 | 3.4 | 132.1+/−8.5 | 128.6+/−5.9 |
3.4. Drug formulation in mixed micelles
Methoxy PEG lipopeptide derivatives containing varied numbers of Fmoc and oleoyl groups were active in solubilizing JP4-039 in saline at a 1:1.5 to 1:15 drug-to-carrier molar ratios. Based on the minimal molar ratio between carrier and drug that is required to form soluble mixed micelles, the conjugate carrying tetra-α-Fmoc-lysyl groups (4) is more efficient than the conjugates 2 and 3 containing two Fmoc lysyl groups, while the mixed micelles formed with the conjugate 1 containing mono-Fmoc lysyl group were unstable over time (Table 2).
Given sufficient carrier-to-drug ratios, PEG-lipopeptide 4 was effective in maintaining a stable formulation for a prolonged period of time (> one month) during which no sign of crystal formation was noticed. A dose-dependent solublization relationship was established for lipopeptide 4 and a fixed amount of JP4-039, with a minimal carrier-to-drug molar ratio around 1.6:1 (Fig. 3). In contrast, comparable PEG-α-Cbz-lysyl lipid conjugates at these ratios could only slow down the crystallization of JP4-039, but failed to form a stable JP4-039-containing micelle solution (not shown). Methoxy-PEG2,000-carbamoyl-POPE 8, a control micelle forming-PEG-lipid conjugate that lacks lysyl domain, was inactive at comparable molar ratios (not shown).
3.5. Cryo-electron microscopy observations
Cryo-electron microscopy (cryo-EM) images for selected lipopeptides confirmed the presence of self-assembled structures with long tubular shape at 20 mg/mL of 3 (not shown) and 4 (Fig. 4). The tubular structures have an electron-light center region of 2.8–4.0 nm in thickness (3.5 ± 0.4 nm, n = 22), presumably the micelle core that is made of lipid chains. The light core is surrounded by an electron-dense peripheral wall, presumably the Fmoc-lysine-containing interface region. The average diameter of the tubular structure, measured from the distance between the mid-points of the dense walls, is ~5.6 ± 0.4 nm (Fig. S3a, in Supporting Info). The thickness of electron-dense regions is ~ 1/3 to 1/2 of the thickness of the electron-light center region. The PEG chains are not electron-dense enough to be revealed with cryo-EM. The reported lipid-anchored PEG5,000-PE conjugates displayed on the surface of liposomes is 10–15 nm in thickness [15]. Assuming that this parameter applies to these tubular-shaped PEG-lipopeptide micelles, the overall diameter including the PEG layer would be in the 27–40 nm range.
Figure 4.
Cryo-EM images of lipopeptide 4 micelles (A), JP4-039-lipopeptide complexes (B), and a proposed model for lipopeptide micelles and drug-lipopeptide complexes (C).
Interestingly, the JP4-039 loaded micelles showed significantly reduced viscosity. The particle sizes measured with laser dynamic light scattering method were also smaller for JP4-039 loaded PEG-lipopeptide 4 than the empty micelles (Table 2). When JP4-039 was present, cryo-EM images showed a mixture of many small dots (~90%, n=388, Fig. 4) and truncated bar-like structures (~10%). The diameters for the dots and bars were slightly less than that of the tubular micelles observed in the sample of lipopeptide alone. The bar structures were variable in length, ranging from ~30 to 300 nm, with a median length under 60 nm as showed in the histograph (Fig S2 in Supporting Info). The size distribution of the JP4-039-loaded micelles on cryo-EM agrees with the results obtained by laser dynamic light scattering (Fig. S2b in Supporting Info). There was no sign of crystals of the free drug (Fig. 4).
3.6. Fluorescence quenching assays
To demonstrate that drug and carrier molecules are physically associated with each other in mixed micelles, we studied the group-group interactions using fluorescence quenching assay. Fig. 5 shows the fluorescence spectra of intrinsic fluorescence originating from Fmoc-groups of lipopeptide 4 (at an excitation wavelength of 300 nm) in the absence JP4-039. A large scale quenching effect was recorded when JP4-039 was added at a drug/carrier molar ratio of 1: 4 ~ 5. The electron-rich nitroxide group is known to be a strong fluorescent quencher for 5-carboxytetramethylrhodamine (5-TAMRA) when placed in close distance to 5-TAMRA-labeled short DNA [16]. Our data therefore strongly suggest that JP4-039 is in a close distance from the Fmoc-groups in lipopeptide 4 micelles.
Figure 5.
Fluorescence quenching studies of lipopeptide 4 alone and lipopeptide 4 with varies amounts of JP4-039.
3.7. Nuclear magnetic resonance (NMR) spectroscopies of Fmoc-containing derivatives
NMR experiments were conducted to further investigate the interaction between Fmoc-containing carriers and JP4-039. Different from the well resolved 1H NMR spectrum of lipopeptide 4 in d6-DMSO, most proton signals originated from the hydrophobic portion (Fmoc groups and aliphatic lipid chains) of lipopeptide 4 prepared as micelle in D2O are severely shielded, possibly attributed to the relatively large size of lipopeptide micelle self-assembly and increased microviscosity of oil core of the micelles (Figs. S3 and S4, Supporting Info). On the other hand, the proton signals of α-Fmoc-ε-tBoc-lysine micelles prepared in D2O (0.1 M in concentration, prepared in 0.1 M potassium bicarbonate) were clearly revealed by 1H NMR (Figure 6c). We have shown that this simple amino acid derivative has the capacity to prevent crystal formation and dissolve JP4-039 in aqueous solution (Figure 1 and Table 1), which provides a valid alternative approach to studying Fmoc/JP4-039 interactions. Our data also show that the presence of JP4-039, even as a minor component (5% by mole ratio) in the mixed micelles, drastically depresses and broadens the corresponding peaks observed in micelles prepared from the lysine derivative alone, possibly due to a well-known paramagnetic relaxation effect of the TEMPO free radical with an unpaired electron on neighboring nuclei via dipolar coupling that affects both the 1H and 13C NMR readings [17] (Figure 6a). Further analysis after peak integration using the peaks that belong to t-Boc and lysine backbone (chemical shift 1.09–1.9 ppm) as an internal reference revealed that the integrated proton signals that belong to Fmoc groups (from 6.7 to 7.8 ppm) were disproportionally suppressed by JP4-039 (Fig. S7, Supporting Info). When we repeated the measurement with the same amount of a water-soluble analog of JP4-039, 4-acetamido-TEMPO (compound C in Figure 6), the suppressing/broadening effect on the entire collection of proton signals were also observed, but to a much less degree (Figure 6b).
Figure 6.
1H-NMR spectra of α–Fmoc-ε-t-Boc-lysine micelles with or without TEMPO free radical derivatives. Micelles were prepared with α–Fmoc-ε-t-Boc-lysine (0.1 M) in 0.1 M KHCO3 alone (c), with JP4-039 at 20:1 mole ratio (a), or with 4-acetamido-TEMPO at 20:1 mole ratio (b).
In addition, the peak intensities of all protons were proportionally affected by this water-soluble (Fig. S8, Supporting Info). The two different stable free radicals demonstrated differential suppression effects with respect to both the magnitude and the selectivity on the NMR proton signals for this amino acid derivative. One explanation could be due to the difference of distribution of the two radicals in the assay systems. More hydrophobic JP4-039 are buried in the center of the micelles, therefore has a closer distance to the hydrophobic Fmoc groups, while the water-soluble 4-acetamido TEMPO is distributed in solution and is more distant from the Fmoc groups, hence exert a lesser suppression effect on the protons in Fmoc groups. Taken together, the fluorescent quenching data and NMR depressing data are in a good agreement to each other and are suggestive that Fmoc groups participate in the interaction between the carrier and JP4-039.
3.8. Surface activity of PEGylated lipopeptides
We have examined the hemolytic activity of plain micelles prepared from PEG-lipid and lipopeptide conjugates on rat red blood cells and compared that to two widely used ethoxylated nonionic surfactants: Triton X-100 and Tween 80. As shown in Fig. 7, while Triton X-100 showed 100% hemolysis at 5 mM, no significant hemolysis (≤2%) was noticed at or below this concentration for Tween 80 and all the PEG-lipid conjugates reported in this work.
Fig 7.
A comparison of hemolytic activity of surfactants on rat red blood cells. Rat red blood cells (1%) were incubated with surfactants at indicated concentrations for 2 hrs at 37 °C. After which supernatant were carefully withdrawn and measured for at OD540 nm and calculated based on OD value under condition total hemolysis occurred.-●-Triton X100, ..○.. Tween 80, -▼-PEG2000-oleic acid ester, -△-lipopeptide 5 (PEG2000-α-Cbz-ε-oleyl lysine);-■-,lipopeptide 1(PEG2000α-Fmoc-ε-oleyl lysine), -□-, lipopeptide 2 (PEG2000α-Fmoc-lysyl-α-Fmoc-lysyl-ε-dioleyl-lysine), -◆-, lipopeptide 3 [PEG2000-lysyl-(α, ε–di-Fmoc-ε-oleyl lysine)2, -◇-, lipopeptide 4 PEG5000-lysyl-[lysyl-(α, ε–di-Fmoc-ε-oleyl lysine)2]2.
3.9. PEGylated α-Cbz-lysyl containing lipopeptides as co-surfactant stabilizes drug-loaded emulsion
In contrast to the good solubilizing activity of α-Fmoc-lysyl containing lipopeptides as stand-alone micelle formulations for JP4-039, lipopeptides containing one to three α-Cbz-lysyl groups in linear configuration failed to form stable mixed-micelles with JP4-039 (not shown). However, we found that they acted as co-surfactants and stabilized the soy phosphatidyl choline-sesame oil emulsion formulation which we have previously found to have poor retention properties for JP4-039 over time (Fig. 8). About 15~30% of drug were dissociated from the emulsions (with and without pegylation) 7 days following the preparation. The drug retention rates were significantly improved when 20% mole of soy phosphatidyl choline was replaced with equal amounts of one of the lipopeptides containing α-Cbz-lysyl. Moreover, the added co-surfactant also speeded up the emulsion preparation by sonication due to enhanced surface activity provided by these pegylated lipopeptides. When given to animals by single injection via i.p. route 24h after the exposure to a lethal dosage of irradiation, JP4-039 formulated in the improved emulsion formulation showed significant radiation protective effects and improved animal survival (both survival time and overall survival rate) over the control group (Fig. 9), confirming that JP4-039 formulated in these formulations are pharmacologically active in vivo. We are currently testing the utility of α-Fmoc-lysyl containing PEG-lipopeptides in emulsion and liposome formulations for JP4-039 and these results will be published elsewhere.
Figure 8.
Effect of co-surfactant on colloidal stability and drug loading capacity of sesame oil-egg PC emulsion containing JP4-039. The rates of drug incorporation for freshly prepared (black bars) and on day 7 (light grey bars) for sesame oil-egg PC emulsion (SOPC), SOPC with 20% PC replaced with PEG-OA, Lipopeptides 5, 6, 7 were determined after retrieval of JP4-039 from these formulations. The % of initial (grey bars) were calculated from these data. Data were presented as mean ± SD (n=3).
Figure 9.
In vivo radiation mitigation activity of JP4-039 formulated in emulsion against whole body irradiation in mice. In vivo radiation mitigation activity against whole body irradiation in mice: All mice were irradiated with a total-body dose of 9.5 Gy at a dose rate of 0.8 Gy/min. The mice were injected i.p. with emulsion alone (solid circle) or JP4-039 formulated in emulsion (open circle, 20 mg/kg, 24 h after irradiation). Mice were followed until they have lost 20% of their body weight or appear moribund, at which time they are euthanized.
4. DISCUSSION
Traditional formulation practices rely heavily on an empirical selection process for the most suitable ingredients from a short list of off-the-shelf starting materials. Many of lipid-based formulations have issues of limited loading capacity and formulation stability. Even though many drugs or candidates are overall hydrophobic by nature, the presence of hydrophilic structural elements in these drug structures determines that the highly hydrophobic environment found in the lipid core is not a thermodynamically favored destination for many of these agents. Inadequate mixing between poorly water-soluble drugs and lipid core of formulation materials can reduce drug-loading capacity or cause formulation instability [3,5]. Most surfactants used in formulations development contain at least a hydrophilic head group and a hydrophobic core, which typically is composed of single or multiple lipid chains. The hydrophobic core is believed to be the key drug-loading region. The interfacial region is usually either missing or rather compact in size relative to the rest of the surfactant molecules and the significance of interfacial region in drug-formulation has been underappreciated. To resolve this drug-carrier incompatibility issue, a portion of less hydrophobic groups (hydrotropic groups) have been incorporated to otherwise relatively hydrophobic polyester backbones to improve paclitaxel formulation in polymer nanoparticles [18–19]. For lipid-based systems, hydrophobic motifs that can better accommodate APIs, such as vitamin E [20], cholic acid, and embelin derivatives [22] have been shown to improve drug loading capacity and formulation stability for paclitaxel, over traditional pure aliphatic chain-containing formulation materials. However these trial-and-error practices may not be universally applicable due to the fixed structural motifs that were used for the synthesis. For example, preliminary study by us has shown that PEG-vitamin E-based micellar formulation, although working well for paclitaxel, was not very effective for the particular agent that we are working on (not shown).
In this work, we have presented a unique “bottom-up”, tailor-designed formulation approach that is quite different from the traditional formulation principle. Rather than relying on the lipid core region as the sole formulation functionality, here we have designed and synthesized a class of new surfactant molecules with an expanded interface region, in which a pre-selected motif capable of interacting with a particular drug candidate was incorporated. The drug formulation functionality of our new surfactants would include both the expanded interface region and the lipid core. These designer surfactants carrying additional drug-interactive motifs at interface location could extend the utility of lipid-based formulations, and more importantly, should make these lipid-based formulations (micelle, liposome, and emulsion) more compatible with moderately hydrophobic, yet poorly water-soluble drugs, which results in formulations with improved drug-loading capacity and stability. The location of interface region would make sense thermodynamically for less hydrophobic drugs, because deep in lipid core would be too hydrophobic and aqueous phase would be too hydrophilic. Two key features were incorporated into our design to promote interfacial drug-carrier interactions: selection of the right drug-interactive motifs and enhancement of drug-carrier interaction through self-assembly phenomena. JP4-039 was used as a model drug to demonstrate the methodologies for the selection of such drug-interacting motifs and the installation of such motifs into surfactant molecules at interface, as well as the use of such tailor-designed surfactants in formulating JP4-039 in micelle and emulsion nanoparticles with improved stability.
Since JP4-039 possesses peptide characteristics to some degree, we searched for structural elements that might interact with JP4-039 from amino acid derivatives. Special attention was paid to lysine, for it has three orthogonally protected functional groups that will simplify the subsequent conjugation maneuver. We experimentally identified Fmoc amine-protecting group as the most potent drug-interactive group for JP4-039. α-Fmoc-ε-tBoc protected lysine is a readily available amino acid derivative widely used for solid phase peptide synthesis. A recent report indicates that dipeptides carrying such group have intrinsic anti-inflammatory activity [23], though more studies are needed to fully characterize the biological activities of such group in vivo.
Fmoc group contains a bulky, fused fluorenylmethyl ring structure capable of providing strong hydrophobic and π-π stacking interactions with other aromatic moieties, including itself. The carbamoyl bond that links the ring structure to lysine can also provide hydrogen-bonding capacity. Fmoc is known to promote parallel interactions of individual short peptides carrying the same group which often leads to the formation of elongated nano-assemblies. Examples include Fmoc-containing short peptides that form interconnected tubular structures and turn into hydrogels [24–26], and our lipopeptides 3 (not shown) and 4 (Fig. 4). The fact that an excess of α-Fmoc-ε-Boc-lysine and α-Fmoc-lysyl-containing lipopeptide conjugates are required to solubilize JP4-039 would suggest a model that involves one JP4-039 surrounded by several Fmoc-containing compounds hold together through a combination of hydrogen bonding, hydrophilic and hydrophobic cooperative interactions among the drug-carrier, and carriers themselves. Our best performer, lipopeptide 4, which has four Fmoc-groups arranged in a constraint manner at the interface and has a high local concentration of Fmoc groups, requires the lowest molar ratio of carrier to drug to achieve a complete solubilization (Table 2, Fig. 3).
The model of Fmoc-JP4-039 interaction was supported by our fluorescence quenching studies (Fig. 5). We also conducted 2-D nuclear magnetic resonance (NMR) spectroscopy to further confirm the interaction between Fmoc and JP4-039. We initially met with some technical difficulties when such interaction was studied with lipopeptide 4/JP4-039 mixed micelles prepared in aqueous solution. Only PEG head group proton signals were detected, while the protons attributed to Fmoc groups and lipid chains are “invisible” due to a shielding effect by the micelle assemblies. Similar shielding phenomenon has been reported with the PEG-cholic acid clusters-based micellar system [21]. We then chose α-Fmoc-ε-Boc-lysine as a simplified system to study Fmoc-JP4-039 interaction because α-Fmoc-ε-Boc-lysine appears to form micelles by itself (Figure S2a, Supporting Info) and is highly effective in preventing JP4-039 from crystal formation in aqueous solution (Figure 1 and Table 1). However, addition of JP4-039 to the system led to severe distortion of proton signals. As little as 5% mole of JP4-039 is sufficient to essentially flatten the signals from neighboring protons, which renders it difficult for determining if chemical shift changes of a specific proton signal is attributed to carrier/drug interaction. The unpaired electron in the TEMPO ring is known to exert strong paramagnetic relaxation effect on neighboring nuclei via dipolar coupling, which can extend over 20 Å in distance [17]. Careful evaluation of the 1H NMR spectrum data for JP4-039-α-Fmoc-ε-Boc-lysine mixed micelles revealed that the presence of TEMPO group differentially affected the different proton signals of α-Fmoc-ε-Boc-lysine. The peak suppressing and flattening effects were significantly more pronounced for Fmoc than for t-Boc group. This overwhelming and somewhat selective suppressing effects caused by TEMPO free radical are clearly affected by distance of the involved groups to the free radical, as we did not observe such large scale suppressing effects with a water-soluble acetamido-TEMPO derivative at equal and even higher TEMPO to α-Fmoc-ε-Boc-lysine molar ratios. Moreover, all protons of α-Fmoc-ε-Boc-lysine were evenly affected by the water-soluble acetamido-TEMPO derivative (Figure 6). The above results strongly support the notion that JP4-039 is contained and surrounded by the Fmoc groups within the micelle assemblies, where it has closer distance to the ring structures than the rest of molecules.
It should be stated that Fmoc may not be the only group involved in the carrier-drug interaction in JP4-039-loaded micelles. Our cryo-EM images show that the drug-loaded micelles have apparent electron-dense region throughout the visible structures, while in empty tubular micelles the core region is electron-light, which would suggest that either JP4-039 molecules may be incorporated into regions that contain both interface and lipid portions through the extensive reorganization process, or alternatively, the dark appearance could be simply due to the projected image of relatively dense shell made of JP4-039 distributed along the interfacially located Fmoc groups. The contribution of lipid core in the mixed micelles remains to be clarified in the future.
One unique feature of our system is its practicality. Both amino acid derivatives and PEGs are readily available in high purity. Chemistries involved in Fmoc and t-Boc protection/deprotection and coupling are all well studied and one can have the flexibility in introducing the motif of choice at the interface region. A highly efficient polymer-assisted liquid phase synthesis scheme was adopted to prepare gram quantities of PEGylated lipopeptides without having to use chromatographic purification steps. The modular design allows one to generate a series of compounds that share the similar general structure and self-assembly properties, yet with the flexibility to change motifs at the interface region. Finally the stepwise process allows a smooth translation from identification of drug-interacting group, tailor-designed surfactant synthesis, to micelle, liposome or emulsion-based drug formulation system. Though the bulk of this work was focused on developing new type of surfactants for formulating JP4-039, this process can be easily extended to the development of various types of new lipidic and polymeric systems for improved in vivo delivery of other therapeutic agents.
Supplementary Material
Acknowledgments
This project was supported by a BARDA contract (HHS0100200800062C), the NIH/NIAID CMCR program (U19 AI068021-06), the NIH/NIGMS CMLD program (P50 GM067082), the NIH/NHLBI R01 HL091828 and NIH/NIGMS R01GM085043.
ABBREVIATIONS
- API
active pharmaceutical ingredients
- t-Boc
N-tert-butoxycarbonyl
- CBz
benzyloxycarbonyl
- CMC
critical micelle concentration
- DCC
N,N′-dicyclohexylcarbodiimide
- DAMP
N,N-dimethylamino pyridine
- Fmoc
9-fluorenylmethyloxycarbonyl
- NHS
N-hydroxysuccinimide
- NMR
Nuclear Magnetic Resonance
- TEA
triethylamine
- TEMPO
(2,2,6,6-Tetramethylpiperidin-1-yl)oxyl
- THF
tetrahydrofuran
References
- 1.Beg S, Swain S, Rizwan M, Irfanuddin M, Malini DS. Bioavailability enhancement strategies: basics, formulation approaches and regulatory considerations. Curr Drug Deliv. 2011;8:691–702. doi: 10.2174/156720111797635504. [DOI] [PubMed] [Google Scholar]
- 2.Nanjwade Bk, Patel DJ, Udhani RA, Manvi FV. Functions of Lipids for Enhancement of Oral Bioavailability of Poorly Water-Soluble Drugs. Sci Pharm. 2011;79:705–727. doi: 10.3797/scipharm.1105-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buse J, El-Aneed A. Properties, engineering and applications of lipid-based nanoparticle drug-delivery systems: current research and advances. Nanomedicine. 2010;5:1237–1260. doi: 10.2217/nnm.10.107. [DOI] [PubMed] [Google Scholar]
- 4.Puri A, Loomis K, Smith B, Lee JH, Yavlovich A, Heldman E, Blumenthal R. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carrier Syst. 2009;26:523–580. doi: 10.1615/critrevtherdrugcarriersyst.v26.i6.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narang AS, Delmarre D, Gao D. Stable drug encapsulation in micelles and microemulsions. Int J Pharm. 2007;345:9–25. doi: 10.1016/j.ijpharm.2007.08.057. [DOI] [PubMed] [Google Scholar]
- 6.Jiang J, Belikova NA, Xiao J, Zhao Q, Greenberger JS, Wipf P, Kagan VE. A mitochondria-targeted nitroxide/hemigramicidin S conjugate protects mouse embryonic cells against gamma irradiation. Int J Radiat Oncol Biol Phys. 2008;70:816–825. doi: 10.1016/j.ijrobp.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang J, Kurnikov I, Belikova NA, Xiao J, Zhao Q, Vlasova IL, Amoscato AA, Braslau R, Studer A, Kagan VE. Structural requirements for optimized delivery, inhibition of oxidative stress, and antiapoptotic activity of targeted nitroxides. J Pharmacol Exp Ther. 2007;320:1050–1060. doi: 10.1124/jpet.106.114769. [DOI] [PubMed] [Google Scholar]
- 8.Rajagopalan MS, Gupta K, Epperly MW, Franicola D, Zhang X, Wang H, Zhao H, Tyurin VA, Kagan VE, Greenberger JS. The Mitochondria-Targeted Nitroxide JP4-039 Augments Potentially Lethal Irradiation Damage Repair. In Vivo. 2009;23:717–726. [PMC free article] [PubMed] [Google Scholar]
- 9.Epperly MW, Goff JP, Li S, Gao X, Wipf P, Dixon T, Wang H, Franicola D, Shen H, Rwigema JC, Kagan VE, Bernard M, Greenberger JS. Intraesophageal administration of GS-nitroxide (JP4-039) protects against ionizing irradiation-induced esophagitis. In Vivo. 2010;24:811–819. [PMC free article] [PubMed] [Google Scholar]
- 10.Epperly MW, Brand R, Stottlemyer J, Dixon TM, Gao X, Li S, Huq S, Wipf P, Falo LD, Greenberger JS. Topical application of GS-nitroxide JP4-039 emulsion mitigation ionizing irradiation induced skin burns. Int J Radiation Oncology Biol Physics. 2010;78:S634–S635. [Google Scholar]
- 11.Goff JP, Epperly MW, Dixon T, Wang H, Franicola D, Shields D, Wipf S, Li P, Gao X, Greenberger JS. Radiobiologic effects of GS-nitroxide (JP4-039) in the hematopoietic syndrome. In Vivo. 2010;25:315–323. [PMC free article] [PubMed] [Google Scholar]
- 12.Frantz MC, Pierce JG, Pierce JM, Li K, Wan Q, Johnson MM, Wipf P. Large-scale asymmetric synthesis of the bioprotective agent JP4-039 and analogs. Org Lett. 2011;13:2318–2321. doi: 10.1021/ol200567p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zalipsky S, Gilon C, Zilkha A. Attachment of drugs to polyethylene glycols. Eur Polym J. 1998;19:1177–1183. [Google Scholar]
- 14.Patist A, Bhagwat SS, Penfield KW, Aikens PP, Shah DO. On the measurement of critical micelle concentrations of pure and technical-grade nonionic surfactants. J Surfactant. 2000;3:53–58. [Google Scholar]
- 15.Kenworthy AK, Hristova K, Needham D, McIntosh TJ. Range and magnitude of the steric pressure between bilayers containing phospholipids with covalently attached poly(ethylene glycol) Biophy J. 1995;68:1921–1936. doi: 10.1016/S0006-3495(95)80369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu P, Clamme JP, Deniz AA. Fluorescence quenching by TEMPO: a sub-30 A single-molecule ruler. Biophys J. 2005;89:L37–L39. doi: 10.1529/biophysj.105.071027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosen PA. Spin labeling of proteins. Methods Enzymol. 1989;177:86–121. doi: 10.1016/0076-6879(89)77007-5. [DOI] [PubMed] [Google Scholar]
- 18.Lee J, Lee SC, Acharya G, Chang CJ, Park K. Hydrotropic solubilization of paclitaxel: analysis of chemical structures for hydrotropic property. Pharm Res. 2003;20:1022–1030. doi: 10.1023/a:1024458206032. [DOI] [PubMed] [Google Scholar]
- 19.Kim JY, Kim S, Pinal R, Park K. Hydrotropic polymer micelles as versatile vehicles for delivery of poorly water-soluble drugs. J Control Release. 2011;152:13–20. doi: 10.1016/j.jconrel.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Dabholkar RD, Sawant RM, Mongayt DA, Devarajan PV, Torchilin VP. Polyethylene glycol-phosphatidylethanolamine conjugate (PEG-PE)-based mixed micelles: some properties, loading with paclitaxel, and modulation of P-glycoprotein-mediated efflux. Int J Pharm. 2006;315:148–157. doi: 10.1016/j.ijpharm.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 21.Luo J, Xiao K, Li Y, Lee JS, Shi L, Tan YH, Xing L, Cheng RH, Liu GY, Lam KS. Well-defined, size-tunable, multifunctional micelles for efficient paclitaxel delivery for cancer treatment. Bioconjug Chem. 2010;21:1216–24. doi: 10.1021/bc1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y, Lu J, Gao X, Li J, Zhao W, Sun M, Stolz DB, Venkataramanan R, Rohan LC, Li S. PEG-derivatized embelin as a dual functional carrier for the delivery of paclitaxel. Bioconjug Chem. 2012;23:1443–51. doi: 10.1021/bc3000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yen CT, Hwang TL, Wu YC, Hsieh PW. Design and synthesis of new N-(fluorenyl-9-methoxycarbonyl) (Fmoc)-dipeptides as anti-inflammatory agents. Eur J Med Chem. 2009;44:1933–1940. doi: 10.1016/j.ejmech.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Gu HW, Yang ZM, Xu B. J Am Chem Soc. 2003;125:13680–13681. doi: 10.1021/ja036817k. [DOI] [PubMed] [Google Scholar]
- 25.Jayawarna V, Ali M, Jowitt TA, Miller AF, Saiani A, Gough JE, Ulijn RV. Adv Mater. 2006;18:611–614. [Google Scholar]
- 26.Mahler A, Reches M, Rechter M, Cohen S, Gazit E. Adv Mater. 2006;18:1365–1370. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.










