Abstract
During the postpartum period, women experience significant changes in their neuroendocrine profiles and social behavior compared to before pregnancy. A common experience with motherhood is a decrease in sexual desire. Although the lifestyle and peripheral physiological changes associated with parturition might decrease a woman’s sexual interest, we hypothesized that there are also hormone-mediated changes in women’s neural response to sexual and infant stimuli with altered reproductive priorities. We predicted that amygdala activation to sexually arousing stimuli would be suppressed in postpartum versus nulliparous women, and altered with intranasal oxytocin administration. To test this, we measured amygdala activation using fMRI in response to sexually arousing pictures, infant pictures, and neutral pictures in 29 postpartum and 30 nulliparous women. Half of the women received a dose of exogenous oxytocin before scanning. As predicted, nulliparous women subjectively rated sexual pictures to be more arousing, and infant pictures to be less arousing, than did postpartum women. However, nulliparous women receiving the nasal oxytocin spray rated the infant photos as arousing as did postpartum women. Right amygdala activation was lower in postpartum versus nulliparous women in response to sexual, infant, and neutral images, suggesting a generalized decrease in right amygdala responsiveness to arousing images with parturition. There was no difference in right amygdala activation with nasal spray application. Postpartum women therefore appear to experience a decrease in sexual interest possibly as a feature of a more generalized decrease in amygdala responsiveness to arousing stimuli.
Keywords: postpartum, amygdala, sexual desire, oxytocin
INTRODUCTION
During the postpartum period women frequently report decreases in sexual desire (Botros, Abraham, Miller, Sand, Gandhi, et al., 2006; De Judicibus & McCabe, 2002; Fischman, Rankin, Soeken, & Lenz, 1986; Glazener, 1997) and to a more variable extent physiological sexual function (Baksu, Davas, Agar, Akyol, & Varolan, 2006; Connolly, Thorp & Pahel, 2005). Postpartum loss of sexual desire is reported across many studies in the first six months postpartum in roughly a third to a half of women (Abdool, Thakar, & Sultan, 2009; Barrett, Pendry, Peacock, Victor, Thakar, et al., 2000) and has been reported to last as long as one year (Pertot, 1981; Pastore, Owens, & Raymond, 2007) to many years following child birth (Botros et al., 2006). The pattern of findings for sexual function as measured by resumption of intercourse and pain with intercourse shows a different pattern following parturition than self-reports of desire. Generally, by six months postpartum most women have resumed sexual intercourse (Connolly et al., 2005; De Judicibus & McCabe, 2002; Hyde et al., 1996) and report orgasm function comparable to before pregnancy (Connolly et al., 2005) even though reports of dyspareunia are quite common in the first six months postpartum (Barrett et al., 2000). The literature to date suggests that physical changes and symptoms during the first six months postpartum, including incontinence, prolapse, and pain, may not be the only, or even most significant, source of decreased sexual interest in women as compared to psychological factors (Botros et al., 2006; De Judicibus & McCabe, 2002; Pertot, 1981).
There are significant neuroendocrinological changes with parturition promoting optimal maternal care that may decrease sexual interest during the first six months to one year postpartum. Neuroimaging work suggests that there are changes in the prefrontal-limbic system, including the amygdala, with motherhood to increase maternal responsiveness to infants (Leibenluft, Gobbini, Harrison, & Haxby, 2004; Lorberbaum, Newman, Dubno, Horwitz, Nahas, et al., 1999; Lorberbaum, Newman, Horwitz, Dubno, Lydiard, et al., 2002; Seifritz, Esposito, Neuhoff, Luthi, Mustovic, et al., 2003; Swain, Lorberbaum, Kose, & Strathearn, 2007, Swain, 2008). Given the role of the amygdala in both sexual behavior (Karama, Lecours, Leroux, Bourgouin, Beaudoin, et al., 2002; Hamann, Herman, Nolan, & Wallen, 2004) and maternal behavior, as well as general emotion and reward processing, we hypothesized that in postpartum women the amygdala would be less responsive to sexual images and more responsive to infant images.
The post-partum period is a reproductive event with unique neuroendocrine characteristics and motivations. Although the mechanisms underlying the postpartum changes in neural and behavioral responses are not entirely clear, the neuropeptide oxytocin is thought to play an important role in postpartum women’s heightened infant responsiveness and decreased response to non-infant stimuli (Carter, Pournajafi-Nazarloo, Kramer, Ziegler, White-Traut et al., 2007; Galbally, Lewis, Ijzendoorn, & Permezel, 2011; Mortimer, 2008). Breast-feeding, which raises oxytocin levels (Altemus, 1995; Carter et al., 2007; Drewett, Bowen-Jones, & Dogterom, 1982) has been associated with a reduction in postpartum women’s sexual desire and frequency of intercourse (Avery, Duckett, & Frantzich, 2000; Forster, Abraham, Taylor, & Llewellyn-Jones, 1994: Glazener, 1997; Hyde et al., 1996). Although oxytocin facilitates peripheral sexual arousal (Salonia, Nappi, Pontillo, Daverio, Smeraldi, et al. 2005), is elevated when sexual arousal does occur (Carmichael, Warburton, Dixon, Davidson, 1987) and increases with orgasm (Blaicher, Gruber, Bieglmayer, Blaicher, Knogler, & Juber, 1999), it is not thought to be a direct driver of sexual motivation and desire that precedes the initiation of sexual behavior (Carter, 1992; Pfaus & Scepkowski, 2005; Pfaus 2009). This is consistent with self-report questionnaire data suggesting that postpartum women seem to enjoy intercourse and experience orgasm when intercourse occurs (Connolly et al., 2005), though they may not desire or initiate it (Woranitat & Taneepanichskul. 2007). Together, this work demonstrates both positive and negative associations of oxytocin with sexual behavior, seemingly varying based on whether the sexual behavior examined is appetitive or consummatory. The current study will examine differences in possible appetitive sexual behavior with parturition and whether there is a causal role for oxytocin in its inhibition.
Studies administering nasal oxytocin to men and nulliparous women further support a role for this hormone in amygdala responsiveness to social stimuli. Oxytocin administration has been associated with decreased amygdala activation in men in response to emotional faces (Domes, Heinrichs, Glascher, Buchel, Braus, et al., 2007; Kirsch, Esslinger, Chen, Mier, Lis, Siddhanti, et al., 2005; Meyer-Lindenberg, Domes, Kirsch, & Heinrichs, 2011) as well as increased amygdala activation in women in response to fearful faces (Domes, Lischke, Berger, Grossmann, Hauenstein, et al., 2010). The authors of the latter study (Domes et al., 2010) argue that increased amygdala activation to oxytocin might facilitate child protection due to the sensitization towards possible threat. In women, therefore oxytocin may be associated with both an increase in amygdala activation to relevant infant stimuli, and a decrease in activation to irrelevant stimuli in the environment, including sexual stimuli. Therefore, in the current study we test the hypothesis that the proposed changes in amygdala activation in response to infant and sexual stimuli are due to specific inhibitory influences of oxytocin on women’s responses to sexual images.
This study aimed to characterize neural activation typical of postpartum women in response to sexually explicit and infant images and examine whether oxytocin is related to amygdala activity in response to these stimuli. We expected postpartum women to report lower arousal to sexual images and increased arousal to infant images. We also expected a lower amygdala response compared to nulliparous women in response to sexually arousing pictures and higher amygdala activation in response to infant images. Differences between nulliparous and postpartum women in both self-reported arousal and amygdala activation were expected to be attenuated with exogenous oxytocin nasal spray.
METHODS
Participants
Participants were 30 nulliparous and 29 postpartum women recruited through flyers, emails, and local organizations. We included only heterosexual women currently in relationships, aged 20–40, and not pregnant. The majority of participants self-reported as White (47; 22 nulliparous), six were Asian (4 nulliparous), three were Black (all nulliparous), one was Hispanic/Latino (postpartum), and two participants self-described themselves as ‘Other’ (1 nulliparous). The majority of women (26) reported an education level at the Bachelor’s degree level, 17 reported completing up to high school (8 postpartum), and 16 had post-graduate degrees (11 postpartum). Average age of participants was 27 years old, however nulliparous women were significantly younger than postpartum women (Mean ± SD nulliparous = 23.8 ± 3.74, Mean ± SD postpartum = 30.21 ± 4.44, p ≤ .001). All women self-reported their health as ‘Good’ or ‘Excellent’ and there was no significant difference across groups. Women reported on average 7 hours of sleep a night, as measured as self-reported hours asleep the previous night (excluding interruptions), with no significant difference between nulliparous and postpartum women (Table 1). We did observe a significant difference in weight (p=.04) and percent body fat (p=.001) in which postpartum women were higher (weight, Mean ± SD nulliparous = 138.21 ± 27.26, Mean ± SD postpartum = 153.72 ± 26.04; Percent body fat, Mean ± SD nulliparous = 25.84 ± 7.76, Mean ± SD postpartum = 33.82 ± 8.89). None of the variables discussed above differed significantly by nasal spray assignment group. All women reported high satisfaction with their partners in terms of their relationship (Scale 1–5, 1=very satisfied, Mean ± SD = 1.4 ± .5) and partner’s help with childcare (Mean ± SD = 1.34 ± .48).
Table 1.
Mean scores (± std. dev) by cohort on the BISF-W, mood, and sleep. Nulliparous women had significantly higher scores for all BISF-W dimensions except D7. There were no differences in measures of anxiety, depression, or sleep
| Nulliparous (N=27) | Postpartum (N=24) | Total (N=51) | |
|---|---|---|---|
| BISF-W | |||
| D1 Thoughts/Desires (range 0–12) *** | 7.71 ± 2.23 | 5.13 ± 2.20 | 6.49 ± 2.55 |
| D2 Arousal (range 0–12) *** | 8.23 ± 2.33 | 5.49 ± 2.40 | 6.94 ± 2.72 |
| D3 Frequency (range 0–12)*** | 5.69 ± 1.89 | 3.20 ± 1.31 | 4.52 ± 2.05 |
| D4 Receptivity/Initiation (range 0–15) * | 9.70± 2.71 | 7.88 ± 3.55 | 8.84 ± 3.24 |
| D5 Pleasure/Orgasm (range 0–12) *** | 6.51 ± 1.48 | 4.03 ± 2.07 | 5.34 ± 2.16 |
| D6 Relationship Satisfaction (range 0–12) ** | 10.52 ± 1.60 | 7.92 ± 2.50 | 9.29 ± 2.44 |
| D7 Problems Affecting Sex (range 0–16) | 2.72 ± 1.59 | 4.97± 2.10 | 3.78 ± 2.15 |
| Composite Score (range −16–75) *** | 45.65 ± 8.51 | 28.66 ± 11.27 | 37.66 ± 13.02 |
| Anxiety (range 0–7) | .32 ± .55 | .25 ± .44 | .29 ± .49 |
| Depression (EPDS) | 5.42 ± 4.51 | 4.24 ± 2.36 | 4.78 ± 3.51 |
| Sleep (Hours) | 7.24 ± 1.46 | 7.45 ± 1.53 | 7.34 ± 1.48 |
Significant difference across cohorts
p ≤ 05;
p ≤ 01;
p ≤ .001
Postpartum respondents were eligible only if they had an infant aged 3–6 months at the time of testing (Mean weeks ± SD = 21.30 ± 6.13; No difference by spray group) and were primarily breast-feeding their infant (> 75% breast-feeding), in order to reduce potential confounds between bottle and breast-feeding women who may have different neuroendocrine states. All postpartum women were breast-feeding primarily (average percent breast-feeding, Mean ± SD = 87% ± 17.55). Sixteen of postpartum women were primiparous (Mean # children = 1.76 ± 1.15). Ten of the postpartum women reported currently using hormonal contraceptives, none of the nulliparous women did. Within postpartum women, eight women reported having resumed menstruation, 21 had not. Differences in contraceptive use and menstruation status did not impact the pattern of findings reported here.
Participants were assigned to either the placebo or oxytocin nasal spray group in a double blind procedure. Because depression is associated with altered neural responses to emotional stimuli (Siegle, Thompson, Carter, Steinhauer, & Thase, 2007), we only included nondepressed women. Screening was done with The Edinburgh Postnatal Depression Scale (Cox, Holden, & Sagovsky, 1987; cut-off score of 10). Due to the strong magnetic fields used for MR imagers, women who had magnetic life-support devices (e.g., pacemakers and aneurysm clips), metal prostheses or other metallic objects (e.g. cochlear implants, steel pins implanted to help repair and strengthen broken bones, metal fragments from previous injuries) were excluded from this research.
Procedure
All procedures of the study were approved by the University’s IRB committee and were in compliance with ethical treatment of human subjects guidelines. After the initial phone screening and scheduling the women were mailed a participant packet for at-home completion before the scheduled test session. This packet contained a consent form detailing the procedures of the study, as well as a questionnaire regarding demographics, health, menstrual cycle, motherhood, relationships with partners, and sexual behavior. The central measure of sexual behavior in the current study was The Brief Index of Sexual Function for Women (BISF-W, Taylor, Rosen, & Leiblum, 1994; Mazer, Leiblum, & Rosen, 2000). The BISF-W is a 22-item self-report questionnaire for assessing female sexual function. Scores overall and across 7 dimensions were calculated according to Mazer et al. (2000). These dimensions were D1 (thoughts/desires), D2 (arousal), D3 (frequency of sexual activity), D4 (receptivity/initiation), D5 (pleasure/orgasm), D6 (relationship satisfaction), and D7 (problems affecting sexual function). Higher scores on the composite and D1–D6 reflect higher sexual function.
Nulliparous respondents were tested during the periovulatory phase of their menstrual cycle (days 8–16) in order to control for fluctuations in hormones and sexual desire. Postpartum participants scheduled their session time around their infant’s usual feeding time to control for the associated changes in oxytocin with breast-feeding (Altemus et al., 1995). Participants were asked to abstain from alcohol, sexual activity, and tobacco use the day of the test session. Postpartum women were requested to bring their infant with them to the test session. On the test day, following the informed consent process, postpartum participants were asked to nurse their infant in a private adjacent room to enhance the comfort of the mothers and infants and control for the time since feeding. Breast-feeding ended approximately one hour and 15 minutes before fMRI scanning. This timing allowed for us to avoid the ceiling and baseline levels present directly before and after breast-feeding (Carter et al. 2007). Following consent (and postpartum nursing), all participants were administered a paper version of the 10-item EPDS to confirm absence of depression. These scores, as well as likert scale ratings of self-reported feelings of anxiety (“Do you currently feel anxious, Scale 0–7; 0 = not at all, 7 = very) were used as the mood variables in the current study. They were then introduced to the task that they would perform in the fMRI scanner and given the opportunity to practice it in the lab. The task involved viewing sexually explicit and infant images taken from publicly available websites, in addition to neutral images from the International Affective Picture Set (IAPS, Lang et al., 2005). The sexual photos used in this study have been used successfully elsewhere and are known to evoke sexual interest in women (Rupp & Wallen, 2007, 2009). During photo presentation participants performed a backward-matching task to ensure attention. For this practice portion participants were shown 10 pictures including each stimulus type for four seconds each with a one second fixation slide in between each picture. If the photo was the same as the image previously viewed they were instructed to hit the space bar, if it was not they were told to do nothing.
After the task introduction, about 30 mins prior to the first run of fMRI scanning, participants received either nasal oxytocin or placebo nasal spray. Participants received an absolute dose of 24 IU, based on previous studies (Ditzen, Schaer, Gabriel, Bodenmann, Ehlert, & Heinrichs, 2009; Heinrichs, Meinlschmidt, Neumann, Wagner, Kirschbaum, et al. 2001; Kirsch et al., 2005; MacDonald, Dadds, Brennan, Meyer-Lindberg et al., 2011, Williams, Levy, & Cauchi, 2011). The oxytocin nasal spray (Syntocinon®, Novartis Pharma, Switzerland) contains a synthetic oxytocin nonapeptide. The spray is most commonly used to facilitate breast-feeding in postpartum women, and is approved by the FDA for use in this sample. Acute overdose of the nasal spray has not been reported as oxytocin is inactivated in the digestive tract by proteolytic enzymes (Syntocinon Nasal Spray Core data sheet). The main risk of exogenous oxytocin is uterine contraction, so all women were asked to take a pregnancy test before drug administration. Allergic reaction to this drug is unlikely, but antihistamines and on-call medical support were present at all sessions as a precaution. The placebo spray contained only the inactive carrier found in the active oxytocin spray and is indistinguishable from the active spray (Meyer-Lindenberg et al., 2011). Oxytocin spray has recently been shown to increase salivary levels of the hormone as soon as 15 minutes after inhalation and levels remain elevated up to four hours (Weisman, Zagoory-Sharon, & Feldman, 2012). A recent study in primates demonstrated increased levels of oxytocin measured in CSF after oxytocin spray inhalation (Chang, Barter, Ebitz, Watson, & Platt, 2012), supporting the possibility of central effects.
Imaging was carried out using a Siemens Magnetom Trio 3T whole body MRI. The MRI session took about one hour, during which the following scans were acquired: 1) one 10-second, three-plane scout/localizer used for choosing slice planes and volumes for the remaining scans; 2) seven approximately 5-minute whole-brain functional blood oxygenation-level dependent (BOLD) scans; and 3) one 5-minute high-resolution whole-brain anatomical scan. Each of the seven functional runs began with 12 secs of rest to ensure a stable baseline signal. During each run, participants viewed in randomized order 64 stimuli from each category of sexual, infant, and neutral pictures. Each stimulus was presented for 2 seconds, followed by a variable inter-stimulus interval (ISI) of 2–6 seconds. As described for the practice task, the participants performed a one-back matching task. Participants then viewed the same stimuli as presented during the fMRI scanning on a laptop and rated them for how ‘aroused they make them feel’ (1–9). In the instructions we clarified ‘arousal’ was not specifically sexual, but was more of an intensity measure of whatever emotion they were feeling. Each rating screen followed the picture as was accompanied by the Self-Assessment Manikin (SAM) figures of the IAPS ratings (Lang et. al, 2005).
Imaging Parameters and Analysis
Images were collected using a 32-channel phased-array head coil. The field of view was 220 × 220 mm, with an in-plane resolution of 128 × 128 pixels and 35 axial slices of 3.4 mm thickness per volume. These parameters produced voxels that were 1.7 × 1.7 × 3.4 mm. Functional images were collected using a gradient echo BOLD echo-planar imaging (EPI) sequence: TE = 24 ms, TR = 2,000 ms, flip angle = 70°. Parallel imaging was used with a iPAT factor of 2. High-resolution T1-weighted anatomical volumes were acquired using a Turbo-flash 3-D sequence: TI = 900 ms, TE = 2.67 ms, TR = 1800 ms, flip angle = 9°, with 192 sagittal slices of 1 mm thickness, a field of view of 224 × 256 mm, and an isometric voxel size of 1 mm3.
Imaging data were analyzed using BrainVoyager™ QX 2.2. Individual anatomical volumes were transformed into a common stereotactic space based on the reference of the Talairach atlas using an eight-parameter affine transformation. All functional volumes were realigned to a reference functional volume, which was the volume collected closest in time to the anatomical volume. Re-alignment was done using an intensity-based motion-correction algorithm. Functional volumes also underwent slice scan-time correction, 3-D spatial Gaussian filtering (FWHM 6 mm), and linear trend removal. Functional volumes were co-registered to the anatomical volume using an intensity-based matching algorithm and normalized to the common stereotactic space using an eight-parameter affine transformation. During normalization, functional data were re-sampled to 3 mm3 isometric voxels. Whole-brain statistical parametric maps were calculated using a random-effects general linear model with predictors based on the timing protocol of the blocked stimulus presentation, convolved with a two-gamma hemodynamic response function. Beta weights were extracted from group ROIs using the VOI/ROI ANCOVA data table tool in BrainVoyager’s volume of interest module. Statistical hypothesis testing was performed on the extracted beta weights using the Repeated Measures ANOVA and multiple regression tools in SPSS. The beta weights represent % BOLD signal change from baseline in response to each stimulus in the amygdala ROI.
Performing analyses on a subset of the data (i.e., only on regions of interest) limits the number of statistical tests relative to a whole-brain analysis and control the Type I error rate while allowing for detection of smaller effect sizes (Poldrack, 2007; Saxe, Brett, & Kanwisher, 2006). Thus, to further analyze the interaction between stimulus, cohort, and nasal spray, we performed another ANOVA on the data from the amygdala region of interest determined from the main effect of the whole-brain analysis. Note that the main effect used to select the ROIs and the interaction effect tested in the ROI analysis are orthogonal contrasts, which protects the analysis from the problem of non-independent ROI analysis (Kriegeskorte, Simmons, Bellgowan, & Baker, 2009).
RESULTS
Questionnaire Data
There was no difference in self-reported anxiety or depression between postpartum or nulliparous women, both groups reporting low on both measures (Anxiety, Score 0–7, Mean ± SD = .29 ± .49; EPDS, Mean ± SD = 4.78 ± 3.51; Table 1). As mentioned, we calculated an overall and seven dimensional scores from the BISF-W (Mazer et al., 2000). Nulliparous women had significantly higher scores on all D1–D6 compared to postpartum women, though no significant difference for D7/problems (Multivariate ANOVA with cohort and group as between subjects factors and age and percent body fat as covariates; Table 1.). All scores were in the normal range (Mazer et al., 2000) and there was no difference across nasal spray groups.
Picture Ratings
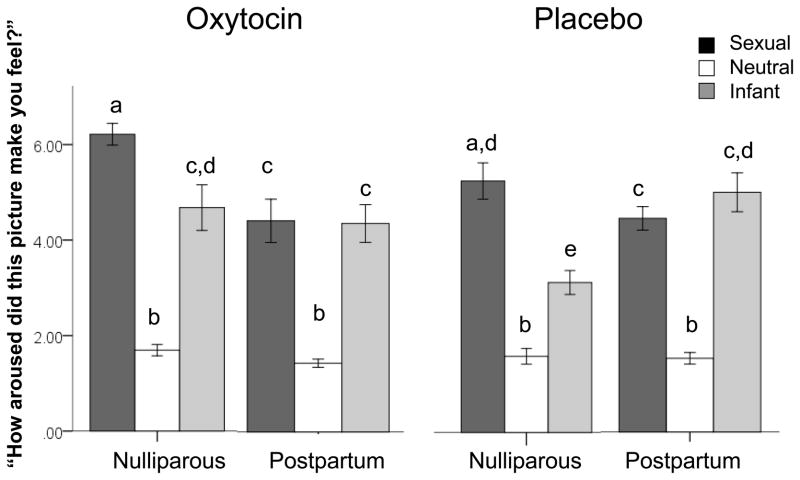
Three women were not included in the self-report data analysis; two women (one in each cohort) rated all pictures as ‘1’ and one postpartum woman did not complete the survey due to time constraints. Overall, pictures of sexual stimuli were rated most arousing (Mean ± SD = 5.09 ± 1.47), followed by infant (Mean ± SD = 4.30 ± 1.55) and then neutral stimuli (Mean ± SD = 1.58 ± .48). We examined if survey ratings for sexual, neutral and infant pictures differed across cohort (nulliparous versus postpartum) or with nasal spray treatment group (Repeated Measures ANOVA across picture type with cohort and nasal spray as between subjects factors and age and % body fat as covariates). There was an interaction of photo type, cohort, and nasal spray cohort (F1,47 = 4.55, p = .04, Figure 1). Post-hoc analyses (One-Way ANOVA) demonstrated that nulliparous women rated sexual stimuli more arousing than postpartum women did, as we predicted (F1,56 = 16.65, p ≤ .001; Nulliparous Mean ± SD = 5.76 ± 1.28; Postpartum Mean ± SD = 4.35 ± 1.32). There was also a trend in which postpartum women rated infant stimuli more arousing than did nulliparous women (F1,55 = 3.63, p = .06; Nulliparous Mean ± SD = 3.93 ± 1.60; Postpartum Mean ± SD = 4.70 ± 1.43). Repeated Measures post-hoc analyses within cohorts showed that nulliparous women found sexual photos significantly more arousing than infant images (p<.001), while postpartum women found them equally arousing (p = .48). Additional post-hoc analyses within nasal spray groups and cohorts also demonstrated an increase in the nulliparous women’s ratings of infant stimuli with oxytocin administration (F1,29 = 7.48, p = .01; Nulliparous Placebo Mean ± SD = 3.23 ± 1.00; Nulliparous Oxytocin Mean ± SD = 4.68 ± 1.79). Ratings of neutral pictures did not differ across cohort or spray group.
Figure 1.
Average ratings of arousal by picture type within cohorts by nasal spray condition. Significant differences are indicated by differences in lettering. Overall, nulliparous women rated sexual pictures to be more arousing than postpartum women did. Postpartum women in the placebo group rated infant pictures more arousing than did nulliparous women in the placebo group. Nulliparous women receiving oxytocin had higher ratings of infant stimuli compared to nulliparous women receiving placebo.
In order to examine relationships between individual factors and subjective ratings of the sexual images we ran stepwise regression analyses across the entire population and within cohorts and groups. Variables entered into the model were percent body fat, age, and BISF Total. For the model conducted across all participants, only women’s scores on the BISF (total) positively predicted subjective ratings of the sexual images (R2 = .12. r = .35, p = .01). BISF-W scores also positively predicted subjective sexual ratings for the model conducted within the Oxytocin nasal spray group (R2 = .30. r = .55, p = .005). Models performed within cohorts and for the placebo participants found no significant predictors.
Whole brain contrasts
fMRI data from six of the women were not usable due to excessive motion artifacts or technical issues at the time of scanning, leaving a total sample of 53 (13 nulliparous placebo, 14 nulliparous oxytocin, 12 postpartum placebo, 14 postpartum oxytocin) for the brain imaging analysis. A whole-brain analysis was conducted using a three-factor 2 × 2 × 2 design with stimulus (sexual, neutral), cohort, and nasal spray as factors. Contrary to our hypotheses, no clusters passed a false discovery rate (FDR) corrected threshold (q = .05) for the main effect of cohort, the main effect of nasal spray, or any of the interaction terms. Across all women we confirmed an increased response to sexual versus neutral stimuli in the right amygdala (Talairach coordinates; 15, −4, −8), hypothalamus (−1, −1, −13), midbrain (1, −25, −7), thalamus (1, −15, 2), insula (39, 3, 24), anterior cingulate cortex (−4, 2, 35), premotor cortex (28, −9, −47) and extended parietal and visual cortical regions (FDR corrected threshold q = .05).
ROI: Amygdala
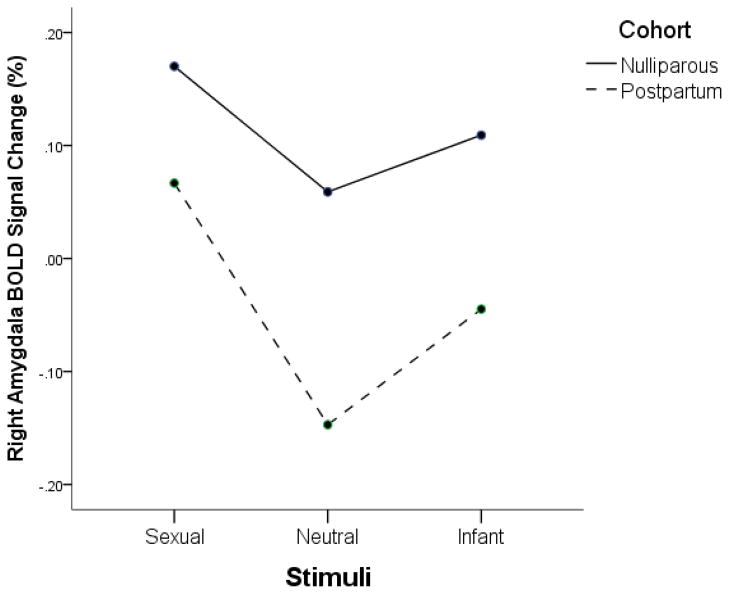
Data extracted from the right Amygdala ROI analysis (Talairach coordinates; 15, −4, −8) were first examined using a Repeated Measures ANOVA across the picture types (sexual, infant, neutral) with cohort and nasal spray as the between subjects factors. The main effect of stimulus (F1,49 = 4.71, p = .04) was not interpreted due to the concern of non-independence with the selection of the ROI (Kriegeskorte et al., 2009). This analysis also showed a main effect of cohort (F1,49 = 4.07, p = .05) in which nulliparous had higher right amygdala responses than did postpartum women overall (Figure 2). There were no significant differences related to nasal spray group.
Figure 2.
BOLD signal change as a function of stimulus and cohort. There was a significant effect of cohort in which nulliparous women had a higher right amygdala response than post partum.
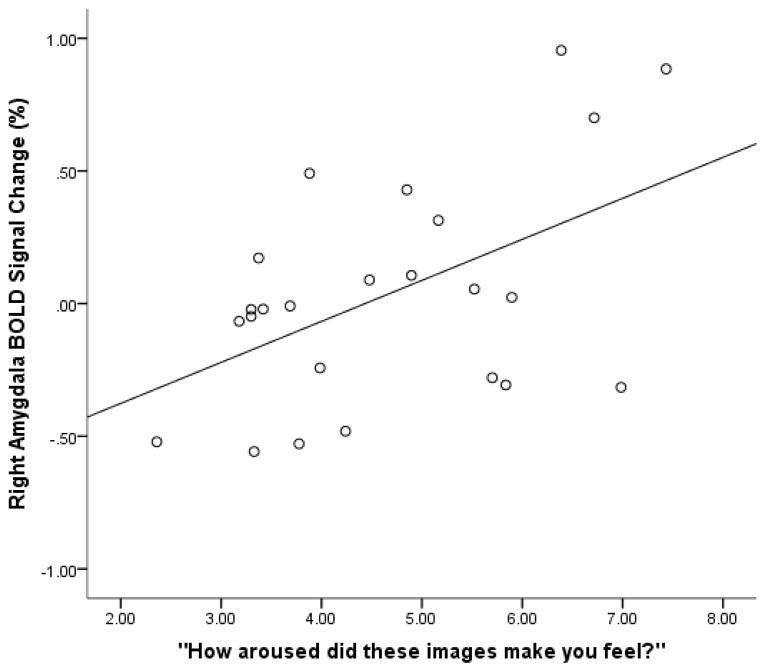
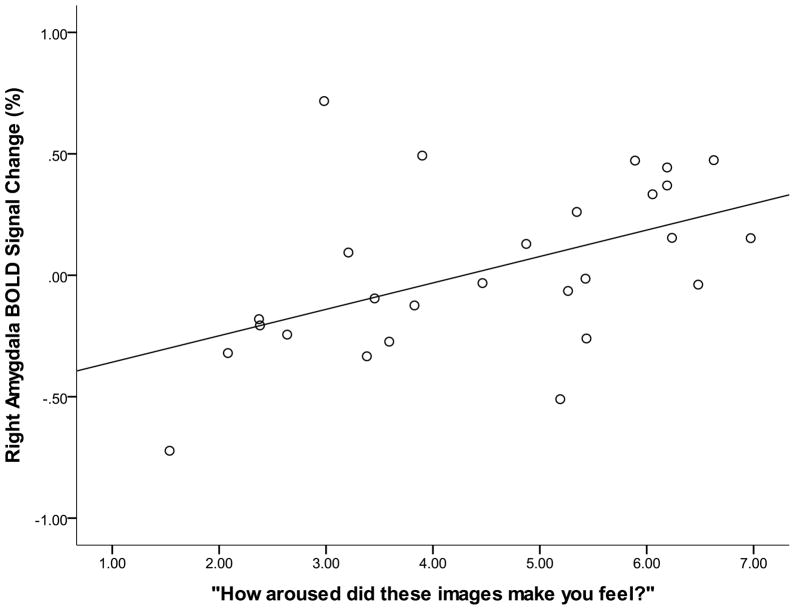
Finally, we were also interested in whether activation in the amygdala was related to women’s self-reports of arousal to the sexual and infant images, the relationship of which we expected to be mediated by spray application. To examine this we conducted two correlation analyses (Pearson Bivariate two-tailed) within nasal spray groups (oxytocin, placebo), for a total of four correlations (infant and sexual stimuli). Variables were amygdala response (sexual or infant) and sexual and infant ratings. For sexual stimuli, we found a positive relationship between amygdala activation and ratings in the placebo (N = 24, R = .51, p = .01; Figure 3) but not oxytocin group (N = 27, R = .01, p = .95). For infant stimuli, in women who received the oxytocin nasal spray there was a significant positive relationship between amygdala activation and ratings of infant images (N = 27, R = .51, p = .007; Figure 4). This relationship between amygdala activation and subjective ratings was not observed in the placebo group (N = 23, R = −.39, p = .06).
Figure 3.
BOLD signal change in placebo participants in response to sexual images in relation to self-reported feelings of arousal in response to the images. There was a significant positive relationship between the right amygdala response and self-reports of arousal for these images within this group (N = 24, R = .51, p = .01).
Figure 4.
BOLD signal change in response to infant images in relation to self-reported feelings of arousal in response to the images in women receiving the intranasal oxytocin. There was a significant positive relationship between the right amygdala response and self-reports of arousal for these images (N = 27, R = .51, p = .007).
DISCUSSION
This study demonstrates differences in amygdala responses to sexual and infant stimuli in postpartum versus nulliparous women. We originally proposed that changes in sexual desire in postpartum women were a byproduct of changes in the functional neuroendocrinology that facilitates maternal behavior, predicted to be demonstrated as lower amygdala responses to sexual stimuli and higher responses to infant stimuli. In our study, postpartum did women self-report lower sexual function, as well as higher ratings of arousal towards infant photos. However, nulliparous women demonstrated higher right amygdala activation compared to postpartum women in response to all types of stimuli presented, sexual, neutral and infant. We interpret these data to suggest that decreases in self-reported feelings of sexual desire in postpartum women are related, in part, to a generalized decrease in amygdala responsiveness to arousing stimuli rather than a sex-stimulus specific change in brain function during the postpartum period. This has implications for the understanding of sexual function and perceptions of dysfunction during the postpartum period, as well as for our understanding of arousal neural systems in general.
Although we did find amygdala response differences between nulliparous and postpartum women in response to infant and sexual stimuli, they were in a different pattern than originally hypothesized. Specifically, amygdala activation in postpartum women was lower in response to both sexual and infant stimuli, rather than increased in response to infant stimuli as predicted. These findings, therefore, do not support an evolutionary trade-off theoretical perspective. These findings are however consistent with previously reported decreases in postpartum women’s peripheral psychophysiological response to arousing stimuli (Altemus, Redwine, Leong, Frye, Porges, et al., 2001; Groer, Davis, & Hemphill, 2002; Heinrichs et al., 2001). It may be that decreased amygdala responsiveness to infants facilitates maternal behavior by buffering new mothers from the stress of the demands and pressures of caring for an infant (Carter, Altemus, & Chrousos, 2001). If so, then the generalized decrease in this region may also decrease arousal to sexual stimuli as a consequence of this change.
The response pattern of decreased amygdala responding may not extend to other categories of stimuli, such as fearful faces and infant-related threats. It has been argued that increased amygdala activation with increased oxytocin might occur to facilitate child protection due to the sensitization towards possible threat (Domes et al., 2010). This interpretation is consistent with the results of the current study in which we used infant stimuli that were generally pleasant. We believe that infant-related threatening stimuli would produce a different response than the non-threatened ‘cute’ infant stimuli used here. It is also unknown whether the same pattern would be observed if we had used pictures of the mother’s own infants versus novel babies. Previous work demonstrated higher right amygdala activation in response to one’s own versus other infants (Leibenkuft et al., 2004). It is possible that postpartum women may have higher amygdala activation in response to their own infants compared to nulliparous women.
In contrast to the fMRI data, our subjective data were generally in support of the proposed directional reproductive trade-off interpretation in that postpartum women reported lower arousal to sexual stimuli in conjunction with higher arousal to infant stimuli compared to placebo nulliparous women. This may suggest that there are other critical neural processes outside of the amygdala for the overall subjective feelings of arousal in response to infants. Alternatively this inconsistency in directionality of findings between subjective and amygdala data may be related to the methodology used, specifically the positive nature of the infant images used. Postpartum women may have experienced more pleasure in response to and interest in infant images, which they could have misinterpreted and self-reported as increased arousal. The study design and data analysis were focused on a hypothesis and theoretical framework addressing oxytocin’s possible inhibitory action on arousal through the amygdala’s response to social stimuli. Therefore the stimuli chosen were specifically quantified for ‘arousal’ rather than ‘attractiveness’ or ‘reward’ or some other motivational variable. For that reason we did not focus ROI analyses on limbic regions related to reward or desire such as the ventral striatum. Although the current study demonstrates important changes in arousal in response to infant and sexual stimuli, we believe that future work should focus on possible oxytocin mediated changes in reward salience of sexual and infant stimuli given known interactions between the dopamine and oxytocin systems (Pfaus, 2009). It is possible that postpartum women’s lower arousal to sexual stimuli and self-reported lower sexual desire are due to oxytocin-related changes in motivated attention and increases reward value towards infants and away from sexual stimuli.
Contrary to our hypotheses, we did not observe differences in amygdala responsiveness to infant or sexual stimuli with oxytocin nasal spray administration. This is inconsistent with a previous study in which administration of nasal oxytocin spray was shown to decrease levels of amygdala activation to infant cries in nulliparous women (Riem, Bakermans-Kranenburg, Pieper, Tops, Boksem, Vermeiren, et al., 2011). We did observe an increase in nulliparous women’s subjective ratings of arousal for infant images with nasal oxytocin administration. However, given the other findings this study does not support an activational or directly state-dependent causal role of oxytocin on postpartum women’s responses to sexual or infant visual stimuli. Thus, we do not believe that this lower arousal response in postpartum women is directly caused by state levels of oxytocin, but instead may reflect longer term changes in postpartum neuroendocrine function warranting further investigation. The finding of an association between amygdala activation and ratings of infant stimuli in the oxytocin, but not placebo, group do suggest some acute role of oxytocin on women’s infant responsiveness, however.
These findings discussed above leave open many questions and possibilities for future research. One hypothesis is that increases in oxytocin do act acutely only transiently in the nulliparous or newly postpartum brain. It is possible that comparing nulliparous to relatively late postpartum women misses the window of time in which oxytocin does act directly and in an activational manner to inhibit arousal to infants, perhaps in the first weeks after birth. The postpartum neuroendocrine system at three-six months postpartum as measured in the current study may be very stable and acclimated to the neuroendocrine state of motherhood and not respond notably to transient fluctuations in oxytocin. Furthermore, differences in responses to sexual stimuli in postpartum women versus nulliparous may not be related to oxytocin or parturition directly, but alternatively may reflect increases in sexual interest in nulliparous women during the follicular phase compared to other times since all nulliparous women were tested during this time. Specific causal attribution of oxytocin and parturition to differences in amygdala responsiveness to sexual stimuli cannot be confidently concluded until this is replicated in another sample of women at different phases of the menstrual cycle. A significant limitation of the study is the absence of serum levels of oxytocin throughout the session, which could help inform the questions raised above. Future work should include repeated measures of oxytocin levels and testing of nulliparous women outside of the follicular phase in order to separate direct state effects of oxytocin from more general neuroendocrine adaptations in the latter postpartum period.
The subjective questionnaire data and arousal ratings that were used in this fMRI study offer interesting replication and insights. Primarily of interest was that nulliparous women reported higher sexual functioning across all six domains of the BISF which address more psychological and motivational issues, in the absence of any difference in scores on D7, which measures physical problems affecting sexual function. This suggests the postpartum period is a period of psychological sexuality adjustment independent of the peripheral physiological changes with parturition. Additionally, postpartum women subjectively rated the sexual pictures to be less arousing than did nulliparous women, indicating lower sexual interest. These findings are also consistent with the idea that postpartum changes in sexual interest were more closely related to centrally mediated processes of desire and motivation as opposed to peripheral physiological problems (Botros et al., 2006; De Judicibus & McCabe, 2002; Pertot, 1981).
Limitations of our study warrant mention in order to inform future work and interpretation of the current results. Although the use of the oxytocin nasal spray methodology is a significant strength, its relative novelty allows for some outstanding questions. The dosage and timing used was based on previous studies in men and women (i.e. Heinrichs et al., 2001; Kirsch et al., 2005) rather than a dose or neuroendocrine response expected to be reflective of postpartum neuroendocrinology. It is not known whether lower or higher doses would produce different patterns of results, or for instance, whether there is some ‘threshold’ over which levels of oxytocin alter responsiveness in women or reach a ceiling effect, and more importantly whether this differs between nulliparous and postpartum women. This may also change with multi-parity, a variable we did not account for in the current study. Future work examining the dose response curve and timing of oxytocin action in humans or primates would be useful for future study designs using nasal oxytocin and for our understanding of oxytocin in the maternal brain. Although there were no differences in total sleep time between nulliparous and postpartum women, we did not measure overall sleep quality or disruption, which may alter neural responses to arousing stimuli. Finally, although our nulliparous and postpartum participants were very similar on many parameters, they did differ in age and use of hormonal contraception, which may have undetected impacts on women’s arousal response given associations of these variables with cortisol responsiveness (Carr, Parker, Madden, MacDonald, & Porter, 1979; Kirschbaum, Platte, Pirke, & Hellhammer, 1996), in addition to possible unmeasured socio-psychological differences between groups such as interest in having children. An ideal follow-up experiment would be a within-subjects design.
In sum, we believe that this study demonstrates important differences in subjective and cognitive responses to sexual and infant images between postpartum and nulliparous women. This study supports the idea that sexual dysfunction in postpartum women may commonly be due to psychological, social, and neuroendocrine changes with the post partum period rather than peripheral physiological problems. This may have important implications in the understanding of postpartum women’s sexuality. From a basic research perspective these findings are further evidence of the extraordinarily complex role of hormones in altering women’s responses to and processing of social stimuli. These findings emphasize the complexity in the mechanisms underlying fluctuations in women’s reproductive priorities. We believe that decreases in sexual desire during the postpartum period may less be considered a dysfunction or problem and more positively as behavioral neuroendocrine change characteristic of the postpartum period warranting further investigation.
Highlights.
Postpartum and nulliparous women given oxytocin viewed sexual images in fMRI.
Nulliparous rated sexual images more arousing than did postpartum women.
Right amygdala activation was lower in postpartum versus nulliparous women.
There was no difference in right amygdala activation with nasal spray application.
Propose a generalized postpartum decrease in amygdala response to arousing images.
Acknowledgments
This study was supported by grant #NIMH R21MH082925 to H. Rupp and J. Heiman. We would like to thank Diane Ebling at the Indiana University Health Center for safety monitoring of the nasal spray. We are grateful to Bloomington Area Birth Services for help with recruitment. The following are thanked for assistance with data collection: L. Reckley, B. Keller, C. Anderson, C. White, and Q. Class. Finally we want to thank the MR operators B. Ward and T. Atwood.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdool Z, Thakar R, Sultan AH. Postpartum female sexual function. Eur J Obstet Gynecol Reprod Biol. 2009;145:133–137. doi: 10.1016/j.ejogrb.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Altemus M. Neuropeptides in anxiety disorders- Effects of lactation. Ann NY Acad Sci. 1995;771:697–707. doi: 10.1111/j.1749-6632.1995.tb44721.x. [DOI] [PubMed] [Google Scholar]
- Altemus M, Redwine LS, Leong Y, Frye CA, Porges SW, Carter SC. Responses to laboratory psychosocial stress in postpartum women. Psychosom Med. 2001;63:814–821. doi: 10.1097/00006842-200109000-00015. [DOI] [PubMed] [Google Scholar]
- Avery MD, Duckett L, Frantzich CR. The experience of sexuality during breast- feeding among primiparous women. J Midwifery Womens Health. 2000;45:227–237. doi: 10.1016/s1526-9523(00)00020-9. [DOI] [PubMed] [Google Scholar]
- Baksu B, Davas I, Agar E, Akyol A, Varolan A. The effect of mode of delivery on postpartum sexual functioning in primiparous women. Int Urogynecol J. 2006;18:401– 406. doi: 10.1007/s00192-006-0156-0. [DOI] [PubMed] [Google Scholar]
- Barrett G, Pendry E, Peacock J, Victor C, Thakar R, Manyonda I. Women’s sexual health after childbirth. Br J Obstet Gyencol. 2000;107:186–195. doi: 10.1111/j.1471-0528.2000.tb11689.x. [DOI] [PubMed] [Google Scholar]
- Blaicher W, Gruber D, Bieglmayer C, Blaicher AM, Knogler W, Huber JC. The role of oxytocin in relation to female sexual arousal. Gynecol Obstet Invest. 1999;47:125–126. doi: 10.1159/000010075. [DOI] [PubMed] [Google Scholar]
- Botros SM, Abraham Y, Miller JR, Sand PK, Gandhi S, Nickolov A, Goldberg RP. Effect of parity on sexual function: An identical twin study. Obstet Gynecol. 2006;107:765–770. doi: 10.1097/01.AOG.0000207677.03235.76. [DOI] [PubMed] [Google Scholar]
- Carmichael MS, Humbert R, Dixen J, Davidson JM. Plasma oxytocin increases in the human sexual response. J Endocrinol Metab. 1987;64:27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- Carr BR, Parker CR, Madden JD, MacDonald PC, Porter JC. Plasma levels of adrenocorticotropin and cortisol in women receiving oral contraceptive steroid treatment. J Endocrinol Metab. 1979;49:346–349. doi: 10.1210/jcem-49-3-346. [DOI] [PubMed] [Google Scholar]
- Carter CS. Oxytocin and sexual behavior. Neurosci Behav Rev. 1992;16:131–144. doi: 10.1016/s0149-7634(05)80176-9. [DOI] [PubMed] [Google Scholar]
- Carter CS, Altemus M, Chrousos GP. Neuroendocrine and emotional changes in the post-partum period. Prog Brain Res. 2001;133:241–249. doi: 10.1016/s0079-6123(01)33018-2. [DOI] [PubMed] [Google Scholar]
- Carter CS, Pournajafi-Nazarloo H, Kramer KM, Ziegler TE, White-Traut R, Bello D, Schwertz D. Oxytocin: Behavioral associations and potential as a salivary biomarker. Ann NY Acad Sci. 2007;1098:312–322. doi: 10.1196/annals.1384.006. [DOI] [PubMed] [Google Scholar]
- Chang SW, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macacca mulatta) Proc Nat Acad Sci. 2012;109:959–964. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly A, Thorp J, Pahel L. Effects of pregnancy and childbirth on postpartum sexual function: a longitudinal prospective study. Int Urogynecol J. 2005;16:263–267. doi: 10.1007/s00192-005-1293-6. [DOI] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression; Development of the 10-iten Edinburgh postnatal depression scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- De Judicibus MA, McCabe MP. Psychological factors and the sexuality of pregnant and postpartum women. J Sex Res. 2002;39:94–103. doi: 10.1080/00224490209552128. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol Psychiatry. 2009;65:728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Glascher J, Buchel C, Braus DF, Herpertz SC. Oxytocin attenuates amygdala responses to emotional faces regardless of valence. Biol Psychiatry. 2007;62:1187–1190. doi: 10.1016/j.biopsych.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Domes G, Lischke A, Berger C, Grossmann A, Hauenstein K, Heinrichs M, Herpertz SC. Effects of intranasal oxytocin on emotional face processing in women. Psychoneuroendocrinology. 2010;35:83–93. doi: 10.1016/j.psyneuen.2009.06.016. [DOI] [PubMed] [Google Scholar]
- Drewett RF, Bowen-Jones A, Dogterom J. Oxytocin Levels during Breast- Feeding in Established Lactation. Horm Behav. 1982;16:245–248. doi: 10.1016/0018-506x(82)90023-x. [DOI] [PubMed] [Google Scholar]
- Fischman SH, Rankin EA, Soeken KL, Lenz ER. Changes in sexual relationships in postpartum couples. J Obstet Gynecol Nurs. 1986;15:58–63. doi: 10.1111/j.1552-6909.1986.tb01369.x. [DOI] [PubMed] [Google Scholar]
- Forster C, Abraham S, Taylor A, Llewellyn-Jones D. Psychological and sexual changes after the cessation of breast-feeding. Obstet Gynecol. 1994;84:872–873. [PubMed] [Google Scholar]
- Galbally M, Lewis AJ, Ijzendoorn M, Permezel M. The role of oxytocin in mother-infant relations: A systematic review of human studies. Harvard Rev Psychiatry. 2011;19:1–14. doi: 10.3109/10673229.2011.549771. [DOI] [PubMed] [Google Scholar]
- Glazener CMA. Sexual function after childbirth: Women’s experiences, persistent morbidity and lack of professional recognition. Br J Obstet Gynecol. 1997;104:330–335. doi: 10.1111/j.1471-0528.1997.tb11463.x. [DOI] [PubMed] [Google Scholar]
- Groer MW, Davis MW, Hemphill J. Postpartum stress: Current concepts and the possible protective role of breastfeeding. J Obstet Gynecol Neo Nurs. 2002;31:411–417. doi: 10.1111/j.1552-6909.2002.tb00063.x. [DOI] [PubMed] [Google Scholar]
- Hamann S, Herman RA, Nolan CL, Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nat Neurosci. 2004;7:411–116. doi: 10.1038/nn1208. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Meinlschmidt G, Neumann I, Wagner S, Kirschbaum C, Ehlert U, Hellhammer DH. Effects of suckling on Hypothalamic-Pituitary-Adrenal Axis responses to psychosocial stress in postpartum lactating women. J Clin Endocrinol Metab. 2001;86:4798–4804. doi: 10.1210/jcem.86.10.7919. [DOI] [PubMed] [Google Scholar]
- Hyde JS, DeLamater JD, Plant EA, Byrd JM. Sexuality during pregnancy and the year postpartum. J Sex Res. 1996;33:143–151. [PubMed] [Google Scholar]
- Karama S, Lecours AR, Leroux JM, Bourgouin P, Beaudoin G, Joubert S, Beauregard M. Areas of brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp. 2002;16:1–13. doi: 10.1002/hbm.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11849–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Platte P, Pirke K, Hellhammer D. Adrenocortical activation following stressful exercise: Further evidence for attenuated free cortisol responses in women using oral contraceptives. Stress Health. 1996;12:137–143. [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PSF, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report. University of Florida; Gainesville: 2005. International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual; p. A-6. [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T, Haxby JV. Mother’s neural activation in response to pictures of their children and other children. Biol Psychiatry. 2004;56:225– 232. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Dubno JR, Horwitz AR, Nahas Z, Teneback CC, Bloomer CW, Bohning DE, Vincent D, Johnson MR, Emmanuel N, Brawman-Mintzer O, Book SW, Lydiard RB, Ballenger JC, George MS. Feasibility of using fMRI to study mothers responding to infant cries. Depress Anxiety. 1999;10:99–104. doi: 10.1002/(sici)1520-6394(1999)10:3<99::aid-da2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, Bohning, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- MacDonald E, Dadds MR, Brennan JL, Williams K, Levy, Cauchi AJ. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011;36:1114–1126. doi: 10.1016/j.psyneuen.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Mazer NA, Leiblum SR, Rosen RC. The Brief Index of Sexual Functioning for Women (BISF-W): a new scoring algorithm and comparison of normative and surgically menopausal populations. Menopause. 2000;7:350–363. doi: 10.1097/00042192-200007050-00009. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature. 2011;12:524–535. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Mortimer AM. The neuroscience of maternal behavior. Curr Psychiatry Rev. 2008;3:129– 135. [Google Scholar]
- Pastore L, Owens A, Raymond C. Postpartum sexuality concerns among first-time parents from one U.S. academic hospital. J Sex Med. 2007;4:115–123. doi: 10.1111/j.1743-6109.2006.00379.x. [DOI] [PubMed] [Google Scholar]
- Pertot S. Postpartum loss of sexual desire and enjoyment. Aust J Psychol. 1981;33:11–18. [Google Scholar]
- Pfaus JG. Pathways of sexual desire. J Sex Med. 2009;6:1506–1533. doi: 10.1111/j.1743-6109.2009.01309.x. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Scepkowski MA. The biologic basis for libido. Curr Sex Health Rep. 2005;2:95–100. [Google Scholar]
- Poldrack RA. Region of interest analysis for fMRI. Soc Cogn Affect Neurosci. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riem MME, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MAS, Vermeiren RRJM, van Ijzendoorn MH, Rombouts SARB. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: A randomized controlled trial. Biol Psychiatry. 2011;70:291–297. doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Rupp HA, Wallen K. Sex Differences in Viewing Sexual Stimuli: An Eye Tracking Study in Men and Women. Horm Behav. 2007;51:524–533. doi: 10.1016/j.yhbeh.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Rupp HA, Wallen K. Sex-specific content preferences for visual sexual stimuli. Arch Sex Behav. 2009;38:417–426. doi: 10.1007/s10508-008-9402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonia A, Nappi RE, Pontillo M, Daverio R, Smeraldi A, Briganti A, Fabbri F, Zanni G, Rigatti P, Montorsi F. Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Horm Behave. 2005;47:164–169. doi: 10.1016/j.yhbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Saxe R, Brett M, Kanwisher N. Divide and conquer: A defense of functional localizers. NeuroImage. 2006;30:1088–1096. doi: 10.1016/j.neuroimage.2005.12.062. [DOI] [PubMed] [Google Scholar]
- Seifritz E, Esposito F, Neuhoff JG, Luthi A, Mustovic H, Dammann G, von Bardeleben U, Radue EW, Cirillo S, Tedeschi G, Di Salle F. Differential sex- independent amygdala response to infant crying and laughing in parents versus nonparents. Biol Psychiatry. 2003;54:1367–1375. doi: 10.1016/s0006-3223(03)00697-8. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD response in unipolar depression: Related and Independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent- infant interactions: psychology, physiology, and in vivo functional neuroimaging. J Child Psychol Psychiatry. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE. Baby stimuli and the parent brain: Functional neuroimaging of the neural substrates of parent-infant attachment. Psychiatry. 2008;5:28–36. [PMC free article] [PubMed] [Google Scholar]
- Taylor JF, Rosen RC, Leiblum SR. Self-report assessment of female sexual function: Psychometric evaluation of the brief index of sexual functioning for women. Arch Sex Behav. 1994;23:627–643. doi: 10.1007/BF01541816. [DOI] [PubMed] [Google Scholar]
- Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man. Chicago: Aldine; 1972. pp. 136–179. [Google Scholar]
- Weisman O, Zagoory-Sharon O, Feldman R. Intranasal oxytocin administration is reflected in human saliva. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.02.014. in press. [DOI] [PubMed] [Google Scholar]
- Woranitat W, Taneepanichskul S. Sexual function during the postpartum period. J Med Assoc Thai. 2007;90:1744–8. [PubMed] [Google Scholar]