Abstract
The selective degeneration of dendrites precedes neuronal cell death in hypoxia-ischemia (HI) and is a neuropathological hallmark of stroke. While it is clear that a number of different molecular pathways likely contribute to neuronal cell death in HI, the mechanisms that govern HI-induced dendrite degeneration are largely unknown. Here, we show that the NAD synthase Nicotinamide mononucleotide adenylyltransferase (Nmnat) functions endogenously to protect Drosophila class IV dendritic arborization (da) sensory neurons against hypoxia-induced dendritic damage. Whereas dendrites of wild-type class IV neurons are largely resistant to morphological changes during prolonged periods of hypoxia (< 1.0% O2), class IV neurons of nmnat heterozygous mutants exhibit significant dendrite loss and extensive fragmentation of the dendritic arbor under the same hypoxic conditions. Although basal levels of autophagy are required for neuronal survival, we demonstrate that autophagy is dispensable for maintaining the dendritic integrity of class IV neurons. However, we find that genetically blocking autophagy can suppress hypoxia-induced dendrite degeneration of nmnat heterozygous mutants in a cell-autonomous manner, suggestive of a self-destructive role for autophagy in this context. We further show that inducing autophagy by overexpression of the autophagy-specific kinase Atg1 is sufficient to cause dendrite degeneration of class IV neurons under hypoxia and that overexpression of Nmnat fails to protect class IV dendrites from the effects of Atg1 overexpression. Our studies reveal an essential neuroprotective role for endogenous Nmnat in hypoxia and demonstrate that Nmnat functions upstream of autophagy to mitigate the damage incurred by dendrites in neurons under hypoxic stress.
Keywords: Nmnat, autophagy, hypoxia-ischemia, dendrite, neurodegeneration, Drosophila
Introduction
Pathological alterations in dendritic structure are an early hallmark of brain injury in hypoxia-ischemia (HI). Transient ischemic episodes can induce rapid morphological changes in neurons, including extensive beading and swelling of dendrites, and the selective degeneration of dendrites during early ischemia likely serves as a precursor to neuronal cell death (Hori and Carpenter, 1994; Hsu and Buzsaki, 1993; Ikonomidou et al., 1989; Kitagawa et al., 1989; Matesic and Lin, 1994). Whereas much attention has focused on the mechanisms underlying neuronal death following HI (Lipton, 1999), relatively little is known of how HI can induce dendritic damage.
Macroautophagy (hereafter referred to as autophagy) is a highly conserved, ubiquitous process that is one of the major pathways responsible for the bulk degradation of proteins and organelles in response to starvation and other cellular stresses (Levine and Klionsky, 2004). Basal levels of autophagy are essential for neuronal survival (Hara et al., 2006; Komatsu et al., 2006), and accordingly, autophagic dysfunction has been linked to a growing number of neurodegenerative disorders (Levine and Kroemer, 2008). Neurons show increased autophagy following cerebral ischemia (Adhami et al., 2006; Carloni et al., 2008; Nitatori et al., 1995; Puyal et al., 2009; Rami et al., 2008; Wen et al., 2008; Zhu et al., 2005); however, the question of whether elevated levels of autophagy are neuroprotective or directly contribute to HI-induced neuronal cell death remains largely unresolved (Nakka et al., 2008; Uchiyama et al., 2008).
Nmnat catalyzes a key step of NAD synthesis and the neuroprotective effects of Nmnat overexpression in response to injury and neurotoxic insults have been well documented (Coleman and Freeman, 2010). Further studies have provided evidence of an evolutionarily conserved role for endogenous Nmnat in axon and dendrite maintenance (Gilley and Coleman, 2010; Wen et al., 2011; Zhai et al., 2006). The recent finding that endogenous Nmnat is upregulated in the brains of adult flies exposed to hypoxia suggests that increasing Nmnat levels may serve as an adaptive response to hypoxic stress (Ali et al., 2011). We previously reported that Nmnat is required for maintaining dendritic coverage of Drosophila dendritic arborization (da) sensory neurons (Wen et al., 2011). In the present study, we set out to determine whether Nmnat functions endogenously to maintain the dendritic integrity of da neurons under hypoxic stress. We show that, while class IV neurons of wild-type larvae exposed to hypoxia (< 1.0% O2) are largely resistant to morphological changes, class IV neurons of nmnat heterozygous mutants exhibit regression and fragmentation of dendrites under the same hypoxic conditions. This hypoxia-induced dendrite degeneration in nmnat heterozygous mutants is unrelated to apoptosis as expression of the caspase inhibitor p35 fails to rescue dendrite phenotypes associated with hypoxia. Surprisingly, we find that reducing Atg1 function suppresses the dendrite degeneration observed in class IV neurons of hypoxia-exposed nmnat mutants and that cell-specific knockdown of autophagy-related genes rescues dendrite phenotypes associated with reduced nmnat function. We further demonstrate that overactivation of autophagy causes dendrite degeneration of class IV neurons under hypoxia and that overexpression of Nmnat fails to rescue autophagy-induced dendritic phenotypes. Our study supports a self-destructive role for autophagy in hypoxia and provides genetic evidence that nmnat functions upstream of autophagy to protect dendrites from damage induced by hypoxic stress.
Material and methods
Fly stocks
The w118 strain was used for all wild-type controls. nmnatΔ4792 and UAS-Nmnat (Zhai et al., 2006), UAS-p35 (Hay et al., 1994), UAS-HSF-eGFP (Yao et al., 2006), UAS-Sima (Zelzer et al., 1997), and ppk-Gal4 (Kuo et al., 2005) are as previously described. Atg1Δ3D, UAS-Atg1, UAS-Atg5-RNAi, UAS-Atg7-RNAi, UAS-Atg12-RNAi, and UAS-mCherry-Atg8 flies were provided by Dr. Thomas Neufeld (University of Minnesota) (Scott et al., 2007; Scott et al., 2004). UAS-GFP-huLC3 and UAS-GFP-Atg5 (Rusten et al., 2004), UAS-Cat, and UAS-SOD1 flies were obtained from the Bloomington Stock Center (Bloomington, IN).
Hypoxia treatment and live imaging
Embryos were collected for 4 hrs on grape juice agar plates supplemented with yeast paste and allowed to develop for 96 hrs at 25°C. Third-instar larvae aged 96-100 hrs after egg laying (AEL) (20-30 per genotype) were rinsed in water and transferred onto a new grape juice agar plate lacking yeast situated within a petri dish. The dish was placed within a plexiglass chamber and exposed to either room air (normoxia) or to < 1.0% O2 (hypoxia) using a calibration mixture (4.94% CO2 and N2 Balance), which was constantly monitored using a Nuvair O2 Quickstick (Oxnard, CA). Larvae were then rinsed in water, mounted under a coverslip in 90% glycerol, and immediately imaged live using a Plan Apo 40x/1.3 NA objective on an Olympus FluoView FV1000 confocal microscope (Olympus, Tokyo, Japan). Confocal image stacks (8-13 images per stack) were acquired at 800x800 pixel resolution and a step size of 1.0 μm and reconstructed into collapsed 2D images using ImageJ software (NIH, Bethesda, MD).
Quantitative and statistical analysis
The total number of dendritic branch points for each neuron was counted manually. The total dendrite length for each neuron was calculated using the NeuronJ tracing tool in ImageJ. The degeneration index (DI) was calculated by summing the lengths of breaks along the dendrites and dividing the sum by the total dendrite length for each neuron. Only dendrite breaks that were greater than 5 μm were measured and used to calculate the DI. Statistical significance was evaluated by comparing all groups (n = 9-12 neurons per genotype) using one-way analysis of variance (ANOVA) with Tukey’s HSD post hoc analysis.
Results
Dendrites of class IV da neurons are largely resistant to hypoxic stress
We investigated the in vivo effects of prolonged hypoxia on dendrite morphology by examining class IV da sensory neurons of Drosophila larvae exposed to varying degrees of hypoxia. Class IV neurons are multi-dendritic sensory neurons of the peripheral nervous system (PNS) that elaborate highly branched dendritic arbors that effectively cover the entire larval body wall (Grueber et al., 2002). Class IV neurons are ideal for these studies because their dendrite arborization patterns are highly stereotyped and their dendritic arbors are largely confined within a two-dimensional space between the epidermis and body wall muscles (Han et al., 2012; Kim et al., 2012), allowing for immediate and direct live visualization of dendrite morphology without any artifacts associated with fixation. We used the class IV neuron-specific ppk-Gal4 reporter driving expression of a green fluorescent protein (GFP)-tagged membrane marker (mCD8::GFP) to examine class IV neurons in wild-type third instar larvae [96-100 hrs after egg laying (AEL)] that were subjected to varying times (4, 8, and 14 hrs) of hypoxia (< 1.0% O2). Despite prolonged hypoxia, GFP-expressing larvae remained viable under these conditions. Surprisingly, the dendritic arborization patterns of class IV neurons were largely unchanged in these larvae when compared with age-matched normoxia (21% O2) controls (data not shown), and a slight, though statistically significant, reduction in the total number of terminal dendritic branches was only observed after 14 hrs of hypoxia exposure (Fig. 1A,D,G). However, the total dendrite length of class IV neurons in hypoxia-exposed larvae at 14 hrs did not differ statistically from normoxia controls (Fig. 1H). Furthermore, despite prolonged hypoxia exposure, class IV dendritic arbors remained intact and did not exhibit any apparent degeneration phenotypes (Fig. 1I), suggesting that these neurons possess an endogenous protection mechanism against hypoxic stress.
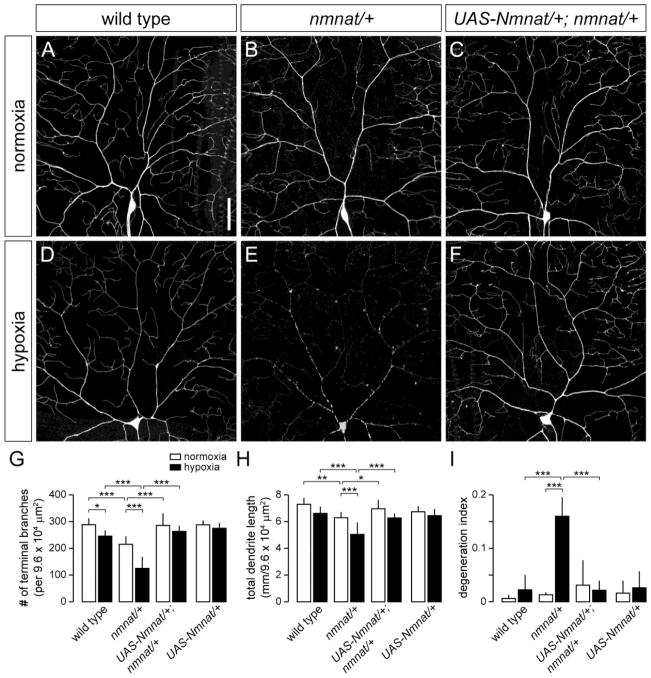
Figure 1. Nmnat protects against dendritic damage in hypoxia-exposed larvae.
(A,B) Dendrite morphology of dorsal class IV ddaC neuron in a wild-type (A) and nmnatΔ4792 heterozygous mutant (nmnat/+) (B) third instar larva (110-114 hrs AEL) after exposure to 14 hrs of normoxia. (C) Selective overexpression of wild-type Nmnat in class IV neurons rescues the dendrite regression phenotype of nmnat heterozygous mutants under normoxia. (D,E) Dendrite morphology of ddaC neuron in a wild-type (D) and nmnat heterozygous mutant (E) third instar larva after exposure to 14 hrs of hypoxia (< 1.0% O2). Class IV neurons of hypoxia-exposed nmnat heterozygotes show extensive breaks along the dendritic arbor. (F) Selective overexpression of wild-type Nmnat in class IV neurons rescues the dendrite degeneration phenotype of nmnat heterozygous mutants under hypoxia. (G–I) Quantification of total number of terminal dendritic branches (G) and total dendrite length (H) per 9.6 x104 μm2 field (mean ± SD), and degeneration index (DI) (I) for ddaC neurons in wild type, nmnat heterozygous mutant (nmnat/+), nmnat heterozygous mutant background overexpressing Nmnat (UAS-Nmnat/+; nmnat/+), and wild-type background overexpressing Nmnat (UAS-Nmnat/+) under normoxia and hypoxia (* p < 0.05, ** p < 0.01, *** p < 0.001). Statistical comparison of UAS-Nmnat/+ with other groups not shown for simplification purposes. Dorsal is up and anterior is to the left in this and all subsequent figures. Scale bar, 50 μm.
Nmnat functions endogenously to maintain dendritic integrity of class IV neurons under hypoxic stress
We previously found that Nmnat is cell-autonomously required for the proper maintenance of class IV dendrites and that it functions as a neuroprotective factor against progressive dendritic loss (Wen et al., 2011). The recent finding that Nmnat is upregulated in response to hypoxia and can mitigate the negative effects of oxidative stress on adult Drosophila lifespan (Ali et al., 2011) led us to investigate whether endogenous Nmnat serves a neuroprotective role in class IV neurons under hypoxia. Consistent with our previous results (Wen et al., 2011), we found that class IV neurons in larvae heterozygous for a loss-of-function mutation in nmnat (nmnatΔ4792) exhibited significant reductions in both the total number of terminal dendritic branches (25.5%) and total dendrite length (13.7%) under normoxia when compared to wild-type controls (Fig. 1B,G,H). However, when nmnatΔ4792 heterozygous mutant larvae were placed under 14 hrs of hypoxia, the total number of terminal dendritic branches and the total dendrite length of class IV neurons were further reduced by 49.2% and 23.8%, respectively, when compared to class IV neurons of wild-type larvae under the same hypoxic conditions (Fig. 1E,G,H). In addition to reduced dendritic branching, class IV neurons in hypoxia-exposed nmnatΔ4792 heterozygotes exhibited extensive beading and fragmentation of the dendritic arbor (Fig. 1E). Both proximal and distal dendrites were affected as these neurons showed breaks at regular intervals along the entire lengths of the dendritic branches (Fig. 1E). We calculated the dendrite degeneration index (DI) for each genotype by summing the lengths of all breaks along the dendrites and dividing that sum by the total dendrite length. Whereas the DI for wild-type class IV neurons in hypoxia was 0.02, the DI for class IV neurons of hypoxia-exposed nmnatΔ4792 heterozygous mutants was 0.16 (Fig. 1I), with 90% of these neurons having a DI greater than 0.10 as opposed to 0% for wild-type.
We then determined whether Nmnat functions cell-autonomously to protect class IV neurons under hypoxia by using ppk-Gal4 to express a wild-type Nmnat transgene specifically in class IV neurons of both wild-type and nmnatΔ4792 heterozygous mutant larvae. While overexpression of Nmnat did not significantly alter the dendritic properties of wild-type class IV neurons under normoxia or hypoxia (Fig. 1G,H), overexpression of Nmnat fully rescued the dendritic phenotypes of class IV neurons in nmnatΔ4792 heterozygotes under both normoxic and hypoxic conditions (Fig. 1C,F–I). Class IV-specific rescue restored total dendrite branching and dendrite lengths to that of wild-type controls and effectively prevented the degeneration of class IV dendrites under hypoxia (Fig. 1G–I). Collectively, these results demonstrate that endogenous Nmnat functions cell-autonomously to maintain the dendritic integrity of class IV neurons under hypoxic stress.
Overexpression of HSF and HIF-1α/Sima rescues hypoxia-induced dendrite degeneration in nmnat mutants
The Drosophila hypoxia-inducible factor 1α (HIF-1α) ortholog Similar (Sima) indirectly upregulates Nmnat expression in response to hypoxia through the induction of heat shock factor (HSF), which functions as a central regulator of nmnat transcription under stress conditions, and overexpression of either HSF or Sima has been shown to upregulate Nmnat in response to hypoxic stress (Ali et al., 2011). We therefore tested whether selective overexpression of HSF or Sima could rescue hypoxia-induced dendritic phenotypes of class IV neurons in nmnatΔ4792 heterozygous mutants. Overexpression of HSF or Sima had no effect on the development or maintenance of class IV dendrites in wild-type larvae under both normoxic and hypoxic conditions (Supplementary Fig. S1I–N). Although overexpression of HSF or Sima partially suppressed the loss of terminal dendritic branches observed in nmnatΔ4792 heterozygotes under normoxia, the total dendrite length remained unchanged in these mutants (Supplementary Fig. S1A–D,I–N). This may be explained by moderate increases in Nmnat expression that are observed upon HSF or Sima overexpression in non-stress conditions (Ali et al., 2011). On the other hand, we found that overexpression of HSF or Sima fully rescued the dendrite regression and degeneration phenotypes of class IV neurons in hypoxia-exposed nmnatΔ4792 heterozygotes (Supplementary Fig. S1E–N), consistent with the ability of either transcription factor to significantly upregulate Nmnat expression upon exposure to stress (Ali et al., 2011). These data provide further evidence that hypoxia engages Nmnat signaling through transcriptional regulation mediated by Sima and HSF.
Impairment of autophagy suppresses hypoxia-induced dendrite degeneration in nmnat mutants
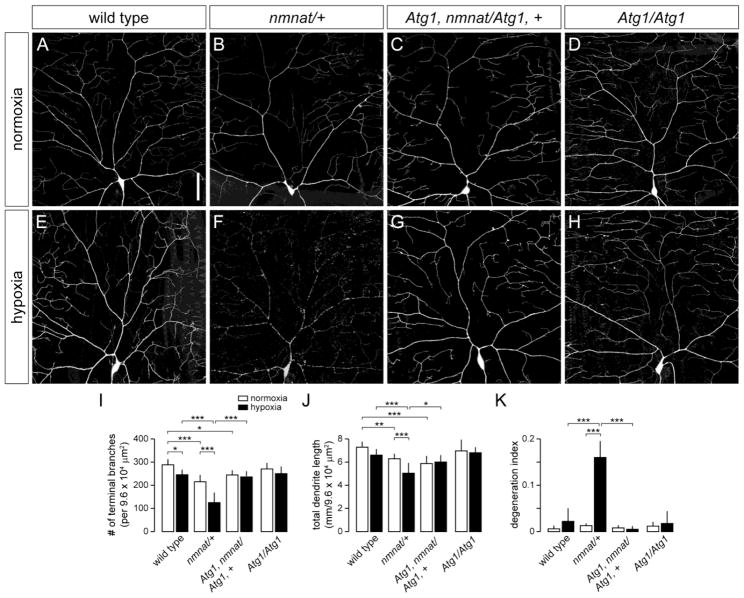
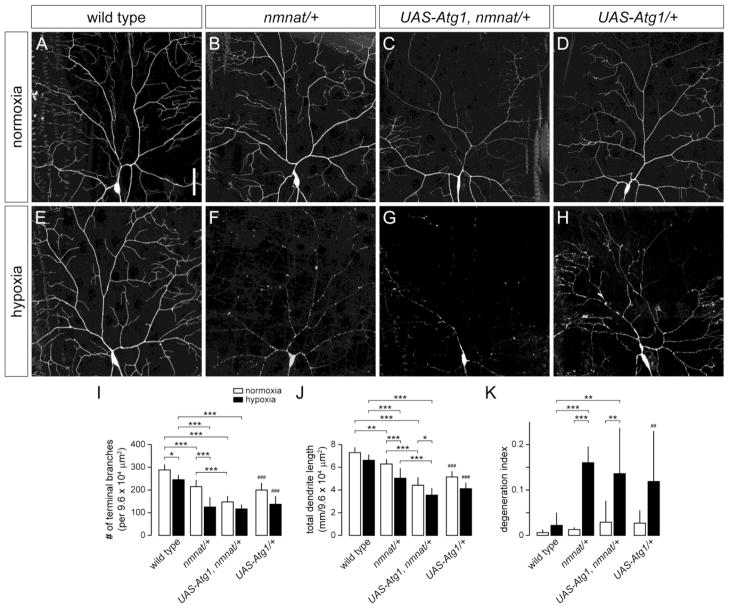
The susceptibility of class IV neurons in nmnatΔ4792 heterozygotes to hypoxia-induced dendritic damage suggests that these neurons demonstrate increased vulnerability to cellular degradation pathways that mediate dendrite degeneration. Autophagy can be induced by HI (Adhami et al., 2006; Carloni et al., 2008; Nitatori et al., 1995; Puyal et al., 2009; Rami et al., 2008; Wen et al., 2008; Zhu et al., 2005) and has been implicated to play a role in neurite degeneration (Knoferle et al., 2010; Wang et al., 2006; Yang et al., 2007), but whether autophagy promotes neuronal survival or destruction in this context remains unclear. We therefore investigated the role of autophagy in class IV neurons under hypoxia and tested whether genetically blocking autophagy could modify dendritic phenotypes associated with reduced nmnat function. We first sought to determine whether hypoxia induces autophagy in class IV neurons by using ppk-Gal4 to selectively express well-established autophagy markers (mCherry-Atg8, GFP-Atg5, or GFP-huLC3) in these neurons (Rusten et al., 2004; Scott et al., 2004). However, expression of these markers either led to overexpression artifacts or was not reliably detected at the single-cell level (data not shown). We therefore tested the endogenous role of autophagy in hypoxia by examining class IV neurons in third-instar larvae homozygous for a loss-of-function Autophagy-specific gene 1 (Atg1) allele (Atg1Δ3D) (Scott et al., 2004). The Atg1 gene encodes for a serine/threonine kinase that is important for the initiation of autophagy and is highly conserved among eukaryotes (Chen and Klionsky, 2011; Scott et al., 2007). We found that loss of Atg1 had no discernible effect on the development of class IV dendrites in normoxic conditions when compared to wild-type controls (Fig. 2A,D,I–K). Furthermore, class IV neurons of hypoxia-exposed Atg1Δ3D homozygous mutants did not exhibit any dendrite branching defects or degeneration phenotypes (Fig. 2E,H,I–K), suggesting that autophagy is dispensable for maintaining the dendritic integrity of these neurons under hypoxic stress. However, when we examined class IV neurons in Atg1Δ3D homozygous mutants with only one functional copy of the nmnat gene, we found that loss of Atg1 fully suppressed both the dendrite regression and degeneration phenotypes of nmnatΔ4792 heterozygotes under hypoxia (Fig. 2F,G,I–K). Loss of Atg1 did not suppress the dendrite maintenance defects found in class IV neurons of nmnatΔ4792 heterozygotes under normoxia (Fig. 2B,C,I–K), pointing to separable neuroprotective roles for Nmnat in neurons under normoxia and hypoxia. These results further suggest that endogenous Nmnat serves to mitigate the detrimental effects of hypoxia-induced autophagy in these neurons.
Figure 2. Loss of Atg1 suppresses hypoxia-induced dendrite degeneration in nmnat mutants.
(A,B) Dendrite morphology of class IV ddaC neuron in a wild-type (A) and nmnat heterozygous mutant (nmnat/+) (B) third instar larva after exposure to 14 hrs of normoxia. (C) Loss of Atg1 fails to suppress the dendrite regression phenotype of class IV neurons in nmnat heterozygous mutants under normoxia. (D) Class IV dendritic arborization patterns are largely unaltered in Atg1 homozygous mutants under normoxia. (E,F) Dendrite morphology of ddaC neuron in a wild-type (E) and nmnat heterozygous mutant (F) third instar larva after exposure to 14 hrs of hypoxia. (G) Loss of Atg1 suppresses the dendrite regression and degeneration phenotypes of class IV neurons in nmnat heterozygous mutants under hypoxia. (H) Class IV dendritic arborization patterns are largely unaltered in Atg1 homozygous mutants under hypoxia. (I–K) Quantification of total number of terminal dendritic branches (I), total dendrite length (J), and degeneration index (DI) (K) for ddaC neurons in wild type, nmnat heterozygous mutant (nmnat/+), Atg1 homozygous mutant with loss of one copy of nmnat (Atg1, nmnat/Atg1, +), and Atg1 homozygous mutant (Atg1/Atg1) under normoxia and hypoxia (* p < 0.05, ** p < 0.01, *** p < 0.001). Statistical comparison of Atg1/Atg1 with other groups not shown for simplification purposes. Scale bar, 50 μm.
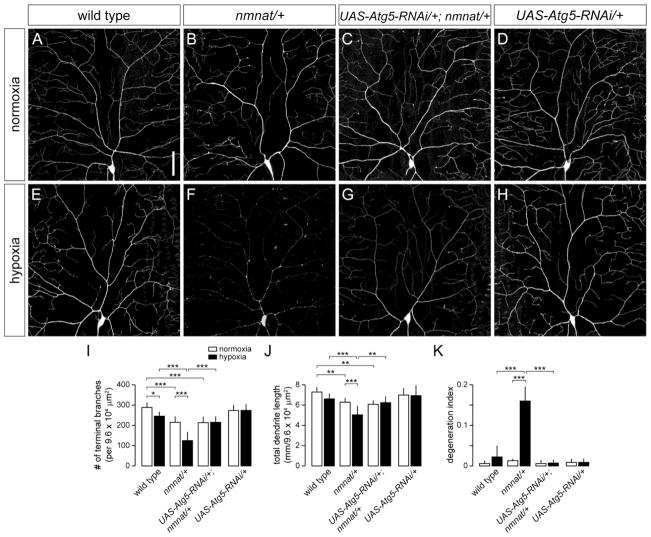
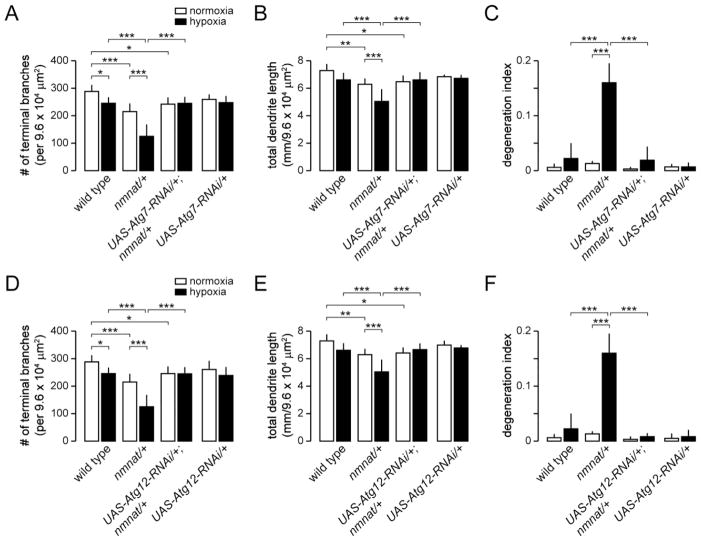
In addition to its function in autophagy, Atg1 has been implicated to play a role in neuronal vesicular transport and autophagy-independent signaling pathways (Alers et al., 2012). We therefore determined the role of other autophagy-related genes in hypoxia-induced dendrite degeneration by using ppk-Gal4 to express RNAi transgenes directed against Autophagy-specific genes in class IV neurons (Scott et al., 2004). We first tested the role of Autophagy-specific gene 5 (Atg5), which is required for the early stages of autophagosome formation (Xie and Klionsky, 2007). Similar to Atg1Δ3D homozygous mutants, we found that RNAi knockdown of Atg5 had no effect on the dendritic branching of class IV neurons under normoxia or hypoxia when compared to wild-type controls (Fig. 3A,D,E,H,I–K), and furthermore, failed to rescue dendritic phenotypes associated with reduced nmnat function in normoxic conditions (Fig. 3B,C,I,J). However, expression of Atg5-RNAi in class IV neurons of nmnatΔ4792 heterozygotes completely suppressed the dendrite regression and degeneration phenotypes normally observed in these neurons under hypoxia (Fig. 3F,G,I–K). We further used ppk-Gal4 to express RNAi transgenes directed against Autophagy-specific genes 7 (Atg7) or 12 (Atg12), two autophagy-related genes that are also required for autophagosome formation (Xie and Klionsky, 2007), in class IV neurons of both wild-type and nmnatΔ4792 heterozygous larvae. We found that, similar to class IV-specific expression of Atg5-RNAi, knockdown of either Atg7 or Atg12 had no effect on the development or maintenance of class IV dendrites under normoxia, but effectively suppressed the dendrite degeneration phenotype of nmnatΔ4792 heterozygotes under hypoxia (Fig. 4). Taken together, these results suggest that autophagy is dispensable for dendrite maintenance under normoxia, but implicate a cell-autonomous role for autophagy in hypoxia-induced dendrite degeneration.
Figure 3. Knockdown of Atg5 suppresses hypoxia-induced dendrite degeneration in nmnat mutants.
(A,B) Dendrite morphology of class IV ddaC neuron in a wild-type (A) and nmnat heterozygous mutant (nmnat/+) (B) third instar larva after exposure to 14 hrs of normoxia. (C) Cell-specific knockdown of Atg5 fails to suppress the dendrite regression phenotype of class IV neurons in nmnat heterozygous mutants under normoxia. (D) Knockdown of Atg5 has no effect on the development or maintenance of class IV neurons under normoxia. (E,F) Dendrite morphology of ddaC neuron in a wild-type (E) and nmnat heterozygous mutant (F) third instar larva after exposure to 14 hrs of hypoxia. (G) Cell-specific knockdown of Atg5 suppresses the dendrite regression and degeneration phenotypes of class IV neurons in nmnat heterozygous mutants under hypoxia. (H) Knockdown of Atg5 has no effect on the development or maintenance of class IV neurons under hypoxia. (I–K) Quantification of total number of terminal dendritic branches (I), total dendrite length (J), and degeneration index (DI) (K) for ddaC neurons in wild type, nmnat heterozygous mutant (nmnat/+), nmnat heterozygous mutant background expressing Atg5-RNAi (UAS-Atg5-RNAi/+; nmnat/+), and wild-type background expressing Atg5-RNAi (UAS-Atg5-RNAi/+) under normoxia and hypoxia (* p < 0.05, ** p < 0.01, *** p < 0.001). Statistical comparison of UAS-Atg5-RNAi/+ with other groups not shown for simplification purposes. Scale bar, 50 μm.
Figure 4. Knockdown of Atg7 and Atg12 suppresses hypoxia-induced dendrite degeneration in nmnat mutants.
(A–B) Quantification of total number of terminal dendritic branches (A), total dendrite length (B), and degeneration index (DI) (C) for ddaC neurons in wild type, nmnat heterozygous mutant (nmnat/+), nmnat heterozygous mutant background expressing Atg7-RNAi (UAS-Atg7-RNAi/+; nmnat/+), and wild-type background expressing Atg7-RNAi (UAS-Atg7-RNAi/+) under normoxia and hypoxia (* p < 0.05, ** p < 0.01, *** p < 0.001). Statistical comparison of UAS-Atg7-RNAi/+ with other groups not shown for simplification purposes. (D-F) Quantification of total number of terminal dendritic branches (D), total dendrite length (E), and degeneration index (DI) (F) for ddaC neurons in wild type, nmnat heterozygous mutant (nmnat/+), nmnat heterozygous mutant background expressing Atg12-RNAi (UAS-Atg12-RNAi/+; nmnat/+), and wild-type background expressing Atg12-RNAi (UAS-Atg12-RNAi/+) under normoxia and hypoxia (* p < 0.05, ** p < 0.01, *** p < 0.001). Statistical comparison of UAS-Atg12-RNAi/+ with other groups not shown for simplification purposes.
Hypoxia-induced dendrite degeneration in nmnat mutants occurs independently of caspase activity
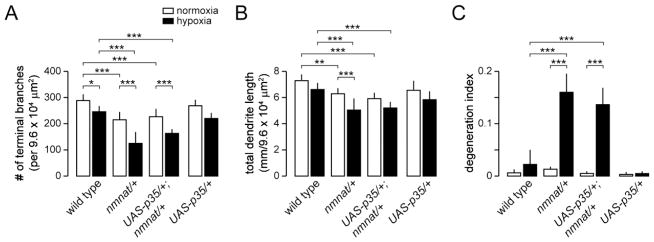
While both autophagy and caspases are thought to contribute to autophagic cell death, recent studies provide evidence that autophagy can mediate cellular destruction independent of caspase activity (Ryoo and Baehrecke, 2010). We therefore determined whether caspases are important for autophagic degradation of class IV neurons under hypoxia by selectively expressing the effector caspase inhibitor p35 in class IV neurons of wild-type and nmnatΔ4792 heterozygotes. We found that the dendritic arborization patterns of class IV neurons expressing p35 were largely unaltered and that expression of p35 failed to suppress the dendrite degeneration phenotypes of class IV neurons of nmnatΔ4792 heterozygotes under hypoxia (Fig. 5). Although these data argue against caspase involvement, we cannot completely exclude the possibility that caspases are important for later events as it was not possible to determine whether dendrite fragments are cleared at later time points because of the reduced viability of larvae that are subjected to greater than 14 hrs of hypoxia. However, these results suggest that autophagy, and not caspases, mediate the initial events that lead to dendrite degeneration under hypoxia.
Figure 5. Hypoxia-induced dendrite degeneration in nmnat mutants is independent of the apoptotic machinery.
(A–B) Quantification of total number of terminal dendritic branches (A), total dendrite length (B), and degeneration index (DI) (C) for ddaC neurons in wild type, nmnat heterozygous mutant (nmnat/+), nmnat heterozygous mutant background expressing p35 (UAS-p35/+; nmnat/+), and wild-type background expressing p35 (UAS-p35/+) under normoxia and hypoxia (* p < 0.05, ** p < 0.01, *** p < 0.001). Statistical comparison of UAS-p35/+ with other groups not shown for simplification purposes.
Increased autophagy induces dendrite degeneration under hypoxia
Our results suggest that hypoxia-induced autophagy causes dendrite degeneration in larvae with reduced nmnat function, but whether elevated levels of autophagy are sufficient to cause dendrite degeneration is unclear. Overexpression of Atg1 has been previously shown to induce high levels of autophagy in larval fat bodies, salivary glands, and neurons (Berry and Baehrecke, 2007; Scott et al., 2007; Shen and Ganetzky, 2009). When we overexpressed Atg1 in class IV neurons of wild-type larvae under normoxia, these neurons exhibited significant reductions in both total number of terminal dendritic branches (30.6%) and total dendrite length (29.7%), but did not exhibit any apparent dendrite degeneration phenotypes according to our criteria (Fig. 6A,D,I–K). While overexpression of Atg1 enhanced the dendritic regression phenotype of class IV neurons in nmnatΔ4792 heterozygotes under normoxia, inducing autophagy had no effect on the dendritic integrity of these mutants (Fig. 6B,C,I–K). When we overexpressed Atg1 in class IV neurons of wild-type larvae under hypoxia, we found that these neurons exhibited significant dendrite loss and extensive fragmentation of the dendritic arbor despite the presence of two functional copies of nmnat (Fig. 6H–K), suggesting overactivation of autophagy can counteract the protective effects of endogenous Nmnat in hypoxia. However, the extent of dendrite degeneration in Atg1-expressing neurons (44% of neurons with a DI > 0.10) was less than what was observed for class IV neurons in nmnatΔ4792 heterozygotes under hypoxia (90% of neurons with a DI > 0.10).
Figure 6. Overexpression of Atg1 induces dendrite degeneration under hypoxia.
(A,B) Dendrite morphology of class IV ddaC neuron in a wild-type (A) and nmnat heterozygous mutant (nmnat/+) (B) third instar larva after exposure to 14 hrs of normoxia. (C) Overexpression of Atg1 enhances the dendrite regression phenotype of nmnat heterozygous mutants under normoxia. (D) Class IV neurons overexpressing Atg1 show reduced dendritic branching. (E,F) Dendrite morphology of ddaC neuron in a wild-type (E) and nmnat heterozygous mutant (F) third instar larva after exposure to 14 hrs of hypoxia. (G) Overexpression of Atg1 enhances dendritic regression, but not dendrite degeneration, phenotype of class IV neurons in nmnat heterozygous mutants under hypoxia. (H) Class IV neurons overexpressing Atg1 show reduced dendritic branching and extensive breaks along the arbors. (I–K) Quantification of total number of terminal dendritic branches (I), total dendrite length (J), and degeneration index (DI) (K) for ddaC neurons in wild type, nmnat heterozygous mutant (nmnat/+), nmnat heterozygous mutant background overexpressing Atg1 (UAS-Atg1, nmnat/+), and wild-type background overexpressing Atg1 (UAS-Atg1/+) under normoxia and hypoxia (* p < 0.05, ** p < 0.01, *** p < 0.001). Statistical comparison of UAS-Atg1/+ shown only with wild-type controls (## p < 0.01, ### p < 0.001). Scale bar, 50 μm.
We further found that overexpression of Atg1 in nmnatΔ4792 heterozygotes did not enhance dendrite degeneration phenotypes observed in these mutants (Fig. 6F,G,K), consistent with the possibility that nmnat and Atg1 act in the same pathway. Although Atg1 overexpression did lead to a further reduction in total dendrite length of class IV neurons in nmnatΔ4792 heterozygotes under hypoxia, this did not differ statistically from class IV neurons overexpressing Atg1 in a wild-type background under the same hypoxic conditions (Fig. 6J).
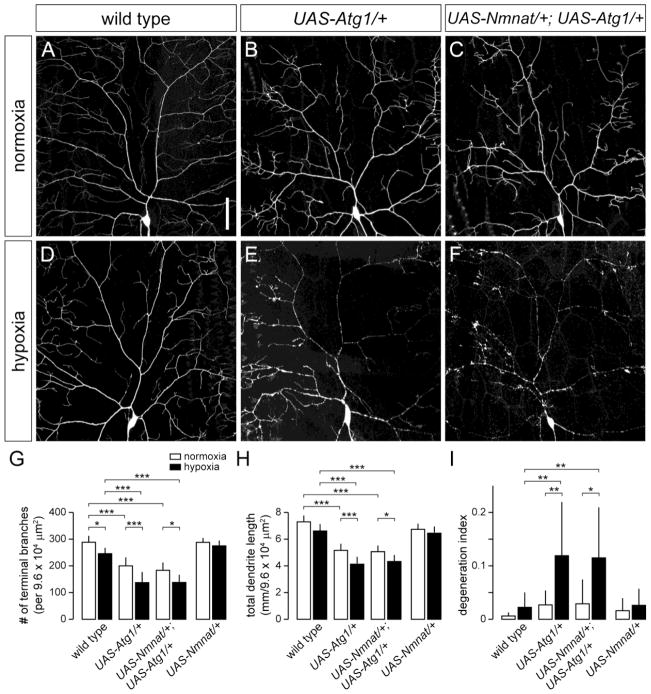
We then tested whether overexpression of Nmnat could prevent the dendrite degeneration induced by Atg1 overexpression in class IV neurons under hypoxia. We found that overexpression of Nmnat did not suppress the loss of dendrites caused by Atg1 overexpression in class IV neurons under normoxia (Fig. 7A–C,G–I), and furthermore, failed to protect Atg1-overexpressing class IV neurons from hypoxia-induced dendrite degeneration (60% of neurons with a DI > 0.10) (Fig. 7D–F,G–I). Collectively, these data suggest that autophagy acts downstream of nmnat and that elevated levels of autophagy lead to dendritic damage of neurons under hypoxia.
Figure 7. Overexpression of Nmnat fails to suppress dendritic phenotypes associated with Atg1 overexpression.
(A,B) Dendrite morphology of class IV ddaC neuron in wild-type (A) or overexpressing Atg1 (B) under normoxia. (C) Overexpression of Nmnat fails to suppress the dendritic branching defect of class IV neurons overexpressing Atg1. (D,E) Dendrite morphology of class IV ddaC neuron in wild-type (D) or overexpressing Atg1 (E) under hypoxia. (F) Overexpression of Nmnat fails to suppress the dendritic branching and degeneration phenotype of class IV neurons overexpressing Atg1. (G–I) Quantification of total number of terminal dendritic branches (G), total dendrite length (H), and degeneration index (DI) (I) for ddaC neurons in wild type, wild-type background overexpressing Atg1 (UAS-Atg1), wild-type background overexpressing Nmnat and Atg1 (UAS-Nmnat/+; UAS-Atg1/+), and wild-type background overexpressing Nmnat (UAS-Nmnat/+) under normoxia and hypoxia (* p < 0.05, ** p < 0.01, *** p < 0.001). Statistical comparison of UAS-Nmnat/+ with other groups not shown for simplification purposes. Scale bar, 50 μm.
Overexpression of ROS scavengers suppresses dendrite degeneration in hypoxia-exposed nmnat mutants
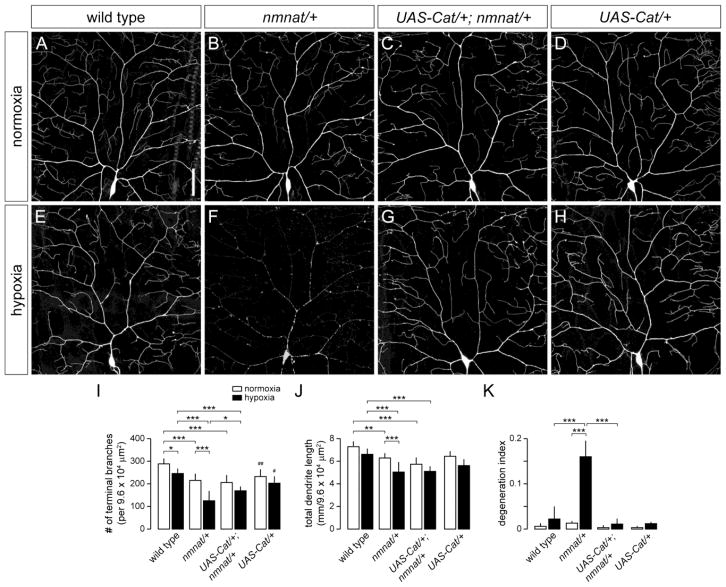
Reactive oxygen species (ROS) are generated in neurons in response to oxygen deprivation and reoxygenation (Abramov et al., 2007), and overproduction of ROS has been implicated to play a role in neuronal damage and cell death associated with ischemia/reperfusion (Abramov et al., 2007; Keller et al., 1998). Furthermore, ROS are potent activators of autophagy (Scherz-Shouval and Elazar, 2011). The previous finding that Nmnat can inhibit ROS accumulation (Press and Milbrandt, 2008), coupled with our data showing that Nmnat acts upstream of autophagy, together support the possibility that Nmnat may suppress autophagy by maintaining low levels of ROS under hypoxic conditions. We therefore asked whether increased levels of ROS contribute to the hypoxia-induced dendrite degeneration in nmnat mutants. To test this idea, we expressed the ROS scavenging enzymes Catalase (Cat) and Superoxide dismutase (SOD) in class IV neurons of both wild-type and nmnatΔ4792 heterozygous larvae. Although overexpression of Cat led to a reduction in terminal dendritic branching of class IV neurons in wild-type larvae under normoxia (Fig. 8A,D,I), the extent of dendritic regression in Cat-overexpressing neurons under hypoxia (13%) did not exceed that of wild-type controls (15%) (Fig. 8E,H,I), suggesting physiological levels of ROS may be important for dendrite development, but that the modest dendritic regression phenotype observed in hypoxia-exposed wild-type larvae is unlikely to be a consequence of increased ROS levels. On the other hand, overexpression of Cat did not enhance or suppress the dendrite maintenance defect of nmnatΔ4792 heterozygous mutants under normoxia (Fig. 8B,C,I,J), but partially suppressed the dendrite regression phenotype of class IV neurons in hypoxia-exposed nmnatΔ4792 heterozygotes, and fully rescued the dendrite fragmentation phenotype normally observed in these neurons under hypoxic conditions (Fig. 8E–K). Overexpression of SOD also rescued the dendrite fragmentation phenotype of hypoxia-exposed nmnatΔ4792 heterozygotes, though to a lesser extent than that observed with Cat overexpression (data not shown). These data suggest that nmnat mutants have elevated levels of ROS under hypoxia and that ROS signaling pathways contribute to the dendrite degeneration phenotype observed in these mutants.
Figure 8. Overexpression of Catalase suppresses the dendrite fragmentation phenotype of hypoxia-exposed nmnat mutants.
(A,B) Dendrite morphology of class IV ddaC neuron in a wild-type (A) and nmnat heterozygous mutant (nmnat/+) (B) third instar larva after exposure to 14 hrs of normoxia. (C) Overexpression of Catalase does not suppress the dendrite regression phenotype of class IV neurons in nmnat heterozygous mutants under normoxia. (D) Dendrite morphology of class IV ddaC neuron overexpressing Catalase under normoxia. (E,F) Dendrite morphology of ddaC neuron in a wild-type (E) and nmnat heterozygous mutant (F) third instar larva after exposure to 14 hrs of hypoxia. (G) Overexpression of Catalase suppresses the dendrite fragmentation phenotype of class IV neurons in nmnat heterozygous mutants under hypoxia. (H) Dendrite morphology of class IV ddaC neuron overexpressing Catalase under hypoxia. (I–K) Quantification of total number of terminal dendritic branches (I), total dendrite length (J), and degeneration index (DI) (K) for ddaC neurons in wild type, nmnat heterozygous mutant (nmnat/+), nmnat heterozygous mutant background expressing Catalase (UAS-Cat/+; nmnat/+), and wild-type background expressing Catalase (UAS-Cat/+) under normoxia and hypoxia (* p < 0.05, ** p < 0.01, *** p < 0.001). Statistical comparison of UAS-Cat/+ shown only with wild-type controls (# p < 0.05, ## p < 0.01). Scale bar, 50 μm.
Discussion
In this study, we found that dendrites of Drosophila da sensory neurons are largely resistant to morphological changes in response to prolonged hypoxia and that Nmnat functions endogenously to maintain the dendritic integrity of class IV neurons under hypoxic stress. We demonstrated that nmnat heterozygous mutants exhibit extensive fragmentation of class IV dendrites when exposed to hypoxia and that nmnat functions in a cell-autonomous manner to prevent hypoxia-induced dendrite degeneration. Dendrite degeneration in nmnat mutants is independent of the apoptotic machinery and can be suppressed by blocking autophagy, suggesting autophagy mediates cellular destruction independent of caspase activity in this context. Finally, our data suggest that Nmnat acts upstream of autophagy pathways, by possibly inhibiting ROS accumulation, to mitigate the damage incurred by dendrites in response to hypoxic stress.
Endogenous Nmnat protects dendrites against hypoxic stress
Dendrites of vertebrate neurons demonstrate a remarkable sensitivity to low oxygen conditions and are highly susceptible to rapid morphological changes even after brief periods of HI (Hori and Carpenter, 1994; Hsu and Buzsaki, 1993; Ikonomidou et al., 1989; Park et al., 1996). CA1 pyramidal neurons typically die several days after a transient ischemic episode (Kirino, 1982), but exhibit widespread beading of dendrites and breakdown of the dendritic cytoskeleton within hours after the ischemic event (Hori and Carpenter, 1994; Hsu and Buzsaki, 1993; Matesic and Lin, 1994). On the other hand, both Drosophila larvae and adults are exceptionally tolerant of hypoxic conditions and CNS neurons demonstrate complete recovery of evoked responses even after prolonged periods of anoxia (Haddad et al., 1997a; Haddad et al., 1997b; Wingrove and O’Farrell, 1999), suggesting Drosophila employ endogenous protection mechanisms to prevent hypoxia-induced changes in dendritic structure that consequently impair neuronal function. However, although understanding of the genetic basis of hypoxia tolerance in Drosophila has significantly progressed (Zhou et al., 2008), the mechanisms by which Drosophila neurons maintain their structural integrity upon hypoxia exposure are still relatively unknown.
Our finding that dendrites of class IV neurons are largely resistant to morphological changes upon hypoxia exposure led us to investigate the neuroprotective mechanisms that are endogenous to these neurons. From previous studies, we found that Nmnat is important for maintaining dendritic coverage of class IV neurons during larval development (Wen et al., 2011). While the enzymatic function of Nmnat in NAD biosynthesis is well known (Magni et al., 2004), Nmnat has garnered significant attention recently due to reports of neuroprotective effects stemming from its overexpression. Indeed, overexpression of Nmnat has been shown to delay injury-induced axonal degeneration (Coleman and Freeman, 2010), protect against neurotoxic insults in both vertebrate and Drosophila models of human neurodegenerative diseases (Ali et al., 2012; Ljungberg et al., 2012; Zhai et al., 2008), reduce the extent of neonatal HI-induced CNS injury (Verghese et al., 2011), and, from our previous work, protect against dendritic regression (Wen et al., 2011). Our finding that Nmnat is important for maintaining the dendritic integrity of class IV neurons under hypoxia provides further support of an evolutionarily conserved role for endogenous Nmnat in the maintenance of neuronal integrity (Gilley and Coleman, 2010; Zhai et al., 2006), and are consistent with recent reports of Nmnat’s function as a stress response protein (Ali et al., 2011). Drosophila Nmnat is upregulated in the brain in response to hypoxia, and while reducing nmnat function was shown to severely compromise the survival of flies exposed to oxidative stress (Ali et al., 2011), the cytoprotective effects of Nmnat upregulation in hypoxia are largely unknown. Our studies here reveal that upregulation of Nmnat is important for maintaining the structural integrity of dendrites in neurons under hypoxia and further support the notion that a critical threshold of endogenous Nmnat is required for its proper protective function against cellular stress.
Autophagy as the major mode of hypoxia-induced dendrite degeneration
Our finding that impairment of autophagy suppresses hypoxia-induced dendrite degeneration in nmnat mutants implicates autophagy as a self-destructive mechanism in HI. Autophagy is well known to be induced in both neonatal and adult brain in response to HI (Adhami et al., 2006; Nitatori et al., 1995; Rami et al., 2008; Wen et al., 2008), but whether autophagy acts as a protective or destructive process remains controversial. While basal levels of autophagy are certainly required for neuronal survival (Hara et al., 2006; Komatsu et al., 2006), genetic and pharmacological evidence indicate that inhibiting autophagy may serve as an effective strategy to reduce HI-induced cellular damage (Koike et al., 2008; Puyal et al., 2009). Our study further argues against a neuroprotective role for autophagy in hypoxia as we found that class IV neurons lacking Atg1 or expressing RNAi transgenes targeting Atg5, Atg7, or Atg12 were largely unaffected when exposed to prolonged hypoxia. Our data are more consistent with a self-destructive role for autophagy in hypoxia as we found that blocking induction of autophagy or subsequent steps in autophagosome formation prevented the hypoxia-induced dendrite degeneration in nmnat mutants. The inability of p35 overexpression to block dendrite degeneration in nmnat mutants further suggests that this autophagic degradation occurs independently of caspase activity. Although autophagy and caspases are intimately linked in autophagic cell death, recent studies suggest that autophagy can also mediate cellular destruction in a caspase-independent manner (Denton et al., 2009).
It is interesting to note that induction of autophagy by overexpression of Atg1 was not sufficient to cause fragmentation of class IV dendrites in either wild-type or nmnat mutant larvae under normoxia and that Atg1 overexpression induced dendrite degeneration only in larvae exposed to hypoxic conditions, suggesting autophagy integrates with other stress response pathways to exert its effects (Kroemer et al., 2010). Furthermore, our finding that overexpression of Atg1 can induce dendrite degeneration in hypoxia-exposed wild-type larvae despite the presence of two functional copies of nmnat, but does not enhance degeneration phenotypes in nmnat heterozygous mutants under hypoxia, suggests that Nmnat acts upstream of autophagy to mitigate its effects on the cell in response to hypoxia. This is consistent with our results showing that overexpression of Nmnat fails to suppress dendrite degeneration induced by Atg1 expression. On the other hand, the inability of Atg1 mutants to suppress the dendrite maintenance defect of nmnat heterozygotes under normoxia suggests that Nmnat protects against dendritic regression and hypoxia-induced degeneration through mechanistically distinct pathways. Furthermore, while loss of Atg1 had no effect on the development or maintenance of class IV dendrites under normoxia, overexpression of Atg1 significantly enhanced the dendrite regression phenotype of nmnat heterozygotes, providing further evidence of separable roles for nmnat and autophagy in normal dendrite development.
How might Nmnat protect against hypoxia-induced dendrite degeneration?
Although the neuroprotective effects of Nmnat are well documented, the mechanisms by which Nmnat achieves this protection remain particularly elusive. Our studies provide a novel link between Nmnat and autophagy and suggest that Nmnat mitigates the self-destructive effects of autophagy induced by hypoxic stress. However, what exactly is the role of Nmnat in this context? The enzymatic activity of Nmnat has been shown to be important for neuroprotection (Coleman and Freeman, 2010), but recent studies point to an additional chaperone function for Nmnat in this role (Ali et al., 2011; Ali et al., 2012; Zhai et al., 2008). Transient cerebral ischemia can cause aggregates of ubiquitinated proteins (Hu et al., 2000), and stress response chaperones such as Hsp70 have been shown to reduce cell death induced by oxygen glucose deprivation (Giffard et al., 2004). This is particularly interesting in light of the recent finding that Nmnat can suppress tau-induced neurodegeneration by promoting the ubiquitination and clearance of toxic tau species (Ali et al., 2012). The recent identification of Nmnat as a stress response chaperone suggests that Nmnat may protect against hypoxia-induced cellular damage by preventing the aggregation of proteins. Although autophagy is a major mode of clearance of protein aggregates (Kirkin et al., 2009), we found that autophagy is largely dispensable for the maintenance of neuronal integrity under hypoxia. Autophagy may therefore be an adaptive response to increased protein aggregation in nmnat mutants under hypoxia leading to increased protein and organelle degradation eventually causing cellular self-destruction. This may further explain why we see dendrite degeneration only when autophagy is induced in hypoxia-exposed larvae.
Our studies further support the possibility that Nmnat protects against dendrite degeneration by maintaining low levels of ROS or by attenuating the deleterious effects of accumulated ROS induced by hypoxia. Our data are consistent with the finding that Nmnat can delay axonal degeneration in neurons treated with rotenone, which induces production of mitochondrial ROS, by preventing ROS accumulation in axons (Press and Milbrandt, 2008). The generation of ROS has been shown to be significantly increased in neurons in response to oxygen deprivation and plays a critical role in neuronal cell death (Abramov et al., 2007). Furthermore, overproduction of ROS is a common pathological feature of most age-related neurodegenerative disorders (Lin and Beal, 2006), as sustained high levels of ROS are known to cause mitochondrial dysfunction, cellular damage, and eventual cell death. In addition to its cytotoxic effects, ROS have also been shown to activate autophagy (Scherz-Shouval et al., 2007). While this is generally thought to promote cell survival in response to oxidative stress (Scherz-Shouval and Elazar, 2007), overactivation of autophagy may cause damage at the cellular level and may also lead to autophagic (type II) cell death. In this manner, Nmnat may function to gate ROS accumulation in response to hypoxia to moderate the levels of autophagy in the neuron.
In summary, our studies demonstrate a cytoprotective function for endogenous Nmnat in hypoxia and provide further evidence for its role as a stress response protein. Our studies further support the idea that targeting autophagy may serve as an effective strategy to reduce cellular damage associated with HI.
Supplementary Material
Acknowledgments
We thank Dr. Thomas Neufeld and the Bloomington Drosophila Stock Center (Indiana University) for Drosophila stocks, Yousuf Ali for technical advice, and the members of our laboratories for critical reading of the manuscript and helpful discussions. This work was supported by a postdoctoral research fellowship from the James & Esther King Biomedical Research Program (Y.W.), NIH NS64269 and the Pew Charitable Trust (R.G.Z.), and NIH NS072588 (M.D.K.).
Abbreviations
- CNS
central nervous system
- da
dendritic arborization
- HI
hypoxia-ischemia
- NAD
nicotinamide adenine dinucleotide
- PNS
peripheral nervous system
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramov AY, Scorziello A, Duchen MR. Three distinct mechanisms generate oxygen free radicals in neurons and contribute to cell death during anoxia and reoxygenation. J Neurosci. 2007;27:1129–1138. doi: 10.1523/JNEUROSCI.4468-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhami F, Liao G, Morozov YM, Schloemer A, Schmithorst VJ, Lorenz JN, Dunn RS, Vorhees CV, Wills-Karp M, Degen JL, Davis RJ, Mizushima N, Rakic P, Dardzinski BJ, Holland SK, Sharp FR, Kuan CY. Cerebral ischemia-hypoxia induces intravascular coagulation and autophagy. Am J Pathol. 2006;169:566–583. doi: 10.2353/ajpath.2006.051066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alers S, Loffler AS, Wesselborg S, Stork B. The incredible ULKs. Cell Commun Signal. 2012;10:7. doi: 10.1186/1478-811X-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali YO, McCormack R, Darr A, Zhai RG. Nicotinamide mononucleotide adenylyltransferase is a stress response protein regulated by the heat shock factor/hypoxia-inducible factor 1alpha pathway. J Biol Chem. 2011;286:19089–19099. doi: 10.1074/jbc.M111.219295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali YO, Ruan K, Zhai RG. NMNAT suppresses Tau-induced neurodegeneration by promoting clearance of hyperphosphorylated Tau oligomers in a Drosophila model of tauopathy. Hum Mol Genet. 2012;21:237–250. doi: 10.1093/hmg/ddr449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DL, Baehrecke EH. Growth arrest and autophagy are required for salivary gland cell degradation in Drosophila. Cell. 2007;131:1137–1148. doi: 10.1016/j.cell.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis. 2008;32:329–339. doi: 10.1016/j.nbd.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Chen Y, Klionsky DJ. The regulation of autophagy - unanswered questions. J Cell Sci. 2011;124:161–170. doi: 10.1242/jcs.064576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MP, Freeman MR. Wallerian degeneration, Wld(s), and Nmnat. Annu Rev Neurosci. 2010;33:245–267. doi: 10.1146/annurev-neuro-060909-153248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton D, Shravage B, Simin R, Mills K, Berry DL, Baehrecke EH, Kumar S. Autophagy, not apoptosis, is essential for midgut cell death in Drosophila. Curr Biol. 2009;19:1741–1746. doi: 10.1016/j.cub.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giffard RG, Xu L, Zhao H, Carrico W, Ouyang Y, Qiao Y, Sapolsky R, Steinberg G, Hu B, Yenari MA. Chaperones, protein aggregation, and brain protection from hypoxic/ischemic injury. J Exp Biol. 2004;207:3213–3220. doi: 10.1242/jeb.01034. [DOI] [PubMed] [Google Scholar]
- Gilley J, Coleman MP. Endogenous Nmnat2 is an essential survival factor for maintenance of healthy axons. PLoS Biol. 2010;8:e1000300. doi: 10.1371/journal.pbio.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- Haddad GG, Sun Y, Wyman RJ, Xu T. Genetic basis of tolerance to O2 deprivation in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1997a;94:10809–10812. doi: 10.1073/pnas.94.20.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad GG, Wyman RJ, Mohsenin A, Sun Y, Krishnan SN. Behavioral and Electrophysiologic Responses of Drosophila melanogaster to Prolonged Periods of Anoxia. J Insect Physiol. 1997b;43:203–210. doi: 10.1016/s0022-1910(96)00084-4. [DOI] [PubMed] [Google Scholar]
- Han C, Wang D, Soba P, Zhu S, Lin X, Jan LY, Jan YN. Integrins regulate repulsion-mediated dendritic patterning of Drosophila sensory neurons by restricting dendrites in a 2D space. Neuron. 2012;73:64–78. doi: 10.1016/j.neuron.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- Hay BA, Wolff T, Rubin GM. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994;120:2121–2129. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- Hori N, Carpenter DO. Functional and morphological changes induced by transient in vivo ischemia. Exp Neurol. 1994;129:279–289. doi: 10.1006/exnr.1994.1170. [DOI] [PubMed] [Google Scholar]
- Hsu M, Buzsaki G. Vulnerability of mossy fiber targets in the rat hippocampus to forebrain ischemia. J Neurosci. 1993;13:3964–3979. doi: 10.1523/JNEUROSCI.13-09-03964.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BR, Martone ME, Jones YZ, Liu CL. Protein aggregation after transient cerebral ischemia. J Neurosci. 2000;20:3191–3199. doi: 10.1523/JNEUROSCI.20-09-03191.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Price MT, Mosinger JL, Frierdich G, Labruyere J, Salles KS, Olney JW. Hypobaric-ischemic conditions produce glutamate-like cytopathology in infant rat brain. J Neurosci. 1989;9:1693–1700. doi: 10.1523/JNEUROSCI.09-05-01693.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JN, Kindy MS, Holtsberg FW, St Clair DK, Yen HC, Germeyer A, Steiner SM, Bruce-Keller AJ, Hutchins JB, Mattson MP. Mitochondrial manganese superoxide dismutase prevents neural apoptosis and reduces ischemic brain injury: suppression of peroxynitrite production, lipid peroxidation, and mitochondrial dysfunction. J Neurosci. 1998;18:687–697. doi: 10.1523/JNEUROSCI.18-02-00687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ME, Shrestha BR, Blazeski R, Mason CA, Grueber WB. Integrins establish dendrite-substrate relationships that promote dendritic self-avoidance and patterning in Drosophila sensory neurons. Neuron. 2012;73:79–91. doi: 10.1016/j.neuron.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- Kirkin V, McEwan DG, Novak I, Dikic I. A role for ubiquitin in selective autophagy. Mol Cell. 2009;34:259–269. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Niinobe M, Mikoshiba K, Hata R, Ueda H, Handa N, Fukunaga R, Isaka Y, Kimura K, et al. Microtubule-associated protein 2 as a sensitive marker for cerebral ischemic damage--immunohistochemical investigation of dendritic damage. Neuroscience. 1989;31:401–411. doi: 10.1016/0306-4522(89)90383-7. [DOI] [PubMed] [Google Scholar]
- Knoferle J, Koch JC, Ostendorf T, Michel U, Planchamp V, Vutova P, Tonges L, Stadelmann C, Bruck W, Bahr M, Lingor P. Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc Natl Acad Sci U S A. 2010;107:6064–6069. doi: 10.1073/pnas.0909794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, Waguri S, Kawahara N, Kuida K, Nagata S, Kominami E, Tanaka K, Uchiyama Y. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol. 2008;172:454–469. doi: 10.2353/ajpath.2008.070876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CT, Jan LY, Jan YN. Dendrite-specific remodeling of Drosophila sensory neurons requires matrix metalloproteases, ubiquitin-proteasome, and ecdysone signaling. Proc Natl Acad Sci U S A. 2005;102:15230–15235. doi: 10.1073/pnas.0507393102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Ljungberg MC, Ali YO, Zhu J, Wu CS, Oka K, Zhai RG, Lu HC. CREB-activity and nmnat2 transcription are down-regulated prior to neurodegeneration, while NMNAT2 over-expression is neuroprotective, in a mouse model of human tauopathy. Hum Mol Genet. 2012;21:251–267. doi: 10.1093/hmg/ddr492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magni G, Amici A, Emanuelli M, Orsomando G, Raffaelli N, Ruggieri S. Structure and function of nicotinamide mononucleotide adenylyltransferase. Curr Med Chem. 2004;11:873–885. doi: 10.2174/0929867043455666. [DOI] [PubMed] [Google Scholar]
- Matesic DF, Lin RC. Microtubule-associated protein 2 as an early indicator of ischemia-induced neurodegeneration in the gerbil forebrain. J Neurochem. 1994;63:1012–1020. doi: 10.1046/j.1471-4159.1994.63031012.x. [DOI] [PubMed] [Google Scholar]
- Nakka VP, Gusain A, Mehta SL, Raghubir R. Molecular mechanisms of apoptosis in cerebral ischemia: multiple neuroprotective opportunities. Mol Neurobiol. 2008;37:7–38. doi: 10.1007/s12035-007-8013-9. [DOI] [PubMed] [Google Scholar]
- Nitatori T, Sato N, Waguri S, Karasawa Y, Araki H, Shibanai K, Kominami E, Uchiyama Y. Delayed neuronal death in the CA1 pyramidal cell layer of the gerbil hippocampus following transient ischemia is apoptosis. J Neurosci. 1995;15:1001–1011. doi: 10.1523/JNEUROSCI.15-02-01001.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Bateman MC, Goldberg MP. Rapid alterations in dendrite morphology during sublethal hypoxia or glutamate receptor activation. Neurobiol Dis. 1996;3:215–227. doi: 10.1006/nbdi.1996.0022. [DOI] [PubMed] [Google Scholar]
- Press C, Milbrandt J. Nmnat delays axonal degeneration caused by mitochondrial and oxidative stress. J Neurosci. 2008;28:4861–4871. doi: 10.1523/JNEUROSCI.0525-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puyal J, Vaslin A, Mottier V, Clarke PG. Postischemic treatment of neonatal cerebral ischemia should target autophagy. Ann Neurol. 2009;66:378–389. doi: 10.1002/ana.21714. [DOI] [PubMed] [Google Scholar]
- Rami A, Langhagen A, Steiger S. Focal cerebral ischemia induces upregulation of Beclin 1 and autophagy-like cell death. Neurobiol Dis. 2008;29:132–141. doi: 10.1016/j.nbd.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Rusten TE, Lindmo K, Juhasz G, Sass M, Seglen PO, Brech A, Stenmark H. Programmed autophagy in the Drosophila fat body is induced by ecdysone through regulation of the PI3K pathway. Dev Cell. 2004;7:179–192. doi: 10.1016/j.devcel.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Ryoo HD, Baehrecke EH. Distinct death mechanisms in Drosophila development. Curr Opin Cell Biol. 2010;22:889–895. doi: 10.1016/j.ceb.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherz-Shouval R, Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol. 2007;17:422–427. doi: 10.1016/j.tcb.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RC, Juhasz G, Neufeld TP. Direct induction of autophagy by Atg1 inhibits cell growth and induces apoptotic cell death. Curr Biol. 2007;17:1–11. doi: 10.1016/j.cub.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott RC, Schuldiner O, Neufeld TP. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell. 2004;7:167–178. doi: 10.1016/j.devcel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Shen W, Ganetzky B. Autophagy promotes synapse development in Drosophila. J Cell Biol. 2009;187:71–79. doi: 10.1083/jcb.200907109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama Y, Koike M, Shibata M. Autophagic neuron death in neonatal brain ischemia/hypoxia. Autophagy. 2008;4:404–408. doi: 10.4161/auto.5598. [DOI] [PubMed] [Google Scholar]
- Verghese PB, Sasaki Y, Yang D, Stewart F, Sabar F, Finn MB, Wroge CM, Mennerick S, Neil JJ, Milbrandt J, Holtzman DM. Nicotinamide mononucleotide adenylyl transferase 1 protects against acute neurodegeneration in developing CNS by inhibiting excitotoxic-necrotic cell death. Proc Natl Acad Sci U S A. 2011;108:19054–19059. doi: 10.1073/pnas.1107325108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang QJ, Ding Y, Kohtz DS, Mizushima N, Cristea IM, Rout MP, Chait BT, Zhong Y, Heintz N, Yue Z. Induction of autophagy in axonal dystrophy and degeneration. J Neurosci. 2006;26:8057–8068. doi: 10.1523/JNEUROSCI.2261-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Y, Parrish JZ, He R, Zhai RG, Kim MD. Nmnat exerts neuroprotective effects in dendrites and axons. Mol Cell Neurosci. 2011;48:1–8. doi: 10.1016/j.mcn.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen YD, Sheng R, Zhang LS, Han R, Zhang X, Zhang XD, Han F, Fukunaga K, Qin ZH. Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy. 2008;4:762–769. doi: 10.4161/auto.6412. [DOI] [PubMed] [Google Scholar]
- Wingrove JA, O’Farrell PH. Nitric oxide contributes to behavioral, cellular, and developmental responses to low oxygen in Drosophila. Cell. 1999;98:105–114. doi: 10.1016/S0092-8674(00)80610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- Yang Y, Fukui K, Koike T, Zheng X. Induction of autophagy in neurite degeneration of mouse superior cervical ganglion neurons. Eur J Neurosci. 2007;26:2979–2988. doi: 10.1111/j.1460-9568.2007.05914.x. [DOI] [PubMed] [Google Scholar]
- Yao J, Munson KM, Webb WW, Lis JT. Dynamics of heat shock factor association with native gene loci in living cells. Nature. 2006;442:1050–1053. doi: 10.1038/nature05025. [DOI] [PubMed] [Google Scholar]
- Zelzer E, Wappner P, Shilo BZ. The PAS domain confers target gene specificity of Drosophila bHLH/PAS proteins. Genes Dev. 1997;11:2079–2089. doi: 10.1101/gad.11.16.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai RG, Cao Y, Hiesinger PR, Zhou Y, Mehta SQ, Schulze KL, Verstreken P, Bellen HJ. Drosophila NMNAT maintains neural integrity independent of its NAD synthesis activity. PLoS Biol. 2006;4:2336–2348. doi: 10.1371/journal.pbio.0040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai RG, Zhang F, Hiesinger PR, Cao Y, Haueter CM, Bellen HJ. NAD synthase NMNAT acts as a chaperone to protect against neurodegeneration. Nature. 2008;452:887–891. doi: 10.1038/nature06721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Xue J, Lai JC, Schork NJ, White KP, Haddad GG. Mechanisms underlying hypoxia tolerance in Drosophila melanogaster: hairy as a metabolic switch. PLoS Genet. 2008;4:e1000221. doi: 10.1371/journal.pgen.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Wang X, Xu F, Bahr BA, Shibata M, Uchiyama Y, Hagberg H, Blomgren K. The influence of age on apoptotic and other mechanisms of cell death after cerebral hypoxia-ischemia. Cell Death Differ. 2005;12:162–176. doi: 10.1038/sj.cdd.4401545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.